 Open Access
Open Access
ARTICLE
Early GLP-1 Agonist Use and Cancer Risk in Type 2 Diabetes: A Real-World Data Cohort Study
1 Institute of Medicine, Chung Shan Medical University, Taichung, 402, Taiwan
2 Department of Emergency Medicine, School of Medicine, Chung Shan Medical University, Taichung, 402, Taiwan
3 Department of Emergency Medicine, Chung Shan Medical University Hospital, Taichung, 402, Taiwan
4 Department of Internal Medicine, Zuoying Armed Forces General Hospital, Kaohsiung, 813, Taiwan
5 Department of Emergency Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei, 114, Taiwan
6 Graduate Institute of Aerospace and Undersea Medicine, College of Biomedical Sciences, National Defense Medical University, Taipei, 114, Taiwan
7 Department of Orthopedic Surgery, Chung Shan Medical University Hospital, Taichung, 402, Taiwan
8 School of Medicine, Chung Shan Medical University, Taichung, 402, Taiwan
9 Department of Medical Research, Chung Shan Medical University Hospital, Taichung, 402, Taiwan
* Corresponding Authors: Yu-Hsun Wang. Email: ; Chao-Bin Yeh. Email:
# These authors contributed equally to this work
(This article belongs to the Special Issue: Therapeutic Challenges in Targeting Cell Death)
Oncology Research 2026, 34(1), 12 https://doi.org/10.32604/or.2025.072875
Received 05 September 2025; Accepted 29 October 2025; Issue published 30 December 2025
Abstract
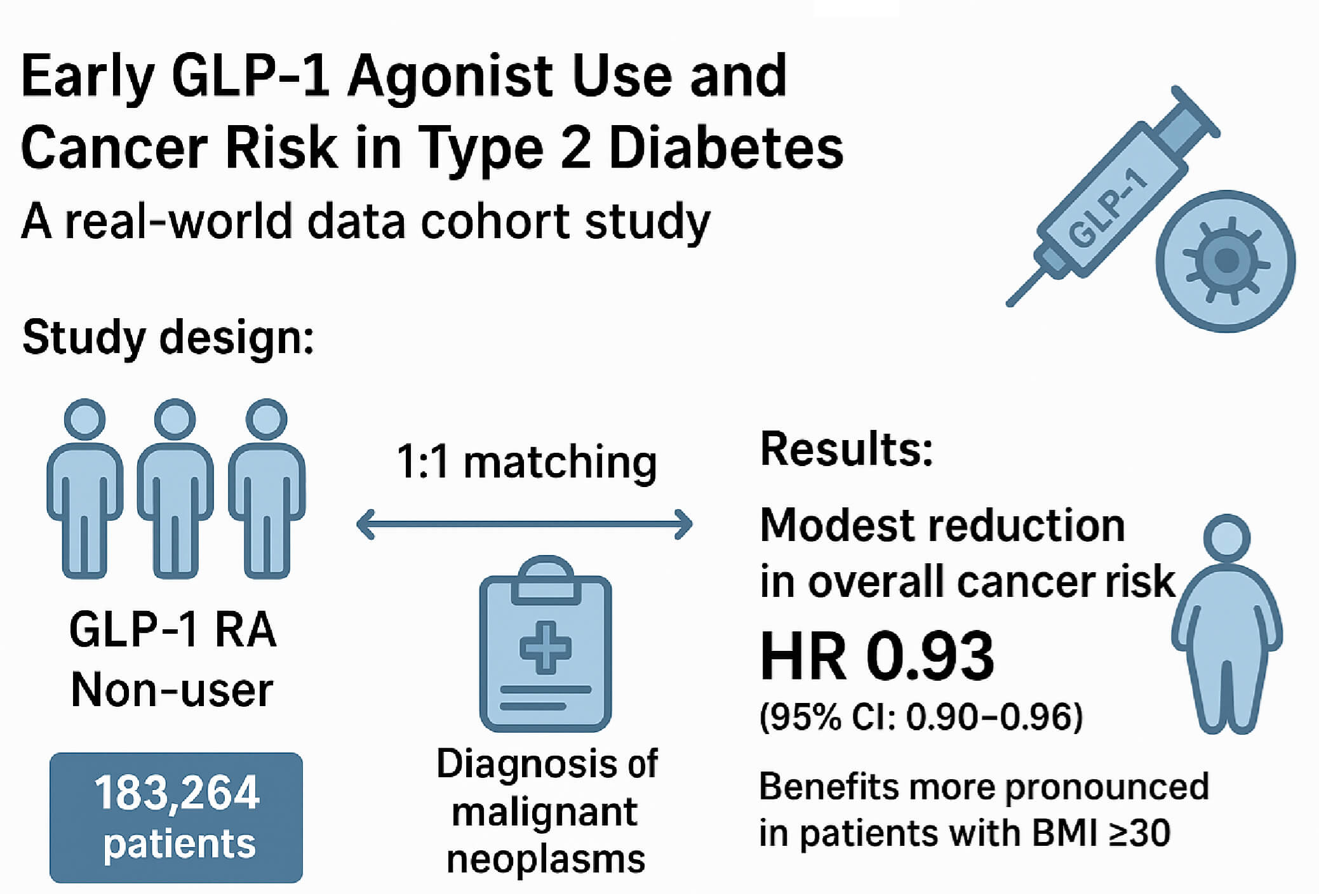
Background: To determine whether initiating a glucagon-like peptide-1 receptor agonist (GLP-1 RA) within 3 months of type 2 diabetes (T2DM) diagnosis alters the subsequent risk of overall and site-specific cancer and whether this association differs by baseline body-mass index (BMI). Methods: This retrospective cohort study used electronic health records from the TriNetX U.S. research network. Adults aged 20 years or older diagnosed with T2DM between 2016 and 2024 were included if they received any hypoglycemic agents within 3 months before and after diagnosis. Following 1:1 propensity score matching, both the GLP-1 RA user and non-user groups included 183,264 patients. The study outcome was defined as a diagnosis of malignant neoplasms. Hazard ratios (HRs) for overall and site-specific cancer risk were estimated using Cox proportional hazards models. Kaplan–Meier analysis and stratified analysis by BMI were performed. Results: Early GLP-1 RA use demonstrated a modest but significant association with reduced overall cancer risk (HR 0.93; 95% CI: 0.90–0.96). Reduced risks were noted for cancers of the digestive (HR 0.81), respiratory (HR 0.66), and female genital (HR 0.87) systems. In stratified analysis, benefits were more pronounced in patients with BMI ≥ 30, particularly for pancreatic and colorectal cancers. Conclusion: Early initiation of GLP-1 receptor agonists in patients with diagnosed T2DM was associated with a modest reduction in overall cancer risk, particularly among individuals with obesity. These findings highlight the dual metabolic and oncologic value of prompt GLP-1 RA therapy.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2026 The Author(s). Published by Tech Science Press.
Copyright © 2026 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools