 Open Access
Open Access
ARTICLE
A Th2-score in the tumor microenvironment as a predictive biomarker of response to Bacillus Calmette Guérin in patients with non-muscle invasive bladder carcinoma: A retrospective study
1 Urology Department, Instituto Alexander Fleming, Ciudad Autónoma de Buenos Aires, 1426, Argentina
2 Immunohistochemistry Department, Instituto Alexander Fleming, Ciudad Autónoma de Buenos Aires, 1426, Argentina
3 Centro de Investigaciones Oncológicas, Fundación Cáncer FUCA, Ciudad Autónoma de Buenos Aires, 1426, Argentina
4 Urology Department, MD Anderson Cancer Center, Houston, TX, 77030, USA
5 Pathology Department, Instituto Alexander Fleming, Ciudad Autónoma de Buenos Aires, 1426, Argentina
* Corresponding Author: María Marcela Barrio,
Oncology Research 2023, 31(2), 207-220. https://doi.org/10.32604/or.2023.028163
Received 08 December 2022; Accepted 15 February 2023; Issue published 10 April 2023
Abstract
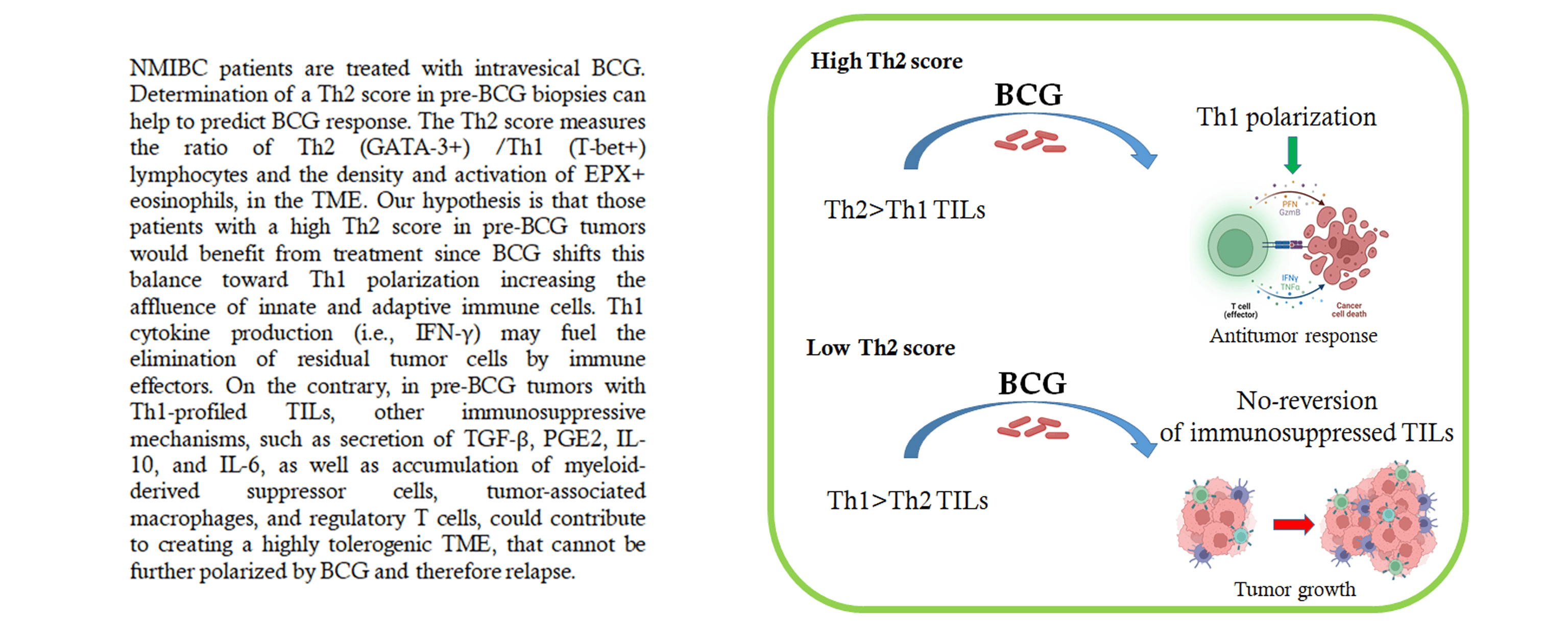
Intravesical Bacillus Calmette Guerin (BCG) is the gold standard therapy for intermediate/high-risk nonmuscle invasive bladder cancer (NMIBC). However, the response rate is ~60%, and 50% of non-responders will progress to muscle-invasive disease. BCG induces massive local infiltration of inflammatory cells (Th1) and ultimately cytotoxic tumor elimination. We searched for predictive biomarker of BCG response by analyzing tumor-infiltrating lymphocyte (TIL) polarization in the tumor microenvironment (TME) in pre-treatment biopsies. Pre-treatment biopsies from patients with NMIBC who received adequate intravesical instillation of BCG (n = 32) were evaluated retrospectively by immunohistochemistry. TME polarization was assessed by quantifying the T-Bet+ (Th1) and GATA-3+ (Th2) lymphocyte ratio (G/T), and the density and degranulation of EPX+ eosinophils. In addition, PD-1/ PD-L1 staining was quantified. The results correlated with BCG response. In most non-responders, Th1/Th2 markers were compared in pre-and post-BCG biopsies. ORR was 65.6% in the study population. BCG responders had a higher G/T ratio and a greater number of degranulated EPX+ cells. Variables combined into a Th2-score showed a significant association with higher scores in responders (p = 0.027). A Th2-score cut-off value >48.1 allowed discrimination of responders with 91% sensitivity but lower specificity. Relapse-free survival was significantly associated with the Th2-score (p = 0.007). In post-BCG biopsies from recurring patients, TILs increased Th2-polarization, probably reflecting BCG failure to induce a pro-inflammatory status and, thus, a lack of response. PD-L1/PD-1 expression was not associated with the response to BCG. Our results support the hypothesis that a preexisting Th2-polarized TME predicts a better response to BCG, assuming a reversion to Th1 polarization and antitumor activity.Graphic Abstract
Keywords
Non–muscle invasive bladder cancer (NMIBC) represents 70% of all initial bladder cancer diagnoses [1,2]. Some high-risk patients (high-grade [HG] pT1 or carcinoma in situ [Cis]) [3] respond to bladder-conservative treatments and behave in a non-lethal fashion, while others are aggressive and tend to progress to muscle invasion and even metastatic disease. pT1 tumors are mostly HG and progress in >50% of cases; deaths occur in 25% of patients during the first five years [3]. Cis is mostly concomitant, with increasing recurrence rates from 43% to 73% [4]. Even if Cis is primary or concomitant, 50% of patients progress to muscle invasion and 20% will ultimately die of metastases if a radical cystectomy is not performed [5]. Intravesical administration of live-attenuated Bacillus Calmette-Guérin (BCG) is the primary adjuvant treatment for high-risk NMIBC patients [6]. Nonetheless, the response rate (RR) to BCG is about 60%, with a 5-year recurrence rate of 30%–40% [7]. Moreover, for those patients presenting either pT1 or Cis, or both tumors, who do not respond to BCG, the risk of progression to invasive disease may reach 50% [8]. Thus, there is still a strong need to find appropriate predictive biomarkers of response to BCG that may help the selection of patients with a low chance of response, before or early after treatment starts, to offer them alternative therapies. Although the exact mechanism of BCG antitumor activity is not fully understood, it is recognized that BCG acts as a localized immunomodulatory agent promoting Th1 immune polarization, and its efficacy must be related to the immune response elicited in the bladder [9].
GATA binding protein 3 (GATA-3) is a transcription factor that is critical for T cell development and Th2 differentiation [10]. T-box transcription factor 21 (T-Bet) is mostly expressed in CD4+ Th1 cells, and its expression is induced by a combination of T cell receptors and IFN-γ signaling [11]. In addition, T-Bet promotes Th1 polarization by preventing GATA-3-mediated Th2 cell development [12].
In the past, eosinophils were inaccurately considered destructive end-stage effector cells (helminth infections and allergy/asthma) due to the release of toxic cationic granule proteins and the production of reactive oxygen species. More recently, eosinophils have been shown to play a fundamental role in remodeling/repair activities, and more importantly, to modulate local immunity [13].
An emerging concept relates to the prognostic and/or predictive value of Th1/Th2 polarization of tumor-infiltrating cells (TILs) before treatment, which may affect the response to BCG immunotherapy. Interestingly, in patients with Cis treated with BCG induction alone [14], those who responded to BCG showed an increased density of intratumor Th2 cells and a higher Th2/Th1 ratio prior to treatment, suggesting that BCG would be effective only when the tumor microenvironment (TME) has shifted from Th2 to Th1.
Programmed death ligand 1 (PD-L1) expressed by tumor and immune cells in the TME suppresses antitumor immune responses and promotes tumor progression [15]. However, PD-L1+ TILs have been correlated with improved overall survival in patients with metastatic urothelial cancer [16]. In a recent study, no association of BCG response with peritumoral PD-L1 expression in pretreatment biopsies was reported [17]. On the contrary, high PD-L1 expression in BCG-induced granulomas has been associated with resistance to therapy in urothelial carcinomas [18]. These data suggest that high numbers of PD-L1+ cells in the bladder could suppress the effectiveness of BCG, a hypothesis also supported by the recent demonstration that combined BCG and anti-PD-L1 treatment increased antitumor immunity in an immunocompetent orthotopic rat bladder cancer model [19]. Thus, the PD-1/PD-L1 axis could be involved in the antitumor immune response of NMIBC.
The aim of our study was to identify potential biomarkers to predict the response to BCG in a high-risk population of NMIBC patients, including those with HG pTa, pT1, and Cis tumors, focusing on Th1/Th2 lymphocyte polarization in the TME. We also explored a possible association of PD-1/PD-L1 expression in NMIBC biopsies with BCG response.
Patients and BCG immunotherapy
This retrospective review of the bladder cancer registry and biopsies from the Urology Department of Instituto Alexander Fleming (Buenos Aires, Argentina) included patients who were diagnosed between 2007 and 2019. Archive tumor samples were analyzed in this study, and all patients provided written informed consent for the use of biopsies for research purposes at the time of surgery. Patients with NMIBC were stratified according to the EAU risk stratification guidelines [20]. We analyzed the initial bladder tumor biopsy obtained via complete transurethral resection (TURB) and post-treatment biopsies when available. Re-TURB was performed when pT1 NMIBC was detected during the first TURB. The patients did not receive any previous treatment before surgery. Adjuvant treatment started 3–4 weeks after TURB and consisted of a standard 6-dose induction course plus a 3-dose maintenance course at 3, 6, 12, 18, 24, 30, and 36 months of BCG (120 mg of Danish strain 1331 SSI) [21]. The patients did not receive any other intravesical therapy during or after TURB. Follow-up cystoscopy, cytology, and TC scans were performed according to the guidelines [20]. Patients were classified as non-responders (NR) if they had a positive biopsy result for bladder carcinoma after BCG induction plus one maintenance course (adequate BCG). BCG responders (R) were defined as those without any recurrence or evidence of disease based on follow-up cystoscopy, urinary cytology, or re-biopsy. Patients with a history of immunosuppressive drug treatment or other possible confounding factors were excluded.
The biopsies were fixed in formalin and paraffin-embedded. Paraffin blocks containing sufficient material were selected for immunohistochemical (IHC) staining with the following anti-human monoclonal Abs: eosinophil peroxidase EPX-mAb (clone MM 25-82.2, kindly provided by Mayo Clinic, Scottsdale, USA), GATA-3 (clone L50-823, Cell Marque, Rocklin, USA), T-Bet (clone EPR9302 RabMab, Abcam, Cambridge, UK), and desmin to confirm muscle indemnity (clone D33, Dako, Glostrup, Denmark). The sections were stained with anti-human PD-L1 (clone SP263, Ventana, Bend USA) and anti-human PD-1 (clone NAT105, Abcam, Cambridge, UK). All markers, except for PD-L1, were determined using standardized automated protocols for LEICA BOND MAX II. PD-L1 expression was determined using the Benchmark ULTRA (Roche, Basel, Switzerland). Sections were examined by optical microscopy (Olympus BX40 microscope, DP2-BSW software), and digitalized images were analyzed using ImageJ software (NIH).
For each immune population, IHC staining was quantified as the average of positive cells in eight high-magnification fields (400×) covering a 1.2 mm2 area. EPX+ eosinophils were quantified at the maximum focus of eosinophilic infiltration (Eo count). Also, EPX-mAb staining was used to identify the maximum level of eosinophilic degranulation (Eodgn). A numerical value was given to each level of eosinophil degranulation based on the normal distribution of the data as follows: 0 = no degranulation; 1 = 1a, small sharp granules or 1b, granules with fuzzy edges; and 2 = multiple granules of imprecise edges far from the cell body. GATA-3+ and T-Bet+ lymphocytes were quantified at the maximum focus of mononuclear cell infiltration. The ratio of Th2 polarized (GATA-3+)/Th1 polarized (T-Bet+) lymphocytes (G/T) was calculated. A Th2-score was defined as:
PD-L1 expression was quantitated by the combined positive score (CPS), which is the number of PD-L1+ cells (tumor cells, lymphocytes, macrophages) divided by the total number of tumor cells, ×100). Percentages >1% were considered positive for PD-L1. To control for inter-observer variability, all IHC counts were performed in duplicate by two double-blinded researchers. Researcher 1 counts were used because the difference between counts was <10%.
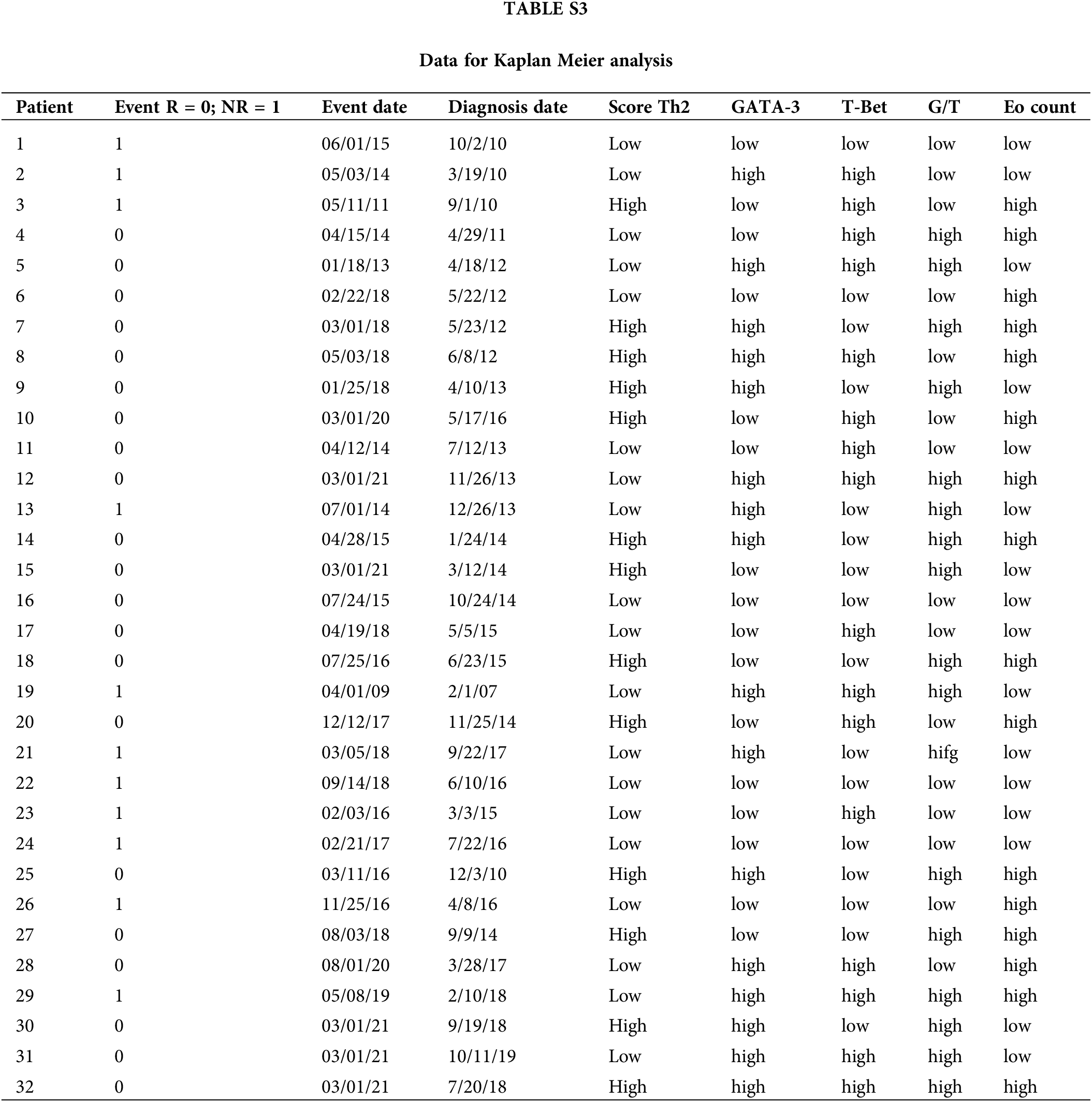
Independent categorical variables were described as percentages and compared using Fisher’s exact test or the chi-square test. Comparisons between R and NR patients were performed using the nonparametric Mann–Whitney U test. Spearman’s correlation coefficient was used to identify associations between continuous variables. GraphPad Prism v10 (GraphPad Software Inc., La Jolla, CA, USA) was used for all statistical analyses. The predictive power of biomarker levels on the BCG response was evaluated by plotting receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC). Recurrence-free survival (RFS) was compared using the Kaplan–Meier method, stratifying patients by the Th2-score and other variables with the log-rank test using R. The p values were two-sided, and p < 0.05 was considered statistically significant.
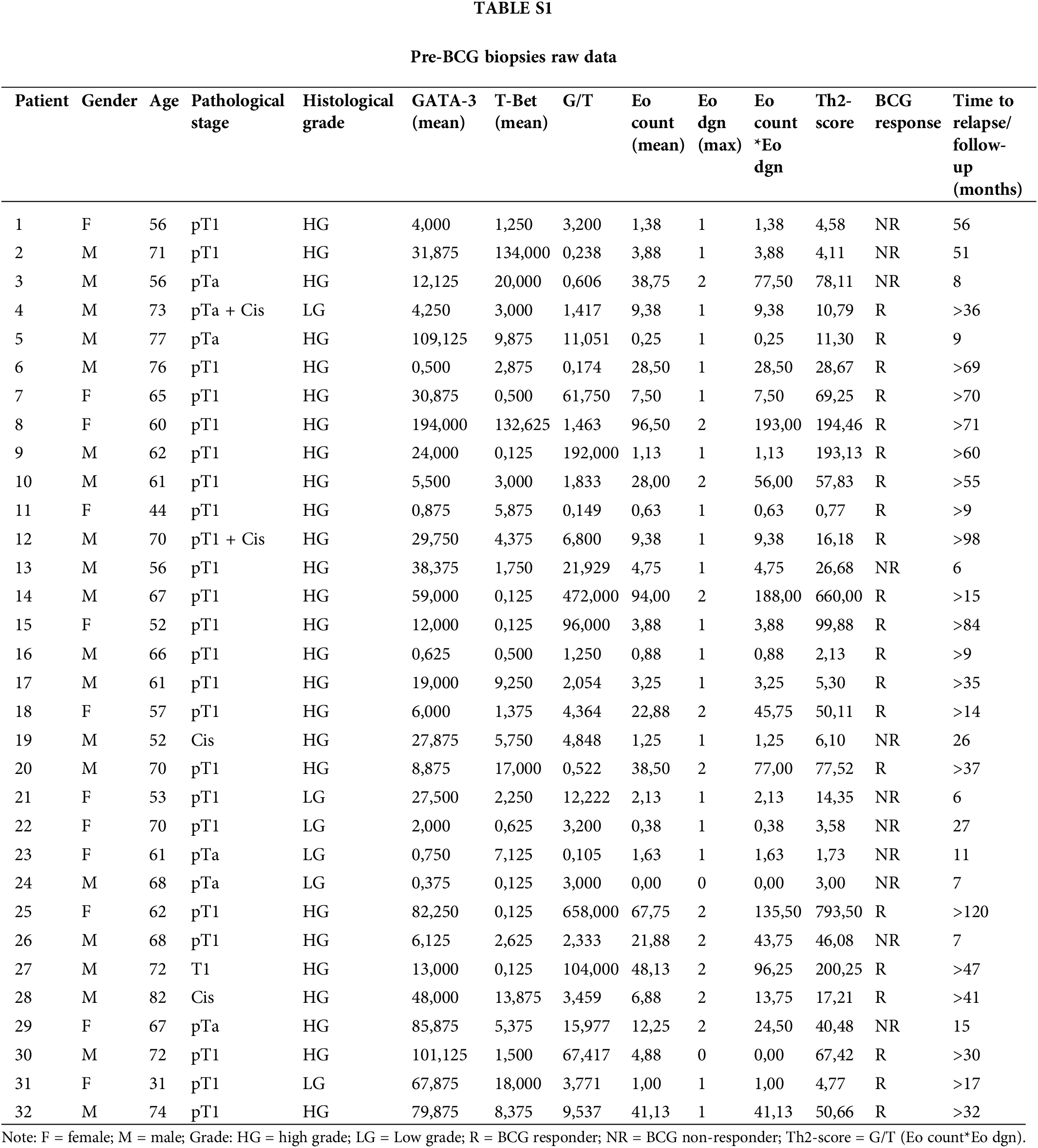
In total, 32 patients were included in this retrospective study (Table 1). The mean age at diagnosis was 64.1 years. The histopathological evaluation confirmed primary high-risk NMIBC according to the EAU guidelines. Six patients showed LG, while 26 (81.2%) had HG cancer, according to the WHO 2004/2016 classification. Most patients had pT1 (24/32) and pTa tumors (6/32). Two patients had only Cis, and two patients had pT1HG + Cis and pTa LG + Cis. One patient had concurrent prostate cancer, which was treated with radical prostatectomy at the time of NMIBC diagnosis.
The median follow-up period for the patients without recurrence was 37 months. The pathological finding at post-BCG control was at pT0 in 24 patients. Overall response rate (ORR) to BCG treatment was 65.6%, as 21/32 patients remained recurrence-free for at least 20 months. BCG NR had a median time to recurrence of 15 months; however, 7/11 (63.3%) recurred before 12 months. ORR was not significantly associated with sex, age, tumor stage, or histological grade in the study population. Three of the 11 patients who experienced BCG failure progressed to muscle-invasive disease.
Tumor-infiltrating lymphocytes profile
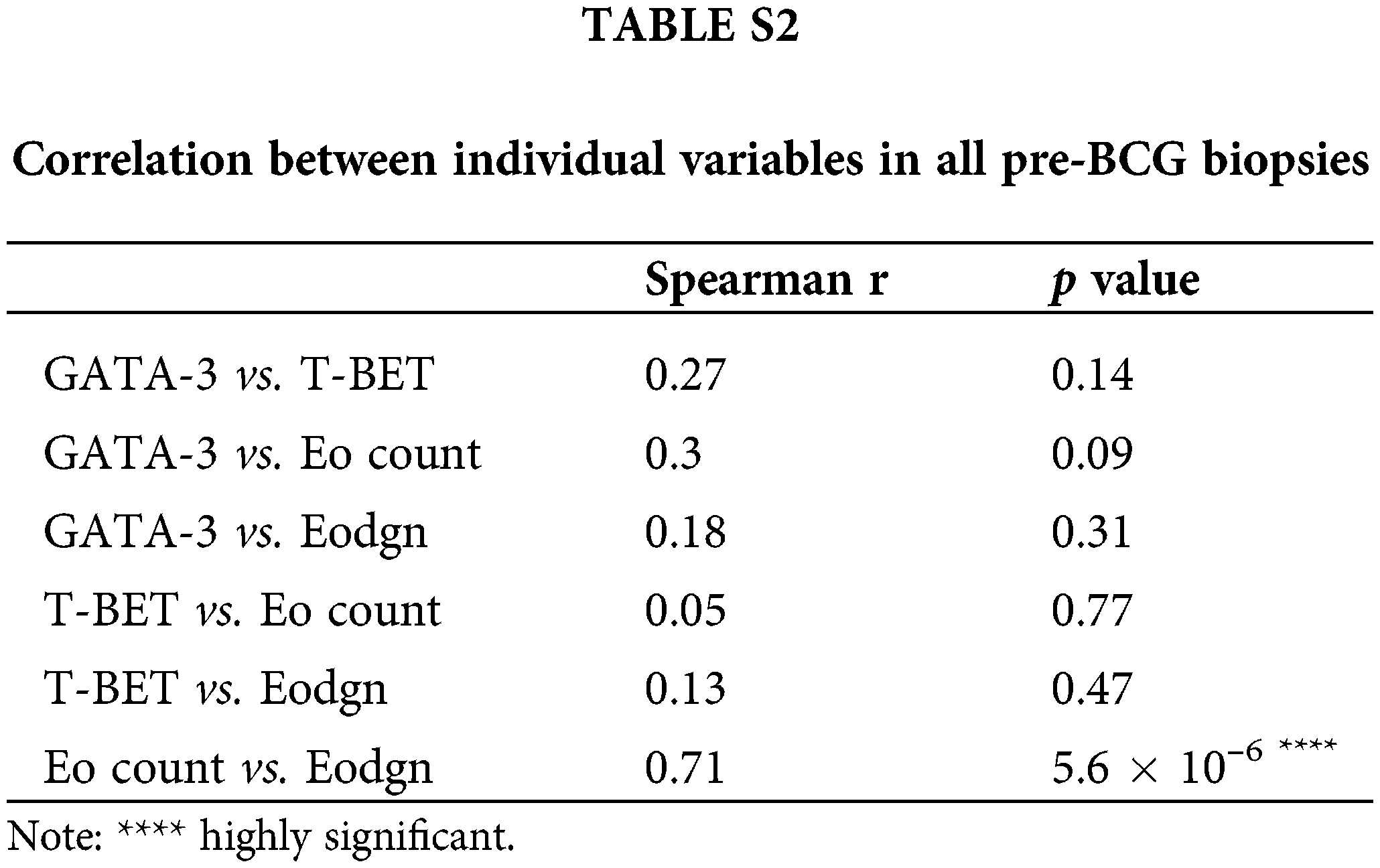
The expression of T-Bet and GATA-3 was detected in the TILs of all pre-BCG treatment biopsies (Fig. 1A). Additionally, eosinophils infiltrating the tumor area were detected in 31/32 biopsies (median Eo count 5.8; range 0–96.5). It was possible to evaluate the extent of Eo degranulation (Eodgn), as a readout of its activation in the TME, because EPX+ granules were evident. A significantly higher infiltration of Th2 cells than Th1 cells was identified, with a median (range) number of total GATA-3+ and T-Bet+ cells of 21.5 (0.5–194) and 2.9 (0.125–134), respectively (p < 0.0005) (Fig. 1B and Suppl. Table S1).
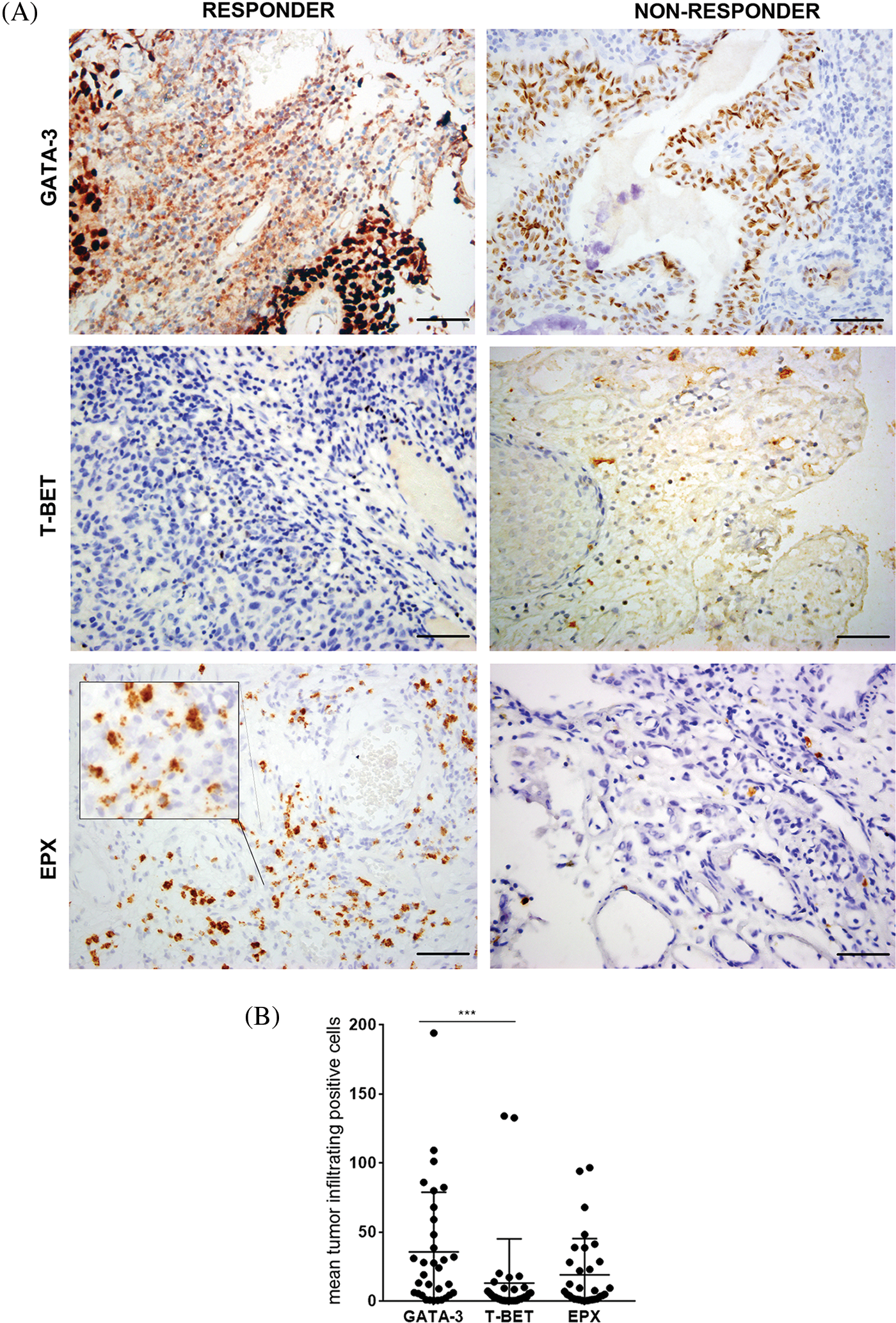
Figure 1: Expression of GATA-3, T-Bet, and EPX in all NMIBC pre-BCG biopsies. (A) Representative pictures of immunohistochemistry staining for GATA-3, T-Bet, and EPX in pre-BCG NMIBC biopsies of R and NR patients. Original magnification 400×. Scale bar = 50 μm. In the case of EPX staining, an inset shows Eodgn in detail. (B) Quantitation of GATA-3+, T-Bet+ TILs, and EPX+ eosinophils EPX in all pre-BCG NMIBC biopsies analyzed. ***Highly significant, p = 0.0005, Mann–Whitney test.
No significant correlation was found between the total number of GATA-3+ and T-Bet+ cells, GATA-3+ and Eo counts, or Eodgn or T-Bet+ and EPX+ cells or Eodgn. Only Eo counts correlated with Eodgn, suggesting that when eosinophils were abundant in the TME, most were activated (Suppl. Table S2).
TIL expression of GATA-3, T-Bet, and EPX (Eo counts or Eodgn) was compared in biopsies from BCG-R and BCG-NR patients. The BCG response was associated with a higher number of GATA-3+ and EPX+ cells, a higher GATA-3/T-Bet ratio (G/T), and a lower number of T-Bet+ cells, although these differences were not statistically significant (Fig. 1, Suppl. Fig. S1).
Th2-score predicts BCG response
When we combined the G/T ratio (TIL polarization) and the product of Eo counts and Eodgn, the BCG response was significantly associated with a higher Th2-score (p = 0.027) (Fig. 2A).
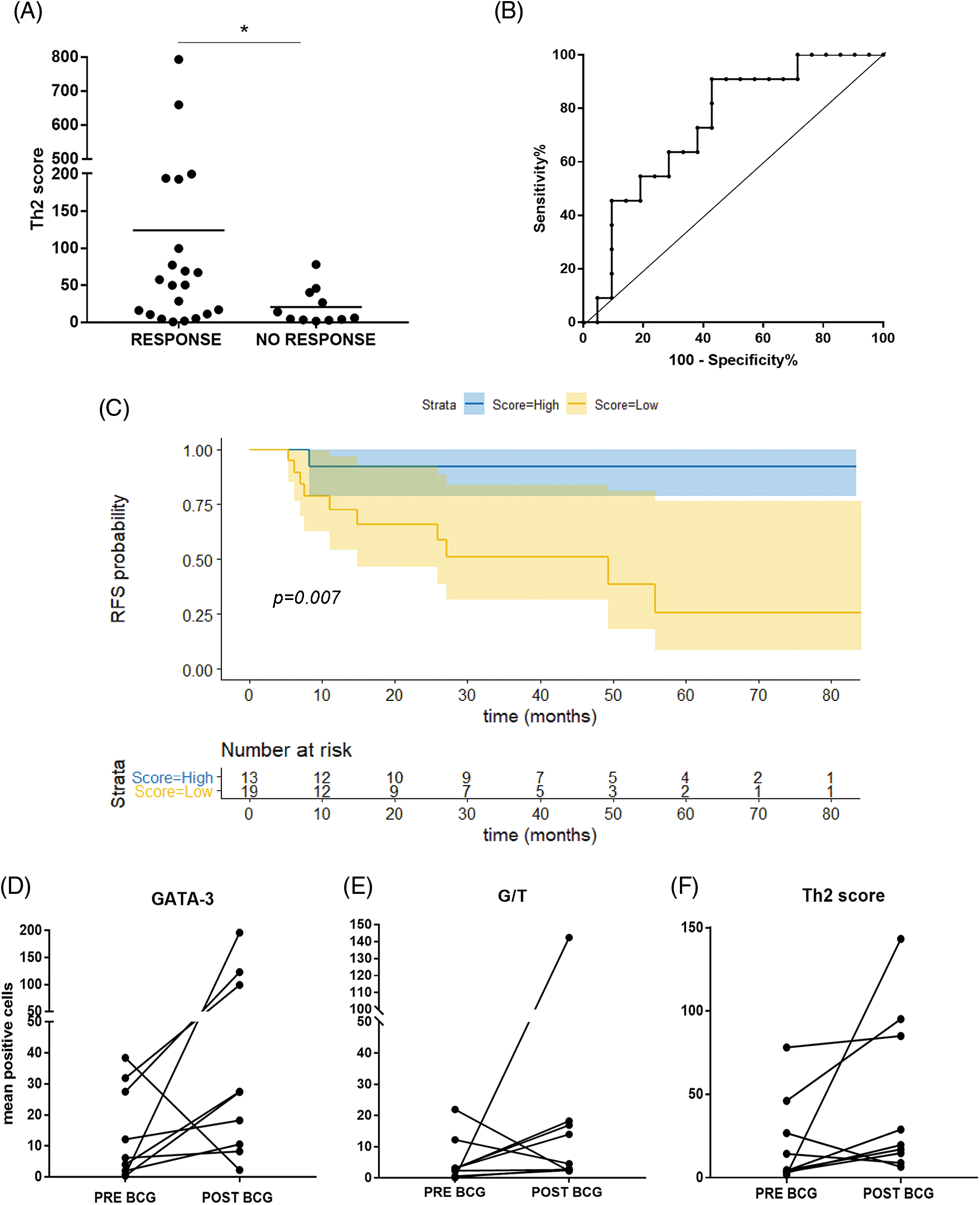
Figure 2: Th2-score in the TME can predict response to BCG in NMIBC patients. (A) Th2-score was higher in BCG-Responder than in BCG-NR non-responder patients (*p = 0.027, Mann–Whitney test); (B) ROC curve for the Th2-score allows discrimination of BCG-R patients (≥48.1) with 91% sensitivity and 57% specificity. (C) Kaplan Meier curve of recurrence-free survival (RFS) in months. Patients were stratified by Th2-score as low or high, based on the ROC curve cut-off (<48.1 = low count; ≥48.1 = high count) (p = 0.007, log-rank test (R); (D–F) Comparison of GATA-3 mean counts, G/T, and Th2-score in pre- and post-BCG biopsies (relapses), respectively.
The AUC of the pre-BCG Th2-score was 0.74 (95% CI 0.56–0.91; p = 0.028). Since the predictive score was intended to identify true R and thus offer BCG treatment with high chances of success, a cutoff value for the Th2-score was set at >48.1, allowing discrimination of R with 91% sensitivity; however, specificity was 57.1% (PPV = 0.526, NPV = 0.923) (Fig. 2B).
For survival analyses, patients were divided into two groups (low vs. high counts) based on the median values of the different variables analyzed (65.5 for age; 21.5 for GATA-3+; 2.9 for T-Bet+; 3.6 for G/T and 5.9; EPX+) (Suppl. Table S3). No significant association was found for the other individual variables (GATA-3+, T-Bet+ cells, or G/T). Patients with high Eo counts tended to have a longer RFS than those with low Eo counts (p = 0.052) (Suppl. Fig. S2). Then, the patients were split into “high-Th2” and “low-Th2” score subgroups according to the cutoff value (48.1). Patients with a high Th2-score on pre-BCG biopsy had a significantly prolonged RFS compared with those with a low Th2-score (p = 0.007) (Fig. 2C).
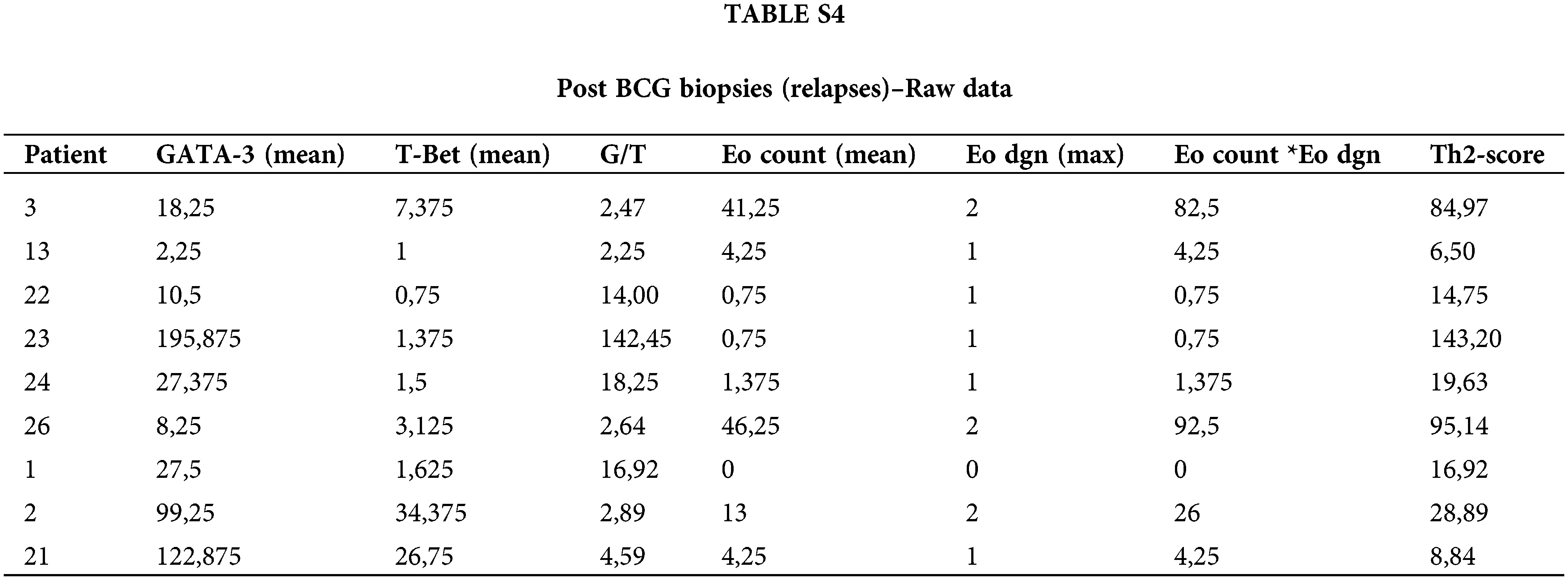
Pre-BCG biopsies were compared to post-BCG biopsies in most NR cases (n = 9). The median time to recurrence was 8 months. Of note, in 8/9 tumors (88.9%), GATA-3+ TILs increased after recurrence (p = 0.055) (Fig. 2D). Accordingly, the G/T and Th2-score were also higher in 7/9 post-BCG biopsies (77.8%) as compared to pre-BCG tumors; however, these differences were not statistically significant (Figs. 2E and 2F, Suppl. Table S4).
PD-1 and PD-L1 expression in NMIBC biopsies and response to BCG
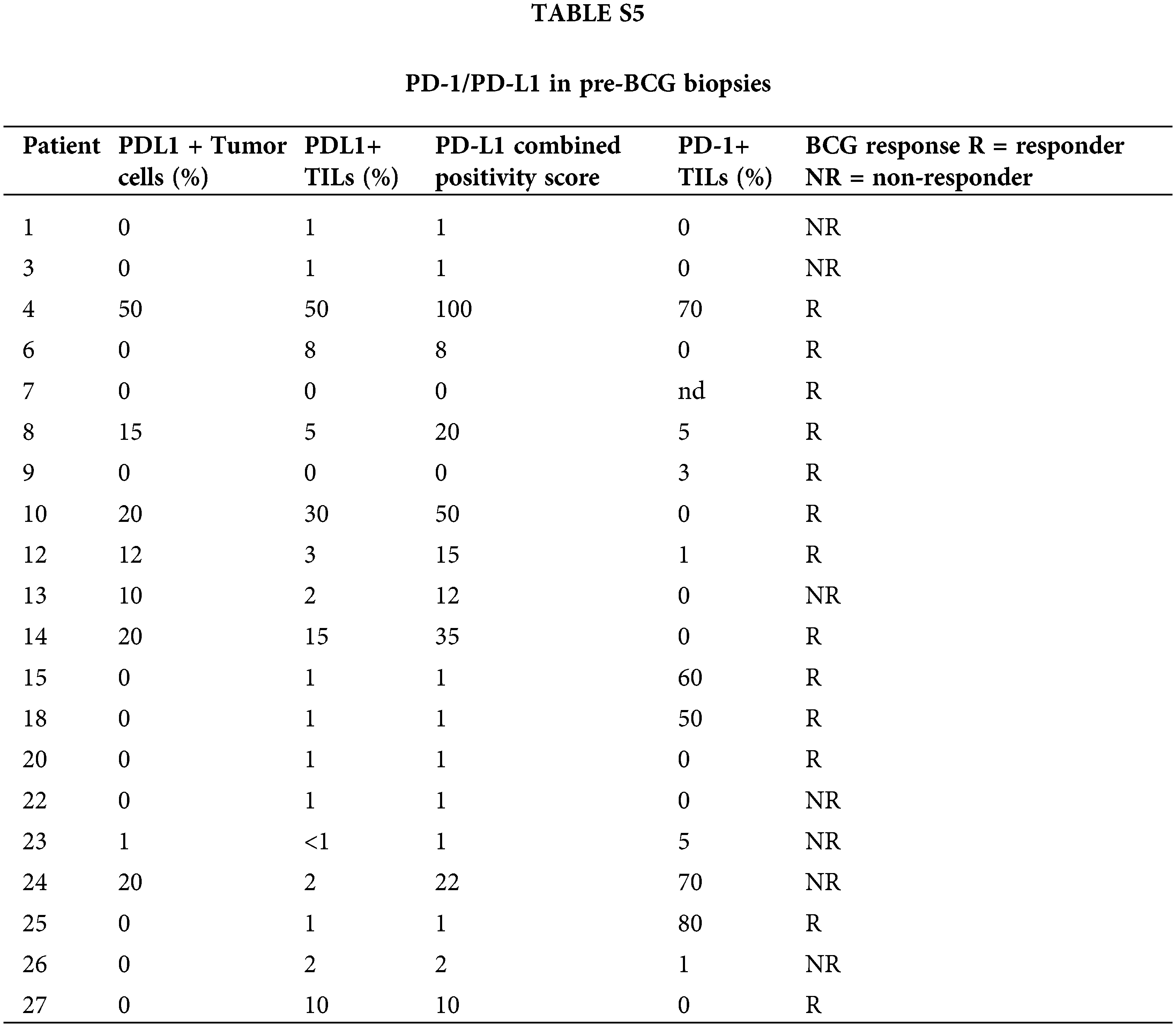
Due to limited tumor availability, PD-L1/PD-1 staining was evaluated in 20 and 19 pre-BCG biopsies, respectively. PD-L1+ tumor cells were observed only in 8/20 tumors (40%), but they were detected in TILs in 15/20 biopsies (75%). No difference was observed in PD-L1+ tumor cells between BCG-R and BCG-NR patients (Fig. 3A), but a higher proportion of biopsies from R expressed PD-L1 in >5% of TILs than in NR (p = 0.051) (Fig. 3B).
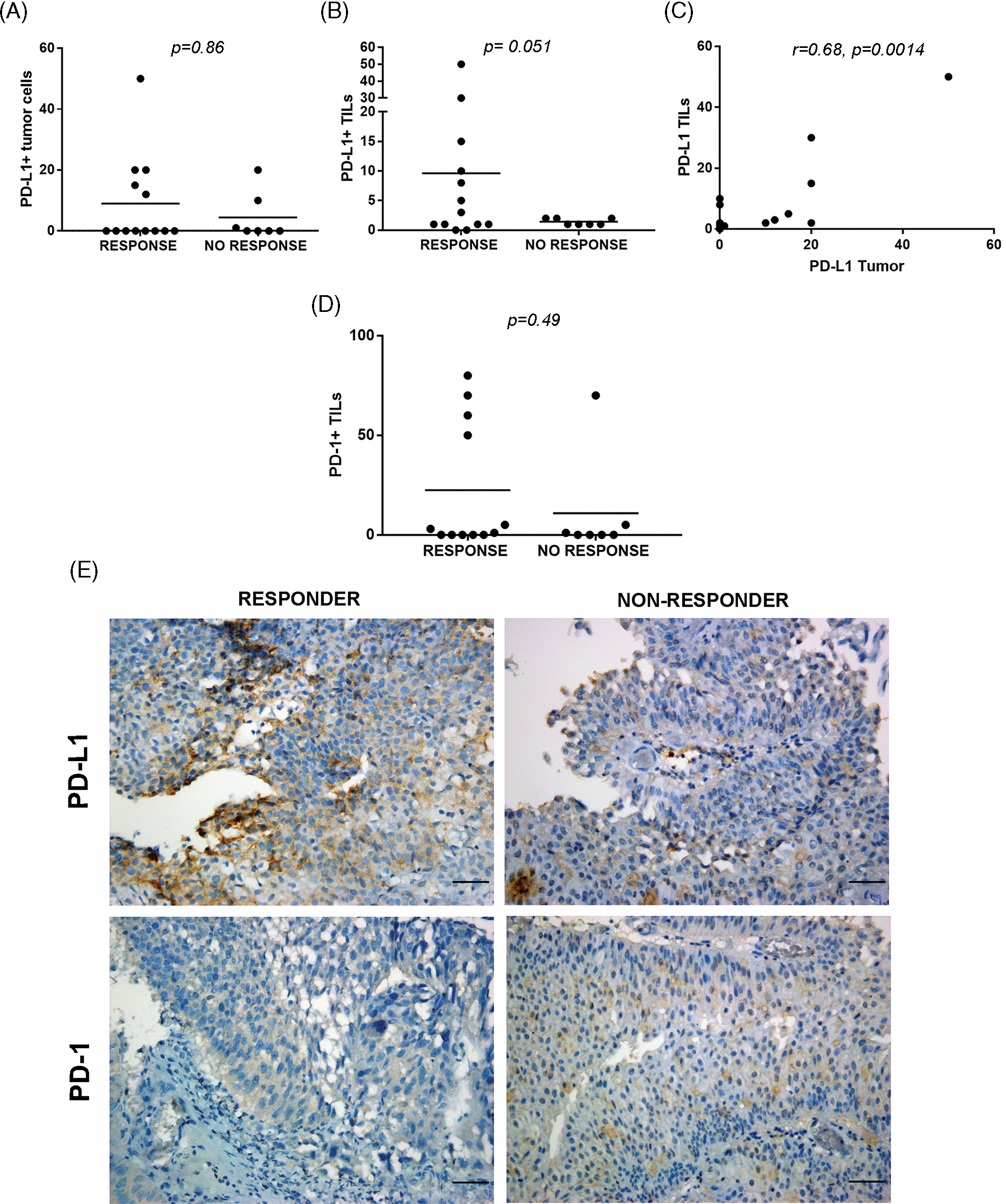
Figure 3: PD-L1/PD-1 expression in pre-BCG TILs of NMIBC biopsies. Quantitation of PD-L1 in tumor cells (A) and TILs (B) performed in BCG R or NR. (C) A positive correlation was found between PD-L1+ tumor cells and PD-L1+ TILs (C, r = 0.68, p = 0.0014, Spearman correlation test). (D) Invasive urothelial carcinoma showing PD-L1+ expression at the plasma membranes (left) and stromal TILs expressing PD-L1 in proximity to tumor cells (right). (E) Representative pictures of immunohistochemical staining of PD-L1 and PD-1 of pre-BCG tumor biopsies of BCG-R and BCG-NR patients. A, B, and D were compared with the Mann–Whitney test. Original magnification 400×. Scale bar = 50 μm.
PD-L1+ tumor cells were correlated with PD-L1+ TILs in all pre-BCG biopsies (r = 0.68, p = 0.0014), but these values were not associated with BCG response (Fig. 3C, Suppl. Table S5). More than 50% PD-1+ TILs were found in 4/12 biopsies from BCG-R patients, but only in 1/7 of BCG-NR, although this difference was not statistically significant (Fig. 3D). Representative images of PD-L1 and PD-1 expression in BCG-R and BCG-NR patients are shown in Fig. 3E. PD-1+ or PD-L1+ TILs were not correlated with GATA-3 or T-Bet expression or with the Th2-score (p > 0.05), regardless of the BCG response.
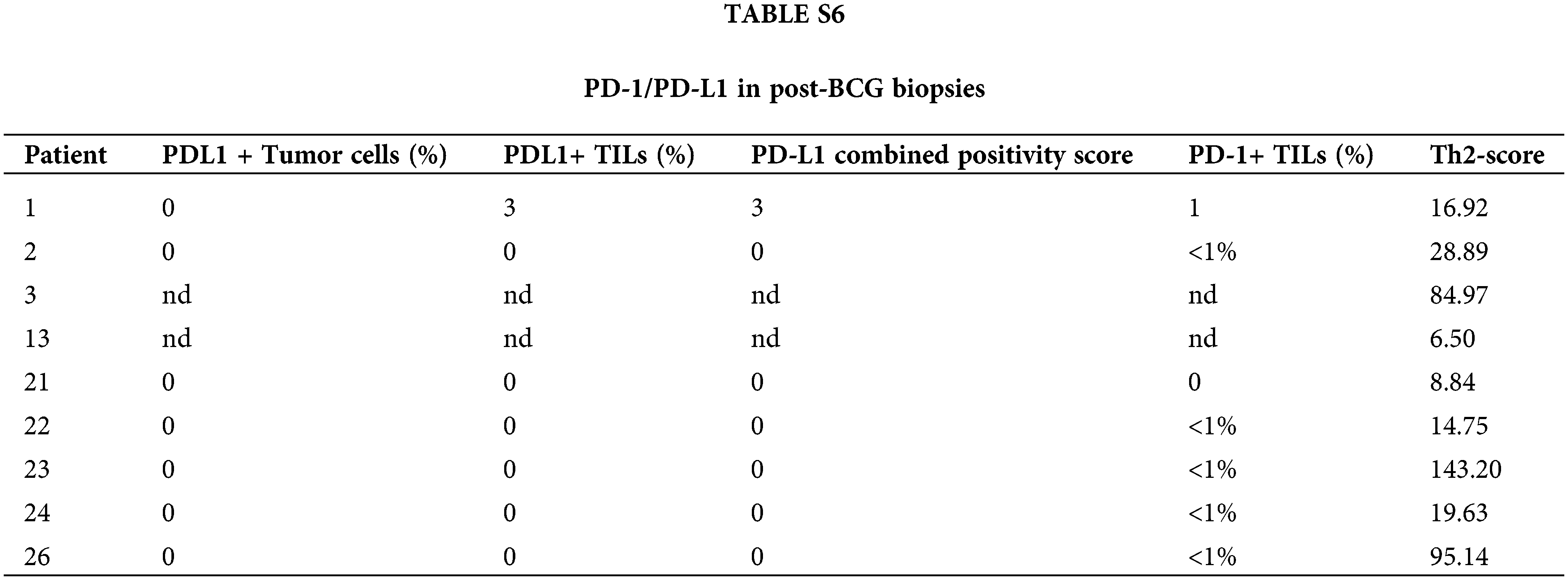
In post-BCG samples of BCG-NR patients, tumor cells and TILs showed no PD-L1 expression (0%–3%), and PD-1+ TILs were <1% (Suppl. Table S6). These results suggest that the PD-1/PD-L1 pathway was not involved in BCG failure.
There is a real need to identify patients with NMIBC that will not benefit from BCG immunotherapy, in order to offer them alternative treatments or clinical trials. In vitro and in vivo evidence supports that BCG acts as a potent Th1 inducer in the TME and is presumably responsible for long-lasting disease control [9,22]. However, the nature of the tumor antigens that are targets of the cytotoxic immune response or whether the anti-BCG immune response is involved in tumor elimination is still unknown. Additionally, experimental evidence has shown a direct effect of BCG in killing bladder cancer cells [23].
In the search for a possible biomarker of response to BCG, we evaluated lymphocyte polarization in the TME, along with the quantitation of eosinophils (associated with a Th2 immune microenvironment), at the maximum focus of infiltration, by IHC in pre-BCG biopsies of patients who had high-risk NMIBC, including HG pTa, LG/HG pT, and Cis tumors, and received adequate BCG. Our goal was to define a Th2-score associated with BCG response and set a cutoff to predict true BCG-R patients with >90% sensitivity. Patients with higher Th2-scores showed significantly higher RFS after BCG treatment. Nunez-Nateras et al. [14] reported that a Th2-score obtained after quantitation of the Th2/Th1 lymphocyte ratio plus eosinophilic infiltration of pre-BCG biopsies was associated with BCG response in 38 NMIBC patients with Cis. The authors hypothesized that a pre-existing Th2 TME would be susceptible to Th1 polarization, and therefore, Th2 status would correlate with the response to BCG treatment. However, in that study, patients received only one course of induction ×6, which is inadequate today. Of note, BCG response was assessed only after a short follow-up (6–8 weeks after BCG induction). Another study confirmed Nunez-Nateras’s findings [24]. However, the small number of patients included (19 BCG-R and 4 BCG-NR) may limit the conclusions, and the fact that only 25% of the patients had Cis makes comparison difficult. Another report on 22 HG pT1 patients (none of them had Cis tumors) showed that BCG-R patients had a significantly lower absolute number of peritumoral T-Bet+ cells and a higher G/T ratio [17]. However, the BCG strain and the treatment scheme were not specified. Furthermore, TILs G/T ratio adjusted for the systemic inflammation (neutrophil-to-lymphocyte ratio) showed a significantly higher association with the BCG response, a prolonged RFS in patients with a lower T-Bet+/Lymphocyte ratio, and higher GTR/NLR. In a later study [25], which included only 19 BCG-R and 4 BCG-NR patients the authors analyzed differences in the expression of GATA-3+ and T-Bet+ TILs (only evaluated in the lamina propria) between BCG-R vs. BCG-NR and found a clear tendency toward increased GATA-3+ T cell counts and GATA-3/T-Bet ratio.
Taken together, our results provide strong evidence that, after surgery, BCG can eradicate NMIBC cells only when the TME converts from Th2 to Th1.
Interestingly, we observed an increase in Th2 TILs in post-BCG biopsies from relapsed patients, probably reflecting the failure of BCG to induce a pro-inflammatory status. Presumably, the residual tumor cells that established strong immunosuppression sustained Th2 polarization.
Emerging data highlight that eosinophils infiltrate multiple tumors and display pleiotropic and even opposing roles (pro- vs. anti-tumorigenic activities) [26]. Furthermore, crosstalk between activated eosinophils and T cells increases CD8+ T cell migration to TME [27]. In addition, in patients treated with immune checkpoint inhibitors, increased eosinophilia is associated with responsiveness to therapy [27]. Thus, a higher pre-BCG Eo count in R patients could represent a Th2 polarized TME that can be shifted to Th1 by BCG administration, and eosinophils may contribute to Th1 cell recruitment. However, it was reported that blood eosinophils in patients with NMIBC could predict disease recurrence during BCG immunotherapy [28]. More data are needed to clarify this point.
Immune checkpoint upregulation is associated with a lack of antitumor cytotoxicity [29]. The upregulation of PD-L1 allows tumor cells to escape immune surveillance; thus, PD-L1 expression in the pretreatment TME could be a mechanism of BCG resistance. In our study, PD-L1+ tumor cells in pre-BCG biopsies were not associated with BCG response, in accordance with a previous report [17]. In our exploratory analysis, however, a higher proportion of BCG-R patients had >5% PD-L1+TILs than those from BCG-NR patients. Our findings are in disagreement with another report that found elevated PD-L1 expression in CD8+ TILs in baseline biopsies of BCG-NR patients [30]. However, we used a different anti-PD-L1 antibody (Ventana clone SP263 instead of Spring Biosciences clone SP142 or DAKO clone 22C3) and analyzed fewer patients. We observed >50% PD-1+TILs in a higher proportion of BCG-R than of BCG-NR patients, although this difference was not statistically significant, probably due to the small number of cases analyzed. In the post-BCG biopsies of relapsed patients, no exhausted PD-1+ TILs were detected. A possible explanation is that prior to BCG, an antitumor immune response was generated in some patients, accounting for the detection of exhausted T cells. However, an induced Th2 TME provides a cytokine balance that precludes the cytotoxic activity of TILs. In addition, PD-L1+ cells in the TME could contribute to the further abrogation of the antitumor response. Although our results suggest that the PD-1/PD-L1 pathway was not involved in BCG failure, this finding must be confirmed in a larger cohort of patients. Treatment with BCG shifts this balance toward Th1 polarization in BCG-R, patients increasing the affluence of innate and adaptive immune cells. Th1 cytokine production (i.e., IFN-γ) may fuel the elimination of residual tumor cells by immune effectors. Instead, in pre-BCG tumors with Th1-profiled TILs, other immunosuppressive mechanisms, such as secretion of TGF-β, PGE2, IL-10, and IL-6, as well as accumulation of myeloid-derived suppressor cells, tumor-associated macrophages, and regulatory T cells, could contribute to creating a highly tolerogenic TME [31]. Due to the small number of tumors available, we were not able to evaluate these immune populations in pre-BCG biopsies.
The limitations of this study are primarily associated with its retrospective design since only FFPE tumor biopsies were available for IHC analyses. In addition, the small number of patients reduced its statistical power. Recently, in the search for a biomarker profile associated with NMIBC patients’ response to BCG, we have initiated a prospective research study to validate the Th2-score combined with the analysis of immune cells and cytokines from urine and blood samples, obtained before and during treatment. Our results may have the potential to translate into a better selection of NMIBC patients to receive BCG treatment.
Acknowledgement: We dedicate this work to our patients. We thank Alejandra Scotti for English editing of the manuscript.
Funding Statement: This work was supported by grants from CONICET, Agencia Nacional de Promoción CientÍfica y Técnica (PICT 2018 00990, FONCYT) de Argentina, Fundación Sales, Fundación Pedro Mosoteguy and Fundación Cáncer FUCA.
Author Contributions: The authors confirm contribution to the paper as follows: Gustavo Villoldo and Maria Marcela Barrio contributed to the conception and design of the study. Material preparation and data collection were performed by JoaquÍn Chemi, Juan Camean, Adrián Burioni Deborah Egea, Mora Amat, and Alberto Villaronga. Immunohistochemistry staining and quantitation were performed by MarÍa Teresa Pombo and Mariana Aris. Data analysis and statistics were performed by Pablo Mandó, Supriya Nagaraju, and José Mordoh. José León Mellado contributed to the preparation of figures and manuscript edition. The first draft of the manuscript was written by MarÍa Marcela Barrio and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Gustavo Villoldo, Alberto Villaronga, JoaquÍn Chemi, and Deborah Egea are surgeons at the Urology Department of the Instituto Alexander Fleming.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article and its supplementary files.
Ethics Approval: This retrospective review of the bladder cancer registry and biopsies from the Urology Department of Instituto Alexander Fleming (Buenos Aires, Argentina) included patients who were diagnosed between 2007 and 2019. In this study, archived tumor samples were analyzed, and all patients signed an informed consent form for the use of biopsies for research purposes at the time of surgery. All the procedures were performed as part of the routine care, in accordance with the relevant guidelines and regulations of Instituto Alexander Fleming. This retrospective study was approved by the Ethics Committee of Instituto Alexander Fleming (CEIAF) under resolution #532. CEIAF approved a waiver of further informed consent signing, in view of the retrospective nature of the study and the lack of identifiable patient information included.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Fleshner, N. E., Herr, H. W., Stewart, A. K., Murphy, G. P., Mettlin, C. et al. (1996). The National Cancer DataBase report on bladder carcinoma. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer, 78(7), 1505–1513. https://doi.org/10.1002/(ISSN)1097-0142 [Google Scholar] [CrossRef]
2. Sylvester, R. J., van der Meijden, A. P.,Lamm, D. L. (2002). Intravesical bacillus Calmette Guerin reduces the risk of progression in patients with superficial bladder cancer: A metaanalysis of the published results of randomized clinical trials. The Journal of Urology, 168(5), 1964–1970. https://doi.org/10.1016/S0022-5347(05)64273-5 [Google Scholar] [PubMed] [CrossRef]
3. Herr, H. W. (1997). Tumour progression and survival in patients with T1G3 bladder tumours: 15-year outcome. British Journal of Urology, 80(5), 762–765. https://doi.org/10.1046/j.1464-410X.1997.00431.x [Google Scholar] [PubMed] [CrossRef]
4. Kiemeney, L. A., Witjes, J. A., Heijbroek, R. P., Debruyne, F. M., Verbeek, A. L. M. (1994). Dysplasia in normal-looking urothelium increases the risk of tumour progression in primary superficial bladder cancer. European Journal of Cancer, 30(11), 1621–1625. https://doi.org/10.1016/0959-8049(94)E0133-O [Google Scholar] [PubMed] [CrossRef]
5. Jakse, G., Hall, R., Bono, A., Höltl, W., Carpentier, P. et al. (2001). Intravesical BCG in patients with carcinoma in situ of the urinary bladder: Long-term results of EORTC GU Group phase II protocol 30861. European Urology, 40(2), 144– 150. https://doi.org/10.1159/000049765 [Google Scholar] [PubMed] [CrossRef]
6. Herr, H. W., Morales, A. (2008). History of bacillus Calmette-Guerin and bladder cancer: An immunotherapy success story. The Journal of Urology, 179(1), 53–56. https://doi.org/10.1016/j.juro.2007.08.122 [Google Scholar] [PubMed] [CrossRef]
7. Griffiths, T. R., Charlton, M., Neal, D. E., Powell, P. H. (2002). Treatment of carcinoma in situ with intravesical bacillus Calmette-Guerin without maintenance. The Journal of Urology, 167(6), 2408–2412. https://doi.org/10.1016/S0022-5347(05)64994-4 [Google Scholar] [CrossRef]
8. Witjes, J. A. (2006). Management of BCG failures in superficial bladder cancer: A review. European Journal of Urology, 49(5), 790– 797. https://doi.org/10.1016/j.eururo.2006.01.017 [Google Scholar] [PubMed] [CrossRef]
9. Kamat, A. M., Briggman, J., Urbauer, D. L., Svatek, R., Nogueras González, G. M. et al. (2016). Cytokine panel for response to intravesical therapy (CyPRITNomogram of changes in urinary cytokine levels predicts patient response to bacillus Calmette-Guérin. European Urology, 69(2), 197–200. https://doi.org/10.1016/j.eururo.2015.06.023 [Google Scholar] [PubMed] [CrossRef]
10. Zeng, W. P. (2013). ‘All things considered’: Transcriptional regulation of T helper type 2 cell differentiation from precursor to effector activation. Immunology, 140(1), 31–38. https://doi.org/10.1111/imm.12121 [Google Scholar] [PubMed] [CrossRef]
11. Lazarevic, V., Glimcher, L. H., Lord, G. M. (2013). T-Bet: A bridge between innate and adaptive immunity. Nature Reviews Immunology, 13(11), 777–789. https://doi.org/10.1038/nri3536 [Google Scholar] [PubMed] [CrossRef]
12. Renkema, K. R., Lee, J. Y., Lee, Y. J., Hamilton, S. E., Hogquist, K. A. et al. (2016). IL-4 sensitivity shapes the peripheral CD8+ T cell pool and response to infection. The Journal of Experimental Medicine, 213(7), 1319–1329. https://doi.org/10.1084/jem.20151359 [Google Scholar] [PubMed] [CrossRef]
13. Grisaru-Tal, S., Itan, M., Klion, A. D., Munitz, A. (2020). A new dawn for eosinophils in the tumour microenvironment. Nature Reviews Cancer, 20(10), 594–607. https://doi.org/10.1038/s41568-020-0283-9 [Google Scholar] [PubMed] [CrossRef]
14. Nunez-Nateras, R., Castle, E. P., Protheroe, C. A., Stanton, M. L., Ocal, T. I. et al. (2014). Predicting response to bacillus Calmette-Guérin (BCG) in patients with carcinoma in situ of the bladder. Urologic Oncology, 32(1), 45.e23–45.e30. https://doi.org/10.1016/j.urolonc.2013.06.008 [Google Scholar] [PubMed] [CrossRef]
15. Ostrand-Rosenberg, S., Horn, L. A., Haile, S. T. (2014). The programmed death-1 immune-suppressive pathway: Barrier to antitumor immunity. The Journal of Immunology, 193(8), 3835–3841. https://doi.org/10.4049/jimmunol.1401572 [Google Scholar] [PubMed] [CrossRef]
16. Bellmunt, J., Mullane, S. A., Werner, L., Fay, A. P., Callea, M. et al. (2015). Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Annals of Oncology, 26(4), 812–817. https://doi.org/10.1093/annonc/mdv009 [Google Scholar] [PubMed] [CrossRef]
17. MartÍnez, R., Tapia, G., De Muga, S., Hernández, A., González Cao, M. et al. (2019). Combined assessment of peritumoral Th1/Th2 polarization and peripheral immunity as a new biomarker in the prediction of BCG response in patients with high-risk NMIBC. Oncoimmunology, 8(8), 1602460. https://doi.org/10.1080/2162402X.2019.1602460 [Google Scholar] [PubMed] [CrossRef]
18. Inman, B. A., Sebo, T. J., Frigola, X., Dong, H., Bergstralh, E. J. et al. (2007). PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: Associations with localized stage progression. Cancer, 109(8), 1499–1505. https://doi.org/10.1002/(ISSN)1097-0142 [Google Scholar] [CrossRef]
19. Wang, Y., Liu, J., Yang, X., Liu, Y., Liu, Y. et al. (2018). Bacillus Calmette-Guérin and anti-PD-L1 combination therapy boosts immune response against bladder cancer. Onco Targets and Therapy, 11, 2891–2899. https://doi.org/10.2147/ott.s165840 [Google Scholar] [PubMed] [CrossRef]
20. Sylvester, R. J., RodrÍguez, O., Hernández, V., Turturica, D., Bauerová, L. et al. (2021). European Association of Urology (EAU) prognostic factor risk groups for non-muscle-invasive bladder cancer (NMIBC) incorporating the WHO 2004/2016 and WHO, 1973 classification systems for grade: An update from the EAU NMIBC guidelines panel. European Urology, 79(4), 480–488. https://doi.org/10.1016/j.eururo.2020.12.033 [Google Scholar] [PubMed] [CrossRef]
21. Lamm, D. L., Blumenstein, B. A., Crissman, J. D., Montie, J. E., Gottesman, J. E. et al. (2000). Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: A randomized southwest oncology group study. The Journal of Urology, 163(4), 1124–1129. https://doi.org/10.1016/S0022-5347(05)67707-5 [Google Scholar] [CrossRef]
22. Paparo, S. R., Fallahi, P. (2017). Bladder cancer and Th1 chemokines. La Clinica terapeutica, 168(1), e59–e63. https://doi.org/10.7417/ct.2017.1984 [Google Scholar] [PubMed] [CrossRef]
23. Yu, D. S., Wu, C. L., Ping, S. Y., Keng, C., Shen, K. H. et al. (2015). Bacille Calmette-Guerin can induce cellular apoptosis of urothelial cancer directly through toll-like receptor 7 activation. The Kaohsiung journal of medical sciences, 31(8), 391–397. https://doi.org/10.1016/j.kjms.2015.05.005 [Google Scholar] [PubMed] [CrossRef]
24. Pichler, R., Fritz, J., Zavadil, C., Schäfer, G., Culig, Z. et al. (2016). Tumor-infiltrating immune cell subpopulations influence the oncologic outcome after intravesical Bacillus Calmette-Guérin therapy in bladder cancer. Oncotarget, 7(26), 39916–39930. https://doi.org/10.18632/oncotarget.9537 [Google Scholar] [PubMed] [CrossRef]
25. Pichler, R., Gruenbacher, G., Culig, Z., Brunner, A., Fuchs, D. et al. (2017). Intratumoral Th2 predisposition combines with an increased Th1 functional phenotype in clinical response to intravesical BCG in bladder cancer. Cancer Immunology Immunotherapy, 66(4), 427–440. https://doi.org/10.1007/s00262-016-1945-z [Google Scholar] [PubMed] [CrossRef]
26. Varricchia, G., Galdiero, M. R., Loffredo, S., Lucarini, V., Marone, G. et al. (2018). Eosinophils: The unsung heroes in cancer? Oncoimmunology, 7(2), e1393134. https://doi.org/10.1080/2162402X.2017.1393134 [Google Scholar] [PubMed] [CrossRef]
27. Grisaru-Tal, S., Dulberg, S., Beck, L., Zhang, C., Itan, M. et al. (2021). Metastasis-entrained eosinophils enhance lymphocyte-mediated anti-tumor immunity. Cancer Research, 81(21), 5555–5571. https://doi.org/10.1158/0008-5472.CAN-21-0839 [Google Scholar] [PubMed] [CrossRef]
28. Temiz, M. Z., Colakerol, A., Ulus, I., Kilic, E., Paslanmaz, F. et al. (2021). Prediction of non-muscle-invasive bladder cancer recurrence during intravesical BCG immunotherapy by use of peripheral blood eosinophil count and percentage: A preliminary report. Cancer Immunology Immunotherapy, 70(1), 245–252. https://doi.org/10.1007/s00262-020-02673-x [Google Scholar] [PubMed] [CrossRef]
29. Boussiotis, V. A. (2016). Molecular and biochemical aspects of the PD-1 checkpoint pathway. The New England Journal of Medicine, 375(18), 1767–1778. https://doi.org/10.1056/NEJMra1514296 [Google Scholar] [PubMed] [CrossRef]
30. Kates, M., Matoso, A., Choi, W., Baras, A. S., Daniels, M. J. et al. (2020). Adaptive immune resistance to intravesical BCG in non-muscle invasive bladder cancer: Implications for prospective BCG unresponsive trials. Clinical Cancer Research, 26(4), 882–891. https://doi.org/10.1158/1078-0432.CCR-19-1920 [Google Scholar] [PubMed] [CrossRef]
31. Crispen, PL., Kusmartsev, S. (2020). Mechanisms of immune evasion in bladder cancer. Cancer Immunology Immunotherapy, 69(1), 3–14. https://doi.org/10.1007/s00262-019-02443-4 [Google Scholar] [PubMed] [CrossRef]
Supplementary Materials
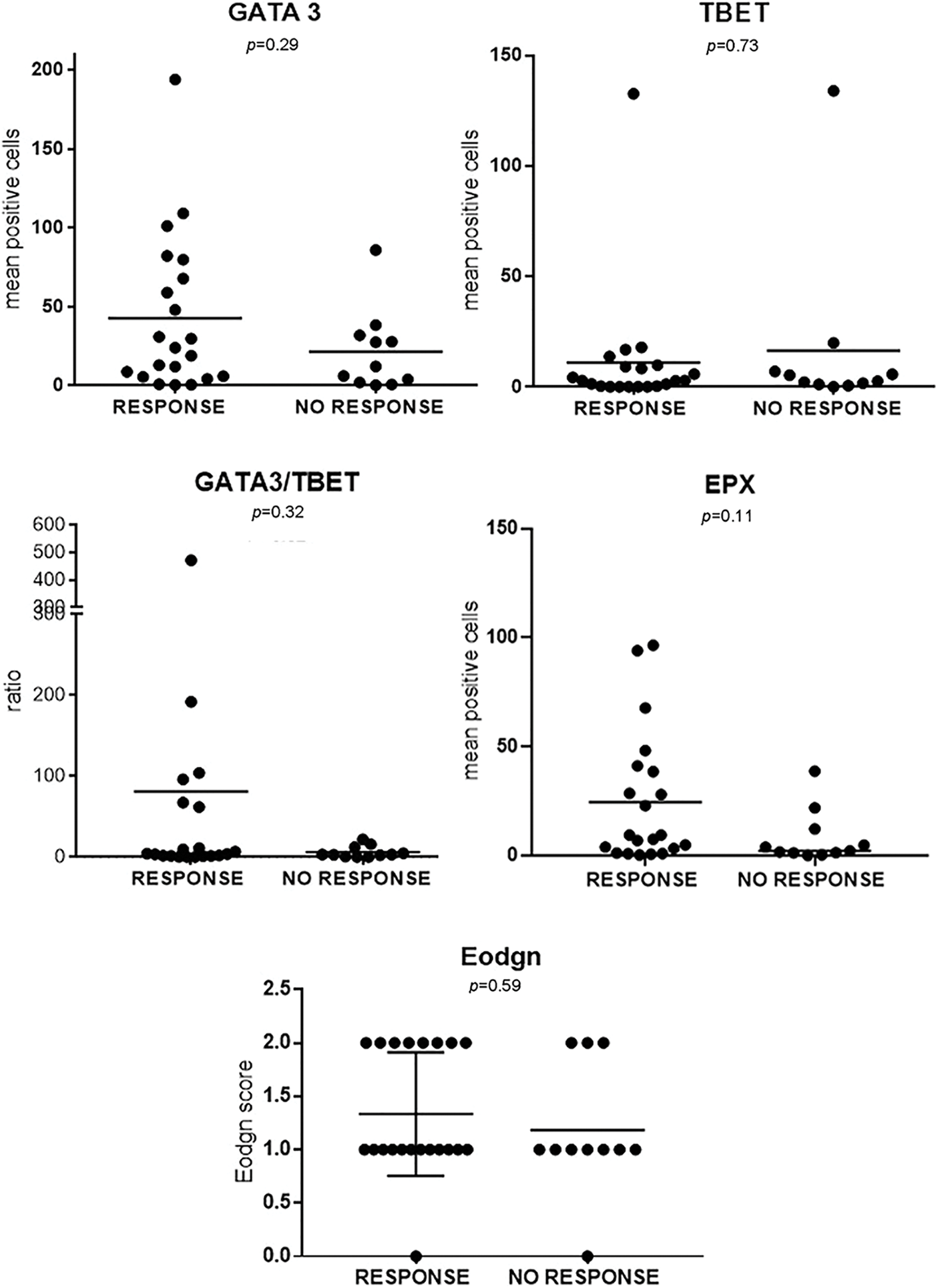
FIGURE S1: Each variable quantitated by IHQ was plotted for BCG-R and BCG-NR patients (n = 32).
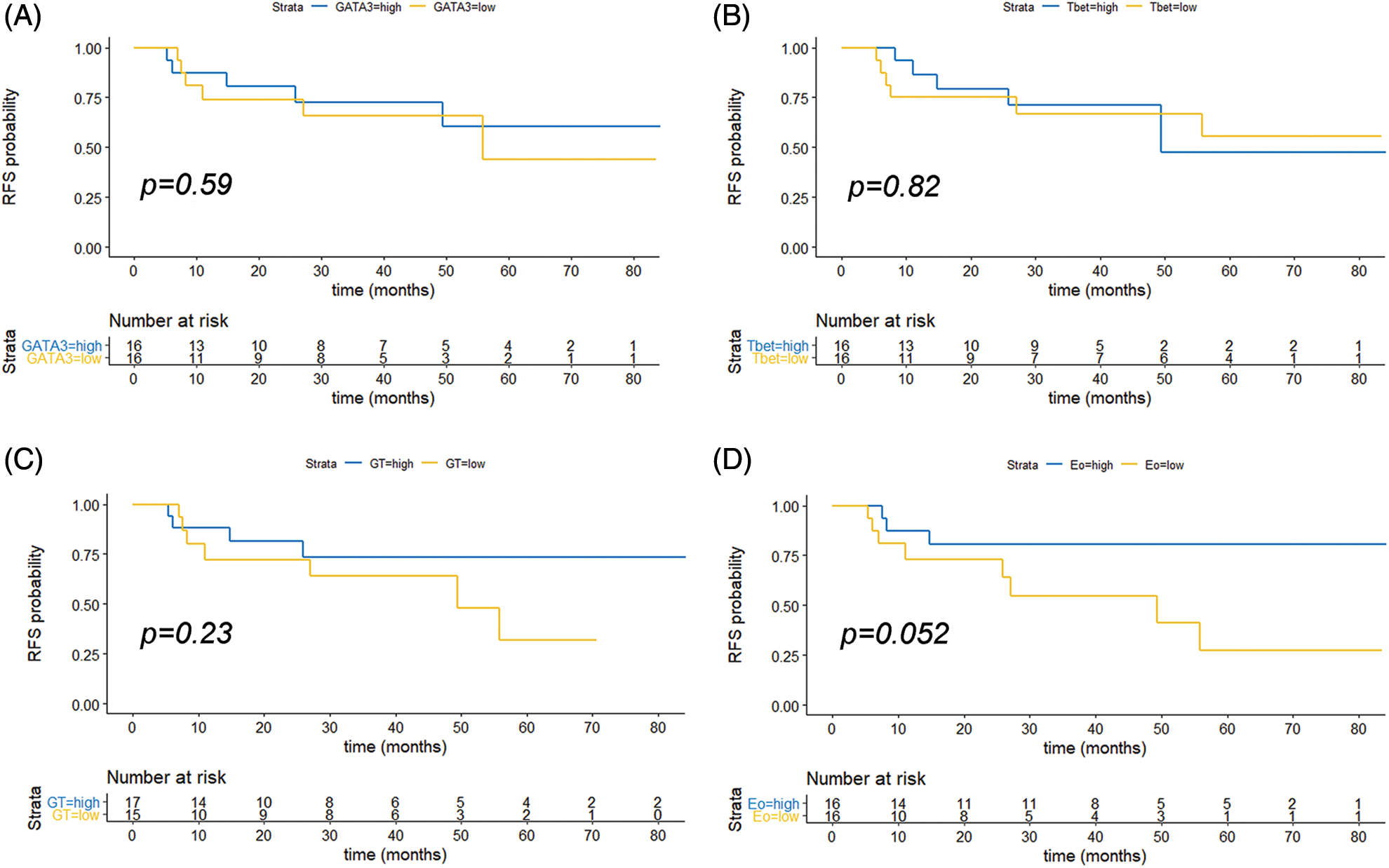
FIGURE S2: Kaplan Meyer curve of recurrence-free survival (RFS) in months. Patients were stratified by GATA-3 (A), T-Bet (B), G/T (C) and Eo counts (D) as low or high, based on the median values of the different variables analyzed (65.5 for age; 21.5 for GATA-3+; 2.9 for T-Bet+; 3.6 for G/T and 5.9 for EPX+). p values are indicated (log rank test, R).
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools