 Open Access
Open Access
ARTICLE
The role of AFAP1-AS1 in mitotic catastrophe and metastasis of triple-negative breast cancer cells by activating the PLK1 signaling pathway
1 Department of Spinal Surgery, Orthopedic Medical Center, Zhujiang Hospital, Southern Medical University, Guangzhou, 510280, China
2 Department of Critical Care Medicine, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, China
3 Department of Breast Surgery, Zhujiang Hospital, Southern Medical University, Guangzhou, 510280, China
4 Department of Gynecology and Obstetrics, Southern Hospital Taihe Branch, Southern Medical University, Guangzhou, 510540, China
5 Department of Clinical Medicine, Nanshan Class, Guangzhou Medical University, Guangzhou, 511436, China
6 Department of Gastroenterology, Shenzhen Hospital, Southern Medical University, Shenzhen, 518110, China
* Corresponding Author: HONGYAN HUANG. Email:
# These authors provided equal contribution to this work
Oncology Research 2023, 31(3), 375-388. https://doi.org/10.32604/or.2023.028256
Received 07 December 2022; Accepted 21 March 2023; Issue published 22 May 2023
Abstract
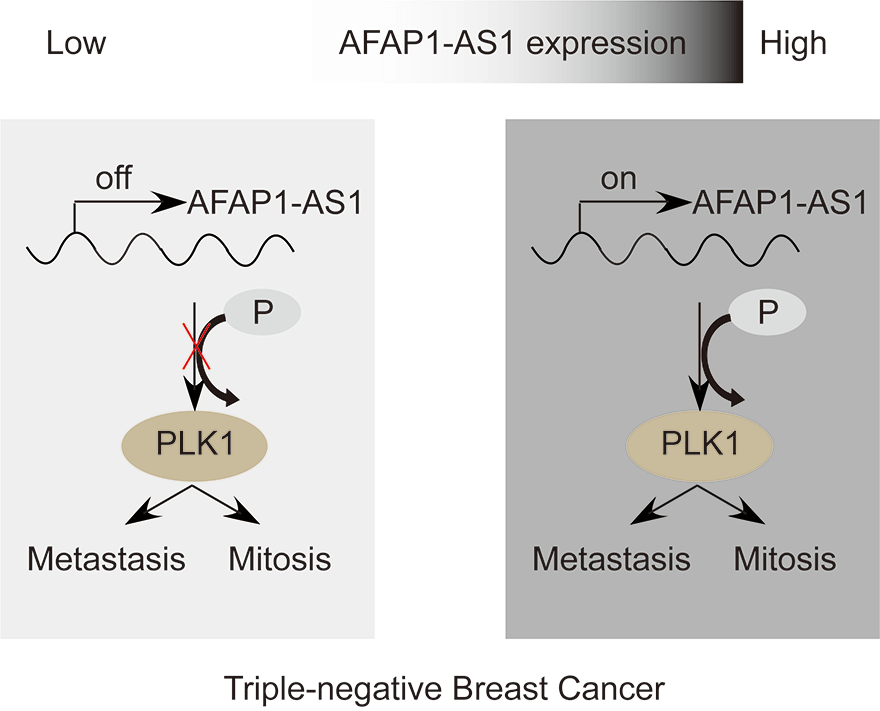
Triple-negative breast cancer (TNBC) is characterized by fast growth, high metastasis, high invasion, and a lack of therapeutic targets. Mitosis and metastasis of TNBC cells are two important biological behaviors in TNBC malignant progression. It is well known that the long noncoding RNA AFAP1-AS1 plays a crucial role in various tumors, but whether AFAP1-AS1 is involved in the mitosis of TNBC cells remains unknown. In this study, we investigated the functional mechanism of AFAP1-AS1 in targeting Polo-like Kinase 1 (PLK1) activation and participating in mitosis of TNBC cells. We detected the expression of AFAP1-AS1 in the TNBC patient cohort and primary cells by in situ hybridization (ISH), northern blot, fluorescent in situ hybridization (FISH) and cell nucleus/cytoplasm RNA fraction isolation. High AFAP1-AS1 expression was negatively correlated with overall survival (OS), disease-free survival (DFS), metastasis-free survival (MFS) and recurrence-free survival (RFS) in TNBC patients. We explored the function of AFAP1-AS1 by transwell, apoptosis, immunofluorescence (IF) and patient-derived xenograft (PDX) models in vitro and in vivo. We found that AFAP1-AS1 promoted TNBC primary cell survival by inhibiting mitotic catastrophe and increased TNBC primary cell growth, migration and invasion. Mechanistically, AFAP1-AS1 activated phosphorylation of the mitosis-associated kinase PLK1 protein. Elevated levels of AFAP1-AS1 in TNBC primary cells increased PLK1 pathway downstream gene expression, such as CDC25C, CDK1, BUB1 and TTK. More importantly, AFAP1-AS1 increased lung metastases in a mouse metastasis model. Taken together, AFAP1-AS1 functions as an oncogene that activates the PLK1 signaling pathway. AFAP1-AS1 could be used as a potential prognostic marker and therapeutic target for TNBC.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools