 Open Access
Open Access
REVIEW
Emerging HER2 Targeting Immunotherapy for Cholangiocarcinoma
1 Division of Hematopoiesis, Joint Research Center for Human Retrovirus Infection & Graduate School of Medical Sciences, Kumamoto University 2-2-1 Honjo, Chuo-ku, Kumamoto, 860-0811, Japan
2 Division of Hematologic Neoplasia, Department of Medical Oncology, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Avenue, Boston, MA 02215, USA
3 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, 50200, Thailand
* Corresponding Author: Seiji Okada. Email:
Oncology Research 2025, 33(9), 2279-2307. https://doi.org/10.32604/or.2025.065319
Received 10 March 2025; Accepted 11 June 2025; Issue published 28 August 2025
Abstract
Cholangiocarcinoma (CCA) is a fatal bile duct malignancy. CCA is intrinsically resistant to standard chemotherapy, responds poorly to it, and has a poor prognosis. Effective treatments for cholangiocarcinoma remain elusive, and a breakthrough in CCA treatment is still awaited. The human epidermal growth factor receptor 2 (HER2) plays an oncogenic role by promoting an aggressive cancer phenotype through multiple pathways. While HER2 has shown increasing potential as an effective target for breast and gastric cancers over the last decade, this has not been the case for CCA. This review explores the possibility of targeting HER2 in CCA immunotherapy. Key findings suggest that HER2 alterations have been reported as one of the signatures associated with a poorer prognosis in liver fluke-associated CCA, the most prevalent subtype in Southeast Asia. Furthermore, we assess recent advances in HER2-targeted therapeutic approaches, presenting the current stage, rationale, and evidence supporting the use of HER2 as a promising therapeutic target for cancer immunotherapy in CCA. We also emphasize the crucial role of animal models in developing anticancer therapies. In summary, focusing on HER2 expression could provide alternative strategies for the HER2-altered CCA cluster.Graphic Abstract
Keywords
Cholangiocarcinoma (CCA) is a deadly and aggressive bile duct malignancy [1–3]. Most CCA patients have a poor prognosis with very low 5-year survival rates, and the median survival rate is less than 1 year [4–6]. Moreover, the response rate to standard chemo-drugs is less than 40%. Tumor recurrence and resistant phenotype to first-line therapy often occur [7–9]. There are still no effective treatment regimens or strategies for CCA. Only R0 surgical resection (completely removed, with no microscopic or macroscopic residual tumor at the surgical margins) [10–12] is considered the best strategy for CCA treatment, whilst only a few patients can be operated on with the resection due to a lack of signs/symptoms [10]. The human epidermal growth factor receptor 2 (HER2) is a member of the human epidermal growth factor receptor (HER) family, which plays oncogenic roles in several malignancies. HER2 promotes cancer cell proliferation, differentiation, and survival via multiple signal transduction pathways [13,14]. In the last decade, there has been increasing potential for using HER2 as an effective target for breast and gastric cancers but not in CCA. Recently, the exploration of CCA cancer biology revealed that HER2 alterations have been dominantly observed in liver fluke-associated CCA clusters and are associated with poorer prognosis [15]. Thus, targeting CCA by focusing on HER2 expression might offer alternative strategies for HER2-altered CCA immunotherapy. This review offers the current understanding and rationale for considering HER2 as a novel, promising target for CCA immunotherapy. It also highlights the vital role of mouse models in bridging basic research to clinical studies.
Cholangiocarcinoma (CCA) refers to a group of biliary malignant tumors that arise from the bile duct epithelial cells. While CCA is currently considered relatively rare, its incidence is rising globally, representing approximately 3% of all gastrointestinal malignancies [6,16] and 15% [4] of primary liver cancers [8–10]. The worldwide incidence [4,5,9] of CCA is approximately 0.3 to 6 per 100,000 per year [4,8,17]. A notable incidence of CCA has been observed in Southeast Asian countries [5], including Laos, Cambodia, and the northeastern region of Thailand [10], with rates of 17.6 for females and 44.3 for males per 100,000 [8,9,18]. More than 8000 cases were annually diagnosed due to liver fluke infection (Ophisthorchis viverrini, Opisthorchis felineus [19], and Clonorchis sinensis [8,20,21]). However, CCA has been considered a rare malignancy in Western countries with liver fluke-negative disease (less than 6 per 100,000). CCA could be anatomically classified into intrahepatic CCA (iCCA) (originating above second-order bile ducts) and extrahepatic CCA (eCCA) (arising below second-order bile ducts). eCCA is further divided at the cystic duct into perihilar CCA (pCCA) and distal CCA (dCCA) [4,13]. Most patients diagnosed with CCA are typically in the advanced stages of the disease, as CCA is often asymptomatic in its early phases. Despite the extensive literature available on CCA, effective treatment targets and early diagnostic markers remain inadequately defined. Currently, R0 resection is considered the gold standard for CCA treatment; however, only approximately 3% of patients are eligible for this surgical option [22]. Consequently, more than 90% of CCA patients experience a poor prognosis, with a median overall survival (mOS) of 4 months, a one-year survival rate of 14.1%, and a two-year survival rate of 7.3% [8,9,23]. While many standard chemotherapies such as gemcitabine, 5-fluorouracil (5-FU) [24], cisplatin, and sorafenib [25] have been used as adjuvant therapy, and demonstrated poor clinical outcomes [17,26]. For instance, 5-FU illustrated a poor response rate ranging from 0% to 40% and a median survival rate of approximately 2 to 12 months [8,27,28]. Clinical trials of targeted therapies of unresected and mixed CCA populations revealed minimal response [29–31]. Recently, the TOPAZ-1 trial [32,33] and the global real-world analysis proposed the combination of immune checkpoint inhibitor, durvalumab plus gemcitabine, and cisplatin as a novel first-line standard care for advanced CCA. The combination significantly improves clinical benefits, with an overall response rate (ORR) of 32.7%, with stable disease in 45.2% of patients. Any grade adverse events (AEs) occurred in 92.9% of patients (grade > 2: 46.6%) [34]. Moreover, resistant phenotype to standard chemo-drugs and tumor recurrence after treatment still occurred [35–37].
3 The Human Epidermal Growth Factor Receptors 2 (HER2) in Cancers
HER2 is a member of the HER family, the tyrosine kinase receptor group which plays oncogenic roles in several cancers such as breast, pancreatic, and gastric cancers [38–40]. The family includes HER1 (known as EGFR), HER2 or ErbB2, HER3, and HER4 (Fig. 1) [41,42]. This receptor family comprises four parts, including the ligand-binding region, transmembrane, C-terminal, and cytoplasmic tyrosine kinase domain. The binding of ligands triggers homo- or hetero-dimerization leading to activation of the tyrosine kinase domain by tyrosine-residue phosphorylation. Although HER family members exhibit similar characteristics, HER2 is distinct due to its absence of specific ligands. Consequently, HER2 signaling predominantly depends on homo- or hetero-dimerization (more potent via hetero-dimerization). HER2-HER3 hetero dimerization is the most prevalent and potent oncogenic stimulator [43,44]. HER2 activation results in the upregulation of several oncogenic downstream pathways, including the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), RAS/RAF, and mitogen-activated protein kinase (MAPK) (Fig. 1), interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), etc., which promote cancer aggressiveness [13,14,40]. HER2 alteration occurs in about 15%–30% of breast cancers and 10%–30% of gastric/gastroesophageal cancers, providing crucial predictive and prognostic markers. HER2 alterations are strongly associated with poorer prognosis in breast cancer [14,45–47]. In recent decades, numerous HER2-targeted agents, including small-molecule tyrosine kinase inhibitors (TKIs), have been developed and approved for the treatment of breast cancer. Afatinib was one of the first evaluated; the drug demonstrated promising anti-breast cancer effects in phase I and II clinical trials but failed in phase III [48–50]. However, tucatinib, pyrotinib, and lapatinib demonstrated better clinical outcomes in phase III [51–53]. HER2-targeted regimens remain an essential option for treating various HER2-altered cancers and may be considered potential targets for CCA treatment regimens in the future, including cases with moderate and low HER2 expression.

Figure 1: HER2 dimerization with other EGFR; HER2 activation leads to the upregulation of several oncogenic cascades such as PI3K/Akt pathways, RAS/RAF, and MAPK pathways [14,40] (Created with BioRender.com)
4 HER2 Alterations and Clinical Significance in CCA
In the past decade, numerous studies have indicated that Cholangiocarcinoma (CCA) exhibits significant heterogeneity and genomic alterations [15,26,54]. Notably, the genetic profiles of CCA depend on the underlying etiology, which is predominantly linked to infections caused by liver flukes, specifically Opisthorchis viverrini and Clonorchis sinensis, commonly found in Southeast Asia and China, respectively [54]. Jusakul et al. [15] demonstrated using comprehensive Omics analysis, copy number, and methylation profiles of 489 CCA cases (both non-fluke- and fluke-related CCAs) that CCA could be classified into four molecular subtypes. Clusters 1 and 2 are almost liver fluke-related CCAs, predominantly with ERBB2 or HER2 alterations, and enrichment with TP53, ARID1A, and BRCA1/2 mutations. Clusters 3 and 4 demonstrated up-regulation of immune-related pathways such as PD-1/PDL2, IDH1/2 mutations, and the fibroblast growth factor receptor (FGFR) family alteration, respectively. Moreover, fluke-related CCAs (cluster1/2) illustrated poorer survival compared with the fluke-negative subtypes. This suggests that chronic liver fluke infection could play a significant role in creating the differing epigenetic and genetic profiles observed between Asian and Western CCAs. Additionally, HER2 alterations may represent a distinctive feature of fluke-positive CCA. Current research indicates that alterations of HER2 may contribute to tumorigenesis in CCA, as demonstrated in transgenic mouse models. Persistent overexpression of HER2 in the basal layer of the biliary tract epithelium resulted in the formation of gallbladder adenocarcinoma in this mouse model [55]. Moreover, the overexpression of HER2 enhanced aggressive phenotypes of fluke-positive CCA cell lines, including proliferation and invasion/migration via PI3K/Akt pathways [56]. Recent in vitro research also reported that high glucose and lactic acidosis conditions promoted aggressiveness of fluke-positive CCA cell lines via the EGFR/STAT3-dependent and ALDH1A3/EGFR axis, respectively [57,58]. These perspectives may indicate that CCAs might be advancing through the EGFR axis. However, further studies are needed to confirm these viewpoints. Notably, the large-scale meta-analysis revealed that 26.5% of CCA patients are HER2 positive [59,60]. The immunohistochemical (IHC) analysis based on the site of the primary tumor revealed that HER2 was positive in 17.4% of eCCA, 4.8% of iCCA, and 19.1% of gallbladder cancer (GBC). Jusakul et al. demonstrated HER2 amplification was more frequent in liver fluke-related CCAs (10.4% of fluke-related CCAs vs. 2.7% of non-fluke CCAs) [15,61]. Chaisaingmongkol et al. found that HER2 and ERBB3 are important oncogenic driver genes in Asian iCCA and hepatocellular carcinoma (HCC) [62]. HER2 amplifications and mutations in MAP2K4, RASA1, and SF3B1 were reported as genetic drivers between iCCA and eCCA [15,26]. In a more recent study of surgically resected 454 CCA cases, HER2 was positive (IHC3+ or IHC2+ plus HER2 gene amplification) in approximately 14.5% of all cases, including 18.5% of dCCA, 3.0% of pCCA, 3.7% of iCCA, and 31.3% of GBC. HER2 heterogeneity (defined as 5% or more of cancer cells exhibiting varying HER2 status) was observed in up to 83% of all cases. It correlated with the tumor histology of GBC, and a reduction in HER2 protein expression in the deeper invasive areas, along with simultaneous dedifferentiation, was commonly observed in HER2-positive cancer cells. The heterogeneity was not correlated with patient outcome; however, it’s important to carefully check HER2 levels using sensitive tests on larger pieces of the tumor. This will further aid in evaluating the impact of HER2 heterogeneity on HER2-targeted therapy in the near future [59,63]. Regarding prognostic significance, fluke-related CCAs (enriched with HER2 alterations) demonstrated poorer prognosis. Likewise, in Lowery et al.’s study, HER2-altered CCAs demonstrated significantly shorter progression-free survival (PFS) under first-line chemotherapy and a significantly lower median overall survival (mOS) compared with HER2 wild-type CCAs [59,64].
5 HER2-Specific Antibody Therapy
Trastuzumab, the humanized monoclonal antibody targeting the extracellular domain 4 (ECD4) of the HER2 receptor [52], was first approved as a therapeutic antibody in 1998 for HER2-positive breast cancer as a single agent or in combination with paclitaxel [65,66]. Since then, it has led to significant improvements in clinical outcomes in several metastatic diseases [67]. Trastuzumab illustrated direct anti-cancer activities through multiple mechanisms such as blocking receptor functions, regulating surface receptor expression and downstream signaling cascades leading to cell cycle arrest in the G1 phase, down-regulating cyclin E, suppressing tumor angiogenesis, impairing DNA repairing processes, which escalate susceptibility to conventional chemotherapy-induced apoptosis [65,68,69]. Not only does trastuzumab exert a direct anti-tumor effect, but it also exerts immunologic responses by facilitating antibody-dependent cell-mediated cytotoxicity (ADCC), the major function of natural killer cells against cancer [70,71]. Other studies demonstrated that macrophage-mediated antibody-dependent cellular phagocytosis (ADCP) is one of Trastuzumab’s mechanisms of action [72]. Recently, Panaampon et al. found that trastuzumab exerts multiple mechanisms against HER2-altered CCA, including direct growth inhibition, complement-dependent cytolysis (CDC), ADCC, and ADCP in both in vitro and CCA patient-derived xenograft (PDX) models [73].
The other HER2-specific monoclonal antibody is pertuzumab, which targets the HER2 extracellular domain 2 (ECD2). The antibody exerts an anti-tumor effect by tightly inhibiting the homodimerization of cytoplasmic tyrosine kinase regions of HER2 and heterodimerization of HER2/HER3, which are important steps for HER2-mediated oncogenic signaling cascades. The drug has been approved by the Food and Drug Administration and the European Medicines Agency for metastatic breast cancer [74,75]. As for combination regimens, it seems that trastuzumab plus pertuzumab or Phesgo, trastuzumab plus pertuzumab plus Hyaluronidase-zzxf, which acts as a dispersion agent to modify the permeability of human connective cancerous tissues, offer better clinical benefits [76]. The FDA approved the regimens in June 2020 for treating HER2-positive both early and metastatic breast cancers, while trastuzumab with tucatinib has been granted accelerated approval for colorectal cancer recently [76,77].
HER2-specific monoclonal antibodies (mAbs) demonstrated favorable clinical outcomes in HER2-positive breast cancer (IHC score 3+ or IHC2+ with gene amplification positive on an in situ hybridization (ISH)). However, HER2-targeted mAbs such as trastuzumab failed to provide clinical benefit in low-HER2-expressing (IHC2+ with ISH- or IHC1+) [78–80], which is reported to be observed in approximately 50% of breast cancer patients and other mass-forming types [81–83]. Some of the trastuzumab resistance mechanisms have been reported, such as overexpressing Akt, TGF-α, IGF1R, VEGF, etc. [84]. Not all tumors that overexpress HER2 exhibit a favorable response to these therapies; many that initially respond later acquire resistance. HER2-targeted drug-resistant cancer cells correlate with increased levels of immunosuppressive molecules such as transforming growth factor-beta 1 (TGFβ1) and programmed cell death ligand 1 (PD-L1), causing resistance to the anti-tumor immune response. Moreover, these molecules are encapsulated within extracellular vesicles (EVs), which facilitate the transfer of resistant phenotypes to drug-sensitive recipient cells. The levels of EV-associated TGFβ1 are also significantly correlated with the therapeutic response to HER2-targeted therapies in HER2-overexpressing cancer [85]. Moreover, other factors in the tumor microenvironment (TME), such as collagen, induce the expression of interleukin-4 induced 1 (IL4I1), contributing to T-cell exhaustion, and promoting immune evasion [86]. Additionally, Intratumor heterogeneity of HER2 expression/amplification within a single tumor present in breast cancer might affect treatment responses to HER2-targeted therapy, including trastuzumab, as the fraction of HER2 non-amplified cells remains and acts as a driver of therapeutic resistance [87]. Notably, the poor response rate and shorter survival of trastuzumab treatments were associated with overall low HER2 expression and intratumor heterogeneity [88,89]. Adverse effects of trastuzumab are associated with a decline in left ventricular ejection fraction (LVEF), which is linked to the expression of HER2 and ErbB4 receptors in cardiomyocytes [90]. In the pivotal clinical trial carried out by Slamon et al., trastuzumab plus anthracyclines, patients with advanced breast cancer experienced substantial clinical benefits; however, this combination resulted in an unacceptably high incidence of heart failure, occurring in 28% of cases [90–92]. With these limitations, we can move forward with the antibody-drug conjugate (ADC).
6 HER2-Specific Antibody-Drug-Conjugate (ADCs)
In the last decade, ADCs have gained popularity in cancer research as they provide an ideal anti-cancer drug delivery system, which significantly increases the maximum therapeutic effect against cancer and reduces undesirable side effects [93]. The ADCs are comprised of potent cytotoxic agents, cancer-specific mAbs, and an appropriate linker to connect the drug molecules. Nowadays, several ADCs have been approved for clinical use. More than 70 ADCs are being investigated in clinical trials now. For HER2-targeting ADCs, only trastuzumab emtansine and trastuzumab deruxtecan are approved ADCs for advanced HER2-expressing breast and gastric cancers. Some HER2-targeting ADCs are now evaluated in late clinical trials (phase III), including trastuzumab duocarmazine (SYD985, T-Duo), BAT8001, and disitamab vedotin (Table 1) [94–96] (https://clinicaltrials.gov/study/NCT03500380) (accessed on 10 June 2025). Trastuzumab dacarbazine (T-Duo) is a second-generation ADC comprising trastuzumab conjugated with a DNA alkylating agent, dacarbazine, with a drug-to-antibody ratio (DAR) of 2.4 to 2.8. The pre-clinical study revealed that a cleavable linker of T-Duo offers an anti-tumor activity to overcome trastuzumab emtansine (T-DM1)-resistant breast cancer in PDX models [97]. Importantly, T-Duo illustrated a favorable profile by significantly prolonging progression-free survival in phase III clinical trials (NCT03262935) and is now being further evaluated in the following steps [98].

T-DM1 is the first HER2-targeted ADC approved by the FDA. The ADC consists of trastuzumab that incorporates a non-cleavable linker with emtansine (DM1), and the cytotoxic microtubule spindle fiber inhibitor with DAR 3.5 [99,100]. T-DM1 demonstrated a favorable anti-tumor effect against HER2 overexpressing breast cancer as an adjuvant, first- and second-line therapy in phase III clinical trials but, less or no effect in low HER2 expression. The main toxicities of T-DM1 are considered from the payload. Fatigue, nausea, thrombocytopenia, rising transaminases, and epistaxis are often reported in adverse events. Almost all of these events generally reported low grades except severe thrombocytopenia (≥grade 3) [99,101]. Thus, patients with cardiac dysfunctions should avoid or adjust appropriate doses or discontinue treatment. BAT8001 is one of the HER2 targeting ADCs (Table 1) comprising trastuzumab biosimilar (BAT0606) connected to a novel covalently uncleavable linker via a stable amide bond to avoid the release of toxic payloads in blood and increase stability to minimize toxicities. The antibody was conjugated with the maytansine derivative, the spindle fiber inhibitor. BAT8001 demonstrated anti-cancer activity by inhibiting the proliferation of HER2-expressing cells in both in vitro and in vivo. Moreover, no adverse effect level has been observed in cynomolgus monkeys. The drug is being evaluated in phase III clinical trials with a favorable safety profile similar to T-DM1 and demonstrates better multiple-dose toxicity than T-DM1 [102,103].
Trastuzumab deruxtecan (T-Dxd) is currently considered the most promising ADC against HER2-positive cancers with a maximum drug antibody ratio (DAR: 8.0). The trastuzumab was homogenously conjugated with 8 molecules of Topoisomerase I (TOP1) inhibitors using an enzymatically cleavable peptide-based linker (Table 1). The linker-drug of T-Dxd demonstrated stability in systemic circulation and selectively targeted HER2-expressing cancer cells by facilitating the antitumor effects of trastuzumab, including ADCC, ADCP, and CDC, and was selectively cleaved by intracellular lysosomal proteases such as cathepsins in cancer cells, allowing for the payload release. Moreover, the Dxd possesses high plasma membrane permeability to exert a potent bystander-killing effect against cancer heterogeneity and the tumor microenvironment [104,105]. T-Dxd not only exerts potent anti-tumor activity against high HER2-expressing cancer but also low-HER2-expressing and tumor microenvironment cells as well [106–108]. These might be an advantage of T-Dxd for fighting against HER2 intratumorally heterogeneities in CCA tumor mass and elimination of tumor microenvironment cells, such as cancer-associated fibroblasts (CAFs). Likewise, the most recent clinical trial (DESTINY-Breast03) demonstrated that T-DXd offers significantly longer progression-free survival than T-DM1 in HER2-altered breast cancer patients [109]. A systematic review revealed that the most common adverse events associated with T-DXd in all grades were nausea, fatigue, vomiting, anemia, constipation, neutropenia, and diarrhea. In terms of adverse events of grade 3 or more, only anemia and neutropenia occurred at a relatively high rate. The rate of nausea was 72.8% to 73%, anemia was 30.4% to 33.2%, while 42.8% of patients were treated with T-DXd, with 19.1% experiencing neutropenia of grade ≥ 3 [110]. Although T-DXd contains trastuzumab, the incidence of cardiotoxicity reported so far has been low. In the DESTINY-Breast01 trial, three patients experienced LVEF; two cases were grade 2 and one was grade 3, and all were asymptomatic; patients recovered or were recovering after therapy interruption. There were no events of cardiac failure associated with the LVEF decrease [92,111].
DHES0815A is another most recent clinically investigating (phase I trial: NCT03451162) HER2-targeted ADC. The humanized 7C2 (hu7C2) HER2-targeting antibody has been chosen as the core immunoglobulin for this antibody-drug conjugate (ADC) to enable a potential combination with current HER2 therapeutic antibodies. The hu7C2 recognizes explicitly the extracellular domain 1 (ECD1) of the HER2 receptor, distinguishing it from trastuzumab (ECD4) and pertuzumab (ECD2), which target different epitopes. Fluorescence-activated cell sorting (FACS) analysis revealed that hu 7C2 illustrated full binding to HER2-positive breast cancer cells in the presence of trastuzumab and pertuzumab. DHES0815A exerted promising anti-tumor activity against HER2-positive cancer cells (both direct- and bystander-killing effects) by the novel cleavable methyl-disulfide linker connected to DNA minor groove crosslinking agent, pyrrolo [2,1- c] [1,4] benzodiazepine monoamide (PBD-MA), the cytotoxic warheads with DAR: 2.0. For ADCC mechanism, trastuzumab illustrated slightly better than huC72, likely due to closer proximity of trastuzumab recognition site (ECD4) which, facilitating closer synapsis of immune effector and cancer cells [112]. The cytotoxic warhead, PBD-MA, demonstrated a reduction in cell viability of both highly proliferative and quiescent cancer cells in an in vitro study [112,113]. This might enhance the potency of the bystander effect against the acquiescent cancer cell population inside the tumor mass. Although DHES0815A has less payload (DAR: 2.0), it demonstrated more potency than T-DM1 (DAR: 3.5). Moreover, an overall preclinical animal model suggested equivalent anti-tumor efficacy between DHES0815A and T-Dxd. The most frequently reported adverse events of DHES0815A from phase I are pruritus, rash, phobia, hyperpigmentation of the skin, nausea, and fatigue [112,114,115]. Currently, several HER2-targeting ADCs are in early phase (I/II) development (Table 2).
Together, HER2-targeting ADCs represent a promising platform for cancer immunotherapy [123]. They demonstrate anti-cancer effects through several mechanisms, including the typical responses linked to the fragment crystallizable (FC) portion of mAbs, such as ADCC, ADCP, and CDC, in addition to their specific cytotoxic payload mechanisms. Advanced linker technology offers various strategies for anti-cancer drug delivery systems to combat HER2-altered cancer, including CCA, while minimizing the unintended release of payloads.
7 HER2 Targets Antibodies, Affibodies, and Nanobodies for Radioimmunotherapy
Not only for precisely delivering cytotoxic agents to cancer cells but mAbs are also applied in cancer theranostics (radionuclide imaging and radiation therapy) as vehicles for radionuclide conjugates. For example, Lutetium-177 (Lu-177)-trastuzumab, which is radiolabeled trastuzumab with a bifunctional chelator, dodecane tetraacetic acid (DOTA), and Lu-177, a medical isotope in targeted radionuclide therapy for treating neuroendocrine- and prostate malignancies [124]. The radioimmunoassay revealed good antigen-binding affinity and specificity of Lu-177-trastuzumab against the HER2 cell surface receptor. Moreover, the patient’s studies illustrated the localization of Lu-177-trastuzumab at both primary and metastatic sites of HER2-positive breast cancer using the combination of Single Photon Emission Computed Tomography scan and Computed Tomography (SPECT/CT) imaging [124]. However, no tumor uptake was detected in HER2-negative patients, indicating the specificity of Lu-177-trastuzumab for HER2-positive cancer diagnosis and probably becoming targeted radionuclide therapy for HER2-positive cancer shortly. Another interesting strategy is near-infrared photoimmunotherapy (NIR-PIT), which is the application of mAbs for carrying the inactive form of photo-absorbing chemical cytotoxic agents to cancer cells. Once the conjugated antibodies bind to cancer cells, near-infrared light is locally provided to activate the photo-absorbing chemical molecules, which results in direct damage to cancer cells and minimizes damage to adjacent normal cells [125,126]. The cell death via NIR-PIT demonstrated distinct characteristics as necrosis, which forms cell swelling, membrane blebbing, and membrane disruption, resulting in vigorous anti-tumor immune responses. IRDye® 700 is one of the most popular photo-absorbing molecules. Sato and the team conducted the dual targeting of EGFR and fibroblast activation protein (FAP) by conjugation of panitumumab, the EGFR targeting antibody, and anti-FAP with IRDye700. The results revealed that dual-targeted EGFR/FAP NIR-PIT significantly offers a therapeutic effect against cancer cells and CAFs simultaneously in vitro and in vivo mice models [127].
Monoclonal antibodies such as trastuzumab or pertuzumab are often used as vehicles for precisely delivering cytotoxic agents or radionuclides to HER2-expressing cancer cells for therapeutic and imaging diagnosis. Although ADCs demonstrated promising clinical profiles against HER2-altered tumors, there are still some insurmountable limitations for mAbs such as heterogenous blood perfusion profiles, slow blood clearance which is also associated with tumor uptake, tumor penetration since the molecular weight of antibodies is relatively high (approximately 160 kDa), extravascular binding resulting in increasing turgor effect (interstitial pressure) leading to heterogenous intratumor distribution or stuck at the periphery of the tumor mass. The relatively large molecular size of mAbs makes them unable to be filtered by the kidney glomerulus and accumulation in the liver, leading to nephrotoxicity or hepatotoxicity. In addition, few therapeutic antibodies can pass through the blood-brain barrier, making it difficult for brain metastasis treatment. Together, these drawbacks initiated further development of smaller molecules of antibody fragments to improve therapeutic efficacy and molecular imaging of cancers by radiolabeled [128,129].
Affibodies are engineered from small antibody fragment molecules that could be applied for theranostics. Affibodies derived from three cysteine-free helical sub-domain scaffolds protein (58 amino acids in length) without disulfide bridges, which cause nonspecific binding to various proteins with a high affinity [130]. Due to their small size, quicker blood clearance, and high affinity, they are attractive for molecular imaging. The first clinical trial of molecular imaging for HER2-positive breast cancer using radiolabeled HER2-targeting affibody ABY-002 was promising. High-quality positron emission tomography (PET) and single-photon emission computed tomography (SPECT) results were acquired 2 h after injection (p.i.) with gallium-68- and indium-11-labeled ABY-003, respectively [131]. Currently, this tracer has been further developed to achieve a better blood clearance profile and reduce image background. Although affibody demonstrates several advantages and properties that make it useful, particularly for molecular imaging, some hurdles must be overcome. The labeling process can lead to increased lipophilicity of the molecule, which might lead to non-specific interaction with normal tissue, as well as the development of affibody, nowadays still expensive [129]. A further important disadvantage is that affibody could be the origin of bacterial scaffold proteins, which risks increasing the immunogenicity of patients after several administrations [132]. Further study is required to improve therapeutic application, which is hampered by the short retention time of the affibody in blood.
Nanobodies are derived from the variable domain of the heavy chain of heavy-chain antibodies (VHH). Heavy chain antibodies (HcAbs) represent a class of antibodies that consist exclusively of two heavy chains and do not contain light chains. HcAbs could be found in camelid species such as Camelus dromedarius, Camelus bactrianus, Lama glama, and sharks [133,134]. Since the lack of light chains, HcAb binds to the antigen by only a single variable domain connected to the FC portion (CH2 and CH3) by a hinge domain. The VHH of HcAb offers a functional equivalent to the fragment antigen-binding region (Fab) of traditional antibodies. Nanobodies provide several advantages to overcome conventional antibodies, such as high affinity, stable molecules, high water solubility, small sizes of 2.5 and 4.0 nm for width and length, with a molecular weight of approximately 15 kDa. Due to their small size, nanobodies are rapidly cleared by the kidneys, resulting in a short biological half-life. Additionally, nanobodies share a high degree of sequence homology with human VH domains, which contributes to their low immunogenicity [128,135]. These properties make nanobodies suitable for tumor tissue penetration and specifically bind to cancer antigens. In the manner of the theranostics tool, nanobodies should be tagged with a short physical half-life of radionuclides. The first clinical study with radioactively labeled nanobody for PET/CT was conducted by Keyaerts and team [136] 68Ga-NOTA-2Rs15d. The favorable biodistribution of the tracer was obtained as accumulation in HER2-positive breast cancer metastases is high, with a very low background compared with normal adjacent tissues. In addition to their use in cancer diagnostics, recent studies have increasingly explored the therapeutic potential of nanobodies. Several therapeutic nanobodies, labeled with various radionuclides, have shown promising anti-cancer effects. For example, 131I-SGMIB-2Rs15d as a single agent or in combination with trastuzumab demonstrated significantly improved median survival in animal models [137]. Interestingly, the study of 225AcDOTA-2Rs15d nanobody alone or in combination with trastuzumab offers prolonged median survival of brain metastatic breast cancer mice [138]. In contrast to conventional antibodies, radiolabeled nanobodies can pass through the blood-brain barrier, offering better options for molecular imaging of brain metastatic lesions and potentially serving as therapeutic agents.
8 Multi-Specific Cell Engager Molecules
Cell engager molecules are the modified antibodies with at least one arm targeting tumor-specific associated antigens. In contrast, other arms engage in activating domains of the immune effector cells, such as CD3 for T-cell recruitment or targeting the low-affinity immunoglobulin gamma Fc region (FcγRIII) known as CD16 for natural killer (NK) cell engagement. A decade before, the first bispecific antibody targeting CD3 and CD19 was first approved by the US FDA in 2014. Since then, gaining momentum of engager molecules in the cancer immunotherapy field until 2022, three Bispecific T-cell engagers were approved later on, four were approved in 2023, and one was just approved in 2024, respectively. T-cell engager technologies are now likely to revolutionize cancer immunotherapy, especially in hematological malignancies, as they offer more potent antitumor activities than classical monoclonal antibody therapies. Several current perspectives of cell engagers have been developed [139], such as bifunctional checkpoint-inhibitory T-cell engagers (CiTEs) [140], Simultaneous multiple interaction T-cell engaging (SMITEs) [141], dual-affinity re-targeting (DART) [142], Trispecific T-cell engagers (TriTE) [143], Trispecific Killer Engagers (TriKE) [144], etc., as summarized in Table 3. More than 100 bispecific T-cell engagers are being investigated in clinical trials [145,146]. Bispecific NK cell engagers are now being examined in the early phase of clinical trials. Furthermore, another binding arm may target additional tumor-specific antigens to increase cancer-specific binding and prevent cancer immune escape or target additional co-stimulatory immune effector cells. The tri-specific engagers were developed.

Cell engager technology offers a promising novel platform for cancer immunotherapy, especially for hematological malignancies. Currently, there are increasing trends for the application in mass-forming cancers by focusing on HER2 or HER3 including zanidatamab, the biparatopic HER2 targeting antibody, and zenocutuzumab (Zeno) which exerts anti-cancer activity by targeting HER2 and HER3 respectively. Moreover, targeting HER2 and CD3 in mass forming such as breast cancer using tri-specific cell engager molecules has been developed by adding further CD28 into another binding arm for co-stimulating T-cell response against HER2-positive breast cancer. The HER2 tri-specific antibody illustrated multiple anti-cancer effects such as T-cell stimulation, and activation of CD8 cytolytic functions, furthermore, they highlighted the importance of CD4-mediated tumor regression in humanized mouse models through the induction of breast cancer cell cycle arrest [143]. The tri-specific antibody also demonstrated favorable pharmacokinetic profiles in non-human primates animal models [143].
Zanidatamab has a special biparatopic structure (bispecific antibody) targeting 2 distinct HER2 epitopes including ECD2 and ECD4. These unique characteristics allow antibodies to bind to HER2 molecules in trans and initiate HER2 reorganization. Zanidatamab exerts several mechanisms of action, such as inhibiting intracellular signaling, ADCC, and ADCP, but strongly induces complement-dependent cytotoxicity against HER2-positive breast, gastric, esophageal, and lung cancer. This functionality is not observed with trastuzumab, pertuzumab, or a combination of both [147]. For in vivo xenograft and PDX models, zanidatamab illustrated better antitumor activity than the combination of trastuzumab and pertuzumab [147]. However, the mechanisms of CCA cells need further study. The antibody is now being evaluated and provides clinical benefit in the clinical trial phase 2 for HER2-altered CCA patients [118,148]. Zanidatamab has shown significant clinical benefits while maintaining a manageable safety profile against HER2-amplified CCA patients. With an ORR of 41% and 68% of disease control rate (DCR). A total of 29 out of 87 participants (33%) experienced side effects such as chills, fever, or high blood pressure. There were no grade 4 treatment-related adverse events and no treatment-related deaths [118].
Zeno, the bispecific humanized antibody (IgG1), contains two different Fabs that target the extracellular domain of HER2 and HER3. The binding against HER3 prevents NRG1 from binding to HER3 in order to prohibit heterodimerization with HER2 [149–151]. Zeno illustrated growth inhibition by cell cycle arrest as well as induction of ADCC against NRG1-altered breast and lung cancer cell lines in preclinical in vitro, SLC3A2-NRG1 and CD74-NRG1 gene fusions lung cancer patient-derived xenograft models (PDXs) showed suppressed growth inhibition and tumor regression. The Zeno is now being FDA-reviewed for use in Neuregulin 1 fusion (NRG1+) non-small cell lung cancer (NSCLC) and NRG1+ pancreatic cancers (PDAC).
Cell engager strategies may offer advantages over traditional therapeutic antibodies, including better tumor penetration because of their smaller size. They can also target multiple tumor-associated antigens, which may improve specificity and prevent the shedding of tumor antigens. Additionally, they may allow for the engagement of co-stimulatory domains of immune effector cells, potentially enhancing the immune response to cancer cells. Targeting HER2 along with other tumor surface molecules, such as FGFR or PD-L1, using multi-specific cell engagers may serve as a viable therapeutic option for HER2-altered CCA shortly.
Adoptive T-cell therapies (ACT) have become one of the pillars of cancer immunotherapy since the PD1/PD-L1 axis was discovered. Three platforms for ACTs currently exist: first, using isolated tumor-specific T-cells from (tumor-infiltrating lymphocytes; TIL) of patients’ samples, which are ex vivo expanded and therapeutically reinfused. Secondly, isolated T-cells from peripheral blood mononuclear cells (PBMCs) were genetically modified to express specific T-cell receptors (TCRs) that target cancer-specific antigens [152]. Third, T-cell modification using a fully synthetic chimeric antigen receptor (CAR) [153,154] with specific characteristics to recognize the desired tumor antigen. Unlike the physiological TCRs, CARs could recognize cancer antigens regardless of the major histocompatibility complex (MHC). This particular advantage appears when MHC expression is lost due to the cancer’s immune evasion mechanism. CAR consists of three primary components: the extracellular domain, which is primarily for recognizing tumor-specific antigens; the hydrophobic transmembrane domain; and the intracellular domain (endodomain) responsible for downstream signal transduction following antigen binding. The cytoplasmic T cell co-receptor, T-cell surface glycoprotein CD3 zeta chain (CD3ζ), contains three immunoreceptor tyrosine-based activation motifs (ITAMs), which are essential components for most CARs [155].
Nowadays, five generations of CAR T-cells have been created based on the composition of the cytoplasmic domains. The first generation of CAR contains only a single CD3ζ region, which lacks co-stimulatory domains such as CD28, CD27, and CD134, and cytokines, such as IL-2 signaling [155,156]. As a result, the first generation of CAR demonstrated low proliferation and cytotoxicity in the initial experiments. The second generation of CAR included co-stimulating parts such as CD28 or CD137 in the cytoplasmic domain to enhance proliferation and activate cytotoxic killing of T-cells [157,158]. The third generation was developed by further extending the co-stimulatory domain from the second generation, including CD134 and CD137. Additionally, the incorporation of cytokine production, specifically interleukin 12 (IL-12), which is consistently expressed upon CAR activation, was designed for the fourth generation, often referred to as T cells redirected for universal cytokine-mediated killing (TRUCK). The activation of this CAR construct offers a tumor-killing effect via multiple synergistic mechanisms such as perforin, granzyme exocytosis, and death ligand-death receptors (Fas-FasL, TRAIL) [156,159]. The fifth generation is now being established and evaluated. The fifth construct is also based on a second-generation construct but comprises a truncated cytoplasmic IL-2 receptor beta chain with a STAT3 binding site. Of this strategy, once tumor antigen activation of the receptors simultaneously with TCR (through CD3ζ regions) along with CD28, the co-stimulatory regions, as well as JAK-STAT3/5 cytokine signaling, fully drive T-cell proliferation and activation [160].
Since the first CAR-T cell was developed, it has demonstrated promising profiles in several clinical trials against CD19-positive hematological malignancies such as acute B-cell lymphoblastic leukemia with unprecedented clinical benefits of up to 81% [161–163]. Now, the FDA has approved six CAR-T cell therapies for clinical use [164–166]. Since CAR-T cell therapies offer an excellent clinical profile against hematological malignancies. Up to 80% ORR and patients are going into complete remission with minimal side effects [167–169]. Nowadays, several CARs have been modified to fight against solid mass-forming malignant antigens such as disialoganglioside (GD2), L1 cell adhesion molecule (CD171), folate-binding receptors, HER2/ERBB2, and vascular endothelial growth factor receptors 2 (VEGFR2) [170]. In contrast to blood cancers, CAR-T cell therapies failed to provide significant clinical benefits in solid tumors and led to severe or lethal side effects reported in several attempts [171–173]. Several challenges have to be considered, such as intra-tumor heterogeneity (ITH), hypoxic conditions, physical barriers, poor vascularization, shedding/loss of tumor antigens, tumor microenvironments, infiltrating T-cells, etc. Together with these reasons, targeting mass-forming cancers with CAR T-cell therapies still has many obstacles to be concerned with. For example, increasing tumor recognition of T-cells, preventing T-cell exhaustion, and increasing tumor-mass infiltration of T-cells. Various strategies have been applied to enhance T-cell tumor recognition and activation, such as CAR T cells secreting cell-engaging molecules, and the cancer vaccine with the mechanisms to emphasize antigen presentation of various solid tumor antigens, enabling protective immunity against cancers [154].
10 The Clinical Trials of HER2 Targeting Immunotherapy in HER2 Altered CCAs
While there isn’t a specific standard just for CCA, the scoring system used for IHC, based on criteria from breast and stomach cancers, defines HER2-positive results as either an IHC score of 3+ or an IHC score of 2+ combined with a positive result from ISH [174]. However, next-generation sequencing (NGS) is recommended for advanced CCA patients. In case of insufficient tumor biopsy samples, circulating tumor DNA (ctDNA) from patients could be used as an alternative way, with an 80% predictive value for HER2 positivity [175]. Recently, several clinical trials demonstrated the benefit of HER2 targeting for immunotherapy in HER2-altered-CCA patients (Table 4). For instance, trastuzumab in combination with tucatinib, a kinase inhibitor, demonstrated a favorable ORR: 46.7% [176]. Moreover, in the KCSG-HB19-19 trial, trastuzumab with FLOFOX regimen (leucovorin, 5-FU, and oxaliplatin) illustrated a favorable disease control rate of 79.4%, however, a lower ORR: 29.4% in 34 patients with HER2-positive CCA (IHC3+ or 2+ with ISH amplification) [177]. Zanidatamab, the biparatotic targeting HER2 antibody, revealed meaningful activity in clinical trials against HER2-positive CCA (IHC3+ or 2+ and ISH+), such a rapid and durable response, safety profile with ORR: 41%, disease control rate (DCR) 68.8% and duration of response (DoR) 12.9 months [118]. Interestingly, blocking the interaction of PD-L1/PD1 using the human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody, durvalumab plus gemcitabine and cisplatin as first-line therapy, demonstrated a shorter overall survival trend in the ERBB2 altered CCA subtype. Supporting the evidence that no clinical benefit to including durvalumab in the strategy [178].
In addition, HER2-targeted ADCs such as T-Dxd exert antitumor activities against a broad range HER2 HER2-expressing breast and gastric cancers in clinical trials and clinical use. Likewise, in CCA, T-Dxd achieved disease control rates up to 75.0% and 81.8% of HER2-low and HER2-positive CCA patients, respectively [180]. Moreover, 36.4% of ORR and 7.4 months of DoR have been observed in 8 HER2-positive CCA patients (five patients for IHC3+, three patients for IHC2+/ISH+ patients, and one HER2-low) [180]. A similar trend has been reported in the DESTINY-panTumor02 clinical trial showed that T-Dxd offers 56.3% ORR in 16 IHC3+ CCA patients [181]. This might be evidenced that T-Dxd possesses potent anti-tumor activity across subtypes and a broad range of HER2-expressing CCAs. However, the mechanisms underlying T-Dxd against CCA have not been fully explored and urgently need further preclinical studies supporting this point of view. Together, these suggest the potential application of specific HER2 targeting immunotherapy for HER2-altered CCA clusters with favorable clinical outcomes and emphasize further developing alternative treatment strategies for CCA patients [118,179,182].
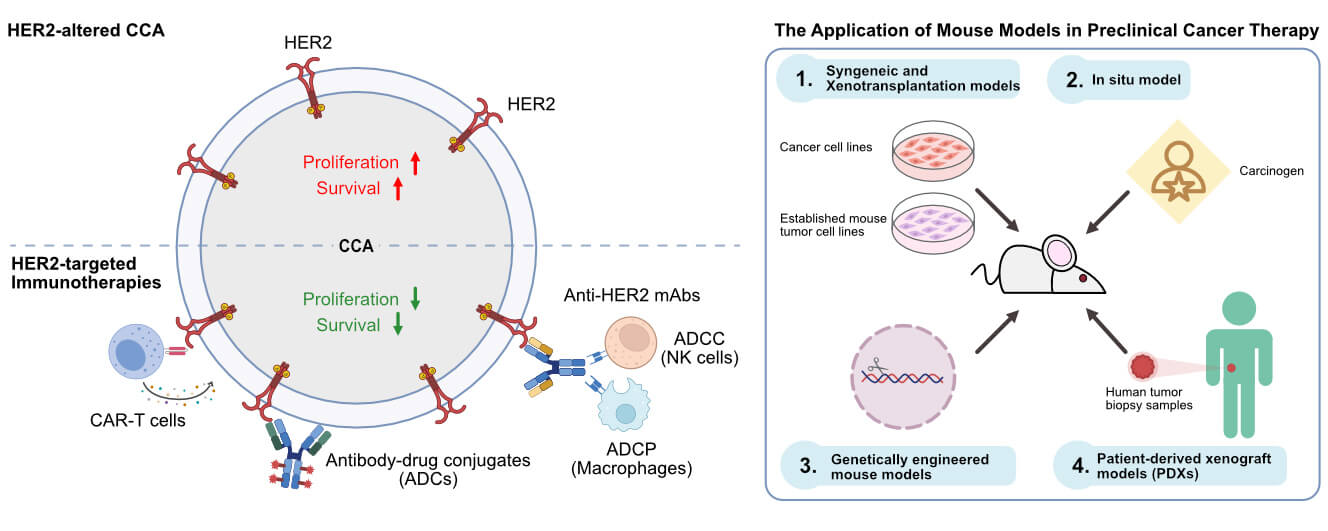
11 The Application of Mouse Models in Preclinical Cancer Therapy
Nowadays, it is undeniable that multi-omics studies have revealed several novel potential therapeutic target molecules for cancer therapy, including CCA. However, only the studies from several in vitro models are insufficient to recapitulate the cancer’s pathophysiological environment and complexity in the human body, as evidenced by the substantial failure rate of compounds during the drug development and discovery processes. To unravel the complexities inherent in cancer, mouse models have become a pivotal bridge between in vitro evaluation and clinical practice. The development of preclinical animal mouse models allows the researcher to understand the multidimensionality of various crucial aspects of cancer. Rodentia mainly comprises mice, rats, and guinea pigs, which are at the forefront of the preclinical animal models, particularly anti-cancer drug developments. Four are prevalently employed for cancer studies, including Syngeneic and Xenotransplantation, in situ model, genetically engineered, and PDXs.
1.) Syngeneic and Xenotransplantation models: Syngeneic transplant, also known as the allograft mouse models, refers to the use of immunodeficient mice as recipients of cancerous cells derived from mice or genetically similar rodents. While the xenograft models refer to the use of immunodeficient mice as recipients of human cancerous cells or primary tumor tissue from patients (transplant cells or tissues between different species) [183,184]. Each transplantation has its advantages and disadvantages. Syngeneic models provide rapid and reproducible tumor growth, which is easy to manipulate. However, it may not fully recapitulate the complexity of human tumors and demonstrate immune phenotypic differences [185]. In addition, the species available for syngeneic models are limited. Xenotransplantation offers various advantages among animal models and human cancers and more accurately reflects original human tumor architecture and microenvironments. However, the mismatch between tumor and host-microenvironment in different species might alter the molecular, immune, and cellular differences. This hinders this model and is also considered one of its weak points.
2.) In situ model: This is the in vivo tumor model that involves the induction of tumorigenesis in mice at the specific original site [186]. These offer more realistic tumor development and progressions, such as local invasion, and distal metastatic spread of the primary site, than the classical Syngeneic models [187]. The methods mainly include the induction of tumor initiation using ultimate chemical carcinogens or utilizing gene editing technology such as CRISPR to induce malignant transformation in mice through knock-out tumor suppressor genes or overexpressing oncogenes. However, one of the seemingly obvious disadvantages is that chemical carcinogens such as Dimethyl-Benz(a)anthracene (DMBA) and Azoxymethane (AOM) have toxic effects on normal adjacent tissue, and gene editing techniques are still relatively costly, complex, and limited in success rates.
3.) Genetically engineered mouse models: refer to mouse models that are established by editing key regulatory genes that relate to human tumorigenesis at the mouse’s genome level to induce spontaneous cancer initiation and pathological progression. This model provides a systemic investigation of the potent oncogenes or tumor suppressor genes, as well as studies the association of targeted anti-cancer drugs and related genes.
4.) Patient-derived xenograft models (PDXs): Xenotransplantation models using in vitro human cancer cell lines in mouse models are not always representative of the fundamental pathophysiology of human tumors. As for cancer cell line transplantation in mice often lacks tumor heterogeneity, intertumoral microenvironment, tumor-related matrix interaction, etc. Cancer cell lines somehow lost their original features of the original cancerous tissues after transplantation [188]. These might contribute to the distinct drug response and make it difficult to predict drug response at the clinical level or lack clinical relevance. Of these, PDX models, which refer to the transplantation of fresh biopsy tumor samples from a patient into immunodeficient mice, could overcome these drawbacks as they preserve the original tumor architecture and tumor microenvironment of the patient’s cancer tissue. PDX models illustrate extreme clinical relevance and predictive value for the translation of preclinical to clinical level. Moreover, this model also offers the potential in sign into treatment efficacy for precision medicine [189]. Nowadays, several immunodeficient mice have been developed and are available for PDX models. For example, NOD/SCID (NOD.Cg-Prkdcscid), which lacks mature B- and T-cells, and has impairment of NK-cells and macrophage functions [190], NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ), which is one of the most widely used mice as it offers a severe immunodeficient phenotype due to no mature B-, T-cells, no NK-cells, and impaired macrophage/dendritic functions [191], and BALB/c Rag2-/-Jak3-/- (BRJ), which is characterized by a lack of mature B- and T-cells as well as NK-cells [192]. Interestingly, BRJ mice have been used as alternative recipients of CCA in both PDXs and CCA cell lines, showing a higher engraftment rate of CCA PDXs up to 75% [193]. Nowadays, BRJ mice have gained popularity in CCA preclinical research in both testing small-molecule inhibitors [194] and immunotherapy [73] as they offer not only a higher success rate of CCA transplantation but also a quality preservation of the original characteristic of the tumor, and the price is relatively lower than other strains.
The PDX platform illustrates a robust preclinical model that bridges preclinical and clinical evaluation. Nevertheless, the PDX model still requires further optimization and development. Human stromal components are still rapidly replaced with murine stromal cells during engraftment and passage of PDX [195]. The immune humanized cancer PDX model (firstly transplanted with human hematopoietic stem cells CD34+ for reconstitution of the human immune cells and followed by PDX transplantation) might be a better relevant model for studying the cancer-related immune response in immunotherapy. Further study is required to improve the PDX model as one of the most promising preclinical platforms.
These insights highlight potential future directions for CCA immunotherapy, focusing on HER2 alterations. As demonstrated in the previous literature, HER2 alteration is dominant in the liver-fluke CCA (most of the Asian CCAs) as well as some small fraction of the Western world’s CCAs, and HER2 alterations are also significantly associated with inferior prognosis of CCA patients. Thus, targeting HER2-altered CCA is urgently needed. Currently, several strategies have been developed for HER2-altered cancers (Fig. 2) such as ADCs which precisely deliver cytotoxic warheads to kill cancer cells directly as well as exert bystander-killing effect against tumor microenvironment cells. Moreover, ADCs such as T-Dxd also possessed the anti-tumor mechanisms of conventional trastuzumab. Not only cytotoxic drug delivery systems but mAbs such as trastuzumab and pertuzumab are now applied for cancer theranostics in radioimmunotherapy as well. Although modification of whole mAbs demonstrated promising clinical profiles against HER2-positive tumors. However, there are still some insurmountable limitations of mAbs such as a more extended blood clearance period, inability to pass through the blood-brain barrier, and the tumor’s physical barrier, etc. These drawbacks minimize the therapeutic efficacy of mAbs against cancer, such as brain metastatic disease, or cause undesirable outcomes, such as nephrotoxicity and hepatotoxicity. Affibodies and nanobodies, which demonstrate quicker blood clearance and better tumor penetration due to their relatively smaller size, have been further developed to address these limitations. Both preclinical and clinical data showed quicker blood clearance and better blood-brain barrier penetration of radiolabeled HER2-targeted nanobodies in molecular imaging of brain metastatic lesions of HER2-positive breast cancer. This might be possible to apply in CCA, not only for molecular imaging but also for therapeutic options, such as nanobody drug conjugate, which should be considered for further study. Raising further with multi-specific cell engager strategies that revolutionize monoclonal antibody therapy by at least one arm targeting tumor-associated antigens, and one or two arms targeting co-stimulatory receptors of immune effector cells, or targeting additional tumor-specific antigens to increase cancer-specific binding and prevent cancer immune escape. Many engaging molecules exert multiple anti-cancer mechanisms in both preclinical and clinical studies. However, further investigation is necessary to avoid or minimize undesirable side effects such as cytokine release syndrome (CRS) as well as the development of CAR cells secreting engager molecules against HER2-altered CCA. In non-fluke-associated cholangiocarcinoma (most Western CCAs), molecular classification has identified specific clusters enriched with PD-1, PD-L1, and PD-L2 expression signatures [15,54,61], which may derive more significant clinical benefit from durvalumab in combination with gemcitabine and cisplatin [34]. We also provided strong evidence from the current stage of literature about clinical trials of targeting HER2-altered CCA using several strategies of immunotherapy that provide favorable clinical outcomes and emerging HER2 as the potential target for CCA immunotherapy shortly.

Figure 2: HER2 targeting immunotherapy platforms Overview of HER2 targeting strategies for immunotherapy against CCA (Created with Affinity Designer 2 software)
Acknowledgement: In this section, we are grateful to all the scientists and clinicians worldwide who dedicate themselves to research and study, and to providing the best treatments and care for patients with CCA.
Funding Statement: This work was supported in part by the e-ASIA Joint Research Program from the Japan Agency for Medical Research and Development (AMED) (grant number: 21jm0210062h0004). The funders had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Author Contributions: Prin Sungwan: Conceptualization, investigation, writing—original draft. Jutatip Panaampon: Conceptualization, investigation, writing—review & editing. Ratchaneewan Sumankan: Writing—original draft. Genki Aoki: Writing—review & editing. Seiji Okada: Conceptualization, investigation, writing—review & editing. supervision, project administration, funding acquisition. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Abbreviations
| ACT | Adoptive T-cell therapies |
| ADC | Antibody-drug-conjugate |
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| ADCP | Antibody-dependent cellular phagocytosis |
| AOM | Azoxymethane |
| CAFs | Cancer-associated fibroblasts |
| CAR | Chimeric antigen receptor |
| CCA | Cholangiocarcinoma |
| CDC | Complement-dependent cytolysis |
| CiTEs | Bifunctional checkpoint-inhibitory T-cell engagers |
| CRS | Cytokine release syndrome |
| ctDNA | Circulating tumor DNA |
| DAR | Drug antibody ratio |
| DART | Dual affinity re-targeting |
| DCR | Disease control rate |
| DMBA | Dimethyl-Benz(a)anthracene |
| DoR | Duration of response |
| ECD2 | Extracellular domain 2 |
| ECD4 | Extracellular domain 4 |
| Fab | Fragment antibody binding region |
| FAP | Fibroblast activation protein |
| FDA | Food and Drug Administration |
| FGFR | Fibroblast growth factor receptor |
| 5-FU | 5-fluorouracil |
| HcAb | Heavy chain antibodies |
| HER2 | Human epidermal growth factor receptor 2 |
| ISH | In-situ hybridization |
| ITH | Intra-tumor heterogeneity |
| NGS | Next-generation sequencing |
| NIR-PIT | Near-infrared photoimmunotherapy |
| NSCLC | Non-small cell lung cancer |
| ORR | Overall response rate |
| PBD-MA | Pyrrolo [2,1- c][1,4]benzodiazepine monoamide |
| PDX | Patient-derived xenograft |
| SE-Asia | Southeast Asia countries |
| SMITEs | Simultaneous multiple interaction BiTEs |
| SPECT/CT | Single Photon Emission Computed Tomography scan and Computed Tomography |
| TAA | Tumor-associated antigens |
| TCR | T-cell receptors |
| T-DM1 | Trastuzumab emtansine |
| T-Dxd | Trastuzumab deruxtecan |
| TIL | Tumor-infiltrating lymphocytes |
| TKIs | Tyrosine kinase inhibitors |
| TOP1 | Topoisomerase I |
| TriKE | Trispecific Killer Engagers |
| TriTE | Trispecific T-cell engagers |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
References
1. Ramírez-Merino N, Aix SP, Cortés-Funes H. Chemotherapy for cholangiocarcinoma: an update. World J Gastrointest Oncol. 2013;5(7):171–6. doi:10.4251/wjgo.v5.i7.171. [Google Scholar] [PubMed] [CrossRef]
2. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. doi:10.1056/nejmoa0908721. [Google Scholar] [PubMed] [CrossRef]
3. Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New horizons for precision medicine in biliary tract cancers. Cancer Discov. 2017;7(9):943–62. doi:10.1158/2159-8290.cd-17-0245. [Google Scholar] [PubMed] [CrossRef]
4. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–88. doi:10.1038/s41575-020-0310-z. [Google Scholar] [PubMed] [CrossRef]
5. Li X, Guan R, Zhang S. Factors contributing to the high malignancy level of cholangiocarcinoma and its epidemiology. Literat Rev Data Biol. 2025;14(4):351. doi:10.3390/biology14040351. [Google Scholar] [PubMed] [CrossRef]
6. Liao Z, Yao Z, Yang Z, Yang S, Gu W, Wang H, et al. Comparative efficacy of targeted therapy, chemotherapy and their combination for advanced cholangiocarcinoma: a systematic review and network meta-analysis. PeerJ. 2025;13(6):e19386. doi:10.7717/peerj.19386. [Google Scholar] [PubMed] [CrossRef]
7. Thongprasert S. The role of chemotherapy in cholangiocarcinoma. Ann Oncol. 2005;16(Supplement 2):ii93–6. [Google Scholar] [PubMed]
8. Qurashi M, Vithayathil M, Khan SA. Epidemiology of cholangiocarcinoma. Eur J Surg Oncol. 2025;51(2):107064. doi:10.1016/j.ejso.2023.107064. [Google Scholar] [PubMed] [CrossRef]
9. Li Y, Yu J, Zhang Y, Peng C, Song Y, Liu S. Advances in targeted therapy of cholangiocarcinoma. Ann Med. 2024;56(1):2310196. doi:10.1080/07853890.2024.2310196. [Google Scholar] [PubMed] [CrossRef]
10. Luvira V, Nilprapha K, Bhudhisawasdi V, Pugkhem A, Chamadol N, Kamsa-ard S. Cholangiocarcinoma patient outcome in northeastern Thailand: single-center prospective study. Asian Pac J Cancer Prev. 2016;17(1):401–6. doi:10.7314/apjcp.2016.17.1.401. [Google Scholar] [PubMed] [CrossRef]
11. Wittekind C, Compton C, Quirke P, Nagtegaal I, Merkel S, Hermanek P, et al. A uniform residual tumor (R) classification: integration of the R classification and the circumferential margin status. Cancer. 2009;115(15):3483–8. doi:10.1002/cncr.24320. [Google Scholar] [PubMed] [CrossRef]
12. Hermanek P, Wittekind C. The pathologist and the residual tumor (R) classification. Pathol-Res Pract. 1994;190(2):115–23. doi:10.1016/s0344-0338(11)80700-4. [Google Scholar] [PubMed] [CrossRef]
13. Jacobi O, Ross JS, Goshen-Lago T, Haddad R, Moore A, Sulkes A, et al. ERBB2 pathway in biliary tract carcinoma: clinical implications of a targetable pathway. Oncol Res Treat. 2021;44(1–2):20–7. doi:10.1159/000511919. [Google Scholar] [PubMed] [CrossRef]
14. Iqbal N, Iqbal N. Human epidermal growth factor receptor 2 (HER2) in cancers: overexpression and therapeutic implications. Mol Biol Int. 2014;2014:852748. doi:10.1155/2014/852748. [Google Scholar] [PubMed] [CrossRef]
15. Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7(10):1116–35. doi:10.1158/2159-8290.CD-17-0368. [Google Scholar] [PubMed] [CrossRef]
16. Kam AE, Masood A, Shroff RT. Current and emerging therapies for advanced biliary tract cancers. Lancet Gastroent Hepatol. 2021;6(11):956–69. doi:10.1016/s2468-1253(21)00171-0. [Google Scholar] [PubMed] [CrossRef]
17. Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13(5):261–80. doi:10.1038/nrgastro.2016.51. [Google Scholar] [PubMed] [CrossRef]
18. Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, et al. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7(1):65. [Google Scholar] [PubMed]
19. Huang YL, Zhang KY, Sun YL, Qian MB, Wang Z. The risk of hepatobiliary complications in Clonorchis and Opisthorchis infection: a systematic review and meta-analysis. Acta Trop. 2024;260(10):107457. doi:10.1016/j.actatropica.2024.107457. [Google Scholar] [PubMed] [CrossRef]
20. Khan AS, Dageforde LA. Cholangiocarcinoma. Surg Clin North Am. 2019;99(2):315–35. [Google Scholar] [PubMed]
21. Sripa B, Suwannatrai AT, Sayasone S, Do DT, Khieu V, Yang Y. Current status of human liver fluke infections in the Greater Mekong Subregion. Acta Trop. 2021;224(5):106133. doi:10.1016/j.actatropica.2021.106133. [Google Scholar] [PubMed] [CrossRef]
22. DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–62. doi:10.1097/01.sla.0000251366.62632.d3. [Google Scholar] [PubMed] [CrossRef]
23. Yoo WG, Sohn WM, Na BK. Current status of Clonorchis sinensis and clonorchiasis in Korea: epidemiological perspectives integrating the data from human and intermediate hosts. Parasitology. 2022;149(10):1296–305. doi:10.1017/s0031182022000798. [Google Scholar] [PubMed] [CrossRef]
24. Elvevi A, Laffusa A, Scaravaglio M, Rossi RE, Longarini R, Stagno AM, et al. Clinical treatment of cholangiocarcinoma: an updated comprehensive review. Ann Hepatol. 2022;27(5):100737. doi:10.1016/j.aohep.2022.100737. [Google Scholar] [PubMed] [CrossRef]
25. Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69(2):223–40. doi:10.2165/00003495-200969020-00006. [Google Scholar] [PubMed] [CrossRef]
26. Jusakul A, Kongpetch S, Teh BT. Genetics of Opisthorchis viverrini-related cholangiocarcinoma. Curr Opin Gastroenterol. 2015;31(3):258–63. doi:10.1097/mog.0000000000000162. [Google Scholar] [PubMed] [CrossRef]
27. Adeva J, Sangro B, Salati M, Edeline J, La Casta A, Bittoni A, et al. Medical treatment for cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):123–42. doi:10.1111/liv.14100. [Google Scholar] [PubMed] [CrossRef]
28. Khosla D, Misra S, Chu PL, Guan P, Nada R, Gupta R, et al. Cholangiocarcinoma: recent advances in molecular pathobiology and therapeutic approaches. Cancers. 2024;16(4):801. doi:10.3390/cancers16040801. [Google Scholar] [PubMed] [CrossRef]
29. Chen JS, Hsu C, Chiang NJ, Tsai CS, Tsou HH, Huang SF, et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann Oncol. 2015;26(5):943–9. doi:10.1093/annonc/mdv035. [Google Scholar] [PubMed] [CrossRef]
30. Lindner P, Rizell M, Hafstrom L. The impact of changed strategies for patients with cholangiocarcinoma in this millenium. HPB Surg. 2015;2015:736049. doi:10.1155/2015/736049. [Google Scholar] [PubMed] [CrossRef]
31. Kamsa-Ard S, Luvira V, Suwanrungruang K, Kamsa-Ard S, Luvira V, Santong C, et al. Cholangiocarcinoma trends, incidence, and relative survival in Khon Kaen, Thailand From 1989 through 2013: a population-based cancer registry study. J Epidemiol. 2019;29(5):197–204. doi:10.2188/jea.je20180007. [Google Scholar] [PubMed] [CrossRef]
32. Wang J, Liu S, Cao Y, Chen Y. Overcoming treatment resistance in cholangiocarcinoma: current strategies, challenges, and prospects. Front Cell Dev Biol. 2024;12:1408852. doi:10.3389/fcell.2024.1408852. [Google Scholar] [PubMed] [CrossRef]
33. Burris HA3rd, Okusaka T, Vogel A, Lee MA, Takahashi H, Breder V, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer (TOPAZ-1patient-reported outcomes from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2024;25(5):626–35. doi:10.1016/s1470-2045(24)00082-2. [Google Scholar] [PubMed] [CrossRef]
34. Rimini M, Fornaro L, Rizzato MD, Antonuzzo L, Rossari F, Satake T, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: a large real-life worldwide population. Eur J Cancer. 2024;208:114199. doi:10.1016/j.annonc.2024.05.299. [Google Scholar] [CrossRef]
35. Strijker M, Belkouz A, van der Geest LG, van Gulik TM, van Hooft JE, de Meijer VE, et al. Treatment and survival of resected and unresected distal cholangiocarcinoma: a nationwide study. Acta Oncol. 2019;58(7):1048–55. doi:10.1080/0284186x.2019.1590634. [Google Scholar] [PubMed] [CrossRef]
36. Groot Koerkamp B, Wiggers JK, Allen PJ, Besselink MG, Blumgart LH, Busch OR, et al. Recurrence rate and pattern of perihilar cholangiocarcinoma after curative intent resection. J Am Coll Surg. 2015;221(6):1041–9. doi:10.1016/j.hpb.2016.01.503. [Google Scholar] [CrossRef]
37. Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Soreide K, Wigmore SJ, et al. Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma. Ann Surg. 2021;273(2):240–50. doi:10.1097/sla.0000000000003801. [Google Scholar] [PubMed] [CrossRef]
38. Boku N. HER2-positive gastric cancer. Gast Cancer. 2014;17(1):1–12. doi:10.1007/s10120-013-0252-z. [Google Scholar] [PubMed] [CrossRef]
39. Lei S, Appert HE, Nakata B, Domenico DR, Kim K, Howard JM. Overexpression of HER2/neu oncogene in pancreatic cancer correlates with shortened survival. Int J Pancreatol. 1995;17(1):15–21. doi:10.1007/bf02788354. [Google Scholar] [PubMed] [CrossRef]
40. Schlam I, Swain SM. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. npj Breast Cancer. 2021;7(1):56. doi:10.1038/s41523-021-00265-1. [Google Scholar] [PubMed] [CrossRef]
41. Rizzo A, Ricci AD, Bonucci C, Tober N, Palloni A, Frega G, et al. Experimental HER2- targeted therapies for biliary tract cancer. Expert Opin Investig Drugs. 2021;30(4):389–99. doi:10.1080/13543784.2021.1854724. [Google Scholar] [PubMed] [CrossRef]
42. Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. Embo J. 1997;16(7):1647–55. doi:10.1093/emboj/16.7.1647. [Google Scholar] [PubMed] [CrossRef]
43. Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–87. doi:10.1038/sj.onc.1210477. [Google Scholar] [PubMed] [CrossRef]
44. Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–16. doi:10.1038/nrm1962. [Google Scholar] [PubMed] [CrossRef]
45. Li C, Yuan Q, Deng T, Xu G, Hou J, Zheng L, et al. Prognosis difference between HER2-low and HER2-zero breast cancer patients: a systematic review and meta-analysis. Breast Cancer. 2023;30(6):965–75. doi:10.1007/s12282-023-01487-w. [Google Scholar] [PubMed] [CrossRef]
46. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi:10.1126/science.3798106. [Google Scholar] [PubMed] [CrossRef]
47. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–12. doi:10.1126/science.2470152. [Google Scholar] [PubMed] [CrossRef]
48. Cortes J, Dieras V, Ro J, Barriere J, Bachelot T, Hurvitz S, et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3a randomised, open-label, multicentre, phase 2 trial. Lancet Oncol. 2015;16(16):1700–10. doi:10.1016/s1470-2045(15)00373-3. [Google Scholar] [PubMed] [CrossRef]
49. Harbeck N, Huang CS, Hurvitz S, Yeh DC, Shao Z, Im SA, et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1an open-label, randomised, phase 3 trial. Lancet Oncol. 2016;17(3):357–66. doi:10.1016/s1470-2045(15)00540-9. [Google Scholar] [PubMed] [CrossRef]
50. Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu Y, et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J Clin Oncol. 2019;37(29):2610–9. doi:10.1200/jco.19.00108. [Google Scholar] [PubMed] [CrossRef]
51. Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597–609. doi:10.1056/nejmoa1914609. [Google Scholar] [PubMed] [CrossRef]
52. Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357(1):39–51. doi:10.1056/nejmra043186. [Google Scholar] [PubMed] [CrossRef]
53. Saura C, Oliveira M, Feng YH, Dai MS, Chen SW, Hurvitz SA, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with >/= 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–49. doi:10.1200/jco.2019.37.15_suppl.1002. [Google Scholar] [CrossRef]
54. Montal R, Sia D, Montironi C, Leow WQ, Esteban-Fabro R, Pinyol R, et al. Molecular classification and therapeutic targets in extrahepatic cholangiocarcinoma. J Hepatol. 2020;73(2):315–27. doi:10.1016/s0168-8278(18)30223-x. [Google Scholar] [CrossRef]
55. Kiguchi K, Carbajal S, Chan K, Beltrán L, Ruffino L, Shen J, et al. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61(19):6971–6. [Google Scholar] [PubMed]
56. Treekitkarnmongkol W, Suthiphongchai T. High expression of ErbB2 contributes to cholangiocarcinoma cell invasion and proliferation through AKT/p70S6K. World J Gastroenterol. 2010;16(32):4047–54. doi:10.1158/1078-0432.tcmusa10-a24. [Google Scholar] [CrossRef]
57. Thamrongwaranggoon U, Detarya M, Seubwai W, Saengboonmee C, Hino S, Koga T, et al. Lactic acidosis promotes aggressive features of cholangiocarcinoma cells via upregulating ALDH1A3 expression through EGFR axis. Life Sci. 2022;302:120648. doi:10.1016/j.lfs.2022.120648. [Google Scholar] [PubMed] [CrossRef]
58. Detarya M, Thaenkaew S, Seubwai W, Indramanee S, Phoomak C, Saengboonmee C, et al. High glucose upregulates FOXM1 expression via EGFR/STAT3 dependent activation to promote progression of cholangiocarcinoma. Life Sci. 2021;271:119114. doi:10.1016/j.lfs.2021.119114. [Google Scholar] [PubMed] [CrossRef]
59. Theocharopoulos C, Ziogas IA, Mungo B, Gogas H, Ziogas DC, Kontis E. HER2-targeted therapies: unraveling their role in biliary tract cancers. Crit Rev Oncol Hematol. 2025;208(6):104655. doi:10.1016/j.critrevonc.2025.104655. [Google Scholar] [PubMed] [CrossRef]
60. Galdy S, Lamarca A, McNamara MG, Hubner RA, Cella CA, Fazio N, et al. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: a potential therapeutic target? Cancer Metastasis Rev. 2017;36(1):141–57. doi:10.1007/s10555-016-9645-x. [Google Scholar] [PubMed] [CrossRef]
61. Putatunda V, Jusakul A, Roberts L, Wang XW. Genetic, epigenetic, and microenvironmental drivers of cholangiocarcinoma. Am J Pathol. 2025;195(3):362–77. doi:10.1016/j.ajpath.2024.10.013. [Google Scholar] [PubMed] [CrossRef]
62. Chaisaingmongkol J, Budhu A, Dang H, Rabibhadana S, Pupacdi B, Kwon SM, et al. Common molecular subtypes among asian hepatocellular carcinoma and cholangiocarcinoma. Cancer Cell. 2017;32(1):57–70.e3. doi:10.1016/j.ccell.2017.05.009. [Google Scholar] [PubMed] [CrossRef]
63. Hiraoka N, Nitta H, Ohba A, Yoshida H, Morizane C, Okusaka T, et al. Details of human epidermal growth factor receptor 2 status in 454 cases of biliary tract cancer. Hum Pathol. 2020;105:9–19. doi:10.1016/j.humpath.2020.08.006. [Google Scholar] [PubMed] [CrossRef]
64. Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clini Cancer Res. 2018;24(17):4154–61. doi:10.1158/1078-0432.ccr-18-0078. [Google Scholar] [PubMed] [CrossRef]
65. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi:10.1056/nejm200103153441101. [Google Scholar] [PubMed] [CrossRef]
66. Sawyers CL. Herceptin: a first assault on oncogenes that launched a revolution. Cell. 2019;179(1):8–12. doi:10.1016/j.cell.2019.08.027. [Google Scholar] [PubMed] [CrossRef]
67. Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2010;28(1):92–8. doi:10.1200/jco.2008.19.9844. [Google Scholar] [PubMed] [CrossRef]
68. Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi:10.1056/NEJMoa052306. [Google Scholar] [PubMed] [CrossRef]
69. Mittendorf EA, Liu Y, Tucker SL, McKenzie T, Qiao N, Akli S, et al. A novel interaction between HER2/neu and cyclin E in breast cancer. Oncogene. 2010;29(27):3896–907. doi:10.1038/onc.2010.151. [Google Scholar] [PubMed] [CrossRef]
70. Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–96. doi:10.1200/jco.2007.14.8957. [Google Scholar] [PubMed] [CrossRef]
71. Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10(17):5650–5. doi:10.1158/1078-0432.ccr-04-0225. [Google Scholar] [PubMed] [CrossRef]
72. Tsao LC, Crosby EJ, Trotter TN, Agarwal P, Hwang BJ, Acharya C, et al. CD47 blockade augmentation of trastuzumab antitumor efficacy dependent on antibody-dependent cellular phagocytosis. JCI Insight. 2019;4(24):e131882. doi:10.1172/jci.insight.131882. [Google Scholar] [PubMed] [CrossRef]
73. Panaampon J, Sungwan P, Fujikawa S, Sampattavanich S, Jirawatnotai S, Okada S. Trastuzumab, a monoclonal anti-HER2 antibody modulates cytotoxicity against cholangiocarcinoma via multiple mechanisms. Int Immunopharmacol. 2024;138(2):112612. doi:10.1016/j.intimp.2024.112612. [Google Scholar] [PubMed] [CrossRef]
74. Robert M, Frenel JS, Bourbouloux E, Berton Rigaud D, Patsouris A, Augereau P, et al. Pertuzumab for the treatment of breast cancer. Expert Rev Anticancer Ther. 2020;20(2):85–95. doi:10.1080/14737140.2019.1596805. [Google Scholar] [PubMed] [CrossRef]
75. Nami B, Maadi H, Wang Z. Mechanisms underlying the action and synergism of trastuzumab and pertuzumab in targeting HER2-positive breast cancer. Cancers. 2018;10(10):342. doi:10.3390/cancers10100342. [Google Scholar] [PubMed] [CrossRef]
76. Gao JJ, Osgood CL, Gong Y, Zhang H, Bloomquist EW, Jiang X, et al. FDA approval summary: pertuzumab, trastuzumab, and hyaluronidase-zzxf injection for subcutaneous use in patients with HER2-positive breast cancer. Clin Cancer Res. 2021;27(8):2126–9. doi:10.1158/1078-0432.ccr-20-3474. [Google Scholar] [PubMed] [CrossRef]
77. Weber GC, Buhren BA, Schrumpf H, Wohlrab J, Gerber PA. Clinical applications of hyaluronidase. Adv Exp Med Biol. 2019;1148(Suppl 2):255–77. doi:10.1007/978-981-13-7709-9_12. [Google Scholar] [PubMed] [CrossRef]
78. Fehrenbacher L, Cecchini RS, Geyer CEJr., Rastogi P, Costantino JP, Atkins JN, et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2+. J Clin Oncol. 2020;38(5):444–53. doi:10.1200/JCO.19.01455. [Google Scholar] [PubMed] [CrossRef]
79. Roy AM, Kumarasamy VM, Dhakal A, O’Regan R, Gandhi S. A review of treatment options in HER2-low breast cancer and proposed treatment sequencing algorithm. Cancer. 2023;129(18):2773–88. doi:10.1002/cncr.34904. [Google Scholar] [PubMed] [CrossRef]
80. Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38(17):1951–62. doi:10.1200/jco.19.02488. [Google Scholar] [PubMed] [CrossRef]
81. Schalper KA, Kumar S, Hui P, Rimm DL, Gershkovich P. A retrospective population-based comparison of HER2 immunohistochemistry and fluorescence in situ hybridization in breast carcinomas: impact of 2007 American Society of Clinical Oncology/College of American Pathologists criteria. Arch Pathol Lab Med. 2014;138(2):213–9. doi:10.5858/arpa.2012-0617-oa. [Google Scholar] [PubMed] [CrossRef]
82. Hamilton E, Shastry M, Shiller SM, Ren R. Targeting HER2 heterogeneity in breast cancer. Cancer Treat Rev. 2021;100(7):102286. doi:10.1016/j.ctrv.2021.102286. [Google Scholar] [PubMed] [CrossRef]
83. Eiger D, Agostinetto E, Saude-Conde R, de Azambuja E. The exciting new field of HER2-low breast cancer treatment. Cancers. 2021;13(5):1015. doi:10.3390/cancers13051015. [Google Scholar] [PubMed] [CrossRef]
84. Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18(6):977–84. doi:10.1093/annonc/mdl475. [Google Scholar] [PubMed] [CrossRef]
85. Martinez VG, Sadhbh ON, Josephine S, Susan B, Aled C, John C, et al. Resistance to HER2-targeted anti-cancer drugs is associated with immune evasion in cancer cells and their derived extracellular vesicles. OncoImmunology. 2017;6(12):e1362530. doi:10.1080/2162402x.2017.1362530. [Google Scholar] [PubMed] [CrossRef]
86. Zhang X, Zhao Y, Chen X. Collagen extracellular matrix promotes gastric cancer immune evasion by activating IL4I1-AHR signaling. Transl Oncol. 2024;49:102113. doi:10.1016/j.tranon.2024.102113. [Google Scholar] [PubMed] [CrossRef]
87. Seol H, Lee HJ, Choi Y, Lee HE, Kim YJ, Kim JH, et al. Intratumoral heterogeneity of HER2 gene amplification in breast cancer: its clinicopathological significance. Mod Pathol. 2012;25(7):938–48. doi:10.1038/modpathol.2012.36. [Google Scholar] [PubMed] [CrossRef]
88. Lee HJ, Seo AN, Kim EJ, Jang MH, Suh KJ, Ryu HS, et al. HER2 heterogeneity affects trastuzumab responses and survival in patients with HER2-positive metastatic breast cancer. Am J Clin Pathol. 2014;142(6):755–66. doi:10.1158/0008-5472.sabcs13-p1-08-38. [Google Scholar] [CrossRef]
89. Allison KH, Dintzis SM, Schmidt RA. Frequency of HER2 heterogeneity by fluorescence in situ hybridization according to CAP expert panel recommendations: time for a new look at how to report heterogeneity. Am J Clin Pathol. 2011;136(6):864–71. doi:10.1309/ajcpxtzskbrip07w. [Google Scholar] [PubMed] [CrossRef]
90. Barish R, Gates E, Barac A. Trastuzumab-induced cardiomyopathy. Cardiol Clin. 2019;37(4):407–18. doi:10.1016/j.ccl.2019.07.005. [Google Scholar] [PubMed] [CrossRef]
91. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365(14):1273–83. doi:10.1056/nejmoa0910383. [Google Scholar] [PubMed] [CrossRef]
92. Dent SF, Morse A, Burnette S, Guha A, Moore H. Cardiovascular toxicity of novel HER2-targeted therapies in the treatment of breast cancer. Curr Oncol Rep. 2021;23(11):128. doi:10.1007/s11912-021-01114-x. [Google Scholar] [PubMed] [CrossRef]
93. Nakada T. Discovery research and translation science of trastuzumab deruxtecan, from non-clinical study to clinical trial. Translat Regula Sci. 2021;3(2):65–71. doi:10.33611/trs.2021-010. [Google Scholar] [CrossRef]
94. Shi F, Liu Y, Zhou X, Shen P, Xue R, Zhang M. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug Deliv. 2022;29(1):1335–44. doi:10.1080/10717544.2022.2069883. [Google Scholar] [PubMed] [CrossRef]
95. Wang J, Liu Y, Zhang Q, Li W, Feng J, Wang X, et al. Disitamab vedotin, a HER2-directed antibody-drug conjugate, in patients with HER2-overexpression and HER2-low advanced breast cancer: a phase I/Ib study. Cancer Commun. 2024;44(7):833–51. doi:10.1002/cac2.12577. [Google Scholar] [PubMed] [CrossRef]
96. Xu Y, Wang Y, Gong J, Zhang X, Peng Z, Sheng X, et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer. 2021;24(4):913–25. doi:10.1007/s10120-021-01168-7. [Google Scholar] [PubMed] [CrossRef]
97. Nadal-Serrano M, Morancho B, Escriva-de-Romani S, Morales CB, Luque A, Escorihuela M, et al. The second generation antibody-drug conjugate SYD985 overcomes resistances to T-DM1. Cancers. 2020;12(3):670. doi:10.3390/cancers12030670. [Google Scholar] [PubMed] [CrossRef]
98. Aftimos PG, Turner N, O’Shaughnessy J, van den Tweel E, Oesterholt M, Escrivá-de-Romaní S, et al. 386MO Trastuzumab duocarmazine versus physician’s choice therapy in pre-treated HER2-positive metastatic breast cancer: final results of the phase III TULIP trial. Ann Oncol. 2023;34:S340–1. doi:10.1016/j.annonc.2023.09.563. [Google Scholar] [CrossRef]
99. Ogitani Y, Aida T, Hagihara K, Yamaguchi J, Ishii C, Harada N, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res. 2016;22(20):5097–108. doi:10.1158/1078-0432.ccr-15-2822. [Google Scholar] [PubMed] [CrossRef]
100. Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–90. doi:10.1158/0008-5472.can-08-1776. [Google Scholar] [PubMed] [CrossRef]
101. Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91. doi:10.1056/nejmoa1209124. [Google Scholar] [PubMed] [CrossRef]
102. Hong R, Xia W, Wang L, Lee K, Lu Q, Jiang K, et al. Safety, tolerability, and pharmacokinetics of BAT8001 in patients with HER2-positive breast cancer: an open-label, dose-escalation, phase I study. Cancer Commun. 2021;41(2):171–82. doi:10.1002/cac2.12135. [Google Scholar] [PubMed] [CrossRef]
103. Wang S, Xu F, Hong R, Xia W, Yu J-C, Tang W, et al. Abstract CT053: bAT8001, a potent anti-HER2 antibody drug conjugate with a novel uncleavable linker to reduce toxicity for patients with HER2-positive tumor. Cancer Res. 2019;79(13_Supplement):CT053. doi:10.1158/1538-7445.am2019-ct053. [Google Scholar] [CrossRef]
104. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016;107(7):1039–46. doi:10.1111/cas.12966. [Google Scholar] [PubMed] [CrossRef]
105. Nakajima S, Mimura K, Matsumoto T, Thar Min AK, Ito M, Nakano H, et al. The effects of T-DXd on the expression of HLA class I and chemokines CXCL9/10/11 in HER2-overexpressing gastric cancer cells. Sci Rep. 2021;11(1):16891. doi:10.1038/s41598-021-96521-2. [Google Scholar] [PubMed] [CrossRef]
106. Joubert N, Beck A, Dumontet C, Denevault-Sabourin C. Antibody-drug conjugates: the last decade. Pharmaceuticals. 2020;13(9):245. doi:10.3390/ph13090245. [Google Scholar] [PubMed] [CrossRef]
107. DiPeri TP, Evans KW, Raso MG, Zhao M, Rizvi YQ, Zheng X, et al. Adavosertib enhances antitumor activity of trastuzumab deruxtecan in HER2-expressing cancers. Clin Cancer Res. 2023;29(21):4385–98. doi:10.1158/1078-0432.CCR-23-0103. [Google Scholar] [PubMed] [CrossRef]
108. Takegawa N, Tsurutani J, Kawakami H, Yonesaka K, Kato R, Haratani K, et al. [fam-] trastuzumab deruxtecan, antitumor activity is dependent on HER2 expression level rather than on HER2 amplification. Int J Cancer. 2019;145(12):3414–24. doi:10.1002/ijc.32408. [Google Scholar] [PubMed] [CrossRef]
109. Cortes J, Hurvitz SA, Im SA, Iwata H, Curigliano G, Kim SB, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in HER2-positive metastatic breast cancer: long-term survival analysis of the DESTINY-Breast03 trial. Nat Med. 2024;30(8):2208–15. doi:10.1038/s41591-024-03021-7. [Google Scholar] [PubMed] [CrossRef]
110. Dowling GP, Daly GR, Keelan S, Boland F, Toomey S, Hill ADK, et al. Efficacy and safety of trastuzumab deruxtecan in breast cancer: a systematic review and meta-analysis. Clin Breast Cancer. 2023;23(8):847–55.e2. doi:10.1016/j.clbc.2023.09.005. [Google Scholar] [PubMed] [CrossRef]
111. Saura C, Modi S, Krop I, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated patients with HER2-positive metastatic breast cancer: updated survival results from a phase II trial (DESTINY-Breast01). Ann Oncol. 2024;35(3):302–7. doi:10.1016/j.annonc.2023.12.001. [Google Scholar] [PubMed] [CrossRef]
112. Lewis GD, Li G, Guo J, Yu SF, Fields CT, Lee G, et al. The HER2-directed antibody-drug conjugate DHES0815A in advanced and/or metastatic breast cancer: preclinical characterization and phase 1 trial results. Nat Commun. 2024;15(1):466. doi:10.1038/s41467-023-44533-z. [Google Scholar] [PubMed] [CrossRef]
113. Thomas JD, Yurkovetskiy AV, Yin M, Bodyak ND, Gumerov DR, Tang S, et al. Discovery of novel polyamide-pyrrolobenzodiazepine hybrids for antibody-drug conjugates. Bioorgan Med Chem Letters. 2022;72:128876. doi:10.1016/j.bmcl.2022.128876. [Google Scholar] [PubMed] [CrossRef]
114. Krop I, Hamilton E, Jung KH, Modi S, Kalinsky KM, Phillips G, et al. Abstract P2-13-25: a phase I dose-escalation study of DHES0815A, a HER2-targeting antibody-drug conjugate with a DNA monoalkylator payload, in patients with HER2-positive breast cancer. Cancer Res. 2022;82(4_Supplement):P2-13-25. doi:10.1158/1538-7445.sabcs21-p2-13-25. [Google Scholar] [CrossRef]
115. Skidmore L, Sakamuri S, Knudsen NA, Hewet AG, Milutinovic S, Barkho W, et al. ARX788, a site-specific anti-HER2 antibody-drug conjugate, demonstrates potent and selective activity in HER2-low and T-DM1-resistant breast and gastric cancers. Mol Cancer Ther. 2020;19(9):1833–43. doi:10.1158/1535-7163.mct-19-1004. [Google Scholar] [PubMed] [CrossRef]
116. Yu J, Fang T, Yun C, Liu X, Cai X. Antibody-drug conjugates targeting the human epidermal growth factor receptor family in cancers. Front Mol Biosci. 2022;9:847835. doi:10.3389/fmolb.2022.847835. [Google Scholar] [PubMed] [CrossRef]
117. Song E, Yao H, Sun M, Zong H, Lin R, Zou W, et al. Abstract PO2-04-03: bL-M07D1, an antibody-drug conjugate directed to HER2 in patients with locally advanced or metastatic Breast Cancer with HER2-positive/low-expression and other solid tumors: results from a first-in-human phase 1 study. Cancer Res. 2024;84(9_Supplement):PO2-04-03. doi:10.1158/1538-7445.sabcs23-po2-04-03. [Google Scholar] [CrossRef]
118. Harding JJ, Fan J, Oh DY, Choi HJ, Kim JW, Chang HM, et al. Zanidatamab for HER2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01a multicentre, single-arm, phase 2b study. Lancet Oncol. 2023;24(7):772–82. doi:10.1016/S1470-2045(23)00242-5. [Google Scholar] [PubMed] [CrossRef]
119. Hamblett K, Barnscher S, Davies R, Hammond P, Hernandez A, Wickman G, et al. Abstract P6-17-13: ZW49, a HER2 targeted biparatopic antibody drug conjugate for the treatment of HER2 expressing cancers. Cancer Res. 2019;79(4_Supplement):P6-17-3. doi:10.1158/1538-7445.sabcs18-p6-17-13. [Google Scholar] [CrossRef]
120. Oh D-Y, Bedard PL, Lee K-W, Han HS, Kang Y-K, Miller WH, et al. Abstract B130: phase 1 study of Zanidatamab Zovodotin (ZW49safety profile and recommended dose (RD) in patients with human epidermal growth factor 2 (HER2)-positive solid cancers. Mol Cancer Ther. 2023;22(12_Supplement):B130. doi:10.1158/1535-7163.targ-23-b130. [Google Scholar] [CrossRef]
121. Park YH, Ahn HK, Kim J-Y, Ahn JS, Im Y-H, Kim S-H, et al. First-in-human phase I study of ALT-P7, a HER2-targeting antibody-drug conjugate in patients with HER2-positive advanced breast cancer. J Clin Oncol. 2020;38(15_suppl):3551. doi:10.1200/JCO.2020.38.15_suppl.3551. [Google Scholar] [CrossRef]
122. Rinnerthaler G, Gampenrieder SP, Greil R. HER2 directed antibody-drug-conjugates beyond T-DM1 in breast cancer. Int J Mol Sci. 2019;20(5):1115. doi:10.3390/ijms20051115. [Google Scholar] [PubMed] [CrossRef]
123. Zimmerman BS, Esteva FJ. Next-generation HER2-targeted antibody-drug conjugates in breast cancer. Cancers. 2024;16(4):800. doi:10.3390/cancers16040800. [Google Scholar] [PubMed] [CrossRef]
124. Bhusari P, Vatsa R, Singh G, Parmar M, Bal A, Dhawan DK, et al. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int J Cancer. 2017;140(4):938–47. doi:10.1002/ijc.30500. [Google Scholar] [PubMed] [CrossRef]
125. Wakiyama H, Kato T, Furusawa A, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy of cancer; possible clinical applications. Nanophotonics. 2021;10(12):3135–51. doi:10.1515/nanoph-2021-0119. [Google Scholar] [PubMed] [CrossRef]
126. Mohiuddin TM, Zhang C, Sheng W, Al-Rawe M, Zeppernick F, Meinhold-Heerlein I, et al. Near infrared photoimmunotherapy: a review of recent progress and their target molecules for cancer therapy. Int J Mol Sci. 2023;24(3):2655. doi:10.3390/ijms24032655. [Google Scholar] [PubMed] [CrossRef]
127. Sato H, Noma K, Ohara T, Kawasaki K, Akai M, Kobayashi T, et al. Dual-targeted near-infrared photoimmunotherapy for esophageal cancer and cancer-associated fibroblasts in the tumor microenvironment. Sci Rep. 2022;12(1):20152. doi:10.21203/rs.3.rs-1367605/v1. [Google Scholar] [CrossRef]
128. Altunay B, Morgenroth A, Beheshti M, Vogg A, Wong NCL, Ting HH, et al. HER2-directed antibodies, affibodies and nanobodies as drug-delivery vehicles in breast cancer with a specific focus on radioimmunotherapy and radioimmunoimaging. Eur J Nucl Med Mol Imaging. 2021;48(5):1371–89. doi:10.1007/s00259-024-06792-w. [Google Scholar] [PubMed] [CrossRef]
129. Fu R, Carroll L, Yahioglu G, Aboagye EO, Miller PW. Antibody fragment and affibody immunoPET imaging agents: radiolabelling strategies and applications. ChemMedChem. 2018;13(23):2466–78. doi:10.1002/cmdc.201800624. [Google Scholar] [PubMed] [CrossRef]
130. Tolmachev V, Orlova A. Affibody molecules as targeting vectors for PET imaging. Cancers. 2020;12(3):651. doi:10.3390/cancers12030651. [Google Scholar] [PubMed] [CrossRef]
131. Baum RP, Prasad V, Müller D, Schuchardt C, Orlova A, Wennborg A, et al. Molecular imaging of HER2-expressing malignant tumors in breast cancer patients using synthetic 111In- or 68Ga-labeled affibody molecules. J Nucl Med. 2010;51(6):892–7. doi:10.2967/jnumed.109.073239. [Google Scholar] [PubMed] [CrossRef]
132. Feldwisch J, Tolmachev V. Engineering of affibody molecules for therapy and diagnostics. Methods Mol Biol. 2012;899:103–26. doi:10.1007/978-1-61779-921-1_7. [Google Scholar] [PubMed] [CrossRef]
133. Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363(6428):446–8. doi:10.1038/363446a0. [Google Scholar] [PubMed] [CrossRef]
134. Greenberg AS, Avila D, Hughes M, Hughes A, McKinney EC, Flajnik MF. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374(6518):168–73. doi:10.1038/374168a0. [Google Scholar] [PubMed] [CrossRef]
135. Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82(1):775–97. doi:10.1146/annurev-biochem-063011-092449. [Google Scholar] [PubMed] [CrossRef]
136. Keyaerts M, Xavier C, Heemskerk J, Devoogdt N, Everaert H, Ackaert C, et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nucl Med. 2016;57(1):27–33. doi:10.2967/jnumed.115.162024. [Google Scholar] [PubMed] [CrossRef]
137. D’Huyvetter M, De Vos J, Xavier C, Pruszynski M, Sterckx YGJ, Massa S, et al. 131I-labeled anti-HER2 camelid sdAb as a theranostic tool in cancer treatment. Clin Cancer Res. 2017;23(21):6616–28. doi:10.1158/1078-0432.ccr-17-0310. [Google Scholar] [PubMed] [CrossRef]
138. Puttemans J, Dekempeneer Y, Eersels JL, Hanssens H, Debie P, Keyaerts M, et al. Preclinical targeted α- and β-Radionuclide therapy in HER2-positive brain metastasis using camelid single-domain antibodies. Cancers. 2020;12(4):1017. doi:10.3390/cancers12041017. [Google Scholar] [PubMed] [CrossRef]
139. Goebeler M-E, Bargou RC. T cell-engaging therapies—BiTEs and beyond. Nat Rev Clin Oncol. 2020;17(7):418–34. doi:10.1038/s41571-020-0347-5. [Google Scholar] [PubMed] [CrossRef]
140. Herrmann M, Krupka C, Deiser K, Brauchle B, Marcinek A, Ogrinc Wagner A, et al. Bifunctional PD-1 × αCD3 × αCD33 fusion protein reverses adaptive immune escape in acute myeloid leukemia. Blood. 2018;132(23):2484–94. doi:10.1182/blood-2018-05-849802. [Google Scholar] [CrossRef]
141. Correnti CE, Laszlo GS, de van der Schueren WJ, Godwin CD, Bandaranayake A, Busch MA, et al. Simultaneous multiple interaction T-cell engaging (SMITE) bispecific antibodies overcome bispecific T-cell engager (BiTE) resistance via CD28 co-stimulation. Leukemia. 2018;32(5):1239–43. doi:10.1038/s41375-018-0014-3. [Google Scholar] [PubMed] [CrossRef]
142. Johnson S, Burke S, Huang L, Gorlatov S, Li H, Wang W, et al. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol. 2010;399(3):436–49. doi:10.1016/j.jmb.2010.04.001. [Google Scholar] [PubMed] [CrossRef]
143. Seung E, Xing Z, Wu L, Rao E, Cortez-Retamozo V, Ospina B, et al. A trispecific antibody targeting HER2 and T cells inhibits breast cancer growth via CD4 cells. Nature. 2022;603(7900):328–34. doi:10.1038/s41586-022-04439-0. [Google Scholar] [PubMed] [CrossRef]
144. GTB-3550 (CD16/IL-15/CD33)Tri-Specific Killer Engager (TriKE®) for the Treatment of High Risk Myelodysplastic Syndromes, Refractory/Relapsed Acute Myeloid Leukemia and Advanced Systemic Mastocytosis [Internet]; 2017 [cited 2025 Jun 10]. Available from: https://clinicaltrials.gov/study/NCT03214666. [Google Scholar]
145. Tapia-Galisteo A, Alvarez-Vallina L, Sanz L. Bi- and trispecific immune cell engagers for immunotherapy of hematological malignancies. J Hematol Oncol. 2023;16(1):83. doi:10.1186/s13045-023-01482-w. [Google Scholar] [PubMed] [CrossRef]
146. Cattaruzza F, Nazeer A, To M, Hammond M, Koski C, Liu LY, et al. Precision-activated T-cell engagers targeting HER2 or EGFR and CD3 mitigate on-target, off-tumor toxicity for immunotherapy in solid tumors. Nat Cancer. 2023;4(4):485–501. doi:10.1038/s43018-023-00536-9. [Google Scholar] [PubMed] [CrossRef]
147. Weisser NE, Sanches M, Escobar-Cabrera E, O’Toole J, Whalen E, Chan PWY, et al. An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat Commun. 2023;14(1):1394. doi:10.1038/s41467-023-37029-3. [Google Scholar] [PubMed] [CrossRef]
148. Meric-Bernstam F, Beeram M, Hamilton E, Oh DY, Hanna DL, Kang YK, et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: a phase 1, dose-escalation and expansion study. Lancet Oncol. 2022;23(12):1558–70. doi:10.1016/s1470-2045(22)00621-0. [Google Scholar] [PubMed] [CrossRef]
149. Geuijen CAW, De Nardis C, Maussang D, Rovers E, Gallenne T, Hendriks LJA, et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 signaling via HER2-guided ligand blockade. Cancer Cell. 2018;33(5):922–36 e10. doi:10.1016/j.ccell.2021.07.015. [Google Scholar] [PubMed] [CrossRef]
150. Schram AM, Odintsov I, Espinosa-Cotton M, Khodos I, Sisso WJ, Mattar MS, et al. Zenocutuzumab, a HER2xHER3 bispecific antibody, is effective therapy for tumors driven by NRG1 gene rearrangements. Cancer Discov. 2022;12(5):1233–47. doi:10.1158/2159-8290.cd-21-1119. [Google Scholar] [PubMed] [CrossRef]
151. de Vries Schultink AHM, Bol K, Doornbos RP, Murat A, Wasserman E, Dorlo TPC, et al. Population pharmacokinetics of MCLA-128, a HER2/HER3 bispecific monoclonal antibody, in patients with solid tumors. Clin Pharmacokinet. 2020;59(7):875–84. doi:10.1007/s40262-020-00858-2. [Google Scholar] [PubMed] [CrossRef]
152. Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–9. doi:10.1126/science.1129003. [Google Scholar] [PubMed] [CrossRef]
153. Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39(1):49–60. doi:10.1016/j.immuni.2013.07.002. [Google Scholar] [PubMed] [CrossRef]
154. Tokarew N, Ogonek J, Endres S, von Bergwelt-Baildon M, Kobold S. Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer. 2019;120(1):26–37. doi:10.1038/s41416-018-0325-1. [Google Scholar] [PubMed] [CrossRef]
155. Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017;5:22. doi:10.1186/s40364-017-0102-y. [Google Scholar] [PubMed] [CrossRef]
156. Klampatsa A, Haas AR, Moon EK, Albelda SM. Chimeric antigen receptor (CAR) T cell therapy for malignant pleural mesothelioma (MPM). Cancers. 2017;9(9):115. doi:10.3390/cancers9090115. [Google Scholar] [PubMed] [CrossRef]
157. Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3(12):939–51. doi:10.1038/nri1248. [Google Scholar] [PubMed] [CrossRef]
158. Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167(11):6123–31. doi:10.4049/jimmunol.167.11.6123. [Google Scholar] [PubMed] [CrossRef]
159. Martinez-Lostao L, Anel A, Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res. 2015;21(22):5047–56. doi:10.1158/1078-0432.ccr-15-0685. [Google Scholar] [PubMed] [CrossRef]
160. Kagoya Y, Tanaka S, Guo T, Anczurowski M, Wang CH, Saso K, et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018;24(3):352–9. doi:10.1038/nm.4478. [Google Scholar] [PubMed] [CrossRef]
161. Tomasik J, Jasinski M, Basak GW. Next generations of CAR-T cells—new therapeutic opportunities in hematology? Front Immunol. 2022;13:1034707. doi:10.3389/fimmu.2022.1034707. [Google Scholar] [PubMed] [CrossRef]
162. Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491–502. doi:10.1016/s0140-6736(21)01222-8. [Google Scholar] [PubMed] [CrossRef]
163. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48. doi:10.1056/NEJMoa1709866. [Google Scholar] [PubMed] [CrossRef]
164. Lin Y, Raje NS, Berdeja JG, Siegel DS, Jagannath S, Madduri D, et al. Idecabtagene Vicleucel (ide-cel, bb2121a BCMA-directed CAR T cell therapy, in patients with relapsed and refractory multiple myeloma: updated results from phase 1 CRB-401 study. Blood. 2020;136(Supplement 1):26–7. doi:10.1182/blood-2020-134324. [Google Scholar] [CrossRef]
165. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–42. doi:10.1056/NEJMoa1914347. [Google Scholar] [PubMed] [CrossRef]
166. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001a multicentre seamless design study. Lancet. 2020;396(10254):839–52. doi:10.1016/s0140-6736(20)31366-0. [Google Scholar] [PubMed] [CrossRef]
167. Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+: CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–38. doi:10.1172/jci85309. [Google Scholar] [PubMed] [CrossRef]
168. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–44. doi:10.1016/j.jtct.2024.08.018. [Google Scholar] [PubMed] [CrossRef]
169. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi:10.1056/nejmoa1804980. [Google Scholar] [PubMed] [CrossRef]
170. Kakarla S, Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J. 2014;20(2):151–5. doi:10.1097/ppo.0000000000000032. [Google Scholar] [PubMed] [CrossRef]
171. Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21(4):904–12. doi:10.1038/mt.2013.17. [Google Scholar] [PubMed] [CrossRef]
172. Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15(4):825–33. doi:10.1038/sj.mt.6300104. [Google Scholar] [PubMed] [CrossRef]
173. Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24(13):e20–2. doi:10.1200/jco.2006.05.9964. [Google Scholar] [PubMed] [CrossRef]
174. Benson AB, D’Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, et al. NCCN guidelines(R) insights: biliary tract cancers, version 2.2023. J Natl Compr Canc Netw. 2023;21(7):694–704. doi:10.6004/jnccn.2023.0035. [Google Scholar] [PubMed] [CrossRef]
175. Shah AN, Sunderraj A, Finkelman B, See SH, Davis AA, Gerratana L, et al. Positive predictive value of ERBB2 copy number gain by tissue or circulating tumor DNA next-generation sequencing across advanced cancers. Oncotarget. 2022;13(1):273–80. doi:10.18632/oncotarget.28188. [Google Scholar] [PubMed] [CrossRef]
176. Nakamura Y, Mizuno N, Sunakawa Y, Canon JL, Galsky MD, Hamilton E, et al. Tucatinib and trastuzumab for previously treated human epidermal growth factor receptor 2-positive metastatic biliary tract cancer (SGNTUC-019a phase II basket study. J Clin Oncol. 2023;41(36):5569–78. doi:10.1200/jco.23.00606. [Google Scholar] [PubMed] [CrossRef]
177. Lee CK, Chon HJ, Cheon J, Lee MA, Im HS, Jang JS, et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: a multi-institutional phase 2 trial of the Korean cancer study group (KCSG-HB19-14). Lancet Gastroenterol Hepatol. 2023;8(1):56–65. doi:10.1016/j.esmoop.2022.100666. [Google Scholar] [CrossRef]
178. Oh DY, He AR, Bouattour M, Okusaka T, Qin S, Chen LT, et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1updated overall survival from a randomised phase 3 study. Lancet Gastroenterol Hepatol. 2024;9(8):694–704. doi:10.1016/j.annonc.2022.07.084. [Google Scholar] [CrossRef]
179. Javle M, Borad MJ, Azad NS, Kurzrock R, Abou-Alfa GK, George B, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathwaya multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021;22(9):1290–300. doi:10.1016/s1470-2045(21)00336-3. [Google Scholar] [PubMed] [CrossRef]
180. Ohba A, Morizane C, Kawamoto Y, Komatsu Y, Ueno M, Kobayashi S, et al. Trastuzumab deruxtecan in human epidermal growth factor receptor 2-expressing biliary tract cancer (HERB; NCCH1805a multicenter, single-arm, phase II trial. J Clin Oncol. 2024;42(27):3207–17. doi:10.1200/jco.23.02010. [Google Scholar] [PubMed] [CrossRef]
181. Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, Gonzalez-Martin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol. 2024;42(1):47–58. doi:10.1200/jco.23.02005. [Google Scholar] [PubMed] [CrossRef]
182. Zeme EL, Van Loon K, Kelley RK, Gordan JD. Emerging therapies for the management of human epidermal growth factor receptor 2-/ERBB2-altered advanced biliary tract cancers. J Clin Oncol. 2024;42(27):3170–6. doi:10.1200/jco.24.00868. [Google Scholar] [PubMed] [CrossRef]
183. Guo H, Xu X, Zhang J, Du Y, Yang X, He Z, et al. The pivotal role of preclinical animal models in anti-cancer drug discovery and personalized cancer therapy strategies. Pharmaceuticals. 2024;17(8):1048. doi:10.3390/ph17081048. [Google Scholar] [PubMed] [CrossRef]
184. Chulpanova DS, Kitaeva KV, Rutland CS, Rizvanov AA, Solovyeva VV. Mouse tumor models for advanced cancer immunotherapy. Int J Mol Sci. 2020;21(11):4118. doi:10.3390/ijms21114118. [Google Scholar] [PubMed] [CrossRef]
185. Wang Z, Wu VH, Allevato MM, Gilardi M, He Y, Luis Callejas-Valera J, et al. Syngeneic animal models of tobacco-associated oral cancer reveal the activity of in situ anti-CTLA-4. Nat Commun. 2019;10(1):5546. doi:10.1038/s41467-019-13471-0. [Google Scholar] [PubMed] [CrossRef]
186. Olson B, Li Y, Lin Y, Liu ET, Patnaik A. Mouse models for cancer immunotherapy research. Cancer Discov. 2018;8(11):1358–65. doi:10.1158/2159-8290.cd-18-0044. [Google Scholar] [PubMed] [CrossRef]
187. Lu C, Yang Y, Zhang M, Li J, Song H, Zhao H, et al. Establishment of an in situ model to explore the tumor immune microenvironment in head and neck squamous cell carcinoma. Head Neck. 2024;46(6):1310–21. doi:10.1002/hed.27707. [Google Scholar] [PubMed] [CrossRef]
188. Kojima Y, Hayakawa F, Morishita T, Sugimoto K, Minamikawa Y, Iwase M, et al. YM155 induces apoptosis through proteasome-dependent degradation of MCL-1 in primary effusion lymphoma. Pharmacol Res. 2017;120(3):242–51. doi:10.1016/j.phrs.2017.04.006. [Google Scholar] [PubMed] [CrossRef]
189. Wang W, Li Y, Lin K, Wang X, Tu Y, Zhuo Z. Progress in building clinically relevant patient-derived tumor xenograft models for cancer research. Animal Model Exp Med. 2023;6(5):381–98. doi:10.1002/ame2.12349. [Google Scholar] [PubMed] [CrossRef]
190. Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154(1):180–91. doi:10.4049/jimmunol.154.1.180. [Google Scholar] [CrossRef]
191. Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–89. doi:10.4049/jimmunol.174.10.6477. [Google Scholar] [PubMed] [CrossRef]
192. Okada S, Vaeteewoottacharn K, Kariya R. Application of highly immunocompromised mice for the establishment of patient-derived xenograft (PDX) models. Cells. 2019;8(8):889. doi:10.3390/cells8080889. [Google Scholar] [PubMed] [CrossRef]
193. Vaeteewoottacharn K, Pairojkul C, Kariya R, Muisuk K, Imtawil K, Chamgramol Y, et al. Establishment of highly transplantable cholangiocarcinoma cell lines from a patient-derived xenograft mouse model. Cells. 2019;8(5):496. doi:10.3390/cells8050496. [Google Scholar] [PubMed] [CrossRef]
194. Sungwan P, Kidoikhammouan S, Thonsri U, Saengboonmee C, Wongkham S, Okada S, et al. Anti-tumor and chemosensitizing effects of the CDK inhibitor dinaciclib on cholangiocarcinoma in vitro and in vivo. In Vivo. 2024;38(5):2284–93. doi:10.21873/invivo.13693. [Google Scholar] [PubMed] [CrossRef]
195. Liu Y, Chanana P, Davila JI, Hou X, Zanfagnin V, McGehee CD, et al. Gene expression differences between matched pairs of ovarian cancer patient tumors and patient-derived xenografts. Sci Rep. 2019;9(1):6314. doi:10.1038/s41598-019-42680-2. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF

 Downloads
Downloads
 Citation Tools
Citation Tools