 Open Access
Open Access
REVIEW
Effectiveness and Safety of Lenvatinib and Everolimus after Immune Checkpoint Inhibitors in Metastatic Renal Cell Cancer: A Systematic Review
1 Medical Oncology Unit, University Hospital of Parma, Parma, 43126, Italy
2 Medical Oncology Unit, University Hospital of Genova, Genova, 16132, Italy
3 Medical Oncology Unit, Portsmouth Hospitals University NHS Trust, Portsmouth, PO6 3LY, UK
4 Pathology Unit, University Hospital of Parma, Parma, 43126, Italy
5 Medical Oncology Unit, Hospital of Macerata, Macerata, 62100, Italy
* Corresponding Author: Giacomo Iovane. Email:
# These two authors contributed equally to this work as the co-last author
Oncology Research 2026, 34(1), 3 https://doi.org/10.32604/or.2025.070523
Received 18 July 2025; Accepted 27 October 2025; Issue published 30 December 2025
Abstract
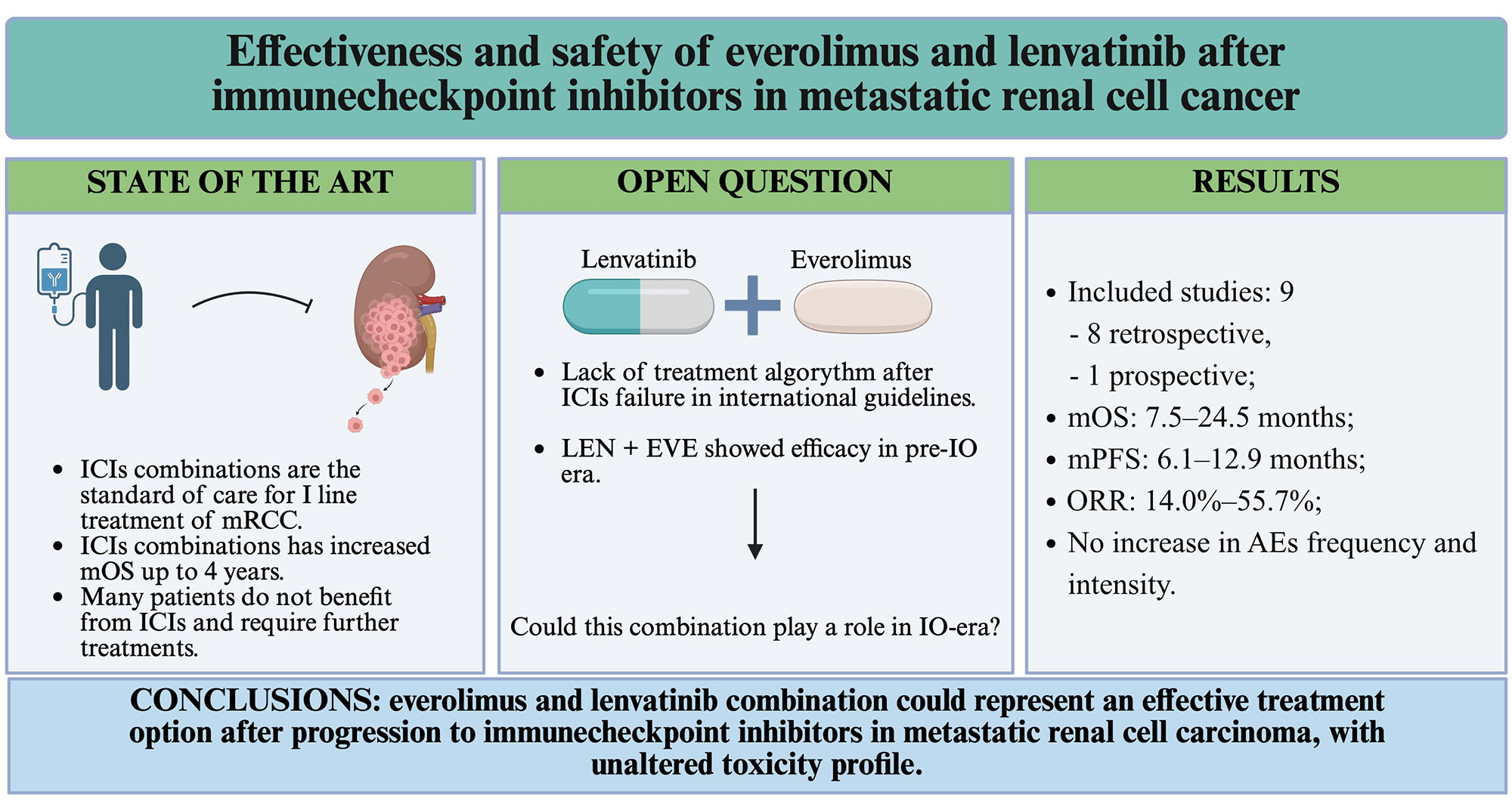
Background: While the treatment of metastatic renal cell carcinoma (mRCC) is evolving due to immune checkpoint inhibitors (ICIs), optimal strategies for later lines of therapy have yet to be defined. The combination of lenvatinib and everolimus represents a viable option, and the present review aimed to summarize its activity, effectiveness, and safety. Methods: A systematic review of the literature was conducted using PubMed, targeting studies published between 2018 and 2025. Eligible studies included English-language prospective and retrospective trials reporting survival outcomes in mRCC patients treated with lenvatinib and everolimus after at least one ICI-containing regimen. Results: Nine studies met the inclusion criteria, encompassing a total of 441 patients. The lenvatinib and everolimus combination was primarily used in the third and subsequent lines of therapy. Median overall survival ranged from 7.5 to 24.5 months, while median progression-free survival was more consistent, between 6.1 and 6.7 months, except for one study reporting 12.9 months. Objective response rates varied widely (14.0%–55.7%). Adverse events of grade ≥ 3 did not exceed the expected rate, with diarrhoea and proteinuria as the most reported events. Dose reductions and treatment discontinuations due to toxicity occurred but were generally lower than in prior pivotal trials. Conclusions: Real-world evidence suggests that lenvatinib and everolimus represent an effective and safe option after ICI failure in mRCC patients. Nevertheless, the lack of randomized phase III trials and the heterogeneity of existing studies highlight the need for more robust prospective research to guide post-ICI therapeutic strategies.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2026 The Author(s). Published by Tech Science Press.
Copyright © 2026 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools