 Open Access
Open Access
REVIEW
Biochar, Properties and Skills with a Focus on Implications for Vineyard Land and Grapevine Performance
Department of Agriculture, Food, Environment and Forestry (DAGRI), University of Florence, Sesto Fiorentino, 50019, Italy
* Corresponding Author: Eleonora Cataldo. Email:
Phyton-International Journal of Experimental Botany 2025, 94(1), 33-64. https://doi.org/10.32604/phyton.2025.059997
Received 21 October 2024; Accepted 12 December 2024; Issue published 24 January 2025
Abstract
Biochar has emerged as a promising tool for enhancing vineyard sustainability by improving soil properties and mitigating climate change impacts. This review highlights key findings on biochar’s role in viticulture, focusing on its effects on soil fertility, water retention, and plant physiology. Field and pot studies demonstrate that biochar amendments enhance soil structure, increase cation exchange capacity (CEC), and promote water availability, leading to improved drought resistance in grapevines. However, the impacts on grape yield, physiology, and quality remain inconclusive, with some studies reporting benefits while others show neutral effects. Future research should focus on optimizing biochar application rates, understanding its interactions with soil microbiota, and assessing long-term impacts on grape production and wine quality. Additionally, addressing potential risks, such as heavy metal contamination and changes in microbial communities, is crucial for its safe and effective use. This review aims to supply a comprehensive assessment of our knowledge about the incidence and consequences of biochar on soil, including its potential use in soil remediation and concerns regarding its possible negative impacts, with a focus on its effects on vine physiology and grape production.Keywords
Abbreviations
| GHG | Greenhouse Gas |
| GWC | Gravimetric Water Content |
| BD | Bulk Density |
| CEC | Cation Exchange Capacity |
| B | Biochar |
| SOM | Soil Organic Matter |
| SOC | Soil Organic Carbon |
| VOCs | Volatile Organic Compounds |
| HMs | Heavy Metals |
| PAHs | Polycyclic Aromatic Hydrocarbons |
Vitis vinifera is a highly important global crop, representing a fundamental resource for many countries from a socio-economic perspective [1]. In 2022, the world’s total grape production was around 80.1 million tons, with China leading as first producer with 15.6 million tons, followed by Italy (8.1 million tons) and France (6.2 million tons). Half of the grape production is utilized for wine [2]. In terms of hectares, the vineyard world’s area is estimated at 7.3 million hectares [3]. Worldwide wine production is estimated to be 258 million hectoliters and global wine exports are valued at around 37.6 billion EUR. This sector is extremely important for some European countries such as Italy, France, and Spain, which together provide 51% of world wine production [4].
Vine cultivation and wine production are demonstrably crucial for the world economy, but, like many other sectors, they are threatened by climate change. Anthropogenic activities have increased greenhouse gas (GHG) emissions, resulting in a rise in heat surface temperature by 1.1°C in recent years, compared with the second half of the nineteenth century [5]. Carbon dioxide is an important GHG, and the agro-food system is responsible for 31% of anthropogenic CO2 emissions across all sectors, accounting for the equivalent of 16 billion tonnes of CO2 [6]. Climate change has also caused an increase in drought events and their duration resulting in water stress in plants [7]. These phenomena affect grapevine phenology, altering quality and yield, leading to lower acidity levels and higher sugar content, particularly, when high temperatures occur near the ripening period [8]. Agriculture therefore contributes to climate change and is affected by it [9]. However, agriculture can help temper the effects of global warming by employing soil management practices that increase the soil’s capacity to sequester carbon from the atmosphere. Soil is one of the most important carbon sinks and has a fundamental role in regulating atmospheric CO2 levels [10].
The amendment of soil with biochar could be proposed as a suitable solution to lessen the repercussions of climate change by reducing atmospheric CO2 levels through carbon storage in the soil [11]. Biochar represents a carbon-rich material produced by biomass, such as organic waste, wood, manure, and agricultural waste, that has undergone the process of pyrolysis [12]. Its carbon content varies depending on the feedstock but is usually around 70%–80% [13]. Biochar is considered a long-term solution to store carbon, with a higher amount of carbon remaining in the soil compared to other organic matter sources [14]. It was estimated that through a large biochar application program, 9.50 billion tons of CO2 may be fixed in the soil by 2100 [15].
This carbonaceous material not only aids in climate change mitigation but is also produced using renewable feedstock, contributing to the reduction of issues like biomass disposal and management. Its production is also a source of renewable energy and its application to soil can offer numerous benefits, improving soil quality and crop yield [16]. Among these proprieties, it was found that biochar improved soil characteristics like porosity, bulk density, water-holding capacity, aeration, cation exchange capacity, pH neuralization in acidic soils, aggregate stability, nutrient availability and uptake, and nitrogen retention [17,18]. It also ameliorates soil fertility by improving enzyme activity and stimulating microbial populations [19]; moreover, it can also help restore polluted soil by reducing the bioavailability of heavy metals, toxic elements, or organic contaminants [20].
Interest in biochar has grown significantly in recent years, as reflected in the increasing number of articles related to the topic. According to the Scopus database, Fig. 1 shows the annual number of articles that mention the word “biochar” in their title, abstract, or keywords.
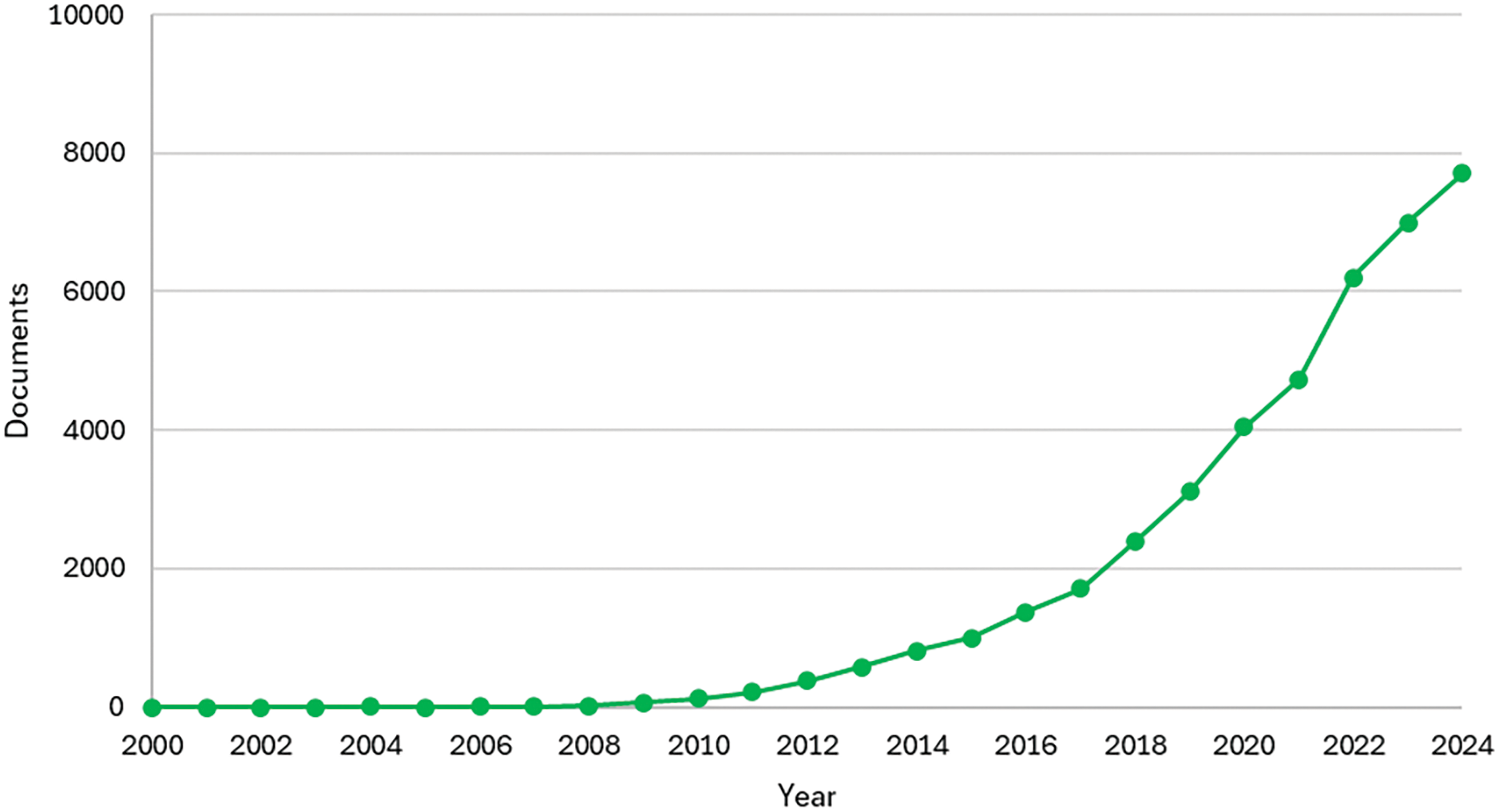
Figure 1: Documents by years. Publications that report the word “biochar” in their title, abstract or keywords, collected from the Scopus website on 20 November 2024
The rise in biochar-related research began around 2009 and has continued to grow each year. Last year, nearly 7000 articles were published, and this year, the number is approaching 8000.
While biochar is relevant to sectors such as energy, chemistry, and even medicine, the field with the most significant interest is Environmental Science, primarily due to biochar’s potential role in climate change mitigation. Agricultural and Biological Sciences occupy the second position, accounting for 12.2% of biochar-related publications (Fig. 2). This interest is related to biochar’s ability to improve soil’s physical, chemical, and biological properties, potentially leading to higher crop yields while also contributing to carbon sequestration from the atmosphere [21,22]. However, the specific use of biochar in viticulture remains underexplored. A search of the Scopus database reveals only 86 articles mentioning both “biochar” and “vineyard,” with limited field experiments. Existing studies often focus on other crops, leaving critical questions unanswered regarding biochar’s long-term impacts on grape yield, soil health, and the quality of wine products. While biochar has been widely studied in general agricultural contexts, its specific application in viticulture remains underexplored. This review addresses this gap by providing a focused analysis of biochar’s potential in vineyards, emphasizing its effects on soil properties, grapevine physiology, and yield under climate stress. By synthesizing the limited field data available and identifying key research needs, this work offers novel insights that can guide future studies and practical applications in sustainable viticulture.
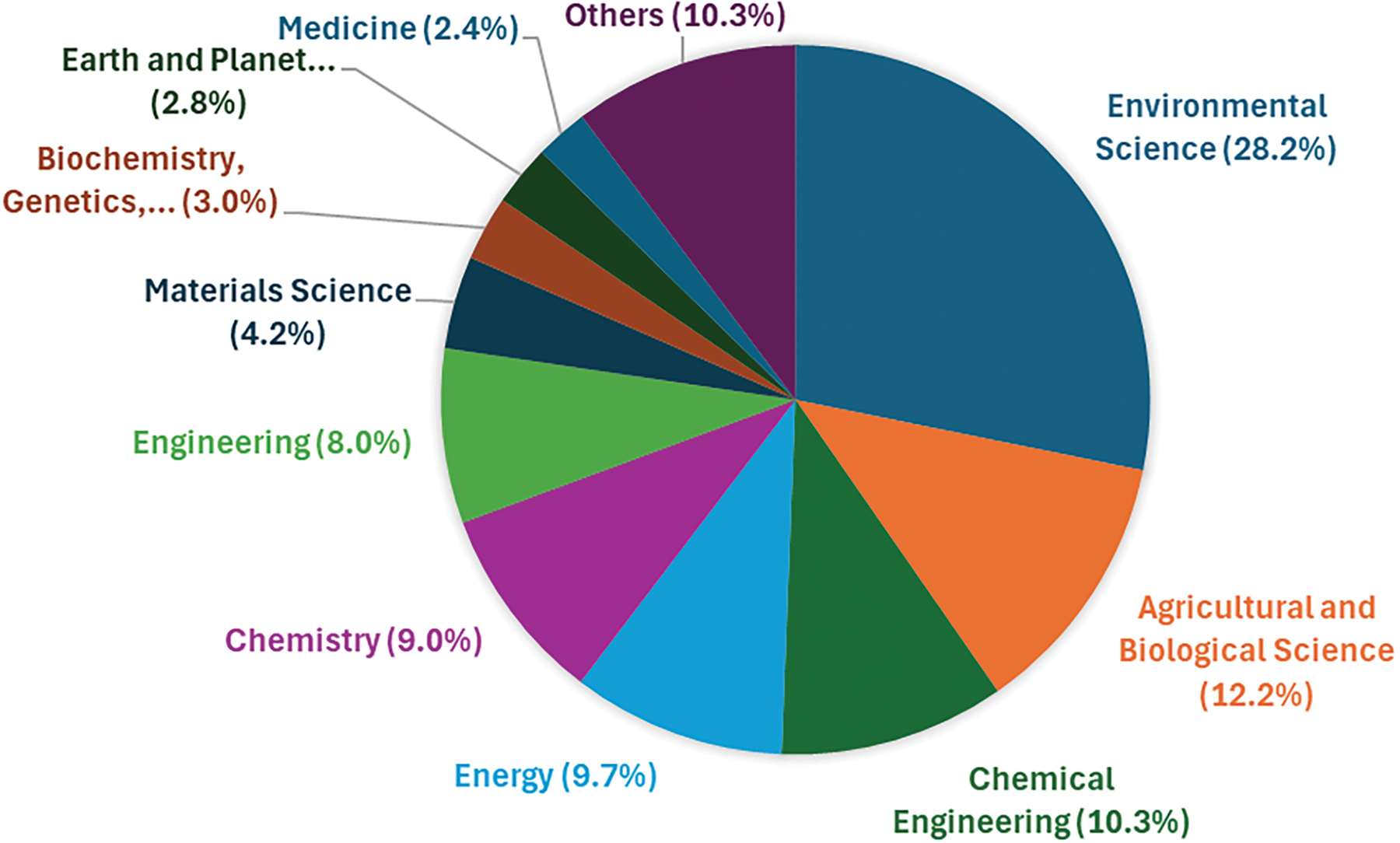
Figure 2: Documents by subject area. Publications that report the word “biochar” in their title, abstract or keywords, divided by subject area, collected from Scopus website on 20 November 2024
In the first part, concerning soil physical properties, around 50% of the analyzed studies focused on crops other than grapevines. This broader approach was intended to provide readers with a comprehensive understanding of biochar’s effects on soil. Fewer studies specifically on viticulture were cited due to the limited number of available studies, although many vineyard-related studies were referenced in multiple sections. For soil chemical properties, 75% of the analyzed studies pertained to viticulture. Studies involving other crops were included to examine a wider range of soil conditions that are difficult to capture solely through vineyard studies. Regarding soil biological properties and pollutant remediation, approximately 40% of the cited studies focused on viticulture. The remaining studies explored biochar’s effects on different soil types to offer a broader perspective on its potential impacts. Finally, in sections specifically addressing vineyards, all analyzed studies directly pertain to this context.
Biochar is a carbon-rich material obtained by heating different organic feedstock such as wood, sewage, manure, or agricultural waste in an enclosed environment with limited or no oxygen present [23]. The utilization of this kind of charcoal to enhance plant growth and productivity has an ancient origin: in the pre-Columbian period, American natives used to distribute biochar in the soil, creating the so-called “Terra preta”, that contained a large volume of fixed carbon and doubled the productivity of crops [24]. Indeed, these soils contain 150 g of carbon per kg of soil, compared to the 20–30 g of carbon per kg of the nearby soils [25].
Different types of feedstocks utilized can lead to biochar production with different characteristics, particularly regarding nutrient content, density, toughness, and porosity [26]. Usually, woody feedstocks generate biochar with high carbon amount and surface area, but with low nutrient content and cation exchange capacity [27]; in addition to this, higher lignin content in the feedstock can lead to a higher yield of biochar [28]. It was reported that biochar generated from manure or organic waste had lower specific surface area and carbon content, but higher cation exchange capacity and nutrient content, H/C, and O/C ratio [29]. These organic feedstocks need to undergo different thermal methods to be converted into biochar, such as pyrolysis, gasification, hydrothermal carbonization, and torrefaction [28].
Pyrolysis is defined as a thermochemical decomposition of feedstock by heating at elevated temperatures, between 300°C and 700°C, with no or little oxygen present [30] which generates fuels, both liquid and gas, as well as biochar [31]. The temperatures and conditions of pyrolysis influence the physical and chemical characteristics of biochar [32]. Slow pyrolysis utilizes moderate temperatures, around 400°C, for a long time at atmospheric pressure leading to the production of a high quantity of biochar, usually more than 30% of the initial biomass [33,34]. Fast pyrolysis is performed rapidly by heating biomass at temperatures higher than 400°C with a lower process duration [35]. This process is focused on the maximization of bio-oil production, so it produces a lesser quantity of biochar, between 10% to 30%, containing 15%–40% of the original biomass carbon [36]. Intermediate pyrolysis utilizes temperatures between 400°C and 500°C for a longer time than in fast pyrolysis, but less than the slow pyrolysis. Biochar yield is around 20%–30% of the initial biomass [37,38].
Gasification utilizes agents like oxygen or steam to perform a partial oxidation of the feedstock. Process temperatures are higher than those of pyrolysis, reaching more than 700°C [39]. The quantity of biochar produced via gasification is usually lower than pyrolysis, around 10% of the initial biomass amount [28,40], with less carbon content, but more stable. This process produces a higher amount of energy than pyrolysis because here biochar is a byproduct, and its production is intentionally reduced to increase syngas production for energy purposes [40].
Hydrothermal carbonization is a pre-treatment process performed in a temperature range between 180°C to 280°C in an inert atmosphere at 2–10 MPa pressure [41]. The solid product of this process is denominated hydrochar and has better performance than raw material as a solid fuel [42].
Torrefaction is performed in a low temperature range (200°C–300°C), in an environment with low or no oxygen present. It is a mild-pyrolysis system that improves some biomass proprieties, so it is often used as pre-treatment to increase biomass energy and quality, producing coal fuels [43].
3 Consequences of Biochar on Land
The belief that biochar in land is an inert material is not entirely correct. In fact, it has a direct impact on soil properties, improving them and leading to an enhancement in soil fertility and crop productivity [44].
Biochar has lower density and higher porosity than soil. Its addition in fields can improve their physical properties enhancing soil porosity, aggregation of particles, water relations, and air content. Nevertheless, these effects are highly contingent on biochar characteristics, soil type, and time [45]. These effects influence plant development and, consequently, yield, by affecting water uptake and root respiration [46]. Below, the consequences of biochar on soil bulk density, porosity and pore distribution, land aggregation, soil water content and plant available water, water infiltration, soil erosion, and soil temperature and albedo are investigated.
Most studies in the literature report a reduction in soil bulk density after biochar application, ranging from 1% to 20% [47]. The degree of decrease depends on the rate of biochar application; in fact, it was reported that higher application doses caused a greater reduction of soil bulk density, compared to lower doses [48]. This reduction is generally more pronounced in soils with larger particles, such as sandy soils [49], as shown in Table 1, where the highest percentage reduction values occur in sandy soils. This type of soil exhibits the highest level of bulk density reduction, at 43% [50].
The reduction in soil bulk density also depends on depth Ventura et al. [51]. After a biochar application of 60 t/ha at 20 cm depth, a significant depletion in soil bulk density was reported, up to 18% in the first 5 cm, and a lower reduction at a depth of 10 cm, where the reduction was up to 9%.
The impoverishment in soil bulk density can be explained by the lower density of biochar compared to soil density. For example, for biochar obtained from different types of wood, bulk density varies between 0.30 g/cm3 and 0.43 g/cm3 [52]. Indeed, bulk density values of Holm-oak biochar ranged around 0.37 and 0.43 g/cm3; Eucalyptus biochar was around 0.30–0.33 g/cm3; biochar from mixed wood sieving was 0.22–0.33 g/cm3; biochar from vineyard pruning was 0.32 g/cm3; and biochar from orchard pruning was 0.43 g/cm3 [49,53–55]. Additionally, the adjunct of biochar to soil leads to an upgrade in biological activity, soil aggregation, and microporosity, which increases bulk density. The high surface area and ion exchange capacity of biochar promote the formation of links among soil organic matter and clay particles, altering soil pore-size distribution and leading to the same result [22].
The reduction in bulk density evidenced in biochar-treated soil appears to remain stable over the years, as demonstrated by Burrell et al. [56], who found that it persisted for at least three years, in a pot experiment using 3% w/w biochar from different feedstock (woodchips, wheat straw, and vineyard prunings).
3.1.2 Porosity and Pore Distribution
As mentioned previously, biochar distribution reduces soil bulk density and, consequently, soil porosity increases [63]. Biochar exhibits high porosity, typically ranging between 70% and 90%. Therefore, its distribution in soil enhances soil porosity, with this increase attributed to the formation of aggregates and interactions among biochar and mineral particles [22]. Generally, the enhancement in soil porosity is directly correlated to the application rate, similar to bulk density [46]. The porosity of biochar hangs on pyrolysis temperature; indeed, biochar produced at 600°C has higher surface area and pore volumes compared to biochar pyrolyzed at 350°C, possibly due to the formation of micropores on the biochar surface [64].
Adekiya et al. [50] reported an increase in soil porosity of 46.5% in the first year of the trial and 65.0% in the second year. This experiment was performed in sandy-loam soil cultivated with cocoyam with biochar from hardwood applied at a 30 t/ha rate. Fu et al. [65] applied four different biochar doses (30, 60, 90, and 120 t/ha) using corn stover biochar pyrolyzed at 550°C, distributing it in two applications in a medium-textured seasonally frozen soil. They found an increase in porosity of 2%, 6.74%, 10.86%, and 19.13%, respectively. Herath et al. [57] used a silt loam soil at permanent pasture and reported a 10% increase in soil porosity with the distribution of 11.3 t/ha of corn stover biochar pyrolyzed at 350°C and a 19% increase using biochar pyrolyzed at 550°C at a rate of 10 t/ha. This study also found an increase in macropore volume of 7% and 20%, respectively, without any change in meso and micro-pore volume. Additionally, Chang et al. [61], in a pot experiment using a typical sandy soil of Florida mixed with different biochar rates (5%, 10%, 15%, and 20% by weight), planted with muscadine grapes, found that porosity increased with the biochar rate, from 45% in the control soil to 67% in the higher biochar rate. Biochar seems to have the ability to increase soil macroporosity, as demonstrated by Zanutel et al. [66], who studied two different soils cultivated with herbaceous crops. Briefly, in sandy loam soil, they observed an increase in macroporosity of 93.55%, and 34.4% for Picea abies derived biochar doses of 13.50 t/ha and 27.00 t/ha, respectively. In the silt-loam soil, increases of 105.7% and 91.4% were observed, respectively. Finally, no effects on meso and micro-porosity were found.
Soil aggregation is fundamental for soil framework as it helps stabilize soil organic matter and improves water-holding capacity, infiltration, and conductivity [67]. Biochar applied to soil looks to enhance aggregate stability, as its derived humic acids facilitate the formation of complex organic matter and minerals. This stabilization is attributed to the hydrophobic polyaromatic groups of humic acids, which prevent water entrance, thus increasing aggregate stability. An augmentation in soil aggregation promotes soil organic matter stability and microbial growth [68].
Ouyang et al. [69] analyzed two different agricultural soils: a silty clay soil and a sandy loam soil, mixed with 2% w/w biochar originating from dairy manure pyrolyzed at 500°C. They found that biochar amendment increased the formation and stabilization of macroaggregates throughout the 90-day incubation period in sandy-loam soil. The maximum increase was observed on day 60, with the number of macroaggregates in the control at 89 ± 1 g/kg soil, while those in the treatment were at 154.9 ± 12 g/kg soil. No significant results were found for macroaggregates in silty clay soil, except during some sampling days and for microaggregates in both soils. Du et al. [70] managed a 5-year field experiment in a fulvic Cambisol cultivated with winter wheat-maize rotation, applying two biochar doses derived from crushed corncob pyrolyzed at 360°C: 4.50 t/ha/year and 9.00 t/ha/year. They found that biochar-amended soil had a higher amount of macroaggregates, with an increase of 49% and 109% compared to control soil, respectively. They also observed an increase in the microaggregates fraction, but only in the higher dose treatment. Han et al. [71] studied the consequences of maize straw biochar pyrolyzed at 300°C, 450°C, and 600°C, at doses of 1% and 3% w/w on a Hydragic Anthrosolos, to assess the biochar result with various pyrolysis temperatures and different doses on soil aggregation. They found an increase in microaggregates in the first three months, ranging from 31.1% to 32.9%–34.9% in the 3% biochar treatments, with no significant differences between them. No difference in macroaggregates fraction was found in this period. After six months, there was an increase in small macroaggregates fraction (2000–250 μm) from 30% to 30.9%–35.4% in 1% biochar treatments and to 32.8%–37.2% in 3% biochar treatments. An increase in large macroaggregates fraction (>2000 μm) was also found. The same result was found after 1 year. The increase in macroaggregates was higher for biochar pyrolyzed at 300°C. Sun et al. [72] carried out a six-year field trial in a Haplic Luvisol amended with maize stover biochar pyrolyzed at 450°C at 15.75, 31.50, and 47.25 t/ha doses. They found an increase in small macroaggregates fraction (2000–250 μm) proportionally with the dose, but a decrease in the higher dose treatment. No effects for large macroaggregates and for microaggregates were found, but a decrease for the silt and clay fraction, especially for the 31.50 t/ha dose, was found. Moreover, an increase in aggregate stability for all the treatments was noted. This study suggests that adding a lower biochar dose can be more helpful for soil aggregation than a higher dose.
In summary, biochar applications to land can promote the formation of macroaggregates by enhancing microbial population and soil organic matter. Its hydrophobicity can also cause particle aggregation and promote interaction between soil components thanks to its wide surface area [73]. The growth of microbial populations further enhances aggregate stability through the production of mucilage, which acts as a powerful glue for aggregates [74].
3.1.4 Plant Available Water Content
In Terra preta lands or Amazonian dark earth, water retention was 18% higher than in nearby soils, likely due to the higher carbon and organic matter content [75], so it was supposed that the high charcoal content could be the cause of this phenomenon. Indeed, is noted that in soil amended with biochar, water retention is influenced by the quantity of water and biochar added and is positively associated with these parameters. However, it is also conditional on the pyrolysis temperature of biochar. Higher temperatures reduce biochar’s water-holding capacity; the optimal pyrolysis temperature to maximize this value appears to be 400°C [76]. The application rate significantly influences soil water content; for instance, Yan et al. [77] found a higher increase in water content with a 40 t/ha than with a 60 t/ha dose. Nevertheless, the studies listed in Table 2 generally reported an increase in soil water content and plant available water with an increase in biochar dose.
Biochar application also appears to increase the plant available water content of the soil. This value is evaluated as the difference between water content at field capacity and the one at permanent wilting point [78].
The contact angle of biochar’s surface with water (that can define its hydrophilicity or hydrophobicity) can influence its interactions with water. Contact angles vary as a result of feedstock and pyrolysis temperature, owing to C-H groups on surfaces. Contact angles greater than 90° lead to hydrophobicity, but when biochar comes into contact with water in soils, the angles tend to reduce their width, leading to hydrophilic characteristics [79].
Baronti et al. [53] worked on an Italian vineyard amended with 22 t/ha of orchard pruning-derived biochar distributed over one year or two consecutive years. They found an increase in water content and available water, especially in the treatment with two biochar distributions, where water content increased by 26% and available water by 45%. The authors suggested that biochar amendment increased water potential the least and improved plant water status due to the amplification of plant available water volume. However, they caution that high water availability increased vine productivity, and a moderate water stress level was beneficial for grape quality. Their results were confirmed by Chang et al. [61] in the previously described study of pot experiment with grapes using different biochar doses and found an increase in soil water-holding capacity with the increase in biochar dose, from 28% in the control to 52% in the higher biochar rate (20% by weight). García-Jaramillo et al. [21], after amending two vineyards in Oregon (USA) with 18 and 35 tons per hectare of biochar, observed a significant increase in gravimetric water content (GWC) at both sites and for both application rates. However, this effect was limited to the subsurface soil, likely because the biochar was incorporated through tillage at a depth of 10 cm. To achieve a more uniform impact throughout the entire rhizosphere, biochar could be integrated into the soil during soil preparation prior to vineyard planting [80]. It was found that soil texture had the most significant influence on soil water retention parameters. As suggested by other studies, biochar appeared to improve available water mostly in coarse-textured soils, while in medium and fine-textured soils the increasing effect was lower or negligible [81,82]. This suggests that a higher amount of biochar is needed in fine-textured soil to appreciate an increase in water content [22]. Confirming this, Chang et al. [83] observed an increase in GWC in their pot experiment using sandy soil mixed with different biochar rates (0%, 5%, 15%, and 20%). In particular, in the first measurement the GWC in the higher biochar dose was 17 times higher than control [84]. They compared different biochar particle sizes and observed that smaller particle sizes gave the maximum increase in soil water content, probably due to the rise in soil microporosity, determining an increase in soil water retention capacity.
Around 80% of agricultural lands are subjected to soil erosion due to land use, deforestation, and unsustainable agricultural practices such as intensive agriculture with high input usage (chemicals for example) and errors in soil management [88]. The annual soil loss has been estimated at 28 billion tons globally, with the agricultural sector being a major contributor due to the soil erosion rate, which exceeds the soil formation rate by 10 to 40 times. Soil conservation is crucial to prevent the loss of the topmost fertile layer of the soil [89]. Biochar application has shown promise in reducing soil erosion by enhancing soil capabilities like aggregate stability, porosity, SOM content, and water infiltration [90], as discussed previously.
The study conducted by Abrol et al. [91] described previously with a 2% w/w mixed wood biochar dose, found a decrease in soil loss in both types of soil: in calcareous loam soil, soil loss was 30% lower with a 2% biochar dose in comparison to the control (ctrl), while in non-calcareous loamy sand soil, there was a depletion of around 50% for both rainfall events compared to the control. Jien et al. [92] worked on a highly weathered acid soil prone to erosion cultivated with pineapple, amended with white lead tree wood biochar pyrolyzed at 700°C, and mixed with soil at rates of 0%, 2.5%, and 5%. Then they simulated an 80 mm/h rainfall and found a decrease in soil erosion rate with the higher biochar rate: soil loss decreased from 1458 ±50 g/m2/h in the control to 730 ± 94.6 g/m2/h and 532 ± 106 g/m2/h in the lower and higher biochar doses, respectively. Shi et al. [93] carried out a four-year field experiment on the mid- and long-term effects of biochar amendment on soil erosion. Biochar was distributed in a 3° sloping soil in four application rates: 25, 50, 75, and 100 t/ha for each year. Soil loss was measured by a sediment collection system at the end of the slope. They found that excessive biochar doses could be futile, while a 50 t/ha dose was optimal, resulting in a maximum 17.5% reduction in soil loss in the third year. Agbede et al. [94] studied a sandy soil cultivated with herbaceous crops amended with biochar from hardwood pyrolyzed at 580°C at rates of 0, 10, 20, and 30 t/ha. They observed a reduction in soil loss with increasing biochar rate. With the control experiencing the highest soil loss at 355.7 kg/ha and 364.4 kg/ha in the first and second years of the experiment, respectively, while the highest biochar application showed lower values at 120.6 kg/ha and 98.8 kg/ha. On average for both years, the application of 30 t/ha of biochar reduced soil loss by 69.90% compared to the control. However, Peng et al. [95] amended a clay loam soil employed for peanut cultivation with rice straw pyrolyzed biochar at 400°C at a rate of 21.15 t/ha and found no significant effect of biochar in reducing soil erosion. The authors suggest that the fine particles and low density of this type of biochar may make it more susceptible to erosion.
It is thought that the rate of biochar application is a salient factor in erosion reduction, and that biochar is more effective in reducing soil erosion in coarse-textured soil, probably due to the greater increase in soil aggregation rate [96].
3.1.6 Soil Temperature and Albedo
Soil temperature can significantly influence soil properties such as the speed of soil organic matter decomposition, water retention, plant water availability, and carbon and nitrogen cycles [97]. Additionally, it can affect biological and microbiological processes such as plant and root growth, germination, evapotranspiration, and soil microbial population growth which in turn affect soil respiration and heat exchange [98]. Some studies showed a considerable decrease in soil reflectance at charcoal-producing sites, indicating that the application of biochar to soil can reduce its albedo, leading to an increase in soil surface temperature and evaporation [99,100]. However, other studies suggest that higher moisture levels and the decrease in soil bulk density resulting from biochar application may reduce soil temperature [101].
Genesio et al. [99] amended a silty loam soil cultivated with wheat with 30 and 60 t/ha of biochar from coppiced woodlands pyrolyzed at 500°C. They found that shortly after biochar application, the average surface albedo of the amended soil was one-third that of the control soil. Nevertheless, this effect decreased with canopy coverage from plants, and by the second year and after two tillages, the effect had disappeared entirely. Zhang et al. [102] distributed 4.5 and 9 t/ha of crushed corn cob biochar pyrolyzed at 360°C on sandy loam soil cultivated with a rotation of winter wheat and maize. They observed that thermal conductivity in the biochar-treated soil was lower than in the control, with decreases of 3.48% for the lower dose and 7.49% for the higher ones. An increase in reflectance in near-ultraviolet and blue-light wavelengths was noted, indicating an increase in photosynthesis of leaves near the soil surface, while a decrease in soil reflectance in the infrared spectrum was observed, probably due to differences in soil color. Soil temperature at 5 cm depth showed a pattern suggesting that biochar amendment can mitigate soil temperature fluctuations, resulting in an average increase in winter of 0.1°C and an average decrease of 0.1°C in summer. Ventura et al. [51] found no effect on soil temperature at 7.50 cm depth after the amending of clay loam soil with fruit tree pruning biochar (10 and 30 t/ha). However, they observed differences between treated and untreated soil surface temperatures, likely due to the dark color of biochar. Yan et al. [77] reported an enhancement in soil surface temperature (0–20 cm) after amending a silty loam maize soil with 0, 20, 40, and 60 t/ha of maize straw biochar, with higher biochar application rates leading to higher soil temperatures. Feng et al. [103] studied silty loam soil employed for corn cultivation under different corn stover-derived biochar application rates (i.e., 0, 10, 20, 30, and 50 t/ha). They observed minimal temperature differences for the lower application rate, from −0.13°C to −0.51°C and for the 20 t/ha, from −0.03°C to +0.69°C, compared to control, while significant differences were found for higher doses: from −0.98°C to +0.72°C for 30 t/ha and from +0.82°C to +2.17°C in the higher dose. Authors suggested that biochar applications exceeding 30 t/ha notably affected soil temperature, particularly increasing minimum nighttime soil temperature, and reducing the difference between day and night. Chang et al. [83] in a greenhouse experiment with grapevines grown in sandy soil amended with varying biochar rates (5%, 10%, 15%, and 20%), observed a decrease in soil temperature as biochar application rates increased. Specifically, on the first day of measurement, soil temperatures in the 10% and 20% treatments were approximately 1°C lower than in the control. Although the effect diminished as the hot season progressed, a downward trend in soil temperature was maintained. Biochar’s impact on soil temperature appears to vary, and further research is needed to assess the influence of biochar on land albedo and temperature and determine under which conditions they may be beneficial for agricultural purposes [77].
Biochar addition to soil leads to an improvement in various soil chemical properties like pH, cation exchange capacity (CEC), nutrient content and retention, and soil organic carbon (SOC), ultimately improving soil fertility and potentially enhancing soil yields [104].
An increase in soil pH is typically observed following biochar distribution. This pH increment is attributed to the alkaline nature of biochar, which differs based on pyrolysis temperature and feedstock. Biochar derived from wood generally exhibits higher pH levels compared to those derived from manure or crop residues [105]. Another factor contributing to pH increases after biochar application is the reduction in exchangeable aluminum (Al3+), which is adsorbed onto negatively charged biochar particles [48]. Functional groups on the biochar surface, such as −COO− or −O−, react with H+ in the land, leading to a drop in soil acidity [106]. Changes in soil pH can alter the nutrient form and facilitate the adsorption of certain nutrients by plants [107].
The extent of pH increase tends to correlate with the biochar application rate, with more pronounced effects observed in acidic soils, compared to alkaline soils [105]. For instance, Hailegnaw et al. [108], studied ten different soil types in the Czech Republic under conventional crop rotation, amended with coniferous wood chips biochar at different doses (0.5%, 2%, 4%, and 8% w/w). They studied seven soils with a pH lower than 6.2 and three soils with a pH higher than 6.2. In the first seven soils, biochar significantly increased pH even at a 2% rate, while in the other three soils pH slightly increased even at an 8% biochar rate. Montagnoli et al. [62] amended an acidic vineyard soil with 30 t/ha of biochar, resulting in an increase in soil pH from 5.05 to 6.41. Biochar appears as a promising approach to facing soil acidification issues, resulting from intensive agricultural practices; this phenomenon leads to aluminum phytotoxicity, nutrient deficiency, and increased heavy metal uptake [109]. Idbella et al. [110] undertook a 10-year experiment in a vineyard in Italy with acidic sandy-clay-loam soil. They applied orchard pruning-derived biochar at 22 t/ha one or two times in the first year. They found an increase in soil pH from 6.33 ± 0.06 to 6.83 ± 0.11 with one biochar application and 7.07 ± 0.1 with the repeating application. Maienza et al. [111] found that biochar (B) application immediately neutralized soil pH value in a vineyard in Italy. Indeed, with an application of 30 t/ha and 30 + 30 t/ha B, after 5 years, the pH in the control was 5.94 ± 0.14, while in the treatments was 6.54 ± 0.13 and 7.31 ± 0.08, respectively. However, Amendola et al. [112] described no significant effect on soil pH after the application of pruning biomass-derived biochar at a 10 t/ha rate in a vineyard in Italy with clay-textured soil and alkaline pH (9.7). Similarly, Bastos et al. [113] in a pot experiment in Portugal, amended a sandy loam soil with 4 and 40 t/ha of biochar from wood chips and found that, after 12 months, the pH in the control and the lower dose was the same (6.5), while in the higher dose, a slight increase (6.7) was observed. These studies confirm that biochar application has no or low impact on pH in alkaline or neutral soils, probably due to its buffering capacity that neutralizes pH changes.
Some studies reported a pH decrease following biochar application under specific conditions. For example, Karimi et al. [114] reported a drop in soil pH after the application of corn residue biochar pyrolyzed at 200°C in a loam calcareous agricultural field in Iran with a pH of 7.7. They found a decrease of 0.22 and 0.30 units with biochar rates of 1% and 2%, respectively. However, slight pH increases were observed using biochar pyrolyzed at higher temperatures. The authors hypothesized that the decrease in pH was due to the production of organic acids by the easy oxidation of biochar generated at low temperatures. Additionally, Chen et al. [115] in a pot experiment using rice grown on a biochar-enriched substrate at an equivalent rate of 5 t/ha, found that over two growing seasons, biochar reduced the topsoil pH by 0.14–0.18 units in the first 6 cm and by 0.05–0.08 units at a depth of 20–30 cm. However, Baronti et al. [60], in a long-term experiment in a vineyard amended with biochar from orchard prunings at 22 or 44 t/ha rates, found that the increase in soil pH lasts at least for ten years. Further studies are needed to better understand the development of soil pH in the soil, which may depend on various factors, from soil type to biochar feedstock.
3.2.2 Cation Exchange Capacity
Cation exchange capacity (CEC) measures the soil’s ability to retain exchangeable cations, thus reducing their loss [116]. Biochar (B) has the potential to enhance soil CEC due to the functional groups present on its surface that promote the formation of a negatively charged soil surface. This effect is particularly notable in coarse-textured soils, while its impact may be less significant in soils with high levels of organic matter and clay [117]. However, the application of B to the soil does not typically result in an immediate increase in soil CEC: time is required for additional functional groups to form. Once these groups are established, they can enhance the soil’s ability to retain ions, reduce nutrient leaching, and make nutrients available for plants [118].
Karimi et al. [119] analyzed the soil of an agricultural loam calcareous field in Iran, mixed with corn residue biochar pyrolyzed at 200°C, 350°C, and 500°C at rates of 1% and 2%. They noted a rise in soil CEC with all biochar treatment, with the highest increase observed in the 2% rate of biochar pyrolyzed at 200°C, where CEC increased from 12.63 ± 0.24 to 14.41 ± 0.24. The authors suggested that this increase could be attributed to the surface functional groups as well as low-weight humic-like compounds released by biochar. For instance, Chang et al. [83], in a pot experiment using a typical sandy soil from Oregon (USA) amended with four biochar rates (5%, 10%, 15%, and 20%) and planted with grapevines, observed an increase in soil CEC as biochar application rates increased. Specifically, CEC in the control was 4.22%, while the treatments resulted in increases to 6.57%, 7.00%, 9.25%, and 9.87%, respectively. Notwithstanding, some studies reported no significant effect on soil CEC following biochar application. For instance, Amendola et al. [112] found no impact on soil CEC after applying 10 t/ha biochar from orchard prunings on alkaline clay soil in an Italian vineyard. In contrast, Rombolà et al. [120] amended an Italian vineyard with sandy-clay-loam soil using one or two biochar applications at a 16.5 t/ha rate. Biochar derived from orchard pruning was pyrolyzed at 500°C. They observed an increase in soil CEC from an average of 11.85 in the control to 18.25 and 23.9 meq/100 g in the one or two biochar applications, respectively. The authors suggested that this increase may be attributed to the retained oxygen in biochar, which appears as carboxyl, carbonyl, or phenolic groups, thereby increasing CEC.
Soil microbial populations are an important part of SOM and carry out its mineralization. Biochar can influence these populations by providing C and N sources [121], thus creating a favorable environment for microorganisms through a reduction in soil bulk density and pH liming effect [122].
The highly porous surface of biochar serves as a habitat for numerous microorganisms to thrive and perform their vital functions. When distributed on soils with low SOM content, biochar typically results in an increase in microbial activity, facilitated by its habitat function and the higher C available [123]. For instance, Idbella et al. [110] amended an Italian vineyard with a double application of 22 t/ha of biochar, and, after ten years, observed a significant increase in soil respiration from 0.35 to 1.04 mg CO2/g dw/d and, consequently, in microbial activity, attributed to the higher soil organic carbon content. This carbon is in a stable form that has become accessible to microorganisms over the years. Similarly, Maienza et al. [111], after amending a vineyard with a single and double dose of 30 t/ha of orchard pruning-derived biochar, reported a significant increase in total microbial biomass, especially in the double-dose treatment, which almost doubled the microbial biomass. Giagnoni et al. [17] also surmised that the increase in soil temperature, due to the darker color of biochar, may stimulate microbial activity and their population growth. In contrast, Andrés et al. [124], after amending with 5 t/ha maize biochar pyrolyzed at 500°C a sandy loam vineyard in Spain, found that biochar reduced the microbial biomass in spring. The authors suggest that this phenomenon could be due to the adsorption of rhizodeposition nutrients on the surface of biochar particles.
As discussed previously, biochar typically induces important changes in soil pH. This can cause a change in the microbial population, especially in the bacteria/fungi ratio, given that fungi are more associated with acidic soils [125], but also with enzymatic activity. In the previously cited study, Idbella et al. [110] found a difference in enzymatic activity in biochar-treated soil, with higher protease activity and a decrease in acid-phosphatase, that may be favored by the increase in soil pH, from 6.33 to 7.07. Additionally, Ameloot et al. [126] amended two silt loam soils with the same crop rotation but different SOM content, with biochar deriving from poultry litter and pine chips, pyrolyzed at 500°C at the equivalent of 20 t/ha dose. They found a significant increase in bacteria/fungi ratio in both soils and an increase in soil pH. However, no change in the composition of the population was reported. In contrast, Maienza et al. [111], in the previously described vineyard experiment, analyzed the abundance of different microbial populations (Gram-negative and positive bacteria, fungi, and) and reported an increase in all these populations in the double-dose treatment. Nevertheless, Amoakwah et al. [127] suggested that pyrolysis temperature can influence the fungal community. Indeed, high temperatures favor the formation of micropores that enhance fungal hyphae penetration, creating a favourable ecosystem for fungi. However, excessively high temperatures can lead to excessive pore volume, creating an inhospitable environment.
Biochar addition can induce changes in the soil microbial population, however, with unpredictable results. It can cause an increase in the population of soil-borne pathogens [128], but it can also have suppressive effects, similar to other organic amendments, on some plant diseases such as potato rot [129]. In this context, in their 10-year vineyard experiment, Idbella et al. [110] found a decrease in the potential soil pathogens Phaeoacremonium and Aspergillus. Moreover, biochar can contain polycyclic aromatic hydrocarbons or heavy metals that can impact soil microbial communities [130]. Therefore, its application on a large scale should be evaluated accurately to avoid environmental disorders and understand the long-term effects of biochar amendment on soils.
Some manuscripts reported no noteworthy effects on the abundance and diversity of microarthropods [113,124]. Bastos et al. [113] suggested that applying less than 40 t/ha of biochar to soils used in intensive viticulture may not impact soil habitat function, but interactions with specific abiotic conditions could lead to some changes, necessitating careful evaluation before biochar application.
Prodana et al. [131] amended a sandy loam vineyard in Portugal with wood chip biochar pyrolyzed at 620°C at 4 and 40 t/ha rates. They observed an increase in the population of the isopod species Porcellinoides pruinosus after biochar application, but, after 18 months, a decrease in its fitness occurred. The authors suggested that this can be attributed to the high usage of chemicals in conventional vineyard management. No changes in the population of the collembola species Folsomia candida were found, however, a slight decrease in young specimens was observed. Authors attributed this change to the higher bioavailability of toxic elements after biochar amendment.
Maienza et al. [132] amended an Italian vineyard with two orchard pruning-derived biochar doses: 22 and 44 t/ha. They observed an increase in the population of Acarina and Collembola, which were the most abundant in all treatments. The authors suggested that this increase could be due to the higher water and nutrient availability, leading to an increase in microbial population which served as feed for these organisms. However, for the double dose, they found a reduction in the diversity of invertebrate populations after biochar application, possibly due to the displacement of organisms adapted to drier soil conditions. In contrast, for the lower biochar dose, a general increase was noted in the populations of the studied organisms.
Briones et al. [133] amended a loamy clay field in the UK, used for bioenergy crop rotation with Miscanthus giganteus-derived biochar pyrolyzed at 450°C (10, 25, and 50 t/ha). They found that biochar amendment had negative effects on earthworms but increased the population of microarthropods, especially at the 25 t/ha dose.
Storing soil organic carbon (SOC) is an indispensable method for sequestering carbon in soils, thus mitigating its release into the atmosphere as CO2, which accounts for approximately 60% of greenhouse gas emissions [134,135]. Several studies reported an enhancement in SOC after biochar application, as shown in Table 3. Typically, SOC increases with the application rate of biochar. This is likely due to the increase in stable organic carbon fraction, which also contributes to carbon sequestration in soil [136].
After applying orchard pruning-derived biochar at a rate of 33 t/ha, Rombolà et al. [120] observed an increase in SOC compared to the control. However, this rate decreased over the years, from 4.79% to 3.49% after three years. The value in the third year was still 3.8 times higher than the control. However, Backer et al. [137] found contrasting results after amending loamy-sandy and sandy-clay-loam soils in Canada under three different cultivations (Panicum virgatum, Glycine max, and Zea mays) with pine wood chips biochar pyrolyzed at 500°C at 10 and 20 t/ha rates. In the sandy-clay-loam soil, no effects were found under soybean cultivation; for corn, an increase of 10.8 g/kg in SOC was found compared to the control but only in the higher rate; for switchgrass, an increase was noted at both rates by 10.8 and 31 g/kg, respectively. In the loamy-sandy soil, no effect on SOC was observed. The authors suggested that this could be due to physical movement or biological degradation of biochar. Abagandura et al. [138] found an increase in SOC with a 10 t/ha biochar rate, as shown in Table 3, but only at the surface depth.
3.4 Contaminated Soils Remediation
Heavy metals present in soils are sources of environmental problems because of their long degradation times, and potential entry into food chains [143]. Biochar’s high CEC, attributed to its negative-charged surface, offers potential for soil remediation by immobilizing heavy metals and pollutants [144]. Additionally, the stimulation activity of biochar may promote the activity of some metal-reducing bacteria [145]. Moreover, the increase in soil pH following B application can mitigate the risk of heavy metals by facilitating their adsorption, oxidation, and complexation in soil [146]. Mineral elements provided by biochar can also combine with heavy metals, reducing their availability [147]. Furthermore, the augment in SOC followed by B application, enhances the formation of bonds between SOM and heavy metals, thereby diminishing their mobility and uptake by plants [148].
Zhang et al. [149] recreated a contaminated soil for growing lettuce using soil with no or with 1% w/w biochar application rate, supplemented with different corn stalk biochar doses (0.5% or 1%). They observed an increase in residual and in organic matter bond fractions of cadmium (Cd) and lead (Pb) of 0.94% and 4.06%, respectively, in soil treated with only fresh biochar, and higher values in the soil with previous biochar applications. Authors suggest that biochar had an immediate effect on heavy metal immobilization, while in the long term, it enhanced the formation of bonds between heavy metals and SOM.
Copper (Cu) is a common heavy metal pollutant in soils, particularly in vineyards where it is used as a fungicide to prevent fungal diseases, leading to its accumulation in soils [150]. Cu can negatively impact soil microbial communities, which consequently interrupts soil processes such as SOM mineralization and nutrient cycles [151]. To address this problem, targeted agricultural practices to reduce the bioavailability of Cu are needed, such as the distribution of biochar, organic matter, or clays to enhance the adsorption of this heavy metal [152]. However, studies on the Cu immobilization capacity of biochar yield contrasting results, highlighting the need for further research in this area.
For example, Mackie et al. [153] after amending a loam vineyard in Switzerland with 8 t/ha biochar from hardwood, found no effects of biochar on total Cu or on its exchangeable fraction. Instead, Soja et al. [154] noted a decrease in the Cu2+ fraction (a relevant form from an ecotoxicological perspective) in two vineyard soils from Austria with different wood chip biochar rates (from 10 to 60 t/ha), with or without a mixture of compost. However, they found no effects on total or exchangeable Cu derived from biochar. In contrast, Pump et al. [155] in a lab experiment where they mixed two different soils (a calcareous soil and an acidic soil) from two vineyards in Australia with 1.5% w/w of softwood chips biochar found interesting differences. In the calcareous soil they found, after three years of incubation, a reduction in exchangeable Cu of 21% compared to control. For the acidic soil, a reduction of 20% was already observed after six weeks, and an even higher reduction was observed after three years. They supposed that biochar aging led to a higher presence of negatively charged surface groups that increase Cu adsorption. These contrasting results may be attributed to the varying properties of biochar depending on its feedstock. Supporting this, Zafeiriou et al. [156] analyzed the effects of two different types of biochar (one derived from sewage sludge and the other from olive tree prunings) on four moderately to slightly acidic vineyard soils. Biochar was mixed with soil at a 20% w/w rate. They found that both types of biochar influenced Cu dynamics in soil, but in different ways: sewage sludge biochar significantly reduced Cu mobility in all soils, by a factor of 1.13 to 3.08 compared to the control. In contrast, biochar from olive tree prunings increased Cu mobility by a factor of 1.10 to 1.40. These findings highlight the need for further studies to determine the effects of different types of biochar on copper. This research is essential to ensure the selection and application of biochars that reduce Cu mobility and promote its immobilization, thereby mitigating the ecological risks associated with this heavy metal.
4 Effects of Biochar on Vitis vinifera
One of the most interesting effects of biochar is its promoting activity for plant growth and yield, due to its ability to enhance soil characteristics, leading to higher soil quality as previously described. Currently, only a limited number of studies about biochar’s impacts on vineyards are available. Further research is required to better understand the influence of biochar amendment on grapevines and on berry quality.
Biochar seems to have the ability to improve root growth in plants, depending on feedstock and pyrolysis conditions [157]. For instance, Montagnoli et al. [62] conducted a study in an experimental Chardonnay vineyard in north Italy, where they applied 30 t/ha biochar from orchard pruning pyrolyzed at 500°C in a loamy sand acidic soil. They found that plants growing in biochar-treated soil showed an earlier pioneer root growth, reaching a peak in the first half of June, while the control plants reached the peak at the end of the month. The different timing was more evident for fibrous roots, which had their peak at the end of June for biochar treatment and at the end of July for the control. Authors suggested that the modification of soil properties with biochar application stimulated faster root growth. This anticipated root growth led to greater canopy growth in biochar-treated plants. Similarly, Chang et al. [61] in a greenhouse experiment in the USA using an acidic sandy soil mixed with southern yellow pine-derived biochar at different percentages (5%, 10%, 15%, and 20%) for Muscadine grapes, found that biochar treatments from 10% enhanced root volume, length, and surface, and increased the number of root forks and crossings, at a similar level for all of the three percentages. Authors suggested that the ameliorating effect of biochar on soil properties and water retention led to higher root growth. Amendola et al. [112] found an intensification in fine root biomass after 10 t/ha B distribution in a vineyard in Tuscany. They found an earlier growth of this kind of root during summer, exactly when plants experienced higher water stress conditions.
Biochar amendment to soil has been proposed as a strategy to mitigate drought stress effects. Baronti et al. [53] amended an Italian vineyard with 22 t/ha of biochar from orchard pruning pyrolyzed at 500°C for one and two years. They found that pre-dawn leaf water potential was less negative in biochar treatments (around −0.25 MPa compared to −0.4 MPa in control in July). Furthermore, stomatal conductance was significantly higher in biochar-treated soils, increasing from less than 150 μmol CO2 m−2s−1 in the control, to more than 160 μmol CO2 m−2s−1 in July, and from around 100 to over 120 μmol CO2 m−2s−1 in august. Additionally, higher photosynthesis rates were observed, rising from less than 10 μmol CO2 m−2s−1 in the control to approximately 12 μmol CO2 m−2s−1 in July, with similar values recorded in August. These findings suggested that biochar application can alleviate drought stress in vineyards. These results are in accordance with Petrillo et al. [85] who, in a pot experiment, mixed a neutral soil with 2% w/w biochar. Two-year-old Pinot noir plants were used for the study and water stress conditions were simulated twice. They reported a higher photosynthesis rate in plants with biochar; while the control values were near zero in the first stress cycle, in the second stress cycle values were higher but not statistically relevant. No difference was found for leaf water potential at midday. In contrast, García-Jaramillo et al. [21] found no effects on such parameters after wood-derived gasification biochar application at 15 and 38 t/ha in two USA vineyards. The authors suggested that the lack of effects may be due to the irrigation practices in the vineyards during the study period. Kanwal et al. [158], in a pot experiment, mixed 2 and 5 g/kg of biochar with soil and transplanted cuttings of Vitis vinifera to analyze the effects of biochar on plant development and physiological parameters under three conditions: no stress, 35% stress, and 70% stress. In all stress conditions, plant height increased with higher biochar doses, particularly in non-stressed plants where biochar-treated plants reached a height of 51.56 cm, compared to control plants, which remained below 40 cm. Under high-stress conditions, the difference was smaller, but biochar-treated plants still outperformed the control. A similar trend was observed for leaf area. This pattern was reflected in stomatal conductance and photosynthesis rates, both of which were significantly higher in biochar-treated plants, especially under moderate stress. For example, stomatal conductance in biochar-treated plants exceeded 250 mmol H2O m−2s−1 (and surpassed 300 mmol H2O m−2s−1 at the higher dose), compared to less than 200 mmol H2O m−2s−1 in the control. Similarly, photosynthesis rates in biochar-treated plants approached 4 μmol CO2 m−2s−1, whereas control plants showed rates around 2.5 μmol CO2 m−2s−1.
Studies about the effect of biochar on grapevine physiology are limited, so it’s not possible to assess if the distribution of such material in soil may improve plant growth. However, Schmidt et al. [159] found no significant effects on plant growth, health, or grape quality after applying 8 t/ha of biochar derived from hardwood and wood chips pyrolyzed at 750°C in a Swiss vineyard. Authors suggested that the lack of effects could be due to plants’ age (around 30 years), which led to a greater root depth. In contrast, Montagnoli et al. [62] observed greater canopy development in biochar-treated vines in their previously described experiment using 30 t/ha of orchard pruning-derived biochar. Additionally, Seif et al. [160], in a pot experiment using one-year-old Vitis vinifera transplants grown in soil mixed with biochar at 1% and 2% (w/w) and irrigated with saline water, observed an increase in plant height from 93 cm in the control to 117.33 cm and 104.33 cm in the biochar treatments, respectively. A similar trend was reported for stem diameter, which measured 4.28 mm in the control and increased to 4.68 mm and 4.62 mm in the treated plants. Leaf area also showed improvement, rising from 87.62 cm2 in the control to 110.11 cm2 and 108.98 cm2 in the two biochar treatments. As a result, plant survivability was higher in biochar-amended soils, reaching 83.33%, compared to 66.67% in the control. This study highlights biochar’s ability to mitigate saline stress by sequestering Na+ ions. These findings are promising and suggest that biochar could facilitate grapevine cultivation in saline environments. Beyond saline stress, Kanwal et al. [158], in the previously mentioned study, reported not only increased plant height under non-stress conditions but also enhanced net photosynthesis. Specifically, photosynthesis rates increased from around 3 μmol CO2 m−2s−1 in the control to nearly 5 μmol CO2 m−2s−1 at the 5% (w/w) biochar dose. These plants also exhibited higher transpiration rates, rising from approximately 3.2 mmol H2O m−2s−1 in the control to about 5 mmol H2O m−2s−1 with 5% biochar. This study underscores the positive impact of biochar on grapevine physiological performance.
Numerous authors have demonstrated that biochar application can enhance plant yield by as much as 10% to 42%, although negative effects have also been reported. However, the response of plant productivity to biochar is highly affected by the characteristics of the biochar itself [118].
Genesio et al. [161] conducted a four-year field trial in central Italy, amending a vineyard with acidic soil and sandy-clay-loam texture using orchard pruning pyrolyzed at 500°C applied 22 and 44 t/ha. They found an increase in yield, especially in the second and third year following biochar application, with improvements ranging from 42.3% to 66.8%. In the first and last year of the experiment, yield increased by 16.1%–35.3%. The yield increase was more pronounced in drier years. However, they found that biochar reapplication did not further improve yield. While no effects were observed on the number of clusters per plant, cluster weight increased by 8.9% to 24.7% in the first and last years, and by 24.3% to a maximum of 49.6% in the second and third years. Moreover, there was an increase in the fresh weight of 50 berries by 8.9%–14.8% in the last year of the experiment. Despite the increase in yield, no effects on grape quality indicators such as °Brix, pH, acidity, and anthocyanins were observed, indicating that grape quality remained consistent. These findings are in consistent with those of Raifer et al. [162] that amended an experimental vineyard in north Italy with different rates (25 and 50 t/ha) of biochar from gasification of coniferous wood. No effects on grape quality and yield per vine were found. However, the authors suggested that these results indicated that biochar can be employed for carbon sequestration and to correct soil pH without impacting grape quality. Similarly, García-Jaramillo et al. [21], amending two different vineyards in the USA with 15 and 38 t/ha of gasification biochar from wood, found no effects on yield and grape quality, although a reduction in wine tannins was found in biochar treatments. The lack of effects on yield was attributed by the authors to the irrigation practices in the vineyards, which mitigated any potential water stress and led to consistent production.
5 Possible Harmful Effects of Biochar on Vitis vinifera
Biochar itself can be a source of pollutants, such as polyaromatic hydrocarbons (PAHs), volatile organic compounds (VOCs), dioxins, furans, or heavy metals (HMs), therefore, its application on vulnerable soils should be carefully evaluated [29,163]. These harmful substances can originate from feedstock or be produced during pyrolysis [29], potentially damaging soil microorganisms [164]. However, only the bioavailable fraction of these substances can cause harmful effects on microorganisms, while other fractions may be so strongly bonded to biochar that they are not bioavailable and thus do not cause negative effects [165]. Additionally, with regard to human health, small biochar particles can have harmful effects on the respiratory system, causing diseases such as pneumoconiosis due to the inhalation of these particles during biochar preparation or soil application [166].
Dangerous compounds can be formed due to the lack of oxygen during pyrolysis. The quantity of these compounds depends on pyrolysis conditions and on feedstock. These compounds can be found especially in biochar made at low temperatures (300°C–400°C) [164]. In fact, higher pyrolysis temperatures (over 400°C) appear to produce biochar with fewer toxic compounds, making it more suitable for soil applications. This effect may be due to high temperatures stabilizing toxic compounds [167]. However, it is important to note that higher pyrolysis temperatures result in lower biochar production.
5.1 Polycyclic Aromatic Hydrocarbons (PAHs)
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds consisting of carbon and hydrogen atoms and two or more condensed aromatic rings. These compounds are known for their carcinogenic, toxic, and mutagenic properties and can accumulate in food chains, posing significant health risks [168]. According to the International Biochar Initiative (IBI), the maximum allowable amount of total PAHs in biochar used for soil amendment is 300 mg/kg of dry weight for ordinary biochar and 6 mg/kg for premium biochar [169]. Moreover, the European Biochar Certification (EBC) stipulated that the content of the 16 priority PAHs in biochar for agricultural use should not exceed 6 + 2.4 mg/kg of dry matter [170].
Pyrolysis temperature appears to be a critical factor in the formation of PAHs. Lower temperatures (below 500°C) tend to produce low molecular weight PAHs, whereas higher temperatures (above 500°C) result in the formation of high molecular weight PAHs [171]. However, determining the optimal temperature for producing biochar with minimal PAHs content remains unclear due to conflicting results [172].
A review by Wang et al. [173] analyzed numerous studies and found that total PAHs in biochar generally increase with rising pyrolysis temperatures. Their findings indicated that only 6% of analyzed biochar produced at temperatures between 600°C–800°C exceeded the IBI’s maximum allowable threshold for ordinary biochar. While, for the lower limit (6 mg/kg), 0% of biochar produced at <200°C exceeded the limit, while 14% of those produced at 200°C–400°C, 35% at 400°C–600°C, 53% at 600°C–800°C, and 83% of biochar produced at >800°C exceeded the limit.
PAHs can lead to soil pollution, categorized into four levels [174]:
I. Non-contaminated soils (under 200 µg/kg),
II. Weakly contaminated soils (between 200 and 600 µg/kg),
III. Contaminated soils (between 600 and 1000 µg/kg),
IV. Heavily contaminated soils (over 1000 µg/kg).
In the previous illustrated study, Wang et al. [173] found that biochar-amended soils exceeded the contamination limit (>600 µg/kg) for 0.84%, 2.52%, 3.36%, 4.2%, and 5.88% with biochar application rates of 10, 20, 30, 40 and 50 t/ha. This suggests that higher biochar application rates can increase soil PAH contamination, though the contamination level also depends on the initial PAH content of the soil and the amount of PAHs absorbed by biochar.
Giagnoni et al. [17] found that, among all studied PAHs, only naphthalene (one of the 16 priority PAHs) increased in biochar-treated soils (22 and 44 t/ha doses), reaching almost 20 ng g soil−1 in the lower dose and almost 35 ng g soil−1 in the higher dose. However, all PAHs levels remained below safety thresholds, indicating no eco-toxicity. Conversely, Rombolà et al. [139] amended an Italian vineyard with 33 t/ha of biochar produced from orchard pruning biomass via slow pyrolysis at 500°C and found higher PAHs content in amended soil (153 ± 38 µg/kg) compared to the control (23 ± 3 µg/kg). However, PAHs levels decreased to 78 ± 20 µg/kg after 35 months. Similarly, Maienza et al. [111], after 30 and 60 t/ha of orchard pruning-derived biochar application, found an increase in PAHs that decreased in both treatments after one year, respectively from 101.7 ± 15.5 to 60.9 ± 13.8 and from 171.9 ± 37.5 to 120.3 ± 13.8 µg/kg, while the level of total PAHs in the control remained stable at 26.8 ± 4.4 µg/kg. However, the values always remained under the limit of Italian law (1000 µg/kg). Authors explained that biochar had a high affinity with PAHs, reducing their availability, but further studies are needed to better assess the long-term content of PAHs in biochar amended soils.
Despite these concerns, some studies suggest that biochar can be used for remediating PAH-contaminated soils. Biochar produced at high temperatures has high adsorption efficiency, forming strong bonds with PAHs, and resulting in low desorption of such compounds from biochar surface, leading to a low bioavailability, although, environmental changes can trigger desorption [175].
Heavy metals can persist in biochar after pyrolysis, especially if the feedstock includes animal excrements, sludges, or contaminated plant material, leading to potential soil pollution [176]. The content of heavy metals in biochar depends on the feedstock and can increase the concentration of these metals in the environment through leaching [177]. Biochar application can also influence the bioavailability of heavy metals, primarily due to changes in soil pH, and texture. Typically, heavy metals’ bioavailability decreases in acidic soils with medium or coarse textures but increases in alkaline and fine-textured soils [178].
Lu et al. [179] analyzed biochar produced from sewage sludge at various pyrolysis temperatures and found that heavy metal content was higher in biochar compared to the original feedstock, with a greater increase correlating with higher pyrolysis temperatures. However, leaching toxicity at pH 5 and 6, which indicates potential toxicity and bioavailability, was lower in biochar than in the feedstock, for biochar produced at temperatures above 300°C. Also, the analysis of bioavailability showed a reduction in bioavailable heavy metals in biochar compared with sewage sludge. These results suggest that pyrolysis can stabilize heavy metals in the original feedstock, reducing their bioavailability. Figueiredo et al. [180] amended a tropical soil in Brazil, under corn cultivation, with sewage sludge biochar pyrolyzed at 300°C and 500°C at a rate of 15 t/ha. They observed no significant change in heavy metal content, except for a slight increase in Mn in soil amended with biochar pyrolyzed at 300°C, probably due to low biochar application rate, leaching, or immobilization in plant roots. No effects were noted in available heavy metals, except for Zn which resulted in more availability in biochar-treated soil. Indeed, Zn was the element with a higher concentration in biochar. However, the concentration of the available heavy metals analyzed resulted low. In this study, authors also noted that higher pyrolysis temperatures decreased the availability of heavy metals. In a study by Zhang et al. [181], non-cultivated soil was amended with 7.5, 15, and 30 t/ha of biochar from sewage sludge, alongside raw sewage sludge, and then sown with corn plants. They found that the content of cadmium, copper, and zinc increased in soil with a higher biochar application rate, exceeding that in sewage sludge treatments. However, metal content in corn plants was lower in biochar treatments, indicating reduced bioavailability. The authors noted the need for further studies to understand the long-term effects of biochar on heavy metal bioavailability in soil.
Maienza et al. [111] in the previously described vineyard experiment using biochar from orchard pruning at 30 or 60 t/ha rates, demonstrated that biochar amendment notably enhanced the amount of total Pb over three years to an average of 83,39 mg/kg for the lower biochar dose and 70.86 mg/kg for the higher dose, compared 18.44 mg/kg in the control. The amount of Cu also increased but only in the higher dose. Despite these increases, heavy metal values always remained below European limits.
Similarly to PAHs, biochar is being studied as a remediation tool for soils contaminated with heavy metals, as discussed in Chapter 4. Indeed, biochar seems to have the ability to immobilize heavy metals reducing their bioavailability [182]. However, the heavy metal content in biochar must be carefully evaluated prior to soil application to prevent pollution from high levels of such pollutants in the feedstock.
Viticulture and the agricultural sector in general are currently threatened by climate change which affects both the quality and quantity of production. Nowadays, winemakers and farmers must overcome this challenge and find practices that are both environmentally friendly and support their income. In this context, biochar appears to be a promising tool to increase plant yield and address climate change, owing to its ability to enhance soil water-holding capacity and soil organic carbon, and reduce soil erosion. Biochar may also be useful for recovering damaged soil that has become less functional due to compaction and loss of fertility, thanks to its ameliorating properties on pH, soil bulk density, porosity, aggregation, and cation exchange capacity. Its remediation properties may assist in the process of restoring polluted soils, making previously unusable lands cultivable once again.
However, some concerns persist regarding the use of this soil improver, since, on the one hand, there is an excess of heterogeneity in its effects due to the origin of the raw material, the pyrolysis conditions, and the size of the particles themselves, and, on the other hand, there is the doubt of possible negative contamination.
With regards to viticulture specifically, the available literature is still limited. In this review, we found that about 75% of the analyzed studies on soil chemical properties focused on viticulture, whereas studies on physical and biological properties often involved other crops due to the scarcity of vineyard-specific research. Despite this, these studies provide valuable insights into biochar’s general effects on soil. Biochar seems to have the potential to increase grape yield, even if many studies found no effects on grape production and quality. However, further studies are required to improve our knowledge of biochar, particularly on its effect on grape plant performance and production, to evaluate if negative effects may occur, before proceeding with a large-scale biochar distribution.
Acknowledgement: Not applicable.
Funding Statement: Authors state no funding involved.
Author Contributions: Eleonora Cataldo conceived and planned the review structure. Pamela Lippi, Giovan Battista Mattii, and Eleonora Cataldo wrote the original draft manuscript. Eleonora Cataldo supervised the work. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Fraga H. Viticulture and winemaking under climate change. Agronomy. 2019;9(12):783. doi:10.3390/agronomy9120783. [Google Scholar] [CrossRef]
2. OIV. Annual assessment of the world vine and wine sector in 2022. International Organisation of Vine and Wine; 2023. Available from: https://www.oiv.int/sites/default/files/documents/OIV_Annual_Assessment-2023.pdf. [Accessed 2024]. [Google Scholar]
3. Conway J. Wine production worldwide in 2022, by country. Statista; 2023. Available from: https://www.statista.com/statistics/240638/wine-production-in-selected-countries-and-regions/. [Accessed 2024]. [Google Scholar]
4. OIV. State of the World Vine and Wine Sector in 2022.pdf. International Organisation of Vine and Wine; 2022. Available from: https://www.oiv.int/sites/default/files/documents/OIV_State_of_the_world_Vine_and_Wine_sector_in_2022_2.pdf. [Accessed 2024]. [Google Scholar]
5. Calvin K, Dasgupta D, Krinner G, Mukherji A, Thorne PW, Trisos C, et al. IPCC, 2023: climate change 2023: synthesis report. In: Core Writing Team, Lee H, Romero J, editors. Contribution of working groups I, II and III to the sixth assessment report of the intergovernmental panel on climate change first. Geneva, Switzerland: Intergovernmental Panel on Climate Change (IPCC); 2023. doi:10.59327/IPCC/AR6-9789291691647. [Google Scholar] [CrossRef]
6. FAO. Greenhouse gas emissions from agrifood systems. Global, regional and country trends, 2000–2020; 2022. FAOSTAT Analytical Brief Series No. 50. Rome, FAO. 1–12. Available from: www.fao.org/food-agriculture-statistics/en/. [Accessed 2024]. [Google Scholar]
7. Knipfer T, Wilson N, Jorgensen-Bambach NE, McElrone AJ, Bartlett MK, Castellarin SD. Cessation of berry growth coincides with leaf complete stomatal closure at pre-veraison for grapevine (Vitis vinifera) subjected to progressive drought stress. Ann Bot. 2023;132(5):979–88. doi:10.1093/aob/mcad144. [Google Scholar] [PubMed] [CrossRef]
8. Serrano AS, Martínez-Gascueña J, Alonso GL, Cebrián-Tarancón C, Carmona MD, Mena Morales A, et al. Variability in the agronomic behavior of 12 white grapevine varieties grown under severe water stress conditions in the La Mancha wine region. Horticulturae. 2023;9(2):243. doi:10.3390/horticulturae9020243. [Google Scholar] [CrossRef]
9. Viveros Santos I, Renaud-Gentié C, Roux P, Levasseur A, Bulle C, Deschênes L, et al. Prospective life cycle assessment of viticulture under climate change scenarios, application on two case studies in France. Sci Total Environ. 2023;880:163288. doi:10.1016/j.scitotenv.2023.163288. [Google Scholar] [PubMed] [CrossRef]
10. Rodrigo García E, Murillo Peña R, Pérez Álvarez EP, Garde Cerdán T, Martínez Vidaurre JM. The influence of climatic conditions and agronomic practices on greenhouse gas emissions in a conventional vineyard (DOCa. Rioja, Spain). Agronomy. 2023;13(9):2199. doi:10.3390/agronomy13092199. [Google Scholar] [CrossRef]
11. Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S. Sustainable biochar to mitigate global climate change. Nat Commun. 2010;1(1):56. doi:10.1038/ncomms1053. [Google Scholar] [PubMed] [CrossRef]
12. Bo X, Zhang Z, Wang J, Guo S, Li Z, Lin H, et al. Benefits and limitations of biochar for climate-smart agriculture: a review and case study from China. Biochar. 2023;5(1):77. doi:10.1007/s42773-023-00279-x. [Google Scholar] [CrossRef]
13. Tan Z, Lin CSK, Ji X, Rainey TJ. Returning biochar to fields: a review. Appl Soil Ecol. 2017;116:1–11. doi:10.1016/j.apsoil.2017.03.017. [Google Scholar] [CrossRef]
14. Lehmann J, Gaunt J, Rondon M. Bio-char sequestration in terrestrial ecosystems—a review. Mitig Adapt Strat Glob Change. 2006;11(2):403–27. doi:10.1007/s11027-005-9006-5. [Google Scholar] [CrossRef]
15. Kuppusamy S, Thavamani P, Megharaj M, Venkateswarlu K, Naidu R. Agronomic and remedial benefits and risks of applying biochar to soil: current knowledge and future research directions. Environ Int. 2016;87:1–12. doi:10.1016/j.envint.2015.10.018. [Google Scholar] [PubMed] [CrossRef]
16. Wang D, Jiang P, Zhang H, Yuan W. Biochar production and applications in agro and forestry systems: a review. Sci Total Environ. 2020;723(4):137775. doi:10.1016/j.scitotenv.2020.137775. [Google Scholar] [PubMed] [CrossRef]
17. Giagnoni L, Maienza A, Baronti S, Vaccari FP, Genesio L, Taiti C, et al. Long-term soil biological fertility, volatile organic compounds and chemical properties in a vineyard soil after biochar amendment. Geoderma. 2019;344(4):127–36. doi:10.1016/j.geoderma.2019.03.011. [Google Scholar] [CrossRef]
18. Oliveira FR, Patel AK, Jaisi DP, Adhikari S, Lu H, Khanal SK. Environmental application of biochar: current status and perspectives. Bioresour Technol. 2017;246(5):110–22. doi:10.1016/j.biortech.2017.08.122. [Google Scholar] [PubMed] [CrossRef]
19. Al-Wabel MI, Hussain Q, Usman ARA, Ahmad M, Abduljabbar A, Sallam AS, et al. Impact of biochar properties on soil conditions and agricultural sustainability: a review. Land Degrad Dev. 2018;29(7):2124–61. doi:10.1002/ldr.2829. [Google Scholar] [CrossRef]
20. Palansooriya KN, Ok YS, Awad YM, Lee SS, Sung J-K, Koutsospyros A, et al. Impacts of biochar application on upland agriculture: a review. J Environ Manag. 2019;234(2):52–64. doi:10.1016/j.jenvman.2018.12.085. [Google Scholar] [PubMed] [CrossRef]
21. García-Jaramillo M, Meyer KM, Phillips CL, Acosta-Martínez V, Osborne J, Levin AD, et al. Biochar addition to vineyard soils: effects on soil functions, grape yield and wine quality. Biochar. 2021;3(4):565–77. doi:10.1007/s42773-021-00118-x. [Google Scholar] [CrossRef]
22. Blanco-Canqui H. Biochar and soil physical properties. Soil Sci Soc Am J. 2017;81(4):687–711. doi:10.2136/sssaj2017.01.0017. [Google Scholar] [CrossRef]
23. Lehmann J, Joseph S. Biochar for environmental management: an introduction. In: Biochar for environmental management; London: Routledge, Taylor & Francis Group; 2015. p. 1–13. [Google Scholar]
24. Atkinson CJ, Fitzgerald JD, Hipps NA. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil. 2010;337(1–2):1–18. doi:10.1007/s11104-010-0464-5. [Google Scholar] [CrossRef]
25. Novotny EH, Hayes MHB, Madari BE, Bonagamba TJ, Azevedo ERD, Souza AAD, et al. Lessons from the Terra Preta de Índios of the Amazon region for the utilisation of charcoal for soil amendment. J Braz Chem Soc. 2009;20(6). doi:10.1590/S0103-50532009000600002. [Google Scholar] [CrossRef]
26. Ippolito JA, Laird DA, Busscher WJ. Environmental benefits of biochar. J Environ Qual. 2012;41(4):967–72. doi:10.2134/jeq2012.0151. [Google Scholar] [PubMed] [CrossRef]
27. Tenic E, Ghogare R, Dhingra A. Biochar—a panacea for agriculture or just carbon? Horticulturae. 2020;6(3):37. doi:10.3390/horticulturae6030037. [Google Scholar] [CrossRef]
28. Kumar A, Bhattacharya T. Biochar: a sustainable solution. Environ Dev Sustain. 2021;23(5):6642–80. doi:10.1007/s10668-020-00970-0. [Google Scholar] [CrossRef]
29. Brtnicky M, Datta R, Holatko J, Bielska L, Gusiatin ZM, Kucerik J, et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci Total Environ. 2021;796(9):148756. doi:10.1016/j.scitotenv.2021.148756. [Google Scholar] [PubMed] [CrossRef]
30. Weber K, Quicker P. Properties of biochar. Fuel. 2018;217:240–61. doi:10.1016/j.fuel.2017.12.054. [Google Scholar] [CrossRef]
31. Gaskin JW, Steiner C, Harris K, Das KC, Bibens B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE. 2008;51(6):2061–9. doi:10.13031/2013.25409. [Google Scholar] [CrossRef]
32. Kloss S, Zehetner F, Dellantonio A, Hamid R, Ottner F, Liedtke V, et al. Characterization of slow pyrolysis biochars: effects of feedstocks and pyrolysis temperature on biochar properties. J Environ Qual. 2012;41(4):990–1000. doi:10.2134/jeq2011.0070. [Google Scholar] [PubMed] [CrossRef]
33. Bruun EW, Ambus P, Egsgaard H, Hauggaard-Nielsen H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol Biochem. 2012;46:73–9. doi:10.1016/j.soilbio.2011.11.019. [Google Scholar] [CrossRef]
34. Ronsse F, Van Hecke S, Dickinson D, Prins W. Production and characterization of slow pyrolysis biochar: influence of feedstock type and pyrolysis conditions. GCB Bioenergy. 2013;5(2):104–15. doi:10.1111/gcbb.12018. [Google Scholar] [CrossRef]
35. Laird DA, Brown RC, Amonette JE, Lehmann J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Wiley InterScience. 2009;3(5):547–62. doi:10.1002/bbb.169. [Google Scholar] [CrossRef]
36. Brewer CE, Hu Y-Y, Schmidt-Rohr K, Loynachan TE, Laird DA, Brown RC. Extent of pyrolysis impacts on fast pyrolysis biochar properties. J Environ Qual. 2012;41(4):1115–22. doi:10.2134/jeq2011.0118. [Google Scholar] [PubMed] [CrossRef]
37. Tinwala F, Mohanty P, Parmar S, Patel A, Pant KK. Intermediate pyrolysis of agro-industrial biomasses in bench-scale pyrolyser: product yields and its characterization. Bioresour Technol. 2015;188:258–64. doi:10.1016/j.biortech.2015.02.006. [Google Scholar] [PubMed] [CrossRef]
38. Torri C, Fabbri D. Biochar enables anaerobic digestion of aqueous phase from intermediate pyrolysis of biomass. Bioresour Technol. 2014;172:335–41. doi:10.1016/j.biortech.2014.09.021. [Google Scholar] [PubMed] [CrossRef]
39. Wang J, Wang S. Preparation, modification and environmental application of biochar: a review. J Clean Prod. 2019;227(12):1002–22. doi:10.1016/j.jclepro.2019.04.282. [Google Scholar] [CrossRef]
40. You S, Ok YS, Chen SS, Tsang DCW, Kwon EE, Lee J, et al. A critical review on sustainable biochar system through gasification: energy and environmental applications. Bioresour Technol. 2017;246(2):242–53. doi:10.1016/j.biortech.2017.06.177. [Google Scholar] [PubMed] [CrossRef]
41. Jian X, Zhuang X, Li B, Xu X, Wei Z, Song Y, et al. Comparison of characterization and adsorption of biochars produced from hydrothermal carbonization and pyrolysis. Environ Technol Innov. 2018;10(1):27–35. doi:10.1016/j.eti.2018.01.004. [Google Scholar] [CrossRef]
42. Wang T, Zhai Y, Zhu Y, Li C, Zeng G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: process conditions, fundamentals, and physicochemical properties. Renew Sustain Energ Rev. 2018;90(1):223–47. doi:10.1016/j.rser.2018.03.071. [Google Scholar] [CrossRef]
43. Silveira EA, Barcelo R, Cruz Lamas G, Paulo De Oliveira Rodrigues P, Santana Chaves B, De Paula Protásio T, et al. Biofuel from agro-industrial residues as sustainable strategy for CO2 mitigation: statistical optimization of pequi seeds torrefaction. Energy Convers Manag. 2024;304:118222. doi:10.1016/j.enconman.2024.118222. [Google Scholar] [CrossRef]
44. Luo C, Yang J, Chen W, Han F. Effect of biochar on soil properties on the Loess plateau: results from field experiments. Geoderma. 2020;369(4):114323. doi:10.1016/j.geoderma.2020.114323. [Google Scholar] [CrossRef]
45. Guo M. Application of biochar for soil physical improvement. In: Guo M, He Z, Uchimiya SM, editors. SSSA Special Publications. Madison, WI, USA: American Society of Agronomy and Soil Science Society of America; 2015. p. 101–22. doi:10.2136/sssaspecpub63.2014.0039.5. [Google Scholar] [CrossRef]
46. Omondi MO, Xia X, Nahayo A, Liu X, Korai PK, Pan G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma. 2016;274(1–2):28–34. doi:10.1016/j.geoderma.2016.03.029. [Google Scholar] [CrossRef]
47. Blanco-Canqui H. Does biochar application alleviate soil compaction? Review and data synthesis Geoderma. 2021;404(4):115317. doi:10.1016/j.geoderma.2021.115317. [Google Scholar] [CrossRef]
48. Singh H, Northup BK, Rice CW, Prasad PVV. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: a meta-analysis. Biochar. 2022;4(1):8. doi:10.1007/s42773-022-00138-1. [Google Scholar] [CrossRef]
49. Verheijen FGA, Zhuravel A, Silva FC, Amaro A, Ben-Hur M, Keizer JJ. The influence of biochar particle size and concentration on bulk density and maximum water holding capacity of sandy vs sandy loam soil in a column experiment. Geoderma. 2019;347(1):194–202. doi:10.1016/j.geoderma.2019.03.044. [Google Scholar] [CrossRef]
50. Adekiya AO, Agbede TM, Olayanju A, Ejue WS, Adekanye TA, Adenusi TT, et al. Effect of biochar on soil properties, soil loss, and cocoyam yield on a tropical sandy loam alfisol. Scientific World J. 2020;2020(2):1–9. doi:10.1155/2020/9391630. [Google Scholar] [PubMed] [CrossRef]
51. Ventura F, Salvatorelli F, Piana S, Pieri L, Pisa PR. The effects of biochar on the physical properties of bare soil. Earth Environ Sci Trans R Soc Edinb. 2012;103(1):5–11. doi:10.1017/S1755691012000059. [Google Scholar] [CrossRef]
52. Downie A, Crosky A, Munroe P. Physical properties of biochar. London: Science and Technology; 2009. [Google Scholar]
53. Baronti S, Vaccari FP, Miglietta F, Calzolari C, Lugato E, Orlandini S, et al. Impact of biochar application on plant water relations in Vitis vinifera (L.). Eur J Agron. 2014;53:38–44. doi:10.1016/j.eja.2013.11.003. [Google Scholar] [CrossRef]
54. Egri D, Pârvulescu OC, Ion VA, Răducanu CE, Calcan SI, Bădulescu L, et al. Vine pruning-derived biochar for agronomic benefits. Agronomy. 2022;12(11):2730. doi:10.3390/agronomy12112730. [Google Scholar] [CrossRef]
55. Pastor-Villegas J, Pastor-Valle JF, Rodríguez JMM, García MG. Study of commercial wood charcoals for the preparation of carbon adsorbents. J Anal Appl Pyrolysis. 2006;76(1–2):103–8. doi:10.1016/j.jaap.2005.08.002. [Google Scholar] [CrossRef]
56. Burrell LD, Zehetner F, Rampazzo N, Wimmer B, Soja G. Long-term effects of biochar on soil physical properties. Geoderma. 2016;282(4):96–102. doi:10.1016/j.geoderma.2016.07.019. [Google Scholar] [CrossRef]
57. Herath HMSK, Camps-Arbestain M, Hedley M. Effect of biochar on soil physical properties in two contrasting soils: an alfisol and an andisol. Geoderma. 2013;209–210(3):188–97. doi:10.1016/j.geoderma.2013.06.016. [Google Scholar] [CrossRef]
58. Usowicz B, Lipiec J, Łukowski M, Marczewski W, Usowicz J. The effect of biochar application on thermal properties and albedo of loess soil under grassland and fallow. Soil Tillage Res. 2016;164:45–51. doi:10.1016/j.still.2016.03.009. [Google Scholar] [CrossRef]
59. Horák J, Šimanský V, Igaz D. Biochar and biochar with N fertilizer impact on soil physical properties in a silty loam haplic luvisol. J Ecol Eng. 2019;20(7):31–8. doi:10.12911/22998993/109857. [Google Scholar] [CrossRef]
60. Baronti S, Magno R, Maienza A, Montagnoli A, Ungaro F, Vaccari FP. Long term effect of biochar on soil plant water relation and fine roots: results after 10 years of vineyard experiment. Sci Total Environ. 2022;851(3):158225. doi:10.1016/j.scitotenv.2022.158225. [Google Scholar] [PubMed] [CrossRef]
61. Chang Y, Rossi L, Zotarelli L, Gao B, Shahid MA, Sarkhosh A. Biochar improves soil physical characteristics and strengthens root architecture in Muscadine grape (Vitis rotundifolia L.). Chem Biol Technol Agric. 2021;8(1):7. doi:10.1186/s40538-020-00204-5. [Google Scholar] [CrossRef]
62. Montagnoli A, Baronti S, Alberto D, Chiatante D, Scippa GS, Terzaghi M. Pioneer and fibrous root seasonal dynamics of Vitis vinifera L. are affected by biochar application to a low fertility soil: a rhizobox approach. Sci Total Environ. 2021;751(3):141455. doi:10.1016/j.scitotenv.2020.141455. [Google Scholar] [PubMed] [CrossRef]
63. Hardie M, Clothier B, Bound S, Oliver G, Close D. Does biochar influence soil physical properties and soil water availability? Plant Soil. 2014;376(1–2):347–61. doi:10.1007/s11104-013-1980-x. [Google Scholar] [CrossRef]
64. Suliman W, Harsh JB, Abu-Lail NI, Fortuna A-M, Dallmeyer I, Garcia-Pérez M. The role of biochar porosity and surface functionality in augmenting hydrologic properties of a sandy soil. Sci Total Environ. 2017;574(9):139–47. doi:10.1016/j.scitotenv.2016.09.025. [Google Scholar] [PubMed] [CrossRef]
65. Fu Q, Zhao H, Li H, Li T, Hou R, Liu D, et al. Effects of biochar application during different periods on soil structures and water retention in seasonally frozen soil areas. Sci Total Environ. 2019;694(2):133732. doi:10.1016/j.scitotenv.2019.133732. [Google Scholar] [PubMed] [CrossRef]
66. Zanutel M, Garré S, Sanglier P, Bielders C. Biochar modifies soil physical properties mostly through changes in soil structure rather than through its internal porosity. Vadose Zone J. 2024;23(1):e20301. doi:10.1002/vzj2.20301. [Google Scholar] [CrossRef]
67. Islam MU, Jiang F, Guo Z, Peng X. Does biochar application improve soil aggregation? A meta-analysis. Soil Tillage Res. 2021;209:104926. doi:10.1016/j.still.2020.104926. [Google Scholar] [CrossRef]
68. Glaser B, Lehmann J, Zech W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fertil Soils. 2002;35(4):219–30. doi:10.1007/s00374-002-0466-4. [Google Scholar] [CrossRef]
69. Ouyang L, Wang F, Tang J, Yu L, Zhang R. Effects of biochar amendment on soil aggregates and hydraulic properties. J Soil Sci Plant Nutr. 2013. doi:10.4067/S0718-95162013005000078. [Google Scholar] [CrossRef]
70. Du Z-L, Zhao J-K, Wang Y-D, Zhang Q-Z. Biochar addition drives soil aggregation and carbon sequestration in aggregate fractions from an intensive agricultural system. J Soils Sediments. 2017;17(3):581–9. doi:10.1007/s11368-015-1349-2. [Google Scholar] [CrossRef]
71. Han L, Zhang B, Chen L, Feng Y, Yang Y, Sun K. Impact of biochar amendment on soil aggregation varied with incubation duration and biochar pyrolysis temperature. Biochar. 2021;3(3):339–47. doi:10.1007/s42773-021-00097-z. [Google Scholar] [CrossRef]
72. Sun Q, Meng J, Lan Y, Shi G, Yang X, Cao D, et al. Long-term effects of biochar amendment on soil aggregate stability and biological binding agents in brown earth. CATENA. 2021;205(12):105460. doi:10.1016/j.catena.2021.105460. [Google Scholar] [CrossRef]
73. Nkoh JN, Baquy MA-A, Mia S, Shi R, Kamran MA, Mehmood K, et al. A critical-systematic review of the interactions of biochar with soils and the observable outcomes. Sustainability. 2021;13(24):13726. doi:10.3390/su132413726. [Google Scholar] [CrossRef]
74. Alghamdi AG. Biochar as a potential soil additive for improving soil physical properties—a review. Arab J Geosci. 2018;11(24):766. doi:10.1007/s12517-018-4056-7. [Google Scholar] [CrossRef]
75. Sohi SP, Krull E, Lopez-Capel E, Bol R. A review of biochar and its use and function in soil. In: Advances in agronomy. Elsevier; 2010. vol. 105, p. 47–82. doi:10.1016/S0065-2113(10)05002-9. [Google Scholar] [CrossRef]
76. Shafie ST, Mohd MA, Hang LL. Effect of pyrolysis temperature on the biochar nutrient and water retention capacity. J Purity Util React Environ. 2012;1(6):323–37. [Google Scholar]
77. Yan Q, Dong F, Li J, Duan Z, Yang F, Li X, et al. Effects of maize straw-derived biochar application on soil temperature, water conditions and growth of winter wheat. Eur J Soil Sci. 2019;70(6):1280–9. doi:10.1111/ejss.12863. [Google Scholar] [CrossRef]
78. Atkinson CJ, Aitkenhead M. How good is the evidence that soil-applied biochar improves water-holding capacity? Soil Use Manag. 2018;34(2):177–86. doi:10.1111/sum.12413. [Google Scholar] [CrossRef]
79. Liu Z, Dugan B, Masiello CA, Gonnermann HM. Biochar particle size, shape, and porosity act together to influence soil water properties. PLoS One. 2017;12(6):e0179079. doi:10.1371/journal.pone.0179079. [Google Scholar] [PubMed] [CrossRef]
80. Zhang J, Amonette JE, Flury M. Effect of biochar and biochar particle size on plant-available water of sand, silt loam, and clay soil. Soil Tillage Res. 2021;212:104992. doi:10.1016/j.still.2021.104992. [Google Scholar] [CrossRef]
81. Rabbi SMF, Minasny B, Salami ST, McBratney AB, Young IM. Greater, but not necessarily better: the influence of biochar on soil hydraulic properties. Eur J Soil Sci. 2021;72(5):2033–48. doi:10.1111/ejss.13105. [Google Scholar] [CrossRef]
82. Razzaghi F, Obour PB, Arthur E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma. 2020;361(2):114055. doi:10.1016/j.geoderma.2019.114055. [Google Scholar] [CrossRef]
83. Chang Y, Rossi L, Zotarelli L, Gao B, Sarkhosh A. Greenhouse evaluation of pinewood biochar effects on nutrient status and physiological performance in muscadine grape (Vitis rotundifolia L.). HortScience. 2021;56(2):277–85. doi:10.21273/HORTSCI15428-20. [Google Scholar] [CrossRef]
84. Alghamdi AG, Alkhasha A, Ibrahim HM. Effect of biochar particle size on water retention and availability in a sandy loam soil. J Saudi Chem Soc. 2020;24(12):1042–50. doi:10.1016/j.jscs.2020.11.003. [Google Scholar] [CrossRef]
85. Petrillo M, Zanotelli D, Lucchetta V, Aguzzoni A, Tagliavini M, Andreotti C. The use of biochar as soil amendment: effects on nitrogen and water availability for potted grapevines. Italus Hortus. 2020;27:28–40. doi:10.26353/j.itahort/2020.2.2840. [Google Scholar] [CrossRef]
86. Qian Z, Tang L, Zhuang S, Zou Y, Fu D, Chen X. Effects of biochar amendments on soil water retention characteristics of red soil at south China. Biochar. 2020;2(4):479–88. doi:10.1007/s42773-020-00068-w. [Google Scholar] [CrossRef]
87. Seyedsadr S, Šípek V, Jačka L, Sněhota M, Beesley L, Pohořelý M, et al. Biochar considerably increases the easily available water and nutrient content in low-organic soils amended with compost and manure. Chemosphere. 2022;293(7):133586. doi:10.1016/j.chemosphere.2022.133586. [Google Scholar] [PubMed] [CrossRef]
88. Polidoro JC, De Freitas PL, Hernani LC, Anjos LHCD, Rodrigues RDAR, Cesário FV, et al. Potential impact of plans and policies based on the principles of conservation agriculture on the control of soil erosion in Brazil. Land Degrad Dev. 2021;32(12):3457–68. doi:10.1002/ldr.3876. [Google Scholar] [CrossRef]
89. Cai W, Huang H, Chen P, Huang X, Gaurav S, Pan Z, et al. Effects of biochar from invasive weed on soil erosion under varying compaction and slope conditions: comprehensive study using flume experiments. Biomass Conv Bioref. 2024;14(5):5771–90. doi:10.1007/s13399-020-00943-3. [Google Scholar] [CrossRef]
90. Blanco-Canqui H. Biochar and water quality. J Environ Qual. 2019;48(1):2–15. doi:10.2134/jeq2018.06.0248. [Google Scholar] [PubMed] [CrossRef]
91. Abrol V, Ben-Hur M, Verheijen FGA, Keizer JJ, Martins MAS, Tenaw H, et al. Biochar effects on soil water infiltration and erosion under seal formation conditions: rainfall simulation experiment. J Soils Sediments. 2016;16(12):2709–19. doi:10.1007/s11368-016-1448-8. [Google Scholar] [CrossRef]
92. Jien S-H, Wang C-S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. CATENA. 2013;110:225–33. doi:10.1016/j.catena.2013.06.021. [Google Scholar] [CrossRef]
93. Shi G, Wu Y, Li T, Fu Q, Wei Y. Mid- and long-term effects of biochar on soil improvement and soil erosion control of sloping farmland in a black soil region, China. J Environ Manag. 2022;320:115902. doi:10.1016/j.jenvman.2022.115902. [Google Scholar] [PubMed] [CrossRef]
94. Agbede TM, Adekiya AO. Influence of biochar on soil physicochemical properties, erosion potential, and maize (Zea mays L.) grain yield under sandy soil condition. Commun Soil Sci Plant Anal. 2020;51(20):2559–68. doi:10.1080/00103624.2020.1845348. [Google Scholar] [CrossRef]
95. Peng X, Zhu QH, Xie ZB, Darboux F, Holden NM. The impact of manure, straw and biochar amendments on aggregation and erosion in a hillslope ultisol. CATENA. 2016;138:30–7. doi:10.1016/j.catena.2015.11.008. [Google Scholar] [CrossRef]
96. Blanco-Canqui H. Does biochar improve all soil ecosystem services? GCB Bioenergy. 2021;13(2):291–304. doi:10.1111/gcbb.12783. [Google Scholar] [CrossRef]
97. Onwuka B. Effects of soil temperature on some soil properties and plant growth. Adv Plants Agric Res. 2018;8(1):34–7. doi:10.15406/apar.2018.08.00288. [Google Scholar] [CrossRef]
98. Wang C, Morrissey EM, Mau RL, Hayer M, Piñeiro J, Mack MC, et al. The temperature sensitivity of soil: microbial biodiversity, growth, and carbon mineralization. ISME J. 2021;15(9):2738–47. doi:10.1038/s41396-021-00959-1. [Google Scholar] [PubMed] [CrossRef]
99. Genesio L, Miglietta F, Lugato E, Baronti S, Pieri M, Vaccari FP. Surface albedo following biochar application in durum wheat. Environ Res Lett. 2012;7(1):014025. doi:10.1088/1748-9326/7/1/014025. [Google Scholar] [CrossRef]
100. Oguntunde PG, Abiodun BJ, Ajayi AE, Van De Giesen N. Effects of charcoal production on soil physical properties in Ghana. Z Pflanzenernähr Bodenk. 2008;171(4):591–6. doi:10.1002/jpln.200625185. [Google Scholar] [CrossRef]
101. Obia A, Cornelissen G, Martinsen V, Smebye AB, Mulder J. Conservation tillage and biochar improve soil water content and moderate soil temperature in a tropical acrisol. Soil Tillage Res. 2020;197(5):104521. doi:10.1016/j.still.2019.104521. [Google Scholar] [CrossRef]
102. Zhang Q, Wang Y, Wu Y, Wang X, Du Z, Liu X, et al. Effects of biochar amendment on soil thermal conductivity, reflectance, and temperature. Soil Sci Soc Am J. 2013;77(5):1478–87. doi:10.2136/sssaj2012.0180. [Google Scholar] [CrossRef]
103. Feng W, Yang F, Cen R, Liu J, Qu Z, Miao Q, et al. Effects of straw biochar application on soil temperature, available nitrogen and growth of corn. J Environ Manag. 2021;277:111331. doi:10.1016/j.jenvman.2020.111331. [Google Scholar] [PubMed] [CrossRef]
104. Sun Z, Hu Y, Shi L, Li G, Pang Z, Liu S, et al. Effects of biochar on soil chemical properties: a global meta-analysis of agricultural soil. Plant Soil Environ. 2022;68(6):272–89. doi:10.17221/522/2021-PSE. [Google Scholar] [CrossRef]
105. Gul S, Whalen JK, Thomas BW, Sachdeva V, Deng H. Physico-chemical properties and microbial responses in biochar-amended soils: mechanisms and future directions. Agric Ecosyst Environ. 2015;206:46–59. doi:10.1016/j.agee.2015.03.015. [Google Scholar] [CrossRef]
106. Dai Z, Zhang X, Tang C, Muhammad N, Wu J, Brookes PC, et al. Potential role of biochars in decreasing soil acidification—a critical review. Sci Total Environ. 2017;581–582(36101):601–11. doi:10.1016/j.scitotenv.2016.12.169. [Google Scholar] [PubMed] [CrossRef]
107. Ding Y, Liu Y, Liu S, Li Z, Tan X, Huang X, et al. Biochar to improve soil fertility. A review. Agron Sustain Dev. 2016;36(2):36. doi:10.1007/s13593-016-0372-z. [Google Scholar] [CrossRef]
108. Hailegnaw NS, Mercl F, Pračke K, Száková J, Tlustoš P. Mutual relationships of biochar and soil pH, CEC, and exchangeable base cations in a model laboratory experiment. J Soils Sediments. 2019;19(5):2405–16. doi:10.1007/s11368-019-02264-z. [Google Scholar] [CrossRef]
109. Huang K, Li M, Li R, Rasul F, Shahzad S, Wu C, et al. Soil acidification and salinity: the importance of biochar application to agricultural soils. Front Plant Sci. 2023;14:1206820. doi:10.3389/fpls.2023.1206820. [Google Scholar] [PubMed] [CrossRef]
110. Idbella M, Baronti S, Giagnoni L, Renella G, Becagli M, Cardelli R, et al. Long-term effects of biochar on soil chemistry, biochemistry, and microbiota: results from a 10-year field vineyard experiment. Appl Soil Ecol. 2024;195(14):105217. doi:10.1016/j.apsoil.2023.105217. [Google Scholar] [CrossRef]
111. Maienza A, Baronti S, Cincinelli A, Martellini T, Grisolia A, Miglietta F, et al. Biochar improves the fertility of a mediterranean vineyard without toxic impact on the microbial community. Agron Sustain Dev. 2017;37(5):47. doi:10.1007/s13593-017-0458-2. [Google Scholar] [CrossRef]
112. Amendola C, Montagnoli A, Terzaghi M, Trupiano D, Oliva F, Baronti S, et al. Short-term effects of biochar on grapevine fine root dynamics and arbuscular mycorrhizae production. Agric Ecosyst Environ. 2017;239(2):236–45. doi:10.1016/j.agee.2017.01.025. [Google Scholar] [CrossRef]
113. Bastos AC, Verheijen FGA, Amaro A, Prodana M, Cardoso DN, Morgado RG, et al. Screening the habitat function of biochar-amended vineyard soils at field plot-scale, based on invertebrate avoidance behaviour. Appl Soil Ecol. 2022;177:104526. doi:10.1016/j.apsoil.2022.104526. [Google Scholar] [CrossRef]
114. Karimi A, Moezzi A, Chorom M, Enayatizamir N. Chemical fractions and availability of Zn in a calcareous soil in response to biochar amendments. J Soil Sci Plant Nutr. 2019;19(4):851–64. doi:10.1007/s42729-019-00084-1. [Google Scholar] [CrossRef]
115. Chen L, Deng R, Li X, Yu M, Xiao H. Responses of wheat production, quality, and soil profile properties to biochar applied at different seasons in a rice-wheat rotation. Phyton-Int J Exp Bot. 2023;92(12):3359–70. doi:10.32604/phyton.2023.046877. [Google Scholar] [CrossRef]
116. Diatta AA, Fike JH, Battaglia ML, Galbraith JM, Baig MB. Effects of biochar on soil fertility and crop productivity in arid regions: a review. Arab J Geosci. 2020;13(14):595. doi:10.1007/s12517-020-05586-2. [Google Scholar] [CrossRef]
117. Clough T, Condron L, Kammann C, Müller C. A review of biochar and soil nitrogen dynamics. Agronomy. 2013;3(2):275–93. doi:10.3390/agronomy3020275. [Google Scholar] [CrossRef]
118. Joseph S, Cowie AL, Van Zwieten L, Bolan N, Budai A, Buss W, et al. How biochar works, and when it doesn’t: a review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy. 2021;13(11):1731–64. doi:10.1111/gcbb.12885. [Google Scholar] [CrossRef]
119. Karimi A, Moezzi A, Chorom M, Enayatizamir N. Application of biochar changed the status of nutrients and biological activity in a calcareous soil. J Soil Sci Plant Nutr. 2020;20(2):450–9. doi:10.1007/s42729-019-00129-5. [Google Scholar] [CrossRef]
120. Rombolà AG, Fabbri D, Baronti S, Vaccari FP, Genesio L, Miglietta F. Changes in the pattern of polycyclic aromatic hydrocarbons in soil treated with biochar from a multiyear field experiment. Chemosphere. 2019;219(14):662–70. doi:10.1016/j.chemosphere.2018.11.178. [Google Scholar] [PubMed] [CrossRef]
121. Zheng Y, Han X, Li Y, Yang J, Li N, An N. Effects of biochar and straw application on the physicochemical and biological properties of paddy soils in northeast China. Sci Rep. 2019;9:16531. doi:10.1038/s41598-019-52978-w. [Google Scholar] [PubMed] [CrossRef]
122. Phares CA, Amoakwah E, Danquah A, Akaba S, Frimpong KA, Mensah TA. Improved soil physicochemical, biological properties and net income following the application of inorganic NPK fertilizer and biochar for maize production. Acta Ecologica Sinica. 2022;42:289–95. doi:10.1016/j.chnaes.2021.12.002. [Google Scholar] [CrossRef]
123. Ameloot N, Graber ER, Verheijen FGA, De Neve S. Interactions between biochar stability and soil organisms: review and research needs. Eur J Soil Sci. 2013;64:379–90. doi:10.1111/ejss.12064. [Google Scholar] [CrossRef]
124. Andrés P, Rosell-Melé A, Colomer-Ventura F, Denef K, Cotrufo MF, Riba M, et al. Belowground biota responses to maize biochar addition to the soil of a Mediterranean vineyard. Sci Total Environ. 2019;660:1522–32. doi:10.1016/j.scitotenv.2019.01.101. [Google Scholar] [PubMed] [CrossRef]
125. Xu W, Whitman WB, Gundale MJ, Chien C, Chiu C. Functional response of the soil microbial community to biochar applications. GCB Bioenergy. 2021;13:269–81. doi:10.1111/gcbb.12773. [Google Scholar] [CrossRef]
126. Ameloot N, Sleutel S, Das KC, Kanagaratnam J, De Neve S. Biochar amendment to soils with contrasting organic matter level: effects on N mineralization and biological soil properties. GCB Bioenergy. 2015;7:135–44. doi:10.1111/gcbb.12119. [Google Scholar] [CrossRef]
127. Amoakwah E, Arthur E, Frimpong KA, Lorenz N, Rahman MA, Nziguheba G, et al. Biochar amendment impacts on microbial community structures and biological and enzyme activities in a weathered tropical sandy loam. Appl Soil Ecol. 2022;172:104364. doi:10.1016/j.apsoil.2021.104364. [Google Scholar] [CrossRef]
128. Kocsis T, Ringer M, Biró B. Characteristics and applications of biochar in soil-plant systems: a short review of benefits and potential drawbacks. Appl Sci. 2022;12:4051. doi:10.3390/app12084051. [Google Scholar] [CrossRef]
129. Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D. Biochar effects on soil biota—a review. Soil Biol Biochem. 2011;43:1812–36. doi:10.1016/j.soilbio.2011.04.022. [Google Scholar] [CrossRef]
130. Deshoux M, Sadet-Bourgeteau S, Gentil S, Prévost-Bouré NC. Effects of biochar on soil microbial communities: a meta-analysis. Sci Total Environ. 2023;902:166079. doi:10.1016/j.scitotenv.2023.166079. [Google Scholar] [PubMed] [CrossRef]
131. Prodana M, Bastos AC, Amaro A, Cardoso D, Morgado R, Machado AL, et al. Biomonitoring tools for biochar and biochar-compost amended soil under viticulture: looking at exposure and effects. Appl Soil Ecol. 2019;137:120–8. doi:10.1016/j.apsoil.2019.01.007. [Google Scholar] [CrossRef]
132. Maienza A, Remelli S, Verdinelli M, Baronti S, Crisci A, Vaccari FP, et al. A magnifying glass on biochar strategy: long-term effects on the soil biota of a Tuscan vineyard. J Soils Sediments. 2023;23(4):1733–44. doi:10.1007/s11368-023-03447-5. [Google Scholar] [CrossRef]
133. Briones MJI, Panzacchi P, Davies CA, Ineson P. Contrasting responses of macro- and meso-fauna to biochar additions in a bioenergy cropping system. Soil Biol Biochem. 2020;145(4):107803. doi:10.1016/j.soilbio.2020.107803. [Google Scholar] [CrossRef]
134. Gross A, Bromm T, Glaser B. Soil organic carbon sequestration after biochar application: a global meta-analysis. Agronomy. 2021;11(12):2474. doi:10.3390/agronomy11122474. [Google Scholar] [CrossRef]
135. Lorenz K, Lal R. Biochar application to soil for climate change mitigation by soil organic carbon sequestration. J Plant Nutr Soil Sci. 2014;177(5):651–70. doi:10.1002/jpln.201400058. [Google Scholar] [CrossRef]
136. Zheng H, Liu D, Liao X, Miao Y, Li Y, Li J, et al. Field-aged biochar enhances soil organic carbon by increasing recalcitrant organic carbon fractions and making microbial communities more conducive to carbon sequestration. Agric Ecosyst Environ. 2022;340:108177. doi:10.1016/j.agee.2022.108177. [Google Scholar] [CrossRef]
137. Backer RGM, Schwinghamer TD, Whalen JK, Seguin P, Smith DL. Crop yield and SOC responses to biochar application were dependent on soil texture and crop type in southern Quebec, Canada. J Plant Nutr Soil Sci. 2016;179(3):399–408. doi:10.1002/jpln.201500520. [Google Scholar] [CrossRef]
138. Abagandura GO, Bansal S, Karsteter A, Kumar S. Soil greenhouse gas emissions, organic carbon and crop yield following pinewood biochar and biochar-manure applications at eroded and depositional landscape positions: a field trial in south Dakota, USA. Soil Use Manag. 2022;38(1):487–502. doi:10.1111/sum.12760. [Google Scholar] [CrossRef]
139. Rombolà AG, Meredith W, Snape CE, Baronti S, Genesio L, Vaccari FP, et al. Fate of soil organic carbon and polycyclic aromatic hydrocarbons in a vineyard soil treated with biochar. Environ Sci Technol. 2015;49(18):11037–44. doi:10.1021/acs.est.5b02562. [Google Scholar] [PubMed] [CrossRef]
140. Šimanský V, Horák J, Igaz D, Jonczak J, Markiewicz M, Felber R, et al. How dose of biochar and biochar with nitrogen can improve the parameters of soil organic matter and soil structure? Biologia. 2016;71(9):989–95. doi:10.1515/biolog-2016-0122. [Google Scholar] [CrossRef]
141. Raya-Moreno I, Cañizares R, Domene X, Carabassa V, Alcañiz JM. Biochar addition to a mediterranean agroecosystem: short-term divergent priming effects. Agriculture. 2024;14(2):242. doi:10.3390/agriculture14020242. [Google Scholar] [CrossRef]
142. Lu H, Bian R, Xia X, Cheng K, Liu X, Liu Y, et al. Legacy of soil health improvement with carbon increase following one time amendment of biochar in a paddy soil—a rice farm trial. Geoderma. 2020;376(11):114567. doi:10.1016/j.geoderma.2020.114567. [Google Scholar] [CrossRef]
143. Wang Y, Wang H-S, Tang C-S, Gu K, Shi B. Remediation of heavy-metal-contaminated soils by biochar: a review. Environ Geotech. 2020;31(4):1–14. doi:10.1680/jenge.18.00091. [Google Scholar] [CrossRef]
144. Bagheri Novair S, Cheraghi M, Faramarzi F, Asgari Lajayer B, Senapathi V, Astatkie T, et al. Reviewing the role of biochar in paddy soils: an agricultural and environmental perspective. Ecotoxicol Environ Saf. 2023;263(9):115228. doi:10.1016/j.ecoenv.2023.115228. [Google Scholar] [PubMed] [CrossRef]
145. Liu M, Almatrafi E, Zhang Y, Xu P, Song B, Zhou C, et al. A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: applications and practical evaluations. Sci Total Environ. 2022;806:150531. doi:10.1016/j.scitotenv.2021.150531. [Google Scholar] [PubMed] [CrossRef]
146. He L, Zhong H, Liu G, Dai Z, Brookes PC, Xu J. Remediation of heavy metal contaminated soils by biochar: mechanisms, potential risks and applications in China. Environ Pollut. 2019;252(1):846–55. doi:10.1016/j.envpol.2019.05.151. [Google Scholar] [PubMed] [CrossRef]
147. Wang Y, Liu Y, Zhan W, Zheng K, Wang J, Zhang C, et al. Stabilization of heavy metal-contaminated soils by biochar: challenges and recommendations. Sci Total Environ. 2020;729(197):139060. doi:10.1016/j.scitotenv.2020.139060. [Google Scholar] [PubMed] [CrossRef]
148. Mansoor S, Kour N, Manhas S, Zahid S, Wani OA, Sharma V, et al. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere. 2021;271(4):129458. doi:10.1016/j.chemosphere.2020.129458. [Google Scholar] [PubMed] [CrossRef]
149. Zhang R-H, Xie Y, Zhou G, Li Z, Ye A, Huang X, et al. The effects of short-term, long-term, and reapplication of biochar on the remediation of heavy metal-contaminated soil. Ecotoxicol Environ Saf. 2022;248(3):114316. doi:10.1016/j.ecoenv.2022.114316. [Google Scholar] [PubMed] [CrossRef]
150. Eon P, Robert T, Goutouly J-P, Aurelle V, Cornu J-Y. Cover crop response to increased concentrations of copper in vineyard soils: implications for copper phytoextraction. Chemosphere. 2023;329:138604. doi:10.1016/j.chemosphere.2023.138604. [Google Scholar] [PubMed] [CrossRef]
151. Karimi B, Masson V, Guilland C, Leroy E, Pellegrinelli S, Giboulot E, et al. Ecotoxicity of copper input and accumulation for soil biodiversity in vineyards. Environ Chem Lett. 2021;19(3):2013–30. doi:10.1007/s10311-020-01155-x. [Google Scholar] [CrossRef]
152. Brunetto G, Bastos De Melo GW, Terzano R, Del Buono D, Astolfi S, Tomasi N, et al. Copper accumulation in vineyard soils: rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere. 2016;162:293–307. doi:10.1016/j.chemosphere.2016.07.104. [Google Scholar] [PubMed] [CrossRef]
153. Mackie KA, Marhan S, Ditterich F, Schmidt HP, Kandeler E. The effects of biochar and compost amendments on copper immobilization and soil microorganisms in a temperate vineyard. Agric Ecosyst Environ. 2015;201:58–69. doi:10.1016/j.agee.2014.12.001. [Google Scholar] [CrossRef]
154. Soja G, Wimmer B, Rosner F, Faber F, Dersch G, Von Chamier J, et al. Compost and biochar interactions with copper immobilisation in copper-enriched vineyard soils. Appl Geochem. 2018;88:40–8. doi:10.1016/j.apgeochem.2017.06.004. [Google Scholar] [CrossRef]
155. Pump C, Keiblinger KM, Scheiblauer E, Johnen S, Lehto NJ, Soja G, et al. Temporal changes in the efficiency of biochar- and compost-based amendments on copper immobilization in vineyard soils. Soil Syst. 2019;3(4):78. doi:10.3390/soilsystems3040078. [Google Scholar] [CrossRef]
156. Zafeiriou I, Karadendrou K, Ioannou D, Karadendrou M-A, Detsi A, Kalderis D, et al. Effects of biochars derived from sewage sludge and olive tree prunings on Cu fractionation and mobility in vineyard soils over time. Land. 2023;12(2):416. doi:10.3390/land12020416. [Google Scholar] [CrossRef]
157. Zou Z, Fan L, Li X, Dong C, Zhang L, Zhang L, et al. Response of plant root growth to biochar amendment: a meta-analysis. Agronomy. 2021;11(12):2442. doi:10.3390/agronomy11122442. [Google Scholar] [CrossRef]
158. Kanwal S, Batool A, Ghufran MA, Khalid A. Effect of dairy manure derived biochar on microbial biomass carbon, soil carbon and Vitis vinifera under water stress conditions. Pak J Bot. 2018;50(5):1713–8. [Google Scholar]
159. Schmidt H-P, Kammann C, Niggli C, Evangelou MWH, Mackie KA, Abiven S. Biochar and biochar-compost as soil amendments to a vineyard soil: influences on plant growth, nutrient uptake, plant health and grape quality. Agric Ecosyst Environ. 2014;191(3):117–23. doi:10.1016/j.agee.2014.04.001. [Google Scholar] [CrossRef]
160. Seif AS, Ibrahim ZA, Beheiry Hr, Abd El-Samad A. Effect of biochar soil application and glycine betaine foliar spraying in mitigating the adverse effects of salinity stress on vegetative growth and survivability of ‘superior’ grapevine cv. transplants. Fayoum J Agric Res Dev. 2020;341:332–47. [Google Scholar]
161. Genesio L, Miglietta F, Baronti S, Vaccari FP. Biochar increases vineyard productivity without affecting grape quality: results from a four years field experiment in Tuscany. Agric Ecosyst Environ. 2015;201(2):20–5. doi:10.1016/j.agee.2014.11.021. [Google Scholar] [CrossRef]
162. Raifer B, Lucchetta V, Loesch M, Chitarrini G, Dordevic N, Matteazzi A, et al. The use of biochar as a soil amendment did not affect wine quality in a müller thurgau vineyard in south Tyrol (Italy). Laimburg J. 2024;6. doi:10.23796/LJ/2024.003. [Google Scholar] [CrossRef]
163. McCormack SA, Ostle N, Bardgett RD, Hopkins DW, Vanbergen AJ. Biochar in bioenergy cropping systems: impacts on soil faunal communities and linked ecosystem processes. GCB Bioenergy. 2013;5(2):81–95. doi:10.1111/gcbb.12046. [Google Scholar] [CrossRef]
164. Pathy A, Ray J, Paramasivan B. Biochar amendments and its impact on soil biota for sustainable agriculture. Biochar. 2020;2(3):287–305. doi:10.1007/s42773-020-00063-1. [Google Scholar] [CrossRef]
165. Godlewska P, Ok YS, Oleszczuk P. THE DARK SIDE OF BLACK GOLD: ecotoxicological aspects of biochar and biochar-amended soils. J Hazard Mater. 2021;403:123833. doi:10.1016/j.jhazmat.2020.123833. [Google Scholar] [PubMed] [CrossRef]
166. Jatav HS, Rajput VD, Minkina T, Singh SK, Chejara S, Gorovtsov A, et al. Sustainable approach and safe use of biochar and its possible consequences. Sustainability. 2021;13(18):10362. doi:10.3390/su131810362. [Google Scholar] [CrossRef]
167. Kamali M, Sweygers N, Al-Salem S, Appels L, Aminabhavi TM, Dewil R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem Eng J. 2022;428(15–18):131189. doi:10.1016/j.cej.2021.131189. [Google Scholar] [CrossRef]
168. Tomczyk B, Siatecka A, Jędruchniewicz K, Sochacka A, Bogusz A, Oleszczuk P. Polycyclic aromatic hydrocarbons (PAHs) persistence, bioavailability and toxicity in sewage sludge- or sewage sludge-derived biochar-amended soil. Sci Total Environ. 2020;747:141123. doi:10.1016/j.scitotenv.2020.141123. [Google Scholar] [PubMed] [CrossRef]
169. IBI. Standardized product definition and product testing guidelines for biochar that is used in soil. International Biochar Initiative. 2015. Available from: https://biochar-international.org/biochar-standards/. [Accessed 2024]. [Google Scholar]
170. EBC. European biochar certificate—guidelines for a sustainable production of biochar; Carbon Standards International (CSIFrick, Switzerland. 2023. Available from: http://european-biochar.org. [Accessed 2024]. [Google Scholar]
171. Yao C, Wang B, Zhang J, Faheem M, Feng Q, Hassan M, et al. Formation mechanisms and degradation methods of polycyclic aromatic hydrocarbons in biochar: a review. J Environ Manag. 2024;357:120610. doi:10.1016/j.jenvman.2024.120610. [Google Scholar] [PubMed] [CrossRef]
172. Odinga ES, Gudda FO, Waigi MG, Wang J, Gao Y. Occurrence, formation and environmental fate of polycyclic aromatic hydrocarbons in biochars. Fundam Res. 2021;1(3):296–305. doi:10.1016/j.fmre.2021.03.003. [Google Scholar] [CrossRef]
173. Wang J, Odinga ES, Zhang W, Zhou X, Yang B, Waigi MG, et al. Polyaromatic hydrocarbons in biochars and human health risks of food crops grown in biochar-amended soils: a synthesis study. Environ Int. 2019;130(1–3):104899. doi:10.1016/j.envint.2019.06.009. [Google Scholar] [PubMed] [CrossRef]
174. Maliszewska-Kordybach B. Polycyclic aromatic hydrocarbons in agricultural soils in Poland: preliminary proposals for criteria to evaluate the level of soil contamination. Appl Geochem. 1996;11(1–2):121–7. doi:10.1016/0883-2927(95)00076-3. [Google Scholar] [CrossRef]
175. Bianco F, Race M, Papirio S, Oleszczuk P, Esposito G. The addition of biochar as a sustainable strategy for the remediation of PAH-contaminated sediments. Chemosphere. 2021;263:128274. doi:10.1016/j.chemosphere.2020.128274. [Google Scholar] [PubMed] [CrossRef]
176. Abukari A, Kaba JS, Dawoe E, Abunyewa AA. A comprehensive review of the effects of biochar on soil physicochemical properties and crop productivity. Waste Dispos Sustain Energy. 2022;4(4):343–59. doi:10.1007/s42768-022-00114-2. [Google Scholar] [CrossRef]
177. Xiang L, Liu S, Ye S, Yang H, Song B, Qin F, et al. Potential hazards of biochar: the negative environmental impacts of biochar applications. J Hazard Mater. 2021;420:126611. doi:10.1016/j.jhazmat.2021.126611. [Google Scholar] [PubMed] [CrossRef]
178. Yuan C, Gao B, Peng Y, Gao X, Fan B, Chen Q. A meta-analysis of heavy metal bioavailability response to biochar aging: importance of soil and biochar properties. Sci Total Environ. 2021;756(5):144058. doi:10.1016/j.scitotenv.2020.144058. [Google Scholar] [PubMed] [CrossRef]
179. Lu T, Yuan H, Wang Y, Huang H, Chen Y. Characteristic of heavy metals in biochar derived from sewage sludge. J Mater Cycles Waste Manag. 2016;18(4):725–33. doi:10.1007/s10163-015-0366-y. [Google Scholar] [CrossRef]
180. de Figueiredo CC, Chagas JKM, da Silva J, Paz-Ferreiro J. Short-term effects of a sewage sludge biochar amendment on total and available heavy metal content of a tropical soil. Geoderma. 2019;344:31–9. doi:10.1016/j.geoderma.2019.01.052. [Google Scholar] [CrossRef]
181. Zhang J, Hu H, Wang M, Li Y, Wu S, Cao Y, et al. Land application of sewage sludge biochar: assessments of soil-plant-human health risks from potentially toxic metals. Sci Total Environ. 2021;756:144137. doi:10.1016/j.scitotenv.2020.144137. [Google Scholar] [PubMed] [CrossRef]
182. Bandara T, Herath I, Kumarathilaka P, Hseu Z-Y, Ok YS, Vithanage M. Efficacy of woody biomass and biochar for alleviating heavy metal bioavailability in serpentine soil. Environ Geochem Health. 2017;39(2):391–401. doi:10.1007/s10653-016-9842-0. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF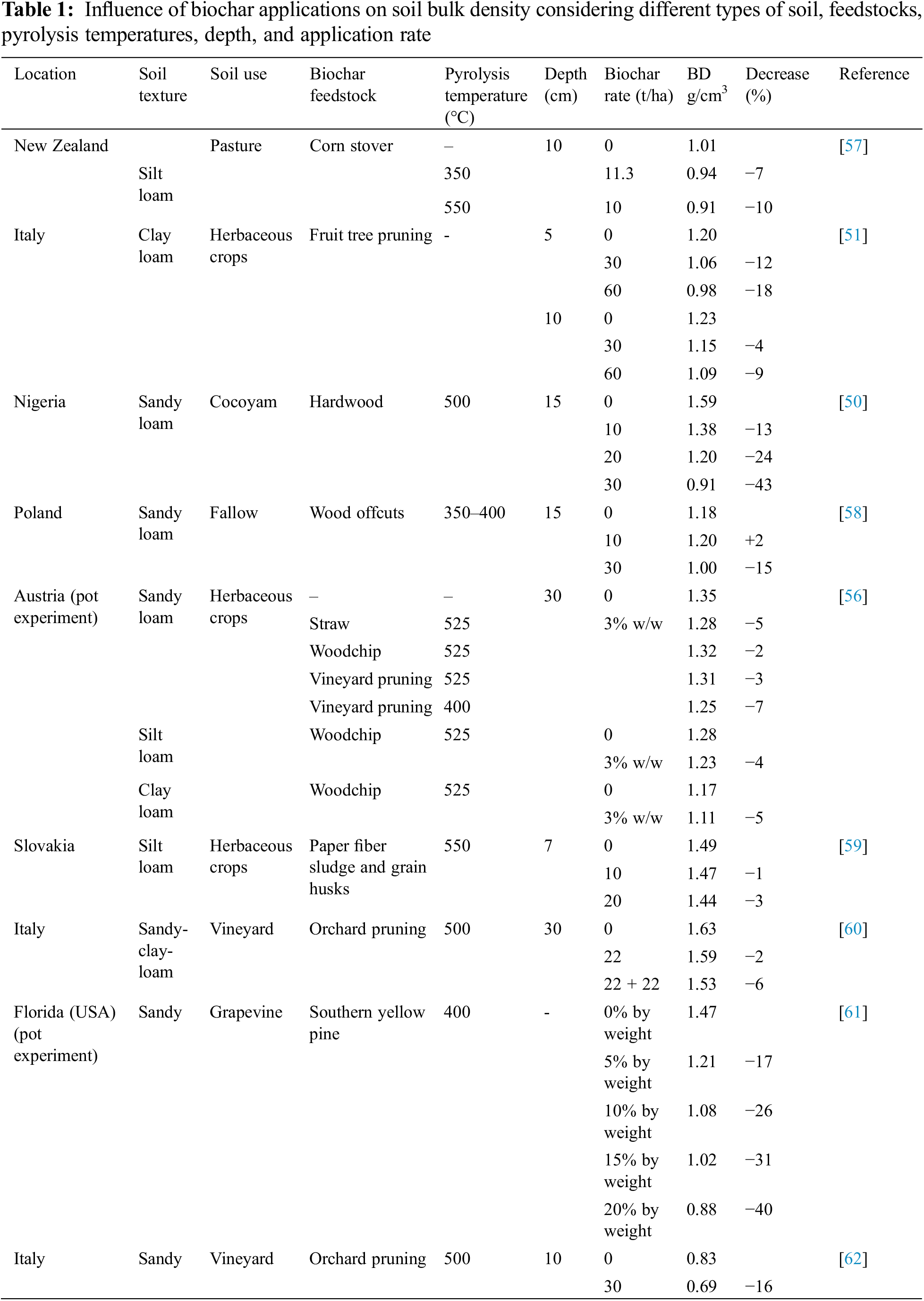
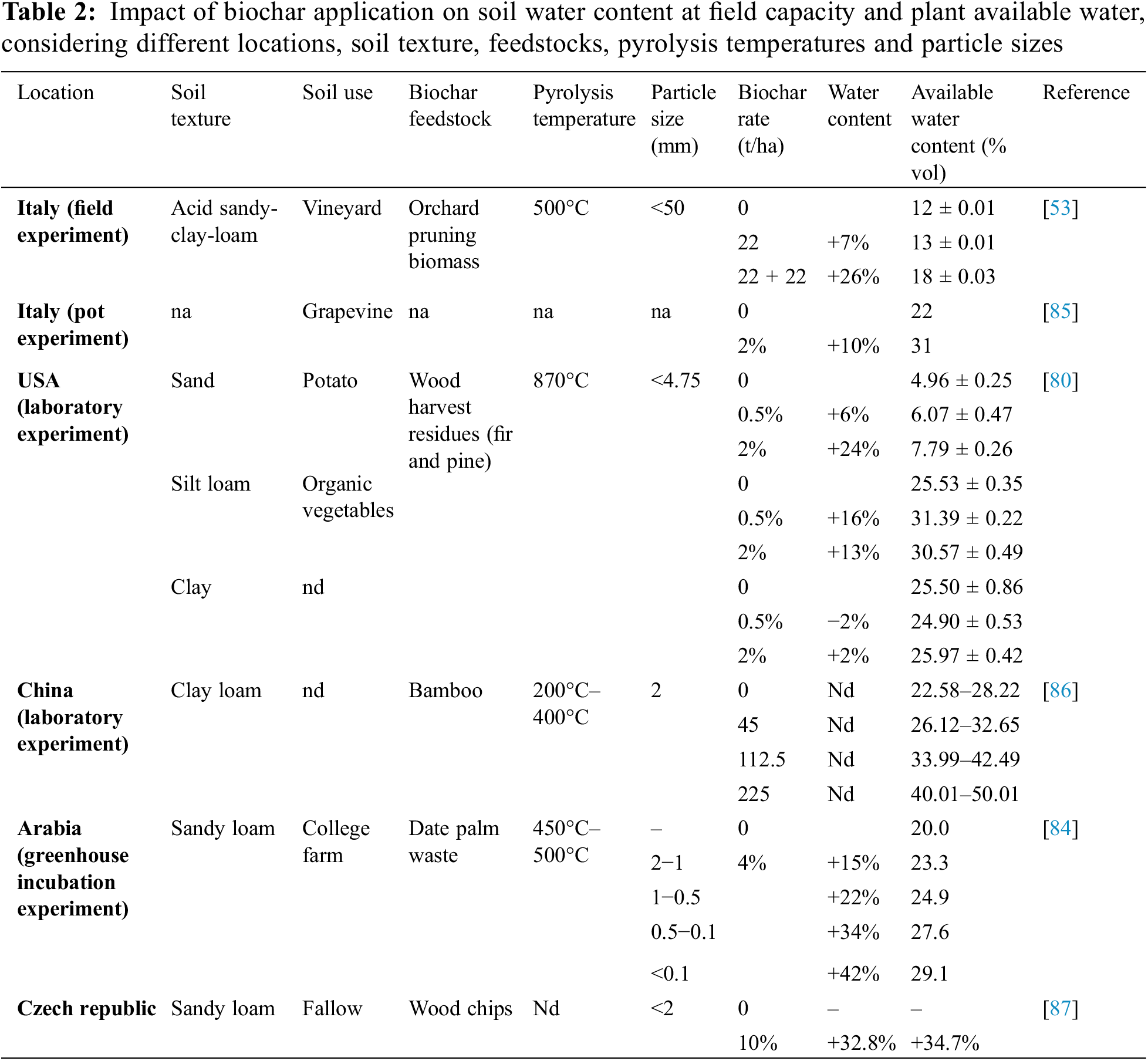
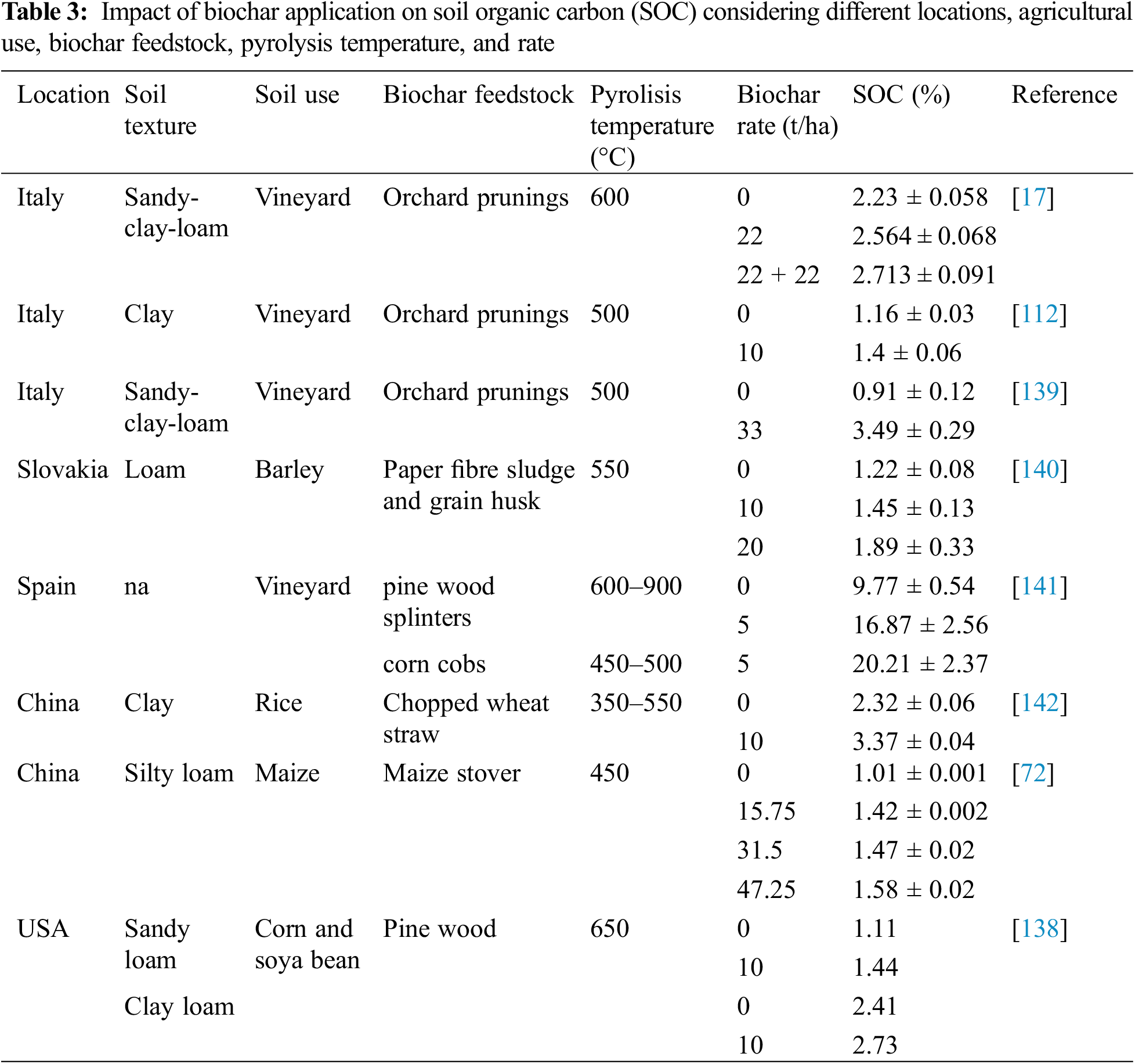
 Downloads
Downloads
 Citation Tools
Citation Tools