 Open Access
Open Access
ARTICLE
Effects of Paclobutrazol Seed Soaking on Non-Structural Carbohydrate and Grain Enrichment in Direct-Seeded Rice
1 Agronomy College, Jilin Agricultural University, Changchun, 130118, China
2 Jilin Academy of Agricultural Sciences, Changchun, 130118, China
* Corresponding Authors: Yanqiu Geng. Email: ; Liying Guo. Email:
# These two authors contributed equally to the article and are co-first authors
(This article belongs to the Special Issue: Crop Managements and Crop Adversity: Strategies, Mechanisms, and Implements)
Phyton-International Journal of Experimental Botany 2025, 94(1), 269-279. https://doi.org/10.32604/phyton.2025.060551
Received 04 November 2024; Accepted 26 December 2024; Issue published 24 January 2025
Abstract
The yield of direct-seeded rice (DSR) was constrained by inadequate grain filling. Recent studies have indicated that paclobutrazol application plays a significant role in enhancing crop agronomic traits and increasing yield. This study aimed to examine the effects of paclobutrazol seed soaking (PSS) on non-structural carbohydrate accumulation and grain enrichment in DSR, potentially providing a theoretical foundation for achieving high-yield DSR cultivation. The experiment utilized two rice varieties, Jiyujing (JYJ) and Jijing305 (JJ305), with seeds soaked in paclobutrazol concentrations of 0 mg L−1 and 100 mg L−1. PSS demonstrated increased chlorophyll content, net photosynthetic rate, and leaf area, as well as an extended photosynthetic function period during the filling stage. It also elevated soluble sugar and starch contents in the flag leaf (during the filling stage) and stem sheath (after heading), decreased starch content in the top panicle while increasing it in the middle and lower panicle during the filling stage, and enhanced spikelet per unit area and seed setting rate, thereby improving DSR yield. In conclusion, PSS enhanced the photosynthetic capacity of DSR during the filling stage, coordinated the filling process of superior and inferior grains, maintained source-sink balance, and facilitated stable and orderly filling, ultimately resulting in improved yield.Keywords
Rice serves as a crucial component in maintaining global food security, providing sustenance for over half of the world’s population [1]. Consequently, ensuring high and stable rice yields is paramount for safeguarding food security. In rice cultivation, transplanting and direct seeding represent the two most prevalent methods. However, labor and water resource scarcity impede the sustainable development of transplanted rice [2,3]. Direct-seeded rice (DSR) involves sowing seeds directly into the field, eliminating the need for traditional transplanting and seedling cultivation. This method has the potential to reduce labor input by approximately 25% and decrease water consumption by 50% [4,5]. Given its labor-saving and efficient nature, DSR holds promise for promoting sustainable agricultural development and is anticipated to be a significant direction for future rice cultivation practices.
Given the constraints of limited arable land, the primary strategy for enhancing crop yield involves increasing productivity per unit area [6,7]. As the global population continues to expand, improving crop yields becomes increasingly crucial to meet the growing food demand. This challenge is further compounded by issues in the grain filling process. The material enrichment phase of grain filling primarily involves the synthesis and accumulation of starch, and maintaining an adequate material source from stem sheaths and leaves is essential for effective grain filling and yield improvement [8,9]. The yield of DSR is particularly hindered by inadequate grain filling, which impedes overall yield increases [10].
Paclobutrazol, a cost-effective plant growth regulator applied in low doses, has gained widespread use in agriculture due to its significant impact on plant growth, development, and stress resistance [11,12]. The application of paclobutrazol has been demonstrated to mitigate lodging risk, enhance photosynthetic capacity, increase dry matter accumulation, and facilitate the transport of photosynthetic products to the panicle, ultimately resulting in higher yields [12–14]. Rice yield stability is primarily determined by seed setting rate (SSR) and/or TS [15,16]. PSS can enhance the yield of DSR by improving SSR and TS [17]. However, there is a lack of systematic research on PSS effects on grain enrichment.
Grain enrichment in rice primarily involves the translocation of photosynthetic assimilates from leaves and storage materials from stem sheaths to the panicle after heading [18]. This two-year field trial utilized Jiyujing (JYJ) and Jijing305 (JJ305) as test materials to examine the effects of PSS on non-structural carbohydrates and grain enrichment. The study focused on assessing photosynthetic characteristics and non-structural carbohydrate content in flag leaves, stem-sheath non-structural carbohydrate content, panicle starch content, and yield under an appropriate concentration of PSS. The results of this investigation aim to provide a theoretical foundation for achieving high-yield cultivation of DSR.
Rice (Oryza sativa L.) varieties JYJ (Ji90-g4; male parent: Qiuguang, female parent: Hui73) and JJ305 (Ji15-30; male parent: Ji09-2624, female parent: 2010Q4-7) were used. The rice seeds were soaked in paclobutrazol (Sichuan Guoguang Agrochemical Co., Ltd., Chengdu, China) at concentrations of 0 mg L−1 and 100 mg L−1 (experimental screening to determine the appropriate concentration) for 24 h to sown. The experiment site, experiment time, fertilization method and water management are the same as Gai et al. [17].
2.2.1 Photosynthetic Characteristics
Flag leaves from main stems were selected at the early (EF), mid (EF), and late (LF) filling stages for the measurement of leaf area and chlorophyll content. The chlorophyll content was calculated using the formula described by Arnon [19]. At the EF, the net photosynthetic rate was measured using a Li-Cor 6400 gas exchange analyzer. The data on chlorophyll content and net photosynthetic rate reference to Gai et al. [17].
2.2.2 Non-Structural Carbohydrates
Flag leaves from the main stems at EF, MF, and LF, as well as stem sheaths at the heading stage (HD), EF, MF, LF, and mature stages (MA) were selected for analysis. Additionally, the main stem panicles, divided into top, middle, and bottom sections based on their length, were sampled at the EF, MF, and LF stages. After drying, the contents of soluble sugar and starch were determined. The determination method followed the protocol described by Yoshida et al. [20].
Plants representative of each plot were collected at MA for analysis of the yield components, and the theoretical yield was calculated. The data on yield reference to Gai et al. [17].
Data were processed by SPSS 23.0 software. Statistical significance was indeed achieved by t-test (p < 0.05). All graphs were constructed with Origin 2021.
3.1 Effects of PSS on Photosynthetic Characteristics of Flag Leaves of DSR
The Flag Leaves of DSR exhibited a progressive decrease in chlorophyll levels, net photosynthetic rate, and leaf area (Fig. 1). In comparison to the control (CK), PSS treatment enhanced chlorophyll content, net photosynthetic rate, and leaf area during the grain filling stage.
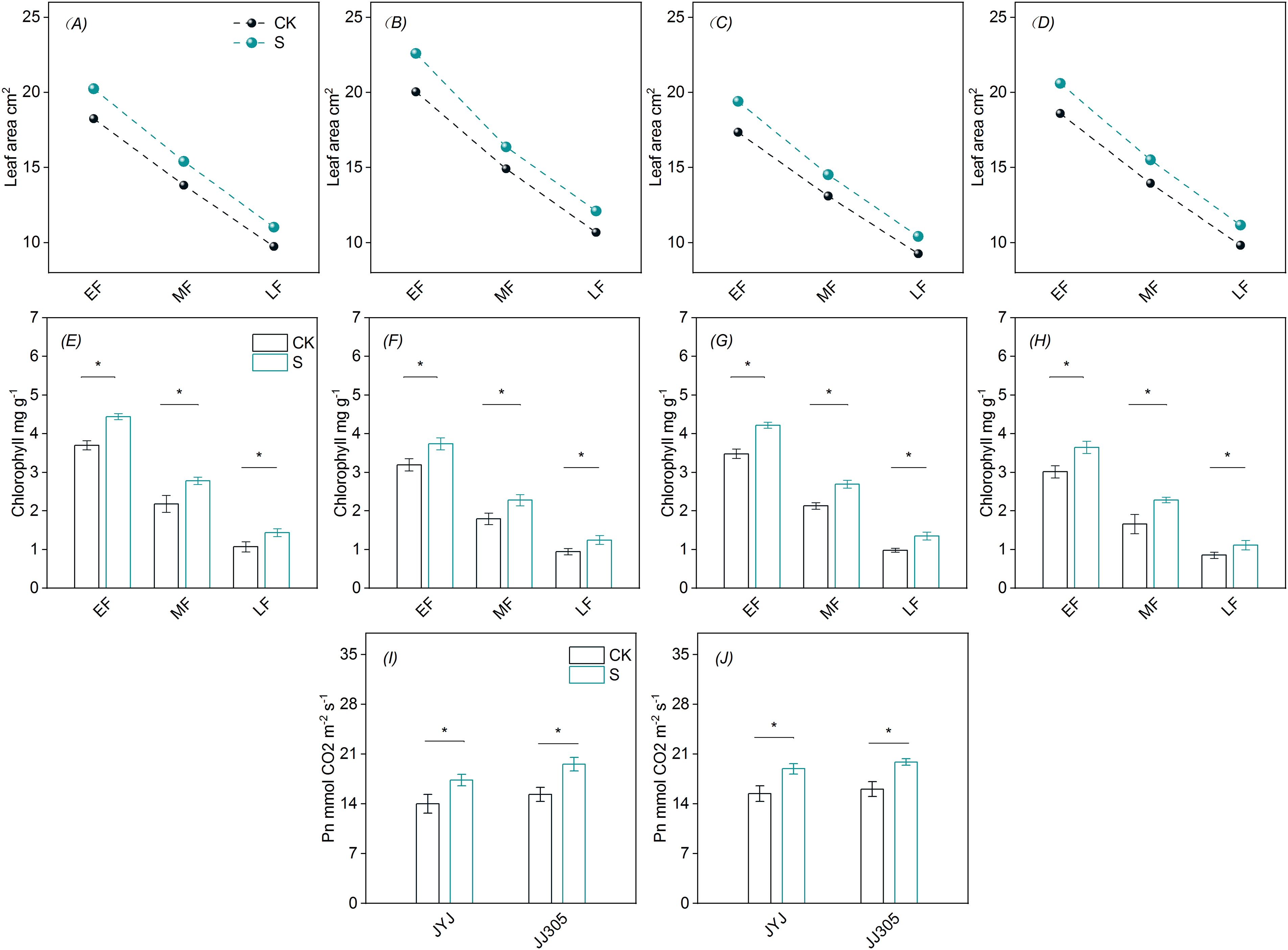
Figure 1: Effects of PSS on photosynthetic characteristics of flag leaves of DSR. A, C, E, G represent rice varieties JYJ; B, D, F, H represent rice varieties JJ305. A, B, E, F, I represent 2021 year; C, D, G, H, J represent 2022 year; * represent p < 0.05
3.2 Effects of PSS on Non-Structural Carbohydrate Content in Flag Leaves of DSR
The soluble sugar and starch contents of flag leaves showed a trend of increasing and then decreasing, and reached the maximum in the MF (Fig. 2). PSS increased the soluble sugar and starch contents at the EF, LF, but decreased at the MF.
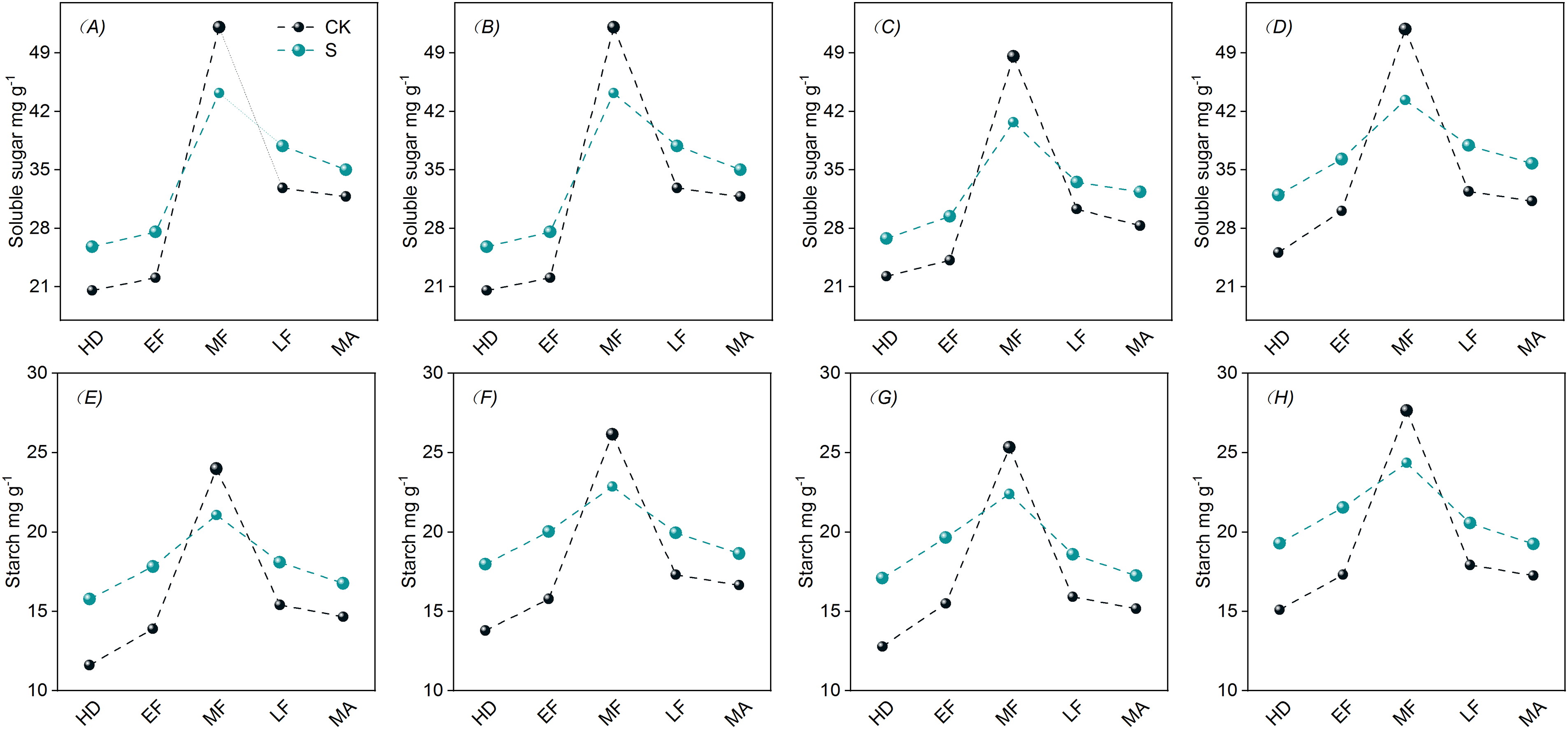
Figure 2: Effects of PSS on non-structural carbohydrate content in flag leaves of DSR. A, E represent 2021 JYJ; B, F represent 2021 JJ305; C, G represent 2022 JYJ; D, H represent 2022 JJ305
3.3 Effects of PSS on Non-Structural Carbohydrate Content in Stem Sheaths of DSR
The soluble sugar levels in the stem sheaths showed a trend of increasing, decreasing, and increasing again, reached the maximum in the EF (Fig. 3). Compared with CK, PSS increased the soluble sugar content of stem sheaths after the HD.
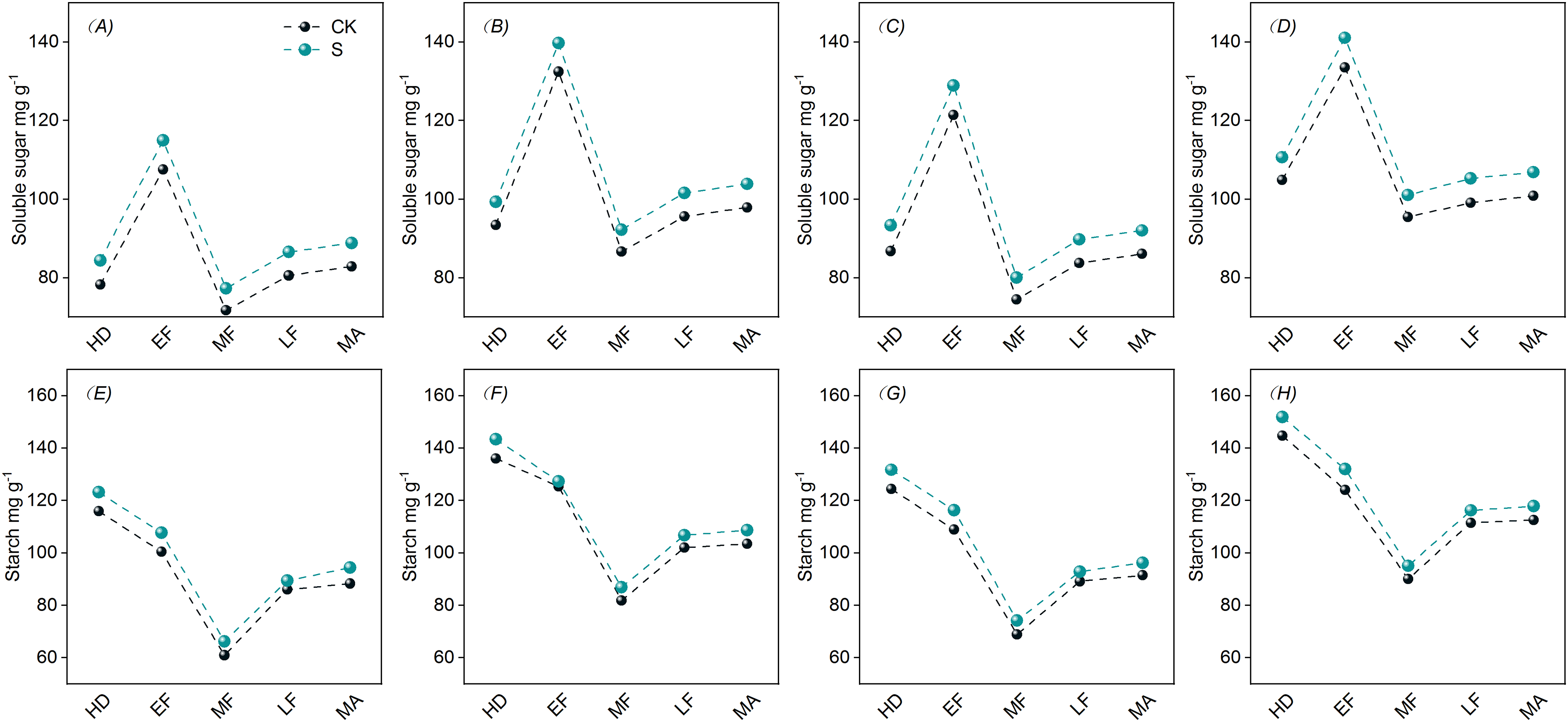
Figure 3: Effects of PSS on non-structural carbohydrate content in stem sheaths of DSR. A, E represent 2021 JYJ; B, F represent 2021 JJ305; C, G represent 2022 JYJ; D, H represent 2022 JJ305
The starch levels in stem sheaths demonstrated an initial decreasing followed by a subsequent increasing, reached the maximum at the HD (Fig. 3). Compared with CK, PSS increased the starch levels in the stem sheaths after the HD.
3.4 Effects of PSS on Starch Content in Panicle of DSR
The starch content in the panicle of DSR exhibited a progressive increase as the reproductive period advanced, with the distribution pattern observed as top panicle > middle panicle > bottom panicle (Fig. 4). In comparison to the CK, PSS treatment resulted in a decrease in starch content in the top panicle, while the middle and bottom panicles showed an increase in starch content.
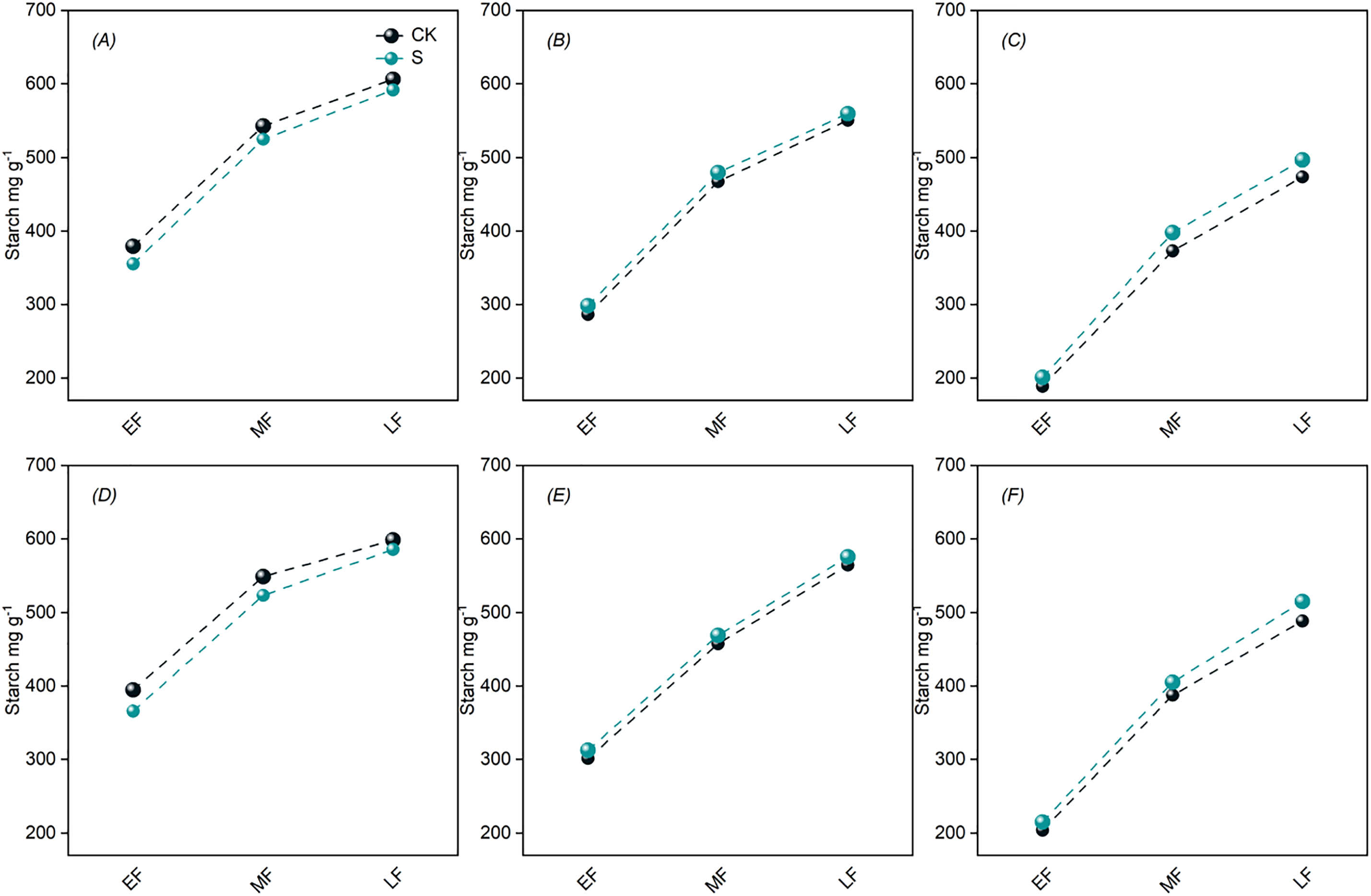
Figure 4: Effects of PSS on starch content in panicle of DSR. A, B, C represent 2022 JYJ; D, E, F represent 2022 JJ305. A, D represent top panicle; B, E represent middle panicle; C, F represent bottom panicle
3.5 Effects of PSS on Yield in DSR
PSS increased the yield of DSR (Fig. 5). Compared with CK, PSS increased the SSR and TS, but decreased the thousand-grain weight (TGW).
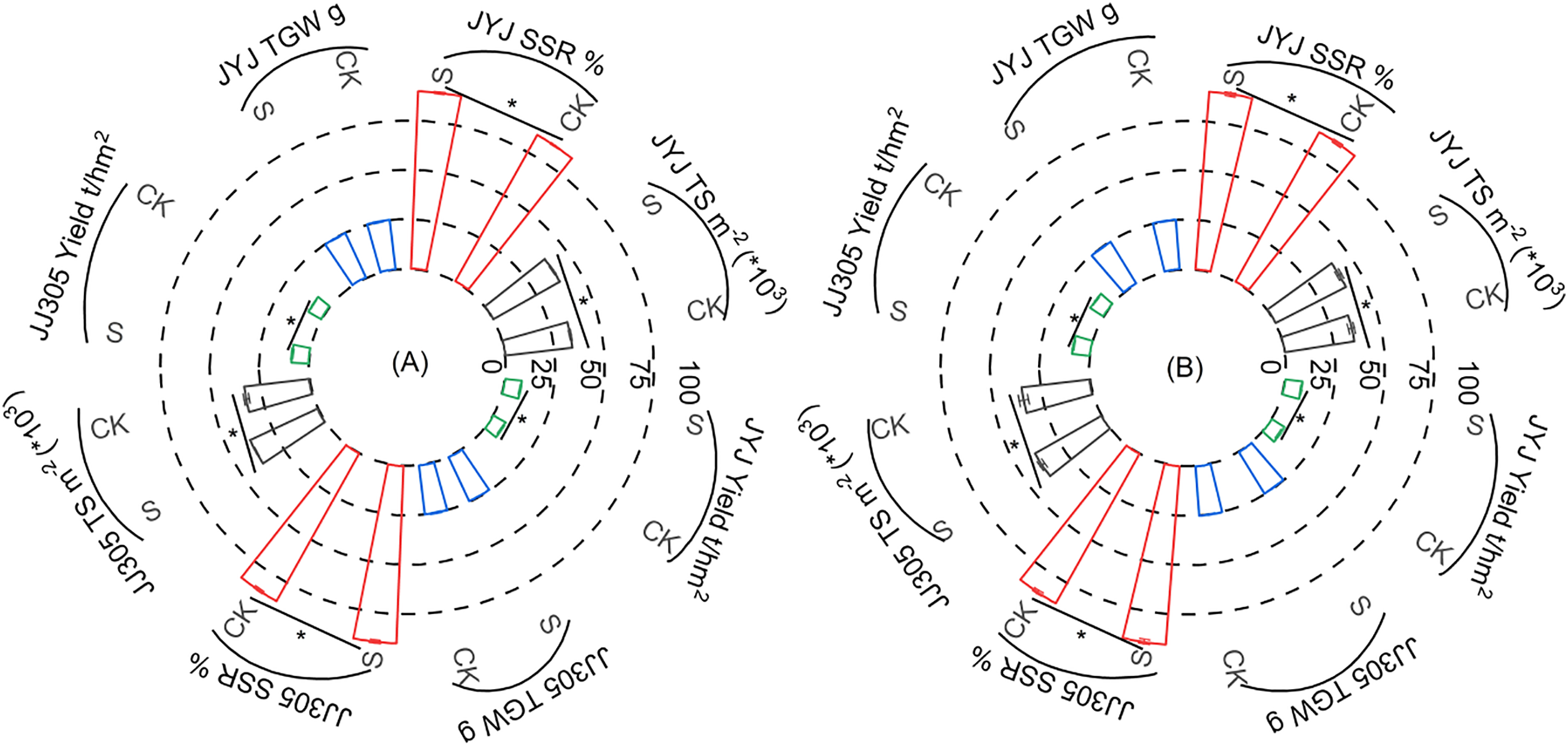
Figure 5: Effects of PSS on yield and yield components in Direct-Seeded Rice. 2021: A; 2022: B. JYJ: Jiyujing; JJ305: Jijing305. The spikelet per unit area: TS; seed setting rate: SSR; thousand-grain weight: TGW; *represent p < 0.05
3.6 Correlation Analysis of Starch Content in Panicle with SSR and TGW
Correlation analysis revealed distinct relationships between starch content in different panicle positions at the LF stage, SSR, and thousand-grain weight (Fig. 6). Starch content in the top panicle exhibited a negative correlation with SSR and a positive correlation with TGW. Conversely, starch content in the middle panicle demonstrated a positive correlation with SSR and a negative correlation with TGW. Notably, starch content in the bottom panicle showed a significant positive correlation with SSR and a negative correlation with TGW.
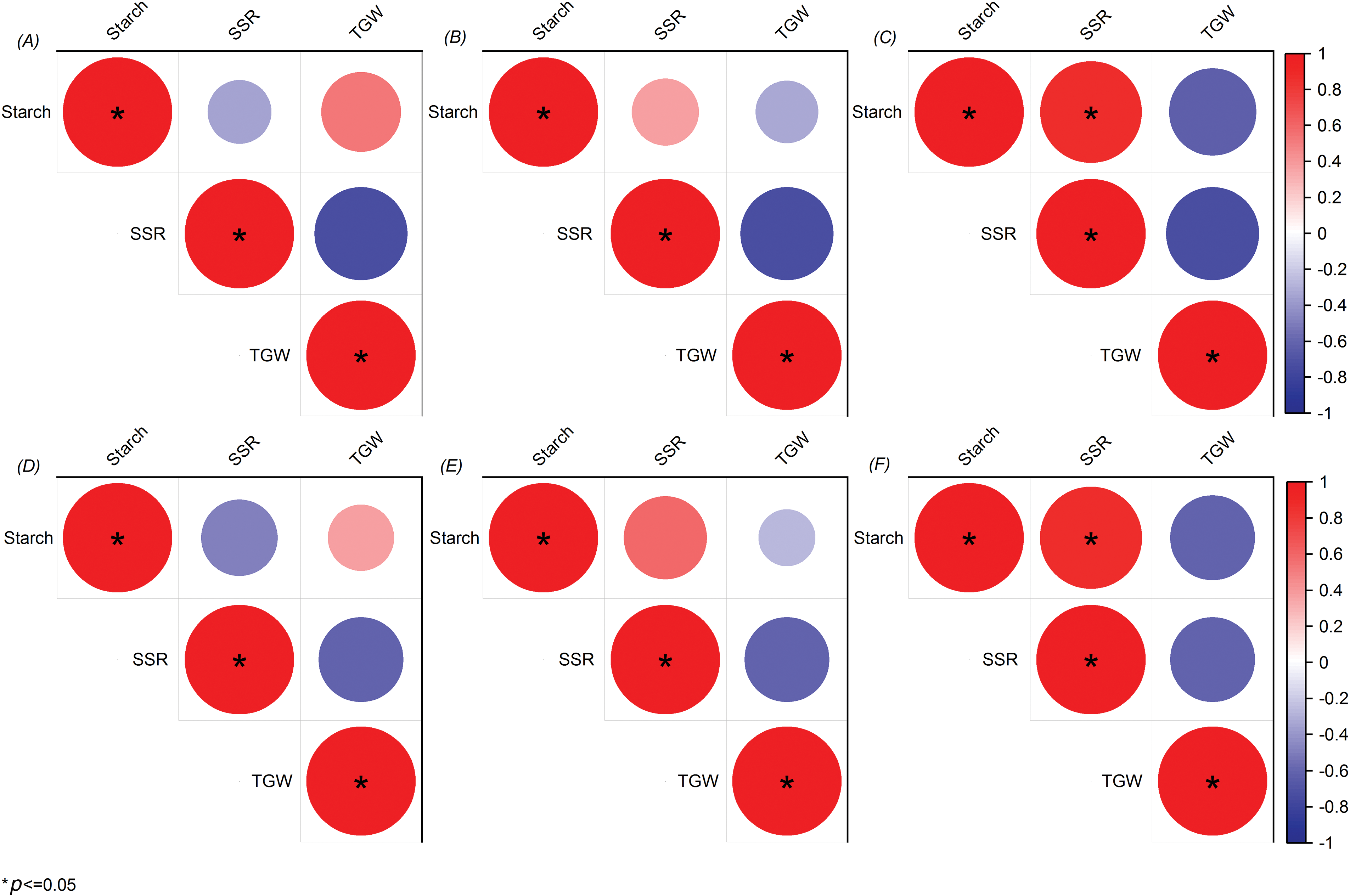
Figure 6: Correlation analysis of starch content in the panicle at LF with SSR and TGW A, B, C represent JYJ; D, E, F represent JJ305. A, D represent top panicle; B, E represent middle panicle; C, F represent bottom panicle; * represent p < 0.05
4.1 Effects of PSS on Material Production of Flag Leaves in DSR
The flag leaf plays a crucial role in rice grain filling, contributing over 50% of the organic matter accumulated in the rice panicle through its photosynthetic products [21–23]. Flag leaf senescence is closely linked to yield, with premature senescence during the filling process potentially shortening the functional period of leaves and limiting the production and transfer of photosynthetic products to the panicles, thus affecting overall yield. Consequently, extending the lifespan of rice flag leaves represents a significant strategy for enhancing rice yield. Previous research has shown that paclobutrazol application increases chlorophyll content, net photosynthesis, antioxidant enzyme activity, and soluble sugar levels [24,25]. In this study, PSS demonstrated the ability to delay leaf senescence and improve flag leaf area, chlorophyll content, and net photosynthetic rate during the filling stage (Fig. 1). This enhancement enabled leaves to maintain higher photosynthetic capacity, ensuring sustained material synthesis and supply in the later stages, thereby providing a sufficient material basis for high DSR yield.
The filling period represents a crucial stage for grain utilization of carbohydrates, directly influencing grain filling and final yield [26,27]. Carbohydrates, the primary products of photosynthesis in plants, are categorized into structural and non-structural types [28]. Non-structural carbohydrates play a vital role in plant growth and development, ensuring normal physiological functions [29]. In this study, PSS increased soluble sugar and starch content in the flag leaves of DSR at the EF and LF stages, providing a sufficient material foundation for grain filling and ultimately promoting grain enrichment (Fig. 2). However, PSS was observed to reduce soluble sugar and starch content in flag leaves at the MF stage (Fig. 2). Two possible explanations for this phenomenon are proposed. Firstly, the plumpness in the stem sheath of DSR may have decreased from EF to MF, with PSS promoting the transfer of materials from leaf to stem sheath, enriching the stem sheath to improve lodging resistance (Fig. 3). Secondly, the grain filling process of DSR from EF to MF requires a significant amount of organic material, and PSS may facilitate the transfer of material from the ‘source’ to the ‘sink’ (Fig. 4).
4.2 Effects of PSS on Non-Structural Carbohydrates in the Stem Sheath of DSR
Non-structural carbohydrates stored in nutrient organs prior to flowering play a crucial role in crop grain filling and significantly impact crop yield [30,31]. In this study, PSS increased the soluble sugar and starch content of stem sheaths at the HD, providing a solid foundation for grain filling (Fig. 3). During the filling period, organic matter from the stem sheaths is translocated to the panicle, resulting in an elevated center of gravity and an increased risk of lodging [32,33]. Zhang et al. demonstrated that increasing the content of non-structural carbohydrates in the stem sheath during the EF stage was beneficial for enhancing rice’s resistance to lodging [34]. In this study, PSS increased the contents of soluble sugar and starch in the stem sheath at the EF stage, while the starch content in the stem sheath at the MF and LF stages was higher (Fig. 3). These findings were conducive to the plumpness of the stem sheath and the improvement of lodging resistance in DSR, which was closely related to the enhancement of photosynthetic capacity and material transport of flag leaves under PSS (Fig. 1 and 2).
4.3 Effects of PSS on Grain Enrichment in DSR
Paclobutrazol application has demonstrated significant importance in enhancing crop agronomic characteristics and increasing yield. Xing et al. showed that the use of paclobutrazol led to a higher SSR, ultimately resulting in a higher yield of rice [35]. In this study, PSS increased the yield of DSR (Fig. 5). SSR, a crucial factor influencing yield, depends on panicle development and grain filling processes [36]. In this study, PSS increased TS in DSR, consequently elevating the demand for photosynthetic products and enhancing their absorption, thereby contributing to improved grain filling (Fig. 5).
The degree of rice grain enrichment is closely associated with its position within the rice spikelet. Generally, grains in the upper and middle parts of the spikelet flower earlier, fill more rapidly, enrich more effectively, and possess higher weights. Conversely, grains in the lower part of the spikelet flower later, fill more slowly, enrich less effectively, and weigh significantly less [37,38]. Grain enrichment primarily involves the synthesis and accumulation of starch, which constitutes approximately 80% of the dry mass of rice grains [9,39]. Xiang et al. found that uniconazole seed soaking decreased the starch content in superior grains and increased it in inferior grains [40]. In this study, PSS decreased starch content in the top panicle but increased it in the middle and bottom panicles, and increased the SSR; starch content in the top panicle negatively correlated with SSR, while starch content in the middle panicle positively correlated with SSR; notably, starch content in the bottom panicle showed a significant positive correlation with SSR (Figs. 4–6). These findings suggest that PSS can coordinate the filling process of superior and inferior grains, thereby promoting grain enrichment. PSS increased the SSR of DSR, thus increasing the number of filled grains per panicle, reducing ineffective tillering, and increasing effective panicles (data not shown). Additionally, this study found that PSS decreased the TGW of DSR, although the difference between treatments was not statistically significant (Fig. 5). This could be attributed to a decrease in the average distribution of organic matter to individual grains due to an increase in sink (Fig. 5). Furthermore, PSS decreased the starch content in the top panicle; starch content in the top panicle positively correlated with TGW, while starch content in middle and bottom panicles negatively correlated with TGW (Figs. 4 and 6). These results indicate that PSS had a minimal negative effect on the seeds in the top panicle.
With a soil half-life exceeding 6 months, residual paclobutrazol may negatively impact plants and pose potential risks to both environmental and human health. PSS can mitigate its direct release into the natural ecosystem and reduce its residual presence in the environment. Si et al. showed that when the concentration of PSS was 10 mg kg−1, no paclobutrazol was detected in storage roots of sweet potato [41].
PSS enhanced the photosynthetic capacity of DSR during the filling stage, maintaining a balance between source and sink, and promoting stable and systematic filling, thereby improving yield. This study further substantiates the agricultural application value of PSS, suggesting a potential novel approach for achieving high-yield cultivation of DSR.
Acknowledgement: Thanks to Tech Science Press for the language editing service for the article.
Funding Statement: This research was supported by the National Key Research and Development Project of China (2022yfd1500501) and Jilin Province Science and Technology Department Outstanding Young Talent Fund Project (20230508001RC).
Author Contributions: Yanqiu Geng and Liying Guo conceived and designed the experiments, Dongsheng Gai, Weiyang Liu, Yong Liu, Pengcheng Fu and Xuanhe Liang performed the experiments, Weiyang Liu, Yong Liu and Xuanhe Liang statistically analyzed the data and made illustrations, Qiang Zhang and Dongsheng Gai wrote the paper. Xiwen Shao reviewed and edited the paper. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials:: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest:: The authors declare no conflicts of interest to report regarding the present study.
References
1. Li HQ, Zhao CJ, Yan BG, Ling L, Meng ZJ. Design and verification of the variable capacity roller-wheel precision rice direct seed-metering device. Agronomy. 2022;12(8):1798. doi:10.3390/agronomy12081798. [Google Scholar] [CrossRef]
2. Nawaz A, Rehman AU, Rehman A, Ahmad S, Siddique KHM, Farooq M. Increasing sustainability for rice production systems. J Cereal Sci. 2022;103:103400. doi:10.1016/j.jcs.2021.103400. [Google Scholar] [CrossRef]
3. Sansen K, Wongboon W, Jairin J, Kato Y. Farmer-participatory evaluation of mechanized dry direct-seeding technology for rice in northeastern Thailand. Plant Prod Sci. 2019;22(1):46–53. doi:10.1080/1343943X.2018.1557530. [Google Scholar] [CrossRef]
4. Liu HY, Hussain SHY, Zheng MM, Peng SB, Huang JL, Cui KH, et al. Dry direct-seeded rice as an alter-native to transplanted-flooded rice in Central China. Agron Sustain Dev. 2014;35(1):285–94. doi:10.1007/s13593-014-0239-0. [Google Scholar] [CrossRef]
5. Kato Y, Katsura K. Rice adaptation to aerobic soils: physiological considerations and implications for agronomy. Plant Prod Sci. 2014;17(1):1–12. doi:10.1626/pps.17.1. [Google Scholar] [CrossRef]
6. Khush GS. What it will take to Feed 5.0 Billion Rice consumers in 2030. Plant Mol Biol. 2005;59(1):1–6. doi:10.1007/s11103-005-2159-5. [Google Scholar] [PubMed] [CrossRef]
7. Horie T, Shiraiwa T, Homma K, Katsura K, Maeda S, Yoshida H. Can Yields of lowland rice resume the increases that they showed in the 1980s? Plant Prod Sci. 2005;8(3):259–74. doi:10.1626/pps.8.259. [Google Scholar] [CrossRef]
8. Zang YG, Wu GZ, Li QQ, Xu YW, Xue MM, Chen XY, et al. Irrigation regimes modulate non-structural carbohydrate remobilization and improve grain filling in rice (Oryza sativa L.) by regulating starch metabolism. J Integr Agric. 2023;23(5):1507–22. doi:10.1016/j.jia.2023.05.012. [Google Scholar] [CrossRef]
9. Zhu TZ, Yin CJ, Zhu TC, Zhou PF, Wu LQ, Wang GJ, et al. Delaying panicle nitrogen application to emergence of 3rd leaf from flag leaf increases the grain-filling ability and yield of large-panicle rice by increasing stem nonstructural carbohydrates at heading. Field Crops Res. 2024;321:109405. doi:10.1016/j.fcr.2024.109405. [Google Scholar] [CrossRef]
10. Namikawa M, Yabiku T, Matsunami M, Matsunami T, Hasegawa T. Nitrogen uptake pattern of dry direct-seeded rice and its contribution to yields in northeastern Japan. Plant Prod Sci. 2023;26(2):101–15. doi:10.1080/1343943X.2023.2185531. [Google Scholar] [CrossRef]
11. Zhang ZZ, Li RX, Chen DY, Chen JY, Xiao OL, Kong ZQ, et al. Effect of paclobutrazol on the physiology and biochemistry of ophiopogon japonicus. Agronomy. 2021;11(8):1533. doi:10.3390/agronomy11081533. [Google Scholar] [CrossRef]
12. Zhang YY, He ZZ, Xing PP, Luo HW, Yan ZS, Tang XR. Effects of paclobutrazol seed priming on seedling quality, photosynthesis, and physiological characteristics of fragrant rice. BMC Plant Biol. 2024;24(1):53. doi:10.1186/s12870-023-04683-0. [Google Scholar] [PubMed] [CrossRef]
13. Kamran M, Ahmad S, Ahmad I, Hussain I, Meng X, Zhang X, et al. Paclobutrazol application favors yield improvement of maize under semiarid regions by delaying leaf senescence and regulating photosynthetic capacity and antioxidant system during grain-filling stage. Agronomy. 2020;10(2):187. doi:10.3390/agronomy10020187. [Google Scholar] [CrossRef]
14. Mehmood MZ, Qadir G, Afzal O, Din AMU, Raza MA, Khan I, et al. Paclobutrazol improves sesame yield by increasing dry matter accumulation and reducing seed shattering under rainfed conditions. Int J Plant Prod. 2021;15(3):337–49. doi:10.1007/s42106-021-00132-w. [Google Scholar] [CrossRef]
15. Jiang KF, Zheng JK, Zhao GL, Zhu YC, Wan XQ, Ding GX. Stability of grain yield traits and their correlation in hybrid rice. Chin J Rice Sci. 2021;15(1):68–70 (In Chinese). [Google Scholar]
16. Balakrishnan D, Subrahmanyam D, Badri J, Raju AK, Rao YV, Beerell K, et al. Genotype x environment interactions of yield traits in backcross introgression lines derived from Oryza sativa cv. Swarna/Oryza nivara. Front Plant Sci. 2016;7:1530. doi:10.3389/fpls.2016.01530. [Google Scholar] [PubMed] [CrossRef]
17. Gai DS, Liu WY, Liang JN, Guo LY, Geng YQ, Zhang Q, et al. The Effects of paclobutrazol seed soaking on biomass production and yield formation in direct-seeded rice. Agronomy. 2023;13(5):1402. doi:10.3390/agronomy13051402. [Google Scholar] [CrossRef]
18. Pan JF, Cui KH, Wei D, Huang JL, Xiang J, Nie LX, et al. Relationships of non-structural carbohydrates accumulation and translocation with yield formation in rice recombinant inbred lines under two nitrogen levels. Physiol Plantarum. 2021;141(4):321–31. doi:10.1111/j.1399-3054.2010.01441.x. [Google Scholar] [PubMed] [CrossRef]
19. Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi:10.1104/pp.24.1.1. [Google Scholar] [PubMed] [CrossRef]
20. Yoshida S, Forno DA, Cock JH, Gomez KA. Laboratory manual for physiological studies of rice. International Rice Research Institute; 1971. [Google Scholar]
21. Lv WK, Hu H, He LP, Zhang XC, Ou XX, Chen HY, et al. Characterization and fine mapping of two white panicle genes with duplicated effect in rice. Int J Agric Biol. 2018;20(12):2805–11. doi:10.17957/IJAB/15.0838. [Google Scholar] [CrossRef]
22. Zhang B, Ye W, Ren D, Tian P, Peng YL, Gao Y, et al. Genetic analysis of flag leaf size and candidate genes determination of a major QTL for flag leaf width in rice. Rice. 2015;8:2. doi:10.1186/s12284-014-0039-9. [Google Scholar] [PubMed] [CrossRef]
23. Shen B, Yu WD, Zhu YJ, Fan YY, Zhuang JY. Fine mapping of a major quantitative trait locus, qFLL6. 2, controlling flag leaf length and yield traits in rice (Oryza sativa L.). Euphytica. 2012;184(1):57–64. doi:10.1007/s10681-011-0539-2. [Google Scholar] [CrossRef]
24. El-Beltagi HS, El-Nady MF, Rezk AA, Tahoon AM, Al-Daej MI, Abdulmajid D, et al. Effects of paclobutrazol seed priming on seedlings quality, physiological and bakanae disease index characteristics of rice (oryza sativa L.). Phyton-Int J Exp Bot. 2024;93(10):2535–56. doi:10.32604/phyton.2024.056734. [Google Scholar] [CrossRef]
25. Wu Y, Liu J, Zhao D, Tao J. Effect of paclobutrazol application on plant growth and flower quality in herbaceous peony. Phyton-Int J Exp Bot. 2022;91(9):2017–32. doi:10.32604/phyton.2022.020643. [Google Scholar] [CrossRef]
26. Jiang ZR, Chen QL, Chen L, Yang HY, Zhu MC, Ding YF, et al. Efficiency of sucrose to starch metabolism is related to the initiation of inferior grain filling in large panicle rice. Front Plant Sci. 2021;12:732867. doi:10.3389/fpls.2021.732867. [Google Scholar] [PubMed] [CrossRef]
27. Yang W, Peng S, Dionisio-Sese ML, Laza RC, Visperas RA. Grain filling duration, a crucial determinant of genotypic variation of grain yield in field-grown tropical irrigated rice. Field Crops Res. 2008;105(3):221–7. doi:10.1016/j.fcr.2007.10.006. [Google Scholar] [CrossRef]
28. Koch KE. Carbohydrate-Modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–40. doi:10.1146/annurev.arplant.47.1.509. [Google Scholar] [PubMed] [CrossRef]
29. Griffiths CA, Paul MJ, Foyer CH. Metabolite transport and associated sugar signalling systems underpinning source/sink interactions. BBABioenerg. 2016;1857:1715–25. doi:10.1016/j.bbabio.2016.07.007. [Google Scholar] [PubMed] [CrossRef]
30. Zhang WY, Qian XY, Li YY, Xu YJ, Wang ZQ, Yang JC. Effects of soil drought on physiological characters and yield of wheat. J Triticeae Crops. 2016;36:491–500 (In Chinese). [Google Scholar]
31. Yang J, Zhang J. Grain filling of cereals under soil drying. New Phytol. 2006;169(2):223–36. doi:10.1111/j.1469-8137.2005.01597.x. [Google Scholar] [PubMed] [CrossRef]
32. Ishimaru K, Togawa E, Ookawa T, Kashiwagi T, Madoka Y, Hirotsu N. New target for rice lodging resistance and its effect in a typhoon. Planta. 2008;227:601–9. doi:10.1007/s00425-007-0642-8. [Google Scholar] [PubMed] [CrossRef]
33. Li LJ, Yuan LM, Wang ZQ, Xu GW, Chen Y. Analysis of physiological causes and countermeasures of lodging in dry rice. Chin J Rice Sci. 2002;(3):28–33 (In Chinese). [Google Scholar]
34. Zhang FZ, Jin ZX, Ma GH, Shang WN, Liu HY, Xu ML, et al. Dynamic changes of lodging resistance and chemical constituents in stem sheath of japonica rice at filling stage. Chin J Rice Sci. 2010;24:264–70 (In Chinese). [Google Scholar]
35. Xing PP, Duan MY, Liu YH, Mo ZW, Lai RF, Tang XR. Enhancement of yield, grain quality characters, 2-Acetyl-1-Pyrroline content, and photosynthesis of fragrant rice cultivars by foliar application of paclobutrazol. J Plant Growth Regul. 2023;42(2):748–58. doi:10.1007/s00344-022-10582-9. [Google Scholar] [CrossRef]
36. Liu K, Deng J, Lu J, Wang XY, Lu BL, Tian XH, et al. High nitrogen levels alleviate yield loss of super hybrid rice caused by high temperatures during the flowering stage. Front Plant Sci. 2019;10:357. doi:10.3389/fpls.2019.00357. [Google Scholar] [PubMed] [CrossRef]
37. Yang JC. Mechanism and regulation of rice filling of weak grain. Acta Agron Sin. 2010;36:2011–9. doi:10.3724/SP.J.1006.2010.02011. [Google Scholar] [CrossRef]
38. Ishimaru T, Matsuda T, Ohsugi R, Yamagishi T. Morphological development of rice caryopses located at the different positions in a panicle from early to middle stage of grain filling. Funct Plant Biol. 2003;30(11):1139–49. doi:10.1071/FP03122. [Google Scholar] [PubMed] [CrossRef]
39. Zeeman SC, Smith SM, Smith AM. The diurnal metabolism of leaf starch. Biochemical J. 2007;401:13–28. doi:10.1042/BJ20061393. [Google Scholar] [PubMed] [CrossRef]
40. Xiang ZF, Yang WY, Ren WJ, Wang XC. Effects of uniconazole on source and sink regulation and yield of rice in late stage. Xi Nan Nong Ye Xue Bao. 2004;(5):604–8 (In Chinese). [Google Scholar]
41. Si CC, Li YJ, Liu HJ, Zhang HY, Meng YY, Shi CY. Impact of paclobutrazol on storage root number and yield of sweet potato (Ipomoea batatas L.). Field Crop Res. 2023;300:109011. doi:10.1016/j.fcr.2023.109011. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools