 Open Access
Open Access
ARTICLE
Significant Changes in Morphological Traits of 422 Barley (Hordeum vulgare L.) Varieties with Different Registration
1 Agricultural Institute Osijek, Osijek, 31000, Croatia
2 Croatian Agency for Agriculture and Food, Osijek, 31000, Croatia
* Corresponding Author: Valentina Spanic. Email:
(This article belongs to the Special Issue: Influence of Biotic and Abiotic Stresses Signals on Plants and their Performance at Different Environments)
Phyton-International Journal of Experimental Botany 2025, 94(2), 317-330. https://doi.org/10.32604/phyton.2025.058201
Received 06 September 2024; Accepted 15 January 2024; Issue published 06 March 2025
Abstract
Enhanced grain yield is achieved in barley by developing varieties incorporating grain yield-related and morphological traits derived from different varieties. The evaluation of 28 morphological characteristics of 422 barley varieties was carried out to assess their changes over time from 1973 to 2023. Most barley yield improvement seems to have been achieved by changes in morphological traits where modern varieties out-yielded older varieties for more than 30% (from 1973 to 2023). According to the Pareto chart, the length of the first segment of the rachis was found to be the most important parameter that changed over time, accompanied by the glaucousness of the flag leaf sheath and spiculation of inner lateral nerves of the dorsal side of the lemma of grain. The importance of leaf attitude manipulation towards the erect type was also elucidated, as the erect type is a more desirable trait due to the better photosynthetic activity of the flag leaves. Further, the hairiness of leaf sheaths of the lowest leaves and glauconite of the sheath of the flag leaf, curvature of the first segment of rachis, and anthocyanin coloration of nerves of the lemma of grain were shifted toward stronger expression as years went by. However, in the newer varieties, the anthocyanin coloration of auricles of the flag leaf was weakened with sparser ear density, with shorter length of the first segment of rachis, and weaker speculation of inner lateral nerves of the dorsal side of the lemma of grain.Keywords
Supplementary Material
Supplementary Material FileThe fourth most-grown cereal worldwide in terms of production is barley (Hordeum vulgare L.) [1,2]. It can be cultivated in different environments due to the large diversity of forms. Barley can be found between 1800 and 3500 m above sea level (m.a.s.l), while is more represented in regions under drought and higher altitudes (above 2800 m) [3]. The global production of barley was about 151.62 million metric tons in the 2022/2023 growing season, increasing from around 145.37 million metric tons in 2021/2022 [4]. Modern types of barley are the result of the selection of wild barley H. vulgare ssp. spontaneum before 10,500 years ago [5]. Hence, it was evident that the genetic gain in grain yield of barley increased up to now [6] where global barley production increased by 30.2% in the last decade [7].
While in the last century, barley was mainly cultivated and used as human food, it is now used for about two-thirds as animal feed, one-third for malt production in beer, and only 2%–3% as human food [8] due to nutritional benefits such as β-glucans in grains. Premium prices for barley for malting depend on grain protein content and grain size but are negatively correlated with grain yield. From a breeding point of view, the main goal of barley selection is the development of barley varieties with high malting quality and enhanced grain yield. However, as mentioned, these two traits are negatively correlated, since high grain yield is under the influence of nitrogen amount, resulting in increased protein content and β-glucan content, which is not desirable for high-quality malt [9]. Considering the significance of barley in several industrial branches, it is crucial to enhance barley grain yields.
Climate change results in unfavorable conditions for the cultivation of many crops, substantially endangering food security [10]. The lower productivity of barley can be the result of many various production challenges under different stresses such as drought and frost, disease, and shoot fly [11]. In general, it is expected to make major crop production larger to meet the food requirements of the growing global population. The barley genetic diversity is a potential foundation of useful morphological traits to enhance grain yield productivity as traits such as date of phenological stages and spike characteristics. Understanding the relationship between phenological stages of barley and environmental factors is crucial for comprehending genotype-by-environment interaction (GEI) [12]. The influence of the environment on the growth and developmental stages could be minimized by the creation of genotypes with good stability that could reduce the possibility of grain yield loss in different environments. To enhance barley productivity; a genetic variation of different morphological traits must be present in the genetic pool. However, knowledge about trait relationships, variance components, mean performance, and genotype × environment interaction is needed by breeders to create genotypes with desired traits in barley. For example, Jeżowski et al. [13] observed a significant influence of the year, genotype, and their interaction on the variability of the morphological traits that were investigated.
Furthermore, the variety descriptors are mainly based on phenotypic descriptors by UPOV [14]. Barley is kept in more than 200 different collections worldwide (∼470,000 accessions) [15] which are the major foundation of plants included in studies of different views of barley genetic diversity. Further, diversity in morphological traits of barley genotypes is important in the breeding process due to the selection of materials with different genetic origins and efficient management of genetic resources [16]. The genetic diversity of barley varieties can be evaluated by morphological traits, grain yield components, and molecular markers. However, the development of varieties with high grain yield requires an intensive knowledge of the existing variations for morphological traits in diverse germplasm, especially those that influence grain yield or its components.
We posited that understanding the relationship between the traits being studied and the years of variety registration could be beneficial for barley breeding, as these traits could be indicators of good grain yield. The main focus of the current research was to investigate how 28 morphological traits changed over time in barley varieties registered from 1973 till 2023. In addition, the relationship among 28 morphological traits was investigated.
2.1 Plant Material and Field Trial
The germplasm used in this investigation consisted of 422 accessions of barley varieties collected and maintained in the referent collection of the Croatian Agency for Agriculture and Food (Croatia). These accessions have originated from a wide range of geographic areas across the EU registered from 1973 to 2023. A field trial was carried out at the experiments of the Croatian Agency for Agriculture and Food in Osijek at 45°32′ N, 18°44′ E longitude and altitude of 94 m above sea level. Varieties were consecutively grown during the period from 2020 to 2022 to observe morphological characteristics. All trials over two growing seasons (2020/21–2021/22) were performed according to CPVO protocol [17] for DUS testing which is adapted to optimize conditions for morphological evaluation of varieties.
The soil at the location where experiments were conducted is eutric cambisol (pHKCl = 6.25, humus = 2.00%–2.20%, P2O5 39.70 mg 100 g−1, H2O = 37.70 mg 100 g−1). The completely randomized block design was applied where one experimental plot consisted of 10 rows, 5 m long, with a 12.5 cm inter-row spacing. Agronomical management practices were performed according to standards for tested locations. To protect plants against aphids, weeds, and diseases, insecticides, herbicides, and fungicides were applied to ensure satisfactory growth of plants for the evaluation of the morphology. One hundred years were randomly harvested with stems during the second part of June in each growing season when barley plants reached physiological maturity.
The 28 morphological traits of barley included traits such as seasonal type and 27 above-ground morphologies, including leaves, ears, and spikelets (Table 1). The traits recorded on a field plot basis were traits from labels A-N, and VV while traits labeled from M-ZZ were evaluated from samples from the field-grown plot. All traits were accessed according to visual assessment according to CPVO protocol [17]. Each barley accession was morphologically characterized and observations on investigated traits were recorded on 20 randomly selected plants in each variety.

Statistica software (version 14) was utilized for various analyses, including descriptive statistics, a Pareto chart using a general regression model, multiple regression analysis, and principal component analysis (PCA). The Pareto chart, presented as a frequency histogram, illustrated the impact of each factor on the response in descending order. A line traversing the columns indicated the threshold for statistical significance (p ≤ 0.05), showing the minimum height a column must reach to be considered statistically significant.
The data on grain yield was sourced from FAOSTAT and USDA [18,19]. Grain yield showed a steady increase globally over the years, rising from 3.19 t ha−1 in the period from 1973 to 1983, to 3.72 t ha−1 from 1984 to 1994, to 3.96 t ha−1 from 1995 to 2005, and reaching 4.40 t ha−1 from 2006 to 2015, finally hitting 4.84 t ha−1 in the period from 2016 to 2023.
In Table S1 and Fig. 1, there are reported mean, minimum, and maximum values, along with standard deviations, for 28 continuous variable descriptors. The hairiness of the leaf sheath of the lowest leaves, number of rows on the ear, development of sterile spikelets on the ear, grain type, hairiness of ventral furrow of grain, shape of the base of the lemma, seasonal type and dimorphic traits were the only characteristics exhibiting dimorphism, while all other traits displayed polymorphism. One trait viz., the intensity of the green color of the plant was trimorphic. A sterile spikelet located in the middle third of the ear, along with the shape and length of the glume, was assessed in relation to the grain of the spikelet, resulting in four distinct states of expression.
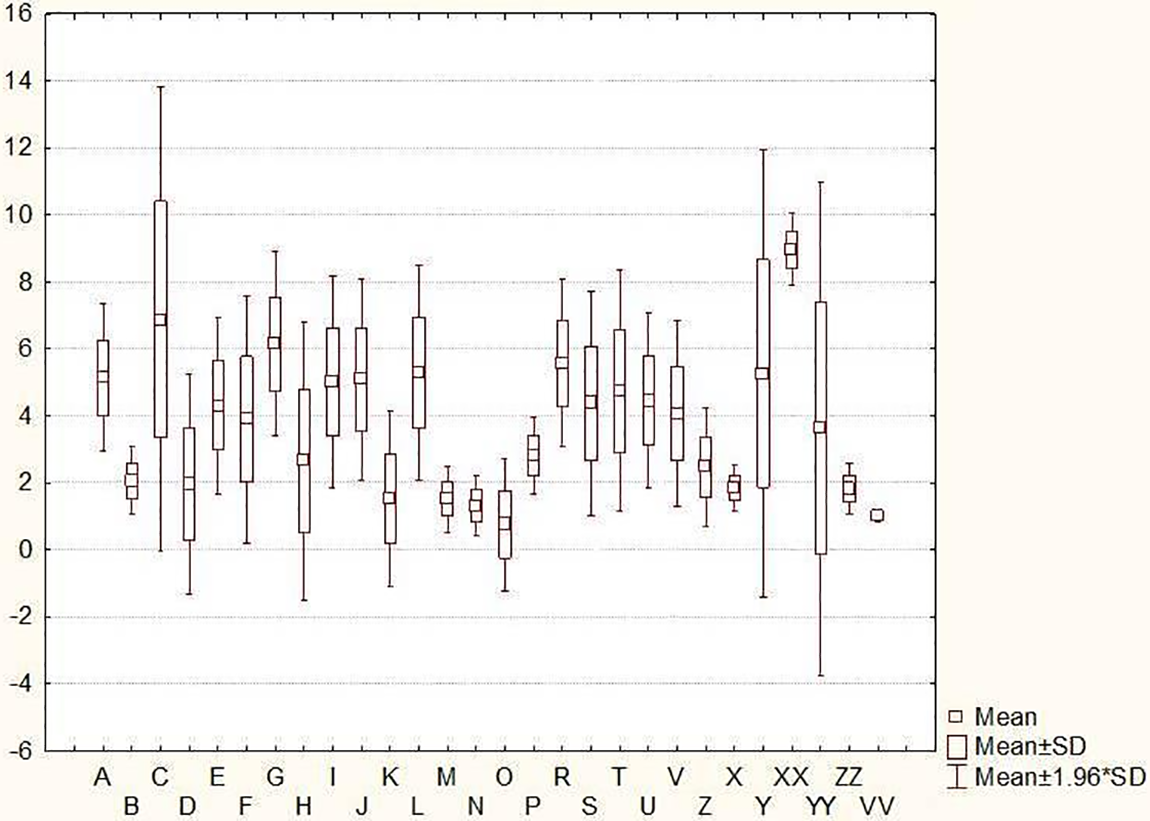
Figure 1: Box plots for the 28 morphological testing traits (A–VV) in 422 barley accessions. For the letter in the X-axis, refer to Table 1. The Y-axis shows the state of expression. In PDF file it could be seen.
3.1 Regression Analysis of Observed Morphological Traits
The Pareto chart indicated the amount of effect of each factor on the response in decreasing order (Fig. 2). From 28 observed traits, a significant change over the years was observed for 10 traits. Those traits were in descendent order: length of the first segment of rachis, glauconite of the sheath of flag leaf, speculation of inner lateral nerves of the dorsal side of the lemma of grain, anthocyanin coloration of nerves of the lemma of grain, hairiness of leaf sheaths of lowest leaves, the curvature of first segment of rachis, attitude of flag leaf, density of ear, seasonal type, and anthocyanin coloration of auricles of flag leaf.
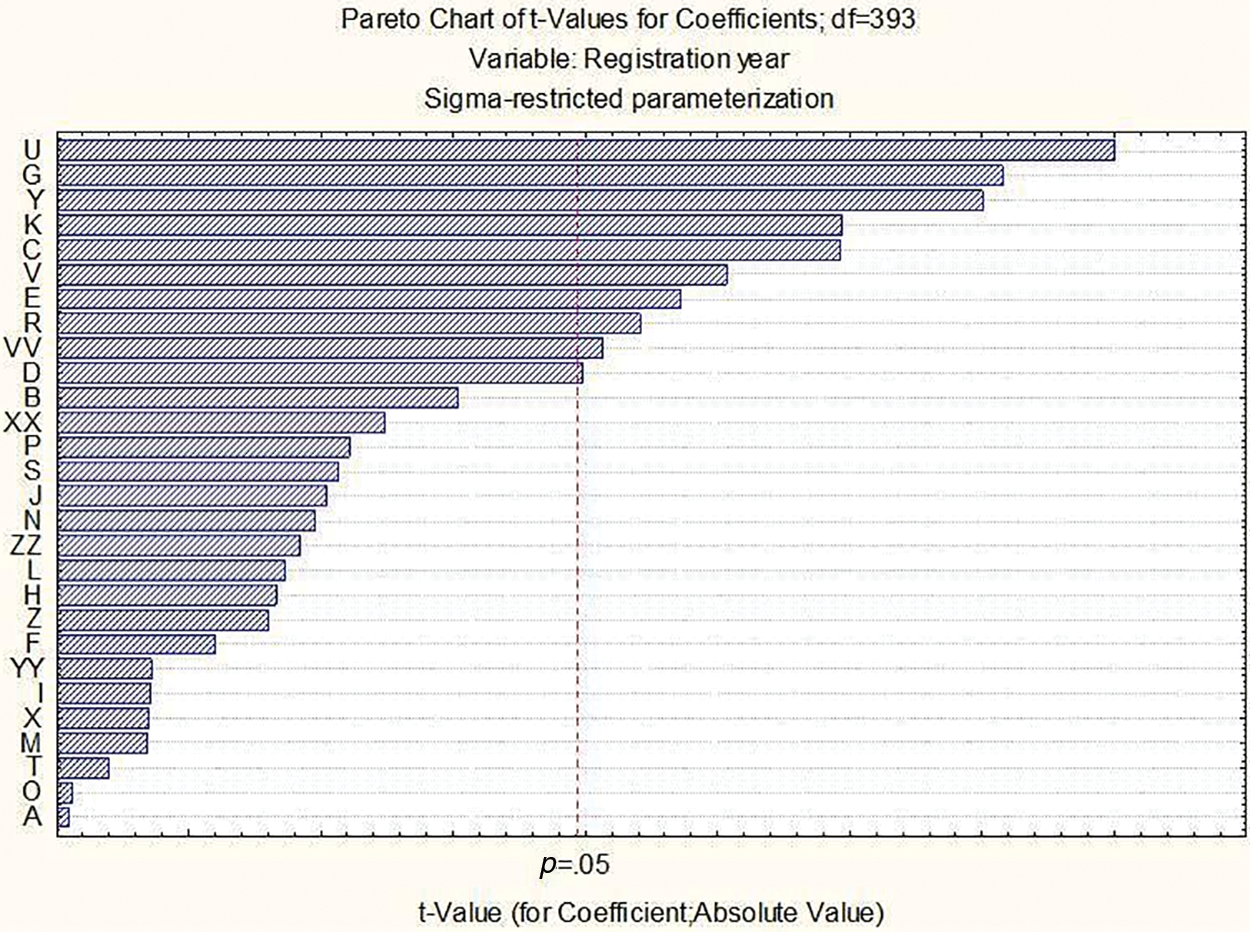
Figure 2: Pareto chart showing the significant changes in observed morphological traits over the years. Explanation of letters (A–VV) can be found in Table 1 (Section 2)
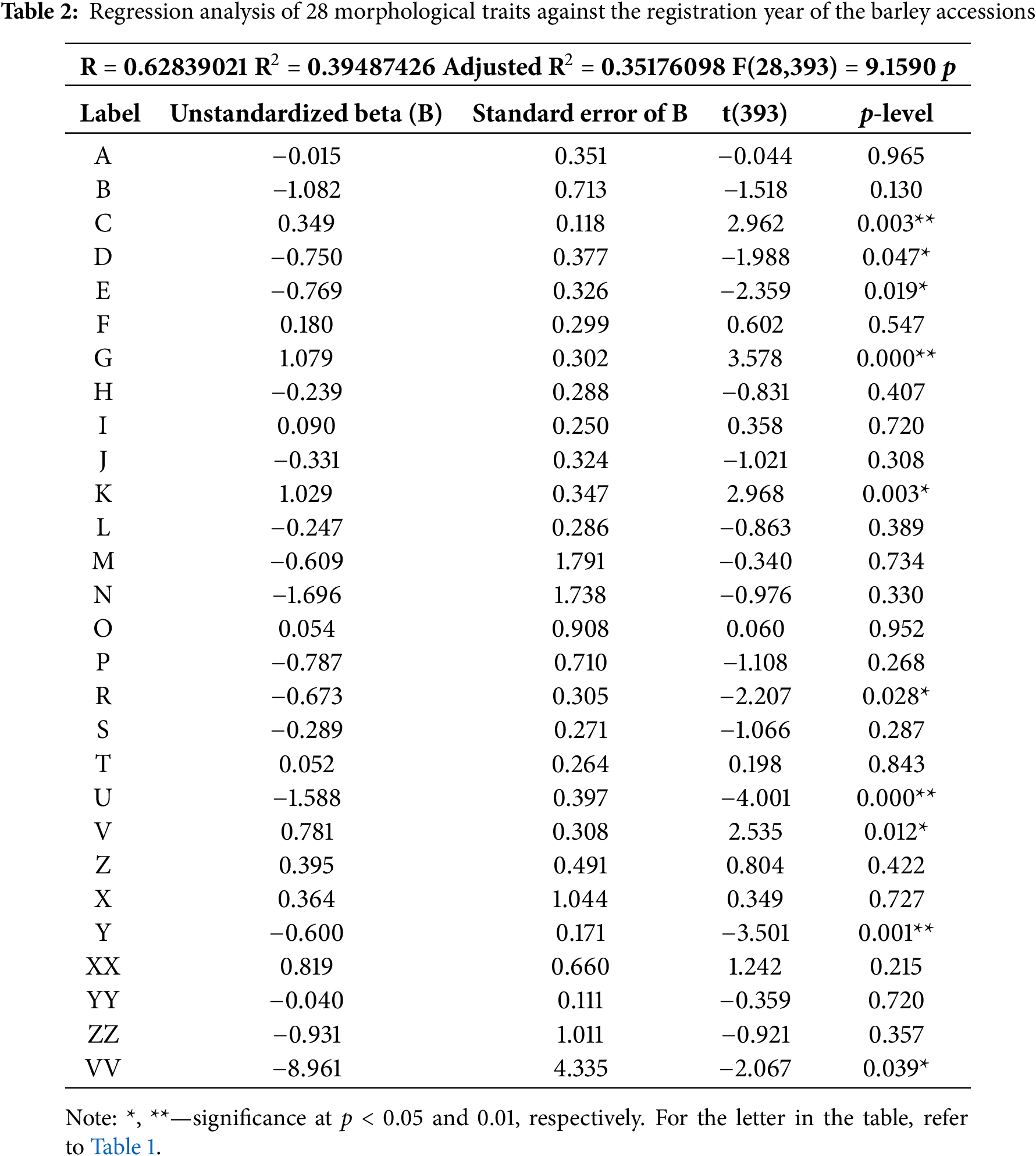
The results of the regression analysis among the variables (Table 2). Four relationships between the observed traits and the year of variety registration showed high significance (p ≤ 0.01), while six traits that affected the year of registration were significant at p < 0.05. The results indicated that any increase in hairiness of leaf sheaths of the lowest leaves, glauconite of the sheath of flag leaf, anthocyanin coloration of nerves of the lemma of grain, and curvature of the first segment of rachis will result in a proportionate increase over the years. On the opposite, proportionate decrease in values of traits such as length of the first segment of rachis, speculation of inner lateral nerves of the dorsal side of the lemma of grain, anthocyanin coloration of auricles of flag leaf, attitude of flag leaf, density of ear, and seasonal type is obtained.
3.2 Principal Component Analysis of 28 Morphological Traits
Principle components analysis (PCA) was used to determine relations among different morphological traits. The first six principal components (PCs) were calculated for 54.0% of the cumulative variability among 422 varieties (Table S2).
Traits related to the ear (number of rows, development of sterile spikelets, and attitude (in mid-third of the ear) of sterile spikelet) the highest contributed to PC1 (Table S3). The highest contribution to PC2 came from the length of awns (compared to ear), length of the first segment of rachis, and time of ear emergence, to PC3 from anthocyanin coloration of tips of awns, anthocyanin coloration of auricles of flag leaf, and anthocyanin coloration of nerves of the lemma of grain. To variability of PC4, the highest contributors were plant growth habit, curvature of the first segment of rachis, and hairiness of leaf sheaths of lowest leaves.
The high relations were obtained between the length of the glume and its relative to grain of median spikelet and hairiness of ventral furrow of grain, plant length and speculation of inner lateral nerves of dorsal side of lemma of grain, shape of base of lemma and shape of ear, ear density and anthocyanin coloration of tips of awns, development of sterile spikelets on-ear and attitude (in mid-third of ear) of sterile spikelet (Fig. 3).
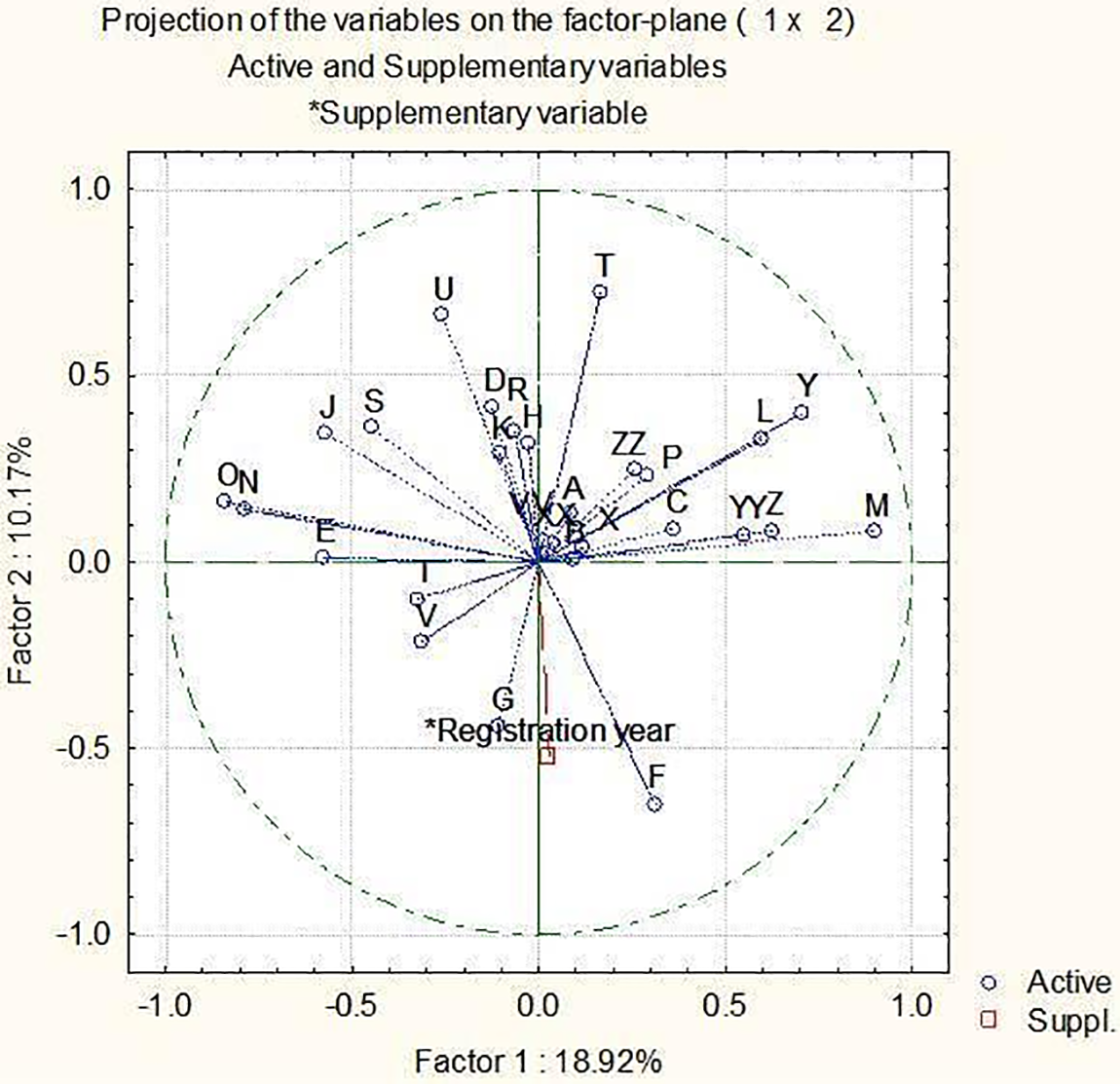
Figure 3: Conduct a principal component analysis using 28 morphological traits, with the registration year treated as a supplementary variable. Please refer to Table 1 for the corresponding letters in the figure
Different varieties of barley adjusted themselves to cold winter temperatures and drought in the terminal phase of development [20]. The same authors reported that earlier heading dates had positive correlation with grain yield in most of the locations, but especially in environments that were low-yielding. The results of the current research showed that the variation in morphological collection within the EU referent collection was large, and thus a better selection according to morphological traits related to grain yield might be expected. This shows the presence of variability in the agro-morphological traits within 422 barley varieties, which provide a wide range for selection of superior varieties by barley breeders. The lowest variation was observed for a few traits such as the hairiness of leaf sheath of lowest leaves, number of rows on the ear, development of sterile spikelets on the ear, grain type, hairiness of ventral furrow of grain, shape of base of lemma, and seasonal type. Ahmed et al. [7] observed low variation in hairiness of leaf sheaths of lowest leaves, anthocyanin coloration of auricles of flag leaf, glauconite of the sheath of flag leaf, ear number of rows, grain husk, grain speculation of inner lateral nerves of the dorsal side of the lemma, grain hairiness of ventral furrow, grain color of aleurone layer and seasonal type. The reason for the low variation of so many traits could be the small number of observed varieties (ten), compared to the current research with 422 observed varieties.
4.1 Morphological and Developmental Traits of the Whole Plant
The growth habit of plants represents the position of tillers and leaves on the plant and this trait is an excellent target for improving canopy architecture, and therefore achieving increased photosynthesis efficiency [21]. According to the current research, the most represented were barley varieties with intermediate and semi-prostrate growth habits. However, for efficient utilization of a higher leaf area per unit of ground area, it is anticipated that growth habits will be more erect.
Further, the intensity of the green color of plants is also related to photosynthesis and plants have a darker intensity of green color because they can absorb red and blue light most efficiently when the darker green light is reflected. Thus, the plant appears green as the green light is not absorbed but reflected. Dark green leaves can promote the activity of photosynthesis, effectively improving grain yield [22]. Most of the barley varieties in the referent collection had medium green color, and only a minority were light or dark green colored. The darker intensity of the green color of the plant might be the trait that in future could enhance grain yield.
The changes in the stage of tillering will influence grain yield as each tiller can form a fertile inflorescence. Furthermore, increased tillering has a negative relation with traits such as plant height [23]. In the current research, the majority of varieties showed medium to medium-long plant height. Thus, in the future, there is a space to improve the grain yield of barley varieties by shortening the plant height.
Almost all barley varieties were winter types except three varieties registered as alternative types. However, in the current research, we only targeted winter barley varieties, as producers prefer winter barley that yields two times what spring barley does [24]. This trait showed a negative relation to the registration year of varieties meaning that alternative types with good grain yield might be preferable that would have lower requirements for low temperatures, compared to winter type.
The leaves have a fundamental role in light interception, efficiency of photosynthesis, and final grain yield [25]. Thus, over the 50 years, breeders put selection pressure on leaf morphology traits as can be seen in significant negative or positive relations of these traits with registration years of barley varieties.
The presence of hairiness of leaf sheaths of the lowest leaves was 2.8-fold times represented in varieties, compared to several varieties with the absence of this trait. Also, it was shown that over time this trait showed a positive relation with the registration year of barley varieties meaning that modern varieties were to a greater extent created with hairiness of leaf sheaths of lowest leaves, compared to older varieties.
Accumulation of anthocyanins is located in vacuoles of different plant cells and tissues such as auricles of flag leaves of barley. Their role is mainly based on adaptation to different environmental stresses [26]. In most varieties, the anthocyanin coloration of auricles of flag leaf was absent or very weak. There was a negative relation of this trait with registration year. The majority of barley varieties showed a semi-erect to the horizontal attitude of the flag leaf. This trait also showed a negative relation with the registration year of varieties. It was suggested that smaller leaf angles from the upper canopy and more horizontally oriented leaves in the lower canopy would make optimal plant architecture [27]. The same authors reported that in barley and other cereals, successive leaves are located on the culm (stem), at 180° to each other. In the current research, it is seen that selection was toward more erect flag leaves which is a more desirable trait.
Further, the glucoside of flag leaf was mostly medium to strong and was in positive relation with the registration year. Breeders paid attention to select varieties with stronger glaucosity of flag leaf. Flag leaves with absent or weak glaucosity have smooth cuticle surfaces where water is retained contributing to the loss of nutrients from the leaf [28]. It was previously reported that glaucosity was associated with drought and heat tolerance and better grain yield under drier conditions [29].
4.3 Morphological Traits of the Barley Ears
The time of ear emergence or heading date of cereals has a high influence on adaptation to different environments and has a large influence on grain yield and quality. The adaptability of barley at different locations is in part caused by the diversity in heading and flowering dates or awn emergence [30]. In the current research, the most represented were early to early medium barley varieties, but there was variability obtained at the time of ear emergence between 422 varieties. Earliness is a positive trait as early maturity of barley under short-day environments is important in many regions with barley production. Further, earliness influences the time and duration of reproductive phases [31]. Also, earliness has an influence on crop adaptation to different stressors including heat and drought influencing grain yield [32].
As already mentioned, anthocyanins are naturally occurring compounds that are synthesized in response to various stresses [33] by scavenging reactive oxygen species (ROS) and reducing oxidative stress. Thus, anthocyanins are desirable to accumulate in awns but in the majority of varieties they were absent or very weak. Still, the improvement of the accumulation of anthocyanins in awns needs to be the target of selection.
The absence of epicuticular wax crystals on the ears contributes to the leaching of nutrients due to the smooth cuticle surface [28]. The majority of observed barley varieties showed medium glaucosity of the ear. Increased glauconite of ears would be a desirable trait as tissues with reduced crystals (weaker glauconite) change color to bright green and increase moisture which is favorable for disease and insect infestation. Although some varieties showed strong glaucosity of the ear, it is desirable to make selection toward varieties having strong or very strong glaucosity. The reason for that is that plant cuticles play an important role in water loss, especially under drought conditions [34].
In the current research, most of the varieties showed horizontal to semi-drooping ear attitudes, although there were more than 100 varieties with erect or semi-erect attitudes. It was previously reported that ear attitudes and plant length had a negative correlation with grain yield, which results in the occurrence that semi-dwarf barley plants with erect ears have a higher yield than tall barley genotypes with recurved ears [35].
The terminology two-row vs. six-row barley is derived from the physical morphology of two different types of ear in barley varieties. There is also existing four-row barley that is unsuitable for brewing. Two-rowed and six-rowed ears of barley were equally represented in the observed collection. Yirgu et al. [11] reported that a higher number of seeds per spike and enhanced productivity at higher elevations and high rainfall areas are obtained in six-rowed barley, while two-rowed barley is frequently produced in marginal areas. Two-row barley is used for the production of quality forage. On the opposite, six-row barley is not suitable for quality forage due to larger grains with a higher test weight and grain weight [36]. Two-row barley, compared to six-row barley is also earlier.
The number of spikelets defines inflorescence architecture and grain yield [37]. In the current referent collection almost two times more there were barley varieties with none or rudimentary development of sterile spikelets in ears. More sterility was expected in six-rowed varieties [38].
Ears varied in size and shape thus producing different numbers of spikelets. But the majority of varieties had the parallel shape of ears with parallel attitude (in mid-third of ear) of sterile spikelet. In the research of Karim et al. [39] the most distinctive morphological trait was ear density which varied from very loose to very compact. In 422 barley varieties, ear density was mostly medium to dense. However, over time it was shown that ear density was significantly negatively related to registration year, resulting in a decrease of ear density as years go by.
The most represented were varieties with medium length of the ear while awns were medium to long. The breeding program tends to have as much as possible long awns as they have a large contribution in photosynthesis accounting for 30%–50% of grain weight in barley [40]. Also, awns may be the protectors against some animals.
In barley, grain number per spike is mainly the result of spike rachis node number, spikelets per node (controlled by row type), and grain number per spikelet [41]. Rachis is an extension of the stem on which the spikelets are found but also rachis is a simple and continuous segmentation of phytomers wherein both vegetative and reproductive organs coexist at opposite ends. In the current research, most of the barley varieties had medium lengths of the first segment of the rachis. Also, a negative relation was obtained between this trait and the registration year of varieties thus showing that selection was shifted toward the shorter length of the first segment, but with increased curvature.
4.4 Morphological Traits of the Barley Ears
In general, the barley grain has a spindle shape, tapering at each end, with a shallow furrow running along the ventral side. As mentioned, anthocyanins influence the regulation of plant development and the interactions between plants and the environments and have a specific role in protection against abiotic stress [42]. Thus, it would be desirable to have strong anthocyanin coloration of nerves of the lemma of grain. However, the majority of barley varieties had an absent or very weak expression of this trait that was positively related to the registration year of varieties, meaning that this trait contributed to the overall grain yield increase in the EU over the years. On the opposite, speculation of inner lateral nerves of dorsal side of the lemma of grain was negatively related to the registration year of varieties, meaning that expression of this trait was going toward absence or weakness as years went by.
The length of the glume and its relative to the grain was mostly equal or longer. Glumes are additional sterile bracts, which are also parts of the spikelet. Rachilla hair type of grain was mostly long. It was previously reported that the characterization of barley germplasm from different countries for morphological traits showed the highest diversity for rachilla hair, growth class, and growth habit [43]. Further, in the current research, almost all the grains were husked with only two varieties having non-husked grains. This means that varieties with a protective layer on the outside of grain were prevalent. The husk has two organs, the lemma and the palea which protect the grain from different external stresses [44]. The harness of the ventral furrow of grain was absent in most varieties, but also it was present in many varieties. The shape of the base of the lemma of most varieties was mostly beveled.
4.5 Relation of Observed Morphological Traits
There is not enough information about the relations of potential morphological traits that can be useful in barley breeding. Thus, we elucidated the most important positive associations of morphological characteristics. The presence of awns may help the ears maintain higher photosynthesis efficiency during the period of grain-filling [9]. However, in the current research, the length of the glume and its relative to the grain was mostly equal, with grain having no hairiness of ventral furrow of grain. Also, the tendency of stronger spiculation of inner lateral nerves of the dorsal side of the lemma of grain was related to taller plants. There might be some explanation in genetics as it was previously reported that tillering and plant height are situated on the similar chromosomal segments hindering phytohormones related to plant stature and sugar-related genes [45]. The beveled shape of the base was related to the parallel shape of the ear. The lemma is the husk organ that can be easily separated from the grains. However, a beveled lemma might protect the grain to a stronger extent.
Medium ear density was related to absent or very weak anthocyanin coloration of the tips of awns. A higher ear density of two-row type of barley is necessary, compared to six-row types to reach the same yield. Further, nitrogen application also influences ear density [46], and nitrogen has a role in the enhancement of anthocyanin synthesis by promoting its biosynthesis pathway [47]. Hence, there is clear evidence that those two traits might be associated with nitrogen fertilization. Further, it was expected that the development of sterile spikelets on the ear and the attitude (in the mid-third of the ear) of sterile spikelets were positively related.
Significant relations between ten out of twenty-eight morphological traits and the registration year of 422 barley varieties were identified which indicated the role of those traits in the improvement of grain yield over time from 1973 till 2023. The Pareto chart showed the highest changes for the length of the first segment of the rachis, glaucosity of the sheath of the flag leaf, and spiculation of inner lateral nerves of the dorsal side of the lemma of grain. Further, speculation of inner lateral nerves of the dorsal side of the lemma of grain was associated with taller plants. However, a large collection of 422 barley accessions could offer a broad range of genetic variability for research in genetics and breeding. In the future, high throughput phenotyping technologies could help in the evaluation of different morphological traits.
Acknowledgement: Not applicable.
Funding Statement: This research was funded by the Ministry of Agriculture.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Valentina Spanic, Ivan Varnica, Goran Jukic, Luka Drenjancevic; data collection: Zvonimir Lalic, Ivica Berakovic; analysis and interpretation of results: Ivica Berakovic, Goran Jukic, Luka Drenjancevic; draft manuscript preparation: Valentina Spanic. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author, V. S., upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/phyton.2025.058201.
References
1. Garcia-Gimenez G, Jobling SA. Gene editing for barley grain quality improvement. J Cereal Sci. 2022;103(40):103394. doi:10.1016/j.jcs.2021.103394. [Google Scholar] [CrossRef]
2. Hafez E, Seleiman M. Response of barley quality traits, yield and antioxidant enzymes to water-stress and chemical inducers’. Int J Plant Prod. 2017;11:477–90. doi:10.22069/ijpp.2017.3712. [Google Scholar] [CrossRef]
3. Tekle HT, Tsehaye Y, Atsbeha G, Abera FA, Chiulele RM. Investigation of genotype x environment interaction for Hordeum vulgare L. ssp. vulgare recombinant inbred lines in multi-environments of Tigray. Ethiopia Ecol Genet Genom. 2024;31(3):100231. doi:10.1016/j.egg.2024.100231. [Google Scholar] [CrossRef]
4. Statista [cited 2024 Nov 15]. Available from: https://www.statista.com/statistics/271973/world-barley-production-since-2008/. [Google Scholar]
5. Stockinger EJ. The breeding of winter-hardy malting barley. Plants. 2021;10(7):1415. doi:10.3390/plants10071415. [Google Scholar] [PubMed] [CrossRef]
6. Mackay I, Horwell A, Garner J, White J, McKee J, Philpott H. Reanalyses of the historical series of UK variety trials to quantify the contributions of genetic and environmental factors to trends and variability in yield over time. Theor Appl Genet. 2011;122(1):225–38. doi:10.1007/s00122-010-1438-y. [Google Scholar] [PubMed] [CrossRef]
7. Ahmed AA, Attya AM, Harb AH, Mostafa S. Genetic variation of barley genotypes using morphological, yield components and molecular markers. J Agric Ecol. 2021;12(2):29–39. [Google Scholar]
8. Baik BK, Ullrich SE. Barley for food: characteristics, improvement, and renewed interest. J Cereal Sci. 2008;48(2):233–42. doi:10.1016/j.jcs.2008.02.002. [Google Scholar] [CrossRef]
9. Chen J, Dai F, Wei K, Zhang G. Relationship between malt qualities and β-amylase activity and protein content as affected by timing of nitrogen fertilizer application. J Zhejiang Univ Sci B. 2006;7(1):79–84. doi:10.1631/jzus.2006.B0079. [Google Scholar] [PubMed] [CrossRef]
10. Khan GR, Alkharabsheh HM, Akmal M, AL-Huqail AA, Ali N, Alhammad BA, et al. Split nitrogen application rates for wheat (Triticum aestivum L.) yield and grain N using the CSM-CERES-wheat model. Agronomy. 2022;12(8):1766. doi:10.3390/agronomy12081766. [Google Scholar] [CrossRef]
11. Yirgu M, Kebede M, Feyissa T, Lakew B, Woldeyohannes AB. Morphological variations of qualitative traits of barley (Hordeum vulgare L.) accessions in Ethiopia. Heliyon. 2022;8(10):e10949. doi:10.1016/j.heliyon.2022.e10949. [Google Scholar] [PubMed] [CrossRef]
12. Bratković K, Luković K, Perišić V, Savić J, Maksimović J, Adžić S, et al. Interpreting the interaction of genotype with environmental factors in barley using partial least squares regression model. Agronomy. 2024;14(1):194. doi:10.3390/agronomy14010194. [Google Scholar] [CrossRef]
13. Jeżowski S, Surma M, Krajewski P, Adamski T. Genotype-environment interaction of barley DH lines in terms of morphological and physical traits of the stem and the degree of lodging. Int Agrophys. 2003;17(2):57–60. [Google Scholar]
14. UPOV. International Convention for the Protection of New Varieties of Plants of December 2, 1961, as Revised at Geneva on November 10, 1972, on October 23, 1978, and on March 19, 1991 [cited 2024 Sep 16]. Available from: https://www.upov.int/edocs/pubdocs/en/upov_pub_221.pdf. [Google Scholar]
15. Knüpffer H, Feuillet C, Muehlbauer GJ. Triticeae genetic resources in ex situ genebank collections. In: Feuillet C, Muehlbauer G, editors. Genetics and genomics of the Triticeae, Plant genetics and genomics: crops and models. 1st ed. New York, NY: Springer USA; 2009. p. 31–79. [Google Scholar]
16. Nyiraguhirwa S, Grana Z, Henkrar F, Ouabbou H, Mohammed I, Udupa SM. Genetic diversity and structure of a barley collection predominantly from the North African region. Cereal Res Commun. 2021;50:647–54. [Google Scholar]
17. CPVO [cited 2024 Sep 16]. Available from: https://cpvo.europa.eu/sites/default/files/documents/hordeum_5_2.pdf. [Google Scholar]
18. FAOSTAT [cited 2024 Sep 16]. Available from: https://www.fao.org/faostat/en/#data/QCL. [Google Scholar]
19. USDA [cited 2024 Sep 16]. Available from: https://ipad.fas.usda.gov/countrysummary/Default.aspx?id=E4&crop=Barley. [Google Scholar]
20. van Oosterom EJ, Acevedo E. Adaptation of barley (Hordeum vulgare L.) to harsh Mediterranean environments. Euphytica. 1992;62(1):29–38. doi:10.1007/BF00036084. [Google Scholar] [CrossRef]
21. Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci. 2015;112(28):8529–36. doi:10.1073/pnas.1424031112. [Google Scholar] [PubMed] [CrossRef]
22. Su X, Yue X, Kong M, Xie Z, Yan J, Ma W, et al. Leaf color classification and expression analysis of photosynthesis-related genes in inbred lines of Chinese cabbage displaying minor variations in dark-green leaves. Plants. 2023;12(11):2124. doi:10.3390/plants12112124. [Google Scholar] [PubMed] [CrossRef]
23. Kuczynska A, Surma M, Adamski T, Mikołajczak K, Krystko-wiak K, Ogrodowicz P. Effects of the semi-dwarfing sdw1/denso gene in barley. J Appl Genet. 2013;54(4):381–90. doi:10.1007/s13353-013-0165-x. [Google Scholar] [PubMed] [CrossRef]
24. Špunar J, Vaculová K, Špunarová M, Nesvadba Z. Comparison of important parameters of spring and winter barley cultivated in sugar beet production area of Czech Republic. Plant Soil Environ. 2002;48(6):237–42. [Google Scholar]
25. Mathan J, Bhattacharya J, Ranjan A. Enhancing crop yield by optimizing plant developmental features. Development. 2016;143(18):3283–94. doi:10.1242/dev.134072. [Google Scholar] [PubMed] [CrossRef]
26. Gordeeva EI, Glagoleva AY, Kukoeva TV, Khlestkina EK, Shoeva OY. Purple-grained barley (Hordeum vulgare L.marker-assisted development of NILs for investigating peculiarities of the anthocyanin biosynthesis regulatory network. BMC Plant Biol. 2019;19(Suppl 1):52. doi:10.1186/s12870-019-1638-9. [Google Scholar] [PubMed] [CrossRef]
27. Shaaf S, Bretani G, Biswas A, Fontana IM, Rossini L. Genetics of barley tiller and leaf development. J Integr Plant Biol. 2019;61(3):226–56. doi:10.1111/jipb.12757. [Google Scholar] [PubMed] [CrossRef]
28. von Wettstein-Knowles P. Ecophysiology with barley eceriferum (cer) mutants: the effects of humidity and wax crystal structure on yield and vegetative parameters. Ann Bot. 2020;126(2):301–13. doi:10.1093/aob/mcaa086. [Google Scholar] [PubMed] [CrossRef]
29. Würschum T, Langer SM, Longin CFH, Tucker MR, Leiser WL. Refining the genetic architecture of flag leaf glaucousness in wheat. Theor Appl Genet. 2020;133(3):981–91. doi:10.1007/s00122-019-03522-x. [Google Scholar] [PubMed] [CrossRef]
30. Ibrahim A, Harrison M, Meinke H, Fan Y, Johnson P, Zhou M. A regulator of early flowering in barley (Hordeum vulgare L.). PLoS One. 2018;13(7):e0200722. doi:10.1371/journal.pone.0200722. [Google Scholar] [PubMed] [CrossRef]
31. Bullrich L, Appendino M, Tranquilli G, Lewis S, Dubcovsky J. Mapping of a thermo-sensitive earliness per se gene on Triticum monococcum chromosome 1Am. Theor Appl Genet. 2002;105(4):585–93. doi:10.1007/s00122-002-0982-5. [Google Scholar] [PubMed] [CrossRef]
32. Mondal S, Singh RP, Crossa J, Huerta-Espino J, Sharma I, Chatrath R, et al. Earliness in wheat: a key to adaptation under terminal and continual high temperature stress in South Asia. Field Crops Res. 2013;51:19–26. doi:10.1016/j.fcr.2013.06.015. [Google Scholar] [CrossRef]
33. Li Z, Ahammed GJ. Plant stress response and adaptation via anthocyanins: a review. Plant Stress. 2023;10:100230. doi:10.1016/j.stress.2023.100230. [Google Scholar] [CrossRef]
34. Monda K, Mabuchi A, Negi J, Iba K. Cuticle permeability is an important parameter for the trade-off strategy between drought tolerance and CO2 uptake in land plants. Plant Signal Behav. 2021;16(6):1908692. doi:10.1080/15592324.2021.1908692. [Google Scholar] [PubMed] [CrossRef]
35. Yang CJ, Russell J, Ramsay L, Thomas W, Powell W, Mackay I. Overcoming barriers to the registration of new plant varieties under the DUS system. Commun Biol. 2021;4(1):302. doi:10.1038/s42003-021-01840-9. [Google Scholar] [PubMed] [CrossRef]
36. Öztürk İ, Tülek B, Avcı R, Kahraman T, Tülek A. Comparison of two and six-rowed barley (Hordeum vulgare L.) genotypes under rainfed conditions for yield, quality and biotic stress tolerance. Ekin J. 2023;9(2):98–105. [Google Scholar]
37. Yuan Z, Persson S, Zhang D. Molecular and genetic pathways for optimizing spikelet development and grain yield. aBIOTECH. 2020;1(4):276–92. doi:10.1007/s42994-020-00026-x. [Google Scholar] [PubMed] [CrossRef]
38. Arisnabarreta S, Miralles DJ. Yield responsiveness in two- and six-rowed barley grown in contrasting nitrogen environments. J Agron Crop Sci. 2006;192(3):178–85. doi:10.1111/j.1439-037X.2006.00203.x. [Google Scholar] [CrossRef]
39. Karim K, Rawda A, Hatem CM, Barek BN. RAPD markers and morpho-physiological characterization of some Tunisian barley ecotypes. Biodivers Conserv. 2010;3(2):1–11. [Google Scholar]
40. Huang B, Wu W, Hong Z. Genetic loci underlying awn morphology in barley. Genes. 2021;12(10):1613. doi:10.3390/genes12101613. [Google Scholar] [PubMed] [CrossRef]
41. Fan C, Xu D, Wang C, Chen Z, Dou T, Qin D, et al. Natural variations of HvSRN1 modulate the spike rachis node number in barley. Plant Commun. 2024;5(1):100670. doi:10.1016/j.xplc.2023.100670. [Google Scholar] [PubMed] [CrossRef]
42. Shi L, Li X, Fu Y, Li C. Environmental stimuli and phytohormones in anthocyanin biosynthesis: a comprehensive review. Int J Mol Sci. 2023;24(22):16415. doi:10.3390/ijms242216415. [Google Scholar] [PubMed] [CrossRef]
43. Javaid A, Ikeda S, Kataoka I, Taketa S. Fine mapping of short rachilla hair gene (srh) in barley and an association study using flanking molecular markers and world germplasms. Asian J Plant Sci. 2009;8(6):400–8. doi:10.3923/ajps.2009.400.408. [Google Scholar] [CrossRef]
44. Grant KR, Brennan M, Hoad SP. The structure of the barley husk influences its resistance to mechanical stress. Front Plant Sci. 2021;11:614334. doi:10.3389/fpls.2020.614334. [Google Scholar] [PubMed] [CrossRef]
45. Alqudah AM, Koppolu R, Wolde GM, Graner A, Schnurbusch T. The genetic architecture of barley plant stature. Front Genet. 2016;7:117. doi:10.3389/fgene.2016.00117. [Google Scholar] [PubMed] [CrossRef]
46. Preiti G, Calvi A, Romeo M, Badagliacca G, Bacchi M. Seeding density and nitrogen fertilization effects on agronomic responses of some hybrid barley lines in a Mediterranean environment. Agronomy. 2021;11(10):1942. doi:10.3390/agronomy11101942. [Google Scholar] [CrossRef]
47. Utasee S, Jamjod S, Lordkaew S, Prom-U-Thai C. Improve anthocyanin and zinc concentration in purple rice by nitrogen and zinc fertilizer application. Rice Sci. 2022;29(5):435–50. doi:10.1016/j.rsci.2022.07.004. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools