 Open Access
Open Access
REVIEW
Enhancing Plant Resilience to Biotic and Abiotic Stresses through Exogenously Applied Nanoparticles: A Comprehensive Review of Effects and Mechanism
1 Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing, 100081, China
2 Date Palm Research Center of Excellence, King Faisal University, Al-Ahsa, 31982, Saudi Arabia
3 Department of Food and Nutrition Sciences, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, 31982, Saudi Arabia
4 Department of Botany, Lahore College for Women University, Lahore, Punjab, 54000, Pakistan
5 Department of Plant Breeding and Genetics, Faculty of Agricultural Sciences, Ghazi University, Dera Ghazi Khan, 32200, Pakistan
6 Department of Botany, The Woman University Multan, 66000, Pakistan
7 Department of Biological Sciences, University of Veterinary and Animal Sciences, Lahore, 54000, Pakistan
8 Center for Water and Environmental Studies, College of Agricultural and Food Sciences, King Faisal University, Al-Ahsa, 31982, Saudi Arabia
* Corresponding Authors: Jalil Ahmad. Email: ; Muhammad Munir. Email:
Phyton-International Journal of Experimental Botany 2025, 94(2), 281-302. https://doi.org/10.32604/phyton.2025.061534
Received 26 November 2024; Accepted 03 February 2025; Issue published 06 March 2025
Abstract
A steady rise in the overall population is creating an overburden on crops due to their global demand. On the other hand, given the current climate change and population growth, agricultural practices established during the Green Revolution are no longer viable. Consequently, innovative practices are the prerequisite of the time struggle with the rising global food demand. The potential of nanotechnology to reduce the phytotoxic effects of these ecological restrictions has shown significant promise. Nanoparticles (NPs) typically enhance plant resilience to stressors by fortifying the physical barrier, optimizing photosynthesis, stimulating enzymatic activity for defense, elevating the concentration of stress-resistant compounds, and activating the expression of genes associated with defense mechanisms. In this review, we thoroughly cover the uptake and translocations of NPs crops and their potential valuable functions in enhancing plant growth and development at different growth stages. Additionally, we addressed how NPs improve plant resistance to biotic and abiotic stress. Generally, this review presents a thorough understanding of the significance of NPs in plants and their prospective value for plant antioxidant and crop development.Keywords
The detrimental impacts of non-living environmental variables on species within a particular ecosystem are called abiotic stress. Significant worldwide concerns include environmental stressors like severe temperature, drought, salinity, and heavy metal contamination. Abiotic stress is more likely to affect plants in the setting of a changing global climate. As a result of global warming, climate change is linked to a marked rise in the frequency and severity of heat waves, droughts, and other abiotic stressors such as flooding, salinity, and freezing temperatures [1]. Likewise, biotic stresses such as viruses, fungus, bacteria, insects, and nematodes can markedly affect the vigor and productivity of crops [2,3].
The use of exogenous compounds is one of the most efficient and environmentally friendly strategies devised to lessen the negative effect of abiotic stress on plants [4]. Nanotechnology is a significant and quickly progressing field of research that has produced numerous innovations [5]. Agriculture’s future needs to be secure and sustainable, and nanotechnology has the potential to help achieve this [6]. Nanotechnology is primarily used in agriculture to monitor product and nutrient levels and apply nano pesticides and fertilizers, strengthen plant resistance to microbial disease and insect pests, and promote growth and productivity [7]. Nanoparticles (NPs) are becoming increasingly important in molecular biology studies because of their special physicochemical qualities. These include extraordinary intracellular stability, nanoscale dimensions (1–100 nm), increased surface area, and enhanced reactivity [8]. NPs made of metals and metal-based compounds have a wide range of physicochemical characteristics and are extensively used in many different sectors. These include agricultural and other industries such as textiles, food preparation, biomedicine, chemical processing, optics, and pharmaceuticals [9,10].
Moreover, metal-based NPs directly affect plant transcriptional regulation and transcript processing [11]. These compounds can bind to specific regions of promoters to alter gene expression, or they can interact with DNA molecules to change the structure of chromatin. Additionally, metal NPs can affect transcript processing via their interactions with RNA-binding proteins and splicing factors. Growth and development of plants are affected by gene expression patterns [12,13]. Metal NPs have a substantial effect on proteins both during and after translation. During protein synthesis, these NPs can affect ribosomal activity, affecting protein quantity and efficiency. They possess the capacity to modify ribosome activity, which affects the synthesis of particular proteins as well as the manner in which protein synthesis proceeds. Furthermore, it has been demonstrated that metal NPs can alter the stability, function, and localization of protein by causing post-translational changes such as phosphorylation, acetylation, and ubiquitination. These alternations are required for controlling a variety of biological functions, such as responses to stress, hormone signaling, and plant growth regulation [14,15]. The lower concentration of NPs caused superoxide dismutase (SOD) and peroxidase (POD) bursts, whereas these enzymes were inhibited at greater doses. Low concentrations of some chemical have harmful effects, but greater concentration have an adverse effect. This phenomenon is called a hormesis effect [16].
This study focuses on understanding how NPs affect plants to improve abiotic and biotic stress tolerance and contribute to sustainable agricultural operations. It summarizes the current understanding and potential applications of NPs in crop plants.
2 Mechanism of Abiotic and Biotic Stress Tolerance by Nanoparticles
To achieve sustainability in agriculture, technologies must be developed to increase food output and reduce crop yield loss. Numerous forms of abiotic stress on plants, such as salt drought, waterlogging, submersion, heavy metal stressors, and mineral and metal toxicity that reduce crop development and yield, are regarded as a significant rising issue in the field of agriculture [17,18]. These variables are primarily responsible for a decline in production. Throughout their lives, plants must deal with a variety of abiotic stressors and develop strong defense mechanisms to withstand them. Research on NPs has reported that they help plants to overcome abiotic stress by their concentration-dependent impact on plant growth and development [18–20]. Additionally, it has been reported that NPs have been found to increase the action of several antioxidant enzymes, including catalase (CAT), POD, and SOD [21]. In order to improve crop plants, NPs cooperate with plants in a variety of ways that result in numerous morphological and physiological changes depending on their chemical composition, size surface coverage, and reactivity. NPs affect plant growth and development in both favorable and harmful ways [22].
NPs represent a viable method for enhancing biotic stress tolerance across different species. This cutting-edge field combines nanotechnology and biological system to develop strategies that strengthen resistance against non-living environmental [23]. Apart from the biosynthesis process, precise and continuous post-synthetic remodeling can regulate the composition and structure of the cell wall during biotic interactions [24]. Their interaction with cell wall material such as cellulose, hemicellulose, and lignin strengthens plant defenses against pathogens [25]. Through adsorption or surface deposition, NPs interact with the plant cell wall to create a barrier that prevents diseases or pests from directly contacting the cell wall, protecting plants from biotic stress [26,27]. NPs can activate defensive responses in plants by interacting with the cell wall, triggering genes relevant to defense, and promoting the production of antimicrobial substances such as protein and phytoalexins that inhibit the development of pathogens [27,28]. Plants produce reactive oxygen species (ROS), trigger phytohormone pathways, and release secondary metabolites in response to biotic stress that inhibit herbivores or prevent the growth of pathogens [29]. NPs, including ZnO and TiO2 have been found to produce ROS upon light exposure [30]. NPs improve biotic stress defense by generating ROS directly within the cell wall. Additionally, by binding to cell wall proteins, they improve their stability and provide improved cell wall defense. The attachment of NPs to cell wall protein provides an added line of resistance in contrast to environmental stresses, with infections [31]. By upregulating genes involved in lignin production and improving the mechanical strength of the cell wall, NPs may affect the metabolic process involved in cell wall biosynthesis [32]. Most defense-related genes are produced by plants exposed to metallic NPs strengthening their cell wall barriers and generating antimicrobial compounds. Through epigenetic processes including DNA methylation and histone acetylation, metal oxide NPs can change gene expression without changing the DNA sequence [33]. This mechanism may result in the activation or suppression of genes essential for cell growth, division, and stress responses. More study is necessary for the best use of metallic NPs since their effects on gene expression differ depending on their characteristics, concentration, exposure time, and plant species [34].
3 Alleviation of Biotic and Abiotic Stress in Crop by Using Nanoparticles
In response to unfavorable environmental conditions, plants initiate biochemical, physiological and transcriptomic changes to alleviate stress [35,36]. These circumstances primarily inhibit their development by generating reactive oxygen species (ROS), which then acts as signal molecules to initiate stress resistance mechanisms.
In agriculture, NPs can be applied in the form of nanosensors, nanofertilizers, nanoherbicides/ nanopesticides/ nanopesticides and nanoremediators [5,37]. However, the mechanism underlying how NPs interact with plants has not been completely elucidated [38,39]. The types of NPs and application of NPs improve growth traits under abiotic and biotic stress (Figs. 1 and 2 and Table 1).

Figure 1: Types of NPs and their function
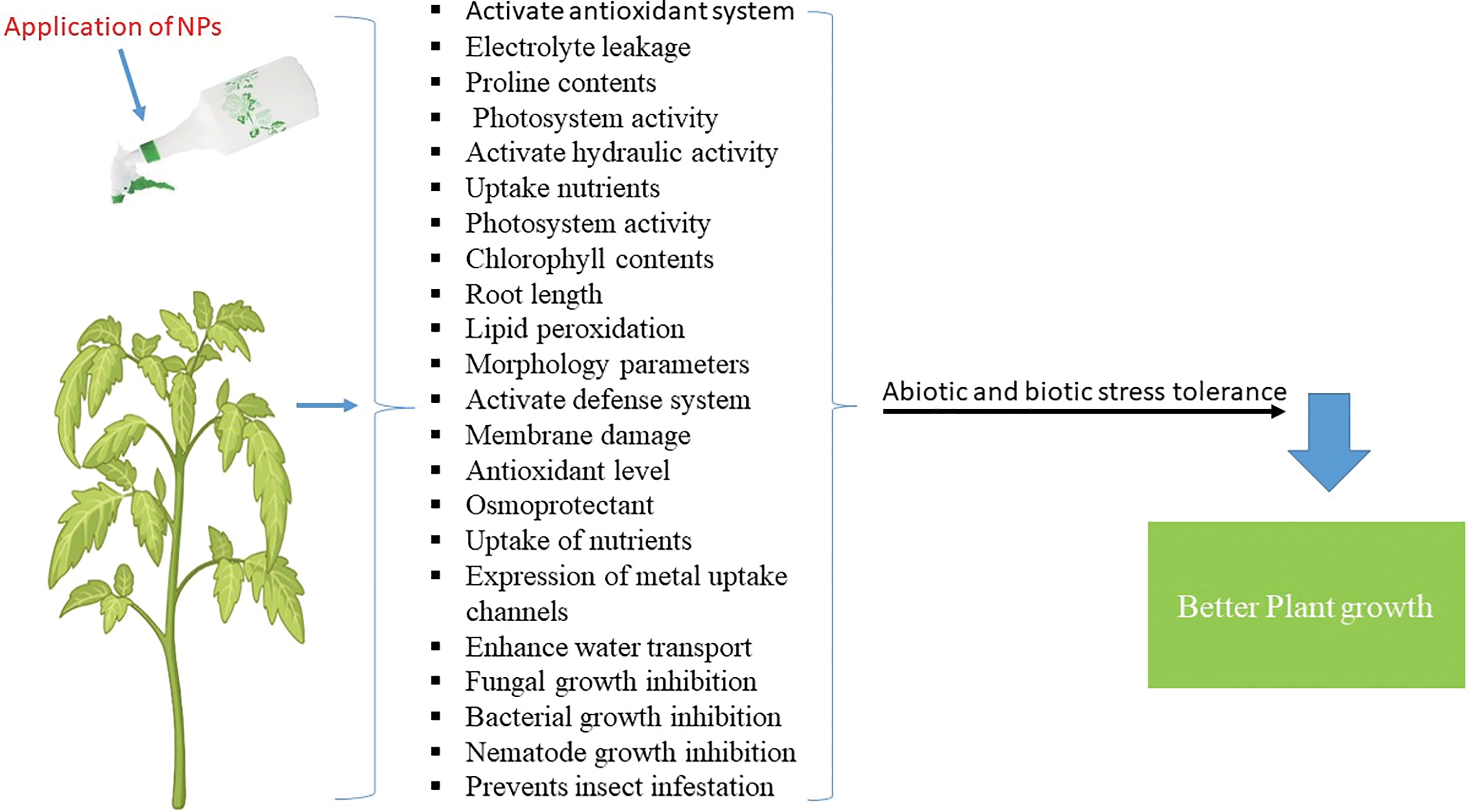
Figure 2: The application of NPs develops growth traits under abiotic and biotic stress
3.1 Nanoparticle Application to Lessen the Effects of Salt Stress
Salt is a significant abiotic stressor that lowers crop quality and productivity on about 45 million hectares of irrigated land [40]. Salinity stress modifies the intake of nutrients, reduces the uptake of water, and causes indirect drought stress through osmotic stress. The buildup of sodium and chloride ions in the cytoplasm cell sources ionic toxicity [41]. The metabolic processes in plant cells are interfered with under such circumstances, which lowers plant development as well as crop efficiency and features overall [42,43]. Agricultural land salinization is primarily driven by two key factors: Overuse of chemicals and climate change.
An alternate strategy to counteract salt stress is to apply various NPs, which lessens the toxicity effects that come with it. Applying FeO-NPs mitigated NaCl stress by enhancing growth, green photosynthetic pigment, and antioxidant enzyme activity in wheat plants. These NPs exhibited the resources to protect cells against ionic stress and reduce the accumulation of sodium ions within cells [44]. Applying ZnO-NPs (0.12 g pot−1) to wheat under salt stress resulted in increased plant height during the vegetative and mature stages longer shoots and spikes, improved fresh and dry weights, and higher levels of chlorophyll A and B, all of which improved grain output [45]. Ye et al. [46] discovered manganese seed priming nanoparticle control the molecular response of pepper plants, shielding them against saline stress. Nanotubes with multi-walled surfaces lessen the development of ROS, thiobarbituric acid, and the Na+/K+ ratio, enhances rapeseed (Brassica napus L.) salt tolerance. However, additional study on both physiochemical and molecular levels is required to clarify how NPs work to improve a plant’s ability to withstand salinity [47]. Hernández-Fuentes et al. [48] revealed that applying Cu-NPs (250 mg L−1) to tomato plant leaves stressed by salt affected the fruit’s antioxidant activity as well as the buildup and disintegration of bioactive compounds. Applying nSiO2 seems to alleviate the deleterious effect of salt by enhancing the epicuticular wax layer [49]. Faiz et al. [50] demonstrated that ZnO-NPs treatment effectively alleviated salt stress in tomato seedlings, boosting antioxidant defenses protein content, catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD). Application of ZnO-NPs to rice not only prevents Na+ from being absorbed, lessening the negative consequences of saline-sodic stress on the grain, but it also makes Zn easier to absorb, increasing rice’s chloroplast pigment content and facilitating effective photosynthesis. Additionally, improves the electron acceptor side of the electron transport chain, which makes it easier for electrons to move between PSII and PSI [51]. The combination of copper oxide and zinc oxide NPs applied topically for nutrient absorption, photosynthesis, and reducing antioxidant activity lowering excess Na+ in radish plants. However, both the metal oxide NPs showed differential responses [52]. The application of SiO2-NPs (30 ± 5 nm) boosted salt-tolerant rice yield by increasing panicle size and grain filling rate. Chlorophyll levels, leaf area index, potassium ion content, dry matter, root system growth, and antioxidant activities were all improved by SiO2 NPs which also reduced malondialdehyde content [53].
3.2 Nanoparticle Application to Lessen the Effects of Drought Stress
Plants normally encounter fluctuating environmental conditions that may be suboptimal for their emergence and development. In the context of ongoing environmental changes and anthropogenic activities, drought and other extreme weather disasters are likely to happen more frequently in the future [54–56]. Crop yields can be severely affected by global warming and drought. It is crucial for the life cycle of plants to have access to water. In general, drought conditions result in reduced water availability for plants; however, a number of variables, such as the length and intensity of the drought, the stage at which develops when it is under water stress, and certain plant characteristics like root architecture, the makeup of the epicuticular wax, and the integrity of cell wall, all affect crop productivity [56,57]. As a result, signal perception and transduction play a complicated and multifaceted role in plants’ response to drought.
Different drought-coping mechanisms have evolved in plants, such as tolerance, avoidance, and escape. The application of nSe alleviated drought stress (DS) adverse effects by boosting photosynthetic pigment production, dry biomass accumulation, RWC, and antioxidant defenses [58]. Applying Se-NPs to leaves at a concentration of 10 ppm substantially improves drought resistance in grapevine saplings. This enhancement is achieved through strengthening antioxidant defense, increasing proline and protein production, and promoting overall plant development. However, Se-NPs concentration below 10 ppm are less effective, while those above this level may result in toxic effects on the plants [59]. Similarly, Javan et al. [60] revealed that fruit firmness in sustained deficit irrigation (SDI) plants decreased by 25.2%, 25.2%, and 21.5% at TiO2-NPs concentrations of 10, 20, and 30 mg L−1, respectively, compared to full irrigation (FI). Cu-NPs application alleviated DS adverse effects, improving wheat growth and yield parameters such as plant height, spike length, 1000 grain weight, stomatal conductance, leaf chlorophyll content, water use efficiency, leaf turgor potential, relative water content, and grain yield [61]. Water-stressed maize seedlings treated with O-CMC NPs showed a considerable increase in chlorophyll content as well as the activity of many antioxidant enzymes, including polyphenol oxidase (PPO), catalase (CAT), Peroxidase (POD), and superoxide dismutase (SOD) [62]. According to Asghari et al. [63], basil under drought stress exhibited improved physiological and phytochemical features when exposed to Se-NPs at concentrations of 50 and 100 ppm. The foliar spray of 10, 20, 30, and 40 Se-NPs to wheat under drought stress conditions improved various growth signs. This improvement were observed in multiple aspects of plant development, including overall plant height, shoot dimensions and mass (both fresh and dry), root characteristics (length and weight in fresh and dry), as well as leaf metrics [64]. Additionally, ZnO-NPs can reduce the buildup of ROS and lipid peroxidation, as well as increase antioxidant activity, in cucumber (Cucumis sativus) seedlings under moderate oxidative stress [65]. Similarly, El-Zohri et al. [66] revealed that tomato plants’ ability to withstand drought stress can be enhanced successfully by tropically applying green ZnO-NPs at lower concentrations. Bisht et al. [67] reported that hematite-NPs decreased ammonia and proline nitrate levels by 22.4%, 25%, and 37%, respectively. Also, 50 mg/L of hematite NPs worked best to control nitrogen and osmolyte breakdown during water stress. Under drought conditions, the combination of Chitosan-Coated Iron Oxide NPs and Kitoplus® advanced stimulant led to an increase in oil yield in a mint plant [68]. Nano-silicon (nSi) application on Kalamata olive tree leaves in a semi-arid environment specifies that treating with 200 mg L−1 notably enhances mechanical strength, growth, and productivity in trees facing moderate stress on physiological and biochemical characteristics under deficit irrigation conditions [69]. In conclusion, prior research has demonstrated promising outcomes after applying NPs to mitigate the negative effects of scarcity stress on plant development and output.
3.3 Nanoparticle Application to Lessen the Effects of Temperature Stress
Among the dynamic environmental elements, one of the most harmful stresses is thought to be the constant rise in temperature [70]. Elevated temperature increases the generation of ROS and triggers oxidative stress in crop plants. This leads to the degradation of membrane lipids, disruption of cellular balance, and impairment of various metabolic functions. Ultimately, these effects result in cell death within the plant [71]. Furthermore, heat stress inhibits carbon fixation, increases the breakdown of chlorophyll, and inhibits photosystem II and electron flow. These results interfere with the photosynthesis process, resulting in diminished plant growth [72]. On the contrary, these plants’ defense mechanisms, secondary metabolism, respiration, and the synthesis of proteins and nucleic acids are all impacted by low temperatures [73,74]. Recent advancements in nanotechnology have improved the efficiency of the agriculture sector with prospective applications to expand plant emergence and change under demanding conditions [75]. In wheat plants, the application of GS-Ag-NPs reduces the effect of heat stress by increasing the flux of carbohydrates metabolism, SOD, and protein, including HSPs, crude fibers, and minerals [76]. The ZnO-NPs application boosted the root dry weight, root–shoot ratio, total dry weight, and chlorophyll a/b ratio in Xiangyaxiangzhan by 10.48%–18.10%, 1.96%–20.31%, 3.25%–6.02%, and 3.70%–6.11% at low temperature, respectively [77]. Wang et al. [78] reported that the application of CH-NPs exposed efficacy in lessening the harmful influences of low-temperature pressure on (Musa acuminate var Baxi) plants through the decline of ROS buildup. Similarly, Djanaguiraman et al. [79] discovered application of Se-NPs in sorghum subjected to elevated temperatures demonstrated efficacy in mitigating adverse effects including membrane degradation, reduced pollen viability, and diminished crop yields, through the initiation of the wheat antioxidant defense system. ZnO-NPs treatment of alfalfa seedlings prior to heat stress changed the ultrastructure of mitochondria, cell walls, and chloroplasts, minimizing heat-induced damage and improving plant growth. Likewise, ZnO-NPs dramatically reduced the decrease in TGS-GUS gene expression that heat caused in Arabidopsis thaliana seedlings that were subjected to 37°C of heat stress [80]. Younis et al. [81] results show that applying Si or Si-NPs to cellular organelles, particularly the nucleus and chloroplasts, successfully repaired the structural harm brought on by heat stress. Furthermore, as demonstrated by higher levels of photosynthetic pigments, improved performance index, and increased photochemical efficiency of photosystem II, both Si and Si-NPs increased photosynthetic capacity. The foliar treatment of nano-ZnO is an effective method to defend mung bean crop from heat stress-induced damage with the minor risk of NPs release into the environment compared to soil application [82]. Similarly, Plaksenkova et al. [83] showed the study concentration of Fe3O4 may positively affect the yield and quality of rocket seedlings, and possibly these NPs could expand the ability of plants to stand against environmental stresses.
3.4 Nanoparticle Application to Lessen the Effect of Heavy Metals
A variety of life forms, including plants, are at risk due to heavy metals (HMs). Crop yields are severely affected by HMs because they disrupt imperative cellular biomolecules severely hampering plant metabolism. NPs are one of the most recent, efficient, and promising strategies for alleviating HMs [84,85].
Faizan et al. [86] revealed that foliar use of ZnO-NPs shortens the negative influence of Cd on tomato plants by increasing the creation of chloroplast pigments, regulating osmoregulation and diminishing the content of O2-, MDA, and H2O2. Likewise, Alomrani et al. [87] revealed that low concentration of Cu-NPs efficiently threatened Solanum melogena from the opposing outcome of heavy metal stress. Cu-NPs showed a key role in plant physiology by stimulating enzyme activity and resistance mechanisms. Similarly, Ahmad et al.’s [88] research showed that applying Si-NPs reduces the amount of Cd adsorbed by tomato roots, reducing heavy metal’s effects. Moreover, it enhances tomato plants under Cd stress regarding growth, yield, and biomolecule indicators. The application of ZnO-NPs initiated efficient heavy metal tolerance mechanisms by triggering multiple biochemical pathways in a coordinated manner to avoid cellular damage from heavy metal oxidative stress-caused toxicity in Leucaena leucocephala [89]. Additionally, Samani et al. [90] revealed that nano silica acts as a beneficial soil amendment, improving nutrient availability and mitigating the harmful impacts of heavy metals on Calendula officinalis. Applying FeO and Se-NPs to wheat reduced oxidative stress and Cd absorption while improving (p < 0.05). gas exchange properties, synthetic and non-synthetic, gene expression, and plant evolution and biomass [91]. Coriander plants exhibited enhanced resistance to Cd stress when titanium dioxide NPs were utilized in seed priming. Similarly, Sardar et al. [92] concluded that TiO2-NP treatment of coriander plants under Cd stress, positively influenced photosynthetic content, total soluble sugar concentration, growth, and yield parameters. Likewise, the application of Ag-NPs might reduce aluminum’s harmful impact on pineapple growth and nutrition at specific concentrations. This is vital for maintaining adequate photosynthetic pigments and a balanced mineral intake during in vitro pineapple cultivation [93]. In lettuce, ZnO-NPs significantly reduced cadmium uptake, with a maximum reduction noticed at a concentration of 100 mg/L of G-ZnO-NPs. The concentrations of zinc (Zn) and cadmium (Cd) were inversely correlated in lettuce shoots [94]. Nevertheless, it is crucial to create innovative nano-remediation techniques to mitigate the detrimental effects of HMs on plant growth and development.
A major contributor to crop losses in agriculture is biotic stress [95]. Living organisms, such as fungi, viruses, bacteria, insect pests, and herbivores, are responsible for causing biotic stress in plants. Unlike abiotic stress, biotic stress severely impedes plant growth through nutritional deficiency, potentially leading to the death of the plant [96,97]. Nanotechnology offers diverse applications for enhancing agricultural productivity, safeguarding crops, and improving the storage, packaging, and transportation of farm products. Additionally, it provides eco-friendly, effective, and secure approaches to control the transmission of plant-based biotic stress [98].
Kaur et al. [99] demonstrated that biosynthesized Ag-NPs exhibit a 46% increase in efficacy and reduced chickpea Fusarium wilt incidence by 73.33% compared to CuOCl. Also, Ag-NPs showed non-toxic properties to chickpea seed germination and the soil microbial community. The application of ZnO-NPs to the artificially inoculated tomato plants with the pathogen Ralstonia. solanacearum significantly enhances plant growth by reducing bacterial soil population and disease severity as compared with the untreated control [100]. The application of CeO2-NPs exhibited significant antifungal activity against Ustilago tritici, the fungal pathogen affecting wheat crops, across all concentrations tested [101]. Similarly, Satti et al. [102] revealed that applying varying concentrations of NPs externally to wheat plants led to decreased disease occurrence and severity index. The most effective suppression of pathogens was achieved when using TiO2 NPS at a concentration of 40 mg L−1. The exogenous treatment of apples (cv.Anna) with chitosan nanoparticles or bulk chitosan significantly enhanced systemic acquired resistance (SAR) against Penicillium expansum infection by enhancing the expression of crucial defense-related genes [103]. The application of ZnO-NPs considerably prevents blast development and improves blast resistance in rice by prompting ROS accumulation and expression of defense-related genes OsNAC4, OsPR10, OsKSL4, and OsPR1b [104]. Additionally, Ghareeb et al. [105] concluded that using ZnONPs improved the quality and quantity of sweet pepper crops while dramatically lowering the severity of Fusarium oxysporum disease. Furthermore, the application of ZnO-NPs to M. incognita resulted in a substantial reduction in the number of nematode galls, egg masses per root, eggs/egg mass, and females by 98%, 99%, 99.9%, and 95.5%, respectively. Foliar application of 0.10 g L−1 MnO2-NPs in conjunction with Pseudomonas putida yielded the most significant reduction in wilt disease indices, galling, and Meloidogyne incognita population, while simultaneously promoting the greatest increase in plant growth parameters. Application of ZnO-NPs using Azadirachta indica leaf extract for biocontrol of diseased lychee fruits [106]. In banana leaves, CS-NP spraying significantly decreased BSV replication by elevating plant defense mechanisms and growth responses. An 18.20-fold increase in expression was achieved with CS-NPs at 400 mg L−1 [107].
Pests pose a significant challenge in agriculture as they have the potential to cause harm to crops and contaminate stored food, resulting in a decline in food quality and the spread of plant diseases [108,109]. The application of silver NPs at low doses could effectively reduce the population of phytophagous mites on tomato plants while minimizing harmful effects on non-target mite species [110]. Additionally, peach tree leaf extracts treated with Ag-NPs and Zn-NPs demonstrated complete rice weevil mortality (Sitophilus oryza L.) and less Rhyzopertha dominica via the fumigation method [111]. Similarly, Al-Azzazy et al. [112] application of ZnO-NPs at varying concentrations ((200, 400, 600, 1000, 1250, and 1500 ppm) against all stages of the date palm mites. As zinc oxide nanoparticle concentrations increased, both tetranychid and phyttoseiid mite mortality rates increased. With minimal effects on Amblyseius swirskii, ZnO-NPs were shown to be highly effective in killing Oligonychus afrasiaticus and Eutetranychus palmatus. Bapat et al. [113] concluded that a higher concentration of Si-NPs in tomatoes inhibited the larval growth of Helicoverpa armigera. Application of Silicon NPs (0.5 and 1 mg L−1) on tomato plants reduced their susceptibility to the Root-knot nematode (Meloidogyne incognita). SiO2-NPs demonstrated a significant protective effect against Caryedon serratus in groundnuts at concentrations of 0.67 and 1.7 mg/kg, and mortality rose with exposure duration [114]. Similarly, Al-Azzazy et al. [115] reported that higher concentrations of Cu-NPs correlated with increased mortality rates in both phytophagous and predatory mites. Cu-NPs demonstrated significant efficacy in eliminating Phyllocoptruta oleivora, Eutetranychus orientalis, and Brevipalpus obovatus while exhibiting minimal effects on Amblyseius swirskii and Euseius scutalis. Nanosilica, with a 30 nm particle size at 0.5 g/kg rice, showed 80% and 97.4% mortality rates against Sitophilus oryzae after 7 and 14 days, respectively [116]. SiO2-NP fumigation of maize grains proved highly active against four common pests: Sitophilus oryzae, Rhizopertha dominica, Tribolium castaneum, and Orizaephilus surinamenisis [117].
Plants rely on NPs to mitigate the adverse effects of environmental stress. NPs may improve plant growth and development by increasing photosynthesis and improving nutrient absorption, even in adverse environments. Nanoparticles have been found to stimulate the production of a range of defensive compounds in plants, which in turn alter the plant’s internal environment and enable it to better withstand various stressors. The utilization of NPs has been shown to improve the ability of plants to endure adverse conditions by activating the antioxidant mechanisms within the plant, thereby minimizing the accumulation of ROS during stress and promoting the expression of genes that provide protection. Abiotic and biotic stress tolerance with NPs is well documented; however, the majority of studies are still in the laboratory. Several concerns have been raised about the potential adverse effects of NPs on the environment and the possibility that NPs could accumulate in edible plant parts due to their extensive usage. Therefore, it is necessary to develop appropriate evaluation methodologies to assess the effects of NPs and nano fertilizers on both biotic and abiotic ecosystem components. It is essential to conduct further research on the development of nanomaterials with low cost, low toxicity, hormesis, ecological safety, and self-degradation properties to commercialize nanotechnology.
Acknowledgement: Thanks to all researchers for their contribution to this work.
Funding Statement: The authors extend their gratitude to the Deanship of Scientific Research (DSR), King Faisal University, Saudi Arabia, for funding the publication of this work (Project number: KFU250560).
Author Contributions: Jalil Ahmad, Muhammad Munir, Conceptualization; Jalil Ahmad, Muhammad Munir and Nashi Alqahtani contributed to writing and original draft preparation. Tahira Alyas, Muhammad Ahmad, Sadia Bashir and Fasiha Qurashi edited the manuscript, and Abdul Ghafoor and Hassan Ali–Dinar supervised, project administration. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: No data was used for the research described in the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Zandalinas SI, Fritschi FB, Mittler R. Global warming, climate change, and environmental pollution: recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021;26(6):588–99. doi:10.1016/j.tplants.2021.02.011. [Google Scholar] [PubMed] [CrossRef]
2. Kumar R, Tiwari RK, Jeevalatha A, Siddappa S, Shah MA, Sharma S, et al. Potato apical leaf curl disease: current status and perspectives on a disease caused by tomato leaf curl New Delhi virus. J Plant Dis Prot. 2021;128(4):897–911. doi:10.1007/s41348-021-00463-w. [Google Scholar] [CrossRef]
3. Lal MK, Tiwari RK, Kumar R, Naga KC, Kumar A, Singh B, et al. Effect of potato apical leaf curl disease on glycemic index and resistant starch of potato (Solanum tuberosum L.) tubers. Food Chem. 2021;359(2):129939. doi:10.1016/j.foodchem.2021.129939. [Google Scholar] [PubMed] [CrossRef]
4. Ahmad J, Hayat F, Khan U, Ahmed N, Li J, Ercisli S, et al. Melatonin: a promising approach to enhance abiotic stress tolerance in horticultural plants. South African J Bot. 2024;164(4):66–76. doi:10.1016/j.sajb.2023.10.045. [Google Scholar] [CrossRef]
5. El-Saadony MT, ALmoshadak AS, Shafi ME, Albaqami NM, Saad AM, El-Tahan AM, et al. Vital roles of sustainable nano-fertilizers in improving plant quality and quantity-an updated review. Saudi J Biol Sci. 2021;28(12):7349–59. doi:10.1016/j.sjbs.2021.08.032. [Google Scholar] [PubMed] [CrossRef]
6. Seleiman MF, Santanen A, Mäkelä PSA. Recycling sludge on cropland as fertilizer—advantages and risks. Resour Conserv Recycl. 2020;155:104647. doi:10.1016/J.RESCONREC.2019.104647. [Google Scholar] [CrossRef]
7. Shang Y, Kamrul Hasan M, Ahammed GJ, Li M, Yin H, Zhou J. Applications of nanotechnology in plant growth and crop protection: a review. Molecules. 2019;24(14):2558. doi:10.3390/molecules24142558. [Google Scholar] [PubMed] [CrossRef]
8. Nejatzadeh F. Effect of silver nanoparticles on salt tolerance of Satureja hortensis L. during in vitro and in vivo germination tests. Heliyon. 2021;7(2):e05981. doi:10.1016/j.heliyon.2021.e05981. [Google Scholar] [PubMed] [CrossRef]
9. Starón A, Dlugosz O, Pulit-Prociak J, Banach M. Analysis of the exposure of organisms to the action of nanomaterials. Materials. 2020;13(12):349. doi:10.3390/ma13020349. [Google Scholar] [PubMed] [CrossRef]
10. Verma SK, Das AK, Gantait S, Kumar V, Gurel E. Applications of carbon nanomaterials in the plant system: a perspective view on the pros and cons. Sci Total Environ. 2019;667:485–99. doi:10.1016/j.scitotenv.2019.02.409. [Google Scholar] [PubMed] [CrossRef]
11. Jośko I, Kusiak M, Xing B, Oleszczuk P. Combined EFfect of Nano-CuO and Nano-ZnO in plant-related system: from bioavailability in soil to transcriptional regulation of metal homeostasis in barley. J Hazard Mater. 2021;416:126230. doi:10.1016/j.jhazmat.2021.126230. [Google Scholar] [PubMed] [CrossRef]
12. Ma C, Chhikara S, Xing B, Musante C, White JC, Dhankher OP. Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sustain Chem Eng. 2013;1(7):768–78. doi:10.1021/sc400098h. [Google Scholar] [CrossRef]
13. Tumburu L, Andersen CP, Rygiewicz PT, Reichman JR. Molecular and physiological responses to titanium dioxide and cerium oxide nanoparticles in arabidopsis. Environ Toxicol Chem. 2017;36(1):71–82. doi:10.1002/etc.3500. [Google Scholar] [PubMed] [CrossRef]
14. Mustafa G, Komatsu S. Plant proteomic research for improvement of food crops under stresses: a review. Mol Omi. 2021;17:860–880. doi:10.1039/D1MO00151E. [Google Scholar] [PubMed] [CrossRef]
15. Mosa KA, Awad A, Yahya RA, Alameeri SN, Ramamoorthy K, Ali MA. Metal nanoparticle implication, transport, and detection in plants. In: Plant metal and metalloid transporters. Singapore: Springer Nature Singapore; 2022. p. 331–360. [Google Scholar]
16. Agathokleous E, Feng ZZ, Iavicoli I, Calabrese EJ. The two faces of nanomaterials: a quantification of hormesis in algae and plants. Environ Int. 2019;131:105044. doi:10.1016/j.envint.2019.105044. [Google Scholar] [PubMed] [CrossRef]
17. Tripathi DK, Singh VP, Kumar D, Chauhan DK. Impact of exogenous silicon addition on chromium uptake, growth, mineral elements, oxidative stress, antioxidant capacity, and leaf and root structures in rice seedlings exposed to hexavalent chromium. Acta Physiol Plant. 2012;34(1):279–89. doi:10.1007/s11738-011-0826-5. [Google Scholar] [CrossRef]
18. Rasheed A, Li H, Tahir MM, Mahmood A, Nawaz M, Shah AN, et al. The role of nanoparticles in plant biochemical, physiological, and molecular responses under drought stress: a review. Front Plant Sci. 2022;13:e0902. doi:10.3389/fpls.2022.976179. [Google Scholar] [PubMed] [CrossRef]
19. Dimkpa CO, Bindraban PS, Fugice J, Agyin-Birikorang S, Singh U, Hellums D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron Sustain Dev. 2017;37(1):1. doi:10.1007/s13593-016-0412-8. [Google Scholar] [CrossRef]
20. Mishra S, Keswani C, Abhilash PC, Fraceto LF, Singh HB. Integrated approach of agri-nanotechnology: challenges and future trends. Front Plant Sci. 2017;8(1896):254477. doi:10.3389/fpls.2017.00471. [Google Scholar] [PubMed] [CrossRef]
21. Lawre S, Raskar S. Effect of titanium dioxide nanoparticles on hydrolytic and antioxidant enzymes during seed germination in onion. Int J Curr Microbiol App Sci. 2014;3:749–60. [Google Scholar]
22. Ma X, Geiser-Lee J, Deng Y, Kolmakov A. Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ. 2010;408(16):3053–61. doi:10.1016/j.scitotenv.2010.03.031. [Google Scholar] [PubMed] [CrossRef]
23. Thabet SG, Alqudah AM. Unraveling the role of nanoparticles in improving plant resilience under environmental stress condition. Plant Soil. 2024;503(1-2):313–30. doi:10.1007/s11104-024-06581-2. [Google Scholar] [CrossRef]
24. Baez LA, Tichá T, Hamann T. Cell wall integrity regulation across plant species. Plant Mol Biol. 2022;109(4-5):483–504. doi:10.1007/s11103-022-01284-7. [Google Scholar] [PubMed] [CrossRef]
25. Jia H, Ma P, Huang L, Wang X, Chen C, Liu C, et al. Hydrogen sulphide regulates the growth of tomato root cells by affecting cell wall biosynthesis under CuO NPs stress. Plant Biol. 2022;24(4):627–35. doi:10.1111/plb.13316. [Google Scholar] [PubMed] [CrossRef]
26. Bidi H, Fallah H, Niknejad Y, Barari Tari D. Iron oxide nanoparticles alleviate arsenic phytotoxicity in rice by improving iron uptake, oxidative stress tolerance and diminishing arsenic accumulation. Plant Physiol Biochem. 2021;163(7):348–57. doi:10.1016/j.plaphy.2021.04.020. [Google Scholar] [PubMed] [CrossRef]
27. Zhao L, Peralta-Videa JR, Varela-Ramirez A, Castillo-Michel H, Li C, Zhang J, et al. Effect of surface coating and organic matter on the uptake of CeO2 NPs by corn plants grown in soil: insight into the uptake mechanism. J Hazard Mater. 2012;225-226:131–8. doi:10.1016/J.JHAZMAT.2012.05.008. [Google Scholar] [PubMed] [CrossRef]
28. Kamel SM, Elgobashy SF, Omara RI, Derbalah AS, Abdelfatah M, El-Shaer A, et al. Antifungal activity of copper oxide nanoparticles against root rot disease in cucumber. J Fungi. 2022;8(9):911. doi:10.3390/jof8090911. [Google Scholar] [PubMed] [CrossRef]
29. Tyagi S, Shah A, Karthik K, Rathinam M, Rai V, Chaudhary N, et al. Reactive oxygen species in plants: an invincible fulcrum for biotic stress mitigation. Appl Microbiol Biotechnol. 2022;106(18):5945–55. doi:10.1007/s00253-022-12138-z. [Google Scholar] [PubMed] [CrossRef]
30. Juárez-Maldonado A, Ortega-Ortíz H, Morales-Díaz AB, González-Morales S, Morelos-Moreno Á, Cabrera-De la Fuente M, et al. Nanoparticles and nanomaterials as plant biostimulants. Int J Mol Sci. 2019;20(1):162. doi:10.3390/ijms20010162. [Google Scholar] [PubMed] [CrossRef]
31. Slavin YN, Bach H. Mechanisms of antifungal properties of metal nanoparticles. Nanomaterials. 2022;12(24):4470. doi:10.3390/nano12244470. [Google Scholar] [PubMed] [CrossRef]
32. Munir N, Gulzar W, Abideen Z, Hancock JT, El-Keblawy A, Radicetti E. Nanotechnology improves disease resistance in plants for food security: applications and challenges. Biocatal Agric Biotechnol. 2023;51(10):102781. doi:10.1016/j.bcab.2023.102781. [Google Scholar] [CrossRef]
33. Wang M, Lai X, Shao L, Li L. Evaluation of immunoresponses and cytotoxicity from skin exposure to metallic nanoparticles. Int J Nanomedicine. 2018;13:4445–59. doi:10.2147/IJN.S170745. [Google Scholar] [PubMed] [CrossRef]
34. Joshi SM, De Britto S, Jogaiah S. Myco-engineered selenium nanoparticles elicit resistance against tomato late blight disease by regulating differential expression of cellular, biochemical and defense responsive genes. J Biotechnol. 2021;325(1):196–206. doi:10.1016/j.jbiotec.2020.10.023. [Google Scholar] [PubMed] [CrossRef]
35. González-García Y, González-Moscoso M, Hernández-Hernández H, Méndez-López A, Juárez-Maldonado A. Induction of stress tolerance in crops by applying nanomaterials. In: Nanotechnology in plant growth promotion and protection; 2021. doi:10.1002/9781119745884.ch8. [Google Scholar] [CrossRef]
36. Esgario JGM, Krohling RA, Ventura JA. Deep learning for classification and severity estimation of coffee leaf biotic stress. Comput Electron Agric. 2020;169:105162. doi:10.1016/j.compag.2019.105162. [Google Scholar] [CrossRef]
37. Elsakhawy T, Omara AE-D, Alshaal T, El-Ramady H, Ghazi A, El-Nahrawy S, et al. Nanomaterials and plant abiotic stress in agroecosystems. Environ Biodivers Soil Secur. 2018;2(1):73–94. doi:10.21608/jenvbs.2018.3897.1030. [Google Scholar] [CrossRef]
38. Saxena R, Tomar RS, Kumar M. Exploring nanobiotechnology to mitigate abiotic stress in crop plants. J Pharm Sci Res. 2015;8:974–80. [Google Scholar]
39. Khan Z, Upadhyaya H. Impact of nanoparticles on abiotic stress responses in plants: an overview. Nanomat Plants, Algae Microorgan. 2018;2:305–22. doi:10.1016/B978-0-12-811488-9.00015-9. [Google Scholar] [CrossRef]
40. Machado RMA, Serralheiro RP. Soil salinity: effect on vegetable crop growth. management practices to prevent and mitigate soil salinization. Horticulturae. 2017;3(2):30. doi:10.3390/horticulturae3020030. [Google Scholar] [CrossRef]
41. Ludwiczak A, Osiak M, Cárdenas-Pérez S, Lubińska-Mielińska S, Piernik A. Osmotic stress or ionic composition: which affects the early growth of crop species more? Agronomy. 2021;11(3):435. doi:10.3390/agronomy11030435. [Google Scholar] [CrossRef]
42. Alam P, Arshad M, Al-Kheraif AA, Azzam MA, Al Balawi T. Silicon nanoparticle-induced regulation of carbohydrate metabolism, photosynthesis, and ROS homeostasis in Solanum lycopersicum subjected to salinity stress. ACS Omega. 2022;7:31834–44. doi:10.1021/acsomega.2c02586. [Google Scholar] [PubMed] [CrossRef]
43. Azzam CR, Zaki SNS, Bamagoos AA, Rady MM, Alharby HF. Soaking maize seeds in zeatin-type cytokinin biostimulators improves salt tolerance by enhancing the antioxidant system and photosynthetic efficiency. Plants. 2022;11(8):1004. doi:10.3390/plants11081004. [Google Scholar] [PubMed] [CrossRef]
44. Manzoor N, Ahmed T, Noman M, Shahid M, Nazir MM, Ali L, et al. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci Total Environ. 2021;769(26):145221. doi:10.1016/j.scitotenv.2021.145221. [Google Scholar] [PubMed] [CrossRef]
45. Adil M, Bashir S, Bashir S, Aslam Z, Ahmad N, Younas T, et al. Zinc oxide nanoparticles improved chlorophyll contents, physical parameters, and wheat yield under salt stress. Front Plant Sci. 2022;13:932861. doi:10.3389/fpls.2022.932861. [Google Scholar] [PubMed] [CrossRef]
46. Ye Y, Cota-Ruiz K, Hernández-Viezcas JA, Valdés C, Medina-Velo IA, Turley RS, et al. Manganese nanoparticles control salinity-modulated molecular responses in Capsicum annuum L. through priming: a sustainable approach for agriculture. ACS Sustain Chem Eng. 2020;8(3):1427–36. doi:10.1021/acssuschemeng.9b05615. [Google Scholar] [CrossRef]
47. Zhao G, Zhao Y, Lou W, Su J, Wei S, Yang X, et al. Nitrate reductase-dependent nitric oxide is crucial for multi-walled carbon nanotube-induced plant tolerance against salinity. Nanoscale. 2019;11(21):10511–23. doi:10.1039/c8nr10514f. [Google Scholar] [PubMed] [CrossRef]
48. Hernández-Fuentes AD, López-Vargas ER, Pinedo-Espinoza JM, Campos-Montiel RG, Valdés-Reyna J, Juárez-Maldonado A. Postharvest behavior of bioactive compounds in tomato fruits treated with cu nanoparticles and NaCl stress. Appl Sci. 2017;7(10):980. doi:10.3390/app7100980. [Google Scholar] [CrossRef]
49. Avestan S, Ghasemnezhad M, Esfahani M, Byrt CS. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy. 2019;9(5):246. doi:10.3390/agronomy9050246. [Google Scholar] [CrossRef]
50. Faiz S, Yasin NA, Khan WU, Shah AA, Akram W, Ahmad A, et al. Role of magnesium oxide nanoparticles in the mitigation of lead-induced stress in Daucus Carota: modulation in polyamines and antioxidant enzymes. Int J Phytoremediation. 2022;24(4):364–72. doi:10.1080/15226514.2021.1949263. [Google Scholar] [PubMed] [CrossRef]
51. Dang K, Wang Y, Tian H, Bai J, Cheng X, Guo L, et al. Impact of ZnO NPs on photosynthesis in rice leaves plants grown in saline-sodic soil. Sci Rep. 2024;14(1):1–15. doi:10.1038/s41598-024-66935-9. [Google Scholar] [PubMed] [CrossRef]
52. Mahawar L, Živčák M, Barboricova M, Kovár M, Filaček A, Ferencova J, et al. Effect of copper oxide and zinc oxide nanoparticles on photosynthesis and physiology of Raphanus sativus L. under salinity stress. Plant Physiol Biochem. 2024;206(3):108281. doi:10.1016/j.plaphy.2023.108281. [Google Scholar] [PubMed] [CrossRef]
53. Jin W, Li L, He W, Wei Z. Application of silica nanoparticles improved the growth, yield, and grain quality of two salt-tolerant rice varieties under saline irrigation. Plants. 2024;13:2452. doi:10.3390/plants13172452. [Google Scholar] [PubMed] [CrossRef]
54. Bailey-Serres J, Parker JE, Ainsworth EA, Oldroyd GED, Schroeder JI. Genetic strategies for improving crop yields. Nature. 2019;575:109–18. doi:10.1038/S41586-019-1679-0. [Google Scholar] [PubMed] [CrossRef]
55. Zia R, Nawaz MS, Siddique MJ, Hakim S, Imran A. Plant survival under drought stress: implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol Res. 2021;242:126626. doi:10.1016/j.micres.2020.126626. [Google Scholar] [PubMed] [CrossRef]
56. Gupta A, Rico-Medina A, Caño-Delgado AI. The physiology of plant responses to drought. Science. 2020;368:266–9. doi:10.1126/science.aaz7614. [Google Scholar] [PubMed] [CrossRef]
57. Nadeem M, Li J, Yahya M, Sher A, Ma C, Wang X, et al. Research progress and perspective on drought stress in legumes: a review. Int J Mol Sci. 2019;20(10):2541. doi:10.3390/ijms20102541. [Google Scholar] [PubMed] [CrossRef]
58. Zeeshan M, Wang X, Salam A, Wu H, Li S, Zhu S, et al. Selenium nanoparticles boost the drought stress response of soybean by enhancing pigment accumulation, oxidative stress management and ultrastructural integrity. Agronomy. 2024;14(7):1372. doi:10.3390/agronomy14071372. [Google Scholar] [CrossRef]
59. Daler S, Korkmaz N, Kılıç T, Hatterman-Valenti H, Karadağ A, Kaya O. Modulatory effects of selenium nanoparticles against drought stress in some grapevine rootstock/scion combinations. Chem Biol Technol Agric. 2024;11(1):1–27. doi:10.1186/s40538-024-00609-6. [Google Scholar] [CrossRef]
60. Javan M, Selahvarzi Y, Sayyad-Amin P, Rastegar S. Potential application of TiO2 nanoparticles to improve the nutritional quality of Strawberry Cv. camarosa under drought stress. Sci Hortic. 2024;330:113055. doi:10.1016/j.scienta.2024.113055. [Google Scholar] [CrossRef]
61. Raza MAS, Amin J, Valipour M, Iqbal R, Aslam MU, Zulfiqar B, et al. Cu-nanoparticles enhance the sustainable growth and yield of drought-subjected wheat through physiological progress. Sci Rep. 2024;14(1):1–10. doi:10.1038/s41598-024-62680-1. [Google Scholar] [PubMed] [CrossRef]
62. Wu H, Du P, Miao X, Hou R, Li S, Zeeshan M, et al. O-Carboxymethyl chitosan nanoparticles: a novel approach to enhance water stress tolerance in maize seedlings. Int J Biol Macromol. 2024;277:134459. doi:10.1016/j.ijbiomac.2024.134459. [Google Scholar] [PubMed] [CrossRef]
63. Asghari J, Mahdavikia H, Rezaei-Chiyaneh E, Banaei-Asl F, Amani Machiani M, Harrison MT. Selenium nanoparticles improve physiological and phytochemical properties of basil (Ocimum basilicum L.) under drought stress conditions. Land. 2023;12(1):164. doi:10.3390/LAND12010164. [Google Scholar] [CrossRef]
64. Ikram M, Raja NI, Javed B, Mashwani ZR, Hussain M, Hussain M, et al. Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress. Green Process Synth. 2020;9:706–14. doi:10.1515/GPS-2020-0067/PDF. [Google Scholar] [CrossRef]
65. Ghani MI, Saleem S, Rather SA, Rehmani MS, Alamri S, Rajput VD, et al. Foliar application of zinc oxide nanoparticles: an effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere. 2022;289:133202. doi:10.1016/j.chemosphere.2021.133202. [Google Scholar] [PubMed] [CrossRef]
66. El-Zohri M, Al-Wadaani NA, Bafeel SO. Foliar sprayed green zinc oxide nanoparticles mitigate drought-induced oxidative stress in tomato. Plants. 2021;10(11):2400. doi:10.3390/plants10112400. [Google Scholar] [PubMed] [CrossRef]
67. Bisht S, Sharma V, Kumari N. Biosynthesized hematite nanoparticles mitigate drought stress by regulating nitrogen metabolism and biological nitrogen fixation in Trigonella foenum-graecum. Plant Stress. 2022;6(1):100112. doi:10.1016/j.stress.2022.100112. [Google Scholar] [CrossRef]
68. Giglou MT, Giglou RH, Esmaeilpour B, Azarmi R, Padash A, Falakian M, et al. A new method in mitigation of drought stress by chitosan-coated iron oxide nanoparticles and growth stimulant in peppermint. Ind Crops Prod. 2022;187(2):115286. doi:10.1016/j.indcrop.2022.115286. [Google Scholar] [CrossRef]
69. Hassan IF, Ajaj R, Gaballah MS, Ogbaga CC, Kalaji HM, Hatterman-valenti HM, et al. Foliar application of nano-silicon improves the physiological and biochemical characteristics of ‘Kalamata’ olive subjected to deficit irrigation in a semi-arid climate. Plants. 2022;11(12):1561. doi:10.3390/plants11121561. [Google Scholar] [PubMed] [CrossRef]
70. Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017;22:53–65. doi:10.1016/j.tplants.2016.08.015. [Google Scholar] [PubMed] [CrossRef]
71. Savicka M, Škute N. Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.) Ekologija. 2010;56. doi:10.2478/v10055-010-0004-x. [Google Scholar] [CrossRef]
72. Li D, Wang M, Zhang T, Chen X, Li C, Liu Y, et al. Glycinebetaine mitigated the photoinhibition of photosystem II at high temperature in transgenic tomato plants. Photosynth Res. 2021;147(3):301–15. doi:10.1007/s11120-020-00810-2. [Google Scholar] [PubMed] [CrossRef]
73. Adhikari L, Baral R, Paudel D, Min D, Makaju SO, Poudel HP, et al. Cold stress in plants: strategies to improve cold tolerance in forage species. Plant Stress. 2022;4:100081. doi:10.1016/j.stress.2022.100081. [Google Scholar] [CrossRef]
74. Aslam M, Greaves JG, Jakada BH, Fakher B, Wang X, Qin Y. AcCIPK5, a pineapple CBL-interacting protein kinase, confers salt, osmotic and cold stress tolerance in transgenic Arabidopsis. Plant Sci. 2022;320:111284. doi:10.1016/j.plantsci.2022.111284. [Google Scholar] [PubMed] [CrossRef]
75. Rana RA, Siddiqui MN, Skalicky M, Brestic M, Hossain A, Kayesh E, et al. Prospects of nanotechnology in improving the productivity and quality of horticultural crops. Horticulturae. 2021;7(10):332. doi:10.3390/horticulturae7100332. [Google Scholar] [CrossRef]
76. Iqbal M, Raja NI, Mashwani Zur R, Yasmeen F, Hussain M, Ejaz M, et al. Insight into carbohydrate metabolism, protein quantification and mineral regulation in wheat (Triticum aestivum L.) by the action of green synthesized silver nanoparticles (AgNPs) against heat stress. J Biomol Struct Dyn. 2024;11(3):1–15. doi:10.1080/07391102.2024.2311333. [Google Scholar] [PubMed] [CrossRef]
77. Mai Y, Ren Y, Deng S, Ashraf U, Tang X, Duan M, et al. Influence of ZnO nanoparticles on early growth stage of fragrant rice at low temperature (LT) stress. J Soil Sci Plant Nutr. 2024;24(1):1317. doi:10.1007/s42729-024-01632-0. [Google Scholar] [CrossRef]
78. Wang A, Li J, Al-Huqail AA, Al-Harbi MS, Ali EF, Wang J, et al. Mechanisms of chitosan nanoparticles in the regulation of cold stress resistance in banana plants. Nanomaterials. 2021;11(10):2670. doi:10.3390/nano11102670. [Google Scholar] [PubMed] [CrossRef]
79. Djanaguiraman M, Belliraj N, Bossmann SH, Prasad PVV. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega. 2018;3(3):2479–91. doi:10.1021/acsomega.7b01934. [Google Scholar] [PubMed] [CrossRef]
80. Wu J, Wang T. Synergistic effect of zinc oxide nanoparticles and heat stress on the alleviation of transcriptional gene silencing in Arabidopsis thaliana. Bull Environ Contam Toxicol. 2020;104(1):49–56. doi:10.1007/s00128-019-02749-0. [Google Scholar] [PubMed] [CrossRef]
81. Younis AA, Khattab H, Emam MM. Impacts of silicon and silicon nanoparticles on leaf ultrastructure and TaPIP1 and TaNIP2 gene expressions in heat stressed wheat seedlings. Biol Plant. 2020;64(14):343–52. doi:10.32615/bp.2020.030. [Google Scholar] [CrossRef]
82. Kareem HA, Saleem MF, Saleem S, Rather SA, Wani SH, Siddiqui MH, et al. Zinc oxide nanoparticles interplay with physiological and biochemical attributes in terminal heat stress alleviation in Mungbean (Vigna radiata L.). Front Plant Sci. 2022;13:139. doi:10.3389/fpls.2022.842349. [Google Scholar] [PubMed] [CrossRef]
83. Plaksenkova I, Jermaļonoka M, Bankovska L, Gavarāne I, Gerbreders V, Sledevskis E, et al. Effects of Fe3O4 nanoparticle stress on the growth and development of rocket Eruca sativa. J Nanomater. 2019;2019(4):1–10. doi:10.1155/2019/2678247. [Google Scholar] [CrossRef]
84. Ahmad B, Zaid A, Zulfiqar F, Bovand F, Dar TA. Nanotechnology: a novel and sustainable approach towards heavy metal stress alleviation in plants. Nanotechnol Environ Eng. 2023;8:27–40. doi:10.1007/s41204-022-00230-8. [Google Scholar] [CrossRef]
85. Naseem Z, Naveed M, Asif M, Alamri S, Nawaz S, Siddiqui MH, et al. Enhancing chromium resistance and bulb quality in onion (Allium cepa L.) through copper nanoparticles and possible health risk. BMC Plant Biol. 2024;24:1–13. doi:10.1186/S12870-024-05460-3/TABLES/3. [Google Scholar] [CrossRef]
86. Faizan M, Faraz A, Mir AR, Hayat S. Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J Plant Growth Regul. 2021;40(1):101–15. doi:10.1007/s00344-019-10059-2. [Google Scholar] [CrossRef]
87. Alomrani SO, Kaleem M, Aslam M, Habib F, Jamal A, Waseem M, et al. Copper nanoparticles alleviate cadmium stress in Solanum melongena through Endogenous melatonin and regulation of some physiochemical attributes. Sci Hortic. 2024;323(1):112546. doi:10.1016/j.scienta.2023.112546. [Google Scholar] [CrossRef]
88. Ahmad A, Javad S, Iqbal S, Shahzadi K, Gatasheh MK, Javed T. Alleviation potential of green-synthesized selenium nanoparticles for cadmium stress in Solanum lycopersicum L: modulation of secondary metabolites and physiochemical attributes. Plant Cell Rep. 2024;43(4):67. doi:10.1007/S00299-024-03197-9. [Google Scholar] [PubMed] [CrossRef]
89. Venkatachalam P, Jayaraj M, Manikandan R, Geetha N, Rene ER, Sharma NC, et al. Zinc oxide nanoparticles (ZnONPs) alleviate heavy metal-induced toxicity in Leucaena leucocephala seedlings: a physiochemical analysis. Plant Physiol Biochem. 2017;110(2):59–69. doi:10.1016/j.plaphy.2016.08.022. [Google Scholar] [PubMed] [CrossRef]
90. Samani M, Ahlawat YK, Golchin A, Alikhani HA, Baybordi A, Mishra S, et al. Nano silica’s role in regulating heavy metal uptake in calendula officinalis. BMC Plant Biol. 2024;24(1):299. doi:10.1186/S12870-024-05311-1. [Google Scholar] [PubMed] [CrossRef]
91. Chen F, Jiang F, Okla MK, Abbas ZK, Al-Qahtani SM, Al-Harbi NA, et al. Nanoparticles synergy: enhancing wheat (Triticum aestivum L.) cadmium tolerance with iron oxide and selenium. Sci Total Environ. 2024;915(4):169869. doi:10.1016/j.scitotenv.2024.169869. [Google Scholar] [PubMed] [CrossRef]
92. Sardar R, Ahmed S, Yasin NA. Titanium dioxide nanoparticles mitigate cadmium toxicity in Coriandrum sativum L. through modulating antioxidant system, stress markers and reducing cadmium uptake. Environ Pollut. 2022;292(4):118373. doi:10.1016/j.envpol.2021.118373. [Google Scholar] [PubMed] [CrossRef]
93. Tejada-Alvarado JJ, Meléndez-Mori JB, Ayala-Tocto RY, Goñas M, Oliva M. Influence of silver nanoparticles on photosynthetic pigment content and mineral uptake in pineapple seedlings grown in vitro under aluminum stress. Agronomy. 2023;13(5):1186. doi:10.3390/agronomy13051186. [Google Scholar] [CrossRef]
94. Timilsina A, Adhikari K, Chen H. Foliar application of green synthesized ZnO nanoparticles reduced Cd content in shoot of lettuce. Chemosphere. 2023;338(1–2):139589. doi:10.1016/j.chemosphere.2023.139589. [Google Scholar] [PubMed] [CrossRef]
95. Chaudhary P, Agri U, Chaudhary A, Kumar A, Kumar G. Endophytes and their potential in biotic stress management and crop production. Front Microbiol. 2022;13:933017. doi:10.3389/fmicb.2022.933017. [Google Scholar] [PubMed] [CrossRef]
96. Gull A, Lone AA, Wani NUI, Gull A, Lone AA, Wani NUI. Biotic and abiotic stresses in plants. Abiotic Biot Stress Plants. 2019. doi:10.5772/INTECHOPEN.85832. [Google Scholar] [CrossRef]
97. Umar OB, Ranti LA, Abdulbaki AS, Bola AL, Abdulhamid AK, Biola MR, et al. Stresses in plants: biotic and abiotic. Curr Trends Wheat Res. 2021. doi:10.5772/INTECHOPEN.100501. [Google Scholar] [CrossRef]
98. Ehsan M, Raja NI, Mashwani Z-R, Fatima N, Abasi F, Wattoo FH, et al. The role of green synthesized nanoparticles in biotic stress resistance in vegetables. Nanopart Plant Biot Stress Manag. 2024;9(2):383–402. doi:10.1007/978-981-97-0851-2_15. [Google Scholar] [CrossRef]
99. Kaur P, Thakur R, Duhan JS, Chaudhury A. Management of wilt disease of chickpea in vivo by silver nanoparticles biosynthesized by rhizospheric microflora of chickpea (Cicer arietinum). J Chem Technol Biotechnol. 2018;93(11):3233–43. doi:10.1002/jctb.5680. [Google Scholar] [CrossRef]
100. Khan RAA, Tang Y, Naz I, Alam SS, Wang W, Ahmad M, et al. Management of Ralstonia Solanacearum in tomato using ZnO nanoparticles synthesized through matricaria chamomilla. Plant Dis. 2021;105(10):3224–30. doi:10.1094/PDIS-08-20-1763-RE. [Google Scholar] [PubMed] [CrossRef]
101. Alotaibi MO, Alotaibi NM, Ghoneim AM, Ain Nul, Irshad MA, Nawaz R, et al. Effect of green synthesized cerium oxide nanoparticles on fungal disease of wheat plants: a field study. Chemosphere. 2023;339(2):139731. doi:10.1016/j.chemosphere.2023.139731. [Google Scholar] [PubMed] [CrossRef]
102. Satti SH, Raja NI, Ikram M, Oraby HF, Mashwani ZUR, Mohamed AH, et al. Plant-based titanium dioxide nanoparticles trigger biochemical and proteome modifications in Triticum aestivum L. under biotic stress of Puccinia Striiformis. Molecules. 2022;27(13):4274. doi:10.3390/molecules27134274. [Google Scholar] [PubMed] [CrossRef]
103. Abdel-Rahman FA, Monir GA, Hassan MSS, Ahmed Y, Refaat MH, Ismail IA, et al. Exogenously applied chitosan and chitosan nanoparticles improved apple fruit resistance to blue mold, upregulated defense-related genes expression, and maintained fruit quality. Horticulturae. 2021;7(8):224. doi:10.3390/horticulturae7080224. [Google Scholar] [CrossRef]
104. Qiu J, Chen Y, Liu Z, Wen H, Jiang N, Shi H, et al. The application of zinc oxide nanoparticles: an effective strategy to protect rice from rice blast and abiotic stresses. Environ Pollut. 2023;331:121925. doi:10.1016/j.envpol.2023.121925. [Google Scholar] [PubMed] [CrossRef]
105. Ghareeb RY, Belal EB, El-Khateeb NMM, Shreef BA. Utilizing bio-synthesis of nanomaterials as biological agents for controlling soil-borne diseases in pepper plants: root-knot nematodes and root rot fungus. BMC Plant Biol. 2024;24(1):112308. doi:10.1186/s12870-024-04760-y. [Google Scholar] [PubMed] [CrossRef]
106. Ahmed J, Ali M, Sheikh HM, Al-Kattan MO, Farhana H, Safaeishakib U, et al. Biocontrol of fruit rot of Litchi Chinensis using zinc oxide nanoparticles synthesized in Azadirachta indica. Micromachines. 2022;13(9):1461. doi:10.3390/mi13091461. [Google Scholar] [PubMed] [CrossRef]
107. Abdelbaset TE, Alahmari AS, Elkammar HF, Alothaim T, Ahmed NG, Abd El Moneim D, et al. Stimulating banana tree resistance to banana streak virus (BSV) disease by chitosan nanoparticles. Eur J Plant Pathol. 2024;169(4):825–40. doi:10.1007/s10658-024-02872-7. [Google Scholar] [CrossRef]
108. Barik TK, Sahu B, Swain V. Nanosilica-from medicine to pest control. Parasitol Res. 2008;103:253–8. doi:10.1007/s00436-008-0975-7. [Google Scholar] [PubMed] [CrossRef]
109. Kandhol N, Singh VP, Peralta-Videa J, Corpas FJ, Tripathi DK. Silica nanoparticles: the rising star in plant disease protection. Trends Plant Sci. 2022;27(1):7–9. doi:10.1016/j.tplants.2021.10.007. [Google Scholar] [PubMed] [CrossRef]
110. Al-Azzazy MM, Ghani SBA, Alhewairini SS. Field evaluation of the efficacy of silver nanoparticles (AgNP) against mites associated with tomato plants in greenhouses. Pakistan J Agric Sci. 2019;56(01):245–59. doi:10.21162/PAKJAS/19.8155. [Google Scholar] [CrossRef]
111. Mosa WFA, El-Shehawi AM, Mackled MI, Salem MZM, Ghareeb RY, Hafez EE, et al. Productivity performance of peach trees, insecticidal and antibacterial bioactivities of leaf extracts as affected by nanofertilizers foliar application. Sci Rep. 2021;11(1):66. doi:10.1038/s41598-021-89885-y. [Google Scholar] [PubMed] [CrossRef]
112. Al-Azzazy MM, Ghani SBA, Alhewairini SS. Acaricidal activity of zinc oxide nanoparticles against mites associated with date palm trees. Pakistan J Agric Sci. 2024;61:351–7. [Google Scholar]
113. Bapat G, Zinjarde S, Tamhane V. Evaluation of silica nanoparticle mediated delivery of protease inhibitor in tomato plants and its effect on insect pest Helicoverpa armigera. Coll Surf B Biointerf. 2020;193:111079. doi:10.1016/j.colsurfb.2020.111079. [Google Scholar] [PubMed] [CrossRef]
114. Diagne A, Diop BN, Ndiaye PM, Andreazza C, Sembene M. Efficacy of silica nanoparticles on groundnut bruchid, Caryedon serratus (Olivier) (Coleoptera, Bruchidae). African Crop Sci J. 2019;27(2):229. doi:10.4314/acsj.v27i2.8. [Google Scholar] [CrossRef]
115. Al-Azzazy MM, Ghani SBA. Field evaluation of the efficacy of copper nanoparticles against mites associated with orange trees. Brazilian J Biol. 2024;84(1):283. doi:10.1590/1519-6984.270451. [Google Scholar] [PubMed] [CrossRef]
116. Kar S, Nayak RN, Sahoo NR, Bakhara CK, Panda MK, Pal US, et al. Rice Weevil management through application of silica nano particle and physico-chemical and cooking characterization of the treated rice. J Stored Prod Res. 2021;94(3):101892. doi:10.1016/j.jspr.2021.101892. [Google Scholar] [CrossRef]
117. El-Naggar ME, Abdelsalam NR, Fouda MMG, Mackled MI, Al-Jaddadi MAM, Ali HM, et al. Soil application of nano silica on maize yield and its insecticidal activity against some stored insects after the post-harvest. Nanomaterials. 2020;10(4):739. doi:10.3390/nano10040739. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools