 Open Access
Open Access
REVIEW
Impact of Soil Microbes and Abiotic Stress on Strawberry Root Physiology and Growth: A Review
1 Institute of Plant Protection, MNS University of Agriculture, Multan, 60000, Pakistan
2 Production Lareault, 90 rue Lareault, Lavaltrie, QC J5T 3H3, Canada
3 Department of Plant Pathology, San Luis Valley Research Center, Colorado State University, Fort Collins, CO 80523, USA
4 Department of Agricultural Science & Engineering, College of Agriculture, Tennessee State University, Nashville, TN 37209, USA
5 Department of Plant Pathology, University of Agriculture Faisalabad, Faisalabad, 38000, Pakistan
6 Department of Agricultural Engineering, Khwaja Fareed University of Engineering and Information Technology, Rahim Yar Khan, 64200, Pakistan
7 Department of Agriculture, Government College University Lahore (GCUL), Lahore, 54000, Pakistan
8 Department of Plant Sciences, Aberdeen Research & Extension Center, University of Idaho, Aberdeen, ID 83210, USA
* Corresponding Authors: Akhtar Hameed. Email: ; Muhammad Saqlain Zaheer. Email:
(This article belongs to the Special Issue: Soil Microbes and Abiotic Stress Factors: Impacts on Root Physiology, Crop Growth, and Hormonal Dynamics)
Phyton-International Journal of Experimental Botany 2025, 94(3), 561-581. https://doi.org/10.32604/phyton.2025.061262
Received 20 November 2024; Accepted 08 February 2025; Issue published 31 March 2025
Abstract
Strawberry (Fragaria ananassa) is well known among consumers because of its attractive color, delicious taste, and nutritional benefits. It is widely grown worldwide, but its production has become a significant challenge due to changing climatic conditions that lead to abiotic stresses in plants, which results in poor root development, nutrient deficiency, and poor plant health. In this context, the major abiotic stresses are temperature fluctuations, water shortages, and high levels of soil salinity. The accumulation of salts in excessive amounts disrupts the osmotic balance and impairs physiological processes. However, drought reduces fruit size, yield, and quality. Similarly, heat and cold stresses directly affect the rate of photosynthesis. Plants respond to these changes by producing growth-promoting hormones to ensure their survival. In the context of these abiotic stresses, beneficial microbes support plant growth. Among these fungi, the most extensively studied are plant growth-promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF). When applied as bioinoculants, they are associated with roots and subsequently improve soil health, fruit quality, and overall crop yield. This review highlights the impacts of abiotic stresses on strawberry roots, growth, and hormonal pathways. Moreover, it focuses on the role of beneficial soil microbes in the mitigation of these responses.Keywords
Strawberry (Fragaria × ananassa Duch.), it is a small soft fruit crop that belongs to the Rosacea family and the Fragaria genus. The modern cultivated strawberry is a hybrid of Fragaria virginia (meadow strawberry) and Fragaria chiloensis (Chilean strawberry) [1]. It is a perennial herbaceous plant with a fibrous root system [2]. It is one of the most widespread fruit crops in the world [3] and is grown commercially throughout temperate and subtropical zones [4], and its global cultivation increased by 9.18 million tons in 2021 [5]. They are well renowned for their antioxidant activities [6] and high mineral contents, including potassium, calcium, and magnesium contents [7]. In addition to nutritional benefits, they offer health benefits such as lowering the risk of cancer, increasing insulin sensitivity, increasing cardiovascular health, and promoting the overall immune system [8]. In addition to being antioxidant and carcinogenic, they are highly anti-hyperglycemic, antidiabetic, and anti-hypersensitive [9]. Additionally, their abundance of bioactive compounds has significantly increased their commercial value [10].
In the past few years, overall global production has reached over 9.1 million tons, with a cost-benefit of approximately 18.41 billion dollars, which has continuously increased since 2010 [11]. They are grown in 73 countries with areas of 390,000 hectares, making them the highest-yielding fruit crop at the global level [12]. China is the largest producer of strawberries, with an annual production of 3.8 million tons, followed by the United States, with an annual production of 1.4 million tons. Similarly, Egypt ranks first in African countries, with a production of 468,000 tons per year [11]. To meet the demands of the growing population, maintaining the quality and production of strawberry plants is necessary. However, its production is limited by poor soil health, nutrient deficiency, and certain abiotic stress factors [13]. In this sense, drought, salinity, and temperature are the main limiting abiotic factors for strawberry production worldwide, as they can influence the anatomical and physiological characteristics of plants by affecting certain molecular pathways and mechanisms [14], which ultimately affects crop production [15], as illustrated in Fig. 1.
Figure 1: A comprehensive mechanism of abiotic stresses affecting plant roots, growth and hormonal pathways
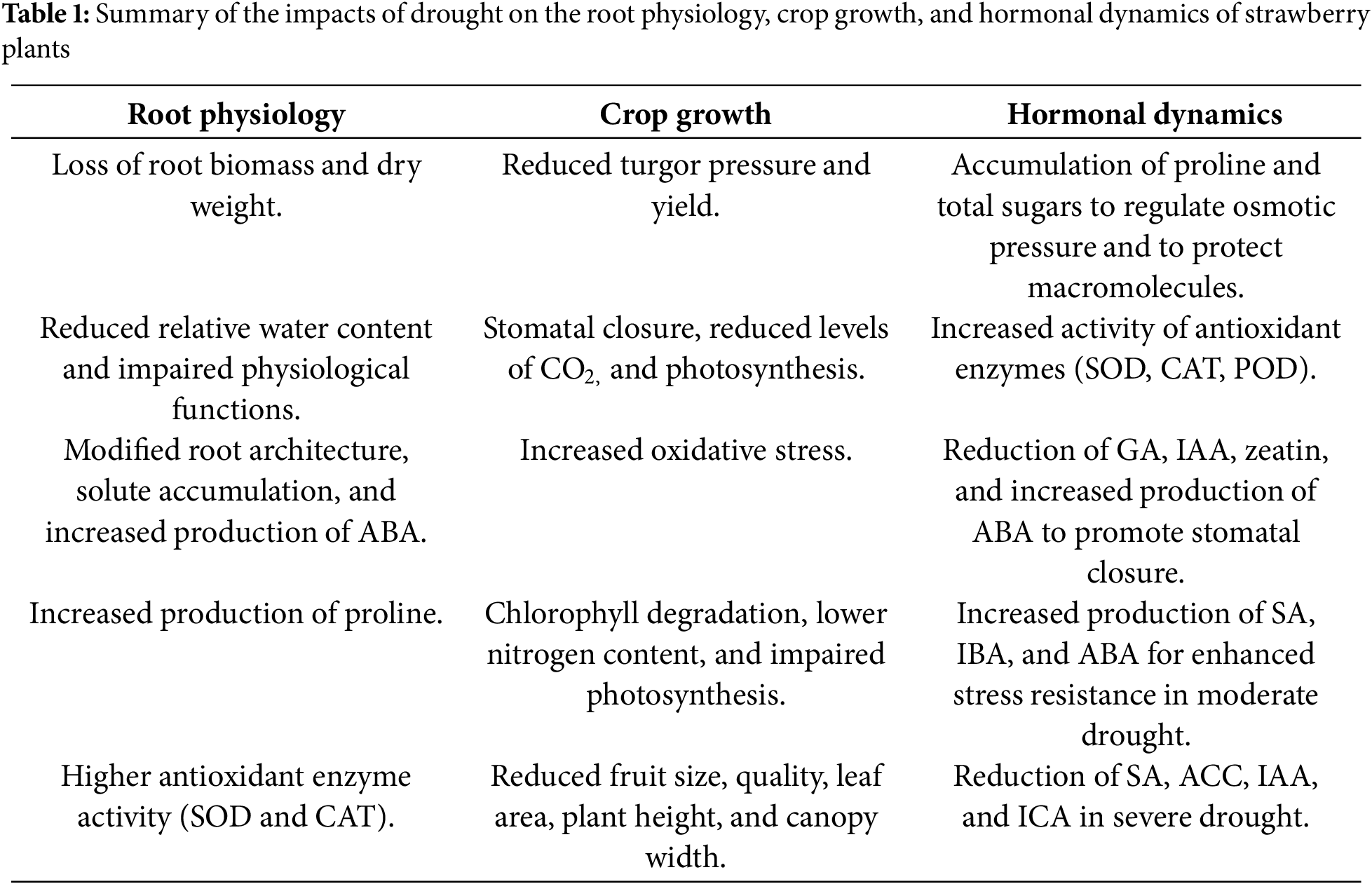
Drought stress is a serious environmental threat that jeopardizes the yield of crops economically [16]. It is a prevalent environmental stress that limits strawberry productivity and affects strawberry morphology as well as enzymatic and physiological activities [17]. Owing to their shallow root system, large leaf area, and juicy texture, strawberry plants are significantly damaged by water deficiency, thereby reducing their biomass and crop yield [18]. Plants greatly suffer from mineral shortcomings due to the unavailability of enough water in the soil and the lower mobilization of minerals within the plants [19]. This detailed review comprehensively explores the impacts of drought on the growth, physiology, and molecular pathways of strawberry plants, the results of which are summarized in Table 1.
2.1 Impacts of Drought on Root Physiology
Strawberries are considered the most drought-sensitive crop [18], resulting in substantial losses in root biomass and dry weight. This sensitivity is not constant across all cultivars, with some cultivars appearing to be more resilient than others [17]. In susceptible cultivars, this stress reduces the relative water content of roots, which is required to maintain turgor pressure and overall plant health. A reduced relative water content impairs the physiological functions of plants [20]. Furthermore, plants respond to drought stress via multiple mechanisms, including modifications in root architecture, root structure, increased solute accumulation, osmotic adjustments, and abscisic acid (ABA) production. Moreover, plants minimize the rate of transpiration by closing their stomata and adaptive root structure, which increases water uptake from the soil. The roots of strawberry plants respond to drought stress by synthesizing proline, a type of osmolyte that increases the stability of proteins and cellular structures under dry conditions [17,20]. Additionally, evidence suggests that water shortage results in an increase in the activities of antioxidant enzymes, such as SOD and CAT, in the roots. These enzymes assist in decreasing oxidative stress during water stress [17,21].
2.2 Impacts of Drought on Crop Growth
The susceptibility of plants to water stress depends upon certain factors, including their growth stage, growth pattern, cultivation variety, weather conditions, and duration of stress. However, they are extremely sensitive to water shortages. In the face of water scarcity, strawberry plants frequently lose turgor and actual yield [18]. Additionally, it severely impacts the physiological processes of plants, such as the transpiration rate, photosynthesis, solute transport, and water balance [22]. Stomatal closure in leaves reduces the level of CO2 under drought stress, which reduces the relative water content and photosynthetic pigments. This results in reduced growth and increased oxidative stress. Water scarcity also causes chlorophyll degradation [23], insufficient nitrogen content [24], and a direct reduction in photosynthesis, severely impacting plant development by hindering cell division, enlargement, and differentiation [23]. Prolonged drought causes a shift in the mechanism of decreased photosynthesis, from stomatal closure to membrane damage in mesophyll cells. This reduces the chlorophyll content and impairs the transport and synthesis of essential compounds [25].
Additionally, reduced leaf gas exchange under drought conditions limits carbon assimilation, ultimately affecting photoassimilation in plants. A decrease in carbon assimilation can inhibit the development of both vegetative and reproductive structures [26,27]. Similarly, reference [28] reported a reduced surface area of leaves, water use efficiency, net assimilation rate, and transpiration due to water shortages. Moreover, the most significant impact of water stress is the reduction in fruit size and yield; however, the extent of this reduction varies among different varieties [18]. Furthermore, inadequate water availability during growth phases lowers fruit quality [12]. Studies have revealed that as the water field capacity decreases from 100% to 25%, parameters such as leaf number, plant height, canopy width, and crown diameter also decline [26].
2.3 Impacts of Drought on Hormonal Dynamics
The increased production of metabolites and various solutes is one of the primary responses of plants to severe drought stress. When plants are under stress, the levels of proline and total sugars begin to increase at relatively high concentrations in certain strawberry species. These solutes perform major functions, including the regulation of osmotic pressure, water retention, and protection of macromolecules from damage [29]. Furthermore, a lack of water increases the levels of anthocyanin and soluble salts, which in turn increase the activity of important enzymes in plants, such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), to neutralize oxidative stress [30]. Drought also led to altered hormonal activities in strawberry plants to mitigate these responses. In this context, the primary hormones are salicylic acid (SA), auxins (IAA, IBA), gibberellins (GA), abscisic acid (ABA), and jasmonic acid (JA). Water shortage typically reduces the production of GA, IAA, and zeatin nucleoside, which results in slower plant growth. When reciprocating, the level of ABA increases. It acts as a signaling molecule that promotes the closure of stomata, reduces the rate of transpiration, and enhances drought resistance. Similarly, under moderate drought conditions, the levels of SA, ABA, and IBA increase, resulting in increased stress resistance. One of the more complex responses to maintain growth is increased levels of GA, JA, and indole-3-carboxylic acid. Moreover, there was an increase in GA, JA, and indole-3-carboxylic acid (ICA) contents, indicating a more complex response to maintain growth. However, severe drought leads to a decline in SA, 1-amminocyclopropane-1-carboxylic acid (ACC), and IAA, suggesting that the plant struggles to maintain normal growth. After rewatering, studies revealed an increase in the levels of ACC, IAA, ICA, and indole-3-propionic acid (IPA) in “Zhangji” strawberry plants, indicating that the recovery phase helps to restore normal growth [31].
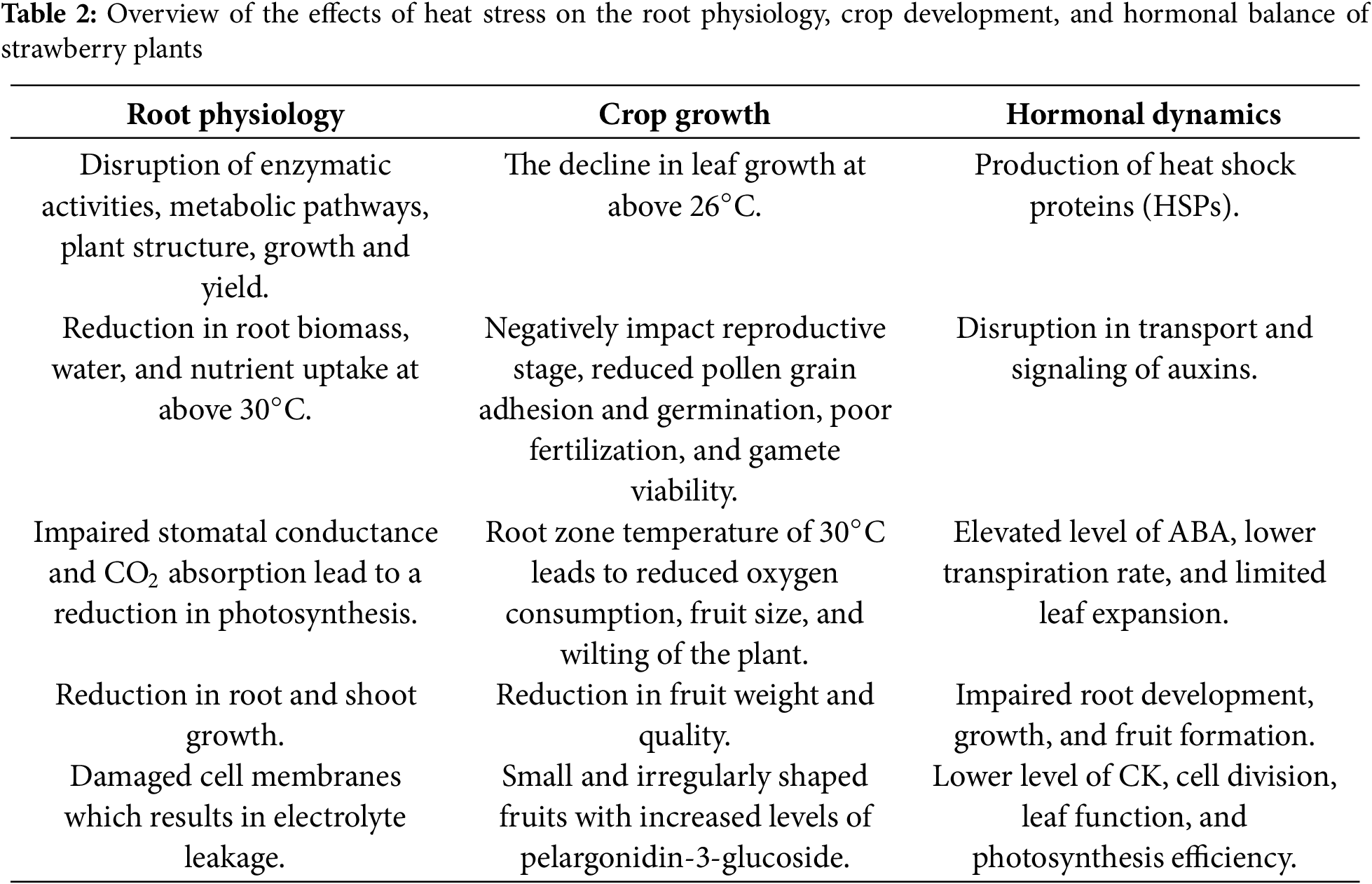
In recent years, temperature stress due to global warming has become a significant factor contributing to yield losses and decreased dry matter content, especially in temperate regions [32]. Compared with the period from 1850–1900, global surface temperatures are projected to rise by more than 1.5°C by the end of the 21st century [33]. When strawberries and other plants are exposed to heat stress, several negative effects can occur, including reduced photosynthesis, damage to cell membranes, cell death, and accelerated aging [34]. However, plants can develop a form of heat tolerance, known as thermotolerance, if they are initially exposed to mild, nonlethal heat stress [35]. In light of these challenges, a detailed review of the impacts of heat stress on strawberries is presented below, along with a summary in Table 2.
3.1 Impacts of Heat Stress on Root Physiology
Both extreme heat and prolonged exposure to moderately high temperatures negatively impact plants by causing severe damage [36], which leads to changes in plant structure, function, and internal processes due to the disruption of enzymes and metabolic pathways that threaten crop yield and sustainability [37]. High temperatures negatively influence several key aspects of strawberry plants, including vegetative growth, root development, pollen health, fruit size, fruit quality, protein levels in leaves, and fruit production [38]. Research conducted by [39] and [38] revealed that temperatures above 30°C reduce root biomass, which hampers the ability of plants to take up water and nutrients effectively. Additionally, high temperatures significantly decrease photosynthesis by affecting stomatal conductance and CO2 absorption, with rates decreasing 5 to 9 times lower than those in plants grown at the optimal temperature of 25°C. This decline is crucial for the healthy growth of both roots and shoots. Additionally, Ref. [39] reported that heat stress causes more damage to cell membranes, leading to increased leakage of electrolytes, which indicates cellular stress and damage.
3.2 Impacts of Heat Stress on Crop Growth
Heat stress significantly impacts strawberry plants, leading to various physiological stresses. Research has revealed that leaf growth increases linearly at temperatures below 26°C, whereas growth decreases linearly at temperatures above this threshold. Optimal growth has been reported to occur between 18.3°C and 27.3°C [40]. High temperature particularly affects strawberry plants during their reproductive stages [41]. Furthermore, elevated temperatures have been linked to a reduction in pollen grains, adhesion, and germination. These results in fewer firm stigmas, causing many fertilization attempts to fail due to abnormal pathways taken by pollen tubes. Consequently, there is an increase in immature and unviable female gametes within the pistil, leading to poor pollen performance shortly after pollination [42]. In addition, a high root zone temperature of 30°C led to decreased oxygen consumption and root cell viability, causing most plants to wilt within two months [43] and reducing fruit size [41]. High-temperature stress also negatively affects photosynthesis and overall crop yield [44]. A previous study revealed that the average individual fruit weight and total fruit weight per plant decreased by 70%–80% at 30°C compared with those of plants grown at 25°C. Moreover, the fruits produced under high temperatures were irregularly shaped, small, and had less intense red coloration. However, these fruits presented significantly increased levels of pelargonidin-3-glucoside, which is the primary phenolic compound of strawberry [45].
3.3 Impacts of Heat Stress on Hormonal Dynamics
In strawberry plants, high-temperature stress triggers the production of heat shock proteins (HSPs), which play key roles in protecting plant cells from heat-induced damage. These proteins stabilize other proteins and cellular structures, reducing the impacts of heat stress [46]. These HSPs interact with plant hormones, particularly ABA, and influence their signaling pathways. This influence induces resistance in plants to survive under stress conditions [46]. Additionally, high temperature affects auxin, a hormone essential for cell elongation and division. Disruption of auxin transport and signaling can result in stunted growth and poor root development [47]. Auxins play a key role in fruit and flower development, so their alteration due to heat stress can lead to reduced flowering and fruit set, negatively impacting overall crop yield. Elevated temperatures are known to interfere with auxin-related processes, such as flowering time and fruit formation. Moreover, the level of ABA increases under heat stress. This hormone is crucial for plants to respond to water stress by promoting stomatal closure, thereby reducing water loss during heat stress. Despite its role in mitigating heat stress, higher levels of ABA hinder plant growth by limiting leaf expansion and root development, which can ultimately reduce the overall yield of strawberry plants. Additionally, high temperatures often lead to a decrease in cytokinin (CK) levels, which are important for cell division and delaying leaf aging. Lower CK levels can result in reduced shoot growth and impaired leaf function [48]. This decrease also affects photosynthesis, leading to reduced chlorophyll content and lower photosystem II efficiency under heat stress [49].
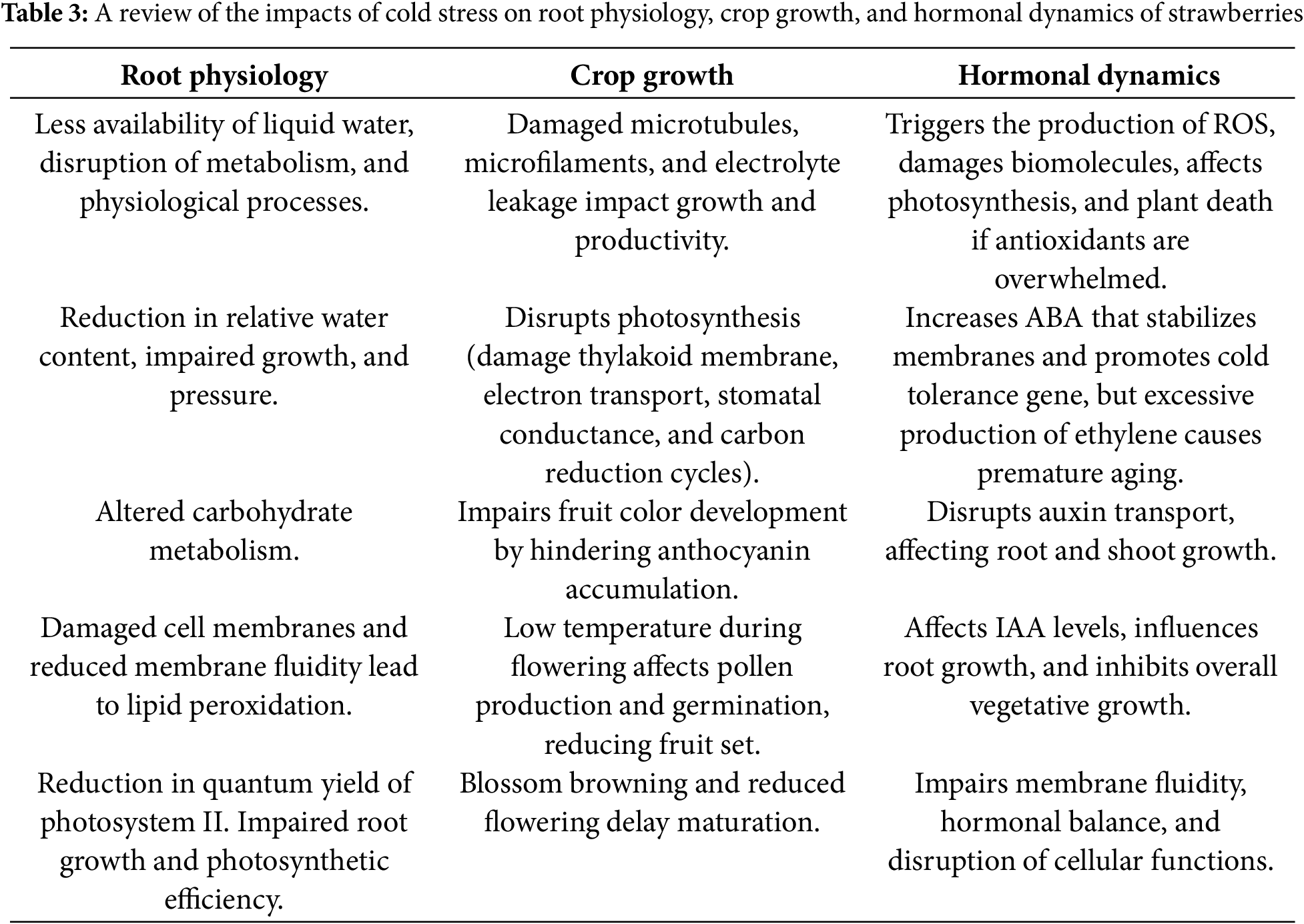
Strawberry seedlings grow best at temperatures between 15°C and 32°C, but temperatures below 10°C can significantly hinder their growth and development. Since strawberries often grow in single-slope greenhouses during the winter and spring, they are frequently exposed to low-temperature and low-light conditions, which negatively affect their growth and fruit quality. Both temperature and light are crucial environmental factors that influence plant growth and development [50], physiological properties, photosynthetic traits [51], and fruit quality [52]. A detailed overview of how low temperature affects the root physiology, crop growth, and hormonal dynamics of strawberry plants is presented in Table 3.
4.1 Impacts of Low-Temperature Stress on Root Physiology
Low-temperature stress affects plants in a way similar to drought, as freezing reduces the availability of liquid water [53], leading to disruptions in physiological processes, metabolism, and plant biology [38]. A study conducted by [54] demonstrated that cold stress reduces the relative water content in strawberry roots. Owing to this reduction, the plant fails to maintain its turgor pressure, cell expansion, and normal growth. This reduction is variable among strawberry cultivars because of genetic differences in terms of cold tolerance. Similarly, there is an alteration in carbohydrate metabolism. Few cultivars respond to cold stress with high levels of soluble carbohydrates. These carbohydrates are used by plants for energy purposes to perform metabolic functions at low temperatures. Similarly, another study conducted by [55] revealed that low temperature affects the structure and function of roots and their ability to take up nutrients, followed by reduced membrane fluidity, lipid peroxidation, and damage to the cell membrane. Additionally, it also results in a reduction in the maximum quantum yield of photosystem II. This reduction reduces photosynthetic efficiency and plant health. Refs. [39] and [38] confirmed that suboptimal temperatures (below 20°C/15°C) restrict root growth, leading to a decline in root biomass and viability.
4.2 Impacts of Low-Temperature Stress on Crop Growth
The exposure of strawberry seedlings to extremely low temperatures leads to significant damage, including depolymerization of microtubules and microfilaments and electrolyte leakage, which can have long-lasting impacts on growth and productivity [55]. It can disrupt photosynthesis by damaging key components, such as the thylakoid membrane, electron transport chain, stomatal conductance, and carbon reduction cycles. For example, stomatal closure after chilling might be a direct response to low temperatures impacting guard cells or an indirect result of increased intercellular CO2 due to the reduced activity of Rubisco [50]. Cold temperatures also interfere with the accumulation of anthocyanin, which is essential for fruit coloration, by hindering the activation of specific proteins under stress, resulting in poor color development [56]. During flowering, temperatures of approximately 8°C and 10°C can temporarily stop pollen production, as observed in the Chandler cultivar, leading to poor fruit set. Pollen germination rates also decrease at temperatures below 16°C, further affecting fertility [57]. Cold conditions during dormancy reduce the number of blossoms and cause the browning of crown tissues, which is crucial for plant growth. Moreover, exposure to freezing temperatures, such as −6°C, can severely limit flower emergence and leaf size [58]. Similarly, short-term extreme cold conditions delay fruit maturation [59]. Additionally, cold stress triggers the production of reactive oxygen species (ROS), which are harmful to biomolecules such as lipids, proteins, and nucleic acids [60]. To combat ROS, plants activate protective mechanisms via the production of antioxidant enzymes such as CAT, SOD, and POD. These enzymes help to manage ROS levels and prevent severe damage. However, if ROS production overwhelms a plant’s defenses, it can lead to irreversible damage, particularly to the photosynthetic machinery and the plant’s nuclear gene expression system [61,62].
4.3 Impacts of Low-Temperature Stress on Hormonal Dynamics
Low-temperature stress causes an increase in reactive oxygen species, which activate the plant’s antioxidant defense system. However, if cold stress becomes too intense, the defense system cannot effectively eliminate excess ROS, leading to severe damage or even plant death [63]. Moreover, it also results in impaired hormonal dynamics. It disrupts auxin transport and signaling pathways, which impairs root and shoot growth [48]. Additionally, cold stress triggers an increase in ABA, which helps plants tolerate cold conditions by stabilizing their cell membranes and regulating genes that respond to cold stress [64]. The expression of ethylene and the transcription factor ICE1 is also upregulated during cold stress, promoting the expression of cold tolerance-related genes. However, too much ethylene causes premature aging of the plant and deterioration of fruit quality. The hormone IAA is a key auxin for cellular growth that responds variably to low temperatures. In some cases, IAA levels increase, supporting root development, but overall vegetative growth may be inhibited as the plant focuses on stress responses [48]. Cold stress further leads to increased lipid peroxidation and reduced membrane fluidity, which impairs cellular functions. This damage is often intensified by hormonal imbalances, which affect a plant’s ability to maintain stability under stress [65].
Salinity is a major environmental stress that limits plant growth by causing imbalances in ions and water within plants, disrupting their metabolism, stability, and development [66]. According to an estimation, approximately one-third of the world’s agricultural land is affected by high salt levels [67]. All plants, particularly strawberry plants, are highly sensitive to salinity [68], with their ideal electrical conductivity ranging between 1 and 1.5 d. Excessive amounts of salts impair the metabolism of strawberry plants [69]. Additionally, when the temperature is high in the absence of rainfall, most of the soil water evaporates and leaves salts behind the soil surface, which leads to their increased concentration, which is referred to as secondary soil salinization. It limits crop growth and reduces yield. All these processes make it important to thoroughly understand the underlying molecular mechanism to makes strawberries resistant to salinity [70], which is summarized in Table 4.
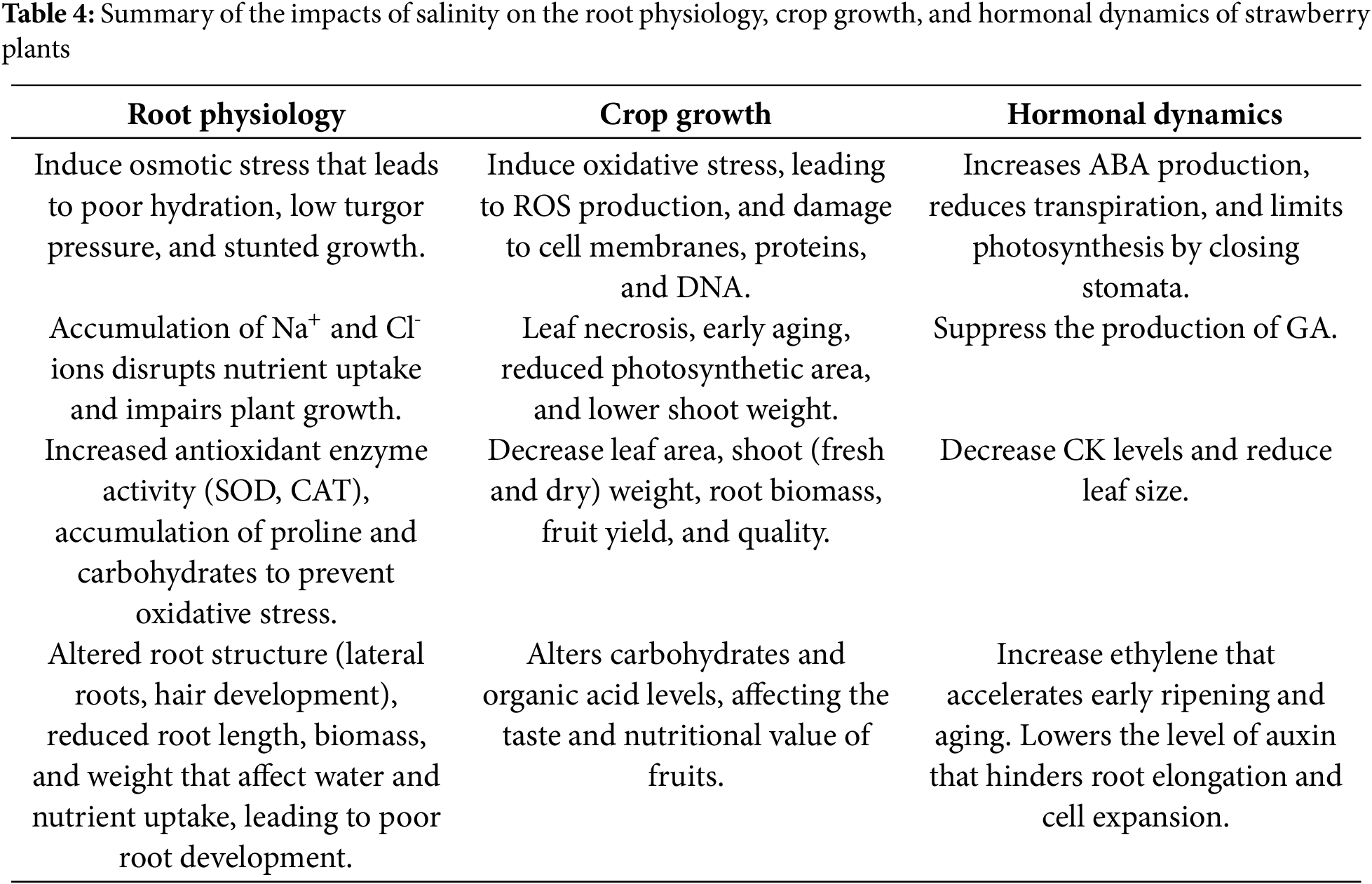
5.1 Impacts of Salinity on Root Physiology
An excessive amount of salt in the root zone leads to osmotic stress in plants. Under these stresses, maintaining plant integrity becomes difficult, resulting in poor hydration, low turgor pressure, stunted growth, and reduced root biomass. In addition, harmful ions such as sodium (Na+) and chloride (Cl-) accumulate. This accumulation prevents the plant from maintaining its nutritional balance. As a result, plants fail to take up essential nutrients such as potassium (K), nitrogen (N), and phosphorus (P), which reduces plant growth and development. In response to such types of oxidative stress, there is increased activity of antioxidant enzymes such as SOD and CAT. Their production protects plants from damage caused by oxidative stress [71]. Furthermore, they resist the accumulation of protective substances such as proline and soluble carbohydrates. They help maintain cellular integrity under saline conditions [71,72]. Moreover, certain changes in root structure, including altered lateral root formation and hair development, have been reported. It promotes the uptake of water and nutrients but also leads to deficiencies in the root system [69,71]. Similarly, reduced root length, biomass, and fresh weight hinder the water and nutrient uptake ability of plants [72,73].
5.2 Impacts of Salinity on Crop Growth
Strawberry plants are known to be highly sensitive to salt stress, which negatively impacts their growth and fruit production [73] and affects plants in three ways: osmotic stress, ionic stress, and oxidative stress. Among these, oxidative stress is considered to be the most harmful [74,75]. This leads to the development of harmful reactive oxygen species (ROS) in plant cells, which damage essential components such as membrane lipids, proteins, and DNA [76]. In strawberries, salt stress causes issues such as leaf necrosis, early leaf aging, and a reduction in the photosynthetic area [77]. Additionally, salinity also reduces the number and size of leaves, shoot weight, and number of branch crowns, all of which result in lower fruit yield [78]. Similarly, vegetative growth, leaf water content, chlorophyll levels, and fruit quality are severely affected by salt stress [79]. Important growth indicators, such as the leaf area, fresh and dry weights of plants above ground mass, and root biomass, decrease significantly under high salinity [72,73]. The decrease in water content due to salinity is crucial because of its ability to maintain turgor pressure. In a study conducted by [73], the Rociera cultivar presented a lower water content under high salinity than did Camarosa, which performed better at moderate salinity levels. Moreover, salinity also leads to reduced fruit yield, with reductions in total yield per plant of approximately 21.5% and 29.1% for Camarosa at medium and high salinities, respectively, and of 17.3% and 22.7% for Rociera under the same conditions [73,80]. To withstand salinity, strawberry plants trigger biochemical responses and the production of osmolytes, including proline and soluble carbohydrates. They maintain the cellular structure and integrity under stress conditions [81]. Moreover, there is an alteration in the levels of carbohydrates and organic acids, neglecting their taste and nutritional value [72,81]. In summary, strawberries are highly sensitive to saline soil, followed by wilting; nutritional imbalances; reductions in fruit yield and quality; toxicity from sodium and chlorine; and ultimately death under severe conditions [29].
5.3 Effects of Salinity on Hormonal Dynamics
The hormonal balance of strawberry plants is disrupted by salt stress, especially GA and ABA. A relatively high level of salinity leads to increased production of ABA. It lowers the rate of transpiration through the closure of stomata but also reduces gaseous exchange and photosynthetic activity. On the other hand, the production of GA (required for growth and development) is suppressed, resulting in stunted growth and fruit yield [82]. Additionally, the level of CK (essential for cell division and growth) decreases, which reduces leaf size and disrupts plant health [71,82]. Similarly, an increase in ethylene production causes early fruit ripening and aging, which decreases the texture, flavor, and quality of fruits. A relatively low level of auxins influences root elongation and cell expansion [73], and plants are unable to take up nutrients and water effectively [83].
In the past few years, interest in the use of microorganisms, including PGPR and AMF, as bioinoculants for the mitigation of abiotic stresses in plants has increased [84]. Their activities in soil improve the physical, biological, and chemical properties of the soil, which influences the growth and development of plants. All these factors lead to healthy plants and improved production. Additionally, associations of AMF and PGPR with roots help maintain a balanced ecosystem for plants that improve soil fertility and nutrient availability in the soil through the breakdown of organic compounds, the development of symbiotic relationships, production of iron carriers, and solubilization of minerals [85]. Moreover, they are also used as biofertilizers. They also play a major role in inducing stress tolerance, nutrient cycling, and disease suppression [86,87]. In addition, they provide strength to the defense system of plants during biotic and abiotic stresses, which helps them survive under these changing climatic conditions, highlighting their importance [88].
6.1 Promotion of Rhizobacteria and Abiotic Stress in Plant Growth
PGPR are beneficial microbes that are found in the areas around roots (rhizosphere), where they interact with roots for nutrient exchange [89]. Studies have revealed that several rhizospheric bacterial species have the potential to improve plant growth, fruit quality, and overall yield [90]. The most prominent strains from different genera are Pseudomonas, Azotobacter, Bacillus, Azospirillum, Erwinia, Serratia, Chromobacterium, Caulobacter, Flavobacterium, Agrobacterium, Arthrobacter, Burkholderia and Micrococcus [91]. Bacillus is well known for its ability to survive for longer periods in soil, even under severe environmental conditions [92]. Although the mechanism of plant growth promotion is unknown, these genes are considered to interact with roots directly or indirectly. According to a study by [92], PGPR stimulates growth by increasing systemic resistance to biotic stress through antibiosis (production of antibiotics) and by competing with harmful microbes for nutrients. Similarly, in a study conducted by [93], PGPR was found to mitigate the harmful effects of salinity on strawberry plants, ensuring that their growth and photosynthesis were not compromised by sodium chloride (NaCl) toxicity. Similarly, Ref. [94] found that Pseudomonas fluorescens (SBU4) and Pseudomonas glycinae (SDK8) are drought tolerant and possess multiple growth-promoting properties, making them suitable as bioinoculants to improve strawberry yield and quality, even during short-term drought stress. In another study, Ref. [95] demonstrated that plant PGPR, especially Pseudomonas and Bacillus, act as early root colonizers, promoting plant growth by increasing seed emergence, increasing plant weight, and improving crop yield. Therefore, we can conclude that although PGPR-based biofertilizers may not completely overcome the negative impacts of abiotic stresses, their influence is still significant, as they have the potential to improve strawberry growth. The detailed mechanism of the interplay of PGPRs in mitigating abiotic stresses is illustrated in Fig. 2.
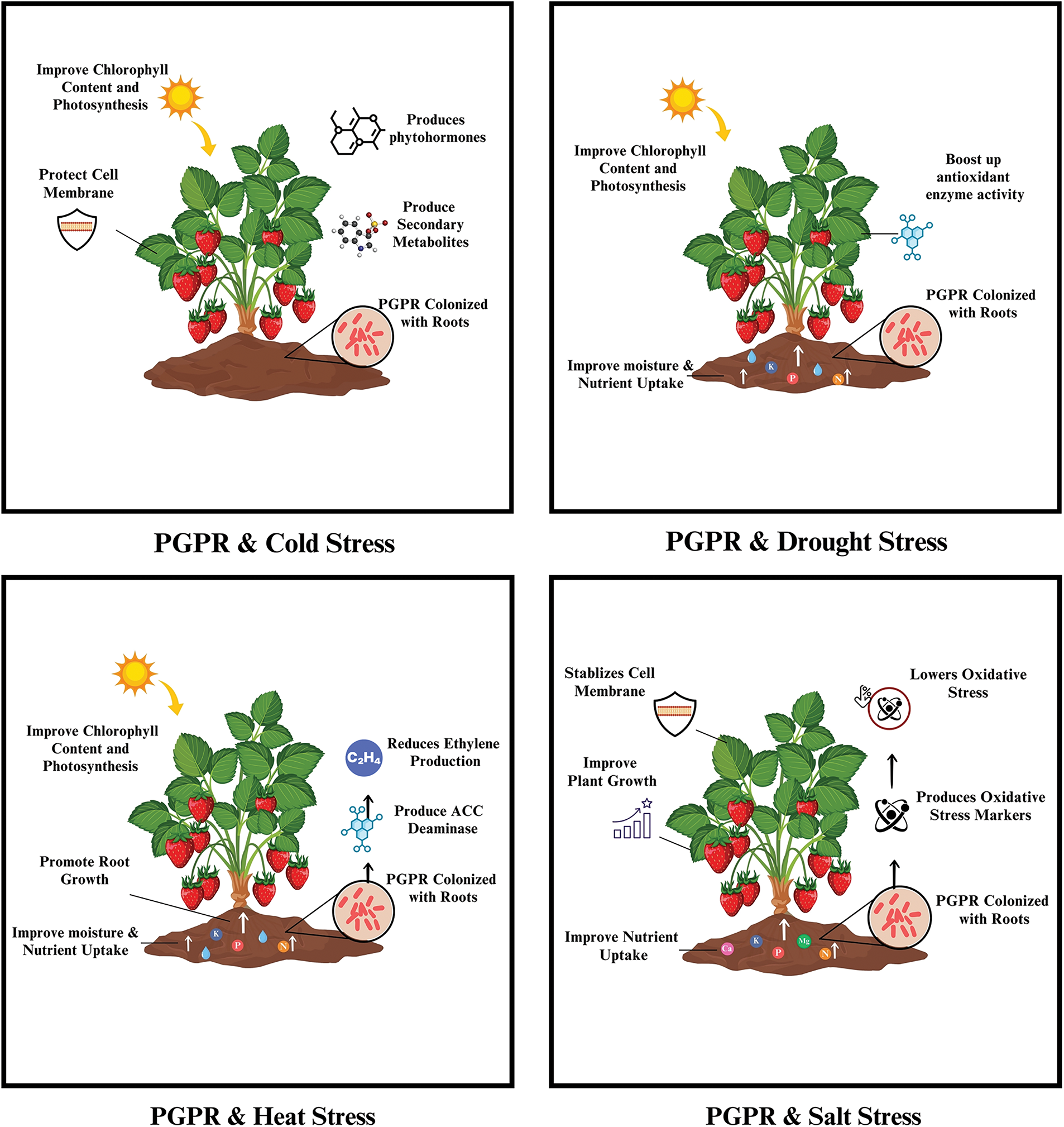
Figure 2: Role of PGPR in the mitigation of abiotic stress
PGPR mitigates cold stress in strawberry plants by increasing the production of beneficial compounds that help stabilize cellular structures under cold stress and promote the synthesis of phytohormones such as IAA. Moreover, PGPR-treated plants present increased production of secondary metabolites, including flavonoids and phenolic compounds, which protect the plants from pathogens during cold stress [96–98]. Additionally, PGPR improves physiological parameters in plants, including chlorophyll content and photosynthesis [97,99,100].
6.1.2 Temperature Stress Suppression
PGPR produces ACC deaminase, an enzyme that breaks down 1-aminocyclopropane-1-carboxylic acid (ACC), which is a precursor to ethylene. Ethylene is directly linked to stress responses in plants, especially under high temperatures. By reducing the level of ethylene, PGPR helps alleviate the negative effects of heat stress, supporting plant growth. Additionally, PGPR improves nutrient uptake under stressful conditions. Some PGPR strains increase root development and water retention, which is important for plants to address drought conditions, particularly during heat waves, when water availability decreases. PGPR also increases the chlorophyll content and improves the nutrient profile of strawberry plants, helping them maintain their photosynthetic efficiency under heat stress [98,101,102].
6.1.3 Salinity Stress Suppression
PGPR plays a key role in increasing the availability and uptake of essential nutrients such as nitrogen, phosphorus, potassium, calcium, and magnesium, even under saline conditions. This nutrient uptake is essential for maintaining plant health when plants are subjected to high salinity [99,103,104]. PGPR treatments reduce electrolyte leakage in plants under saline stress, which indicates better membrane stability and less cellular damage [100,105,106]. A study conducted by [101] revealed that plants treated with PGPR presented lower levels of oxidative stress markers, including malondialdehyde and hydrogen peroxide. Moreover, the growth and yield parameters were greater than those of the untreated plants.
6.1.4 Drought Stress Suppression
The inoculation of plant roots with PGPR increases the chlorophyll content and photosynthetic activity in leaves, root length, root surface area, and water and nutrient uptake during water shortage [97]. PGPR can increase the activity of antioxidant enzymes such as SOD, CAT, and POD [107–109]. These enzymes help mitigate the oxidative damage caused by drought stress, thereby protecting plant cells and enhancing overall plant health [102,110,111]. PGPR inoculation has been shown to maintain better water status in strawberry plants under drought conditions.
6.2 Arbuscular Mycorrhizal Fungi and Abiotic Stress Mitigation
AMF in association with roots plays a primary role in stress mitigation and nutrient and moisture uptake. The role of these genes in maintaining plant health is illustrated in Fig. 3. They capture essential nutrients and water from the soil through their mycelial network and transport them to plants side by side. This interaction of AMF with the host plant is considered a symbiotic relationship [103,112]. In addition, the colonization of plants by AMF alters stomatal behavior by increasing stomatal conductance up to 24% compared with that of non-colonized plants, which exhibit greater production [104,113,114].
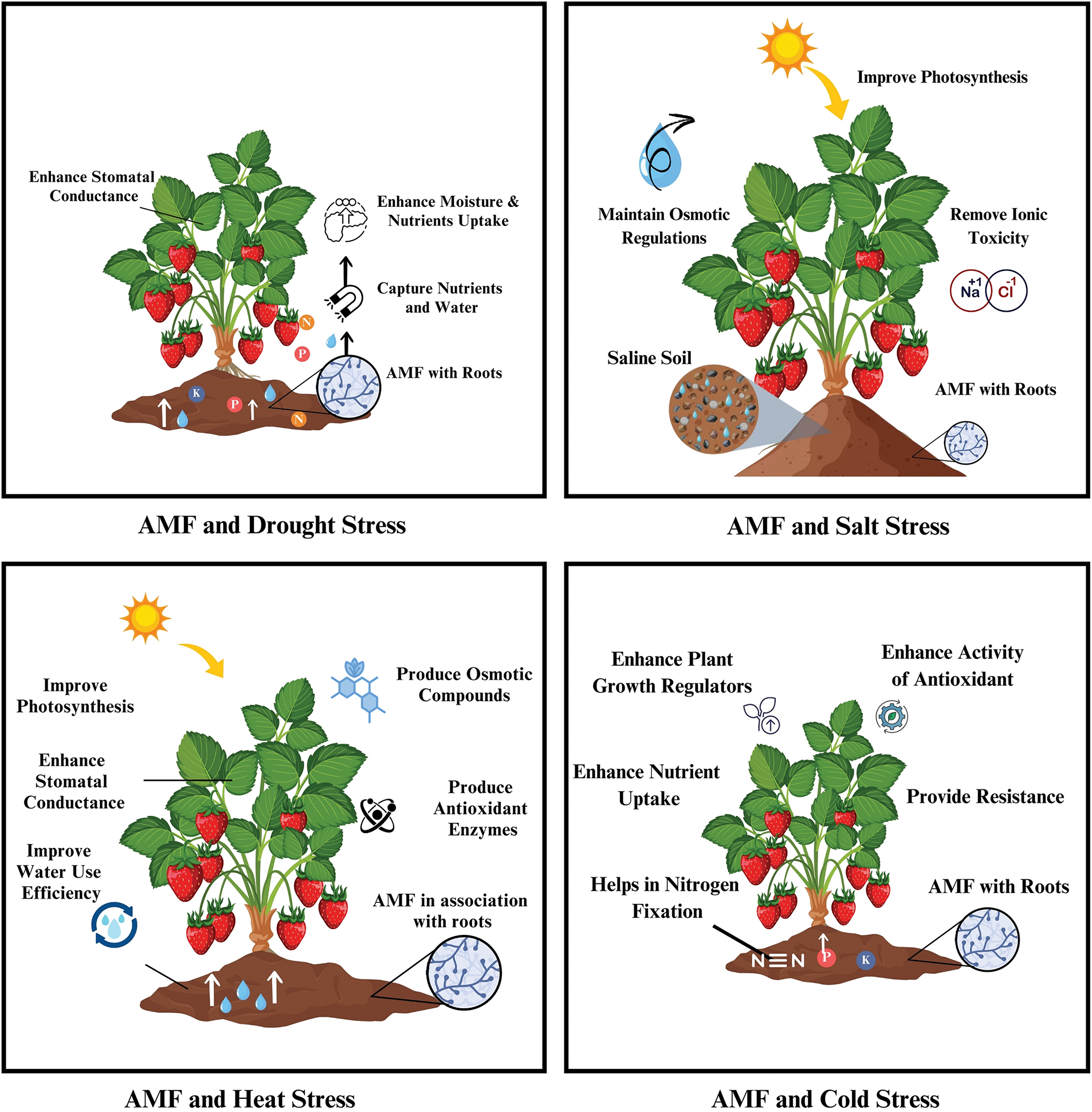
Figure 3: Role of AMF in the mitigation of abiotic stress
AMF, along with other rhizospheric microbes, develop resistance in plants to cold stress through the stimulation of processes such as increased nitrogen fixation, reduced ethylene production, and the production of plant growth regulators, including ABA, auxins, and GA [112–114]. Moreover, they increase the activity of antioxidant enzymes, nutrient uptake, and resistance to low temperatures [105,115–117]. In the face of heat stress, they improve plant biomass, relative water content, and water use efficiency; the production of antioxidant enzymes such as ascorbate POD, CAT, and SOD; and the synthesis of osmotic compounds such as trehalose, glomalin, and proline [118–120]. All these activities increase the rates of photosynthesis, chlorophyll content, stomatal conductance, and carotenoid content. The colonization of these plants improves nutrient assimilation and metabolism and promotes recovery from heat stress [106]. Similarly, AMF also helps mitigate saline stress via osmotic regulation and the maintenance of cellular integrity, turgor pressure, and cellular functions [120–122]. They prevent ionic toxicity by balancing the levels of sodium and potassium ions. All these factors lead to improved photosynthetic activity and water use efficiency and ultimately to healthy plants in saline soil [107].
Abiotic stresses significantly influence the root physiology, crop growth, and hormonal dynamics of strawberry plants. In this context, soil microbes act as primary supporters of plant growth, the most prominent of which are PGPR and AMF. They trigger root development, nutrient uptake, stress tolerance, plant growth, and the production of stress-responsive hormones such as ABA and JA. All these activities of beneficial soil microbes help mitigate environmental challenges. It can be concluded that the application of beneficial microbes can increase the production and stress tolerance of plants. Through a thorough understanding of this relationship, it is possible to develop, ecofriendly and innovative approaches for stress mitigation that will be able to minimize food security issues and maximize yield. The study highlights the potential of soil microbes, particularly plant growth-promoting rhizobacteria (PGPR) and mycorrhizal fungi, to mitigate the negative effects of abiotic stress on strawberry growth and root physiology. Their application in strawberry cultivation can enhance nutrient uptake, root architecture, and resilience to stressors like drought and salinity. Farmers are encouraged to adopt microbial inoculants as biofertilizers or biostimulants, which are eco-friendly alternatives to chemical inputs. Additionally, integrating these microbes with practices like organic mulching, optimized irrigation, and stress-tolerant strawberry varieties can further boost productivity under challenging environmental conditions. Field trials are recommended to fine-tune the dosages and combinations of microbial treatments for specific regions and stress scenarios to ensure maximum benefits and economic viability.
Acknowledgement: We acknowledge the use of ChatGPT (OpenAI, San Francisco, CA, USA) for improving the language clarity and readability of this manuscript.
Funding Statement: Not applicable.
Author Contributions: Hira Akhtar wrote the introduction and drought stress section, and Akhtar Hameed wrote the heat stress and drew figures. Rana Binyamin wrote the section heat stress and made tales. Kashif Riaz wrote the low-temperature stress section and made different tales, Hafiz Muhammad Usman Aslam wrote the salinity portion. Faizan Ali wrote the low-temperature stress section. Subhan Ali and Zuniara Akash wrote the section interplay of soil microbes. Muhammad Saqlain Zaheer and Kamran Ikram also wrote the section interplay of soil microbes and improved the technical and English language of the paper. Yasir Niaz and Hafiz Haider Ali wrote the section cold stress suppression and verified the data. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Declaration of Generative AI and AI-Assisted Technologies in the Writing Process: During the preparation of this manuscript the authors used Chat GPT and Grammarly in order to improved the technical and English language of the paper. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
References
1. Finn CE, Retamales JB, Lobos GA, Hancock JF. The chilean strawberry (Fragaria chiloensisover 1000 years of domestication. HortScience. 2013;48(4):418–21. doi:10.21273/HORTSCI.48.4.418. [Google Scholar] [CrossRef]
2. Lewers KS, Fleisher DH, Daughtry CS. Low tunnels as a strawberry breeding tool and season-extending production system. Int J Fruit Sci. 2017;17(3):233–58. doi:10.1080/15538362.2017.1305941. [Google Scholar] [CrossRef]
3. Carole D, Derricka M, Désiré M, Souaibou A, Célestine ME, Joël BN, et al. Strawberry (Fragaria spp.cultivation, production, consumption, and marketing in cameroon. Agric Sci. 2024;15:449–71. doi:10.4236/as.2024.154027. [Google Scholar] [CrossRef]
4. Hancock JF. Strawberries: crop production science in horticulture. Oxon: CABI Publishing; 1999. p. 109–12. [Google Scholar]
5. Zacharaki AK, Monaghan JM, Bromley JR, Vickers LH. Opportunities and challenges for strawberry cultivation in urban food production systems. Plants People Planet. 2024;6(3):611–21. doi:10.1002/ppp3.10475. [Google Scholar] [CrossRef]
6. Tulipani S, Marzban G, Herndl A, Laimer M, Mezzetti B, Battino M. Influence of environmental and genetic factors on health-related compounds in strawberry. Food Chem. 2011;124(3):906–13. doi:10.1016/j.foodchem.2010.07.018. [Google Scholar] [CrossRef]
7. Perin EC, Messias RD, Galli V, BorowskI JM, Souza ER, Avilao LO, et al. Mineral content and antioxidant compounds in strawberry fruit submitted to drough stress. J Food Sci Technol. 2019;39(1):245–54. doi:10.1590/fst.09717. [Google Scholar] [CrossRef]
8. Lakshmi MA, Sharm V, Singh RD. Medicinal properties of strawberry: a short review. Proc Chem Biol Med Sci. 2021;22(33–34):99–105. [Google Scholar]
9. Villamil-Galindo E, Van de Velde F, Piagentini AM. Strawberry agro-industrial by-products as a source of bioactive compounds: effect of cultivar on the phenolic profile and the antioxidant capacity. Bioresour Bioprocess. 2021;8(1):61. doi:10.1186/s40643-021-00416-z. [Google Scholar] [PubMed] [CrossRef]
10. Samtani JB, Rom CR, Friedrich H, Fennimore SA, Finn CE, Petran A, et al. The status and future of the strawberry industry in the United States. Horttechnology. 2019;29(1):11–24. doi:10.21273/HORTTECH04135-18. [Google Scholar] [CrossRef]
11. Ngouana LS, Tonfack LB, Temegne CN, Agendia AP, Youmbi E. Current status of strawberry (Fragaria spp.) cultivation and marketing in Cameroon. J Agric Res. 2023 Dec 1;14:100761. doi:10.1016/j.jafr.2023.100761. [Google Scholar] [CrossRef]
12. Hernandez-Martinez NR, Blanchard C, Wells D, Salazar-Gutiérrez MR. Current state and future perspectives of commercial strawberry production: a review. Sci Hortic. 2023;312(10):111893. doi:10.1016/j.scienta.2023.111893. [Google Scholar] [CrossRef]
13. Scherr KD, Jamieson MA. Abiotic and biotic drivers of strawberry productivity across a rural urban gradient. BAAE. 2021;57(3):65–77. doi:10.1016/j.baae.2021.09.007. [Google Scholar] [CrossRef]
14. Al-Khayri JM, Rashmi R, Surya Ulhas R, Sudheer WN, Banadka A, Nagella P, et al. The role of nanoparticles in response of plants to abiotic stress at physiological, biochemical, and molecular levels. Plants J. 2023 Jan 7;12(2):292. doi:10.3390/plants12020292. [Google Scholar] [PubMed] [CrossRef]
15. Liang J, Zheng J, Wu Z, Wang H. Strawberry FaNAC2 it enhances tolerance to abiotic stress by regulating proline metabolism. Plants J. 2020;9(11):1417. doi:10.3390/plants9111417. [Google Scholar] [PubMed] [CrossRef]
16. Rathod A, Verma NS. Impact of abiotic stress on agronomical crops. In: Frontiers of agronomy. Rohini, ND, USA: Elite Publishing House; 2023. 27 p. [Google Scholar]
17. Zahedi SM, Hosseini MS, Fahadi Hoveizeh N, Kadkhodaei S, Vaculík M. Physiological and biochemical responses of commercial strawberry cultivars under optimal and drought stress conditions. Plants J. 2023;12(3):496. doi:10.3390/plants12030496. [Google Scholar] [PubMed] [CrossRef]
18. Adak N, Gubbuk H, Tetik N. Yield, quality and biochemical properties of various strawberry cultivars under water stress. J Sci Food Agric. 2018;98(1):304–11. doi:10.1002/jsfa.8471. [Google Scholar] [PubMed] [CrossRef]
19. Hosseini MS, Samsampour D, Zahedi SM, Zamanian K, Rahman MM, Mostofa MG, et al. Melatonin alleviates drought impact on growth and essential oil yield of lemon verbena by enhancing antioxidant responses, mineral balance, and abscisic acid content. Physiol Plant. 2021;172(2):1363–75. doi:10.1111/ppl.13335. [Google Scholar] [PubMed] [CrossRef]
20. Morales-Quintana L, Moya M, Santelices-Moya R, Cabrera-Ariza A, Rabert C, Pollmann S, et al. Improvement in the physiological and biochemical performance of strawberries under drought stress through symbiosis with Antarctic fungal endophytes. Front Microbiol. 2022;13:939955. doi:10.3389/fmicb.2022.939955. [Google Scholar] [PubMed] [CrossRef]
21. Zahedi SM, Moharrami F, Sarikhani S, Padervand M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci Rep. 2020;10(1):17672. doi:10.1038/s41598-020-74273-9. [Google Scholar] [PubMed] [CrossRef]
22. Bhusal N, Han SG, Yoon TM. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci Hortic. 2019;246:535–43. doi:10.1016/j.scienta.2018.11.021. [Google Scholar] [CrossRef]
23. Elkeilsh A, Awad YM, Soliman MH, Abu-Elsaoud A, Abdelhamid MT, El-Metwally IM. Exogenous application of β-sitosterol mediated growth and yield improvement in water-stressed wheat (Triticum aestivum) involves up-regulated antioxidant system. J Plant Res. 2019;132:881–901. doi:10.1007/s10265-019-01143-5. [Google Scholar] [PubMed] [CrossRef]
24. Klamkowski K, Treder W. Response to drought stress of three strawberry cultivars grown under greenhouse conditions. J Fruit Ornam Plant Res. 2008;16(16):179–88. [Google Scholar]
25. Passioura JB, Condon AG, Richards RA. Water deficits, the development of leaf area and crop productivity. In: Smith JAC, Griffiths H, editors. Water deficits: plant responses from cell to community. Oxford: BIOS Scientific Publishers; 2000. p. 253–64. [Google Scholar]
26. Boyer JS. Plant productivity and environment. Science. 1982;218(4571):443–8. doi:10.1126/science.218.4571.443. [Google Scholar] [PubMed] [CrossRef]
27. Gehrmann H. Growth, yield, and fruit quality of strawberries as affected by water supply. Acta Hortic. 1984;171:463–9. [Google Scholar]
28. Nair AS, Abraham TK, Jaya DS. Studies on the changes in lipid peroxidation and antioxidants in drought stress induced cowpea (Vigna unguiculata L.) varieties. J Environ Biol. 2008;29(5):689–91. [Google Scholar] [PubMed]
29. Sun Y, Niu G, Wallace R, Masabni J, Gu M. Relative salt tolerance of seven strawberry cultivars. Horticulture. 2015;1(1):27–43. doi:10.3390/horticulturae1010027. [Google Scholar] [CrossRef]
30. El-Beltagi HS, Ismail SA, Ibrahim NM, Shehata WF, Alkhateeb AA, Ghazzawy HS, et al. Unravelling the effect of triacontanol in combating drought stress by improving growth, productivity, and physiological performance in strawberry plants. Plants J. 2022;11(15):1913. doi:10.3390/plants11151913. [Google Scholar] [PubMed] [CrossRef]
31. Jiang L, Song R, Wang X, Wang J, Wu C. Transcriptomic and metabolomic analyses provide new insights into the response of strawberry (Fragaria× ananassa Duch.) to drought stress. Horticulture. 2024;10(7):734. doi:10.3390/horticulturae10070734. [Google Scholar] [CrossRef]
32. Malhi GS, Kaur M, Kaushik P. Impact of climate change on agriculture and its mitigation strategies: a review. Sustainability. 2021;13(3):1318. doi:10.3390/su13031318. [Google Scholar] [CrossRef]
33. Huang J, Li Q, Song Z. Historical global land surface air apparent temperature and its future changes based on CMIP6 projections. Sci Total Environ. 2022;816:151656. doi:10.1016/j.scitotenv.2021.151656. [Google Scholar] [PubMed] [CrossRef]
34. Dos Santos TB, Ribas AF, de Souza SG, Budzinski IG, Domingues DS. Physiological responses to drought, salinity, and heat stress in plants: a review. Stresses. 2022 Feb 16;2(1):113–35. doi:10.3390/stresses2010009. [Google Scholar] [CrossRef]
35. Sharma M, Negi S, Kumar P, Srivastava DK, Choudhary MK, Irfan M. Fruit ripening under heat stress: the intriguing role of ethylene-mediated signaling. Plant Sci J. 2023;335:111820. doi:10.1016/j.plantsci.2023.111820. [Google Scholar] [PubMed] [CrossRef]
36. Masouleh SS, Sassine YN. Molecular and biochemical responses of horticultural plants and crops to heat stress. Ornam Hortic. 2020;26(2):148–58. doi:10.1016/j.plantsci.2023.111820. [Google Scholar] [CrossRef]
37. Nadeem M, Li J, Wang M, Shah L, Lu S, Wang X, et al. Unraveling field crops sensitivity to heat stress: mechanisms, approaches, and future prospects. J Agron. 2018;8(7):128. doi:10.3390/agronomy8070128. [Google Scholar] [CrossRef]
38. Kadir S, Sidhu G, Al-Khatib K. Strawberry (Fragaria xananassa Duch.) growth and productivity as affected by temperature. HortScience. 2006;41(6):1423. [Google Scholar]
39. Menzel CM. A review of fruit development in strawberry: high temperatures accelerate flower development and decrease the size of the flowers and fruit. J Horticultur Sci Biotechnol. 2023;98(4):409–31. doi:10.1080/14620316.2023.2166599. [Google Scholar] [CrossRef]
40. Menzel CM. Temperatures above 30°C decrease leaf growth in strawberry under global warming. J Horticultur Sci Biotechnol. 2024;7:1–24. [Google Scholar]
41. Ledesma NA, Nakata M, Sugiyama N. Effect of high temperature stress on the reproductive growth of strawberry cvs.‘Nyoho’ and ‘Toyonoka’. Sci Hortic. 2008;116(2):186–93. doi:10.1016/j.scienta.2007.12.010. [Google Scholar] [CrossRef]
42. Zini LM, Galati BG, Carrera CS. High temperatures during late floral bud stages decrease fertilization in strawberry (Fragaria × ananassapollen-pistil interaction and anatomical evidences. Plant Biosyst. 2023;157(2):367–78. doi:10.1080/11263504.2023.2165566. [Google Scholar] [CrossRef]
43. Dash PK, Chase CA, Agehara S, Zotarelli L. Alleviating heat stress during early-season establishment of containerized strawberry transplants. J Berry Res. 2022;12(1):19–40. doi:10.3233/JBR-210702. [Google Scholar] [CrossRef]
44. Balasooriya HN, Dassanayake KB, Seneweera S, Ajlouni S. Interaction of elevated carbon dioxide and temperature on strawberry (Fragaria × ananassa) growth and fruit yield. Int J Agricul Biosyst Eng. 2018;12(9):279–87. [Google Scholar]
45. Balasooriya BLHN, Dassanayake K, Ajlouni S. High temperature effects on strawberry fruit quality and antioxidant contents. Acta Hortic. 2020;1278:225–34. [Google Scholar]
46. Ullah I, Toor MD, Yerlikaya BA, Mohamed HI, Yerlikaya S, Basit A, et al. High-temperature stress in strawberry: understanding physiological, biochemical and molecular responses. Planta. 2024;260(5):118. doi:10.1007/s00425-024-04544-6. [Google Scholar] [PubMed] [CrossRef]
47. Bhattacharya A. Physiological processes in plants under low temperature stress. Singapore: Springer; 2022. 734 p. [Google Scholar]
48. Lv J, Zheng T, Song Z, Pervaiz T, Dong T, Zhang Y, et al. Strawberry proteome responses to controlled hot and cold stress partly mimic post-harvest storage temperature effects on fruit quality. Front Nutr. 2022;8:812666. doi:10.3389/fnut.2021.812666. [Google Scholar] [PubMed] [CrossRef]
49. Manafi H, Baninasab B, Gholami M, Talebi M, Khanizadeh S. Exogenous melatonin alleviates heat-induced oxidative damage in strawberry (Fragaria × ananassa Duch. cv. Ventana) Plant. J Plant Growth Regul. 2022;41:52–64. doi:10.1007/s00344-020-10279-x. [Google Scholar] [CrossRef]
50. Allen DJ, Ort DR. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001;6(1):36–42. doi:10.1016/S1360-1385(00)01808-2. [Google Scholar] [PubMed] [CrossRef]
51. Singh SP, Singh P. Effect of temperature and light on the growth of algae species: a review. Renew Sustain Energy Rev. 2015;50:431–44. doi:10.1016/j.rser.2015.05.024. [Google Scholar] [CrossRef]
52. Li H, Li T, Gordon RJ, Asiedu SK, Hu K. Strawberry plant fruiting efficiency and its correlation with solar irradiance, temperature and reflectance water index variation. Environ Exp Bot. 2010;68(2):165–74. doi:10.1016/j.envexpbot.2009.12.001. [Google Scholar] [CrossRef]
53. Zhang Y, Tang HR, Luo Y, Hou YX. Responses of antioxidant enzymes and compounds in strawberry (Fragaria × ananassa ‘Toyonaka’) to cold stress. New Zeal J Crop Hort. 2009;37(4):383–90. doi:10.1080/01140671.2009.9687594. [Google Scholar] [CrossRef]
54. Zareei E, Karami F, Gholami M, Ershadi A, Avestan S, Aryal R, et al. Physiological and biochemical responses of strawberry crown and leaf tissues to freezing stress. BMC Plant Biol. 2021;21:532. doi:10.1186/s12870-021-03300-2. [Google Scholar] [PubMed] [CrossRef]
55. Jiang N, Yang Z, Zhang H, Xu J, Li C. Effect of low temperature on photosynthetic physiological activity of different photoperiod types of strawberry seedlings and stress diagnosis. Agronomy. 2023;13(5):1321. doi:10.3390/agronomy13051321. [Google Scholar] [CrossRef]
56. Mao W, Han Y, Chen Y, Sun M, Feng Q, Li L, et al. Low temperature inhibits anthocyanin accumulation in strawberry fruit by activating FvMAPK3-induced phosphorylation of FvMYB10 and degradation of Chalcone Synthase 1. Plant Cell. 2022;34(4):1226–49. doi:10.1093/plcell/koac006. [Google Scholar] [PubMed] [CrossRef]
57. Risser G. Effect of low temperatures on pollen production and germination in strawberry. Acta Hortic. 1996;439:651–8. doi:10.17660/ActaHortic.1997.439.109. [Google Scholar] [CrossRef]
58. Marini RP, Boyce BR. Influence of low temperatures during dormancy on growth and development of Catskill’ strawberry plants. J Amer Soc Hort Sci. 1997;104:159–62. [Google Scholar]
59. Cui M, Pham MD, Hwang H, Chun C. Flower development and fruit malformation in strawberries after short-term exposure to high or low temperature. Sci Hortic. 2021;288:110308. doi:10.1016/j.scienta.2021.110308. [Google Scholar] [CrossRef]
60. Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch. 2010;48(12):909–30. doi:10.1016/j.plaphy.2010.08.016. [Google Scholar] [PubMed] [CrossRef]
61. Xu PL, Guo YK, Bai JG, Shang L, Wang XJ. Effects of long-term chilling on ultrastructure and antioxidant activity in leaves of two cucumber cultivars under low light. Physiol Plant. 2008;132(4):467–78. doi:10.1111/j.1399-3054.2007.01036.x. [Google Scholar] [PubMed] [CrossRef]
62. Suzuki N, Koussevitzky SH, Mittler RO, Miller GA. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012;35(2):259–70. doi:10.1111/j.1365-3040.2011.02336.x. [Google Scholar] [PubMed] [CrossRef]
63. Yong Z, Tang H-R, Luo Y. Variation in antioxidant enzyme activities of two strawberry cultivars with short-term low temperature stress. World J Agric Sci. 2008;4(4):458–62. [Google Scholar]
64. Cao K, Zhang Z, Fan H, Tan Y, Xu H, Zhou X. Comparative transcriptomic analysis reveals gene expression in response to cold stress in Rhododendron aureum Georgi. Theor Exp Plant Physiol. 2022;34(3):347–66. doi:10.1007/s40626-022-00248-y. [Google Scholar] [CrossRef]
65. Roussos PA, Ntanos E, Tsafouros A, Denaxa NK. Strawberry physiological and biochemical responses to chilling and freezing stress and application of alleviating factors as countermeasures. J Berry Res. 2020;10(3):437–57. doi:10.3233/JBR-190494. [Google Scholar] [CrossRef]
66. Balasubramaniam T, Shen G, Esmaeili N, Zhang H. Plants’ response mechanisms to salinity stress. Plants. 2023;12(12):2253. doi:10.3390/plants12122253. [Google Scholar] [PubMed] [CrossRef]
67. Hossain A, Krupnik TJ, Timsina J, Mahboob MG, Chaki AK, Farooq M, et al. Agricultural land degradation: processes and problems undermining future food security. In: Pessarakli M, editor. Environment, climate, plant and vegetation growth. Cham: Springer International Publishing; 2020. p. 17–61. [Google Scholar]
68. Crizel RL, Perin EC, Siebeneichler TJ, Borowski JM, Messias RS, Rombaldi CV, et al. Abscisic acid and stress induced by salt: effect on the phenylpropanoid, L-ascorbic acid and abscisic acid metabolism of strawberry fruits. Plant Physiol Biochem. 2020;152(30):211–20. doi:10.1016/j.plaphy.2020.05.003. [Google Scholar] [PubMed] [CrossRef]
69. Medrano Macias J, Lopez Caltzontzit MG, Rivas Martinez EN, Narvaez Ortiz WA, Benavides Mendoza A, Martinez Lagunes P. Enhancement to salt stress tolerance in strawberry plants by iodine products application. Agronomy. 2021;11(3):602. doi:10.3390/agronomy11030602. [Google Scholar] [CrossRef]
70. Amil-Ruiz F, Blanco-Portales R, Munoz-Blanco J, Caballero JL. The strawberry plant defense mechanism: a molecular review. Plant Cell Physiol. 2011;52(11):873–903. doi:10.1093/pcp/pcr136. [Google Scholar] [PubMed] [CrossRef]
71. Zeid IM, Mohamed FH, Metwali EM. Responses of two strawberry cultivars to NaCl-induced salt stress under the influence of ZnO nanoparticles. Saudi J Biol Sci. 2023;30(4):103623. doi:10.1016/j.sjbs.2023.103623. [Google Scholar] [PubMed] [CrossRef]
72. Ntanos E, Kekelis P, Assimakopoulou A, Gasparatos D, Denaxa NK, Tsafouros A, et al. Amelioration effects against salinity stress in strawberry by bentonite-zeolite mixture, glycine betaine, and Bacillus amyloliquefaciens in terms of plant growth, nutrient content, soil properties, yield, and fruit quality characteristics. Appl Sci. 2021;11(19):8796. doi:10.3390/app11198796. [Google Scholar] [CrossRef]
73. Denaxa NK, Nomikou A, Malamos N, Liveri E, Roussos PA, Papasotiropoulos V. Salinity effect on plant growth parameters and fruit bioactive compounds of two strawberry cultivars, coupled with environmental conditions monitoring. Agronomy. 2022;12(10):2279. doi:10.3390/agronomy12102279. [Google Scholar] [CrossRef]
74. Tanveer M, Shabala S. Targeting redox regulatory mechanisms for salinity stress tolerance in crops. In: Kumar V, Wani S, Suprasanna P, Tran LS, editors. Salinity responses and tolerance in plants. 1st ed. 2018; Cham: Springer. Vol. 1. p. 213–34. [Google Scholar]
75. Khan WU, Tanveer M, Shaukat R, Ali M, Pirdad F. An overview of salinity tolerance mechanism in plants. In: Ahmad P, editor. Salt and drought stress tolerance in plants: signaling networks and adaptive mechanisms. Cham: Springer; 2020. p. 1–6. [Google Scholar]
76. Kalia R, Sareen S, Nagpal A, Katnoria J, Bhardwaj R. ROS-induced transcription factors during oxidative stress in plants: a tabulated review. In: Sharma P, Dubey RS, editors. Reactive oxygen species and antioxidant systems in plants: role and regulation under abiotic stress. 1st ed.Cham: Springer; 2017. p. 129–58. [Google Scholar]
77. Ondrasek G, Rengel Z, Maurović N, Kondres N, Filipović V, Savić R, et al. Growth and element uptake by salt-sensitive crops under combined NaCl and Cd stresses. Plants. 2021;10(6):1202. doi:10.3390/plants10061202. [Google Scholar] [PubMed] [CrossRef]
78. Haque MA, Sakimin SZ. Planting arrangement and effects of planting density on tropical fruit crops-A Review. Horticulturae. 2022;8(6):485. doi:10.3390/horticulturae8060485. [Google Scholar] [CrossRef]
79. Garriga M, Muñoz CA, Caligari PD, Retamales JB. Effect of salt stress on genotypes of commercial (Fragaria x ananassa) and Chilean strawberry (F. chiloensis). Sci Hortic. 2015;195:37–47. doi:10.1016/j.scienta.2015.08.036. [Google Scholar] [CrossRef]
80. Keutgen AJ, Keutgen N. Influence of NaCl salinity stress on fruit quality in strawberry. In: Proceedings of the International Symposium on Managing Greenhouse Crops in Saline Environment; 2003 Jul 9; Israel. Leuven, Belgium: International Society for Horticultural Science; 2003. p. 155–7. [Google Scholar]
81. Zahedi SM, Abdelrahman M, Hosseini MS, Hoveizeh NF, Tran LS. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ Pollut. 2019;253:246–58. doi:10.1016/j.envpol.2019.04.078. [Google Scholar] [PubMed] [CrossRef]
82. Atta K, Mondal S, Gorai S, Singh AP, Kumari A, Ghosh T, et al. Impacts of salinity stress on crop plants: improving salt tolerance through genetic and molecular dissection. Front Plant Sci. 2023;14:1241736. doi:10.3389/fpls.2023.1241736. [Google Scholar] [PubMed] [CrossRef]
83. Li S, Chang L, Sun R, Dong J, Zhong C, Gao Y, et al. Combined transcriptomic and metabolomic analysis reveals a role for adenosine triphosphate-binding cassette transporters and cell wall remodeling in response to salt stress in strawberry. Front Plant Sci. 2022;13:996765. doi:10.3389/fpls.2022.996765. [Google Scholar] [PubMed] [CrossRef]
84. Gamalero E, Glick BR. Recent advances in bacterial amelioration of plant drought and salt stress. J Biol. 2022 Mar 12;11(3):437. doi:10.3390/biology11030437. [Google Scholar] [PubMed] [CrossRef]
85. Chamberlain LA, Whitman T, Ané JM, Diallo T, Gaska JM, Lauer JG, et al. Corn-soybean rotation, tillage, and foliar fungicides: impacts on yield and soil fungi. Field Crops Res. 2021;262:108030. doi:10.1016/j.fcr.2020.108030. [Google Scholar] [CrossRef]
86. Marco S, Loredana M, Riccardo V, Raffaella B, Walter C, Luca N. Microbe-assisted crop improvement: a sustainable weapon to restore holobiont functionality and resilience. Hortic Res. 2022;9:160. doi:10.1093/hr/uhac160. [Google Scholar] [PubMed] [CrossRef]
87. Gupta R, Anand G, Gaur R, Yadav D. Plant-microbiome interactions for sustainable agriculture: a review. Physiol Mol Biol Plants. 2021;27(1):165–79. doi:10.1007/s12298-021-00927-1. [Google Scholar] [PubMed] [CrossRef]
88. Jung SC, Martinez-Medina A, Lopez-Raez JA, Pozo MJ. Mycorrhiza-induced resistance and priming of plant defenses. J Chem Ecol. 2012;38(6):651–64. doi:10.1007/s10886-012-0134-6. [Google Scholar] [PubMed] [CrossRef]
89. Deng S, Wipf HM, Pierroz G, Raab TK, Khanna R, Coleman-Derr D. A plant growth-promoting microbial soil amendment dynamically alters the strawberry root bacterial microbiome. Sci Rep. 2019;9(1):17677. doi:10.1038/s41598-019-53623-2. [Google Scholar] [PubMed] [CrossRef]
90. Lazcano C, Boyd E, Holmes G, Hewavitharana S, Pasulka A, Ivors K. The rhizosphere microbiome plays a role in the resistance to soil-borne pathogens and nutrient uptake of strawberry cultivars under field conditions. Sci Rep. 2021;11(1):3188. doi:10.1038/s41598-021-82768-2. [Google Scholar] [PubMed] [CrossRef]
91. Verma M, Mishra J, Arora NK. Plant growth-promoting rhizobacteria: diversity and applications. Environ Biotechnol. 2019;2019:129–73. doi:10.1007/978-981-10-7284-0. [Google Scholar] [CrossRef]
92. Hashem A, Tabassum B, Abd_Allah EF. Bacillus subtilis: a plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J Biol Sci. 2019;26(6):1291–7. doi:10.1016/j.sjbs.2019.05.004. [Google Scholar] [PubMed] [CrossRef]
93. de Souza Ribeiro M, Rodrigues FA, De Araujo RC, Nadal MC, Andrade GV, Pasqual M, et al. Growth-promoting bacteria induce salt stress tolerance in strawberry plants. J Plant Growth Regul. 2023;42(12):7606–13. doi:10.1007/s00344-023-11036-6. [Google Scholar] [CrossRef]
94. Dhiman VK. Characterization of drought tolerant plant growth promoting rhizobacteria and their evaluation on strawberry (Fragaria × ananassa Duch.) [Ph.D. thesis]. Nauni, Solan, India: University of Horticultural Sciences; 2020. [Google Scholar]
95. Goyal R, Sindhu S, Godara A. Effect of rhizobacterium on growth, yield and quality of Strawberry. Indian J Ecol. 2020;47(1):92–5. [Google Scholar]
96. Hosseini A, Hosseini M, Schausberger P. Plant growth-promoting rhizobacteria enhance defense of strawberry plants against spider mites. Front Plant Sci. 2022;12:783578. doi:10.3389/fpls.2021.783578. [Google Scholar] [PubMed] [CrossRef]
97. Raturi P, Rai R, Sharma AK, Singh AK, Dimri DC, Bains G. Effects of plant growth-promoting rhizobacteria (PGPR) and arbuscular mycorrhizal fungi (AMF) on morpho-physiological parameters of strawberry cv. chandler under different moisture levels. Int J Environ Clim. 2023;13(9):2707–13. doi:10.9734/ijecc/2023/v13i92502. [Google Scholar] [CrossRef]
98. Paliwoda D, Mikiciuk G, Chudecka J, Tomaszewicz T, Miller T, Mikiciuk M, et al. Effects of inoculation with plant growth-promoting rhizobacteria on chemical composition of the substrate and nutrient content in strawberry plants growing in different water conditions. Agric J. 2023;14(1):46. doi:10.3390/agriculture14010046. [Google Scholar] [CrossRef]
99. Yavuz A, Erdogan U, Turan M, Argın S, Kocaman A. Synergistic strategies for overcoming salt stress in strawberry farming: the use of organic fertilizers and plant growth promoting rhizobacteria (PGPR). Appl Fruit Sci. 2024;66(5):1787–97. doi:10.1007/s10341-024-01169-7. [Google Scholar] [CrossRef]
100. Garza-Alonso CA, Olivares-Sáenz E, González-Morales S, Cabrera-De la Fuente M, Juárez-Maldonado A, González-Fuentes JA, et al. Strawberry biostimulation: from mechanisms of action to plant growth and fruit quality. Plants. 2022;11(24):3463. doi:10.3390/plants11243463. [Google Scholar] [PubMed] [CrossRef]
101. Kul R. Integrated application of plant growth promoting rhizobacteria and biochar improves salt tolerance in eggplant seedlings. Turk J Agric. 2022;46(5):677–702. doi:10.55730/1300-011X.3035. [Google Scholar] [CrossRef]
102. Paliwoda D, Mikiciuk G, Mikiciuk M, Kisiel A, Sas-Paszt L, Miller T. Effects of rhizosphere bacteria on strawberry plants (Fragaria × ananassa Duch.) under water deficit. Int J Mol Sci. 2022;23(18):10449. doi:10.3390/ijms231810449. [Google Scholar] [PubMed] [CrossRef]
103. Kakouridis A, Hagen JA, Kan MP, Mambelli S, Feldman LJ, Herman DJ, et al. Routes to roots: direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. New Phytol. 2022;236(1):210–21. doi:10.1111/nph.18281. [Google Scholar] [PubMed] [CrossRef]
104. Augé RM. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza. 2001;11(1):3–42. doi:10.1007/s005720100097. [Google Scholar] [CrossRef]
105. Kushwaha P, Kashyap PL, Kuppusamy P. Microbes for cold stress resistance in plants: mechanism, opportunities, and challenges. In: Yadav S, editor. Microbiological advancements for higher altitude agro-ecosystems & sustainability. Cham: Springer; 2020. p. 269–92. [Google Scholar]
106. Duc NH, Csintalan Z, Posta K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol Bioch. 2018;132:297–307. doi:10.1016/j.plaphy.2018.09.011. [Google Scholar] [PubMed] [CrossRef]
107. Zong J, Zhang Z, Huang P, Yang Y. Arbuscular mycorrhizal fungi alleviates salt stress in Xanthoceras sorbifolium through improved osmotic tolerance, antioxidant activity, and photosynthesis. fmicb. 2023 Mar 16;14:1138771. doi:10.3389/fmicb.2023.1138771. [Google Scholar] [PubMed] [CrossRef]
108. Yang X, Wang J, Xia X, Zhang Z, He J, Nong B, et al. OsTTG1, a WD40 repeat gene, regulates anthocyanin biosynthesis in rice. Plant J. 2021;107(1):198–214. doi:10.1111/tpj.15285. [Google Scholar] [PubMed] [CrossRef]
109. Zhao Y, Wang H, Song B, Xue P, Zhang W, Peth S, et al. Characterizing uncertainty in process-based hydraulic modeling, exemplified in a semiarid Inner Mongolia steppe. Geoderma. 2003;440(1):116713. doi:10.1016/j.geoderma.2023.116713. [Google Scholar] [CrossRef]
110. Han Y, Zhang Y, Yang Z, Zhang Q, He X, Song Y, et al. Improving aerobic digestion of food waste by adding a personalized microbial inoculum. Curr Microbiol. 2004;81(9):277. doi:10.1007/s00284-024-03796-5. [Google Scholar] [PubMed] [CrossRef]
111. Junaid Nazir M, Zhang X, Du D, Yu F. How physical disturbance and nitrogen addition affect the soil carbon decomposition? Phyton-Int J Exp Bot. 2022;91(9):2087–97. doi:10.32604/phyton.2022.021412. [Google Scholar] [CrossRef]
112. Zhang S, Jian Y, Yan B, Jin J, Wu J, Liang C, et al. Land consolidation with seedling cultivation could decrease soil microbial PLFA diversity. Phyton-Int J Exp Bot. 2022;91(8):1745–56. doi:10.32604/phyton.2022.021076. [Google Scholar] [CrossRef]
113. Wahid F, Sharif M, Fahad S, Ali A, Adnan M, Rafiullah et al. Mycorrhiza and phosphate solubilizing bacteria: potential bioagents for sustainable phosphorus management in agriculture. Phyton-Int J Exp Bot. 2022;91(2):257–78. doi:10.32604/phyton.2022.016512. [Google Scholar] [CrossRef]
114. Eren AH. Genome-wide identification of ALDH gene family under salt and drought stress in Phaseolus vulgaris. Phyton-Int J Exp Bot. 2024;93(11):2883–907. doi:10.32604/phyton.2024.058627. [Google Scholar] [CrossRef]
115. Porcelli CA, Rubio G, Boem FHG, Lavado RS. The effect of water and salt stress on Paspalum dilatatum, a constituent of pampas natural grasslands. Phyton-Int J Exp Bot. 2024;93(8):2009–18. doi:10.32604/phyton.2024.052874. [Google Scholar] [CrossRef]
116. Ji X, Jiang Z, Wang J, Dong L, Deng X. Genome-wide identification and expression analysis of the GSK3 gene family in sunflower under various abiotic stresses. Phyton-Int J Exp Bot. 2024;93(8):1839–50. doi:10.32604/phyton.2024.052809. [Google Scholar] [CrossRef]
117. Rehman B, Zulfiqar A, Attia H, Sardar R, Saleh MA, Alamer KH, et al. Seed priming with potassium nitrate can enhance salt stress tolerance in maize. Phyton-Int J Exp Bot. 2024;93(8):1819–38. doi:10.32604/phyton.2024.048780. [Google Scholar] [CrossRef]
118. Farhan M, Sathish M, Kiran R, Mushtaq A, Baazeem A, Hasnain A, et al. Plant nitrogen metabolism: balancing resilience to nutritional stress and abiotic challenges. Phyton-Int J Exp Bot. 2024;93(3):581–609. doi:10.32604/phyton.2024.046857. [Google Scholar] [CrossRef]
119. Zhang R, Chen T, Chen Z, Chen H, Wei X, Kamran M, et al. The combination of Achnatherum inebrians extracts and soil microorganisms inhibited seed germination and seedling growth in Elymus nutans. Phyton-Int J Exp Bot. 2024;93(3):567–80. doi:10.32604/phyton.2024.047485. [Google Scholar] [CrossRef]
120. Mao C, He J, Wen X, Xiang Y, Feng J, Shu Y. Correlation and pathway analysis of the carbon, nitrogen, and phosphorus in soil-microorganism-plant with main quality components of tea (Camellia sinensis). Phyton-Int J Exp Bot. 2024;93(3):487–502. doi:10.32604/phyton.2024.048246. [Google Scholar] [CrossRef]
121. Liang X, An W, Li Y, Wang Y, Qin X, Cui Y, et al. Impact of different rates of nitrogen supplementation on soil physicochemical properties and microbial diversity in goji berry. Phyton-Int J Exp Bot. 2024;93(3):467–86. doi:10.32604/phyton.2024.047628. [Google Scholar] [CrossRef]
122. Tahjib-Ul-Arif M, Asaduzzaman M, Shirazy BJ, Khan MSU, Rahman AMS, Murata Y, et al. Seed priming improves chilling stress tolerance in rice (Oryza sativa L.) seedlings. Phyton-Int J Exp Bot. 2024;93(11):3013–27. doi:10.32604/phyton.2024.058710. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools