 Open Access
Open Access
ARTICLE
Total Phenolic and Flavonoid Contents of Cymbocarpum widemannii and Their Antioxidant, Antimicrobial, DNA Damaging Activities
1 Department of Biology, Faculty of Art and Science, Siirt University, Siirt, 56100, Türkiye
2 Department of Agricultural Biotechnology, Faculty of Agriculture, Siirt University, Siirt, 56100, Türkiye
3 Department of Crop and Animal Production, Kurtalan Vocational School, Siirt University, Siirt, 56100, Türkiye
4 Department of Food Engineering, Faculty of Engineering and Architecture, Siirt University, Siirt, 56100, Türkiye
* Corresponding Author: Behcet Inal. Email:
(This article belongs to the Special Issue: Functional Plant Extracts and Bioactive Metabolites)
Phyton-International Journal of Experimental Botany 2025, 94(3), 781-791. https://doi.org/10.32604/phyton.2025.062171
Received 11 December 2024; Accepted 20 February 2025; Issue published 31 March 2025
Abstract
The use of conventional herbal medicines is a rapidly expanding phenomenon in developed nations. For instance, 30%–50% of all drug use in China is attributed to traditional herbal preparations. Current study evaluated the antioxidant (DPPH, FRAP), total phenolic and flavonoid content, antibacterial activity, and DNA damage protective potential of aqueous and methanolic extracts obtained from the aerial parts and roots of Cymbocarpum wiedemannii Boiss., an endemic plant in Turkey. In antioxidant analyses, the methanolic extract of the aerial parts showed the highest %DPPH (73.38) and IC50 (3.46 mg/mL) values. The FRAP analysis revealed the highest iron-reducing capacity in the methanolic extract of the aerial parts (108.10 ± 0.11 mg FeSO4/mL). The aqueous extract of the aerial parts exhibited the highest total phenolic content (1.69 ± 0.02 mg gallic acid/mL), while the methanolic extract of the aerial parts had the highest total flavonoid content (13.53 ± 0.09 mg rutin/mL). Antibacterial activity tests showed no significant effect at a concentration of 1 mg/mL for the samples. DNA protective effects were tested on pBR322 plasmid DNA, demonstrating that both aerial and root extracts could protect DNA from Fenton reaction-induced damage. In conclusion, C. wiedemannii exhibits potential bioactive properties, particularly in terms of its antioxidant and DNA protective effects.Graphic Abstract
Keywords
Since ancient times, humans have known about plants in the Apiaceae family. Early civilizations easily differentiated the distinctive fragrances, flavors, and edibility (or toxicity) of numerous Apiaceae species flourishing in their regions, resulting in their use for varied purposes. Evidence of this comes from the presence of coriander, cumin, and fennel in a Mycenaean text dating from the 17th–15th centuries BC [1]. Theophrastus of Eresus, a Greek botanist and student of Aristotle, stands out for his meticulous identification of Apiaceae species. His writings mention anise, coriander, dill, caraway, and fennel more frequently than any other plants within this family. Later, Dioscorides’ renowned Greek Herbal had over 50 Apiaceae plants in its “Herbs” section [2]. Notably, 40 of these plants are now well-described in important historical texts [3].
The Apiaceae family is distinguished by the diverse odors and flavors of its member species. This unique characteristic stems from their rich variety of phytochemical compounds. Early humans utilized Apiaceae plants in various ways, including food, beverages, sweeteners, medicines, and industrial applications. Most, if not all, Apiaceae species are rich in bioactive compounds with potential pharmacological applications. These compounds exhibit a wide range of properties, making them valuable for antispasmodic, carminative, cosmetic, diuretic, laxative, sedative or stimulant, gastric, and topical uses. However, caution is advised, as some Apiaceae taxa, such as hemlock (Oenanthe crocata), poison hemlock (Conium maculatum), and water hemlock (Cicuta spp.) are highly toxic [4].
Plants possess sophisticated defense mechanisms, including the production of Reactive Oxygen Species (ROS) to combat pests and harmful microorganisms [5]. This natural defense mechanism can translate into potential health benefits for humans. Numerous studies have shown that plant extracts, rich in secondary metabolites, exhibit antimicrobial and antioxidant activities [6,7]. These findings suggest that secondary metabolites may play a significant role in the evolution of broad-spectrum treatments for various diseases [7]. A recent study by [8] employed RSH-GC/MS analysis to identify volatile components in Cymbocarpum wiedemannii, detecting 14 compounds. Plants have a long past about their using in conventional medicine due to potential biological properties. While some plant-based remedies are already integrated into alternative therapies, the discovery of side effects associated with conventional drugs has intensified the search for safer alternative treatments [9]. Natural plant compounds derived from plants can be divided into several categories. They can function as biologically active agents for direct therapeutic use, as precursor compounds with specific biological properties to produce more effective derivatives, as unique structural frameworks that can be converted into druggable molecules, or as chemical markers for standardizing crude plant extracts. Additionally, plant extracts can be utilized in the creation of herbal formulations. Organic chemicals identified and described from plant sources have long been utilized in the treatment of many diseases, and they continue to play an important role in contemporary medicine. These chemicals are utilized both in their natural state, as pure pharmaceuticals or herbal remedies, and as precursor molecules for the manufacture of enhanced synthetic and semi-synthetic analogs with higher pharmacological potential. Important active compounds utilized clinically today include morphine, codeine, noscapine, papaverine, quinine, artemisinin, and paclitaxel [10,11]. Unfortunately, knowledge on the biological features of Cymbocarpum wiedemannii is extremely sparse. Therefore, the present study aimed to lay the groundwork for future pharmacological and pharmaceutical research on this plant. It is important to understand the role of free radicals in human health. Various molecules, ranging from single compounds like hydrogen peroxide (H2O2) to complex polymers, can act as radicals and damage vital cellular components like DNA, proteins, and lipids. These interactions are linked to the development of many diseases, including cancers, cardiovascular diseases, and cataracts [7]. The hydroxyl radical (OH) is a highly reactive molecule that can damage various biomolecules, including plasmid DNA, and plays a key role in oxidative DNA damage. The Fenton reaction, triggered by Hydrogen peroxide (H2O2) and transition metals like (Fe2+) and copper (Cu2+), produces hydroxyl radicals [12]. The reaction mimics the intracellular production of hydroxyl radicals from iron and forms the basis of the DNA-nicking assay. The assay replicates conditions found within living organisms by generating hydroxy radicals that can damage the plasmid DNA structure. During the Fenton reaction, hydroxy radical production disrupts the supercoiled form (Form I) of plasmid DNA, converting it to open circular (Form II) and nicked linear (Form III) forms. According to Kitts, Wijewickreme [13], these altered DNA structures exhibit distinct mobility during gel electrophoresis, allowing for their separation and visualization. Supporting this concept, previous research by Leba, Brunschwig [14] demonstrated that blocking the Fenton reaction with organic solvents can prevent DNA strand breaks, highlighting the role of hydroxyl radicals in this process [14]. DNA exists in different forms depending on the degree of damage to its double helix. The intact, double-stranded helical structure (supercoil) is known as Form I. Exposure to free radicals and other DNA-damaging interactions might cause one strand to break. The transition from the tightly packed Form I to the more relaxed, open circular Form II lowers mobility during gel electrophoresis. Further damage can break the other strand, resulting in the linear Form III, which migrates the slowest among the three forms. When stained with ethidium bromide and visualized under UV light, these different DNA forms appear as distinct bands on agarose gel [15].
Although numerous plant species have been studied for antioxidant, antibacterial, and DNA protective capabilities, no such research have been undertaken on C. widemannii, which we explored in this paper. So this study aimed to investigate the antioxidant and antibacterial activity of extracts from C. wiedemannii Boiss (Turkish name: Tüysüz aşotu), along with their ability to protect DNA from damage. The antioxidant capacity of the extracts was assessed using DPPH (2,2-diphenyl-1-picrylhydrazyl) and FRAP (Ferric Reducing Antioxidant Power) assays, while total phenolic and total flavonoid content were also determined. Additionally, the antibacterial activity and DNA protective effects of both methanol and aqueous extracts were investigated.
2.1 Plant Tissue Extract Preparation
The plant sample was collected from its natural habitat responsibly to ensure minimal impact on the natural population. The plant is located inside the 3425 m, 38°55'24'' K, 42°51'07'' D geographical coordinates. In the laboratory, the obtained plant components were dried in a darkened environment to preserve their bioactive chemicals. As tissues, both above-ground and root parts were then ground into a fine powder using a mixer. Four grams of powder were weighed and placed in separate flasks, each containing 40 mL of either water or methanol. These mixtures were shaken at 37°C for 24 h to facilitate extraction of the desired compounds. Following filtration through coarse filter paper, the solvents were removed using an evaporator. The concentrated plant extracts were then dried completely in an oven at 37°C within the same containers. Finally, solutions were prepared at appropriate concentrations from the dried extracts and used in subsequent experiments [16]. All analysis results in the study were performed using 1 mg/mL plant extract.
2.2 Determination of Total Phenolic Content in Tissues
The Folin-Ciocalteu (FCR) method was used to estimate the total phenolic content of the extracts. The method relies on the reaction between FCR reagent and phenolic compounds in the presence of sodium carbonate (Na2CO3), which produces a characteristic green color. One milliliter of each sample was added to separate tubes, followed by 1 mL of FCR reagent. The mixtures were incubated at room temperature for 3 min. Then, 1 mL of a saturated Na2CO3 (7%) was added. Foaming and green color creation were observed at this phase The tubes were then incubated in the dark for 90 min at room temperature. Finally, the absorbance of the samples was measured at 760 nm wavelength using a spectrophotometer [16].
Blind; 1 mL solvent + 1 mL FCR + 1 mL 7% Na2CO3; Standard was prepared with solutions of Gallic acid at different concentrations (0.05–1 mg/mL).
2.3 Determination of Total Flavonoid Content in Tissues
The flavonoid concentration in the extracts was evaluated by reading the response of NaNO2 extracts with AlCl3 at 510 nm wavelength. After adding 400 μL of 80% methanol to 1 mL of the extract (separate for every concentration adjusted), 30 μL of 5% NaNO2 was added and left to incubate for 6 min. At the end of the time, 30 μL of 10% AlCl36H2O was added, shaken well, left for six minutes, then 400 μL NaOH (1 M) was added and incubated for 15 min. The absorbance of the resulting solution was measured at a wavelength of 510 nm using a spectrophotometer [16]. Blind: 80% methanol + 30 μL 5% NaNO2 + 30 μL 10% AlCl3.6H2O + 400 μL NaOH (1 M) Standard Regression Curve was plotted for numerous concentrations (0.1–1 mg/mL) of Rutin.
2.4 Assessing Antioxidant Activity in Samples
One milliliter of each prepared plant extract concentration was pipetted into separate tubes. Then, 4 mL of a 0.001 M DPPH solution (prepared in absolute methanol) was added to each tube. The mixtures were thoroughly mixed and incubated for 30 min to allow the DPPH radical to react with potential antioxidant compounds present in the extracts. The absorbance of each sample was measured at 517 nm using a spectrophotometer. 1 mg/mL extract concentration was used for DPPH activity and a control sample was prepared by adding 1 mL of methanol to 4 mL of DPPH [16,17].
where AK is the control absorbance and A1 is the sample absorbance.
To assess the antioxidant activity of the extract, the FRAP method was employed with modifications based on the protocol [18]. A freshly prepared FRAP solution was used, containing sodium acetate buffer (300 mM, pH 3.6), 10 mM TPTZ (2,4,6-Tris (2-pyridyl)-triazine), and ferric chloride solution (20 mM) prepared in 40 mM HCl, in a 10:1:1 ratio. For each sample, 100 μL of the concentrated extract was mixed with 3 mL of FRAP solution. The last concentration used for FRAP analysis was 1 mg/mL extract. The mixtures were incubated at 37°C for 4 min with mixing at one-minute intervals. Absorbance was then measured at 593 nm wavelength using a spectrophotometer. Ferrous sulfate was used as the standard for calibration [16]. The results were expressed as mM FeSO4 per gram of dried sample weight.
2.5 Determination of Antimicrobial Activity
The disc diffusion approach was employed to assess the antimicrobial activity of the extracts against various microorganisms. This method relies on the diffusion of the extract from a paper disc into an agar plate containing the test organism (Staphylococcus aureus ATCC 6538P and Bacillus cereus ATCC 7064, gram-positive bacteria, Escherichia coli W3110, gram-negative bacteria and Candida albicans ATCC 10231, yeast). Determination of antimicrobial activity was done according to previous studies [19–21].
Approximately 24 h before the assay, bacteria, and fungi were subcultured using nutrient broth (NB) and yeast peptone dextrose agar media. Hinton Agar and two-fold Muller Hinton Broth (×2 MHB) were utilized to assess the minimum inhibitory concentration (MIC) and the zone of inhibition [22]. A precise scale weighed 4 g of Nutrient Broth as a liquid media. Then it was placed in an Erlenmeyer filled with 500 mL of pure water. After that, the mouth of the Erlenmeyer was cotton wooled, coated in aluminum foil, and prepared using an autoclave or microwave equipment. According to a prior study, the processes for media preparation were completed [23].
2.7 Assessment of Activity of DNA Damage
The capacity of various concentrations of the extract of C. wiedemannii Boiss to preserve pBR322 plasmid DNA from the damaging effects of free •OH radicals generated by Fenton’s reagent was assessed by DNA damage protection assay as conducted in different studies [24,25]. The combination of reactions contained 6 μL of plasmid DNA, 3 μL of Fenton’s reagent (30 mM H2O2, 50 mM Ascorbic acid, and 80 mM FeCl3), and 6 μL of above-ground or root extract (10 µg/mL). The final volume was adjusted to 15 µL with ddH2O. Negative or positive controls replaced Fenton’s reagent or plant extract with water, respectively. After incubation at 37°C for 30 min, 15 µL of the volume was mixed with 3 µL of loading dye (Thermo 6x loading dye) and loaded onto a 0.5% agarose gel consisting of 0.25 g agarose and 1 µL ethidium bromide in 50 mL TAE. Electrophoresis was performed at 80 V for 1 h, followed by gel visualization using a Biorad Gel Doc and Image Lab software.
Values were presented as mean ± standard deviation. Data visualization and statistical analyses were carried out using Origins 6.1 (OriginLab, Northampton, MA, USA). Statistical analysis achieved in the current study was addressed using Student’s t-test, with significance set at p < 0.05.
3.1 Antioxidant Activity Analysis
Phenolic compounds are recognized for their significant antioxidant activity, principally because of their capacity to release harmful hydrogen atoms or electrons, thereby neutralizing free radicals and reducing oxidative stress. The presence of hydroxyl groups in their structure increases their radical scavenging potential, making them effective in preventing oxidative stress-related damage. In addition, phenolic can chelate metal ions and reduce their catalytic role in the formation of reactive oxygen species (ROS). The strong correlation between phenolic content and antioxidant capacity is widely recognized, with higher phenolic concentrations generally leading to increased antioxidant activity [26].
The DPPH method was carried out determination the free radical scavenging activity of the plant extracts. At a concentration of 1 mg/mL, the aboveground water extract exhibited the highest free radical scavenging capacity (47%), followed by the aboveground methanol extract (73%), root water extract (29%), and root methanol extract (26%) (Fig. 1). Lower Ic50 values indicate greater antioxidant capacity. The Ic50 values for extracts were determined to be 2.9, 3.8, 3.5, and 3.9 mg/mL for the aboveground water, aboveground methanol, and root methanol extracts, respectively (Fig. 1).
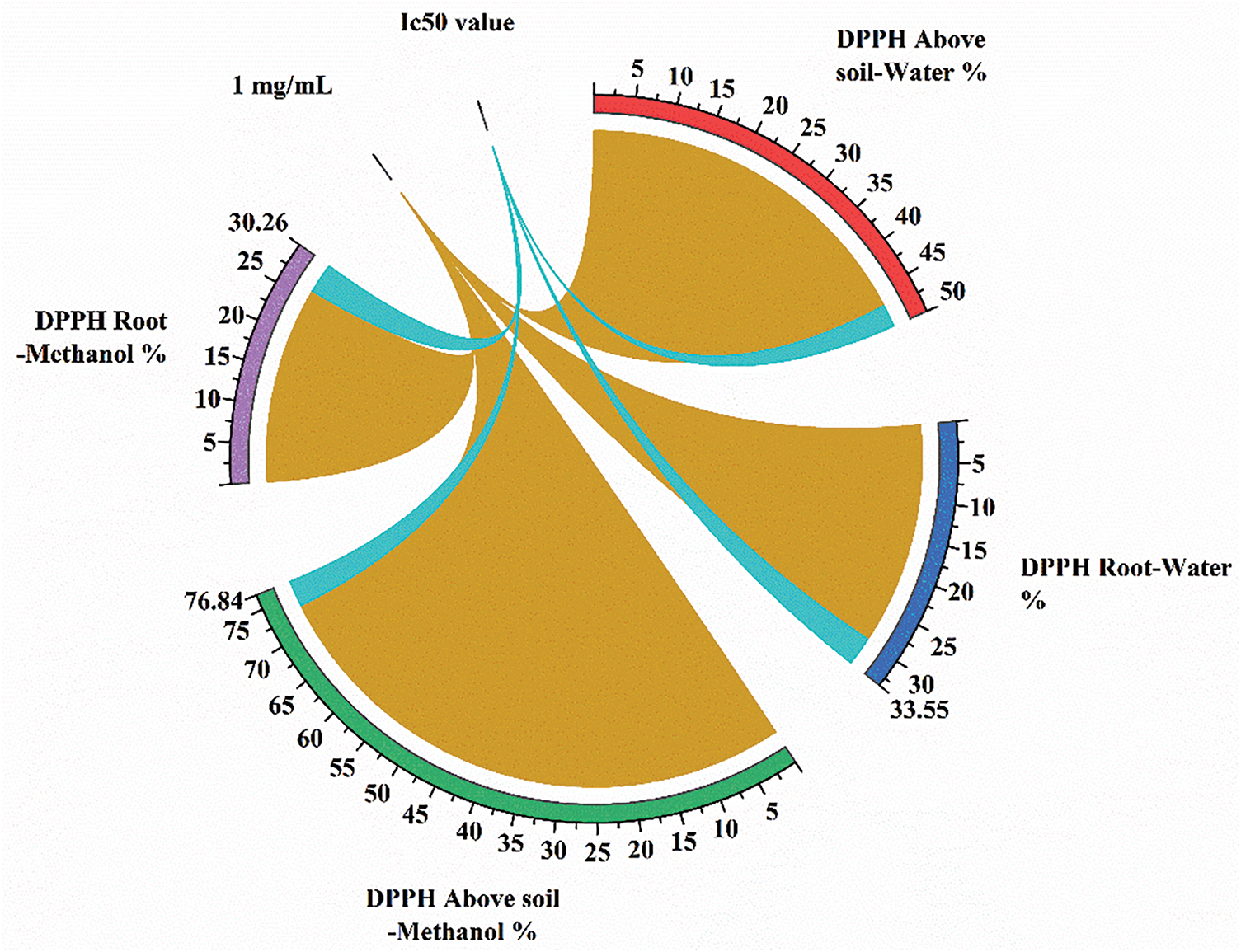
Figure 1: Percent DPPH analysis result graph
The FRAP assay was performed at a concentration of 1 mg/mL for all samples. The above-ground methanol extract samples displayed the highest FRAP capacity, indicating strong decreasing power (Fig. 2).
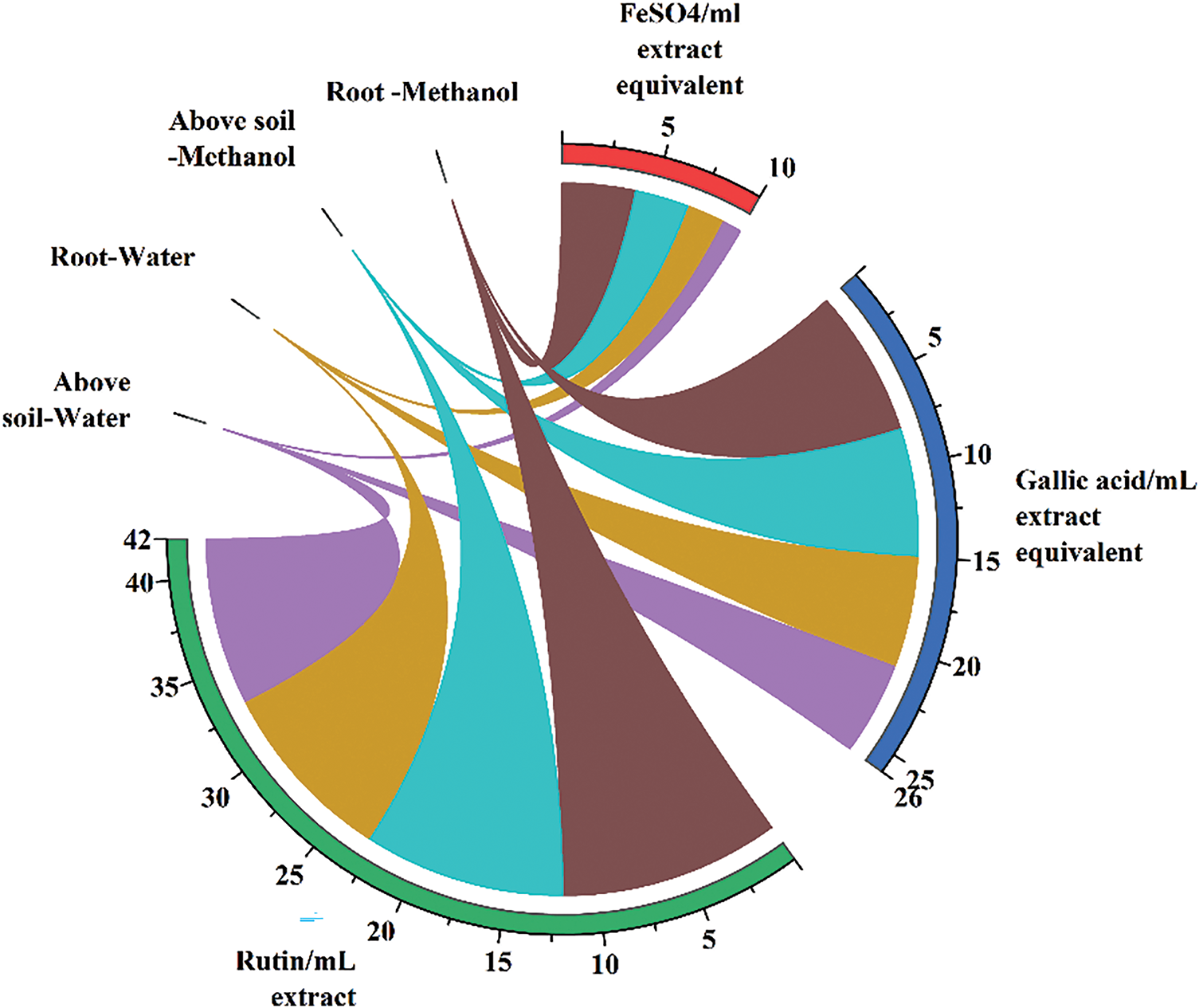
Figure 2: Total FRAP (FeSO4), phenolic and flavonoid
Karakay et al. evaluated the health effects of essential oils derived from Cymbocarpum erythraeum [9]. Their research indicated that the oils have not only antioxidant capabilities, but also other intriguing biological impacts. These included the ability to kill cancer cells (Cytotoxic activity) and inhibit enzymes crucial for nerve function (anticholinesterase activity). The antioxidant capacity of the essential oils was measured using DPPH, TBA, and TOS assays. The Ellman method was employed to assess their anticholinesterase activity against acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). Cytotoxic activity was evaluated on human cancer cell lines (U-87MG and PC-3) using the MTT assay. The study identified the root essential oil as particularly effective in targeting PC-3 cells, exhibiting nearly 50% cell death at a concentration of 0.03 mh/L. In contrast, the essential oil from the aerial parts (including flowers) demonstrated a stronger cytotoxic effect against U-87MG cancer cells (34.62% at 0.03 mg/L). Furthermore, the root oil exhibited the most potent inhibition of both AChE and BuChE with values of 113 and 197 μM, respectively. Chemical analysis using GC-FID and GC-MS techniques revealed the key components of both root and aerial oils. (E)-2-decenal (52.1%), (E)-2-dodecenal (36.1%), and (E)-2-tetradecenal (22.3%) were identified as the most abundant constituents. The study also highlighted that (E)-2-dodecenal, the main compound in root oil, possesses individual inhibitory effects against AChE and BuChE (IC50 values of 100 and 136 μM, respectively) and antioxidant activity, further supporting its potential as a bioactive agent. By scavenging reactive oxygen species (ROS) and shielding cellular structures, phenolic chemicals are essential for improving oxidative stress tolerance in plants. Crude extracts from phenolic-rich fruits, vegetables, herbs, and grains are becoming more and more popular in the food industry due to their potent antioxidant qualities. These natural extracts are prized for their possible health benefits, such as lowering the risk of oxidative stress-related illnesses in people, in addition to their capacity to maintain food quality [27].
3.2 The Total Amount of Flavonoids and Phenolic Found in Tissues
The total phenolic motif of the extracts of the sample was identified as gallic acid equivalent (mg gallic acid/mL extract). The highest phenolic content was discovered in the above-ground aqueous extracts, while the root aqueous extract exhibited the lowest value (Fig. 2).
Camele et al. [28] conducted independent hydrodistillations on crushed fruits and vegetative components of C. wiedemannii. The oils were then analyzed using GC/MS [28]. The research yielded the identification of 56 components, accounting for 96% of the fruit oil content. In comparison, 33 chemicals representing 94% of the oil were described in the oil recovered from the aboveground sections.
Fidan et al. [8] investigated the volatile components of the endemic, fragrant plant C. wiedemannii taxon using an automated robotic sample handling of chromatography-mass spectrometry technique (RSH-GC/MS). This analysis identified a total of 14 distinct volatile components. Among these essential oils, Butanal, 2-methyl- (Area 48.69%) had the highest rate, while Octanal (Area 0.54%) had the lowest.
Butanal, 2-methyl, one of the main components, has been found to have antimicrobial activity [28]. The second and third main components, à-Pinene and Linalol, have been reported to have antimicrobial, antifungal, and anti-inflammatory activities, respectively [29,30]. In a prior study, Cymbocarpum erythraeum was used, and the results revealed that this plant has excellent antioxidant activity. It was also expected that C. erythraeum could serve as a natural alternative to synthetic medications, offering a viable option for herbal drug development in neurological and cancer treatments [9].
3.3 Identification of DNA Damage Repair
This experiment aimed at the protective impact of plant extract content on pBR322 plasmid DNA contrary to damage caused by free -OHradicals. The Fenton reaction generates -OH radicals, which are known to cause oxidative breaks in DNA strands, converting the circular plasmid DNA into a linear form [31]. When exposed to Fenton’s reagent, the plasmid DNA primarily undergoes the production of reactive species and -OH radicals. These radicals then induce strand breaks in the plasmid DNA. The natural supercoiled form (Form I) of pBR322 plasmid DNA is composed of an open circular form (Form II) with a single strand break and a linear form (Form III) with additional breaks [27,32].
The effect of the herbal extract on the plasmid DNA against the Fenton agent is shown in Fig. 3. Lane 1 shows the control plasmid DNA with minimal single-stranded breaks (Form II). In line 2, Fenton’s reagent exposure induces DNA strand breaks, converting the supercoiled form (Form I) to an open circular form (Form II). Lane 3 demonstrates that methanol exposure alongside the Fenton reagent further fragments the DNA (Forms I and II), likely due to methanol’s ability to break DNA at multiple sites [33,34]. Lanes 4 and 5 show partial restoration of the fragmented DNA (Forms I and II) when treated with methanol-prepared aboveground and root extracts, respectively, suggesting some protective effects. However, lanes 6 and 7 reveal that water-extracted C. wiedemannii extracts from both aboveground and root parts lacked a protective effect against Fenton’s reagent-induced DNA damage (Fig. 3).
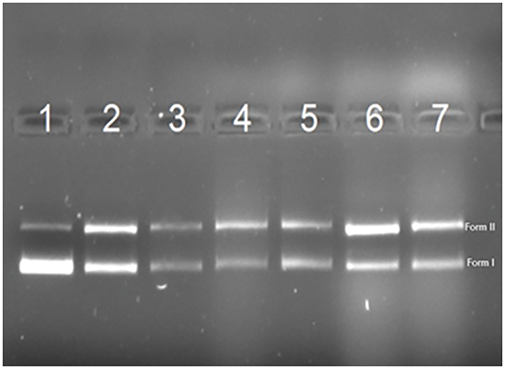
Figure 3: Effect of different plant tissues on DNA damage repair (1) pDNA (pBR322) + Water, (2) pDNA + Fenton + Water, (3) pDNA + Fenton + Methanol, (4) pDNA + Fenton + above the soil-Methanol Extract, (5) pDNA + Fenton + Root-Methanol Extract, (6) pDNA + Fenton + above soil-Water Extract, (7) pDNA + Fenton + Root-Water Extract
Previous research has explored the potential of plant extracts for DNA protection against oxidative damage. A previous study reported that methanol extracts from the leaves and flowers of Ferulago cassia Boiss. Exhibited DNA protective effects, suggesting their potential as an anticancer agent [35]. Similarly, Anand, Mahadeva [36] demonstrated that a methanolic extract of Centella asiatica (L) Urb, belonging to the Apiaceae family, possessed various biological properties, including antioxidant activity, reducing power and the ability to protect DNA from damage [36].
This study evaluated the antimicrobial activity of a Cymbocarpum erythraeum extract against various microorganisms at a concentration of 1 mg/mL but showed no significant inhibitory effects. This lack of activity could be due to the specific microorganisms tested or the extract concentration. Heidari, Manayi [37] found potential antimicrobial properties in Cymbocarpum erythraeum. They isolated various secondary metabolites and observed that the plant’s hexane extracts effectively inhibited all tested Helicobacter pylori strains. This prior study also identified four potentially active compounds: isoquercetin, rutin, β-sitosterol, and 2-desenol. These contrasting findings suggest that Cymbocarpum erythraeum extracts might have selective antimicrobial properties.
Phytochemical compounds play an increasingly important role in medicine today. They are being utilized in preventive medicine, body defense mechanisms, and even as first-line treatments for various diseases, offered in the form of herbal products. Therefore, identifying the chemical content profiles of these known medicinal plants, as well as exploring new, undiscovered plant sources, is crucial for further advancement.
Our study focused on the C. wiedemannii taxon. Analyses of extracts obtained using different solvents revealed promising biological activities. Specifically, the methanol extracts from both the above-ground and root tissues revealed the ability to prevent DNA damage. According to the results of the FRAP experiment and significant DPPH scavenging activity, this study indicates the strong antioxidant capabilities of Cymbocarpum wiedemannii, notably in its methanol-ethanol extract from aerial sections. A substantial relationship between phenolic components and antioxidant potential is suggested by the high total phenolic and flavonoid content. Furthermore, the protective activity of aerial and root extracts on pBR322 plasmid DNA against Fenton’s reagent suggests prospective DNA damage prevention qualities, supporting the plant’s potential function in preventing diseases linked to oxidative stress. Based on these promising findings, we believe further in-depth investigations are warranted. Further studies could explore a wider range of extract concentrations and different solvent systems to improve the bioactive chemicals’ extraction from Cymbocarpum wiedemannii. It is anticipated that more comprehensive data on the natural ingredients of plants will provide researchers with a new path to verify and identify this natural functional food as a suitable pharmacological drug, such as antioxidants, DNA protection, and antimicrobial potential of plant ingredients in the coming years.
Acknowledgement: Our sincere appreciation goes out to Siirt University’s BİYAL Laboratory.
Funding Statement: No funding support was received from any institution.
Author Contributions: Behcet Inal was instrumental in designing the study and carrying out molecular tests, Mehmet Fidan performed the identification, collection, and antioxidant analyses of plant material. Ulutas Mehmet Sefa performed molecular analyses, and Mesut Sırrı performed antioxidant analyses. Bülent Hallaç performed antimicrobial analyses. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials Availability: The corresponding author can provide the data used in the current study upon request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Abbreviations
| ROS | Reactive Oxgen Species |
| DNA | Deoxyribonucleic Acid |
| H2O2 | Hydrogen peroxide |
| FRAP | Ferric Reducing Antioxidant Power |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| AChE | Acetylcholinesterase |
| BuChE | Butyrylcholinesterase |
References
1. Chadwick J. The decipherment of Linear B. Cambridge, UK: Cambridge University Press; 1990. [Google Scholar]
2. Gunther RT. The Greek herbal of dioscorides. New York, NY, USA: Hafner Pub. Co; 1959. [Google Scholar]
3. Evergetis E, Haroutounian SA. The Umbelliferae (Apiaceae) of dioscorides annotated in codex neapolitanus graecus #1. J Ethnopharmacol. 2015;175:549–66. doi:10.1016/j.jep.2015.10.016. [Google Scholar] [PubMed] [CrossRef]
4. Reduron J. Taxonomy, origin and importance of the Apiaceae family. CABI Wallingford, UK: Carrots and related Apiaceae crops; 2020. p. 1–8. [Google Scholar]
5. Dinakar C, Abhaypratap V, Yearla SR, Raghavendra AS, Padmasree K. Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta. 2010;231(2):461–74. doi:10.1007/s00425-009-1067-3. [Google Scholar] [PubMed] [CrossRef]
6. Sibanda T, Okoh A. The challenges of overcoming antibiotic resistance: plant extracts as potential sources of antimicrobial and resistance modifying agents. Afr J Biotechnol. 2007;6:2886–96. [Google Scholar]
7. Sicari V, Pellicanò T, Giuffrè A, Zappia C, Capocasale M, Poiana M. Physical chemical properties and antioxidant capacities of grapefruit juice (Citrus paradisi) extracted from two different varieties. Int Food Res J. 2018;25(5):1978–84. [Google Scholar]
8. Fidan M, Teğin I, Gök M. Cymbocarpum wiedemannii Boiss. Taksonunun Topraküstü Kısımlarının RSH-GC/MS ile analizi. In: IV. International Siirt Conference on Scientific Research; 2023 Nov 17–18; Siirt, Türkiye: IKSAD Publishing House. [Google Scholar]
9. Karakaya S, Özdemir Ö, Koca M, Demirci B, Aksakal Ö, Turkez H, et al. Cytotoxic effect and molecular docking studies of essential oils of Cymbocarpum erythraeum (DC.) Boiss. (Apiaceae) as potential inhibitors of cholinesterase. J Essent Oil Res. 2020;32(5):436–48. doi:10.1080/10412905.2020.1787884. [Google Scholar] [CrossRef]
10. Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17(3):215–34. doi:10.1039/a902202c. [Google Scholar] [PubMed] [CrossRef]
11. Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4(3):206–20. doi:10.1038/nrd1657. [Google Scholar] [PubMed] [CrossRef]
12. Goldstein S, Meyerstein D, Czapski G. The Fenton reagents. Free Radic Biol Med. 1993;15(4):435–45. doi:10.1016/0891-5849(93)90043-t. [Google Scholar] [PubMed] [CrossRef]
13. Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203(1–2):1–10. doi:10.1023/a:1007078414639. [Google Scholar] [PubMed] [CrossRef]
14. Leba LJ, Brunschwig C, Saout M, Martial K, Vulcain E, Bereau D, et al. Optimization of a DNA nicking assay to evaluate Oenocarpus bataua and Camellia sinensis antioxidant capacity. Int J Mol Sci. 2014;15(10):18023–39. doi:10.3390/ijms151018023. [Google Scholar] [PubMed] [CrossRef]
15. Zhang H, Barceló JM, Lee B, Kohlhagen G, Zimonjic DB, Popescu NC, et al. Human mitochondrial topoisomerase I. Proc Natl Acad Sci U S A. 2001;98(19):10608–13. doi:10.1073/pnas.191321998. [Google Scholar] [PubMed] [CrossRef]
16. Fidan M. Assessment of biological activity and element analysis of Psylliostachys spicata (Willd.) nevski. J Anim Plant Sci. 2018;28(6):1635–40. [Google Scholar]
17. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–200. doi:10.1038/1811199a0. [Google Scholar] [CrossRef]
18. Müller L, Fröhlich K, Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAPABTS bleaching assay (αTEACDPPH assay and peroxyl radical scavenging assay. Food Chem. 2011;129(1):139–48. [Google Scholar]
19. Tegin İ., Yabalak E, Hallaç B, Sabancı N, Fidan M, Sadık B. Unlocking the potential of Allium dictyoprasum C.A. Meyer ex kunth: quantum chemical insights into radical scavenging, chemical composition, phenolic content, and antimicrobial activity. Int J Environ Health Res. 2024;3(3):1–18. doi:10.1080/09603123.2024.2382304. [Google Scholar] [PubMed] [CrossRef]
20. Hallaç B, Kilinççeker O, Acar Z. Siirt’te yetişen zivzik narlarından (Punica granatum L.) elde edilen kabukların bazı gıda patojenlerine karşı antimikrobiyal etkilerinin belirlenmesi, determination of antimicrobial effects against some food pathogens of peels obtained from zivzik pomegranates (Punica granatum L.) grown in siirt. Yüzüncü Yıl Üniversitesi Fen Bilimleri Enstitüsü Dergisi. 2022;27(3):695–703. doi:10.53433/yyufbed.1120314. [Google Scholar] [CrossRef]
21. Ponce AG, Fritz R, del Valle C, Roura SI. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT Food Sci Technol. 2003;36(7):679–84. doi:10.1016/S0023-6438(03)00088-4. [Google Scholar] [CrossRef]
22. Gangegoda S, Abeywardhana S, Sigera S, Nirmani AAEB, Peiris DC. Antioxidant and antimicrobial properties of Codium fragile (Suringar) methanol extract: Insights from molecular docking analysis. Algal Res. 2024;82:103619. doi:10.1016/j.algal.2024.103619. [Google Scholar] [CrossRef]
23. Coban AY, Demırpek U, Yıldırım T, Caycı YT, Kocagoz T, Durupınar B. Rapid detection of methicillin resistance in Staphylococcus aureus isolates; evaluation of colorimetric Quicolor ES agar and determination of breakpoint inhibition zone diameters of cefoxitin. World J Microbiol Biotechnol. 2011;27(8):1901–4. doi:10.1007/s11274-011-0649-y. [Google Scholar] [CrossRef]
24. Lee JC, Kim HR, Kim J, Jang YS. Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem. 2002;50(22):6490–6. doi:10.1021/jf020388c. [Google Scholar] [PubMed] [CrossRef]
25. Tian B, Hua Y. Concentration-dependence of prooxidant and antioxidant effects of aloin and aloe-emodin on DNA. Food Chem. 2005;91(3):413–8. doi:10.1016/j.foodchem.2004.06.018. [Google Scholar] [CrossRef]
26. Shi L, Zhao W, Yang Z, Subbiah V, Suleria HAR. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ Sci Pollut Res Int. 2022;29(54):81112–29. doi:10.1007/s11356-022-23337-6. [Google Scholar] [PubMed] [CrossRef]
27. Akkemik E, Fidan M, Balaban M, Inal B. ICP-OES and LC-ESI-MS/MS analyses, enzyme inhibition and DNA protection potential of Pelargonium quercetorum Agnew. Studia UBB Chemia. 2022;67(4):197–213. doi:10.24193/subbchem.2022.4.13. [Google Scholar] [CrossRef]
28. Camele I, Sadeek SA, Racioppi R, Elshafie HS. Antimicrobial activity of diffusible and volatile metabolites emitted by Beauveria bassiana: chemical profile of volatile organic compounds (VOCs) using SPME-GC/MS analysis. Plants. 2023;12(15):2854. doi:10.3390/plants12152854. [Google Scholar] [PubMed] [CrossRef]
29. de Cássia da S, Sá R, Andrade LN, de Sousa DP. A review on anti-inflammatory activity of monoterpenes. Molecules. 2013;18(1):1227–54. doi:10.3390/molecules18011227. [Google Scholar] [PubMed] [CrossRef]
30. Guzmán-Gutiérrez SL, Bonilla-Jaime H, Gómez-Cansino R, Reyes-Chilpa R. Linalool and β-pinene exert their antidepressant-like activity through the monoaminergic pathway. Life Sci. 2015;128:24–9. doi:10.1016/j.lfs.2015.02.021. [Google Scholar] [PubMed] [CrossRef]
31. von Sonntag C. Radiation chemistry in the 1990s: pressing questions relating to the areas of radiation biology and environmental research. Int J Radiat Biol. 1994;65(1):19–26. doi:10.1080/09553009414550031. [Google Scholar] [CrossRef]
32. Yayıntaş ÖT, Demir N. Determination of antioxidant activity and DNA damage protection of Marchantia polymorpha L. Fresenius Environ Bull. 2021;4:3420–6. [Google Scholar]
33. Park SC, Lim JY, Jeen YT, Keum B, Seo YS, Kim YS, et al. Ethanol-induced DNA damage and repair-related molecules in human intestinal epithelial Caco-2 cells. Mol Med Rep. 2012;5(4):1027–32. doi:10.3892/mmr.2012.754. [Google Scholar] [PubMed] [CrossRef]
34. Brooks PJ. DNA damage, DNA repair, and alcohol toxicity—a review. Alcohol Clin Exp Res. 1997;21(6):1073–82. [Google Scholar] [PubMed]
35. Yilmaz E, Ege M, Misirli D, Sonmez GD, Kilic O, Elmastas M. Phytochemical analysis, DNA barcoding and DNA protective activity of Ferulago cassia Boiss. Pak J Bot. 2023;55(6):2221–9. doi:10.30848/pjb2023-6(31). [Google Scholar] [CrossRef]
36. Anand T, Mahadeva N, Phani KG, Farhath K. Antioxidant and DNA damage preventive properties of Centella asiatica (L) urb. Pharmacogn J. 2010;2(17):53–8. doi:10.1016/S0975-3575(10)80010-0. [Google Scholar] [CrossRef]
37. Heidari S, Manayi A, Saeidnia S, Mighani H, Monsef Esfahani HR, Gohari AR, et al. Chemical constituents of Cymbocarpum erythraeum (DC.) boiss., and evaluation of its anti-Helicobacter pylori activity. Turk J Pharm Sci. 2018;15(1):103–6. doi:10.4274/tjps.96168. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools