 Open Access
Open Access
ARTICLE
Biological Potential and Chemical Characterization of Juniperus oxycedrus Leaves and Seed Cones
1 High Institute of Nursing Professions and Health Techniques, Beni Mellal, 23000, Morocco
2 Valorization of Medicinal and Aromatic Plants and Environment Team, Faculty of Sciences, Moulay Ismail University, Meknes, 50000, Morocco
3 Bioactive and Environmental Health Laboratory, Faculty of Sciences, Moulay Ismail University, Meknes, 50000, Morocco
4 Laboratory of Engineering and Applied Technologies, Higher School of Technology, M’ghila Campus, Sultan Moulay Slimane University, Beni Mellal, 23000, Morocco
5 Team of Microbiology and Health, Laboratory of Chemistry-Biology Applied to the Environment, Moulay Ismail University, Faculty of Science, Meknes, 50000, Morocco
6 Faculty of Medicine and Pharmacy, Mohammed First University, Oujda, 60000, Morocco
7 Higher Institute of Nursing Professions and Health Techniques of Laâyoune, Dakhla, 73000, Morocco
8 Biological Engineering Laboratory, Faculty of Science and Techniques, Sultan Moulay Silmane University, Beni Mellal, 23000, Morocco
9 Department of Pharmacology and Toxicology, College of Pharmacy, Qassim University, Qassim, 51452, Saudi Arabia
10 Department of Medical Laboratories, College of Applied Medical Sciences, Qassim University, Buraydah, 51452, Saudi Arabia
11 Department of Biology, College of Sciences, Princess Nourah bint Abdulrahman University, Riyadh, 11671, Saudi Arabia
12 Department of Biology, College of Science, Qassim University, Qassim, 51452, Saudi Arabia
13 LIMAS, Department of Chemical Sciences, Faculty of Sciences Dhar El Mahraz, Sidi Mohamed Ben Abdellah University, Fez, 30003, Morocco
14 Laboratory of Pharmacology and Toxicology, Biopharmaceutical and Toxicological Analysis Research Team, Faculty of Medicine and Pharmacy, University Mohammed V, Rabat, 10100, Morocco
15 Ecology and Environment Laboratory, Faculty of Sciences Ben m’sik, Hassan II University, Casablanca, 20670, Morocco
* Corresponding Authors: Samiah Hamad Al-Mijalli. Email: ; Emad M. Abdallah. Email:
(This article belongs to the Special Issue: Innovative Approaches in Experimental Botany: Essential Oils as Natural Therapeutics)
Phyton-International Journal of Experimental Botany 2025, 94(3), 657-677. https://doi.org/10.32604/phyton.2025.062289
Received 15 December 2024; Accepted 28 February 2025; Issue published 31 March 2025
Abstract
Juniperus oxycedrus (J. oxycedrus) is a traditional culinary spice and medicinal herb with a longstanding history of ethnopharmacological applications across diverse cultures. While prior research has explored the biological activities and phytochemical constituents of extracts derived from its leaves and seed cones, the present study systematically investigates their mineral and phenolic profiles alongside their multifunctional bioactive potential. Inductively coupled plasma-atomic emission spectroscopy (ICP-AES) analysis revealed a substantial abundance of essential macro- and microelements. Reversed-phase high-performance liquid chromatography (RP-HPLC) further identified high concentrations of phenolic acids (e.g., p-coumaric acid) and flavonoids (e.g., rutin and quercetin). The extracts exhibited potent radical scavenging activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH), robust antioxidant capacity against hydrogen peroxide, and significant inhibition of xanthine oxidase (XO) activity. Notably, both extracts demonstrated marked antibacterial efficacy. In silico molecular docking studies suggested that the antimicrobial activity may stem from the phenolic constituents, which exhibited favorable binding affinities to the active site of bacterial target proteins. These findings underscore J. oxycedrus as a promising reservoir of bioactive natural compounds, warranting further exploration for therapeutic and nutraceutical applications.Keywords
The surge in research dedicated to phytochemistry and natural medicine has sparked a burgeoning interest in medicinal plants, owing to their potential applications in both the fields of medicine and food [1]. One such plant that has garnered attention is Juniperus oxycedrus, which thrives naturally in rocky regions of the Mediterranean and Near Eastern countries. Juniper seed cones, derived from this remarkable conifer, have long been celebrated in European and Scandinavian cuisines for imparting a distinctive flavor to meat dishes, particularly wild birds and game [2]. Moreover, they are an essential component of the beloved spirit, gin. Notably, the historical use of J. oxycedrus in traditional medicine has addressed a range of health issues, including obesity, hyperglycemia, tuberculosis, pneumonia, and bronchitis [3].
Emerging studies have further elucidated the therapeutic potential of Juniperus oxycedrus, with experimental evidence indicating that oral administration of its fruit and leaf extracts in streptozotocin-induced diabetic rat models elicited a statistically significant reduction in fasting blood glucose levels. Concurrently, these interventions demonstrated a marked attenuation of lipid peroxidation biomarkers, notably malondialdehyde (MDA), in both hepatic and renal tissues, suggesting antioxidative protective effects against oxidative stress-associated pathologies [4]. In Turkey, J. oxycedrus seed cones and leaves have been employed in the treatment of diabetes [4]. Intriguingly, polyphenols, the main class of natural antioxidants found in the compounds isolated from J. oxycedrus, have come to the forefront. Another study demonstrated that J. oxycedrus root bark exhibits substantial antioxidant activity when assessed in vitro, despite having fewer phenolic compounds than the needles [5]. Furthermore, extracts derived from the leaves and seed cones of J. oxycedrus have exhibited the ability to inhibit the growth of various microorganisms [6].
Notably, previous studies have indicated that polyphenol extracts generally exhibit greater antibacterial efficacy against Gram-positive bacteria compared to Gram-negative bacteria [7–9]. Previous in silico analyses, such as those by Lafraxo et al. [10], have demonstrated that the essential oil from J. thurifera bark exhibits inhibitory activity against the enzyme NADPH oxidase. Building on this, the present study explores the molecular docking of key compounds from J. oxycedrus to investigate their binding affinity and potential therapeutic relevance against Staphylococcus aureus. Indeed, this comprehensive study is designed to characterize aqueous extracts of J. oxycedrus leaves (JOL-AE) and seed cones (JOSC-AE) from Morocco. Also, the current study aimed to analyze the phenolic and flavonoid content, identify specific phenolic compounds using high-performance liquid chromatography (HPLC), and determine the mineral composition through inductively coupled plasma atomic emission spectrometry (ICP-AES). Antioxidant activities were evaluated against the DPPH• radical and hydrogen peroxide (H2O2), while the inhibitory potential against xanthine oxidase was assessed. Additionally, antibacterial activity against specific bacterial strains was examined. The findings of this study are expected to enhance the understanding of the medicinal and culinary applications of Juniperus oxycedrus, potentially supporting its broader utilization across various fields.
The reagents and standards, including Quercetin, Ascorbic Acid, 2,2-diphenyl-1-picrylhydrazyl (DPPH•), Potassium dihydrogen phosphate (KH2PO4), Sodium hydrogen phosphate (Na2HPO4), Potassium ferricyanide (K3[Fe(CN)6]), Trichloroacetic acid (C2HCl3O2), 2,3,5-triphenyltetrazolium chloride (TTC), and Iron(III) chloride hexahydrate (FeCl3·6H2O), were sourced from Sigma-Aldrich. Chloramphenicol was obtained from Thermo Scientific Chemicals. Mueller-Hinton agar, Mueller-Hinton broth, and Dimethyl sulfoxide (DMSO) were procured from Merck (Darmstadt, Germany). Methanol was supplied by Biosolve (Dieuze, France). Distilled water was produced using a Milli-Q water purification system (Millipore, Bedford, MA, USA).
Leaves and seed cones of Juniperus oxycedrus were collected in May 2021 from the forest of the Middle Atlas Mountains in Taza City, Morocco (33°57′11.14″ N, 4°3′4.68″ O). Identification of the species level was performed by Professor Hamid Khamar of the Scientific Institute of Rabat, Morocco, and assigned the code number RAB108846. Following collection, the plant material was transported to the laboratory, washed with distilled water, and dried in the dark at room temperature for 60 days. The stems were then removed, and only the leaves and seed cones were retained for further analysis. The dried material was subsequently ground to a fine powder with a mean particle size of less than 910 μm. Also, voucher specimens were deposited at the Laboratory of Environmental and Health, Faculty of Sciences, Moulay Ismail University, Meknes, Morocco.
2.3 Preparation of Plant Aqueous Extract
Aqueous extracts of J. oxycedrus leaves (JOL-AE) and seed cones (JOSC-AE) were prepared by boiling approximately 10 g of the powdered material in 500 mL of distilled water for 30 min. The resulting extracts were filtered using a Büchner funnel and subsequently freeze-dried. The lyophilized extracts were stored in a desiccator, protected from light, until further experimentation. The extraction yields were 29.96% for the leaves and 39.21% for the seed cones.
2.4 Chemical Characterizations
2.4.1 Determination of Total Phenol Content
The total phenolic content of J. oxycedrus extracts was determined using the Folin–Ciocalteu method, as previously described by [11] with some modifications. In brief, 500 µL of various concentrations of each sample, solubilized in methanol, were mixed with 2.5 mL of Folin–Ciocalteu reagent (10%) dissolved in ultrapure water. After 1 min, 2.5 mL of Na2CO3 (7.5%) was added to the mixture, and the final solution was incubated in the dark at 25°C for 30 min. A blank was prepared by mixing 500 µL of methanol with 2.5 mL of Folin–Ciocalteu reagent and 2.5 mL of 7.5% Na2CO3. Absorbance was then measured at 765 nm. Gallic acid was used as a standard under the same conditions to construct the calibration curve. The total phenolic content was expressed as milligrams of gallic acid equivalent per gram of dried extract weight (mg GAE/g DW extract). Three independent experiments were performed in triplicate, and results were reported as the mean ± standard error.
2.4.2 Determination of Total Flavonoids Content
The total flavonoid content of J. oxycedrus extracts was assessed using a modified version of the method outlined by [11]. In this procedure, 2.5 mL of the plant extract, dissolved in ultrapure water, was combined with 2.5 mL of a 2% aluminum chloride (AlCl3) solution. The mixture was then incubated at room temperature in darkness for 1 h. The absorbance was recorded at 420 nm using a spectrophotometer. A calibration curve was constructed using quercetin as the reference standard under identical conditions. The flavonoid content was expressed in milligrams of quercetin equivalent per gram of dried extract weight (mg QE/g extract). Each experiment was conducted in triplicate, with results presented as the mean ± standard error.
2.4.3 Qualitative Analysis by RP-HPLC
Chromatographic analysis was conducted following a modified version of the method reported by [12]. The separation was performed using an EC NUCLEOSIL column (5 μm, C18, 100-5, 250 mm × 4.6 mm; Macherey-Nagel, Germany) under specific conditions. The mobile phase consisted of water containing 1% formic acid (A) and acetonitrile (B), with a gradient program starting at 7% B, increasing to 25% B at 40 min, 50% B at 85 min, and reaching 100% B at 120 min. The flow rate was set at 0.5 mL/min, and UV detection was carried out at 280 nm. The injection volume was 20 μL. ChromNAV 2.0-JASCO software was used for system control and data acquisition.
2.4.4 Quantification of Phenolic Acids and Flavonoids
The quantification of phenolic compounds was performed through high-performance liquid chromatography with ultraviolet detection (HPLC-UV) employing an EC NUCLEOSIL column (5 μm, C18, 100-5, 250 × 4.6 mm; Macherey-Nagel, Germany) under chromatographic conditions consistent with those outlined in the identification protocol. Spectral data for all chromatographic peaks were acquired at a detection wavelength of λ = 280 nm. Data processing was conducted using the HPLC software ChromNAV 2.0 (JASCO). Concentrations of phenolic acids and flavonoids present in the aqueous plant extracts were determined through interpolation against calibration curves generated using corresponding reference standards.
2.4.5 Quantification of Mineral Contents
The quantification of minor and major mineral elements in digested plant material was conducted via inductively coupled plasma atomic emission spectroscopy (ICP-AES), following a protocol adapted from [13]. Briefly, 1 g of powdered Juniperus oxycedrus seed cones and leaves was subjected to digestion by heating at 110°C in a mixture of 5 mL concentrated nitric acid (HNO3) and 15 mL hydrochloric acid (HCl). Following complete digestion, the samples were cooled to ambient temperature, and the final volume was adjusted to 100 mL using ultrapure water. Digestates were analyzed in duplicate, and trace metal concentrations were directly quantified using an Agilent 5110 ICP-OES Spectrometer (PerkinElmer, Inc., Waltham, MA, USA). Instrumental parameters were maintained as follows: plasma gas flow rate at 12 L/min, auxiliary gas flow rate at 1 L/min, and nebulizer gas flow rate at 0.7 L/min. The sample introduction rate was set to 1.5 mL/min, and radio frequency (RF) power was stabilized at 1200 W to ensure optimal plasma stability and analytical precision. Spectral data acquisition and calibration were performed under these standardized conditions to ensure reproducibility.
2.5.1 Assessment of DPPH Radical Scavenging Activity
The DPPH• (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity was assessed using a protocol adapted with minor modifications from [14–17]. Briefly, 1.5 mL of methanolic sample solutions was combined with 1.5 mL of DPPH methanolic solution (0.2 mM) and incubated under dark conditions at ambient temperature for 60 min. Absorbance measurements were subsequently recorded at a wavelength of 517 nm using a Synergy 168 H1 spectrophotometer (BioTek, El Segundo, CA, USA). Three separate assays were conducted in triplicate, and the results were compared to those of ascorbic acid, which served as the positive control and was tested under identical conditions.
2.5.2 Hydrogen Peroxide (H₂O₂) Scavenging Activity of Antioxidants
Excessive concentrations of reactive oxygen species (ROS) in the human body have been associated with various pathological conditions. Among these, hydrogen peroxide (H2O2), when present at high levels, poses a significant threat to cellular integrity [18]. This molecule is primarily generated through the catalytic conversion of the superoxide radical (O2•−) by the enzyme superoxide dismutase (SOD). To assess hydrogen peroxide (H2O2) scavenging activity, the method described by [19,20] was followed. A buffer solution (pH 7.4) containing 40 mmol/L hydrogen peroxide was prepared. Subsequently, 1 mL of the aqueous extract solution or standard (ascorbic acid) at varying concentrations was mixed with 0.6 mL of the H2O2 solution (40 mmol/L). After a 10-min incubation period, the absorbance of hydrogen peroxide was measured using spectrophotometry at a wavelength of λ = 230 nm. The experiment was conducted in three independent trials.
2.5.3 Assessment of Xanthine Oxidase Inhibitory Activity
Xanthine oxidase (XO) is recognized as a critical enzyme mediating the oxidation of hypoxanthine to xanthine, as well as the subsequent formation of uric acid. These catalytic processes are effectively suppressed in the presence of Allopurinol or phenolic compounds [21]. The inhibition of xanthine oxidase was evaluated using a modified protocol adapted from [22,23]. In this assay, a mixture was prepared by combining 1 mL of the test extract or Allopurinol (standard) with 2.9 mL of phosphate buffer (pH 7.5), followed by the addition of 0.1 mL of xanthine oxidase enzymatic solution (0.2 units/mL) and 1 mL of 0.5 mM xanthine solution. The resultant solution was incubated at 25°C for 15 min. To terminate the enzymatic reaction, 1 mL of HCl was introduced, after which the absorbance of the mixture was recorded at a wavelength of 295 nm. A control solution was prepared under identical conditions, excluding the enzymatic component. Allopurinol was employed as the positive control.
Four bacterial strains, comprising two Gram-negative (Escherichia coli ATCC 25922 and Salmonella Typhimurium ATCC 700408) and two Gram-positive (Staphylococcus aureus ATCC 29213 and Bacillus subtilis ATCC 6633), were utilized in the bioassays. The bacterial strains were cultivated on Mueller Hinton Agar (Biokar, Beauvais, France) and incubated at 37°C for 24 h. Fresh 24 h cultures were prepared for each experimental trial.
To evaluate the inhibition diameters of the studied extracts against the tested bacteria, we used the disc diffusion assay as previously described [24] with some modifications. Bacterial suspensions standardized to a 0.5 McFarland turbidity (approximately 108 CFU/mL) were prepared and inoculated via uniform swabbing onto Petri dishes containing Mueller-Hinton Agar (Biokar, Beauvais, France). Subsequently, 20 µL of each test extract was applied to sterile 6 mm diameter filter paper discs (Whatman No. 4), which were then aseptically positioned onto the agar surface. Sterile distilled water and chloramphenicol (30 µg/disc) served as negative and positive controls, respectively. All plates were incubated aerobically at 37°C for 24 h, after which the diameters of the zones of inhibition (including disk diameter) were measured in millimeters.
2.6.3 Determination of MIC and MBC
The minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) were assessed using a standardized microdilution technique in 96-well microtiter plates, as outlined in CLSI guidelines [17,25], with minor adaptations. Serial two-fold dilutions of the extract (40 μL) were prepared in sterile distilled water, followed by the addition of 40 μL of bacterial suspension (106 CFU/mL) and 120 μL of Müller Hinton broth (Biokar, Beauvais, France) to each well. A negative control, comprising sterile distilled water and extract, and a positive control, containing bacterial suspension and broth alone, were included. The plates were incubated at 37°C for 24 h. Subsequently, 40 μL of 0.2 g/mL 2,3,5-triphenyl tetrazolium chloride (TTC) was introduced into each well, followed by an additional 30 min incubation at 37°C. The MIC was defined as the lowest concentration at which no visible bacterial growth was observed. For MBC determination, 5 μL aliquots from non-turbid wells were aseptically transferred onto Müller Hinton Agar (Biokar, Beauvais, France), incubated at 37°C for 24 h, and the lowest concentration yielding no bacterial colony formation was recorded as the MBC.
2.7.1 ADMET In Silico Pharmacokinetics
The implementation of computational drug discovery methodologies may significantly diminish the necessity for experimental investigations while concurrently enhancing the likelihood of successful therapeutic development [26,27].
To evaluate the pharmacokinetic profiles of quercetin and rutin for their prospective pharmaceutical applications, ADMET (absorption, distribution, metabolism, excretion, and toxicity) profiling was employed. The online pkCSM platform was utilized to predict ADMET characteristics and patient tolerability.
Based on established criteria, intestinal absorption was classified as optimal at values exceeding 90%, whereas values below 30% were indicative of suboptimal bioavailability. The volume of distribution (VDss) was categorized as elevated at thresholds above 0.45 L/kg, reflecting favorable systemic dispersion. For blood-brain barrier (BBB) permeation, log BB values surpassing 0.3 denoted efficient trans-barrier penetration, while values below −0.1 suggested limited cerebral distribution. Central nervous system (CNS) permeability was assessed using standardized thresholds, with log PS values greater than −2 signifying adequate CNS penetration and values below −3 correlating with restricted access. These parameters collectively serve as critical benchmarks for the systematic evaluation of drug candidate viability through ADMET profiling [28,29].
In this section, we focus on antibacterial activity and have chosen S. aureus because this bacteria strain is involved in a wide range of pathologies, with varying degrees of severity. These microorganisms have been identified as primary etiological agents of healthcare-associated infections (nosocomial infections), though their transmission is not restricted to clinical settings, with community-acquired infections being increasingly documented. Ubiquitously distributed across human and animal reservoirs, they constitute commensal components of cutaneous microbiota and demonstrate preferential colonization of external mucous membranes. Notably, environmental persistence has been well-documented in diverse ecological niches, including aqueous systems (non-potable water sources), terrestrial substrates, and contaminated fomites.
The most active compounds, rutin and quercetin, were analyzed by DFT using Gaussian 09 software (Frisch et al., 2004). The conformation was then optimized using the B3LYP/6-31G (d, p) method (# opt B3LYP/6-31G geom= connectivity) to obtain the most stable form, which was then saved under the extension (ligand.pdb) (Table 1), to be used for molecular anchoring with the 1mwt active site.
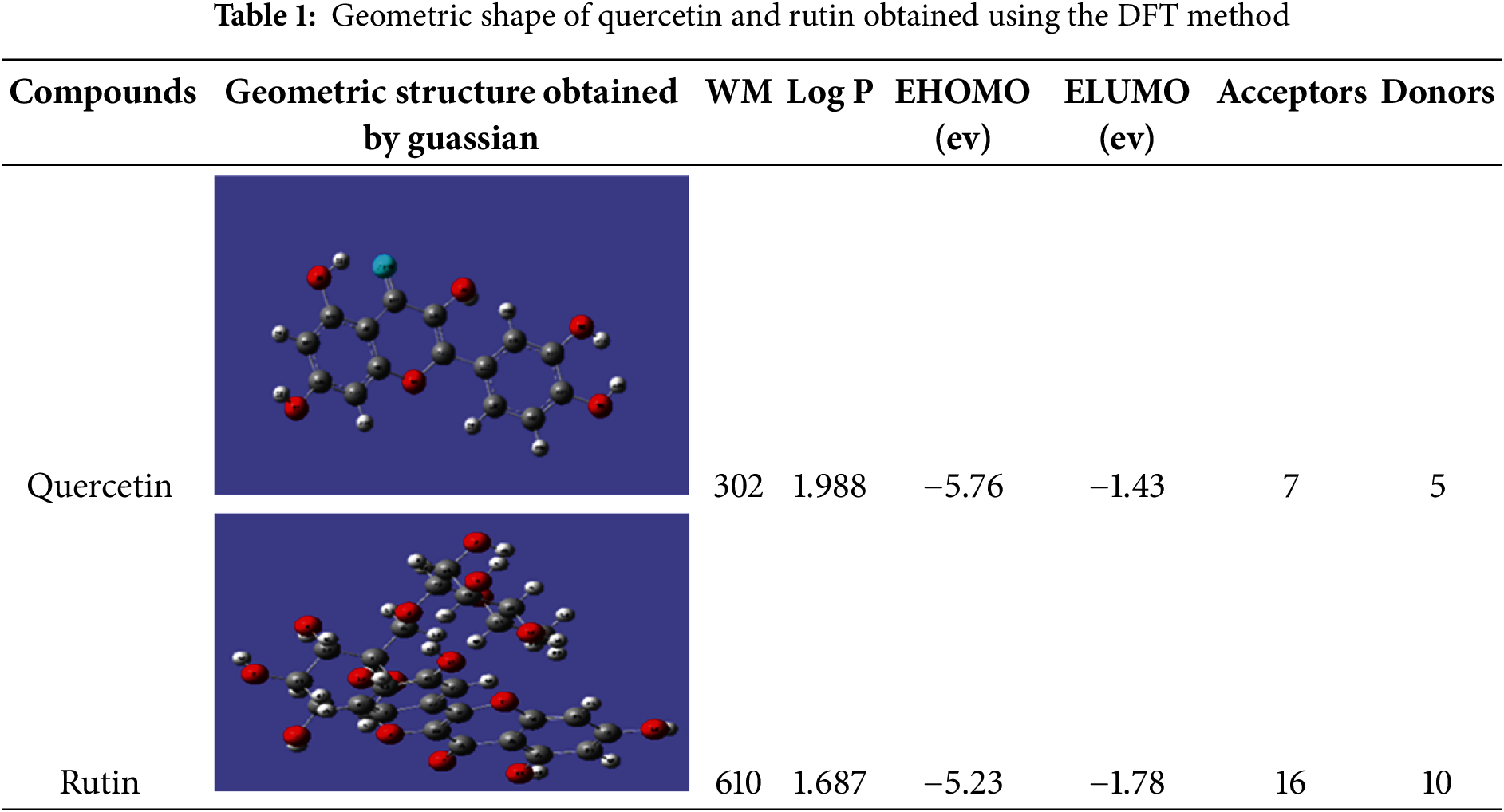
2.7.4 Preparation of the Active Site
The three-dimensional structural data of the target protein (PDB ID: 1MWT) were acquired from the RCSB Protein Data Bank. Structural refinement involved the removal of the co-crystallized penicillin ligand from the PLpro catalytic pocket, enabling topological analysis of ligand-interacting residues and subsequent delineation of the active site. Solvent molecules were computationally excised, and hydrogen atoms were parametrized using AutoDockTools [30] followed by molecular visualization via Discovery Studio Visualizer [31]. Ligand-protein interaction energies were quantified using the AUTOGRID37 algorithm [30]; to generate a three-dimensional affinity grid (Fig. 1). Binding free energies were estimated through empirical scoring functions, with conformational ensembles ranked based on thermodynamic stability. Computational parameters included a grid resolution of 0.35 Å and a cubic grid box spanning 60 × 60 × 60 Å3 to ensure comprehensive sampling of the catalytic domain.
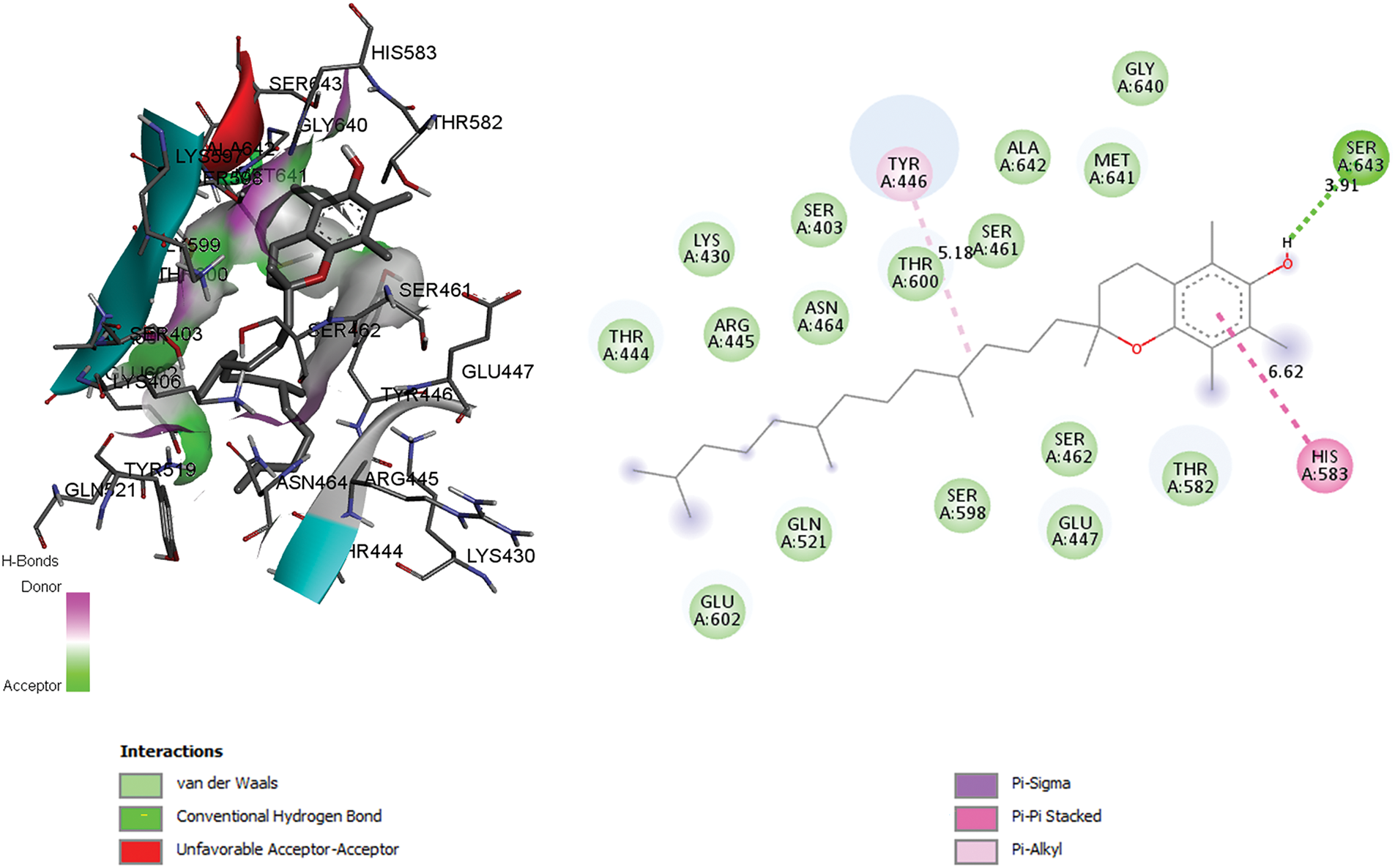
Figure 1: 2D and 3D anchor positions showing interactions of the co-crystallized compound (PENICILLIN G C16 H20 N2 O4 S) with penicillin G acyl and penicillin-binding protein 2a from Staphylococcus aureus strain 27r (binding energy 9.6 kcal/mol). This figure was created with Discovery Studio 3.5
GraphPad Prism 6 software (San Diego, CA, USA) was utilized for the analysis of biological data. As the data demonstrated normal distribution and homogeneity of variance, an unpaired t-test with Welch’s correction was performed to evaluate significant differences among the samples and relative to the control. A p-value of less than 0.05 was considered indicative of statistical significance.
3.1 Chemical Characterizations
3.1.1 Determination of Trace Elements Using ICP-AES
Macro-elements and microelements are the two categories of plants’ mineral composition. These components play a crucial role in the cell’s vital biological processes. The elemental composition of the leaves and seed cones of J. oxycedrus is presented in Table 2 below; the concentrations were expressed in mg/kg of the dry weight of the plant material. Macro-elements (S, K, Ca, Mg, P, Na, Fe), microelements (B, Cu, Mn, Zn, V), and heavy metals (Cd, Pb, Ni, Mo, Cr, As, Co) were determined in the studied organs of this plants. Our results show that sulfur (S) is the most abundant macro-element in both samples. The concentration of this element was 43.860 and 39.660 mg/kg respectively for leaves and seed cones. Calcium (Ca) and potassium (K) were the second most abundant elements. The concentration of potassium was 4530 and 7560 mg/kg, while that of calcium was 18820 and 5420 mg/kg respectively for leaves and seed cones. For the other macroelements, the concentrations varied between 730 and 860 mg/kg for magnesium (Mg), 584 and 710 mg/kg for phosphorus (P), 116.97 and 185.67 mg/kg for sodium (Na), and 39.51 and 217.85 mg/kg for iron (Fe).
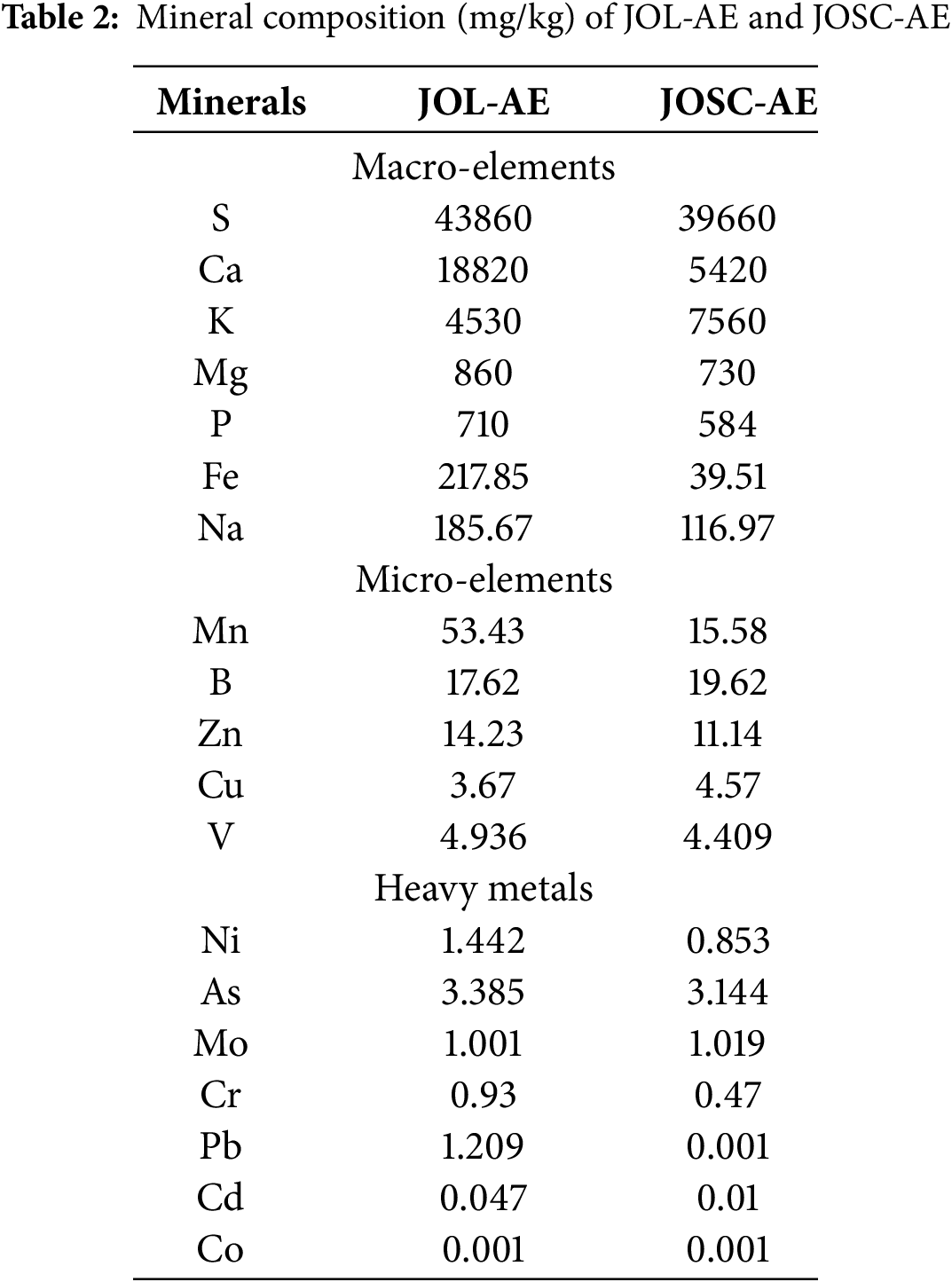
The quantification of microelements showed that the most abundant element is manganese (Mn) with a concentration of 53.43 and 15.58 mg/kg respectively for the leaves and the seed cones of J. oxycedrus. Followed by boron (B) with a concentration of 17.62 and 19.62 mg/kg respectively for leaves and seed cones. The concentrations of the other microelements varied between 3.67 and 4.57 mg/kg for copper (Cu), 11.14 and 14.23 mg/kg for zinc (Zn), and 4.409 and 4.936 mg/kg for vanadium (V).
Both samples showed low concentrations of heavy metal residues in the organs studied (Table 2). Their concentrations varied between 0.047 and 0.01 mg/kg for Cadmium (Cd), 0.001 and 1.209 mg/kg for Lead (Pb), 0.853 and 1.442 mg/kg for Nikel (Ni), 1.001 and 1.019 mg/kg for molybdenum (Mo), 0.47 and 0.93 mg/kg for chromium (Cr), 3.144 and 3.385 mg/kg for arsenic (As) and 0.001 and 0.001 mg/kg for cobalt (Co).
Elevated concentrations of copper (Cu: 7.10 mg/kg), manganese (Mn: 27.79 mg/kg), chromium (Cr: 2.87 mg/kg), nickel (Ni: 10.43 mg/kg), and iron (Fe: 187.95 mg/kg) were reported in Juniperus oxycedrus seed cones from Turkey by [32], exceeding values quantified in the present investigation. In contrast, reduced zinc (Zn: 7.70 mg/kg) concentrations were documented relative to current findings. The seed cones and leaves of J. oxycedrus can be categorized as nutritious foods since they contain enough amounts of microelements and a high concentration of macronutrients. Additionally, the absence or presence of extremely low amounts of heavy metals in these plants is crucial for their therapeutic usage without toxicity.
3.1.2 Identification of Phenolic Compounds
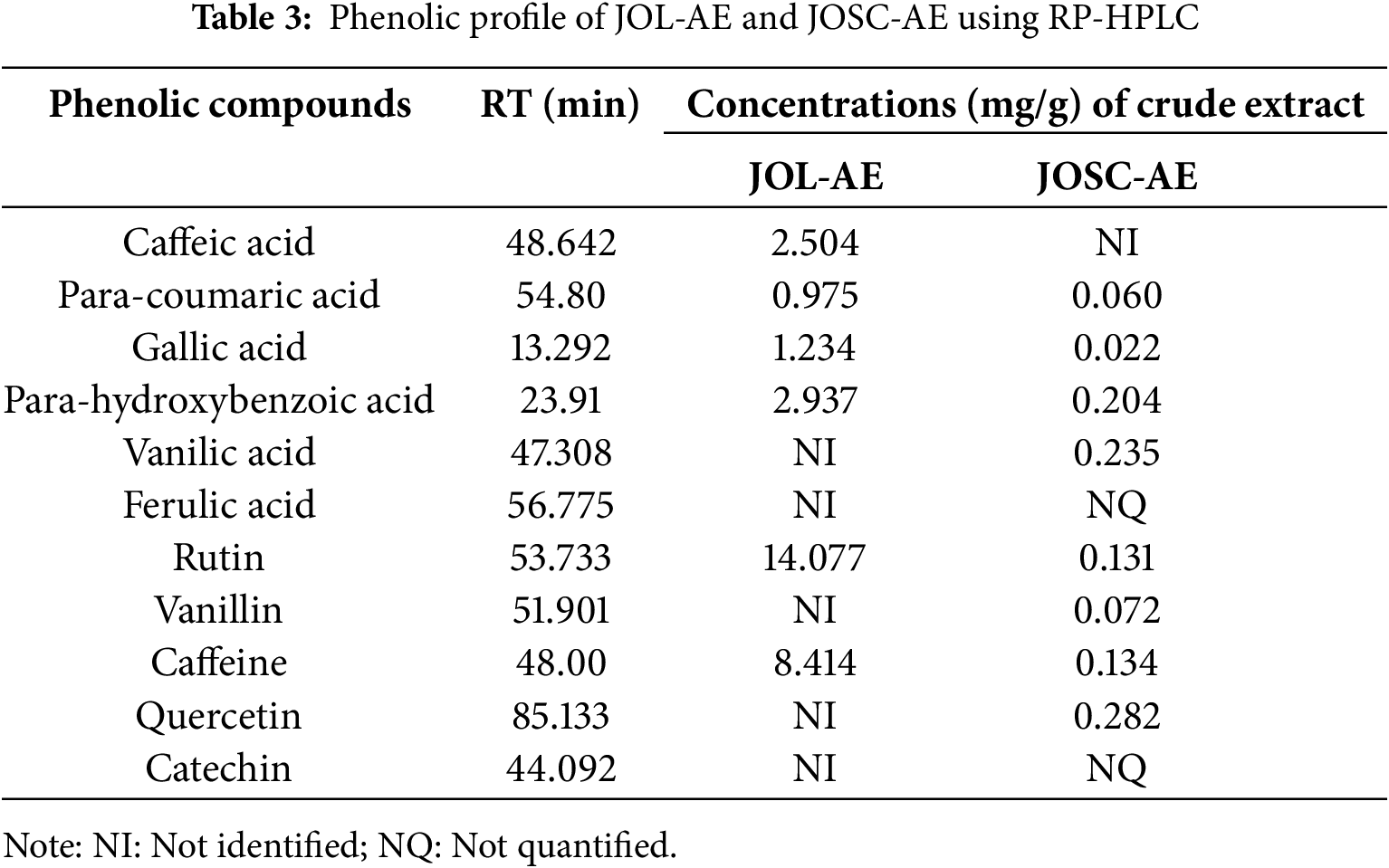
The presence of phenolic acids and flavonoids in the aqueous extracts of Juniperus oxycedrus leaves and seed cones is identified through HPLC analysis. Six compounds were identified in JOL-AE, namely, para-hydroxybenzoic acid, caffeic acid, gallic acid, para-coumaric acid, caffeine, and rutin. While 10 were detected in JOSC-AE, which are Gallic Acid, Para-hydroxybenzoic Acid, Vanillic Acid, Para-coumaric Acid, Ferulic Acid, Quercetin, Catechin, Rutin, Caffeine and Vanillin (Table 3). To ensure the superposition of each standard with the corresponding molecule, we overloaded the extract with standards corresponding to the suspected molecules and we observed an increase in their concentrations.
3.1.3 Quantification of Phenolic Compounds
The quantification of the identified compounds showed that the JOL-AE (Fig. 2) is more concentrated in phenolic compounds than that of the JOSC-AE (Fig. 3). The main compound in JOL-AE was rutin with a concentration of (14.007 mg/g of raw extract), followed by caffeine (8.414 mg/g), parahydroxybenzoic acid (2.937 mg/g), caffeic acid (2.504 mg/g), gallic acid (1.234 mg/g) and paracoumaric acid (0.957 mg/g) (Table 3). For JOSC-AE, the concentrations were (0.282 mg/g of extract) for quercetin, (0.235 mg/g) for vanillic acid, (0.204 mg/g) for para-hydroxybenzoic acid, (0.134 mg/g) for caffeine, (0.131 mg/g) for Rutin, (0.072 mg/g) for vanillin, (0.060 mg/g) for para-coumaric acid, (0.022 mg/g) for gallic acid (Table 3).
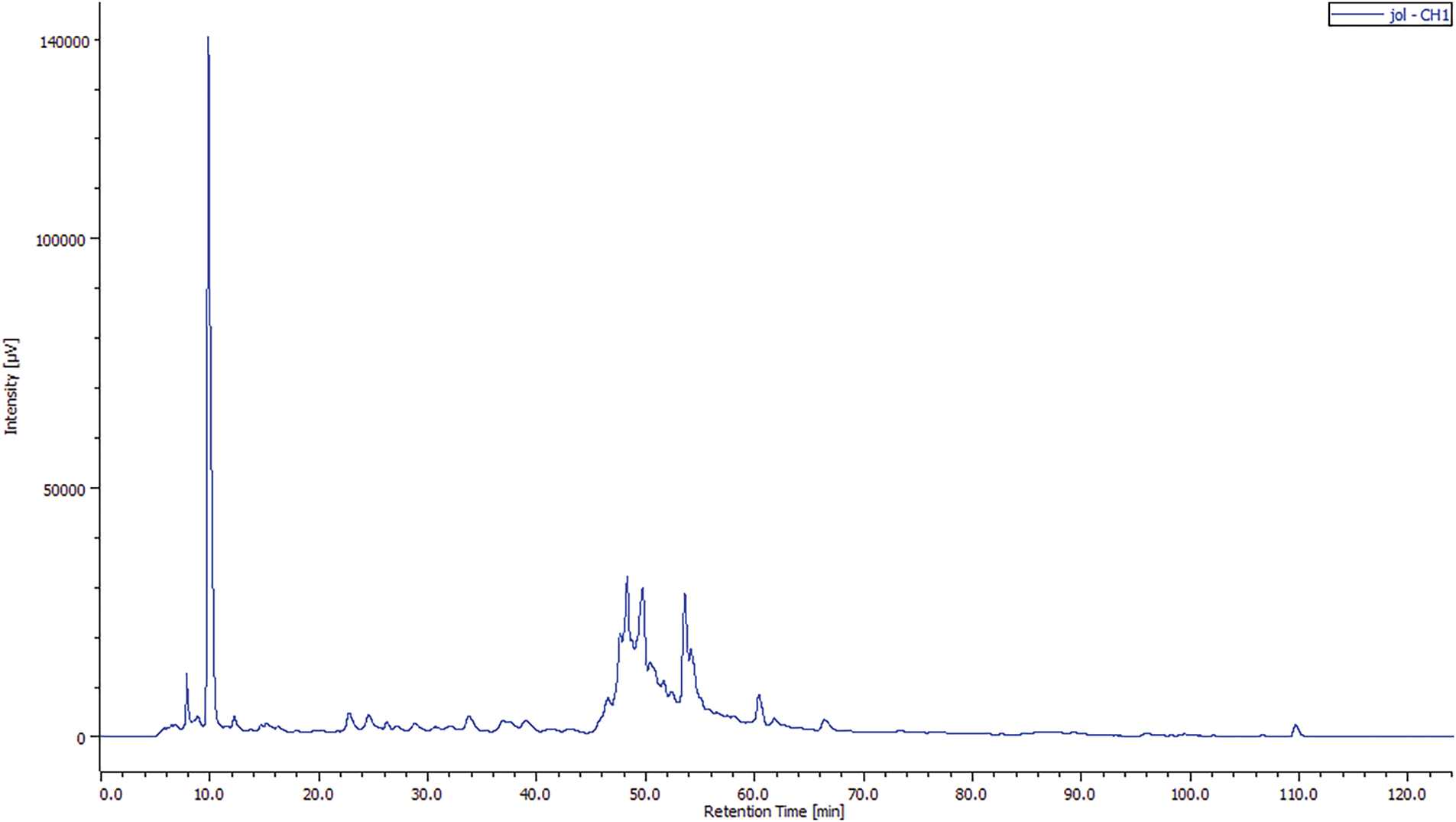
Figure 2: Chromatogram of the phenolic profile of JOL-AE by HPLC-UV
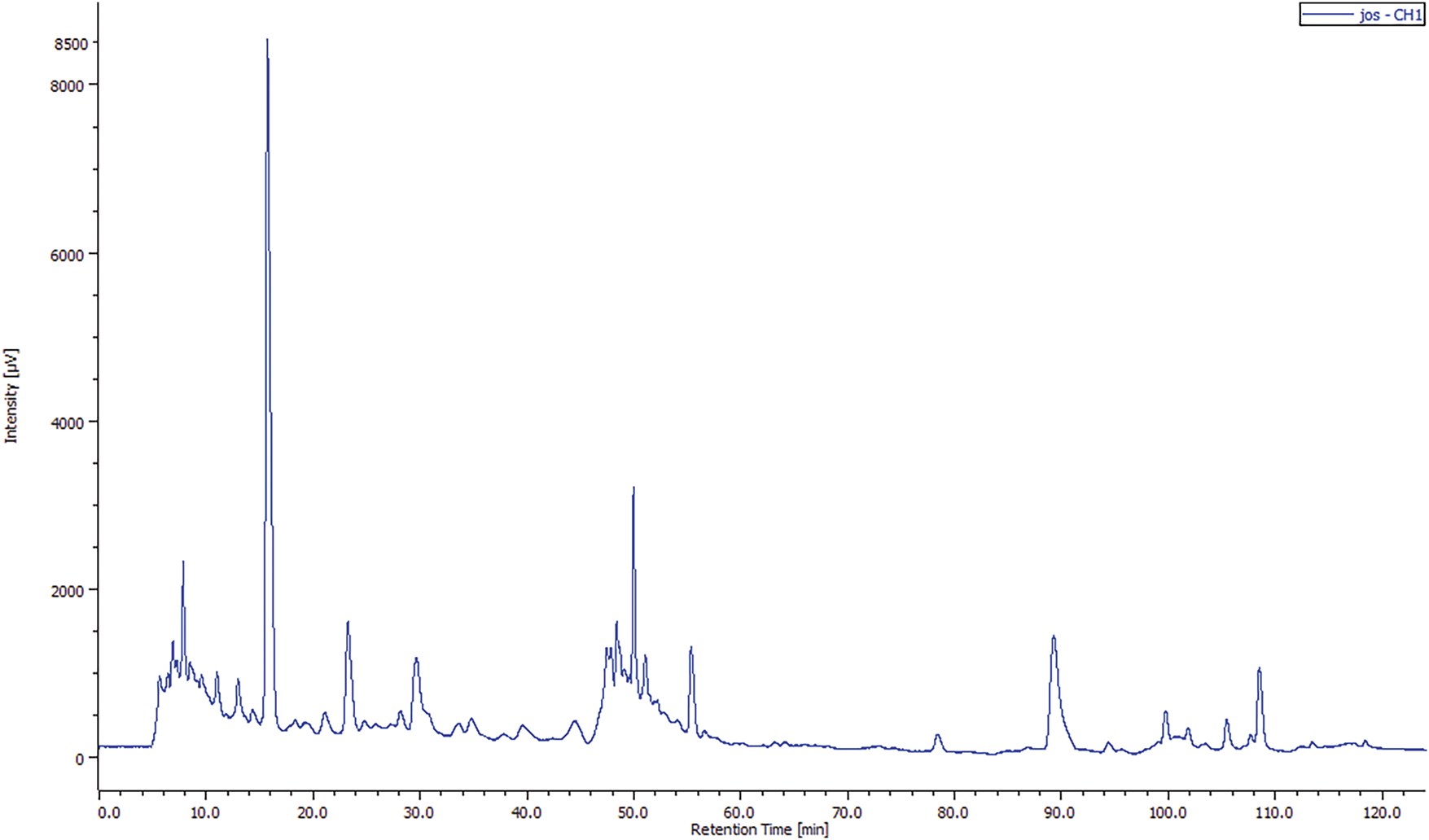
Figure 3: Chromatogram of the phenolic profile of JOSC-AE by HPLC-UV
While some research has been conducted on this species, only a limited number focus on its typical consumption method (infusion). In a previous study by [33] who worked on J. oxycedrus collected in Amasya/Turkey. These authors noted that rutin was identified as the main compound of the methanolic extract of JOL (0.11 mg per g of extract), but its concentration was only 0.01 mg per g for seed cones extract. We also noted that the phenolic profile of the aqueous extracts of seed cones and leaves is close to that reported by [13] where they mentioned the presence of caffeic acid, para-coumaric acid, para-hydroxybenzoic acid, and rutin in the extracts of J. oxycedrus from Taza city, Morocco. These researchers also mentioned that rutin is the main compound of the leaf’s aqueous extract with a concentration of 10.805 mg per gramme of dry extract and that the concentration of the identified compounds is very low in the seed cones extract.
3.1.4 Total Polyphenol and Flavonoid Quantification
Phenolic compounds are generally the best-known secondary metabolites in plants. These molecules are characterized by significant antioxidant activity and other beneficial biological effects for human health.
The phytochemical analysis of both extracts revealed varying levels of phenolic compounds (Table 4). Notably, JOL-AE exhibited the highest phenolic content (79.38 ± 0.82 mg GAE/g dw), whereas JOSC-AE had the lowest (6.59 ± 0.27 mg GAE/g dw). A similar trend was observed for flavonoid content, with JOL-AE containing 29.47 ± 0.2 mg QE/g dw, compared to 2.69 ± 0.08 mg QE/g dw in JOSC-AE.
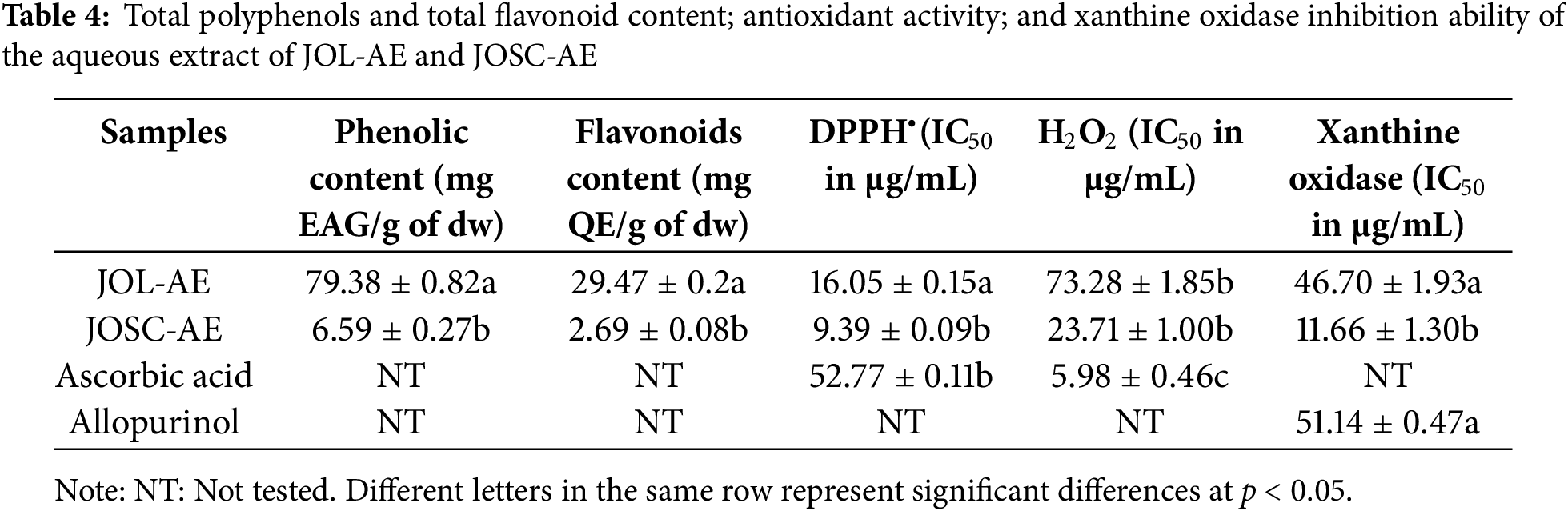
Comparing our results with those obtained by other researchers. For J. oxycedrus leaves we observed that our sample showed a lower content of total phenolic compounds with a concentration of 79.38 ± 0.82 mg EAG/g of crude extract than that obtained by [13] who found a value of 147.29 mg EAG/g crude extract. On the other hand, for total flavonoids, our results showed higher content for needles extract with a content of 29.47 ± 0.2 mg EQ/g of crude extract than those reported by [34] who found a concentration of 1.68 ± 0.01 mg of EQ/g of crude extract.
For JOSC-AE, few studies have focused on the identification and dosage of the phenolic compounds contained in the various aqueous extracts of this plant. Our results show that the concentration of total polyphenols is 6.59 ± 0.27 mg EAG/g of crude extract, while other authors found a higher value of 28.11 mg EAG/g of crude extract [13]. For flavonoids, our results show that this extract is poor in total flavonoids, the concentration obtained is 2.69 ± 0.08 mg of EQ/g of crude extract, this value closely approximates that obtained by [13] who found a concentration of 3.2 mg of EQ/g for the aqueous extract and of 8.3 mg of EQ/g for the methanolic extract. This difference may be caused by the use of another extraction method by these authors, as well as we cannot ignore that the subspecies were not the same, which influences the concentration of secondary metabolites of the studied samples.
3.2.1 DPPH• Radical Scavenging Activity
The evaluation of antioxidant activity in plant extracts necessitates the inclusion of the 2,2-diphenyl-1-picrylhydrazyl (DPPH•) radical scavenging assay as a fundamental screening method. By the protocols detailed in the methodology, Juniperus oxycedrus aqueous extracts (JOL-AE and JOSC-AE) were subjected to DPPH• radical neutralization analysis. A concentration-dependent inhibitory effect was observed across experimental replicates. It was demonstrated that both extracts exhibited potent radical scavenging capacity, with half-maximal inhibitory concentrations (IC50) quantified at 9.39 ± 0.09 μg/mL for JOSC-AE and 16.05 ± 0.15 μg/mL for JOL-AE (Fig. 4; Table 4). Higher concentrations were excluded from the analysis due to solubility constraints. Notably, the scavenging efficacy of both extracts surpassed that of the reference antioxidant ascorbic acid, which yielded an IC50 value of 52.77 ± 0.11 μg/mL.
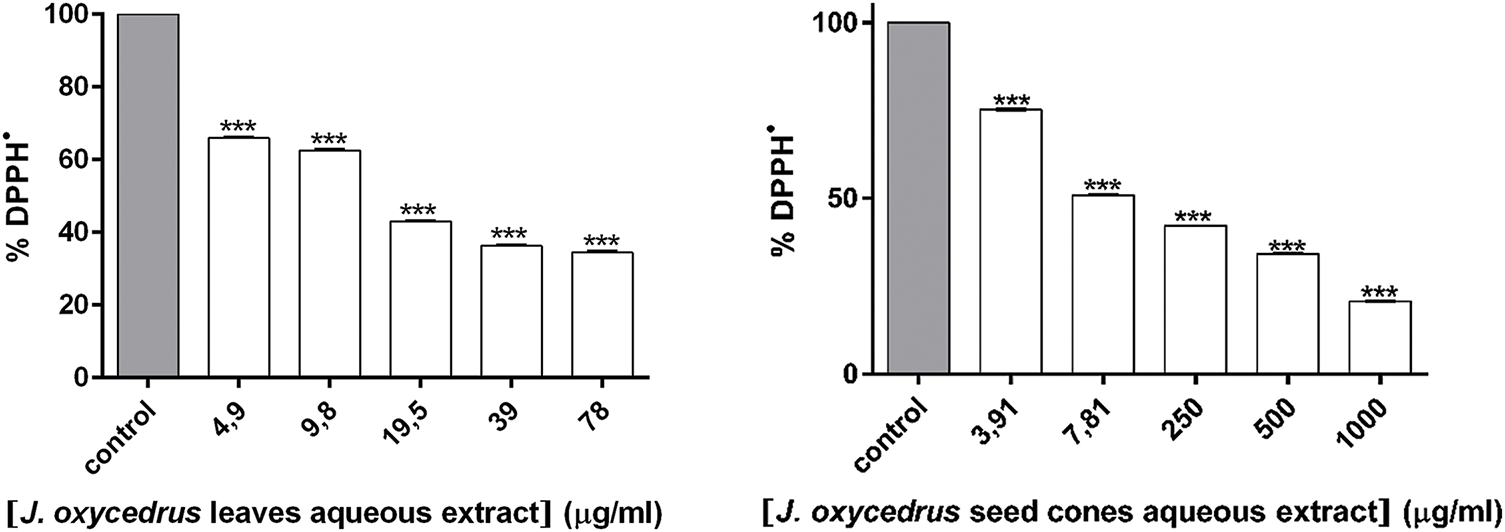
Figure 4: DPPH• scavenging activity of JOL-AE and JOSC-AE. *** represent significant difference as determined by unpaired t-test with Welch’s correction (p < 0.001)
For JOL-AE our results showed a better scavenging capacity against the DPPH• radical (IC50 value of 16.05 ± 0.15 μg extract/mL) than that reported by [35] who obtained an IC50 value of 140 ± 0.01 µg/mL, and similar activity to that obtained by [36] who reported an IC50 value of 17.91 ± 0.37 μg extract/mL, these authors used the same chemical systems and the same extraction method as well. On the other hand, [5] studied the hydro-methanolic extract (80%) and obtained a higher capacity to scavenge the radical DPPH• with an IC50 value of (10.95 ± 0.7 μg/mL). Conversely, the ability of JOSC-AE was better than that reported by [13] who obtained an IC50 value of 960 ± 0.07 μg/mL. These authors used the same chemical systems and the same extraction methods. Similarly, reference [37] studied the ability of the ethanolic extract to scavenge the DPPH• radical and obtained a lower activity than ours with an IC50 value of 64.49 ± 0.23 μg of extract/mL. The observed variations in bioactivities may be partially attributed to discrepancies in the mass/solvent volume ratio, solvent polarity, and extraction duration employed across studies, as these parameters were not standardized. Furthermore, potential intersubspecific differences, which directly affect phytochemical constituents and their resultant pharmacological properties, cannot be disregarded as a contributory factor.
3.2.2 Antioxidant Activity against Hydrogen Peroxide (H2O2)
The antioxidant power of the JOL-AE and JOSC-AE was studied against oxygen peroxide (H2O2), the obtained results show that both extracts have a strong activity against Hydrogen peroxide with IC50 values of 23.71 ± 1.00 μg/mL and 73.28 ± 1.85 μg/mL respectively for JOSC-AE and JOL-AE. The ability of both extracts to scavenge the H2O2 was lower than that obtained by ascorbic acid which showed an IC50 value of 5.98 ± 0.46 μg/mL. The IC50 values obtained are presented in Table 4.
The chemical analysis showed the presence of many phenolic compounds, which are known for their antioxidant activity against Oxygen and nitrogen reactive species, which make sense of the obtained results. Other researchers [38] proved the inhibition of reactive oxygen species production in the presence of hydroethanolic extract of J. oxycedrus leaves using concentrations of 1.25, 2.5, and 5 µg/mL.
3.3 Inhibition of Xanthine Oxidase In-Vitro
The ability of JOL-AE and JOSC-AE to inhibit xanthine oxidase activity was verified. Indeed, the seed cones extract has a good inhibitory activity against this enzyme with an IC50 value of 11.66 ± 1.3 μg/mL (Table 4), which was higher than that obtained by the leaf extract with an IC50 value of 46.7 ± 1.93 μg/mL. However, both extracts showed stronger activity than that obtained by the positive control (Allopurinol) which showed an IC50 value of 51.14 ± 0.47 (μg/mL).
To the best of our knowledge, the inhibition activity of JOL-AE and JOSC-AE against xanthine oxidase has never performed before. Nevertheless, the activity of the studied extracts against XO is very interesting and may be due to the presence of flavonoids such as quercetin and rutin, especially J. oxycedrus leaves, which are known for this higher XO inhibition. The lowest concentrations of these flavonoids in J. oxycedrus seed cones proved that other bioactive compounds, such as terpenoids, phenolic acids, or other secondary metabolites, may also play a significant role in the observed activity (XO inhibition).
Several previous studies have mentioned that rutin and quercetin inhibit the activity of the XO enzyme [29,39]. Also, according to Cos et al. [29], a flavonoid compound with hydroxyl groups at C5 and C7 and a double bond between C2 and C3 is required for a strong inhibitory effect on xanthine oxidase. Moreover, Nagao et al. have stated that hydroxyl groups at the C5 and C7 of flavonoids were necessary for inhibiting the activity of xanthine oxidase but not a hydroxyl group at the C3 of quercetin’s flavone structure [40]. Our results suggested that the amount and type of flavonoids and phenolic compounds in the extracts could influence their ability to inhibit XO activity. Our findings also agreed with those of previous reports suggesting that polyphenols and other minerals in aqueous extracts could interact synergically to increase their total antioxidant activity [41,42].
The antibacterial activity of the studied extracts was carried out by disc diffusion and broth micro-dilution assays, and results were presented in Table 5 and Fig. 5. Disc diffusion assay revealed that JOL-AE had the higher antibacterial activity with inhibition diameters ranging between 12.8 ± 0.3 mm and 17.2 ± 1.1 mm, while JOSC-AE had inhibition diameters between 11.2 ± 0.3 mm and 15.2 ± 0.8 mm. Moreover, broth micro-dilution assay showed that JOL-AE has the highest antibacterial activity with MICs of 6.25, 12.5, 3.12, and 1.56 mg/mL against E. coli, S. Typhimurium, S. aureus, and B. subtilis, respectively. While JOSC-AE showed MIC values of 12.5, 25.0, 6.25, and 6.25 mg/mL, respectively (Table 5). The ratio (MBC/MIC ≤ 4) indicates that these extracts had bactericidal activity against the tested bacteria [43].
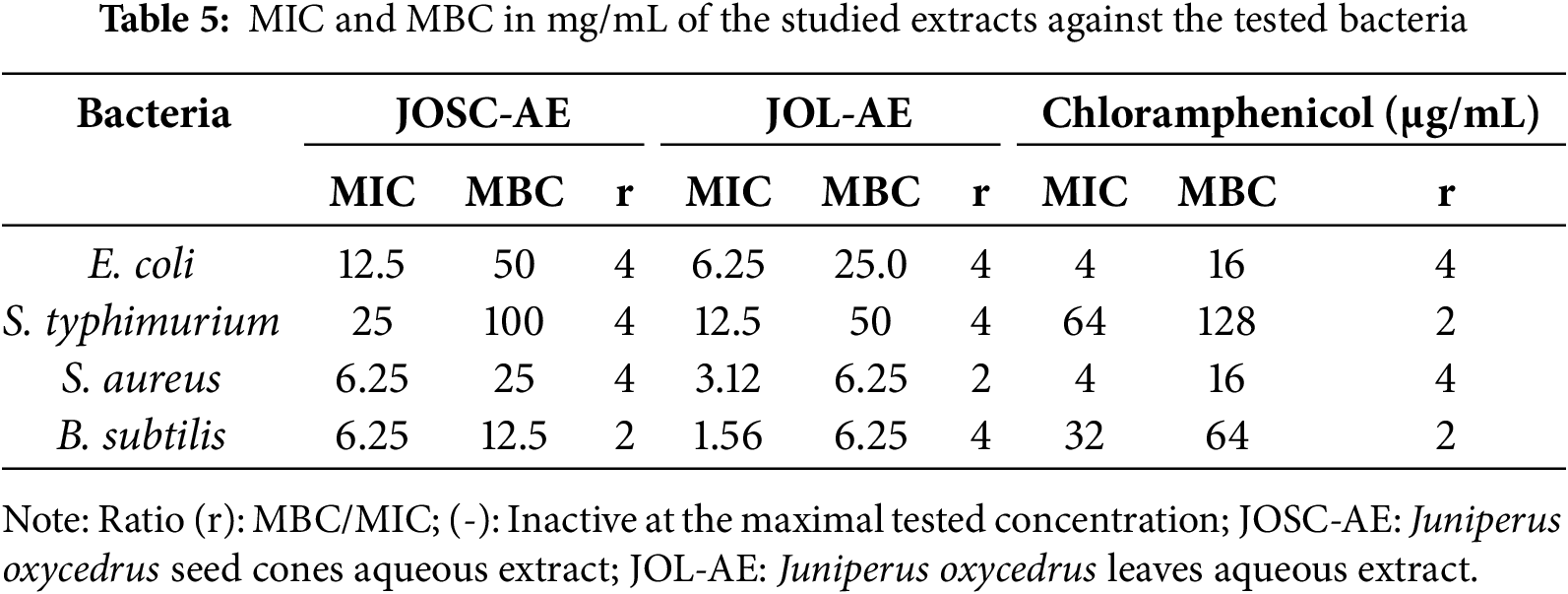
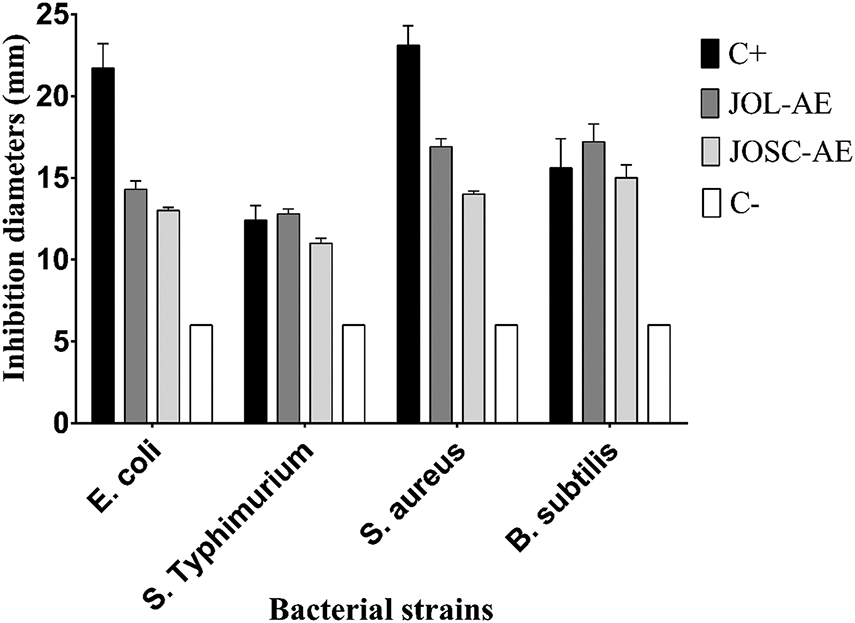
Figure 5: Inhibition diameters (mm) of the studied aqueous extracts against the tested bacteria. C+: positive control (Chloramphenicol; 30 µg); C-: negative control (sterile distilled water)
A previous Turkish study showed that the aqueous extract of J. oxycedrus displayed a bacteriostatic activity versus S. aureus (MIC of 78.12 µg/mL), while it was ineffective against Gram-negative [35]. However, Karaman and their colleagues showed that aqueous leaf extract of J. oxycedrus collected from Turkey was inactive against a panel of Gram-positive and Gram-negative bacteria when tested at a concentration of 300 µg/disc [44]. Taviano et al. [6] reported that methanolic extract of two J. oxycedrus subspecies (oxycedrus and macrocarpa (Sibth. & Sm)) from Turkey have a good antibacterial activity against S. aureus with a MIC value of 625.00 μg/mL [6]. These authors used a different solvent for extraction. Furthermore, we cannot ignore that the subspecies are unlikely to be identical, which also affects the chemical composition and thus the biological activity.
The antibacterial activity of plant extracts has been postulated to be associated with their distinct phytochemical profiles [45]. It is noteworthy that variations in the anatomical origin of the plant material and the quantitative variability of phenolic constituents are critical determinants of the antimicrobial efficacy observed in such extracts. Consequently, the antibacterial effects reported for Juniperus oxycedrus extracts may be mechanistically linked to the relative abundance of phenolic compounds, which are known to modulate bioactive properties through structure-dependent interactions [46]. In this regard, previous studies demonstrated that p-coumaric acid had strong antibacterial action against S. aureus and E. coli with MIC values of 20 and 80 μg/mL, respectively [47]. Additionally, previous studies have demonstrated the antibacterial capacity of caffeic acid, caffeine, and their derivatives; in addition to their synergic effect to enhance the antimicrobial activity of the conventional antibiotics against the drug-resistant bacteria [25,48,49]. Moreover, Rutin and its derivatives showed interesting antibacterial activity against bacteria and yeast (Al-Majmaie et al., 2019), and enhance the antibacterial activities of flavonoids against Bacillus cereus and Salmonella Enteritidis [50]. The observed variations in the phytochemicals and biological activities of Juniperus extracts are not only attributable to differences in geographic origin but could also stem from taxonomic distinctions between species. Prof. Adams’ seminal work established that the eastern J. oxycedrus, commonly found in Turkey and surrounding regions, is a distinct species now recognized as Juniperus deltoides [51]. This species is taxonomically separate from the western J. oxycedrus, prevalent in the western Mediterranean, and from J. macrocarpa. The distinction between these species underscores the importance of precise taxonomic identification when interpreting comparative studies on Juniperus species [52]. The recognition of J. deltoides as a separate species highlights the potential for significant biochemical and ecological differences, which may influence the composition and activity of the extracts. Therefore, our study’s findings should be viewed within this context to avoid misleading interpretations, as these taxonomic distinctions may partially explain the differences observed.
The significant barrier-penetration potential of both compounds has been demonstrated in prior studies. Drug metabolism, defined as the biotransformation processes mediated by enzymatic activity within biological systems, was investigated (Table 6). Notably, inhibition of cytochrome P450 3A4 (CYP3A4) emerged as a critical pharmacological consideration, with the selected compounds exhibiting dual functionality as both substrates and competitive inhibitors of this enzyme. Pharmacokinetic evaluation further revealed reduced total clearance values, indicative of prolonged systemic retention, with one compound, Quercetin, demonstrating favorable pharmacokinetic persistence. Concurrent toxicity assessments confirmed the absence of adverse biological effects within the tested parameters. Collectively, these findings suggest that both compounds exhibit favorable pharmacokinetic profiles, characterized by efficient penetration, metabolic stability, and low toxicity, warranting further investigation for therapeutic applications.
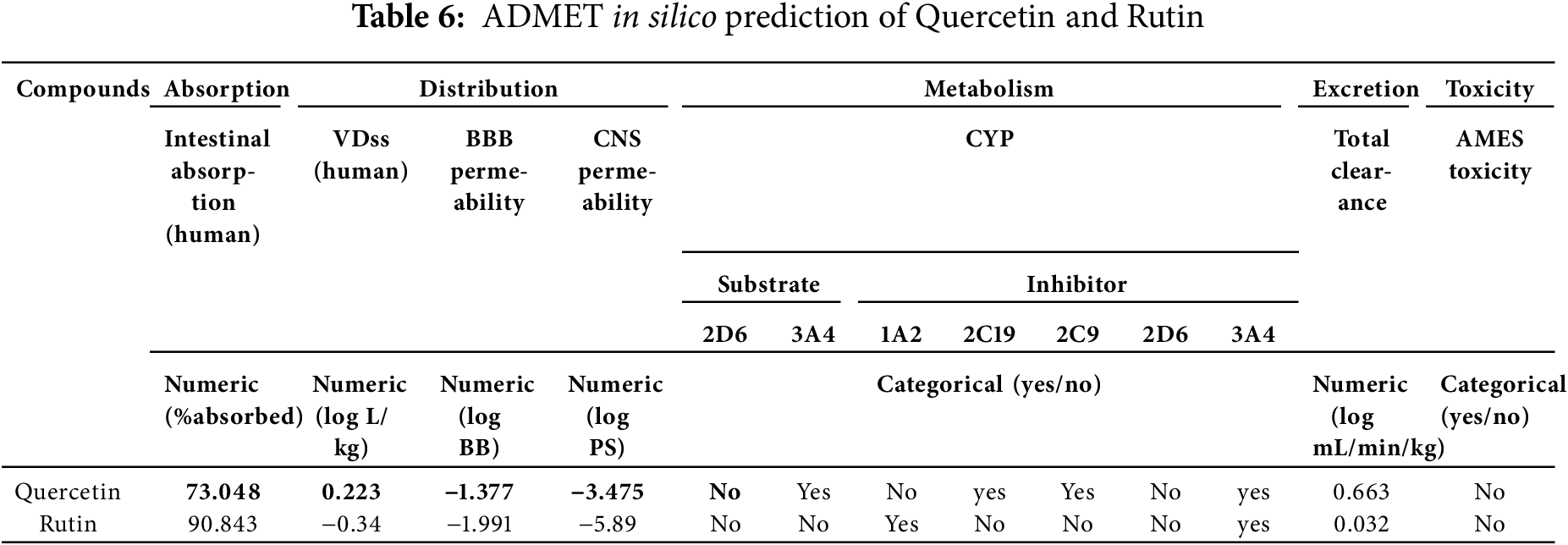
Quercetin exhibits a good binding pattern with the protein’s active site (Table 7), which is ensured by three hydrogen bonds with Ser403, Ser598, Gly640 having a bond length of 4.11 Å, 3.44 Å, 4.00 Å, respectively, this type of interaction gives the molecule a high degree of stability in the active site, especially as it is oxygen-rich and has a higher electronegativity, making it more reactive and receptive to electrons, as can be seen with the bonds formed with the amino acid Serine. Interactions between residues Tyr446, His583, and Ala642, as well as other significant Pi-Alkyl interactions, are of great importance for the stability of the complex, particularly those with a distance less than 6 A°. The other residues forming the complex are therefore mainly the result of Van der Waals interactions: Lys406, Tyr519, Gln521, Asn464, Glu602, Thr600, Thr582, Ser643, Gly599, and Ser462.
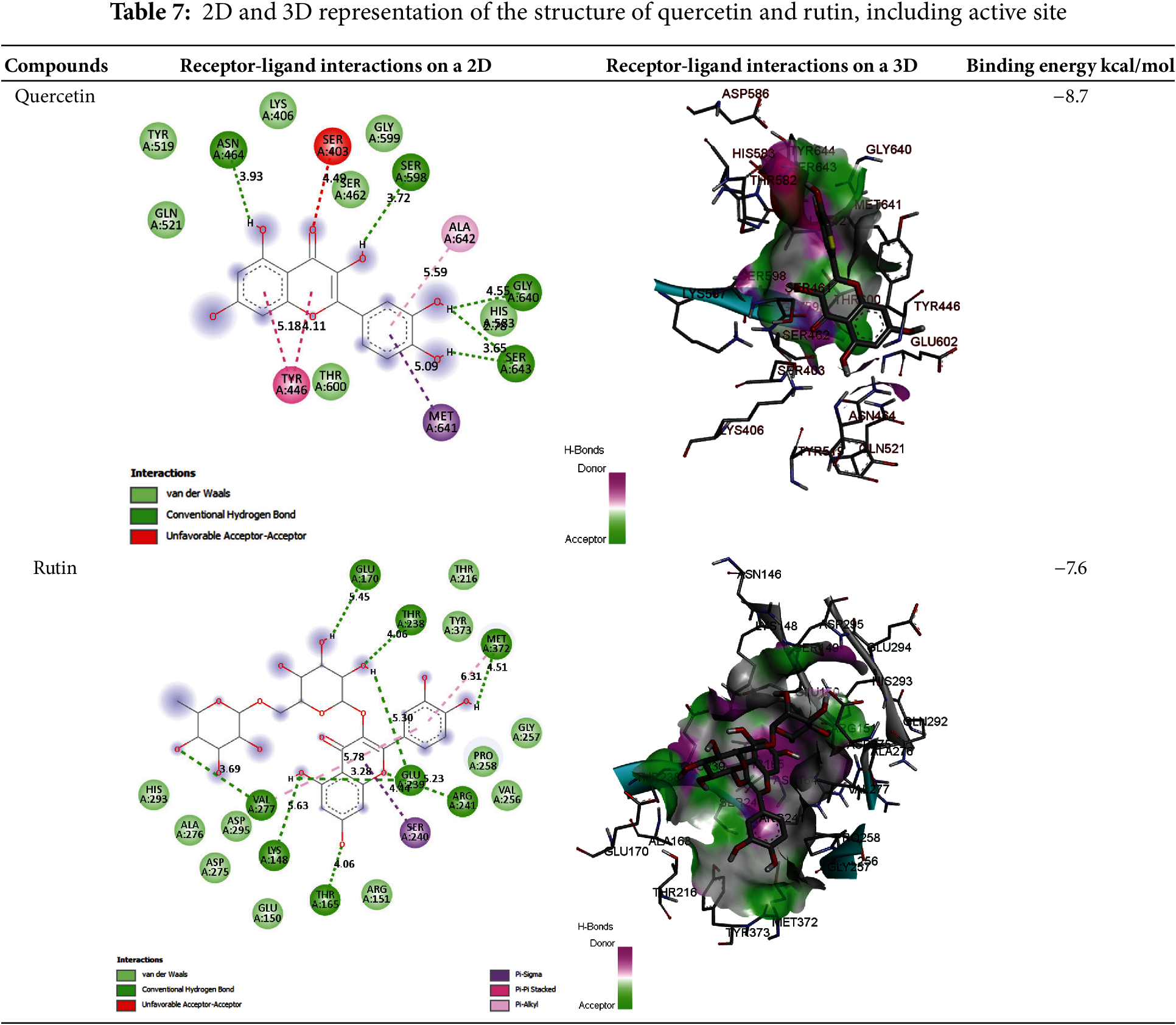
In addition, the Rutin also binds well to the PLpro catalytic pocket of penicillin G acyl-Penicillin, involving eight hydrogen bonds with Glu170, Thr238, Met372, Glu239, Arg241, Thr165, Lys148 and Val277 with a bond length of 5.45 Å, 4.06 Å, 4.51 Å, 5.23 Å, 3.28 Å, 2.88 Å, 4.06 Å, 3.69 Å, respectively, and other important hydrophobic interactions via Ser240 with a distance of 4.44 A°, other residues interact with the active site via Van der Waals interactions such as Asp164, Val165, Met208, Met 243, Ser 245, Ala246, Pro247, Pro248, Gly266, Thr308 (Table 7) This type of binding is called dipole-dipole interaction and is based on electrostatic attraction/repulsion between quercetin with fixed dipoles and the active site.
A comparison of the attacks of the two most active molecules shows that quercetin attacks virtually the same active site, while rutin attacks a different region of the protein, which explains the high antibacterial efficacy of both extracts. To sum up this theoretical section, the results obtained are very similar to the experimental results, leading us to propose these molecules as highly effective drugs against this type of bacteria.
The aim of the present study was to determine the antioxidant, anti-inflammatory, and antimicrobial potential, as well as the mineral composition of J. oxycedrus from the region of Taza Morocco. The aqueous extract showed strong antioxidant activity against DPPH• and H2O2 and a high potential to inhibit the catalyzing power of xanthine oxidase. The chemical composition showed the richness of this plant on phenolic acids, flavonoids, and mineralogical components with absence/minor concentrations of heavy metals. The presence of bioactivity appears to be linked in part to the major phenolic compounds, as these metabolites possess crucial structural features that influence biological activities. These findings collectively promote the use of this plant material. Furthermore, because of its abundance in bioactive natural compounds, its richness of benefit minerals in the absence of heavy metals incorporating this extract into dietary supplements and pharmaceutical formulations could prove beneficial in addressing diseases linked to inflammation, bacterial infections, and oxidative stress. J. oxycedrus extracts can serve as a valuable resource for the development of pharmaceutical products targeting oxidative stress and microbial infections. Furthermore, their natural origin and bioactive profile position them as excellent candidates for incorporation into nutraceutical formulations, offering health benefits through dietary supplements. Future studies should focus on scaling up the extraction process, validating the safety and efficacy through in vivo models, and exploring formulation strategies for clinical applications.
Acknowledgement: Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R158), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Conceptualization: Mohamed reda Kachmar; methodology: Mohamed reda Kachmar, Abdelaziz Ed-dra, Safaâ Kachmar, Hammou Anarghou, Sulaiman Mohammed Alnasser, Fahad M. Alshabrmi, Samiah Hamad Al-Mijalli, Emad M. Abdallah, Nidal Naceiri Mrabti; formal analysis: Emad M. Abdallah; data analysis: Abdelmounaim Laabar, Mourad Chikhaoui, Galman Aziz; investigation: Toufik Bouddine, Nidal Naceiri Mrabti, Abdelmounaim Laabar, Mourad Chikhaoui, Galman Aziz; resources: Said Chakir; data curation: Mohamed reda Kachmar, Toufik Bouddine, Mourad Chikhaoui; writing—original draft preparation: Mohamed reda Kachmar, Fahad M. Alshabrmi; writing—review and editing: Toufik Bouddine, Hammou Anarghou, Abdelaziz Ed-dra, Said Chakir visualization, Mohamed reda Kachmar, Abdelaziz Ed-dra, Safaâ Kachmar; supervision: Lhoussain Hajji, Said Chakir; project administration, Said Chakir. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: No datasets were generated or analyzed during this study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Abbreviations
| H2O2 | Hydrogen peroxide |
| XO | Xanthine oxidase |
| JOSC-AE | Juniperus oxycedrus seed cones aqueous extract |
| JOL-AE | Juniperus oxycedrus leaves aqueous extract |
| ICP-AES | Inductively coupled plasma atomic emission spectrometry |
| HPLC | High-performance liquid chromatography |
| TTC | 2,3,5-triphenyltetrazolium chloride |
| FeCl3·6H2O | Iron(III) chloride hexahydrate |
| RF | Radio frequency |
| MICs | Minimum inhibitory concentrations |
| MBCs | Minimum bactericidal concentrations |
| ADMET | Absorption, distribution, metabolism, excretion, and toxicity |
| BBB | Blood-brain barrier |
| CNS | Central nervous system |
References
1. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. doi:10.3389/fphar.2013.00177. [Google Scholar] [PubMed] [CrossRef]
2. Loizzo MR, Tundis R, Conforti F, Saab AM, Statti GA, Menichini F. Comparative chemical composition, antioxidant and hypoglycaemic activities of Juniperus oxycedrus ssp. oxycedrus L. berry and wood oils from Lebanon. Food Chem. 2007;105:572–8. doi:10.1016/j.foodchem.2007.04.015. [Google Scholar] [CrossRef]
3. De Medina F, Gámez M, Jiménez I, Jiménez J, Osuna J, Zarzuelo A. Hypoglycemic activity of juniper berries. Planta Med. 1994;60:197–200. doi:10.1055/s-2006-959457. [Google Scholar] [PubMed] [CrossRef]
4. Orhan N, Berkkan A, Orhan DD, Aslan M, Ergun F. Effects of Juniperus oxycedrus ssp. oxycedrus on tissue lipid peroxidation, trace elements (Cu, Zn, Fe) and blood glucose levels in experimental diabetes. J Ethnopharmacol. 2011;133:759–64. doi:10.1016/j.jep.2010.11.002. [Google Scholar] [PubMed] [CrossRef]
5. Chaouche TM, Haddouchi F, Ksouri R, Medini F, Atik-Bekara F. In vitro evaluation of antioxidant activity of the hydro-methanolic extracts of Juniperus oxycedrus subsp. oxycedrus. Phytothérapie. 2013;11:244–9. doi:10.1007/s10298-013-0779-5. [Google Scholar] [CrossRef]
6. Taviano MF, Marino A, Trovato A, Bellinghieri V, Melchini A, Dugo P, et al. Juniperus oxycedrus L. subsp. oxycedrus and Juniperus oxycedrus L. subsp. macrocarpa (Sibth. & Sm.) Ball.“berries” from Turkey: comparative evaluation of phenolic profile, antioxidant, cytotoxic and antimicrobial activities. Food Chem Toxicol. 2013;58:22–9. doi:10.1016/j.fct.2013.03.049. [Google Scholar] [PubMed] [CrossRef]
7. Benkhaira N, Zouine N, Fadil M, Koraichi SI, El Hachlafi N, Jeddi M, et al. Application of mixture design for the optimum antibacterial action of chemically-analyzed essential oils and investigation of the antiadhesion ability of their optimal mixtures on 3D printing material. Bioprinting. 2023;34:e00299. doi:10.1016/j.bprint.2023.e00299. [Google Scholar] [CrossRef]
8. Okoro IO, Osagie A, Asibor EO. Antioxidant and antimicrobial activities of polyphenols from ethnomedicinal plants of Nigeria. Afr J Biotechnol. 2010;9(20):2989–93. [Google Scholar]
9. Oumaskour K, Boujaber N, Etahiri S, Assobhei O. Screening of antibacterial and antifungal activities in green and brown algae from the coast of Sidi Bouzid (El Jadida, Morocco). Afr J Biotechnol. 2012;11:16831–7. [Google Scholar]
10. Lafraxo S, El Moussaoui A, Bin Jardan A, El Barnossi Y, Chebaibi A, Baammi M, et al. GC-MS profiling, in vitro antioxidant, antimicrobial, and in silico NADPH oxidase inhibition studies of essential oil of juniperus thurifera bark. In: El Sayed M, editors. Evidence-based complementary and alternative medicine. Vol. 2022. 2022. p. 1–13. [Google Scholar]
11. Mrabti HN, Doudach L, Kachmar MR, Khali Z, Mrabti NN, Benrahou KB. Phenolic content, antibacterial, antioxidant, and toxicological investigations of Erodium guttatum (Geraniaceae) collected from the Northeast of Morocco. Turk J Bot. 2021;45:739–49. doi:10.3906/bot-2107-29. [Google Scholar] [CrossRef]
12. Kachmar MR, El Majdoub YO, Oliveira AP, Bouymajane A, Mrabti HN, Bouddine T, et al. Juniperus oxycedrus leaves and berries extracts: HPLC-PDA-ESI/MS2 phenolic characterization and in vitro anti-inflammatory effects. Phytomedicine Plus. 2024;4:100528. doi:10.1016/j.phyplu.2024.100528. [Google Scholar] [CrossRef]
13. Ben Mrid R, Bouchmaa N, Bouargalne Y, Ramdan B, Karrouchi K, Kabach I, et al. Phytochemical characterization, antioxidant and in vitro cytotoxic activity evaluation of Juniperus oxycedrus Subsp. oxycedrus needles and berries. Molecules. 2019;24:502. doi:10.3390/molecules24030502. [Google Scholar] [PubMed] [CrossRef]
14. El Hachlafi N, Elbouzidi A, Batbat A, Taibi M, Jeddi M, Addi M, et al. Chemical composition and assessment of the anti-inflammatory, antioxidant, cytotoxic and skin enzyme inhibitory activities of Citrus sinensis (L.) osbeck essential oil and its major compound limonene. Pharmaceuticals. 2024;17:1652. doi:10.3390/ph17121652. [Google Scholar] [PubMed] [CrossRef]
15. Al-Mijalli SH, Mrabti HN, Elbouzidi A, Ashmawy NS, Batbat A, Abdallah EM, et al. Thymus serpyllum L. essential oil: phytochemistry and in vitro and in silico screening of its antimicrobial, antioxidant and anti-inflammatory properties. Phyton-Int J Exp Bot. 2025;94:209–27. doi:10.32604/phyton.2025.060438. [Google Scholar] [CrossRef]
16. Nouioura G, El Fadili M, El Hachlafi N, Maache S, Mssillou I, Abuelizz A, et al. Coriandrum sativum L., essential oil as a promising source of bioactive compounds with GC/MS, antioxidant, antimicrobial activities: in vitro and in silico predictions. Front Chem. 2024;12:1369745. doi:10.3389/fchem.2024.1369745. [Google Scholar] [PubMed] [CrossRef]
17. Chebbac K, Moussaoui AE, Bourhia M, Salamatullah AM, Alzahrani A, Guemmouh R. Chemical analysis and antioxidant and antimicrobial activity of essential oils from Artemisia negrei L. against drug-resistant microbes. Evid Based Complement Alternat Med. 2021;2021:5902851. doi:10.1155/2021/5902851. [Google Scholar] [PubMed] [CrossRef]
18. Sroka Z, Cisowski W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem Toxicol. 2003;41:753–8. doi:10.1016/s0278-6915(02)00329-0. [Google Scholar] [PubMed] [CrossRef]
19. Chen H, Bai Z, Tao S, Li M, Jian L, Zhang Y, et al. Optimization of enzyme-assisted microwave extraction, structural characterization, antioxidant activity and in vitro protective effect against H2O2-induced damage in HepG2 cells of polysaccharides from roots of Rubus crataegifolius Bunge. Int J Biol Macromol. 2024;276:133969. doi:10.1016/j.ijbiomac.2024.133969. [Google Scholar] [PubMed] [CrossRef]
20. Que Y, Zhang Y, Liang F, Wang L, Yang Y, Zhang J, et al. Structural characterization, antioxidant activity, and fermentation characteristics of Flammulina velutipes residue polysaccharide degraded by ultrasonic assisted H2O2-Vc technique. Ultrason Sonochem. 2024;111:107085. doi:10.1016/j.ultsonch.2024.107085. [Google Scholar] [PubMed] [CrossRef]
21. Osman NI, Sidik NJ, Awal A, Adam NAM, Rezali NI. In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. and total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. J Intercult Ethnopharmacol. 2016;5:343. doi:10.5455/jice.20160731025522. [Google Scholar] [PubMed] [CrossRef]
22. Dhammaraj T, Kotseekieo P, Chotikarn T, Phosrithong N, Praison W, Prasomsub T, et al. In vitro investigation of xanthine oxidase inhibitory and antioxidant activities of 3,4,5-trihydroxycinnamic acid. J Herbmed Pharmacol. 2024;13:439–49. doi:10.34172/jhp.2024.49420. [Google Scholar] [CrossRef]
23. Trinh PTN, Truc NC, Danh TT, Trang NTT, Le Hang DT, Hung QT. A study on the antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activity of the Artemisia vulgaris L. extract and its fractions. J Ethnopharmacol. 2024;334:118519. doi:10.1016/j.jep.2024.118519. [Google Scholar] [PubMed] [CrossRef]
24. Benkhaira N, El Hachlafi N, Jeddi M, Abdnim R, Bnouham M, Koraichi SI, et al. Unveiling the phytochemical profile, in vitro bioactivities evaluation, in silico molecular docking and ADMET study of essential oil from Clinopodium nepeta grown in Middle Atlas of Morocco. Biocatal Agricult Biotechnol. 2023;54:102923. doi:10.1016/j.bcab.2023.102923. [Google Scholar] [CrossRef]
25. Reshetnikov DV, Burova LG, Rybalova TV, Bondareva EA, Patrushev SS, Evstropov AN, et al. Synthesis and antibacterial activity of caffeine derivatives containing amino-acid fragments. Chem Nat Compd. 2022;58:908–15. doi:10.1007/s10600-022-03826-3. [Google Scholar] [CrossRef]
26. Ferreira LL, Andricopulo AD. ADMET modeling approaches in drug discovery. Drug Discov Today. 2019;24:1157–65. doi:10.1016/j.drudis.2019.03.015. [Google Scholar] [PubMed] [CrossRef]
27. Elbouzidi A, Taibi M, Laaraj S, Loukili EH, Haddou M, El Hachlafi N, et al. Chemical profiling of volatile compounds of the essential oil of grey-leaved rockrose (Cistus albidus L.) and its antioxidant, anti-inflammatory, antibacterial, antifungal, and anticancer activity in vitro and in silico. Front Chem. 2024;12:1334028. doi:10.3389/fchem.2024.1334028. [Google Scholar] [PubMed] [CrossRef]
28. Pliska V, Testa B, van de Waterbeemd H, Mannhold R, Kubinyi H, Timmerman H. Lipophilicity in drug action and toxicology. Hoboken, NJ, USA: VCH Weinheim; 1996. [Google Scholar]
29. Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, et al. Structure−activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod. 1998;61:71–6. doi:10.1021/np970237h. [Google Scholar] [PubMed] [CrossRef]
30. Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. doi:10.1002/jcc.21256. [Google Scholar] [PubMed] [CrossRef]
31. Kouranov A, Xie L, de la Cruz J, Chen L, Westbrook J, Bourne PE, et al. The RCSB PDB information portal for structural genomics. Nucleic Acids Res. 2006;34:D302–5. doi:10.1093/nar/gkj120. [Google Scholar] [PubMed] [CrossRef]
32. Ozkaya A, Ciftci H, Yilmaz O, Zafer Tel A, Cil E, Cevrimli BS. Vitamin, trace element, and fatty acid levels of Vitex agnus-castus L., Juniperus oxycedrus L., and Papaver somniferum L. plant seeds. J Chem. 2013;2013:845743. doi:10.1155/2013/845743. [Google Scholar] [CrossRef]
33. Yaglioglu AS, Eser F. Screening of some Juniperus extracts for the phenolic compounds and their antiproliferative activities. S Afr J Bot. 2017;113:29–33. doi:10.1016/j.sajb.2017.07.005. [Google Scholar] [CrossRef]
34. Guenane H, Gherib A, Bakchiche B, Carbonell-Barrachina ÁA, Hernández F, Cano-Lamadrid M. Antioxidant capacity, mineral content and essential oil composition from select algerian medicinal plants. Scient Study Res Chem Chem Eng, Biotechnol, Food Indus. 2017;18:275–89. [Google Scholar]
35. Miceli N, Marino A, Köroğlu A, Cacciola F, Dugo P, Mondello L, et al. Comparative study of the phenolic profile, antioxidant and antimicrobial activities of leaf extracts of five Juniperus L. (Cupressaceae) taxa growing in Turkey. Nat Prod Res. 2020;34:1636–41. doi:10.1080/14786419.2018.1523162. [Google Scholar] [PubMed] [CrossRef]
36. El Jemli M, Kamal R, Marmouzi I, Zerrouki A, Cherrah Y, Alaoui K. Radical-scavenging activity and ferric reducing ability of Juniperus thurifera (L.J. oxycedrus (L.J. phoenicea (L.) and Tetraclinis articulata (L.). Adv Pharmacol Sci. 2016;2016:6392656. doi:10.1155/2016/6392656. [Google Scholar] [PubMed] [CrossRef]
37. Živić N, Milošević S, Dekić V, Dekić B, Ristić N, Ristić M, et al. Phytochemical and antioxidant screening of some extracts of Juniperus communis L. and Juniperus oxycedrus L. Czech J Food Sci. 2019;37(5):351–8. doi:10.17221/28/2019-CJFS. [Google Scholar] [CrossRef]
38. Tavares L, McDougall GJ, Fortalezas S, Stewart D, Ferreira RB, Santos CN. The neuroprotective potential of phenolic-enriched fractions from four Juniperus species found in Portugal. Food Chem. 2012;135:562–70. doi:10.1016/j.foodchem.2012.05.023. [Google Scholar] [PubMed] [CrossRef]
39. Ahmad NS, Farman M, Najmi MH, Mian KB, Hasan A. Pharmacological basis for use of Pistacia integerrima leaves in hyperuricemia and gout. J Ethnopharmacol. 2008;117:478–82. doi:10.1016/j.jep.2008.02.031. [Google Scholar] [PubMed] [CrossRef]
40. Nagao A, Seki M, Kobayashi H. Inhibition of xanthine oxidase by flavonoids. Biosci Biotechnol Biochem. 1999;63:1787–90. doi:10.1271/bbb.63.1787. [Google Scholar] [PubMed] [CrossRef]
41. Ordonez AAL, Gomez JD, Vattuone MA. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006;97:452–8. doi:10.1016/j.foodchem.2005.05.024. [Google Scholar] [CrossRef]
42. Shahidi F, Wanasundara UN, Amarowicz R. Natural antioxidants from low-pungency mustard flour. Food Res Intern. 1994;27:489–93. doi:10.1016/0963-9969(94)90244-5. [Google Scholar] [CrossRef]
43. Ed-Dra A, Nalbone L, Filali FR, Trabelsi N, El Majdoub YO, Bouchrif B, et al. Comprehensive evaluation on the use of Thymus vulgaris essential oil as natural additive against different serotypes of Salmonella enterica. Sustainability. 2021;13:4594. doi:10.3390/su13084594. [Google Scholar] [CrossRef]
44. Karaman I, Şahin F, Güllüce M, Öǧütçü H, Şengül M, Adıgüzel A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J Ethnopharmacol. 2003;85:231–5. doi:10.1016/S0378-8741(03)00006-0. [Google Scholar] [PubMed] [CrossRef]
45. Ennajar M, Bouajila J, Lebrihi A, Mathieu F, Abderraba M, Raies A, et al. Chemical composition and antimicrobial and antioxidant activities of essential oils and various extracts of Juniperus phoenicea L. (Cupressacees). J Food Sci. 2009;74(7):M364–71. doi:10.1111/j.1750-3841.2009.01277.x. [Google Scholar] [PubMed] [CrossRef]
46. Zaazaa L, Naceiri Mrabti H, Ed-Dra A, Bendahbia K, Hami H, Soulaymani A, et al. Determination of mineral composition and phenolic content and investigation of antioxidant, antidiabetic, and antibacterial activities of Crocus sativus L. Aqueous stigmas extracts. Adv Pharmacol Pharm Sci. 2021;2021:7533938. doi:10.1155/2021/7533938. [Google Scholar] [PubMed] [CrossRef]
47. Chen S-L, Yu H, Luo H-M, Wu Q, Li C-F, Steinmetz A. Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chin Med. 2016;11:37. doi:10.1186/s13020-016-0108-7. [Google Scholar] [PubMed] [CrossRef]
48. Khan F, Bamunuarachchi NI, Tabassum N, Kim Y-M. Caffeic acid and its derivatives: antimicrobial drugs toward microbial pathogens. J Agric Food Chem. 2021;69:2979–3004. doi:10.1021/acs.jafc.0c07579. [Google Scholar] [PubMed] [CrossRef]
49. Woziwodzka A, Krychowiak-Maśnicka M, Gołuński G, Łosiewska A, Borowik A, Wyrzykowski D, et al. New life of an old drug: caffeine as a modulator of antibacterial activity of commonly used antibiotics. Pharmaceuticals. 2022;15:872. doi:10.3390/ph15070872. [Google Scholar] [PubMed] [CrossRef]
50. Arima H, Ashida H, Danno G. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci Biotechnol Biochem. 2002;66:1009–14. doi:10.1271/bbb.66.1009. [Google Scholar] [PubMed] [CrossRef]
51. Adams RP, Terzioğlu S, Mataraci T. Taxonomy of Juniperus oxycedrus var. spilinanus in Turkey: leaf terpenoids and SNPS from nrDNA and petN. Phytologia. 2010;92:156–66. [Google Scholar]
52. Adams RP, Morris JA, Pandey RN, Schwarzbach AE. Cryptic speciation between Juniperus deltoides and Juniperus oxycedrus (Cupressaceae) in the Mediterranean. Biochem Syst Ecol. 2005;33:771–87. doi:10.1016/j.bse.2005.01.001. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools