 Open Access
Open Access
ARTICLE
Coffea arabica var. Borbon Biochemical Response to Chitosan Oligosaccharides Foliar Exposure
1 Laboratory of Biotecnology, Instituto de Ciencias Agrícolas de la Universidad Autónoma de Baja California (ICA-UABC), Nuevo León, 21705, Baja California, México
2 Department of Molecular Biology, Tecnológico Nacional de México, Instituto Tecnológico de Tuxtla Gutierrez, Tuxtla Gutierrez, 29050, Chiapas, México
* Corresponding Author: Daniel González-Mendoza. Email:
(This article belongs to the Special Issue: Plant Responses to Abiotic Stress Mechanisms)
Phyton-International Journal of Experimental Botany 2025, 94(3), 641-655. https://doi.org/10.32604/phyton.2025.062871
Received 30 December 2024; Accepted 20 February 2025; Issue published 31 March 2025
Abstract
The biochemical response of Coffea arabica var. Borbon to chitosan and chitosan oligosaccharides (COS) was evaluated in one-year-old plants under greenhouse conditions. COS solutions were synthesized through chemical and physical hydrolysis using acetic acid, hydrogen peroxide, and microwave irradiation. The obtained COS had an average molecular weight (Mw) of 3549.90 ± 0.33 Daltons (Da), a deacetylation degree (DD) of 76.64 ± 1.12%, and a polymerization degree (PD) of 18.91 ± 0.0018. Solutions of chitosan and COS were applied to C. arabica var. Borbon at concentrations of 0.25, 0.5, and 1 wt%. The experimental design was conducted using a completely randomized design with four replications. The biochemical responses assessed included soluble protein content, phenylalanine ammonia-lyase (PAL), chitinase, β-1,3-glucanase, peroxidase, catalase, and chlorophyll fluorescence. The application of COS demonstrated significant differences (α = 0.05) in protein concentration, with the activity of β-1,3-glucanase, chitinase, and catalase being 1.5, 7.5, and 3.9 times higher, respectively, while showing similar behavior to chitosan in PAL activity, both up to 4.4 times higher than the distilled water control and lower than chitosan in peroxidase activity. Treatments with chitosan yielded a higher photochemical efficiency of Photosystem II (PSII). The application of COS suggests a viable foliar alternative to active plant defense mechanisms without the risk of phytotoxicity.Keywords
Abbreviations
| CAT | Catalase |
| CERK1 | Chitin Elicitor Receptor Kinase 1 |
| CHI | Chitosan |
| Chl | Chlorophyll |
| CLR | Coffee Leaf Rust |
| COS | Chitosan oligosaccharides |
| CytPM-COS | Cytosinpeptidemycin and COS |
| DA | Degree of Acetylation |
| DAMPS | Damage Associated Molecular Patterns |
| DD | Deacetylation Degree |
| Fv/Fm | Potential photochemical yield |
| GlcN | D-glucosamine |
| GlcNAc | N-acetyl-D-glucosamine |
| HR | Hypersensitive Response |
| ISR | Induced Systemic Resistance |
| MW | Molecular Weight average |
| PAL | Phenylalanine Ammonium Lyase |
| PD | Polymerization Degree |
| POX | Peroxidases |
| PPO | Polyphenol oxidase |
| PR | Pathogenesis Related proteins |
| PRRS | Pattern Recognition Receptors |
| PSII | Photosystem II |
| PTI | PAMPs or DAMPs triggered immunity |
| ROS | Reactive Oxygen Species |
| SAR | Systemic Acquired Resistance |
| SOD | Superoxide Dismutase |
| SPC | Soluble Protein Content |
The global coffee trade is one of the most important commodity markets worldwide [1]. In 2023, coffee production reached 171.4 million bags (60 kg), with Brazil as the leading coffee-producing country, followed by Vietnam. Mexico ranked 10th in global coffee production [2], with 81%–82% of its output concentrated in the states of Chiapas, Veracruz, and Puebla [3]. In 2023, approximately 87% of Mexico’s total coffee production was Coffea arabica, while the remaining percentage was Coffea canephora, commonly known as arabica and robusta, respectively [2]. C. arabica generates higher income in Mexico due to its cup qualities, but it also presents production challenges due to its susceptibility to pests and diseases [4]. For instance, Coffee Leaf Rust (CLR), caused by Hemileia vastatrix, an obligate biotrophic fungus, and Coffee Berry Disease (CBD), caused by Colletotrichum kahawae Waller & Bridge [5], are among the most threatening diseases for coffee plantations. H. vastatrix infections in C. arabica plantations can cause yield losses of up to 70% for farmers. In 2016, Mexico reported a 50% loss in C. arabica production due to the severity of the CLR epidemic [6]. Several coffee varieties grown in Mexico, such as Typica, Bourbon, Caturra, Mundo Novo, and Garnica, are known for their susceptibility to CLR [7].
Some alternatives for controlling phytopathogens in coffee plantations involve cultural practices as well as chemical and biological control methods. Cultural practices involve modifying plantation management to create conditions that limit the spread of phytopathogens [6,8]. These measures include reducing plantation density and implementing a canopy crop program to minimize microenvironments and direct contact between plants [9], maintaining a younger population of coffee plants, and ensuring proper plant nutrition with acidified soil to enhance nutrient uptake [8]. Additionally, coffee plantation management could also include establishing physical barriers to protect the plant population from wind, adverse temperature conditions, and the spread of pathogen inoculum [8,10]. Natural barriers also facilitate the implementation of these physical barriers and biological control strategies by enriching of soil microbiota [8,10,11].
Chemical control involves the application of exogenous substances to coffee plants, either soil treatment or foliar spraying. Some pesticides used in coffee plantations belong to chemical families such as carbamates, neonicotinoids, triazoles, and organophosphates [12]. These substances can act as protective or systemic fungicides [13]. Exogenous elicitors are other substances used to control phytopathogens, which do not necessarily possess direct fungicidal activity [14]. Elicitors are molecules recognized by patter recognition receptors (PRRs) that activate the plant’s defense mechanisms. Elicitors can be sourced from microbial secretions or from the wound tissues of host plants. Some alternatives for controlling phytopathogens include foliar applications of exogenous elicitors related to the target pathogen as a protective strategy [6].
The primary mechanism in biological and chemical control by elicitors involves activating the plant host defense mechanisms. Plants react to microorganism invasion with biochemical changes associated with biotic stress signaling, which alters their physiology. For instance, available oxygen can be utilized to produce Reactive Oxygen Species (ROS) that inhibit pathogen growth [5,15]. Additional defense strategies include cell wall thickening, oxidative bursts, hypersensitive responses (HR), the synthesis of antimicrobial compounds like phenolics, flavonoids, and lignins, and the expression of antifungal pathogenesis-related proteins (PR) such as chitinases and glucanases, as part of their immune response [5,15–17].
Chitosan is derived from chitin under alkaline conditions through a process known as deacetylation. While both chitosan and chitin are made up of the same monomer units, they differ in their proportions. They consist of N-acetyl-D-glucosamine (GlcNAc) and D-glucosamine (GlcN) linked by a β-1,4-glycosidic bond. Chitin is primarily composed of GlcNAc units, whereas chitosan mainly comprises GlcN units [18,19]. The synthesis of chitosan oligosaccharides (COS) involves breaking glycosidic bonds and depolymerizing chitosan through methods such as acid hydrolysis, oxidative hydrolysis, mechanical hydrolysis, or enzymatic hydrolysis [19]. COS is defined as chitosan with a degree of polymerization (DP) of less than 20 and a molecular weight (Mw) of under 3900 [20]. COS has gained popularity in agricultural applications due to its enhanced water solubility, low viscosity, and stability at neutral pH [19,21].
Chitosan is recognized by PRRs and induces the transcription of defense-related genes, such as β-1,3-glucanases, chitinases, PAL, PR1, and antioxidant enzymes [22]. It also activates the SA pathway and SAR response through the activation of gene NPR1 transcription, a key gene that regulates plant defense mechanisms across wide variety of plants [22].
The molecular and enzymatic effects of chitosan and chitosan derivatives have been studied in various C. arabica varieties to mitigate biotic and abiotic stressors [23]. Previous studies have identified key physicochemical parameters that influence the molecular and biochemical responses of C. arabica as the source of chitosan, production method, viscosity, molecular weight, and degree of polymerization [22]. In contrast, fewer studies have been conducted to describe the molecular and biochemical impact of COS on C. arabica varieties. This highlights the need for a properly characterization of COS and understanding of how plants respond to its application.
Various biochemical assays can be achieved to properly understand how the exposed plants responded to an exogenous elicitor such as chitosan and derivatives. Beside them can be found gene expression, enzymatic activity, photopigments concentration, and chlorophyll (Chl) fluorescence. Chl fluorescence is widely used in plant studies to examine the effects of biotic and abiotic factors on the plants [24–26]. The relationship between variable and maximum fluorescence is referred to as maximum PSII efficiency and is employed to assess the photosynthetic effectiveness of PSII. The expected range for Fv/Fm is about 0.80 to 0.83. In coffee plants, the optimal value is 0.85; however, this value may decrease to 0.79 under stress conditions [21,27].
Chl fluorescence serves as a sensitive tool used to assess and monitor the physiological and biochemical state of plants. It is employed to observe how external factors (e.g., stressors, application of chemicals, changes in the rhizosphere microbiome) affect the stability and efficacy of PSII [25,26]. This technique can help elucidate and understand potential fluctuations in PSII due to inhibition in the electron transfer chain or physical damage in the chloroplast [25,26].
Previous studies have demostrated that the foliar application of chitosan increases the expression of plant defense genes and enzymatic activity in C. arabica [17,22]. The application of COS foliar activated plant defense mechanisms in the Arabidopsis ecotype Columbia [28], in Nicotiana glutinosa using a Cytosinpeptidemycin and COS system (CytPM-COS) [29], and in Passiflora spp. [30]. However, the effects of foliar application of chitosan oligosaccharides have not been fully studied in C. arabica varieties, except for the effectiveness of COS against H. vastatrix, evaluated in foliar discs [31]. The objective of this study was to evaluate the biochemical responses of C. arabica var. Borbon to the foliar application of chitosan oligosaccharide (COS). This research aims to enhance the understanding of COS applications and its potential to strengthen the resistance of Coffea spp. against plant pathogens.
2.1 Synthesis of Chitosan Oligosaccharides (COS)
COS was synthesized according to [19] from low molecular weight chitosan with a deacetylation level greater than 75%, which was dissolved in 1% acetic acid at a concentration of 3 wt% while stirring at 500 rpm and 70°C until the chitosan was fully dissolved. Oxidative and mechanical hydrolysis were performed to break the β-1,4-glycosidic bond by adding 1% hydrogen peroxide dropwise at 25°C while stirring at 300 rpm, followed by microwave application at 700 W for 1.5 min [21].
2.2 Chitosan Oligosaccharides Characterization
2.2.1 Deacetylation Degree (DD)
Deacetylation Degree determination was performed by Fourier Transform Infrared Spectroscopy (FTIR) according to [17,32,33] by FTIR with Attenuated Total Reflectance (ATR) (Agilent 4300 Handheld FTIR, USA) from 4000 to 650 cm−1 with 2 cm−1 resolution, 10 μL of each sample was dried over ATR. The DD was obtained by Eqs. (1) and (2), where A1320 and A1420 are the absorbance of chitosan at wavelengths 1320 and 1420 cm−1, respectively, and DA is the Degree of Acetylation [17].
2.2.2 Molecular Weight Determination
Molecular weight determination was carried out using intrinsic viscosity, as described in [34]. The viscosities of chitosan and COS were measured with an Ostwald capillary viscometer at 25°C, using a solvent system of 0.3 M acetic acid and 0.2 M sodium acetate. The molecular weight was calculated utilizing the Mark-Houwink Eq. (3), where [η] represents the intrinsic viscosity, MV denotes the viscosity average molecular weight, and K and a are constants specific to a given solvent system. The Degree of Polymerization (PD) was determined from the average molecular weight and the proportional contributions of the molecular weights of chitosan monomers N-acetyl-D-glucosamine (GlcNAc) and D-glucosamine (GlcN).
2.3 Plant Materials and Experimental Design
A local producer in Angel Albino Corzo, Chiapas, Mexico, provided Coffea arabica var Borbon seeds. The seeds were previously disinfected with 20% commercial sodium hypochlorite for 5 min and rinsed with 20 mL of sterile distilled water three times to eliminate traces of sodium hypochlorite. They were then dried at room temperature, sown in sterile sand, and transplanted two months before the analysis to ensure the acclimatization of the plants. Six-month-old transplanted plants were grown in polyethylene bags measuring 6.7 cm × 6 cm × 26 cm (Length × Width × Height) with a substrate mixture of peat moss and agrolite in a 3:1 ratio. Under greenhouse conditions, the plants were watered twice a week with 100 mL of water. Sixty-eight uniform plants were selected to perform the COS foliar application. The selected plants were randomly exposed to different treatments, including a distilled water control and different concentrations of chitosan and synthesized COS (1%, 0.75%, 0.5%, and 0.25%) through foliar application of 2 mL 24 h before each analysis. All treatments were evaluated with four replicates.
The second pair of leaves from each plant was collected to perform protein extraction as outlined in [35]. The resulting protein extract was used to assess the soluble protein content (SPC) using the Bradford method. The SPC values obtained were utilized to determine enzyme-specific activity. The enzymatic activities assessed included PAL, β-1,3-glucanases, chitinases, peroxidase (POD), and catalase (CAT). SPC and enzymatic activities were analyzed using UV-Vis spectroscopy (Nanodrop One
Protein extraction was performed according to [35] by homogenizing 50 mg of collected leaves in liquid nitrogen using pre-chilled mortar and pestles and macerating for 4 h at 4°C in an extraction buffer composed of Tris-HCl 0.1 pH 8, ascorbic acid 0.1 wt%, glycerin 10%, polyvinylpyrrolidone (PVP) 1 wt%, and β-mercaptoethanol 5%. The homogenized samples were centrifuged at 15,000× g for 15 min. The supernatant from each sample was then transferred to microcentrifuge tubes and stored at −4°C until further use.
2.4.2 Phenylalanine Ammonia Lyase (PAL) Activity
PAL activity was determined as detailed in [36], with a few modifications. 5 μL of enzyme extract were added to 145 μL of Tris-HCl buffer solution (pH 8.8) at 50 mM, along with 50 μL of 50 mM L-phenylalanine, and incubated in a water bath at 37°C for 20 min. The absorbance was measured at 280 nm.
2.4.3 β-1,3-Glucanases Activity
The activity of β-1,3-glucanases was evaluated using a colorimetric method as described in [35], with some modifications. 8 μL of enzymatic extract were added to 885 μL of a sodium acetate buffer solution (pH 5, 50 mM) along with 7 μL of laminarin at 0.15 wt%. The samples were incubated in a water bath at 40°C for 10 min, then placed in an ice bath for 5 min. Finally, 335 μL of 3,5-Dinitrosalicylic acid at 96 mM were added to the samples and incubated in a water bath at 90°C for 10 min, followed by another ice bath for 5 min. The absorbance was measured at 515 nm.
Chitinase activity was evaluated following the method described by [37], with minor modifications. Briefly, the catalytic activity of chitinases in the presence of chitin as a substrate produces N-acetylglucosamine. To initiate the reaction, 5 μL of the enzymatic extract was added to 445 μL of sodium acetate buffer solution (pH 5) at 0.5 mM, along with 50 μL of chitin at 0.05 wt%. The solution was incubated at 40°C for 30 min, followed by the addition of 1 mL of potassium ferrocyanide, which was then kept in a water bath at 95°C for 15 min. To stop the reaction, the samples were placed in an ice bath. Absorbance was measured at 420 nm.
2.4.5 Peroxidase Activity (POD)
POD activity was conducted as described in [38], with minor modifications. A total of 33 μL of enzymatic extract was added to 1 mL of reaction media consisting of a sodium phosphate buffer solution (10 mM, pH 6), guaiacol (0.25 wt%), and hydrogen peroxide (10 mM). The absorbance was measured at 470 nm every 20 s for 1 min.
CAT activity was performed according to [38], with minor modifications. An enzymatic extract of 11.11 μL was added to 1 mL of reaction media consisting of 1 M Tris-HCl (pH 8), 5 mM EDTA (ethylenediaminetetraacetic acid), and 30 mM. The absorbance was measured at 240 nm every 10 s for 1 min to monitor the CAT catalytic performance in the presence of H2O2.
Chlorophyll fluorescence measurements were recorded according to [39,40]. Using a chlorophyll meter, FluorPen FP 100 (Photon Systems Instruments, Czech Republic), measurements were taken from a dark-adapted second pair of leaves for 5 min. The data were recorded at 24, 48, and 72 h after COS foliar exposure, with four replicates for each treatment. Potential photochemical yield (Fv/Fm) values were extracted from OJIP curves.
Statistical analysis was performed using a one-way analysis of variance (ANOVA), followed by Tukey’s test (p < 0.05) with four replicates. Additionally, Principal Component Analysis (PCA) was performed for groups of COS and chitosan concentrations related to the studied enzymatic activities. R Studio was utilized for statistical analysis and to generate graphical representations.
Varieties of Coffea arabica and Coffea canephora have the highest economic impact on the global coffee indusdry. However, Coffea arabica plantations have faced biotic and abiotic stresses, such as climate change and disease advances concerning agricultural communities and coffee markets [41]. In response to these issues, it is essential to develop new sustainable technologies to alleviate the effects of biotic and abiotic stress factors in coffee plantations. This includes the synthesis and foliar application of chitosan oligosaccharides (COS) [21]. Chitosan and its derivatives are widely studied as elicitors of plant immune responses. Unfortunately, there is limited research and information regarding the potential application of COS in Coffea arabica plants.
3.1 Characterization of Chitosan Oligosaccharides
In this study, the microwave-assisted synthesis of COS was successfully conducted in the presence of acetic acid and hydrogen peroxide, according to the COS characterization results [20]. The synthesized COS exhibited an average molecular weight of 3547.90 ± 0.33 Da based on intrinsic viscosity values ([η] = 23.3 ± 0.18 cP), a polymerization degree (PD) of 18.91 ± 0.0018, and a degree of deacetylation (DD%) of 76.64 ± 1.12, compared to chitosan’s original maximum supplier values of 190,000 Da based on viscosity (300 cP) and a measured DD% of 76.67 ± 2.32. This illustrates the effect of chemical and physical hydrolysis on the viscosity and molecular weight of oligosaccharides, resulting in reductions of 92.23% ± 0.12 and 98.13% ± 0.00017, respectively, without influencing the degree of deacetylation (DD%) of 0.91% ± 0.97, which aligns with previously reported findings [19] due to the synthesis conditions that only favored the glycosidic bond rupture.
COS physicochemical properties define its bioactivity and impact on exposed plants [21,42]. Completely deacetylated COS is less capable of being recognized by plant receptors and activating the plant immune system [21]. The elicitor function of COS in activating plant defense mechanisms closely relates to PD. To enhance COS bioactivity, applied COS should have a PD of at least 4 [42].
Additionally, molecular weight and the method of application are key factors influencing the bioassimilation of chitosan and its derivatives. The molecular weight of the used chitin derivative influences its water solubility and viscosity, making it a vital factor in the mobilization and availability of COS within plant tissues [43,44].
The FTIR spectra of chitosan and chitosan oligosaccharides (Fig. 1) exhibit similar behavior, with changes in transmittance intensity corresponding to glycosidic bond breakage [19]. Both polysaccharides display chatacteristic peaks related to saccharide structures at 1151.3, 1060.7, 1018.5, and 895.81 cm−1 associated with C-O and β-1,4-glycosidic bond stretching vibrations [45–47]. The peak at 1660.2 cm−1 is linked to C-N vibration, while those at 1626, 1453, and 1420 cm−1 relate to amide I (C=O); the peak at 1320 cm−1 pertains to amide III [32,45,46,48]. Sharp bands at 1400.7 and 1376.5 cm−1 are assigned to C-H bending and stretching vibrations [49], and the absorption at 1251.8 cm−1 corresponds to C-O-C stretching vibrations. The peaks at 3200.6, 2861, and 2921 cm−1 are associated with -OH vibration and C-H stretching [48,49].
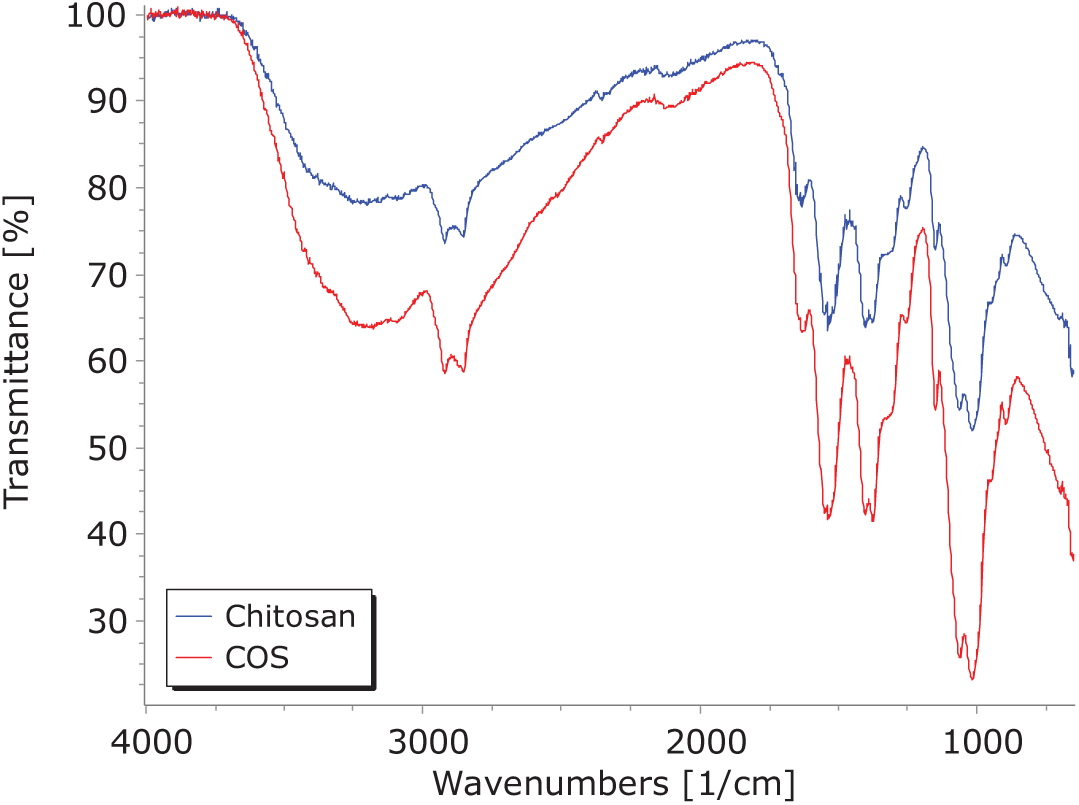
Figure 1: FTIR spectrum of chitosan and chitosan oligosaccharides (COS)
The primary mechanism involving elicitor molecules, such as chitosan and COS entails activating the plant’s defense responses. In reaction to microbial invasion, plants undergo biochemical changes associated with biotic stress signaling, which modifies their physiology. For instance, available oxygen can be utilized to generate Reactive Oxygen Species (ROS), which inhibit pathogen growth [5,15]. Additional defense strategies encompass cell wall thickening, oxidative bursts, hypersensitive responses (HR), the synthesis of antimicrobial compounds like phenolics, flavonoids, and lignins, and the expression of antifungal pathogenesis-related proteins (PR) such as chitinases and glucanases [5,15–17].
The foliar application of chitosan has been well-documented to activate defense mechanisms associated with Systemic Acquired Resistance (SAR) responses. Chitin and its derivatives, such as chitosan and chitosan oligosaccharides, are recognized by plants as Microbial Associated Molecular Patterns (MAMPs) via transmembrane proteins called Pattern Recognition Receptors (PRRs) and the CERK1 receptor (Chitin Elicitor Receptor Kinase 1) [50]. The activation of defense mechanisms by exogenous elicitors like chitosan and chitosan oligosaccharides, which function as MAMPs, is linked to the expression of pathogenesis-related proteins (PR proteins), including β-1,3-glucanases and chitinases. These enzymes play a key rol fall under the category of PR proteins with catabolic activity; these enzymes degrade β-1,3-glucan and chitin in the fungal cell walls of pathogens, respectively [51].
Chitinases and β-1,3-glucanase activity lead to the release of low molecular weight saccharides, including oligosaccharides and polysaccharides, which are recognized by PRRs as elicitors or MAMPs. This recognition activates immune signaling pathways, triggering Pattern Triggered Immunity (PTI) [52–55]. The activation of PTI is associated with plant defense mechanisms, involving enzymatic pathways such as Phenylalanine ammonia-lyase (PAL), polyphenol oxidase (PPO), superoxide dismutase (SOD), catalase (CAT), and peroxidases (POX) [15]. The overall plant defense response (both enzymatic and non-enzymatic) near the wound site includes the SAR mechanism [56].
Systemic Acquired Resitance SAR mechanisms can be triggered by external compounds that function as signaling molecules, MAMPs, or Damage-Associated Molecular Patterns (DAMPs). These include salicylic acid, jasmonic acid, polysaccharides, and oligosaccharides such as chitosan, alginate, and carrageenan, along with their oligosaccharide derivatives, to induce the expression of defense genes [17,18,57,58].
The foliar application of COS increases the total soluble protein content related to various metabolic processes induced by COS application; in contrast, chitosan decreases protein content compared to the control treatment. Additionally, chitosan increases total soluble protein content depending on the applied concentration. The accumulation of total soluble protein can be linked to cell redox homeostasis under induced stress for Reactive Oxygen Species (ROS) scavenging [59]. Notable, COS applications exhibits higher 1,3-glucanase activity than the control and chitosan treatments.
The differences in β-1,3-glucanase and chitinase activity suggest improved assimilation of the applied exogenous elicitor, COS, compared to other chitosan derivatives. PR proteins accumulate in the apoplast to inhibit the extracellular growth of pathogens. For instance, β-1,3-glucanase (PR2) and chitinases (PR3) break down penetration structures such as the intercellular hyphae and haustoria walls of phytopathogens by catalyzing the hydrolysis of chitin and β-glucans [16,53,58].
The results (Fig. 2) indicate a significant increase in soluble protein content (SPC) in C. arabica var. Borbon treated with COS at 0.25%, 0.75%, and 1%, compared to control plants treated with distilled water. In contrast, a notable reduction in SPC was observed in plants treated with Chitosan at 0.25% and 0.5%. Plants treated with COS at 0.5% and Chitosan at 0.5% did not display a significant difference compared to control plants (p < 0.05). All enzymatic activity results (Fig. 2) are expressed as specific activity based on SPC results.
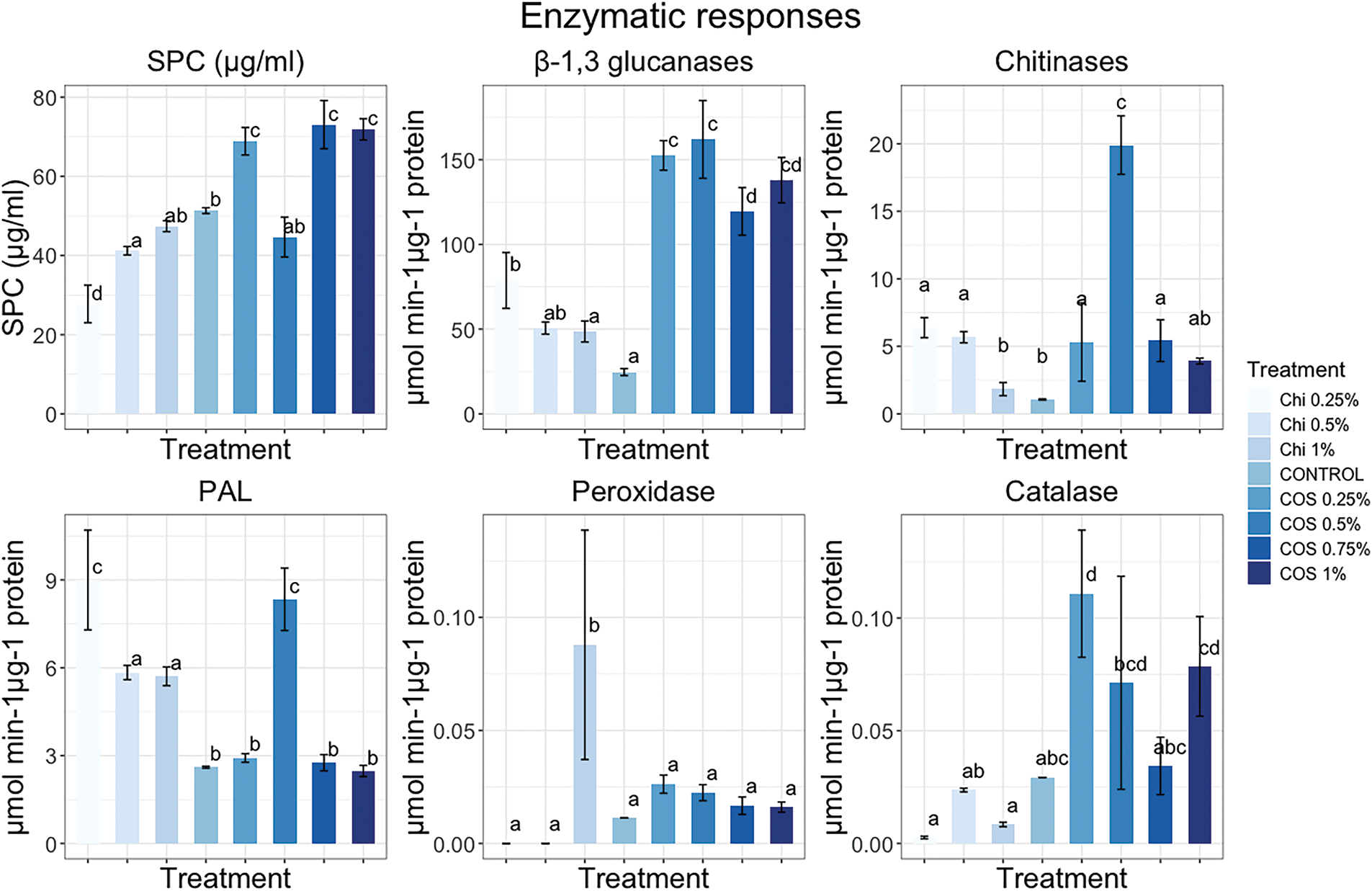
Figure 2: Enzymatic responses of Coffea arabica var. Bourbon to foliar exposure of chitosan, chitosan oligosaccharides, and distilled water used as a control. The evaluated specific responses were soluble protein content by Bradford methodology, β-1,3-glucanase, chitinases, PAL, peroxidases, and catalase
C. arabica var. Borbon plants treated with COS at concentrations of 0.25%, 0.5%, 0.75%, and 1%, along with chitosan at 0.25%, exhibit an increase in β-1,3-glucanase activity (Fig. 2) compared to control plants (p < 0.05). Conversely, plants treated with chitosan at 0.5% and 1%, which did not show a significant difference (p < 0.05). The plants treated with COS at 0.25% and 0.5% demonstrate the highest β-1,3-glucanase activity, as well as those treated with chitosan at 1%. The chitinase activity results (Fig. 2) indicate a significant increase (p < 0.05) in plants treated with COS at 0.25%, 0.5%, 0.75%, and 1% and chitosan at 0.25%, 0.5%, and 1% compared to control plants. The plants treated with COS at 0.5% display the highest chitinase activity.
Treatments with COS 0.5% and Chitosan 0.25% exhibited higher PAL activity (Fig. 2), followed by Chitosan 0.5% and 1%. In contrast, COS at 0.25%, 0.75%, and 1% did not show significant differences (p < 0.05) when compared to control plants. Moreover, only Chitosan 1% demonstrated a significant increase (p < 0.05) in POD activity (Fig. 2) compared to the control and plants treated with COS 0.25%, 0.5%, 0.75%, 1%, and Chitosan 0.25% and 0.5%. Higher CAT (Catalase) activity (Fig. 2) was observed in plants treated with COS 0.25%, while no significant differences (p < 0.05) were observed in plants treated with COS at 0.5%, 0.75%, 1%, or Chitosan at 0.25%, 0.5%, and 1%.
Phenylalanine ammonia-lyase (PAL) plays regulatory rol in the synthesis of phenylpropanoid compounds [21]. The increase in β-1,3-glucanase and chitinase activity has been associated with PAL activity; while, the rise of reactive oxygen species (ROS) at low concentrations, such as hydrogen peroxide (H2O2), acts as secondary messengers in the phenylpropanoid pathway [21,51]. The increase in PAL activity is linked to lignin synthesis, which strengthens plant cell walls. By engaging PAL in the synthesis of cinnamic acid from phenylalanine, the production of cinnamic acid contributes to the synthesis of phenolic compounds like lignin and flavonoids [21,43]. The results suggest a correlation between β-1,3-glucanases, chitinases, and antioxidant enzymes such as catalase (CAT) and peroxidase (POD) with PAL activity in plants treated with COS 0.5 wt%. The observed enzymatic activities suggest that COS 0.5 wt% treatment holds promise for enhancing SAR and ISR responses (Induced Systemic Resistance). Coffee plants treated with COS 0.5 wt% exhibit higher enzyme activity levels of β-1,3-glucanases, chitinases, POD, CAT, and PAL, which may correlate with a potentially better response and greater efficacy in controlling biotrophic pathogens [21,43,51].
Principal Component Analysis (PCA) results show that PC1 accounts for 41.3% of the variance, while PC2 explains 34.25% (Fig. 3). It demonstrates how the chitosan groups at 0.25%, 0.5%, and 1% are clustered together near the control (C) group, suggesting a certain similarity among them. The dispersed and clustered groups of COS at 0.25%, 0.75%, and 1% reveal specific differences between COS and chitosan. The enzyme activity vectors indicate that antioxidant enzymes, such as POD, for chitosan groups compared to COS, but show slight differences when contrasted with the control group. Furthermore, PAL activity exhibits more influence for chitosan groups as well as the COS 0.5% group. In contrast, the COS 0.25%, 0.75%, and 1% groups demonstrate higher influences in chitinases (CHI), β-1,3-glucanase (GLU), and catalase (CAT).
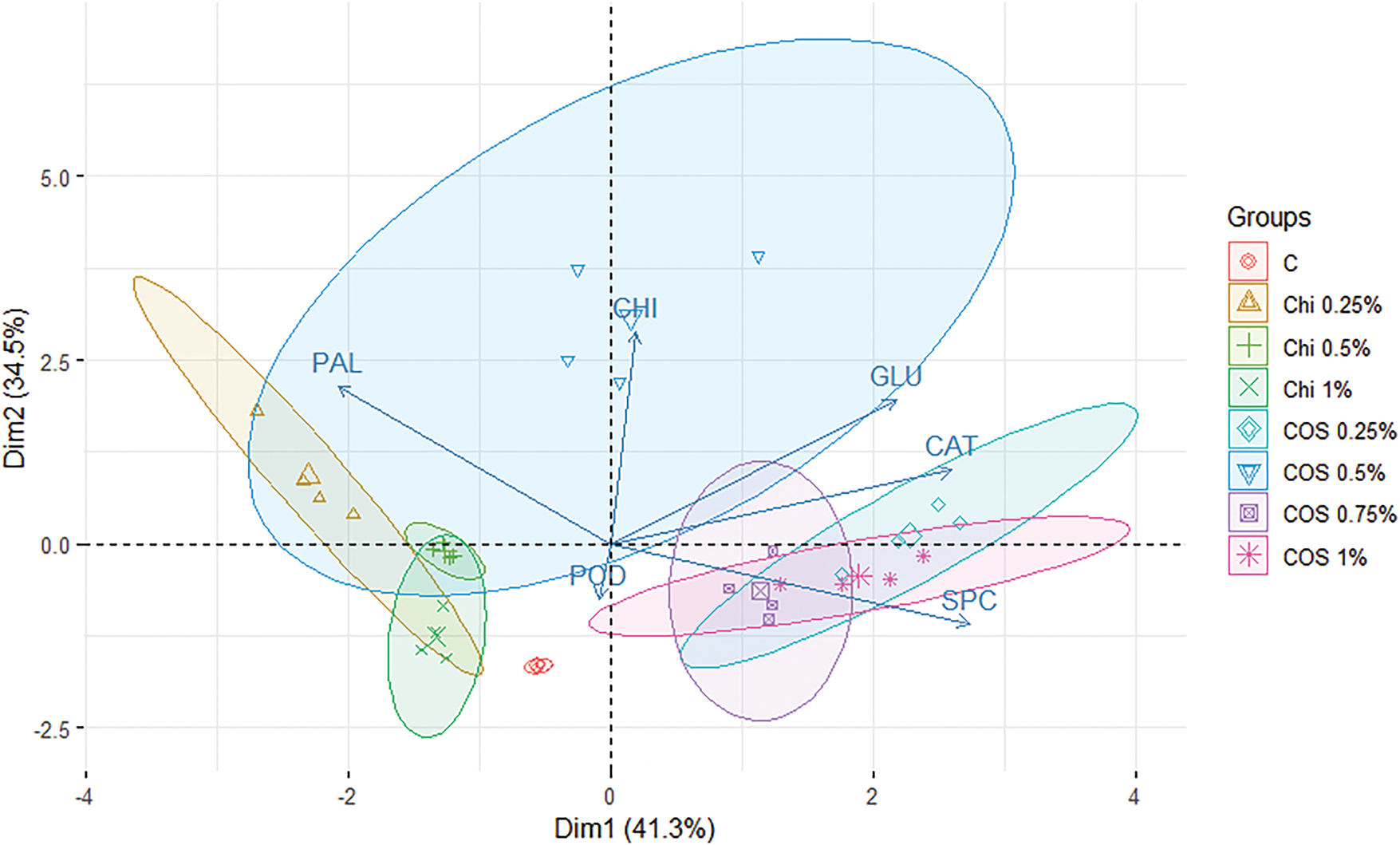
Figure 3: Principal Component Analysis (PCA) for enzymatic responses of Coffea arabica var. Borbon by exposure to chitosan (Chi) and chitosan oligosaccharides (COS) at different concentrations, compared to control C plants treated with distilled water
Overall, β-1,3-glucanase, chitinases, PAL, and CAT activities were significantly higher in COS plants treated compared to those treated with chitosan. These differences may be attributed to the variations in bioavailability between the molecules. The bioavailability of these molecules could be enhanced by the increased hydrophilicity of COS along with the substantial reduction in the viscosity of chitosan to COS, from 300 cP to 23.3 ± 0.18 cP. These changes may improve the mobility of the molecules and potentially enhance their bioavailability in coffee plants. Additionally, the reduction in the molecular weight of chitosan compared to COS improves the bioassimilation of the molecules, as demonstrated by the increase in enzymatic activity [43,44]. The variability in defense enzyme activity in coffee plants resulting from foliar exposure to COS and Chi treatments can be attributed to differences in molecular weight, demonstrating improved bioassimilation for COS treatments, particularly for COS 0.5 wt% with Mw of 3549.90 ± 0.33 Da compared with the Mw of Chi of 190,000 Da.
Chlorophyll fluorescence (Chl fluorescence) is a non-invasive technique used to measure the activity of PSII. It measures the fraction of absorbed light energy that is not utilized in photochemical reactions and is instead re-emitted as fluorescence. When a plant is exposed to photosynthetically active radiation (PAR), approximately 97% of this energy is utilized in photochemical reactions, while the remainder is released as fluorescence and heat [59,60]. Photochemical potential yield (Fv/Fm) values (Fig. 4) demonstrate slight differences between treatments at 24, 48, and 72 h after exposure. Twenty-four hours post-exposure, the Fv/Fm values indicate a minor decrease in COS 0.5%, but no treatment exhibited a significant difference (p < 0.05) from the control treatment. At 48 and 72 h after exposure, neither treatment displayed a significant difference (p < 0.05).
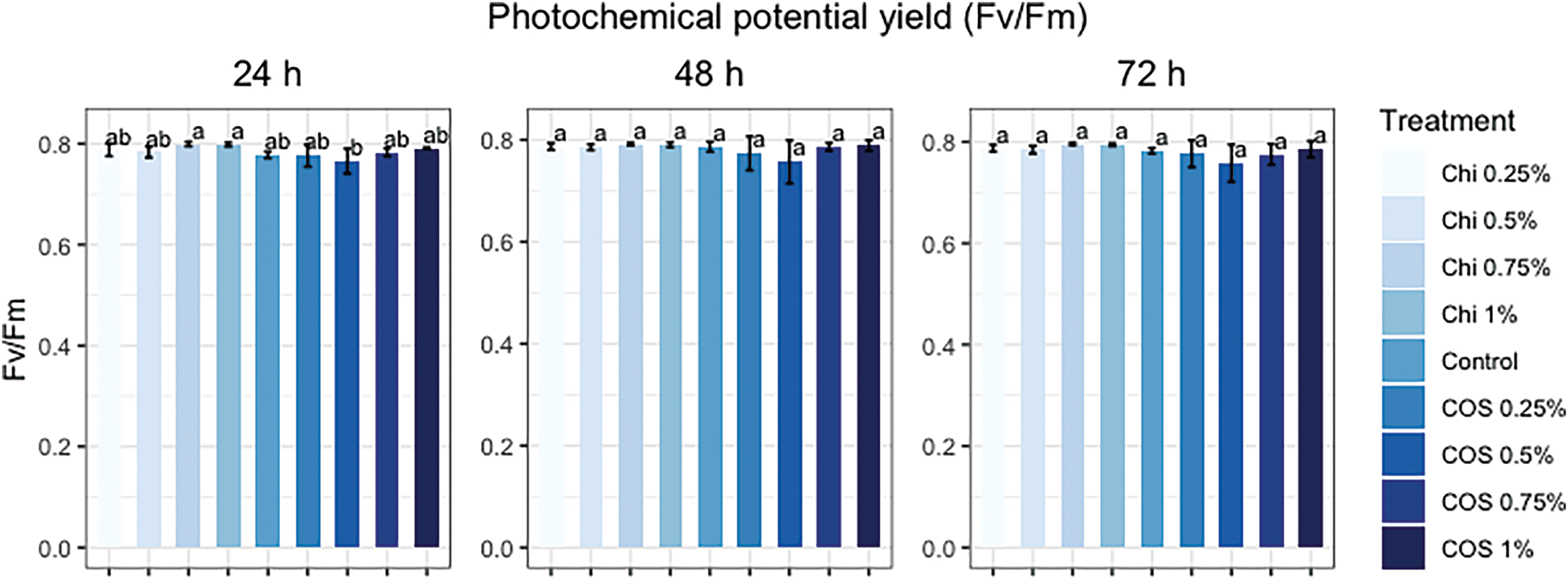
Figure 4: Photochemical potential yield (Fv/Fm) in Coffea arabica var. Borbon exposed to foliar applications of chitosan (Chi) and chitosan oligosaccharides (COS) at various concentrations compared to control plants treated with distilled water
Fig. 4 suggests that COS and chitosan (Chi) exert a transient effect on the photochemical efficiency of PSII within the first 24 h following foliar application. A slight increase in Fv/Fm of PSII in the 0.75% and 1% treatments. This can be related to the increase in photopigments (chlorophyll a, b, and carotenoids) observed in the Coffea canephora var. Robusta assay with the foliar application of chitosan with 80% DD and 750,000 Da, along with COS with 2000 Da and DP 8–16 [61]. The increase in Fv/Fm may also be connected to photopigment concentrations and the impact of nutrient uptake from the treatments on the plants. This was suggested in an assay conducted on Coffea canephora Pierre Var. Robusta treated with chitosan nanoparticles (600 kDa, 85% DD) and chitosan oligomers, which could be linked to the property of chitosan derivatives to enhance magnesium and nitrogen levels in the leaves [23].
The conducted study suggests the application of exogenous chitosan oligosaccharides (COS) is safe for Coffea arabica var. Borbon plants. Foliar application of COS with an average molecular weight of 3549.90 ± 0.33 and a Deacetylation Degree (DD%) of 76.64 ± 1.12 demonstrates a higher availability of amide I and III in FTIR spectrograms compared to chitosan samples. Thus, this can be associated with the increased specific enzymatic activity (β-1,3-glucanases, chitinases, PAL, and CAT) observed in coffee plants treated with COS. The COS treatment that yielded better responses was COS 0.5 wt%. However, coffee plants treated with chitosan exhibited a higher potential photochemical yield 24 h after application in comparison to both the control and COS treatments. Further research are needed to clarify the effect of foliar application of COS in coffee plants and its potential to mitigate the development of certain diseases like Coffee Leaf Rust (CLR).
Acknowledgement: The authors are grateful to the Universidad Autonoma de Baja California and Tecnologico Nacional de Mexico, Campus Tuxtla Gutierrez for the support provided to conduct this work. The authors also would like to express their gratitude to Consejo Nacional de Humanidades, Ciencias y Tecnologías de Mexico (Conahcyt).
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Alexis Salazar-Navarro, Daniel González-Mendoza, Víctor Ruíz-Valdiviezo; data collection: José Joya-Dávila, Olivia Tzintzun-Camacho, Ulin Basilio-Cortes; analysis and interpretation of results: Dagoberto Duran-Hernández, Henry López-Lopez, Onecimo Grimaldo-Juárez; draft manuscript preparation: Alexis Salazar-Navarro, Daniel González-Mendoza, Víctor Ruíz-Valdiviezo. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data are available from the corresponding author upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Panggabean YBS, Arsyad M, Mahyuddin N. Coffee farming business development: e-commerce technology utilization. IOP Conf Ser: Earth Environ Sci. 2021;807(3):032011. doi:10.1088/1755-1315/807/3/032011. [Google Scholar] [CrossRef]
2. USDA. Coffee: world markets and trade. [cited 2024 Dec 16]. Available from: https://public.govdelivery.com/accounts/USDAFAS/subscriber/new. [Google Scholar]
3. SIAP. Panorama agroalimentario. Ciudad de México, México: Gobierno de México; 2023. [Google Scholar]
4. Joya Dávila JG, Gutiérrez Miceli FA, Luján Hidalgo MC, Serrano Gómez LA, Ruíz Sesma B. Cambios bioquímicos y morfométricos en Coffea arabica posterior a un tratamiento con metanosulfonato de etilo. Rev De Cienc Biológicas Y De La Salud. 2023;XXV(3):36–41. (In Spanish). [cited 2025 Jan 1]. Available from: http://biotecnia.unison.mx. [Google Scholar]
5. De Nardi B, Dreos R, Del Terra L, Martellossi C, Asquini E, Tornincasa P, et al. Differential responses of Coffea arabica L. leaves and roots to chemically induced systemic acquired resistance. Genome. 2006;49(12):1594–605. doi:10.1139/g06-125. [Google Scholar] [PubMed] [CrossRef]
6. Salazar-Navarro A, Ruiz-Valdiviezo V, Joya-Dávila J, Gonzalez-Mendoza D. Coffee leaf rust (Hemileia vastatrix) disease in coffee plants and perspectives by the disease control. Phyton. 2024;93(5):923–49. doi:10.32604/phyton.2024.049612. [Google Scholar] [CrossRef]
7. Spinoso-Castillo JL, Escamilla-Prado E, Aguilar-Rincón VH, Morales Ramos V, de los Santos GG, Pérez-Rodríguez P, et al. Genetic diversity of coffee (Coffea spp.) in Mexico evaluated by using DArTseq and SNP markers. Genet Resour Crop Evol. 2020;67(7):1795–806. doi:10.1007/s10722-020-00940-5. [Google Scholar] [CrossRef]
8. Ferrucho RL, Marín-Ramírez GA, Gaitan A. Integrated disease management for the sustainable production of Colombian coffee. Agronomy. 2024;14(6):1286. doi:10.3390/agronomy14061286. [Google Scholar] [CrossRef]
9. Koutouleas A, Collinge DB, Ræbild A. Alternative plant protection strategies for tomorrow’s coffee. Plant Pathol. 2023;72(3):409–29. doi:10.1111/ppa.13676. [Google Scholar] [CrossRef]
10. Alejandro Salazar Navarro A, Manuel Ruíz-Valdiviezo V, Gregorio Joya-Dávila J, González-Mendoza D. Potential of ectomycorrhizal and endomycorrhizal fungi in Coffea spp. plantations. Coffee Sci. 2024;19:1–10. doi:10.25186/.v19i.2242. [Google Scholar] [CrossRef]
11. Duong B, Marraccini P, Maeght JL, Vaast P, Lebrun M, Duponnois R. Coffee microbiota and its potential use in sustainable crop management. A review. Front Sustain Food Syst. 2020;4:607935. doi:10.3389/fsufs.2020.607935. [Google Scholar] [CrossRef]
12. Merhi A, Kordahi R, Hassan HF. A review on the pesticides in coffee: usage, health effects, detection, and mitigation. Front Public Health. 2022;10:1004570. [Google Scholar] [PubMed]
13. de Resende MLV, Pozza EA, Reichel T, Botelho DMS. Strategies for coffee leaf rust management in organic crop systems. Agronomy. 2021;11(9):1865. doi:10.3390/agronomy11091865. [Google Scholar] [CrossRef]
14. Selwal N, Rahayu F, Herwati A, Latifah E, Supriyono, Suhara C, et al. Enhancing secondary metabolite production in plants: exploring traditional and modern strategies. J Agric Food Res. 2023;14:100702. doi:10.1016/j.jafr.2023.100702. [Google Scholar] [CrossRef]
15. Kaur S, Samota MK, Choudhary M, Choudhary M, Pandey AK, Sharma A, et al. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol Mol Biol Plants. 2022;28(2):485–504. doi:10.1007/s12298-022-01146-y. [Google Scholar] [PubMed] [CrossRef]
16. Guerra-Guimarães L, Silva MC, Struck C, Loureiro A, Nicole M, Rodrigues CJ, et al. Chitinases of Coffea arabica genotypes resistant to orange rust Hemileia vastatrix. Biologia Plant. 2009;53(4):702–6. doi:10.1007/s10535-009-0126-8. [Google Scholar] [CrossRef]
17. López-Velázquez JC, Haro-González JN, García-Morales S, Espinosa-Andrews H, Navarro-López DE, Montero-Cortés MI, et al. Evaluation of the physicochemical properties of chitosans in inducing the defense response of Coffea arabica against the fungus Hemileia vastatrix. Polymers. 2021;13(12):1940. doi:10.3390/polym13121940. [Google Scholar] [PubMed] [CrossRef]
18. Moenne A, González A. Chitosan-, alginate-carrageenan-derived oligosaccharides stimulate defense against biotic and abiotic stresses, and growth in plants: a historical perspective. Carbohydr Res. 2021;503:108298. doi:10.1016/j.carres.2021.108298. [Google Scholar] [PubMed] [CrossRef]
19. Salazar-Navarro AA, Rivera-Reyna NE, González-Mendoza D. Synthesis of silica chitosan oligosaccharides nanoparticles. Agro Prod. 2023;16(11):107–14. [Google Scholar]
20. Liang S, Sun Y, Dai X. A review of the preparation, analysis and biological functions of chitooligosaccharide. Int J Mol Sci. 2018;19(8):2197. doi:10.3390/ijms19082197. [Google Scholar] [PubMed] [CrossRef]
21. Mukhtar Ahmed KB, Khan MMA, Siddiqui H, Jahan A. Chitosan and its oligosaccharides, a promising option for sustainable crop production—a review. Carbohydr Polym. 2020;227:115331. doi:10.1016/j.carbpol.2019.115331. [Google Scholar] [PubMed] [CrossRef]
22. López-Velázquez JC, García-Morales S, López-Sánchez GP, Montero-Cortés MI, Uc-Várguez A, Qui-Zapata JA. High-density chitosan induces a biochemical and molecular response in Coffea arabica during infection with Hemileia vastatrix. Int J Mol Sci. 2023;24(22):16165. doi:10.3390/ijms242216165. [Google Scholar] [PubMed] [CrossRef]
23. Van SN, Dinh Minh H, Nguyen Anh D. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal Agric Biotechnol. 2013;2(4):289–94. doi:10.1016/j.bcab.2013.06.001. [Google Scholar] [CrossRef]
24. Murchie EH, Lawson T. Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot. 2013;64(13):3983–98. doi:10.1093/jxb/ert208. [Google Scholar] [PubMed] [CrossRef]
25. ALKahtani MDF, Attia KA, Hafez YM, Khan N, Eid AM, Ali MAM, et al. Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with chitosan and plant growth promoting rhizobacteria. Agronomy. 2020;10(8):1180. doi:10.3390/agronomy10081180. [Google Scholar] [CrossRef]
26. Suárez JC, Vanegas JI, Contreras AT, Anzola JA, Urban MO, Beebe SE, et al. Chlorophyll fluorescence imaging as a tool for evaluating disease resistance of common bean lines in the western Amazon region of Colombia. Plants. 2022;11(10):1371. doi:10.3390/plants11101371. [Google Scholar] [PubMed] [CrossRef]
27. de Souza BP, Martinez HEP, de Carvalho FP, Loureiro ME, Sturião WP. Gas exchanges and chlorophyll fluorescence of young coffee plants submitted to water and nitrogen stresses. J Plant Nutr. 2020;43(16):2455–65. doi:10.1080/01904167.2020.1771589. [Google Scholar] [CrossRef]
28. Jia X, Qin H, Bose SK, Liu T, He J, Xie S, et al. Proteomics analysis reveals the defense priming effect of chitosan oligosaccharides in Arabidopsis-Pst DC3000 interaction. Plant Physiol Biochem. 2020;149:301–12. doi:10.1016/j.plaphy.2020.01.037. [Google Scholar] [PubMed] [CrossRef]
29. Guo Y, Dong Y, Xu C, Xie Q, Xie Y, Xia Z, et al. Novel combined biological antiviral agents Cytosinpeptidemycin and Chitosan oligosaccharide induced host resistance and changed movement protein subcellular localization of tobacco mosaic virus. Pestic Biochem Physiol. 2020;164:40–6. doi:10.1016/j.pestbp.2019.12.006. [Google Scholar] [PubMed] [CrossRef]
30. Zhang L, Yu L, Zhao Z, Li P, Tan S. Chitosan oligosaccharide as a plant immune inducer on the Passiflora spp. (passion fruit) CMV disease. Front Plant Sci. 2023;14:1131766. doi:10.3389/fpls.2023.1131766. [Google Scholar] [PubMed] [CrossRef]
31. Silva-Castro I, Barreto RW, Rodriguez MCH, Matei PM, Martín-Gil J. Control of coffee leaf rust by chitosan oligomers and Propolis. Agric Life Life Agric Conf Proc. 2018;1(1):311–5. doi:10.2478/alife-2018-0046. [Google Scholar] [CrossRef]
32. de Queiroz Antonino RSCM, Lia Fook BRP, de Oliveira Lima VA, de Farias Rached RÍ, Lima EPN, da Silva Lima RJ, et al. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus Vannamei Boone). Mar Drugs. 2017;15(5):E141. doi:10.3390/md15050141. [Google Scholar] [PubMed] [CrossRef]
33. Zając A, Hanuza J, Wandas M, Dymińska L. Determination of N-acetylation degree in chitosan using Raman spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2015;134:114–20. doi:10.1016/j.saa.2014.06.071. [Google Scholar] [PubMed] [CrossRef]
34. Kasaai MR. Calculation of Mark–Houwink–Sakurada (MHS) equation viscometric constants for chitosan in any solvent-temperature system using experimental reported viscometric constants data. Carbohydr Polym. 2007;68(3):477–88. doi:10.1016/j.carbpol.2006.11.006. [Google Scholar] [CrossRef]
35. Luján-Hidalgo MC, Jiménez-Aguilar LA, Ruiz-Lau N, Reyes-Zambrano SJ, Gutiérrez-Miceli FA. Cambios bioquímicos en respuesta al ataque de roya en plantaciones de café. Polibotánica. 2020;49:149–60. (In Spanish). doi:10.18387/polibotanica.49.10. [Google Scholar] [CrossRef]
36. Reichel T, de Resende MLV, Monteiro ACA, Freitas NC, Dos Santos Botelho DM. Constitutive defense strategy of coffee under field conditions: a comparative assessment of resistant and susceptible cultivars to rust. Mol Biotechnol. 2022;64(3):263–77. doi:10.1007/s12033-021-00405-9. [Google Scholar] [PubMed] [CrossRef]
37. Castro R, Álvarez A, Machado E, Mendoza M, Gómez R, García P. Caracterización de una quitinasa extracelular producida por Serratia sp. BIOMI-363706 usando quitina coloidal como sustrato. Rev Soc Quím Perú. 2011;77(2):101–8. (In Spanish). [Google Scholar]
38. Santos-Espinoza AM, González-Mendoza D, Ruiz-Valdiviezo VM, Luján-Hidalgo MC, Jonapa-Hernández F, Valdez-Salas B, et al. Changes in the physiological and biochemical state of peanut plants (Arachis hypogaea L.) induced by exposure to green metallic nanoparticles. Int J Phytoremediat. 2021;23(7):747–54. doi:10.1080/15226514.2020.1856037. [Google Scholar] [PubMed] [CrossRef]
39. Valdez-Salas B, Beltran-Partida E, Mendez-Trujillo V, González-Mendoza D. Silver nanoparticles from Hpytus suaveolens and their effect on biochemical and physiological parameter in mesquite plants. Biocatal Agric Biotechnol. 2020;28:101733. doi:10.1016/j.bcab.2020.101733. [Google Scholar] [CrossRef]
40. González-Mendoza D, Troncoso-Rojas R, Gonzalez-Soto T, Grimaldo-Juarez O, Ceceña-Duran C, Duran-Hernandez D, et al. Changes in the phenylalanine ammonia lyase activity, total phenolic compounds, and flavonoids in Prosopis glandulosa treated with cadmium and copper. An Acad Bras Cienc. 2018;90(2):1465–72. doi:10.1590/0001-3765201820170622. [Google Scholar] [PubMed] [CrossRef]
41. Rhiney K, Guido Z, Knudson C, Avelino J, Bacon CM, Leclerc G, et al. Epidemics and the future of coffee production. Proc Natl Acad Sci U S A. 2021;118(27):e2023212118. [cited 2025 Jan 1]. Available from: https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2023212118/-/DCSupplemental. [Google Scholar] [PubMed]
42. Zhang X, Li K, Liu S, Zou P, Xing R, Yu H, et al. Relationship between the degree of polymerization of chitooligomers and their activity affecting the growth of wheat seedlings under salt stress. J Agric Food Chem. 2017;65(2):501–9. doi:10.1021/acs.jafc.6b03665. [Google Scholar] [PubMed] [CrossRef]
43. Ji H, Wang J, Chen F, Fan N, Wang X, Xiao Z, et al. Meta-analysis of chitosan-mediated effects on plant defense against oxidative stress. Sci Total Environ. 2022;851(Pt 1):158212. doi:10.1016/j.scitotenv.2022.158212. [Google Scholar] [PubMed] [CrossRef]
44. Román-Doval R, Torres-Arellanes SP, Tenorio-Barajas AY, Gómez-Sánchez A, Valencia-Lazcano AA. Chitosan: properties and its application in agriculture in context of molecular weight. Polymers. 2023;15(13):2867. doi:10.3390/polym15132867. [Google Scholar] [CrossRef]
45. Sun T, Zhou D, Xie J, Mao F. Preparation of chitosan oligomers and their antioxidant activity. Eur Food Res Technol. 2007;225(3–4):451–6. [Google Scholar]
46. Sun T, Qin Y, Xu H, Xie J, Hu D, Xue B, et al. Antibacterial activities and preservative effect of chitosan oligosaccharide Maillard reaction products on Penaeus vannamei. Int J Biol Macromol. 2017;105(Pt 1):764–8. doi:10.1016/j.ijbiomac.2017.07.100. [Google Scholar] [PubMed] [CrossRef]
47. Xia W, Wei XY, Xie YY, Zhou T. A novel chitosan oligosaccharide derivative: Synthesis, antioxidant and antibacterial properties. Carbohydr Polym. 2022;291:119608. doi:10.1016/j.carbpol.2022.119608. [Google Scholar] [PubMed] [CrossRef]
48. Hai NTT, Thu LH, Nga NTT, Hoa TT, Tuan LNA, Van Phu D, et al. Preparation of chitooligosaccharide by hydrogen peroxide degradation of chitosan and its effect on soybean seed germination. J Polym Environ. 2019;27(9):2098–104. doi:10.1007/s10924-019-01479-y. [Google Scholar] [CrossRef]
49. Wang SM, Huang QZ, Wang QS. Study on the synergetic degradation of chitosan with ultraviolet light and hydrogen peroxide. Carbohydr Res. 2005;340(6):1143–7. doi:10.1016/j.carres.2005.02.009. [Google Scholar] [PubMed] [CrossRef]
50. Dreischhoff S, Das IS, Jakobi M, Kasper K, Polle A. Local responses and systemic induced resistance mediated by ectomycorrhizal fungi. Front Plant Sci. 2020;11:590063. doi:10.3389/fpls.2020.590063. [Google Scholar] [PubMed] [CrossRef]
51. Adamuchio-Oliveira LG, Mazaro SM, Mógor G, Sant’Anna-Santos BF, Mógor ÁF. Chitosan associated with chelated copper applied on tomatoes: enzymatic and anatomical changes related to plant defense responses. Sci Hortic. 2020;271:109431. doi:10.1016/j.scienta.2020.109431. [Google Scholar] [CrossRef]
52. do Céu Silva M, Guerra-Guimarães L, Diniz I, Loureiro A, Azinheira H, Pereira AP, et al. An overview of the mechanisms involved in coffee-Hemileia vastatrix interactions: plant and pathogen perspectives. Agronomy. 2022;12(2):326. doi:10.3390/agronomy12020326. [Google Scholar] [CrossRef]
53. Silva MDC, Várzea V, Guerra-Guimarães L, Azinheira HG, Fernandez D, Petitot AS, et al. Coffee resistance to the main diseases: leaf rust and coffee berry disease. Braz J Plant Physiol. 2006;18(1):119–47. [cited 2023 Apr 11]. Available from: http://www.scielo.br/j/bjpp/a/dTHqD9SqgdJtq9wHCf9Hp6R/?format=html. [Google Scholar]
54. Hou S, Liu Z, Shen H, Wu D. Damage-associated molecular pattern-triggered immunity in plants. Front Plant Sci. 2019;10:646. doi:10.3389/fpls.2019.00646. [Google Scholar] [PubMed] [CrossRef]
55. Patel ZM, Mahapatra R, Jampala SSM. Role of fungal elicitors in plant defense mechanism. In: Molecular aspects of plant beneficial microbes in agriculture. 1st ed. Amsterdam, The Netherlands: Elsevier; 2020. p. 143–58. [Google Scholar]
56. Barka GD, Caixeta ET, de Almeida RF, Alvarenga SM, Zambolim L. Differential expression of molecular rust resistance components have distinctive profiles in Coffea arabica—Hemileia vastatrix interactions. Eur J Plant Pathol. 2017;149(3):543–61. doi:10.1007/s10658-017-1202-0. [Google Scholar] [CrossRef]
57. Alemu K, Adugna G, Lemessa F, Muleta D. Induction of systemic resistance in Arabica coffee (Coffea arabica L.) against coffee berry disease (Colletotrichum kahawae Waller & Bridge) mediated through plant defense activator. Int J Pest Manage. 2019;65(4):313–23. doi:10.1080/09670874.2018.1506190. [Google Scholar] [CrossRef]
58. Guzzo SD, Martins EMF. Local and systemic induction of β-1, 3-glucanase and chitinase in coffee leaves protected against Hemileia vastatrix by Bacillus thuringiensis. J Phytopathol. 1996;144(9–10):449–54. doi:10.1111/j.1439-0434.1996.tb00322.x. [Google Scholar] [CrossRef]
59. Attia MS, Osman MS, Mohamed AS, Mahgoub HA, Garada MO, Abdelmouty ES, et al. Impact of foliar application of chitosan dissolved in different organic acids on isozymes, protein patterns and physio-biochemical characteristics of tomato grown under salinity stress. Plants. 2021;10(2):388. doi:10.3390/plants10020388. [Google Scholar] [PubMed] [CrossRef]
60. Moreno SG, Vela HP, Alvarez MOS. La fluorescencia de la clorofila a como herramienta en la investigación de efectos tóxicos en el aparato fotosintético de plantas y algas. Rev De Educ Bioquímica. 2008;27(4):119–29. [Google Scholar]
61. Dzung NA, Khanh VTP, Dzung TT. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr Polym. 2011;84(2):751–5. doi:10.1016/j.carbpol.2010.07.066. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools