 Open Access
Open Access
ARTICLE
Interplay of Temporal Variation in Nectar Parameters and Pollinator-Mediated Adaptations in Epimedium wushanense
1 College of Life Sciences, China West Normal University, Nanchong, 637000, China
2 School of Ecology and Environment, Tibet University, Lasha, 850000, China
3 College of Environment Science and Engineering, China West Normal University, Nanchong, 637000, China
* Corresponding Author: Qiumei Quan. Email:
(This article belongs to the Special Issue: Advances in Ornamental Plants: Micropropagation, Plant Biotechnology, Chromosome Doubling, Mutagenesis, Plant Breeding, Environmental Stress Tolerance, and Postharvest Physiology)
Phyton-International Journal of Experimental Botany 2025, 94(5), 1519-1532. https://doi.org/10.32604/phyton.2025.064112
Received 05 February 2025; Accepted 16 April 2025; Issue published 29 May 2025
Abstract
This study investigates the diurnal patterns of nectar secretion, sugar content, and caloric value in Epimedium wushanense, and their interaction mechanisms with pollinator behavior under varying environmental conditions. Nectar secretion exhibited a diurnal pattern, peaking between 11:00 and 13:00, with progressive increases in both volumes (19.07 ± 1.66 μL/day) and caloric value (6.03 ± 0.55 cal/day) over four consecutive days, culminating in maximal production on Day 4 (p < 0.05). Floral bagging significantly altered nectar traits (Mann-Whitney U test, p < 0.05), with bagged inflorescences demonstrating 61.82% higher nectar volume productivity relative to unbagged controls. Pollinator visitation, primarily by Bombus trifasciatus and Bombus grahami, was strongly correlated with nectar sugar concentration and distribution, peaking during midday when temperatures and humidity were optimal. Notably, B. trifasciatus displayed legitimate pollination behavior, while B. grahami exhibited nectar robbing. Bombus grahami peaked at 15:00 (7.67 ± 0.33 visits) under 22.8°C/58% RH, outperforming Bombus trifasciatus (5.67 ± 0.27 at 13:00; p < 0.05), highlighting differential pollinator effectiveness. Temperature negatively impacted unbagged nectar volume and caloric value but positively influenced bagged nectar, suggesting adaptive resource allocation strategies. These findings underscore the intricate relationship between environmental factors, nectar dynamics, and pollinator behavior, revealing how E. wushanense optimizes reproductive success through temporal and ecological adaptations. This study provides critical insights into the ecological mechanisms driving plant-pollinator interactions and resource allocation in changing environments.Keywords
Floral rewards serve as key mediators of plant-pollinator interactions, with nectar provisioning playing a pivotal role in governing the evolutionary trajectories of these mutualisms [1,2]. As a primary attractant in animal-pollinated plants, nectar provides essential nutritional resources that shape pollinator behavior and reproductive success. In insect-pollinated species, nectar secretion dynamics are closely tied to the physiological needs and foraging patterns of pollinators, playing a critical role in facilitating effective pollination [3]. Pollination, a cornerstone of reproductive success in many crops, is driven by floral rewards, with nectar serving as a key determinant of pollinator visitation [4]. For instance, diurnal pollinators are attracted to plants that secrete nectar during daylight hours, while nocturnal pollinators favor species that produce nectar at night [5]. These patterns underscore the coevolutionary relationships between nectar traits—such as production, sugar composition, and concentration—and pollinator preferences. Birds, for example, favor nectar with higher sugar content but lower concentration, whereas bees are drawn to nectar with moderate to high sugar concentrations [6]. Pollinators exhibit remarkable sensitivity to variations in nectar characteristics, and the optimal foraging theory provides a robust framework for understanding their energy-maximizing strategies [7]. Nectar sugars, derived from photosynthetic processes or starch reserves, serve as a direct energy source for pollinators, highlighting the intricate link between plant physiology and pollinator behavior [8]. By modulating nectar secretion, plants can optimize pollinator attraction and reproductive outcomes, thereby enhancing their fitness [9].
However, nectar production entails significant energy costs, necessitating a balance between attracting pollinators and minimizing resource expenditure [10]. Studies on Blandfordia nobilis reveal that while nectar removal can increase net production, it often reduces seed production, suggesting a trade-off between nectar investment and reproductive success [11]. Uncollected nectar is frequently reabsorbed by plants, further emphasizing the need for efficient nectar secretion strategies [12]. Two primary theories explain nectar secretion dynamics, one posits a stable standing crop to meet pollinator preferences, while the other suggests rhythmic secretion patterns tailored to specific pollinator behaviors [13]. Temporal segregation of pollinator visits, with peaks occurring early in the day, allows pollinators to access resources before depletion by nectar robbers [14]. Additionally, environmental factors such as temperature and humidity significantly influence nectar secretion, with high temperatures and dry conditions generally enhancing production, while rain and low temperatures suppress it [15]. These findings underscore the complex interplay between plant physiology, pollinator behavior, and environmental conditions in shaping nectar-mediated plant-pollinator interactions.
The genus Epimedium (Berberidaceae) comprises over 60 species globally, renowned for their medicinal properties, ornamental value, and potential as ground cover plants. Natural variations and interspecific transitions have been documented in western Sichuan Province, highlighting the ecological and evolutionary complexity of this genus [16–18]. Among these species, Epimedium wushanense stands out for its unique floral morphology, characterized by panicle-type inflorescences and four-petaled flowers with nectar spurs. These spurs, which contain distinct nectar droplets, provide an excellent model for investigating nectar secretion dynamics, including volume and sugar concentration (Fig. 1). Nectar secretion in E. wushanense occurs within specialized spur-like structures that attract key pollinators such as bees and butterflies through high sugar concentrations [19,20]. This trait critically shapes pollinator foraging behavior, driving cross-pollination success and reproductive fitness [21,22]. Despite its importance, three unresolved questions limit our understanding: (i) Temporal dynamics: How do nectar volume, caloric value, and spur morphology vary across diurnal cycles and flowering stages? (ii) Environmental regulation: What is the role of temperature and humidity in modulating nectar production under natural (unbagged) vs. pollinator-excluded (bagged) conditions? (iii) Pollinator-mediated feedback: How do interactions with legitimate pollinators (Bombus trifasciatus) vs. nectar robbers (Bombus grahami) influence post-visitation nectar replenishment? To address these gaps, we integrated time-resolved nectar sampling, controlled bagging experiments, and behavioral observations. This study investigates the temporal dynamics of nectar secretion and its interaction with pollinator behavior in E. wushanense. We hypothesize that nectar parameters and pollinator activity are modulated by environmental factors, and adaptive resource allocation occurs under varying conditions. This work advances mechanistic models of plant-pollinator mutualisms while providing actionable insights for conserving medicinal Epimedium species.

Figure 1: The inflorescence (a) and nectar spur (b) in E. wushanense
2.1 Study Sites and Study Species
The study took place from March to April 2022 at Jincheng Mountain Forest Park (106°28′ E, 30°45′ N), at an elevation of 646–768 m in northeastern Sichuan, China. Epimedium wushanense typically forms panicle-type inflorescences (Fig. 1a). Flowering occurs from March to late April, with anthesis lasting 3–4 days, from petal blooming to withering. The flower features four pale yellow conical spurs, each 2.5–3.5 cm long, with a shallow purple cup-shaped base (Fig. 1b). The plant has four stamens, each about 4 mm in length, and a pistil around 5 mm long. The ovary typically contains around 10 ovules.
2.2 Nectar Production during Flower Lifespan
40 plants were randomly selected, and flowers nearing bloom were bagged. On each of the first four days of flowering, ten flowers were sampled between 9:00 a.m. and 11:30 a.m. to measure nectar volume and sugar concentration using microcapillary tubes and a pocket refractometer, respectively. After measurement, flowers were re-bagged.
2.3 Daily Dynamics of Nectar Production
Nectar secretion and sugar concentration were measured in both bagged (pollinator-excluded) and unbagged flowers at two-hour intervals from 9:00 a.m. to 17:00 p.m. Temperature and relative humidity were recorded concurrently. Forty plants were selected, and 80–100 flowers were tagged, with half bagged and half left unbagged. Nectar volume was measured using microcapillary tubes, and sugar concentration was determined with a refractometer. Caloric values (cal) were calculated using the formula: Caloric value = nectar volume (μL) × sugar concentration (%) × 0.04, where 0.04 represents the energy conversion factor (cal/μL) for sucrose solutions [18].
During the peak blooming period, pollination observations were conducted over a 5-day period in E. wushanense. The observations included recording the pollinator type, pollination behavior, visit frequency, and pollination time. Additionally, images of the pollinators were captured using a Nikon DSLR D7000 with 16.2 megapixels. The flower visitors were captured for subsequent identification and measurements in the laboratory.
2.5 The Dynamics of Nectar Secretion in Relation to Pollinator Visitation
For the pollination dynamics assessment, ten plants were randomly selected and bagged to prevent external interference. During the initial flowering period, fifty-six spurs from fourteen flowers were tagged, and nectar locations within the spurs were marked with a 0.5 mm gel ink pen. The flowers were then exposed to pollinators (N = 55, fifty-six spurs from fourteen flowers were tagged, with one spur excluded due to physical damage during handling, leaving 55 spurs for analysis). After pollinator visitation, the nectar positions were re-marked to observe changes in nectar secretion. The spurs were re-bagged to continue monitoring secretion dynamics. Observations were made five hours later, as well as on the following day at 9:00 a.m. and 17:00 p.m., to record nectar position changes.
All data, including nectar secretion volume, sugar content, caloric value, and pollinator efficiency, were analyzed using R version 4.4.2. Normality and homoscedasticity of the variables were tested using the Shapiro-Wilk test and Levene’s test, respectively. For non-normally distributed data, the Kruskal-Wallis test and Mann-Whitney U test were applied. Normal and normally transformable data were analyzed using one-way analysis of variance (ANOVA) with post-hoc comparisons conducted using Duncan’s multiple range test. To minimize experimental bias and increase the reliability of results, randomization and replication were incorporated into the sampling and experimental design. The repeated measures ANOVA, assessing the effects of bagging, time periods (diurnal intervals (e.g., 9:00–11:00)), flowering stages (sequential days (Days 1–4) post-anthesis), and their interactions on nectar secretion volume. All statistical tests used two-tailed pvalues,with significance thresholds set at p < 0.05 unless otherwise noted.
3.1 Nectar Parameters of E. Wushanense
To eliminate the influence of external factors, we analyzed the nectar characteristics of bagged plants. The nectar secretion volume of E. wushanense increased progressively over time, with an average daily secretion volume of 19.07 ± 1.66 μL. The maximum secretion occurred on Day 4, showing significant increases of 221.72% and 39.92% compared to Day 1 and Day 2, respectively. Although there was a difference between Days 3 and 4, it was not statistically significant (p > 0.05, Fig. 2a), indicating a slowing down of nectar secretion. The average sugar content of the nectar was 31.52 ± 0.77%, and no significant differences in sugar content were observed during the flowering period (p > 0.05, Fig. 2b). The average caloric value of the nectar was 6.03 ± 0.55 cal, with the highest value on Day 4, showing significant increases of 3.88% and 2.09% compared to Days 1 and 2 (p < 0.05, Fig. 2c). Differences between Days 3 and 4 were observed but were not statistically significant (p > 0.05, Fig. 2c). Nectar spur length gradually increased from Day 1 to Day 3 (mean ± SE: 13.08 ± 0.18 mm), with growth rate slowing on Day 4 and no significant difference observed between Days 3 and 4 (p > 0.05). This elongation trend mirrored nectar volume dynamics, as both parameters were significantly longer in Days 3–4 compared to Days 1–2 (Fig. 2d).

Figure 2: Temporal variation of nectar volume (a), nectar sugar concentration (b), nectar caloric value (c) and nectar length in spurs (d) in E. wushanense (mean ± SE). Different lowercase letters indicate significant differences (ANOVA, p < 0.05)
3.2 Daily Variation of Nectar Parameters with Different Bagging Methods
The combined effects of bagging methods (pollinator exclusion) and temporal factors (diurnal temperature fluctuations and flowering progression) significantly regulated nectar secretion volume and caloric value. According to the results of the repeated measures ANOVA, bagging methods, time periods, and flowering stages significantly affected nectar secretion volume and caloric value, but had a smaller impact on nectar sugar content (Table 1). Specifically, the interactions between bagging methods, time periods, and flowering stages significantly influenced nectar secretion volume and caloric value (p < 0.05). Notably, the interaction between bagging methods and different time periods, as well as between bagging methods and flowering stages, had a highly significant effect on nectar volume and caloric value (p < 0.001). However, the effects of bagging methods, time periods, and flowering stages on nectar sugar content were not significant (p > 0.05). Overall, bagging methods had a considerable impact on nectar secretion volume and caloric content, while the effects of time periods and flowering stages were more complex, suggesting that various growth stages and environmental factors, such as temperature changes and flowering progression, significantly regulate nectar production and quality.
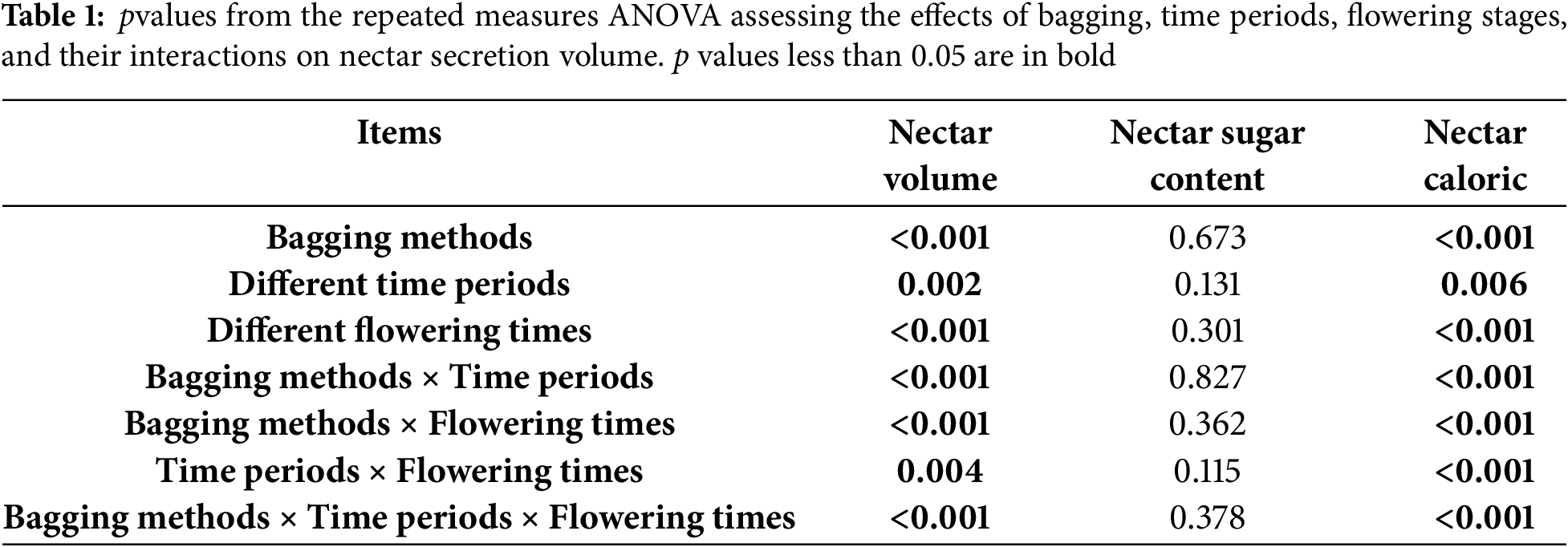
We compared the changes in nectar parameters between bagged and unbagged flowers over different time periods, and the results showed that in the bagged flowers, nectar secretion showed a gradual increase throughout the day (Fig. 3a). No significant difference in nectar secretion was observed between 9:00 and 11:00, but at 13:00, the nectar secretion was significantly higher than at 9:00. No significant difference was found between 15:00 and 17:00 (One-way ANOVA with Duncan’s analysis, p < 0.05). The highest nectar secretion occurred between 11:00 and 13:00. In contrast, the unbagged flowers exhibited a clear decline in nectar secretion throughout the day. No significant differences were observed between 9:00 and 11:00, while nectar secretion peaked at 13:00 and then gradually decreased (One-way ANOVA with Duncan’s analysis, p < 0.05). When comparing nectar secretion between bagged and unbagged flowers, no significant difference was found at 9:00 (Mann-Whitney U test, p > 0.05), but significant differences were observed between 11:00 and 17:00 (Mann-Whitney U test, p < 0.05, Fig. 3a). Nectar sugar content in E. wushanense showed no significant differences across the five collection times in both bagged and unbagged flowers (One-way ANOVA with Duncan’s analysis, p > 0.05) (Fig. 3b). In both types of flowers, nectar sugar content exhibited an upward trend, peaking at 13:00, followed by a gradual decrease. Both one-way ANOVA and nonparametric tests revealed no significant differences between bagged and unbagged flowers at any of the five collection times. The trend in nectar caloric value closely mirrored that of nectar volume (Fig. 3c).
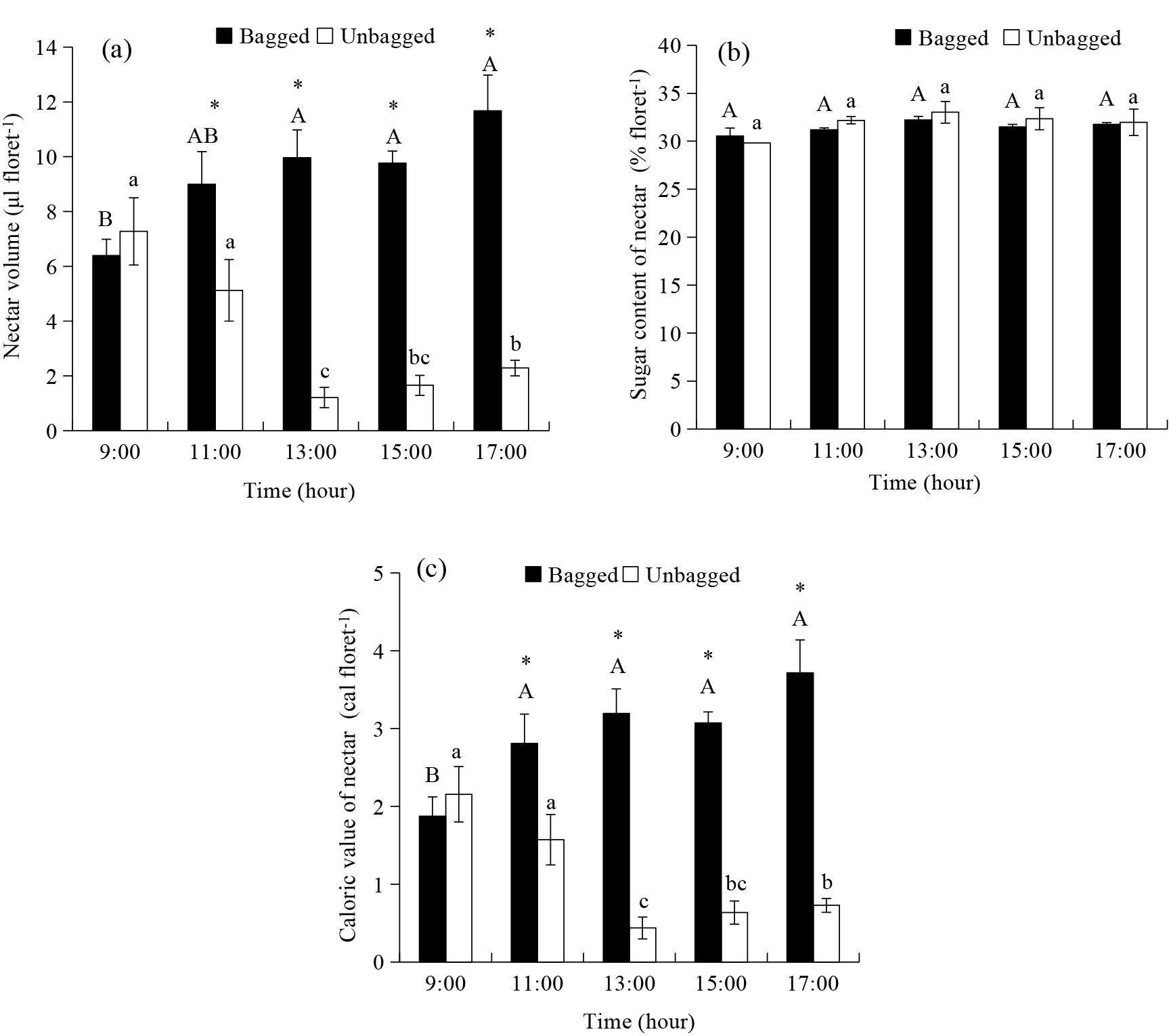
Figure 3: Temporal variation of nectar volume, nectar sugar concentration and caloric value of E. wushanense between different bagging methods and different time periods. (a) Nectar volume; (b) Sugar concentration; (c) Caloric value. Different uppercase or lowercase letters among the various bagging methods indicate significant differences between different time periods (ANOVA, p < 0.05) and * indicate significant differences between bagging methods (Mann-Whitney U test, p < 0.05)
3.3 Diurnal Variation in Pollinator Visitation and Environmental Correlations
Among the flower visitors of E. wushanense, Bombus trifasciatus (Fig. 4a) and Bombus grahami (Fig. 4b) are common visitors that feed on nectar. B. trifasciatus displayed legitimate pollination behavior by tightly gripping a spur with its front legs and using its proboscis to suck nectar, while pollen grains adhered to its neck hairs during the visitation. These pollen grains were later deposited onto the stigmas when the bumblebee visited flowers on different plants of E. wushanense. In contrast, B. grahami exhibited opportunistic feeding behavior, piercing holes in the middle of the spurs to consume nectar, bypassing the anthers. No pollen grains were found on their bodies during microscopic analysis, indicating that they were not effective pollinators.

Figure 4: The flower visitors of E. wushanense that feed on nectar. (a) is effective pollinators (B. trifasciatus) of E. wushanense, (b) is not effective pollinators (B. grahami) of E. wushanense
The diurnal variations in temperature and humidity during nectar collection from E. wushanense are illustrated in Fig. 5a. During the observation period, the mean daily temperature ranged from 17.21 ± 0.15°C to 22.77 ± 0.18°C, while the mean daily relative humidity fluctuated between 58.10 ± 0.47% and 82.57 ± 0.86%. The visitation frequencies of the pollinators, B. grahami and B. trifasciatus, exhibited distinct diurnal patterns (Fig. 5b). B. grahami visitation frequencies increased progressively from 1.17 ± 0.167 visits at 9:00 to a peak of 7.67 ± 0.333 visits at 15:00, followed by a slight decrease to 2.33 ± 0.333 visits at 17:00. In contrast, B. trifasciatus demonstrated a more stable visitation frequency, with a slight increase from 2.00 ± 0.277 visits at 9:00 to a peak of 5.67 ± 0.267 visits at 13:00, and a subsequent decrease to 1.67 ± 0.133 visits by 17:00. Statistical analysis revealed a significant increase in the visitation frequency of both pollinators during midday hours, particularly between 13:00 and 15:00, the peak visitation frequency of B. grahami occurred at 15:00, significantly higher than that of B. trifasciatus. (p < 0.05, Fig. 5b). Pollinator activity aligned with environmental trends, suggesting a potential association between optimal conditions and foraging behavior. Overall, these findings highlight a clear temporal pattern in pollinator activity that aligns with the diurnal variation in temperature and humidity, emphasizing the importance of environmental factors in shaping pollinator visitation behavior.

Figure 5: Temporal variation of temperature, relative humidity, and visitation frequency of E. wushanense. (a) is temperature and humidity, (b) is pollinator visitation frequency. Different uppercase or lowercase letters among the visitors indicate significant differences between different time periods (ANOVA, p < 0.05) and * indicate significant differences between different visitors (Mann-Whitney U test, p < 0.05)
The correlation analysis of environmental and biological variables in E. wushanense revealed significant relationships among temperature, nectar characteristics, and pollinator visitation frequencies (Fig. 6). Temperature exhibited strong negative correlations with unbagged nectar volume (r = −0.98) and calories (r = −0.97), indicating reduced nectar production and energy content at higher temperatures, while showing positive correlations with bagged nectar volume (r = 0.99) and calories (r = 0.98), suggesting differential resource allocation under varying conditions. Pollinator visitation frequencies (B. grahami and B. trifasciatus) were positively correlated with nectar sugar concentration (r = 0.57 and r = 0.46, respectively) and distribution (r = 0.80 and r = 0.55, respectively), highlighting a preference for flowers with higher sugar content and evenly distributed nectar. Notably, B. trifasciatus showed a stronger association with bagged nectar sugar concentration (r = 0.65), implying that bagged flowers may offer more reliable resources. Unbagged nectar volume and calories were highly correlated (r = 1.00), but both were negatively correlated with bagged nectar variables (r = −0.93), indicating a trade-off between open and bagged flower resource allocation. Hierarchical clustering grouped temperature, bagged nectar volume, and calories into a tightly correlated cluster, while unbagged nectar variables and pollinator visitation frequencies formed distinct clusters, reflecting their unique roles in the system. These findings underscore the sensitivity of plant-pollinator interactions to environmental variability, with temperature and humidity fluctuations significantly influencing nectar production and pollinator behavior.
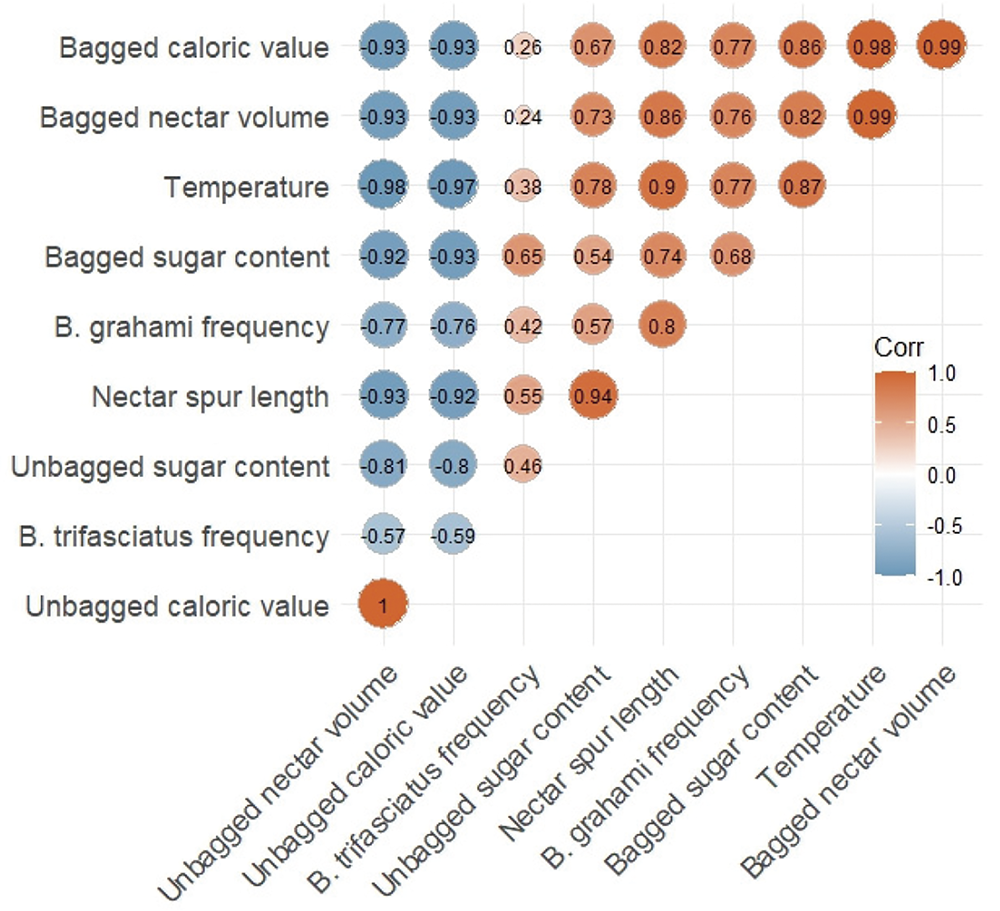
Figure 6: The correlation heatmap between nectar parameters and environmental factor
3.4 The Nectar Secretion Dynamic Visited by Pollinators
Nonparametric analyses revealed significant differences in nectar secretion across five time intervals following Bombus visitation (Kruskal-Wallis test, p < 0.05, Fig. 7). Initially, nectar secretion decreased sharply after visitation, with only minimal nectar produced shortly thereafter. However, nectar secretion increased significantly five hours post-visitation (Mann-Whitney U test, p < 0.05, Fig. 7). Furthermore, nectar secretion on the second day after Bombus visitation was significantly higher than that on the first day (p < 0.05). No significant differences were observed between morning and afternoon nectar secretion rates (p > 0.05). Despite this, the second day post-visitation showed a marked increase in nectar secretion compared to the first day. Although nectar secretion resumed in 55 spurs (from 14 flowers), the spurs exhibited rapid withering, particularly on the second day after visitation.
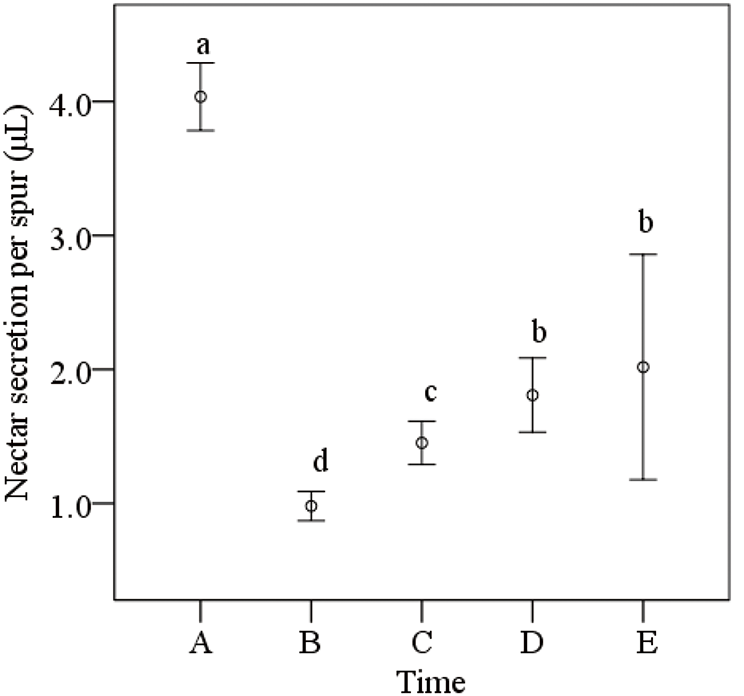
Figure 7: Temporal variation of nectar secretion after Bombus was visited (A, April 13th 9:00, B, visited by Bombus. April 13th 9:00, C, April 13th 16:30, D, April 14th 9:00 a.m., E, April 14th 17:00). Different lowercase letters among the visitors indicate significant differences between different time periods (ANOVA, p < 0.05)
4.1 Temporal Dynamics of Nectar Secretion
The nectar secretion dynamics of E. wushanense revealed a progressive increase in nectar volume over time, peaking on Day 4, which aligns with patterns observed in other plant species. In these species, nectar production typically increases as flowers mature, influenced by both developmental stages and environmental factors such as temperature and humidity [23]. However, the increase in nectar volume in E. wushanense over time does not suggest that nectar production is directly influenced by the developmental stages of the flowers themselves. Instead, this pattern may reflect an adaptive strategy to maximize pollinator attraction during peak flowering periods [24]. Interestingly, throughout the flowering period, no significant differences were observed in the sugar content of the nectar, indicating that sugar concentration may be regulated independently of nectar volume. This could be an adaptive mechanism to ensure consistent energy rewards for pollinators despite fluctuations in nectar volume [25]. This stability in nectar composition aligns with findings in other plant species, where environmental factors did not substantially alter the sugar content during the flowering period [26]. The peak on Day 4 reflects adaptive investment during peak flowering, while subsequent decline aligns with resource depletion post-pollination [27,28]. This suggests that the nectar secretion dynamics in E. wushanense are not only shaped by the plant’s internal maturation processes but also by external environmental conditions that influence nectar production and its subsequent depletion. Bagging methods significantly influenced nectar secretion volume and caloric value, highlighting the role of environmental factors such as temperature and humidity in regulating nectar production [29]. The interaction between bagging methods and time periods suggests that microclimatic conditions created by bagging can alter resource allocation patterns in flowers [30,31]. Notably, the lack of significant differences in sugar content between bagged and unbagged flowers indicates that sugar concentration is less sensitive to environmental fluctuations compared to nectar volume and caloric value. This finding is consistent with studies showing that sugar content is often tightly regulated to ensure pollinator fidelity.
4.2 Pollinator Behavior and Environmental Interactions
The study found clear diurnal patterns in the visitation frequency of B. trifasciatus and B. grahami, with peak activity occurring between 13:00 and 15:00. Temperature and humidity fluctuations during the observation period were strongly linked to these patterns, supporting previous research that highlights the role of environmental conditions in influencing pollinator behavior. Specifically, optimal temperature and humidity conditions typically correlate with higher visitation rates, enhancing foraging efficiency [32]. The increased midday visitation coincided with higher nectar secretion, underscoring the relationship between pollinator activity and nectar availability. Interestingly, B. grahami showed no pollen on its body during visits to E. wushanense, indicating that its feeding behavior is opportunistic and does not contribute to pollination, unlike the legitimate pollination activity of B. trifasciatus [33,34]. The peak visitation from both species aligns with optimal environmental conditions for foraging, suggesting that pollinators time their visits to maximize energy intake [35]. The significant increase in visitation between 13:00 and 15:00 corresponds to the highest nectar secretion and caloric values. Moreover, the strong correlation between visitation frequency and nectar sugar concentration highlights the importance of nectar quality in shaping pollinator preferences and interactions [36].
The contrasting correlations between temperature and bagged/unbagged nectar suggest that pollinators play a crucial role in mediating resource allocation under environmental stress. In particular, the negative correlation between temperature and unbagged nectar volume indicates that higher temperatures may induce stress in plants, reducing nectar production. Conversely, the positive correlation between temperature and bagged nectar variables suggests that controlled environments can mitigate the adverse effects of temperature fluctuations on nectar yield [37,38]. This finding aligns with previous studies, including those on Psittacanthus robustus, which demonstrated how controlled environmental conditions can buffer temperature-induced stress on nectar secretion [39]. Moreover, the close correlation between temperature, bagged nectar volume, and caloric value emphasizes the interconnectedness of environmental factors and plant physiological responses [40]. This highlights the sensitivity of plant-pollinator systems to environmental variability, underscoring the importance of considering these factors in conservation and management, particularly in the context of climate change. Additional analysis revealed strong relationships between environmental factors (temperature and humidity), nectar characteristics (volume and calories), and pollinator visitation frequency. Furthermore, the positive correlation between pollinator visitation and nectar sugar concentration supports the idea that pollinators prefer flowers with higher sugar content, a preference also observed in earlier studies on other species [41].
4.3 The Impact of Bombus Visitation on Nectar Secretion Dynamics
The nonparametric analysis of nectar secretion following Bombus visitation revealed a sharp decrease in nectar production immediately after visitation, followed by a significant increase five hours later. This dynamic suggests a potential feedback mechanism whereby nectar production is stimulated following pollinator visitation, as observed in other species [42]. This phenomenon may be a result of mechanical or chemical cues from the pollinator, which trigger a physiological response in the plant. Previous studies on Cucurbita and Solanum spp. have demonstrated similar patterns of nectar secretion responses to pollinator visits, with plants increasing nectar production in anticipation of future pollinator visits [43,44]. Moreover, the observation that nectar secretion was significantly higher on the second day after visitation suggests a lasting effect of pollinator activity on nectar production, further supporting the idea of plant-pollinator interaction shaping nectar dynamics over time [45]. Our findings suggest that understanding nectar secretion timing can guide habitat management to optimize pollinator visits, thus supporting plant-pollinator interactions and reproductive success [46]. This approach is especially important for species like E. wushanense, which rely on specific pollinator interactions. Expanding this research to consider broader ecological factors will enhance conservation strategies and ecosystem resilience [47].
The nectar secretion dynamics of E. wushanense are shaped by both intrinsic developmental processes and extrinsic environmental factors, with nectar volume and caloric value peaking during mid-flowering stages. Bagging experiments revealed that microclimatic conditions significantly influence nectar production, while sugar content remains stable, ensuring consistent energy rewards for pollinators. Pollinator visitation, particularly by B. trifasciatus, aligns with optimal environmental conditions and nectar availability, highlighting the intricate interplay between plant physiology and pollinator behavior. The observed feedback mechanism, where nectar production increases post-visitation, underscores the adaptive nature of plant-pollinator interactions. These findings emphasize the sensitivity of such systems to environmental variability, with implications for conservation under climate change scenarios.
Acknowledgement: We thank the Jinchengshan National Foreast Park of Sichuan Province for their support and help during the fieldwork. Prof. Shi Aiming’s team for identification of flower-visiting insects.
Funding Statement: This research was supported by Sichuan Science and Technology Program (No. 2023NSFSC1282), Talent Foundation of China West Normal University (Nos. 17YC142 and 17YC137), the National General Cultivation Project of China West Normal University (No. 19B029), Youth Foundation Specialization of West China Normal University (No. 22KB004).
Author Contributions: Qiumei Quan and Yunxiang Li contributed to the study conception and design. Data collection and experiments were performed by Lanying Chen. Yifu Cai and Lanying Chen analyzed the data. The first draft of the manuscript was written by Lanying Chen. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All data included in this study are available upon request by contact with the corresponding author.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Paiva ÉAS, Ballego-Campos I, Gibernau M. True nectar or stigmatic secretion? Structural evidence elucidates an old controversy regarding nectaries in Anthurium. Am J Bot. 2021;108(1):37–50. doi:10.1002/ajb2.1595. [Google Scholar] [PubMed] [CrossRef]
2. Albuquerque NL, Milet-Pinheiro P, Cruz DD, Navarro DF, Machado IC. Pollination of the strongly scented Sarcoglottis acaulis (Orchidaceae) by male orchid bees: nectar as resource instead of perfume. Plant Biol. 2021;23(5):719–27. doi:10.1111/plb.13297. [Google Scholar] [PubMed] [CrossRef]
3. Castro-Cárdenas N, Martén-Rodríguez S, Vázquez-Santana S, Cornejo-Tenorio G, Navarrete-Segueda A, Ibarra-Manríquez G. Putting the puzzle together: the relationship between floral characters and pollinator morphology determines pollination mode in the fig-fig wasp mutualism. Plant Biol. 2024;26(7):1131–43. doi:10.1111/plb.13712. [Google Scholar] [PubMed] [CrossRef]
4. Kim YK, Lee S, Song JH, Kim MJ, Yunusbaev U, Lee ML, et al. Comparison of biochemical constituents and contents in floral nectar of Castanea spp. Molecules. 2020;25(18):4225. doi:10.3390/molecules25184225. [Google Scholar] [PubMed] [CrossRef]
5. Nakajima R, Okamiya H, Shimokawa S, Yamamoto K, Kato H, Murakami N. Comparison of floral traits and pollinator assemblages of insular and mainland varieties of Lilium auratum. Plant Species Biol. 2018;33(4):276–88. doi:10.1111/1442-1984.12222. [Google Scholar] [CrossRef]
6. Schmidt-Lebuhn AN, Schwerdtfeger M, Kessler M, Lohaus G. Phylogenetic constraints vs. ecology in the nectar composition of Acanthaceae. Flora Morphol Distrib Funct Ecol Plants. 2007;202(1):62–9. doi:10.1016/j.flora.2006.02.005. [Google Scholar] [CrossRef]
7. Pyke GH. Floral nectar: pollinator attraction or manipulation? Trends Ecol Evol. 2016;31(5):339–41. doi:10.1016/j.tree.2016.02.013. [Google Scholar] [PubMed] [CrossRef]
8. Konarska A, Chmielewski P. Taxonomic traits in the microstructure of flowers of parasitic Orobanche picridis with particular emphasis on secretory structures. Protoplasma. 2020;257(1):299–317. doi:10.1007/s00709-019-01438-3. [Google Scholar] [PubMed] [CrossRef]
9. Cecala JM, Lau PW, Leong JM. Floral bagging differentially affects handling behaviours and single-visit pollen deposition by honey bees and native bees. Ecol Entomol. 2020;45(5):1099–107. doi:10.1111/een.12890. [Google Scholar] [CrossRef]
10. Henning T, Weigend M. Total control—pollen presentation and floral longevity in Loasaceae (blazing star family) are modulated by light, temperature and pollinator visitation rates. PLoS One. 2012;7(8):e41121. doi:10.1371/journal.pone.0041121. [Google Scholar] [PubMed] [CrossRef]
11. Agostini K, Sazima M, Galetto L. Nectar production dynamics and sugar composition in two Mucuna species (Leguminosae, Faboideae) with different specialized pollinators. Naturwissenschaften. 2011;98(11):933–42. doi:10.1007/s00114-011-0844-6. [Google Scholar] [PubMed] [CrossRef]
12. Nepi M, Stpiczynska M. Do plants dynamically regulate nectar features through sugar sensing? Plant Signal Behav. 2008;3(10):874–6. doi:10.4161/psb.3.10.6228. [Google Scholar] [PubMed] [CrossRef]
13. Adgaba N, Al-Ghamdi A, Tadesse Y, Getachew A, Awad AM, Ansari MJ, et al. Nectar secretion dynamics and honey production potentials of some major honey plants in Saudi Arabia. Saudi J Biol Sci. 2017;24(1):180–91. doi:10.1016/j.sjbs.2016.05.002. [Google Scholar] [PubMed] [CrossRef]
14. Souza CV, Nepi M, Machado SR, Guimarães E. Floral biology, nectar secretion pattern and fruit set of a threatened Bignoniaceae tree from Brazilian tropical forest. Flora. 2017;227:46–55. doi:10.1016/j.flora.2016.12.007. [Google Scholar] [CrossRef]
15. Zhang JY, Sun H, Zhao LM, Zhang CB, Yan H, Peng B, et al. Nectar secretion of RN-type cytoplasmic male sterility three lines in soybean [Glycine max (L.) Merr]. J Integr Agric. 2018;17(5):1085–92. [Google Scholar]
16. Sheng M, Chen Q, Wang L, Tian X. Hybridization among Epimedium (Berberidaceae) species native to China. Sci Hortic. 2011;128(3):342–51. doi:10.1016/j.scienta.2011.01.020. [Google Scholar] [CrossRef]
17. Chen YY, Li YX, Quan QM, Liao XL, Cheng XG. Genetic diversity and relationship among 7 species of genus Epimedium in Sichuan revealed by ISSR analysis. Bull Bot Res. 2012;32(2):208–12. (In Chinese). [Google Scholar]
18. Qian YF, Du W, Chen LY, Quan QM, Li YX. Floral traits and pollination biology of Epimedium chlorandrum Stearn (Berberidaceae). J Plant Ecol. 2023;16(4):rtad003. doi:10.1093/jpe/rtad003. [Google Scholar] [CrossRef]
19. Chen LY, Xiao X, Xiao J. Floral traits and reproductive characters of different large-flowered taxa Epimedium (Berberidaceae). Bull Bot Res. 2019;39(6):808–16. (In Chinese). [Google Scholar]
20. Bareke T, Addi A. Floral nectar secretion dynamics of Pavonia urens (Malvaceae) and honey production potential. Nusant Biosci. 2024;16(1):89–95. [Google Scholar]
21. Pattrick JG, Symington HA, Federle W, Glover BJ. Bumblebees negotiate a trade-off between nectar quality and floral biomechanics. iScience. 2023;26(11):108071. doi:10.1016/j.isci.2023.108071. [Google Scholar] [PubMed] [CrossRef]
22. de-Oliveira-Nogueira CH, Souza UF, Machado TM, Figueiredo-de-Andrade CA, Mônico AT, Sazima I, et al. Between fruits, flowers and nectar: the extraordinary diet of the frog Xenohyla truncata. Food Webs. 2023;35:e00281. doi:10.1016/j.fooweb.2023.e00281. [Google Scholar] [CrossRef]
23. McCallum KP, McDougall FO, Seymour RS. A review of the energetics of pollination biology. J Comp Physiol B Biochem Syst Environ Physiol. 2013;183(7):867–76. doi:10.1007/s00360-013-0760-5. [Google Scholar] [PubMed] [CrossRef]
24. Pyke GH. What does it cost a plant to produce floral nectar? Nature. 1991;350(6313):58–9. doi:10.1038/350058a0. [Google Scholar] [CrossRef]
25. Nicolson SW. Sweet solutions: nectar chemistry and quality. Philos Trans R Soc Lond B Biol Sci. 2022;377(1853):20210163. doi:10.1098/rstb.2021.0163. [Google Scholar] [PubMed] [CrossRef]
26. Liu Y, Dunker S, Durka W, Dominik C, Heuschele JM, Honchar H, et al. Eco-evolutionary processes shaping floral nectar sugar composition. Sci Rep. 2024;14(1):13856. doi:10.1038/s41598-024-64755-5. [Google Scholar] [PubMed] [CrossRef]
27. Han Y, Lipeizhong W, Liang X, Cai Z, Liu W, Dou J, et al. Effects of various nectar and pollen plants on the survival, reproduction, and predation of Neoseiulus bicaudus. Insects. 2024;15(3):190. doi:10.3390/insects15030190. [Google Scholar] [PubMed] [CrossRef]
28. Nikolova T, Yordanova M, Petrova V. Influence of meteorological conditions on the production of nectar and pollen of Cucurbita pepo var. giromontia. Bulg J Agric Sci. 2019;25(2):310–3. [Google Scholar]
29. Domingos-Melo A, Cocucci AA, Tschapka M, Machado IC. A negative association between nectar standing crop and pollen transfer suggests nectar functions as a manipulator of pollinating bats. Ann Bot. 2023;131(2):361–72. doi:10.1093/aob/mcac154. [Google Scholar] [PubMed] [CrossRef]
30. Galen C, Plowright RC. Contrasting movement patterns of nectar-collecting and pollen-collecting bumble bees (Bombus terricola) on fireweed (Chamaenerion angustifolium) inflorescences. Ecol Entomol. 1985;10(1):9–17. doi:10.1111/j.1365-2311.1985.tb00530.x. [Google Scholar] [CrossRef]
31. Devkota K, Ferreira AB, Timberlake TP, dos Santos CF. The impact of pollinator decline on global protein production: implications for livestock and plant-based products. Glob Ecol Conserv. 2024;50:e02815. doi:10.1016/j.gecco.2024.e02815. [Google Scholar] [CrossRef]
32. Nuru A, Al-Ghamdi AA, Tena YT, Shenkut AG, Ansari MJ, Al-Maktary A. Floral phenology, nectar secretion dynamics, and honey production potential, of two lavender species (Lavandula dentata, and L. pubescens) in southwestern Saudi Arabia. J Apic Sci. 2015;59(2):135–44. doi:10.1515/jas-2015-0028. [Google Scholar] [CrossRef]
33. Page ML, Williams NM. Honey bee introductions displace native bees and decrease pollination of a native wildflower. Ecology. 2023;104(2):e3939. doi:10.1002/ecy.3939. [Google Scholar] [PubMed] [CrossRef]
34. Oliveira PE, Rech AR. Floral biology and pollination in Brazil: history and possibilities. Acta Bot Bras. 2018;32(3):321–8. doi:10.1590/0102-33062018abb0255. [Google Scholar] [CrossRef]
35. Hameed O, Ugine T, Westbrook A, Losey J. Consumption of nectar-like sugar solutions promotes longevity and fecundity in the ladybird beetles Harmonia axyridis and Hippodamia convergens. Arthropod-Plant Interact. 2024;18(4):763–70. doi:10.1007/s11829-024-10086-1. [Google Scholar] [CrossRef]
36. Mitchell RJ, Waser NM. Adaptive significance of Ipomopsis aggregata nectar production: pollination success of single flowers. Ecology. 1992;73(2):633–8. doi:10.2307/1940769. [Google Scholar] [CrossRef]
37. Zimmerman M, Pyke GH. Experimental manipulations of Polemonium foliosissimum: effects on subsequent nectar production, seed production and growth. J Ecol. 1988;76(3):777. doi:10.2307/2260573. [Google Scholar] [CrossRef]
38. Neece J, Brokaw J, Coker A, Bruninga-Socolar B. Seeding density of wildflower mixes affects nectar production in a focal plant species. Restor Ecol. 2023;31(7):e13912. doi:10.1111/rec.13912. [Google Scholar] [CrossRef]
39. Guerra TJ, Galetto L, Silva WR. Nectar secretion dynamic links pollinator behavior to consequences for plant reproductive success in the ornithophilous mistletoe Psittacanthus robustus. Plant Biol. 2014;16(5):956–66. doi:10.1111/plb.12146. [Google Scholar] [PubMed] [CrossRef]
40. Göttlinger T, Lohaus G. Comparative analyses of the metabolite and ion concentrations in nectar, nectaries, and leaves of 36 bromeliads with different photosynthesis and pollinator types. Front Plant Sci. 2022;13:987145. doi:10.3389/fpls.2022.987145. [Google Scholar] [PubMed] [CrossRef]
41. Totland O. Environment-dependent pollen limitation and selection on floral traits in an alpine species. Ecology. 2001;82(8):2233. doi:10.2307/2680228. [Google Scholar] [CrossRef]
42. Zhao Z, Hou M, Wang Y, Du G. Phenological variation of flower longevity and duration of sex phases in a protandrous alpine plant: potential causes and fitness significance. BMC Plant Biol. 2020;20(1):137. doi:10.1186/s12870-020-02356-w. [Google Scholar] [PubMed] [CrossRef]
43. Barman M, Tenhaken R, Dötterl S. Negative and sex-specific effects of drought on flower production, resources and pollinator visitation, but not on floral scent in monoecious Cucurbita pepo. New Phytol. 2024;244(3):1013–23. doi:10.1111/nph.20016. [Google Scholar] [PubMed] [CrossRef]
44. Dalmazzo M, Zumoffen L, Ghiglione C, Roig-Alsina A, Chacoff N. Diversity and biological traits of bees visiting flowers of Cucurbita maxima var. zapallito differ between biodiversity-based and conventional management practices. Environ Monit Assess. 2023;196(1):6. doi:10.1007/s10661-023-12161-1. [Google Scholar] [PubMed] [CrossRef]
45. Luizzi VJ, Harrington AH, Bronstein JL, Arnold AE. Nectar robbers and simulated robbing differ in their effects on nectar microbial communities. Plant Species Biol. 2024;39(3):126–37. doi:10.1111/1442-1984.12446. [Google Scholar] [CrossRef]
46. Wang WJ, Lin YT, Chen HF, Huang MY, Ren ZX, Zhao JL. Diurnal nectar secretion dynamics in Roscoea cautleoides (Zingiberaceae) reveal the role of non-sugar chemicals in plant-pollinator interaction. Curr Plant Biol. 2025;41:100443. doi:10.1016/j.cpb.2025.100443. [Google Scholar] [CrossRef]
47. Benvenuti S. Weed role for pollinator in the agroecosystem: plant-insect interactions and agronomic strategies for biodiversity conservation. Plants. 2024;13(16):2249. doi:10.3390/plants13162249. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools