 Open Access
Open Access
REVIEW
Advances in PGPR-Mediated Plant-Pathogen Control for Food Security and Ecosystem Stability
Department of Horticulture and Life Science, Yeungnam University, Gyeongsan, 38541, Republic of Korea
* Corresponding Authors: Sajid Ali. Email: ; Yong-Sun Moon. Email:
(This article belongs to the Special Issue: Multi-Level Mechanisms in Plant-Pathogen Interactions)
Phyton-International Journal of Experimental Botany 2025, 94(5), 1419-1451. https://doi.org/10.32604/phyton.2025.064284
Received 11 February 2025; Accepted 22 April 2025; Issue published 29 May 2025
Abstract
This review focused on the role of plant growth-promoting rhizobacteria (PGPR) in enhancing plant growth and protecting against pathogens, highlighting their mechanisms of action, ecological benefits, and challenges. PGPR mediate plant growth through several mechanisms, including nutrient acquisition, production of antimicrobial compounds and induction of systemic resistance. These mechanisms are critical in improving crop yields, especially under stressful conditions. This review examines the molecular mechanisms of PGPR-mediated plant pathogen control, cellular mechanisms of PGPR in plant pathogen control, ecological and environmental benefits of PGPR application. Despite their potential, PGPR application is limited by environmental variability, inconsistent efficacy, and challenges in formulation and commercialization. The review discusses these challenges and also provides solutions. Additionally, the review outlines the latest advancements in PGPR strain selection and their genetic modifications for enhanced resilience and biocontrol efficacy. PGPR are particularly crucial in addressing global food security challenges, exacerbated by climate change, and the need for sustainable agricultural practices. PGPR have been shown to increase crop yields by 20%–30% in drought-prone regions and reduce pesticide use by up to 50%, contributing to more sustainable farming. As research advances, PGPR can play a key role in reducing chemical input dependency and promoting long-term agricultural sustainability. This review examines the role of PGPR in pathogen control and highlights their potential to enhance agricultural sustainability.Keywords
Plant-pathogen interactions represent a critical dimension of agricultural science, greatly influencing crop productivity and ecosystem stability [1]. The constant co-evolution of plants and pathogens—including bacteria, fungi, viruses, and nematodes—has shaped the trajectory of agricultural systems for centuries. Plant diseases caused by these pathogens continue to threaten food security significantly, reducing yields and adversely affecting crop quality globally [2]. Estimates suggest that plant diseases contribute to approximately 30% of annual crop losses globally, resulting in direct economic losses exceeding $200 billion annually, with staple crops such as wheat, maize, and rice being particularly vulnerable. These losses not only affect food availability but also have far-reaching consequences on farmers’ livelihoods, especially in developing regions [3]. In addition to direct yield reductions, plant diseases also contribute to the degradation of ecosystem services, such as soil fertility, carbon sequestration, and water regulation, thus threatening the stability of ecosystems and the agricultural systems dependent on them. Maintaining plant health is crucial for ensuring ecosystem stability [4]. Healthy plants provide critical services, including nutrient cycling, habitats for biodiversity, and support for the physical structure of soils, which are essential for long-term ecological and agricultural sustainability. For instance, plants contribute to soil aeration and water retention, enhance biodiversity by providing food and shelter for other organisms, and facilitate carbon sequestration, which helps mitigate climate change [5]. However, plant diseases can disrupt these vital functions, leading to a cascade of ecological imbalances. Pathogenic attacks on plants result in several symptoms, ranging from root rot, blight, wilting, and stunted growth to complete plant death. These effects undermine plant health and hinder the plant’s ability to perform ecosystem functions. Consequently, plant diseases represent a double-edged sword, threatening both the productivity of crops and the stability of the ecosystems that support them [6,7].
Conventional strategies for managing plant diseases have traditionally relied on chemical pesticides and fertilizers. While these methods have been effective in controlling certain plant pathogens, they have several significant drawbacks [8]. For example, the widespread use of chemical treatments can result in environmental degradation, including soil and water contamination, and the development of resistance in pathogens [9]. Pesticide resistance, in particular, has become a growing concern, with several pathogen species evolving mechanisms to evade chemical control, making management even more difficult. Additionally, chemical inputs often disrupt the delicate balance of beneficial soil microbes, negatively affecting soil health and plant growth [10]. Thus, the need for sustainable and eco-friendly alternatives to chemical pesticides has never been more urgent. In this context, plant growth-promoting rhizobacteria (PGPR) have emerged as a promising solution, offering an environmentally friendly and cost-effective approach to disease management [11]. PGPR are a diverse group of microorganisms that colonize the rhizosphere of plants and promote plant growth through a variety of mechanisms. These bacteria enhance plant growth and development by increasing nutrient availability, producing phytohormones, and protecting plants from pathogens through direct and indirect mechanisms [12]. One of the key roles of PGPR in plant health is their ability to induce systemic resistance in plants, making them more resistant to a broad spectrum of pathogens. PGPR can also produce a range of antimicrobial compounds, including antibiotics, volatile organic compounds (VOCs), and hydrolytic enzymes, which help inhibit pathogen growth [13]. Moreover, PGPR can outcompete harmful pathogens in the rhizosphere for space and resources, thus reducing the likelihood of infection. These multifaceted benefits of PGPR make them powerful agents in enhancing plant resistance to diseases, thus reducing the need for chemical pesticides and supporting sustainable agricultural practices [14].
The significance of PGPR in modern agriculture cannot be overstated, particularly in the context of global challenges such as climate change, population growth, and food security [15]. The world’s population is projected to reach 9.7 billion by 2050, placing immense pressure on agricultural systems to produce sufficient food. Climate change is expected to exacerbate existing challenges, with more frequent and severe droughts, floods, and temperature extremes placing additional stress on crops, making them more vulnerable to pathogens [16]. In this context, PGPR offer a sustainable solution, as they are eco-friendly, cost-effective, and capable of enhancing plant growth and pathogen resistance. In addition to promoting plant health, PGPR contribute to soil fertility by increasing nutrient availability and enhancing soil microbial diversity, which are crucial for long-term agricultural sustainability [17]. Several plant pathogens have been successfully managed using PGPR. For example, Pseudomonas fluorescens, a widely studied PGPR, has been shown to control soilborne pathogens such as Fusarium species, Pythium spp., and Rhizoctonia solani by producing antibiotics and competing for space in the rhizosphere [18]. Similarly, Bacillus subtilis and Bacillus thuringiensis have demonstrated efficacy in controlling foliar pathogens, including Alternaria spp., Botrytis cinerea, and Xanthomonas spp., through lipopeptide and VOC production. Furthermore, PGPR also enhance resistance to viral pathogens, such as Tobacco mosaic virus, by inducing systemic acquired resistance (SAR) in plants. The ability of PGPR to reduce the severity of diseases caused by these pathogens is crucial for maintaining crop productivity and reducing the reliance on chemical pesticides [19,20].
The global impact of plant diseases on crop productivity is vast and multifaceted. It is estimated that plant pathogens are responsible for 20%–30% of global crop losses, with some crops suffering up to 50% yield loss in regions heavily affected by diseases [21]. For example, the wheat yellow rust epidemic in 2010 resulted in an estimated yield loss of 9 million metric tons, while rice blast disease, caused by Magnaporthe oryzae, results in an annual global rice production loss of 10%–30%. These losses contribute to the growing food insecurity in many parts of the world, particularly in developing countries where agriculture plays a central role in the economy [22,23]. Moreover, plant diseases affect biodiversity by reducing the population sizes of native plant species, which can have cascading effects on ecosystems. The decline of plant species due to pathogen pressure disrupts the food web and decreases habitat availability for other organisms, leading to further biodiversity loss [24]. In the context of food security, the importance of PGPR cannot be overstated. With increasing pressure to enhance agricultural productivity, PGPR offer an innovative approach to disease management that is both environmentally sustainable and effective. By enhancing plant resistance to pathogens, PGPR reduce the need for chemical pesticides, thereby promoting healthier soils, reducing water contamination, and preventing pesticide resistance development [25]. PGPR application has been shown to improve crop yields by 10%–30% in various agricultural systems, making them a valuable tool for farmers worldwide [26,27]. Additionally, PGPR support rhizosphere heath by improving soil structure, increasing nutrient cycling, and promoting microbial diversity. These benefits contribute to the long-term sustainability of agricultural ecosystems, ensuring that crops can withstand the challenges posed by climate change and the increasing demand for food [17,28]. The direct and indirect mechanisms through which PGPR augment plant growth promotion and plant protection against pathogens are illustrated in Fig. 1.
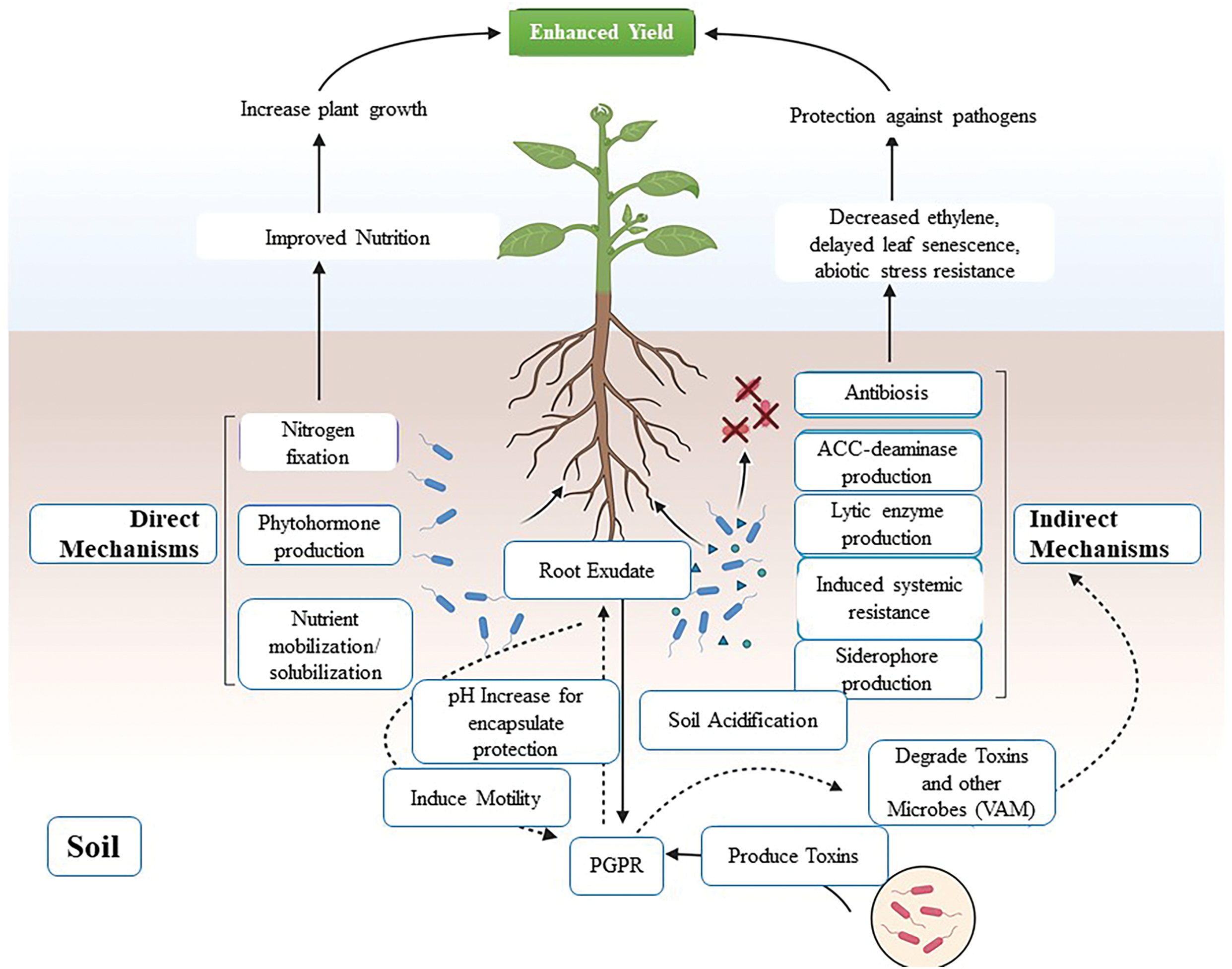
Figure 1: Illustrates the direct and indirect mechanisms through which PGPR augment plant growth promotion and provide protection against pathogens
This review aims to provide a comprehensive overview of the molecular and ecological mechanisms underlying PGPR-mediated plant-pathogen control, with an emphasis on the types of pathogens that PGPR are effective against. It also explores the current state of research on PGPR application in agriculture, identifying key PGPR strains used for managing plant diseases, and discussing the challenges and future directions for their widespread use. Additionally, this review examines the potential of PGPR to enhance plant resistance to a wide range of pathogens and their role in promoting sustainable agricultural practices, highlighting their potential to reduce reliance on chemical pesticides and fertilizers. By synthesizing current research and offering insights into future developments, this review contributes to the advancement of PGPR-based solutions in agriculture, helping to ensure global food security and the stability of ecosystems in the face of ongoing environmental challenges.
2 Molecular Mechanisms of PGPR-Mediated Plant-Pathogen Control
PGPR benefit plants through several molecular and biochemical mechanisms, each contributing to the plant’s enhanced resistance to pathogens. These mechanisms enable PGPR to function as natural biocontrol agents, reducing the need for chemical pesticides and enhancing the sustainability of agricultural practices [29,30]. A key aspect of PGPR-mediated disease resistance is the induction of systemic resistance in plants, production of antimicrobial compounds, modulation of plant hormones, and formation of biofilms that aid in pathogen suppression (Fig. 2). PGPR mediates pathogen suppression through various mechanisms [13]. These include competition for nutrients and space in the rhizosphere, the production of antimicrobial compounds such as antibiotics and volatile organic compounds (VOCs), and the activation of plant immune responses such as Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR) [31]. Biofilm formation further enhances PGPR colonization and pathogen suppression [29]. Fig. 2 summarizes these mechanisms. Understanding these processes at the molecular level offers critical insight into how PGPR can be effectively utilized in crop protection and sustainable farming.
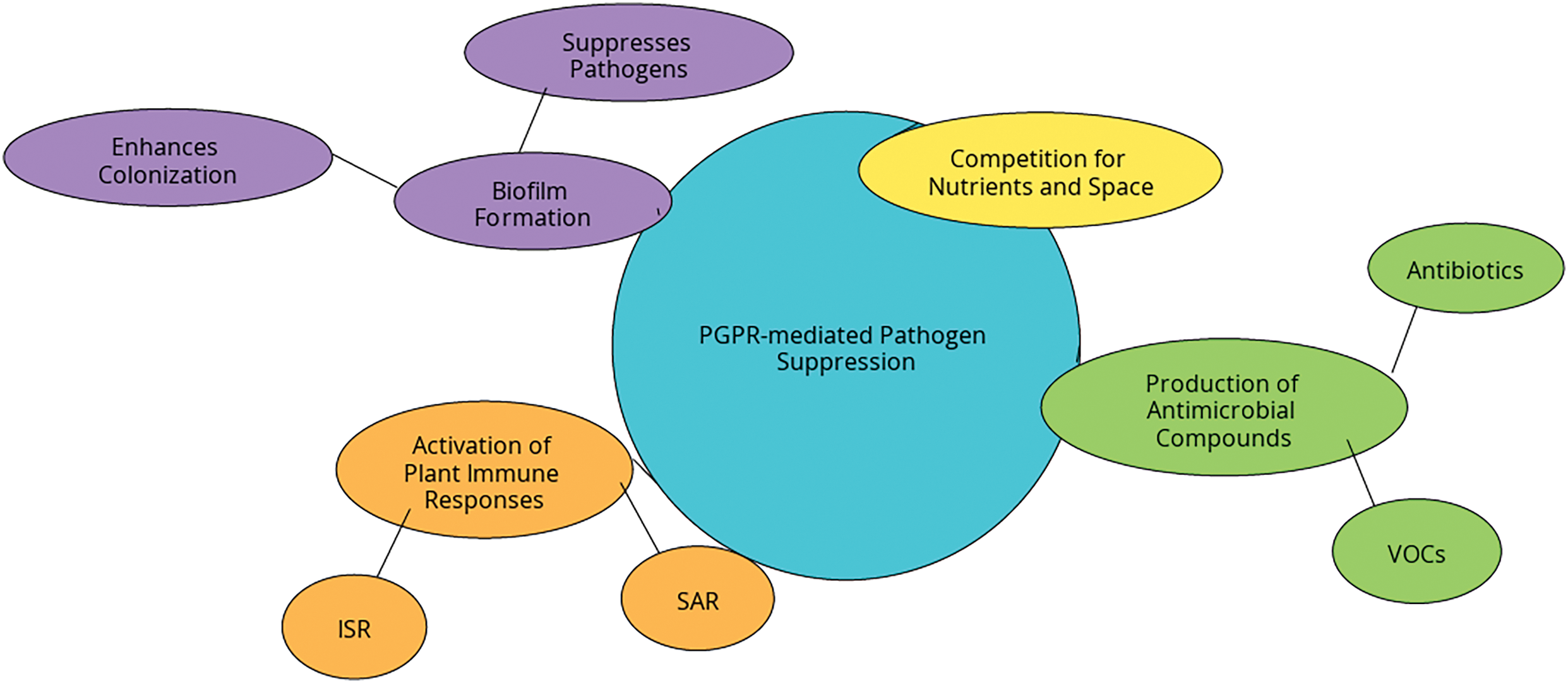
Figure 2: The flowchart illustrates the various mechanisms through which plant growth-promoting rhizobacteria (PGPR) mediate pathogen suppression
In addition to the general concepts described, certain antimicrobial compounds produced by PGPR strains are essential for suppressing pathogens. For example, Pseudomonas aeruginosa and Pseudomonas fluorescens produces phenazines, which exhibit a strong antimicrobial activity by disrupting pathogen cellular functions and inhibit biofilm production and leading to inhibiting the colonization of the pathogen [32,33]. Another well-known PGPR, Bacillus subtilis, synthesizes lipopeptides, such as surfactin, that exhibit broad-spectrum antimicrobial activity by destabilizing microbial cell membranes [34,35]. Pseudomonas chlororaphis produces volatile organic compounds (VOCs), such as 2,4-diacetylphloroglucinol (DAPG), which inhibit pathogen growth by causing cellular damage to plant pathogens. Additionally, HCN prevents the growth of insects, microbes and other plant diseases. It is produced from glycine by HCN synthase in a variety of Pseudomonas species. According to the majority of fluorescent Pseudomonads a few species of the genus Chromobacterium, Burkholderia, and some Rhizobia, reported for cyanide synthesis in the bacteria [36]. PGPR like Trichoderma harzianum secrete hydrolytic enzymes (e.g., chitinase and β-1,3-glucanase), which degrade fungal cell walls and reduce pathogen virulence. PGPR also activate plant immune responses, including the induction of Systemic Acquired Resistance (SAR) or Induced Systemic Resistance (ISR). These reactions occur when plant receptors identify PGPR-associated molecular patterns (PAMPs). This results in the activation of signaling pathways, such as calcium influx, MAPK signaling, and the generation of reactive oxygen species (ROS). These pathways then activate genes linked to defense and improve the plant’s resistance to infections [37,38].
PGPR-ISR is one of the most widely studied mechanisms by which PGPR confer protection against plant pathogens. ISR refers to the enhanced defense of a plant against pathogens that is triggered by an initial interaction with beneficial microorganisms, such as PGPR, leading to a plant-wide immune response [14]. This defense response occurs without the need for the pathogen to directly infect the plant, thus enhancing the plant’s resistance to subsequent pathogen attacks. The molecular basis of ISR involves the activation of the plant’s innate immune system, which includes both local and systemic responses that prepare the plant for future pathogen challenges [39,40].
At the core of ISR is the interaction between PGPR and plant immune receptors, particularly pattern recognition receptors (PRRs) that detect pathogen-associated molecular patterns (PAMPs). PRRs are part of the plant’s immune system and are responsible for recognizing conserved microbial features such as flagellin, chitin, or lipopolysaccharides [41,42]. When PGPR interact with plant roots, they often produce signaling molecules, including PAMPs, that are recognized by these receptors. The binding of PGPR-derived PAMPs to PRRs triggers a cascade of signaling events that activate the plant’s immune response [14,43]. These events are often mediated by mitogen-activated protein kinase (MAPK) pathways, calcium signaling, and reactive oxygen species (ROS) generation, all of which contribute to the activation of defense genes. This process induces the production of pathogenesis-related (PR) proteins, which include enzymes such as chitinases and glucanases, capable of degrading pathogen cell walls and inhibiting pathogen growth [44,45].
Research has shown that PGPR can activate ISR in many plant species. For example, in Arabidopsis thaliana, treatment with Pseudomonas fluorescens induces ISR against Pythium ultimum by activating genes involved in the defense response [46]. Similarly, in tomato plants, Bacillus subtilis application induces ISR, leading to increased resistance to Fusarium oxysporum and other soilborne pathogens. The magnitude of ISR activation can vary depending on the PGPR strain, plant species, and pathogen type. Quantitative studies suggest that PGPR application can reduce disease incidence by up to 40% in some crops, providing a robust alternative to chemical pesticide use [47,48].
Another key mechanism by which PGPR protect plants from pathogens is through the production of antimicrobial compounds. These compounds are produced by PGPR as part of their natural defense strategies against competing microorganisms in the rhizosphere [18]. PGPR synthesize a variety of antimicrobial compounds, including antibiotics, enzymes, and VOCs, all of which play a role in pathogen suppression. The production of these compounds is often species-specific, and the effectiveness of PGPR as biocontrol agents can be linked to the types of antimicrobial compounds they produce [49].
Antibiotics produced by PGPR include compounds such as phenazines, which are produced by Pseudomonas species, and bacillomycin, produced by Bacillus species. These antibiotics are toxic to a broad spectrum of plant pathogens, including fungi, bacteria, and viruses [50]. For example, Pseudomonas fluorescens produces phenazines, which inhibit the growth of Fusarium spp. and other soilborne pathogens. Similarly, Bacillus subtilis produces a range of lipopeptides, including surfactin and iturin, which exhibit strong antifungal activity against pathogens such as Rhizoctonia solani and Alternaria solani [51]. In addition to antibiotics, PGPR also produce hydrolytic enzymes, such as chitinases, glucanases, and proteases, which break down the cell walls of pathogens and inhibit their growth. Enzyme production is often induced by PAMPs, which are detected by plant PRRs, further enhancing the plant’s immune response [52,53].
VOCs are another class of antimicrobial compounds produced by PGPR that play an important role in pathogen suppression. VOCs, including aldehydes, alcohols, ketones, and terpenes, are emitted by PGPR into the surrounding environment and can act in many ways to inhibit pathogen growth [54]. For instance, Pseudomonas putida and Bacillus amyloliquefaciens produce VOCs such as 2,3-butanediol and acetoin, which inhibit the growth of fungal pathogens such as Fusarium spp. and Botrytis cinerea. VOCs can also affect the growth of bacterial pathogens by disrupting their cell membranes and inhibiting their ability to form biofilms. The production of these antimicrobial compounds by PGPR represents a key mechanism in their ability to suppress pathogen growth and promote plant health [20,55].
In addition to antimicrobial compound production, PGPR can modulate plant hormone signaling to enhance plant growth and defense responses. Plant hormones, including auxins, cytokinins, and ethylene, play central roles in regulating various aspects of plant growth, development, and stress responses. PGPR can influence the levels and activity of these hormones, thus improving plant health and resistance to pathogens [13,56].
Auxins, such as indole-3-acetic acid (IAA), are among the most important plant hormones involved in regulating root development. PGPR are known to produce IAA, which can promote root growth and increase the plant’s ability to absorb water and nutrients [57,58]. Additionally, IAA can influence the plant’s defense response by modulating the expression of defense-related genes. For example, Pseudomonas putida produces IAA, which enhances root growth and increases the plant’s resistance to root pathogens such as Pythium spp. and Rhizoctonia spp. Cytokinins, another group of plant hormones, are involved in promoting cell division and differentiation [59]. PGPR can produce cytokinins, which enhance plant growth and improve pathogen resistance by modulating the plant’s immune response [60]. In Arabidopsis plants treated with Bacillus amyloliquefaciens, cytokinin production was linked to increased resistance to Fusarium spp. by enhancing the expression of defense genes [61].
Ethylene, a plant hormone involved in stress responses and pathogen defense, can also be modulated by PGPR. PGPR can produce ethylene or enhance its production in plants, which in turn activates the plant’s defense response against pathogens [62]. For example, Pseudomonas fluorescens can stimulate ethylene production in plants, which activates the expression of defense-related genes and increases resistance to various pathogens. Conversely, PGPR can also modulate ethylene levels to prevent overactivation of defense responses that could harm plant growth [63].
Biofilm formation is another critical mechanism through which PGPR suppress plant pathogens and enhance root colonization. Biofilms are clusters of bacteria encased in a self-produced extracellular matrix that protects the bacteria from environmental stressors and enhances their ability to compete with other microorganisms [64]. In the rhizosphere, PGPR form biofilms on plant roots, creating a physical barrier that protects plants from pathogen colonization. This biofilm formation is especially important for soil-borne pathogens, which must invade the root system to cause disease [64]. The formation of biofilms by PGPR allows them to outcompete pathogens for space and nutrients, thereby preventing pathogen establishment. For example, Pseudomonas fluorescens and Bacillus subtilis form biofilms that reduce the ability of Fusarium spp. and Rhizoctonia solani to colonize plant roots [65].
The impact of biofilms extends beyond competition with pathogens. Biofilms also help PGPR maintain a stable and long-lasting presence in the rhizosphere, increasing their effectiveness as biocontrol agents. In addition, biofilms enhance the ability of PGPR to survive under nutrient-limited conditions and help them resist environmental stresses, such as desiccation and changes in pH. Furthermore, biofilm formation by PGPR can stimulate plant immune responses, further enhancing plant resistance to pathogens [66,67].
Conclusively, PGPR utilize a multifaceted array of molecular mechanisms to protect plants from pathogens. These mechanisms include the induction of systemic resistance, production of antimicrobial compounds, modulation of plant hormone signaling, and biofilm formation [13]. Each of these mechanisms contributes to enhanced pathogen resistance, reduced disease symptoms, and growth maintenance. Understanding these mechanisms is essential for optimizing PGPR use in agricultural systems to provide a sustainable alternative to chemical pesticides and contribute to the overall health and productivity of crops [39,68]. As research continues to uncover the complexity of PGPR interactions with plants and pathogens, these beneficial microorganisms will likely play an increasingly important role in integrated pest management strategies and sustainable agricultural practices worldwide [69].
3 Cellular Mechanisms of PGPR in Plant-Pathogen Interaction
The cellular mechanisms through which PGPR mediate plant-pathogen interactions are a fundamental area of study in plant biology, as they offer insights into how beneficial microorganisms can protect plants from a variety of pathogens. These mechanisms involve complex signaling pathways, interactions with the plant’s immune system, and modifications to the root environment, which enhance plant resistance to soil-borne pathogens [18,70]. Specifically, PGPR initiates pathogen suppression through various cellular mechanisms. Upon recognition of PAMPs by plant receptors, several defense responses are activated, including MAPK signaling, calcium signaling, and ROS production. These responses trigger the induction of systemic resistance, which enhances plant immunity across the whole plant [71]. Additionally, PGPR directly inhibit pathogen growth and compete for resources in the rhizosphere. The ability of PGPR to improve plant health is underpinned by their influence on plant immune signaling, enhancement of root defense mechanisms, and modulation of the rhizosphere microbiome. These interactions collectively contribute to plant growth promotion and disease suppression, providing a sustainable alternative to chemical pesticides and fertilizers (Table 1) [72,73].
PGPR strains have demonstrated significant potential in managing various plant diseases by targeting diverse pathogens and employing multiple mechanisms of action. For instance, Pseudomonas fluorescens effectively controls soilborne pathogens like Ralstonia solanacearum, Rhizoctonia solani, in Fusarium ox-ysporum in tomatoes by producing antibiotics (phenazines), excluding pathogens competitively, and activating systemic resistance [74]. Similarly, Bacillus amyloliquefaciens suppresses bacterial and fungal pathogens such as Botrytis pelargonii and Alternaria alternata in crops like peppers, thus reducing disease severity by up to 50% through lipopeptide production, systemic resistance induction, and niche competition [74,75]. Other notable rhizobacteria include Bacillus, Pseudomonas, and Serratia lead to disease reduction in potatoes against Phytophthora infestans through antibiosis, and, indirectly, through the induction of plant defense systems [76]. Additionally, strains like Bacillus velezensis (SM-39), and Bacillus cabrialesii (SM-93) demonstrate effective pathogen control against Fusarium spp., Macrophomina phaseolina, and Rhizoctonia solani in Triticum aestivum [77]. Pseudomonas strain IALR1619 also exhibit significant reductions in disease severity, leveraging antifungal compound production, suppress Pythium ultimum in Cucumis sativus [78]. Collectively, these PGPR strains exemplify the promise of eco-friendly alternatives to chemical pesticides, offering sustainable solutions for integrated pest management by reducing disease severity and promoting plant health.
The plant immune system plays a crucial role in recognizing and responding to pathogen attack. When plants are exposed to pathogens, they rely on sophisticated signaling pathways to activate defense responses that inhibit pathogen growth and limit infection [86]. One of the most well-established pathways involved in plant immune responses is the mitogen-activated protein kinase (MAPK) cascade. MAPKs are a family of enzymes that transduce external signals into a range of intracellular responses, ultimately leading to the activation of genes that promote plant defense [87]. When PGPR are introduced to plants, they can trigger MAPK signaling pathways by delivering microbe-associated molecular patterns (MAMPs), such as flagellin or chitin, which are recognized by the plant’s PRRs. This recognition initiates a signaling cascade that activates a broad array of defense mechanisms, including ROS production, defense-related protein synthesis, and SAR induction [88,89].
In addition to MAPK signaling, calcium signaling is another essential mechanism that mediates plant immune responses. The interaction between PGPR and plant roots can lead to calcium ion (Ca2+) elevation in plant cells, which acts as a secondary messenger in plant immune signaling [90]. Increased cytosolic Ca2+ levels activate various downstream signaling components, including calcium-dependent protein kinases (CDPKs), which in turn activate further immune responses. Calcium signaling is particularly important in the activation of genes involved in pathogen defense, such as those encoding PR proteins, antimicrobial compounds, and cell wall-modifying enzymes. For instance, Pseudomonas fluorescens stimulates calcium influx in plant roots, which enhances plant resistance to pathogens like Pythium spp. and Rhizoctonia solani [91,92].
ROS, including hydrogen peroxide (H2O2) and superoxide anions (O2−), are another class of signaling molecules that play a pivotal role in plant defense. PGPR can trigger ROS generation in plants as part of the initial immune response to pathogen invasion. ROS not only act as signaling molecules to activate further defense responses, but they also have direct antimicrobial properties that help limit pathogen growth [93]. ROS accumulation in plant cells creates an oxidative burst, which is associated with pathogen growth inhibition through membrane damage and enzyme inactivation. Additionally, ROS are involved in cell wall reinforcement through the cross-linking of lignin and other cell wall components, thus fortifying the plant’s physical defenses [94]. In Arabidopsis thaliana treated with Bacillus subtilis, ROS production has been linked to enhanced resistance to Fusarium oxysporum and Botrytis cinerea, demonstrating the role of ROS in PGPR-mediated pathogen defense [95].
Cross-talk between PGPR and plant immune systems further amplifies plant resistance to pathogens. PGPR can induce both local and systemic immune responses in plants, which are coordinated by intricate networks of signaling pathways [89]. For example, when PGPR such as Pseudomonas fluorescens or Bacillus subtilis interact with plant roots, they activate a combination of local responses at the infection site and systemic responses throughout the plant [96]. This interaction primes the plant to respond more rapidly and effectively to subsequent pathogen attacks. Studies have shown that PGPR can induce both PAMP-triggered immunity (PTI) and effector-triggered immunity (ETI) in plants, which are two complementary arms of the immune system. PTI is triggered by the recognition of conserved microbial signatures, such as flagellin, while ETI is activated when the plant detects specific microbial effectors that are secreted and enter plant cells. The simultaneous activation of both PTI and ETI results in a stronger and more coordinated immune response, allowing the plant to fend off a broader range of pathogens [14,97].
PGPR-mediated root defense is another critical aspect of their ability to protect plants from soil-borne pathogens. The root system is often the first site of pathogen attack, as soilborne pathogens must infiltrate the root tissues to cause disease [98]. PGPR can enhance root defense through several mechanisms, including resource competition, antimicrobial compound secretion, and root environment modification. One of the most important roles of PGPR in root defense is their ability to outcompete pathogens for space and nutrients in the rhizosphere, thus preventing pathogen colonization [14,29]. For instance, Bacillus subtilis and Pseudomonas fluorescens are known to produce antifungal metabolites and compete with pathogens such as Fusarium spp. and Rhizoctonia solani for available niches in the root zone [96].
Furthermore, PGPR can improve root health by promoting root growth and development, which enhances the plant’s ability to resist pathogen attack. PGPR promote root growth through the production of phytohormones, including auxins, cytokinins, and gibberellins [18]. These hormones stimulate cell division, elongation, and differentiation in root tissues, leading to the development of a more extensive root system. A larger root mass increases the plant’s ability to take up water and nutrients, making it more resistant to drought and nutrient stress, which are often predisposing factors for pathogen infection [99,100]. Moreover, enhanced root growth enables plants to maintain better root health, reducing their susceptibility to root-borne pathogens. In Arabidopsis and tomato plants treated with Pseudomonas and Bacillus strains, root growth was significantly enhanced, resulting in increased resistance to root rot diseases caused by Pythium and Fusarium species [101,102].
The rhizosphere is a dynamic environment that hosts a diverse microbial community, including both beneficial and harmful microorganisms. PGPR play a pivotal role in shaping the rhizosphere microbiome, which in turn influences plant health and disease resistance. PGPR can alter the composition and diversity of the rhizosphere microbiota by promoting the growth of beneficial microorganisms while inhibiting the growth of pathogens [18,103]. One of the key ways PGPR influence the rhizosphere microbiome is by producing antimicrobial compounds that selectively inhibit pathogen growth. This antagonistic interaction between PGPR and harmful pathogens helps maintain a balanced microbial community in the rhizosphere, thereby preventing harmful pathogens from dominating [29]. For example, Bacillus amyloliquefaciens produces lipopeptides, which inhibit the growth of Fusarium oxysporum and Rhizoctonia solani, while promoting the growth of beneficial bacteria that support plant growth [104].
PGPR also interact with other beneficial microorganisms, such as mycorrhizal fungi and nitrogen-fixing bacteria, to enhance plant health. Mycorrhizal fungi form symbiotic relationships with plant roots, improving nutrient uptake, particularly phosphorus, while also enhancing pathogen resistance through the production of antifungal compounds [105]. PGPR can facilitate these symbiotic relationships by promoting mycorrhizal colonization of plant roots and stimulating the production of mycorrhizal spores [106]. Similarly, PGPR can interact with nitrogen-fixing bacteria, such as Rhizobium spp., which form nodules on legume roots and provide essential nitrogen to the plant. These interactions between PGPR, mycorrhizal fungi, and nitrogen-fixing bacteria enhance plant growth and resistance to pathogens, contributing to overall plant health and ecosystem stability [107].
Antagonistic interactions between PGPR and harmful pathogens are a key feature of PGPR’s role in plant disease suppression. PGPR not only compete with pathogens for space and nutrients but also directly suppress pathogen growth through the production of antimicrobial compounds, VOCs, and enzymes [108]. These interactions have been studied extensively regarding soilborne pathogens, where PGPR such as Pseudomonas fluorescens and Bacillus subtilis have been shown to reduce pathogen colonization and suppress disease symptoms. For example, Pseudomonas fluorescens produces phenazines, which inhibit the growth of Fusarium spp., while Bacillus subtilis secretes iturin, which suppresses the growth of Rhizoctonia solani [109]. These antagonistic interactions are often complemented by the PGPR’s ability to induce plant immune responses, further enhancing disease resistance [108,110].
Thus, PGPR exert their beneficial effects on plants through a variety of cellular mechanisms that involve intricate interactions with the plant’s immune system as well as rhizosphere microbiome modulation and root defense enhancement. The ability of PGPR to trigger immune signaling pathways, promote root health, and outcompete pathogens in the rhizosphere is central to their role in plant pathogen control [111,112]. Understanding these cellular mechanisms at a deeper level provides valuable insights into how PGPR can be utilized as a sustainable and effective tool for enhancing plant health and managing plant diseases. Continued research into PGPR-mediated mechanisms of pathogen control will lead to more targeted and efficient applications of PGPR in agriculture, ultimately contributing to the development of more resilient and sustainable agricultural systems [113].
4 Ecological and Environmental Benefits of PGPR Application
The application of PGPR in agriculture offers numerous ecological and environmental benefits that are increasingly recognized as essential for promoting sustainable farming practices. PGPR provide a promising alternative to chemical inputs, contributing to a reduction in the reliance on chemical pesticides and fertilizers [114,115]. By fostering plant growth, enhancing disease resistance, and improving soil health, PGPR help maintain or even increase crop productivity while minimizing negative environmental impacts. The integration of PGPR into agricultural systems holds significant promise for addressing pressing environmental concerns such as soil degradation, water contamination, and the loss of biodiversity, thereby contributing to long-term ecosystem stability [116,117].
One of the primary benefits of PGPR application is the reduction of chemical inputs in agricultural systems. Chemical pesticides and fertilizers have long been the mainstay of modern agriculture, helping to protect crops from pests and diseases while ensuring high yields [118]. However, the widespread and excessive use of these chemicals has led to several environmental issues, including soil degradation, water contamination, and the development of pesticide resistance in pathogens and pests. PGPR offer an eco-friendly alternative to these chemicals by enhancing plant health, promoting nutrient uptake, and protecting crops from pathogens naturally [119]. For instance, PGPR such as Pseudomonas fluorescens and Bacillus subtilis reduce the need for chemical pesticides by inducing systemic resistance in plants and outcompeting pathogens in the rhizosphere. Studies have demonstrated that PGPR use can lead to a reduction in pesticide application by up to 30%–50%, thus decreasing the environmental burden associated with pesticide use [120,121].
The environmental impact of reduced pesticide use is significant, particularly in terms of improving soil and water quality. Pesticide runoff from agricultural fields is a major source of water pollution, affecting aquatic ecosystems and contaminating drinking water sources [122]. The accumulation of pesticides in the soil can also disrupt soil microbial communities, leading to a decline in soil fertility and the development of resistant pest populations. By reducing the need for chemical pesticides, PGPR helps mitigate these environmental risks [123]. PGPR application has been associated with improved soil health, including increased microbial diversity and activity, which are essential for maintaining soil fertility and structure. For example, PGPR such as Rhizobium spp. and Azospirillum spp. promote nitrogen fixation in the soil, reducing the need for synthetic nitrogen fertilizers [18]. This not only reduces the environmental footprint of agriculture but also promotes the sustainability of farming systems by enhancing nutrient cycling and improving soil organic matter content [124].
PGPR also contributes significantly to sustainable agricultural practices, such as organic farming and integrated pest management (IPM). In organic farming, the use of synthetic chemical inputs is restricted, and PGPR can serve as valuable tools for managing plant diseases, improving nutrient availability, and promoting plant growth without resorting to chemical fertilizers or pesticides [125]. For instance, Trichoderma spp., a well-known PGPR, has been used extensively in organic farming systems to control soilborne pathogens and improve plant growth. In IPM systems, PGPR are often integrated with other biocontrol agents, such as natural predators or parasitoids, to provide a holistic approach to pest and disease management [108]. The use of PGPR in these systems not only reduces the reliance on chemical inputs but also promotes the health and stability of agroecosystems by maintaining a balanced microbial community and enhancing plant resilience to environmental stress [126].
The role of PGPR in promoting sustainable agriculture goes beyond disease control and nutrient management. PGPR also plays a crucial role in improving soil health, which is fundamental for the long-term sustainability of agricultural systems. By enhancing the activity of beneficial soil microorganisms, PGPR contributes to the overall health of the rhizosphere, promoting the decomposition of organic matter, enhancing nutrient cycling, and improving soil structure [127,128]. For example, PGPR such as Bacillus amyloliquefaciens and Pseudomonas putida stimulate the activity of soil enzymes involved in the breakdown of organic materials, which helps recycle nutrients and improve soil fertility. Additionally, PGPR application increases soil organic carbon content and improves soil aggregation, both of which are critical for maintaining soil structure and preventing erosion [121].
The long-term benefits of PGPR for ecosystem stability are also evident in their ability to promote biodiversity. Biodiversity is essential for the resilience and functioning of ecosystems, as it enhances ecosystem services such as pollination, pest control, and nutrient cycling. PGPR application has been shown to have positive effects on soil biodiversity, particularly by promoting the growth of beneficial microorganisms and enhancing microbial community structure [26]. PGPR can alter the composition of the rhizosphere microbiome by stimulating the growth of beneficial bacteria and fungi while suppressing the growth of harmful pathogens [29]. For example, Pseudomonas spp. increases the diversity of soil bacteria by promoting the growth of other beneficial microbes and suppressing pathogenic species such as Fusarium spp. and Rhizoctonia solani. This shift in microbial community composition can enhance plant health and resilience to disease, contributing to the overall biodiversity of the soil ecosystem [18].
The impact of PGPR on soil biodiversity goes beyond bacteria and fungi because it can also influence the abundance and diversity of other soil organisms, such as earthworms and nematodes, which play key roles in soil health and nutrient cycling [129]. Studies have shown that PGPR application can increase earthworm populations, which in turn improve soil structure and nutrient availability. PGPR such as Bacillus subtilis have been shown to stimulate the growth of beneficial nematodes, which help control root-damaging pests and enhance soil health. By promoting the activity and diversity of these soil organisms, PGPR contributes to the overall health and resilience of soil ecosystems, enhancing their capacity to withstand environmental stresses such as drought or pathogen invasion [130,131].
Beyond the rhizosphere, PGPR also promote biodiversity at the ecosystem level. By enhancing plant health and promoting sustainable agricultural practices, PGPR help maintain the balance of ecosystems, including agroecosystems, wetlands, and forest ecosystems [132]. For example, PGPR can enhance the establishment and growth of native plants in disturbed ecosystems, such as agricultural fields or degraded lands, thereby contributing to habitat restoration and ecosystem recovery [133]. The increased plant growth resulting from PGPR application can also lead to enhanced carbon sequestration, further contributing to ecosystem stability by mitigating climate change. In agroecosystems, PGPR can help maintain biodiversity by reducing the need for chemical inputs, which can harm non-target species such as pollinators, birds, and beneficial insects [134].
The contribution of PGPR to ecosystem balance and resilience is particularly important regarding climate change, which poses significant challenges to agriculture and biodiversity. Climate change is expected to exacerbate the frequency and intensity of extreme weather events, such as droughts, floods, and temperature extremes, which can severely affect crop productivity and ecosystem functioning [135]. PGPR can help mitigate the impacts of these stresses by enhancing plant resilience to environmental fluctuations and improving soil health. By promoting drought tolerance, improving nutrient availability, and enhancing pathogen resistance, PGPR can help plants withstand the negative effects of climate change, thus contributing to the resilience of agricultural systems and ecosystems [136,137]. The ecological and environmental benefits of PGPR application are profound and multifaceted. By reducing the reliance on chemical pesticides and fertilizers, PGPR help mitigate the environmental risks associated with conventional agricultural practices, including water contamination, soil degradation, and pesticide resistance [101]. The use of PGPR in sustainable farming systems contributes to the health of the soil ecosystem by promoting biodiversity, enhancing nutrient cycling, and improving soil structure [138]. Moreover, PGPR play a crucial role in promoting long-term ecosystem stability by enhancing plant health, reducing the environmental impact of farming, and contributing to the resilience of ecosystems in the face of climate change [139]. The integration of PGPR into agricultural systems offers a promising pathway for achieving more sustainable and environmentally friendly agricultural practices, ensuring food security and ecosystem health for future generations [140].
5 PGPR in Pathogen Control for Major Crops
PGPR have emerged as a significant tool for managing plant pathogens across various crops, offering both direct and indirect benefits in pathogen control. These beneficial microorganisms play a critical role in protecting crops against soil-borne and foliar pathogens, as well as in enhancing disease resistance in perennial crops and those vulnerable to climate change-induced stresses [141]. The integration of PGPR into agricultural systems offers a sustainable alternative to chemical pesticides, thus reducing environmental impacts while promoting plant health and ecosystem stability. Their application has proven effective in managing major plant diseases caused by fungi, bacteria, and viruses, demonstrating their broad utility across different crop types and environmental conditions [137,142].
PGPR have shown significant efficacy in the control of soil-borne pathogens. Soil-borne pathogens like Fusarium spp., Pythium spp., and Rhizoctonia solani are responsible for considerable crop losses, particularly in crops such as tomatoes, cucumbers, and rice. PGPR can suppress the growth of these pathogens through several mechanisms, including the production of antibiotics, competition for nutrients and space, and the induction of systemic resistance in plants [143]. For instance, Pseudomonas fluorescens has been extensively studied for its ability to control Fusarium wilt in tomatoes and cucumbers. For example, one field study demonstrated a 40–60% reduction in disease severity in tomato plants treated with P. fluorescens compared to that of the untreated controls [144]. This reduction in disease incidence is primarily attributed to the antibiotic production by P. fluorescens, including phenazines and 2,4-diacetylphloroglucinol, which directly inhibit the growth of Fusarium spp. [145]. Similarly, Bacillus subtilis has been successfully used to control Rhizoctonia solani in soybean and rice, with reductions in root rot symptoms of up to 50%. These PGPR outcompete the pathogen for space and nutrients, while also producing lipopeptides that have direct antifungal activity [146].
PGPR also play a crucial role in controlling foliar pathogens, which are responsible for a variety of destructive diseases in crops. Foliar pathogens, such as Xanthomonas spp. (causing bacterial spot), Phytophthora infestans (causing late blight), and downy mildew, can cause severe damage to crops like tomatoes, peppers, and potatoes [21]. The use of PGPR in managing these diseases has proven highly effective in reducing reliance on chemical pesticides. For example, Bacillus subtilis has been widely applied for managing bacterial leaf spot caused by Xanthomonas spp. in peppers and tomatoes [109]. In a field trial, the application of B. subtilis led to a 30%–50% reduction in bacterial spot incidence, significantly lowering the need for chemical bactericides. In addition to its direct antibacterial activity, B. subtilis also enhances plant immune responses, priming the plant for a quicker and more robust response to pathogen attack [147]. Similarly, Trichoderma harzianum, a well-known PGPR, has been used to control Phytophthora infestans, the causal agent of late blight in potatoes and tomatoes. Specifically, T. harzianum competes with the pathogen for space on the leaf surface and produces hydrolytic enzymes that degrade the pathogen’s cell wall, effectively reducing disease severity. Additionally, the use of PGPR to manage foliar pathogens not only reduces disease incidence but also promotes plant growth by enhancing nutrient availability and improving overall plant health.
In perennial crops, PGPR enhance disease resistance, making them a valuable tool in the management of long-lived crops such as fruit trees, vines, and woody perennials. Perennial crops are particularly vulnerable to repeated pathogen infections due to their long life cycle, making effective pathogen control crucial for maintaining productivity [148]. For example, in grapevines, Pseudomonas putida and Bacillus amyloliquefaciens have been used to reduce Phytophthora spp. infections, a common cause of root rot. In field trials, the application of these PGPR resulted in a 25%–40% reduction in disease incidence, improving both plant health and yield [149]. In apple orchards, Bacillus subtilis has been employed to control Venturia inaequalis, the fungus responsible for apple scab. A study showed that B. subtilis applications reduced apple scab severity by up to 50%, leading to healthier trees and higher fruit yield. Thus, PGPR not only protect against pathogens but also promote plant growth, improve nutrient uptake, and enhance the overall health of perennial crops, which is crucial for their long-term productivity and resilience [150].
The role of PGPR in controlling pathogens in crops affected by climate change-induced stress is becoming increasingly important. Climate change is expected to intensify the frequency and severity of extreme weather events, such as droughts, floods, and temperature extremes, which can weaken plant defenses and exacerbate pathogen susceptibility [151]. PGPR offer a promising solution to mitigate the effects of these stresses, while also improving plant resilience to pathogen outbreaks. For example, Azospirillum brasilense and Bacillus spp. have been used to enhance drought tolerance in crops like maize and wheat. In maize, Bacillus spp. significantly improved root development and water uptake, leading to a 20%–30% increase in plant growth under water-limited conditions [152]. Similarly, B. pumilus improved wheat growth under heat stress, reducing yield losses by approximately 25% compared to that of the non-inoculated plants. These PGPR not only enhance drought tolerance but also help reduce pathogen infections that tend to proliferate under stressed conditions [153]. In one study, Pseudomonas fluorescens reduced the severity of Fusarium spp. infections in wheat plants exposed to heat stress, with a 30%–40% reduction in disease symptoms compared to that of the untreated controls [154].
PGPR also contribute to pathogen control by enhancing plant immune responses under abiotic stress conditions [155]. The ability of PGPR to induce SAR and ISR in plants enhances the plant’s ability to resist pathogens even during environmental stresses. For example, Pseudomonas fluorescens and Bacillus subtilis can prime plants to activate their defense mechanisms more rapidly upon pathogen attack, thereby improving disease resistance [156]. In a study on soybean, B. subtilis application led to a 50% reduction in Fusarium root rot under drought conditions, demonstrating the combined effect of PGPR in both stress tolerance and disease control [157].
Finally, PGPR offer significant benefits for pathogen control across major crops, ranging from soil-borne pathogens such as Fusarium and Rhizoctonia to foliar disease-causing pathogens like Xanthomonas and Phytophthora infestans [158]. Through mechanisms such as nutrient competition, antimicrobial compound production, and plant immunity induction, PGPR reduce the incidence and severity of diseases, leading to improved plant health and higher yields (Fig. 3). PGPR application has been shown to improve both crop yields and pathogen management in several crops [159]. Fig. 3 illustrates the impact of PGPR on crop yield and disease severity. The application of PGPR in perennial crops helps protect against long-term infections and promotes resilience in fruit trees, vines, and other long-lived crops. Moreover, PGPR is a valuable tool for managing crops affected by climate change-induced stresses, improving drought tolerance, and mitigating pathogen outbreaks under extreme weather conditions [148,160]. As research continues to uncover the full potential of PGPR in pathogen control, their widespread adoption in sustainable agriculture will contribute to the development of more resilient and environmentally friendly farming systems, thereby reducing reliance on chemical pesticides and improving crop productivity.
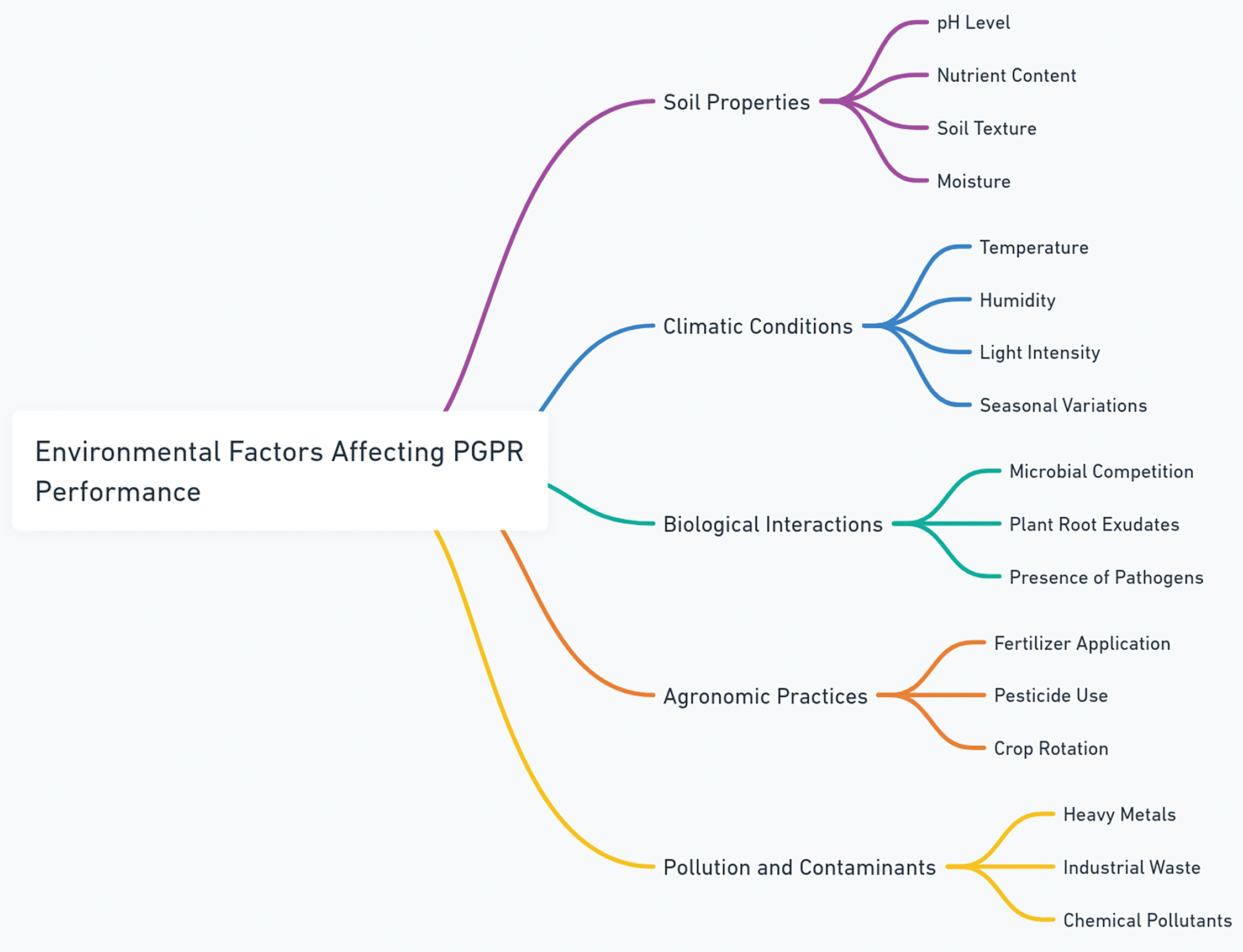
Figure 3: The performance of plant growth-promoting rhizobacteria (PGPR)-based treatments is influenced by various environmental conditions
6 Challenges and Limitations in PGPR Application
While PGPR offer promising solutions for pathogen control and sustainable agriculture, there are several challenges and limitations in their widespread application. These challenges range from variability in efficacy to difficulties in scaling up production and overcoming regulatory barriers. Understanding these limitations is essential for optimizing PGPR use in agricultural systems and ensuring that their benefits are realized in diverse field conditions [108,161].
One of the primary challenges in PGPR application is the variability in their efficacy across different environments (Fig. 3). Environmental factors, including soil pH, temperature, and moisture levels, influence PGPR performance [162]. Fig. 3 presents a mind-map summarizing these factors and their impact on PGPR efficacy.
Environmental conditions such as temperature, humidity, and soil pH can significantly impact the survival and activity of PGPR in the field (Table 2). For instance, PGPR strains that thrive in temperate climates may not perform well in tropical or arid environments [163]. In a study on Pseudomonas fluorescens application to control Fusarium wilt in tomatoes, the treatment efficacy varied by region, with a 40% reduction in disease severity in temperate climates but only a 15% reduction in tropical climates. Similarly, soil properties such as texture, organic matter content, and pH can influence the effectiveness of PGPR [144]. Soils with low organic matter or high salinity may not support the growth of certain PGPR strains, leading to reduced pathogen suppression. For example, in saline soils, the performance of Bacillus subtilis in controlling Rhizoctonia solani was significantly lower, with a 20% reduction in disease severity compared to a 50% reduction in non-saline soils [164].
While the efficacy of PGPR is often influenced by environmental conditions, several strategies can be employed to mitigate these challenges including microencapsulation and controlled-release formulations. In microencapsulation, PGPR are encapsulated in protective coatings, has been shown to enhance the survival and activity of PGPR under stressful environmental conditions [172,173]. This technique offers protection from UV radiation, extreme temperatures, and soil desiccation, ensuring that PGPR can persist in the rhizosphere and maintain pathogen-suppressing activity. Additionally, controlled-release formulations (CRFs) allow for the gradual release of PGPR over time, providing sustained protection against pathogens and improving the long-term efficacy of PGPR applications. These formulations also help to synchronize PGPR activity with the plant’s growth cycle, further optimizing their performance [174,175].
Plant pathogen diversity also complicates PGPR use in pathogen control. Different PGPR strains exhibit varying levels of effectiveness against different pathogens. While some PGPR are effective against a wide range of pathogens, others are more specific in their activity [137]. For example, Trichoderma harzianum suppresses a wide range of fungal pathogens, including Phytophthora infestans and Fusarium oxysporum, but may not be effective against bacterial pathogens such as Xanthomonas spp. [176]. In contrast, Pseudomonas fluorescens is more effective against bacterial pathogens but may offer limited control over fungal diseases. The strain-specific nature of PGPR means that choosing the right PGPR for the target pathogen is crucial for successful disease management [177]. Additionally, environmental stress factors, such as drought, may reduce the effectiveness of PGPR by impairing their ability to induce systemic resistance in plants. Thus, the variability in PGPR efficacy highlights the need for tailored approaches that take into account environmental and pathogen-specific factors [137].
Another major challenge in the widespread use of PGPR in agriculture is the scaling up of production and PGPR application on a commercial scale [178]. The mass production of PGPR strains that are effective, stable, and economically viable remains a significant hurdle. While PGPR can be cultured in laboratory settings, scaling up production to meet the demands of large-scale agricultural use requires considerable investment in infrastructure, technology, and resources [179]. The cost of producing PGPR at a commercial scale is often high, and the quality of the product can vary between batches. For example, a study on the mass production of Bacillus subtilis for use in tomato disease control found that while the product was effective at small-scale trials, large-scale production led to inconsistent results, with some batches showing reduced efficacy due to variations in microbial concentration and formulation stability [162,180].
The formulation of PGPR products also poses challenges. PGPR formulations must be stable under varying environmental conditions, such as changes in temperature, humidity, and soil pH. Many PGPR strains are sensitive to desiccation, UV radiation, and extreme temperatures, which can compromise their viability during storage and application [181]. For instance, Pseudomonas fluorescens strains may lose up to 50% of their viability when stored for extended periods at ambient temperatures. To ensure the stability of PGPR formulations, protective agents such as cryoprotectants, stabilizers, and encapsulation techniques are often used [182]. However, these add to the cost of production and may not always provide long-term stability, particularly in the field where conditions can fluctuate. Inconsistent formulations and reduced stability can undermine the efficacy of PGPR-based treatments, leading to poor results when applied to crops [183].
In addition to these technical challenges, there are significant regulatory and commercialization barriers that hinder the widespread adoption of PGPR in agriculture. Regulatory frameworks for PGPR-based biocontrol agents vary widely across regions, and many countries do not have established guidelines for their registration and use [162]. Unlike chemical pesticides, which are subject to extensive regulation and testing, PGPR products are often considered to be “natural” and may not fall under the same regulatory scrutiny. This lack of clear regulatory standards can create uncertainty for producers and consumers alike. For instance, in some regions, PGPR products may not be registered as biocontrol agents, which limits their market access and restricts their use in commercial agriculture [183,184].
The regulatory process for registering PGPR-based products can also be lengthy and expensive. In the European Union, for example, the registration of biocontrol products can take several years and require extensive testing to ensure safety and efficacy [185]. This regulatory delay can prevent farmers from accessing effective PGPR treatments in a timely manner, particularly when facing an outbreak of a plant pathogen. Moreover, the cost of registration and testing can be prohibitive for small-scale producers, limiting the availability of PGPR products on the market [186]. The lack of standardized guidelines for PGPR also presents challenges in ensuring the quality and consistency of PGPR products. Without clear regulations, manufacturers may produce substandard products that fail to meet efficacy and safety standards, undermining consumer confidence in PGPR as a viable biocontrol solution [187].
In addition to regulatory challenges, economic and market barriers further complicate the commercialization of PGPR products. Despite the growing demand for sustainable agricultural practices, the market for PGPR-based biocontrol agents remains a niche, with limited consumer awareness and acceptance [185]. Farmers may be hesitant to adopt PGPR-based products due to concerns about their effectiveness, ease of use, and cost compared to that of conventional chemical pesticides. Additionally, the upfront costs of PGPR products may be higher than those of synthetic chemicals, particularly for small-scale farmers who may not see immediate returns on their investment [188]. Furthermore, the lack of widespread education and training on the use of PGPR in agriculture can limit their adoption. For instance, in some regions, farmers may lack awareness of PGPR-based treatments and may continue to rely on chemical inputs due to established practices and marketing efforts by pesticide companies [189]. Overcoming these economic and market barriers requires not only greater awareness and education but also the development of cost-effective and user-friendly PGPR products that can compete with traditional chemical inputs [190].
7 Future Directions and Outlook
The future of PGPR as biocontrol agents and agricultural tools is promising, with several areas of research requiring further exploration to optimize their use in diverse agricultural systems. Advances in PGPR strain selection, integration with other biocontrol strategies, applications in precision agriculture, and the necessity for large-scale trials are all critical factors for realizing the full potential of PGPR in sustainable farming [108]. These areas of research offer valuable opportunities to improve pathogen management, increase crop yields, and minimize environmental impacts, aligning with the growing demand for eco-friendly agricultural practices [191].
Advances in PGPR strain selection represent one of the most crucial areas for enhancing PGPR effectiveness in agricultural applications. The isolation and identification of PGPR strains with superior biocontrol capabilities are essential for optimizing their performance in diverse environmental conditions. Consequently, various techniques are employed for isolating effective PGPR strains, including culture-based methods, molecular tools, and high-throughput screening techniques [192,193]. However, culture-based methods, while still widely used, often fail to capture the full diversity of PGPR populations in the rhizosphere. More advanced molecular techniques, such as 16S rRNA gene sequencing and metagenomics, enable researchers to identify novel PGPR strains with potential biocontrol properties from complex microbial communities [194]. High-throughput screening of microbial libraries also allows the rapid assessment of the antimicrobial potential of different PGPR strains, facilitating the selection of the most effective candidates. For example, screening Bacillus species for their ability to suppress Fusarium spp. led to the identification of strains that reduce disease severity in crops like tomatoes by up to 50% [195,196].
Genetic engineering has also emerged as a promising tool to enhance the properties of PGPR strains. Through genetic modifications, researchers can introduce traits that improve their biocontrol potential, such as enhanced production of antimicrobial compounds, increased resistance to environmental stressors, or improved root colonization [197]. A notable example is the genetic engineering of Pseudomonas fluorescens to overexpress antifungal compounds like phenazines, which significantly enhances its ability to suppress soil-borne pathogens such as Fusarium spp. In addition, genetic engineering can be used to optimize PGPR strains for specific environmental conditions, such as high salinity or extreme temperatures, expanding their applicability across diverse agricultural systems [198]. More recently, the study of Liu et al. [199] revealed that Bacillus subtilis (HS3) and Bacillus mycoides (EC18) are rhizospheric bacteria with plant growth-promoting activity. The CRISPR-Cas9 system was employed to investigate the plant-microbe interaction mechanisms of these isolates and their role in biocontrol. Their results demonstrated that fengycin and surfactin contribute to the antifungal activity of B. subtilis, which also emits several volatile organic compounds, including 2,3-butanediol, promoting plant growth. Confocal laser scanning microscopy of the GFP-labeled strain revealed that HS3 selectively colonizes root hairs of Lolium perenne in a hydroponic system. Finally, they concluded that the CRISPR-Cas9 system developed for these environmental isolates is broadly applicable and will aid in elucidating Bacillus and other plant-microbe interaction. The application of genetic engineering to PGPR represents an exciting frontier for improving the efficacy and resilience of biocontrol agents [200]. Similarly, synthetic microbial communities (SMC) have emerged as a promising strategy for plant disease management, leveraging the collective capabilities of multiple microbial species. Advances in omics technologies and tools such as artificial intelligence have significantly improved the design and efficiency of SMCs, enabling synergistic interactions that can effectively control phytopathogens. However, the complexity of plant-associated systems, along with the numerous variables influencing SMC performance, presents challenges in developing a universal biocontrol approach. Future research should focus on refining the design principles and addressing the critical considerations for the successful application of SMCs in plant disease management [201].
Integrating PGPR with other biocontrol strategies is another key avenue for enhancing pathogen management in agriculture. Combining PGPR with other biocontrol agents, such as biopesticides and mycorrhizal fungi, can provide synergistic effects that improve disease suppression and plant health [202]. Biopesticides, such as Beauveria bassiana and Trichoderma harzianum, are fungal-based agents that have been successfully used in conjunction with PGPR to target a wide range of pathogens. For example, Trichoderma spp. and Pseudomonas fluorescens have been shown to work together in suppressing Rhizoctonia solani, with combined applications leading to a 40–60% reduction in disease severity compared to single-agent treatments [203,204]. Using PGPR with mycorrhizal fungi is another promising strategy, as mycorrhizal fungi enhance nutrient uptake and increase plant resistance to root pathogens, while PGPR suppress pathogenic microbes in the rhizosphere [205]. This combination improved plant growth and yield by 20–30% in crops such as maize and wheat. Integrating PGPR with other biocontrol agents, such as mycorrhizae and biopesticides, not only improves pathogen control but also enhances soil health and biodiversity, thus supporting sustainable agricultural practices (Fig. 4) and offering a promising approach for enhancing pathogen management [108]. Fig. 4 illustrates how these agents can work synergistically to improve pathogen control.
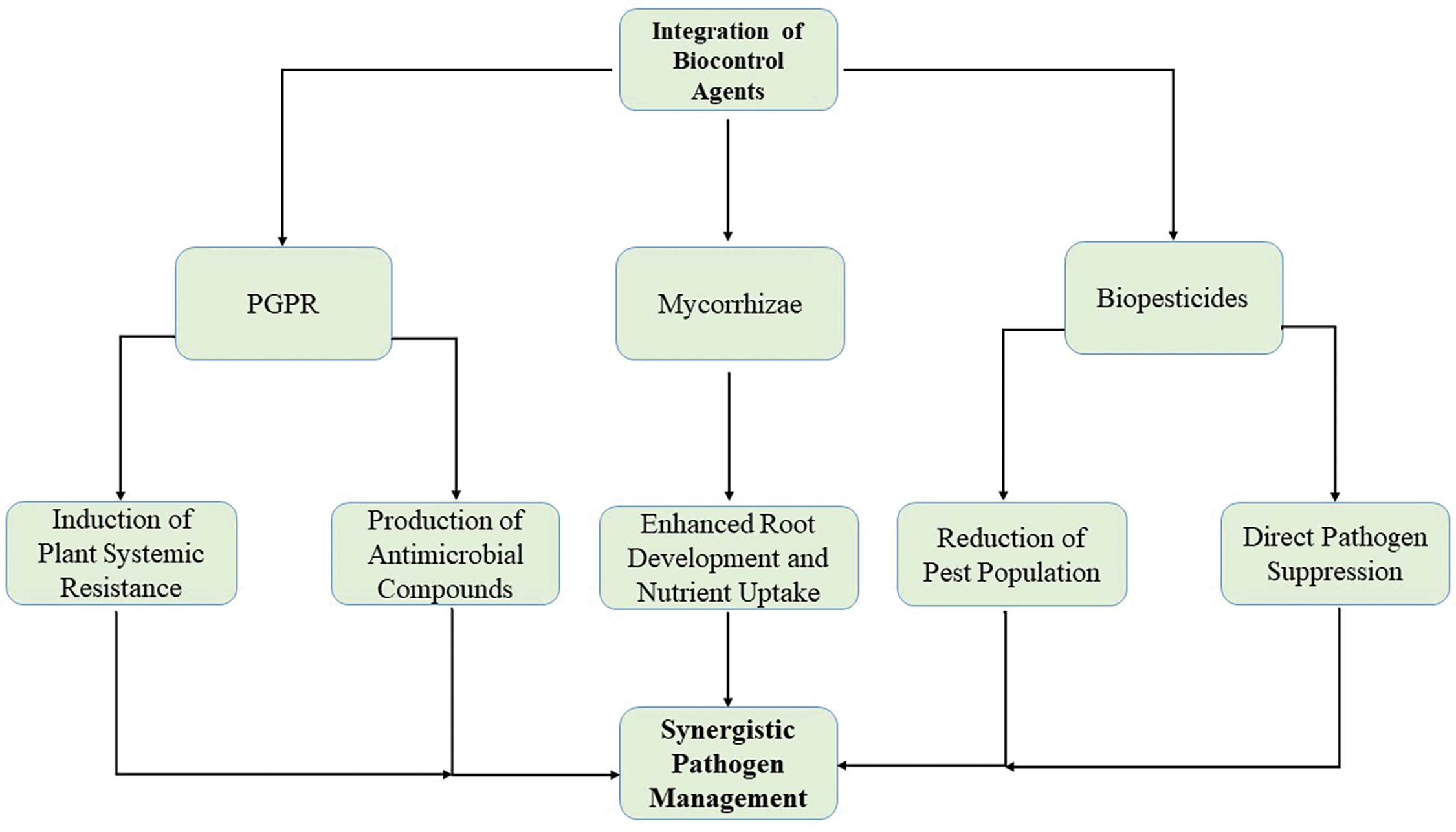
Figure 4: Integrating plant growth-promoting rhizobacteria (PGPR) with other biocontrol agents offers a promising approach to enhancing pathogen management
PGPR’s potential in precision agriculture represents an innovative approach to enhancing crop management and optimizing resource use. Precision agriculture leverages advanced technologies, such as remote sensing, drones, and sensor networks, to monitor and manage agricultural processes with high precision [206]. In the context of PGPR application, these technologies can be used to track PGPR distribution, monitor plant health, and identify areas with high pathogen pressure. For instance, drones equipped with multispectral imaging can detect plant stress caused by pathogen infection, allowing for the targeted application of PGPR in areas where they are most needed. Additionally, sensor networks can be used to monitor soil conditions, such as moisture, pH, and temperature, to determine the optimal conditions for PGPR survival and activity [206,207]. By combining PGPR with precision agriculture technologies, farmers can improve disease management while minimizing resource inputs, leading to more efficient and sustainable farming practices. For example, the use of PGPR combined with precision irrigation systems has been shown to increase water use efficiency by 15%–25% in crops such as rice and wheat, while simultaneously enhancing pathogen resistance [208].
While much progress has been made in the development and application of PGPR, large-scale field trials are essential for evaluating the effectiveness of PGPR-based treatments in real-world agricultural settings. Many studies on PGPR efficacy have been conducted in controlled greenhouse conditions or small-scale field trials, but the performance of PGPR in larger, more variable environments remains an uncertainty [209]. Large-scale trials across different agroecological zones are necessary to assess the impact of PGPR on crop productivity, disease resistance, and soil health under diverse field conditions. For example, trials in both temperate and tropical regions have shown varying levels of PGPR effectiveness, with some strains performing better in cooler climates and others excelling in hotter environments [210]. These trials provide valuable insights into the environmental factors that influence PGPR performance, allowing researchers to identify the most effective PGPR strains for specific crops and regions. Furthermore, large-scale trials help to determine the optimal application rates and timings for PGPR, ensuring that they provide consistent and sustainable benefits in the field [205].
Collaboration between researchers, farmers, and policymakers is essential for the successful implementation of PGPR-based solutions at the farm level. Researchers must continue to generate evidence on the efficacy and environmental benefits of PGPR, while farmers require practical information on how to integrate PGPR into their farming systems. Policymakers play a key role in supporting the adoption of PGPR by establishing clear regulatory frameworks and providing incentives for the use of biocontrol agents. For example, in the European Union, the use of PGPR-based products has been supported through regulatory reforms that streamline the registration process for biocontrol agents [211]. Similarly, government initiatives to promote sustainable farming practices, such as subsidies for organic farming, can encourage the adoption of PGPR-based treatments. Collaboration between these stakeholders is crucial for translating scientific research into practical solutions that can benefit farmers, improve crop yields, and promote environmental sustainability.
In conclusion, PGPR offer substantial benefits in managing plant pathogens and enhancing agricultural productivity. PGPR contribute to pathogen control through multiple mechanisms, including nutrient competition, antimicrobial compound production, and systemic resistance induction in plants. Additionally, PGPR not only suppress pathogens but also enhance plant health by improving root growth, nutrient uptake, and resilience to abiotic stresses like drought, thus further enhancing crop productivity. Despite these advantages, several challenges remain, including variability in PGPR efficacy due to environmental factors and pathogen species, as well as difficulties in scaling up production and ensuring formulation stability. Additionally, regulatory barriers and the need for widespread adoption present obstacles to the commercial use of the PGPR. However, ongoing research into strain selection, genetic engineering, and the integration of PGPR with other biocontrol strategies is expected to improve their performance and expand their use in diverse agricultural systems. PGPR play a critical role in addressing global food security challenges by enhancing crop resilience and reducing dependence on chemical inputs. As climate change exacerbates environmental stresses, PGPR’s ability to improve disease resistance and promote growth under stressful conditions is increasingly valuable. PGPR have been shown to increase crop yields in drought-prone regions and reduce chemical pesticide use, thus contributing to sustainable agricultural practices and improved environmental outcomes. Conclusively, PGPR offer a promising solution for managing plant diseases, improving crop yields, and supporting sustainable agriculture. As research continues to optimize their use and overcome existing challenges, PGPR can significantly contribute to global food security and environmental sustainability, making them an integral part of the future of agriculture.
Acknowledgement: Not applicable.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Sajid Ali, Yong-Sun Moon; data collection: Sajid Ali; analysis and interpretation of results: Sajid Ali, Yong-Sun Moon; draft manuscript preparation: Sajid Ali, Yong-Sun Moon. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Gorshkov V, Tsers I. Plant susceptible responses: the underestimated side of plant-pathogen interactions. Biol Rev Camb Philos Soc. 2022;97(1):45–66. doi:10.1111/brv.12789. [Google Scholar] [PubMed] [CrossRef]
2. Rajesh N, Gupta MK, Gouda G, Donde R, Sabarinathan S, Dash GK, et al. Plant pathogen co-evolution in rice crop. In: Gupta MK, Behera L, editors. Applications of bioinformatics in rice research. Singapore: Springer; 2021. p. 297–314. doi:10.1007/978-981-16-3997-5_14. [Google Scholar] [CrossRef]
3. Panno S, Davino S, Caruso AG, Bertacca S, Crnogorac A, Mandić A, et al. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy. 2021;11(11):2188. doi:10.3390/agronomy11112188. [Google Scholar] [CrossRef]
4. Rehman A, Farooq M, Lee DJ, Siddique KHM. Sustainable agricultural practices for food security and ecosystem services. Environ Sci Pollut Res Int. 2022;29(56):84076–95. doi:10.1007/s11356-022-23635-z. [Google Scholar] [PubMed] [CrossRef]
5. Yadav AN, Kour D, Kaur T, Devi R, Yadav A, Dikilitas M, et al. Biodiversity, and biotechnological contribution of beneficial soil microbiomes for nutrient cycling, plant growth improvement and nutrient uptake. Biocatal Agric Biotechnol. 2021;33:102009. doi:10.1016/j.bcab.2021.102009. [Google Scholar] [CrossRef]
6. Nigam R. Plant diseases and food security in the 21st century. Bhopal, India: AG Publishing House; 2024. 245 p. [Google Scholar]
7. Bohra Y, Singh A, Kaur A, Rautela A, Verma RK, Sharma VK, et al. Emerging pathogens: a consequence of climate change or ecological imbalance? Plant Dis Res. 2023;38(1):47–58. doi:10.5958/2249-8788.2023.00015.X. [Google Scholar] [CrossRef]
8. Van Bruggen AHC, Gamliel A, Finckh MR. Plant disease management in organic farming systems. Pest Manag Sci. 2016;72(1):30–44. doi:10.1002/ps.4145. [Google Scholar] [PubMed] [CrossRef]
9. Akanmu AO, Babalola OO, Venturi V, Ayilara MS, Adeleke BS, Amoo AE, et al. Plant disease management: leveraging on the plant-microbe-soil interface in the biorational use of organic amendments. Front Plant Sci. 2021;12:700507. doi:10.3389/fpls.2021.700507. [Google Scholar] [PubMed] [CrossRef]
10. Siddiqui JA, Fan R, Naz H, Bamisile BS, Hafeez M, Ghani MI, et al. Insights into insecticide-resistance mechanisms in invasive species: challenges and control strategies. Front Physiol. 2023;13:1112278. doi:10.3389/fphys.2022.1112278. [Google Scholar] [PubMed] [CrossRef]
11. Hasan A, Tabassum B, Hashim M, Khan N. Role of plant growth promoting rhizobacteria (PGPR) as a plant growth enhancer for sustainable agriculture: a review. Bacteria. 2024;3(2):59–75. doi:10.3390/bacteria3020005. [Google Scholar] [CrossRef]
12. Laishram B, Devi OR, Dutta R, Senthilkumar T, Goyal G, Paliwal DK, et al. Plant-microbe interactions: pgpm as microbial inoculants/biofertilizers for sustaining crop productivity and soil fertility. Curr Res Microb Sci. 2024;8(8):100333. doi:10.1016/j.crmicr.2024.100333. [Google Scholar] [PubMed] [CrossRef]
13. Seth K, Vyas P, Deora S, Gupta AK, Meena M, Swapnil P, et al. Understanding plant-plant growth-promoting rhizobacteria (PGPR) interactions for inducing plant defense. In: Swapnil P, Meena M, Zehra A, editors. Plant-microbe interaction—recent advances in molecular and biochemical approaches. Amsterdam, The Netherlands: Elsevier; 2023. p. 201–26. doi:10.1016/b978-0-323-91876-3.00010-5. [Google Scholar] [CrossRef]
14. Zhu L, Huang J, Lu X, Zhou C. Development of plant systemic resistance by beneficial rhizobacteria: recognition, initiation, elicitation and regulation. Front Plant Sci. 2022;13:952397. doi:10.3389/fpls.2022.952397. [Google Scholar] [PubMed] [CrossRef]
15. Patani A, Patel M, Islam S, Yadav VK, Prajapati D, Yadav AN, et al. Recent advances in Bacillus-mediated plant growth enhancement: a paradigm shift in redefining crop resilience. World J Microbiol Biotechnol. 2024;40(2):77. doi:10.1007/s11274-024-03903-5. [Google Scholar] [PubMed] [CrossRef]
16. De Wrachien D, Schultz B, Goli MB. Impacts of population growth and climate change on food production and irrigation and drainage needs: a world-wide view. Irrig Drain. 2021;70(5):981–95. doi:10.1002/ird.2597. [Google Scholar] [CrossRef]
17. Hakim S, Naqqash T, Nawaz MS, Laraib I, Siddique MJ, Zia R, et al. Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front Sustain Food Syst. 2021;5:617157. doi:10.3389/fsufs.2021.617157. [Google Scholar] [CrossRef]
18. Wang H, Liu R, You MP, Barbetti MJ, Chen Y. Pathogen biocontrol using plant growth-promoting bacteria (PGPRrole of bacterial diversity. Microorganisms. 2021;9(9):1988. doi:10.3390/microorganisms9091988. [Google Scholar] [PubMed] [CrossRef]
19. Syed Nabi RB, Shahzad R, Tayade R, Shahid M, Hussain A, Ali MW, et al. Evaluation potential of PGPR to protect tomato against Fusarium wilt and promote plant growth. PeerJ. 2021;9(12):e11194. doi:10.7717/peerjtt.11194. [Google Scholar] [CrossRef]
20. Grahovac J, Pajčin I, Vlajkov V. Bacillus VOCs in the context of biological control. Antibiotics. 2023;12(3):581. doi:10.3390/antibiotics12030581. [Google Scholar] [CrossRef]
21. Mehmood N, Saeed M, Zafarullah S, Hyder S, Rizvi ZF, Gondal AS, et al. Multifaceted impacts of plant-beneficial Pseudomonas spp. in managing various plant diseases and crop yield improvement. ACS Omega. 2023;8(25):22296–315. doi:10.1021/acsomega.3c00870. [Google Scholar] [PubMed] [CrossRef]
22. Pequeno DNL, Ferreira TB, Fernandes JMC, Singh PK, Pavan W, Sonder K, et al. Production vulnerability to wheat blast disease under climate change. Nat Clim Chang. 2024;14(2):178–83. doi:10.1038/s41558-023-01902-2. [Google Scholar] [CrossRef]
23. Odeph MA. Distribution and socio-economic impact of blast pathogen, molecular characterization of associated fungi and screening for disease tolerance in kenyan finger millet (Eleusine coracana) [dissertation]. Nairobi, Kenya: Jomo Kenyatta University of Agriculture and Technology; 2023. [Google Scholar]
24. Rogers HS, Donoso I, Traveset A, Fricke EC. Cascading impacts of seed disperser loss on plant communities and ecosystems. Annu Rev Ecol Evol Syst. 2021;52(1):641–66. doi:10.1146/annurev-ecolsys-012221-111742. [Google Scholar] [CrossRef]
25. Sarker PK, Paul AS, Karmoker D. Mitigating climate change and pandemic impacts on global food security: dual sustainable agriculture approach (2S approach). Planta. 2023;258(6):104. doi:10.1007/s00425-023-04257-2. [Google Scholar] [PubMed] [CrossRef]
26. Shah A, Nazari M, Antar M, Msimbira LA, Naamala J, Lyu D, et al. PGPR in agriculture: a sustainable approach to increasing climate change resilience. Front Sustain Food Syst. 2021;5:667546. doi:10.3389/fsufs.2021.667546. [Google Scholar] [CrossRef]
27. Mufti R, Bano A. PGPR-induced defense responses in the soybean plant against charcoal rot disease. Eur J Plant Pathol. 2019;155(3):983–1000. doi:10.1007/s10658-019-01828-6. [Google Scholar] [CrossRef]
28. Wei X, Xie B, Wan C, Song R, Zhong W, Xin S, et al. Enhancing soil health and plant growth through microbial fertilizers: mechanisms, benefits, and sustainable agricultural practices. Agronomy. 2024;14(3):609. doi:10.3390/agronomy14030609. [Google Scholar] [CrossRef]
29. Santoyo G, Urtis-Flores CA, Loeza-Lara PD, Orozco-Mosqueda MDC, Glick BR. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology. 2021;10(6):475. doi:10.3390/biology10060475. [Google Scholar] [PubMed] [CrossRef]
30. Vocciante M, Grifoni M, Fusini D, Petruzzelli G, Franchi E. The role of plant growth-promoting rhizobacteria (PGPR) in mitigating plant’s environmental stresses. Appl Sci. 2022;12(3):1231. doi:10.3390/app12031231. [Google Scholar] [CrossRef]
31. Baloch FB, Zeng N, Gong H, Zhang Z, Zhang N, Baloch SB, et al. Rhizobacterial volatile organic compounds: implications for agricultural ecosystems’ nutrient cycling and soil health. Heliyon. 2024;10(23):e40522. doi:10.1016/j.heliyon.2024.e40522. [Google Scholar] [PubMed] [CrossRef]
32. Chin-A-Woeng TFC, Bloemberg GV, Lugtenberg BJJ. Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol. 2003;157(3):503–23. doi:10.1046/j.1469-8137.2003.00686.x. [Google Scholar] [PubMed] [CrossRef]
33. Serafim B, Bernardino AR, Freitas F, Torres CAV. Recent developments in the biological activities, bioproduction, and applications of Pseudomonas spp. phenazines. Molecules. 2023;28(3):1368. doi:10.3390/molecules28031368. [Google Scholar] [PubMed] [CrossRef]
34. Moryl M, Spętana M, Dziubek K, Paraszkiewicz K, Różalska S, Płaza GA, et al. Antimicrobial, antiadhesive and antibiofilm potential of lipopeptides synthesised by Bacillus subtilis, on uropathogenic bacteria. Acta Biochim Pol. 2015;62(4):725–32. doi:10.18388/abp.2015_1120. [Google Scholar] [PubMed] [CrossRef]
35. Wang Y, He Y, Zhang H, Ma X. Purification and characterization of lipopeptides produced by Bacillus subtilius and their antibacterial effects on Escherichia coli and Staphylococcus aureus. Process Biochem. 2024;146(4):44–55. doi:10.1016/j.procbio.2024.06.033. [Google Scholar] [CrossRef]
36. Lee JH, Anderson AJ, Kim YC. Root-associated bacteria are biocontrol agents for multiple plant pests. Microorganisms. 2022;10(5):1053. doi:10.3390/microorganisms10051053. [Google Scholar] [PubMed] [CrossRef]
37. Falardeau J, Wise C, Novitsky L, Avis TJ. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J Chem Ecol. 2013;39(7):869–78. doi:10.1007/s10886-013-0319-7. [Google Scholar] [PubMed] [CrossRef]
38. Maurya S, Thakur R, Vighnesh R, Suresh S, Dang A, Raj D, et al. Eco-friendly management of plant pathogens through secondary metabolites released by fluorescent pseudomonads. J Pure Appl Microbiol. 2024;18(3):1471–88. doi:10.22207/jpam.18.3.40. [Google Scholar] [CrossRef]
39. Kaur S, Samota MK, Choudhary M, Choudhary M, Pandey AK, Sharma A, et al. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol Mol Biol Plants. 2022;28(2):485–504. doi:10.1007/s12298-022-01146-y. [Google Scholar] [PubMed] [CrossRef]
40. Tiwari M, Pati D, Mohapatra R, Sahu BB, Singh P. The impact of microbes in plant immunity and priming induced inheritance: a sustainable approach for crop protection. Plant Stress. 2022;4(2):100072. doi:10.1016/j.stress.2022.100072. [Google Scholar] [CrossRef]
41. Kumari R, Pandey E, Bushra S, Faizan S, Pandey S. Plant growth promoting rhizobacteria (PGPR) induced protection: a plant immunity perspective. Physiol Plant. 2024;176(5):e14495. doi:10.1111/ppl.14495. [Google Scholar] [PubMed] [CrossRef]
42. Chakraborty BN, Chakraborty U. Molecular detection of fungal pathogens and induction of phytoimmunity using bioinoculants. Indian Phytopathol. 2021;74(2):307–22. doi:10.1007/s42360-021-00351-1. [Google Scholar] [CrossRef]
43. Jaroszuk-Ściseł J, Nowak A. Bacterial cyclodipeptides in triggers plant immunity potential. In: Sharma V, Salwan R, Jedryczka M, editors. The chemical dialogue between plants and beneficial microorganisms. Amsterdam, The Netherlands: Elsevier; 2023. p. 31–47. doi:10.1016/b978-0-323-91734-6.00021-1. [Google Scholar] [CrossRef]
44. Mondal S. Heavy metal stress-induced activation of mitogen-activated protein kinase signalling cascade in plants. Plant Mol Biol Report. 2023;41(1):15–26. doi:10.1007/s11105-022-01350-w. [Google Scholar] [CrossRef]
45. Ravi B, Foyer CH, Pandey GK. The integration of reactive oxygen species (ROS) and calcium signalling in abiotic stress responses. Plant Cell Environ. 2023;46(7):1985–2006. doi:10.1111/pce.14596. [Google Scholar] [PubMed] [CrossRef]
46. Choudhary DK, Prakash A, Johri BN. Induced systemic resistance (ISR) in plants: mechanism of action. Indian J Microbiol. 2007;47(4):289–97. doi:10.1007/s12088-007-0054-2. [Google Scholar] [PubMed] [CrossRef]
47. Samaras A, Roumeliotis E, Ntasiou P, Karaoglanidis G. Bacillus subtilis MBI600 promotes growth of tomato plants and induces systemic resistance contributing to the control of soilborne pathogens. Plants. 2021;10(6):1113. doi:10.3390/plants10061113. [Google Scholar] [CrossRef]
48. Choudhary DK, Johri BN. Interactions of Bacillus spp. and plants—with special reference to induced systemic resistance (ISR). Microbiol Res. 2009;164(5):493–513. doi:10.1016/j.micres.2008.08.007. [Google Scholar] [PubMed] [CrossRef]
49. Ali GS, Norman D, El-Sayed AS. Soluble and volatile metabolites of plant growth-promoting rhizobacteria (PGPRs) role and practical applications in inhibiting pathogens and activating induced systemic resistance (ISR). Adv Bot Res. 2015;75(5):241–84. doi:10.1016/bs.abr.2015.07.004. [Google Scholar] [CrossRef]
50. Wang Y, Pei Y, Wang X, Dai X, Zhu M. Antimicrobial metabolites produced by the plant growth-promoting rhizobacteria (PGPRBacillus and Pseudomonas. Adv Agrochem. 2024;3(3):206–21. doi:10.1016/j.aac.2024.07.007. [Google Scholar] [CrossRef]
51. Kenawy A, Dailin DJ, Abo-Zaid GA, Malek RA, Ambehabati KK, Zakaria KHN, et al. Biosynthesis of antibiotics by PGPR and their roles in biocontrol of plant diseases. In: Aayyed RZ, editor. Plant growth promoting rhizobacteria for sustainable stress management. Singapore: Springer; 2019. p. 1–35. doi:10.1007/978-981-13-6986-5_1. [Google Scholar] [CrossRef]
52. Panicker S, Sayyed RZ. Hydrolytic enzymes from PGPR against plant fungal pathogens. In: Sayyed RZ, Singh A, Ilyas N, editors. Antifungal metabolites of rhizobacteria for sustainable agriculture. Berlin/Heidelberg, Germany: Springer; 2022. p. 211–38. doi:10.1007/978-3-031-04805-0_10. [Google Scholar] [CrossRef]
53. Duhan L, Kumari D, Verma R, Pasrija R. Fungal hydrolytic enzymes produced by plant growth-promoting rhizobacteria (PGPR). In: Secondary metabolites and volatiles of pgpr in plant-growth promotion. Cham: Springer; 2022. p. 313–33. doi:10.1007/978-3-031-07559-9_16. [Google Scholar] [CrossRef]
54. Naz R, Khushhal S, Asif T, Mubeen S, Saranraj P, Sayyed RZ. Inhibition of bacterial and fungal phytopathogens through volatile organic compounds produced by Pseudomonas sp. In: Sayyed RZ, Uarrota VG, editors. Secondary metabolites and volatiles of PGPR in plant-growth promotion. Berlin/Heidelberg, Germany: Springer; 2022. p. 95–118. doi:10.1007/978-3-031-07559-9_6. [Google Scholar] [CrossRef]
55. Rani A, Rana A, Dhaka RK, Singh AP, Chahar M, Singh S, et al. Bacterial volatile organic compounds as biopesticides, growth promoters and plant-defense elicitors: current understanding and future scope. Biotechnol Adv. 2023;63(6):108078. doi:10.1016/j.biotechadv.2022.108078. [Google Scholar] [PubMed] [CrossRef]
56. Orozco-Mosqueda MDC, Santoyo G, Glick BR. Recent advances in the bacterial phytohormone modulation of plant growth. Plants. 2023;12(3):606. doi:10.3390/plants12030606. [Google Scholar] [PubMed] [CrossRef]
57. Pantoja-Guerra M, Valero-Valero N, Ramírez CA. Total auxin level in the soil-plant system as a modulating factor for the effectiveness of PGPR inocula: a review. Chem Biol Technol Agric. 2023;10(1):6. doi:10.1186/s40538-022-00370-8. [Google Scholar] [CrossRef]
58. Ali S, Moon YS. Unveiling auxin’s role in seed dormancy, germination and possible impact of IAA producing PGPR in seed bio-priming and plant growth. Pak J Bot. 2025;57(2):515–22. doi:10.30848/PJB2025-2(16). [Google Scholar] [CrossRef]
59. Singh A, Yajnik KN, Mogilicherla K, Singh IK. Deciphering the role of growth regulators in enhancing plant immunity against herbivory. Physiol Plant. 2024;176(6):e14604. doi:10.1111/ppl.14604. [Google Scholar] [PubMed] [CrossRef]
60. Kapoor B, Kumar P, Sharma R, Kumar A. Regulatory interactions in phytohormone stress signaling implying plants resistance and resilience mechanisms. J Plant Biochem Biotechnol. 2021;30(4):813–28. doi:10.1007/s13562-021-00739-0. [Google Scholar] [CrossRef]
61. Tsai SH, Hsiao YC, Chang PE, Kuo CE, Lai MC, Chuang HW. Exploring the biologically active metabolites produced by Bacillus cereus for plant growth promotion, heat stress tolerance, and resistance to bacterial soft rot in Arabidopsis. Metabolites. 2023;13(5):676. doi:10.3390/metabo13050676. [Google Scholar] [PubMed] [CrossRef]
62. Shekhawat K, Fröhlich K, García-Ramírez GX, Trapp MA, Hirt H. Ethylene: a master regulator of plant-microbe interactions under abiotic stresses. Cells. 2022;12(1):31. doi:10.3390/cells12010031. [Google Scholar] [PubMed] [CrossRef]
63. Zhu L, Qian N, Sun Y, Lu X, Duan H, Qian L. Pseudomonas fluorescens DN16 enhances cucumber defense responses against the necrotrophic pathogen Botrytis cinerea by regulating thermospermine catabolism. Front Plant Sci. 2021;12:645338. doi:10.3389/fpls.2021.645338. [Google Scholar] [CrossRef]
64. Rafique M, Hayat K, Mukhtar T, Khan A, Afridi M, Hussain T, et al. Bacterial biofilm formation and its role against agricultural pathogens. In: Vilas AM, editor. The battle against microbial pathogens: basic science, technological advances and educational programs. Norristown, PA, USA: Formatex research Center; 2015. p. 373–82. [Google Scholar]
65. Kumar S, Anjali, Arutselvan, Masurkar R, Singh P, Tripathi UB, et al. Bacillus subtilis-mediated induction of disease resistance and promotion of plant growth of vegetable crops. In: Mageshwaran V, Singh UB, Saxena AK, Singh HB, editors. Applications of bacillus and bacillus derived genera in agriculture, biotechnology and beyond. Singapore: Springer; 2024. p. 165–211. doi:10.1007/978-981-99-8195-3_9. [Google Scholar] [CrossRef]
66. Rafique M, Naveed M, Mumtaz MZ, Niaz A, Alamri S, Siddiqui MH, et al. Unlocking the potential of biofilm-forming plant growth-promoting rhizobacteria for growth and yield enhancement in wheat (Triticum aestivum L.). Sci Rep. 2024;14(1):15546. doi:10.1038/s41598-024-66562-4. [Google Scholar] [PubMed] [CrossRef]
67. Çam S. Co-inoculation of biofilm-and exopolysaccharide-producing rhizobacteria promoting wheat development by boosting plant nutrients in a nutrient-limited soil. J Plant Nutr. 2024;47(20):4033–47. doi:10.1080/01904167.2024.2394131. [Google Scholar] [CrossRef]
68. Andersen EJ, Ali S, Byamukama E, Yen Y, Nepal MP. Disease resistance mechanisms in plants. Genes. 2018;9(7):339. doi:10.3390/genes9070339. [Google Scholar] [PubMed] [CrossRef]
69. Vishwakarma K, Kumar N, Shandilya C, Mohapatra S, Bhayana S, Varma A. Revisiting plant-microbe interactions and microbial consortia application for enhancing sustainable agriculture: a review. Front Microbiol. 2020;11:560406. doi:10.3389/fmicb.2020.560406. [Google Scholar] [PubMed] [CrossRef]
70. Rosier A, Medeiros FHV, Bais HP. Defining plant growth promoting rhizobacteria molecular and biochemical networks in beneficial plant-microbe interactions. Plant Soil. 2018;428(1):35–55. doi:10.1007/s11104-018-3679-5. [Google Scholar] [CrossRef]
71. Ahn IP, Lee SW, Kim MG, Park SR, Hwang DJ, Bae SC. Priming by rhizobacterium protects tomato plants from biotrophic and necrotrophic pathogen infections through multiple defense mechanisms. Mol Cells. 2011;32(1):7–14. doi:10.1007/s10059-011-2209-6. [Google Scholar] [PubMed] [CrossRef]
72. Tripathi A, Pandey VK, Jain D, Singh G, Brar NS, Taufeeq A, et al. An updated review on significance of PGPR-induced plant signalling and stress management in advancing sustainable agriculture. J Agric Food Res. 2024;16(4):101169. doi:10.1016/j.jafr.2024.101169. [Google Scholar] [CrossRef]
73. Thepbandit W, Athinuwat D. Rhizosphere microorganisms supply availability of soil nutrients and induce plant defense. Microorganisms. 2024;12(3):558. doi:10.3390/microorganisms12030558. [Google Scholar] [PubMed] [CrossRef]
74. Suresh P, Vellasamy S, Almaary KS, Dawoud TM, Elbadawi YB. Fluorescent pseudomonads (FPs) as a potential biocontrol and plant growth promoting agent associated with tomato rhizosphere. J King Saud Univ Sci. 2021;33(4):101423. doi:10.1016/j.jksus.2021.101423. [Google Scholar] [CrossRef]
75. Kazerooni EA, Maharachchikumbura SSN, Al-Sadi AM, Kang SM, Yun BW, Lee IJ. Biocontrol potential of Bacillus amyloliquefaciens against Botrytis pelargonii and Alternaria alternata on Capsicum annuum. J Fungi. 2021;7(6):472. doi:10.3390/jof7060472. [Google Scholar] [CrossRef]
76. Daayf F, Adam L, Fernando WGD. Comparative screening of bacteria for biological control of potato late blight (strain US-8using invitro, detached-leaves, and whole-plant testing systems. Can J Plant Pathol. 2003;25(3):276–84. doi:10.1080/07060660309507080. [Google Scholar] [CrossRef]
77. Mulk S, Wahab A, Yasmin H, Mumtaz S, El-Serehy HA, Khan N, et al. Prevalence of wheat associated Bacillus spp. and their bio-control efficacy against Fusarium root rot. Front Microbiol. 2022;12:798619. doi:10.3389/fmicb.2021.798619. [Google Scholar] [PubMed] [CrossRef]
78. Amaradasa BS, Mei C, He Y, Chretien RL, Doss M, Durham T, et al. Biocontrol potential of endophytic Pseudomonas strain IALR1619 against two Pythium species in cucumber and hydroponic lettuce. PLoS One. 2024;19(2):e0298514. doi:10.1371/journal.pone.0298514. [Google Scholar] [PubMed] [CrossRef]
79. Akintokun AK, Taiwo MO. Biocontrol potentials of individual specie of rhizobacteria and their consortium against phytopathogenic Fusarium oxysporum and Rhizoctonia solani. Int J Sci Res Environ Sci. 2016;4(7):219–27. doi:10.12983/ijsres-2016-p0219-0227. [Google Scholar] [CrossRef]
80. Dobrzyński J, Jakubowska Z, Kulkova I, Kowalczyk P, Kramkowski K. Biocontrol of fungal phytopathogens by Bacillus pumilus. Front Microbiol. 2023;14:1194606. doi:10.3389/fmicb.2023.1194606. [Google Scholar] [PubMed] [CrossRef]
81. Jiang N, Qiu J, Tian D, Shi H, Liu Z, Wen H, et al. Mixture of Bacillus amyloliquefaciens and Bacillus pumilus modulates community structures of rice rhizosphere soil to suppress rice seedling blight. Rice Sci. 2025;32(1):118–30. doi:10.1016/j.rsci.2024.09.001. [Google Scholar] [CrossRef]
82. Postma J, Nijhuis EH. Pseudomonas chlororaphis and organic amendments controlling Pythium infection in tomato. Eur J Plant Pathol. 2019;154(1):91–107. doi:10.1007/s10658-019-01743-w. [Google Scholar] [CrossRef]
83. Jelušić A, Popović T, Dimkić I, Mitrović P, Peeters K, Višnjevec AM, et al. Changes in the winter oilseed rape microbiome affected by Xanthomonas campestris pv. campestris and biocontrol potential of the indigenous Bacillus and Pseudomonas isolates. Biol Control. 2021;160:104695. doi:10.1016/j.biocontrol.2021.104695. [Google Scholar] [CrossRef]
84. Zhang D, Yu S, Yang Y, Zhang J, Zhao D, Pan Y, et al. Antifungal effects of volatiles produced by Bacillus subtilis against Alternaria solani in potato. Front Microbiol. 2020;11:1196. doi:10.3389/fmicb.2020.01196. [Google Scholar] [PubMed] [CrossRef]
85. Balthazar C, Novinscak A, Cantin G, Joly DL, Filion M. Biocontrol activity of Bacillus spp. and Pseudomonas spp. against Botrytis cinerea and other Cannabis fungal pathogens. Phytopathology. 2022;112(3):549–60. doi:10.1094/PHYTO-03-21-0128-R. [Google Scholar] [PubMed] [CrossRef]
86. Dodds PN, Rathjen JP. Plant immunity: towards an integrated view of plant-pathogen interactions. Nat Rev Genet. 2010;11(8):539–48. doi:10.1038/nrg2812. [Google Scholar] [PubMed] [CrossRef]
87. Taj G, Agarwal P, Grant M, Kumar A. MAPK machinery in plants: recognition and response to different stresses through multiple signal transduction pathways. Plant Signal Behav. 2010;5(11):1370–8. doi:10.4161/psb.5.11.13020. [Google Scholar] [PubMed] [CrossRef]
88. Thomas L, Singh I. Microbe-mediated biotic stress signaling and resistance mechanisms in plants. In: Giri B, Sharma MP, editors. Plant stress biology. Singapore: Springer; 2020. p. 297–334. doi:10.1007/978-981-15-9380-2_10. [Google Scholar] [CrossRef]
89. Ansari MM, Bisht N, Singh T, Chauhan PS. Symphony of survival: insights into cross-talk mechanisms in plants, bacteria, and fungi for strengthening plant immune responses. Microbiol Res. 2024;285(1):127762. doi:10.1016/j.micres.2024.127762. [Google Scholar] [PubMed] [CrossRef]
90. Manzoor H. Calcium signaling in plant defense: involvement of subcellular compartments and glutamate receptors [dissertation]. Dijon, France: Université de Bourgogne; 2012. [Google Scholar]
91. Negi NP, Prakash G, Narwal P, Panwar R, Kumar D, Chaudhry B, et al. The calcium connection: exploring the intricacies of calcium signaling in plant-microbe interactions. Front Plant Sci. 2023;14:1248648. doi:10.3389/fpls.2023.1248648. [Google Scholar] [PubMed] [CrossRef]
92. Shi X, Bao J, Lu X, Ma L, Zhao Y, Lan S, et al. The mechanism of Ca2+ signal transduction in plants responding to abiotic stresses. Environ Exp Bot. 2023;216(5):105514. doi:10.1016/j.envexpbot.2023.105514. [Google Scholar] [CrossRef]
93. Zandi P, Schnug E. Reactive oxygen species, antioxidant responses and implications from a microbial modulation perspective. Biology. 2022;11(2):155. doi:10.3390/biology11020155. [Google Scholar] [PubMed] [CrossRef]
94. Sahu PK, Jayalakshmi K, Tilgam J, Gupta A, Nagaraju Y, Kumar A, et al. ROS generated from biotic stress: effects on plants and alleviation by endophytic microbes. Front Plant Sci. 2022;13:1042936. doi:10.3389/fpls.2022.1042936. [Google Scholar] [PubMed] [CrossRef]
95. Su F, Zhao B, Dhondt-Cordelier S, Vaillant-Gaveau N. Plant-growth-promoting rhizobacteria modulate carbohydrate metabolism in connection with host plant defense mechanism. Int J Mol Sci. 2024;25(3):1465. doi:10.3390/ijms25031465. [Google Scholar] [PubMed] [CrossRef]
96. Dimkić I, Janakiev T, Petrović M, Degrassi G, Fira D. Plant-associated Bacillus and Pseudomonas antimicrobial activities in plant disease suppression via biological control mechanisms—a review. Physiol Mol Plant Pathol. 2022;117(25):101754. doi:10.1016/j.pmpp.2021.101754. [Google Scholar] [CrossRef]
97. Manoharan B, Narayanasamy S, Joshi JB, Jegadeesan S, Qi S, Dai Z, et al. Molecular events and defence mechanism against biotic stress induced by bio-priming of beneficial microbes. In: Bastas KK, Kumar A, Sivakumar U, editors. Microbial biocontrol: molecular perspective in plant disease management. Singapore: Springer; 2023. p. 61–87. doi:10.1007/978-981-99-3947-3_3. [Google Scholar] [CrossRef]
98. Grover M, Bodhankar S, Sharma A, Sharma P, Singh J, Nain L. PGPR mediated alterations in root traits: way toward sustainable crop production. Front Sustain Food Syst. 2021;4:618230. doi:10.3389/fsufs.2020.618230. [Google Scholar] [CrossRef]
99. Jia Z, Giehl RFH, von Wirén N. Nutrient-hormone relations: driving root plasticity in plants. Mol Plant. 2022;15(1):86–103. doi:10.1016/j.molp.2021.12.004. [Google Scholar] [PubMed] [CrossRef]
100. Ma Y, Xu J, Qi J, Zhao D, Jin M, Wang T, et al. Crosstalk among plant hormone regulates the root development. Plant Signal Behav. 2024;19(1):2404807. doi:10.1080/15592324.2024.2404807. [Google Scholar] [PubMed] [CrossRef]
101. Muhammad M, Wahab A, Waheed A, Mohamed HI, Hakeem KR, Li L, et al. Harnessing bacterial endophytes for environmental resilience and agricultural sustainability. J Environ Manage. 2024;368:122201. doi:10.1016/j.jenvman.2024.122201. [Google Scholar] [PubMed] [CrossRef]
102. Tanveer S, Ilyas N, Akhtar N, Sayyed RZ, Almalki WH. Induction of regulatory mechanisms by plant growth promoting rhizobacteria in crops facing drought stress. Crop Pasture Sci. 2023;74(9):856–70. doi:10.1071/cp22263. [Google Scholar] [CrossRef]
103. Chen J, Sharifi R, Khan MSS, Islam F, Bhat JA, Kui L, et al. Wheat microbiome: structure, dynamics, and role in improving performance under stress environments. Front Microbiol. 2022;12:821546. doi:10.3389/fmicb.2021.821546. [Google Scholar] [PubMed] [CrossRef]
104. Al-Mutar DMK, Alzawar NSA, Noman M, Azizullah, Li D, Song F. Suppression of Fusarium wilt in watermelon by Bacillus amyloliquefaciens DHA55 through extracellular production of antifungal lipopeptides. J Fungi. 2023;9(3):336. doi:10.3390/jof9030336. [Google Scholar] [PubMed] [CrossRef]
105. Hnini M, Rabeh K, Oubohssaine M. Interactions between beneficial soil microorganisms (PGPR and AMF) and host plants for environmental restoration: a systematic review. Plant Stress. 2024;11:100391. doi:10.1016/j.stress.2024.100391. [Google Scholar] [CrossRef]
106. Noceto PA, Bettenfeld P, Boussageon R, Hériché M, Sportes A, van Tuinen D, et al. Arbuscular mycorrhizal fungi, a key symbiosis in the development of quality traits in crop production, alone or combined with plant growth-promoting bacteria. Mycorrhiza. 2021;31(6):655–69. doi:10.1007/s00572-021-01054-1. [Google Scholar] [PubMed] [CrossRef]
107. Fahde S, Boughribil S, Sijilmassi B, Amri A. Rhizobia: a promising source of plant growth-promoting molecules and their non-legume interactions: examining applications and mechanisms. Agriculture. 2023;13(7):1279. doi:10.3390/agriculture13071279. [Google Scholar] [CrossRef]
108. El-Saadony MT, Saad AM, Soliman SM, Salem HM, Ahmed AI, Mahmood M, et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: mechanisms, challenges and future perspectives. Front Plant Sci. 2022;13:923880. doi:10.3389/fpls.2022.923880. [Google Scholar] [PubMed] [CrossRef]
109. Sivasakthi S, Usharani G, Saranraj P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)—Pseudomonas fluorescens and Bacillus subtilis: a review. Afr J Agric Res. 2014;9(16):1265–77. doi:10.5897/AJAR2013.7914. [Google Scholar] [CrossRef]
110. Abdelaziz AM, Hashem AH, El-Sayyad GS, El-Wakil DA, Selim S, Alkhalifah DHM, et al. Biocontrol of soil borne diseases by plant growth promoting rhizobacteria. Trop Plant Pathol. 2023;48(2):105–27. doi:10.1007/s40858-022-00544-7. [Google Scholar] [CrossRef]
111. Khan N, Ali S, Shahid MA, Mustafa A, Sayyed RZ, Curá JA. Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: a review. Cells. 2021;10(6):1551. doi:10.3390/cells10061551. [Google Scholar] [PubMed] [CrossRef]
112. Mohanram S, Kumar P. Rhizosphere microbiome: revisiting the synergy of plant-microbe interactions. Ann Microbiol. 2019;69(4):307–20. doi:10.1007/s13213-019-01448-9. [Google Scholar] [CrossRef]
113. Bhat MA, Kumar V, Bhat MA, Wani IA, Dar FL, Farooq I, et al. Mechanistic insights of the interaction of plant growth-promoting rhizobacteria (PGPR) with plant roots toward enhancing plant productivity by alleviating salinity stress. Front Microbiol. 2020;11:1952. doi:10.3389/fmicb.2020.01952. [Google Scholar] [PubMed] [CrossRef]
114. Mohanty P, Singh PK, Chakraborty D, Mishra S, Pattnaik R. Insight into the role of PGPR in sustainable agriculture and environment. Front Sustain Food Syst. 2021;5:667150. doi:10.3389/fsufs.2021.667150. [Google Scholar] [CrossRef]
115. Mishra P, Singh PP, Singh SK, Verma H. Sustainable agriculture and benefits of organic farming to special emphasis on PGPR. In: Kumar A, Singh AK, Choudhary KK, editors. Role of plant growth promoting microorganisms in sustainable agriculture and nanotechnology. Amsterdam, The Netherlands: Elsevier; 2019. p. 75–87. doi:10.1016/b978-0-12-817004-5.00005-1. [Google Scholar] [CrossRef]
116. Yang P, Condrich A, Scranton S, Hebner C, Lu L, Ali MA. Utilizing plant growth-promoting rhizobacteria (PGPR) to advance sustainable agriculture. Bacteria. 2024;3(4):434–51. doi:10.3390/bacteria3040030. [Google Scholar] [CrossRef]
117. Upadhyay SK, Rajput VD, Kumari A, Espinosa-Saiz D, Menendez E, Minkina T, et al. Plant growth-promoting rhizobacteria: a potential bio-asset for restoration of degraded soil and crop productivity with sustainable emerging techniques. Environ Geochem Health. 2023;45(12):9321–44. doi:10.1007/s10653-022-01433-3. [Google Scholar] [PubMed] [CrossRef]
118. Bhosale SB, Patil MB. Revolutionizing agriculture: the growth of organic farming in India. J Chem Health Risks. 2024;14(2):1950–61. [Google Scholar]
119. Bhunia S, Bhowmik A, Mallick R, Mukherjee J. Agronomic efficiency of animal-derived organic fertilizers and their effects on biology and fertility of soil: a review. Agronomy. 2021;11(5):823. doi:10.3390/agronomy11050823. [Google Scholar] [CrossRef]
120. Verma PP, Shelake RM, Das S, Sharma P, Kim JY. Plant growth-promoting rhizobacteria (PGPR) and fungi (PGPFpotential biological control agents of diseases and pests. In: Singh DP, Gupta VK, Prabha R, editors. Microbial interventions in agriculture and environment. Singapore: Springer; 2019. p. 281–311. doi:10.1007/978-981-13-8391-5_11. [Google Scholar] [CrossRef]
121. Saeed Q, Wang X, Haider FU, Kučerik J, Mumtaz MZ, Holatko J, et al. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: a comprehensive review of effects and mechanisms. Int J Mol Sci. 2021;22(19):10529. doi:10.3390/ijms221910529. [Google Scholar] [PubMed] [CrossRef]
122. Tudi M, Ruan HD, Wang L, Lyu J, Sadler R, Connell D, et al. Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health. 2021;18(3):1112. doi:10.3390/ijerph18031112. [Google Scholar] [PubMed] [CrossRef]
123. Romeh AAA. Remedial potential of plant growth promoting rhizobacteria (PGPR) for pesticide residues: recent trends and future challenges. In: Siddiqui S, Meghvansi MK, Chaudhary KK, editors. Pesticides bioremediation. Berlin/Heidelberg, Germany: Springer; 2022. p. 381–97. doi:10.1007/978-3-030-97000-0_14. [Google Scholar] [CrossRef]
124. Holka M, Kowalska J, Jakubowska M. Reducing carbon footprint of agriculture—can organic farming help to mitigate climate change? Agriculture. 2022;12(9):1383. doi:10.3390/agriculture12091383. [Google Scholar] [CrossRef]
125. Pandiyan A, Sarsan S, Guda Sri Durga G, Ravikumar H. Biofertilizers and biopesticides as microbial inoculants in integrated pest management for sustainable agriculture. In: Singh RP, Manchanda G, Panosyan H, editors. Microbial essentialism. Amsterdam, The Netherlands: Elsevier; 2024. p. 485–518. doi:10.1016/b978-0-443-13932-1.00010-6. [Google Scholar] [CrossRef]
126. Sahu PK, Singh DP, Prabha R, Meena KK, Abhilash PC. Connecting microbial capabilities with the soil and plant health: options for agricultural sustainability. Ecol Indic. 2019;105(3):601–12. doi:10.1016/j.ecolind.2018.05.084. [Google Scholar] [CrossRef]
127. Prasad M, Srinivasan R, Chaudhary M, Choudhary M, Jat LK. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture, PGPR amelioration in sustainable agriculture. In: Singh AK, Singh PK, editors. PGPR amelioration in sustainable agriculture. Amsterdam, The Netherlands: Elsevier; 2019. p. 129–57. doi:10.1016/b978-0-12-815879-1.00007-0. [Google Scholar] [CrossRef]
128. Hayat R, Ahmed I, Ali Sheirdil R. An overview of plant growth promoting rhizobacteria (PGPR) for sustainable agriculture. In: Ashraf M, Ozturk M, Ahmad MSA, Aksoy A, editors. Crop production for agricultural improvement. Berlin/Heidelberg, Germany: Springer; 2012. p. 557–79. doi:10.1007/978-94-007-4116-4_22. [Google Scholar] [CrossRef]
129. Roufaida K, Rayene K. Contribution to study the role of earthworms to enhancing plant rhizosphere with PGPR bacteria [dissertation]. Tebessa, Algeria: University Larbi Tébessi–Tébessa; 2024. [Google Scholar]
130. Schonbeck M. Soil health and organic farming. Santa Cruz, CA, USA: Organic Farming Research Foundation; 2017. [Google Scholar]
131. del Barrio-Duque A, Ley J, Samad A, Antonielli L, Sessitsch A, Compant S. Beneficial endophytic bacteria—Serendipita indica interaction for crop enhancement and resistance to phy-topathogens. Front Microbiol. 2019;10:2888. doi:10.3389/fmicb.2019.02888. [Google Scholar] [PubMed] [CrossRef]
132. Panda SK, Das S. Potential of plant growth-promoting microbes for improving plant and soil health for biotic and abiotic stress management in mangrove vegetation. Rev Environ Sci Bio/Technol. 2024;23(3):801–37. doi:10.1007/s11157-024-09702-6. [Google Scholar] [CrossRef]
133. Singh AK, Sisodia A, Sisodia V, Padhi M. Role of microbes in restoration ecology and ecosystem services. In: Singh JS, Singh DP, editors. New and future developments in microbial biotechnology and bioengineering. Amsterdam, The Netherlands: Elsevier; 2019. p. 57–68. doi:10.1016/b978-0-444-64191-5.00004-3. [Google Scholar] [CrossRef]
134. Fathi A, Modara B, Taha AH. Role of plant growth promoting rhizobacteria (PGPR) and biochar to soil carbon sequestration and plant performance in climate resilience—a review. Res Crop Ecophysiol. 2023;18(2):147–59. doi:10.3390/plants13050613. [Google Scholar] [PubMed] [CrossRef]
135. Toor MD, Ur Rehman M, Abid J, Nath D, Ullah I, Basit A, et al. Microbial ecosystems as guardians of food security and water resources in the era of climate change. Water Air Soil Pollut. 2024;235(11):741. doi:10.1007/s11270-024-07533-3. [Google Scholar] [CrossRef]
136. Al-Turki A, Murali M, Omar AF, Rehan M, Sayyed RZ. Recent advances in PGPR-mediated resilience toward interactive effects of drought and salt stress in plants. Front Microbiol. 2023;14:1214845. doi:10.3389/fmicb.2023.1214845. [Google Scholar] [PubMed] [CrossRef]
137. Meena M, Swapnil P, Divyanshu K, Kumar S, Harish, Tripathi YN, et al. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: current perspectives. J Basic Microbiol. 2020;60(10):828–61. doi:10.1002/jobm.202000370. [Google Scholar] [PubMed] [CrossRef]
138. Khoshru B, Nosratabad AF, Mahjenabadi VAJ, Knežević M, Hinojosa AC, Fadiji AE, et al. Multidimensional role of Pseudomonas: from biofertilizers to bioremediation and soil ecology to sustainable agriculture. J Plant Nutr. 2025;48(6):1016–42. doi:10.1080/01904167.2024.2416078. [Google Scholar] [CrossRef]
139. Zumbal G, Anum B, Ullah U, Khan J, Iqbal F, Ullah Q. Integrating plant growth-promoting rhizobacteria (PGPR) for sustainable agriculture in pakistan: enhancing crop yields, soil health, and environmental resilience. GSAR J Agric Vet Sci. 2024;1(1):42–52. [Google Scholar]
140. He S, Li L, Lv M, Wang R, Wang L, Yu S, et al. PGPR: key to enhancing crop productivity and achieving sustainable agriculture. Curr Microbiol. 2024;81(11):377. doi:10.1007/s00284-024-03893-5. [Google Scholar] [PubMed] [CrossRef]
141. Arora NK, Tewari S, Singh R. Multifaceted plant-associated microbes and their mechanisms diminish the concept of direct and indirect PGPRs. In: Arora NK, editor. Plant microbe symbiosis: fundamentals and advances. Berlin/Heidelberg, Germany: Springer; 2013. p. 411–49. doi:10.1007/978-81-322-1287-4_16. [Google Scholar] [CrossRef]
142. Syed Ab Rahman SF, Singh E, Pieterse CMJ, Schenk PM. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018;267:102–11. doi:10.1016/j.plantsci.2017.11.012. [Google Scholar] [PubMed] [CrossRef]
143. Gogoi P, Kakoti P, Saikia J, Sarma RK, Yadav A, Singh BP, et al. Plant growth-promoting rhizobacteria in management of soil-borne fungal pathogens. In: Singh BP, Singh G, Kumar K, Nayak SC, Srinivasa N, editors. Management of fungal pathogens in pulses. Berlin/Heidelberg, Germany: Springer; 2020. doi:10.1007/978-3-030-35947-8_1. [Google Scholar] [CrossRef]
144. Matumwabirhi K. Effectiveness of Trichoderma spp., Bacillus spp. And pseudomonas fluorescens in the management of early blight of tomatoes [master’s thesis]. Nairobi, Kenya: University of Nairobi; 2020. [Google Scholar]
145. Mishra J, Mishra I, Arora NK. 2,4-diacetylphloroglucinol producing Pseudomonas fluorescens JM-1 for management of ear rot disease caused by Fusarium moniliforme in Zea mays L. 3 Biotech. 2022;12(6):138. doi:10.1007/s13205-022-03201-7. [Google Scholar] [PubMed] [CrossRef]
146. Fischer S, Príncipe A, Alvarez F, Cordero P, Castro M, Godino A, et al. Fighting plant diseases through the application of Bacillus and Pseudomonas strains. In: Aroca R, editor. Symbiotic endophytes. Berlin/Heidelberg: Springer; 2013. p. 165–93. doi:10.1007/978-3-642-39317-4_9. [Google Scholar] [CrossRef]
147. Dimopoulou A, Theologidis I, Varympopi A, Papafotis D, Mermigka G, Tzima A, et al. Shifting perspectives of translational research in bio-bactericides: reviewing the Bacillus amyloliquefaciens paradigm. Biology. 2021;10(11):1202. doi:10.3390/biology10111202. [Google Scholar] [PubMed] [CrossRef]
148. Swarnakar S, Pratim A. Cross-talk between the microorganisms and genetic drivers of drought stress responses: present understanding and prospects for crop improvement under drought environment. Agric Res J. 2021;12(5):1833–50. [Google Scholar]
149. Bustamante MI. Biocontrol strategies against grapevine trunk diseases using endophytic and rhizospheric bacteria and reassessment of the etiology of aspergillus vine canker and sour rot of grapes in California [dissertation]. Davis, CA, USA: UC Davis-University of California; 2023. [Google Scholar]
150. Okoro CA, El-Hasan A, Voegele RT. Integrating biological control agents for enhanced management of apple scab (Venturia inaequalisinsights, risks, challenges, and prospects. Agrochemicals. 2024;3(2):118–46. doi:10.3390/agrochemicals3020010. [Google Scholar] [CrossRef]
151. Sangiorgio D, Cellini A, Donati I, Pastore C, Onofrietti C, Spinelli F. Facing climate change: application of microbial biostimulants to mitigate stress in horticultural crops. Agronomy. 2020;10(6):794. doi:10.3390/agronomy10060794. [Google Scholar] [CrossRef]
152. Moreno-Galván A, Romero-Perdomo FA, Estrada-Bonilla G, Meneses CHSG, Bonilla RR. Dry-caribbean Bacillus spp. strains ameliorate drought stress in maize by a strain-specific antioxidant response modulation. Microorganisms. 2020;8(6):823. doi:10.3390/microorganisms8060823. [Google Scholar] [PubMed] [CrossRef]
153. Shahid M, Zeyad MT, Syed A, Singh UB, Mohamed A, Bahkali AH, et al. Stress-tolerant endophytic isolate Priestia aryabhattai BPR-9 modulates physio-biochemical mechanisms in wheat (Triticum aestivum L.) for enhanced salt tolerance. Int J Environ Res Public Health. 2022;19(17):10883. doi:10.3390/ijerph191710883. [Google Scholar] [PubMed] [CrossRef]
154. Meena B. Biological control of pest and diseases using fluorescent pseudomonads. In: Sahayaraj K, editor. Basic and applied aspects of biopesticides. Berlin/Heidelberg, Germany: Springer; 2014. p. 17–29. [Google Scholar]
155. Khan N, Bano A, Ali S, Babar MA. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020;90(2):189–203. doi:10.1007/s10725-020-00571-x. [Google Scholar] [CrossRef]
156. Kamle M, Borah R, Bora H, Jaiswal AK, Singh RK, Kumar P. Systemic acquired resistance (SAR) and induced systemic resistance (ISRrole and mechanism of action against phytopathogens. In: Hesham AE, Upadhayay RS, Sharma GD, Manoharachary C, editors. Fungal biotechnology and bioengineering. Berlin/Heidelberg, Germany: Springer; 2020. p. 457–70. doi:10.1007/978-3-030-41870-0_20. [Google Scholar] [CrossRef]
157. Rashad YM, El-Sharkawy HHA, Hafez M, Bourouah M, Abd-ElGawad AM, Youssef MAA, et al. Fostering resistance in common bean: synergistic defense activation by Bacillus subtilis HE18 and Pseudomonas fluorescens HE22 against Pythium root rot. Rhizosphere. 2024;29(13):100851. doi:10.1016/j.rhisph.2024.100851. [Google Scholar] [CrossRef]
158. Muhammad M, Ahmad MW, Basit A, Ullah S, Mohamed HI, Nisar N, et al. Plant growth-promoting rhizobacteria and their applications and role in the management of soilborne diseases. In: Abd-Elsalam KA, Mohamed HI, editors. Bacterial secondary metabolites. Amsterdam, The Netherlands: Elsevier; 2024. p. 59–82. doi:10.1016/b978-0-323-95251-4.00001-6. [Google Scholar] [CrossRef]
159. Hashem A, Tabassum B, Abd-Allah EF. Bacillus subtilis: a plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J Biol Sci. 2019;26(6):1291–7. doi:10.1016/j.sjbs.2019.05.004. [Google Scholar] [CrossRef]
160. De Pace C, Ricciardi L, Kumar A, Pavan S, Lotti C, Dixit S, et al. Identification of traits, genes, and crops of the future. In: Kole C, editor. Genomics and breeding for climate-resilient crops. Berlin/Heidelberg: Springer; 2013. p. 27–177. doi:10.1007/978-3-642-37045-8_3. [Google Scholar] [CrossRef]
161. Hossain MA, Hossain MS, Akter M. Challenges faced by plant growth-promoting bacteria in field-level applications and suggestions to overcome the barriers. Physiol Mol Plant Pathol. 2023;126(2):102029. doi:10.1016/j.pmpp.2023.102029. [Google Scholar] [CrossRef]
162. Tabassum B, Khan A, Tariq M, Ramzan M, Iqbal Khan MS, Shahid N, et al. Bottlenecks in commercialisation and future prospects of PGPR. Appl Soil Ecol. 2017;121(1):102–17. doi:10.1016/j.apsoil.2017.09.030. [Google Scholar] [CrossRef]
163. Coy RM. Plant growth-promoting rhizobacteria (PGPR) mediate interactions between abiotic and biotic stresses in cool-and warm-season grasses [dissertation]. Auburn, AL, USA: Auburn University; 2017. [Google Scholar]
164. Ganeshan G, Kumar AM. Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases. J Plant Interact. 2005;1(3):123–34. doi:10.1080/17429140600907043. [Google Scholar] [CrossRef]
165. Adeniji A, Huang J, Li S, Lu X, Guo R. Hot viewpoint on how soil texture, soil nutrient availability, and root exudates interact to shape microbial dynamics and plant health. Plant Soil. 2024;36:103063. doi:10.1007/s11104-024-07020-y. [Google Scholar] [CrossRef]
166. Chowdhury N, Hazarika DJ, Goswami G, Sarmah U, Borah S, Boro RC, et al. Acid tolerant bacterium Bacillus amyloliquefaciens MBNC retains biocontrol efficiency against fungal phytopathogens in low pH. Arch Microbiol. 2022;204(2):124. doi:10.1007/s00203-021-02741-5. [Google Scholar] [PubMed] [CrossRef]
167. Yi Y, Luan P, Liu S, Shan Y, Hou Z, Zhao S, et al. Efficacy of Bacillus subtilis XZ18-3 as a biocontrol agent against Rhizoctonia cerealis on wheat. Agriculture. 2022;12(2):258. doi:10.3390/agriculture12020258. [Google Scholar] [CrossRef]
168. Liu J, Zhang J, Zhu M, Wan H, Chen Z, Yang N, et al. Effects of plant growth promoting rhizobacteria (PGPR) strain Bacillus licheniformis with biochar amendment on potato growth and water use efficiency under reduced irrigation regime. Agronomy. 2022;12(5):1031. doi:10.3390/agronomy12051031. [Google Scholar] [CrossRef]
169. Ahmad HA, Farhana L, Haroon U, Saleem H, Anar M, Akbar M, et al. Rhizospheric bacterial strain Pseudomonas putida and fungal strain Penicillium chrysogenum alleviate Fusarium wilt of tomato by improving key growth attributes. Eur J Plant Pathol. 2024;7(9):276. doi:10.1007/s10658-024-02989-9. [Google Scholar] [CrossRef]
170. Das I, Singh AP. Effect of PGPR and organic manures on soil properties of organically cultivated mungbean. Bioscan. 2014;9(1):27–9. [Google Scholar]
171. Balla A, Silini A, Cherif-Silini H, Bouket AC, Alenezi FN, Belbahri L. Recent advances in encapsulation techniques of plant growth-promoting microorganisms and their prospects in the sustainable agriculture. Appl Sci. 2022;12(18):9020. doi:10.3390/app12189020. [Google Scholar] [CrossRef]
172. Rojas-Padilla J, de-Bashan LE, Parra-Cota FI, Rocha-Estrada J, de Los Santos-Villalobos S. Microencapsulation of Bacillus strains for improving wheat (Triticum turgidum subsp durum) growth and development. Plants. 2022;11(21):2920. doi:10.3390/plants11212920. [Google Scholar] [PubMed] [CrossRef]
173. Saberi-Riseh R, Moradi-Pour M, Mohammadinejad R, Thakur VK. Biopolymers for biological control of plant pathogens: advances in microencapsulation of beneficial microorganisms. Polymers. 2021;13(12):1938. doi:10.3390/polym13121938. [Google Scholar] [PubMed] [CrossRef]
174. Riseh RS, Tamanadar E, Hajabdollahi N, Vatankhah M, Thakur VK, Skorik YA. Chitosan microencapsulation of rhizobacteria for biological control of plant pests and diseases: recent advances and applications. Rhizosphere. 2022;23(6):100565. doi:10.1016/j.rhisph.2022.100565. [Google Scholar] [CrossRef]
175. Ali M, Cybulska J, Frąc M, Zdunek A. Application of polysaccharides for the encapsulation of beneficial microorganisms for agricultural purposes: a review. Int J Bio-Log Macromol. 2023;244(3):125366. doi:10.1016/j.ijbiomac.2023.125366. [Google Scholar] [PubMed] [CrossRef]
176. Amira MB, Lopez D, Mohamed AT, Khouaja A, Chaar H, Fumanal B, et al. Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biol Control. 2017;110:70–8. doi:10.1016/j.biocontrol.2017.04.008. [Google Scholar] [CrossRef]
177. Landa BB, Montes-Borrego M, Navas-Cortés JA. Use of PGPR for controlling soilborne fungal pathogens: assessing the factors influencing its efficacy. In: Maheshwari DK, editor. Bacteria in agrobiology: disease management. Berlin/Heidelberg: Springer; 2012. p. 259–92. doi:10.1007/978-3-642-33639-3_10. [Google Scholar] [CrossRef]
178. Lobo CB, Tomás MSJ, Viruel E, Ferrero MA, Lucca ME. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol Res. 2019;219:12–25. doi:10.1016/j.micres.2018.10.012. [Google Scholar] [PubMed] [CrossRef]
179. Yadav A, Yadav K. Challenges and opportunities in biofertilizer commercialization. SVOA Microbiol. 2024;5(1):1–14. doi:10.58624/svoamb.2024.05.037. [Google Scholar] [CrossRef]
180. Bagga D, Chauhan S, Bhavanam A, Nikhil GN, Meena SS, Mohanty A. Recent advancements in fermentation strategies for mass production and formulation of biofertilizers: towards waste valorization. J Soil Sci Plant Nutr. 2024;24(3):5868–97. doi:10.1007/s42729-024-01947-y. [Google Scholar] [CrossRef]
181. Patel JS, Kumar G, Bajpai R, Teli B, Rashid M, Sarma BK. PGPR formulations and application in the management of pulse crop health. In: Rakshit A, Meena VS, Parihar M, Singh HB, Singh AK, editors. Biofertilizers. Amsterdam, The Netherlands: Elsevier; 2021. p. 239–51. doi:10.1016/b978-0-12-821667-5.00012-9. [Google Scholar] [CrossRef]
182. Pour MM, Saberi-Riseh R, Mohammadinejad R, Hosseini A. Investigating the formulation of alginate gelatin encapsulated Pseudomonas fluorescens (VUPF5 and T17-4 strains) for controlling Fusarium solani on potato. Int J Biol Macromol. 2019;133(3):603–13. doi:10.1016/j.ijbiomac.2019.04.071. [Google Scholar] [PubMed] [CrossRef]
183. Backer R, Rokem JS, Ilangumaran G, Lamont J, Praslickova D, Ricci E, et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci. 2018;9:1473. doi:10.3389/fpls.2018.01473. [Google Scholar] [PubMed] [CrossRef]
184. Chandler D, Bailey AS, Tatchell GM, Davidson G, Greaves J, Grant WP. The development, regulation and use of biopesticides for integrated pest management. Philos Trans R Soc Lond B Biol Sci. 2011;366(1573):1987–98. doi:10.1098/rstb.2010.0390. [Google Scholar] [PubMed] [CrossRef]
185. Peter AJ, Amalraj ELD, Talluri VR. Commercial aspects of biofertilizers and biostimulants development utilizing rhizosphere microbes: global and Indian scenario. In: Sharma SK, Singh UB, Sahu PK, Singh HV, Sharma PK, editors. Rhizosphere microbes. Singapore: Springer; 2020. p. 655–82. doi:10.1007/978-981-15-9154-9_27. [Google Scholar] [CrossRef]
186. Keswani C, Prakash O, Bharti N, Vílchez JI, Sansinenea E, Lally RD, et al. Re-addressing the biosafety issues of plant growth promoting rhizobacteria. Sci Total Environ. 2019;690(2):841–52. doi:10.1016/j.scitotenv.2019.07.046. [Google Scholar] [PubMed] [CrossRef]
187. Soumare A, Boubekri K, Lyamlouli K, Hafidi M, Ouhdouch Y, Kouisni L. From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: status and needs. Front Bioeng Biotechnol. 2020;7:425. doi:10.3389/fbioe.2019.00425. [Google Scholar] [PubMed] [CrossRef]
188. Freyer B, Ellssel P, Nyakanda F, Saussure S. Exploring the off-farm production, marketing and use of organic and biofertilisers in Africa: a scoping study. Brussels, Belgium: European Union; 2024. [Google Scholar]
189. Sen K, Patra P, Mallick S, Islam SS, Dutta S, Midya S. From pollution to prosperity: the role of PGPRs in bioremediation. In: Adhikary PP, Shit PK, Laha J, editors. Soil, water pollution and mitigation strategies. Berlin/Heidelberg, Germany: Springer; 2024. p. 191–221. doi:10.1007/978-3-031-63296-9_7. [Google Scholar] [CrossRef]
190. Nair KP. Technological advancements in coconut, arecanut and cocoa research: a century of service to the global farming community by the central plantation crops research institute, Kasaragod, Kerala state, India. In: Nair KP, editor. Tree crops. Berlin/Heidelberg, Germany: Springer; 2020. p. 377–536. doi:10.1007/978-3-030-62140-7_11. [Google Scholar] [CrossRef]
191. Elnahal ASM, El-Saadony MT, Saad AM, Desoky EM, El-Tahan AM, Rady MM, et al. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: a review. Eur J Plant Pathol. 2022;162(4):759–92. doi:10.1007/s10658-021-02393-7. [Google Scholar] [CrossRef]
192. Kumar A, Patel JS, Meena VS, Srivastava R. Recent advances of PGPR based approaches for stress tolerance in plants for sustainable agriculture. Biocatal Agric Biotechnol. 2019;20(4):101271. doi:10.1016/j.bcab.2019.101271. [Google Scholar] [CrossRef]
193. Injamum-Ul-Hoque M, Imran M, Zainurin N, Shaffique S, Kang SM, Ahsan SM, et al. Isolation and identification of multi-traits PGPR for sustainable crop productivity under salinity stress. Sustainability. 2024;16(21):9263. doi:10.3390/su16219263. [Google Scholar] [CrossRef]
194. Clagnan E, Costanzo M, Visca A, Di Gregorio L, Tabacchioni S, Colantoni E, et al. Culturomics- and metagenomics-based insights into the soil microbiome preservation and application for sustainable agriculture. Front Microbiol. 2024;15:1473666. doi:10.3389/fmicb.2024.1473666. [Google Scholar] [PubMed] [CrossRef]
195. Gurusinghe S, Brooks TL, Barrow RA, Zhu X, Thotagamuwa A, Dennis PG, et al. Technologies for the selection, culture and metabolic profiling of unique rhizosphere microorganisms for natural product discovery. Molecules. 2019;24(10):1955. doi:10.3390/molecules24101955. [Google Scholar] [PubMed] [CrossRef]
196. Raymaekers K, Ponet L, Holtappels D, Berckmans B, Cammue BPA. Screening for novel biocontrol agents applicable in plant disease management-a review. Biolog Control. 2020;144:104240. doi:10.1016/j.biocontrol.2020.104240. [Google Scholar] [CrossRef]
197. Viebahn M, Smit E, Glandorf DCM, Wernars K, Bakker PAHM. Effect of genetically modified bacteria on ecosystems and their potential benefits for bioremediation and biocontrol of plant diseases—a review. In: Lichtfouse E, editor. Climate change, intercropping, pest control and beneficial microorganisms. Berlin/Heidelberg, Germany: Springer; 2009. p. 45–69. doi:10.1007/978-90-481-2716-0_4. [Google Scholar] [CrossRef]
198. Beyari EA. Alternatives to chemical pesticides: the role of microbial biocontrol agents in phytopathogen management: a comprehensive review. J Plant Pathol. 2025;107(1):291–314. doi:10.1007/s42161-024-01808-8. [Google Scholar] [CrossRef]
199. Liu T, Zheng Y, Wang L, Wang X, Wang H, Tian Y. Optimizing surfactin yield in Bacillus velezensis BN to enhance biocontrol efficacy and rhizosphere colonization. Front Microbiol. 2025;16:1551436. doi:10.3389/fmicb.2025.1551436. [Google Scholar] [PubMed] [CrossRef]
200. Tilgam J, Verma S, Choudhury S, Singh D, Das S. Genetic enhancement of biocontrol agent as effective management of soilborne disease. In: Singh UB, Kumar R, Singh HB, editors. Detection, diagnosis and management of soil-borne phytopathogens. Singapore: Springer; 2023. p. 127–58. doi:10.1007/978-981-19-8307-8_6. [Google Scholar] [CrossRef]
201. Karmakar B, Thakuria D, Begum RH, Joga RJ. Recent advances in experimental design of synthetic microbial communities for biocontrol application. BioControl. 2025;70(2):229–44. doi:10.1007/s10526-024-10295-w. [Google Scholar] [CrossRef]
202. Ayaz M, Li CH, Ali Q, Zhao W, Chi YK, Shafiq M, et al. Bacterial and fungal biocontrol agents for plant disease protection: journey from lab to field, current status, challenges, and global perspectives. Molecules. 2023;28(18):6735. doi:10.3390/molecules28186735. [Google Scholar] [PubMed] [CrossRef]
203. Kumar D, Singh PN, Willer H, Sharma SK, Singh UB, Lagashetti AC, et al. Fungal biofertilizers and biopesticides and their roles in sustainable agriculture. In: Singh SK, Kumar D, Shamim M, Sharma R, editors. Applied mycology for agriculture and foods. New York, NY, USA: Apple Academic Press; 2023. p. 165–216. doi:10.1201/9781003369868-9. [Google Scholar] [CrossRef]
204. Singh S, Balodi R, Meena PN, Singhal S. Biocontrol activity of Trichoderma harzianum, Bacillus subtilis and Pseudomonas fluorescens against Meloidogyne incognita, Fusarium oxysporum and Rhizoctonia solani. Indian Phytopathol. 2021;74(3):703–14. doi:10.1007/s42360-021-00368-6. [Google Scholar] [CrossRef]
205. Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv. 2014;32(2):429–48. doi:10.1016/j.biotechadv.2013.12.005. [Google Scholar] [PubMed] [CrossRef]
206. Qasim M. Beneficial microbes in plant health: from biocontrol agent to plant growth promoting rhizobacteria. Int J Res Adv Agric Sci. 2023;2(3):39–49. [Google Scholar]
207. Bhat MA, Mishra AK, Jan S, Bhat MA, Kamal MA, Rahman S, et al. Plant growth promoting rhizobacteria in plant health: a perspective study of the underground interaction. Plants. 2023;12(3):629. doi:10.3390/plants12030629. [Google Scholar] [PubMed] [CrossRef]
208. Zuluaga MYA, Fattorini R, Cesco S, Pii Y. Plant-microbe interactions in the rhizosphere for smarter and more sustainable crop fertilization: the case of PGPR-based biofertilizers. Front Microbiol. 2024;15:1440978. doi:10.3389/fmicb.2024.1440978. [Google Scholar] [PubMed] [CrossRef]
209. Sarker A, Ansary MWR, Hossain MN, Islam T. Prospect and challenges for sustainable management of climate change-associated stresses to soil and plant health by beneficial rhizobacteria. Stresses. 2021;1(4):200–22. doi:10.3390/stresses1040015. [Google Scholar] [CrossRef]
210. Koskey G, Mburu SW, Awino R, Njeru EM, Maingi JM. Potential use of beneficial microorganisms for soil amelioration, phytopathogen biocontrol, and sustainable crop production in smallholder agroecosystems. Front Sustain Food Syst. 2021;5:606308. doi:10.3389/fsufs.2021.606308. [Google Scholar] [CrossRef]
211. Mawar R, Manjunatha BL, Sanyal A, Sharma SK, Singh HB, Dubey SC, et al. Current regulatory requirements for PGPM products for management of seed, soil and plant health: an overview. In: Mawar R, Sayyed RZ, Sharma SK, Sattiraju KS, editors. Plant growth promoting microorganisms of arid region. Singapore: Springer; 2023. p. 349–63. doi:10.1007/978-981-19-4124-5_16. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF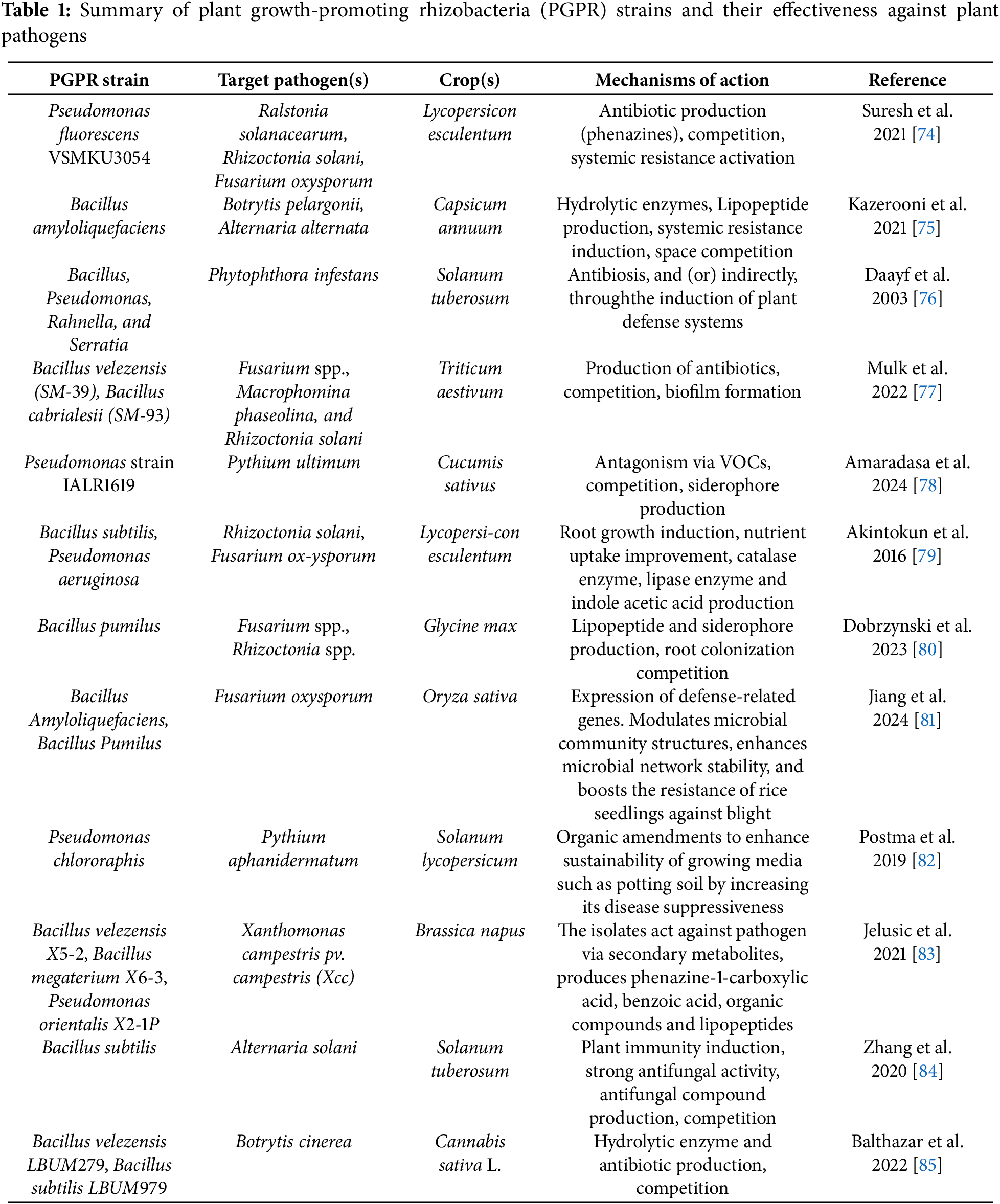
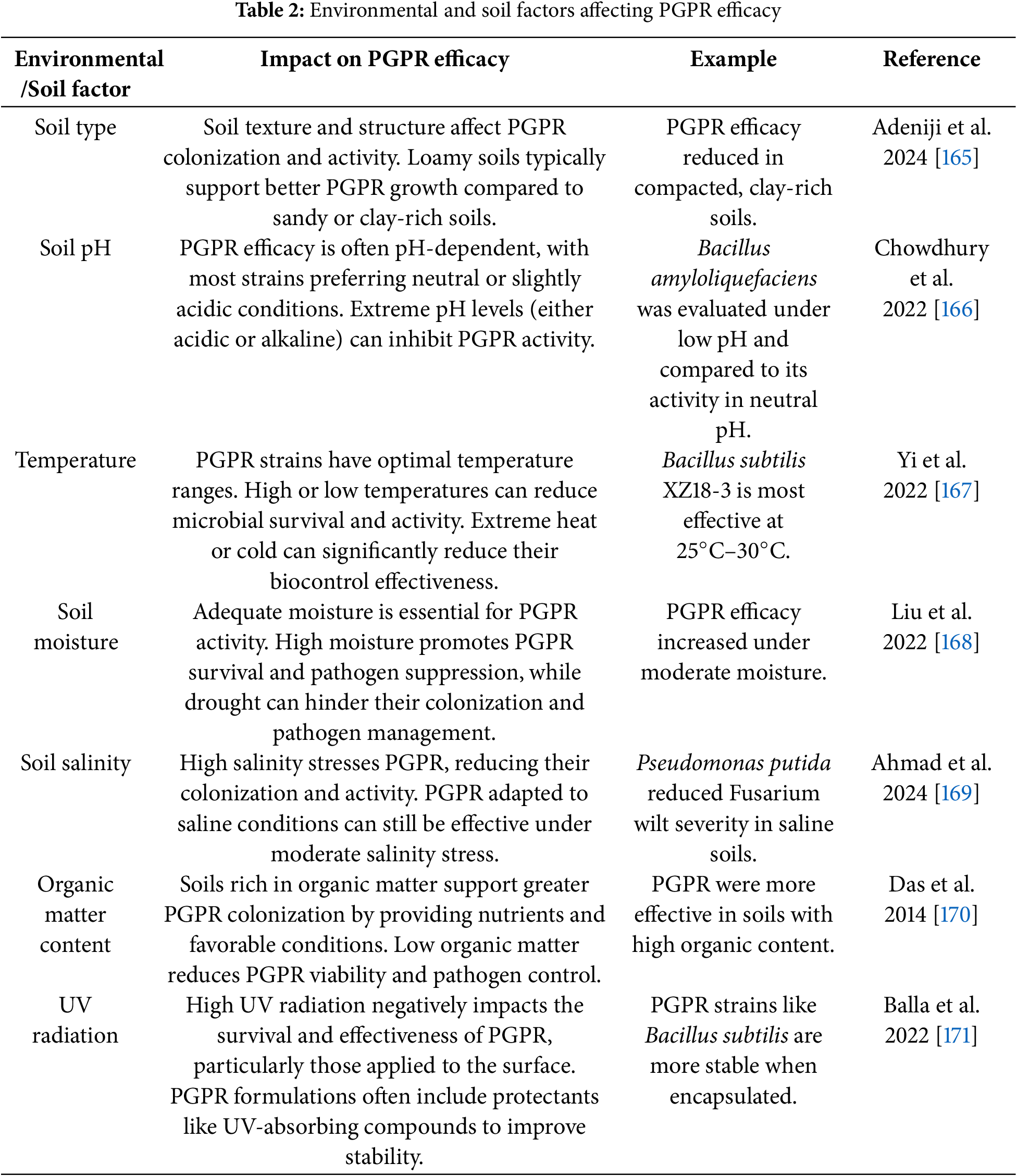
 Downloads
Downloads
 Citation Tools
Citation Tools