 Open Access
Open Access
CASE REPORT
Long-term follow-up of metastatic renal myoepithelial carcinoma: case report and literature review
1 Department of Urology, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY 10467, USA
2 Medicine, Mohammed VI University Hospital, Faculty of Medicine of Oujda, Mohammed First University, Oujda, 60000, Morocco
3 Department of Medicine (Hematology/Oncology), Northwestern University Feinberg School of Medicine, Chicago, IL 60611, USA
* Corresponding Authors: Niraj Shenoy. Email: ; Ahmed Aboumohamed. Email:
Canadian Journal of Urology 2025, 32(3), 167-172. https://doi.org/10.32604/cju.2025.063279
Received 10 January 2025; Accepted 06 May 2025; Issue published 27 June 2025
Abstract
Background: Primary renal myoepithelial carcinoma is an exceptionally rare malignancy with limited data on optimal treatment, particularly in metastatic settings. Case Description: In 2020, Shenoy reported a dramatic response in a case of metastatic myoepithelial carcinoma with Ewing sarcoma breakpoint region 1-POU class 5 homeobox 1 (EWSR1-POU5F1) fusion arising from the left kidney using the Ewing Sarcoma vincristine, doxorubicin, cyclophosphamide/ifosfamide, etoposide (VDC/IE) chemotherapy regimen. Ten months post-treatment, the patient showed ~90% reduced disease burden on imaging. Subsequent treatment included consolidation vincristine, cyclophosphamide/ifosfamide, etoposide (VC/IE) chemotherapy, surgical resection of the remnant tumor, and follow-up imaging. Conclusion: The patient has been disease-free for 44 months off treatment and 5 years post-treatment initiation. To our knowledge, this is the first report of long-term disease-free survival in metastatic primary renal myoepithelial carcinoma. We also review the literature on this rare disease.Keywords
Supplementary Material
Supplementary Material FileMyoepithelial carcinoma (MEC) is an exceedingly rare malignancy, most commonly arising in the salivary glands and soft tissues of the head and neck.1 Primary renal MEC is exceptionally uncommon, with fewer than ten reported cases in the literature.2–4 Due to its rarity, the clinical course of renal MEC remains poorly understood, presenting significant diagnostic and therapeutic challenges.
Recent molecular studies have identified recurrent Ewing sarcoma breakpoint region 1 (EWSR1) gene rearrangements in MEC, with the EWSR1-POU class 5 homeobox 1 (EWSR1-POU5F1) fusion particularly associated with aggressive behavior in soft tissue and visceral myoepithelial tumors.5 However, the molecular characteristics of renal MEC remain insufficiently defined due to the limited number of cases.
Currently, there are no established treatment guidelines for renal MEC, making management highly case-dependent. Therapeutic strategies often involve a combination of surgical resection and systemic therapy. However, the choice of chemotherapeutic regimen and responses to therapy are highly variable, and the long-term prognosis remains uncertain.
Here, we present a long-term follow-up of Shenoy6 previously reported case of metastatic primary renal MEC with EWSR1-POU5F1 fusion, demonstrating a sustained response to chemotherapy followed by surgical resection. To our knowledge, this is the first documented case of long-term disease-free survival in metastatic renal MEC, highlighting the potential efficacy of aggressive multimodal therapy. Additionally, we review the existing literature to provide further context on this rare and aggressive malignancy.
Below is a brief review of the case, including the initial pathologic assessment, the chemotherapeutic regimen decision, and the profound treatment response reported by Shenoy6
A 22-year-old African American male presented with a two-month history of right-sided abdominal pain, fatigue, and weight loss. Imaging studies revealed a large primary left renal mass extending across the midline to the porta hepatis, with involvement of the aorta and inferior vena cava (IVC). Additionally, liver metastases and extensive lymphadenopathy (mesenteric, retroperitoneal, and pelvic) were observed on computed tomography (CT). A CT-guided 7-core mass biopsy confirmed the histopathologic diagnosis of high-grade *mesenchymal-epithelial carcinoma (MEC)* harboring an *EWSR1-POU5F1* fusion.
Histologic examination demonstrated a poorly differentiated round cell tumor with scant cytoplasm, arranged in nests surrounded by desmoplastic stroma. Immunohistochemical staining was positive for cytokeratin (AE1/AE3) and CD56, with focal weak expression of Pax8. The tumor tested negative for desmin, Wilms tumor protein (WT1-1 and WT1-C, send-out test), chromogranin, synaptophysin, cytokeratin 903 (CK903), CD99, CD45, and Bcl2. Notably, INI-1 (SMARCB1) expression was retained (send-out test). The case was referred to Memorial Sloan Kettering for further consultation, where an EWSR1 rearrangement was confirmed.
Laboratory findings showed a markedly elevated lactate dehydrogenase (LDH) level (2140 U/L), indicative of aggressive disease progression, consistent with the patient’s cachectic state. Given the patient’s rapidly deteriorating clinical status, chemotherapy was initiated on an urgent basis, soon after the histopathologic report of high-grade myoepithelial carcinoma with EWSR1-POU5F1 fusion was available. The Ewing’s sarcoma VDC/IE combination chemotherapy regimen was selected based on the factors outlined in Shenoy’s 2020 report.6
At the time of the previous report, approximately 10 months post-treatment initiation, the patient had achieved a ~90% reduction in disease burden on imaging and was asymptomatic from a cancer standpoint. While the planned regimen was VDC/IE per the Adolescent and Young Adult Ewing Sarcoma (AEWS 1031) Study Regimen A (administered every 2 weeks for a total of 17 cycles—6 induction and 11 consolidation), several delays occurred due to grade 3 to 4 neutropenia. The majority of cycles were administered at intervals of ≥3 weeks, with increasing intervals during the consolidation phase.
However, no episodes of febrile neutropenia occurred throughout the treatment course, and no growth factors were administered. Given the prolonged marrow recovery time, renal dysfunction (Cr > 2), and the persistently low fluorodeoxyglucose (FDG) uptake in the relatively small remnant primary tumor mass (which had remained stable in size over several preceding months), a multidisciplinary decision was made to pause systemic treatment at ~16 months post-initiation and proceed with surgical resection of the primary mass. The plan included likely resuming further systemic treatment after surgery.
He underwent open left radical nephrectomy and tumor debulking approximately 18 months post-treatment initiation. Intraoperatively, significant fibrotic tissue along the lateral aspect of the aorta up to the renal hilum was noted and removed. The left upper pole and associated fibrotic tissue were adherent to the spleen. Pre-aortic and left para-aortic lymph nodes were removed en bloc with the left kidney from the origin of the inferior mesenteric artery to the origin of the superior mesenteric artery. The postoperative course was unremarkable, and the patient was discharged on postoperative day (POD) 3.
Remarkably, surgical pathology revealed no residual carcinoma, only markedly atrophic renal parenchyma with extensive treatment effects (fibrosis), along with negative margins and lymph nodes. Given the pathology findings, a decision was made not to re-initiate systemic therapy at that time and to monitor with imaging. Follow-up magnetic resonance imaging (MRI) 4 months postoperatively revealed no evidence of residual or recurrent malignancy in the surgical bed, including a stable 4 mm hepatic lesion adjacent to the gallbladder fossa, which remained unchanged on a subsequent MRI follow-up 9 months postoperatively (Figure 1). A chest CT at 9 months postoperatively also showed no evidence of disease.
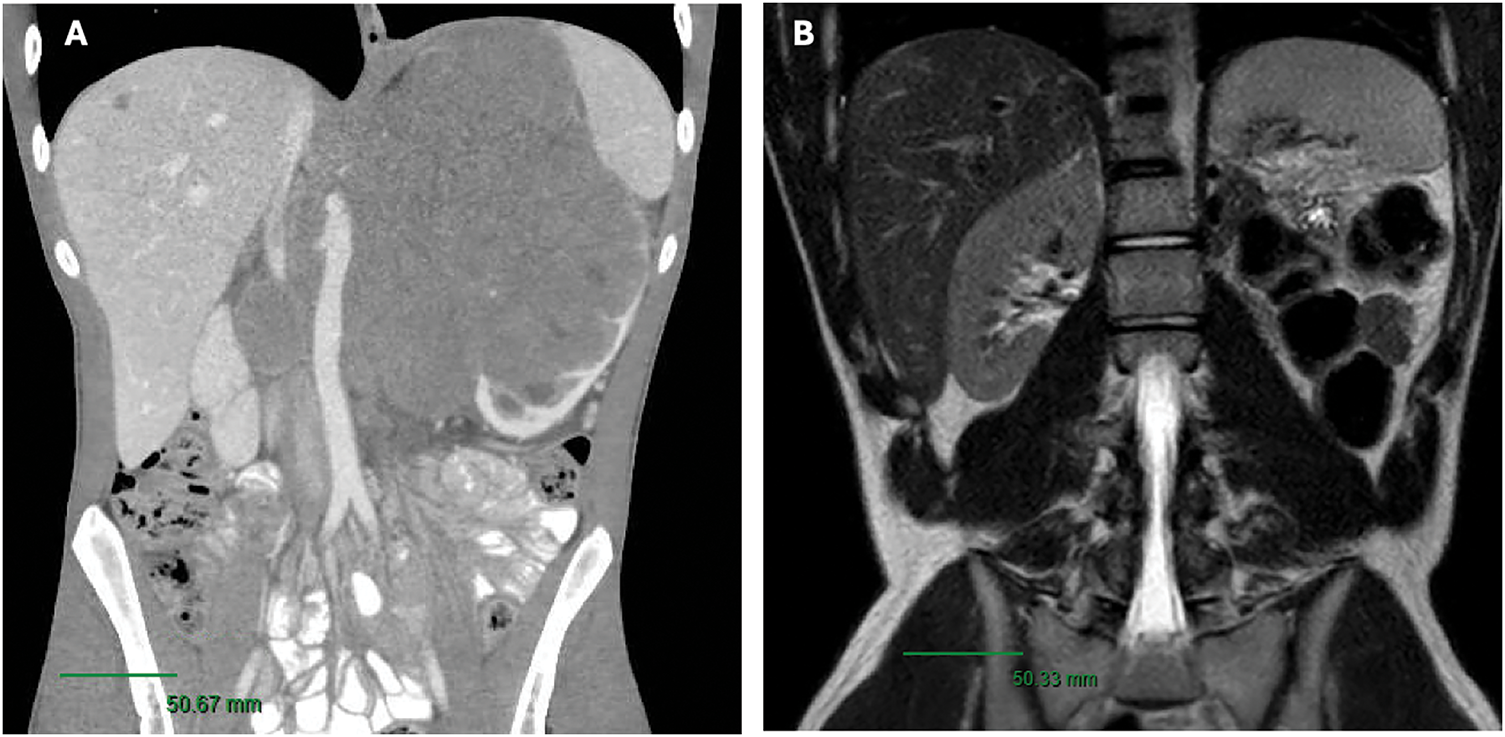
FIGURE 1. (A) Pre-treatment CT Scan (B) Post-left radical nephrectomy MRI/pelvis revealing no suspicious masses or abdomen evidence of recurrence within the nephrectomy bed. Scale bar, 50 mm
His case was discussed at the Genitourinary Tumor Board for assessment of his persistent hepatic lesion, and a multidisciplinary evaluation concluded that the lesion was likely scar tissue; thus, no further treatment was pursued. At the time of the last follow-up, 44 months postoperatively, he continued to do well and remains on surveillance, with no evidence of disease on repeat chest CT and abdominal MRI, and no residual liver lesion identified.
Primary renal myoepithelial carcinoma is an exceptionally rare and aggressive tumor, with EWSR1 rearrangements identified in a minority of cases. Diagnosing this malignancy can be particularly challenging due to its morphologic diversity and similarity to its soft tissue counterpart, displaying a mix of cellular and stromal components. Multiple cell types (epithelioid, spindled, plasmacytoid, and clear) are often present in a wide array of configurations (e.g., cords, trabeculae, solid sheets, and ducts), with the cellular component integrated into a myxoid/chondromyxoid to hyalinized stroma with potential chondroid and osseous differentiation.
Immunohistochemical analysis is essential for diagnosis, as tumor cells variably express myoepithelial markers such as p63, CD10, and smooth muscle actin. In certain cases, immunohistochemistry may not provide a definitive diagnosis, making molecular studies essential for confirmation. This challenge is exemplified by Nishith and Chowdhury, who detailed the diagnosis of a 67-year-old man with a right supraclavicular mass, where an extensive immunohistochemical analysis was supplemented by fluorescent in situ hybridization (FISH) to establish the final diagnosis.
We present the long-term follow-up of a previously reported case of successfully treated metastatic, primary renal MEC with EWSR-POU5F1 fusion, with VDC/IE combination chemotherapy followed by surgical extirpation, with no evidence of disease at 38 months after initial diagnosis. At the time of Shenoy’s initial report6, the patient was approximately 10 months post-treatment initiation with approximately 90% disease burden reduction on imaging. This report describes the subsequent management with continuation of consolidation VC/IE, surgical resection, and follow-up surveillance imaging with 5 years after treatment initiation.
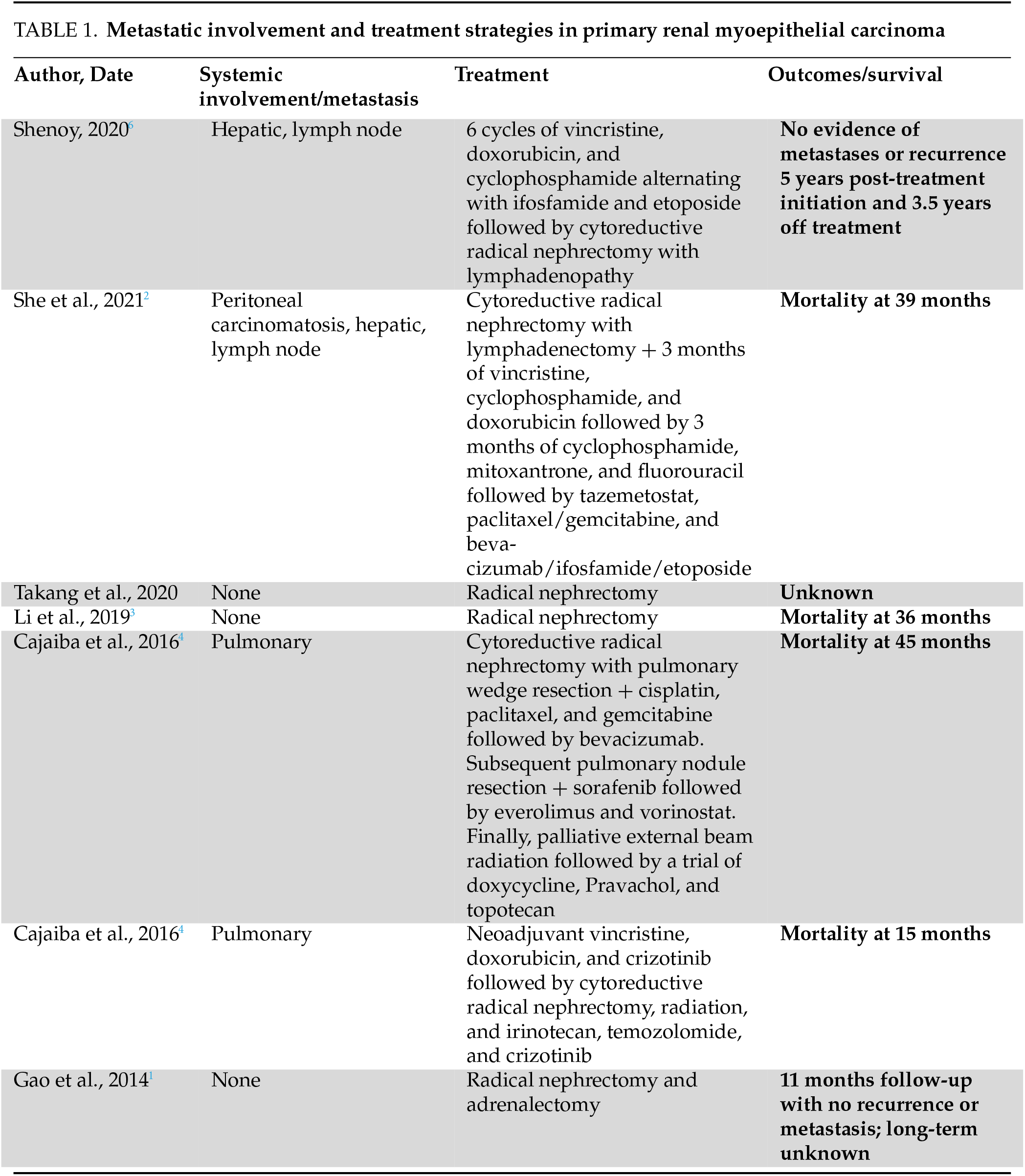
A review of the literature reveals only a handful of documented cases of primary renal MEC, with no standard treatment algorithm and an overall poor long-term prognosis (Table 1).
Other primary renal MEC reports with metastatic disease at presentation
In a report by She et al.2, a 19-year-old girl presented with bleeding secondary to primary renal MEC with EWSR1 gene rearrangement (fusion partner not reported), with hepatic and lymph node metastases. She underwent initial cytoreductive nephrectomy and lymphadenectomy followed by three cycles of vincristine, cyclophosphamide, and doxorubicin chemotherapy. The patient experienced an initial response but quickly developed disease progression with worsening liver metastases and distant lymph node involvement, at which point the regimen was changed to cyclophosphamide, mitoxantrone, 5-fluorouracil (5-FU), and bleomycin. Progression continued with extracapsular hepatic involvement, peritoneal carcinomatosis, and extensive abdominal tumor seeding. She subsequently underwent a trial of tazemetostat and multiple courses of oral cyclophosphamide, paclitaxel/gemcitabine, and bevacizumab/ifosfamide/etoposide. Unfortunately, disease dissemination proceeded, and she died of metastatic disease and renal failure at 39 months post-diagnosis.
Two pediatric cases aged 4 and 6 years old were reported by Cajaiba et al.4, both with primary renal MEC with EWSR1 gene rearrangement presenting with pulmonary metastases at the time of initial diagnosis. Both underwent a combination of cytoreductive nephrectomy, radiation, and multiple rounds of chemotherapy and immunotherapy with poor outcomes and mortality at 15 and 45 months. These two cases were the first reports of primary renal MEC. The first patient underwent cytoreductive radical nephrectomy with pulmonary wedge resection and a regimen of cisplatin, paclitaxel, and gemcitabine followed by bevacizumab. She subsequently underwent pulmonary nodule resection and received sorafenib, followed by everolimus and vorinostat. Finally, palliative external beam radiation and a trial of doxycycline, pravastatin, and topotecan were attempted, with the patient eventually succumbing to disease dissemination and death 45 months after the initial diagnosis. The second patient underwent neoadjuvant vincristine, doxorubicin, and crizotinib followed by cytoreductive radical nephrectomy and radiation. A course of irinotecan, temozolomide, and crizotinib was additionally trialed until death at 15 months.
Primary renal MEC reports with localized disease at presentation
A recent case was described by Li et al.3 involving an 85-year-old man who presented with hematuria and flank pain, was found to have a localized left renal mass without lymphadenopathy, and underwent a left radical nephrectomy with surgical pathology revealing primary renal epithelial-myoepithelial carcinoma. In this instance, the patient did not undergo chemotherapy and eventually developed multiple distant metastases, leading to multi-organ failure at 3 years post-diagnosis.
Another case of localized disease utilizing solely surgical extirpation was presented by Gao et al.1 in a 56-year-old male patient who underwent right nephrectomy and adrenalectomy with no evidence of recurrence or metastases at 11 months follow-up.
Primary renal MEC is a rare, aggressive clinical entity with significant heterogeneity of disease and treatment course. The rarity of the disease adds a challenge to developing standardized management strategies, both in the metastatic and localized settings. We present a follow-up of a previously reported case of successfully treated metastatic primary renal MEC with the VDC/IE combination chemotherapy regimen followed by surgical extirpation of the remnant primary tumor mass, with no evidence of disease at long-term follow-up. To the author’s knowledge, this is the first report of long-term disease-free survival in a case of metastatic primary renal myoepithelial carcinoma.
Acknowledgement
The authors would like to sincerely thank our multidisciplinary and cross-institutional team for their efforts in providing an excellence of patient care and comprehensive collaboration. We thank the patient and his family for their participation, trust, and support throughout this study. Their willingness to contribute has been invaluable and is deeply appreciated.
Funding Statement
Niraj Shenoy’s research was supported by the Albert Einstein Cancer Center Core Grant (2P30CA013330-47).
Author Contributions
Priya Dave: conception, performance of work, interpretation of data, writing the article, had access to the data; Ghizlane Yaakoubi: performance of work, writing the article, had access to the data; Justin Loloi: conception, performance of work, interpretation of data, writing the article, had access to the data; Niraj Shenoy: conception, performance of work, interpretation of data, writing the article, had access to the data; Ahmed Aboumohamed: conception, performance of work, interpretation of data, writing the article, had access to the data. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
The datasets and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
This study was approved by IRB of Albert Einstein College of Medicine [#2023-15147]. All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from the patient and family prior to their inclusion in the study.
Conflicts of Interest
The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials
The supplementary material is available online at https://www.techscience.com/doi/10.32604/cju.2025.063279/s1.
References
1. Gao H-X, Liu C-X, Zou H et al. Parachordoma/myoepithelioma of the kidney: first report of a myxoid mimicry in an unusual location. Int J Clin Exp Pathol 2014;7(3):1258–1265. [Google Scholar] [PubMed]
2. She Y-H, Chen Y-T, Huang Y-M et al. Primary renal myoepithelial carcinoma with EWSR-1 gene rearrangement-case report and literature review. Acta Nephrologica 2021;35(2):105–111. [Google Scholar]
3. Li Q, Mou Z, Yang K, Jiang H. A first case report of primary epithelial myoepithelial carcinoma-like renal tumor showing a perivascular pseudorosette-like pattern: description of morphologic, immunohistochemical, and genetic features. Medicine 2019;98(39):e17245. doi:10.1097/md.0000000000017245. [Google Scholar] [PubMed] [CrossRef]
4. Cajaiba MM, Jennings LJ, Rohan SM et al. Expanding the spectrum of renal tumors in children: primary renal myoepithelial carcinomas with a novel EWSR1-KLF15 fusion. Am J Surg Pathol 2016;40(3):386–394. doi:10.1097/pas.0000000000000545. [Google Scholar] [PubMed] [CrossRef]
5. Suurmeijer AJH, Dickson BC, Swanson D et al. A morphologic and molecular reappraisal of myoepithelial tumors of soft tissue, bone, and viscera with EWSR1 and FUS gene rearrangements. Genes Chromosomes Cancer 2020;59(6):348–356. doi:10.1002/gcc.22835. [Google Scholar] [PubMed] [CrossRef]
6. Shenoy N. Aggressive myoepithelial carcinoma with EWSR1-POU5F1 fusion highly responsive to Ewing sarcoma combination chemotherapy. Cancer 2020;126(24):5198–5201. doi:10.1002/cncr.33220. [Google Scholar] [PubMed] [CrossRef]
7. Takang CMC, Vivian O, Tafere S, Marciales W, Lingamurthy M. An unusual case of epithelial-myoepithelial carcinoma of the kidney: primary vs. metastasis? A diagnostic dilemma. In: Florida B, editor. Manatee memorial hospital. Southern Medical Association; 2020. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools