 Open Access
Open Access
ARTICLE
Robotic-assisted super-extended pelvic lymph node dissection for prostate cancer: safety and pathologic findings
1 Department of Urology, UConn Health, Farmington, CT 06030, USA
2 Research Program, Hartford Hospital, Hartford Healthcare, Hartford, CT 06106, USA
3 Division of Urology, Hartford Healthcare Medical Group, Hartford Healthcare, Hartford, CT 06106, USA
4 Pathology Department, Hartford Hospital, Hartford Healthcare, Hartford, CT 06106, USA
* Corresponding Author: Tara McLaughlin Proto. Email:
Canadian Journal of Urology 2025, 32(3), 189-198. https://doi.org/10.32604/cju.2025.063773
Received 23 January 2025; Accepted 25 April 2025; Issue published 27 June 2025
Abstract
Introduction: We examined the pathology and safety outcomes associated with the extent of pelvic lymph node dissection in patients with high-risk prostate cancer undergoing radical prostatectomy. Materials and Methods: We retrospectively identified men with prostate cancer who underwent robot-assisted radical prostatectomy with pelvic lymph node dissection between May 2016 and September 2021. Cases were categorized using Current Procedural Terminology (CPT) codes (38571) for extended lymph node dissection and super-extended lymph node dissection (38572). Using logistic regression, we compared the groups on a number of factors, including recurrence. Results: Super-extended lymph node dissection had significantly higher median prostate-specific antigen and National Comprehensive Cancer Network risk classification prior to surgery. Significant differences were observed in the pathologic T stage and pathology grade group. Time on robot was significantly longer for the super-extended group, while estimated blood loss was lower. No differences were observed in length of stay or any complication-related variable. Super-extended had significantly higher node positivity (36.1% vs. 7.6%, p < 0.001) and recurrence. 10.0% of super-extended cases had node positivity in the aortic bifurcation, the common iliac, or the pre-sacral chains that would have been missed with an extended dissection. 2.2% of patients had node positivity in these chains only. Conclusions: Super-extended lymph node dissection is safe and feasible for patients with high-risk prostate cancer. Further research is needed to better understand its clinical benefit and to further inform optimal patient selection.Keywords
For patients undergoing radical prostatectomy (RP) for prostate cancer, National Comprehensive Cancer Network (NCCN) guidelines call for an extended pelvic lymph node dissection (ELND), including the removal of nodes from the obturator as well as the external and internal iliac regions.1 Although ELND is the standard of care for most men undergoing RP, a great deal of variability exists between surgeons and centers, and the therapeutic benefits of the various degrees of extension remain to be determined. A higher number of dissected nodes has been associated with improved cancer-specific and biochemical recurrence-free survival rates.2–8 The potential risks of ELND (relative to standard pelvic LND (lymph node dissection)) include longer operative time, higher rates of intraoperative complications, longer hospital stay, and a greater rate of lymphocele development.3,4,9,10 Despite these risks, some investigators have examined the feasibility of a super-extended pelvic lymph node dissection (sELND), further extending the dissection to include nodes located in aortic bifurcation, common iliac, and pre-sacral areas.11
This retrospective study examined the safety, pathologic findings, and rates of disease recurrence associated with sELND in prostate cancer patients undergoing RP in higher risk categories as determined by NCCN classification. Further, we quantified node positivity in the aortic bifurcation, the common iliac, and the pre-sacral locations that would be missed in a standard ELND. Finally, we compared sELND to ELND on recurrence rates and the need for salvage treatment as of the date of the latest prostate-specific antigen (PSA) measurement.
Starting in May 2016, patients categorized as high or very high-risk according to NCCN guidelines (including some with unfavorable, intermediate risk prostate cancer) were offered sELND during RP. This project was approved by the Hartford HealthCare Institutional Review Board with a waiver of informed consent (E-HHC-2019-0190). We queried our IRB-approved prostate cancer database and the electronic medical record to identify men with prostate cancer who underwent robot-assisted radical prostatectomy (RARP) with pelvic lymph node dissection (PLND) between May 2016 and September 2021. Patients who received prior radiation therapy to the prostate (salvage prostatectomies) were excluded. Cases were further categorized using Current Procedural Terminology (CPT) codes (38571 for ELND and 38572 sELND).
Using a standard transperitoneal approach, RARP/PLND was performed by one surgeon with 24 years of experience performing these procedures. The LND extended to the bifurcation of the superior internal and external iliac artery, the genitofemoral nerve laterally, the obturator nerve posteriorly, the pelvic sidewall laterally, and the bladder/sigmoid medially. In sELND, the dissection was extended up to the aortic bifurcation. Electrocautery and clips were used as necessary to obtain the specimens.
Prostate and seminal vesicle specimens were received fresh. Surgical margins were inked, and the specimen was fixed in 10% neutral buffered formalin. Lymph nodes were submitted whole or thinned if larger than 0.5 cm in thickness, allowing for microscopic enumeration of both positive and negative lymph nodes. Sections were fixed in neutral buffered formalin and subjected to standard histology processing, with 4–5 micron thick sections mounted on glass slides and stained with hematoxylin and eosin (H&E), according to standard protocols.
Slides were examined microscopically, and the following parameters were reported: histologic type, Gleason patterns and grade group, tumor quantification, presence/absence of intraductal carcinoma, extraprostatic extension, gross bladder neck invasion, seminal vesicle invasion, lymphovascular invasion, perineural invasion, tumor invasion of other adjacent structures, and margin status. We recorded the total number of nodes, the number of positive nodes, and the presence of extranodal extension per regional node packet.
Data were extracted from our prospectively maintained database; medical records were used as a supplemental source. In addition to the primary outcome, lymph node metastasis, secondary outcomes included the distribution of positive nodes, 90-day Clavien complications, recurrence (defined as PSA > 0.2 ng/mL) or PSA > 0.02 ng/mL, and receipt of salvage treatment at any time post-surgery. Recurrence included persistent disease (i.e., PSA never reaching undetectable levels). We determined the distribution of positive nodes; those that could not be placed into a specific anatomic location (i.e., “right obturator” vs. “right iliac”) were not included in the more specific group but were included in the broader grouping (i.e., “combined ELND nodes”). Other data collected included robot time, estimated blood loss (EBL), margin status, and length of stay (LOS). Extracted data also included age at RARP/PLND, ethnicity, body mass index (BMI), pre-operative PSA, clinical stage, NCCN risk classification, pathologic stage and grade group, receipt of neoadjuvant androgen deprivation therapy (ADT), and the number of lymph nodes removed.
We compared the extended vs. super-extended groups on continuous measures using Wilcoxon Ranked Sum tests and t-tests as appropriate to their underlying distributions. Wilcoxon Ranked Sum tests were used for ordinal variables. Chi-square tests of proportion, Fisher’s Exact or Fisher-Freeman-Halton, were used for categorical measures depending on a number of categories and cell frequencies. Clavien classification was analyzed ordinally, with grades I through V (differentiating III a vs. b) and a seventh value of 0 added to denote patients with no complications. The associations between the extent of lymph node dissection (ELND vs. sELND) and major outcomes, detection of lymph node metastasis, complications, and cancer recurrence were analyzed via logistic regression, controlling for confounds identified through univariate analyses. For the logistic regression, pre-operative PSA was categorized into <4 ng/mL, 4–10 ng/mL vs. >10 ng/mL; stage was dichotomized into ≤pT2 vs. >pT2; grade group was dichotomized into ≤ grade group 2 vs. >2; and NCCN risk was dichotomized into <high-risk vs. ≥high-risk. Clinical T stage was dichotomized into one vs. greater than one. We used Kaplan Meier and Cox regression to compare the two PLND groups on time to recurrence with Cox controlling for possible confounds. Subgroup analysis on the rate of recurrence between groups was performed on patients with NCCN unfavorable intermediate or worse disease, as there were no patients with low or favorable intermediate disease in the sELND group. Data were compiled in Excel. SPSS version 26 (IBM, Armonk, NY, USA) was used for all analyses. A p value < 0.05 was used to indicate statistical significance.
Pre-operative characteristics (Table 1). The sELND (n = 180) and ELND (n = 540) groups differed significantly on PSA, clinical stage, and NCCN risk classification before surgery and on rates of neoadjuvant ADT. We reviewed the charts of 22 patients who received neoadjuvant ADT: 3 received it to shrink a very large prostate prior to surgery, 2 received it due to previous initiation of ADT by a past provider with no obvious clinical indication; the remaining 17 received ADT based on the preliminary reports from the Cancer and Leukemia Group B (CALGB) 90203 trial12 (neoadjuvant ADT/docetaxel) or pivoted from ADT/radiation (XRT) to surgery before receiving XRT. No other pre-operative differences were observed.
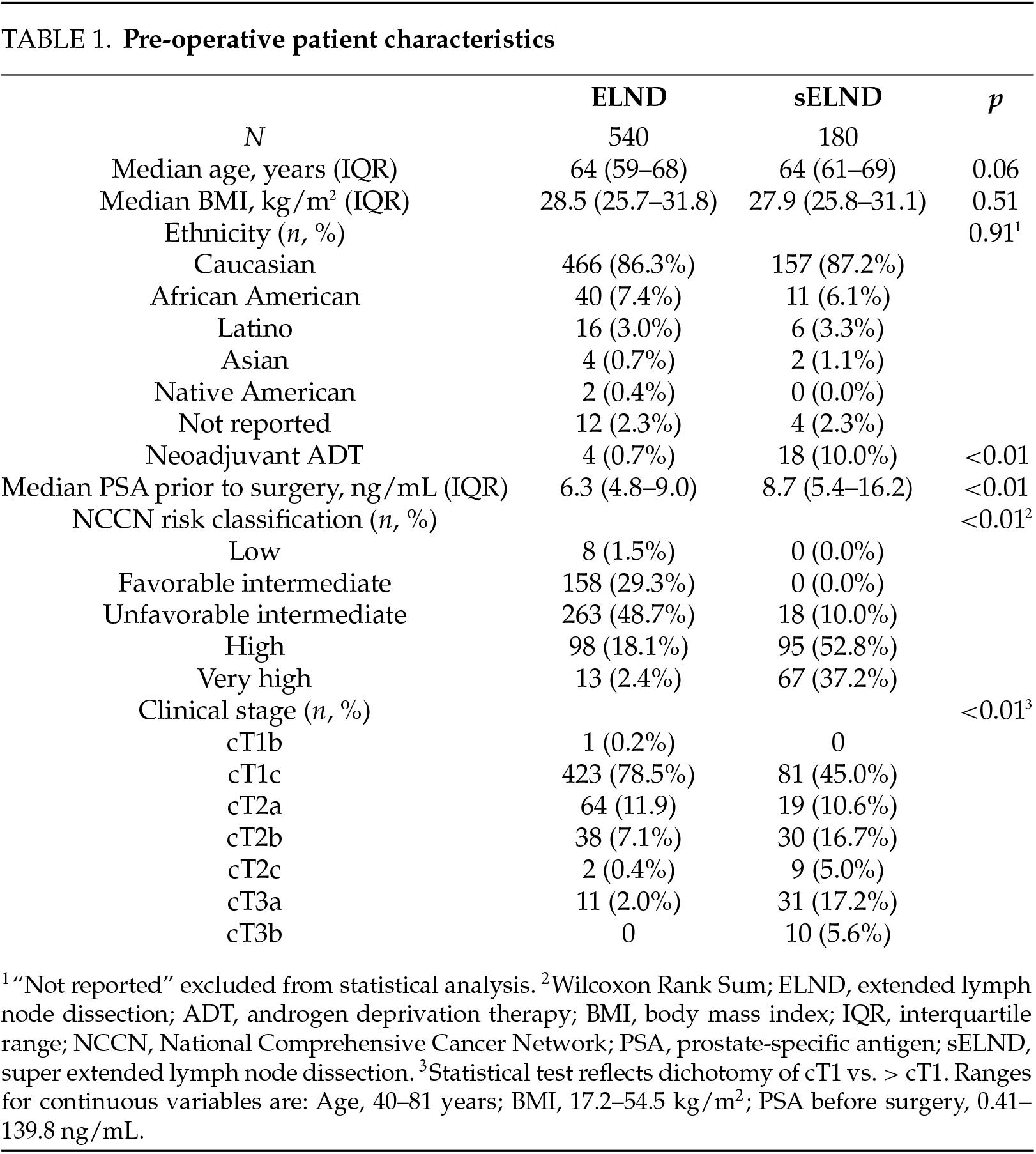
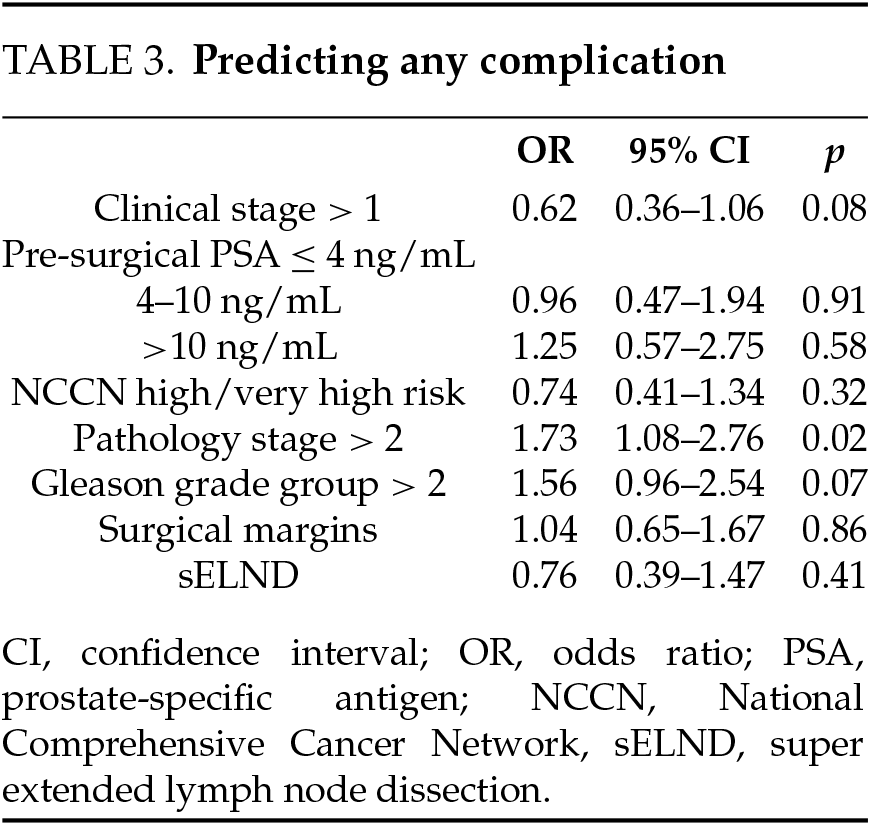
Peri/post-operative characteristics (Table 2). Time on robot was significantly longer for the super-extended group; EBL was lower. Significant differences were observed on the pathology stage, pathology grade group, and surgical margins, with worse disease more frequent in the sELND group. No differences were observed on length of stay (LOS) or any complication-related variable. Higher pathologic T stage (OR = 1.73; 95% CI 1.08–2.76; p = 0.02) was significantly associated with higher rates of any complication (Table 3). No associations were observed between pathology stage, grade group, pre-operative PSA, NCCN category with Clavien IIIa or higher complications.
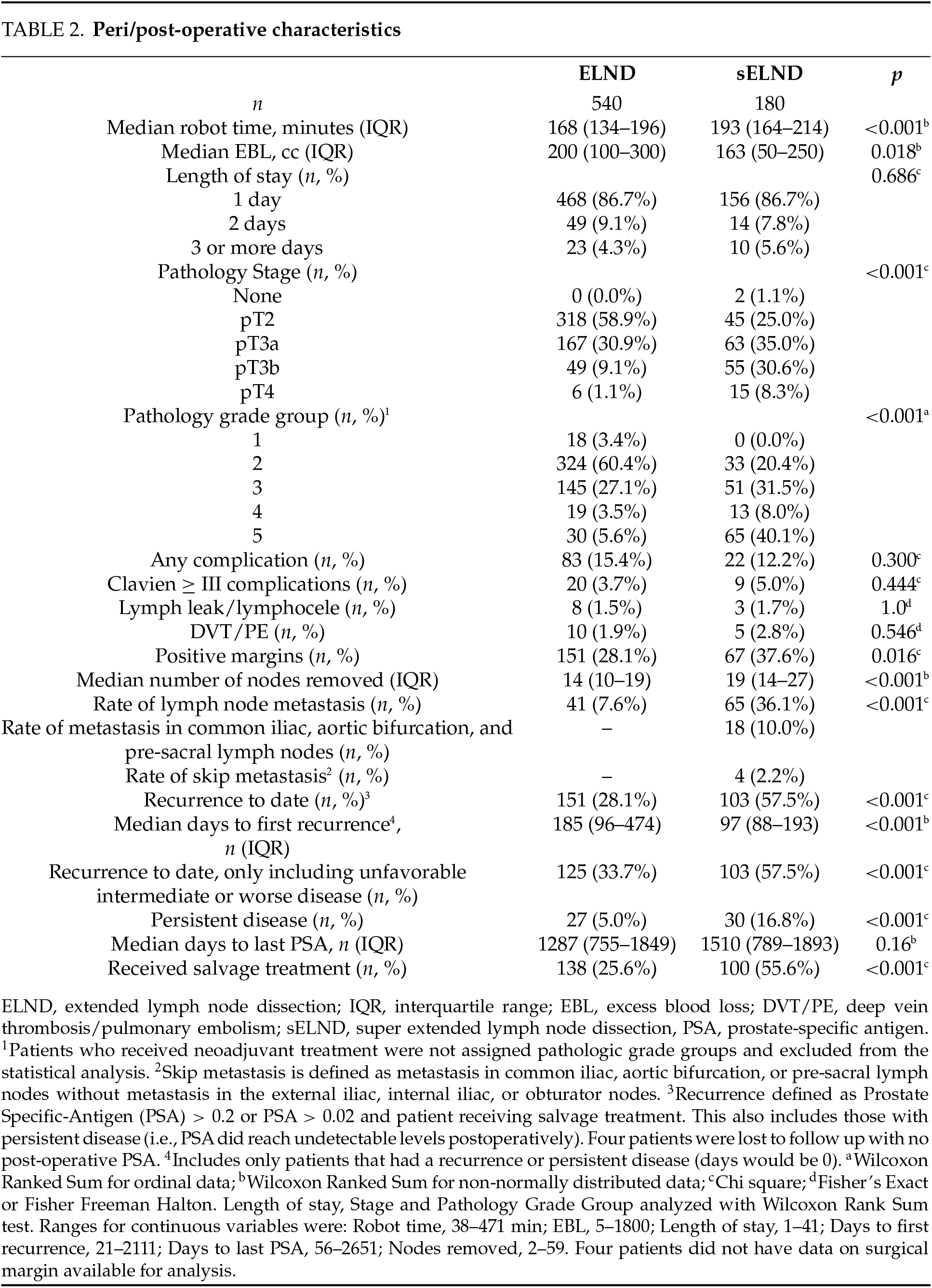
Lymph node metastasis detection. The super-extended group had significantly higher node positivity (36.1% vs. 7.6%, p < 0.001) (Table 2). The median number of nodes removed was significantly higher for the super-extended group (19 vs. 14, p < 0.001). Figure 1 depicts the percentage of patients with node positivity in each template location: 18 patients (10.0%) in the super-extended group had node positivity in the aortic bifurcation, common iliac, or pre-sacral chains that would have been missed with an ELND; 4 of these patients (2.2%) had node positivity uniquely in these chains with negative nodes in the obturator and the external and internal iliac areas.
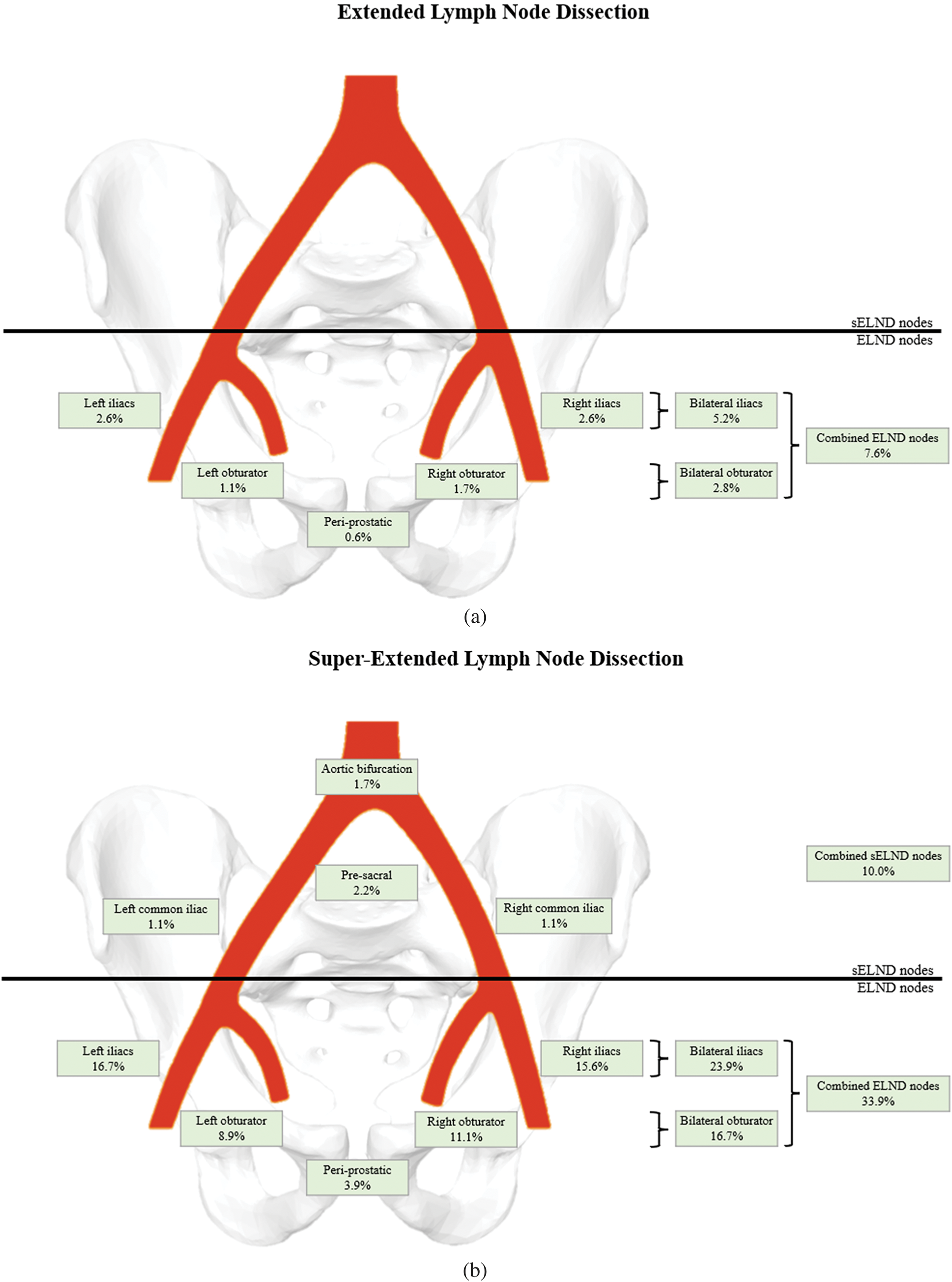
FIGURE 1. Percentage of patients with positive nodes in various anatomic locations for each group (a. Extended lymph node dissection; b. Super-extended lymph node dissection)
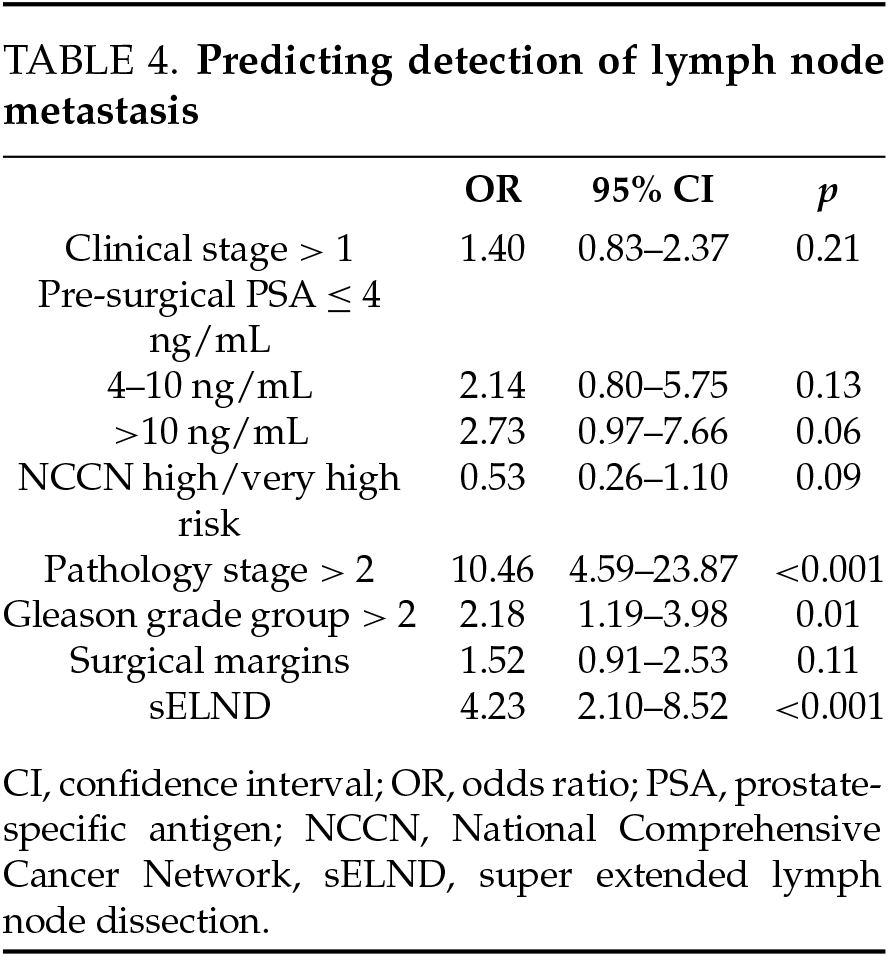
In multivariate analysis, the extent of the LND was a significant predictor of lymph node involvement independent of pathology stage, clinical stage, grade group, pre-operative PSA, NCCN category, and margin status. Lymph node involvement was more likely to be detected in the super-extended group (OR = 4.23 (2.10–8.52); 95% CI, 2.10–8.52; p < 0.001) (Table 4). Gleason grade group > 2, pathologic T stage > 2, and pre-operative PSA > 10 were also independent predictors of lymph involvement.
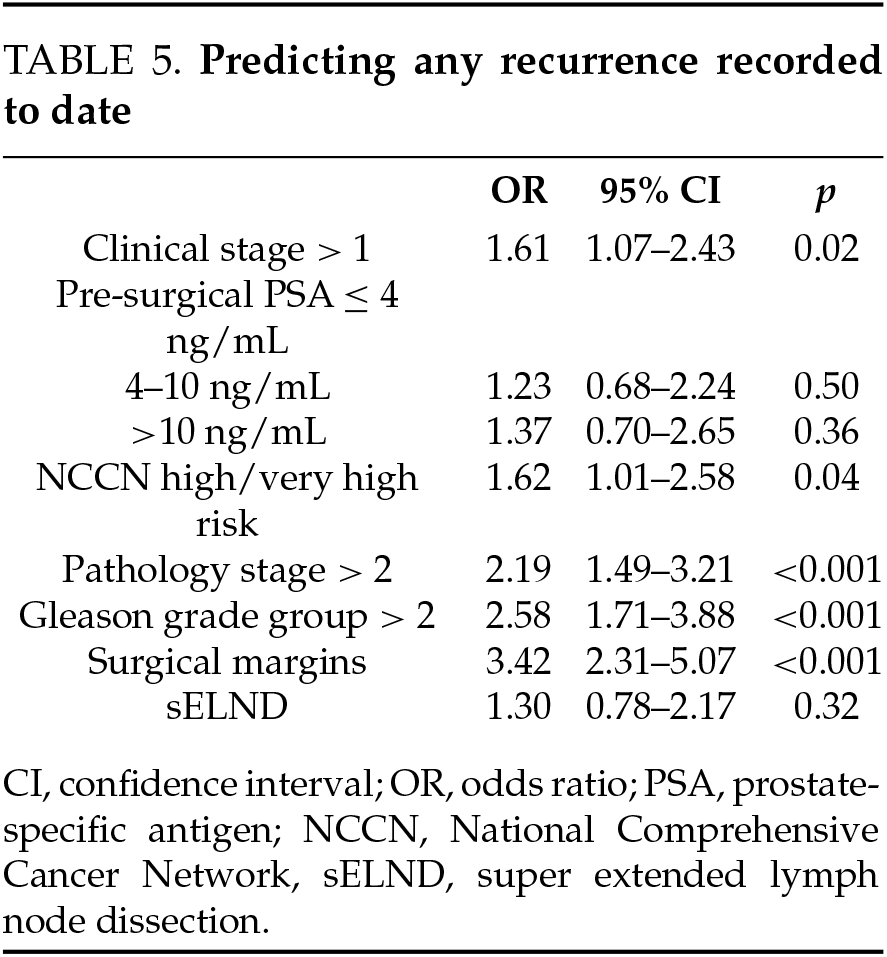
Disease recurrence (Table 2). The median length of follow-up was 3.7 years; 4 patients were lost to follow-up. During this time, the sELND group had significantly higher rates of recurrence (57.5% vs. 28.1%; p < 0.001) and of persistent disease and salvage treatment. After accounting for the pathology stage, clinical stage, grade group, pre-operative PSA, NCCN category, and margin status, the extent of lymph node dissection was not a significant predictor of recurrence (Table 5). In subgroup analysis, the extent of lymph node dissection was still not significant when only patients with unfavorable intermediate disease or worse were included.
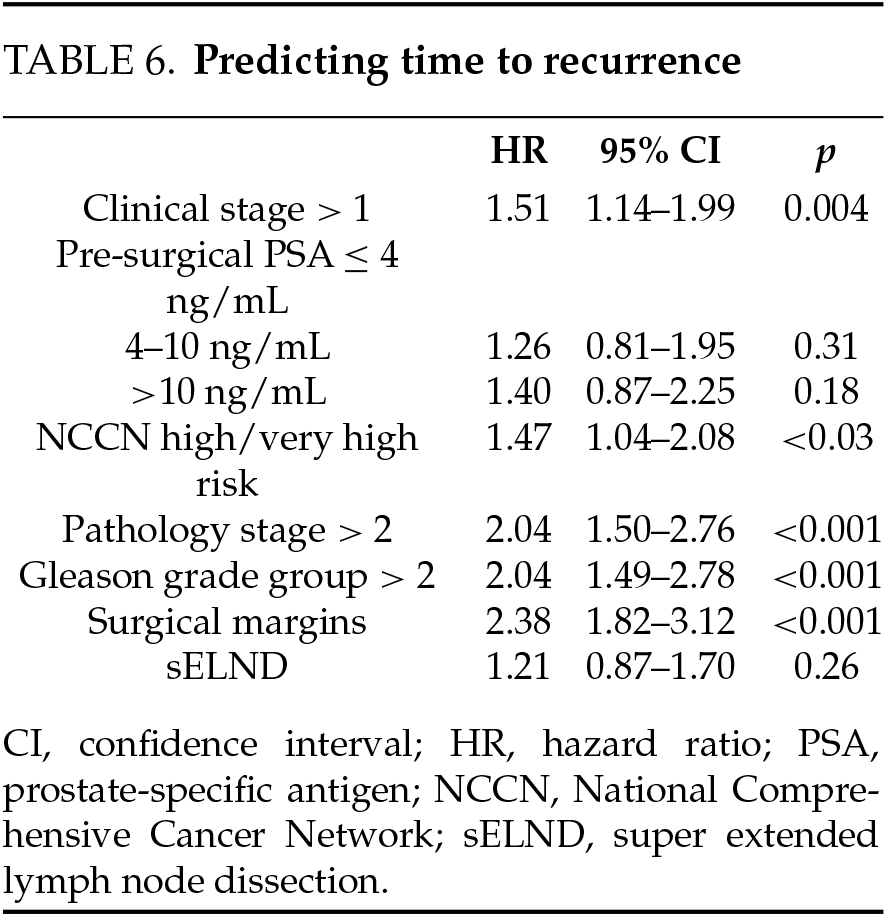
Time to recurrence. Among those who recurred, recurrence occurred earlier in the sELND group (median 97 days vs. 185 days; p < 0.001). The extent of dissection did not predict time to recurrence when other factors were included in multivariate Cox Regression. Clinical T stage, pathologic Gleason grade group > 2, pathologic T stage > 2, and NCCN ≥ high-risk and positive surgical margins were independent predictors of time to recurrence (Table 6).
The importance of extended lymph node dissection, for both prognostication and therapeutic benefit, is a subject of debate in the context of many urologic malignancies. Randomized trials are currently examining the clinical benefit of ELND for prostate cancer, and we eagerly await results.13 A similar discussion swirls around the appropriate anatomic boundaries and benefit of PLND as a component of bladder cancer treatment and whether node dissection up to the inferior mesenteric artery has a therapeutic benefit for those with this condition.14 Given that the lymphatic drainage of the prostate is similar to the bladder, sELND may hold some prognostic or therapeutic benefit for prostate cancer.
Since the benefit of sELND for prostate cancer is unclear, it is important to understand the impact that the procedure may have on morbidity. Contrary to some previous reports3,4,9, we did not see higher rates of Clavien
Given the practice of offering sELND to those in higher NCCN risk categories, it is not surprising that sELND had a much higher rate of node positivity compared to ELND (36.1% vs. 7.6%, p < 0.001). Independent of the relative disease profiles, our findings suggest the detection of lymph node metastasis was improved in the sELND group. 10.0% of patients in the super-extended group had node positivity in the aortic bifurcation, the common iliac, or the pre-sacral chains, which would have been missed by ELND. Perhaps even more importantly, 2.2% of patients in the super-extended group had metastasis uniquely outside the ELND template and would have had reported no lymph node metastasis (pN0) by ELND had sELND not been performed.
We compared two groups of patients: those in which sELND found an increased number/volume of positive nodes compared to ELND (10.0%) and those in which sELND found nodal metastasis that would have been missed by ELND (2.2%). In the first group, although lymph node positive status (pN+) or pN0 remains unchanged, the volume of positive nodes may change adjuvant management. Furthermore, one could argue that the involvement of the common iliac and aortic bifurcation lymph nodes represents disease outside the pelvis with upstaging to pM1a. These patients might be considered for metastatic disease protocols.
In their investigation of adjuvant radiotherapy in patients with prostate cancer, Abdollah et al. demonstrated that men with 3–4 positive lymph nodes and those with 2 or fewer positive lymph nodes (with Gleason 7 to 10, stage pT3b/pT4 or positive surgical margins) benefit from the addition of adjuvant radiotherapy to ADT.15 Additionally, current guidelines suggest that patients designated pN1 by ELND be offered observation when two or fewer nodes show microscopic involvement with a PSA < 0.1 ng/mL in the absence of extranodal extension.1 Thus, the volume of positive nodes determined by PLND may impact treatment decisions, particularly using three or more positive nodes as a cut-off. Therefore, extending the dissection to sELND may allow the surgeon to better quantify nodal status and improve adjuvant management. Current positive node guidelines may need to be adjusted when sELND templates are applied. In the second group, some patients are upstaged from pN0 to pN+ by sELND. Given the more appropriate designation of pN1 by sELND, adjuvant androgen deprivation therapy and radiation therapy should be discussed with these men.1
We observed significantly higher rates of recurrence in the sELND group and noted that sELND significantly predicted time to recurrence. After controlling for covariates relating to worse disease in the sELND group, the extent of the lymph node dissection was no longer a significant predictor of recurrence. Regardless, the retrospective nature of our study makes it difficult to comment on the therapeutic benefits of a sELND. Previous studies have investigated outcomes as they relate to the extent of lymph node dissection. Abdollah et al. have demonstrated that removal of more lymph nodes during RP is significantly associated with cancer-specific survival and that the benefits of ELND may not appear until 20–30 months of follow-up.7 A therapeutic benefit of ELND compared to limited PLND was also demonstrated by Bivalacqua et al., who noted improved oncologic outcomes at 10 years in patients undergoing ELND.16 Additionally, Dursun et al. studied 103,250 patients with intermediate and high-risk prostate cancer using the number of excised nodes as a proxy for the extent of lymph node dissection.17 They found that the removal of 20 or more nodes in high-risk patients was associated with better survival outcomes after a minimum of 5-year follow-up. As previously discussed, node dissection does not always predict better outcomes. Preisser et al. failed to note a difference in oncologic outcomes at 120 months when they compared intermediate/high-risk prostate cancer patients who underwent RP with PLND to those who underwent RP without PLND.5 When Droghetti et al. used a radiotracer to visualize sentinel lymph nodes in the prostate before RP.18 They noted that 55.8% of patients had at least one sentinel node outside of an extended dissection template. Widening the dissection to include those nodes improved oncologic outcomes. It is not clear if any of these findings can be extrapolated to sELND. Prospectively enrolling patients into studies with longer follow-up measurements is needed to better understand the therapeutic benefit of sELND.
Future work should also aim to identify the most appropriate candidates for sELND. At present, there is no imaging technique that can accurately identify patients who will benefit from PLND; the optimal candidacy is instead determined by clinical characteristics.19,20 Novel models continue to be developed, including those that take into account multiparametric magnetic resonance imaging (MRI) findings and MRI-targeted biopsy results.21–23 Other proposed models support improved selection with the inclusion of prostate-specific membrane antigen (PSMA) positron emission tomography (PET).24 As PSMA PET was not widely available in our institution between 2016 and 2021, it is unclear what impact modern imaging would have on our results. Additionally, as artificial intelligence (AI) becomes more routinely integrated into clinical practice, this may also help contribute to patient selection. AI-identified histologic features from biopsy specimens have been correlated with prognostic variables, such as biochemical recurrence.25 Accurately determining the population of patients who would benefit most from sELND is an important step to implementing this procedure.
This study had several limitations. First, patients were not randomized to extended vs. super-extended groups. As the treatment decision was arrived at through shared decision-making, bias could have been present on the part of the surgeon and the patient. The sELND group inherently included patients with more serious diseases, predisposing them to recurrence and complicating analyses regarding the therapeutic benefits of sELND. Second, this study involved one institution and included cases performed by a single surgeon. Thus, the findings may not be generalizable to the entire population undergoing sELND. The future generalizability of results from investigations into the use of sELND could be improved by implementing a standardized sELND technique, such as a monoblock.26
We observed that 2.2% of patients with lymph node positivity in the super-extended group would have been designated pN0 if the dissection was limited to the extended template and that 10.0% of patients had node positivity outside of the standard extended template. This study supports the safety and feasibility of sELND for patients with high-risk prostate cancer. Further investigation should be undertaken to establish the clinical benefit of the procedure and to further inform optimal patient selection.
Acknowledgement
Not applicable.
Funding Statement
The authors received no specific funding for this study.
Author Contributions
The authors confirm contribution to the paper as follows: Conceptualization, Joseph Wagner, Ryan Daigle, Ilene Staff; methodology, Joseph Wagner, Ryan Daigle, Ilene Staff, Jonathan Earle, Joseph Tortora, Kevin Pinto, Rosa Negron; formal analysis, Ilene Staff, Jonathan Earle; investigation, Ryan Daigle, Ilene Staff; data curation, Joseph Tortora, Kevin Pinto, Rosa Negron; writing—original draft preparation, Ryan Daigle; writing—review and editing, Ryan Daigle, Ilene Staff, Tara McLaughlin Proto, Joseph Tortora, Kevin Pinto, Rosa Negron; visualization, Ryan Daigle, Ilene Staff; supervision, Joseph Wagner, Tara McLaughlin Proto; project administration, Joseph Wagner, Tara McLaughlin Proto. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
Data are available upon request.
Ethics Approval
This project was approved by the Hartford HealthCare Institutional Review Board with a waiver of informed consent (E-HHC-2019-0190).
Conflicts of Interest
Joseph Wagner is a consultant for Covidien and participates on the speaker’s bureau for Genomic Health.
References
1. National Comprehensive Cancer Network. Prostate Cancer (Version 4. 2022). Plymouth Meeting; PA, USA:NCCN; 2022. [Google Scholar]
2. Mandel P, Kriegmair MC, Bogdan K et al. Association between lymph node counts and oncological outcomes in lymph node positive prostate cancer. Eur Urol Focus 2017;3(2–3):248–255. doi:10.1016/j.euf.2016.02.018. [Google Scholar] [PubMed] [CrossRef]
3. Fossati N, Willemse PM, Van den Broeck T et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: a systematic review. Eur Urol 2017;72(1):84–109. doi:10.1016/j.eururo.2016.12.003. [Google Scholar] [PubMed] [CrossRef]
4. Chopra S, Alemozaffar M, Gill I, Aron M. Extended lymph node dissection in robotic radical prostatectomy: current status. Indian J Urol 2016;32(2):109–114. doi:10.4103/0970-1591.163303. [Google Scholar] [PubMed] [CrossRef]
5. Preisser F, van den Bergh RCN, Gandaglia G et al. Effect of extended pelvic lymph node dissection on oncologic outcomes in patients with D’Amico intermediate and high risk prostate cancer treated with radical prostatectomy: a multi-institutional study. J Urol 2020;203(2):338–343. doi:10.1097/JU.0000000000000504. [Google Scholar] [PubMed] [CrossRef]
6. Lestingi JFP, Guglielmetti GB, Trinh QD et al. Extended versus limited pelvic lymph node dissection during radical prostatectomy for intermediate-and high-risk prostate cancer: early oncological outcomes from a randomized phase 3 trial. Eur Urol 2021;79(5):595–604. doi:10.1016/j.eururo.2020.11.040. [Google Scholar] [PubMed] [CrossRef]
7. Abdollah F, Gandaglia G, Suardi N et al. More extensive pelvic lymph node dissection improves survival in patients with node-positive prostate cancer. Eur Urol 2015;67(2):212–219. doi:10.1016/j.eururo.2014.05.011. [Google Scholar] [PubMed] [CrossRef]
8. Chenam A, Ruel N, Pal S et al. Biochemical recurrence after robot-assisted extended pelvic lymphadenectomy for prostate cancer. Can J Urol 2018;25(3):9340–9348. [Google Scholar] [PubMed]
9. Briganti A, Chun FK, Salonia A et al. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur Urol 2006;50(5):1006–1013. doi:10.1016/j.eururo.2006.08.015. [Google Scholar] [PubMed] [CrossRef]
10. Mistretta FA, Boeri L, Grasso AA et al. Extended versus standard pelvic lymphadenectomy during robot-assisted radical prostatectomy: the role of extended template as an independent predictor of lymph node invasion with comparable morbidity burden. Minerva Urol Nefrol 2017;69(5):475–485. doi:10.23736/S0393-2249.17.02838-7. [Google Scholar] [PubMed] [CrossRef]
11. Gandaglia G, Zaffuto E, Fossati N et al. Identifying candidates for super-extended staging pelvic lymph node dissection among patients with high-risk prostate cancer. BJU Int 2018;121(3):421–427. doi:10.1111/bju.14066. [Google Scholar] [PubMed] [CrossRef]
12. Eastham JA, Heller G, Halabi S et al. CALGB 90203 (allianceradical prostatectomy (RP) with or without neoadjuvant chemohormonal therapy (CHT) in men with clinically localized, high-risk prostate cancer (CLHRPC). J Clin Oncol 2019;37(15_suppl):5079. doi:10.1200/JCO.2019.37.15_suppl.5079. [Google Scholar] [CrossRef]
13. Benfante N, Carroll E, Carruthers J et al. A randomized trial on pelvic lymph node dissection versus no lymph node dissection at radical prostatectomy: report of a trial in progress. J Clin Oncol 2022;40(16_suppl):TPS5116. doi:10.1200/JCO.2022.40.16_suppl.TPS5116. [Google Scholar] [CrossRef]
14. Gschwend JE, Heck MM, Lehmann J et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur Urol 2019;75(4):604–611. doi:10.1016/j.eururo.2018.09.047. [Google Scholar] [PubMed] [CrossRef]
15. Abdollah F, Karnes RJ, Suardi N et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol 2014;32(35):3939–3947. doi:10.1200/JCO.2013.54.7893. [Google Scholar] [PubMed] [CrossRef]
16. Bivalacqua TJ, Pierorazio PM, Gorin MA, Allaf ME, Carter HB, Walsh PC. Anatomic extent of pelvic lymph node dissection: impact on long-term cancer-specific outcomes in men with positive lymph nodes at time of radical prostatectomy. Urology 2013;82(3):653–658. doi:10.1016/j.urology.2013.03.086. [Google Scholar] [PubMed] [CrossRef]
17. Dursun F, Elshabrawy A, Wang H et al. Impact of extent of lymphadenectomy on all-cause mortality in patients with intermediate-and high-risk prostate cancer managed with radical prostatectomy. In: 22nd Annual Meeting of the Society of Urologic Oncology; December 1–3, 2021; Orlando, FL, USA. [Google Scholar]
18. Droghetti M, Ozman O, Berrens AC et al. Location-based oncological outcomes of sentinel node dissection in radical prostatectomy. J Urol 2024;212(3):409–419. doi:10.1097/JU.0000000000004051. [Google Scholar] [PubMed] [CrossRef]
19. Sierra PS, Lestingi JFP, Albuquerque EV et al. Robot-assisted extended pelvic lymph node dissection in prostate cancer. When and how? Arch Esp Urol 2019;72(3):257–265. [Google Scholar] [PubMed]
20. Tafuri A, Rizzetto R, Amigoni N et al. Predictors of lymph node invasion in patients with clinically localized prostate cancer who undergo radical prostatectomy and extended pelvic lymph node dissection: the role of obesity. Urol Int 2021;105(5–6):362–369. doi:10.1159/000510008. [Google Scholar] [PubMed] [CrossRef]
21. Gandaglia G, Ploussard G, Valerio M et al. A novel nomogram to identify candidates for extended pelvic lymph node dissection among patients with clinically localized prostate cancer diagnosed with magnetic resonance imaging-targeted and systematic biopsies. Eur Urol 2019;75(3):506–514. doi:10.1016/j.eururo.2018.10.012. [Google Scholar] [PubMed] [CrossRef]
22. Gandaglia G, Martini A, Ploussard G et al. External validation of the 2019 briganti nomogram for the identification of prostate cancer patients who should be considered for an extended pelvic lymph node dissection. Eur Urol 2020;78(2):138–142. doi:10.1016/j.eururo.2020.03.023. [Google Scholar] [PubMed] [CrossRef]
23. Draulans C, Everaerts W, Isebaert S et al. Development and external validation of a multiparametric magnetic resonance imaging and international society of urological pathology based add-on prediction tool to identify prostate cancer candidates for pelvic lymph node dissection. J Urol 2020;203(4):713–718. doi:10.1097/JU.0000000000000652. [Google Scholar] [PubMed] [CrossRef]
24. Ferraro DA, Muehlematter UJ, Garcia Schüler HI et al. 68Ga-PSMA-11 PET has the potential to improve patient selection for extended pelvic lymph node dissection in intermediate to high-risk prostate cancer. Eur J Nucl Med Mol Imaging 2020;47(1):147–159. doi:10.1007/s00259-019-04511-4. [Google Scholar] [PubMed] [CrossRef]
25. Marletta S, Eccher A, Martelli FM et al. Artificial intelligence-based algorithms for the diagnosis of prostate cancer: a systematic review. Am J Clin Pathol 2024;161(6):526–534. doi:10.1093/ajcp/aqad182. [Google Scholar] [PubMed] [CrossRef]
26. Mattei A, Würnschimmel C, Baumeister P et al. Standardized and simplified robot-assisted superextended pelvic lymph node dissection for prostate cancer: the monoblock technique. Eur Urol 2020;78(3):424–431. doi:10.1016/j.eururo.2020.03.032. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools