 Open Access
Open Access
REVIEW
Botulinum toxin A in idiopathic overactive bladder: a narrative review of 5410 cases
Department of Urology A, Ibn Sina University Hospital, Bettouga Avenue, Rabat, 10000, Morocco
* Corresponding Author: Salim Lachkar. Email:
Canadian Journal of Urology 2025, 32(3), 145-165. https://doi.org/10.32604/cju.2025.064912
Received 27 February 2025; Accepted 23 April 2025; Issue published 27 June 2025
Abstract
Introduction: When conservative treatments fail, botulinum toxin A (BoNT-A) is an option for refractory idiopathic overactive bladder (OAB). This review evaluates the efficacy, safety, and predictive factors for BoNT-A in this situation. Material and Methods: A literature search up to January 2025 was performed using PubMed, Google Scholar, and Embase to assess efficacy, safety, and predictors of adverse events (AE) related to BoNT-A. The risk of bias was assessed using the Risk of Bias 2 (RoB 2) tool for randomized studies and the Critical Appraisal Skills Programme (CASP) checklist for cohort studies. The quality of the review was evaluated based on the Oxford criteria, following the Strengthening the Assessment of Narrative Review Articles (SANRA) guidelines, and by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for systematic reviews. Results: 31 studies were included, involving 5410 patients. BoNT-A improves OAB symptoms even after reinjections. Higher doses do not enhance efficacy but increase AE. AE includes high post-void residual (PVR), clean intermittent self-catheterization (CISC), and Urinary Tract Infection (UTI). Predictors of CISC include age, male gender, hysterectomy, ≥3 vaginal deliveries, mixed incontinence, prior mid-urethral sling (MUS), high PVR, low Pressure at Pdet at First Micturition (PIP1) in women, low Bladder Compliance Index (BCI) in men, and high Bladder Outlet Obstruction Index (BOOI). Diabetes and heart failure increase PVR. UTIs are more frequent in women and men with benign prostatic hyperplasia, with CISC increasing the risk fivefold. Severe complications are rare. Predictors of poor response include male gender, high BOOI, low urinary flow, and diabetes. Discussion: BoNT-A is effective for OAB, especially for incontinence. AE is dose-dependent and limits treatment adherence. Their link with poor response remains unclear. Conclusion: BoNT-A effectively treats refractory idiopathic OAB, improving symptoms and quality of life with repeated injections.Keywords
Overactive bladder (OAB), defined by the International Continence Society (ICS), is characterized by urgency, with or without urinary incontinence (UI), frequent urination, or nocturia, without apparent pathology.1 The predominant cause is detrusor overactivity (DO), diagnosed urodynamically as involuntary detrusor contractions during bladder filling. DO manifests as neurogenic (associated with neurological conditions) and non-neurogenic (often termed idiopathic). Prevalent, overactive bladder (OAB) affects up to 16% of young adults, impacting quality of life and healthcare costs, with recognized risk factors including age, gender, menopause, and obesity.2
Primary OAB treatment includes pharmaceutical options like anticholinergics and beta-3 adrenergic receptor agonists when behavioral therapies fail. Anticholinergics, the gold standard, have high discontinuation rates due to side effects.3 When conservative therapies prove ineffective, minimally invasive treatments like posterior tibial nerve stimulation, sacral neuromodulation, and intradetrusor injections of botulinum toxin A (BoNT-A) can be considered. In 2013, BoNT-A received validation from the Food and Drug Administration4 for refractory idiopathic OAB management.
The existing literature on BoNT-A for refractory idiopathic OAB is highly heterogeneous,5 with inconsistent definitions of efficacy, poor response, and varied outcome measures.6 There is no standardized approach to assessing patient-reported outcomes (PROs) or quality of life,7 and follow-up intervals differ widely across studies,8 limiting the ability to compare results. These gaps hinder clear conclusions about the treatment’s effectiveness and risks.
This review provides an overview of the field, focusing on the efficacy, safety, and factors that may predict poor response and adverse events following BoNT-A treatment for refractory idiopathic OAB.
This analysis was conducted by the principles outlined in the Scale for the Assessment of Narrative Review Articles (SANRA) and the guideline recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A prospective protocol was developed before initiating this review to ensure a structured and systematic approach.
A comprehensive search was performed up to January 2025, using PubMed, Google Scholar, and Embase databases. No restrictions were applied regarding language or publication date. The research question was framed using the PICO methodology:
• P (Population): Adult patients with idiopathic OAB
• I (Intervention): BoNT-A
• C (Comparison): Placebo or other treatments (e.g., anticholinergics)
• O (Outcome): Improvement in symptoms of OAB, including voiding frequency, urgency, incontinence episodes, nocturia, and quality of life
To address this, Medical Subject Heading (MeSH) terms and relevant keywords were used individually to ensure a thorough search. The specific search terms included: “Botox” OR “botulinum toxin A”, “OAB” OR “idiopathic overactive bladder” OR “overactive bladder”, “effectiveness” OR “efficacy”, “side effect” OR “adverse effect”, “predictors”, “outcome”.
References from relevant studies were also manually screened to identify additional pertinent research. Once potential studies were identified, duplicate entries were removed, and the articles underwent an additional screening process to ensure they met the predefined criteria. Figure 1 shows the study selection flow chart based on PRISMA guidelines.
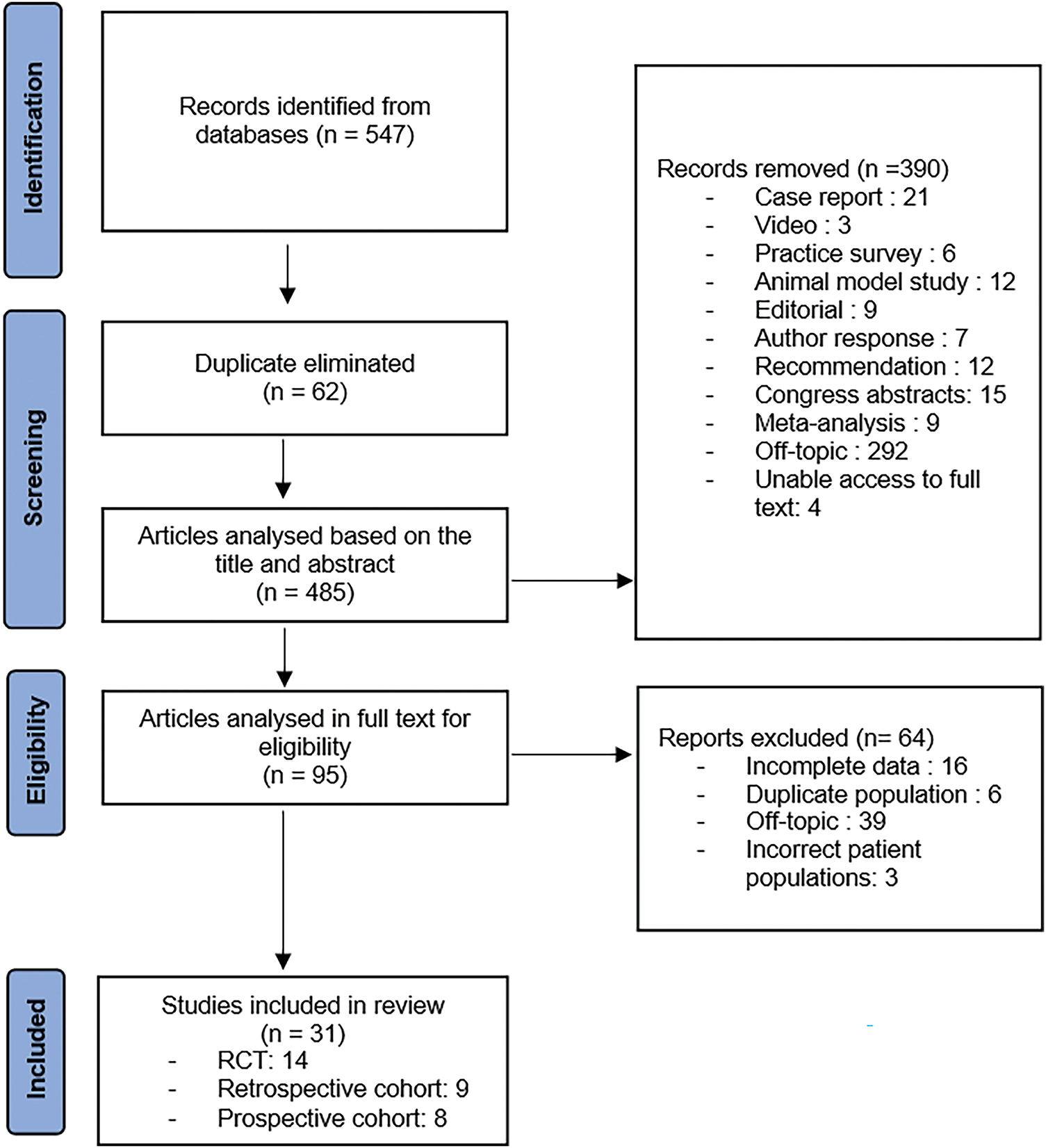
FIGURE 1. Study selection flow chart
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) Adults aged 18 years or older. (2) Patients with idiopathic overactive bladder. (3) Treatment with intradetrusor injections of BoNT-A, regardless of the dose. (4) Randomized controlled trials (RCTs), cohort studies, or case-control studies. (5) Comparisons including (a) Efficacy or AE of different doses of BoNT-A compared to placebo or (b) responses to BoNT-A, with a focus on either good and poor responders or comparisons between patients reporting AE and those who did not.
The exclusion criteria included: (1) Children. (2) Neurogenic overactive bladder. (3) Use of botulinum toxin other than type A. (4) Non-comparative studies, reviews, commentaries, conference abstracts, editorials, practice surveys, guidelines, and case reports.
The following outcome measures were assessed: efficacy of BoNT-A (symptom improvement and urodynamic criteria), adverse events AE (tolerance, need for self-catheterization, elevated post-void residual volume (PVR), and urinary tract infections (UTI)), poor response to BoNT-A, and predictive factors for AE.
Study selection and data extraction
The study selection process consisted of two phases. The first phase involved screening titles and abstracts to identify potentially relevant studies. In the second phase, a full-text review was conducted for selected articles. Two independent reviewers assessed eligibility based on predefined criteria, with any disagreements resolved through discussion or consultation with a third reviewer.
The data extracted included: study description (first author, year, type of study, sample size, toxin dose, injection method, inclusion criteria, and follow-up), efficacy outcome criteria, average variation in parameters (such as frequency, urinary incontinence, and urodynamics), AE, and their predictive factors.
We assessed the quality of the studies using the Oxford criteria (OCEBM Levels of Evidence).
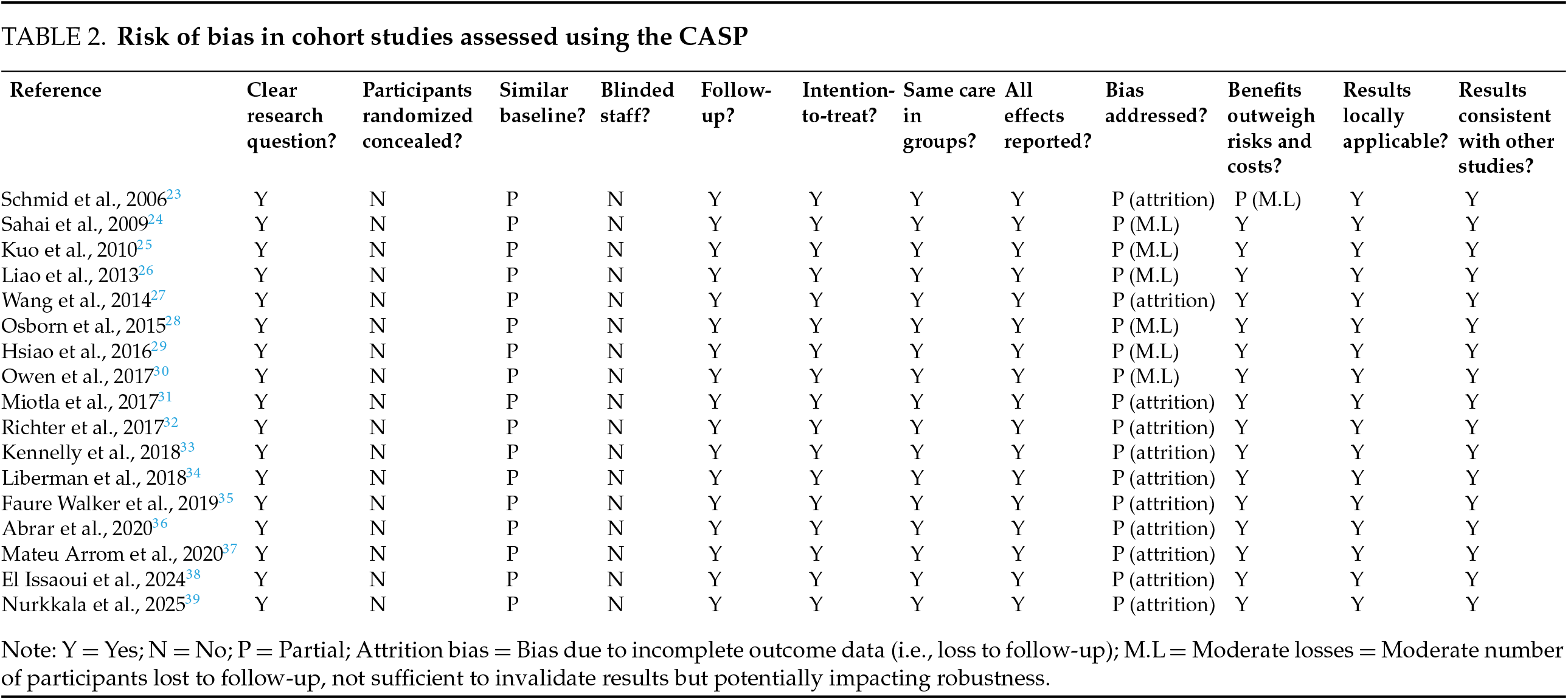
We used bias analysis using the Risk of Bias 2 (RoB 2) tool for randomized studies and the Critical Appraisal Skills Programme (CASP) checklist for cohort studies (Tables 1 and 2).
The extracted data were analyzed and presented in tabular and graphical formats. Descriptive statistics were employed for quantitative analysis. A comprehensive narrative synthesis was conducted to systematically summarize the findings from the included studies.
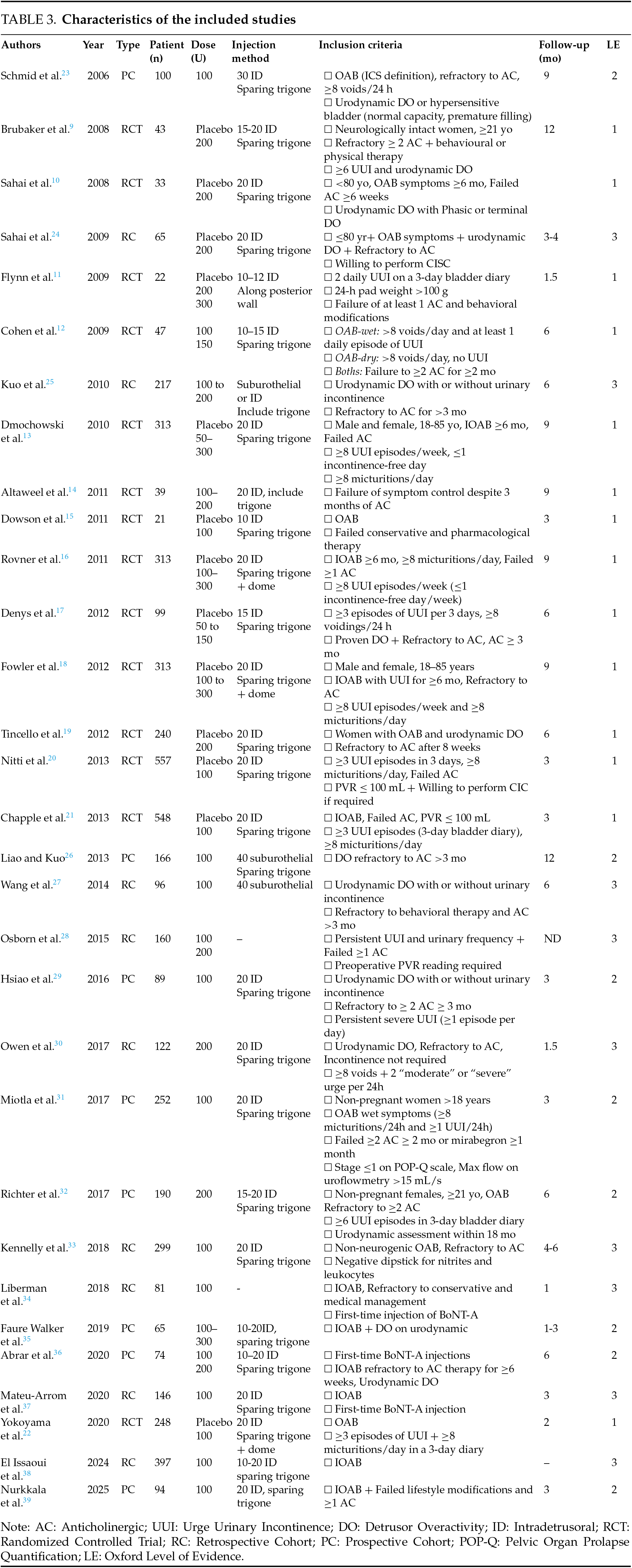
Characteristics of included studies
The initial search identified 547 articles across three databases. After removing 62 duplicates, 485 studies underwent title/abstract screening, resulting in the exclusion of 386 records. Four additional articles were excluded due to unavailability of full texts. Following full-text assessment of the remaining 95 articles, 64 studies were excluded based on eligibility criteria (Figure 1).
The final analysis included 31 studies: 14 randomized controlled trials (RCTs), 9 retrospective cohort studies (RCs), and 8 prospective non-randomized cohort studies (PCs), encompassing a total of 5410 patients. All studies were published in English between 2006 and 2025. The characteristics of the included studies are presented in Table 3.
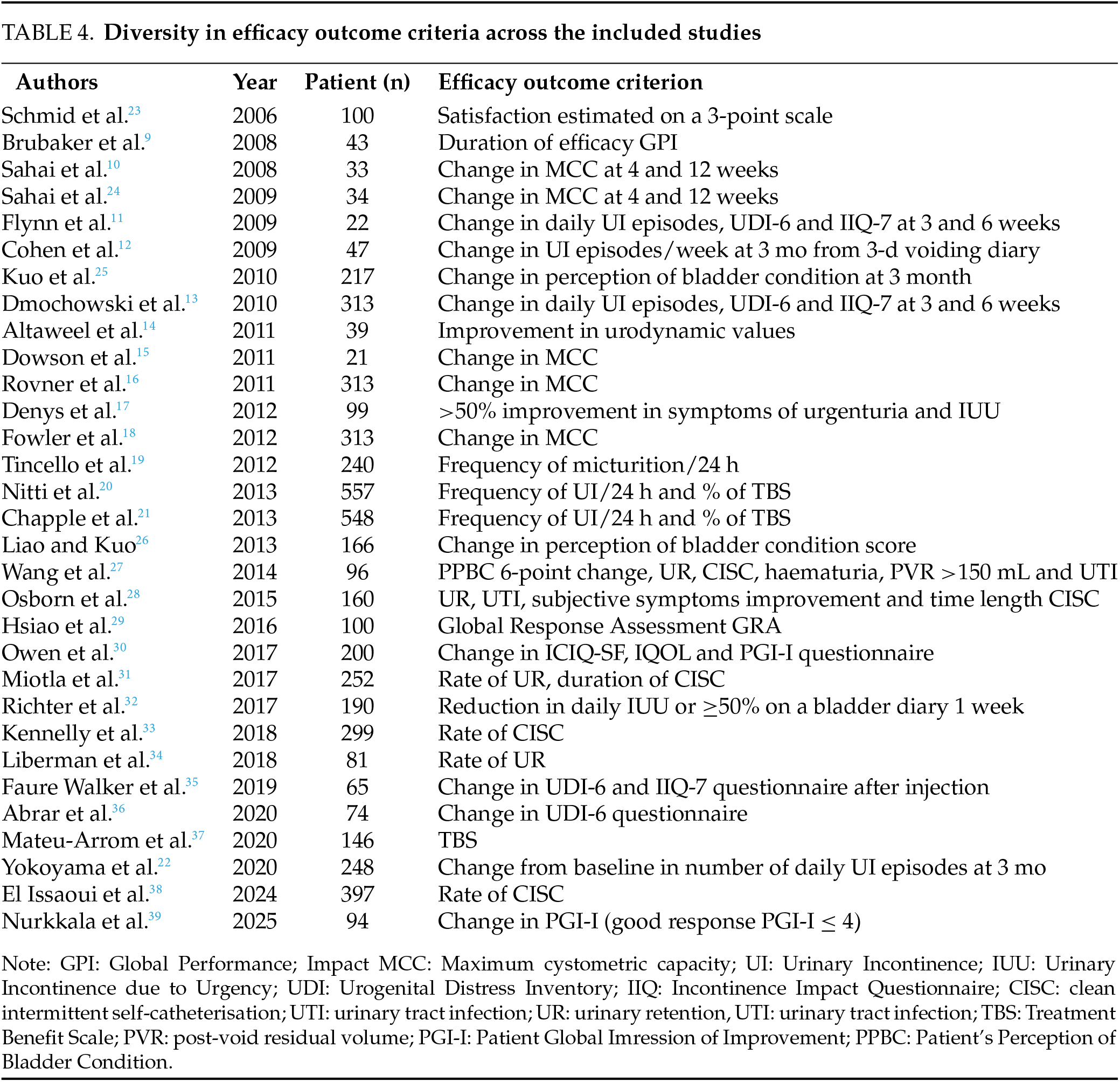
Variability in outcome measures
The 31 included studies evaluate the efficacy of BoNT-A injections. 25 studies use different scores to assess symptom improvement. This variability poses challenges. Urgency, a key symptom of OAB, remains difficult to assess objectively due to its subjective nature and patient variability. Patient expectations also influence treatment perceptions, with partial symptom relief sometimes perceived as failure if complete resolution was expected. 6 studies utilize urodynamic parameters to provide objective measurements. However, these assessments are invasive, poorly tolerated, and impractical for long-term monitoring due to BoNT-A’s temporary effects. This variability in assessment criteria introduces potential bias. Table 4 highlights these differences in outcome measures.
All studies demonstrate greater efficacy of BoNT-A at 3 months compared to placebo. Regarding voiding frequency, 16 studies report an average reduction of −2.55 micturitions per 24 h with BoNT-A across all doses, compared to −0.73 with placebo, with a maximum reduction of over –5 micturitions in 3 studies (Figure 2). For urinary incontinence (UI), 16 studies, show a reduction of −2.57 episodes per 24 h with BoNT-A, compared to 0.64 with placebo, with a maximum reduction of −4.5 episodes in Flynn11 and Tincello’s19 studies. Up to 25% of patients achieve complete continence (Figure 3). Additionally, BoNT-A improves nocturia, patient satisfaction, and overall quality of life.21 The effects of BoNT-A appear quickly, with urgency episodes typically reduced by the 8th day and peaking between the 2nd and 8th week.23,29 Long-term follow-up in Nitti20 and Chapple’s21 studies found an average efficacy duration of approximately 24 weeks with a 100U BoNT-A dose.
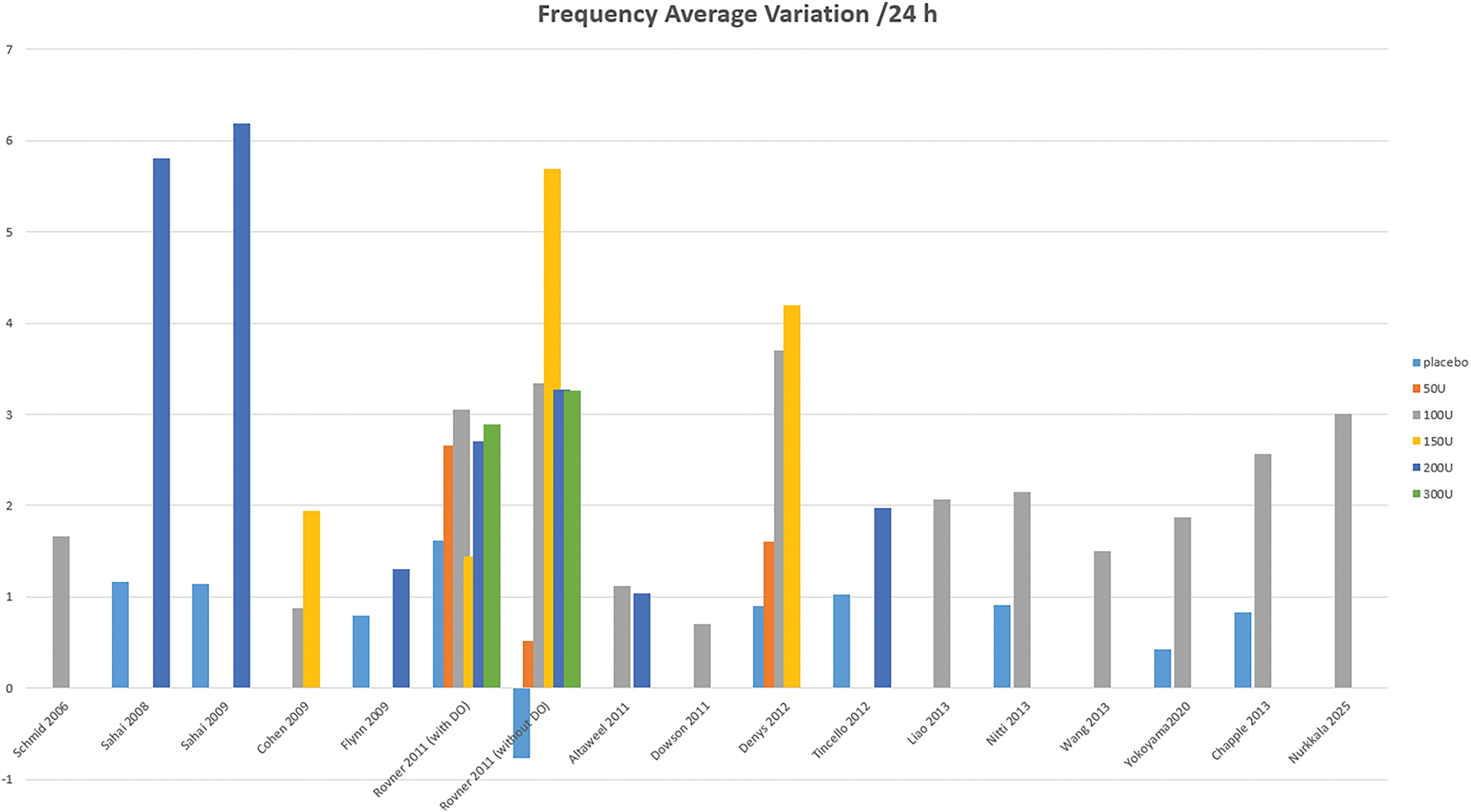
FIGURE 2. Variation of voiding frequency over a 24-h period
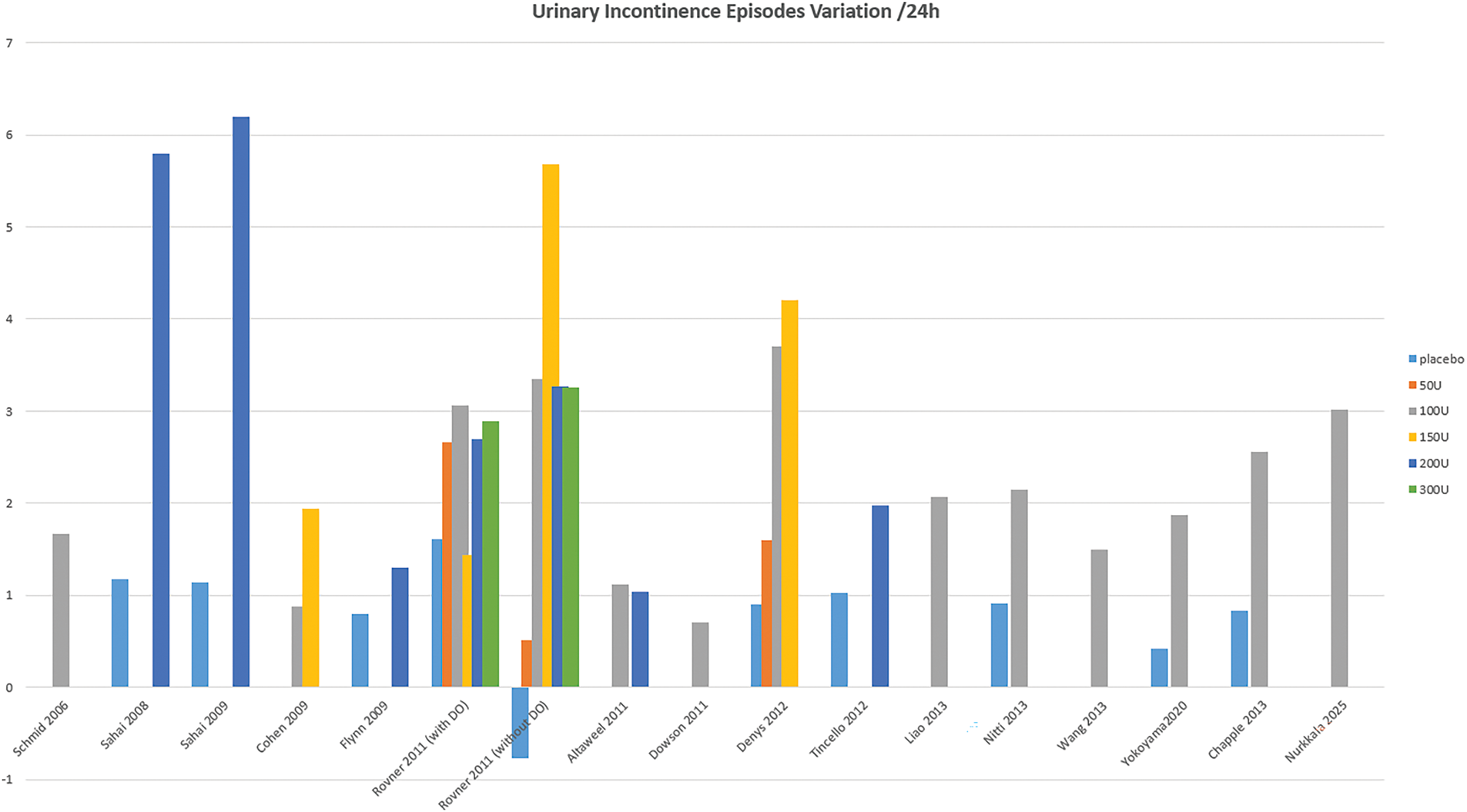
FIGURE 3. Variation in the frequency of urinary incontinence episodes over a 24-h period
Urodynamic changes post injection
9 studies assess changes in urodynamic parameters post-injection, with mixed results. Only 4 studies10,16,17,30 report a significant decrease in maximum detrusor pressure (Pdet max), averaging −10 cmH2O, although not for all doses. All studies show an increase in maximum cystometric capacity (MCC), ranging from +71 to +138 mL, though not consistently across all doses. The results for Volume at the first detrusor contraction (VFDC) vary: 3 studies10,16,32 report significant improvements (+23.1 to +59 mL), 2 show no significant change,14,15 and 2 find improvement only at higher doses (above 150U).10,16 Significant differences in uninhibited detrusor contractions are noted in 2 studies17,23 (Table 5).
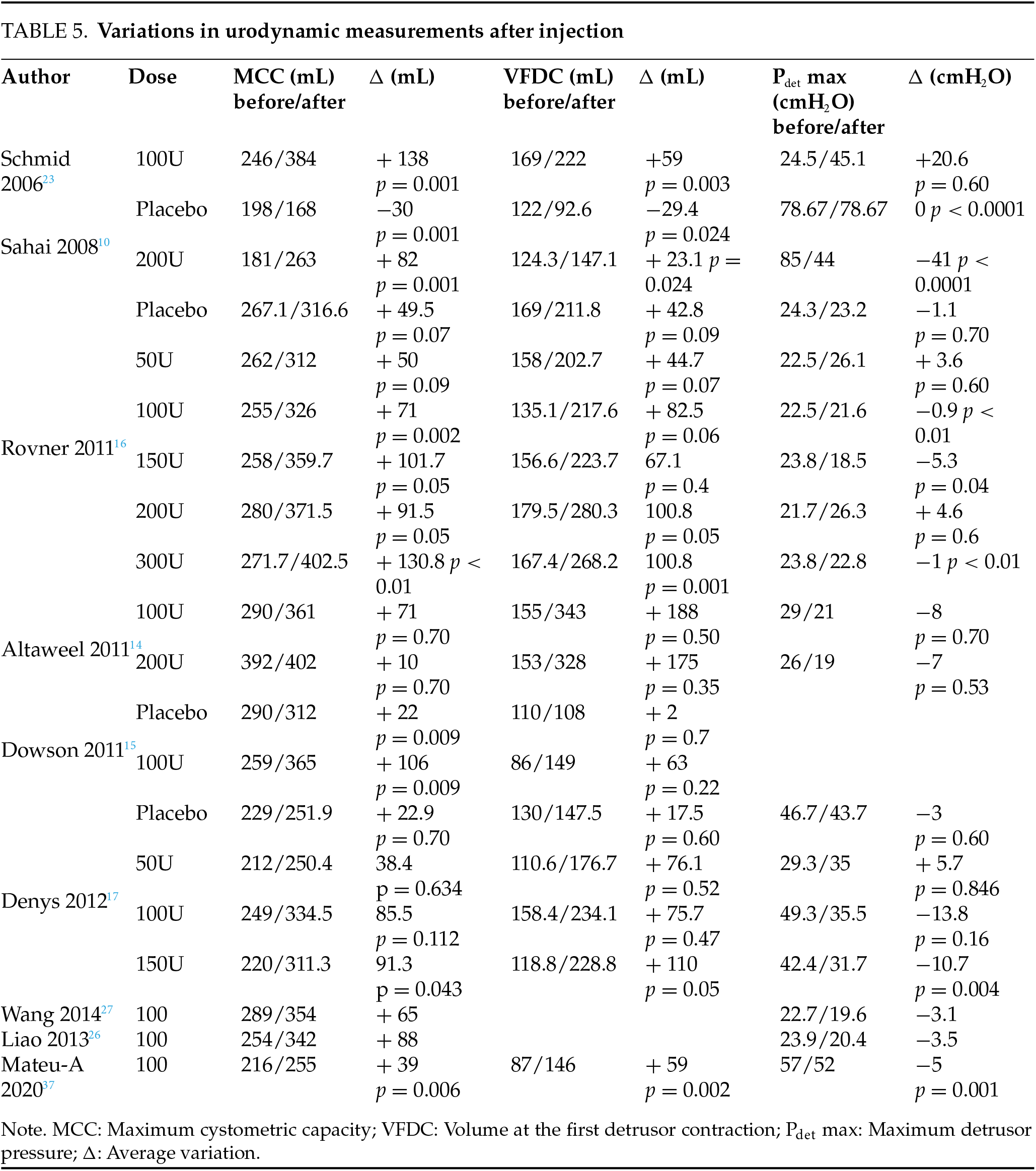
The BoNT-A dose significantly impacts efficacy. A 100-unit dose generally outperforms a 50-unit dose, while doses between 100 and 300 units show comparable efficacy at 3 months.12,14,17,37 Brubaker’s study9 found that a 200-unit dose reduced UI by 60%, while in 2 studies, a 100-unit dose reduced UI episodes by 50%.20,21 Doses above 150 units do not enhance efficacy but increase the risk of complications16 (Figures 2 and 3). A 50-unit dose improves symptoms but demonstrates minimal urodynamic changes, with results similar to placebo.13,16 However, objective results from micturition diaries or urodynamic studies do not always align with subjective patient-reported outcomes.40
However, BoNT-A is not effective for everyone. 14 studies report the rate of poor responders with 25.02% in our study. There is a lack of a consistent definition of a poor response complicating direct comparisons. The most common criterion is less than 50% improvement in urgency and UI episodes. Other studies utilize scores or urodynamic criteria (Table 6). A 100-case prospective cohort23 used a broad criterion of no urodynamic or subjective change, which could lead to the overestimation of poor responses by not accounting for subtle improvements. One RCT11,14 focused on no MCC change, though this approach may neglect other important outcomes like quality of life. Several studies18,25,26 used patient-reported outcomes like a significant decrease in PPBC, which is subject to patient bias. Liao et al.25 required a PPBC decrease at 12 months. This long-term assessment might miss earlier markers of failure, influenced by individual perceptions of bladder function. Symptom-based definitions,14,29,31 like <20% improvement in urgency29 or <50% reduction in urgency frequency,14 yet may be influenced by recall bias or fluctuating symptoms, not fully reflecting the complexity of treatment responses. Similarly, a recent RCT33 and a prospective study of 74 cases35 focused on symptom improvement thresholds using subjective measures (e.g., UDI-6), which may not reflect underlying physiological changes. Finally, Mateu-A et al.36 used the TBS score, a more structured approach, though it may obscure individual patient experiences.
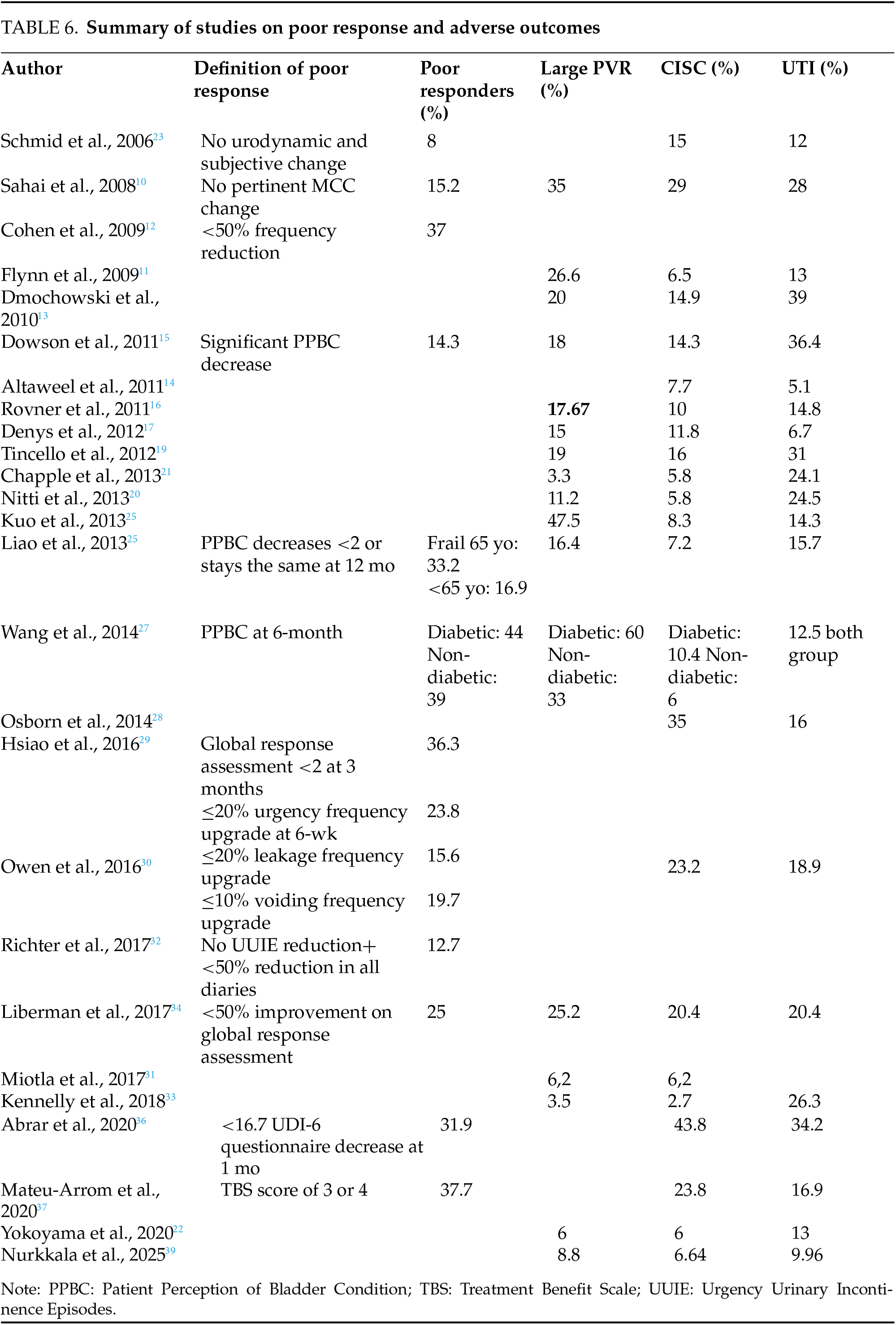
23 studies have investigated the occurrence of AE. Generally, BoNT-A is well tolerated and safe in routine practice, with less than 5% of severe complications.23 High PVR is the most commonly reported AE, documented in 17 studies with an overall rate of 22.4% in our work, and exceeding 25% in 6 studies.10,11,24,25,29 This variation can be attributed to differing definitions of significant PVR (≥150 mL, ≥200 mL, or greater). PVR typically occurs within the first month post-injection20,26 and is dose-dependent, with a notable increase observed at doses starting from 150 units of toxin.16 At a dose of 100 U, less than 10% of studies report PVR,20,21,22,28,35 whereas at 200 U, over 20% report PVR (Table 6).
Clean intermittent self-catheterization
The rates of CISC across the 23 studies vary widely, from 6.2%30 to 43.8%,35 with an average of 14.02% in our study. This variability can be explained by differences in the criteria used to define the need for CISC, which are generally based on clinical signs and/or PVR. No standardized protocol currently exists for determining when to initiate CISC.30 The need for CISC decreases with the toxin dose: rates exceed 40% at 300 units, around 30% at 200 units, less than 11% at 100 units, and only 3% at 50 units.16,24 When required, CISC typically lasts six weeks or less20 (Table 6).
The frequency of UTIs shows also considerable variation, as studies employ different definitions. Among the 23 studies, the average UTI rate is 19.4%, with a range from 5.1%17 to 36.4%.18 The occurrence of UTI may exacerbate PVR, with a risk ranging from 10% to 30%, depending on the PVR threshold.14,16,17 Managing UTI is essential due to potential complications. In a series of 299 cases, the presence or absence of UTI was the primary safety criterion32 (Table 6).
Local AE following BoNT-A injections include macroscopic hematuria, with reported occurrences ranging from 0.2%15 to 23.1%,17 with an intermediate occurrence of 3.6%.24 Dysuria is reported less frequently,23,24 moderately (10%,37) or more frequently (46.5%.34) Pain at the injection site and non-bacterial cystitis are also noted.37
Rare systemic AE due to toxin diffusion include respiratory depression,41 muscle weakness,42 fatigue (2%),43 skin rash,44 nasopharyngitis,37 and gastroparesis.16 Rare cases of urinary retention,16 pyelonephritis,44 and bilateral hydronephrosis24,44 have been documented. Importantly, no cases of mortality have been reported in multiple studies.43–48
Predictive factors of poor response
10 studies have identified factors associated with a poor response to BoNT-A injections (Table 7).

Male gender is a predictive factor for a poor response, as reported in the studies by Hsiao (OR = 3.75, 95% CI 1.40–10.06, p = 0.009)29 and Abrar (OR = 5.45, 95% CI 1.83–16.47, p = 0.002).36 Up to 36% of males show an inadequate response to BoNT-A injections,29 possibly due to voiding dysfunction linked to benign prostatic hyperplasia.41 Although the efficacy of BoNT-A has been widely studied in women, there is less research on its use in men. Improvement in quality-of-life scores is statistically more significant in women.42 Table 7 summarizes predicting factors of poor response. Age is another factor associated with poor response, identified in 4 studies.12,26,30,32 Cohen et al.12 define the threshold at 55 years (p = 0.03)12 but only in univariate analysis, while Liao et al.26 set it at 65 years in the “Frailty” subgroup defined by specific criteria (see Table 7). Advanced age is particularly predictive of poor continence response.12
A high body mass index (BMI) has been linked to a less favorable treatment response, particularly for the PGI score, although the precise BMI threshold for this effect remains debated.30 Smoking is another factor for poor response, found in one study (OR = 2.89, 95% CI 1.08–7.73, p = 0.034) regarding a reduction in urgency episodes (≤20%).30 The presence of diabetes is not a factor for poor response in a study specifically comparing this criterion.27
Urodynamic parameters prior to injection may also predict a poor response, as indicated in 3 studies. Significant factors include elevated Pdet max (>110 cmH2O),24 high bladder outlet obstruction index (BOOI),36 low detrusor compliance (<10 mL/cmH2O),30 and reduced MCC (<100 mL).30 Schmid additionally highlighted bladder wall fibrosis, observed on vesical biopsies, as a contributing factor to a poor response.23
Post-injection complications have been correlated with a poor response, such as CISC,35 UTI, and hematuria, potentially due to bladder inflammation and exacerbation of urinary symptoms.25 Richter et al.32 proposed using indices correlated with poor response, such as the Health Utilities Index Mark 3 (HUI-3) (per 0.30-point increase; 95% CI 0.23–0.77, p < 0.001) and the Functional Comorbidity Index (FCI) (OR = 0.84, 95% CI 0.71–0.99).
Predictive factors for adverse events
Post-void residual and clean intermittent self-catheterization
Predictors for the necessity of CISC have been reported in 9 studies, (Table 7). 2 studies identified age as a negative predictive factor, with a threshold starting at 68 years31 or specifically in the subgroup of “≥65 years fragile elderly”.26 Male gender is a second predictive factor in 2 studies (Kuo et al.25 OR = 9.2, 95% CI 1.5–34.0, p = 0.013; Abrar et al.36: OR = 5.14, 95% CI 1.41–18.72, p = 0.013), potentially due to the association between age and benign prostatic hyperplasia. Certain gynecological histories appear to be predictive of the risk of CISC. A history of hysterectomy increases the risk by 4.5 times (95% CI 1.09–18.8, p = 0.038),36 likely related to denervation of the bladder wall or bladder neck, resulting in reduced sensation during filling and increased bladder capacity.43 A history of ≥3 vaginal deliveries (OR = 6.86, p < 0.01)31 and anterior colporrhaphy (OR = 3.71, 95% CI 1.52–9.06)38 are also associated with increased risk. Additionally, comorbidities such as diabetes mellitus27 and congestive heart failure6 are linked to higher PVR values after toxin injection. Diabetes mellitus lead to cystopathy, detrusor underactivity and elevated PVR.27 However, aside from PVR >150 mL, diabetes does not significantly affect symptoms or urodynamic parameters after three months.27,37 Finally, the presence of mixed UI and a history of mid-urethral sling procedures appears to be a risk factor for urinary retention.38 Several urodynamic factors are statistically linked to the need for CISC, including a PVR ≥ 100 mL,25,28 large bladder capacity,28 reduced maximum urinary flow rate,24 and in women a low projected isovolumetric pressure (PIP1) ≤50.24 In men, factors such as a low bladder contraction index (BCI) ≤ 120 and a high bladder outlet obstruction index (BOOI) are associated with the need for CISC.24
3 studies25,27,40 examined factors related to UTI. Women (p = 0.002) have a threefold higher susceptibility to post-injection UTI,25 as do men with benign prostatic hyperplasia. Post-injection CISC increases the risk by a factor of five36 (95% CI: 1.38–20.00, p = 0.015). For women, a low pre-injection (PIP1) serves as a predictive factor for UTI.36 In men, a decrease in the bladder contraction index (BCI) is not correlated with an increased risk of UTI.25 Furthermore, the presence of a substantial PVR (PVR) has been associated with an increased likelihood of UTI, although the exact threshold varies between studies.9,25 Interestingly, diabetes mellitus does not appear to elevate the risk of infections post-injection.27
Table 7 summarizes predicting factors of adverse events.
We observed variability in BoNT-A efficacy measures. Twenty-five studies used different scores for symptom improvement, while six studies relied on urodynamic parameters (Table 4). The lack of standardized definitions complicates assessment and has been widely discussed in the literature. A meta-analysis including 38 RCTs reported 62 different BoNT-A outcome measures.7 In another systematic review of 19,994 participants, 15 different QoL scores were identified, with OAB-q, PPBC, I-QOL, and IIQ-7 being the most common.49 We propose defining efficacy as a >50% improvement in urinary urgency and urge urinary incontinence (if present), as assessed by a bladder diary, or a >10-point change in I-QOL. We prefer clinical criteria over urodynamic parameters due to the invasive, poorly tolerated nature of urodynamics and their impracticality for long-term monitoring, given BoNT-A’s temporary effects. We chose urgency and urinary incontinence as key symptoms of OAB, as they most significantly impact patients’ QoL.8 We selected I-QOL due to its demonstrated strong reliability, validity, and responsiveness in QoL assessment, as shown in a meta-analysis of 19,994 cases.49 The 10-point change in I-QOL is an extrapolation based on an RCT by Yalcin et al.,50 who identified thresholds for the minimal clinically important difference in I-QOL. In their systematic review, Abrar et al.6 recommend using the concept of the “minimally important difference”,51 which represents the smallest significant change in QoL, combined with a voiding diary as an objective benchmark (51). The CHORUS Groups, an international collaboration for harmonizing outcomes in urogynaecology, are developing unified Core Outcome Sets (COS) and Core Outcome Measure Sets (COMS) for future research.52
In our study, the efficacy of BoNT-A in OAB is well-documented, with the strongest evidence. Ten RCTs (Level 1) report a reduction in voiding frequency compared to placebo (Figure 2), and eleven RCTs a UI episodes drop (Figure 3). High-quality RCTs10,16,23 also consistently report nocturia improvement. These conclusions are consistent with the existing literature. BoNT-A reduces micturition frequency (−0.7 to −2.8/day) and urgency episodes (30%–69%), similar to anticholinergics.53 However, it has a stronger impact on UI episodes, with reductions of 55%–79%.46 At 3 months, it doubles the continence rate (23% vs. 11%, p < 0.003),54 significantly improving quality of life. BoNT-A is especially effective in patients intolerant to anticholinergics.46
BoNT-A’s efficacy and AE are dose-dependent. Doses above 150 U do not provide additional benefits but increase adverse effects. In our study, a 313-case study comparing 50 to 300 U showed no significant improvement with higher doses, but increased adverse effects.16 Similarly, two RCTs (100 vs. 150 U)14 and (100 vs. 200 U)17 found no significant difference. in UUI reduction (67% vs. 75%)14 or complete dryness (p = 0.10),14 QoL (p = 0.001)17 with significant differences in PVR (p = 0.002).14 Additionally, a 99-case RCT (100 U vs. 150 U) showed that 100 U had reasonable efficacy and a lower risk of high PVR (p = 0.0003).20 These results are consistent with a recent pilot study studying predicting elevated postvoid residual urine volume.55
Repetitive BoNT-A injections consistently lead to positive clinical outcomes and sustained quality of life, supported by strong evidence.44 The benefits of reinjections are similar to those of the initial treatment,23 and repeated injections do not negatively affect bladder wall integrity,56 despite the need for CISC. Anxiety and depression scores improve after the second injection and remain stable.57 Over the years, with an average of six injections, 74% to 83% of patients report high satisfaction due to reduced incontinence episodes.23
BoNT-A injections are generally well tolerated.34 In our study, we found an AE rate of 21.7%, consistent with the literature. A 2019 European report on idiopathic OAB indicates an AE rate of 26% after the first injection and 22% after the second.56 A time-based analysis of post-injection adverse effects showed that PVR peaks at week 2, increasing, then declining by week 36.14 In 3 RCTs, CISC was most common within the first month.16,19,20 UTIs generally occur in the first 2 weeks, concomitant with the increase in PVR,11,18 related to urinary stasis.15 No specific time-based data on adverse effects from repeated injections were found in long-term series.58–61
These main AEs are typically mild to moderate, transient, and manageable with standard antibiotics and clean intermittent CISC.44,45 Despite this, these AEs have a significant impact on treatment adherence. A 5-year follow-up study48 revealed that, aside from cases of total or partial ineffectiveness (37%), reasons for discontinuing treatment included the need for CISC (11%) and UTI (9%), leading to a 25% long-term treatment discontinuation rate among patients. Overall, the rate of treatment discontinuation varies across long-term studies: 18.9% at 5 years in a French multi-center study,58 25% at 6 years in a real-life study,61 and up to 38.9% at 4 years in a 90-case retrospective analysis.59
In our review, we identified several predictive factors for AE following BoNT-A injections (Table 7). The strength of these associations varies by study design, sample size, and statistical methods. Only one Level 1 RCT11 according to the Oxford Levels of Evidence scale investigated predictive factors for AE, finding that pre-injection Qmax <15 predicts CISC (p = 0.003), supported by a prospective large cohort35 with an OR of 0.91, though selection bias limits causality.
Male gender is a consistent predictor of CISC in two large cohorts: one retrospective Level 3 with 217 cases25 and one prospective Level 2 with 146 cases,35 with ORs ranging from 5.14 (p = 0.013) to 9.2 (p = 0.13), likely due to concomitant bladder outlet obstruction. Prospective cohort studies offer stronger causal evidence compared to retrospective studies, which are prone to biases.62 These data are supported by a recent meta-analysis, which, however, highlighted low evidence and limited information regarding the safety of BoNT-A for male OAB.
Preoperative PVR is also widely reported as a factor for CISC, with confirmation from 3 large retrospective cohorts15,25,26 and one prospective controlled study.35 However, its significance diminishes when associated with frailty (p = 0.18) or diabetes (p = 0.07), indicating moderate uncertainty.
The role of age as a predictor for CISC is unclear. Only one Level 2 prospective cohort30 found it significant in 252 cases, with potential selection bias. However, a meta-analysis focusing specifically on the elderly population shows an increased risk of CISC over 65 years old (RD: 0.154; 95% CI: 0.058 to 0.251).63
Gynecological historywas identified as a predictor in two large Level 2 (n = 146)35 and Level 3 (n = 397)38 cohorts, with high ORs (4.55 for hysterectomy2,35 3.71 for anterior colporrhaphy).38 However, wide confidence intervals (1.09–18.8 for hysterectomy2,35 1.52–9.06 for colporrhaphy38) reduce precision, necessitating further prospective studies. We did not find other studies in the literature reporting these conclusion.
We found in a 122 case Level 3 study that high BMI prédit une poor answer (OR = 1.07, 95% CI 1.0–1.16, p = 0.065).29 This contrasts with the results of a retrospective study from the literature involving 185 cases, which specifically studied injections in individuals with high BMI.64
Urodynamic parameters, such as lower female PIP1 and MCC/10 mL increments, are strongly associated with AEs. Studies with satisfactory quality (Levels 235 and 338) report consistent ORs with narrow confidence intervals, supporting their predictive value. These results are also found in a large meta-analysis.6
We identified age as a predictor of poor response in one RCT14 and a level 2 prospective cohort.25 In contrast, literature data from a pooled analysis of Moore’s trial65 showed no statistically significant differences in efficacy between patients over and under 65 years of age. A 2024 meta-analysis including only elderly patients highlights the need to weigh the benefits of BoNT-A for UI against its risks in this population, particularly due to their increased risk of infections and urinary retention.63
Tincello et al.19 found that DO does not impact BoNT-A efficacy, suggesting that urodynamic confirmation may not be necessary. In a study by Mateu-Arrom et al.,36 a higher BOOI predicted poor response in men, not due to bladder contractility but rather to urethral resistance, which appears to be a more important factor in treatment outcomes. This suggests that urodynamic testing may still be required for patient selection. Level 212 and level 335 included studies that suggested that reduced detrusor contractility may predict the need for CISC. However, three studies found no link between CISC rates and the Bladder Contractility Index.10,16,33
The pre-injection PVR remains a subject of debate. While high-level evidence includes studies that found no association between pre-injection PVR and CISC,12,19,25 many other robust evidence consider a high pre-injection PVR as an exclusion criterion in their study design.23,24,27 This discrepancy is explained by a dual semantic issue widely discussed in the literature, as highlighted by two large meta-analyses66,67 linking outcome variability to differences in initiation criteria for CISC across studies and the definition of what constitutes a high PVR.
Liao et al.26 found that diabetes increases PVR (60.4% vs. 33.3%; p = 0.007), likely due to cytopathic and detrusor underactivity. These data contradict those from the literature, including a retrospective cohort of 565 patients,68 in which diabetic patients had a similar rate of high PVR and urinary retention requiring CISC as non-diabetic patients.
We found that statistically significant risk factors for UTI include female gender25 and a CISC,35 consistent with findings by Everaert et al.69 Further studies are needed to explore methods for preventing post-injection UTI.45
The relationship between AEs and poor treatment response remains unclear, possibly due to exacerbation of bladder inflammation and worsening of lower urinary tract symptoms.70 Future perspectives are being explored for cases of poor response to BoNT-A. Integrating BoNT-A with rehabilitative strategies has shown promising results in spastic diplegia, reducing spasticity and improving gait.71 This combined approach could also benefit OAB by pairing BoNT-A with behavioral or physical therapies to enhance bladder control. However, further studies are needed.
This review has several limitations that should be considered. Most studies included providing level 3 evidence, with many being retrospective cohorts, which inherently carry a risk of bias, particularly about selection and recall biases. Variations in Botulinum Toxin-A doses across studies, coupled with the lack of standardized definitions for poor response and CISC initiation, further complicate direct comparisons. Additionally, many studies suffer from small sample sizes, and some may involve overlapping populations, which could lead to potential confounding. Importantly, while the moderate quality of studies is acknowledged, the potential impact of publication bias, often observed in studies with positive results, has not been thoroughly discussed. The absence of long-term data further limits the generalizability of findings. Another key limitation is the lack of data on covariates such as comorbidities and concomitant medications. Only two studies addressed comorbidities: one found no link between diabetes and treatment response or infection risk,26 and another linked frailty in older adults to poor response, without details on polypharmacy.25 The remaining studies did not report these factors, limiting the assessment of their role in adverse events and treatment outcomes. Future studies should account for these covariates. Despite these limitations, consistent trends, particularly in short-term efficacy and safety, were observed across the studies.
In summary, this comprehensive review highlights the efficacy of intradetrusorial BoNT-A injections for refractory idiopathic detrusor overactivity. Results consistently demonstrate significant symptom improvement, enhanced quality of life, and urodynamic benefits. Factors like age, gender, and the potential need for CISC influence treatment response. Despite challenges, successive injections maintain positive outcomes and manage AE, affirming BoNT-A as a viable, sustainable therapeutic option. This knowledge guides clinical decisions, with room for further research to refine this promising approach.
Acknowledgement
The authors have no acknowledgments to declare.
Funding Statement
The authors received no specific funding for this study.
Author Contributions
The authors confirm contribution to the paper as follows: Study conception and design: Salim Lachkar, Ahmed Ibrahimi; Data collection: Salim Lachkar, Ahmed Ibrahimi, Imad Boualaoui; Analysis and interpretation of results: Salim Lachkar, Ahmed Ibrahimi; Draft manuscript preparation: Salim Lachkar, Hachem El Sayegh, Yassine Nouini; Final manuscript review and approval. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author, Salim Lachkar, upon reasonable request.
Ethics Approval
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest to report regarding the present study.
References
1. Abrams P, Artibani W, Cardozo L et al. Reviewing the ICS, 2002 terminology report: the ongoing debate. Neurourol Urodyn 2009;28(4):287. doi:10.1002/nau.20737. [Google Scholar] [PubMed] [CrossRef]
2. Zhang L, Cai N, Mo L, Tian X, Liu H, Yu B. Global prevalence of overactive bladder: a systematic review and meta-analysis. Int Urogynecol J 2025 févr 14;21(2):167. doi:10.1007/s00192-024-06029-2. [Google Scholar] [PubMed] [CrossRef]
3. Al-Dossari R, Kalra M, Adkison J, Nguyen BM. Non-surgical management of urinary incontinence. J Am Board Fam Med 2024;37(5):909–918. doi:10.3122/jabfm.2023.230471R1. [Google Scholar] [PubMed] [CrossRef]
4. Food and Drug Administration. Highlights of prescribing information 2017 [cited 2025 Mar 8]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103000s5302lbl.pdf. [Google Scholar]
5. Câncio Martins Bissaia Barreto JA, Táboas Simões MI, Gomes Engenheiro G, Ferreira Matos JI, Rodrigues Leal JA. The role of botulinum toxin in the management of nonneurogenic overactive bladder in children: highlights for clinical practice. A systematic review. Curr Urol 2024 mars;18(1):1–6. doi:10.1097/CU9.0000000000000124. [Google Scholar] [PubMed] [CrossRef]
6. Abrar M, Pindoria N, Malde S, Chancellor M, DeRidder D, Sahai A. Predictors of poor response and adverse events following botulinum toxin a for refractory idiopathic overactive bladder: a systematic review. Eur Urol Focus 2021;7(6):1448–1467. doi:10.1016/j.euf.2020.06.013. [Google Scholar] [PubMed] [CrossRef]
7. Moussa R, Rada MP, Durnea C et al. Outcome reporting in randomized controlled trials (RCTs) on the pharmacological management of idiopathic overactive bladder (OAB) in women; a systematic review for the development of core outcome sets (COS). Int Urogynecol J 2022 mai;33(5):1243–1250. doi:10.1007/s00192-021-05040-1. [Google Scholar] [PubMed] [CrossRef]
8. Liang P, Yu L, Xia B, Zhang D. Comparative efficacy and safety of mirabegron and vibegron in female patients with overactive bladder: a systematic review and meta-analysis of randomized controlled trials. Urology 2025 févr 17;111(1):804. [Google Scholar]
9. Brubaker L, Richter HE, Visco A et al. Refractory idiopathic urge urinary incontinence and botulinum a injection. J Urol 2008 juill 1;180(1):217–222. doi:10.1016/j.juro.2008.03.028. [Google Scholar] [PubMed] [CrossRef]
10. Sahai A, Khan MS, Le Gall N, Dasgupta P. Urodynamic assessment of poor responders after botulinum toxin-a treatment for overactive bladder. Urology 2008 mars 1;71(3):455–459. doi:10.1016/j.urology.2007.11.039. [Google Scholar] [PubMed] [CrossRef]
11. Flynn MK, Amundsen CL, Perevich M, Liu F, Webster GD. Outcome of a randomized, double-blind, placebo controlled trial of botulinum a toxin for refractory overactive bladder. J Urol 2009 juin;181(6):2608–2615. doi:10.1016/j.juro.2009.01.117. [Google Scholar] [PubMed] [CrossRef]
12. Cohen BL, Barboglio P, Rodriguez D, Gousse AE. Preliminary results of a dose-finding study for botulinum toxin-A in patients with idiopathic overactive bladder: 100 versus 150 units. Neurourol Urodyn 2009 mars;28(3):205–208. doi:10.1002/nau.20611. [Google Scholar] [PubMed] [CrossRef]
13. Dmochowski R, Chapple C, Nitti VW et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol 2010 déc;184(6):2416–2422. doi:10.1016/j.juro.2010.08.021. [Google Scholar] [PubMed] [CrossRef]
14. AlTaweel W, Mokhtar A, Rabah D. Prospective randomized trial of 100u vs 200u botox in the treatment of idiopathic overactive bladder. Urol Ann 2011;3(2):66. doi:10.4103/0974-7796.82170. [Google Scholar] [PubMed] [CrossRef]
15. Dowson C, Sahai A, Watkins J, Dasgupta P, Khan MS. The safety and efficacy of botulinum toxin-A in the management of bladder oversensitivity: a randomised double-blind placebo-controlled trial: botulinum toxin-A and bladder oversensitivity. Int J Clin Pract 2011 juin;65(6):698–704. doi:10.1111/j.1742-1241.2011.02663.x. [Google Scholar] [PubMed] [CrossRef]
16. Rovner E, Kennelly M, Schulte-Baukloh H, Zhou J, Haag-Molkenteller C, Dasgupta P. Urodynamic results and clinical outcomes with intradetrusor injections of onabotulinumtoxina in a randomized, placebo-controlled dose-finding study in idiopathic overactive bladder. Neurourol Urodyn 2011 avr;30(4):556–562. doi:10.1002/nau.21021. [Google Scholar] [PubMed] [CrossRef]
17. Denys P, Le Normand L, Ghout I et al. Efficacy and safety of low doses of onabotulinumtoxina for the treatment of refractory idiopathic overactive bladder: a multicentre, double-blind, randomised, placebo-controlled dose-ranging study. European Urol 2012 mars;61(3):520–529. doi:10.1016/j.eururo.2011.10.028. [Google Scholar] [PubMed] [CrossRef]
18. Fowler CJ, Auerbach S, Ginsberg D et al. OnabotulinumtoxinA improves health-related quality of life in patients with urinary incontinence due to idiopathic overactive bladder: a 36-week, double-blind, placebo-controlled, randomized, dose-ranging trial. Eur Urol 2012 juill;62(1):148–157. doi:10.1016/j.eururo.2012.03.005. [Google Scholar] [PubMed] [CrossRef]
19. Tincello DG, Kenyon S, Abrams KR et al. Botulinum toxin A versus placebo for refractory detrusor overactivity in women: a randomised blinded placebo-controlled trial of 240 women (the RELAX Study). European Urol 2012 sept;62(3):507–514. doi:10.1016/j.eururo.2011.12.056. [Google Scholar] [PubMed] [CrossRef]
20. Nitti VW, Dmochowski R, Herschorn S et al. OnabotulinumtoxinA for the treatment of patients with overactive bladder and urinary incontinence: results of a phase 3, randomized, placebo controlled trial. J Urol 2013 juin;189(6):2186–2193. doi:10.1016/j.juro.2012.12.022. [Google Scholar] [PubMed] [CrossRef]
21. Chapple C, Sievert K-D, MacDiarmid S et al. OnabotulinumtoxinA 100 U significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: a randomised, double-blind, placebo-controlled trial. European Urol 2013 août;64(2):249–256. doi:10.1016/j.eururo.2013.04.001. [Google Scholar] [PubMed] [CrossRef]
22. Yokoyama O, Honda M, Yamanishi T et al. OnabotulinumtoxinA (botulinum toxin type A) for the treatment of Japanese patients with overactive bladder and urinary incontinence: results of single-dose treatment from a phase III, randomized, double-blind, placebo-controlled trial (interim analysis). Int J of Urology 2020 Mars;27(3):227–234. doi:10.1111/iju.14176. [Google Scholar] [PubMed] [CrossRef]
23. Schmid DM, Sauermann P, Werner M et al. Experience with 100 cases treated with botulinum-A toxin injections in the detrusor muscle for idiopathic overactive bladder syndrome refractory to anticholinergics. J Urol 2006 juill;176(1):177–185. doi:10.1016/S0022-5347(06)00590-8. [Google Scholar] [PubMed] [CrossRef]
24. Sahai A, Dowson C, Khan MS, Dasgupta P. Improvement in quality of life after botulinum toxin-A injections for idiopathic detrusor overactivity: results from a randomized double-blind placebo-controlled trial. BJU Int 2009 Juin;103(11):1509–1515. doi:10.1111/j.1464-410X.2009.08402.x. [Google Scholar] [PubMed] [CrossRef]
25. Kuo HC, Liao CH, Chung SD. Adverse events of intravesical botulinum toxin A injections for idiopathic detrusor overactivity: risk factors and influence on treatment outcome. Eur Urol 2010 déc 1;58(6):919–926. doi:10.1016/j.eururo.2010.09.007. [Google Scholar] [PubMed] [CrossRef]
26. Liao CH, Kuo HC. Increased risk of large post-void residual urine and decreased long-term success rate after intravesical onabotulinumtoxinA injection for refractory idiopathic detrusor overactivity. J Urol 2013 mai 1;1189(5):1804–1810. doi:10.1016/j.juro.2012.11.089. [Google Scholar] [PubMed] [CrossRef]
27. Wang C, Liao C, Kuo H. Diabetes mellitus does not affect the efficacy and safety of intravesical onabotulinumtoxina injection in patients with refractory detrusor overactivity. Neurourol Urodyn 2014 oct;33(8):1235–1239. doi:10.1002/nau.22494. [Google Scholar] [PubMed] [CrossRef]
28. Osborn DJ, Kaufman MR, Mock S, Guan MJ, Dmochowski RR, Reynolds WS. Urinary retention rates after intravesical onabotulinumtoxinA injection for idiopathic overactive bladder in clinical practice and predictors of this outcome. Neurourol Urodyn 2015 sept;34(7):675–678. doi:10.1002/nau.22642. [Google Scholar] [PubMed] [CrossRef]
29. Hsiao SM, Lin HH, Kuo HC. Factors associated with therapeutic efficacy of intravesical onabotulinumtoxinA injection for overactive bladder syndrome. Hills RK, éditeur. PLoS One 2016 janv 29;11(1):e0147137. doi:10.1371/journal.pone.0147137. [Google Scholar] [PubMed] [CrossRef]
30. Owen RK, Abrams KR, Mayne C, Slack M, Tincello DG. Patient factors associated with onabotulinum toxin A treatment outcome in women with detrusor overactivity. Neurourol Urodyn 2017 févr;36(2):426–431. doi:10.1002/nau.22948. [Google Scholar] [PubMed] [CrossRef]
31. Miotla P, Cartwright R, Skorupska K et al. Urinary retention in female OAB after intravesical Botox injection: who is really at risk? Int Urogynecol J 2017 juin;28(6):845–850. doi:10.1007/s00192-016-3212-4. [Google Scholar] [PubMed] [CrossRef]
32. Richter HE, Amundsen CL, Erickson SW et al. Characteristics associated with treatment response and satisfaction in women undergoing onabotulinumtoxinA and sacral neuromodulation for refractory urgency urinary incontinence. J Urol 2017 oct;198(4):890–896. doi:10.1016/j.juro.2017.04.103. [Google Scholar] [PubMed] [CrossRef]
33. Kennelly M, Green L, Alvandi N et al. Clean intermittent catheterization rates after initial and subsequent treatments with onabotulinumtoxinA for non-neurogenic overactive bladder in real-world clinical settings. Curr Med Res Opin 2018 oct 3; 34(10):1771–1776. doi:10.1080/03007995.2018.1443061. [Google Scholar] [PubMed] [CrossRef]
34. Liberman D, Milhouse O, Johnson-Mitchell M, Siegel SW. Real-world retention rates after intravesical onabotulinumtoxinA for idiopathic overactive bladder. Female Pelvic Med Reconstr Surg 2018 nov;24(6):404–407. doi:10.1097/SPV.0000000000000496. [Google Scholar] [PubMed] [CrossRef]
35. Faure Walker NA, Syed O, Malde S, Taylor C, Sahai A. Onabotulinum toxin A injections in men with refractory idiopathic detrusor overactivity. Urol Janv 2019 janv;123:242–246. doi:10.1016/j.urology.2018.09.016. [Google Scholar] [PubMed] [CrossRef]
36. Abrar M, Stroman L, Malde S, Solomon E, Sahai A. Predictors of poor response and adverse events following botulinum Toxin-A for refractory idiopathic overactive bladder. Urology 2020 janv;135:32–37. doi:10.1016/j.urology.2019.08.054. [Google Scholar] [PubMed] [CrossRef]
37. Mateu Arrom L, Mayordomo Ferrer O, Sabiote Rubio L et al. Treatment response and complications after intradetrusor onabotulinumtoxinA injection in male patients with idiopathic overactive bladder syndrome. J Urol 2020 févr;203(2):392–397. doi:10.1097/JU.0000000000000525. [Google Scholar] [PubMed] [CrossRef]
38. El Issaoui M, Elissaoui S, Elmelund M, Klarskov N. Predictive factors for clean intermittent catheterization after intravesical onabotulinumtoxinA injections in women with overactive bladder: a danish retrospective cohort study. Int Urogynecol J 2025 janv;36(1):107–115. doi:10.1007/s00192-024-05960-8. [Google Scholar] [PubMed] [CrossRef]
39. Nurkkala M, Salo H, Piltonen T, Sova H, Rossi HR. Efficacy of 100-U Onabotulinumtoxin A treatment in female idiopathic overactive bladder—a prospective follow-up study. Int Urogynecol J 2025 janv 31;36(3):685–693. doi:10.1007/s00192-025-06047-8. [Google Scholar] [PubMed] [CrossRef]
40. Abrams P, Artibani W, Gajewski JB, Hussain I. Assessment of treatment outcomes in patients with overactive bladder: importance of objective and subjective measures. Urology 2006 août;68(2 Suppl):17–28. doi:10.1016/j.urology.2006.05.044. [Google Scholar] [PubMed] [CrossRef]
41. Duthie JB, Vincent M, Herbison GP, Wilson DI, Wilson D. Botulinum toxin injections for adults with overactive bladder syndrome. In: Cochrane database of systematic reviews. Chichester, UK: John Wiley & Sons, Ltd., 2011 [cité 2025 févr 26]. p. CD005493.pub3. doi:10.1002/14651858.CD005493.pub3. [Google Scholar] [CrossRef]
42. Mangera A, Andersson K-E, Apostolidis A et al. Contemporary management of lower urinary tract disease with botulinum toxin A: a systematic review of botox (onabotulinumtoxinA) and dysport (abobotulinumtoxinA). Eur Urol 2011 oct;60(4):784–795. doi:10.1016/j.eururo.2011.07.001. [Google Scholar] [PubMed] [CrossRef]
43. Deffieux X, Fatton B, Denys P et al. Intra-detrusor injection of botulinum toxin for female refractory idiopathic overactive bladder syndrome. J Gynecol Obstet Biol Reprod 2014 oct;43(8):572–580. [Google Scholar]
44. Gamé X, Karsenty G, Ruffion A et al. Idiopathic overactive bladder and BOTOX(®literature review. Prog Urol 2015 juin;25(8):461–473. doi:10.1016/j.purol.2015.01.006. [Google Scholar] [PubMed] [CrossRef]
45. Harris S, Rizzolo D. Botulinum toxin as a treatment for refractory overactive bladder. JAAPA 2016 févr;29(2):1–4. doi:10.1097/01.JAA.0000476217.57808.c4. [Google Scholar] [PubMed] [CrossRef]
46. Makovey I, Davis T, Guralnick ML, O’Connor RC. Botulinum toxin outcomes for idiopathic overactive bladder stratified by indication: lack of anticholinergic efficacy versus intolerability. Neurourol Urodyn 2011 nov;30(8):1538–1540. doi:10.1002/nau.21150. [Google Scholar] [PubMed] [CrossRef]
47. Chermansky C, Schurch B, Rahnama’i MS et al. How can we better manage drug-resistant OAB/DO? ICI-RS 2018. Neurourol Urodyn 2019 déc;38 Suppl 5(S5):S46–S55. doi:10.1002/nau.24055. [Google Scholar] [PubMed] [CrossRef]
48. Marcelissen TAT, Rahnama’i MS, Snijkers A, Schurch B, De Vries P. Long-term follow-up of intravesical botulinum toxin—a injections in women with idiopathic overactive bladder symptoms. World J Urol 2017 Févr;35(2):307–311. doi:10.1007/s00345-016-1862-y. [Google Scholar] [PubMed] [CrossRef]
49. Usman Ali M, Fong KNK, Kannan P et al. Measures of quality of life of people with neurogenic overactive bladder: a systematic review of psychometric properties. Eur J Obstet Gynecol Reprod Biol 2024 Janv;292(Suppl.):40–57. doi:10.1016/j.ejogrb.2023.11.010. [Google Scholar] [PubMed] [CrossRef]
50. Yalcin I, Patrick DL, Summers K, Kinchen K, Bump RC. Minimal clinically important differences in incontinence quality-of-life scores in stress urinary incontinence. Urology 2006 Juin;67(6):1304–1308. doi:10.1016/j.urology.2005.12.006. [Google Scholar] [PubMed] [CrossRef]
51. Copay AG, Subach BR, Glassman SD, Polly DW, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J 2007;7(5):541–546. doi:10.1016/j.spinee.2007.01.008. [Google Scholar] [PubMed] [CrossRef]
52. Doumouchtsis SK, Nama V, Falconi G et al. Developing core outcome sets (COS) and core outcome measures sets (COMS) in cosmetic gynecological interventions: protocol for a development and usability study. JMIR Res Protoc 2021 nov 15;10(11):e28032. doi:10.2196/28032. [Google Scholar] [PubMed] [CrossRef]
53. Novara G, Galfano A, Secco S et al. A systematic review and meta-analysis of randomized controlled trials with antimuscarinic drugs for overactive bladder. Eur Urol 2008 oct;54(4):740–763. doi:10.1016/j.eururo.2008.06.080. [Google Scholar] [PubMed] [CrossRef]
54. Visco AG, Brubaker L, Richter HE et al. Anticholinergic versus botulinum toxin A comparison trial for the treatment of bothersome urge urinary incontinence: ABC trial. Contemp Clin Trials 2012 Janv;33(1):184–196. doi:10.1016/j.cct.2011.09.019. [Google Scholar] [PubMed] [CrossRef]
55. Franco I, Schwartz M, Cline K, Glazier D, Patel A. Predicting elevated postvoid residual urine volume following onabotulinumtoxinA treatment for overactive bladder: a pilot study. Low Urin Tract Symptoms 2025 janv;17(1):e70004. doi:10.1111/luts.70004. [Google Scholar] [PubMed] [CrossRef]
56. de Santé Haute Autorité. Avis de la Commission de la transparence—BOTOX. [cited 2025 Apr 17]. Available from: https://www.has-sante.fr/upload/docs/evamed/CT-17237_BOTOX_HVI_PIS_NE_EPIBoreal_Avis2_modifiele09052019_CT17237.pdf. [Google Scholar]
57. Jayarajan J, Radomski SB. Pharmacotherapy of overactive bladder in adults: a review of efficacy, tolerability, and quality of life. Res Rep Urol 2013 déc 6;6:1–16. [Google Scholar]
58. Delaval S, Dequirez PL, Hentzen C et al. Intravesical injections of botulinum neurotoxin A to treat overactive bladder and/or detrusor overactivity related to multiple sclerosis: 5-Year continuation rate and specific risk factors for discontinuation—a study from the neuro-urology committee of the French association of urology. Mult Scler 2023 Juill;29(8):1024–1032. [Google Scholar]
59. Bernstein S, Schwartz M, Ifantides KB. Patient persistence to onabotulinumtoxinA treatment for overactive bladder using a reduced injection-site paradigm: a retrospective chart review study. Neurourol Urodyn 2025 févr;44(2):338–344. [Google Scholar]
60. Mohamed-Ahmed R, Lor KY, Taithongchai A, Rantell A, Araklitis G, Robinson D. Long term safety, continuation rates and subjective and objective success of posterior tibial nerve stimulation for overactive bladder. Continence 2024 sept 1;11:101341. [Google Scholar]
61. Manso M, Soares JD, Henriques M, Botelho F, Silva C, Cruz F. Efficacy, satisfaction, and compliance: insights from 15 years of botulinum toxin use for female urgency urinary incontinence. Toxins 2024 Aug;16(8):332. [Google Scholar]
62. Kim S. Overview of clinical study designs. Clin Exp Emerg Med 2024 mars;11(1):33–42. [Google Scholar]
63. Chen YH, Kuo JH, Huang YT, Lai PC, Ou YC, Lin YC. Evaluating the efficacy and safety of botulinum toxin in treating overactive bladder in the elderly: a meta-analysis with trial sequential analysis of randomized controlled trials. Toxins 2024 nov 8;16(11):484. [Google Scholar]
64. Aalami Harandi A, Nauheim J, Abraham NE. Risk of retention after OnabotulinumtoxinA injection for overactive bladder in a diverse urban population with high BMI and comorbidity rates. Urogynecology 2023 janv 1;29(1):41–47. [Google Scholar]
65. Moore C, Kaufmann A, Joshi M, Nardo C, Zheng Y, Herschorn S. MP76-12 overactive bladder patients ≥65 years of age have a similar efficacy and safety profile with onabotulinumtoxina as patients <65 years of age. J Urology [Internet] 2014 avr;191(4S). [cité 2025 févr 26]. http://www.jurology.com/doi/10.1016/j.juro.2014.02.2405. [Google Scholar]
66. Stavrou S, Paynter JA, Carins T, Qin KR, Brennan J. Variation in the definitions of urinary retention in studies of intravesical botulinum toxin for idiopathic overactive bladder: a narrative systematic review. Neurourol Urodyn 2025 Apr;44(4):860–877. [Google Scholar]
67. Castaneda PR, Chen A, Kuhlmann P, Anger JT, Eilber KS. Variation in defining retention after onabotulinum toxin A for overactive bladder: a systematic review. Urogynecology 2024 sept 1;30(9):736–741. [Google Scholar]
68. Takashima Y, Handler S, Laus K et al. The correlation of diabetes mellitus and urinary retention from intravesical onabotulinumtoxinA injection for overactive bladder. Urogynecology 2023 mai 1;29(5):511–519. [Google Scholar]
69. Everaert K, Gruenenfelder J, Schulte-Baukloh H et al. Impact of onabotulinumtoxinA on quality of life and practical aspects of daily living: a pooled analysis of two randomized controlled trials. Int J Urol 2015 déc;22(12):1131–1137. [Google Scholar]
70. Jiang YH, Ong HL, Kuo HC. Predictive factors of adverse events after intravesical suburothelial onabotulinumtoxina injections for overactive bladder syndrome—a real-life practice of 290 cases in a single center. Neurourol Urodyn 2017 janv;36(1):142–147. [Google Scholar]
71. Donati D, Farì G, Giorgi Fet al. Effectiveness of integrating botulinum toxin type A with rehabilitative strategies for managing spastic diplegia in children: scope review. OBM Neurobiol 2024 oct;8(4):1–19. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF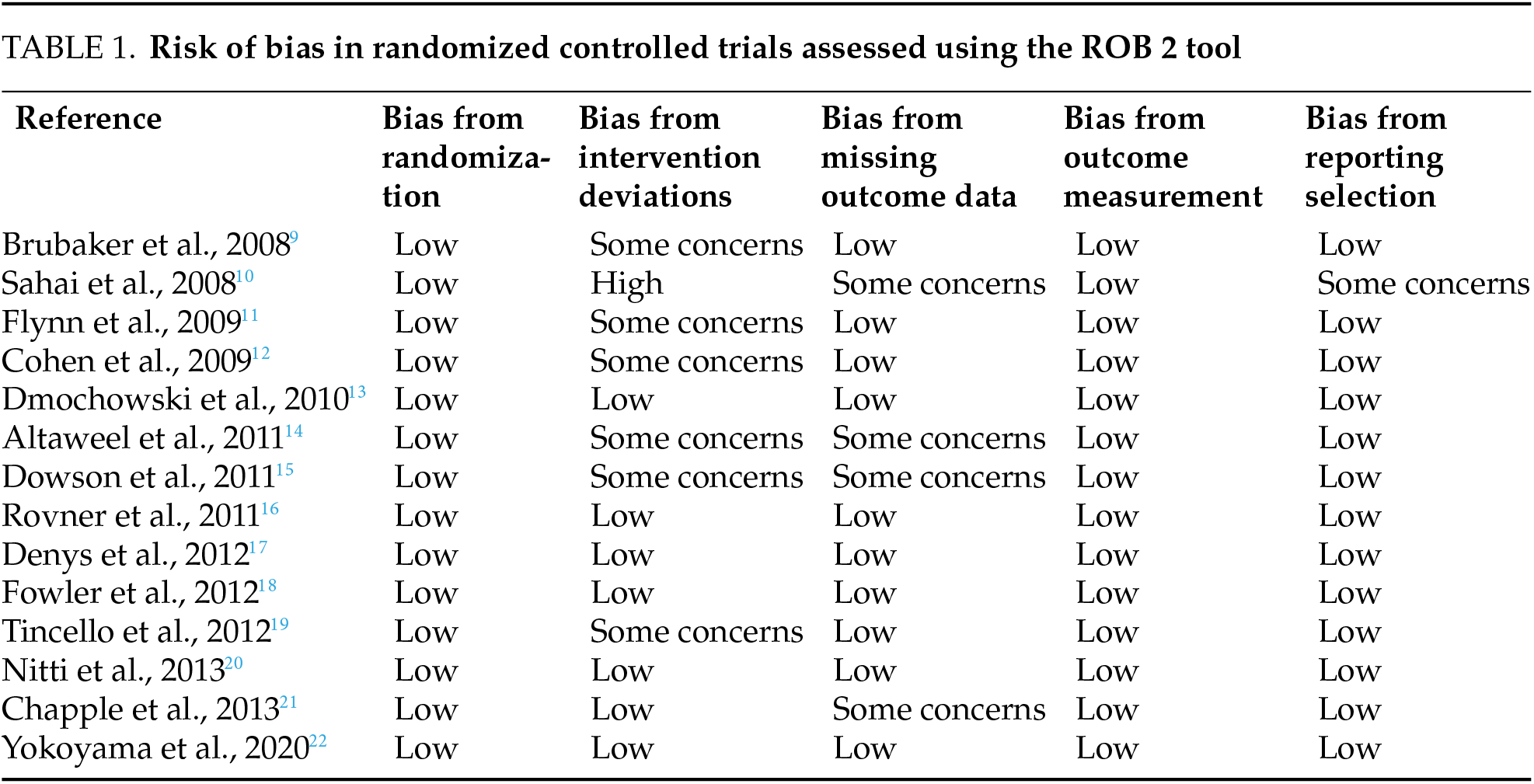
 Downloads
Downloads
 Citation Tools
Citation Tools