 Open Access
Open Access
CASE REPORT
Successful treatment of rare vaso-vesical fistula with minimally invasive measures despite prior history of radiotherapy: a case report
1 Department of Urology, NYU Langone Hospital–Long Island, Mineola, NY 11501, USA
2 Department of Urology, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA
* Corresponding Author: Jordan L. Mendelson. Email:
Canadian Journal of Urology 2025, 32(6), 673-676. https://doi.org/10.32604/cju.2025.063770
Received 23 January 2025; Accepted 12 May 2025; Issue published 30 December 2025
Abstract
Stereotactic body radiotherapy (SBRT) for prostate cancer is a generally well-tolerated treatment but can rarely lead to complications such as fistula formation. We report a 69-year-old male on maintenance ibrutinib for chronic lymphocytic leukemia who developed a fistula between his bladder and vas deferens in the setting of ascending scrotal infection. Despite his prior history of SBRT, the fistula was successfully treated with minimally invasive measures. A combination of abscess debridement, urinary diversion, and broad-spectrum antibiotics helped to achieve fistula resolution. The unique presentation described herein highlights the importance of early aggressive intervention for source control and infection management in patients with complex pelvic infections post-SBRT.Keywords
Stereotactic body radiotherapy (SBRT) administers targeted hypofractionated radiation doses to tumors, to minimize damage to surrounding healthy tissues.1 Though generally well tolerated, SBRT is associated with several potential adverse events, such as fistula formation. One recent meta-analysis examining an aggregate of more than 7600 patients found that the pooled prevalence of radiation-induced fistula formation across six cohort studies was just 0.2%.2 Though rectovesical fistulae are likely the most common type of fistula to form following radiotherapy for prostate cancer, fistulae may form between any pelvic structures, such as the distal ureters, small bowel, urethra, or pubic symphysis.3 One large, multi-institutional study examining more than 2000 patients treated with SBRT for low- and intermediate-risk prostate cancer (PCa) found that just one patient developed a fistula following radiotherapy.4
Though some fistulae between the genitourinary tract and neighboring structures may be treated conservatively, others may require surgical intervention.3 Fistulae may create a conduit for the spread of bacteria within the pelvis, which may lead to abscess formation and may even progress to the development of Fournier’s gangrene if left untreated.5,6 Herein, we present a case of fistula formation between the bladder and vas deferens which presented in the setting of ascending scrotal infection and was successfully treated with minimally invasive measures despite the patient’s prior history of SBRT.
A 69-year-old male with a past medical history significant for Gleason grade group 3 prostate cancer diagnosed in 2022 (cT1N0M0, stage IIC) and treated with SBRT in March–April 2023 and chronic lymphocytic leukemia (CLL) in remission on maintenance ibrutinib had been diagnosed with epididymo-orchitis approximately one month before presentation. Though he initially responded to a course of cefuroxime, his symptoms soon worsened, prompting his presentation to an outside emergency department (ED). After an ultrasound revealed a small scrotal abscess, his antibiotic regimen was switched to ciprofloxacin. Several weeks later, there was still minimal-to-no improvement noted. He was subsequently referred to the ED.
Further questioning revealed an additional medical history of hypertension, hyperlipidemia, shingles, and Ramsay-Hunt syndrome. His surgical history was notable only for bilateral inguinal hernia repairs with mesh in the remote past. The patient never smoked cigarettes, though he was a daily marijuana user and imbibed approximately two alcoholic beverages per day.
Physical exam was significant for severe scrotal induration, erythema, and fluctuance of the left hemiscrotum with pus expressible from a pinpoint area of drainage. Vital signs were within normal limits. Labs were significant only for leukocytosis to 27.9. He underwent a CT scan of his abdomen and pelvis, which revealed extensive inflammation of the left seminal vesicle, left vas deferens, and left spermatic cord, which coalesced into a large, multi-loculated left scrotal abscess with locules of air within. Additionally, there appeared to be focal invasion of the left vas deferens into the left lateral wall of the urinary bladder without intraluminal air to suggest frank fistulization and several prostatic and peri-prostatic abscesses (Figure 1). The combination of findings was interpreted as a potential impending or atypical appearance of Fournier’s gangrene.
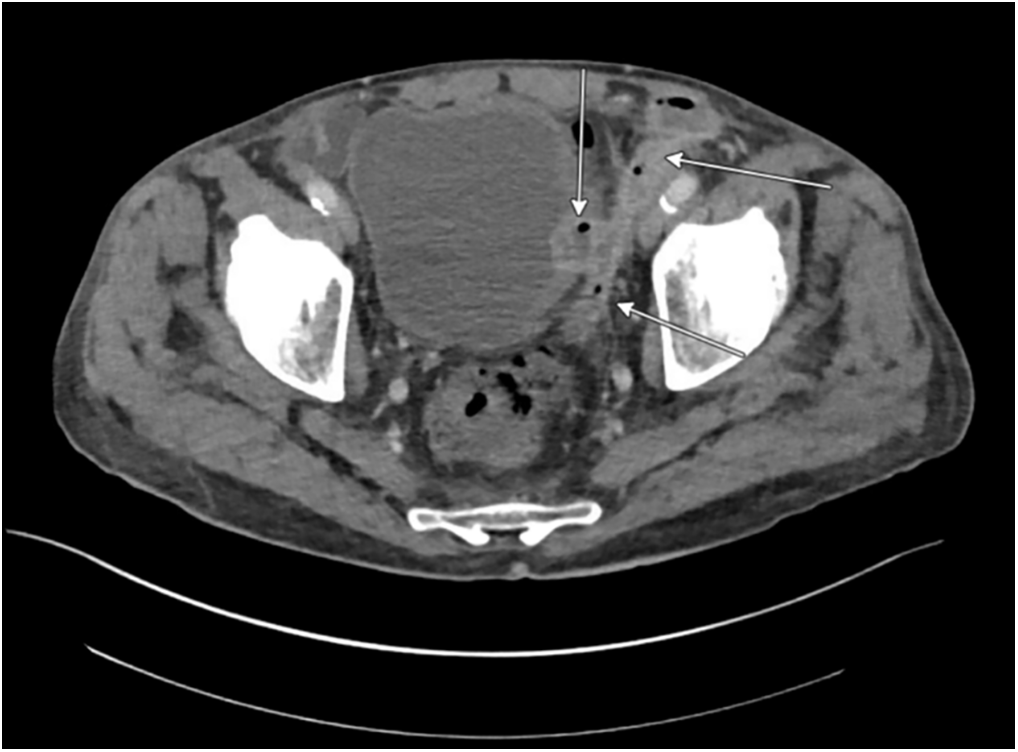
FIGURE 1. Initial CT A/P with IV contrast
The initial intervention included a bedside incision and drainage of the scrotal abscess. A cruciate incision was made in the anterior-inferior scrotum and copious amounts of foul-smelling, purulent fluid were expressed and sent for culture. Multiple septations were dissected bluntly. The debrided area was then packed with ½” iodoform gauze. An 18F Foley catheter was then placed with the return of clear yellow urine. The patient was started on Vancomycin and Meropenem per Infectious Disease recommendations. Fluid culture subsequently grew Staphylococcus epidermidis. The scrotal packing was exchanged daily. The patient’s clinical picture began to improve, with decreasing pain and induration. The patient’s leukocytosis improved from 27.9 to 18.9 over the course of several days.
On hospital day four, a repeat CT scan revealed an interval decrease in the sizes of the various pelvic abscesses. There was a new, tiny focus of gas within the bladder along the left lateral wall adjacent to the left vas deferens, highly suspicious for a developing fistula (Figure 2).
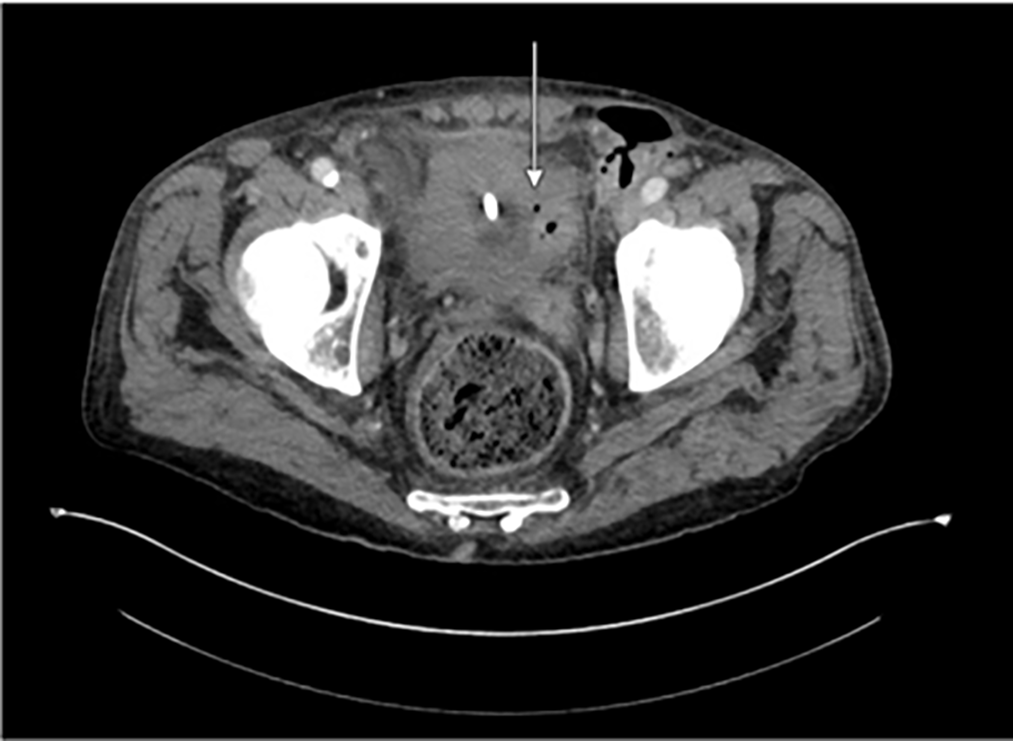
FIGURE 2. Repeat CT A/P with IV contrast on hospital day #4
Despite clinical improvement, the patient was taken to the operating room on hospital day six due to his persistent leukocytosis (16.5) and imaging findings of persistent abscesses. At that time, he underwent cystoscopy, placement of a suprapubic catheter, transperineal aspiration of several prostatic abscesses under ultrasound guidance, and additional debridement of his scrotal abscess, which required a counter-incision in the left groin. Various fluid cultures from the operating room grew coagulase-negative Staphylococcus, Corynebacterium, and gram-positive cocci in pairs.
Over the course of the next several days, the patient continued to improve clinically with daily packing changes. His persistent leukocytosis downtrended slightly to 14.3. Repeat CT on hospital day eight showed a slight interval decrease in the size of the left vas deferens’ extension into the bladder (Figure 3). The patient passed a trial of void on hospital day ten and had clinically stabilized. Repeat cystoscopy on hospital day thirteen showed marked improvement of the prior induration and inflammatory changes of the left lateral wall of the bladder. His white blood cell count finally normalized on hospital day fourteen. The patient was discharged the following day with a PICC line on Vancomycin for an additional 27 days (to complete a total 42-day course per Infectious Disease).
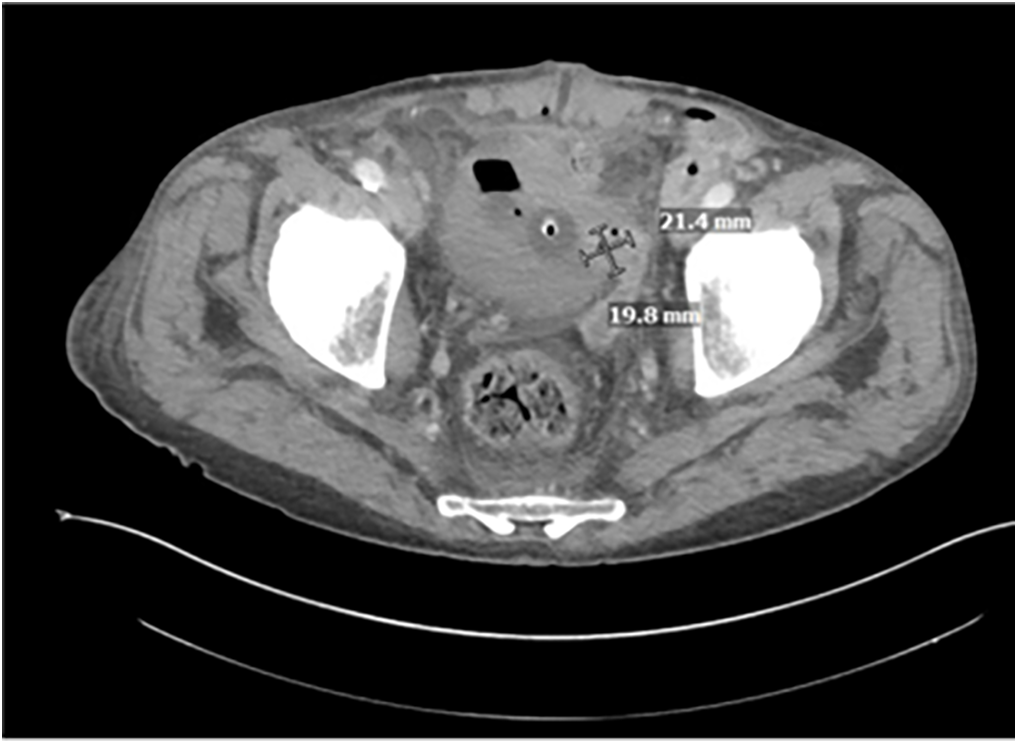
FIGURE 3. CT A/P with IV contrast hospital day 8
Repeat CT three weeks later revealed diffuse improvement of the suspected fistulous connection from the left vas deferens to the bladder and various pelvic abscesses. The patient exhibited significant clinical improvement at that time and continued to recover appropriately without further issues. The patient consented to the inclusion of anonymized photos and publications.
Our patient initially presented with left epididymo-orchitis and his infection progressed despite appropriate antibiotic coverage. He subsequently developed a scrotal abscess and then developed additional pelvic abscesses in various locations, concerning for an atypical presentation of Fournier’s gangrene with gas visualized on CT. He developed a fistula between his left vas deferens and the bladder, likely due to ascending infection. He may have been predisposed to worsening infection due to both his maintenance ibrutinib immunotherapy for CLL and his prior history of SBRT for localized PCa. Complicated urinary tract infections, including scrotal abscesses, are seen in patients with CLL on ibrutinib and those with prior pelvic radiotherapy.7 Immunosuppression and radiation-related tissue changes may increase infection risk and complicate treatment.8 Despite this, his fistula healed with minimally invasive measures.
Based on an extensive review of the literature, the development of a vaso-vesical fistula is an exceedingly rare occurrence. The primary principle of management is similar to that of all urinary tract fistulae: urinary diversion to both maintain a low-pressure system and reduce extravasation of urine.3 Once identified, debridement of the infectious source and urinary diversion with a suprapubic tube and Foley catheter were initiated. This, combined with broad-spectrum antibiotics, enabled the fistula tract to sufficiently heal based on both repeat cystoscopy and repeat imaging. While fistula formation is a known, uncommon sequela of radiotherapy, this case highlights a very unusual site and context of fistula formation, underscoring its importance.
Toxicities may overlap in patients receiving radiotherapy while on ibrutinib, though data on this is limited. Studies suggest that concurrent treatment may increase tissue fragility and infection risk, warranting further investigation.7 Surgical management after radiotherapy is challenging due to fibrosis, vascular changes, and impaired healing.9 These factors increase operative difficulty and must be considered when planning intervention.
This case also highlights the importance of early, aggressive, and—if necessary—repeated debridement of scrotal and pelvic abscesses to gain source control. Our patient was not clinically improving despite appropriate antibiotics, so he was admitted to the hospital for broad-spectrum IV antibiotics and debridement of his abscesses. The minimally invasive measures described herein successfully treated both his pelvic abscesses and his vaso-vesical fistula without any further complications. Key takeaways from this case include the risk of complications in patients with prior radiotherapy and concurrent ibrutinib use, prompting future studies to explore these combined effects of radiotherapy and novel agents to improve patient outcomes in similar cases.
Acknowledgement
The authors have no further acknowledgements.
Funding Statement
The authors received no specific funding for this study.
Author Contributions
The authors confirm contribution to the paper as follows: Study conception and design: Anthony Corcoran, Phillip Westbrook, Jordan L. Mendelson; Data collection: Jordan Kassab, Katie Yang, Jordan L. Mendelson; Draft manuscript preparation: Jordan L. Mendelson, Jordan Kassab; Manuscript revision and editing: Jordan L. Mendelson, Anthony Corcoran, Phillip Westbrook. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials
Not applicable.
Ethics Approval
Not applicable. As per the New York University School of Medicine IRB policy, this manuscript does not involve human subjects and IRB review is not required. The patient consented to the inclusion of anonymized photos and publications.
Conflicts of Interest
The authors declare no conflicts of interest to report regarding the present study.
References
1. Fuller DB. Prostate stereotactic body radiotherapy—methods, rationale, outcomes, and future directions. In: Stereotactic Body Radiotherapy. London, UK: Springer; 2014. p. 195–224. doi:10.1007/978-0-85729-597-2_11. [Google Scholar] [CrossRef]
2. Sadighian M, Hakam N, Amend G et al. Radiation-induced fistulas in patients with prior pelvic radiotherapy for prostate cancer: a systematic review and meta-analysis. Urology 2023;176:121–126. doi:10.1016/j.urology.2023.03.015. [Google Scholar] [PubMed] [CrossRef]
3. Lobo N, Kulkarni M, Hughes S, Nair R, Khan MS, Thurairaja R. Urologic complications following pelvic radiotherapy. Urology 2018;122:1–9. doi:10.1016/j.urology.2018.07.017. [Google Scholar] [PubMed] [CrossRef]
4. Kishan AU, Dang A, Katz AJ et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open 2019;2(2):e188006. doi:10.1001/jamanetworkopen.2018.8006. [Google Scholar] [PubMed] [CrossRef]
5. Simpson JA, Banerjea A, Scholefield JH. Management of anal fistula. BMJ 2012;345:e6705. doi:10.1136/bmj.e6705. [Google Scholar] [PubMed] [CrossRef]
6. Yanar H, Taviloglu K, Ertekin C et al. Fournier’s gangrene: risk factors and strategies for management. World J Surg 2006;30(9):1750–1754. doi:10.1007/s00268-005-0777-3. [Google Scholar] [PubMed] [CrossRef]
7. Varughese T, Taur Y, Cohen N et al. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin Infect Dis 2018;67(5):687–692. doi:10.1093/cid/ciy175. [Google Scholar] [PubMed] [CrossRef]
8. Nepon H, Safran T, Reece EM, Murphy AM, Vorstenbosch J, Davison PG. Radiation-induced tissue damage: clinical consequences and current treatment options. Semin Plast Surg 2021;35(3):181–188. doi:10.1055/s-0041-1731464. [Google Scholar] [PubMed] [CrossRef]
9. Ho CI, Ballard HJ, Arscott WT et al. Concurrent use of novel agents and radiation is tolerated in lymphoma patients. Blood 2019;134(Supplement_1):2905. doi:10.1182/blood-2019-124195. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools