 Open Access
Open Access
REVIEW
Possible therapeutic role of short-chain fatty acids from skin commensal bacteria in UVB-induced skin carcinogenesis
1 Centre for Biomaterials, Cellular & Molecular Theranostics (CBCMT), Vellore Institute of Technology (VIT), Vellore, 632014, India
2 School of Biosciences and Technology (SBST), Vellore Institute of Technology (VIT), Vellore, 632014, India
* Corresponding Author: RAUNAK KUMAR DAS. Email:
(This article belongs to the Special Issue: Application of Deep Learning in Cancer)
BIOCELL 2023, 47(10), 2195-2205. https://doi.org/10.32604/biocell.2023.030383
Received 03 April 2023; Accepted 01 June 2023; Issue published 08 November 2023
Abstract
Solar ultraviolet B (UVB) radiation is a major skin cancer-causing agent. Initiation, promotion, and progression are the diverse phases of UVB-induced carcinogenesis. Exposure to UVB causes abnormalities in a series of biochemical and molecular pathways: thymine dimer formation, DNA damage, oxidative stress, inflammatory responses, and altered cell signaling, eventually resulting in tumor formation. The increased skin cancer rates urge researchers to develop more efficient drugs, but synthetic chemotherapeutic drugs have more contrary effects and drug resistance issues, which have been reported recently. The current review focuses on the relationship between microbes and cancer. Human skin acts as a barrier against the external environment and serves as a protective shield for its inhabitant microbiota, collectively called skin microbes. The gut microbiome plays a vital role in cancer therapy. Production of short-chain fatty acids (SCFAs) such as butyrate, acetate, and propionate by intestinal microbes has anti-cancer properties against various cancer cell lines. Yet, the knowledge of SCFAs produced by skin microbes remains yet to be elucidated exhaustively. In this review, we strive to summarize the findings of studies performed to date regarding the anti-cancer properties of SCFA against various cancer cell lines and provide insight into future directions in the skin microbiome field.Graphic Abstract
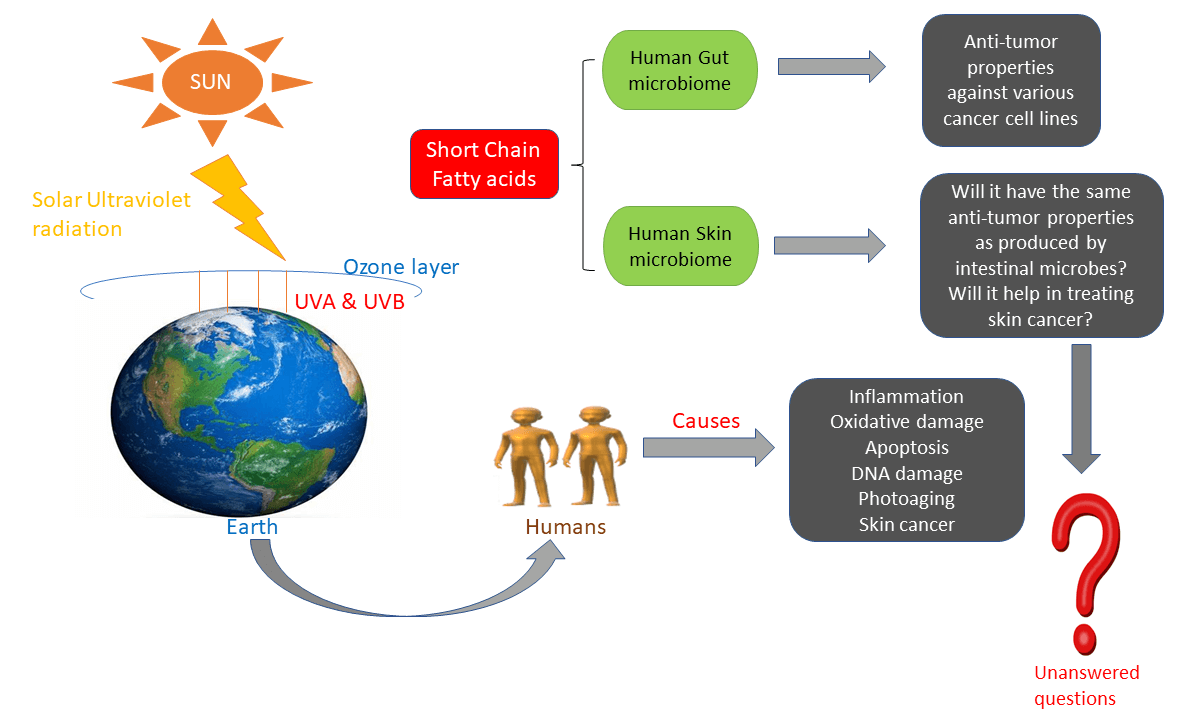
Keywords
In the United States, about 40%–50% of all diagnosed cancers represent skin cancer (Bray et al., 2013) and are extensively classified into (a) melanomas and (b) non-melanoma skin cancers (NMSCs) (Simões et al., 2015). NMSCs include basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) (Apalla et al., 2017). The aggressive form of skin cancer is melanoma which spreads to different areas of the body and accounts for around 70% of skin cancer-related deaths (Nikolaou and Stratigos, 2014; Stockert and Blázquez-Castro, 2022). BCCs have rare metastatic characteristics, whereas SCCs display a high metastatic rate (Didona et al., 2018). Skin is generally susceptible to injury, as it is exposed to pathogens, solar ultraviolet radiation (UVR), and various chemicals that finally lead to the development of skin cancers (Ng et al., 2018). Of all possible exposures, UVR from sunlight has been perceived as the primary causal agent of skin cancer (Solar and Ultraviolet Radiation, 2011). Both UVA and UVB are carcinogens. Especially, UVB directly causes DNA damage, prompting the development of massive damage between adjacent pyrimidine sites and the generation of reactive oxygen species (ROS) (Levav-Cohen et al., 2014). Preventive measures should be taken to overcome the global increase in skin cancer rates (Domingues et al., 2018). This review addresses the harmful side of UVB in skin cancer and the necessity of short-chain fatty acids (SCFAs) in managing them.
Harmful effects of ultraviolet B on the skin
The sun produces electromagnetic radiation that encompasses a broad range of wavelengths. Among them, only a few wavelengths are able to pass through the ozone layer and reach the earth surface; these include ultraviolet (UV) radiation, infrared (IR), and visible light (VL). Solar UV radiations occur in the wavelength range of around 200–400 nm; however, only UVA (400–315 nm) and UVB (315–280 nm) can reach the earthbound surface, while UVC (280–100 nm) is completely absorbed by the ozone layer (Svobodová et al., 2003). Fig. 1 demonstrates how extreme exposure to UVB harms the capacity of basal keratinocytes for maintaining skin homeostasis and will prompt different skin diseases, which incorporate erythema, edema, sunburn, keratinocyte hyperplasia, and causes DNA mutations, photo-aging, and skin cancer (Strozyk and Kulms, 2013).
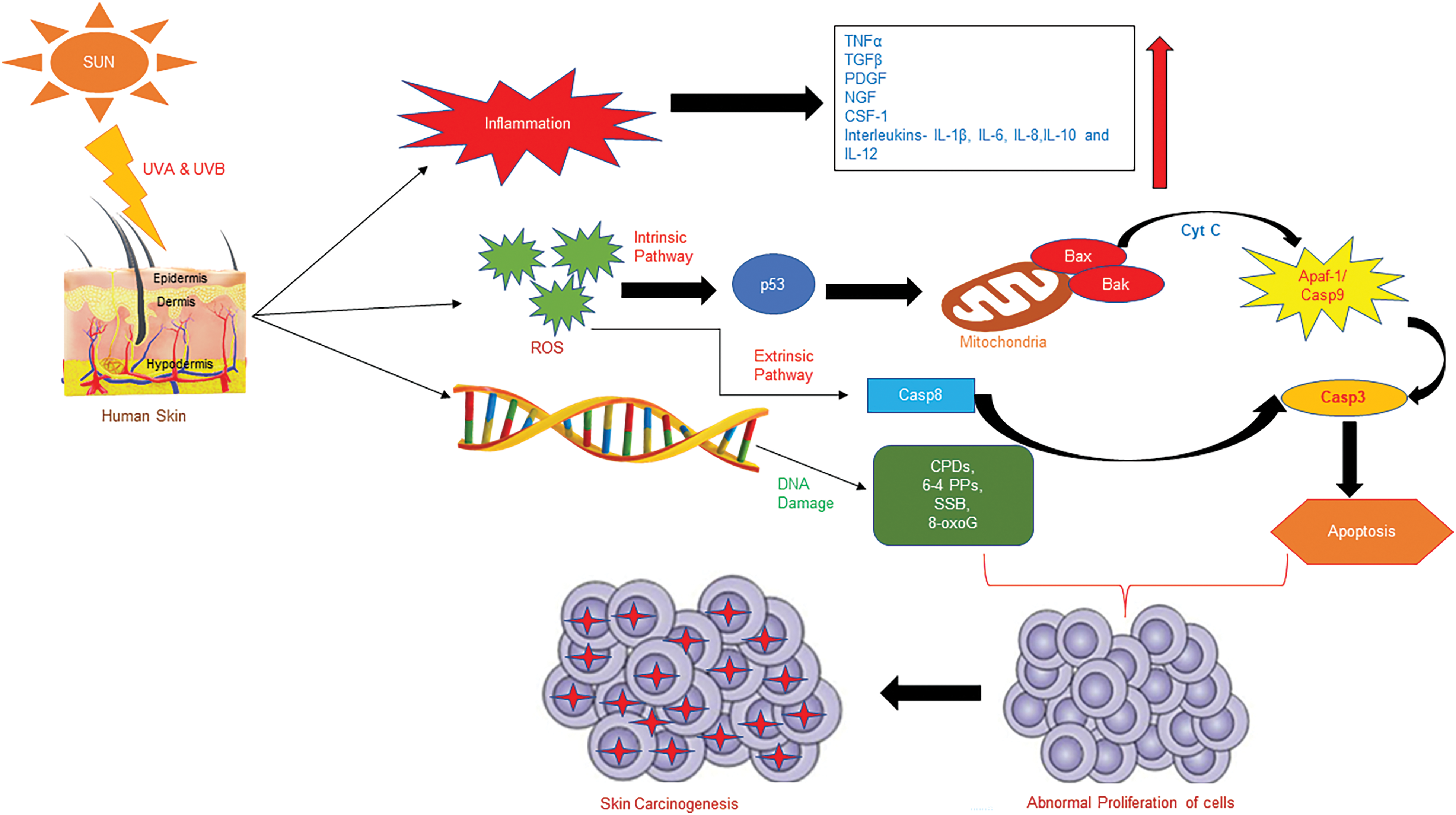
Figure 1: Effects of ultraviolet radiation on human skin. TNFα, tumor necrosis factor alpha; TGFβ, transforming growth factor beta; PDGF, platelet-derived growth factor; NGF, nerve growth factor; CSF-1, colony stimulating factor-1; Cyt C, cytochrome C; APAF-1, apoptotic protease activating factor-1; Casp9, caspase 9; Casp3, caspase 3; Casp8, caspase 8; SSB, single strand break; 8-oxoG, 8-hydroxy-2′-deoxyguanine.
UVB radiation instigated Different DNA damage classes, specifically cyclobutane pyrimidine dimers (CPDs), pyrimidine (6-4) photoproducts (6-4PPs), DNA strand breaks, and DNA cross-links (Brash, 1997). CPDs are the most cytotoxic lesions which are responsible for cell death, but 6-4PPs cause more prominent alterations in the structure of the DNA double helix (Kciuk et al., 2020) and UVA does not induce 6-4PPs. However, longer UV wavelengths cause more DNA strand breaks (Sczepanski et al., 2009). Upon exposure to UVA or UVB, 6-4PPs undergo photoisomerization to Dewar isomers (Kciuk et al., 2020). Tandem pyrimidine residues form at the site of UV-induced DNA damage (Rochette et al., 2003). After DNA damage, cells respond by promptly halting cell division to prevent further DNA damage, which allows the DNA repair mechanism to begin. If the DNA damage induced by UVB is not fixed, it brings about mutations in the genome, prompting skin carcinogenesis (Brash et al., 1991). NER is essential in the repair of CPDs and 6-4 PPs (Sugasawa et al., 1998). The p53 mutations, which include T
The chief cause of both BCC and SCC is exposure to UVR. Almost all BCC occur on UVR-exposed body sites, and SCC originates from a malignant mutation of keratinocytes in the epidermis and skin adnexa and is capable of metastasis (Lo and Fisher, 2014). Early diagnosis of both BCC and SCC can be treated, but the metastatic potential of BCC and SCC is really lethal (Kumar et al., 2015). Studies have proven that UV-induced DNA damage is the chief source of SCCs (Brash et al., 1991). Tumor growth was controlled by Ras proteins which incorporate H-ras, K-ras, and N-ras (Bos, 1989). Any changes in Ras oncogene leads to NMSCs (Pierceall et al., 1991). Hedgehog signaling, which is one of the very important pathways in embryonic development and furthermore identified to be associated with cancer promotion, was regulated by genomic phenylthiocarbamide protein (Wicking et al., 1997).
Vulnerability to ultraviolet radiation is the fundamental source of skin carcinogenesis that disrupts cutaneous cells by reactive oxygen species (ROS) overproduction (Liu-Smith et al., 2017). Direct exposure of the epidermis to UVR can lead to oxidative stress through NADPH oxidase activation or by prompting lipid peroxidation, which initiates ROS. Substantial increase in ROS by UVB irradiation triggers nuclear DNA damage via the development of cyclobutane pyrimidine dimers (CPDs), pyrimidine (6-4) photoproducts, and 8-hydroxy-2′-deoxyguanine (8-OHdG) (Gilbert et al., 2012). 8-OHdG, a biomarker for oxidative DNA damage, binds with adenine rather than cytosine, suggesting that oxidative damage can be tumorigenic (Agar et al., 2004). ROS-mediated carcinogenesis is of two types–genotoxic and non-genotoxic(Benedetti et al., 2021). ROS-mediated genotoxicity causes protooncogene actuation (BRAF, N-Ras, Ras-related C3 botulinum toxin substrate 1, phosphatase and tensin homolog etc.), tumor suppressor gene deactivation (p53 and protein patched homolog 1), genomic instability, and epigenetic modification. These alterations may further lead to mutations (Farhood et al., 2019; Xian et al., 2019). Fig. 2 represents the non-genotoxicity-intervened carcinogenesis that circuitously affects DNA via activation of various pathways-oxidative-stress related pathways and antioxidant stress pathways (Farhood et al., 2019; Xian et al., 2019).
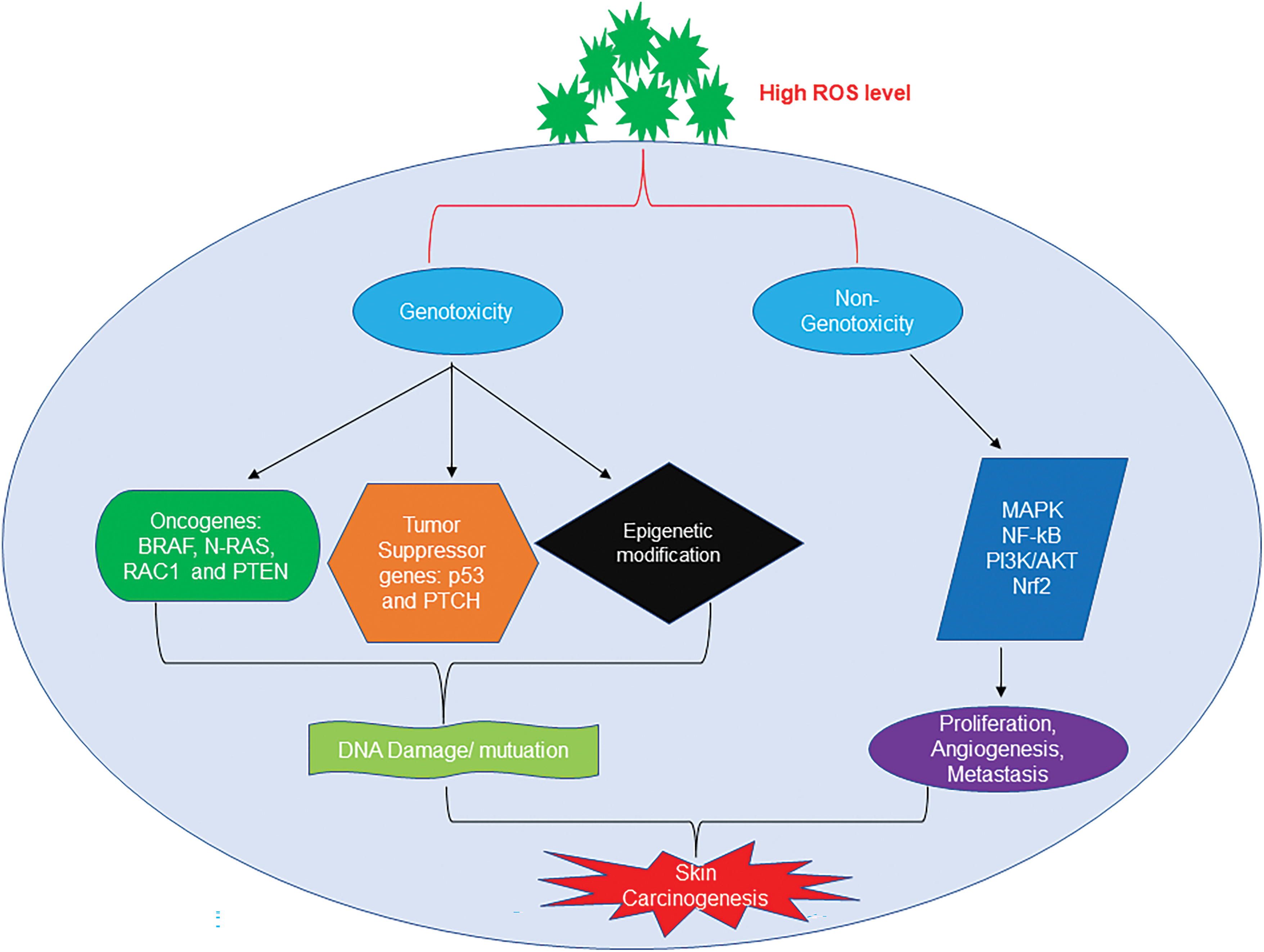
Figure 2: Reactive oxygen species (ROS)-mediated skin carcinogenesis. Increased ROS levels undergo genotoxicity and non-genotoxicity pathways and result in cancer.
ROS induced by UVB is produced through various mechanisms. The very first mechanism is the induction of ROS by the enzyme catalase. Through the catalytic activity, the enzyme catalase degrades hydrogen peroxide into water and oxygen (de Jager et al., 2017). The other antioxidant enzymes, such as glutathione and superoxide dismutase (SOD), are involved in scavenging ROS (Benedetti et al., 2021; Kora et al., 2023). Due to increased ROS production by UVB, these antioxidants fail to scavenge ROS, which altogether results in photocarcinogenesis (Xian et al., 2019). ROS production leads to the activation of mitogen-activated protein kinases (MAPKs), such as extracellular-signal-regulated kinase (ERK) and c-Jun N-terminal kinases (JNK), further leading to the downstream activation of transcription factor AP-1. The regulation of genes involved in the cell cycle, proliferation, and apoptosis is controlled by activator protein 1 and nuclear factor κB (NF-kB) (Bickers and Athar, 2006).
Overexposure to UVR, particularly UVB, can cause inflammation of the skin. Inflammation is the self-defensive response of the body and is classified into-(i) acute inflammation-caused as a result of exposure to any causative agents, which starts rapidly and becomes severe within a few; (ii) chronic inflammation, which can last for a few weeks to several months. Studies have shown that there is a strong relationship between UVR-spurred inflammation and cancer in the skin. Cancer mostly forms at the site of chronic inflammation (Balkwill and Mantovani, 2001). Exposure to UVB primes the secretion of various cytokines and chemokines from different skin cells, i.e., keratinocytes and Langerhans cells secretes a number of cytokines, such as transforming growth factor beta (TGFβ), tumor necrosis factor-alpha (TNFα), growth factors such as platelet-derived growth factor (PDGF), nerve growth factor (NGF), colony stimulating factor-1 (CSF-1), etc., and interleukins such as IL-1β, IL-6, IL-8, IL-10, and IL-12. UVB irradiation induces keratinocytes to deliver these cytokines, and the liberation of this production has been depicted in several skin cancers (van Kempen et al., 2003).
The activation of p38, MAPK, and Akt by UVB helps in the endurance of keratinocytes and opposes apoptosis that results in the accumulation of DNA damage, which may prompt malignant growth (Lee et al., 2011). UVB instigates the PI3K pathway for cell survival and aggravation, accomplished as a downstream movement of epithelial growth factor receptor (Wan et al., 2001). DNA damage by UVB is sufficient to intervene in NF-kB stimulation (Abeyama et al., 2000). Besides, (Simon et al., 1994) showed that UVB can directly actuate NF-kB from chromosomal DNA damage. Inhibitors of nuclear factor-kB kinase alpha (IKKα) and NF-kB assume a major function in maintaining skin homeostasis. Decrease in the outflow of IKKα promotes UVB-induced chronic inflammation and the process of carcinogenesis in mice models (van Kempen et al., 2003). IKKα similarly has a role in the promotion of SCC (Van Waes et al., 2007).
Solar UVB is a potent genotoxic agent that leads to apoptosis, portrayed by membrane blebbing and nuclear fragmentation (Su et al., 2015). Skin cells activate apoptosis to overcome the damage caused by UVB (Van Waes et al., 2007). UVB irradiation, a strong inducer of apoptosis in cultured cells, triggers both intrinsic and extrinsic pathways (Su et al., 2015). However, a few cells escape and may result in tumorigenesis. Henceforth, for the protection of normal cells from UVB, apoptosis aids as an essential process (Elmore, 2007; Zhang et al., 2023).
(i) Intrinsic pathway: UVB-triggered cell death generally occurs through the intrinsic apoptotic pathway, which is initiated by p53. Transformed p53 often exists in non-melanoma skin cancers (Rodust et al., 2009). In mitochondria, p53 increases the production of the pro-apoptotic protein, such as Bcl-2 associated X-protein (Bax), and diminishes the action of anti-apoptotic proteins, for example, B-cell lymphoma 2 (Bcl-2). The modification of the outer mitochondrial membrane structure by UVB gives rise to an imbalance in Bax/Bcl-2 ratio and delivers cytochrome c. Once delivered, cytochrome c and the apoptotic protease activating factor-1 form the apoptosome. This protein complex recruits and enacts caspase 9 and 3, bringing about apoptosis (Singh et al., 2019).
(ii) Extrinsic pathway: The multimerization of CD95 (Fas/APO-1) by UVB brings out its attachment to the adapter protein Fas-associated protein with death domain, followed by the activation of caspase cascade from caspase 8 to caspase 3 (Bang et al., 2003). After UVB irradiation, the TNF receptor is grouped and internalized in keratinocytes. TRAIL receptors, including TRAIL-R1 and TRAIL-R2, serve as lure receptors because of their competitive binding to cease apoptosis. UVB irradiation may change this equilibrium and may induce TRAIL-mediated apoptosis by the hindrance of binding with lure receptors (Qin et al., 2004).
Epithelial-mesenchymal transition (EMT) and skin carcinogenesis
In the property of invasiveness, EMT plays a leading role in which the phenotypic changes combined with EMT contribute to tumor heterogeneity and therapeutic resistance (Shu et al., 2013; Veloz et al., 2021). EMT is facilitated by EMT-inducing transcription factors (EMT-TFs) and is connected with normal organ advancement, wound healing, and the intrusiveness of cancer cells (Sato et al., 2016). TGF-β is the key regulator persuader of EMT; also, the epidermal growth factor (EGF), fibroblast growth factor, hepatocyte growth factor, Wnt, and ECM components have been proven to prompt this action (Nieto and Cano, 2012). EMT-TFs can be classified into two groups, in which one group suppresses the expression of E-cadherin, Snail, Slug, ZEB1, ZEB2, E47, KLF8, and Brachyury directly correlate with the E-cadherin gene promoter to arrest gene expression. In contrast, the other group-Twist1, FOXC2, Goosecoid, E2-2, SIX1, and PRRX1 trigger the EMT without direct attachment to the E-cadherin gene promoter (De Craene and Berx, 2013; Yang et al., 2021). N-cadherin, fibronectin, and vimentin are the mesenchyme-associated genes whose expression is initiated by EMT-TFs. EMT prompts the up-regulation of N-cadherin and simultaneous down-regulation of E-cadherin, termed as “Cadherin Switch Field” (Tyagi et al., 2015). In various human cancers, overexpression of EMT-TFs has been identified in various human cancers (De Craene and Berx, 2013). UVB-irradiated HaCaT cells exhibit amplified aggressiveness with exaggerated migration and invasive potential, and mesenchymal phenotypes, which validate that UVB causes EMT and is related to skin carcinogenesis (Tyagi et al., 2015).
Short-chain fatty acids in biological research
Fatty acids (FA) are essential components for energy metabolism, stability of cell membranes, and modulation in multiple biological processes. Based on the hydrocarbon chain length, FA is classified into short-, medium- and long-chain fatty acids (Fung et al., 2011). The FA are mainly produced in the diet. SCFA can be easily absorbed, recycled, and metabolized in the body to make the body functional. Fig. 3 presents the pathway for SCFA production.
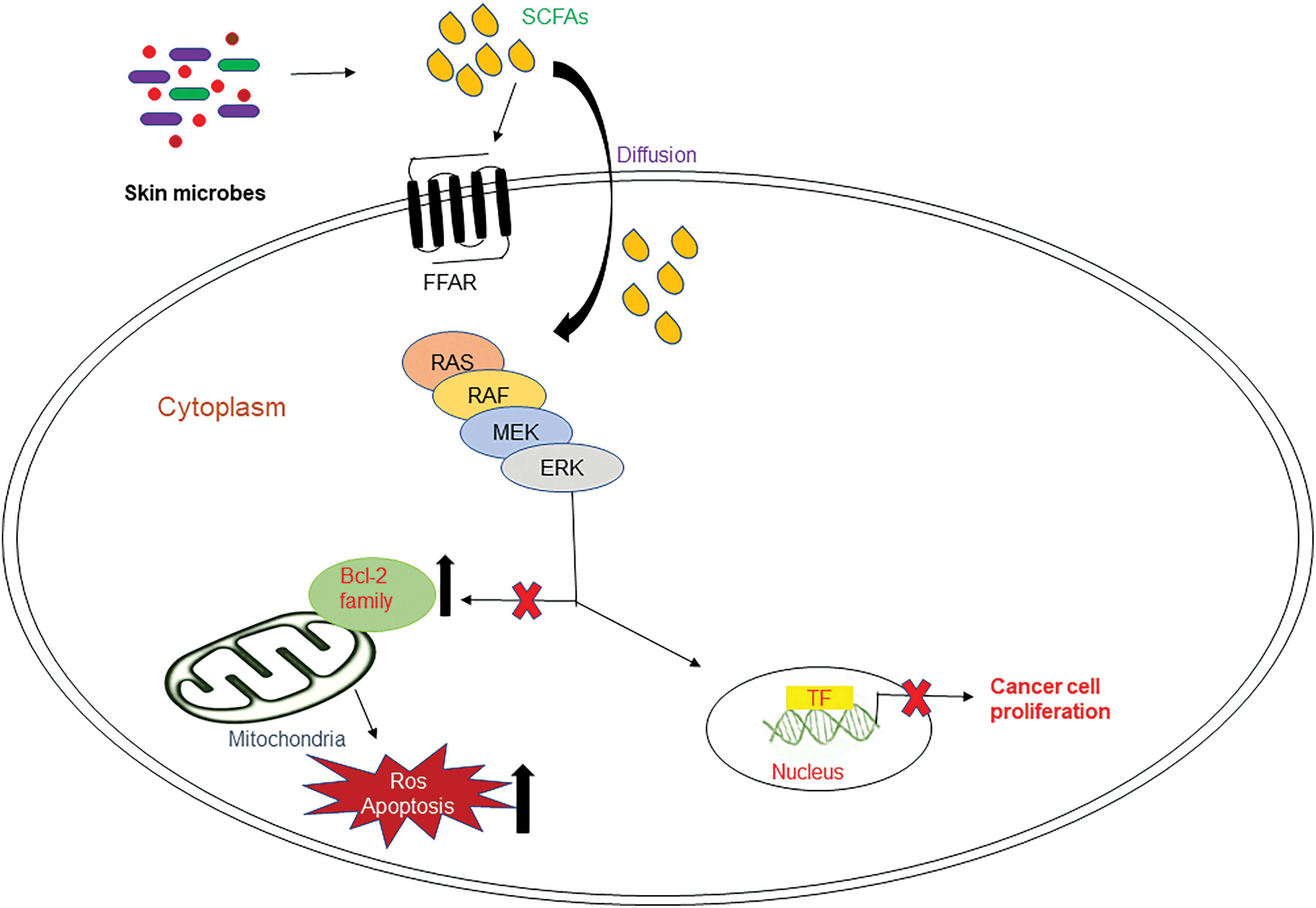
Figure 3: Production of small chain fatty acid. Intestinal microbes hydrolyze indigestible carbohydrates and produce short-chain fatty acids (SCFAs).
Even though the uptake of long-chain fatty acids (LCFA) and medium-chain fatty acids (MCFA) occur through similar mechanisms, there exist some differences. Both LCFA and MCFA are absorbed to some extent, and are the rate-limiting step for MCFA and LCFA is passaging across the brush-border membrane and unstirred water layer (UWL), respectively (Schönfeld and Wojtczak, 2016; Xu et al., 2021). For cellular uptake, intracellular transport, and metabolism, LCFA needs free fatty acid-binding proteins. However, SCFAs require no or much fewer free fatty acid binding proteins for their intracellular transport and metabolism. Also, SCFAs are secreted endogenously, whereas MCFAs and LCFAs are exogenous (Schönfeld and Wojtczak, 2016).
Based on the efficiency and bioavailability of SCFA, it is broadly used in research. Acetate (C2), propionate (C3), and butyrate (C4) are most abundantly secreted SCFAs via the gut flora as an end product of anaerobic fermentation (Fig. 3) (Parada Venegas et al., 2019). In the gut, Bacteroidetes and Firmicutes are the predominant bacterial phyla, whereas Actinobacteria, Proteobacteria, and Verrucomicrobia are the minor phyla. These microbes ferment most of the dietary substances and produce an array of metabolites, in which many are beneficial (Kobayashi et al., 2018). SCFAs have been linked to beneficial effects on human health associated with their metabolic and signaling properties. Fig. 4 represents the regulatory functions of SCFAs produced by gut flora will depend on specific receptors expressed in different cell types, which are mainly free fatty acid receptors (Keshari et al., 2019).
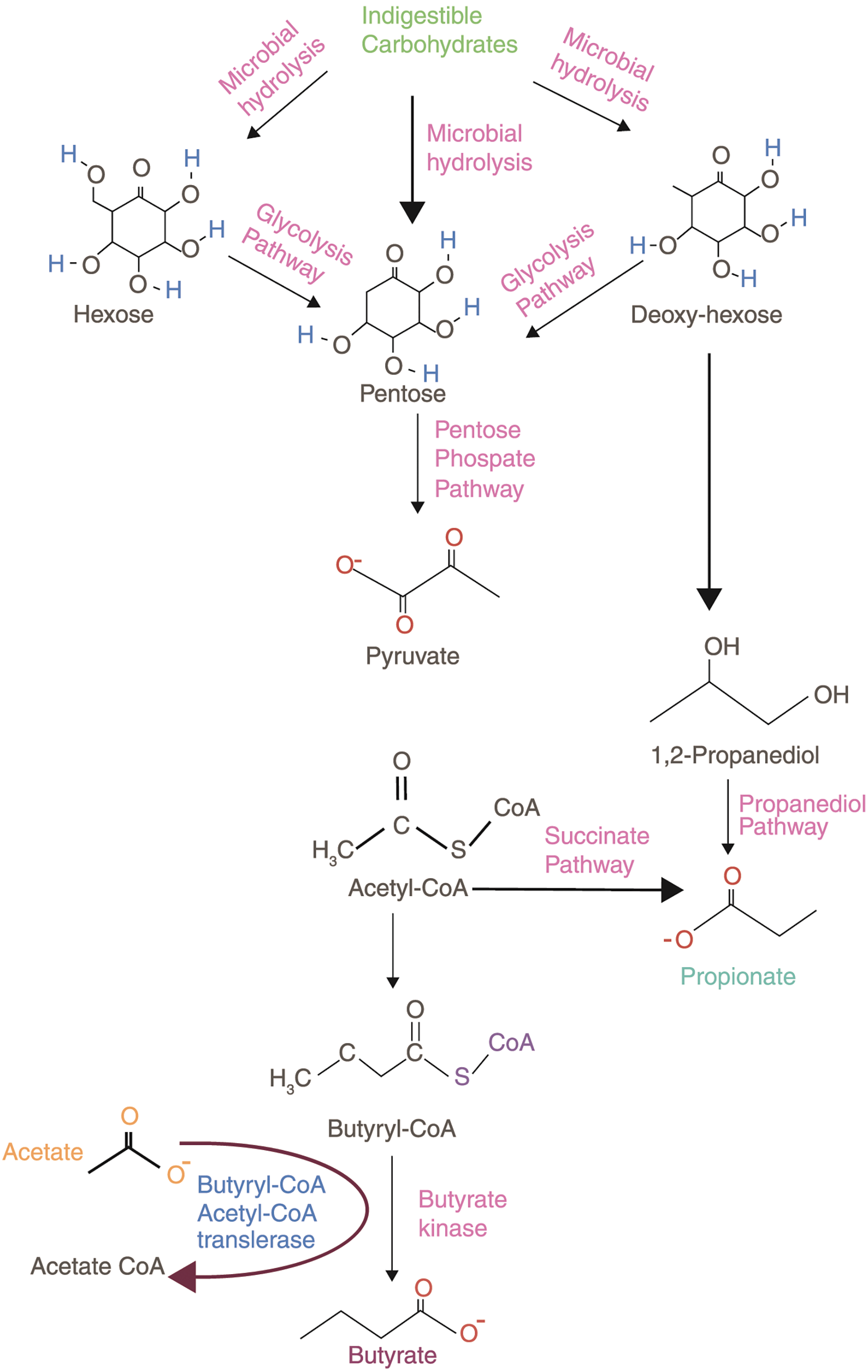
Figure 4: Short-chain fatty acids and their receptors. FFAR1: free fatty receptor 1; FFAR2: free fatty acid receptor 2; FFAR3: free fatty acid receptor 3; GPR40: G-protein coupled receptor 40; GPR41: G-protein coupled receptor 41; GPR43: G-protein coupled receptor 43; GPR109A: G-protein coupled receptor 109A; olfr78: olfactory receptor 78.
Each SCFAs bind to its specific free fatty acid receptors and mediates the cyclic adenosine monophosphate (cAMP) or extracellular-signal-regulated kinase 1/2 (ERK 1/2) signaling via G-protein dependent or independent pathways (Marinissen and Gutkind, 2001). The epithelial cells have free fatty acid receptors FFAR3 or GPR41, FFAR2 or GPR43, and GPR109A. Enteric neurons and intestinal leukocytes express GPR41 and GPR43, respectively, while intestine endocrine cells express both GPR41 and GPR43 (Sivaprakasam et al., 2016). The list of SCFAs receptors and their functions are briefed in Table 1.
Evidence for the benefits of SCFAs
The comprehensive anti-tumor properties of acetate, propionate, and butyrate are mentioned in this review. Butyrate renders protection against carcinogenesis by delivering 70% energy to colonocytes and sustains intestinal barrier functions, reducing inflammation (Bedford and Gong, 2018). Butyrate is produced by specific gut bacteria that come under the order Clostridiales, such as Lachnospiraceae (Coprococcus, Eubacterium, Anaerostipes, and Roseburia), Ruminococcaceae (Faecalibacterium and Subdoligranulum) and Erysipelotrichaceae (Holdemanella) (Louis et al., 2014; Flint, 2016). Many investigations have shown that butyrate exhibits anti-cancer activity through various signaling pathways that control cell survival and apoptosis in multiple cancer cells (Candido et al., 1978). Detailed information on the effects of butyrate on oncogenic signaling pathways is provided in an earlier publication (Chen et al., 2019). In vivo investigations revealed that butyrate diminishes the rate of colon cancer (McIntyre et al., 1993). Gonçalves et al. (2011) indicated that butyrate is a breast cancer-resistant protein (BCRP) substrate. Butyrate-induced apoptosis in a colon cancer cell line HCT116 (Fung et al., 2011). The anti-cancer effect of butyrate was explored in a breast cancer cell line MCF-7 (Yonezawa et al., 2007). The viability of U937 leukemia cells was decreased by butyrate to about 60% (Pulliam et al., 2016). 12-0-tetradecanoylphorbol-13-acetate (TPA) induced skin tumors in mice were reduced by topical application of butyric acid (Gupta and Mehrotra, 1997). This provides us the evidence that butyrate has anti-cancer properties against multiple cancer cell types.
Acetate is one of the most important SCFAs and has been less explored than propionate and butyrate. Also, acetate is the net fermentation end product for most gut bacteria, while butyrate and propionate are produced by very specific species (Martin-Gallausiaux et al., 2021). Acetate impedes proliferation and stimulates apoptosis in colon cancer cells, also acetate-induced apoptosis in CRC cells, further roots to mitochondrial alterations (Marques et al., 2013). The primary end products of Propionibacteria were acetate and propionate, which destroys two human adenocarcinoma cell lines by apoptosis through co-cultures with the dairy species Propionibacterium freudenreichii and Propionibacterium acidipropionici (Jan et al., 2002).
The anti-inflammatory properties of acetate and propionate were proved in human monocytes and in vivo colitis models (Cox et al., 2009; Maslowski et al., 2009). Propionate is considered the most powerful endogenic agonist for both G-protein coupled receptors, free fatty acid receptor 3 (FFA3) and FFA2 (Brown et al., 2003; Le Poul et al., 2003). Propionate is produced by Bacteroidetes and some Firmicutes, such as the Negativicutes (Veillonella and Phascolarctobacterium). Some other Firmicutes, belonging to Negativicutes (Megasphaera), Lachnospiraceae (Coprococcus), Ruminococcaceae, Proteobacteria, and Lachnospiraceae species (Martin-Gallausiaux et al., 2021). In vitro studies have shown that propionate reduces BaF3 cell proliferation through a cAMP level-dependent pathway, and FFA2 activation changes BaF3 cell growth which shows that propionate decreases cancer cell proliferation in the liver. An ongoing investigation (Høgh et al., 2020) revealed that the propionate causes metabolic changes bringing about the natural-killer group 2, member D (NKG2D) ligand surface expression, which renders as a potential immune activating anti-cancer therapy. Also, studies by Kim et al. (2019) have demonstrated that propionate prompts cell apoptosis and cell cycle arrest in lung cancer.
The anti-cancer properties of these SCFAs against colorectal cancer cells has been listed in numerous publications (Gu et al., 2012; Kobayashi et al., 2018; Ohara and Suzutani, 2018; You et al., 2018). In human colon cells and neutrophils, the anti-proliferative capacity of SCFAs has been related to the capacity of SCFA to hinder histone deacetylase function (Aoyama et al., 2010). The most remarkable histone deacetylase inhibitor is butyrate, though propionate exhibited the intermediate profile and acetate didn’t impact the deacetylase activity (Aoyama et al., 2010). Studies by (Maslowski et al., 2009; Pirozzi et al., 2018; Keshari et al., 2019) have shown that butyric acid from skin commensal bacteria reduces inflammation by binding to its free fatty acid receptors. Fig. 5 shows the possible molecular mechanisms of small-chain fatty acids in cancer cell inhibition (He et al., 2020).
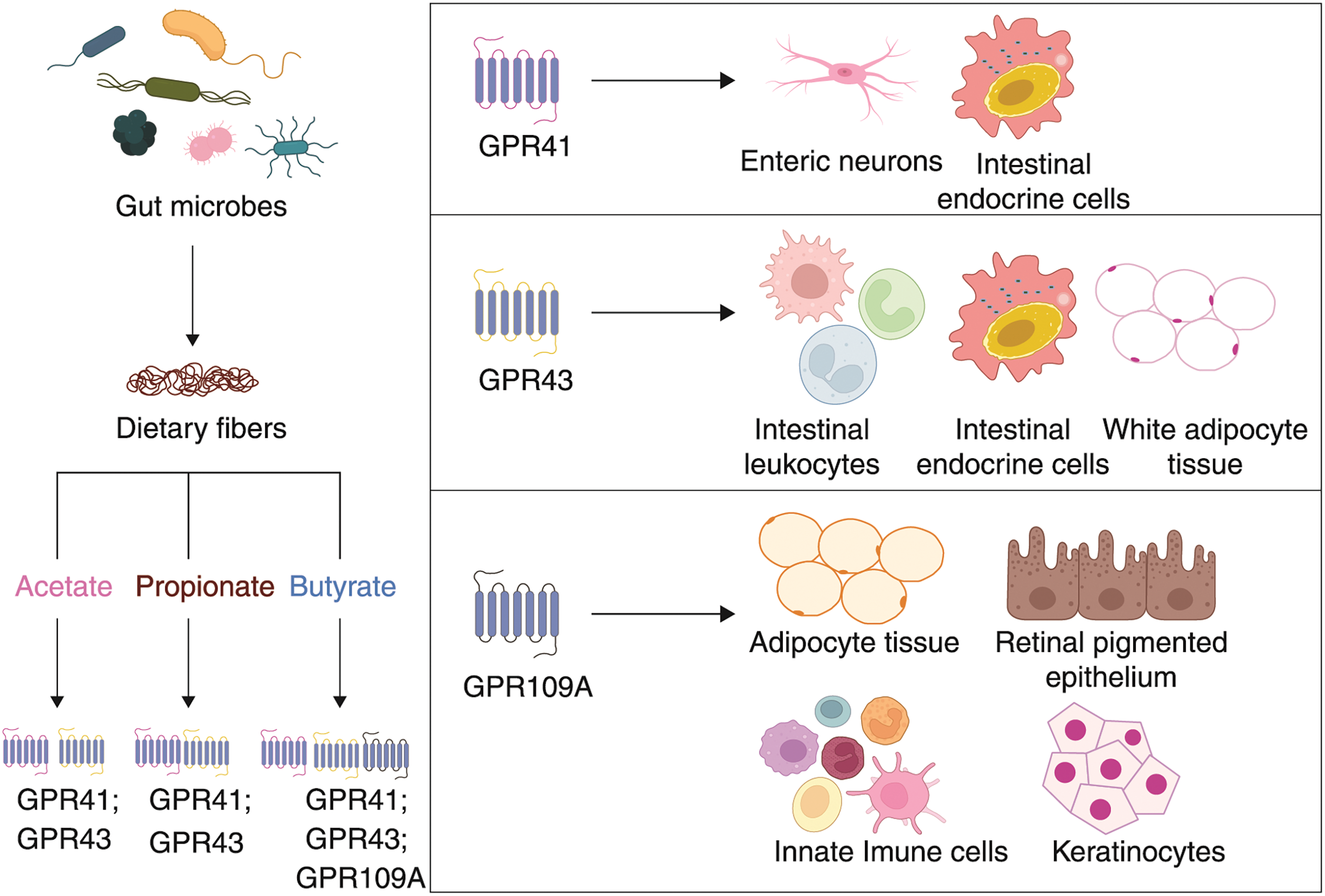
Figure 5: Possible mechanism of small chain fatty acids in cancer in the RAS-RAF-ERK kinase (MEK)-extracellular-signal-regulated kinase (ERK) signal transduction cascade. Skin commensal bacteria produce SCFAs that bind to specific FFARs and inhibit Bcl-2 family proteins, thereby increasing ROS and apoptosis, altogether inhibiting cancer cell proliferation.
Being a very delicate organ, skin cells are very susceptible to UVR. The studies elaborated above clearly indicate the potential of UVB in prompting skin cancer. An increase in skin cancer rates triggers researchers to develop novel and efficient therapeutic strategies to treat skin cancer. The benefits of SCFAs in various cancer cell lines were listed out in this review. Although SCFAs produced by gut microbes play a vital role in reducing inflammation and carcinogenesis, the time required for them to travel the skin exceeds the optimal duration (Chen et al., 2019). Thus, an effective methodology in which a considerable amount of SCFAs reaching the skin in enough time need to be discovered.
The skin is a resilient organ that provides diverse microbial habitats. The role of skin microbes on the skin remain understudied, and their microbiome are largely unknown. Along with SCFA, MCFA also have anti-inflammatory properties; however, the lower absorption capacity of MCFA remains a drawback. SCFAs are not just secreted by gut commensal bacteria, and even skin microbiome ferments glycerol in the skin and produces SCFAs (Sahuri-Arisoylu et al., 2021). Glycerol metabolism in microorganisms has been investigated for >50 years. Therefore, the period of SCFAs drifting from gut to skin will be minimized if it is already produced by the skin commensals. MCFA and LCFA are not produced by the skin microbes, and it is only available through the diet. The knowledge of the underlying mechanisms of SCFAs from skin commensals is not yet clear. Hence, we propose that understanding the functions of SCFAs which are secreted by skin microbes, will be more supportive in studying their effects on skin cancer. Considering all the published reports, SCFAs are non-toxic to skin cells; the role of SCFAs from skin microbiome in skin cancer needs to be discovered with the actual aim of avoiding the intense effects of chronic UVB. Overall, a better understanding of skin commensals could lead the way to reduce skin cancer burden.
Acknowledgement: We would like to thank the Centre for Biomaterials, Cellular & Molecular Theranostics, School of Biosciences and Technology, Vellore Institute of Technology, Vellore.
Funding Statement: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions: Conceptualization, literature search, and data analysis, writing-original draft preparation: Pavithra S; Reviewing and editing, approval, investigation: Dr. Raunak Kumar Das. All authors read and approved the final manuscript.
Availability of Data and Materials: Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Abeyama K, Eng W, Jester JV, Vink AA, Edelbaum D, Cockerell CJ, Bergstresser PR, Takashima A (2000). A role for NF-kB—dependent gene transactivation in sunburn. Journal of Clinical Investigation 105: 1751–1759. https://doi.org/10.1172/JCI9745 [Google Scholar] [PubMed] [CrossRef]
Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM (2004). The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America 101: 4954–4959. https://doi.org/10.1073/pnas.0401141101 [Google Scholar] [PubMed] [CrossRef]
Aoyama M, Kotani J, Usami M (2010). Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26: 653–661. https://doi.org/10.1016/j.nut.2009.07.006 [Google Scholar] [PubMed] [CrossRef]
Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Loannides D (2017). Epidemiological trends in skin cancer what does the future hold. Dermatology Practical & Conceptual 7: 1–6. https://doi.org/10.5826/dpc.0702a01 [Google Scholar] [PubMed] [CrossRef]
Balkwill F, Mantovani A (2001). Inflammation and cancer: Back to Virchow. Lancet 357: 539–545. https://doi.org/10.1016/S0140-6736(00)04046-0 [Google Scholar] [CrossRef]
Bang B, Gniadecki R, Larsen JK, Baadsgaard O, Skov L (2003). In vivo UVB irradiation induces clustering of Fas (CD95) on human epidermal cells. Experimental Dermatology 12: 791–798. https://doi.org/10.1111/j.0906-6705.2003.00091.x [Google Scholar] [PubMed] [CrossRef]
Bedford A, Gong J (2018). Implications of butyrate and its derivatives for gut health and animal production. Animal Nutrition 4: 151–159. https://doi.org/10.1016/j.aninu.2017.08.010 [Google Scholar] [PubMed] [CrossRef]
Benedetti M, Giuliani ME, Mezzelani M, Nardi A, Pittura L, Gorbi S, Regoli F (2021). Emerging environmental stressors and oxidative pathways in marine organisms: Current knowledge on regulation mechanisms and functional effects. BIOCELL 46: 37–49. https://doi.org/10.32604/biocell.2022.017507 [Google Scholar] [CrossRef]
Bickers DR, Athar M (2006). Oxidative stress in the pathogenesis of skin disease. Journal of Investigative Dermatology 126: 2565–2575. https://doi.org/10.1038/sj.jid.5700340 [Google Scholar] [PubMed] [CrossRef]
Bos JL (1989). Ras oncogenes in human cancer: A review. Cancer Research 49: 4682–4689. [Google Scholar] [PubMed]
Brash DE (1997). Sunlight and the onset of skin cancer. Trends in Genetics 13: 410–414. https://doi.org/10.1016/S0168-9525(97)01246-8 [Google Scholar] [PubMed] [CrossRef]
Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Pontén J (1991). A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America 88: 10124–10128. https://doi.org/10.1073/pnas.88.22.10124 [Google Scholar] [PubMed] [CrossRef]
Bray F, Ren JS, Masuyer E, Ferlay J (2013). Global estimates of cancer prevalence for 27 sites in the adult population in 2008. International Journal of Cancer 132: 1133–1145. https://doi.org/10.1002/ijc.27711 [Google Scholar] [PubMed] [CrossRef]
Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L et al. (2003). The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. Journal of Biological Chemistry 278: 11312–11319. https://doi.org/10.1074/jbc.M211609200 [Google Scholar] [PubMed] [CrossRef]
Candido EPM, Reeves R, Davie JR (1978). Sodium butyrate inhibits histone deacetylation in cultured cells. Cell 14: 105–113. https://doi.org/10.1016/0092-8674(78)90305-7 [Google Scholar] [PubMed] [CrossRef]
Chen J, Zhao KN, Vitetta L (2019). Effects of intestinal microbial-elaborated butyrate on oncogenic signaling pathways. Nutrients 11: 1–26. https://doi.org/10.3390/nu11051026 [Google Scholar] [PubMed] [CrossRef]
Coakley MF, Hurt DE, Weber N, Mtingwa M, Fincher EC et al. (2014). The NIH 3D print exchange: A public resource for bioscientific and biomedical 3D prints. 3D Printing and Additive Manufacturing 1: 137–140. https://doi.org/10.1089/3dp.2014.1503 [Google Scholar] [PubMed] [CrossRef]
Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L et al. (2009). Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E2 and cytokines. World Journal of Gastroenterology 15: 5549–5557. https://doi.org/10.3748/wjg.15.5549 [Google Scholar] [PubMed] [CrossRef]
De Craene B, Berx G (2013). Regulatory networks defining EMT during cancer initiation and progression. Nature Reviews Cancer 13: 97–110. https://doi.org/10.1038/nrc3447 [Google Scholar] [PubMed] [CrossRef]
de Jager TL, Cockrell AE, Du Plessis SS (2017). Ultraviolet light induced generation of reactive oxygen species. Advances in Experimental Medicine and Biology 996: 15–23. https://doi.org/10.1007/978-3-319-56017-5 [Google Scholar] [CrossRef]
Didona D, Paolino G, Bottoni U, Cantisani C (2018). Non melanoma skin cancer pathogenesis overview. Biomedicines 6: 1–15. https://doi.org/10.3390/biomedicines6010006 [Google Scholar] [PubMed] [CrossRef]
Domingues B, Lopes JM, Soares P, Pópulo H (2018). Melanoma treatment in review. ImmunoTargets and Therapy 7: 35–49. https://doi.org/10.2147/itt.s134842 [Google Scholar] [PubMed] [CrossRef]
Elmore S (2007). Apoptosis: A review of programmed cell death. Toxicologic Pathology 35: 495–516. https://doi.org/10.1080/01926230701320337 [Google Scholar] [PubMed] [CrossRef]
Farhood B, Najafi M, Salehi E, Hashemi Goradel N, Nashtaei MS, Khanlarkhani N, Mortezaee K (2019). Disruption of the redox balance with either oxidative or anti-oxidative overloading as a promising target for cancer therapy. Journal of Cellular Biochemistry 120: 71–76. https://doi.org/10.1002/jcb.27594 [Google Scholar] [PubMed] [CrossRef]
Flint HJ (2016). Gut microbial metabolites in health and disease. Gut Microbes 7: 187–188. https://doi.org/10.1080/19490976.2016.1182295 [Google Scholar] [PubMed] [CrossRef]
Fung KY, Brierley GV, Henderson S, Hoffmann P, McColl SR, Lockett T, Head R, Cosgrove L (2011). Butyrate-induced apoptosis in HCT116 colorectal cancer cells includes induction of a cell stress response. Journal of Proteome Research 10: 1860–1869. https://doi.org/10.1021/pr1011125 [Google Scholar] [PubMed] [CrossRef]
Gilbert R, Martin R, Clark J (2012). Non-melanoma and melanoma skin cancer. In: Stell and Maran’s Textbook of Head and Neck Surgery and Oncology. CRC Press. https://doi.org/10.1201/b13389-44 [Google Scholar] [CrossRef]
Gonçalves P, Gregório I, Martel F (2011). The short-chain fatty acid butyrate is a substrate of breast cancer resistance protein. American Journal of Physiology-Cell Physiology 301: 984–995. https://doi.org/10.1152/ajpcell.00146.2011 [Google Scholar] [PubMed] [CrossRef]
Gu S, Tian Y, Chlenski A, Salwen HR, Lu Z, Raj JU, Yang Q (2012). Valproic acid shows a potent antitumor effect with alteration of DNA methylation in neuroblastoma. Anti-Cancer Drugs 23: 1054–1066. https://doi.org/10.1097/CAD.0b013e32835739dd [Google Scholar] [PubMed] [CrossRef]
Gupta KP, Mehrotra NK (1997). Differential effects of butyric acid on mouse skin tumorigenesis. Biomedical and Environmental Sciences 10: 436–441. [Google Scholar] [PubMed]
He J, Zhang P, Shen L, Niu L, Tan Y et al. (2020). Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. International Journal of Molecular Sciences 21: 1–16. https://doi.org/10.3390/ijms21176356 [Google Scholar] [PubMed] [CrossRef]
Høgh RI, Møller SH, Jepsen SD, Mellergaard M, Lund A, Pejtersen M, Fitzner E, Andresen L, Skov S (2020). Metabolism of short-chain fatty acid propionate induces surface expression of NKG2D ligands on cancer cells. FASEB Journal 34: 15531–15546. https://doi.org/10.1096/fj.202000162R [Google Scholar] [PubMed] [CrossRef]
Jan G, Belzacq AS, Haouzi D, Rouault A, Métivier D, Kroemer G, Brenner C (2002). Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death and Differentiation 9: 179–188. https://doi.org/10.1038/sj.cdd.4400935 [Google Scholar] [PubMed] [CrossRef]
Kciuk M, Marciniak B, Mojzych M, Kontek R (2020). Focus on UV-induced DNA damage and repair—disease relevance and protective strategies. International Journal of Molecular Sciences 21: 1–33. https://doi.org/10.3390/ijms21197264 [Google Scholar] [PubMed] [CrossRef]
Keshari S, Balasubramaniam A, Myagmardoloonjin B, Herr DR, Negari IP, Huang CM (2019). Butyric acid from probiotic staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptor. International Journal of Molecular Sciences 20: 4477. https://doi.org/10.3390/ijms20184477 [Google Scholar] [PubMed] [CrossRef]
Kim K, Kwon O, Ryu TY, Jung CR, Kim J, Min JK, Kim DS, Son MY, Cho HS (2019). Propionate of a microbiota metabolite induces cell apoptosis and cell cycle arrest in lung cancer. Molecular Medicine Reports 20: 1569–1574. https://doi.org/10.3892/mmr.2019.10431 [Google Scholar] [PubMed] [CrossRef]
Klungland A, Rosewell I, Hollenbach S, Larsen E, Daly G, Epe B, Seeberg E, Lindahl T, Barnes DE (1999). Accumulation of premutagenic DNA lesions in mice defective in removal of oxidative base damage. Proceedings of the National Academy of Sciences of the United States of America 96: 13300–13305. https://doi.org/10.1073/pnas.96.23.13300 [Google Scholar] [PubMed] [CrossRef]
Kobayashi M, Mikami D, Uwada J, Yazawa T, Kamiyama K, Kimura H, Taniguchi T, Iwano M (2018). A short-chain fatty acid, propionate, enhances the cytotoxic effect of cisplatin by modulating GPR41 signaling pathways in HepG2 cells. Oncotarget 9: 31342–31354. https://doi.org/10.18632/oncotarget.25809 [Google Scholar] [PubMed] [CrossRef]
Kora D, Dey A, Pal B, Roy UK, Dey N, Bhatacharjee T, Bhattacharjee S (2023). ROS-hormone interaction in regulating integrative défense signaling of plant cell. BIOCELL 47: 503–521. https://doi.org/10.32604/biocell.2023.025744 [Google Scholar] [CrossRef]
Kumar R, Deep G, Agarwal R (2015). An overview of ultraviolet B radiation-induced skin cancer chemoprevention by silibinin. Current Pharmacology Reports 1: 206–215. https://doi.org/10.1007/s40495-015-0027-9 [Google Scholar] [PubMed] [CrossRef]
Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V et al. (2003). Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. Journal of Biological Chemistry 278: 25481–25489. https://doi.org/10.1074/jbc.M301403200 [Google Scholar] [PubMed] [CrossRef]
Lee ER, Kim JH, Choi HY, Jeon K, Cho SG (2011). Cytoprotective effect of eriodictyol in UV-irradiated keratinocytes via phosphatase-dependent modulation of both the p38 MAPK and Akt signaling pathways. Cellular Physiology and Biochemistry 27: 513–524. https://doi.org/10.1159/000329973 [Google Scholar] [PubMed] [CrossRef]
Levav-Cohen Y, Goldberg Z, Tan KH, Alsheich-Bartok O, Zuckerman V, Haupt S, Haupt Y (2014). The p53-Mdm2 loop: A critical juncture of stress response. Sub-Cellular Biochemistry 85: 161–186. https://doi.org/10.1007/978-94-017-9211-0 [Google Scholar] [CrossRef]
Liu-Smith F, Jia J, Zheng Y (2017). UV-induced molecular signaling differences in melanoma and non-melanoma skin cancer. Advances in Experimental Medicine and Biology 996: 27–40. https://doi.org/10.1007/978-3-319-56017-5 [Google Scholar] [CrossRef]
Lo JA, Fisher DE (2014). The melanoma revolution: From UV carcinogenesis to a new era in therapeutics. Science 346: 945–949. https://doi.org/10.1126/science.1253735 [Google Scholar] [PubMed] [CrossRef]
Louis P, Hold GL, Flint HJ (2014). The gut microbiota, bacterial metabolites and colorectal cancer. Nature Reviews Microbiology 12: 661–672. https://doi.org/10.1038/nrmicro3344 [Google Scholar] [PubMed] [CrossRef]
Lu H, Stratton CW, Tang YW (2020). Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. Journal of Medical Virology 92: 401–402. https://doi.org/10.1002/jmv.25678 [Google Scholar] [PubMed] [CrossRef]
Lukasova M, Malaval C, Gille A, Kero J, Offermanns S (2011). Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. The Journal of Clinical Investigation 121: 1163–1173. https://doi.org/10.1172/JCI41651 [Google Scholar] [PubMed] [CrossRef]
Marinissen MJ, Gutkind JS (2001). G-protein-coupled receptors and signaling networks: Emerging paradigms. Trends in Pharmacological Sciences 22: 368–376. https://doi.org/10.1016/S0165-6147(00)01678-3 [Google Scholar] [PubMed] [CrossRef]
Marques C, Oliveira CSF, Alves S, Chaves SR, Coutinho OP, Côrte-Real M, Preto A (2013). Acetate-induced apoptosis in colorectal carcinoma cells involves lysosomal membrane permeabilization and cathepsin D release. Cell Death and Disease 4: e507. https://doi.org/10.1038/cddis.2013.29 [Google Scholar] [PubMed] [CrossRef]
Martin-Gallausiaux C, Marinelli L, Blottière HM, Larraufie P, Lapaque N (2021). SCFA: Mechanisms and functional importance in the gut. Proceedings of the Nutrition Society 80: 37–49. https://doi.org/10.1017/S0029665120006916 [Google Scholar] [PubMed] [CrossRef]
Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F et al. (2009). Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286. https://doi.org/10.1038/nature08530 [Google Scholar] [PubMed] [CrossRef]
McIntyre A, Gibson PR, Young GP (1993). Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut 34: 386–391. https://doi.org/10.1136/gut.34.3.386 [Google Scholar] [PubMed] [CrossRef]
Ng CY, Yen H, Hsiao HY, Su SC (2018). Phytochemicals in skin cancer prevention and treatment: An updated review. International Journal of Molecular Sciences 19: 941. https://doi.org/10.3390/ijms19040941 [Google Scholar] [PubMed] [CrossRef]
Nieto MA, Cano A (2012). The epithelial-mesenchymal transition under control: Global programs to regulate epithelial plasticity. Seminars in Cancer Biology 22: 361–368. https://doi.org/10.1016/j.semcancer.2012.05.003 [Google Scholar] [PubMed] [CrossRef]
Nikolaou V, Stratigos AJ (2014). Emerging trends in the epidemiology of melanoma. British Journal of Dermatology 170: 11–19. https://doi.org/10.1111/bjd.12492 [Google Scholar] [PubMed] [CrossRef]
Ohara T, Suzutani T (2018). Intake of Bifidobacterium longum and Fructooligosaccharides prevents Colorectal Carcinogenesis. Euroasian Journal of Hepato-Gastroenterology 8: 11–17. https://doi.org/10.5005/jp-journals-10018-1251 [Google Scholar] [PubMed] [CrossRef]
Parada Venegas D, de la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA (2019). Short chain fatty acids (SCFAs)mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology 10: 277. https://doi.org/10.3389/fimmu.2019.00277 [Google Scholar] [PubMed] [CrossRef]
Pierceall WE, Goldberg LH, Tainsky MA, Mukhopadhyay T, Ananthaswamy HN (1991). Ras gene mutation and amplification in human nonmelanoma skin cancers. Molecular Carcinogenesis 4: 196–202. https://doi.org/10.1002/mc.2940040306 [Google Scholar] [PubMed] [CrossRef]
Pirozzi C, Francisco V, Guida FD, Gómez R, Lago F, Pino J, Meli R, Gualillo O (2018). Butyrate modulates inflammation in chondrocytes via GPR43 receptor. Cellular Physiology and Biochemistry 51: 228–243. https://doi.org/10.1159/000495203 [Google Scholar] [PubMed] [CrossRef]
Pulliam SR, PellomJr ST, Shanker A, Adunyah SE (2016). Butyrate regulates the expression of inflammatory and chemotactic cytokines in human acute leukemic cells during apoptosis. Cytokine 84: 74–87. https://doi.org/10.1016/j.cyto.2016.05.014 [Google Scholar] [PubMed] [CrossRef]
Qin JZ, Bacon P, Panella J, Sitailo LA, Denning MF, Nickoloff BJ (2004). Low-dose UV-radiation sensitizes keratinocytes to TRAIL-induced apoptosis. Journal of Cellular Physiology 200: 155–166. https://doi.org/10.1002/jcp.20017 [Google Scholar] [PubMed] [CrossRef]
Rochette PJ, Therrien JP, Drouin R, Perdiz D, Bastien N, Drobetsky EA, Sage E (2003). UVA-induced cyclobutane pyrimidine dimers form predominantly at thymine±thymine dipyrimidines and correlate with the mutation spectrum in rodent cells. Nucleic Acids Research 31: 2786–2794. https://doi.org/10.1093/nar/gkg402 [Google Scholar] [PubMed] [CrossRef]
Rodust PM, Stockfleth E, Ulrich C, Leverkus M, Eberle J (2009). UV-induced squamous cell carcinoma—a role for antiapoptotic signalling pathways. British Journal of Dermatology 161: 107–115. https://doi.org/10.1111/j.1365-2133.2009.09458.x [Google Scholar] [PubMed] [CrossRef]
Sahuri-Arisoylu M, Mould RR, Shinjyo N, Bligh SWA, Nunn AVW, Guy GW, Thomas EL, Bell JD (2021). Acetate induces growth arrest in colon cancer cells through modulation of mitochondrial function. Frontiers in Nutrition 8: 1–10. https://doi.org/10.3389/fnut.2021.588466 [Google Scholar] [PubMed] [CrossRef]
Sato R, Semba T, Saya H, Arima Y (2016). Concise review: Stem cells and epithelial-mesenchymal transition in cancer: Biological implications and therapeutic targets. Stem Cells 34: 1997–2007. https://doi.org/10.1002/stem.2406 [Google Scholar] [PubMed] [CrossRef]
Schönfeld P, Wojtczak L (2016). Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. Journal of Lipid Research 57: 943–954. https://doi.org/10.1194/jlr.R067629 [Google Scholar] [PubMed] [CrossRef]
Sczepanski JT, Jacobs AC, Majumdar A, Greenberg MM (2009). Scope and mechanism of interstrand cross-link formation by the C4′-oxidized abasic site NIH public access. Journal of the American Chemical Society 131: 11132–11139. https://doi.org/10.1021/ja903404v [Google Scholar] [PubMed] [CrossRef]
Shu M, Wang Y, Yu J, Kuo S, Coda A, Jiang Y, Gallo RL, Huang CM (2013). Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS One, 8. https://doi.org/10.1371/journal.pone.0055380 [Google Scholar] [PubMed] [CrossRef]
Simon MM, Aragane Y, Schwarz A, Luger TA, Schwarz T (1994). UVB light induces nuclear factor κB (NFκB) activity independently from chromosomal DNA damage in cell-free cytosolic extracts. Journal of Investigative Dermatology 102: 422–427. https://doi.org/10.1111/1523-1747.ep12372194 [Google Scholar] [PubMed] [CrossRef]
Simões MCF, Sousa JJS, Pais AACC (2015). Skin cancer and new treatment perspectives: A review. Cancer Letters 357: 8–42. https://doi.org/10.1016/j.canlet.2014.11.001 [Google Scholar] [PubMed] [CrossRef]
Singh R, Letai A, Sarosiek K (2019). Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nature Reviews Molecular Cell Biology 20: 175–193. https://doi.org/10.1038/s41580-018-0089-8 [Google Scholar] [PubMed] [CrossRef]
Sivaprakasam S, Prasad PD, Singh N (2016). Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Journal of Investigative Dermatology 102: 422–427. https://doi.org/10.1016/j.pharmthera.2016.04.007 [Google Scholar] [PubMed] [CrossRef]
Solar and Ultraviolet Radiation (2011). IARC monographs on the evaluation of carcinogenic risks to humans. International Agency for Research on Cancer 100: 35–101. [Google Scholar]
Stockert JC, Blázquez-Castro A (2022). Biomedical overview of melanin. Updating molecular modeling, synthesis mechanism, and supramolecular properties regarding melanoma therapy. BIOCELL 46: 1391–1415. https://doi.org/10.32604/biocell.2022.019493 [Google Scholar] [CrossRef]
Strozyk E, Kulms D (2013). The role of AKT/mTOR pathway in stress response to UV-irradiation: Implication in skin carcinogenesis by regulation of apoptosis, autophagy and senescence. International Journal of Molecular Sciences 14: 15260–15285. https://doi.org/10.3390/ijms140815260 [Google Scholar] [PubMed] [CrossRef]
Su Z, Yang Z, Xu Y, Chen Y, Yu Q (2015). Apoptosis, autophagy, necroptosis, and cancer metastasis. Molecular Cancer 14: 1–14. https://doi.org/10.1186/s12943-015-0321-5 [Google Scholar] [PubMed] [CrossRef]
Sugasawa K, Ng JM, Masutani C, Iwai S, van der Spek PJ, Eker AP, Hanaoka F, Bootsma D, Hoeijmakers JH (1998). Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Molecular Cell 2: 223–232. https://doi.org/10.1016/S1097-2765(00)80132-X [Google Scholar] [PubMed] [CrossRef]
Svobodová A, Psotová J, Walterová D (2003). Natural phenolics in the prevention of UV-induced skin damage. A review. Biomedical Papers of the Medical Faculty of the University Palacktfytf Olomouc Czechoslovakia 147: 137–145. https://doi.org/10.5507/bp.2003.019 [Google Scholar] [CrossRef]
Tang C, Ahmed K, Gille A, Lu S, Gröne HJ, Tunaru S, Offermanns S (2015). Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nature Medicine 21: 173–177. https://doi.org/10.1038/nm.3779 [Google Scholar] [PubMed] [CrossRef]
Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N et al. (2014). Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature Medicine 20: 159–166. https://doi.org/10.1038/nm.3444 [Google Scholar] [PubMed] [CrossRef]
Tyagi N, Bhardwaj A, Srivastava SK, Arora S, Marimuthu S, Deshmukh SK, Singh AP, Carter JE, Singh S (2015). Development and characterization of a novel in vitro progression model for UVB-induced skin carcinogenesis. Scientific Reports 5: 1–11. https://doi.org/10.1038/srep13894 [Google Scholar] [PubMed] [CrossRef]
van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM (2003). The tumor microenvironment: A critical determinant of neoplastic evolution. European Journal of Cell Biology 82: 539–548. https://doi.org/10.1078/0171-9335-00346 [Google Scholar] [PubMed] [CrossRef]
Van Waes C, Yu M, Nottingham L, Karin M (2007). Inhibitor-κB kinase in tumor promotion and suppression during progression of squamous cell carcinoma. Clinical Cancer Research 13: 4956–4959. https://doi.org/10.1158/1078-0432.CCR-07-1287 [Google Scholar] [PubMed] [CrossRef]
Veloz L, Bosch SA, Aparicio G, Zolessi FR (2021). Cell extrusion in development and cancer, what MARCKS the difference for epithelial integrity. BIOCELL 46: 639–644. https://doi.org/10.32604/biocell.2022.018798 [Google Scholar] [CrossRef]
Wan YS, Wang ZQ, Shao Y, Voorhees JJ, Fisher GJ (2001). Ultraviolet irradiation activates PI 3-kinase/AKT survival pathway via EGF receptors in human skin in vivo. International Journal of Oncology 18: 461–466. https://doi.org/10.3892/ijo.18.3.461 [Google Scholar] [PubMed] [CrossRef]
Wicking C, Shanley S, Smyth I, Gillies S, Negus K et al. (1997). Most germ-line mutations in the nevoid basal cell carcinoma syndrome lead to a premature termination of the patched protein, and no genotype-phenotype correlations are evident. American Journal of Human Genetics 60: 21–26. [Google Scholar] [PubMed]
Wikonkal NM, Brash DE (1999). Ultraviolet radiation induced signature mutations in photocarcinogenesis. Journal of Investigative Dermatology Symposium Proceedings 4: 6–10. https://doi.org/10.1038/sj.jidsp.5640173 [Google Scholar] [PubMed] [CrossRef]
Xian D, Lai R, Song J, Xiong X, Zhong J (2019). Emerging perspective: Role of increased ROS and redox imbalance in skin carcinogenesis. Oxidative Medicine and Cellular Longevity 16: 8127362. https://doi.org/10.1155/2019/8127362 [Google Scholar] [PubMed] [CrossRef]
Xu E, Chen C, Fu J, Zhu L, Shu J, Jin M, Wang Y, Zong X (2021). Dietary fatty acids in gut health: Absorption, metabolism and function. Animal Nutrition 7: 1337–1344. https://doi.org/10.1016/j.aninu.2021.09.010 [Google Scholar] [PubMed] [CrossRef]
Yang X, Xu Y, Mei S, Li J (2021). Elp3 modulates neural crest and colorectal cancer migration requiring functional integrity of HAT and SAM domains. BIOCELL 46: 463–470. https://doi.org/10.32604/biocell.2021.014834 [Google Scholar] [CrossRef]
Yonezawa T, Kobayashi Y, Obara Y (2007). Short-chain fatty acids induce acute phosphorylation of the p38 mitogen-activated protein kinase/heat shock protein 27 pathway via GPR43 in the MCF-7 human breast cancer cell line. Cellular Signalling 19: 185–193. https://doi.org/10.1016/j.cellsig.2006.06.004 [Google Scholar] [PubMed] [CrossRef]
You P, Wu H, Deng M, Peng J, Li F, Yang Y (2018). Brevilin A induces apoptosis and autophagy of colon adenocarcinoma cell CT26 via mitochondrial pathway and PI3K/AKT/mTOR inactivation. Biomedicine and Pharmacotherapy 98: 619–625. https://doi.org/10.1016/j.biopha.2017.12.057 [Google Scholar] [PubMed] [CrossRef]
Zhang Y, Zhou X, Zhang C, Lai D, Liu D, Wu Y (2023). Vitamin B3 inhibits apoptosis and promotes autophagy of islet β cells under high glucose stress. BIOCELL 47: 859–868. https://doi.org/10.32604/biocell.2023.026429 [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF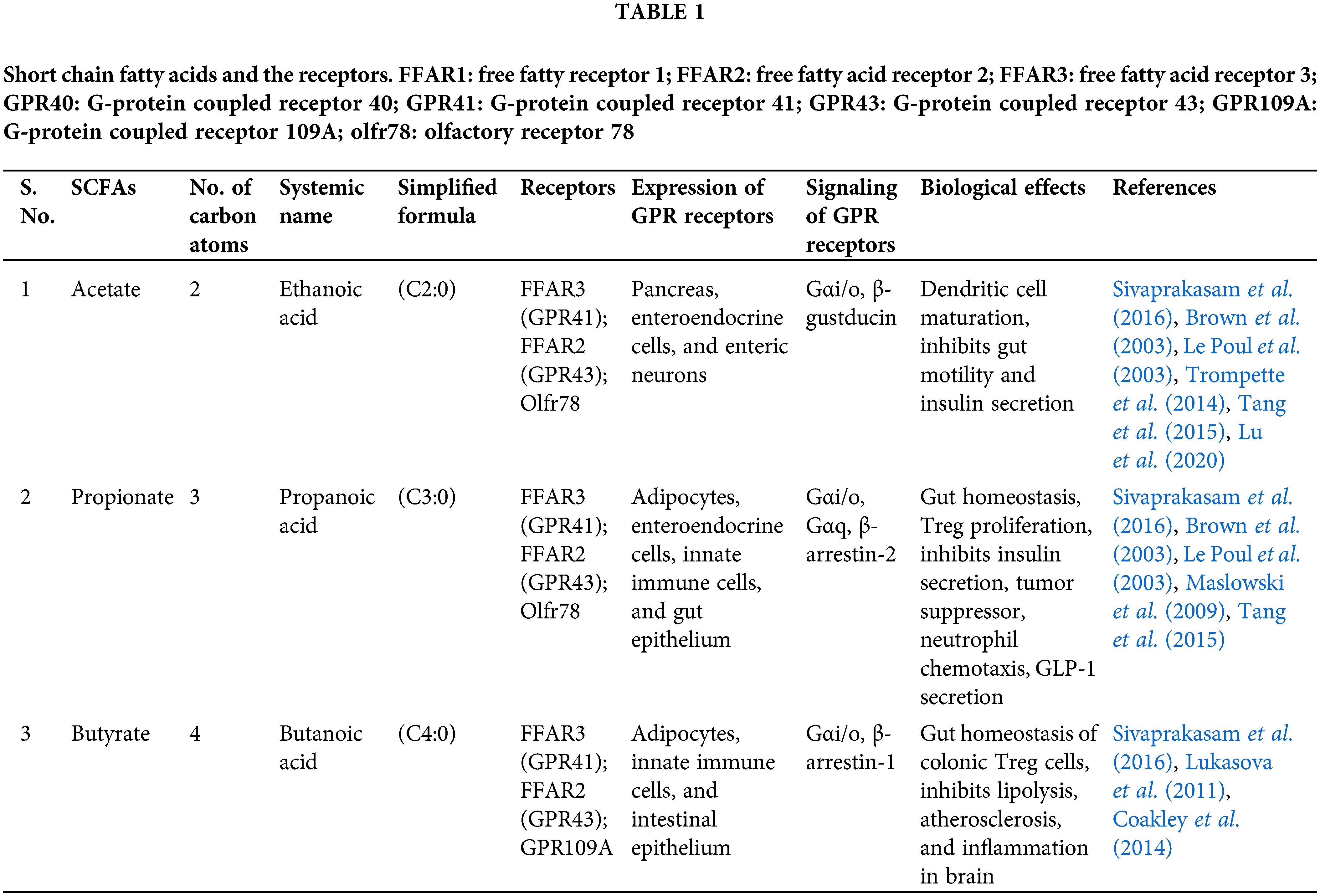
 Downloads
Downloads
 Citation Tools
Citation Tools