 Open Access
Open Access
ARTICLE
Suppression of cell pyroptosis by omeprazole through PDE4-mediated autophagy in gastric epithelial cells
1 Department of Pediatrics, The First School of Clinical Medicine of Jinan University, The First Affiliated Hospital, Jinan University, Guangzhou, China
2 Department of Gastroenterology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
3 Department of Gastroenterology, Guangdong Provincial Clinical Research Center for Child Health, Guangzhou Medical University, Guangzhou, China
4 Department of Nephrology, Zhuhai Maternal and Child Health Hospital, Zhuhai, China
* Corresponding Authors: WANFU XU. Email: ; SITANG GONG. Email:
# These authors contributed equally to this work
BIOCELL 2023, 47(12), 2709-2719. https://doi.org/10.32604/biocell.2023.044295
Received 27 July 2023; Accepted 18 October 2023; Issue published 27 December 2023
Abstract
Introduction: Helicobacter pylori is a risk factor for the development of peptic ulcers with autophagy dysfunction. Omeprazole was widely known as the first-line regimen for H. pylori-associated gastritis. Objectives: The objective of this work was to assess the role of omeprazole on cell pyroptosis and autophagy. Methods: The clinical samples were collected. Quantitative polymerase chain reaction, western blotting, enzyme linked immunosorbent assay, and immunofluorescence (IF) analysis were conducted to reveal the mechanism of omeprazole on cell pyroptosis and autophagy. Results: The results revealed that omeprazole could decrease cell pyroptosis, which was attributed to the downregulation of cleaved caspase-1 expression, resulting in the inhibition of gasdermin E and interleukin-18/1β maturation and secretion as well as the resolution of inflammation. Mechanistically, omeprazole treatment led to drastic downregulation of mammalian target of rapamycin (mTOR) activity was observed in BGC823 cells, leading to enhanced autophagy characterized by increased LC3II expression, which further reduced cell pyroptosis. This omeprazole-mediated phenomenon was enhanced after phosphodiesterase-4 (PDE4) inhibitor dipyridamole (DIP) treatment. In addition, activation of mTOR by MHY1485 could rescue the suppression of cell pyroptosis induced by omeprazole. Most importantly, IF analysis suggested that phosphorylation of mTOR and PDE4 activity and caspase-1 were enhanced in H. pylori-infected gastric mucosa. Conclusion: These findings indicate that omeprazole suppresses cell pyroptosis through PDE4-mediated autophagy in gastric epithelial cells, and DIP enhanced the omeprazole-mediated inhibition of cell pyroptosis, implying that DIP is an alternative combined therapy strategy in improving the treatment of patients with H. pylori infection.Keywords
Omeprazole, a proton-pump inhibitor (PPIs), has been used in the management and treatment of several conditions, including peptic ulcer disease, gastrointestinal reflux, and helicobacter pylori (H. pylori) infection (Shah and Gossman, 2021). H. pylori, including virulence factor CagA, has been reported to promote invasion and migration in gastric cancer cells and interleukin (IL)-1β production in neutrophils, respectively (Zhang et al., 2022; Jang et al., 2020; Pachathundikandi et al., 2020). NLRP3 inflammasome activation, the critical component of cell pyroptosis (Zhivaki and Kagan, 2021), derived mature IL-1β and IL-18 production to trigger inflammation (Pachathundikandi et al., 2020; Pachathundikandi et al., 2016). In general, pathogens was recognized by the NOD-like receptors (NLPs), which further triggers caspase-1 activation (Malireddi et al., 2020). The activation of caspase-1 could cleave IL-1β and IL-18 into mature form as well as gasdermin D (GSDMD), leading to the formation of cell membrane pores to release IL-1β and IL-18 to aggravate inflammation (Shi et al., 2015). Interestingly, that work showed that omeprazole ameliorated cisplatin (CP)-induced renal injury through inhibition of the toll-like receptor 4/ nuclear factor-κB/nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 (NLRP3) signaling pathway (Gao et al., 2020b), suggesting the role of omeprazole in alleviation of gastritis. However, whether NLRP3 inflammation is involved in the mechanism of omeprazole-regulated gastritis remains to be identified.
AMP-activated protein kinase (AMPK) is a critical regulator of energy homeostasis, which was sensitivity to increased AMP:ATP ratio caused by phosphodiesterase (PDEs) inhibition (Giricz et al., 2012; Xi et al., 2016). The upstream kinases liver kinase B1 and AMPK activation in the catalytic subunit at threonine 172 (T172) could inhibit mTOR activity, which further triggered autophagy characterized by increased LC3II/LC3I and decreased p62 expression through ULK1 (Yeo, 2019; Sun et al., 2018; Nazio and Cecconi, 2017). The enhancement of autophagy could alleviate pyroptosis (Yu et al., 2022; Tang et al., 2022). Blockade of autophagic flow through the AKT/mTOR activation pathway led to an excessive accumulation of ROS, which further activated the NLRP3 inflammasome (Yu et al., 2022), suggesting that the activation of autophagy could reduce pyroptosis, alleviating inflammation, which might be a potent target in the treatment of inflammatory diseases. Interestingly, a further study demonstrated that icariside II, a phosphodiesterase 5 inhibitor, attenuated cerebral ischemia/reperfusion injury by inhibiting glycogen synthase kinase-3β (GSK-3β)-mediated activation of autophagy (Gao et al., 2020a). These findings established a possible link between PDE, cell pyroptosis, and autophagy.
Recently, more and more work about the function of PPI in gastrointestinal function has been reported. The previous studies from our lab have demonstrated that another PPI, rabeprazole, inhibited gastric epithelial cell proliferation, which was attributed to the inhibitory effect of Rabeprazole on Signal transducer and activator of transcription 3 (STAT3)-mediated glycolysis (Zhou et al., 2021), further work showed that rabeprazole inhibited cell pyroptosis, leading to decrease inflammatory reaction (Xie et al., 2021), and destroyed gastric barrier function by inhibition of FOXF1/STAT3-mediated ZO-1 expression (Yang et al., 2023). In addition to rabeprazole, we also demonstrated that omeprazole suppressed de novo lipogenesis in gastric epithelial cells by reducing fatty acid synthase and ATP citrate lyase expression (Chen et al., 2020). However, the mechanism through which omeprazole regulates gastritis remains unknown. Thus, clarifying the regulation mechanism of omeprazole-regulated gastritis might provide new targets. Herein, omeprazole was found to suppress cell pyroptosis through mTOR-mediated autophagy, which was enhanced in response to DIP stimulation. Most importantly, phosphorylation of mTOR and caspase-1 were remarkedly increased in H. pylori-positive gastric mucosa. These findings suggested that omeprazole could suppress cell pyroptosis by triggering autophagy mediated by mTOR and DIP combined with omeprazole treatment could be an effective therapy in patients with gastritis.
Omeprazole (IO0030) was purchased from (Solarbio, Beijing, China). TRIzol (15596) was from Invitrogen (Carlsbad, CA, USA). First-Strand cDNA Synthesis Kit (AORT-0020) and All-in-One qPCR Mix (QP002) were from GeneCopoeia (Rockville, MD, USA). Dipyridamole (DIP; S1895) was obtained from Selleck (Shanghai, China). MHY1485 (HY-B0795) was purchased from (MedChemExpress, NJ, USA). Other reagents were obtained from Biosharp (Hefei, China). The antibodies used in this study were mTOR (ab134903, abcam, Cambridge, UK, 1:2000 for western blotting, WB), phosphodiesterase-4 (PDE4; ab14628, Abcam Cambridge, UK, 1:2000 for WB); PDE4B/C/D phospho (Ser133/119/190) (YP0668, Immunoway, SuZhou, China, 1:2000 for WB, 1:400 for immunofluorescence, IF); mTOR (phosphor s2448)(ab109268, Abcam, Cambridge, UK, 1:2000 for WB, 1:400 for IF), caspase-1 (ab179515, Abcam, Cambridge, UK, 1:1000 for WB,1:300 for IF); NLRP3 (ab270449, Abcam, Cambridge, UK, 1:2000 for WB); IL-18 (A1115, Abclonal, Wuhan, China, 1:2000 for WB); IL-1β (A19635, Abclonal, Wuhan, China, 1:1000 for WB); GSDMD (A17308, Abclonal, Wuhan, China, 1:2000 for WB); gasdermin E (GSDME; 13075-1-AP, Proteintech, Chicago, IL, 1:1000 for WB), α-tubulin (AC007,1:4000 for WB); β-actin (AC026, 1:5000 for WB). IL-1β enzyme-linked immunosorbent assay (ELISA) kit (ab229384) and IL-18 ELISA kit (ab215539) as well as lactate dehydrogenase (LDH) assay kit (ab102526) were obtained from Abcam (Cambridge, UK).
Cell culture, treatment, and transfection
GES-1 and BGC823 cells were cultured in Dulbecco’s Modified Eagle Medium (Kalamazoo, MI, USA) supplied with 10% fetal bovine serum. For treatment, cells were incubated with omeprazole at a final concertation of 300 mM for 48 h. DIP (DIP) was used at a final concentration of 4 µM, for transfection, mRFP-GFP-LC3 plasmid was purchased from (YouBio, Changsha, China) and transfected into cells with lipofectamine 3000 (Invitrogen, catalog no. L3000001).
Quantitative polymerase chain reaction (qPCR) analysis
As described in an earlier study (Wang et al., 2021), after stimulation with or without omeprazole in a 6-well plate, 1 mL TRIzol was added to replace with cell medium to lyse cell. The whole RNA was extracted to convert into cDNA and amplified by qPCR assay using a cDNA Synthesis Kit combined with an All-in-One qPCR Mix, respectively. Primer used as followed: IL-1β: Forward: 5′-ATGATGGCTTATTACAGTGGCAA-3′, Reverse: 5′-GTCGGAGATTCGTAGCTGGA-3′; IL-18: Forward: 5′-TCTTCATTGACCAAGGAAATCGG-3′, Reverse: 5′-TCCGGGGTGCATTATCTCTAC-3′; GSDMD: Forward: 5′-GTGTGTCAACCTGTCTATCAAGG-3′, Reverse: 5′-CATGGCATCGTAGAAGTGGAAG-3′; NLRP3: Forward: 5′-GATCTTCGCTGCGATCAACAG-3′, Reverse: 5′-CGTGCATTATCTGAACCCCAC-3′; ASC: Forward: 5′-TGGATGCTCTGTACGGGAAG-3′, Reverse: 5′-CCAGGCTGGTGTGAAACTGAA-3′; Caspase-1: Forward: 5′-CCTTAATATGCAAGACTCTCAAGGA-3′, Reverse: 5′-TAAGCTGGGTTGTCCTGCACT-3′; UBC: Forward: 5′-ATTTGGGTCGCGGTTCTTG-3′ and Reverse: 5′-TGCCTTGACATTCTCGATGGT-3′.
Enzyme-linked immunosorbent assay
After treatment, the supernatant was harvested to centrifuge to remove debris, and the concentration of IL-1β and IL-18 in the content were measured and quantitated by the corresponding ELISA according to the kit protocol, respectively.
LDH (lactate dehydrogenase) is released due to the cell membrane is destroyed caused by pyroptosis, and the severity of pyroptosis can be reflected by the LDH release level. After treatment with or without omeprazole, the supernatants from BGC823 cells were collected to detect using an LDH assay kit. Relative cell death was calculated as described in the study by Xie et al. (2021).
This was performed as described earlier (Xu et al., 2017). Briefly, the lysate was harvested and the proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. After incubation with Tris-buffered saline Tween-20 with 5% milk, the membrane was incubated with appropriate antibodies at 4°C for 24 h, followed by washing with PBST for three times for 5 min. The membrane was further incubated for another 1 h at room temperature with a horseradish peroxidase-conjugated secondary antibody. The protein was detected using an enhanced chemiluminescence kit (Perkin Elmer, catalog no. NEL105001EA).
Immunofluorescent staining was performed as described in our previous study (Xu et al., 2017). Fixed and permeabilized cells were treated overnight at 4°C with primary antibodies, followed by incubation for 1 h with secondary antibodies conjugated with Alexa-488 or 594 at room temperature. Coverslips were mounted onto the glass slides with the prolong gold antifade reagent. The stained cells were imaged with a Leica TCS SP8 inverted fluorescence microscope (Leica Microsystems).
Data was displayed as mean ± SD and performed by GraphPad Prism V software (La Jolla, CA, USA). A p-value < 0.05 was considered statistically significant.
Suppression of cell pyroptosis in gastric epithelial cells by Omeprazole
In our previous study, we found that omeprazole could decrease de novo lipogenesis in gastric epithelial cells (Chen et al., 2020), and saturated fatty acid was reported to trigger caspase-4/5 in human monocytes to induce IL-1β and IL-18 release (Pillon et al., 2016). These findings focused us to explore the possible role of omeprazole in cell pyroptosis. The result from the LDH release assay revealed that omeprazole treatment alone led to a decrease in the enhanced LDH release caused by LPS in BGC823 and GES-1 cells (Fig. 1A). Further results from qPCR and WB analyses showed that omeprazole treatment could inhibit IL-18 and IL-1β expression at mRNA and protein level (Figs. 1B and 1C). Consequently, the content of IL-18/1β in supernatant from BGC823 cells was drastically reduced in response to omeprazole stimulation (Fig. 1D). Most importantly, a pivotal executioner of cell pyroptosis (Poh et al., 2019; Zhang et al., 2019) GSDME, not GSDMD, was significantly inhibited in gastric epithelial cells treated with omeprazole (Fig. 1E). These finding suggested that omeprazole has a potential function in suppressing cell pyroptosis in gastric epithelial cells, which could alleviate inflammation.
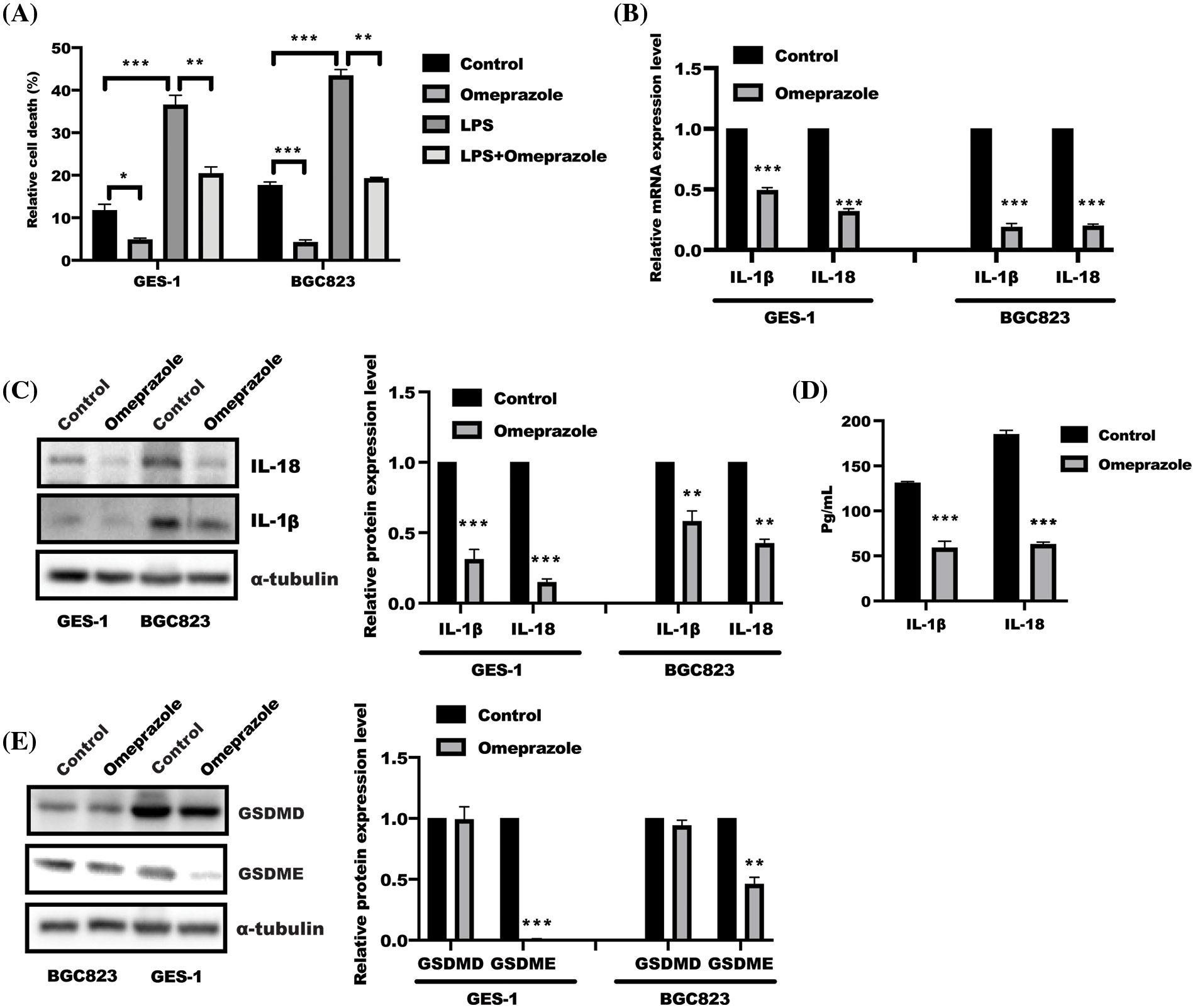
Figure 1: Omeprazole attenuated gasdermin (GSDM) E-mediated cell pyroptosis. (A) Test of lactate dehydrogenase (LDH) level was conducted to show relative cell death in GES-1 and BGC823 cells after omeprazole incubation. Data was exhibited as the mean ± SD. The statistical difference was examined using two-way ANOVA, n = 3, *p < 0.05, **p < 0.01, ***p < 0.001. (B) RNA was isolated from GES-1 and BGC823 cells stimulated with or without omeprazole (300 µM) for 24 h; quantitative polymerase chain reaction was employed to test interleukin (IL)-1β/IL-18 mRNA level, and data were displayed as the mean ± SD. The statistic difference was determined by one sample t-test, n = 3, ***p < 0.001. (C) Cells treated with or without omeprazole for 24 h, were subjected to western blotting to analyze IL-1β/IL-18 expression; the band intensity was measured and determined by t-test, and data were displayed as the mean ± SD. n = 3, **p < 0.01, ***p < 0.001. (D) The content of IL-1β and IL-18 in BGC823 cells received as indicated stimulation for 24 h was tested by enzyme linked immunosorbent assay; data were displayed as the mean ± SD, and determined by t-test, n = 3, **p < 0.01, ***p < 0.001 vs. con group; (E) GES-1 and BGC823 cells were treated with or without omeprazole for 24 h, and GSDME and GSDMD expressions were examined by western blotting; the band intensity was measured and examined using t-test, and data were presented as the mean ± SD. n = 3, **p < 0.01, ***p < 0.001.
Inhibition of nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 inflammasomes activation by omeprazole
NLRP3 inflammasome activation is an important event for GSDME and IL-18/1β maturation. Thus, NLRP3 inflammasome attracted our attention in assessing the effect of omeprazole on NLRP3 inflammasome (Liu et al., 2021; Li et al., 2021). As expected, the results from quantitative PCR displayed that omeprazole stimulation resulted in a strong inhibition of NLRP3 and Caspase 1 activation, while no remarkable change was observed in the expression of apoptosis-associated speck-like protein containing a CARD (ASC) mRNA in BGC823 and GES-1 cells (Fig. 2A). In line with this, we observed that the expression of NLRP3 and caspase-1, not ASC, was inhibited in BGC823 and GES-1 cells after omeprazole treatment (300 µM) (Fig. 2B). Moreover, omeprazole stimulation significantly reversed NLRP3 inflammasomes activation in the presence of LPS (500 ng/mL) in BGC823 cells (Fig. 2C). These findings suggest that omeprazole suppressed cell pyroptosis in gastric epithelial cells to ameliorate inflammation by reducing caspase-1-mediated NLRP3 inflammasome activation.
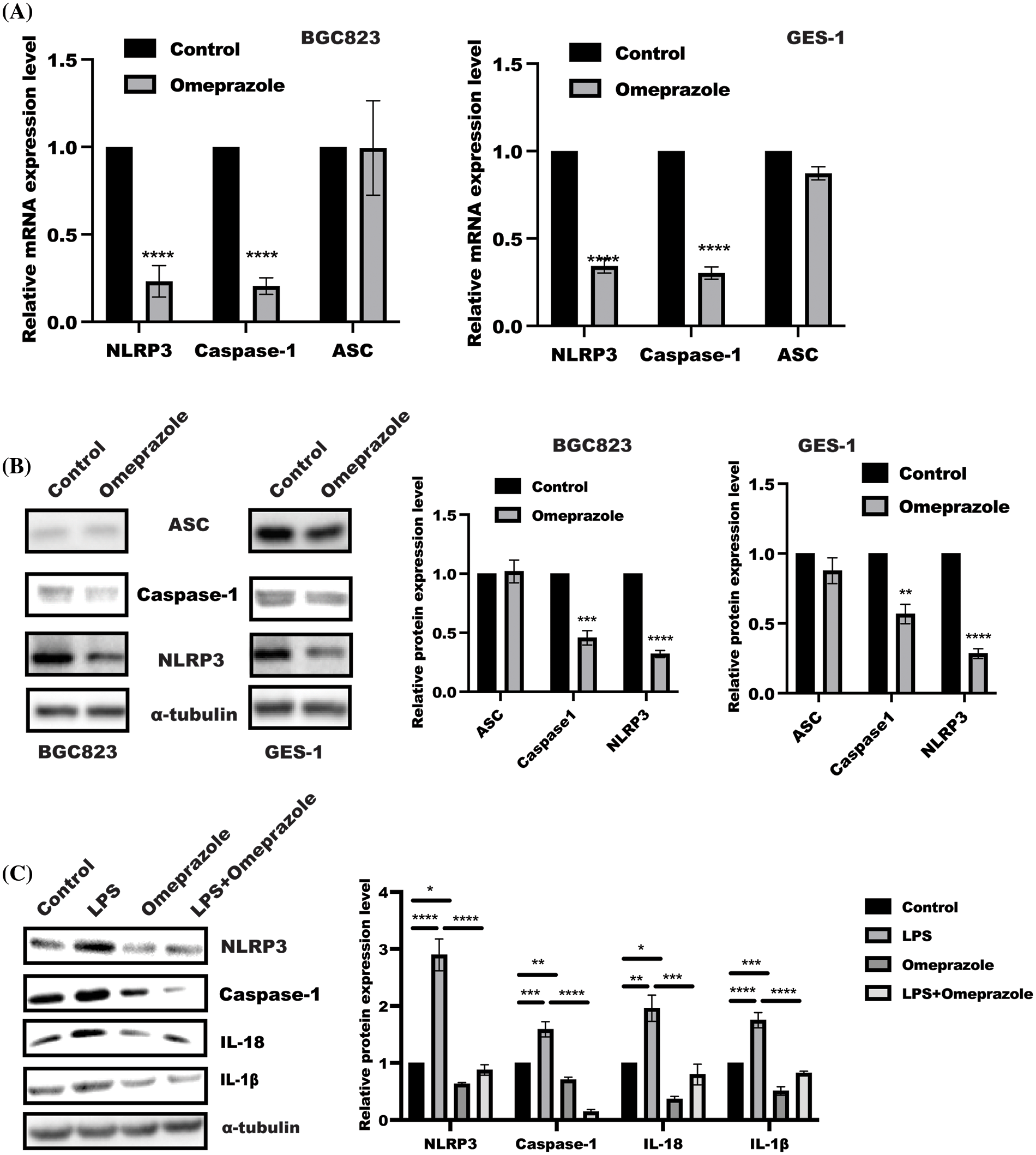
Figure 2: Omeprazole inhibited nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 (NLRP3) inflammasomes activation. (A) Quantitative polymerase chain reaction was used to examine the levels of NLRP3, apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1 mRNA level in BGC823 (left panel) and GES-1 cells (right panel) in the presence or absence of omeprazole (300 µM), respectively. Data were presented as the mean ± SD. n = 3, one sample t-test, **p < 0.01, ***p < 0.001 vs. control group; (B) BGC823 and GES-1 cells were stimulated with or without Omeprazole (300 µM) for 24 h, and sodium dodecyl sulfate polyacrylamide gel electrophoresis was used to determine the expressions of ASC, NLRP3, and caspase-1. The quantitation of band density was displayed as mean ± SD. n = 3, one sample t-test, **p < 0.01, ***p < 0.001, ****p < 0.0001: (C) After incubation with omeprazole (300 µM) for 1 h, followed by LPS stimulation for further 24 h, BGC823 cells were lysed to the expression of indicated proteins was examined, the quantitation was performed and analyzed by two-way ANOVA. Data were displayed as mean ± SD. n = 3, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Suppression of cell pyroptosis by omeprazole through autophagy induction
Autophagy has been well demonstrated to block pyroptosis (Guo et al., 2021; Yu et al., 2019; Lin et al., 2021). To investigate whether autophagy is involved in omeprazole-mediated pyroptosis, we examined the expression of several autophagy-related genes in response to omeprazole treatment. As presented in Fig. 3A, real-time PCR and WB analysis of BGC823 and GES-1 cells treated with omeprazole for 48 h, respectively, showed significant induction of autophagy characterized by enhanced LC3II and ULK1 expression as well as decreased p62 expression, which was attributed to suppression of mTOR phosphorylation caused by omeprazole treatment (Figs. 3A and 3B). To further monitor the effect of omeprazole on autophagy flux, the mRFP-GFP-LC3 plasmid was transfected into BGC823 cells. As expected, the number of autophagosomes (indicated as yellow puncta) was increased caused by omeprazole stimulation (Fig. 3C).
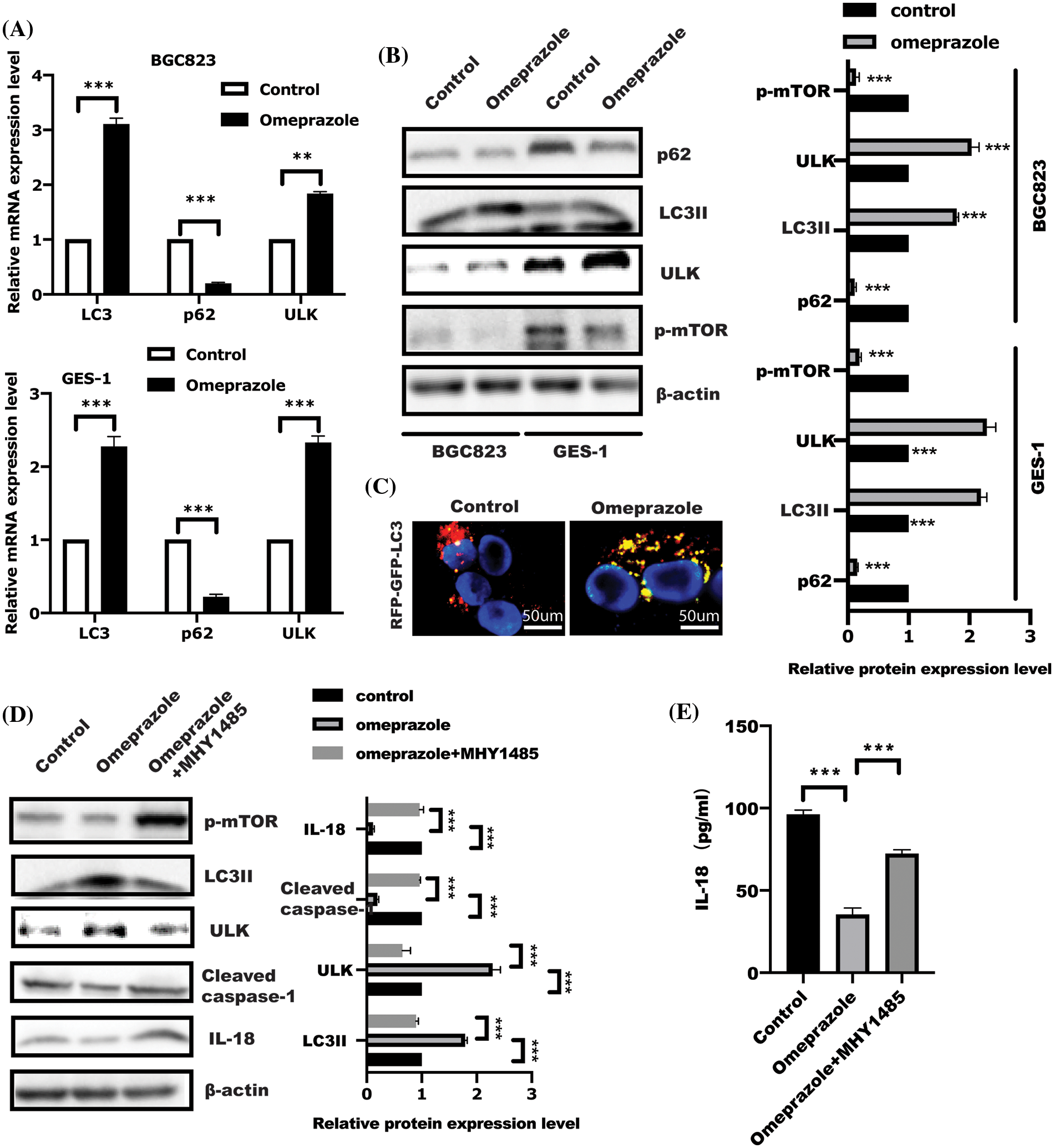
Figure 3: Omeprazole regulated cell pyroptosis is dependent on the mammalian target of rapamycin (mTOR)-mediated autophagy. (A) After stimulation for 48 h, BGC823 and GES-1 cells were isolated to extract total RNA to examine the expression of indicated genes. Data were displayed as mean ± SD. The significance was determined using one sample t-test. n = 3, **p < 0.01, ***p < 0.001. (B) As described in (A), the whole cell lysate was collected to test indicated protein by immunoblotting; band intensity was quantified and analyzed using a t-test, n = 3, ***p < 0.001. (C) Autophagy flux analysis in BGC823 cells using LC3-GFP-RFP, after omeprazole treatment for 48 h. (D) After incubation with MHY1485 (10 µM) for 1 h, BGC823 cells were stimulated with omeprazole for a further 48 h; western blotting was conducted to examine indicated protein and quantitation was performed by one-way ANOVA with multiple comparisons, followed by Bonferroni post hoc test for significance. n = 3, ***p < 0.001. (E) IL-18 level in indicated group was examined. Data were displayed as the mean ± SD, and determined using one-way ANOVA with multiple comparisons, followed by the Bonferroni post hoc test. n = 3, ***p < 0.001.
Initiation of autophagosome formation is regulated by mTOR inhibition. Insulin receptor withdrawal, amino acid starvation, and low ATP, result in mTOR inhibition, resulting in ULK1 activation and initiation of autophagy (Zhang, 2015). Next, MHY1485, an activator of mTOR, was used to further confirm omeprazole-regulated autophagy in an mTOR-dependent manner. As expected, MHY1485 treatment in BGC823 cells led to a reversal of omeprazole-mediated autophagy and also counterbalanced reduced caspase-1 expression caused by omeprazole (Fig. 3D), which further restored IL-18 level (Fig. 3E). This suggested that omeprazole might trigger mTOR-mediated autophagy to attenuate cell pyroptosis in BGC823 cells.
Dipyridamole enhanced omeprazole-mediated autophagy and pyroptosis
AMP-activated protein kinase (AMPK)/mTOR act as a cellular energy sensor, which is controlled in response to increased AMP:ATP ratio (Szrejder et al., 2020; Jayarajan et al., 2019). Intracellular cAMP degradation is mediated by PDE4 (Schick and Schlegel, 2022); this incited us to ask whether PDE4 is involved in omeprazole-induced autophagy. As shown in Fig. 4A, mTOR phosphorylation and PDE4 activity were significantly attenuated in BGC823 cells treated with omeprazole and DIP, an inhibitor of PDE4, respectively, which could induce autophagy. In addition, the levels of cleaved caspase-1 and GSDME were significantly reduced, while that of LC3II was enhanced in BGC823 cells treated with DIP along with omeprazole compared with omeprazole treatment alone (Fig. 4B). The level of IL-1β and IL-18 was also markedly decreased in response to DIP combined with omeprazole (Fig. 4C), Thus, the inhibition of PDE4 by DIP enhanced omeprazole-mediated autophagy and pyroptosis through mTOR.
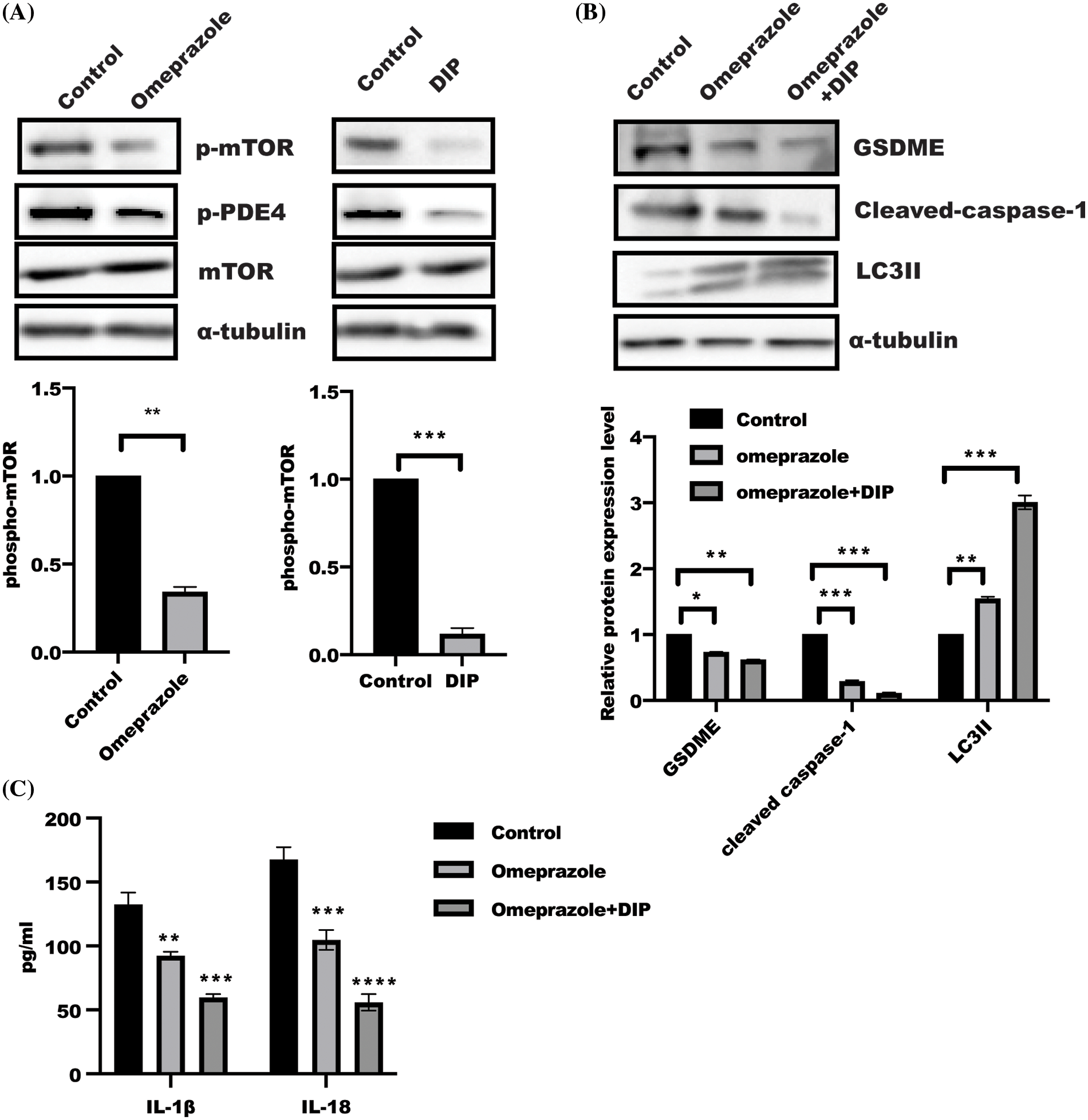
Figure 4: The synergistic activity of dipyridamole (DIP) and omeprazole regulated mTOR activity. (A) Phosphorylation of mTOR in BGC823 receiving omeprazole and DIP stimulation was determined by western blotting. Data were exhibited as the mean ± SD. One sample t-test was applied for quantification, n = 3, ***p < 0.001, **p < 0.01. (B) BGC823 cells were incubated as indicated for 48 h, and protein were detected by immunoblotting. Data were displayed as mean ± SD. And quantified by one-way ANOVA with multiple comparisons, n = 3. ***p < 0.001, **p < 0.01, *p < 0.05. (C) The Enzyme linked immunosorbent assay was used to test IL-1β and IL-18 levels in BGC823 cells treated as described in (B). Data was displayed as mean ± SD. and determined by one-way ANOVA with multiple comparisons, n = 3. ****p < 0.0001, ***p < 0.01, **p < 0.01.
Phosphorylation of PDE4/mTOR and caspase-1 were enhanced in gastric mucosa in patients with H. pylori infection
Our above-mentioned experiments showed that omeprazole could enhance mTOR-mediated autophagy, which further suppressed cell pyroptosis. Based on this, we examined the relationship between mTOR phosphorylation and caspase-1 in clinical samples. A total of 38 gastric mucosa samples, including 24 (63.2%) boys and 14 (36.8%) girls in ages between 3 to 12 years (median age = 7.5), were enrolled from Guangzhou Women and Children’s Medical Center after clinical ethical review. Detailed clinical information of subjects could be obtained based on reasonable request.
Next, we examined the possible changes in patients who received omeprazole treatment and found that phosphorylation of mTOR and caspase1 expression as well as PDE4 phosphorylation were significantly reduced in gastric mucosa with H. pylori infection after omeprazole treatment (Figs. 5A–5C). These findings suggest impaired autophagy and enhanced cell pyroptosis in the patients with H. pylori-infected gastric mucosa, and omeprazole not only inhibited acid secretion, but was also able to suppress inflammation by suppressing pyroptosis via PDE4/mTOR-mediated autophagy.
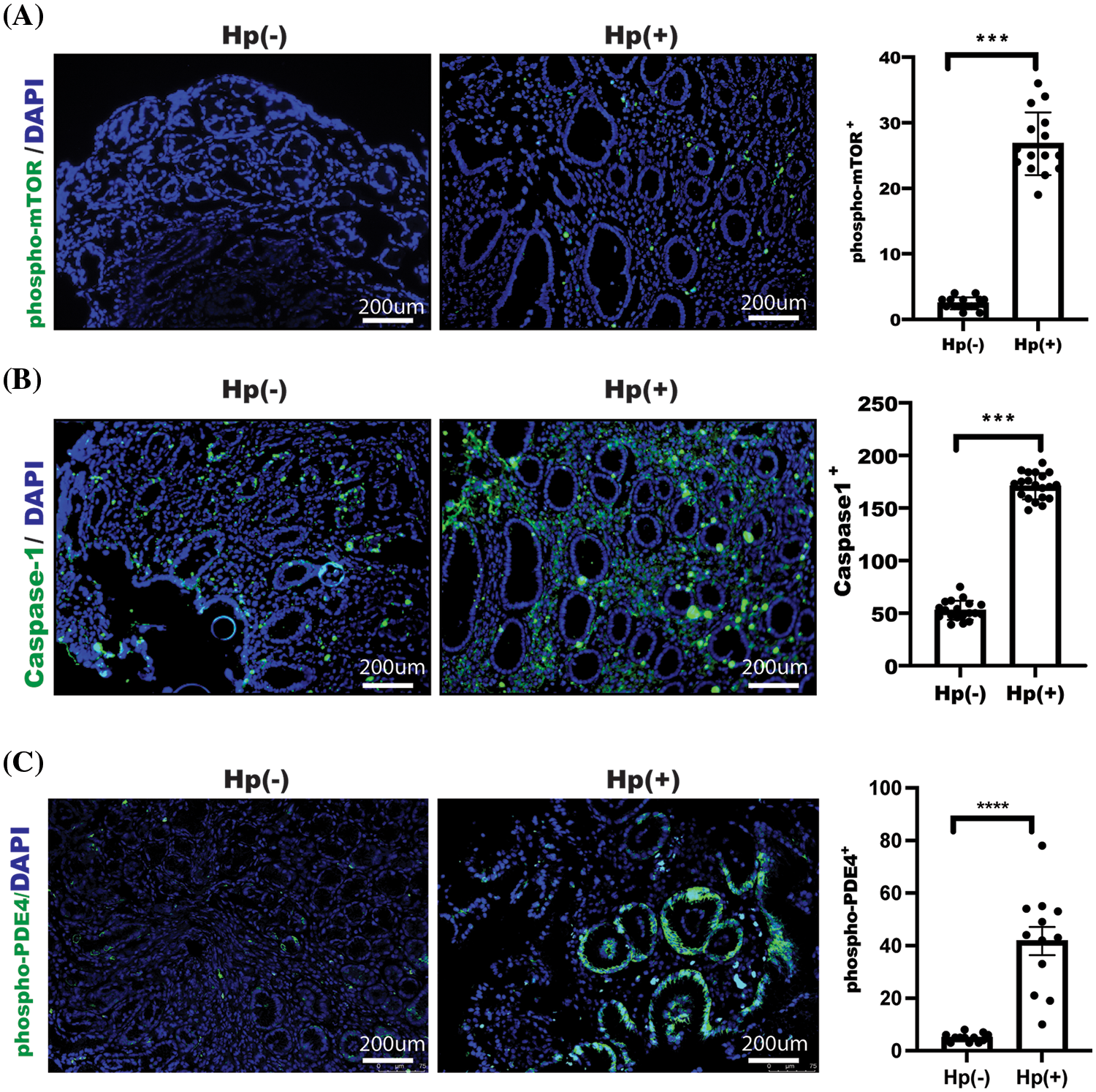
Figure 5: Caspase-1 and phosphorylation of mTOR as well as phosphodiesterase 4 (PDE4) activation were enhanced in gastric mucosa with H. pylori-infectious patients. Images of phosphorylation of mTOR (A), Caspase-1 expression (B), and phosphorylation of PDE4 (C) were acquired and examined, and indicated proteins were assessed and determined using t-test, n = 3, data was displayed as the mean ± SD, ***p < 0.001, ****p < 0.0001.
Until now, except for the inhibition of the proton pump, there are limited reports on the biological function and mechanism of action of omeprazole in gastrointestinal disease. Our previous study has revealed that omeprazole suppressed fatty acid synthase and ATP citrate lyase expression, leading to decreased lipogenesis (Chen et al., 2020). In this work, we further show that omeprazole inhibited cell pyroptosis by enhancing mTOR-mediated autophagy. Omeprazole treatment suppressed NLRP3 inflammasomes activation, resulting in inhibition of casapse-1 activity and IL-18/1β production and alleviated gastritis, which was attributed to increased mTOR-induced autophagy. The inhibition of PDE4 by DIP enhanced omeprazole-triggered autophagy, leading to suppressed pyroptosis to a great extent. Taken together, these findings reveal the biological function of omeprazole in alleviating inflammation through activation of autophagy mediated by PDE4.
Our lab previously showed that omeprazole can interfere with de novo lipid synthesis through fatty acid synthase and ATP citrate lyase (Chen et al., 2020). Interestingly, fatty acids (FA), the product of de novo lipid synthesis, is a critical component of membranes, energy fuels, and precursors of signaling components. Generation of saturated fatty acids and monounsaturated fatty acids (MUFA) could support tumorigenesis through remodeling tumor microenvironment, while polyunsaturated fatty acid (PUFA) may be inhibitory to tumor growth (Choi et al., 2019; Ide et al., 2013). These findings implied that omeprazole could decrease MUFAs through de novo lipid synthesis in gastric epithelial cells, leading to attenuation of gastritis. Interestingly, cell pyroptosis is a kind of cell death accompanying aggravated inflammation, which led us to ask whether cell pyroptosis is involved in omeprazole-regulated gastritis. As expected, we observed that omeprazole alleviated inflammation through inhibition of NLRP3 inflammasome activation by reducing NLRP3 and caspase1 expression. However, our study has some limitations which remain to be addressed in our next work, such as the possible function of omeprazole in metabolic reprogramming, including glycolysis, polyol pathway, and glutamine, and whether omeprazole-mediated metabolism is involved in H. pylori-associated gastritis. Moreover, in addition to caspase-1-mediated canonical pyroptosis, the function of omeprazole in caspase-4 and caspase-5-induced non-canonical pyroptosis remains to be addressed in the future work.
PDEs constitute a large family of enzymes that catalyze the degradation of cAMP and/or cGMP, and are important in modulating the intracellular concentrations of the cyclic nucleotides and in controlling their downstream signal transduction, involving cAMP-response element-binding protein (CREB) and NF-κB (Yuan et al., 2022; Chinn et al., 2022). PDE4B has attracted more attention because of its close relationship with inflammatory disease (Amata et al., 2014). Our previous study demonstrated that inhibition of PDE4 by DIP alleviated intestinal inflammatory responses in patients with irritable bowel disease through the increase in CD8+CD39+ T cells mediated by PKA/CREB (Huang et al., 2019). Roflumilast, another PDE4 inhibitor, has been shown to trigger autophagy through the enhancement of p-AMPK, p-ULK1, beclin-1, and LC3II/I expression, and decreased expressions of P62 level and p-mTOR proteins (Zaki et al., 2023; Mukherjee et al., 2022). The interaction between ATG8 and GSDMD destroyed autophagy and triggered cell pyroptosis in BEAS-2B cells (Xu et al., 2023). In addition to autophagy, whether there is another possible pathway involved in omeprazole-mediated pyroptosis, such as ferroptosis and mitophagy, needs to be investigated.
Recently, our work showed that inhibition of PDE4 by DIP or roflumilast could promote intestinal epithelial cell differentiation to induce mucosal healing through the inhibition of ferroptosis in inflammatory bowel disease (Pan et al., 2023). In the current study, we found that PDE4 inhibition by DIP enhanced omeprazole-mediated cell pyroptosis inhibition through the inhibition of mTOR phosphorylation. DIP induced an increase in AMP/ATP ratio, leading to the activation of AMPK to trigger autophagy, which further inhibited pyroptosis. However, how omeprazole regulates PDE4 activity remains to be identified, and will be the focus of our next study. Further discussion is required to address whether omeprazole treatment caused gastric epithelial cells mucosal healing and development of immune system cells, including gastric intestinal metaplasia and proliferation. Moreover, the critical issue of how omeprazole suppresses PDE4 phosphorylation will be addressed in our next work. Taken together, these findings extended the biological function of omeprazole in the treatment of gastritis, including H. pylori-induced gastritis. However, personalized treatment is also critical for patients due to clinical variations, including differences in doctors and patients, in addition to some of the challenges such as lack of technological skills, poor time management, and lack of infrastructure, particularly in the age of COVID-19 (Nimavat et al., 2021). In addition, a follow-up or assessment could be performed to analyze the post-treatment recovery.
This study shows that omeprazole alleviates inflammation by inhibiting mTOR-mediated autophagy in gastric epithelial cells based on cell pyroptosis. The results also extend the function of DIP in omeprazole-regulated cell pyroptosis and provide the novel therapeutic strategy of using DIP combined with omeprazole for improved treatment of gastritis. However, in the future, further work is required to address these issues, such as determining the dosage and side effects of DIP and the appropriate time to remove or add DIP into prescription.
Acknowledgement: We thank the doctors in the department for kindly supporting our work.
Funding Statement: This work was supported by National Natural Science Foundation of China (No. 82200607), Guangdong Basic and Applied Basic Research Foundation (Nos. 2020A1515110109, 2021A1515012194, 2023A1515030064), Basic and Applied Research Project of Guangzhou Municipal Science and Technology Project (No. 202201020631), Guangzhou Medical Key Disciplines and Specialties (No. 011006003). Guangzhou Key Laboratory of Pediatric Inflammatory Bowel Disease (No. 2023A03J0866), National Health Commission Key Laboratory of Tropical Disease Prevention and Control (2022NHCTDCKFKT21001).
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: W.X, S.G, and L.G; data collection: L.Y, H.S, and X.L; analysis and interpretation of results: W.P, and L.X and W.D; draft manuscript preparation: W.X, L.G, and S.G. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The datasets generated during and/or analyses during the current study are available from the corresponding author upon reasonable request.
Ethics Approval: Upon the declaration of Helsinki, the study was reviewed and approved by The Medical Ethics Committee for Clinical Ethical Review in Guangzhou Women and Children’s Medical Center (No. 461800). The child’s caregiver gave written informed consent for his clinical records that it is not open; however, it could be reached based on reasonable inquiries.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Amata E, Bland ND, Hoyt CT, Settimo L, Campbell PK, Pollastri MP (2014). Repurposing human PDE4 inhibitors for neglected tropical diseases: Design, synthesis and evaluation of cilomilast analogues as Trypanosoma brucei PDEB1 inhibitors. Bioorganic & Medicinal Chemistry Letters 24: 4084–4089. [Google Scholar]
Chen P, Li L, Wang H, Zhao J, Cheng Y et al. (2020). Omeprazole, an inhibitor of proton pump, suppresses De novo lipogenesis in gastric epithelial cells. Biomedicine & Pharmacotherapy 130: 110472. [Google Scholar]
Chinn AM, Salmeron C, Lee J, Sriram K, Raz E, Insel PA (2022). PDE4B is a homeostatic regulator of cyclic AMP in dendritic cells. Frontiers in Pharmacology 13: 833832. [Google Scholar] [PubMed]
Choi S, Yoo YJ, Kim H, Lee H, Chung H, Nam MH, Moon JY, Lee HS, Yoon S, Kim WY (2019). Clinical and biochemical relevance of monounsaturated fatty acid metabolism targeting strategy for cancer stem cell elimination in colon cancer. Biochemical and Biophysical Research Communications 519: 100–105. [Google Scholar] [PubMed]
Gao J, Long L, Xu F, Feng L, Liu Y, Shi J, Gong Q (2020a). Icariside II, a phosphodiesterase 5 inhibitor, attenuates cerebral ischaemia/reperfusion injury by inhibiting glycogen synthase kinase-3β-mediated activation of autophagy. British Journal of Pharmacology 177: 1434–1452. [Google Scholar] [PubMed]
Gao H, Wang X, Qu X, Zhai J, Tao L, Zhang Y, Song Y, Zhang W (2020b). Omeprazole attenuates cisplatin-induced kidney injury through suppression of the TLR4/NF-κB/NLRP3 signaling pathway. Toxicology 440: 152487. [Google Scholar] [PubMed]
Giricz Z, MentzerJr, RM, Gottlieb RA (2012). Autophagy, myocardial protection, and the metabolic syndrome. Journal of Cardiovascular Pharmacology 60: 125–132. [Google Scholar] [PubMed]
Guo R, Wang H, Cui N (2021). Autophagy regulation on pyroptosis: Mechanism and medical implication in Sepsis. Mediators of Inflammation 2021: 9925059. [Google Scholar] [PubMed]
Huang B, Chen Z, Geng L, Wang J, Liang H et al. (2019). Mucosal profiling of pediatric-onset colitis and IBD reveals common pathogenics and therapeutic pathways. Cell 179: 1160–1176. [Google Scholar] [PubMed]
Ide Y, Waki M, Hayasaka T, Nishio T, Morita Y et al. (2013). Human breast cancer tissues contain abundant phosphatidylcholine (36:1) with high stearoyl-CoA desaturase-1 expression. PLoS One 8: e61204. [Google Scholar] [PubMed]
Jang AR, Kang MJ, Shin JI, Kwon SW, Park JY et al. (2020). Unveiling the crucial role of type IV secretion system and motility of Helicobacter pylori in IL-1β production via NLRP3 inflammasome activation in Neutrophils. Front Immunol 11: 1121. [Google Scholar] [PubMed]
Jayarajan V, Appukuttan A, Aslam M, Reusch P, Regitz-Zagrosek V, Ladilov Y (2019). Regulation of AMPK activity by type 10 adenylyl cyclase: Contribution to the mitochondrial biology, cellular redox and energy homeostasis. Cellular and Molecular Life Sciences 76: 4945–4959. [Google Scholar] [PubMed]
Li L, Qian K, Sun Y, Zhao Y, Zhou Y, Xue Y, Hong X (2021). Omarigliptin ameliorated high glucose-induced nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome activation through activating adenosine monophosphate-activated protein kinase α (AMPK α) in renal glomerular endothelial cells. Bioengineered 12: 4805–4815. [Google Scholar] [PubMed]
Lin L, Zhang MX, Zhang L, Zhang D, Li C, Li YL (2021). Autophagy, pyroptosis, and ferroptosis: New regulatory mechanisms for atherosclerosis. Frontiers in Cell and Developmental Biology 9: 809955. [Google Scholar] [PubMed]
Liu W, Zhang G, Sun B, Wang S, Lu Y, Xie H (2021). Activation of NLR family, domain of pyrin containing 3 inflammasome by nitrous oxide through thioredoxin-interacting protein to induce nerve cell injury. Bioengineered 12: 4768–4779. [Google Scholar] [PubMed]
Malireddi RKS, Kesavardhana S, Karki R, Kancharana B, Burton AR, Kanneganti TD (2020). RIPK1 distinctly regulates Yersinia-induced inflammatory cell death, PANoptosis. Immunohorizons 4: 789–796. [Google Scholar] [PubMed]
Mukherjee P, Bagchi A, Banerjee A, Roy H, Bhattacharya A, Biswas A, Chatterji U (2022). PDE4 inhibitor eliminates breast cancer stem cells via noncanonical activation of mTOR. Journal of Cellular Biochemistry 123: 1980–1996. [Google Scholar] [PubMed]
Nazio F, Cecconi F (2017). Autophagy up and down by outsmarting the incredible ULK. Autophagy 13: 967–968. [Google Scholar] [PubMed]
Nimavat N, Singh S, Fichadiya N, Sharma P, Patel N, Kumar M, Chauhan G, Pandit N (2021). Online medical education in India-different challenges and probable solutions in the age of COVID-19. Advances in Medical Education and Practice 12: 237–243. [Google Scholar] [PubMed]
Pachathundikandi S, Blaser N, Bruns H, Backert S (2020). Helicobacter pylori avoids the critical activation of NLRP3 inflammasome-mediated production of oncogenic mature IL-1β in human immune cells. Cancers 12: 803. [Google Scholar] [PubMed]
Pachathundikandi S, Muller A, Backert S (2016). Inflammasome activation by Helicobacter pylori and its implications for persistence and immunity. Current Topics in Microbiology and Immunology 397: 117–131. [Google Scholar] [PubMed]
Pan W, Xiang L, Liang X, Du W, Zhao J, Zhang S, Zhou X, Geng L, Gong S, Xu W (2023). Vitronectin destroyed intestinal epithelial cell differentiation through activation of PDE4-mediated ferroptosis in inflammatory bowel disease. Mediators of Inflammation 2023: 6623329. https://doi.org/10.1155/2023/6623329 [Google Scholar] [PubMed] [CrossRef]
Pillon NJ, Chan KL, Zhang S, Mejdani M, Jacobson MR, Ducos A, Bilan PJ, Niu W, Klip A (2016). Saturated fatty acids activate caspase-4/5 in human monocytes, triggering IL-1β and IL-18 release. American Journal of Physiology-Endocrinology and Metabolism 311: E825–E835. [Google Scholar] [PubMed]
Poh L, Kang SW, Baik SH, Ng GYQ, She DT et al. (2019). Evidence that NLRC4 inflammasome mediates apoptotic and pyroptotic microglial death following ischemic stroke. Brain Behavior and Immunity 75: 34–47. [Google Scholar] [PubMed]
Schick MA, Schlegel N (2022). Clinical implication of Phosphodiesterase-4-inhibition. International Journal of Molecular Sciences 23: 1209. [Google Scholar]
Shah N, Gossman W (2021). Omeprazole. Treasure Island (FLStatPearls. [Google Scholar]
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015). Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665. [Google Scholar] [PubMed]
Sun L, Zhao M, Liu A, Lv M, Zhang J, Li Y, Yang X, Wu Z (2018). Shear stress induces phenotypic modulation of vascular smooth muscle cells via AMPK/mTOR/ULK1-mediated autophagy. Cellular and Molecular Neurobiology 38: 541–548. [Google Scholar] [PubMed]
Szrejder M, Rachubik P, Rogacka D, Audzeyenka I, Rychlowski M, Angielski S, Piwkowska A (2020). Extracellular ATP modulates podocyte function through P2Y purinergic receptors and pleiotropic effects on AMPK and cAMP/PKA signaling pathways. Archives of Biochemistry and Biophysics 695: 108649. [Google Scholar] [PubMed]
Tang H, Ye Y, Li L, Zhou Y, Hou L, Ren S, Xu Y (2022). A20 alleviated caspase-1-mediated pyroptosis and inflammation stimulated by Porphyromonas gingivalis lipopolysaccharide and nicotine through autophagy enhancement. Human Cell 35: 803–816. [Google Scholar] [PubMed]
Wang Y, Zhou X, Zou K, Chen G, Huang L et al. (2021). Monocarboxylate transporter 4 triggered cell pyroptosis to aggravate intestinal inflammation in inflammatory bowel disease. Frontiers in Immunology 12: 644862. [Google Scholar] [PubMed]
Xi G, Rosen CJ, Clemmons DR (2016). IGF-I and IGFBP-2 stimulate AMPK activation and autophagy, are required for osteoblast differentiation. Endocrinology 157: 268–281. [Google Scholar] [PubMed]
Xie J, Fan L, Xiong L, Chen P, Wang H et al. (2021). Rabeprazole inhibits inflammatory reaction by inhibition of cell pyroptosis in gastric epithelial cells. BMC Pharmacology and Toxicology 22: 44. [Google Scholar] [PubMed]
Xu L, Shi Z, Pan Z, Wu R (2023). METTL3 promotes hyperoxia-induced pyroptosis in neonatal bronchopulmonary dysplasia by inhibiting ATG8-mediated autophagy. Clinics 78: 100253. [Google Scholar] [PubMed]
Xu W, Zhang Z, Zou K, Cheng Y, Yang M et al. (2017). MiR-1 suppresses tumor cell proliferation in colorectal cancer by inhibition of Smad3-mediated tumor glycolysis. Cell Death & Disease 8: e2761. [Google Scholar]
Yang F, Li L, Zhou Y, Pan W, Liang X et al. (2023). Rabeprazole destroyed gastric epithelial barrier function through FOXF1/STAT3-mediated ZO-1 expression. Clinical and Experimental Pharmacology and Physiology 50: 516–526. https://doi.org/10.1111/1440-1681.13769 [Google Scholar] [PubMed] [CrossRef]
Yeo EJ (2019). Hypoxia and aging. Experimental and Molecular Medicine 51: 1–15. [Google Scholar]
Yu P, Wang HY, Tian M, Li AX, Chen XS, Wang XL, Zhang Y, Cheng Y (2019). Eukaryotic elongation factor-2 kinase regulates the cross-talk between autophagy and pyroptosis in doxorubicin-treated human melanoma cells in vitro. Acta Pharmacologica Sinica 40: 1237–1244. [Google Scholar] [PubMed]
Yu Z, Xie X, Su X, Lv H, Song S et al. (2022). ATRA-mediated-crosstalk between stellate cells and Kupffer cells inhibits autophagy and promotes NLRP3 activation in acute liver injury. Cell Signal 93: 110304. [Google Scholar] [PubMed]
Yuan F, Ren H, Tan W, Wang Y, Luo H (2022). Effect of phosphodiesterase-4 inhibitor rolipram on colonic hypermotility in water avoidance stress rat model. Neurogastroenterology & Motility 34: e14317. [Google Scholar]
Zaki ES, Sayed RH, Saad MA, El-Yamany MF (2023). Roflumilast ameliorates ovariectomy-induced depressive-like behavior in rats via activation of AMPK/mTOR/ULK1-dependent autophagy pathway. Life Sciences 327: 121806. [Google Scholar] [PubMed]
Zhang J (2015). Teaching the basics of autophagy and mitophagy to redox biologists--mechanisms and experimental approaches. Redox Biology 4: 242–259. [Google Scholar] [PubMed]
Zhang X, Li C, Chen D, He X, Zhao Y, Bao L, Wang Q, Zhou J, Xie Y (2022). H. pylori CagA activates the NLRP3 inflammasome to promote gastric cancer cell migration and invasion. Inflammation Research 71: 141–155. [Google Scholar] [PubMed]
Zhang D, Qian J, Zhang P, Li H, Shen H, Li X, Chen G (2019). Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. Journal of Neuroscience Research 97: 645–660. [Google Scholar] [PubMed]
Zhivaki D, Kagan JC (2021). NLRP3 inflammasomes that induce antitumor immunity. Trends in Immunology 42: 575–589. [Google Scholar] [PubMed]
Zhou Y, Chen S, Yang F, Zhang Y, Xiong L et al. (2021). Rabeprazole suppresses cell proliferation in gastric epithelial cells by targeting STAT3-mediated glycolysis. Biochemical Pharmacology 188: 114525. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools