 Open Access
Open Access
ARTICLE
Magnetic mitohormesis: A non-invasive therapy for inflammatory disorders?
Department of Surgery, Yong Loo Lin School of Medicine, Institute of Health Technology and Innovation, iHealthtech, Biolonic Currents Electromagnetic Pulsing Systems Laboratory, BICEPS, Healthy Longevity Translational Research Programme, NUS Centre for Cancer Research, National University of Singapore, Singapore
* Corresponding Author: Alfredo Franco-Obregón,
BIOCELL 2023, 47(2), 239-244. https://doi.org/10.32604/biocell.2023.025357
Received 07 July 2022; Accepted 25 August 2022; Issue published 18 November 2022
Abstract
An organism’s survival depends on its ability to adapt to stress. Mitochondria are the cellular integrators of environmental stressors that ultimately translate their responses at the organismal level, and are thus central to the process whereby organisms adapt to their respective environments. Mitochondria produce molecular energy via oxidative phosphorylation that then allows cells to biosynthetically respond and adapt to changes in their environment. Reactive oxygen species (ROS) are by-products of oxidative phosphorylation that can be either beneficial or damaging, depending on the context; ROS are hence both the conveyors of environmental stress as well as cellular “adaptogens”. Mitohormesis refers to the process whereby low levels of oxidative stress spur survival adaptations, whereas excessive levels stymie survival. Low energy and frequency pulsing electromagnetic fields have been recently shown capable of stimulating mitochondrial respiration and ROS production and instilling mitohormetic survival adaptations, similarly to, yet independently of, exercise, opening avenues for the future development of Magnetic Mitohormetic interventions for the improvement of human health. This viewpoint explores the possibilities and nuances of magnetic-based therapies as a form of clinical intervention to non-invasively activate magnetic mitohormesis for the management of chronic diseases.Keywords
Human health and mitochondrial function are inextricably coupled statuses (Nunnari and Suomalainen, 2012; Louzada et al., 2020). Nearly all of life’s processes require cellular-based energy production to be executed. These include growth, repair, and immunological defense. The requisite energy for biosynthesis is predominantly supplied by the mitochondria with the participation of molecular oxygen to serve as the final electron acceptor during cellular respiration. The capacity of molecular oxygen to successfully accept respiratory electrons (to form water) places an upper limit on the respiratory capacity that, when exceeded, results in the production of potentially deleterious respiratory by-products. In this respect, mitochondria are the greatest producers of reactive oxygen species (ROS) (Oyewole and Birch-Machin, 2015). Approximately 0.2–2% of the electrons processed via the mitochondrial electron transport chain are unable to fully reduce molecular oxygen and ultimately produce superoxide or hydrogen peroxide, the two most predominant ROS species (Geto et al., 2020). In cases where the existing antioxidant defenses of the mitochondria are inadequate to neutralize constitutive ROS production, or under circumstances where energy requirement exceeds the antioxidant capacity of the mitochondria, ROS levels can rise sufficiently to oxidatively damage proteins and nucleic acids or cause lipid peroxidation within cellular membrane-delimited domains that, on one level, compromise the functioning and viability of the directly implicated cell, while on another level result in the release of mitochondrial DNA and mitochondrial breakdown products into the extracellular environment. Due to its close proximity to the source of ROS production and the absence of nuclear-like DNA repair mechanisms, the mitochondrial genome is particularly susceptible to oxidative damage wherein mutations are not corrected and perpetuated, ultimately compromising energy production, cellular repair, and membrane integrity and result in the escape of mitochondrial components into the general circulation where they induce systemic inflammation. Systemic inflammation, in turn, undermines tissue regeneration and maintenance as well as disrupts systemic metabolism and immunity. Accordingly, a deterioration in mitochondrial respiratory and antioxidant efficiency is associated with accelerated aging (Wang and Hekimi, 2015; Gallage and Gil, 2016; Ferri et al., 2020; Lima et al., 2022).
The muscular mitochondrial pool is critically important for organismal health and longevity (Russell et al., 2014; Hood et al., 2019; De Mario et al., 2021; Fealy et al., 2021). Indeed, metabolism, systemic inflammatory status and resilience to disease all have mitochondrial origins that can be linked back to skeletal muscle (Smith et al., 2018; Louzada et al., 2020; Fealy et al., 2021; Amorim et al., 2022). Moreover, as immunity is a function of systemic inflammation, it also links back to the muscular mitochondrial pool (Ubaida-Mohien et al., 2019; Wallings et al., 2020; Lisci et al., 2021). Cancer progression is also influenced by systemic inflammatory status and hence, is also subject to modulation by the muscular mitochondrial response (Geto et al., 2020; Stine et al., 2021). Indeed, organismal health, in general, parallels muscle health.
Counter to conventional wisdom, ROS are not exclusively evil. In a microcosmic parallel to physical exercise, whereby muscular activity improves physical performance, mitochondria adapt to their own usage. In essence, mitochondria respond to the same ROS they produce during oxidative energy production by enhancing mitochondrial function, and moreover, they underlie exercise adaptations on the organismal level. Key to this adaptive process is Pparg coactivator-1α (PGC-1α), the ROS/Redox-responsive master gene involved in mitochondriogenesis and exercise-based physical adaptations (Thirupathi and de Souza, 2017; Louzada et al., 2020). Stimulated mitochondrial energy production during physical activity hence, reinforces mitochondrial respiratory fitness by increasing mitochondrial number and expanding the interconnected mitochondrial network (Hood et al., 2019; Geto et al., 2020; Philp et al., 2021). Mitochondrial fusion facilitates the sharing of mitochondrial metabolites, energy substrates, and mitochondrial DNA, enhancing mitochondrial resistance to oxidative stress as well as forestalling mitochondrial fragmentation in preparation for clearance via mitophagy. Routine aerobic exercise also enhances the mitochondrial antioxidant defenses and efficiency of oxidation phosphorylation (ATP production) and results in a shift in substrate utilization towards lipids, all of which are metabolically beneficial conditions. By contrast, low levels of physical activity are associated with the reduced mitochondrial number, attenuated respiratory efficiency, mitochondrial fragmentation, mitophagy, and extracellular expulsion. A sedentary lifestyle is hence characterized by mitochondrial molecular profiles known to be associated with systemic inflammation and metabolic imbalance.
Mitohormesis refers to the process whereby an organism adapts to mitochondrial ROS (Ristow and Schmeisser, 2014). Energy-consuming processes that are not pathologically inflammatory in nature, generally promote organ health and maintenance with functional consequences. In this manner, normal exercise benefits muscle energetics and systemic health. This adaptive process is short-circuited in persons that are physically incapacitated due to frailty, age, disease, or trauma. Developing methods to re-engage muscular mitochondrial-based adaptations in the clinically immobilized has thus been a major focus of the physical rehabilitation sciences. A noted caveat to these efforts is the fact that mitochondrial energy expenditure is more metabolically relevant than mere movement per se, and therefore, assisted movement of the body by a physical therapist would not be sufficient to fully re-engage the response. A manner to non-invasively activate mitochondrial respiration would be beneficial.
Muscular Mitohormesis: Harnessing the Innate Endocrine Function of Skeletal Muscle
Muscle, as our largest unified tissue mass, is also our greatest unified source of mitochondria. Exercise or physical movement, by virtue of their requirement for mitochondrial energy production, is thus the most natural manner to induce systemic mitohormesis with a positive consequence over systemic inflammation. This role of muscles is largely mediated via the actions of its secretome (Louzada et al., 2020). The production of mitochondrial ROS has been shown to be a key stimulus in activating growth factor pathways within muscles (Auten and Davis, 2009; Scheele et al., 2009). Energy-dependent muscle secretome release and distribution to peripheral tissues (and muscle) is a key reason behind the described healthful benefits of exercise. In these collateral tissues, the muscle secretome influences tissue as well as mitochondrial homeostasis (Romanello, 2020), particularly in adipose and bone tissues (Kirk et al., 2020; Gomarasca et al., 2020). Adipose tissue in sedentary individuals releases proinflammatory cytokines (adipokines) into the systemic circulation, and via this pathway, adipose tissue inflammation gives rise to system-wide metabolic disorders. On the other hand, myokines released from the muscle in response to exercise promote adipose browning characterized by enhanced mitochondriogenesis, thermogenesis, lipolysis, and a shift in adipokine secretory profile. Adipose tissue responds to myokine conditioning by attenuating inflammatory adipokine secretion, thereby reinforcing muscle and systemic metabolism. In this manner, cytokine-mediated muscle-adipose crosstalk is a major regulator of systemic inflammatory status, metabolic balance, and microbiome diversity (Li et al., 2017; Leal et al., 2018, 2021; Gomarasca et al., 2020; Suriano et al., 2020; Zhang and Sun, 2021). Accordingly, evidence supports the role of the muscle secretome in immunometabolism and its importance for the control of tumor growth and chronic inflammation (Bay and Pedersen, 2020). A detailed discussion of the muscle secretome components mediating systemic immunomodulation and anti-inflammatory roles is beyond the scope of this viewpoint and has been comprehensively discussed elsewhere (Bay and Pedersen, 2020; Louzada et al., 2020).
Muscular Magnetic Mitohormesis & Muscle Secretome Activation
Low amplitude and extremely low-frequency pulsed electromagnetic fields (PEMFs) were shown capable of inducing mitochondrial respiration and mitohormetic responses in muscle, both in vitro (Yap et al., 2019) and in vivo (Tai et al., 2020), downstream of PGC-1α activation. The employed Helmholtz coil systems create a three-dimensional volume of field uniformity (Crocetti et al., 2013; Wong et al., 2022) that is essential for achieving optimal biological efficacy (Parate et al., 2017; Yap et al., 2019; Madanagopal et al., 2021). Muscle was found to be most responsive to PEMFs at an amplitude of 1.5 milliTesla (mT) delivered once a week for 10 min (Yap et al., 2019), whereas smaller or greater amplitude PEMFs, shorter or longer duration, or more frequent exposures, did not render additional benefits or were even less effective. These weak magnetic fields are only ~20–30 times greater in amplitude than the standing geomagnetic field of the Earth and are in the extremely low-frequency range (Hz–kHz) and hence, are non-ionizing. Analogous PEMFs were shown to protect against inflammatory stress (Parate et al., 2020). Moreover, as the fields work on the quantum physical level to stimulate mitochondrial electron transport (Usselman et al., 2016), they are too weak to act as a vicarious form of mechanical stimulation. That is, by inadvertently causing muscle fiber or cell contraction, they exert no mechanical stress. Nonetheless, as they do activate mitochondrial respiration and downstream ROS production, generating mitohormetic levels of oxidative stress, prudence is advised, and overexposure should be avoided.
In isolated muscle cells, Magnetic Mitohormesis was associated with increased mitochondriogenesis, mitochondrial respiration, and reduced apoptosis (Yap et al., 2019). In mice, muscular Magnetic Mitohormesis was shown to improve running performance after as little as five weeks of weekly exposure (10 min/wk for a total of 50 min of exposure), enhance muscle oxidative capacity (increase type muscle fiber expression governing aerobic/endurance activities), increase muscle and adipose mitochondriogenesis, induce adipose browning, improve insulin sensitivity, augment fatty acid oxidation, and induce shifts in the microbiome indicative of leaner phenotype as previously described with exercise training (Tai et al., 2020). The observed effects are correlated with the magnetically-induced activation of the muscle secretome (Wong et al., 2022), as previously demonstrated in the stem cell niche with anti-inflammatory attributes (Parate et al., 2020). Indeed, Magnetic Mitohormesis recapitulates many of the hallmark metabolic indices typically associated with habitual exercise (Fig. 1).
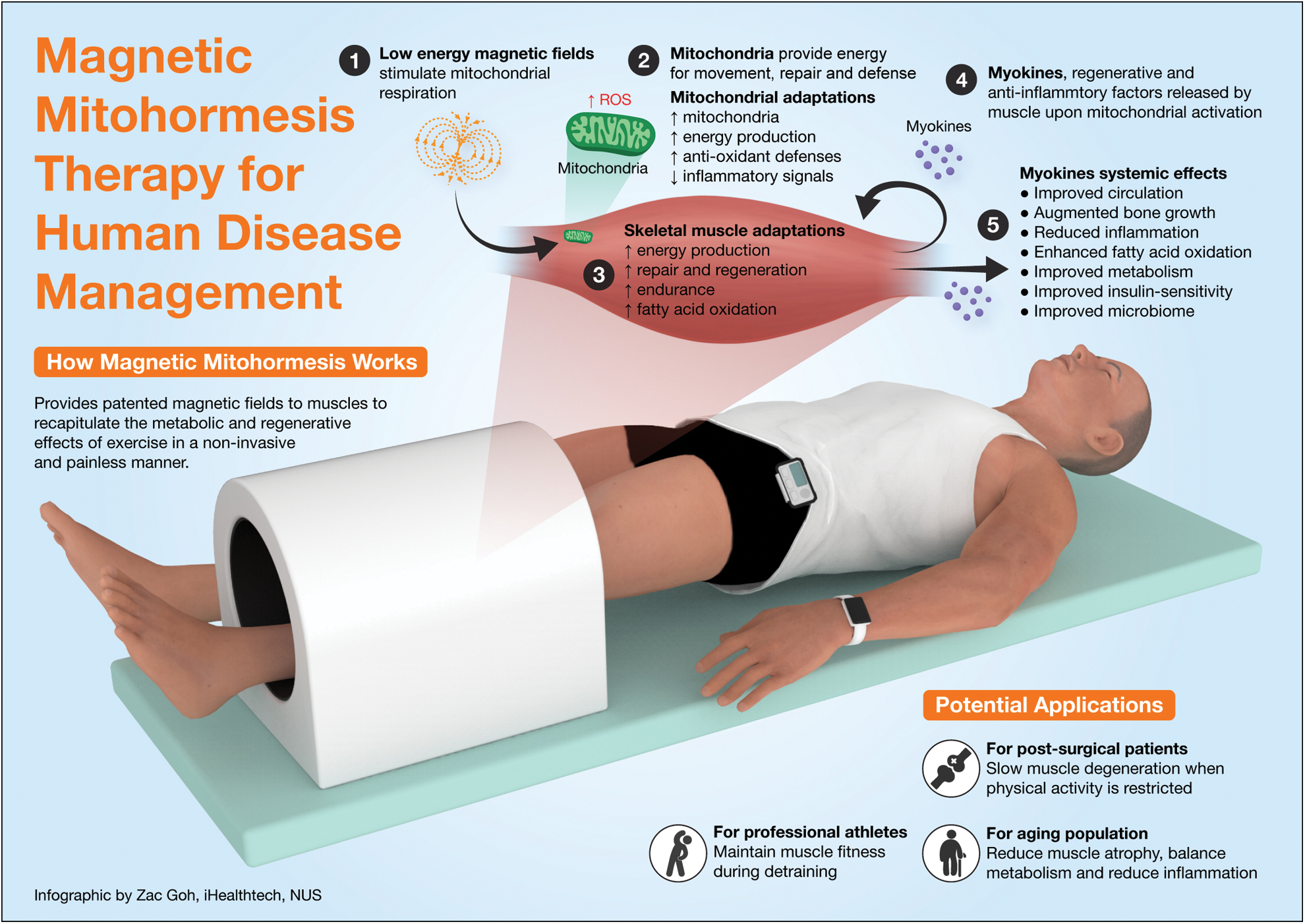
Figure 1: Magnetic mitohormesis, mechanisms, and responses. Adapted from NUH Médico (MCI (P) 121/03/2020).
Mitochondrial Fitness and COVID-19 Vulnerability
Measures are currently being urgently sought to curtail the damaging consequences of the global COVID-19 pandemic. COVID-19 is commonly associated with damage to the respiratory system, endothelial inflammation, and multiple organ failure that are triggered by excessive production of proinflammatory cytokines (Filgueira et al., 2021). On the other hand, physical activity induces the production of myokines that mitigate low-grade systemic inflammation. Moreover, long COVID is similarly linked to systemic inflammatory status and is likewise ameliorated by muscular respiratory fitness (Burtscher et al., 2020; Ranasinghe et al., 2020; Sies and Ursini, 2021). Available evidence thus supports that vulnerability to COVID-19 is improved by physical and mitochondrial fitness.
Therefore, the development of safe and non-exertional methods to improve mitochondrial fitness may serve to manage persistent COVID-19 symptoms. Although this notion is provocative to contemplate, it remains to be shown in large-scale randomized clinical trials whether analogous magnetic therapies will one day prove a viable COVID-19 intervention.
The magnetic paradigm discussed here has been shown to invoke mitohormetic responses in muscle cells and stem cell classes associated with the production of ROS and the correspondent cellular responses, including enhanced mitochondriogenesis, improved survival, and enhanced tissue differentiation (Yap et al., 2019; Parate et al., 2020; Tai et al., 2020; Celik et al., 2021; Madanagopal et al., 2021; Wong et al., 2022). It is widely agreed that actively stimulating muscular mitochondrial energy production has clear health benefits. Physical exercise is the best method to achieve this objective as it also gives rise to a fuller breadth of collateral responses, contributed by the mechanobiological stimulation of muscle and bone, as well as the direct neurological and endocrine engagement during the execution of the exercise. However, if one is limited in physical capacity due to age, disease, or general frailty, then magnetic mitohormesis may serve as a valid and safe alternative to help sustain metabolic balance; treatment in animals and humans requires only 10 min per week. Moreover, the absence of mechanical stress afforded by Magnetic Mitohormesis may be a valuable asset in some clinical scenarios where muscle loading is ill-advised or unfeasible. The provocative implications are that analogous magnetic field therapies may one day be exploitable as a manner to forestall the onset, or in the management, of chronic diseases and await clinical validation.
Acknowledgement: The author would like to acknowledge funding support by a charitable donation by the Lee Kong Chian MedTech Initiative, Singapore, and funding from the Institute for Health Innovation & Technology, iHealthtech, at the National University of Singapore. The author would also like to thank the members of the Biolonic Currents Electromagnetic Pulsing Systems (BICEPS) Laboratory, without whom this research would not have been possible. Finally, the author would also like to acknowledge Zac Goh of the iHealthtech, the National University of Singapore, for the graphical design.
Availability of Data and Materials: Not applicable.
Author Contribution: The author confirms sole responsibility for the interpretation of the findings and manuscript preparation.
Ethics Approval: Not applicable.
Funding Statement: This work is supported by Lee Kong Chian MedTech Initiative, Singapore (N-176-000-045-001) and the Institute for Health Innovation & Technology, iHealthtech, at the National University of Singapore. The publication cost of this article is funded by Lee Kong Chian MedTech Initiative, Singapore.
Conflicts of Interest: AFO is an inventor on patent WO 2019/17863 A1, System, and Method for Applying Pulsed Electromagnetic Fields, as well as a contributor to QuantumTxPte, Ltd., which elaborates electromagnetic field devices for human use.
Note Added in Proof: Since the publication of this viewpoint a manuscript has been published in support of the metabolic attributes of Magnetic Mitohormesis. Stephenson MC, Krishna L, Selvan RMP, Tai YK, Wong CJK, Yin JN, Toh SJ, Torta F, Triebl A, Frӧhlich J, Beyer C, Li JZ, Tan SS, Wong CK, Chinnasamy D, Sivappiragasam S/O Pakkiri L, Drum CL, Wenk MR, Totman JJ, Franco-Obregón A (2022), Magnetic field therapy enhances muscle mitochondrialbioenergetics and attenuates systemic ceramide levels following ACL reconstruction: Southeast asian randomized-controlled pilot trial. Journal of Orthopaedic Translation. https://doi.org/10.1016/j.jot.2022.09.011.
References
Amorim JA, Coppotelli G, Rolo AP, Palmeira CM, Ross JM, Sinclair DA (2022). Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nature Reviews Endocrinology 18: 243–258. DOI 10.1038/s41574-021-00626-7. [Google Scholar] [CrossRef]
Auten RL, Davis JM (2009). Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatric Research 66: 121–127. DOI 10.1203/PDR.0b013e3181a9eafb. [Google Scholar] [CrossRef]
Bay ML, Pedersen BK (2020). Muscle-organ crosstalk: Focus on immunometabolism. Frontiers in Physiology 11: 567881. DOI 10.3389/fphys.2020.567881. [Google Scholar] [CrossRef]
Burtscher J, Cappellano G, Omori A, Koshiba T, Millet GP (2020). Mitochondria: In the cross fire of SARS-CoV-2 and immunity. iScience 23: 101631. DOI 10.1016/j.isci.2020.101631. [Google Scholar] [CrossRef]
de Mario A, Gherardi G, Rizzuto R, Mammucari C (2021). Skeletal muscle mitochondria in health and disease. Cell Calcium 94: 102357. DOI 10.1016/j.ceca.2021.102357. [Google Scholar] [CrossRef]
Celik C, Franco-Obregón A, Lee EH, Hui JHP, Yang Z (2021). Directionalities of magnetic fields and topographic scaffolds synergise to enhance MSC chondrogenesis. Acta Biomaterialia 119: 169–183. DOI 10.1016/j.actbio.2020.10.039. [Google Scholar] [CrossRef]
Crocetti S, Beyer C, Schade G, Egli M, Fröhlich J, Franco-Obregón A (2013). Low intensity and frequency pulsed electromagnetic fields selectively impair breast cancer cell viability. PLoS One 8: e72944. DOI 10.1371/journal.pone.0072944. [Google Scholar] [CrossRef]
Fealy CE, Grevendonk L, Hoeks J, Hesselink MKC (2021). Skeletal muscle mitochondrial network dynamics in metabolic disorders and aging. Trends in Molecular Medicine 27: 1033–1044. DOI 10.1016/j.molmed.2021.07.013. [Google Scholar] [CrossRef]
Ferri E, Marzetti E, Calvani R, Picca A, Cesari M, Arosio B (2020). Role of age-related mitochondrial dysfunction in sarcopenia. International Journal of Molecular Sciences 21: 5236. DOI 10.3390/ijms21155236. [Google Scholar] [CrossRef]
Filgueira TO, Castoldi A, Santos LER, de Amorim GJ, de Sousa Fernandes MS, Anastácio WLDN, Campos EZ, Santos TM, Souto FO (2021). The relevance of a physical active lifestyle and physical fitness on immune defense: Mitigating disease burden, with focus on COVID-19 consequences. Frontiers in Immunology 12: 587146. DOI 10.3389/fimmu.2021.587146. [Google Scholar] [CrossRef]
Gallage S, Gil J (2016). Mitochondrial dysfunction meets senescence. Trends in Biochemical Sciences 41: 207–209. DOI 10.1016/j.tibs.2016.01.005. [Google Scholar] [CrossRef]
Geto Z, Molla MD, Challa F, Belay Y, Getahun T (2020). Mitochondrial dynamic dysfunction as a main triggering factor for inflammation associated chronic non-communicable diseases. Journal of Inflammation Research 13: 97–107. DOI 10.2147/JIR.S232009. [Google Scholar] [CrossRef]
Gomarasca M, Banfi G, Lombardi G (2020). Myokines: The endocrine coupling of skeletal muscle and bone. Advances in Clinical Chemistry 94: 155–218. DOI 10.1016/bs.acc.2019.07.010. [Google Scholar] [CrossRef]
Hood DA, Memme JM, Oliveira AN, Triolo M (2019). Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annual Review of Physiology 81: 19–41. DOI 10.1146/annurev-physiol-020518-114310. [Google Scholar] [CrossRef]
Kirk B, Feehan J, Lombardi G, Duque G (2020). Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Current Osteoporosis Reports 18: 388–400. DOI 10.1007/s11914-020-00599-y. [Google Scholar] [CrossRef]
Leal LG, Lopes MA, BatistaJr ML (2018). Physical exercise-induced myokines and muscle-adipose tissue crosstalk: A review of current knowledge and the implications for health and metabolic diseases. Frontiers in Physiology 9: 1307. DOI 10.3389/fphys.2018.01307. [Google Scholar] [CrossRef]
Leal DV, Ferreira A, Watson EL, Wilund KR, Viana JL (2021). Muscle-bone crosstalk in chronic kidney disease: The potential modulatory effects of exercise. Calcified Tissue International 108: 461–475. DOI 10.1007/s00223-020-00782-4. [Google Scholar] [CrossRef]
Li F, Li Y, Duan Y, Hu CAA, Tang Y, Yin Y (2017). Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine & Growth Factor Reviews 33: 73–82. DOI 10.1016/j.cytogfr.2016.10.003. [Google Scholar] [CrossRef]
Lisci M, Barton PR, Randzavola LO, Ma CY, Marchingo JM, Cantrell DA, Paupe V, Prudent J, Stinchcombe JC, Griffiths GM (2021). Mitochondrial translation is required for sustained killing by cytotoxic T cells. Science 374: eabe9977. DOI 10.1126/science.abe9977. [Google Scholar] [CrossRef]
Lima T, Li TY, Mottis A, Auwerx J (2022). Pleiotropic effects of mitochondria in aging. Nature Aging 2: 199–213. DOI 10.1038/s43587-022-00191-2. [Google Scholar] [CrossRef]
Louzada RA, Bouviere J, Matta LP, Werneck-de-Castro JP, Dupuy C, Carvalho DP, Fortunato RS (2020). Redox signaling in widespread health benefits of exercise. Antioxidants & Redox Signaling 33: 745–760. DOI 10.1089/ars.2019.7949. [Google Scholar] [CrossRef]
Madanagopal TT, Tai YK, Lim SH, Fong CHH, Cao T, Rosa V, Franco-Obregón A (2021). Pulsed electromagnetic fields synergize with graphene to enhance dental pulp stem cell-derived neurogenesis by selectively targeting TRPC1 channels. European Cells and Materials 41: 216–232. DOI 10.22203/eCM.v041a16. [Google Scholar] [CrossRef]
Nunnari J, Suomalainen A (2012). Mitochondria: In sickness and in health. Cell 148: 1145–1159. DOI 10.1016/j.cell.2012.02.035. [Google Scholar] [CrossRef]
Oyewole AO, Birch-Machin MA (2015). Mitochondria-targeted antioxidants. FASEB Journal 29: 4766–4771. DOI 10.1096/fj.15-275404. [Google Scholar] [CrossRef]
Parate D, Franco-Obregón A, Frӧhlich J, Beyer C, Abbas AA, Kamarul T, Hui JHP, Yang Z (2017). Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Scientific Reports 7 9421. DOI 10.1038/s41598-017-09892-w. [Google Scholar] [CrossRef]
Parate D, Kadir ND, Celik C, Lee EH, Hui JHP, Franco-Obregón A, Yang Z (2020). Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Stem Cell Research & Therapy 11: 46. DOI 10.1186/s13287-020-1566-5. [Google Scholar] [CrossRef]
Philp AM, Saner NJ, Lazarou M, Ganley IG, Philp A (2021). The influence of aerobic exercise on mitochondrial quality control in skeletal muscle. The Journal of Physiology 599: 3463–3476. DOI 10.1113/JP279411. [Google Scholar] [CrossRef]
Ranasinghe C, Ozemek C, Arena R (2020). Exercise and well-being during COVID 19—time to boost your immunity. Expert Review of Anti-Infective Therapy 18: 1195–1200. DOI 10.1080/14787210.2020.1794818. [Google Scholar] [CrossRef]
Ristow M, Schmeisser K (2014). Mitohormesis: Promoting health and lifespan by increased levels of reactive oxygen species (ROS). Dose Response 12: 288–341. DOI 10.2203/dose-response.13-035.Ristow. [Google Scholar] [CrossRef]
Romanello V (2020). The interplay between mitochondrial morphology and myomitokines in aging sarcopenia. International Journal of Molecular Sciences 22: 91. DOI 10.3390/ijms22010091. [Google Scholar] [CrossRef]
Russell AP, Foletta VC, Snow RJ, Wadley GD (2014). Skeletal muscle mitochondria: A major player in exercise, health and disease. Biochimica et Biophysica Acta 1840: 1276–1284. DOI 10.1016/j.bbagen.2013.11.016. [Google Scholar] [CrossRef]
Scheele C, Nielsen S, Pedersen BK (2009). ROS and myokines promote muscle adaptation to exercise. Trends in Endocrinology and Metabolism 20: 95–99. DOI 10.1016/j.tem.2008.12.002. [Google Scholar] [CrossRef]
Sies H, Ursini F (2021). Homeostatic control of redox status and health. International Union of Biochemistry and Molecular Biology Life 74: 24–28. DOI 10.1002/iub.2519. [Google Scholar] [CrossRef]
Smith RL, Soeters MR, Wüst RCI, Houtkooper RH (2018). Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocrine Reviews 39: 489–517. DOI 10.1210/er.2017-00211. [Google Scholar] [CrossRef]
Stine ZE, Schug ZT, Salvino JM, Dang CV (2021). Targeting cancer metabolism in the era of precision oncology. Nature Reviews Drug Discovery 3: 1–22. DOI 10.1038/s41573-021-00339-6. [Google Scholar] [CrossRef]
Suriano F, Van Hul M, Cani PD (2020). Gut microbiota and regulation of myokine-adipokine function. Current Opinion in Pharmacology 52: 9–17. DOI 10.1016/j.coph.2020.03.006. [Google Scholar] [CrossRef]
Tai YK, Ng C, Purnamawati K, Yap JLY, Yin JN et al. (2020). Magnetic fields modulate metabolism and gut microbiome in correlation with PGC-1α expression: follow-up to an in vitro magnetic mitohormetic study. FASEB Journal 34: 11143–11167. DOI 10.1096/fj.201903005RR. [Google Scholar] [CrossRef]
Thirupathi A, de Souza CT (2017). Multi-regulatory network of ROS: The interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. Journal of Physiology and Biochemistry 73: 487–494. DOI 10.1007/s13105-017-0576-y. [Google Scholar] [CrossRef]
Ubaida-Mohien C, Lyashkov A, Gonzalez-Freire M, Tharakan R, Shardell M et al. (2019). Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. eLife 8: e49874. DOI 10.7554/eLife.49874. [Google Scholar] [CrossRef]
Usselman RJ, Chavarriaga C, Castello PR, Procopio M, Ritz T, Dratz EA, Singel DJ, Martino CF (2016). The quantum biology of reactive oxygen species partitioning impacts cellular bioenergetics. Scientific Reports 6: 38543. DOI 10.1038/srep38543. [Google Scholar] [CrossRef]
Wallings RL, Herrick MK, Tansey MG (2020). Linking mitochondria to the immune response. eLife 9: e56214. DOI 10.7554/eLife.56214. [Google Scholar] [CrossRef]
Wang Y, Hekimi S (2015). Mitochondrial dysfunction and longevity in animals: Untangling the knot. Science 350: 1204–1207. DOI 10.1126/science.aac4357. [Google Scholar] [CrossRef]
Wong CJK, Tai YK, Yap JLY, Fong CHH, Loo LSW et al. (2022). Brief exposure to directionally-specific pulsed electromagnetic fields stimulates extracellular vesicle release and is antagonized by streptomycin: a potential regenerative medicine and food industry paradigm. Biomaterials 287: 121658. DOI 10.1016/j.biomaterials.2022.121658. [Google Scholar] [CrossRef]
Yap JLY, Tai YK, Frohlich J, Fong CHH, Yin JN et al. (2019). Ambient and supplemental magnetic fields promote myogenesis via a TRPC1-mitochondrial axis: Evidence of a magnetic mitohormetic mechanism. FASEB Journal 13: 12853–12872. DOI 10.1096/fj.201900057R. [Google Scholar] [CrossRef]
Zhang L, Sun Y (2021). Muscle-bone crosstalk in chronic obstructive pulmonary disease. Frontiers in Endocrinology 12: 724911. DOI 10.3389/fendo.2021.724911. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools