 Open Access
Open Access
ARTICLE
Analysis of the personalized treatment and the relevant prognostic factors in children with medulloblastoma
1 Department of Neurosurgery, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, 610072, China
2 Chinese Academy of Sciences Sichuan Translational Medicine Research Hospital, Chengdu, 610072, China
* Corresponding Author: RUXIANG XU. Email:
# These authors contributed equally to this study
BIOCELL 2023, 47(5), 1065-1073. https://doi.org/10.32604/biocell.2023.025924
Received 05 August 2022; Accepted 28 November 2022; Issue published 10 April 2023
Abstract
Purpose: The present study summarized cases of children (n = 32) with medulloblastoma (MB) who were treated using stratified therapy based on risk grading and also discussed the factors affecting prognosis. Methods: According to the risk stratification criteria, the cases were divided into the following four risk groups: low, standard, high, and very high. The 5-year overall survival (OS) and progression-free survival (PFS) rates were summarized. Further, the effects on the prognosis of tumor size, tumor stage, degree of resection, treatment mode, metastatic recurrence, molecular typing, and risk stratification were analyzed. Results: In the present study, following surgery, 3 cases abandoned radiotherapy (RT) and chemotherapy (CHT), 7 cases (<3 years of age) received only CHT, and 22 cases received combined RT and CHT. Total and near-total tumor resections were performed in 29 cases (90.6%). Subtotal resections were performed in 3 cases, and there were no surgery-related deaths. The average follow-up duration was 47 months. The average 5-year PFS and OS rates were 57.3% ± 7.2% and 68.7% ± 8.6%, respectively. The OS and PFS rates were significantly correlated with tumor-risk stratification, molecular staging, tumor stage, treatment mode, and recurrence after surgery (p < 0.01). The degree of tumor resection, pathological type, and the presence of preoperative implantation were secondary factors affecting the prognosis (p < 0.05). Age was correlated with the PFS rate. There was no correlation between age/tumor location/tumor size and prognosis (p > 0.05). Favorable prognostic factors in the low- and standard-risk groups were stage M0, wingless-type MB, postoperative RT combined with CHT, no postoperative recurrence, age ≥3 years, and total tumor resection. Conclusions: Personalized treatment strategies based on the risk stratification of MB and postoperative stratified comprehensive treatment could help improve the prognosis for MB.Keywords
Pediatric medulloblastoma (MB) is the most common and most malignant primary brain tumor occurring in children. Adjuvant cranial and spinal irradiation (CSI) and chemotherapy (CHT) are the standard treatment modes after tumor resection for this malignancy (Hoff et al., 2009). However, the treatment sensitivity of different molecular subtypes of MB is unknown. Although there has been a significant improvement in postoperative survival rates in recent years, existing therapeutic strategies often ignore the individual differences between children. However, these variations have the potential to develop more precise therapies and improve prognoses. Therefore, this aspect has emerged as the main topic in MB research. Accordingly, developing therapeutic regimens based on risk stratification may help to significantly improve the prognosis. To perform a traditional risk stratification, we used a combination of magnetic resonance imaging (MRI) and CSF cytology to classify MB into the standard risk (SR) or high risk (HR) groups. Patients with MB who were older than 3 years of age, were classed as M0, those who had a postoperative residual tumor of <1.5 cm2, and had histological non-interstitial changes were classified as SR, and the rest were classified as HR (Gajjar et al., 2006).
The present study devised personalized treatment plans for children with MB (n = 32) from several studies according to the risk stratification of the tumor. These treatment plans achieved better efficacy. The following sections summarize and analyze the results.
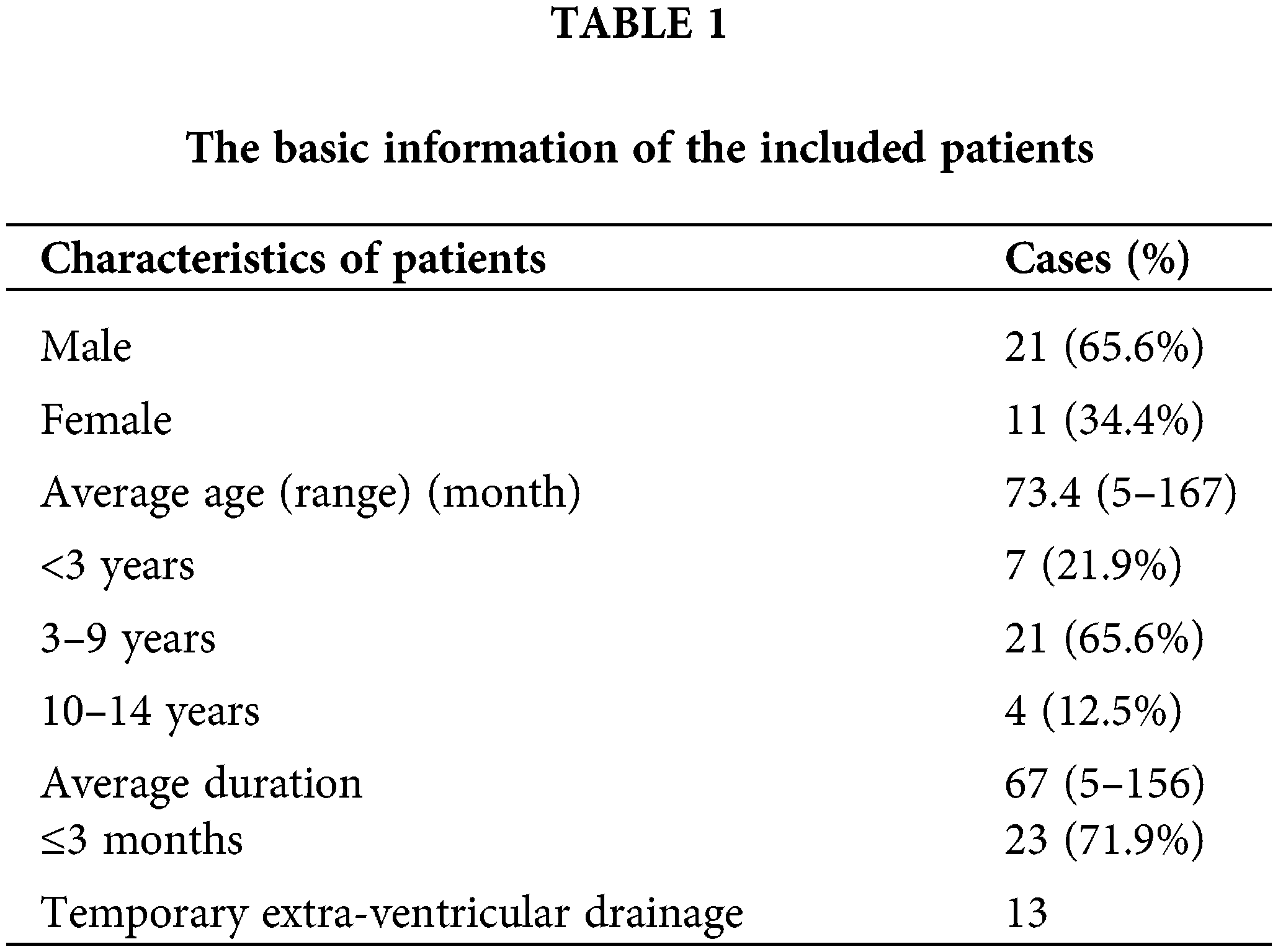
This study retrospectively selected subjects with MB (n = 32) who had a confirmed diagnosis via histopathology and molecular typing and had been surgically treated by the authors of the respective publications with comprehensive adjuvant therapy. Surgical and adjuvant treatment protocols were approved by the ethics committee of the hospital, and all records were anonymous. Clinical data were collected using medical charts, and follow-up data were collected during telephone interviews or outpatient follow-up appointments. Table 1 presents the basic information about the patients who participated in this study.
The inclusion criteria were as follows:
① Patients with perfect preoperative computed tomography (CT) MRI examinations, those who underwent surgical treatment, those with confirmation of MB by postoperative immunohistochemical examination, and those with a clear molecular typing diagnosis.
② Patients ≤14 years of age whose legal guardians had been informed of all treatment options and risks and had provided written informed consent.
③ Patients who were followed up by an outpatient visit or a telephone interview (the final follow-up date was December 30, 2019).
The exclusion criteria were as follows:
① Patients with MB of ectopic origin that protruded into the posterior cranial fossa and those with recurrent MB.
② Patients who failed to complete more than 12 months of postoperative follow-up appointments.
Clinical and imaging evaluation
Of the 32 cases in the present study, 21 (65.6%) evidenced signs and symptoms of cranial hypertension, 12 (37.5%) showed ataxia, and 5 (15.6%) revealed cranial nerve dysfunction. Of the children aged <3 years, 5 (15.6%) showed progressive cranial enlargement. Cranial CT and MRI scans were obtained before and after surgery. During the preoperative CT examination, small cystic lesions (<0.25 of the tumor diameter) were found in 3 cases.
Generally, the head MRI documented relatively clear borders, a low and equal signal in T1WI, and a high and equal signal in T2WI. Additionally, there was mild or obvious homogeneous enhancement or heterogeneous enhancements of different thicknesses.
An entire spinal MRI scan was conducted to evaluate the existence of implant metastasis. In 29 cases (90.6%), the tumors were located in the midline. In these cases, the main body of the tumor was located in the cerebellar vermis in 9 cases and the fourth ventricle in 20 cases. In 3 cases (9.4%), the main body of the tumor was confined to the cerebellar hemisphere. In 7 cases, the fourth ventricle was pushed forward, and there was a cerebrospinal fluid (CSF) zone between the tumor and the floor of the fourth ventricle. Of the 25 cases with no CSF zone between the tumor and the brain stem, 7 (21.9%) invaded the floor of the fourth ventricle, and 3 (9.4%) invaded the dorsal side of the brain stem.
The tumors were classified according to their maximum diameters. There were 17 medium-type tumors (2–4 cm), 13 large-type tumors (4.1~6 cm), and 2 huge-type tumors (>6 cm). In 11 cases, there was lateral growth located in the fourth ventricle. In 7 cases, the tumor protruded into the foramen magnum via the median foramen of the fourth ventricle, and chronic sub-tonsillar herniation was present. In 2 cases, the tumor protruded into one side of the pontine cerebellar angle cistern via the Luschka foramen.
Before surgery, 4 cases had enhanced implanted metastases on the spinal cord. A lumbar puncture for CSF-exfoliated tumor cytology study was conducted prior to the surgery in 9 cases, while it was conducted after the surgery in 23 cases. Exfoliated tumor cells were found in the CSF in 7 cases.
The 32 cases of MB were classified into four histological types according to the 2021 classification criteria of the World Health Organization (WHO) for MB: classic MB (CMB), large cell/anaplasticity (LC/A), MB with extensive nodularity (MBEN), and desmoplastic/nodular MB (DNMB). They were further divided into four molecular subtypes according to molecular typing criteria (Ellison et al., 2011).
Therapeutic mode and definition
Postoperative adjuvant RT and CHT were grouped according to the results of molecular typing, and the postoperative adjuvant RT regimen was implemented according to the grouping. The 32 cases were divided into three categories according to the treatment plan. The operation group (OP) (n = 3) underwent a tumor resection without any RT or CHT. The OP + CHT group received only adjuvant CHT after tumor resection (n = 7, <3 years of age). The OP + RT + CHT group (n = 22) received all the therapeutic measures for MB, including surgery, RT, and CHT.
Risk stratification and stratified treatment
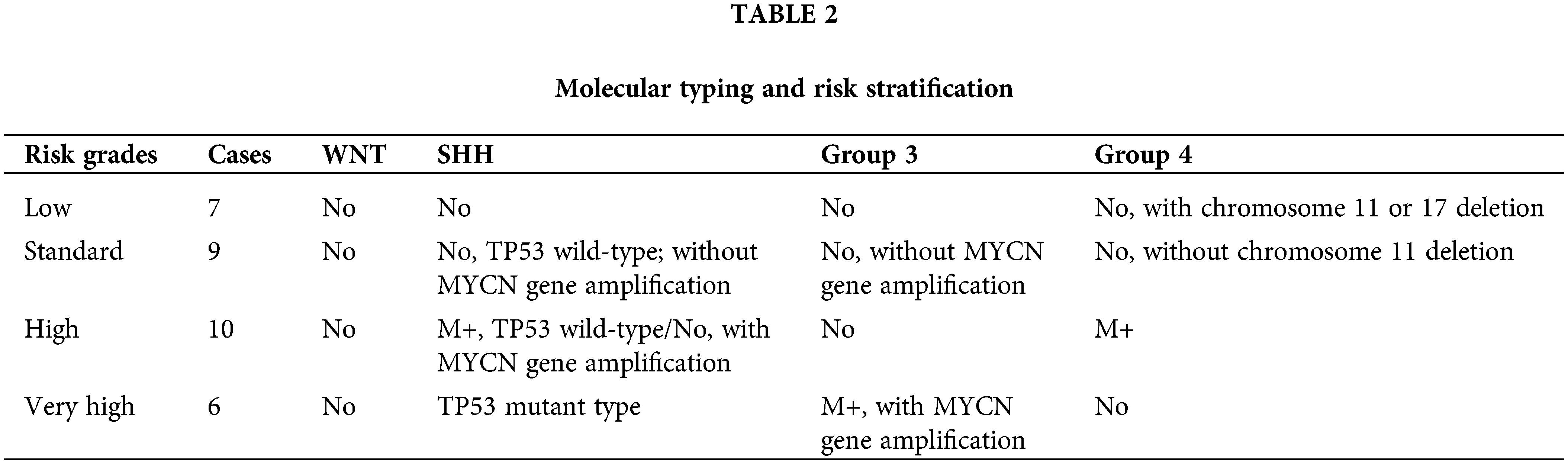
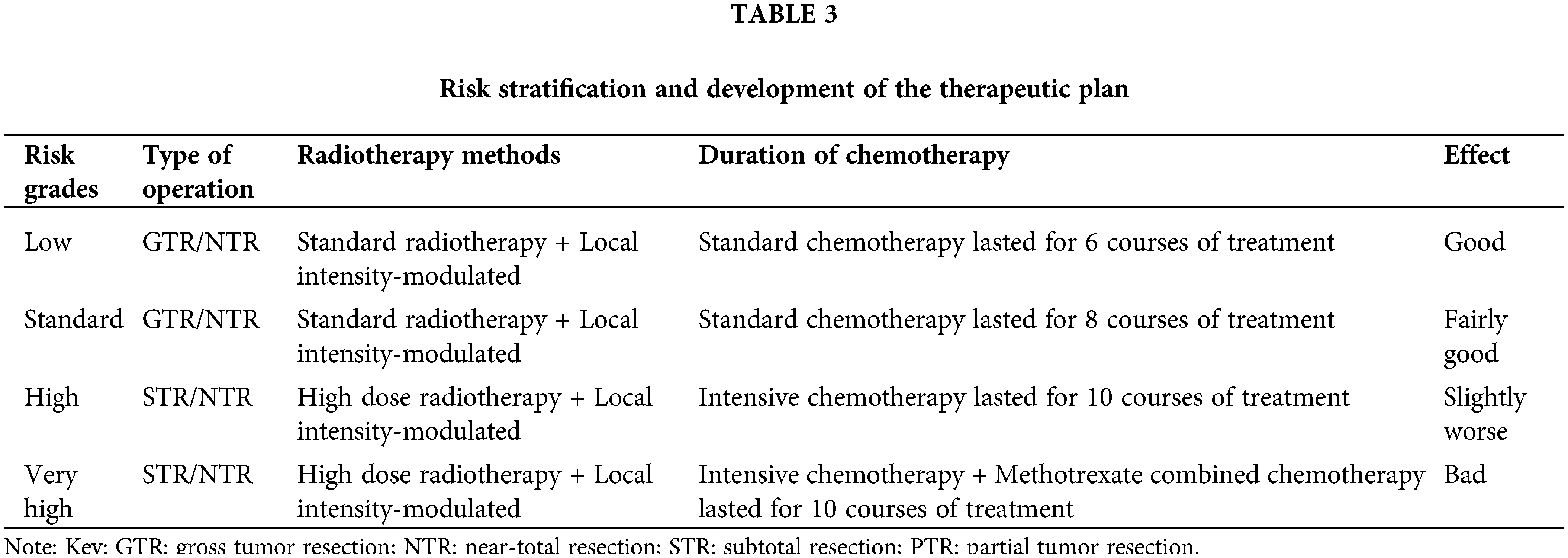
The presence of tumor implantation metastasis was assessed using the results of preoperative whole brain and whole spinal cord MRI, lumbar pool CSF cytology, and two-week postoperative lumbar pool CSF cytology. The staging of MB for implantation metastasis was performed using the Chang classification criteria (Madhogarhia et al., 2022). The risk stratification of MB was divided into four levels according to the 2016 molecular staging criteria of the WHO, the risk stratification criteria of Ramaswamy et al. (2016), and the presence or absence of metastatic implantation in the patients (Table 2). Postoperatively, different adjuvant treatment protocols were developed according to the risk stratification (Table 3).
The surgical strategy and selection of the type of cerebellar medullary fissure opening were formulated according to the preoperative tumor size, location, and invasion range. The principle of first separating the tumor–cerebellar surface followed by the tumor–brainstem surface from the base up was followed. Intra-tumor decompression was performed initially; then, the tumor was gradually resected in pieces, and finally, the tumor wall was separated and resected.
Here, 17 cases of medium-sized (2–4 cm) and 11 cases of large-sized (4–6 cm; lateral or tumor growth toward the foramen magnum) tumors were treated using the unilateral cerebellar medulla approach, while the bilateral cerebellar medulla method was used in 2 cases of large sized (4.0–6.0 cm) and 2 cases of huge (>6 cm) tumors. Temporary extraventricular drainage was performed before surgery in 13 patients with significant hydrocephalus. Although all 32 cases in the group had intraoperative access to CSF circulation, 2 cases still required a postoperative ventriculoperitoneal shunt.
Radiotherapy and chemotherapy regimens
According to the risk stratification, all groups received personalized RT and CHT regimens after RT (Rutkowski et al., 2005, 2009); the criteria for these regimens are shown in Table 3. A total of 22 patients with MB aged >3 years without severe malignancy received both CHT + RT postoperatively. In the very high-risk group, 3 cases the combined treatment was abandoned postoperatively in three cases in patients aged 3 years or older and were classified as the surgery-only group (overall abandonment rate: 9.4%). Standard RT was defined as CSI receiving a radiation dose of 23.4 Gy. High-dose RT was defined as CSI receiving a radiation dose of 36–39.60 Gy, and local intensity-modulated RT was defined as a primary site of the tumor receiving a radiation dose of 54–55.80 Gy. The low-risk and standard-risk groups received standard RT for CSI and intensity-modulated irradiation to the primary tumor site, while the high-risk and very high-risk groups received high-dose irradiation and intensity-modulated irradiation to the primary tumor site.
In standard CHT, a combination of cisplatin (or carboplatin), vincristine, and cyclophosphamide (or lomustine) is administered over a 28-day course, as follows: cisplatin (75 mg/m2, day 1) + vincristine (1.50 mg/m2, days 1, 7, and 14) + cyclophosphamide (1,000 mg/m2, days 21–22). Intensive CHT combines standard CHT with etoposide (2.50 mg/kg, days 1–3), which is administered intravenously in alternating cycles during each course of treatment. In our study, 6 courses of standard CHT were delivered to the low-risk group, and 8 courses were delivered to the standard-risk group. The high-risk group was treated with 10 courses of intensive CHT, and the very high-risk group received intensive CHT with the addition of methotrexate (5 g/m2, days 1–2) for 10 courses (Table 3). Four children in the high-risk and very high-risk groups aged <3 years were treated with intensive systemic CHT. After receiving this treatment, autologous stem cell transplantation was also required.
Follow-up and evaluation criteria
The follow-up assessments for all 32 cases included post-surgery imaging. The median follow-up time was 47 months (33–91 months). The children were observed for RT and/or CHT tolerance after individual chemoradiation and/or CHT, and adverse RT effects were recorded. Follow-up consultations were conducted via an outpatient MRI review and a follow-up telephone interview. In the first year, the follow-up period was reviewed every 3 months after surgery; after the second year, this increased to every 6 months. An enhanced MRI was reviewed once to detect tumor recurrence and/or CSF implantation metastasis. The appearance of new lesions in the original surgical area after total tumor resection or the increased size of residual lesions (regarded as recurrence) was confirmed via postoperative CT and MRI examinations. The post-surgery disappearance or reduction of the original symptoms was regarded as improvement, and the aggravation of the original symptoms or the appearance of new symptoms after surgery was regarded as aggravation.
During the follow-up period, 22 cases improved after treatment and returned to normal, 5 cases had neurological dysfunction or developed new symptoms (including 4 cases of relapse), and 5 cases died. Patients with progression or exacerbation were referred to an authority.
Overall survival (OS) refers to the time (months) from the date of surgery to the date of death (from any cause) or the last follow-up appointment. Progression-free survival (PFS) refers to the time from tumor resection to any of the following: the first recurrence at any site, disease progression, the occurrence of tumor metastasis, death from any cause, or the final follow-up review. The influence of various factors on the prognosis was determined via a statistical analysis of the 5-year PFS and OS rates.
Tumor characteristics and the extent of resection
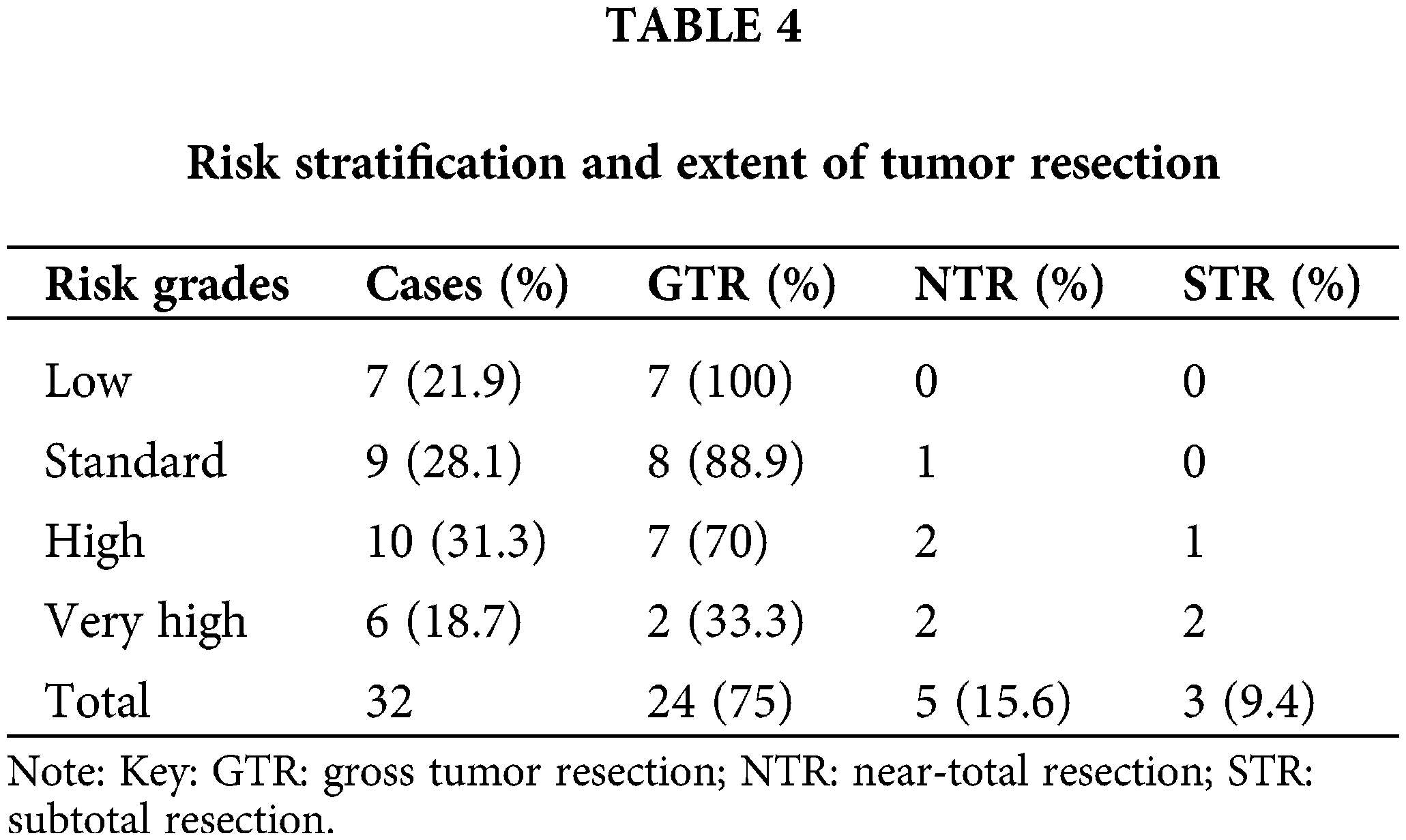
The degree of tumor resection was divided into gross tumor resection (GTR) (no residual tumor), near-total resection (NTR) (remaining tumor <1.5 cm2), and subtotal resection (STR) (remaining tumor ≥1.5 cm2). As documented in Table 4, 24 cases had GTR, 5 had NTR, and 3 had STR. Therefore, 29 cases (90.6%) had either GTR or NTR. There were no cases of surgery-related deaths. All three cases with STR could not receive GTR because the tumor was embedded in the brainstem. In patients with tumors invading the base of the fourth ventricle and the cerebellar peduncle, GTR or NTR was performed intra-operatively. During the follow-up period, tumor recurrence occurred in 4 cases.
Analysis of the factors influencing prognosis
Of the 32 cases in the present study, 3 cases were assigned to the OP group due to the absence of post-surgical comprehensive adjuvant therapy; 7 cases in the OP + CHT group; and 22 cases in the OP + RT + CHT group with the completion of all recommended treatment procedures. A total of 25 cases (78.1%) had no metastasis (M0), while metastasis was present in 7 cases (21.9%), including 4 cases with preoperative spinal cord implantation (enhanced nodules) and 3 cases with positive CSF cytology. The estimated 5-year PFS and OS rates for all patients were 57.3% ± 7.2% and 68.7% ± 8.6%, respectively. As shown in Table 5, the risk stratification of the tumor, molecular staging, treatment modality, tumor stage, and metastatic recurrence after surgery were the main influencing factors for MB in children. The 5-year OS was significantly higher in the low-risk and standard-risk groups compared with the high-risk and very high-risk groups. Additionally, the 5-year OS and PFS rates were significantly higher in the OP + RT + CHT group than in the OP group, and they were higher than in the OP + RT group. The metastasis (M) stage was correlated with tumor recurrence and tumor-free survival time. The PFS and OS were significantly better in the M0 group than in the M+ group.
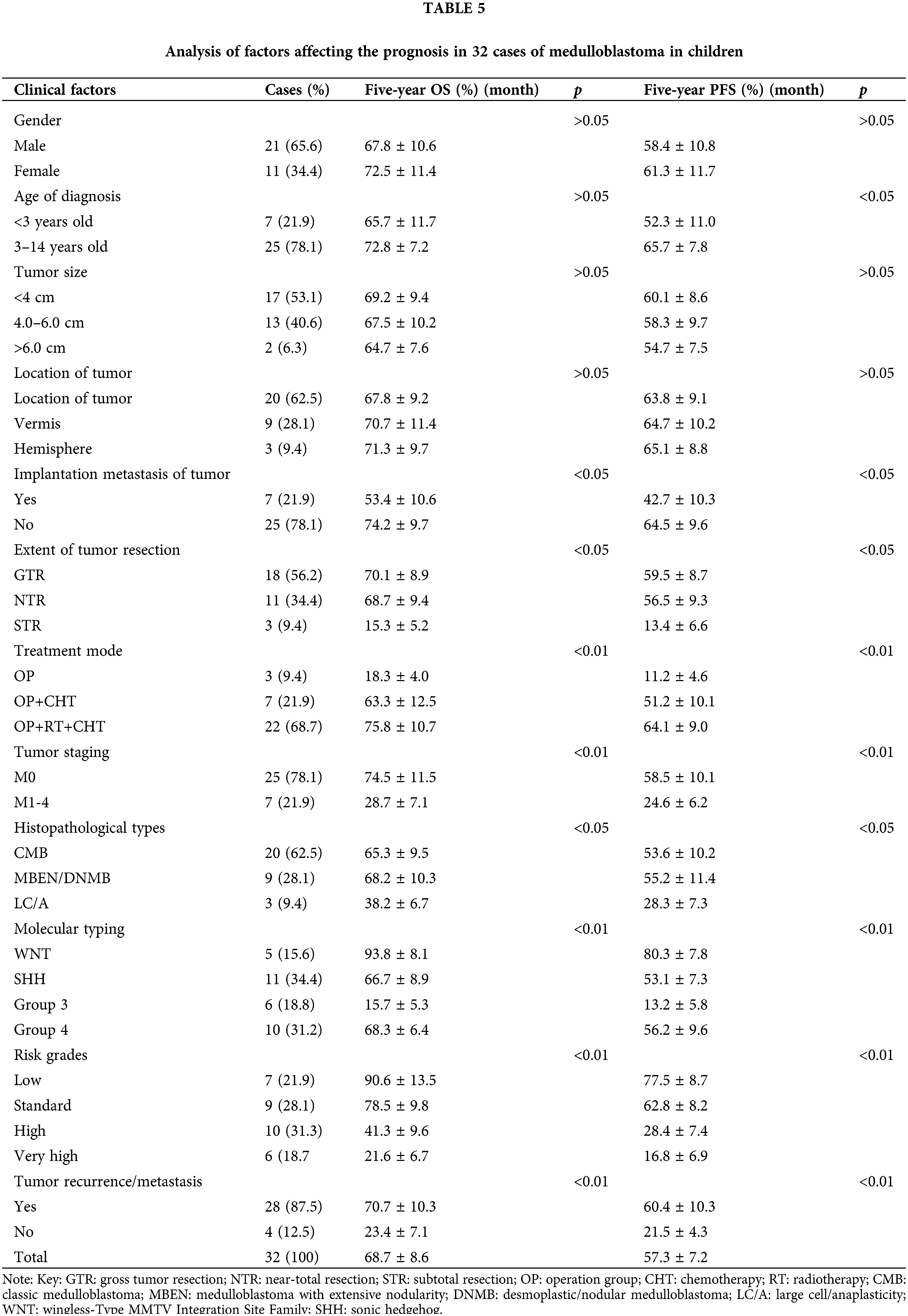
The histological type, degree of resection, and presence of preoperative implantation metastasis of the tumor impacted the OS and PFS in children with MB. The PFS and OS rates were significantly better in patients with CMB and DNMB/MBEN compared with patients with LC/A-MB. The 5-year OS and PFS rates were higher in those who received GTR or NTR compared with those with STR. Age had a certain effect on the PFS of children with MB, and the 5-year OS rate was also higher in patients >3 years of age than in those <3 years; however, this difference was not statistically significant.
Adverse reactions relevant to the therapy
Two cases required a postoperative ventriculo–abdominal shunt. There were 11 (34.35%) cases with postoperative neck pain, 5 (15.6%) with intracranial infection, 5 (15.6%) with subdural effusion, 3 with ataxia or balance disorders, 2 with cranial nerve palsy (6.3%), 1 with transient cerebellar mutism (3.1%), and 3 (9.4%) with other complications, all of which were cured after symptomatic treatments. Following RT, 13 cases developed granulocytopenia, and 4 required the subcutaneous injection of recombinant human granulocyte-stimulating factor and the transfusion of red blood cells and platelets. In 4 children in the high-risk (<3 years of age) and very high-risk groups, autologous stem cell transplantation was performed after intensive systemic CHT. Allergies developed in 4 cases during CHT, including 2 cases of bacteremia, 1 case of rhinorrhea and gingival hemorrhage, and 1 case of epilepsy. All of these allergies improved after symptomatic supportive treatments.
The primary treatment for MB is maximum tumor resection, and the optimal survival rate and highest quality of life are ensured using moderate-risk CSI and CHT. Jiang et al. (2016) reported that the 5-year PFS and OS rates were 47.1% ± 4.6% and 54.6% ± 4.6%, respectively. Bokun et al. (2018) reported the average 3-, 5-, and 10-year OS rates in 87 pediatric patients with MB to be 76.4%, 66.2%, and 59.2%, respectively, with average PFS rates of 75.8%, 62.8%, and 56.6%, respectively. Rutkowski et al. (2010) reported the 8-year event-free survival (EFS) and OS rates in 260 MB cases (<5 years of age) to be 39% ± 3% and 56% ± 3%, respectively. Padovani et al. (2007) reported that the 5- and 10-year OS rates in 253 cases with MB were 72% and 55%, respectively. In the present study, the 32 cases of MB had 5-year OS and PFS rates of 68.7% ± 8.6% and 57.3% ± 7.2%, respectively. These results were consistent with those reported previously by other researchers.
The impact of risk-stratified treatment on prognosis
The risk stratification is not comprehensive enough to reflect the diversity of treatments. This is because different molecular subtypes of MB are significantly heterogeneous and can be identified using transcriptional analyses (Taylor et al., 2012). In addition to the amplification of MYC and MYCN genes, it is necessary to perform molecular typing based on MB. Risk stratification based on molecular typing is more accurate, and in this study, the authors used molecular biological characteristics to establish the risk-grading criteria, which formed the basis for the stratified treatment design.
Since the molecular genetic profiles of MB with identical pathological typing can differ, the prognoses between individuals with identical pathological typing can also vary. In the present study, treatment was administered according to the risk stratification of MB. In the low- and intermediate-risk groups, the 5-year OS rates were 90.6% ± 13.5% and 77.5% ± 8.7%, respectively, while the PFS rates were 78.5% ± 9.8% and 62.8% ± 8.2%, respectively. These rates were significantly higher than in the high-risk and very high-risk groups. Gandola et al. (2009) administered hyper-segmented RT combined with CHT in a high-risk group, with 5-year PFS and OS rates of 72% and 73%, respectively. This indicates that patients in high-risk groups require higher doses of therapy. Further elucidation of the pathogenesis and tumor drivers of MB groups 3 and 4 (Hovestadt et al., 2019) may influence and guide future molecularly stratified treatments.
The degree of resection in MB is strongly correlated with prognosis. Rutkowski (Bokun et al., 2018) reported a significant difference in survival between patients who underwent complete and incomplete tumor resection. The 8-year EFS and OS rates for complete and incomplete tumor resection were 54% ± 5% and 27% ± 5%, and 77% ± 4% and 50% ± 6%, respectively. Zhang et al. (2014) suggested that GTR was a significantly favorable factor affecting both OS and EFS. Further, PFS is believed to be significantly higher with GTR than STR (Thompson et al., 2016), and both GTR and NTR are correlated with enhanced PFS and OS (Kocakaya et al., 2016; Ning et al., 2015; Gudrunardottir et al., 2014). In the present study, the 5-year OS and PFS rates for patients receiving GTR, NTR, and STR were 70.1% ± 8.9%, 59.5% ± 8.7%, and 68.7% ± 9.4% and 56.5% ± 9.3%, 15.3% ± 5.2%, and 13.4% ± 6.6%, respectively. Therefore, it is recommended that patients with tumors should receive GTR at the time of their initial surgery, although the potential risks of GTR must be evaluated intra-operatively against the residual tumor.
The importance of GTR was recognized as early as the Cushing era, and patients who underwent radical resection had better survival rates (Gudrunardottir et al., 2014). While maximum safe resection remains the surgical objective, in terms of PFS and OS, GTR offers no definitive benefits over NTR. A renewed recognition of the extent of tumor resection (Thompson et al., 2018) has the potential to change the intra-operative strategy. According to the results of the authors, the extent of surgical resection was consistent with this strategy (Table 3). Considering the high risk of the incidence of cerebellar mutism after GTR, the excessive resection of small residual tumors overlying the brainstem should be avoided (Thompson et al., 2018). Therefore, the degree of GTR and NTR should be personalized based on intra-operative conditions.
The impact of tumor location and the extent of invasion (i.e., the involvement of the brainstem or the floor of the fourth ventricle) on the prognosis of MB is controversial, and many studies have yielded differing results (Coltin et al., 2021). The literature reported a better prognosis for hemispheric MB than MB in the midline(8-year EFS rates of 58% ± 6% vs. 33% ± 3% and 8-year OS rates of 71% ± 6% vs. 51% ± 4%) (Bokun et al., 2018). However, Rutkowski et al. (Rutkowski et al., 2010) suggested that the prognosis was unaffected by the location of the tumor (midline vs. hemisphere).
In the present study, the results revealed no clear correlation between tumor location and prognosis. The location of the tumor impacted only the surgical difficulty and not the prognosis. Tumors involving the ventricular floor did not affect the extent of tumor resection, which was influenced only by tumors that had invaded the brainstem. Although brainstem invasion was independent of the tumor location, it was dependent on the mode of tumor invasion. The same postoperative outcome could be obtained with both NTR and GTR. However, in the present study, the sample size was too small, and perhaps insufficient, to reveal the significance.
Patients in the M0 stage have a better prognosis than in the M+ stage in children both older and younger than 3 years of age. A report by Bokun et al. (2018) of 87 MB cases in children gave the average 3-, 5-, and 10-year OS rates of those in the M0 stage as 86.4%, 74%, and 63.1%, respectively, while these rates in the M+ stage were 48.9%, 44.0%, and 37.7%, respectively. These results revealed that the survival rate in the M0 stage was significantly higher than in the M+ stage. Rutkowski et al. (2010) evaluated 260 cases of MB and found a significantly higher survival rate in 20 cases in stage M1 over 55 cases in stage M2/M3. In the present study, the 5-year OS and PFS rates for 25 cases in stage M0 were 74.5% ± 11.5% and 58.5% ± 10.1%, respectively, while for the M+ stage, these rates were 28.7% ± 7.1% and 24.6% ± 6.2%, respectively. These results indicate that the tumor stage is correlated with the prognosis.
The metastasis of MB is an independent risk factor for prognosis and recurrence often signals a shorter life expectancy for the patient. with a 5-year survival rate of <10% after recurrence, even in patients who were initially at standard risk (Johnston et al., 2018). The 5-year PFS for MB in stage M0 is >95% for the wingless (WNT) type compared with ~50%–60% for group 3 and ~70%–80% for SHH-MB or patients in group 4. This is provided that the maximum safety standards for surgery and postoperative CSI and CHT are followed (Madhogarhia et al., 2022). Bowers et al. (2007) reported 41 cases of recurrent MB with a median time to recurrence of 1.8 years.
Group 3 has a more invasive nature with a higher incidence of metastasis since tumor cells are easily shed from the primary tumor to the soft meningeal space. In the present study, the 5-year OS and PFS rates in the 28 cases without recurrence/metastasis were 70.7% ± 10.3% and 60.4% ± 10.3%, respectively; these rates were clearly higher than the OS and PFS rates for the four cases with metastasis (23.4% ± 7.1% and 21.5% ± 4.3%, respectively) (Table 5). This observation is similar to that of Rutkowski et al. (2010). To prevent the recurrence of soft meninges or metastases visible on MRI, RT to the entire neuraxis is required, not just the primary tumor bed.
Pathological type and prognosis
The literature reported a poorer prognosis for the LC/A type compared with the CMB type (Eberhart and Burger, 2003) and a better prognosis for the MBEN type (Rutkowski et al., 2010). In infants, the MBEN type is usually the SHH-TP53 wild type, which has a good prognosis even under a regimen of CHT alone. Patients aged <3 years with MB have a poor prognosis if they have significant postoperative residuals, type LC/A pathological tissue, are classed as stage M+, and have postoperative metastatic recurrence (von Bueren et al., 2011). Atallah et al. (2019) reported 44 cases of MB in children and concluded that LC/A, being in a high-risk group, and CSF with implants were correlated with poorer OS and PFS rates. The 8-year survival was highest in DNMB/MBEN, followed by children with CMB, while LC/A had the lowest survival rate (Rutkowski et al., 2010). The results of the present study also indicated that the 5-year survival rate in CMB and MBEN/DNMB was significantly higher than in LC/A (p < 0.05), with MBEN/DNMB having the best prognosis. However, the difference between MBEN/DNMB and CMB was not statistically significant (Table 5). These results could be caused by the small sample size.
Molecular typing and prognosis
The prognosis of MB is not only correlated with the pathological type but also depends on the molecular typing and metastatic status of the disease (Khatua and Zaky, 2014). The WNT type has the best prognosis, while the SHH-type with a TP53 mutation is an extremely high-risk subtype with a very poor prognosis even under intense treatment (Schwalbe et al., 2017). The prognosis for group 4 is essentially the same as for the SHH wild type, and certain group-4 cases and low-risk patients with the deletion of chromosome 11 or the addition of chromosome 17 have a very good prognosis (Schwalbe et al., 2017). The worst prognosis is for patients in group 3 (Taylor et al., 2012)., especially in conjunction with MYC amplification and/or pathological histological type LC/A, which was also discouraging in the prospective trial group (Madhogarhia et al., 2022). There may be overtreatment of the WNT type, but the best way to reduce adjuvant therapy for this type remains unclear (Zhang et al., 2014). In the present study, the 5-year OS and PFS rates for the WNT type were 93.8% ± 8.1% and 80.3% ± 7.8%, respectively, which are significantly higher than in the SHH type and group 4. However, in group 3 they were only 15.7% ± 5.3% and 13.2% ± 5.8%, respectively (p < 0.01). These results also indicated that the WNT type has the best prognosis, while group 3 has the worst prognosis, which is consistent with the results of the majority of current studies.
Therapeutic Mode and Prognosis
In the present study, the OP + RT + CHT group had the best prognosis, with 5-year OS and PFS rates of 75.8% ± 10.7% and 64.1% ± 9.0%, respectively. These rates were significantly higher than those in the OP and OP + CHT groups. Postoperative treatment with RT and CHT was a significantly favorable independent factor for improving the OS and the EFS. Postoperative CHT significantly affected survival in the CMB, SHH, and WNT subgroups, although it did not have the same effect in the subgroups for DMB and non-SHH/WNT (Zhang et al., 2014). Though the present study did not investigate in detail the effect of each treatment mode group in terms of pathology type and age, it indicated that the effect of the treatment mode on prognosis was significant.
The use of RT has shown significant effects on tumor control. There is a tendency for MB to develop implant metastases, and the most successful RT approach is CSI with local intensive irradiation of the primary tumor site. In the low- and standard-risk groups, CSI of 23.4 Gy plus enhanced irradiation of at least 55 Gy to the tumor bed in conjunction with adjuvant CHT resulted in a 5-year survival rate of 80%. In the high-risk and very high-risk groups, the CSI was increased to 36–39 Gy, with enhanced irradiation of the tumor bed at 55 Gy; this was followed by CHT with cisplatin and cyclophosphamide, resulting in a 5-year survival rate of 60%–65%. Patients with no metastases and tumors who underwent GTR or NTR received a CSI radiation dose of 23.4 Gy and a tumor bed enhancement therapy dose of 54.0 Gy (Packer and Vezina, 2008).
In conclusion, survival rates could be improved with treatment based on the appropriate risk stratification according to different molecular typing and risk grading approaches. The prognosis of MB was shown to be primarily dependent on the molecular typing of the tumor, the risk stratification, the existence of implant metastases, and the mode of treatment. Secondary influencing factors were the pathological type of the tumor, the age of the patient, and the degree of tumor resection. A better prognosis was found in patients with GTR, patients with the M0 stage, those in low- and intermediate-risk groups, and those receiving standardized post-surgery radio CHT.
Funding Statement: The work was funded by the Key Research and Development Project of the Science and Technology Department of Sichuan Province (No. 2021YFS0010).
Author Contributions: LHC, HTZ and YX conceived the idea and conceptualised the study. KS and WC collected the data. LHC and YX analysed the data. LHC and RXX drafted the manuscript, then HTZ and RXX reviewed the manuscript. All authors read and approved the final draft.
Availability of Data and Materials: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Atallah A, Rady MR, Kamal HM, El-Mansy N, Alsawy MFM, Hegazy A, Zaghloul M (2019). Telovelar approach to pediatric fourth ventricle tumors: Feasibility and outcome. Turkish Neurosurgery 29: 497–505. https://doi.org/10.5137/1019-5149.JTN.24078-18.3 [Google Scholar] [PubMed] [CrossRef]
Bokun J, Grujicic D, Skender-Gazibara M, Paripovic L, Pekmezovic T et al. (2018). Management and treatment of children with medulloblastoma in Serbia, a middle-income country. JBUON 23: 1156–1162. [Google Scholar] [PubMed]
Bowers DC, Gargan L, Weprin BE, Mulne AF, Elterman RD, Munoz L, Giller CA, Winick NJ (2007). Impact of site of tumor recurrence upon survival for children with recurrent or progressive medulloblastoma. Journal of Neurosurgery 107: 5–10. https://doi.org/10.3171/PED-07/07/005 [Google Scholar] [PubMed] [CrossRef]
Coltin H, Sundaresan L, Smith KS, Skowron P, Massimi L et al. (2021). Subgroup and subtype-specific outcomes in adult medulloblastoma. Acta Neuropathologica 142: 859–871. https://doi.org/10.1007/s00401-021-02358-4 [Google Scholar] [PubMed] [CrossRef]
Eberhart CG, Burger PC (2003). Anaplasia and grading in medulloblastomas. Brain Pathology 13: 376–385. https://doi.org/10.1111/j.1750-3639.2003.tb00037.x [Google Scholar] [PubMed] [CrossRef]
Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C et al. (2011). Medulloblastoma: Clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathologica 121: 381–396. https://doi.org/10.1007/s00401-011-0800-8 [Google Scholar] [PubMed] [CrossRef]
Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE et al. (2006). Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96Long-term results from a prospective, multicentre trial. Lancet Oncology 7: 813–820. https://doi.org/10.1016/S1470-2045(06)70867-1 [Google Scholar] [PubMed] [CrossRef]
Gandola L, Massimino M, Cefalo G, Solero C, Spreafico F et al. (2009). Hyperfractionated accelerated radiotherapy in the Milan strategy for metastatic medulloblastoma. Journal of Clinical Oncology 27: 566–571. https://doi.org/10.1200/JCO.2008.18.4176 [Google Scholar] [PubMed] [CrossRef]
Gudrunardottir T, Lannering B, Remke M, Taylor MD, Wells EM, Keating RF, Packer RJ (2014). Treatment developments and the unfolding of the quality of life discussion in childhood medulloblastoma: A review. Childs Nervous System 30: 979–990. https://doi.org/10.1007/s00381-014-2388-5 [Google Scholar] [PubMed] [CrossRef]
Hoff KV, Hinkes B, Gerber NU, Deinlein F, Mittler U et al. (2009). Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT’91. European Journal of Cancer 45: 1209–1217. https://doi.org/10.1016/j.ejca.2009.01.015 [Google Scholar] [PubMed] [CrossRef]
Hovestadt V, Smith KS, Bihannic L, Filbin MG, Shaw ML et al. (2019). Resolving medulloblastoma cellular architecture by single-cell genomics. Nature 572: 74–79. https://doi.org/10.1038/s41586-019-1434-6 [Google Scholar] [PubMed] [CrossRef]
Jiang T, Zhang Y, Wang J, Du J, Ma Z, Li C, Liu R, Zhang Y (2016). Impact of tumor location and fourth ventricle infiltration in medulloblastoma. Acta Neurochirurgica 158: 1187–1195. https://doi.org/10.1007/s00701-016-2779-3 [Google Scholar] [PubMed] [CrossRef]
Johnston DL, Keene D, Strother D, Taneva M, Lafay-Cousin L et al. (2018). Survival following tumor recurrence in children with medulloblastoma. Journal of Pediatric Hematology Oncology 40: e159–e163. https://doi.org/10.1097/MPH.0000000000001095 [Google Scholar] [PubMed] [CrossRef]
Khatua S, Zaky W (2014). The biologic era of childhood medulloblastoma and clues to novel therapies. Future Oncology 10: 637–645. https://doi.org/10.2217/fon.13.185 [Google Scholar] [PubMed] [CrossRef]
Kocakaya S, Beier CP, Beier D (2016). Chemotherapy increases long-term survival in patients with adult medulloblastoma—A literature-based meta-analysis. Neuro Oncology 18: 408–416. https://doi.org/10.1093/neuonc/nov185 [Google Scholar] [PubMed] [CrossRef]
Madhogarhia R, Haldar D, Bagheri S, Familiar A, Anderson H et al. (2022). Radiomics and radiogenomics in pediatric neuro-oncology: A review. Neurooncology Advances 4: vdac083. https://doi.org/10.1093/noajnl/vdac083 [Google Scholar] [PubMed] [CrossRef]
Ning MS, Perkins SM, Dewees T, Shinohara ET (2015). Evidence of high mortality in long term survivors of childhood medulloblastoma. Journal of Neuro-Oncology 122: 321–327. https://doi.org/10.1007/s11060-014-1712-y [Google Scholar] [PubMed] [CrossRef]
Packer RJ, Vezina G (2008). Management of and prognosis with medulloblastoma: Therapy at a crossroads. Archives of Neurology 65: 1419–1424. https://doi.org/10.1001/archneur.65.11.1419 [Google Scholar] [PubMed] [CrossRef]
Padovani L, Sunyach MP, Perol D, Mercier C, Alapetite C et al. (2007). Common strategy for adult and pediatric medulloblastoma: A multicenter series of 253 adults. International Journal of Radiation Oncology Biology Physics 68: 433–440. https://doi.org/10.1016/j.ijrobp.2006.12.030 [Google Scholar] [PubMed] [CrossRef]
Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC et al. (2016). Risk stratification of childhood medulloblastoma in the molecular era: The current consensus. Acta Neuropathologica 131: 821–831. https://doi.org/10.1007/s00401-016-1569-6 [Google Scholar] [PubMed] [CrossRef]
Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M et al. (2005). Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. New England Journal of Medicine 352: 978–986. https://doi.org/10.1056/NEJMoa042176 [Google Scholar] [PubMed] [CrossRef]
Rutkowski S, Gerber NU, von Hoff K, Gnekow A, Bode U et al. (2009). Treatment of early childhood medulloblastoma by postoperative chemotherapy and deferred radiotherapy. Neuro Oncology 11: 201–210. https://doi.org/10.1215/15228517-2008-084 [Google Scholar] [PubMed] [CrossRef]
Rutkowski S, von Hoff K, Emser A, Zwiener I, Pietsch T et al. (2010). Survival and prognostic factors of early childhood medulloblastoma: An international meta-analysis. Journal of Clinical Oncology 28: 4961–4968. https://doi.org/10.1200/JCO.2010.30.2299 [Google Scholar] [PubMed] [CrossRef]
Schwalbe EC, Lindsey JC, Nakjang S, Crosier S, Smith AJ et al. (2017). Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: A cohort study. Lancet Oncology 18: 958–971. https://doi.org/10.1016/S1470-2045(17)30243-7 [Google Scholar] [PubMed] [CrossRef]
Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ et al. (2012). Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathologica 123: 465–472. https://doi.org/10.1007/s00401-011-0922-z [Google Scholar] [PubMed] [CrossRef]
Thompson EM, Bramall A, Herndon IIJE, Taylor MD, Ramaswamy V (2018). The clinical importance of medulloblastoma extent of resection: A systematic review. Journal of Neuro-Oncology 139: 523–539. https://doi.org/10.1007/s11060-018-2906-5 [Google Scholar] [PubMed] [CrossRef]
Thompson EM, Hielscher T, Bouffet E, Remke M, Luu B et al. (2016). Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: A retrospective integrated clinical and molecular analysis. Lancet Oncology 17: 484–495. https://doi.org/10.1016/S1470-2045(15)00581-1 [Google Scholar] [PubMed] [CrossRef]
von Bueren AO, von Hoff K, Pietsch T, Gerber NU, Warmuth-Metz M et al. (2011). Treatment of young children with localized medulloblastoma by chemotherapy alone: Results of the prospective, multicenter trial HIT, 2000 confirming the prognostic impact of histology. Neuro Oncology 13: 669–679. https://doi.org/10.1093/neuonc/nor025 [Google Scholar] [PubMed] [CrossRef]
Zhang ZY, Xu J, Ren Y, Li KK, Ng HK, Mao Y, Zhong P, Yao Y, Zhou LF (2014). Medulloblastoma in China: Clinicopathologic analyses of SHH, WNT, and non-SHH/WNT molecular subgroups reveal different therapeutic responses to adjuvant chemotherapy. PLoS One 9: e99490. https://doi.org/10.1371/journal.pone.0099490 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools