 Open Access
Open Access
ARTICLE
Flavonoids in safflower extract reduce cisplatin-induced damage to human follicle dermal papilla cells by inhibiting DNA damage and Rad17/Chk1/Cdc25C signaling
1 Department of Research, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, 231, Taiwan
2 Department of Chinese Medicine, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, 231, Taiwan
3 School of Post-Baccalaureate Chinese Medicine, Tzu Chi University, Hualien, 970, Taiwan
4 Department of Life Sciences, National Science and Technology Council, Taipei, 106, Taiwan
5 Department of Dermatology, Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, New Taipei City, 231, Taiwan
6 School of Medicine, Tzu Chi University, Hualien, 970, Taiwan
* Corresponding Authors: CHUN-HUA WANG. Email: ,
(This article belongs to the Special Issue: Cellular Signal Transduction in Biological Activities)
BIOCELL 2023, 47(8), 1793-1802. https://doi.org/10.32604/biocell.2023.030093
Received 22 March 2023; Accepted 08 May 2023; Issue published 28 August 2023
Abstract
Background: Cisplatin is a chemotherapeutic agent commonly used clinically for the treatment of various human cancers. Patients often reduce the use of cisplatin due to its side effects, which in turn affects its treatment. This study explored the mechanism of action of safflower extract as an adjuvant traditional Chinese medicine for chemotherapy. Methods: Primary human follicle dermal papilla cells (HFDPCs) were used as target cells for cisplatin-induced damage to hair cells. Western blotting was used to investigate the molecular targets of cisplatin and safflower extract in causing HFDPCs damage. Cell survival and cell cycle were analyzed by mitochondrial staining reagent WST-1 and propidium iodide. Results: Cisplatin could reduce the viability of HFDPCs without causing cell death. Cisplatin increased the level of phospho-Rad17 in HFDPCs and activated the Chk1/Cdc25C signaling to reduce the expression of Cdc2 protein, thereby arresting the cells in the G2/M phase. The combination of safflower extract and the flavonoids could effectively inhibit the signal transduction of Rad17/Chk1/Cdc25 in cisplatin-treated cells and reduce the cell population in the G2/M phase. Finally, we also confirmed that safflower extract could effectively inhibit the damage to HFDPCs caused by cisplatin, mainly at the level of reducing the DNA damage caused by cisplatin. Conclusions: Safflower extract can be used as an adjuvant Chinese medicine for chemotherapy to reduce the damage caused by chemotherapy to normal hair follicle cells.Keywords
Hair loss crises are a common occurrence that affects the scalp or the entire body. The symptoms may be transient or long-lasting. Additionally, hair loss symptoms may differ between men and women; various contributing factors may be family history of genetic disease, changes in hormonal regulation, infection, medication, medical treatment (e.g., radiation therapy), or stress, among others (York et al., 2020). Chemotherapy-induced alopecia (CIA), continues to be a problem in clinical oncology (Palamaras et al., 2011; Trueb, 2010). More than 50% of cancer chemotherapy patients perceive CIA as a painful side effect leading to poor treatment outcomes (Choi et al., 2014; Wils et al., 2019). The incidence of chemotherapy-induced hair loss varies with the type of chemotherapy drug given, the dosage used, and the route of administration.
Safflower is a traditional Chinese herbal medicine obtained from the dried corolla of Carthamus tinctorius L. In traditional Chinese medicine, the use of safflower promotes blood circulation and removes blood stasis (Adamska and Biernacka, 2021; Lu et al., 2021; Yao et al., 2016). Safflower extract was shown to promote the expression of vascular endothelial growth factor and keratinocyte growth factor in dermal papilla cells, thereby promoting the growth of living animal hair (Junlatat and Sripanidkulchai, 2014). Safflower oils on the market, such as HT26, are promoted as products for hair care, although there is no scientific evidence for these products to demonstrate their efficacy and targets.
Hair loss caused by chemotherapy is the direct consequence of the toxic effect of chemotherapy drugs on the rapidly dividing cells of hair follicles, in which dermal papilla cells (DPCs) play a key role in the hair growth cycle (Matsuzaki and Yoshizato, 1998; Higgins et al., 2013; Chen et al., 2020). These cells are also often used as target cells to study the cellular mechanism of hair follicle tissue growth (Madaan et al., 2018; Yang and Cotsarelis, 2010). Cisplatin, a chemotherapy drug, promotes DPC apoptosis through the activation of free radicals (Luanpitpong et al., 2011). The use of chemotherapy drugs induces the death of follicle cells in the dermal papilla and may lead to hair loss.
In this study, we found that cisplatin caused DNA damage in primary human follicle dermal papilla cells (HFDPCs) and further activated the Rad17 signal transduction pathway. The activation of the Rad17 pathway may lead to cell cycle arrest and decreased cell viability in HFDPCs. Safflower extract can reduce the DNA damage caused by cisplatin on HFDPCs and the activation of Rad17 pathway caused by cisplatin. This suggests that it can be used as an auxiliary traditional Chinese medicine for chemotherapy to reduce its side effects.
Preparation of safflower extract and materials
Ten grams of safflower powder (Shun Ten Pharmaceutical Co., Ltd., New Taipei City, Taiwan) was dissolved in 100 mL of distilled water. After incubation at 70°C for 30 min, the supernatant was collected by centrifugation at 14,000 × g and stored at 4°C after filtration through a 0.45 μm pore size filter. Hydroxysafflor yellow A, safflower yellow A, and luteolin were purchased from ChemFaces (Wuhan, Hubei, PRC) with a purity of 98%. Cis-diammineplatinum dichloride (cisplatin) was obtained from Sigma (St. Louis, MO, USA).
HFDPCs were purchased from PromoCell (Heidelberg, Germany). HFDPCs were subcultured with a medium purchased from the manufacturer (ready-to-use follicle dermal papilla cell growth medium). The HFDPCs selected for this study were from the 4th to 10th cell doubling generations. The alkaline phosphatase activity of the dermal papilla hair follicle cells was continuously maintained (Tsai et al., 2019).
HFDPCs from the 4th to 10th cell doubling passages were seeded in 24-well culture dishes in triplicate at 3 × 104 cells per well, and the cells were cultured in follicle dermal papilla cell growth medium. The cells were treated with 10–100 μg/mL safflower extract or 25 μg/mL flavonoids found in safflower extract for 24 h and then treated with 100 μM cisplatin for 24 h. The WST-1 reagent (100 μL) was added, and the cells were incubated at 37°C for 4–6 h. The supernatant was transferred to a 96-well dish, and the absorbance at 450 and 650 nm was read with an EIA reader (Infinite F200, Tecan, Durham, NC, USA) to estimate the cell viability of the cells.
HFDPCs from the 4th to 10th cell doubling passages were seeded in 24-well culture dishes in triplicate with a cell number of at 3 × 104 cells per well, and the cells were cultured in Dulbecco’s modified Eagle medium containing 1% fetal calf serum. After treatment with 10–100 μg/mL safflower extract for 24 h and then treated with 100 μM cisplatin for 24 h, the cell supernatant was collected. After removing the residual cell pellet by centrifugation at 1500 × g for 5 min, 20 μL of the cell culture supernatant was removed and analyzed for lactate dehydrogenase (LDH) activity with the Cytotoxicity Detection Kit (Sigma) to estimate cell death.
HFDPCs from the 4th to 10th cell doubling passages were plated in 10-cm culture dishes at a cell number of 1 × 106 and cultured for 24 h. After treatment with 50 μg/mL safflower extract or 25 μg/mL flavonoids found in safflower extract for 24 h and then treated with 100 μM cisplatin for 24 h, RIPA buffer containing protease inhibitor cocktail (Roche Diagnostics, Germany) and phosphatase inhibitors (50 mM Tris [pH 8.0], 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium deoxycholate) was used to lyse the cells. Cell lysates were collected, and the protein concentration was measured. Proteins from 20–60 μg of cell extracts were separated on 10%–12% polyacrylamide gels, transferred to PVDF membranes, and allowed to react with various primary antibodies for 12 h at 4°C. Then, the corresponding horseradish peroxidase-conjugated secondary antibodies (Calbiochem, Darmstadt, Germany) was added at room temperature for 2 h, and the Amersham ECL kit (Amersham, Bucks, UK) was used for enzyme coloration, which was visualized by placing in a luminometer for image development and film scanning. The antibodies used in this study were phospho-Rad17 (Ser635), Rad17, phospho-Chk1 (Ser345), Chk1, phospho-cdc25C (Ser216), cdc25C, cyclin A, cyclin B, and phospho-Histone H2A.X (Ser139), obtained from Cell Signaling Technology (Beverly, MA, USA). Antibodies recognizing actin were purchased from Sigma‒Aldrich. Antibodies recognizing Cdc2 were purchased from Proteintech (IL, USA).
HFDPCs from the 4th to 10th cell doubling passages were planted in 10-cm culture dishes at a cell number of 1 × 106 and cultured for 24 h. After treatment with 50 μg/mL safflower extract or 25 μg/mL flavonoids found in safflower extract for 24 h and then treated with 100 μM cisplatin for 48 h, cells were harvested and fixed in ice-cold 80% ethanol at −20°C for at least 12 h. Cells were stained with propidium iodide solution (20 μg/mL propidium iodide, 0.1% Triton-X 100 and 0.2 mg/mL RNase A) and analyzed by flow cytometry (Cytomics FC 500; Becton Dickinson, Franklin Lakes, NJ, USA). Finally, the percentage of cell cycle phase distribution was analyzed with Multicycle for Windows (Becton Dickinson) software.
The data of each group were obtained in at least three replicates and expressed as the mean ± standard deviation. One-way ANOVA with Dunnett’s post hoc test was used for statistical analysis, and p values < 0.05 were considered significantly different.
Safflower extract reduces the cisplatin-induced decrease in dermal papilla cell viability
Like previous results (Luanpitpong et al., 2011), cisplatin reduced the viability of HFDPCs. When HFDPCs were pretreated with safflower extract or treated with cisplatin simultaneously, increasing the dose of safflower extract reduced the effect of cisplatin on HFDPC viability. However, post-treatment with safflower extract did not alter the effect of cisplatin on HFDPC viability (Fig. 1A). To further clarify whether cisplatin reduces the cell viability of HFDPCs related to cell apoptosis, we analyzed the content of LDH released by cells. Cisplatin did not increase the amount of LDH released from cells (Fig. 1B), implying that cisplatin does not cause cell apoptosis. In addition, we also found that the cell morphology of HFDPCs did not change in cells treated with cisplatin or safflower extract (Fig. 1C). The above results imply that cisplatin reduces the viability of HFDPCs not due to cell death. Only high doses (50–100 μg/mL) of safflower extract significantly inhibited the effect of cisplatin on HFDPCs. For possible clinical use in vivo in the future, we adopted a concentration of 50 μg/mL in subsequent experiments to examine the effect of safflower extract on cisplatin-regulated intracellular signal transduction in HFDPCs.
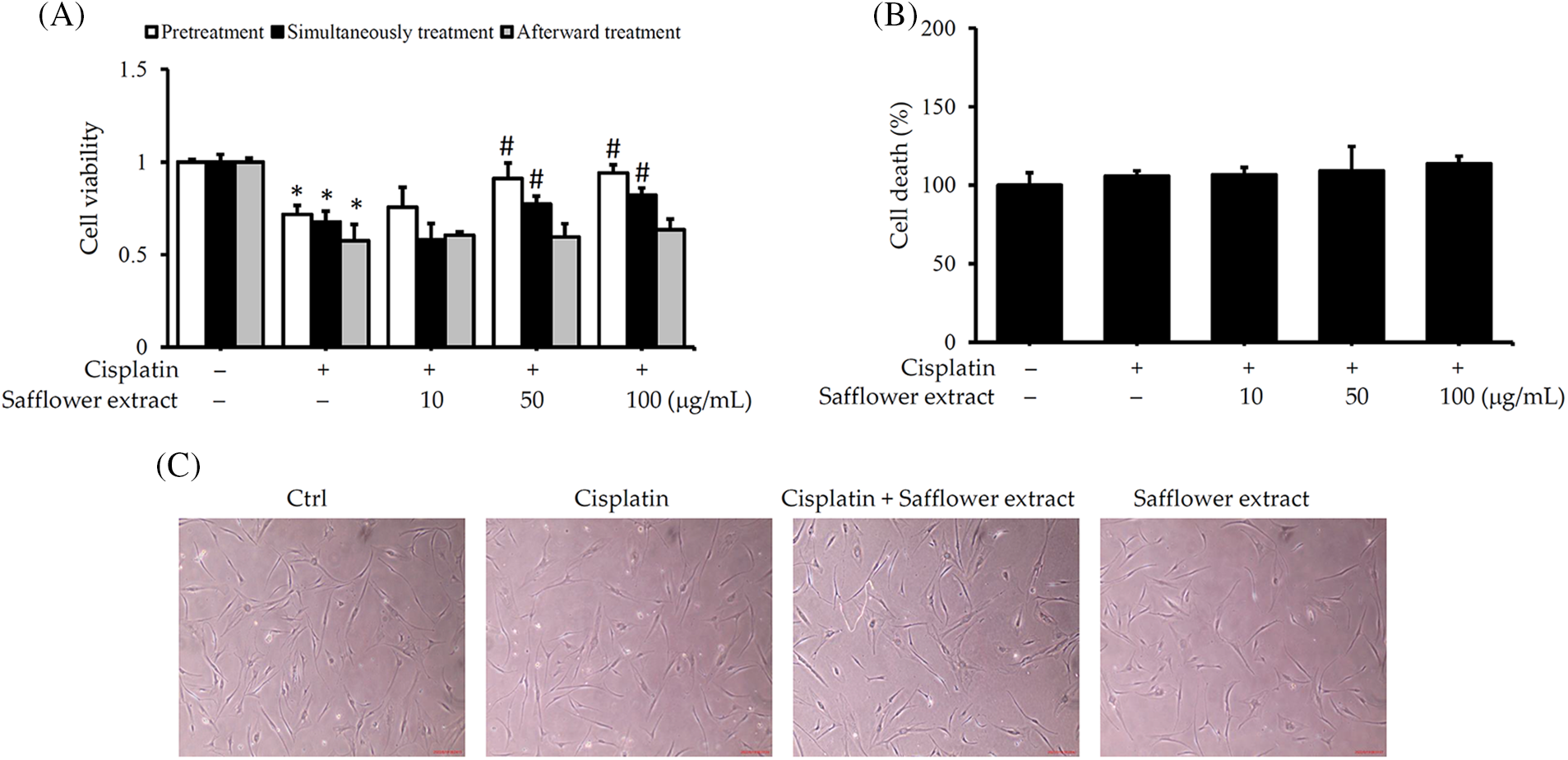
Figure 1: Safflower extract reduces the effect of cisplatin on the viability of primary human follicle dermal papilla cells (HFDPCs). HFDPCs were seeded in triplicate in 24-well dishes and cultured overnight. Cells were then treated with safflower extract at concentrations ranging from 10–100 μg/mL for 24 h and subsequently, treated with 100 μM cisplatin for 24 h in the pretreatment group, with both safflower extract and 100 μM cisplatin for 24 h in the simultaneous treatment group, or with 100 μM cisplatin for 24 h followed by safflower extract at concentrations ranging from 10–100 μg/mL for 24 h in the afterward treatment group. Cell viability (A) and cell death (B) were determined by the WST-1 assay and the release of lactate dehydrogenase. (C) Observation of cell type under an optical microscope at 40× magnification. * indicates p < 0.05 compared with the control group, # indicates p < 0.05 compared with the cisplatin-treated group.
Safflower extract reduces the level of phospho-Rad17 in primary human follicle dermal papilla cells induced by cisplatin
Cisplatin mainly affects intracellular DNA repair and correction systems, and previous literature studies have pointed out that the Rad17 signal transduction pathway plays an important role in cells affected by cisplatin (Belmonte-Fernandez et al., 2023; Wang et al., 2014; Zhou et al., 2013). In order to further explore the target of cisplatin in inhibiting the cell viability of dermal papilla hair follicle cells, we analyzed the expression of Rad17 in the cells. The results in Fig. 2 show that cisplatin can increase the content of phospho-Rad17 in HFDPCs, but it will not affect the total protein expression of Rad17. Safflower extract reduced the level of cisplatin-induced phospho-Rad17 in cells. The levels of phospho-Rad17 and total Rad17 in HFDPCs treated with safflower extract alone were not affected (Fig. 2).
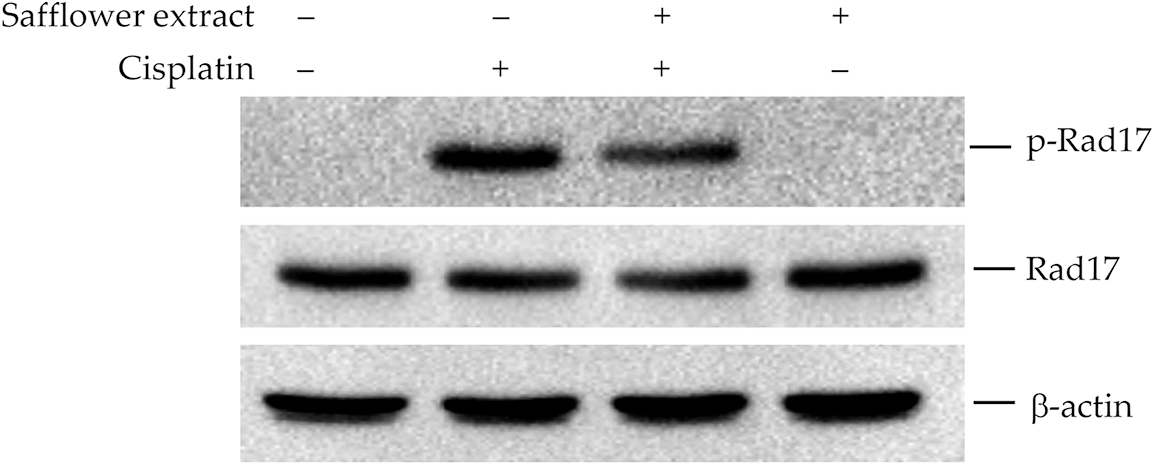
Figure 2: Effect of cisplatin on Rad17 protein expression in primary human follicle dermal papilla cells (HFDPCs). HFDPCs were pretreated with 50 μg/mL safflower extract for 24 h and then treated with 100 μM cisplatin for 24 h. Cell lysates were immunoblotted using phospho-specific and total Rad17 antibodies.
Safflower extract reduces cisplatin-activated Chk1/Cdc25C signaling in HFDPCs
Phosphorylation of Rad17 was found to be involved in the activation of Chk1 (Wang et al., 2006), and Chk1/Cdc25C/Cdc2 was involved in the regulation of cell cycle G2→M (Aleem and Arceci, 2015; Donzelli and Draetta, 2003; Liu et al., 2020). Therefore, we further analyzed the effect of cisplatin on the expression of cell cycle regulatory proteins in HFDPCs. As shown in Fig. 3A, cisplatin increased the expression levels of phospho-Chk1 (S345) and phospho-Cdc25C (S216) proteins in HFDPCs. Cisplatin also decreased the level of Cdc2 in cells but did not change the expression levels of cyclin A and cyclin B (Fig. 3B). Safflower extract reduced cisplatin-induced expression levels of phospho-Chk1 (S345) and phospho-Cdc25C (S216) and partially restored the expression level of Cdc2 in cells (Fig. 3). Treatment with safflower extract alone did not affect the level of phosphorylated Chk1/Cdc25C in cells.
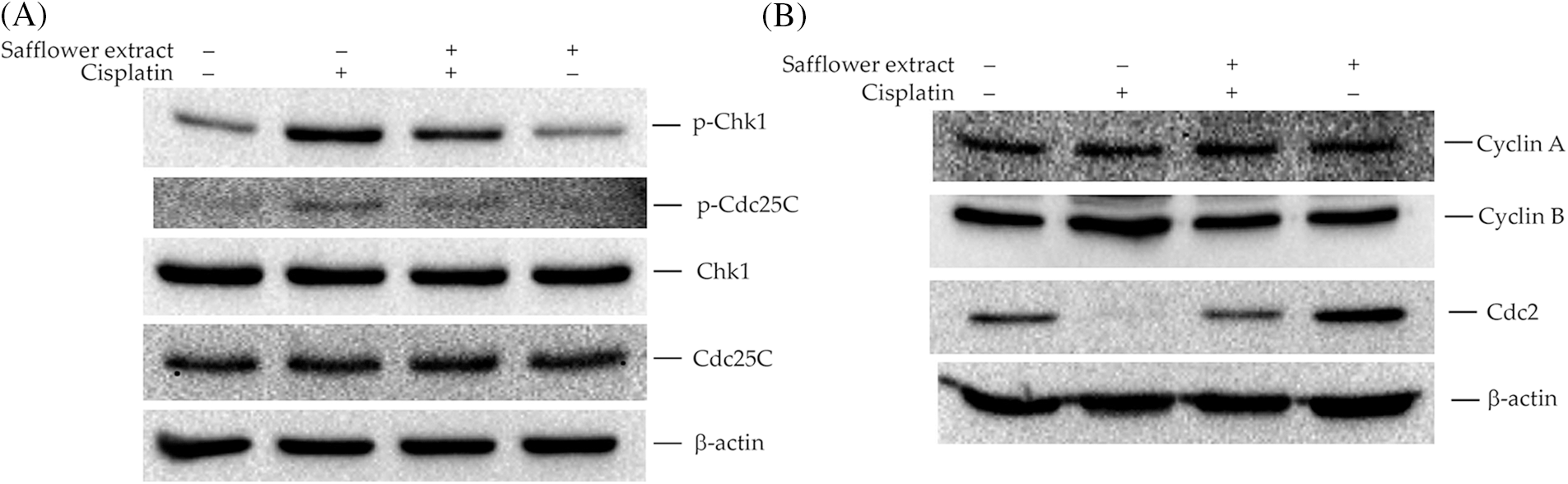
Figure 3: Effects of cisplatin and safflower extract on the expression levels of Chk1/Cdc25C signaling proteins in primary human follicle dermal papilla cells (HFDPCs). HFDPCs were pretreated with 50 μg/mL safflower extract for 24 h and then treated with 100 μM cisplatin for 24 h. The levels of Chk1 and Cdc25C (A) and cell cycle-related proteins (B) were analyzed by western blotting.
Safflower extract reduces cisplatin-induced primary human follicle dermal papilla cells cell cycle arrest in the G2/M phase
As shown above, cisplatin activates the Chk1/Cdc25C pathway and leads to a decrease in the expression of the Cdc2 protein. Cdc2 protein mainly regulates the G2/M phase of the cell cycle, so we further analyzed whether cisplatin can affect the cell cycle of HFDPCs. Cisplatin indeed increased the cell population of HFDPCs in the G2/M phase (Fig. 4). Cells treated with safflower extract significantly reduced the number of cells in the G2/M phase caused by cisplatin (Figs. 4A and 4B). However, treatment with safflower extract alone did not affect the cell cycle of HFDPCs.
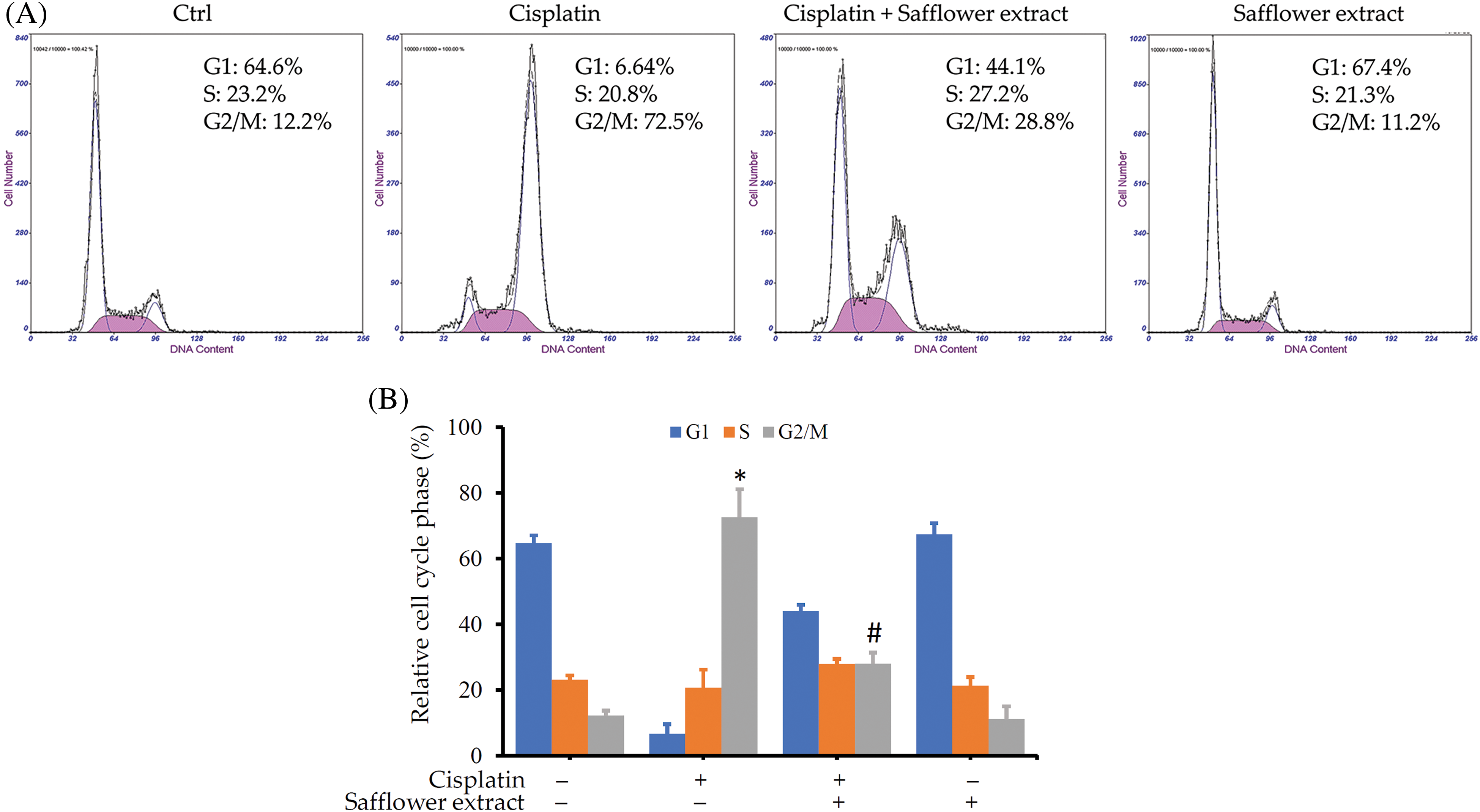
Figure 4: Effects of cisplatin and safflower extract on the cell cycle of primary human follicle dermal papilla cells (HFDPCs). HFDPCs were pretreated with 50 μg/mL safflower extract for 24 h and then treated with 100 μM cisplatin for 48 h. After fixation and staining with propidium iodide, the cell cycle was analyzed by flow cytometry. Shown is the representative chart (A). Experimental results are summarized as the mean (±SD) percentage (B). * indicates p < 0.05 compared with the control group, # indicates p < 0.05 compared with the cisplatin-treated group.
Flavonoid compounds found in safflower extract reduce cisplatin-stimulated phospho-Rad17/Chk1/Cdc25C signals in primary human follicle dermal papilla cells
To clarify the active compounds of safflower extract in resisting the effect of cisplatin on HFDPCs, we purchased the commercially available and most potentially active flavonoid compounds in safflower extract (Mani et al., 2020; Yu et al., 2013) as well as other flavonoid compounds. We then analyzed whether these compounds have the same effect as safflower extract in inhibiting cisplatin from reducing the cell viability of HFDPCs. The results in Fig. 5A showed that the flavonoid compounds in safflower extract, such as hydroxysafflor yellow A, safflower yellow A, and luteolin, as well as the flavonoid compound 7-hydroxyflavone in the non-safflower extract, can inhibit the decline of HFDPCs cell viability caused by cisplatin (Fig. 5A). We observed that cisplatin treatment increased the level of phospho-Rad17 in HFDPCs, while the flavonoid compounds found in safflower extract were able to reduce the expression of phospho-Rad17 induced by cisplatin (Fig. 5B). We also further analyzed the effects of these flavonoid compounds on Chk1/Cdc25C signal transduction and downstream cyclin A/cyclin B/Cdc2 expression. These flavonoid compounds all inhibited increases in phospho-Chk1 and phospho-Cdc25C (Fig. 6A) and the decreased expression of Cdc2 (Fig. 6B) induced by cisplatin.
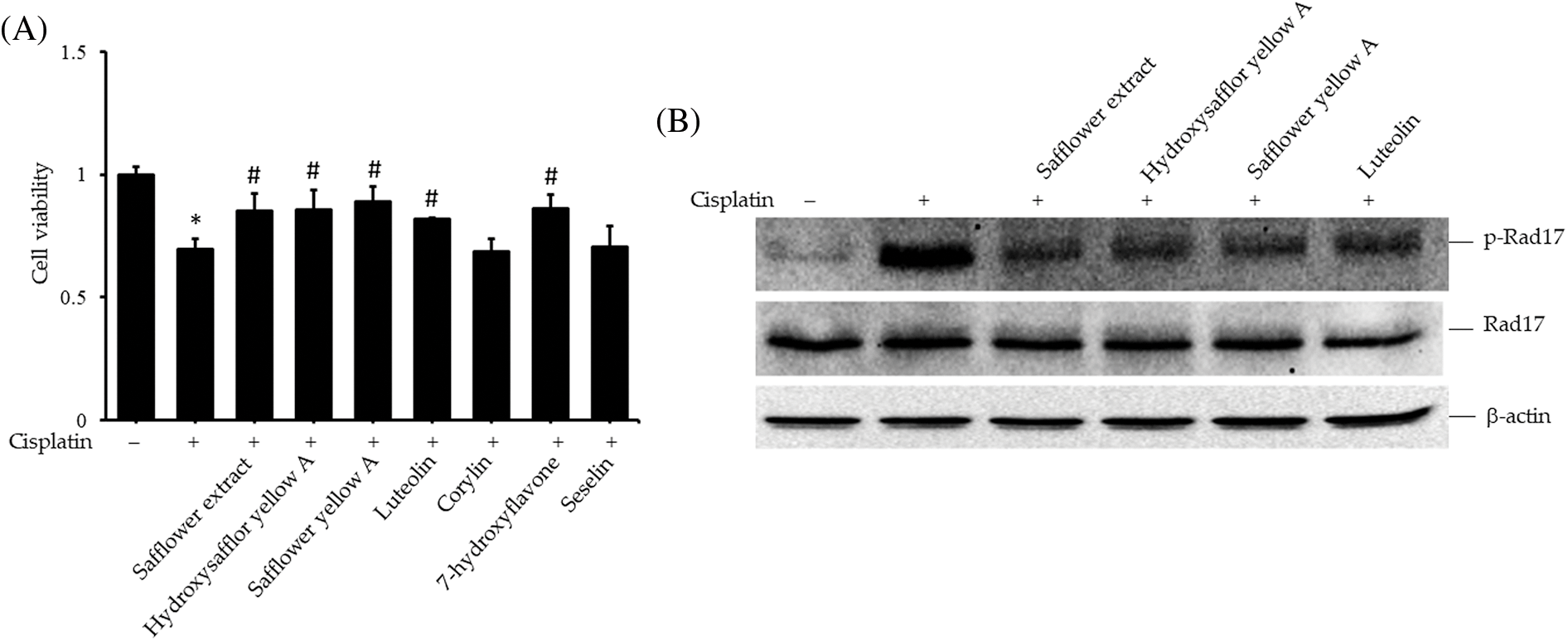
Figure 5: Effects of flavonoids found in safflower extract on cisplatin-induced reduction of primary human follicle dermal papilla cells (HFDPC) viability and induced Rad17 expression levels. Cells were then pretreated with 50 μg/mL safflower extract or 25 μg/mL flavonoids for 24 h and then treated with 100 μM cisplatin for 24 h. Cell viability was determined by WST-1 assay (A). HFDPCs were pretreated with 50 μg/mL safflower extract or 25 μg/mL flavonoids found in safflower extract for 24 h and then treated with 100 μM cisplatin for 24 h. Cell lysates were immunoblotted using phospho-specific and total Rad17 antibodies (B). * indicates p < 0.05 compared with the control group, # indicates p < 0.05 compared with the cisplatin-treated group.
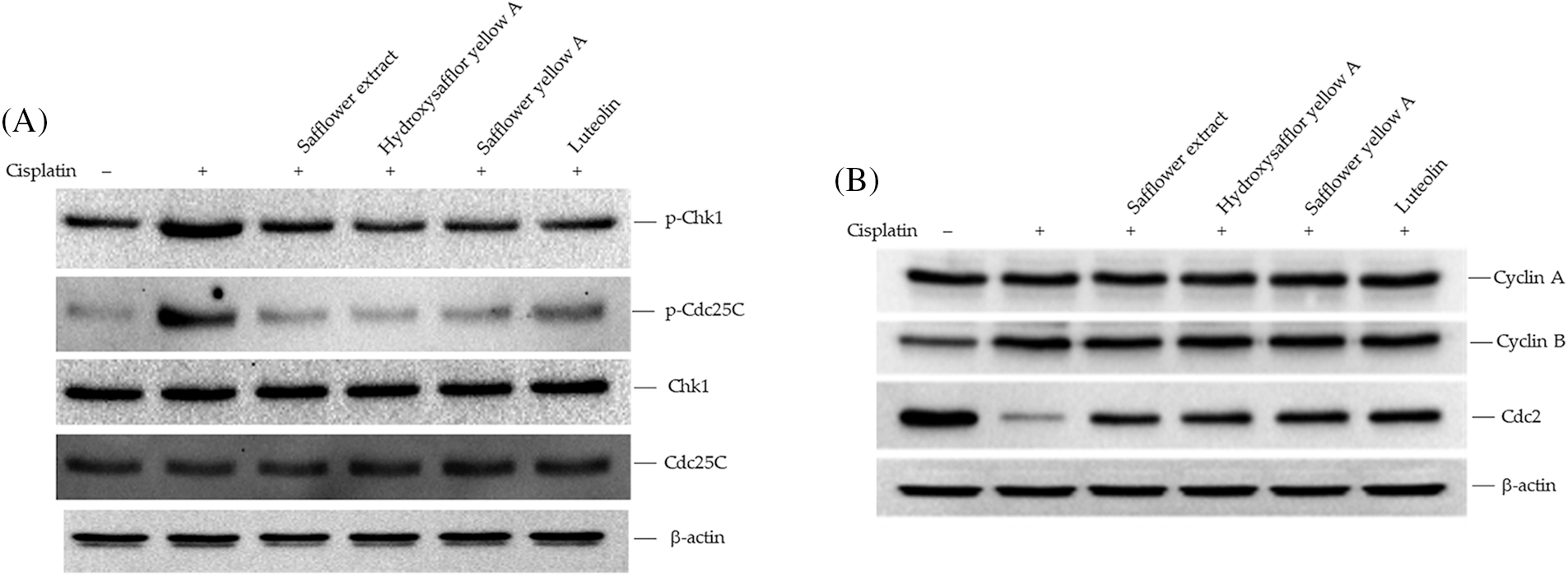
Figure 6: Effects of flavonoids found in safflower extract on the expression levels of Chk1/Cdc25C signaling proteins induced by cisplatin in primary human follicle dermal papilla cells (HFDPCs). HFDPCs were pretreated with 50 μg/mL safflower extract or 25 μg/mL flavonoids found in safflower extract for 24 h and then treated with 100 μM cisplatin for 24 h. The levels of Chk1 and Cdc25C (A) and cell cycle-related proteins (B) were analyzed by western blotting.
Flavonoid compounds found in safflower extract reduce cisplatin-induced primary human follicle dermal papilla cell cycle arrest in the G2/M phase
In addition to the protein expression in the Rad17 signaling pathway, we also further explored whether these flavonoid compounds found in safflower extract can also alter the cell cycle of HFDPCs affected by cisplatin. The addition of flavonoid compounds, such as safflower extract, reduced the increase in the number of cells in the G2/M phase of HFDPCs caused by cisplatin (Figs. 7A and 7B). These results suggested that flavonoid compounds may be related to the inhibition of cisplatin by safflower extract on the growth of HFDPCs.
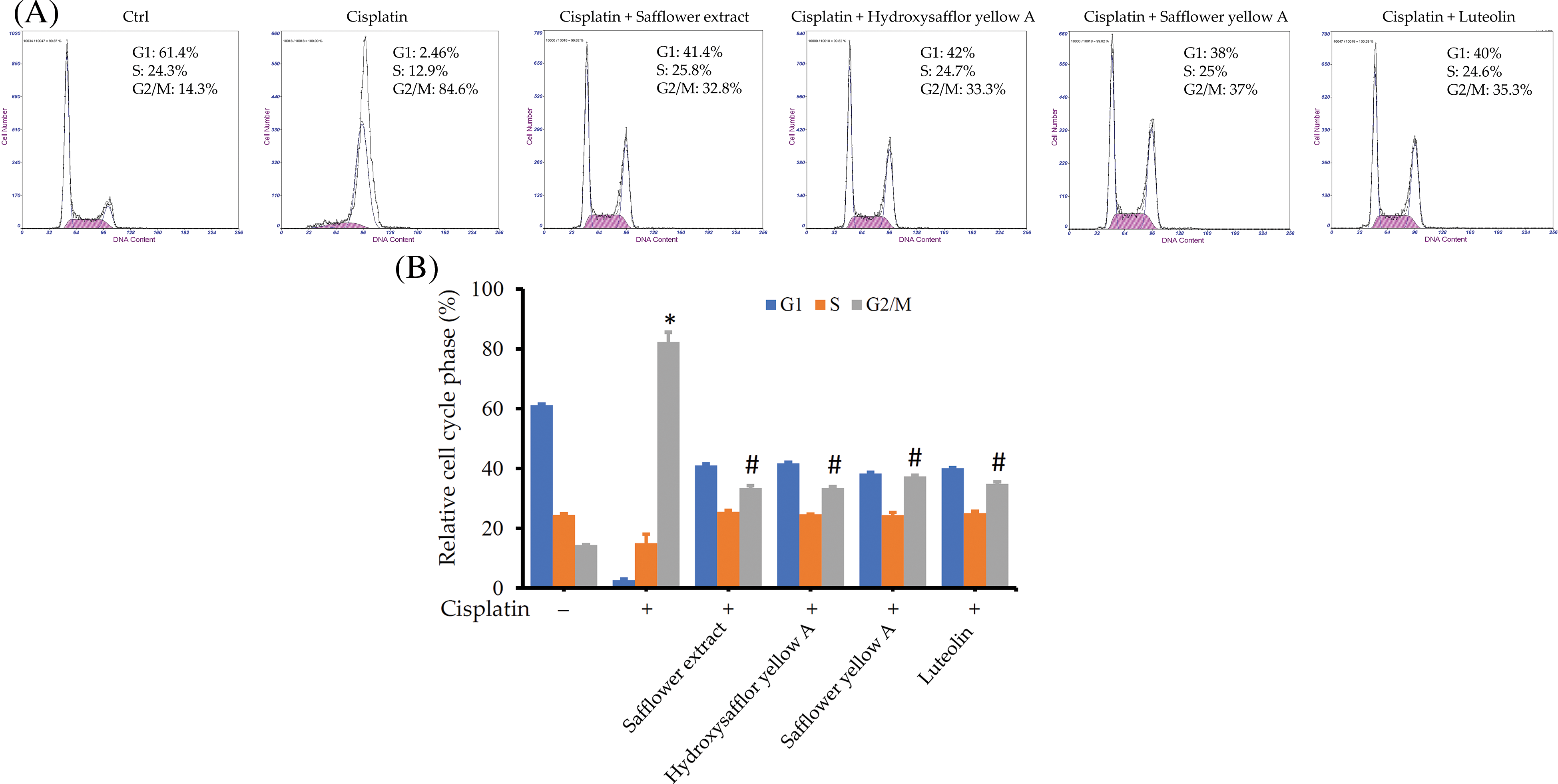
Figure 7: Effects of flavonoids found in safflower extract and cisplatin on the cell cycle of primary human follicle dermal papilla cells (HFDPCs). HFDPCs were pretreated with 50 μg/mL safflower extract or 25 μg/mL flavonoids found in safflower extract for 24 h and then treated with 100 μM cisplatin for 48 h. After fixation and staining with propidium iodide, the cell cycle was analyzed by flow cytometry. Shown is the representative chart (A). Experimental results are summarized as the mean (±SD) percentage (B). * indicates p < 0.05 compared with the control group, # indicates p < 0.05 compared with the cisplatin-treated group.
Safflower extract reduces the cisplatin-induced DNA damage of primary human follicle dermal papilla cells
The main targets of cisplatin on cells are the formation of DNA bridges, interference with DNA repair, and the subsequent formation of intracellular free radicals leading to apoptosis of cancer cells (Dasari and Tchounwou, 2014; Go and Adjei, 1999; Tchounwou et al., 2021). We, therefore, investigated whether safflower extract alleviates the effects of cisplatin on cells at the level of DNA damage. The expression of γ-H2AX was used to reflect DNA damage (Collins et al., 2020; Mah et al., 2010; Podhorecka et al., 2010). As shown in Fig. 8, the addition of cisplatin increased the level of γ-H2AX in the cells, reflecting that cisplatin can indeed damage DNA in HFDPCs. The addition of safflower extract and the flavonoid compounds found in the safflower extract significantly reduced the expression of cisplatin-induced γ-H2AX. These results indicated that the effect of safflower extract against cisplatin on cells may be at the level of DNA damage.
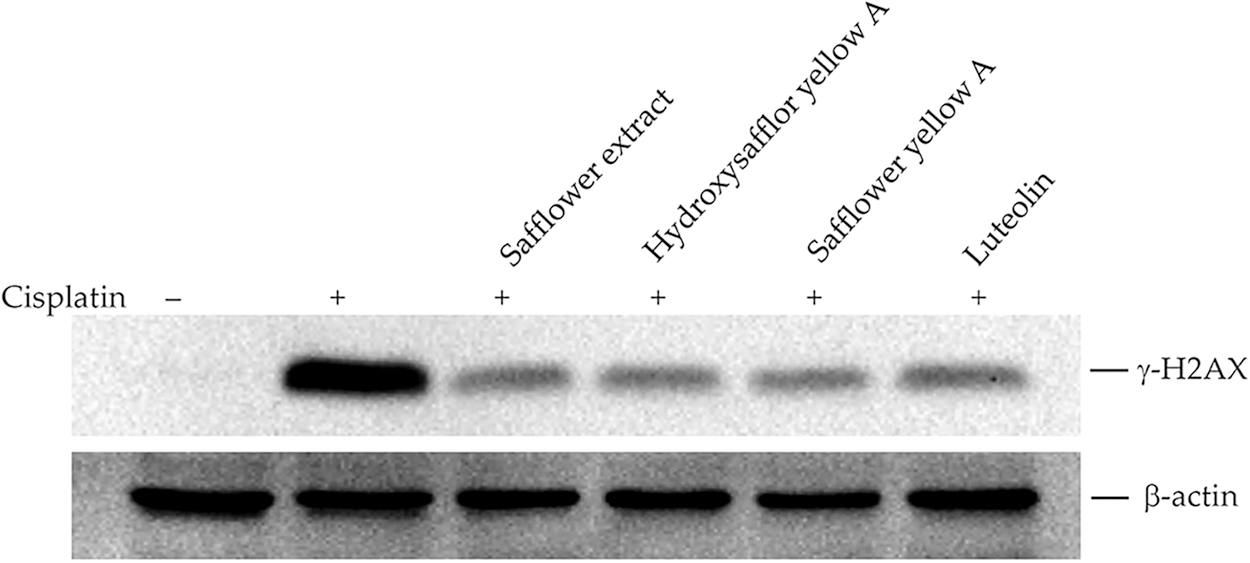
Figure 8: Effects of cisplatin and safflower extract on the expression level of γ-H2AX in primary human follicle dermal papilla cells (HFDPCs). HFDPCs were pretreated with 50 μg/mL safflower extract or 25 μg/mL flavonoids found in safflower extract for 24 h and then treated with 100 μM cisplatin for 24 h. The level of γ-H2AX was analyzed by western blotting.
Cisplatin is a commonly used chemotherapeutic agent in clinical practice. Its side effects mainly include ototoxicity and hair loss, and these side effects cause patients to reduce their willingness to use cisplatin, thereby affecting the curative effect of the drug. At present, there are many treatments of a combination of Chinese and Western medicines. For example, by integrating chemotherapy drugs with traditional Chinese medicines such as Scutellaria barbata and Solanum nigrum to increase the medical treatment willingness and compliance with chemotherapy patients. These traditional Chinese medicines have the ability to kill cancer cells (Fong et al., 2008; Perez et al., 2010; Wang et al., 2015; Uen et al., 2018; Liu et al., 2019), but their effects on hair growth and ear nerve protection have not been reported in the relevant literature, suggesting that the use of these traditional Chinese medicines is mainly to reduce the dose of chemotherapy agents to alleviate the discomfort caused by chemotherapy.
Cisplatin was first used clinically to arrest the growth of gastric cancer cells in the clinic (Petrelli et al., 2013; Sun et al., 2021). Gastric cancer cell lines are cisplatin-sensitive cells (IC50 of 1–10 μM) (Mora-Lagos et al., 2020), implying that the use of clinical drug doses should be less harmful to HFDPCs. Moreover, more than 60% of patients with hair loss due to chemotherapy drugs can usually recover completely within 6 months after the treatment (Bhoyrul et al., 2021). These clinical data show that chemotherapy drugs do not cause irreversible damage to hair growth and support our findings that although cisplatin affects the growth of HFDPCs, it does not cause cell death. We found that cisplatin inhibited the growth of HFDPCs mainly through DNA damage, triggering the phosphorylation of the Rad17 protein and activating the Chk1/Cdc25C signaling pathway, resulting in a decrease in the expression of the Cdc2 protein and arresting the cells in the G2/M phase. The flavonoid compounds found in safflower extract can inhibit the DNA damage in HFDPCs caused by cisplatin so that the downstream Rad17/Chk1/Cdc25 signaling pathway will not be activated by cisplatin, allowing the cells to continue to proliferate (Fig. 9). In the past, studies have also shown that flavonoid compounds can indeed reduce DNA damage (Tiwari and Mishra, 2017, 2022), thereby reducing the generation of cancer cells.
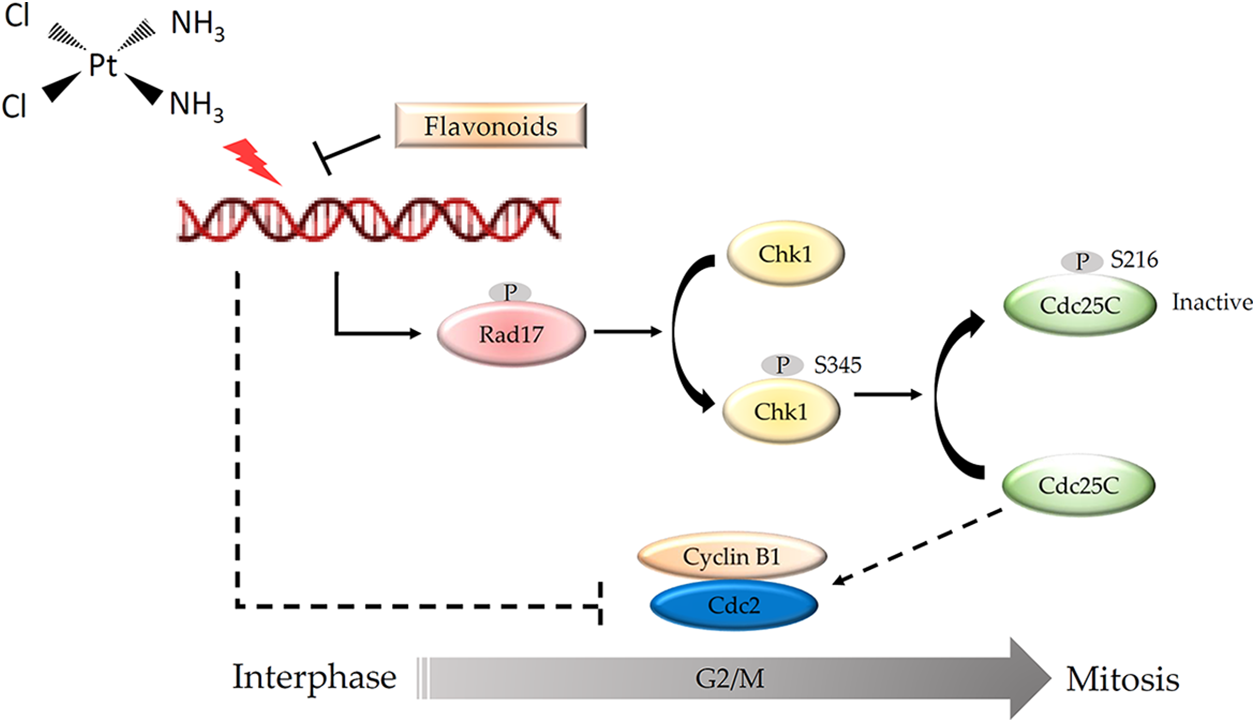
Figure 9: Schematic diagram of the mechanism by which flavonoids found in safflower extract inhibits cisplatin-induced G2/M cell cycle arrest in primary human follicle dermal papilla cells (HFDPCs). Cisplatin damages the DNA of HFDPCs in the dermal papilla, thereby increasing the phosphorylation of Rad, Chk1, and Cdc25C proteins and reducing the expression of Cdc2, thus arresting the cell cycle at the G2/M phase. Flavonoids found in safflower extract can inhibit the effect of cisplatin on DNA, thereby attenuating the effect of cisplatin on the cell cycle. The DNA structure was obtained by BioRender software.
Safflower extract can promote hair growth in vivo (Junlatat and Sripanidkulchai, 2014); however, in our study, the use of safflower extract alone did not increase the cell viability of dermal papilla cells, implying that the role of safflower extract in vivo may be to promote blood circulation and improve the local environment deep in the hair. The curative effect of promoting hair regeneration using safflower extract should be able to improve the phenomenon of hair loss caused by chemotherapy. However, since flavonoid compounds can reduce the DNA damage caused by cisplatin, whether they affect the ability of cisplatin to kill cancer cells still needs further investigation. A literature review presented that the flavonoids in many plant extracts can promote the apoptosis of gastric cancer cell lines (Lee et al., 2012; Bhosale et al., 2021; Abusaliya et al., 2022). These studies demonstrated that flavonoids can stall the progression of the G2→M cell cycle in AGS gastric cancer cell lines and then cause apoptosis. Although their cellular targets are still not clear, flavonoids may have different activities in normal cells and cancer cells. However, whether these results can support flavonoids as adjuvant Chinese medicine for chemotherapy still needs to be confirmed through more research.
In addition to safflower extract, other plant extracts can also promote hair regeneration, such as Dicerocaryum senecioides, Camellia sinensis (L.) Kuntze, or Coffea arabica L., among others (Kwon et al., 2007; Rambwawasvika et al., 2019; Bussoletti et al., 2020). Several potential active ingredients in hair growth-promoting plant extracts, including steroids, flavonoids, and triterpenoids, have been identified. Among these, flavonoids have been found to be the most effective in promoting hair regeneration in BalB/c mice (Rambwawasvika et al., 2019). In vivo experiments have shown that flavonoids, such as the flavonoid-rich extract of Pinus thunbergii bark, can also promote hair regeneration, which promotes hair growth in C57BL/6 mice by regulating the expression of inflammatory substances and promoting growth factors (Her et al., 2022). Similarly, the addition of flavonoid-rich extract of Equisetum hyemale L. was found to promote hair regrowth in Sprague Dawley rats with chemically-induced alopecia (David et al., 2019).
In addition to flavonoids, other potential active substances, such as tocopherols, carotenoids, phytosterols, and saponins, are also found in safflower extract (Mani et al., 2020). Tocopherol and carotenoids have been shown to reduce the toxicity of cisplatin on ear nerve cells (Leonetti et al., 2003; Firdous and Kuttan, 2012; Kim et al., 2016). Carotenoids and saponins can reduce acute kidney injury caused by cisplatin (Sindhu and Kuttan, 2013; Li et al., 2021). Saponins can also mitigate intestinal damage caused by the drug (Hu et al., 2021). Compared with other chemotherapeutic drugs, the main function of traditional Chinese medicine is to kill cancer cells and thus reduce the dose of chemotherapeutic drugs needed. The main function of safflower extract used clinically, is to directly reduce the damage caused by chemotherapeutic drugs to normal tissues or cells.
Although many studies currently use dermal papilla cells to simulate hair growth conditions, one of the many experimental limitations is that these cells cannot accurately reflect the in vivo environment. In a 2D culture environment, dermal papilla cells rapidly lose their hair inductive potential and, therefore cannot be cultured for a long time. However, now many 3D culture technologies are available for dermal papilla cells combined with special scaffolds that can simulate the in vivo environment and support long-term culture (Betriu et al., 2020; Bejaoui et al., 2022; Yoon et al., 2022). This is beneficial for studying various drugs. Therefore, the use of a 3D culture environment to study the effects of safflower extract on cisplatin-induced damage to HFDPCs will help to explore more cellular mechanisms and better approximate the in vivo situation.
In summary, cisplatin inhibits hair growth, possibly by causing DNA damage in HFDPCs and causing cell cycle arrest through the Rad17/Chk1/Cdc25c signaling. Safflower extract can reduce the DNA damage caused by cisplatin in HFDPCs, thereby reducing the decrease in cell viability caused by cisplatin.
Our research demonstrated that the safflower extract and flavonoids contained in the extract can alleviate cisplatin-induced decrease in the viability of dermal papilla cells, and the main mechanism of action is to reduce the level of DNA damage caused by cisplatin. In conclusion, safflower extract or its flavonoid compounds can be developed as chemotherapy adjuvant drugs, which can slow down the side effects of hair loss caused by chemotherapy and increase the willingness of patients to take chemotherapy.
Acknowledgement: The authors thank the Core Laboratory of Taipei Tzu Chi Hospital for support.
Funding Statement: This study was supported by the Taipei Tzu Chi Hospital through grants from the Buddhist Tzu Chi Medical Foundation under the Numbers TCRD-TPE-110-13 and TCRD-TPE-111-23, Taipei, Taiwan.
Author Contributions: Draft manuscript preparation: FMT and CHW; experimental design: PHL, CHW, and LKW; experimentation: LKW and FMT; analysis of results: CYK and MLC. All authors reviewed the final version of the manuscript.
Availability of Data and Materials: All data generated or analyzed during this study are included in this published article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
Abusaliya A, Ha SE, Bhosale PB, Kim HH, Park MY, Vetrivel P, Kim GS (2022). Glycosidic flavonoids and their potential applications in cancer research: A review. Molecular & Cellular Toxicology 18: 9–16. https://doi.org/10.1007/s13273-021-00178-x [Google Scholar] [CrossRef]
Adamska I, Biernacka P (2021). Bioactive substances in safflower flowers and their applicability in medicine and health-promoting foods. International Journal of Food Science 2021: 6657639. https://doi.org/10.1155/2021/6657639 [Google Scholar] [PubMed] [CrossRef]
Aleem E, Arceci RJ (2015). Targeting cell cycle regulators in hematologic malignancies. Frontiers in Cell and Developmental Biology 3: 16. https://doi.org/10.3389/fcell.2015.00016 [Google Scholar] [PubMed] [CrossRef]
Bejaoui M, Oliva AK, Ke MS, Ferdousi F, Isoda H (2022). 3D spheroid human dermal papilla cell as an effective model for the screening of hair growth promoting compounds: Examples of minoxidil and 3,4,5-Tri-O-caffeoylquinic acid (TCQA). Cells 11: 2093. https://doi.org/10.3390/cells11132093 [Google Scholar] [PubMed] [CrossRef]
Belmonte-Fernandez A, Herrero-Ruiz J, Galindo-Moreno M, Limon-Mortes MC, Mora-Santos M, Saez C, Japon MA, Tortolero M, Romero F (2023). Cisplatin-induced cell death increases the degradation of the MRE11-RAD50-NBS1 complex through the autophagy/lysosomal pathway. Cell Death & Differentiation 30: 488–499. https://doi.org/10.1038/s41418-022-01100-1 [Google Scholar] [PubMed] [CrossRef]
Betriu N, Jarrosson-Moral C, Semino CE (2020). Culture and differentiation of human hair follicle dermal papilla cells in a soft 3D self-assembling peptide scaffold. Biomolecules 10: 684. https://doi.org/10.3390/biom10050684 [Google Scholar] [PubMed] [CrossRef]
Bhosale PB, Vetrivel P, Ha SE, Kim HH, Heo JD, Won CK, Kim SM, Kim GS (2021). Iridin induces G2/M phase cell cycle arrest and extrinsic apoptotic cell death through PI3K/AKT signaling pathway in AGS gastric cancer cells. Molecules 26: 2802. https://doi.org/10.3390/molecules26092802 [Google Scholar] [PubMed] [CrossRef]
Bhoyrul B, Asfour L, Lutz G, Mitchell L, Jerjen R, Sinclair RD, Holmes S, Chaudhry IH, Harries MJ (2021). Clinicopathologic characteristics and response to treatment of persistent chemotherapy-induced alopecia in breast cancer survivors. JAMA Dermatology 157: 1335–1342. https://doi.org/10.1001/jamadermatol.2021.3676 [Google Scholar] [PubMed] [CrossRef]
Bussoletti C, Tolaini MV, Celleno L (2020). Efficacy of a cosmetic phyto-caffeine shampoo in female androgenetic alopecia. Giornale Italiano di Dermatologia e Venereologia 155: 492–499. https://doi.org/10.23736/S0392-0488.18.05499-8 [Google Scholar] [PubMed] [CrossRef]
Chen CL, Huang WY, Wang EHC, Tai KY, Lin SJ (2020). Functional complexity of hair follicle stem cell niche and therapeutic targeting of niche dysfunction for hair regeneration. Journal of Biomedical Science 27: 43. https://doi.org/10.1186/s12929-020-0624-8 [Google Scholar] [PubMed] [CrossRef]
Choi EK, Kim IR, Chang O, Kang D, Nam SJ et al. (2014). Impact of chemotherapy-induced alopecia distress on body image, psychosocial well-being, and depression in breast cancer patients. Psycho-Oncology 23: 1103–1110. https://doi.org/10.1002/pon.3531 [Google Scholar] [PubMed] [CrossRef]
Collins PL, Purman C, Porter SI, Nganga V, Saini A et al. (2020). DNA double-strand breaks induce H2Ax phosphorylation domains in a contact-dependent manner. Nature Communications 11: 3158. https://doi.org/10.1038/s41467-020-16926-x [Google Scholar] [PubMed] [CrossRef]
Dasari S, Tchounwou PB (2014). Cisplatin in cancer therapy: Molecular mechanisms of action. European Journal of Pharmacology 740: 364–378. https://doi.org/10.1016/j.ejphar.2014.07.025 [Google Scholar] [PubMed] [CrossRef]
David P, Santiago C, Castillo A, De Guzman G (2019). Hair regenerative activities of flavonoid rich extract of Equisetum hyemale L. (Equisetaceae) in chemically induced alopecia in Sprague Dawley rats. Journal of Pharmacy & Pharmacognosy Research 7: 323–330. https://jppres.com/jppres/pdf/vol7/jppres19.557_7.5.323.pdf [Google Scholar]
Donzelli M, Draetta GF (2003). Regulating mammalian checkpoints through Cdc25 inactivation. EMBO Reports 4: 671–677. https://doi.org/10.1038/sj.embor.embor887 [Google Scholar] [PubMed] [CrossRef]
Firdous AP, Kuttan R (2012). Amelioration of cisplatin-induced toxicity in mice by carotenoid meso-zeaxanthin. Human and Experimental Toxicology 31: 710–717. https://doi.org/10.1177/0960327111431707 [Google Scholar] [PubMed] [CrossRef]
Fong S, Shoemaker M, Cadaoas J, Lo A, Liao W, Tagliaferri M, Cohen I, Shtivelman E (2008). Molecular mechanisms underlying selective cytotoxic activity of BZL101, an extract of Scutellaria barbata, towards breast cancer cells. Cancer Biology and Therapy 7: 577–586. https://doi.org/10.4161/cbt.7.4.5535 [Google Scholar] [PubMed] [CrossRef]
Go RS, Adjei AA (1999). Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. Journal of Clinical Oncology 17: 409–422. https://doi.org/10.1200/JCO.1999.17.1.409 [Google Scholar] [PubMed] [CrossRef]
Her Y, Lee TK, Sim H, Lee JC, Kim DW et al. (2022). Pinus thunbergii bark extract rich in flavonoids promotes hair growth in dorsal skin by regulating inflammatory cytokines and increasing growth factors in mice. Molecular Medicine Reports 25: 100. https://doi.org/10.3892/mmr.2022.12616 [Google Scholar] [PubMed] [CrossRef]
Higgins CA, Chen JC, Cerise JE, Jahoda CA, Christiano AM (2013). Microenvironmental reprogramming by three-dimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proceedings of the National Academy of Sciences of the United States of America 110: 19679–19688. https://doi.org/10.1073/pnas.1309970110 [Google Scholar] [PubMed] [CrossRef]
Hu JN, Yang JY, Jiang S, Zhang J, Liu Z, Hou JG, Gong XJ, Wang YP, Wang Z, Li W (2021). Panax quinquefolium saponins protect against cisplatin evoked intestinal injury via ROS-mediated multiple mechanisms. Phytomedicine 82: 153446. https://doi.org/10.1016/j.phymed.2020.153446 [Google Scholar] [PubMed] [CrossRef]
Junlatat J, Sripanidkulchai B (2014). Hair growth-promoting effect of Carthamus tinctorius floret extract. Phytotherapy Research 28: 1030–1036. https://doi.org/10.1002/ptr.5100 [Google Scholar] [PubMed] [CrossRef]
Kim SK, Im GJ, An YS, Lee SH, Jung HH, Park SY (2016). The effects of the antioxidant alpha-tocopherol succinate on cisplatin-induced ototoxicity in HEI-OC1 auditory cells. International Journal of Pediatric Otorhinolaryngology 86: 9–14. https://doi.org/10.1016/j.ijporl.2016.04.008 [Google Scholar] [PubMed] [CrossRef]
Kwon OS, Han JH, Yoo HG, Chung JH, Cho KH, Eun HC, Kim KH (2007). Human hair growth enhancement in vitro by green tea epigallocatechin-3-gallate (EGCG). Phytomedicine 14: 551–555. https://doi.org/10.1016/j.phymed.2006.09.009 [Google Scholar] [PubMed] [CrossRef]
Lee DH, Park KI, Park HS, Kang SR, Nagappan A et al. (2012). Flavonoids isolated from Korea Citrus aurantium L. induce G2/M phase arrest and apoptosis in human gastric cancer AGS cells. Evidence-Based Complementary and Alternative Medicine 2012: 515901. https://doi.org/10.1155/2012/515901 [Google Scholar] [PubMed] [CrossRef]
Leonetti C, Biroccio A, Gabellini C, Scarsella M, Maresca V et al. (2003). Alpha-tocopherol protects against cisplatin-induced toxicity without interfering with antitumor efficacy. International Journal of Cancer 104: 243–250. https://doi.org/10.1002/(ISSN)1097-0215 [Google Scholar] [CrossRef]
Li Q, Zhang Y, Yang Y, Huang S, Zou X, Wei C, Liang T, Zhong X (2021). Panax notoginseng saponins reduces the cisplatin-induced acute renal injury by increasing HIF-1α/BNIP3 to inhibit mitochondrial apoptosis pathway. Biomedicine and Pharmacotherapy 142: 111965. https://doi.org/10.1016/j.biopha.2021.111965 [Google Scholar] [PubMed] [CrossRef]
Liu W, Yang B, Yang L, Kaur J, Jessop C et al. (2019). Therapeutic effects of ten commonly used chinese herbs and their bioactive compounds on cancers. Evidence-Based Complementary and Alternative Medicine 2019: 1–10. https://doi.org/10.1155/2019/6057837 [Google Scholar] [PubMed] [CrossRef]
Liu K, Zheng M, Lu R, Du J, Zhao Q, Li Z, Li Y, Zhang S (2020). The role of CDC25C in cell cycle regulation and clinical cancer therapy: A systematic review. Cancer Cell International 20: 213. https://doi.org/10.1186/s12935-020-01304-w [Google Scholar] [PubMed] [CrossRef]
Lu PH, Kuo CY, Chan CC, Wang LK, Chen ML, Tzeng IS, Tsai FM (2021). Safflower extract inhibits adp-induced human platelet aggregation. Plants 10: 1192. https://doi.org/10.3390/plants10061192 [Google Scholar] [PubMed] [CrossRef]
Luanpitpong S, Nimmannit U, Chanvorachote P, Leonard SS, Pongrakhananon V, Wang L, Rojanasakul Y (2011). Hydroxyl radical mediates cisplatin-induced apoptosis in human hair follicle dermal papilla cells and keratinocytes through Bcl-2-dependent mechanism. Apoptosis 16: 769–782. https://doi.org/10.1007/s10495-011-0609-x [Google Scholar] [PubMed] [CrossRef]
Madaan A, Verma R, Singh AT, Jaggi M (2018). Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. International Journal of Cosmetic Science 40: 429–450. https://doi.org/10.1111/ics.12489 [Google Scholar] [PubMed] [CrossRef]
Mah LJ, El-Osta A, Karagiannis TC (2010). gammaH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 24: 679–686. https://doi.org/10.1038/leu.2010.6 [Google Scholar] [PubMed] [CrossRef]
Mani V, Lee SK, Yeo Y, Hahn BS (2020). A metabolic perspective and opportunities in pharmacologically important safflower. Metabolites 10: 253. https://doi.org/10.3390/metabo10060253 [Google Scholar] [PubMed] [CrossRef]
Matsuzaki T, Yoshizato K (1998). Role of hair papilla cells on induction and regeneration processes of hair follicles. Wound Repair and Regeneration 6: 524–530. https://doi.org/10.1046/j.1524-475X.1998.60605.x [Google Scholar] [PubMed] [CrossRef]
Mora-Lagos B, Cartas-Espinel I, Riquelme I, Parker AC, Piccolo SR et al. (2020). Functional and transcriptomic characterization of cisplatin-resistant AGS and MKN-28 gastric cancer cell lines. PLoS One 15: e0228331. https://doi.org/10.1371/journal.pone.0228331 [Google Scholar] [PubMed] [CrossRef]
Palamaras I, Misciali C, Vincenzi C, Robles WS, Tosti A (2011). Permanent chemotherapy-induced alopecia: A review. Journal of the American Academy of Dermatology 64: 604–606. https://doi.org/10.1016/j.jaad.2010.03.020 [Google Scholar] [PubMed] [CrossRef]
Perez AT, Arun B, Tripathy D, Tagliaferri MA, Shaw HS et al. (2010). A phase 1B dose escalation trial of Scutellaria barbata (BZL101) for patients with metastatic breast cancer. Breast Cancer Research and Treatment 120: 111–118. https://doi.org/10.1007/s10549-009-0678-5 [Google Scholar] [PubMed] [CrossRef]
Petrelli F, Zaniboni A, Coinu A, Cabiddu M, Ghilardi M, Sgroi G, Barni S (2013). Cisplatin or not in advanced gastric cancer: A systematic review and meta-analysis. PLoS One 8: e83022. https://doi.org/10.1371/journal.pone.0083022 [Google Scholar] [PubMed] [CrossRef]
Podhorecka M, Skladanowski A, Bozko P (2010). H2AX phosphorylation: Its role in DNA damage response and cancer therapy. Journal of Nucleic Acids 2010: 920161. https://doi.org/10.4061/2010/920161 [Google Scholar] [PubMed] [CrossRef]
Rambwawasvika H, Dzomba P, Gwatidzo L (2019). Hair growth promoting effect of dicerocaryum senecioides phytochemicals. International Journal of Medicinal Chemistry 2019: 7105834. https://doi.org/10.1155/2019/7105834 [Google Scholar] [PubMed] [CrossRef]
Sindhu ER, Kuttan R (2013). Carotenoid lutein protects the kidney against cisplatin-induced acute renal failure. Journal of Environmental Pathology, Toxicology and Oncology 32: 21–28. https://doi.org/10.1615/JEnvironPatholToxicolOncol.2013004933 [Google Scholar] [PubMed] [CrossRef]
Sun MY, Xu B, Wu QX, Chen WL, Cai S, Zhang H, Tang QF (2021). Cisplatin-resistant gastric cancer cells promote the chemoresistance of cisplatin-sensitive cells via the exosomal RPS3-mediated PI3K-Akt-Cofilin-1 signaling axis. Frontiers in Cell and Developmental Biology 9: 618899. https://doi.org/10.3389/fcell.2021.618899 [Google Scholar] [PubMed] [CrossRef]
Tchounwou PB, Dasari S, Noubissi FK, Ray P, Kumar S (2021). Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. Journal of Experimental Pharmacology 13: 303–328. https://doi.org/10.2147/JEP.S267383 [Google Scholar] [PubMed] [CrossRef]
Tiwari P, Mishra KP (2017). Role of flavonoids in DNA damage and carcinogenesis prevention. Journal of Carcinogenesis & Mutagenesis 8: 1000297. https://doi.org/10.4172/2157-2518.1000297 [Google Scholar] [CrossRef]
Tiwari P, Mishra KP (2022). Role of plant-derived flavonoids in cancer treatment. Nutrition and Cancer 75: 1–20. https://doi.org/10.1080/01635581.2022.2135744 [Google Scholar] [PubMed] [CrossRef]
Trueb RM (2010). Chemotherapy-induced alopecia. Current Opinion in Supportive and Palliative Care 4: 281–284. https://doi.org/10.1097/SPC.0b013e3283409280 [Google Scholar] [PubMed] [CrossRef]
Tsai FM, Li CH, Wang LK, Chen ML, Lee MC, Lin YY, Wang CH (2019). Extracellular Signal-regulated kinase mediates ebastine-induced human follicle dermal papilla cell proliferation. BioMed Research International 2019: 6360503. https://doi.org/10.1155/2019/6360503 [Google Scholar] [PubMed] [CrossRef]
Uen WC, Lee BH, Shi YC, Wu SC, Tai CJ, Tai CJ (2018). Inhibition of aqueous extracts of Solanum nigrum (AESN) on oral cancer through regulation of mitochondrial fission. Journal of Traditional and Complementary Medicine 8: 220–225. https://doi.org/10.1016/j.jtcme.2017.05.011 [Google Scholar] [PubMed] [CrossRef]
Wang CW, Chen CL, Wang CK, Chang YJ, Jian JY, Lin CS, Tai CJ, Tai CJ (2015). Cisplatin-, doxorubicin-, and docetaxel-induced cell death promoted by the aqueous extract of solanum nigrum in human ovarian carcinoma cells. Integrative Cancer Therapies 14: 546–555. https://doi.org/10.1177/1534735415588826 [Google Scholar] [PubMed] [CrossRef]
Wang Q, Goldstein M, Alexander P, Wakeman TP, Sun T, Feng J, Lou Z, Kastan MB, Wang XF (2014). Rad17 recruits the MRE11-RAD50-NBS1 complex to regulate the cellular response to DNA double-strand breaks. EMBO Journal 33: 862–877. https://doi.org/10.1002/embj.201386064 [Google Scholar] [PubMed] [CrossRef]
Wang X, Zou L, Lu T, Bao S, Hurov KE, Hittelman WN, Elledge SJ, Li L (2006). Rad17 phosphorylation is required for claspin recruitment and Chk1 activation in response to replication stress. Molecular Cell 23: 331–341. https://doi.org/10.1016/j.molcel.2006.06.022 [Google Scholar] [PubMed] [CrossRef]
Wils R, Jacob AR, Daniel ES, Chacko RT, Reka S (2019). Distress and coping in cancer patients experiencing chemotherapy-induced alopecia. Indian Journal of Continuing Nursing Education 20: 60–64. https://doi.org/10.4103/IJCN.IJCN_4_19 [Google Scholar] [CrossRef]
Yang CC, Cotsarelis G (2010). Review of hair follicle dermal cells. Journal of Dermatological Science 57: 2–11. https://doi.org/10.1016/j.jdermsci.2009.11.005 [Google Scholar] [PubMed] [CrossRef]
Yao D, Wang Z, Miao L, Wang L (2016). Effects of extracts and isolated compounds from safflower on some index of promoting blood circulation and regulating menstruation. Journal of Ethnopharmacology 191: 264–272. https://doi.org/10.1016/j.jep.2016.06.009 [Google Scholar] [PubMed] [CrossRef]
Yoon YC, Ahn BH, Min JW, Lee KR, Park SH, Kang HC (2022). Stimulatory effects of extracellular vesicles derived from Leuconostoc holzapfelii that exists in human scalp on hair growth in human follicle dermal papilla cells. Current Issues in Molecular Biology 44: 845–866. https://doi.org/10.3390/cimb44020058 [Google Scholar] [PubMed] [CrossRef]
York K, Meah N, Bhoyrul B, Sinclair R (2020). A review of the treatment of male pattern hair loss. Expert Opinion on Pharmacotherapy 21: 603–612. https://doi.org/10.1080/14656566.2020.1721463 [Google Scholar] [PubMed] [CrossRef]
Yu SY, Lee YJ, Kim JD, Kang SN, Lee SK, Jang JY, Lee HK, Lim JH, Lee OH (2013). Phenolic composition, antioxidant activity and anti-adipogenic effect of hot water extract from safflower (Carthamus tinctorius L.) seed. Nutrients 5: 4894–4907. https://doi.org/10.3390/nu5124894 [Google Scholar] [PubMed] [CrossRef]
Zhou Z, Jing C, Zhang L, Takeo F, Kim H, Huang Y, Liu Z, Wan Y (2013). Regulation of Rad17 protein turnover unveils an impact of Rad17-APC cascade in breast carcinogenesis and treatment. Journal of Biological Chemistry 288: 18134–18145. https://doi.org/10.1074/jbc.M113.456962 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools