 Open Access
Open Access
REVIEW
Macrophage polarization in cardiac transplantation: Insights into immune modulation and therapeutic approaches
1 Department of Cardiovascular Surgery, The First Affiliated Hospital of Guangxi Medical University, Nanning, 530021, China
2 The First Clinical Medical College of Guangxi Medical University, Nanning, 530021, China
3 Department of Cardiovascular Surgery, Beijing Anzhen Hospital of Capital Medical University, Beijing, 100029, China
* Corresponding Authors: BAOSHI ZHENG. Email: ; CHENG LUO. Email:
(This article belongs to the Special Issue: Exploring Mitochondria: Unraveling Structure, Function, and Implications in Health and Disease)
BIOCELL 2025, 49(1), 61-78. https://doi.org/10.32604/biocell.2024.056981
Received 04 August 2024; Accepted 31 October 2024; Issue published 24 January 2025
Abstract
The role and regulatory mechanisms of macrophage polarization in cardiac transplantation have gained significant attention. Macrophages can polarize into either the M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotype in response to environmental cues. M1 macrophages facilitate transplant rejection by releasing inflammatory mediators and activating T cells, whereas M2 macrophages support graft survival by secreting anti-inflammatory factors and promoting tissue repair. Mitochondrial quality control regulation plays a crucial role in macrophage polarization, which may influence graft survival and immune responses. This review provides an overview of the current understanding of mitochondrial quality control-regulated macrophage polarization in cardiac transplantation, its effects on graft outcomes, and potential therapeutic strategies to modulate this process to enhance transplant success rates. The review was conducted by systematically analyzing recent studies and integrating findings from key research articles to synthesize a comprehensive understanding of this emerging field.Keywords
Cardiac transplantation is a critical reatment for patients with end-stage heart failure, offering a lifesaving solution when other treatments fail. Since the first successful heart transplant in 1967 by South African surgeon Christiaan Barnard [1], this procedure has continuously evolved. Despite challenges such as immune rejection, advances in immunosuppressive therapy and surgical techniques have markedly improved success and survival rates, solidifying cardiac transplantation as a key intervention for severe cardiovascular diseases [2].
Studying macrophage polarization in the immune system has become increasingly important in cardiac transplantation research [3]. Macrophages are crucial immune system components that play a key role in inflammation, infection, and tissue repair [4]. Their polarization states include the M1 (pro-inflammatory) and M2 (anti-inflammatory) types, which perform specific functions under different physiological conditions [5]. Studying macrophage polarization can yield a deeper understanding of the molecular mechanisms of immune regulation, providing new targets and strategies for disease treatment [3]. In cardiac transplantation, understanding the regulatory mechanisms of macrophage polarization aids the optimization of post-transplant immune responses, increasing surgical success rates and presenting more effective methods for treating heart disease patients. Furthermore, this comprehensive research will provide a new scientific basis for medical practice and heart disease treatment.
This review aims to provide a comprehensive overview of the regulatory mechanisms and the role of mitochondrial quality control-regulated macrophage polarization in cardiac transplantation. By synthesizing recent studies, the review seeks to highlight potential therapeutic strategies that modulate macrophage polarization to improve graft survival and reduce rejection. The focus is on exploring the interplay between macrophage polarization and immune modulation in the context of cardiac transplantation to offer insights for future research and clinical practice.
Given the essential role of the mitochondria in cellular energy production and the overall health of transplanted hearts, mitochondrial quality control (MQC) is a critical aspect of cardiac transplantation. The MQC mechanisms encompass mitochondrial biogenesis, mitophagy, and mitochondrial dynamics regulation through fusion and fission processes [6]. Effective MQC ensures the removal of damaged mitochondria and the maintenance of a healthy mitochondrial population, which is vital for cell survival and function, particularly in the high-energy-demand environment of the heart [7]. Recent studies have demonstrated that manipulating MQC significantly influences macrophage polarization, thereby affecting cardiac transplantation outcomes [8,9]. For example, interventions that enhance mitochondrial biogenesis or promote dysfunctional mitochondria clearance shift macrophages towards the M2 phenotype, which is associated with anti-inflammatory and tissue repair functions [10]. This shift reduces the inflammatory response that leads to transplant rejection, and promotes tissue repair and graft survival. However, despite these advances, the specific mechanisms by which MQC influences macrophage polarization remain inadequately understood, and conflicting results have emerged regarding the optimal strategies to modulate these processes for therapeutic benefit [9].
Understanding the interplay between MQC and macrophage polarization opens new avenues for therapeutic strategies to improve transplant outcomes [6]. Targeting MQC pathways may enable the development of treatments that enhance graft tolerance and longevity, thereby improving the overall success rates of cardiac transplantation [11]. Recent scientific advancements in macrophage polarization and MQC research have provided a more comprehensive understanding of their roles in inflammation, infection, and tissue repair [12]. Researchers have highlighted the pivotal roles of M1 and M2 macrophages in these processes and how MQC can modulate these functions to improve graft outcomes [10]. This growing body of knowledge suggests new directions for future heart disease treatment and immune regulation, emphasizing the importance of integrated approaches that consider immune modulation and cellular quality control [7]. Nevertheless, significant gaps remain in our understanding of how to precisely control MQC to achieve desired macrophage phenotypes in different transplantation contexts [12], yet further research is needed to resolve these uncertainties and optimize clinical applications.
Immunological Basis of Cardiac Transplantation
Cellular mechanisms of transplant rejection
The major histocompatibility complex (MHC) is a critical transplantation antigen component essential for antigen presentation and recognition [13]. MHC molecules interact with T cells by presenting antigen fragments, triggering an immune response [14]. In addition to MHC, donor heart antigens include other cell membrane molecules and intracellular proteins [13]. These antigens are recognized by the immune system, eliciting both antibody-mediated and T cell-mediated immune responses [15]. The immune system recognizes donor antigens through MHC molecules, activating T cells, which play a crucial role in both direct and indirect pathways of graft rejection [13]. In the indirect pathway, T cells recognize allopeptides presented by the host’s antigen-presenting cells (APCs). However, there is also a direct pathway where T cells recognize intact donor MHC molecules directly on donor cells. This direct recognition plays a critical role in acute graft rejection, as it can trigger a rapid and potent immune response [13]. Both pathways contribute significantly to transplant rejection by initiating immune responses that damage the donor heart tissue [14].
T cell activation plays a pivotal role in the immune response during cardiac transplantation, with both primary and secondary signals essential for their function [16]. The intricate pathways involving co-stimulatory molecules such as CD28 and CD40L are vital for T cell-mediated immunity [16,17]. However, in the context of this review, which focuses on macrophage polarization, it is important to recognize that T cells interact closely with macrophages to shape the overall immune environment in the transplanted heart. This interplay between T cells and macrophages influences both acute and chronic rejection processes [18]. Recent research has highlighted the role of innate allorecognition by monocyte-derived dendritic cells (mo-DCs) and macrophages in transplant rejection [19]. Specifically, the CD47/SIRPa axis has been identified as a critical pathway through which strain polymorphisms influence the recognition of allogeneic MHC molecules, leading to enhanced macrophage activation and immune response [20]. Furthermore, the engagement of PIR-A and PIR-B with MHC class I molecules has been shown to modulate the immune response, where PIR-A promotes and PIR-B inhibits immune signaling [21]. This balance plays a significant role in determining the outcome of graft survival or rejection. Studies by Lakkis and colleagues demonstrate that blocking the PIR-A pathway can mitigate rejection by reducing macrophage-mediated inflammation, while PIR-B engagement helps to dampen immune responses, thus contributing to graft tolerance [22]. In particular, alloreactive T cells, especially Th1 cells, can also activate macrophages either via direct contact during alloantigen presentation by macrophages to T cells in the graft, or via Th1 cell production of interferon gamma (IFNγ), which enhances macrophage polarization toward M1-like and augments macrophage function [23]. Regulatory T cells (Tregs) are also critical in controlling immune responses and inducing immune tolerance. Antibody-mediated rejection (AMR) is a significant cardiac transplantation complication, occurring in approximately 10%–20% of heart transplant patients [24]. AMR can manifest early (acute AMR) or late (chronic AMR) post-transplantation and is associated with the progression of cardiac allograft vasculopathy (CAV) and adverse transplant outcomes [25,26]. The AMR pathological mechanism involves the deposition of donor-specific antibodies (DSA) against human leukocyte antigen (HLA) molecules, especially HLA class II, although HLA class I associations also occur [27].
The histological features of AMR in cardiac transplantation include microvascular inflammation, macrophage adhesion within capillaries, and endothelial cell swelling [28]. These features highlight the importance of endothelial cell and macrophage interactions in AMR pathogenesis. Adenovirus-mediated interleukin-10 (IL-10) treatment reduced pathological damage, perivascular fibrosis, apoptosis, and inflammation and increased the ratio of Treg–TIGIT+ Treg cells, Arg-1+ cells, and CD206+ cells in a murine heart transplantation model [29]. Mechanistic studies suggested that IL-10 activates SOCS5 by negatively regulating microRNA-155, promoting macrophage M2 polarization, which presents new therapeutic possibilities for chronic rejection post-cardiac transplantation [29,30].
DSA are crucial in cardiac transplantation, serving as key factors in both acute and chronic rejection. They are generated when the recipient’s immune system recognizes donor HLA molecules, a process mediated by APCs that present donor antigens to T cells [31]. DSA binding to HLA antigens on the endothelial cells of the transplanted heart activates the classical complement pathway, leading to the deposition of complement components like C1q, C4b, and C3b [32,33]. This results in the formation of the membrane attack complex (MAC), directly damaging myocardial cells and causing tissue injury [33]. Activated CD4+ helper T cells provide the necessary signals to activate and differentiate B cells [34]. The B cells that receive these signals proliferate and differentiate into plasma cells, producing DSA against donor HLA antigens [35]. The DSA generation and action mechanisms are similar to those in other organ transplants but have a more pronounced effect on the transplanted heart [31]. Additionally, DSA binding can trigger antibody-dependent cell-mediated cytotoxicity (ADCC), recruiting natural killer (NK) cells and macrophages, which recognize and bind the Fc portion of antibodies through their Fc receptors, releasing cytotoxic substances such as perforin and granzymes and exacerbating myocardial cell damage [36,37].
Advances in immune tolerance research
Research on immune tolerance in cardiac transplantation covers molecular mechanisms, applications in autoimmune diseases and organ transplantation, and emerging research areas. Immune tolerance refers to the recipient’s immune system accepting the graft without long-term immunosuppression, thereby avoiding rejection [38]. The molecular mechanism of immune tolerance primarily relies on the Treg function [39]. Tregs inhibit effector T cell activation by secreting inhibitory cytokines such as IL-10 and transforming growth factor-β (TGF-β) [40,41]. Immune checkpoint molecules such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death 1 (PD-1) are also critical in maintaining Treg function and stability [42]. Additionally, blocking co-stimulatory signals, such as by using CTLA-4-Ig (abatacept), prevented full T cell activation by inhibiting CD28 signaling, promoting immune tolerance [43].
The application of immune tolerance is equally important in autoimmune diseases. Modulating Treg quantity and function alleviates diseases such as type 1 diabetes and systemic lupus erythematosus [44]. For example, increasing the number of Tregs significantly reduced symptoms and slowed the progression of these diseases [45,46]. The application of immune tolerance is also crucial in organ transplantation, especially cardiac transplantation [47,48]. Traditional immunosuppressive therapy effectively reduces acute rejection but long-term use can lead to infections, cancer, and other complications [38]. Inducing immune tolerance reduces the dependence on long-term immunosuppression [49]. The concept of microchimerism has gained wide research attention. Microchimerism induces tolerance without long-term immunosuppression by introducing a small number of donor hematopoietic stem cells into the recipient to form a donor–recipient mixed chimerism [50,51]. Animal models and preliminary clinical studies have demonstrated that this method successfully induced graft tolerance and maintained long-term transplant function [52,53].
Research on immune tolerance in cardiac transplantation has progressed substantially, focusing on reducing rejection and prolonging graft survival [54,55]. One major research direction is the application of Tregs, which are crucial in maintaining the immune balance and preventing autoimmunity [56–58]. Enhancing Treg numbers or function inhibits harmful immune responses against the graft [44]. Clinical studies have demonstrated that Treg therapy effectively reduced rejection [59]. Another important advancement is inducing immune tolerance through co-stimulatory blockade [60]. Co-stimulatory molecules such as CD28 and CD40L are critical for T cell activation, and blocking these pathways prevents full T cell activation, thereby reducing graft rejection [61]. For example, CTLA-4-Ig (abatacept) demonstrated efficacy in clinical trials by blocking CD28 signaling [62]. Furthermore, the potential of gene editing technologies such as CRISPR/Cas9 in regulating immune responses is being explored [63]. For example, specifically modifying immune-related genes induced immune tolerance more precisely [64].
Lastly, microbiome research has revealed the potential role of gut microbiota in immune regulation [65]. A healthy gut microbiota balance maintains immune system stability, regulating inflammation and immune tolerance [66]. Adjusting the gut microbiota of transplant recipients could be a new strategy to promote transplant tolerance [67]. Overall, advances in immune tolerance research for cardiac transplantation offer new hope for improving transplant outcomes. These studies aid the understanding of how the immune system interacts with the graft and provide a basis for developing new treatments to reduce rejection and enhance long-term graft survival.
Immune cell interaction is crucial for understanding cardiac transplant rejection. The post-transplant immune response is a complex process involving interactions among immune cells, particularly T cells, B cells, APCs, and NK cells [68]. The recipient’s APCs capture and process the HLA antigens of the transplanted heart [69]. These APCs present the processed antigens as peptide–MHC complexes to the T cells [70]. CD4+ helper T cells recognize antigen peptides on MHC class II molecules, while CD8+ cytotoxic T cells recognize antigen peptides on MHC class I molecules, which is key to T cell activation [71]. Activated CD4+ helper T cells secrete cytokines such as IL-2, IFN-γ, and tumor necrosis factor-α (TNF-α), which promote T and B cell proliferation and differentiation [72]. Aided by antigens and T cells, the B cells differentiate into plasma cells that produce DSA against donor HLA [73].
DSA are major mediators of AMR and bind to HLA antigens on the endothelial cells of the transplanted heart, triggering complement system activation [33]. The classical complement pathway activation leads to the deposition of complement components such as C1q, C4b, and C3b, forming the MAC, which directly disrupts cell membranes, causing cell lysis and tissue damage [74,75]. DSA can also bind their Fc portion to Fc receptors on NK cells and macrophages, inducing antibody-dependent cell-mediated cytotoxicity (ADCC) [76]. NK cells and macrophages release cytotoxic substances (perforin and granzymes), causing myocardial cell damage and graft function loss [77]. The interaction of these immune cells significantly affects the cardiac transplantation outcome [78]. Acute rejection typically occurs early post-transplantation due to DSA and cell-mediated mechanisms, leading to rapid graft function decline [79]. Chronic rejection is characterized by long-term immune attack and results in chronic graft damage and eventual graft failure [80]. Understanding these cellular interaction mechanisms is crucial for developing effective immunosuppressive strategies and improving the long-term success rate of cardiac transplantation (Fig. 1).
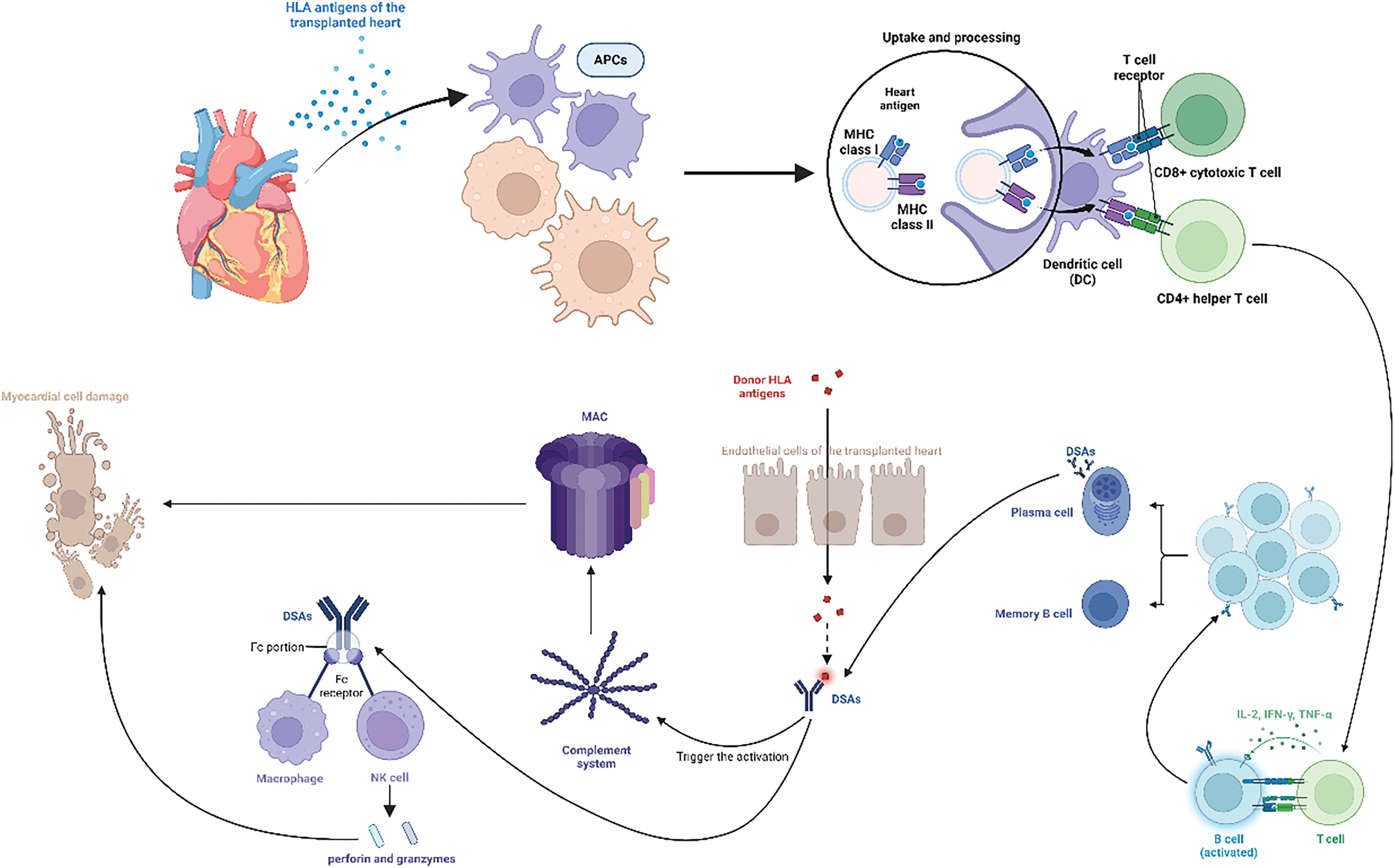
Figure 1: This figure illustrates the interactions among immune cells during cardiac transplantation. Interactions among immune cells during cardiac transplantation. Human leukocyte antigens (HLAs) from the transplanted heart are captured and processed by recipient antigen-presenting cells (APCs), which present the antigens to T cells as peptide–major histocompatibility complex (MHC) complexes. CD4+ helper T cells recognize MHC class II molecules, while CD8+ cytotoxic T cells recognize MHC class I molecules. Activated CD4+ T cells secrete cytokines, promoting T and B cell proliferation and differentiation. B cells become plasma cells that produce donor-specific antibodies (DSAs). DSAs bind to HLAs on endothelial cells, activating the complement system and forming the membrane attack complex (MAC), causing cell lysis and tissue damage. DSAs also bind to Fc receptors on natural killer (NK) cells and macrophages, inducing antibody-dependent cell-mediated cytotoxicity (ADCC) and releasing perforin and granzymes, leading to myocardial cell damage and graft function loss. Understanding these interactions is crucial for developing effective immunosuppressive strategies and improving cardiac transplantation outcomes. Created in BioRender. Jingwei, J. (2024) BioRender.com/v34u205 (accessed 10 September 2024).
Biological Characteristics of Macrophages
Macrophages originate from bone marrow-derived monocyte precursors and other locations [81]. Bone marrow precursor cells enter tissues via the circulatory system, where they differentiate into mature macrophages [82]. Embryonic-derived macrophages present during embryonic development contribute to immune system establishment, maintenance, and function throughout development [83]. These macrophages reside in the bone marrow and various tissues and organs, such as Kupffer cells in the liver, microglia in the brain, and Langerhans cells in the skin, performing specific immune defense and regulatory functions [84].
Macrophages are among the most diverse, active, and heterogeneous cells in the body, executing a wide range of physiological functions, including antigen processing, inflammatory response, tissue homeostasis, repair, and regeneration [85]. Macrophages have diverse origins and primarily include bone marrow-derived myeloid precursor cells and embryonic-derived macrophages [86]. Bone marrow-derived macrophages are continuously replenished in adults through monocyte, dendritic cell, and neutrophil development [87]. Contrastingly, embryonic-derived macrophages migrate to peripheral organs during embryonic development from the yolk sac and fetal liver, reside in tissues in adulthood, and maintain their numbers through self-renewal, relying minimally on input from circulating blood [88].
In inflamed tissues, macrophages are sourced from circulating macrophages and locally proliferating macrophages [5]. Specifically, both donor-derived tissue-resident macrophages and recipient infiltrating macrophages coexist in the graft, potentially exhibiting significant differences in function and interaction in transplantation [89]. Donor macrophages may influence graft acceptance and rejection through interactions with the recipient’s immune system [89].
Macrophage polarization is a complex and dynamic process regulated by signaling molecules, gene regulation, cell surface marker changes, and functional transformations [90]. Differentiated macrophages exhibit M1 or M2 polarization states, playing distinct roles in inflammation and tissue repair [91]. Macrophages can be classically activated into M1 macrophages or alternatively activated into M2 macrophages based on different signaling molecules [92].
However, recent studies have challenged the oversimplified M1/M2 macrophage polarization paradigm, revealing a more intricate spectrum of polarization states influenced by the tissue environment and signaling cues [93–95]. Emerging research indicates that monocytes and macrophages do not merely polarize into static M1 or M2 phenotypes. Instead, they exhibit a dynamic range of activation states, including low-grade inflammatory memory states, which can persist and influence tissue homeostasis and disease outcomes [96,97]. Latest studies have demonstrated that macrophages exposed to chronic, subclinical inflammatory stimuli can develop a type of “innate memory” [98,99]. This memory state enables them to respond more robustly to subsequent challenges, contributing to chronic inflammation and disease progression [98,99].
M1 macrophages, induced by IFN-γ, TNF-α, and toll-like receptors (TLR) ligands, primarily engage in immune defense and inflammatory responses [100]. Conversely, M2 macrophages, which are differentiated under the influence of IL-4 and IL-13, secrete anti-inflammatory cytokines like IL-10 and transforming growth factor beta (TGF-β), playing key roles in tissue repair and the regulation of inflammatory responses [101,102]. These cells produce inflammatory cytokines, such as IL-1β, TNF-α, and IL-6, promoting inflammation by releasing pro-inflammatory factors, nitric oxide, and reactive oxygen species (ROS), which are essential for pathogen clearance and immune response [103]. M1 macrophages highly express MHC class II antigens, CD80, CD86, CCR7, and the chemokines CCL8/15/20 and CXCL9/10/11/13, enhancing their pro-inflammatory function [104–106]. M2 macrophages can be divided into the M2a, M2b, M2c, and M2d subtypes depending on the inducing factors [107]. M2a cells highly express CD206 and CD209 and are primarily anti-inflammatory cells; M2b cells are induced in the presence of immune complexes and IL-1 receptor antagonists, expressing chemokines and regulatory cytokines such as IL-10 and TGF-β; M2c cells express CD163, TLR-1/8, and IL-21 receptor and are involved in tissue repair and regeneration; M2d cells differentiate in the tumor microenvironment, exhibiting immunosuppressive functions that promote tumor growth and metastasis [107,108].
Overall, M1 macrophages participate in immune defense and inflammatory responses through pro-inflammatory actions, while M2 macrophages maintain tissue homeostasis and immune regulation through anti-inflammatory and tissue repair functions [109]. M1 and M2 macrophage balance and regulation are crucial for maintaining normal immune function and pathogen defense [110]. Additionally, recent studies have demonstrated that macrophage polarization is not fixed but dynamically switches under different environmental stimuli, providing new insights for macrophage-targeted therapies in various pathological states [111].
In gene regulation, transcription factors such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and signal transducer and activator of transcription 1 (STAT1) are key in M1 polarization, while STAT6 and peroxisome proliferator-activated receptor gamma (PPARγ) are critical for M2 polarization. M1 macrophages upregulate the inducible nitric oxide synthase (iNOS) gene upon activation, whereas M2 macrophages upregulate the arginase 1 (Arg1) gene [112,113]. These gene expression changes reflect the functional transformation of macrophages under different polarization states. The transcription factors such as interferon regulatory factor 5 (IRF5) and krupple-like factor 4 (KLF4) are also important in macrophage polarization, promoting M1 and M2 polarization, respectively [4].
Regarding cell surface marker changes, M1 macrophages typically express higher levels of CD86 and MHC class II molecules, which enhance antigen presentation and T cell activation [114]. M2 macrophages express higher levels of CD206 (mannose receptor) and CD163 (scavenger receptor), which are associated with anti-inflammatory and tissue repair functions [115]. Functionally, M1 macrophages have strong microbial killing capabilities and pro-inflammatory properties, mainly through ROS and reactive nitrogen species (RNS) production, to kill pathogens and initiate inflammatory responses, rendering them crucial in anti-infection and anti-tumor immunity [103]. Contrastingly, M2 macrophages play a primary role in tissue repair and anti-inflammatory responses, suppressing inflammation and repairing damage by secreting anti-inflammatory cytokines and promoting tissue regeneration [116]. M2 macrophages also participate in clearing apoptotic cells and reconstructing tissue architecture [117]. Macrophage phenotypic switching is dynamic and can be flexibly regulated in different microenvironments [118]. According to the study by Liu et al., increased M2 transcripts, including Arg1, Mrc1, and Mmp12, were positively correlated with graft deterioration in a mouse kidney allotransplantation model [119]. These M2 macrophages might have originated from in situ M1 macrophages, further demonstrating macrophage plasticity [120].
Macrophages are critical in clearing cellular debris, pathogens, and other foreign substances and in antigen presentation to activate and regulate immune responses, performing vital immune defense functions. This phenotypic plasticity enables macrophages to adapt to various physiological and pathological conditions [121]. Macrophages can reversibly switch between the M1 and M2 phenotypes during immune responses and tissue repair, where they are regulated by signaling molecules and cytokines, including extracellular matrix, inflammatory factors, and cytokines [122]. Additionally, M2 macrophages function in the tumor microenvironment by secreting anti-inflammatory cytokines such as IL-10 and TGF-β, suppressing immune responses and promoting tumor growth and metastasis [123].
Role of Macrophages in Cardiac Transplantation
Localization and dynamic regulation of macrophages
Cardiac transplantation immunological studies have demonstrated that macrophages are densely distributed in the vessel walls, myocardium, and pericardium of the transplanted heart, playing crucial roles [124]. These cells help the transplanted tissue adapt to the new environment by clearing pathogens and regulating inflammation and immune responses in the early stages [12,125]. They also sense allogeneic antigens in the transplanted vessels, actively participating in the inflammatory process and affecting endothelial function and permeability [126]. The macrophage numbers temporarily increase in the early post-transplant period to defend against pathogens and aid the adaptation of the transplanted tissue [127]. However, chronic rejection and persistent immune responses over time lead to further increases in macrophage numbers [128]. This accumulation is a hallmark of chronic rejection and promotes chronic inflammation and fibrosis by secreting cytokines and growth factors, severely affecting the long-term survival of the transplanted heart [129,130]. Therefore, studying the dynamic changes and functions of macrophages in transplanted hearts is crucial for understanding and managing transplant rejection [89].
Role of macrophages in transplant rejection
Macrophages play multiple key roles in transplant rejection. First, they initiate the rejection response by recognizing allogeneic antigens from the donor tissue and presenting antigen fragments to other immune cells, activating T cells and other immune effector cells [131,132]. The activated macrophages release inflammatory mediators such as TNF-α and IL-1β, leading to inflammatory responses in the transplanted tissue and promoting the progression of rejection [133]. Macrophages are activated during ischemia-reperfusion injury and the release of damage-associated molecular patterns (DAMPs), secreting pro-inflammatory cytokines that sustain local inflammation, causing early graft injury and upregulating co-stimulatory molecules to activate T cells and initiate immune responses [134,135]. Macrophages in grafts engulf necrotic cells, secrete pro-inflammatory cytokines and chemokines, release ROS, and activate adaptive immune cells [136,137]. Macrophage infiltration is a significant factor in chronic rejection, leading to tissue fibrosis and graft vasculopathy, characterized by progressive neointima formation and vascular occlusion [138]. Reducing macrophage numbers significantly decreases graft damage and inflammatory responses (Fig. 2) [139].
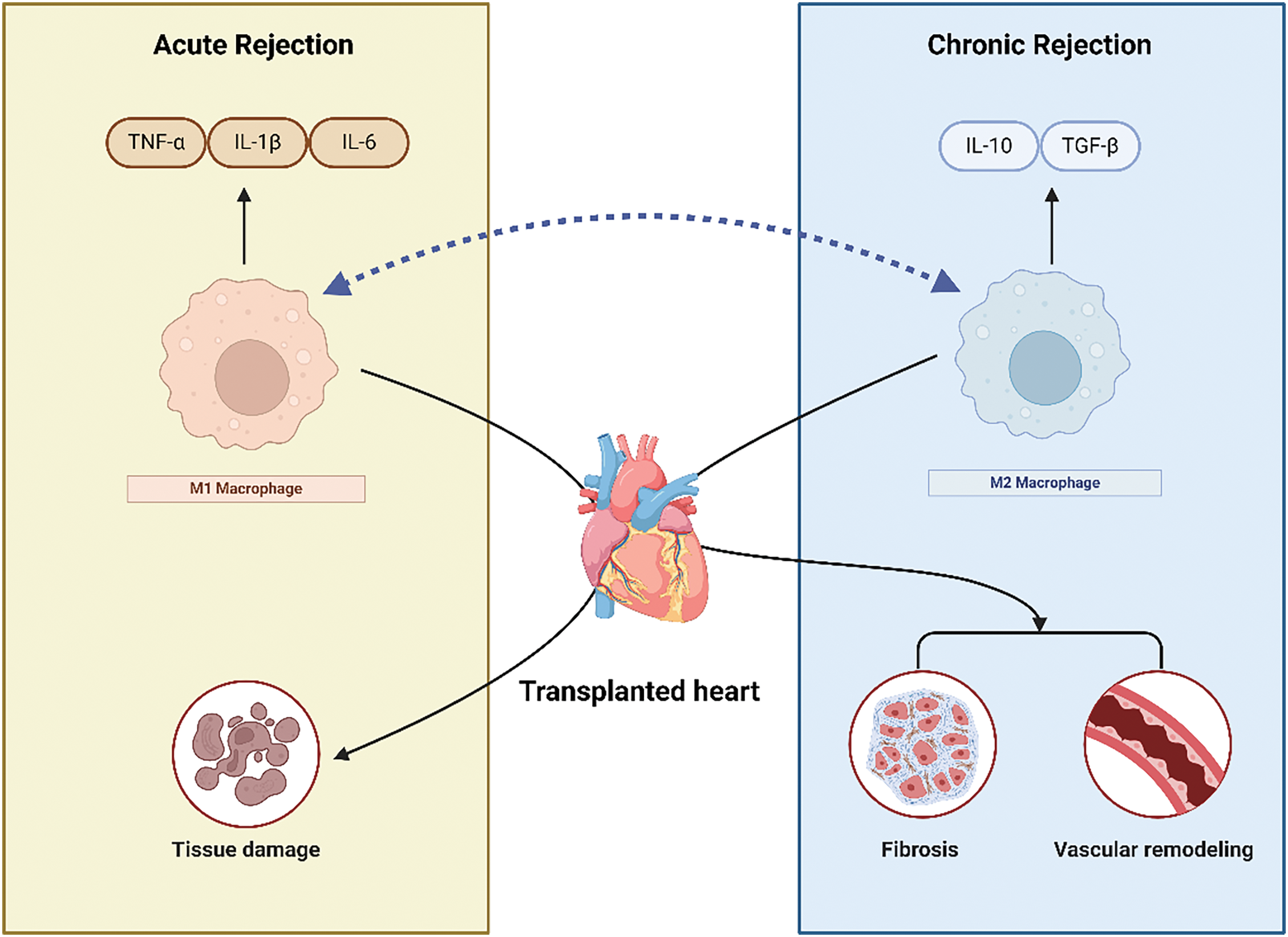
Figure 2: This figure illustrates the distinct roles of M1 and M2 macrophages during acute and chronic cardiac transplant rejection. In the acute phase, M1 macrophages are activated by pro-inflammatory signals such as IFN-γ, releasing inflammatory cytokines (TNF-α, IL-1β, IL-6) and reactive oxygen species (ROS), which contribute to tissue damage and transplant rejection. M1 macrophages also enhance antigen presentation and activate T cells, further promoting immune responses. In contrast, during chronic rejection, M2 macrophages, activated by anti-inflammatory cytokines (IL-4, IL-13), secrete IL-10 and TGF-β, promoting tissue repair and fibrosis. While M2 macrophages aid in suppressing inflammation, their excessive activity can lead to chronic fibrosis and vascular remodeling, contributing to long-term graft dysfunction. The bidirectional arrow between M1 and M2 macrophages highlights the plasticity of macrophage polarization, indicating their ability to switch phenotypes based on environmental cues. Created in BioRender. Jingwei, J. (2024) BioRender.com/m41n448 (accessed 10 September 2024).
Donor-specific alloantibodies are key in chronic graft damage, with macrophages considered key effector cells in antibody-mediated graft injury [140]. In a mouse cardiac transplantation model, recipient mice treated with carrageenan exhibited a 30%–80% reduction in macrophages, while the T, B, and NK cell functions remained largely unchanged, significantly reducing cardiac graft vasculopathy [141]. Further studies revealed that infiltrating macrophages in grafts predominantly adopted the M2 phenotype in chronic rejection [142]. Human kidney transplant biopsy studies demonstrated that approximately 92% of infiltrating macrophages exhibited the M2 phenotype (CD68+CD206+) 1 year post-transplant, with their proportion positively correlated with the degree of kidney fibrosis [143].
Despite evidence indicating M2 cell induction in chronic graft rejection, eicosanoid, ROS, and nitric oxide production might also induce graft vasculopathy, suggesting M1 macrophage involvement [130]. IFN-γ was crucial for inducing graft vasculopathy in a mouse cardiac transplantation model [144]. IFN-γ induced iNOS expression in macrophages and upregulated MHC class II, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) expression, confirming the role of M1 macrophages in rejection [145]. Recent evidence indicates that donor-specific alloantibodies are key in chronic graft damage [140].
Role of macrophages in immune tolerance
Macrophages participate in graft rejection and are essential in tissue damage and repair. They can exacerbate transplant tissue damage or promote tissue healing [130]. Additionally, macrophages regulate the activity of T cells, B cells, and other immune cells by presenting antigens and secreting immunomodulatory factors, influencing the immune balance [46]. During rejection and inflammation, macrophages exhibit anti-inflammatory characteristics by secreting anti-inflammatory cytokines (e.g., IL-10) to suppress excessive inflammatory responses [146]. Macrophages have critical repair roles in tissue damage caused by injury or inflammation, promoting healing and reconstructing damaged tissue [147]. Under specific conditions, macrophages can present an anti-inflammatory and immunosuppressive phenotype, secreting anti-inflammatory cytokines (e.g., IL-10), regulating T cell activity, and promoting immune tolerance [148]. Macrophages in an immune tolerance environment may exhibit an M2 anti-inflammatory phenotype, helping reduce rejection and maintaining the immune balance [149].
Macrophages are significant in post-transplant rejection and contribute to graft survival. Traditionally, macrophages were considered the primary pro-inflammatory and immune-activating effector cells, particularly crucial in transplant rejection [150]. However, recent studies have revealed the dual role of macrophages, indicating that they can also exert immunosuppressive and pro-tolerant functions under specific conditions [151]. Macrophages activated in vitro with macrophage colony-stimulating factor (M-CSF) and IFN-γ can acquire immunosuppressive properties, forming regulatory macrophages (Mregs), a distinct subgroup that expresses markers different from M1 and M2 macrophages [143,152]. Mregs have unique functions and may inhibit polyclonal T cell proliferation and survival through an iNOS-dependent pathway, exerting immunomodulatory effects [153,154]. According to the study by Tran and Thomson (2020), in a mouse cardiac transplantation model, mice treated with Mregs exhibited significantly prolonged survival, indicating the importance of Mregs in post-transplant immunoregulation [155].
Mreg-based therapy has extended to clinical trials, demonstrating that patients receiving donor-derived Mreg treatment within 24 weeks post-kidney transplantation maintained stable kidney function within 6 years post-transplant [156]. This result indicates the potential application of Mregs in long-term graft survival and function stability post-transplantation [156]. The success of Mreg therapy is an important basis for developing new immunomodulatory strategies, potentially improving the long-term outcomes of organ transplantation and patient prognosis [157]. Some studies have noted that macrophage accumulation in transplanted organs is a significant feature of rejection, and colony stimulating factor 1 (CSF1) produced by neutrophils is crucial in regulating macrophage polarization and proliferation [158]. This process is especially important for inducing transplant tolerance, as CSF1 promotes the development of inhibitory Ly6Clow macrophages, which are associated with non-responsiveness to transplanted organs and observed in long-term surviving transplant recipients [159]. Therefore, neutrophil-derived CSF1 mediates immune tolerance to transplanted organs by affecting macrophage function, significantly affecting transplant medicine development [159].
Research indicates that macrophages play roles beyond mediating rejection in organ transplantation, promoting graft survival through various mechanisms [143]. Tissue-resident macrophage subpopulations expressing TIM4 and CD169 create a favorable immunosuppressive environment by inducing Foxp3+ Tregs and decomposing the inflammatory mediator ATP into anti-inflammatory adenosine, promoting graft survival [160]. This mechanism is significant for maintaining long-term graft survival, particularly in the early post-transplant stages when the immune response is most intense [160]. Moreover, M2 macrophages have demonstrated significant immunoregulatory capabilities by upregulating PD-L1 expression and responding to CTLA4-Ig treatment, extending the survival time for heart and islet transplants [161]. These macrophages reduced rejection by inhibiting T cell activity and promoting immune tolerance. For example, CTLA4-Ig treatment significantly prolonged graft survival time in a mouse cardiac transplantation model [162]. This effect is primarily due to CTLA4-Ig’s ability to inhibit the interaction between T cell CD28 and dendritic cell CD80/CD86, thereby reducing T cell priming and activation [163].
The intravenous injection of soluble fibronectin induced macrophage polarization to the M2 phenotype, significantly improving graft survival [142]. This method protects grafts from acute rejection damage by regulating local and systemic immune responses [142]. In a rat cardiac transplantation model, regenerated M2 macrophages after macrophage clearance alleviated chronic rejection by reducing T lymphocyte proliferation and altering IFN-γ, TNF-α, monocyte chemoattractant protein-1 (MCP-1), and IL-10 expression [164]. Bone marrow-derived suppressor cells (MDSCs) have potent immunosuppressive properties and are involved in tumor immune evasion [165]. Recent studies indicate that the macrophage receptor MerTK drives MDSC induction after recognizing and clearing donor apoptotic cells, crucial for islet allograft survival [166]. However, cytomegalovirus (CMV) infection inhibits MDSC induction, disrupting recipient immune tolerance and complicating post-transplant immunoregulation [167].
In summary, these studies reveal multiple mechanisms by which macrophages promote graft survival. Further research may identify more macrophage subpopulations and regulatory pathways that improve graft prognosis. This knowledge is expected to provide a basis for developing new immunomodulatory strategies, enhancing the long-term success rate of organ transplantation.
Role of macrophage polarization in cardiac transplantation
Macrophage polarization plays a pivotal role in regulating immune responses during cardiac transplantation. The M1 and M2 polarization states are reversible and regulated by microenvironmental factors, allowing for a dynamic balance that can shift depending on the needs of the immune response [148]. Additionally, the interaction between M1 and M2 macrophages affects the immune balance, regulating the balance between inflammatory responses and tissue repair [168]. Specifically, M2 macrophages play immunoregulatory and anti-inflammatory roles by inhibiting M1 macrophage activity [169]. Macrophage polarization is important in transplant rejection. M1 macrophages primarily act by releasing inflammatory mediators, presenting antigens, and activating T cells. Polarized M1 macrophages release large amounts of inflammatory mediators, such as TNF-α and IL-1β, initiating immune inflammation and promoting transplant rejection [170]. Additionally, M1 macrophages exacerbate rejection by enhancing antigen presentation capabilities and T cell activation [170].
In contrast, M2 macrophages primarily participate in anti-inflammatory and tissue repair functions and inhibit T cell activity [171]. Polarized M2 macrophages aid inflammatory response suppression, promote the repair and healing of transplanted tissue, and regulate rejection by inhibiting T cell activity, maintaining the immune balance [172]. M2 macrophages have immunosuppressive and regulatory roles in immune tolerance environments, suppressing excessive immune responses [143]. Furthermore, M2 macrophages release anti-inflammatory factors such as IL-10, regulating T cell activity and promoting the immune balance [173]. Regulating macrophage polarization involves multiple signaling pathways. Extracellular signals include the immunomodulatory cytokines (e.g., IL-4, IL-13) and inflammatory factors (e.g., IFN-γ) that induce macrophage polarization towards the M2 or M1 state [174]. Intracellular signaling involves transcription factors such as STAT6 and STAT1 playing critical roles in macrophage polarization, respectively promoting M2 and M1 formation [175]. Additionally, epigenetic modifications, such as histone deacetylation and methylation, also participate in regulating macrophage polarization [176]. These mechanisms collectively regulate macrophage polarization and are crucial in the transplant rejection and immune tolerance processes.
Understanding the mechanisms that govern macrophage polarization in the transplanted heart provides crucial insights into potential therapeutic strategies. By targeting pathways that favor M2 polarization and suppress M1 activity, it may be possible to enhance graft survival and reduce rejection. In this context, interventions aimed at shifting macrophages toward the M2 phenotype are being explored as promising approaches to improve cardiac transplantation outcomes.
Regulation mechanisms of macrophage polarization in cardiac transplantation
In cardiac transplantation, the macrophage polarization state critically affects graft survival and function. Macrophages can switch to different functional states based on environmental signals, primarily dividing into M1 or M2 macrophages [175]. M1 macrophages are activated upon encountering the recipient’s allogeneic antigens and secrete large amounts of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β, which are involved in acute rejection and tissue damage [177]. Contrastingly, M2 macrophages are activated under the stimulation of anti-inflammatory cytokines such as IL-4 and IL-13, secreting IL-10 and TGF-β, promoting tissue repair and anti-inflammatory responses, and aiding long-term graft survival. M1 macrophage numbers increase in the early stages of cardiac transplantation to respond to surgical injury and pathogens [178]. Over time, regulating macrophage polarization towards the M2 phenotype becomes crucial for maintaining graft stability. Modulating macrophage polarization significantly influences the graft immune environment and rejection response. For example, using certain immunosuppressants or biologics promotes M2 macrophage generation and inhibits M1 macrophage activation, thereby reducing rejection [179]. Additionally, the local microenvironment of the transplanted heart, such as hypoxic conditions and metabolic products, also affects macrophage polarization [178]. Hypoxia-inducible factor (HIF) promotes M2 macrophage polarization under hypoxic conditions, aiding anti-inflammatory and repair processes [180]. These results suggest that regulating macrophage polarization could provide new strategies for immune management post-cardiac transplantation (Fig. 3).
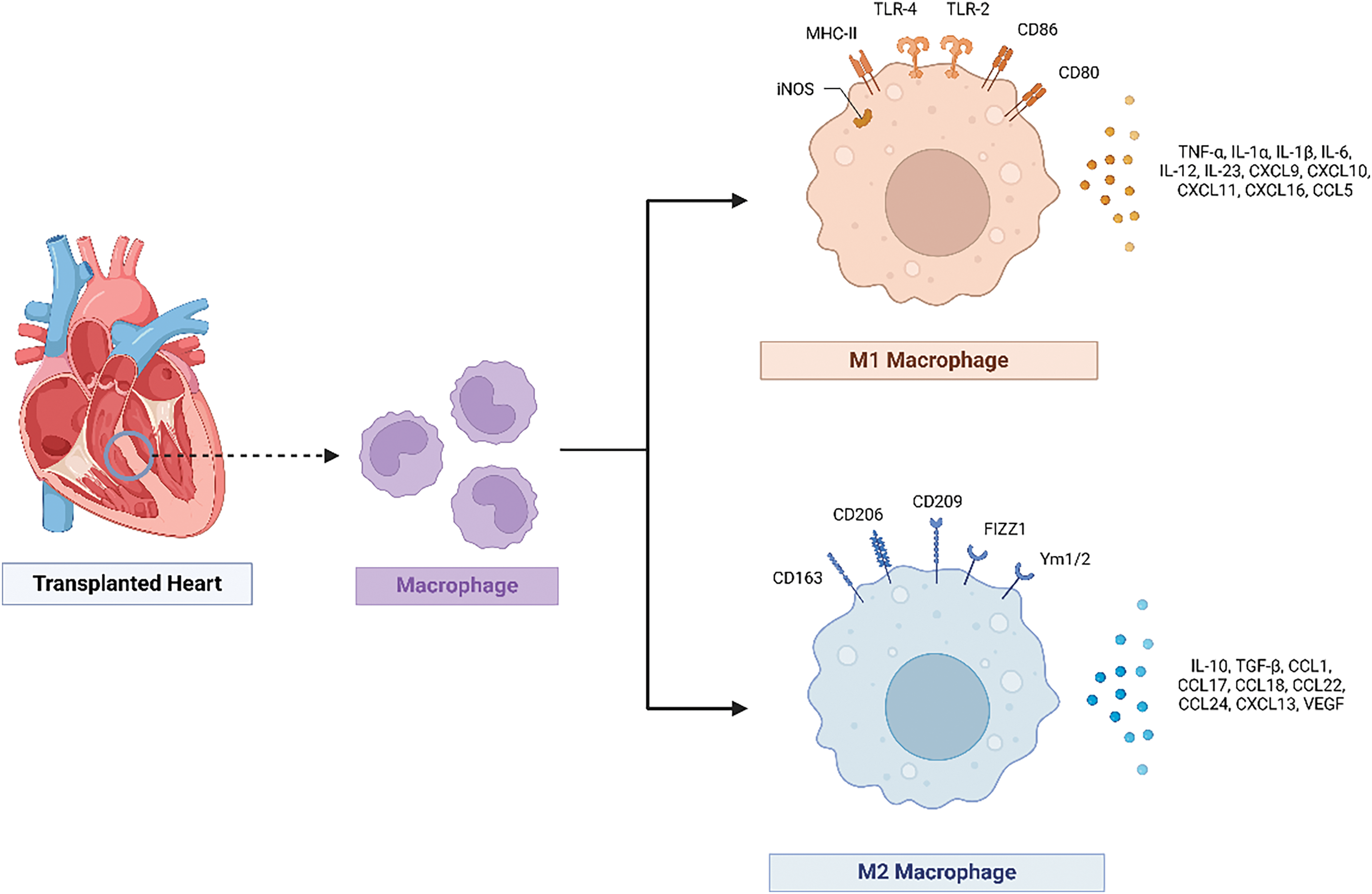
Figure 3: This figure shows the polarization of macrophages in cardiac transplantation. The polarization of macrophages in cardiac transplantation. Macrophages can polarize into M1 (proinflammatory) or M2 (anti-inflammatory) states. M1 macrophages secrete cytokines such as TNF-α, IL-6, and IL-1β, contributing to acute rejection. M2 macrophages release IL-10 and transforming growth factor-beta (TGF-β), promoting tissue repair and graft survival. Regulating macrophage polarization towards the M2 phenotype is crucial for long-term graft stability. Created in BioRender. Jingwei, J. (2024) BioRender.com/t82a854 (accessed 10 September 2024).
Interaction mechanisms between macrophage polarization and natural active drugs in cardiovascular MQC
Macrophage polarization and MQC are critical factors that closely influence graft survival and function in cardiac transplantation. Macrophages can polarize into M1 or M2 phenotypes based on environmental signals [174]. M1 macrophages primarily initiate inflammatory responses by producing pro-inflammatory cytokines and ROS, while M2 macrophages alleviate inflammation and promote graft tolerance by secreting anti-inflammatory cytokines and facilitating tissue repair [111]. In MQC, mitochondrial biogenesis, mitophagy, and the fusion and fission processes are crucial for maintaining mitochondrial health and function [181,182]. Peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1α) regulates mitochondrial biogenesis, the PINK1–Parkin pathway regulates mitophagy, and mitochondrial dynamic-related proteins such as MFN1 and DRP1 regulate mitochondrial fusion and fission [183,184].
Natural active drugs such as resveratrol and quercetin affect cardiovascular MQC and macrophage polarization through various mechanisms. For example, resveratrol enhances PGC-1α activity, promoting mitochondrial biogenesis and mitophagy by activating the sirtuin 1(SIRT1) and AMP-activated protein kinase (AMPK) pathways, thereby improving mitochondrial function [185,186]. Resveratrol reduces the M1 macrophage pro-inflammatory response and promotes the M2 macrophage anti-inflammatory response by inhibiting the NF-κB pathway [187,188]. Quercetin activates the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway and increases the expression of antioxidant enzymes, reducing ROS production and protecting mitochondria from oxidative damage [189,190]. Additionally, quercetin promotes M2 macrophage polarization and reduces inflammatory responses by regulating signaling pathways such as the NF-κB and STAT3 pathways [191].
The link between these processes is primarily reflected in the interaction between inflammation and MQC. ROS and pro-inflammatory cytokines produced by M1 macrophages directly damage the mitochondria, leading to mitochondrial dysfunction and apoptosis [192–195]. Healthy mitochondria influence macrophage polarization by regulating cellular energy metabolism and ROS production, maintaining the anti-inflammatory phenotype of M2 macrophages [182]. Combining natural active drugs can achieve dual regulation of macrophage polarization and MQC, reducing graft rejection in cardiac transplantation. Natural active drugs may regulate immune responses, potentially promote M2 macrophage polarization, and might improve mitochondrial biogenesis and mitophagy, which could enhance mitochondrial function and cellular energy metabolism. This comprehensive treatment strategy is expected to improve the success rate of cardiac transplantation and the long-term survival of grafts (Fig. 4).
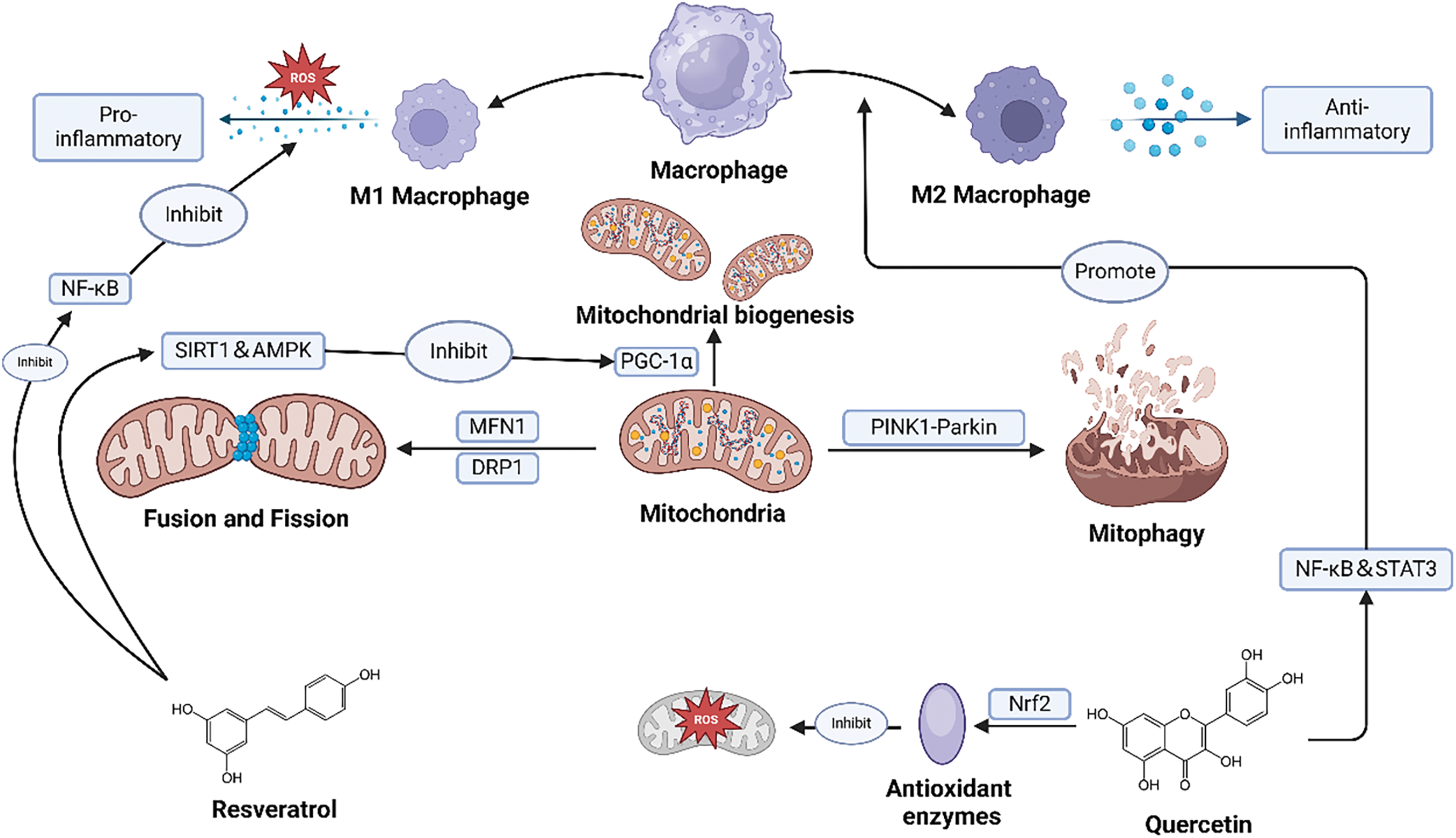
Figure 4: This figure illustrates the interactions between macrophage polarization, mitochondrial quality control, and natural active compounds in heart transplantation. Interactions between macrophage polarization, mitochondrial quality control, and natural active compounds in heart transplantation. M1 macrophages trigger inflammation by producing proinflammatory cytokines and reactive oxygen species (ROS), damaging mitochondria. In contrast, M2 macrophages decrease inflammation by secreting anti-inflammatory factors and promoting tissue repair. Mitochondrial quality control includes PGC-1α-regulated biogenesis, PINK1-Parkin pathway-regulated mitophagy, and MFN1/DRP1-regulated fusion and fission. Natural compounds like resveratrol and quercetin enhance mitochondrial function and promote M2 polarization. These combined effects help decrease graft rejection, improving the long-term survival and function of the graft. Created in BioRender. Jingwei, J. (2024) BioRender.com/r77s642 (accessed 10 September 2024).
Clinical applications of macrophage polarization in cardiac transplantation
The macrophage polarization state has significant clinical applications in managing cardiac transplantation. Regulating macrophage polarization significantly affects the occurrence of transplant rejection and long-term graft survival [196]. The M1 and M2 macrophage states play different roles at different post-transplantation stages. In the early stages of transplantation, macrophages primarily exhibit the M1 phenotype, secreting pro-inflammatory cytokines, and participating in acute rejection and tissue damage [197]. However, the key to long-term survival is polarizing macrophages to the M2 phenotype, thereby reducing inflammation, and promoting tissue repair and anti-inflammatory responses [198].
Immunosuppressants, such as tacrolimus and sirolimus, play a critical role in reducing the risk of acute and chronic rejection in heart transplant patients. While their precise mechanisms in modulating macrophage polarization remain under investigation, their overall immunosuppressive effects are crucial in maintaining graft survival and function [199]. Additionally, emerging technologies such as exosomes and microRNAs have demonstrated potential in regulating macrophage polarization [200]. For example, specific microRNAs delivered through exosomes post-cardiac transplantation induced M2 macrophage polarization, reducing inflammation and tissue damage [201]. Local microenvironment regulation is also a key factor. Hypoxic conditions promote M2 macrophage polarization by upregulating HIF, enhancing their anti-inflammatory and repair functions [202]. This regulatory mechanism demonstrated significant effects in experimental models and may be applied clinically to manage the immune system of heart transplant patients, improving graft long-term survival rates and function.
Research on macrophage polarization in cardiac transplantation reveals its critical role in transplant rejection and immune tolerance. The macrophage polarization state affects acute rejection long-term survival and chronic rejection. M1 macrophages play a primary role in the early stages of transplantation by releasing pro-inflammatory cytokines and enhancing antigen presentation to activate T cells, exacerbating rejection [197]. However, M2 macrophages gradually become crucial as the transplantation progresses, exerting anti-inflammatory and repair functions and promoting long-term graft survival [198].
Regulating macrophage polarization, particularly through emerging technologies such as immunosuppressants, exosomes, and microRNAs, can effectively reduce rejection and increase graft survival rates. Local microenvironments, such as hypoxic conditions and metabolic products, are also essential in macrophage polarization, providing new strategies for immune management post-cardiac transplantation. Despite significant progress in the regulatory mechanisms and clinical applications of macrophage polarization, many issues require further research. For example, how macrophage polarization can be precisely regulated and how these strategies can be applied in different clinical contexts remain critical research areas. Additionally, understanding the specific roles and mechanisms of macrophages in different organ transplantation types will provide a more comprehensive scientific basis for optimizing post-transplant immune management.
Moreover, future research should leverage emerging technologies such as single-cell analyses and advanced informatics, including artificial intelligence (AI) and machine learning (ML). Single-cell analyses provide the ability to dissect the heterogeneity of immune cells at an unprecedented resolution, which is critical for understanding the complex dynamics among various immune cell populations involved in cardiac transplantation. AI and ML techniques can be applied to manage and interpret the vast amounts of data generated, allowing for the identification of novel biomarkers and therapeutic targets. These technologies hold great potential to advance our understanding of immune regulation in transplantation, leading to more personalized and effective therapeutic strategies that improve graft survival and patient outcomes.
In summary, macrophage polarization research brings new hope to cardiac transplantation. An in-depth understanding and effective regulation of macrophage polarization states is expected to significantly improve transplant patients’ prognosis, increase transplantation success rates, and enhance long-term survival rates. Continued research in this field will provide new treatment methods and strategies for clinical practice, advancing the development of transplant medicine.
Acknowledgement: All figures were created with BioRender.com (accessed 10 September 2024).
Funding Statement: This research was supported by Guangxi Natural Science Foundation (2023GXNSFAA026128).
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Jingwei Jiang, Bo Jia; draft manuscript preparation: Jingwei Jiang, Bo Jia; literature search and reference organization: Chuan Wang, Chen Fang; critical analysis and interpretation: Yugu Li, Guoxing Ling; review and editing: Baoshi Zheng, Cheng Luo; supervision: Baoshi Zheng, Cheng Luo. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: No data was used for the research described in the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Brink JG, Hassoulas J. The first human heart transplant and further advances in cardiac transplantation at Groote Schuur Hospital and the University of Cape Town—with reference to: the operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town. Cardiovasc J Afr. 2009;20(1):31–5. [Google Scholar] [PubMed]
2. Hayes D, Harhay MO, Cherikh WS, Chambers DC, Khush KK, Hsich E, et al. The international thoracic organ transplant registry of the international society for heart and lung transplantation: twenty-third pediatric lung transplantation report—2020; focus on deceased donor characteristics. J Heart Lung Transplantation. 2020;39(10):1038–49. doi:10.1016/j.healun.2020.07.007. [Google Scholar] [PubMed] [CrossRef]
3. Wynn TA, Chawla A, Pollard JW, Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–55. doi:10.1038/nature12034. [Google Scholar] [PubMed] [CrossRef]
4. Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Investig. 2012;122(3):787–95. doi:10.1172/JCI59643. [Google Scholar] [PubMed] [CrossRef]
5. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili S-A, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–40. doi:10.1002/jcp.v233.9. [Google Scholar] [CrossRef]
6. Pour PA, Hosseinian S, Kheradvar A. Mitochondrial transplantation in cardiomyocytes: foundation, methods, and outcomes. Am J Physiol-Cell Physiol. 2021;321(3):C489–503. doi:10.1152/ajpcell.00152.2021. [Google Scholar] [PubMed] [CrossRef]
7. Atici AE, Crother TR, Noval Rivas M. Mitochondrial quality control in health and cardiovascular diseases. Front Cell Dev Biol. 2023;11:1290046. doi:10.3389/fcell.2023.1290046. [Google Scholar] [PubMed] [CrossRef]
8. Yang Y, Li T, Li Z, Liu N, Yan Y, Liu B. Role of mitophagy in cardiovascular disease. Aging Dis. 2020;11(2):419. doi:10.14336/AD.2019.0518. [Google Scholar] [PubMed] [CrossRef]
9. Marchi S, Guilbaud E, Tait SWG, Yamazaki T, Galluzzi L, Marchi S, et al. Mitochondrial control of inflammation. Nat Rev Immunol. 2022;23(3):159–73. doi:10.1038/s41577-022-00760-x. [Google Scholar] [PubMed] [CrossRef]
10. Chang X, Liu R, Li R, Peng Y, Zhu P, Zhou H. Molecular mechanisms of mitochondrial quality control in ischemic cardiomyopathy. Int J Biol Sci. 2023;19(2):426–48. doi:10.7150/ijbs.76223. [Google Scholar] [PubMed] [CrossRef]
11. Ajoolabady A, Chiong M, Lavandero S, Klionsky DJ, Ren J. Mitophagy in cardiovascular diseases: molecular mechanisms, pathogenesis, and treatment. Trends Mol Med. 2022;28(10):836–49. doi:10.1016/j.molmed.2022.06.007. [Google Scholar] [PubMed] [CrossRef]
12. Chen R, Zhang H, Tang B, Luo Y, Yang Y, Zhong X, et al. Macrophages in cardiovascular diseases: molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 2024;9(1):130. doi:10.1038/s41392-024-01840-1. [Google Scholar] [PubMed] [CrossRef]
13. Neefjes J, Jongsma MLM, Paul P, Bakke O, Neefjes J, Jongsma MLM, et al. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11(12):823–36. doi:10.1038/nri3084. [Google Scholar] [PubMed] [CrossRef]
14. Wieczorek M, Abualrous ET, Sticht J, Álvaro-Benito M, Stolzenberg S, Noé F, et al. Major Histocompatibility Complex (MHC) class I and MHC class II proteins: conformational plasticity in antigen presentation. Front Immunol. 2017;8:292. [Google Scholar] [PubMed]
15. Alexander SI, Clayton PA, Chadban SJ. Organ transplantation in Australia. Transplantation. 2017;101(5):891–2. doi:10.1097/TP.0000000000001621. [Google Scholar] [PubMed] [CrossRef]
16. Ville S, Poirier N, Blancho G, Vanhove B. Co-Stimulatory blockade of the CD28/CD80-86/CTLA-4 balance in transplantation: impact on memory T cells? Front Immunol. 2015;6:411. [Google Scholar] [PubMed]
17. Perrin S, Magill M. The inhibition of CD40/CD154 costimulatory signaling in the prevention of renal transplant rejection in nonhuman primates: a systematic review and meta analysis. Front Immunol. 2022;13:861471. doi:10.3389/fimmu.2022.861471. [Google Scholar] [PubMed] [CrossRef]
18. Chaintreuil P, Kerreneur E, Bourgoin M, Savy C, Favreau C, Robert G, et al. The generation, activation, and polarization of monocyte-derived macrophages in human malignancies. Front Immunol. 2023;14:1178337. doi:10.3389/fimmu.2023.1178337. [Google Scholar] [PubMed] [CrossRef]
19. Gui Z, Al Moussawy M, Sanders SM, Abou-Daya KI. Innate allorecognition in transplantation: ancient mechanisms with modern impact. Transplantation. 2024;108(7):1524–31. [Google Scholar] [PubMed]
20. Murata Y, Saito Y, Kotani T, Matozaki T. CD47-signal regulatory protein α signaling system and its application to cancer immunotherapy. Cancer Sci. 2018;109(8):2349–57. doi:10.1111/cas.2018.109.issue-8. [Google Scholar] [CrossRef]
21. Lin S-J, Huang J-Y, Le P-T, Lee C-T, Chang C-C, Yang Y-Y, et al. Expression of the AHPND toxins PirAvp and PirBvp is regulated by components of the vibrio parahaemolyticus Quorum Sensing (QS) system. Int J Mol Sci. 2022;23(5):2889. doi:10.3390/ijms23052889. [Google Scholar] [PubMed] [CrossRef]
22. Dai H, Lan P, Zhao D, Abou-Daya K, Liu W, Chen W, et al. PIRs mediate innate myeloid cell memory to nonself MHC molecules. Science. 2020;368(6495):1122–7. doi:10.1126/science.aax4040. [Google Scholar] [PubMed] [CrossRef]
23. Wen Q, Kong Y, Zhao H-Y, Zhang Y-Y, Han T-T, Wang Y, et al. G-CSF-induced macrophage polarization and mobilization may prevent acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019;54(9):1419–33. doi:10.1038/s41409-019-0449-9. [Google Scholar] [PubMed] [CrossRef]
24. Chih S, Chruscinski A, Ross HJ, Tinckam K, Butany J, Rao V. Antibody-mediated rejection: an evolving entity in heart transplantation. J Transplant. 2012;2012(1):210210. [Google Scholar] [PubMed]
25. Coutance G, Ouldamar S, Rouvier P, Saheb S, Suberbielle C, Bréchot N, et al. Late antibody-mediated rejection after heart transplantation: mortality, graft function, and fulminant cardiac allograft vasculopathy. J Heart Lung Transplant. 2015;34(8):1050–7. doi:10.1016/j.healun.2015.03.002. [Google Scholar] [PubMed] [CrossRef]
26. Hammond ME, Zollinger C, Vidic A, Snow G, Stehlik J, Alharethi R, et al. Donor causes of death and sex and their relationship to AMR and CAV in heart transplant recipients. J Clin Med. 2023;12(24):7629. doi:10.3390/jcm12247629. [Google Scholar] [PubMed] [CrossRef]
27. Yamamoto T, Watarai Y, Takeda A, Tsujita M, Hiramitsu T, Goto N, et al. De Novo Anti-HLA DSA characteristics and subclinical antibody-mediated kidney allograft injury. Transplantation. 2016;100(10):2194–202. doi:10.1097/TP.0000000000001012. [Google Scholar] [PubMed] [CrossRef]
28. Afzali B, Chapman E, Racapé M, Adam B, Bruneval P, Gil F, et al. Molecular assessment of microcirculation injury in formalin-fixed human cardiac allograft biopsies with antibody-mediated rejection. Am J Transplant. 2017;17(2):496–505. doi:10.1111/ajt.13956. [Google Scholar] [PubMed] [CrossRef]
29. Kong G, Chen Y, Liu Z, Wang Y, Li H, Guo C. Adenovirus-IL-10 relieves chronic rejection after mouse heart transplantation by inhibiting miR-155 and activating SOCS5. Int J Med Sci. 2023;20(2):172–85. doi:10.7150/ijms.77093. [Google Scholar] [PubMed] [CrossRef]
30. Pasca S, Jurj A, Petrushev B, Tomuleasa C, Matei D. MicroRNA-155 implication in M1 polarization and the impact in inflammatory diseases. Front Immunol. 2020;11:625. doi:10.3389/fimmu.2020.00625. [Google Scholar] [PubMed] [CrossRef]
31. Marco I, García JCL-A, Martín JG, Sánchez AS, Carmena MDG-C, Sierra EM, et al. De novo donor-specific antibodies after heart transplantation: a comprehensive guide for clinicians. J Clin Med. 2023;12(23):7474. doi:10.3390/jcm12237474. [Google Scholar] [PubMed] [CrossRef]
32. Murata K, Baldwin WM. Mechanisms of complement activation, C4d deposition, and their contribution to the pathogenesis of antibody-mediated rejection. Transplant Rev. 2009;23(3):139–50. doi:10.1016/j.trre.2009.02.005. [Google Scholar] [PubMed] [CrossRef]
33. Grafals M, Thurman JM. The role of complement in organ transplantation. Front Immunol. 2019;10:2380. doi:10.3389/fimmu.2019.02380. [Google Scholar] [PubMed] [CrossRef]
34. Louis K, Tabib T, Macedo C, Wang J, Cantalupo P, Chandran U, et al. High dimensional profiling of immune responses to kidney transplant reveals heterogeneous T helper 1 and B cell effectors associated with rejection. Am J Transplant. 2024. doi:10.1016/j.ajt.2024.10.009. [Google Scholar] [PubMed] [CrossRef]
35. Ma N, Wu W-B, Zhao X-Y, Xu L-P, Zhang X-H, Wang Y, et al. Targeting TFH cells is a novel approach for donor-specific antibody desensitization of allograft candidates: an in vitro and in vivo study. Haematologica. 2024;109(4):1233. [Google Scholar] [PubMed]
36. Zahavi D, AlDeghaither D, O’Connell A, Weiner LM. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antibody Therapeutics. 2018;1(1):7–12. doi:10.1093/abt/tby002. [Google Scholar] [PubMed] [CrossRef]
37. Delpire B, Van Loon E, Naesens M. The Role of Fc gamma receptors in antibody-mediated rejection of kidney transplants. Transpl Int. 2022;35:10465. doi:10.3389/ti.2022.10465. [Google Scholar] [PubMed] [CrossRef]
38. Mattina A, Baidal D, Marfil-Garza B, Occhipinti M, Sordi V. Editorial: diabetes, transplantation and regenerative medicine. Front Clin Diabetes Healthc. 2024;5:1388904. doi:10.3389/fcdhc.2024.1388904. [Google Scholar] [PubMed] [CrossRef]
39. Zhou B, Zhang M, Ma H, Wang Y, Qiu J, Liu Y, et al. Distinct palmitoylation of Foxp3 regulates the function of regulatory T cells via palmitoyltransferases. Cell Mol Immunol. 2024;21(7):1–3. doi:10.1038/s41423-024-01166-6. [Google Scholar] [PubMed] [CrossRef]
40. Li C, Jiang P, Wei S, Xu X, Wang J, Li C, et al. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19(1):1–23. doi:10.1186/s12943-019-1085-0. [Google Scholar] [CrossRef]
41. Sojka DK, Huang Y-H, Fowell DJ. Mechanisms of regulatory T-cell suppression—a diverse arsenal for a moving target. Immunology. 2008;124(1):13–22. doi:10.1111/j.1365-2567.2008.02813.x. [Google Scholar] [PubMed] [CrossRef]
42. Kim J-H, Kim BS, Lee S-K. Regulatory T cells in tumor microenvironment and approach for anticancer immunotherapy. Immune Netw. 2020;20(1):e4. doi:10.4110/in.2020.20.e4. [Google Scholar] [PubMed] [CrossRef]
43. Charbonnier L-M, Vokaer B, Lemaître PH, Field KA, Leo O, Moine AL. CTLA4-Ig restores rejection of MHC Class-II mismatched allografts by disabling IL-2-expanded regulatory T cells. Am J Transplant. 2012;12(9):2313–21. doi:10.1111/j.1600-6143.2012.04184.x. [Google Scholar] [PubMed] [CrossRef]
44. Carbone F, Colamatteo A, La Rocca C, Lepore MT, Russo C, De Rosa G, et al. Metabolic plasticity of regulatory T cells in health and autoimmunity. J Immunol. 2024;212(12):1859–66. doi:10.4049/jimmunol.2400079. [Google Scholar] [PubMed] [CrossRef]
45. Jiang Z, Zhu H, Wang P, Que W, Zhong L, Li X-K, et al. Different subpopulations of regulatory T cells in human autoimmune disease, transplantation, and tumor immunity. MedComm. 2022;3(2):e137. doi:10.1002/mco2.137. [Google Scholar] [PubMed] [CrossRef]
46. Thome AD, Atassi F, Wang J, Faridar A, Zhao W, Thonhoff JR, et al. Ex vivo expansion of dysfunctional regulatory T lymphocytes restores suppressive function in Parkinson’s disease. npj Parkinson’s Dis. 2021;7(1):41. doi:10.1038/s41531-021-00188-5. [Google Scholar] [PubMed] [CrossRef]
47. Nykänen AI, Keshavjee S, Liu M. Creating superior lungs for transplantation with next-generation gene therapy during ex vivo lung perfusion. J Heart Lung Transplant. 2024;43(5):838–48. doi:10.1016/j.healun.2024.01.016. [Google Scholar] [PubMed] [CrossRef]
48. Kim C-H. Induction of Xenograft Tolerance and Chimerism as an Alternative Preve. In: Glycoimmunology in xenotransplantation. Singapore: Springer; 2024. [Google Scholar]
49. Wang X, Xie M, Li T, Shi J, Wu M, Zhang S, et al. Comparative ability of various immunosuppressants as adjuvants on the activity of T1D vaccine. Vaccines. 2024;12(10):1117. doi:10.3390/vaccines12101117. [Google Scholar] [PubMed] [CrossRef]
50. Wu S-L, Pan C-E. Tolerance and chimerism and allogeneic bone marrow/stem cell transplantation in liver transplantation. World J Gastroenterol. 2013;19(36):5981. doi:10.3748/wjg.v19.i36.5981. [Google Scholar] [PubMed] [CrossRef]
51. Koporc Z, Bigenzahn S, Blaha P, Fariborz E, Selzer E, Sykes M, et al. Induction of mixed chimerism through transplantation of CD45-congenic mobilized peripheral blood stem cells after nonmyeloablative irradiation. Biol Blood Marrow Transplant. 2006;12(3):284–92. doi:10.1016/j.bbmt.2005.11.011. [Google Scholar] [PubMed] [CrossRef]
52. Mathew JM, Leventhal JR, Miller J. Microchimerism in promoting graft acceptance in clinical transplantation. Curr Opin Organ Transplant. 2011;16(4):345–52. doi:10.1097/MOT.0b013e3283489a42. [Google Scholar] [PubMed] [CrossRef]
53. Huelsboemer L, Kauke-Navarro M, Reuter S, Stoegner VA, Feldmann J, Hirsch T, et al. Tolerance induction in vascularized composite allotransplantation—a brief review of preclinical models. Transpl Int. 2023;36:10955. doi:10.3389/ti.2023.10955. [Google Scholar] [PubMed] [CrossRef]
54. Cui D, Zhang Y, Tan C, Liu Z. Chailing decoction generated Foxp3+ regulatory T cells and suppressed activity of mTOR signaling pathway in a murine cardiac transplantation model. Med Discov. 2024;3(5):1157. doi:10.52768/2993-1142/1157. [Google Scholar] [CrossRef]
55. Zhou X, Xu Q, Li W, Dong N, Stomberski C, Narla G, et al. Protein Phosphatase 2A activation promotes heart transplant acceptance in mice. Transplantation. 2024;108(3):e36–48. [Google Scholar] [PubMed]
56. Sanders JM, Jeyamogan S, Mathew JM, Leventhal JR. Foxp3+ regulatory T cell therapy for tolerance in autoimmunity and solid organ transplantation. Front Immunol. 2022;13:1055466. doi:10.3389/fimmu.2022.1055466. [Google Scholar] [PubMed] [CrossRef]
57. Mashayekhi K, Khazaie K, Faubion WA, Kim GB. Biomaterial-enhanced treg cell immunotherapy: a promising approach for transplant medicine and autoimmune disease treatment. Bioact Mater. 2024;37:269–98. [Google Scholar] [PubMed]
58. Ferreira LMR, Muller YD, Bluestone JA, Tang Q, Ferreira LMR, Muller YD, et al. Next-generation regulatory T cell therapy. Nat Rev Drug Discov. 2019;18(10):749–69. doi:10.1038/s41573-019-0041-4. [Google Scholar] [PubMed] [CrossRef]
59. Aiyengar A, Romano M, Burch M, Lombardi G, Fanelli G. The potential of autologous regulatory T cell (Treg) therapy to prevent Cardiac Allograft Vasculopathy (CAV) in paediatric heart transplant recipients. Front Immunol. 2024;15:1444924. doi:10.3389/fimmu.2024.1444924. [Google Scholar] [PubMed] [CrossRef]
60. Lobo CS, Mendes MIP, Pereira DA, Gomes-da-Silva LC, Arnaut LG, Lobo CS, et al. Photodynamic therapy changes tumour immunogenicity and promotes immune-checkpoint blockade response, particularly when combined with micromechanical priming. Sci Rep. 2023;13(1):11667. doi:10.1038/s41598-023-38862-8. [Google Scholar] [PubMed] [CrossRef]
61. Riella LV, Sayegh MH. T-cell co-stimulatory blockade in transplantation: two steps forward one step back!. Expert Opin Biol Ther. 2013;13(11):1557–68. doi:10.1517/14712598.2013.845661. [Google Scholar] [PubMed] [CrossRef]
62. Krummey SM, Hartigan CR, Liu D, Ford ML. CD28-Dependent CTLA-4 expression fine-tunes the activation of human Th17 cells. iScience. 2020;23(4):100912. doi:10.1016/j.isci.2020.100912. [Google Scholar] [PubMed] [CrossRef]
63. Schelker RC, Fioravanti J, Mastrogiovanni F, Baldwin JG, Rana N, Li P, et al. LIM-domain-only 4 (LMO4) enhances CD8+ T-cell stemness and tumor rejection by boosting IL-21-STAT3 signaling. Signal Transduct Target Ther. 2024;9(1):199. doi:10.1038/s41392-024-01915-z. [Google Scholar] [PubMed] [CrossRef]
64. Simeonov DR, Marson A, Simeonov DR, Marson A. CRISPR-based tools in immunity. Ann Rev Immunol. 2019;37(1):571–97. doi:10.1146/annurev-immunol-042718-041522. [Google Scholar] [PubMed] [CrossRef]
65. Kim Y-C, Sohn K-H, Kang H-R. Gut microbiota dysbiosis and its impact on asthma and other lung diseases: potential therapeutic approaches. Korean J Intern Med. 2024;39(5):746–58. doi:10.3904/kjim.2023.451. [Google Scholar] [PubMed] [CrossRef]
66. Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F, Di Vincenzo F, et al. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2023;19(2):275–93. doi:10.1007/s11739-023-03374-w. [Google Scholar] [PubMed] [CrossRef]
67. Kazemian N, Ramezankhani M, Sehgal A, Khalid FM, Kalkhoran AHZ, Narayan A, et al. The trans-kingdom battle between donor and recipient gut microbiome influences fecal microbiota transplantation outcome. Sci Rep. 2020;10(1):18349. doi:10.1038/s41598-020-75162-x. [Google Scholar] [PubMed] [CrossRef]
68. Handelsman S, Overbey J, Chen K, Lee J, Haj D, Li Y, et al. PD-L1’s role in preventing alloreactive T cell responses following hematopoietic and organ transplant. Cells. 2023;12(12):1609. doi:10.3390/cells12121609. [Google Scholar] [PubMed] [CrossRef]
69. DeWolf S, Sykes M. Alloimmune T cells in transplantation. J Clin Investig. 2017;127(7):2473–81. doi:10.1172/JCI90595. [Google Scholar] [PubMed] [CrossRef]
70. Marino J, Paster J, Benichou G. Allorecognition by T lymphocytes and allograft rejection. Front Immunol. 2016;7:582. [Google Scholar] [PubMed]
71. Kang S-S, Isser A, Schneck J. 1215 Using nanoparticles as artificial antigen presenting cells to activate human CD4 T cells for immunotherapy. J Immunother Cancer. 2023;11(Suppl 1):6086. doi:10.1136/jitc-2023-SITC2023.1215. [Google Scholar] [CrossRef]
72. Tay RE, Richardson EK, Toh HC, Tay RE, Richardson EK, Toh HC. Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Ther. 2020;28(1):5–17. doi:10.1038/s41417-020-0183-x. [Google Scholar] [PubMed] [CrossRef]
73. Süsal C, Döhler B, Ruhenstroth A, Morath C, Slavcev A, Fehr T, et al. Donor-specific antibodies require preactivated immune system to harm renal transplant. eBioMedicine. 2016;9:366–71. doi:10.1016/j.ebiom.2016.06.006. [Google Scholar] [PubMed] [CrossRef]
74. Janeway C, Travers P, Walport M, Shlomchik M. Immunobiology: the immune system in health and disease. New York, NY, USA: Garland Science; 2001. [Google Scholar]
75. Xie CB, Jane-Wit D, Pober JS. Complement Membrane Attack Complex: new roles, mechanisms of action, and therapeutic targets. Am J Pathol. 2020;190(6):1138–50. doi:10.1016/j.ajpath.2020.02.006. [Google Scholar] [PubMed] [CrossRef]
76. Diebold M, Farkash EA, Barnes J, Regele H, Kozakowski N, Schatzl M, et al. Natural killer cell presence in antibody-mediated rejection. Transpl Int. 2024;37:13209. doi:10.3389/ti.2024.13209. [Google Scholar] [PubMed] [CrossRef]
77. Liu K, Han B. Role of immune cells in the pathogenesis of myocarditis. J Leukocyte Biol. 2023;115(2):253–75. doi:10.1093/jleuko/qiad143. [Google Scholar] [PubMed] [CrossRef]
78. Li G, Chen J, Wang Z, Kang S, Liu Y, Ai X, et al. CD47 blockade reduces ischemia/reperfusion injury in murine heart transplantation and improves donor heart preservation. Int Immunopharmacol. 2024;132:111953. doi:10.1016/j.intimp.2024.111953. [Google Scholar] [PubMed] [CrossRef]
79. Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, et al. National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant. 2004;4(7):1033–41. doi:10.1111/j.1600-6143.2004.00500.x. [Google Scholar] [PubMed] [CrossRef]
80. Cao J, Ling Q. Liver transplantation and immune tolerance: setting the stage for optimal post-transplant status. iLIVER. 2024;3(2):100097. doi:10.1016/j.iliver.2024.100097. [Google Scholar] [CrossRef]
81. Hoeffel G, Ginhoux F. Ontogeny of tissue-resident macrophages. Front Immunol. 2015;6:486. [Google Scholar] [PubMed]
82. Hume DA. Differentiation and heterogeneity in the mononuclear phagocyte system. Mucosal Immunol. 2008;1(6):432–41. doi:10.1038/mi.2008.36. [Google Scholar] [PubMed] [CrossRef]
83. Mosser DM, Hamidzadeh K, Goncalves R. Macrophages and the maintenance of homeostasis. Cell Mol Immunol. 2021;18(3):579–87. doi:10.1038/s41423-020-00541-3. [Google Scholar] [PubMed] [CrossRef]
84. Freitas-Lopes MA, Mafra K, David BA, Carvalho-Gontijo R, Menezes GB. Differential location and distribution of hepatic immune cells. Cells. 2017;6(4):48. doi:10.3390/cells6040048. [Google Scholar] [PubMed] [CrossRef]
85. Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–62. doi:10.1016/j.immuni.2016.02.015. [Google Scholar] [PubMed] [CrossRef]
86. Luque-Martin R, Mander PK, Leenen PJM, Winther MPJ. Classic and new mediators for in vitro modelling of human macrophages. J Leukoc Biol. 2020;109(3):549–60. [Google Scholar] [PubMed]
87. Lavin Y, Merad M. Macrophages: gatekeepers of tissue integrity. Cancer Immunol Res. 2013;1(4):201–9. doi:10.1158/2326-6066.CIR-13-0117. [Google Scholar] [PubMed] [CrossRef]
88. Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. 2019;20(1):29–39. doi:10.1038/s41590-018-0272-2. [Google Scholar] [PubMed] [CrossRef]
89. Kopecky BJ, Dun H, Amrute JM, Lin C-Y, Bredemeyer AL, Terada Y, et al. Donor macrophages modulate rejection after heart transplantation. Circulation. 2022;146(8):623–38. doi:10.1161/CIRCULATIONAHA.121.057400. [Google Scholar] [PubMed] [CrossRef]
90. Xia T, Fu S, Yang R, Yang K, Lei W, Yang Y, et al. Advances in the study of macrophage polarization in inflammatory immune skin diseases. J Inflamm. 2023;20(1):33. doi:10.1186/s12950-023-00360-z. [Google Scholar] [PubMed] [CrossRef]
91. Du Q, Dickinson A, Nakuleswaran P, Maghami S, Alagoda S, Hook AL, et al. Targeting Macrophage polarization for reinstating homeostasis following tissue damage. Int J Mol Sci. 2024;25(13):7278. doi:10.3390/ijms25137278. [Google Scholar] [PubMed] [CrossRef]
92. Tarique AA, Logan J, Thomas E, Holt PG, Sly PD, Fantino E. Functional, and plasticity features of classical and alternatively activated human macrophages. Am J Respir Cell Mol Biol. 2015;53(5):676–88. doi:10.1165/rcmb.2015-0012OC. [Google Scholar] [PubMed] [CrossRef]
93. Liu X, Zhang J, Zeigler AC, Nelson AR, Lindsey ML, Saucerman JJ. Network analysis reveals a distinct axis of macrophage activation in response to conflicting inflammatory cues. J Immunol. 2021;206(4):883–91. doi:10.4049/jimmunol.1901444. [Google Scholar] [PubMed] [CrossRef]
94. Alvarez MM, Liu JC, Trujillo-de Santiago G, Cha B-H, Vishwakarma A, Ghaemmaghami AM, et al. Delivery strategies to control inflammatory response: modulating M1-M2 polarization in tissue engineering applications. J Control Release. 2016;240:349–63. doi:10.1016/j.jconrel.2016.01.026. [Google Scholar] [PubMed] [CrossRef]
95. Xue J-D, Gao J, Tang A-F, Feng C. Shaping the immune landscape: multidimensional environmental stimuli refine macrophage polarization and foster revolutionary approaches in tissue regeneration. Heliyon. 2024;10(17):e37192. doi:10.1016/j.heliyon.2024.e37192. [Google Scholar] [PubMed] [CrossRef]
96. Dick SA, Wong A, Hamidzada H, Nejat S, Nechanitzky R, Vohra S, et al. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci Immunol. 2022;7(67):eabf7777. doi:10.1126/sciimmunol.abf7777. [Google Scholar] [PubMed] [CrossRef]
97. Naler LB, Hsieh Y-P, Geng S, Zhou Z, Li L, Lu C. Epigenomic and transcriptomic analyses reveal differences between low-grade inflammation and severe exhaustion in LPS-challenged murine monocytes. Commun Biol. 2022;5(1):102. doi:10.1038/s42003-022-03035-2. [Google Scholar] [PubMed] [CrossRef]
98. Geng S, Zhang Y, Yi Z, Lu R, Li L. Resolving monocytes generated through TRAM deletion attenuate atherosclerosis. JCI Insight. 2021;6(20):e149651. doi:10.1172/jci.insight.149651. [Google Scholar] [PubMed] [CrossRef]
99. Pradhan K, Yi Z, Geng S, Li L. Development of exhausted memory monocytes and underlying mechanisms. Front Immunol. 2021;12:778830. doi:10.3389/fimmu.2021.778830. [Google Scholar] [PubMed] [CrossRef]
100. Xie Y, Zhou X, Zhang J, Yu H, Song Z. Immunomodulatory responses of differentially polarized macrophages to fungal infections. Int Immunopharmacol. 2022;111:109089. doi:10.1016/j.intimp.2022.109089. [Google Scholar] [PubMed] [CrossRef]
101. Giri PS, Rath SN. Macrophage polarization dynamics in biomaterials: implications for in vitro wound healing. ACS Appl Bio Mater. 2024;7(4):2413–22. doi:10.1021/acsabm.4c00066. [Google Scholar] [PubMed] [CrossRef]
102. Ji Z, Jiang X, Li Y, Song J, Chai C, Lu X. Neural stem cells induce M2 polarization of macrophages through the upregulation of interleukin-4. Exp Ther Med. 2020;20(6):148. doi:10.3892/etm. [Google Scholar] [CrossRef]
103. Pérez S, Rius-Pérez S. Macrophage polarization and reprogramming in acute inflammation: a redox perspective. Antioxidants. 2022;11(7):1394. doi:10.3390/antiox11071394. [Google Scholar] [PubMed] [CrossRef]
104. Poltavets AS, Vishnyakova PA, Elchaninov AV, Sukhikh GT, Fatkhudinov TK. Macrophage modification strategies for efficient cell therapy. Cells. 2020;9(6):1535. doi:10.3390/cells9061535. [Google Scholar] [PubMed] [CrossRef]
105. Chávez-Galán L, Olleros ML, Vesin D, Garcia I. Much more than M1 and M2 Macrophages, There are also CD169+ and TCR+ Macrophages. Front Immunol. 2015;6:263. [Google Scholar]
106. Radandish M, Khalilian P, Esmaeil N. The role of distinct subsets of macrophages in the pathogenesis of MS and the impact of different therapeutic agents on these populations. Front Immunol. 2021;12:667705. doi:10.3389/fimmu.2021.667705. [Google Scholar] [PubMed] [CrossRef]
107. Wang LX, Zhang SX, Wu HJ, Rong XI, Guo J. M2b macrophage polarization and its roles in diseases. Journal of Leukocyte Biology. 2018;106(2):345–58. [Google Scholar] [PubMed]
108. Gharavi AT, Hanjani NA, Movahed E, Doroudian M. The role of macrophage subtypes and exosomes in immunomodulation. Cell Mol Biol Lett. 2022;27(1):83. doi:10.1186/s11658-022-00384-y. [Google Scholar] [PubMed] [CrossRef]
109. Chang-Hoon L, Eun Young C. Macrophages and Inflammation. J Rheum Dis. 2018;25(1):11–8. doi:10.4078/jrd.2018.25.1.11. [Google Scholar] [CrossRef]
110. Tan H-Y, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev. 2016;2016(1):2795090. doi:10.1155/omcl.v2016.1. [Google Scholar] [CrossRef]
111. Chen X, Tang J, Shuai W, Meng J, Feng J, Han Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm Res. 2020;69(9):883–95. doi:10.1007/s00011-020-01378-2. [Google Scholar] [PubMed] [CrossRef]
112. Yang X, Chang Y, Wei W. Emerging role of targeting macrophages in rheumatoid arthritis: focus on polarization, metabolism and apoptosis. Cell Prolif. 2020;53(7):e12854. doi:10.1111/cpr.v53.7. [Google Scholar] [CrossRef]
113. Yao Q, Liu J, Zhang Z, Li F, Zhang C, Lai B, et al. Peroxisome proliferator-activated receptor γ (PPARγ) induces the gene expression of integrin αVβ5 to promote macrophage M2 polarization. J Biol Chem. 2018;293(43):16572–82. doi:10.1074/jbc.RA118.003161. [Google Scholar] [PubMed] [CrossRef]
114. Li Y, Wang R, Gao Q. The roles and targeting of tumor-associated macrophages. Front Biosci. 2023;28(9):207. doi:10.31083/j.fbl2809207. [Google Scholar] [PubMed] [CrossRef]
115. Toki D, Zhang W, Hor KLM, Liuwantara D, Alexander SI, Yi Z, et al. The role of macrophages in the development of human renal allograft fibrosis in the first year after transplantation. Am J Transplant. 2014;14(9):2126–36. doi:10.1111/ajt.12803. [Google Scholar] [PubMed] [CrossRef]
116. Zhang X, Hasani-Sadrabadi MM, Dashtimighadam E, Fahimipour F, Shokeen B, Bezouglaia O, et al. Dissolvable microneedle patch enables local delivery of immunomodulatory microparticles containing bifunctional molecules for periodontal tissue regeneration. Med-X. 2024;2(1):11. doi:10.1007/s44258-024-00023-5. [Google Scholar] [CrossRef]
117. Zuo W, Sun R, Ji Z, Ma G. Macrophage-driven cardiac inflammation and healing: insights from homeostasis and myocardial infarction. Cell Mol Biol Lett. 2023;28(1):81. doi:10.1186/s11658-023-00491-4. [Google Scholar] [PubMed] [CrossRef]
118. Zhao C, Medeiros TX, Sové RJ, Annex BH, Popel AS. A data-driven computational model enables integrative and mechanistic characterization of dynamic macrophage polarization. iScience. 2021;24(2):102112. doi:10.1016/j.isci.2021.102112. [Google Scholar] [PubMed] [CrossRef]
119. Liu Y, Kloc M, Li XC. Macrophages as effectors of acute and chronic allograft injury. Curr Transplant Rep. 2016;3(4):303–12. doi:10.1007/s40472-016-0130-9. [Google Scholar] [PubMed] [CrossRef]
120. O’Brien EM, Spiller KL. Pro-inflammatory polarization primes Macrophages to transition into a distinct M2-like phenotype in response to IL-4. J Leukocyte Biol. 2021;111(5):989–1000. [Google Scholar] [PubMed]
121. Vicenzi S, Tran T, Avsharian L, Hartman J, Rapp A, Crews L. Tuning the innate immune multiple myeloma microenvironment by modulating IRF4. Blood. 2023;142:6604. doi:10.1182/blood-2023-187814. [Google Scholar] [CrossRef]
122. Mamilos A, Winter L, Schmitt VH, Barsch F, Grevenstein D, Wagner W, et al. Macrophages: from simple phagocyte to an integrative regulatory cell for inflammation and tissue regeneration—a review of the literature. Cells. 2023;12(2):276. doi:10.3390/cells12020276. [Google Scholar] [PubMed] [CrossRef]
123. Deswal B, Bagchi U, Santra MK, Garg M, Kapoor S. Inhibition of STAT3 by 2-methoxyestradiol suppresses M2 polarization and protumoral functions of macrophages in breast cancer. BMC Cancer. 2024;24(1):1129. doi:10.1186/s12885-024-12871-w. [Google Scholar] [PubMed] [CrossRef]
124. Chen C, Wang J, Liu C, Hu J. Cardiac resident macrophages: key regulatory mediators in the aftermath of myocardial infarction. Front Immunol. 2023;14:78. doi:10.3389/fimmu.2023.1207100. [Google Scholar] [PubMed] [CrossRef]
125. Lafuse WP, Wozniak DJ, Rajaram MVS. Role of cardiac macrophages on cardiac inflammation, Fibrosis and Tissue Repair. Cells. 2021;10(1):51. [Google Scholar]
126. Li Q, Lan P. Activation of immune signals during organ transplantation. Signal Transduct Target Ther. 2023;8(1):110. doi:10.1038/s41392-023-01377-9. [Google Scholar] [PubMed] [CrossRef]
127. Famulski KS, Kayser D, Einecke G, Allanach K, Badr D, Venner J, et al. Alternative macrophage activation‐associated transcripts in T-cell-mediated rejection of mouse kidney allografts. Am J Transplant. 2010;10(3):490–7. doi:10.1111/j.1600-6143.2009.02983.x. [Google Scholar] [PubMed] [CrossRef]
128. Ronca V, Wootton G, Milani C, Cain O. The immunological basis of liver allograft rejection. Front Immunol. 2020;11:2155. doi:10.3389/fimmu.2020.02155. [Google Scholar] [PubMed] [CrossRef]
129. Hurskainen M, Ainasoja O, Lemström KB. Failing heart transplants and rejection—a cellular perspective. J Cardiovasc Dev Dis. 2021;8(12):180. [Google Scholar] [PubMed]
130. Ordikhani F, Pothula V, Sanchez-Tarjuelo R, Jordan S, Ochando J. Macrophages in organ transplantation. Front Immunol. 2020;11:582939. doi:10.3389/fimmu.2020.582939. [Google Scholar] [PubMed] [CrossRef]
131. Baldwin WMIII, Larsen CP, Fairchild RL. Innate immune responses to transplants: a significant variable with cadaver donors. Immunity. 2001;14(4):369–76. doi:10.1016/S1074-7613(01)00117-0. [Google Scholar] [PubMed] [CrossRef]
132. Xu XC, Goodman J, Sasaki H, Lowell J, Mohanakumar T. Activation of natural killer cells and macrophages by porcine endothelial cells augments specific T-cell xenoresponse. Am J Transplant. 2002;2(4):314–22. doi:10.1034/j.1600-6143.2002.20405.x. [Google Scholar] [PubMed] [CrossRef]
133. Kloc M, Uosef A, Kubiak JZ, Ghobrial RM. Macrophage proinflammatory responses to microorganisms and transplanted organs. Int J Mol Sci. 2020;21(24):9669. doi:10.3390/ijms21249669. [Google Scholar] [PubMed] [CrossRef]
134. Terry AQ, Kojima H, Sosa RA, Kaldas FM, Chin JL, Zheng Y, et al. Disulfide-HMGB1 signals through TLR4 and TLR9 to induce inflammatory macrophages capable of innate-adaptive crosstalk in human liver transplantation. Am J Transplant. 2023;23(12):1858–71. doi:10.1016/j.ajt.2023.08.002. [Google Scholar] [PubMed] [CrossRef]
135. Dholakia S, De Vlaminck I, Khush KK. Adding insult on injury: immunogenic role for donor-derived cell-free DNA? Transplantation. 2020;104(11):2266–71. doi:10.1097/TP.0000000000003240. [Google Scholar] [PubMed] [CrossRef]
136. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [Google Scholar] [PubMed]
137. Xu M, Chen Z, Chen K, Ma D, Chen L, DiPietro LA. Phagocytosis of apoptotic endothelial cells reprograms macrophages in skin wounds. J Immunol Regener Med. 2021;12:100038. doi:10.1016/j.regen.2021.100038. [Google Scholar] [PubMed] [CrossRef]
138. Simonson MS, Robinson AV, Schulak JA, Hricik DE. Inhibition of endothelin-1 improves survival and vasculopathy in rat cardiac transplants treated with cyclosporine. Transplantation. 2002;73(7):1054–9. doi:10.1097/00007890-200204150-00007. [Google Scholar] [PubMed] [CrossRef]
139. Tang Y, Xiao Z, Pan L, Zhuang D, Cho K-S, Robert K, et al. Therapeutic targeting of retinal immune microenvironment with CSF-1 receptor antibody promotes visual function recovery after ischemic optic neuropathy. Front Immunol. 2020;11:585918. doi:10.3389/fimmu.2020.585918. [Google Scholar] [PubMed] [CrossRef]
140. Sutanto H, Maimunah U, Fetarayani D. Donor-specific antibodies and their impact on antibody-mediated rejection post-liver transplantation: a comprehensive review. J Liver Transplant. 2024;14:100214. doi:10.1016/j.liver.2024.100214. [Google Scholar] [CrossRef]
141. Kitchens WH, Chase CM, Uehara S, Cornell LD, Colvin RB, Russell PS, et al. Macrophage depletion suppresses cardiac allograft vasculopathy in mice. Am J Transplant. 2007;7(12):2675–82. doi:10.1111/j.1600-6143.2007.01997.x. [Google Scholar] [PubMed] [CrossRef]
142. Gao C, Wang X, Lu J, Li Z, Jia H, Chen M, et al. Mesenchymal stem cells transfected with sFgl2 inhibit the acute rejection of heart transplantation in mice by regulating macrophage activation. Stem Cell Res Ther. 2020;11(1):241. doi:10.1186/s13287-020-01752-1. [Google Scholar] [PubMed] [CrossRef]
143. Tan L, Guo Y, Feng C, Hou Y, Xie X, Zhao Y. The dual regulatory roles of macrophages in acute allogeneic organ graft rejection. Engineering. 2022;10(1):21–9. doi:10.1016/j.eng.2021.10.015. [Google Scholar] [CrossRef]
144. Koglin J, Glysing-Jensen T, Gadiraju S, Russell ME. Attenuated cardiac allograft vasculopathy in mice with targeted deletion of the transcription factor STAT4. Circulation. 2000;101(9):1034–9. doi:10.1161/01.CIR.101.9.1034. [Google Scholar] [PubMed] [CrossRef]
145. Sektioglu IM, Carretero R, Bender N, Bogdan C, Garbi N, Umansky V, et al. Macrophage-derived nitric oxide initiates T-cell diapedesis and tumor rejection. OncoImmunology. 2016;5(10):e1204506. doi:10.1080/2162402X.2016.1204506. [Google Scholar] [PubMed] [CrossRef]
146. Saraiva M, Vieira P, O’Garra A. Biology and therapeutic potential of interleukin-10. J Exp Med. 2019;217(1):e20190418. doi:10.1084/jem.20190418. [Google Scholar] [PubMed] [CrossRef]
147. Zhao C, Yang Z, Li Y, Wen Z. Macrophages in tissue repair and regeneration: insights from zebrafish. Cell Regen. 2024;13(1):12. doi:10.1186/s13619-024-00195-w. [Google Scholar] [PubMed] [CrossRef]
148. Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8(1):207. doi:10.1038/s41392-023-01452-1. [Google Scholar] [PubMed] [CrossRef]
149. Magatti M, Vertua E, Cargnoni A, Silini A, Parolini O. The immunomodulatory properties of amniotic cells: the two sides of the coin. Cell Transplant. 2018;27(1):31–44. doi:10.1177/0963689717742819. [Google Scholar] [PubMed] [CrossRef]
150. Delves PJ. Cellular components of the immune system MSD manual professional edition: MSD manual; 2024. Available from: https://www.msdmanuals.com/en-sg/professional/immunology-allergic-disorders/biology-of-the-immune-system/cellular-components-of-the-immune-system. [Accessed 2024]. [Google Scholar]
151. Ma R-Y, Black A, Qian B-Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 2022;43(7):546–63. doi:10.1016/j.it.2022.04.008. [Google Scholar] [PubMed] [CrossRef]
152. Pham HL, Hoang TX, Kim JY. Human regulatory macrophages derived from THP-1 cells using arginylglycylaspartic acid and vitamin D3. Biomedicines. 2023;11(6):1740. doi:10.3390/biomedicines11061740. [Google Scholar] [PubMed] [CrossRef]
153. Zhuang J, Hou J. The role of regulatory myeloid cell therapy in renal allograft rejection. Front Immunol. 2021;12:625998. doi:10.3389/fimmu.2021.625998. [Google Scholar] [PubMed] [CrossRef]
154. Gan X, Gu J, Ju Z, Lu L. Diverse roles of immune cells in transplant rejection and immune tolerance. Engineering. 2022;10:44–56. doi:10.1016/j.eng.2021.03.029. [Google Scholar] [CrossRef]
155. Tran LM, Thomson AW. Detection and monitoring of regulatory immune cells following their adoptive transfer in organ transplantation. Front Immunol. 2020;11:614578. doi:10.3389/fimmu.2020.614578. [Google Scholar] [PubMed] [CrossRef]
156. Schulze M, Hutchinson JA, Brem-Exner BG, Riquelme P, Roelen D, Sotnikova A, et al. A Cell-based Approach to the Minimisation of Immunosuppression in Renal Transplantation: 1486. Transplantation. 2008;86(2S):497. doi:10.1097/01.tp.0000331303.85234.e4. [Google Scholar] [CrossRef]
157. Fortunato M, Morali K, Passeri L, Gregori S. Regulatory cell therapy in organ transplantation: achievements and open questions. Front Immunol. 2021;12:641596. doi:10.3389/fimmu.2021.641596. [Google Scholar] [PubMed] [CrossRef]
158. Braza MS, Conde P, Garcia M, Cortegano I, Brahmachary M, Pothula V, et al. Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance. Am J Transplant. 2018;18(5):1247–55. doi:10.1111/ajt.14645. [Google Scholar] [PubMed] [CrossRef]
159. Li X, Wu J, Zhu S, Wei Q, Wang L, Chen J. Intragraft immune cells: accomplices or antagonists of recipient-derived macrophages in allograft fibrosis? Cell Mol Life Sci. 2023;80(7):195. doi:10.1007/s00018-023-04846-0. [Google Scholar] [PubMed] [CrossRef]
160. Thornley TB, Fang Z, Balasubramanian S, Larocca RA, Gong W, Gupta S, et al. Fragile TIM-4-expressing tissue resident macrophages are migratory and immunoregulatory. J Clin Investigat. 2014;124(8):3443–54. doi:10.1172/JCI73527. [Google Scholar] [PubMed] [CrossRef]
161. Campa-Carranza JN, Paez-Mayorga J, Chua CYX, Nichols JE, Grattoni A. Emerging local immunomodulatory strategies to circumvent systemic immunosuppression in cell transplantation. Expert Opin Drug Deliv. 2022;19(5):595–610. doi:10.1080/17425247.2022.2076834. [Google Scholar] [PubMed] [CrossRef]
162. Wu C, Zhao Y, Xiao X, Fan Y, Kloc M, Liu W, et al. Graft-infiltrating macrophages adopt an M2 phenotype and are inhibited by purinergic receptor P2X7 antagonist in chronic rejection. Am J Transplant. 2016;16(9):2563–73. doi:10.1111/ajt.13808. [Google Scholar] [PubMed] [CrossRef]
163. Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018;131(1):58–67. doi:10.1182/blood-2017-06-741033. [Google Scholar] [PubMed] [CrossRef]
164. Liu X, Lu Y, Lian Y, Chen Z, Xia J, Meng L, et al. Macrophage depletion improves chronic rejection in rats with allograft heart transplantation. Transplant Proc. 2020;52(3):992–1000. doi:10.1016/j.transproceed.2019.12.037. [Google Scholar] [PubMed] [CrossRef]
165. Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol. 2014;41(2):174–84. doi:10.1053/j.seminoncol.2014.02.003. [Google Scholar] [PubMed] [CrossRef]
166. Mehrotra P, Ravichandran KS. Drugging the efferocytosis process: concepts and opportunities. Nature Reviews Drug Discovery. 2022;21(8):601–20. doi:10.1038/s41573-022-00470-y. [Google Scholar] [PubMed] [CrossRef]
167. Dangi A, Zhang L, Zhang X, Luo X. Murine CMV induces type 1 IFN that impairs differentiation of MDSCs critical for transplantation tolerance. Blood Adv. 2018;2(6):669–80. doi:10.1182/bloodadvances.2017012187. [Google Scholar] [PubMed] [CrossRef]
168. Wang C, Cao M, Jiang X, Yao Y, Liu Z, Luo D. Macrophage balance fraction determines the degree of immunosuppression and metastatic ability of breast cancer. Int Immunopharmacol. 2021;97:107682. doi:10.1016/j.intimp.2021.107682. [Google Scholar] [PubMed] [CrossRef]
169. Seyedizade SS, Afshari K, Bayat S, Rahmani F, Momtaz S, Rezaei N, et al. Current status of M1 and M2 macrophages pathway as drug targets for inflammatory bowel disease. Arch Immunol Ther Exp. 2020;68(2):10. doi:10.1007/s00005-020-00576-4. [Google Scholar] [PubMed] [CrossRef]
170. Yu S, Lu J. Macrophages in transplant rejection. Transpl Immunol. 2022;71:101536. doi:10.1016/j.trim.2022.101536. [Google Scholar] [PubMed] [CrossRef]
171. Hu Q, Lyon CJ, Fletcher JK, Tang W, Wan M, Hu TY. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharmaceutica Sinica B. 2021;11(6):1493–512. doi:10.1016/j.apsb.2020.12.014. [Google Scholar] [PubMed] [CrossRef]
172. Panzer SE. Macrophages in transplantation: a matter of plasticity, polarization, and diversity. Transplantation. 2022;106(2):257–67. doi:10.1097/TP.0000000000003804. [Google Scholar] [PubMed] [CrossRef]
173. Li D, Gao S. The interplay between T lymphocytes and macrophages in myocardial ischemia/reperfusion injury. Mole Cell Biochem. 2024;479(8):1925–36. doi:10.1007/s11010-023-04822-z. [Google Scholar] [PubMed] [CrossRef]
174. Ghafouri-Fard S, Abak A, Tavakkoli Avval S, Shoorei H, Taheri M, Samadian M. The impact of non-coding RNAs on macrophage polarization. Biomed Pharmacother. 2021;142:112112. doi:10.1016/j.biopha.2021.112112. [Google Scholar] [PubMed] [CrossRef]
175. He L, Jhong J-H, Chen Q, Huang K-Y, Strittmatter K, Kreuzer J, et al. Global characterization of macrophage polarization mechanisms and identification of M2-type polarization inhibitors. Cell Rep. 2021;37(5):109955. doi:10.1016/j.celrep.2021.109955. [Google Scholar] [PubMed] [CrossRef]
176. Lee H-T, Oh S, Ro DH, Yoo H, Kwon Y-W. The key role of DNA methylation and histone acetylation in epigenetics of atherosclerosis. J Lipid Atheroscler. 2020;9(3):419–34. doi:10.12997/jla.2020.9.3.419. [Google Scholar] [PubMed] [CrossRef]
177. Sun S, Chen R, Dou X, Dai M, Long J, Wu Y, et al. Immunoregulatory mechanism of acute kidney injury in sepsis: a narrative review. Biomed Pharmacother. 2023;159:114202. doi:10.1016/j.biopha.2022.114202. [Google Scholar] [PubMed] [CrossRef]
178. Li W, Xu Z, Zou B, Yang D, Lu Y, Zhang X, et al. Macrophage regulation in vascularization upon regeneration and repair of tissue injury and engineered organ transplantation. Fundam Res. 2024. doi:10.1016/j.fmre.2023.12.015. [Google Scholar] [CrossRef]
179. Li T, Zhang Z, Bartolacci JG, Dwyer GK, Liu Q, Mathews LR, et al. Graft IL-33 regulates infiltrating macrophages to protect against chronic rejection. J Clin Investig. 2020;130(10):5397–412. doi:10.1172/JCI133008. [Google Scholar] [PubMed] [CrossRef]
180. Guo D, Liu H, Zhao S, Lu X, Wan H, Zhao Y, et al. Synergistic rheumatoid arthritis therapy by interrupting the detrimental feedback loop to orchestrate hypoxia M1 macrophage polarization using an enzyme-catalyzed nanoplatform. Bioact Mater. 2024;41:221–38. [Google Scholar] [PubMed]
181. Khaliulin I, Hamoudi W, Amal H. The multifaceted role of mitochondria in autism spectrum disorder. Mol Psychiatr. 2024. doi:10.1038/s41380-024-02725-z. [Google Scholar] [PubMed] [CrossRef]
182. Cardinali DP, Vigo DE. Chronobiotic and cytoprotective activity of melatonin in the cardiovascular system. Doses matter. npj Biol Timing Sleep. 2024;1(1):7. doi:10.1038/s44323-024-00007-z. [Google Scholar] [CrossRef]
183. Xu K, Saaoud F, Shao Y, Lu Y, Yang Q, Jiang X, et al. A new paradigm in intracellular immunology: mitochondria emerging as leading immune organelles. Redox Biol. 2024;76:103331. doi:10.1016/j.redox.2024.103331. [Google Scholar] [PubMed] [CrossRef]
184. Brogyanyi T, Kejík Z, Veselá K, Dytrych P, Hoskovec D, Masařik M, et al. Iron chelators as mitophagy agents: potential and limitations. Biomed Pharmacother. 2024;179:117407. doi:10.1016/j.biopha.2024.117407. [Google Scholar] [PubMed] [CrossRef]
185. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. doi:10.1016/j.cell.2013.05.039. [Google Scholar] [PubMed] [CrossRef]
186. Lian B, Zhang J, Yin X, Wang J, Li L, Ju Q, et al. SIRT1 improves lactate homeostasis in the brain to alleviate parkinsonism via deacetylation and inhibition of PKM2. Cell Rep Med. 2024;5(8):101684. doi:10.1016/j.xcrm.2024.101684. [Google Scholar] [PubMed] [CrossRef]
187. Wu J, Tian Z, Wang B, Liu J, Bi R, Zhan N, et al. Exploring resveratrol against Alzheimer’s disease and Parkinson’s disease through integrating network pharmacology, bioinformatics, and experimental validation strategy in vitro. Heliyon. 2024;10(18):e37908. doi:10.1016/j.heliyon.2024.e37908. [Google Scholar] [PubMed] [CrossRef]
188. Sun Y, Sun H, Zhang Z, Tan F, Qu Y, Lei X, et al. New insight into oxidative stress and inflammatory responses to kidney stones: potential therapeutic strategies with natural active ingredients. Biomed Pharmacother. 2024;179:117333. doi:10.1016/j.biopha.2024.117333. [Google Scholar] [PubMed] [CrossRef]
189. Zhai X, Wang Z, Gao J. Quercetin alleviates microglial-induced inflammation after traumatic brain injury via the PGC-1α/Nrf2 pathway dependent on HDAC3 inhibition. Brain Res Bull. 2024;217:111080. doi:10.1016/j.brainresbull.2024.111080. [Google Scholar] [PubMed] [CrossRef]
190. Ebadpour N, Mahmoudi M, Kamal Kheder R, Abavisani M, Baridjavadi Z, Abdollahi N, et al. From mitochondrial dysfunction to neuroinflammation in Parkinson’s disease: pathogenesis and mitochondrial therapeutic approaches. Int Immunopharmacol. 2024;142:113015. doi:10.1016/j.intimp.2024.113015. [Google Scholar] [PubMed] [CrossRef]
191. Xu J, Li Y, Yang X, Li H, Xiao X, You J, et al. Quercetin inhibited LPS-induced cytokine storm by interacting with the AKT1-FoxO1 and Keap1-Nrf2 signaling pathway in macrophages. Sci Rep. 2024;14(1):20913. doi:10.1038/s41598-024-71569-y. [Google Scholar] [PubMed] [CrossRef]
192. de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41(3):374–403. doi:10.1016/j.ccell.2023.02.016. [Google Scholar] [PubMed] [CrossRef]
193. Yue T, Zhang W, Pei H, Danzeng D, He J, Yang J, et al. Monascus pigment-protected bone marrow-derived stem cells for heart failure treatment. Bioact Mater. 2024;42:270–83. [Google Scholar] [PubMed]
194. Sandhir R, Halder A, Sunkaria A. Mitochondria as a centrally positioned hub in the innate immune response. BBA—Mol Basis Dis. 2017;1863(5):1090–7. doi:10.1016/j.bbadis.2016.10.020. [Google Scholar] [PubMed] [CrossRef]
195. He T, Pu J, Ge H, Liu T, Lv X, Zhang Y, et al. Elevated circulating LncRNA NORAD fosters endothelial cell growth and averts ferroptosis by modulating the miR-106a/CCND1 axis in CAD patients. Sci Rep. 2024;14(1):24223. doi:10.1038/s41598-024-76243-x. [Google Scholar] [PubMed] [CrossRef]
196. Hashimoto Y, Negishi J, Funamoto S, Kimura T, Kobayashi H, Oshika T, et al. Preparation, physico-biochemical characterization, and proteomic analysis of highly transparent corneal extracellular matrices for lamellar keratoplasty and tissue-engineered cornea construction. Mater Today Bio. 2024;28(2):101241. doi:10.1016/j.mtbio.2024.101241. [Google Scholar] [PubMed] [CrossRef]
197. Zhao L, Jin S, Wang S, Zhang Z, Wang X, Chen Z, et al. Tertiary lymphoid structures in diseases: immune mechanisms and therapeutic advances. Signal Transduct Target Ther. 2024;9(1):225. doi:10.1038/s41392-024-01947-5. [Google Scholar] [PubMed] [CrossRef]
198. Yarmohammadi F, Karimi G. Serum and glucocorticoid-regulated kinase 1 (SGK1) as an emerging therapeutic target for cardiac diseases. Pharmacol Res . 2024;208:107369. doi:10.1016/j.phrs.2024.107369. [Google Scholar] [PubMed] [CrossRef]
199. Maldini CR, Messana AC, Bendet PB, Camblin AJ, Musenge FM, White ML, et al. Immunosuppressant therapy averts rejection of allogeneic FKBP1A-disrupted CAR-T cells. Mol Ther. 2024;32(10):3485–503. doi:10.1016/j.ymthe.2024.06.022. [Google Scholar] [PubMed] [CrossRef]
200. Yang Y, Chen X-Q, Jia Y-X, Ma J, Xu D, Xiang Z-L. Circ-0044539 promotes lymph node metastasis of hepatocellular carcinoma through exosomal-miR-29a-3p. Cell Death Dis. 2024;15(8):630. doi:10.1038/s41419-024-07004-x. [Google Scholar] [PubMed] [CrossRef]
201. Liao H-J, Yang Y-P, Liu Y-H, Tseng H-C, Huo T-I, Chiou S-H, et al. Harnessing the potential of mesenchymal stem cells-derived exosomes in degenerative diseases. Regen Ther. 2024;26(Suppl 1):599–610. doi:10.1016/j.reth.2024.08.001. [Google Scholar] [PubMed] [CrossRef]
202. Liu R, Li X, Ma H, Yang Q, Shang Q, Song L, et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superoxide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Radic Biol Med. 2020;161:339–50. doi:10.1016/j.freeradbiomed.2020.10.029. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools