 Open Access
Open Access
REVIEW
How does Hedgehog signaling participate in the cross-interaction of hormones and testis development?
The Sperm Laboratory, College of Life Sciences, Zhejiang University, Hangzhou, 310058, China
* Corresponding Author: WAN-XI YANG. Email:
BIOCELL 2025, 49(1), 93-107. https://doi.org/10.32604/biocell.2024.058299
Received 09 September 2024; Accepted 15 November 2024; Issue published 24 January 2025
Abstract
Hedgehog (HH) signaling has been researched for decades and Hedgehog has 3 homologs: Sonic Hedgehog (Shh), Indian Hedgehog (Ihh), and Desert Hedgehog (Dhh). Dhh is the one involved in male gonad and germ cell development. The distribution of molecules in Hedgehog signaling in testis indicated that Hedgehog signaling executes important functions during testis development. The patients with Dhh signaling deficiency develop dysgenesis of gonads and hormone production which demands further exploration of gonad HH signaling. Some results proved the indispensable roles of HH signaling in gonad and germ cell development and the interaction with hormones. This review evaluates HH functions in the testis and how HH affects and is affected by hormones and provides novel insights about HH signaling to the readers.Graphic Abstract
Keywords
The hedgehog (HH) gene was first discovered in Drosophila which encodes a protein participating in cell-cell communication during segmental patterning [1,2]. Hedgehog proteins were secreted as a morphogen to conduct cells’ proliferation and development through short- and long-range signaling [3,4]. In vertebrate animals, there are 3 types of HH protein: Sonic Hedgehog (Shh), Indian Hedgehog (Ihh), and Desert Hedgehog (Dhh), they play different roles in different parts of the body during development.
The gradient of Shh directs subsequent morphogenesis of limb buds [5–7] and neural development [8], Shh takes part in the development of the cerebellum [9], bladder [10], neural tube [11] and the differentiation of neural cells, neural stem cells, oligodendrocyte precursor cells and astrocytes [12–16]. Some neurodegenerative diseases are related to Shh [17–19]. In general, the Shh takes part in the development of the nervous system. Ihh was expressed by prehypertrophic and hypertrophic chondrocytes which participate in regulating the endochondral ossification [20,21], the activation of Ihh signaling could drive resting zone chondrocytes to convert into osteoblasts eventually [22,23]. Ihh plays roles in the formation and specialization of craniofacial, bone, and cartilage [24–26]. Ihh takes charge of bone and cartilage formation. Dhh plays an important role in gonads especially testis development and hormone secretion [27,28], and even the evolution of scrotal testis [29]. But their roles are not always strictly separated: Shh could be involved in the formation and patterning of craniofacial structures [30]; Ihh may participate in the proliferation of colonic tumor cells [31]; Dhh functions in perineurium development [32] and participates in nerve injury and neuropathies [33,34], also the differentiation of chondrocytes [35]. The coordination of Ihh and Shh can mediate epithelial-stromal cross-talk with the function of primary cilia during decidualization in mice [36]. The relationship of the three HH components and molecular mechanisms in HH signaling had been reviewed before [37].
In addition to the typical functions that were reviewed previously, some research indicates that the HH signaling and the role Dhh plays in testis development have a close relationship with hormones and their production [38]. As the producer of testosterone in male vertebrates, the normal development and function of Leydig cells contribute a lot to male gonad development [39]. However, the null mutation of Dhh suppressed the production of testosterone by Leydig cells and spermatogenesis [40,41], which could be the defects of steroidogenic enzyme expression levels in Leydig cells [42]. Some in vitro research even showed that the HH signal pathway could regulate the steroidogenesis enzymes through a combination with their promoters [43]. More and more research indicated that there is a deep relationship between HH signaling, hormone production, and testis development.
During the past years of research on HH signaling and gonads, researchers have found the indispensable relations between them. Since cholesterol is needed during the processing of mature HH, some researchers discovered a close relationship between HH signaling and androgens [44]. Others found the important functions of HH in steroidogenic glands [45,46], gonadal development, and some clinical cases [27,47]. However, this previous research didn’t realize that HH signaling has other molecular relationships in gonads. That is HH has relations with different kinds of hormones, especially a deep interaction with androgens. The interactions of HH signaling with hormones and the signaling functions in gonad development are not separated but related. In this review, we will take several parts to focus on HH functions in the testis and discuss why HH signaling is so important for testis development and hormones, dig into the deeper interaction of HH signaling and hormones, and propose our theory about HH signaling in testis. We searched for different types of HHs in different species and summarized the available information in Table 1.
HH Signaling Influences Male Gonad and Germ Cell Development
Tissue localization of HH signaling components
To understand HH signaling, researchers did so many experiments and most of them concentrated on the downstream of HH. It will be easier to understand these issues by having a brief review of the main members downstream of HH in this signaling pathway. Ptc (Patched) takes the first position in the downstream of protein HH. Ptc suppresses the activation of Smoothened (Smo), the combination of HH and Ptc could initiate the degradation of Ptc and cancel the suppression of Smo by Ptc [59,60]. In drosophila, active Smoothened combines with kinesin Cos2 [61], and Cos2 regulates the activity of transcription factor Ci (Cubitus interruptus) [62]. In vertebrate cells, there are 3 formations of the transcription factor downstream of HH signaling homolog to Ci: Gli 1, 2, and 3 (glioma-associated oncogene family members 1, 2, and 3) [63], and Cos2’s homologous protein are Kif7 and Kif27 [64,65]. Interestingly, Glis’ regulation in vertebrates is not through Kif7 or Kif27. It is primary cilia that play the regulatory role of Cos2 in drosophila [66], Kif7 and Kif27 regulate the Smoothened, primary cilia, and Gli complex by organizing the cilium tip compartment instead [67,68].
Several members of HH signaling were detected after these years of research. HH protein has 3 homologous in vertebrate animals, but in testis, especially for mammalian animals, Dhh plays a more important role [47]. Expression of Dhh was initiated after the expression of sex-determining region Y (Sry) in precursors of Sertoli cells and will last in adulthood during the development of testis [69]. In some research, Dhh was detected in the cytoplasm of Sertoli cells, late condensing spermatids, and Leydig cells in Chinese tongue sole testis [70]. So Sertoli cells could be the main source of Dhh protein in the testis at first, germ cells, and Leydig cells could be the target of Dhh since HH was secreted to execute its function.
Ptc1 mRNA is specifically localized in the germ line in the testis [71]. However, further research showed that its protein localization indicated that Ptc1 was expressed in late spermatocytes, round spermatids, and Leydig cells, but not in other cells in the testis [72]. Ptc2 mRNA was detected in spermatogonia and spermatocytes [73], and Ptc3 protein was localized on the mid-piece of sperm [74]. As the receptor of HH protein, distributions of Ptc proteins and their mRNA give us a view on HH target cells primarily: Leydig cell and germ cell are HH protein target cell types in testis. Fortunately, the distribution of Smo protein made a supplementary support for this proposal. Smo protein was expressed in late spermatocytes, spermatids, and Leydig cells, but not in other cells [72].
Gli mRNAs were detected in Sertoli cells [75] and predominantly in spermatogonia and spermatocytes in adult testis [73]. Gli1 protein is expressed in Sertoli cells, spermatogonia, and spermatid [76]. Our proposed distributions of HH signaling members in testis are shown in Fig. 1.
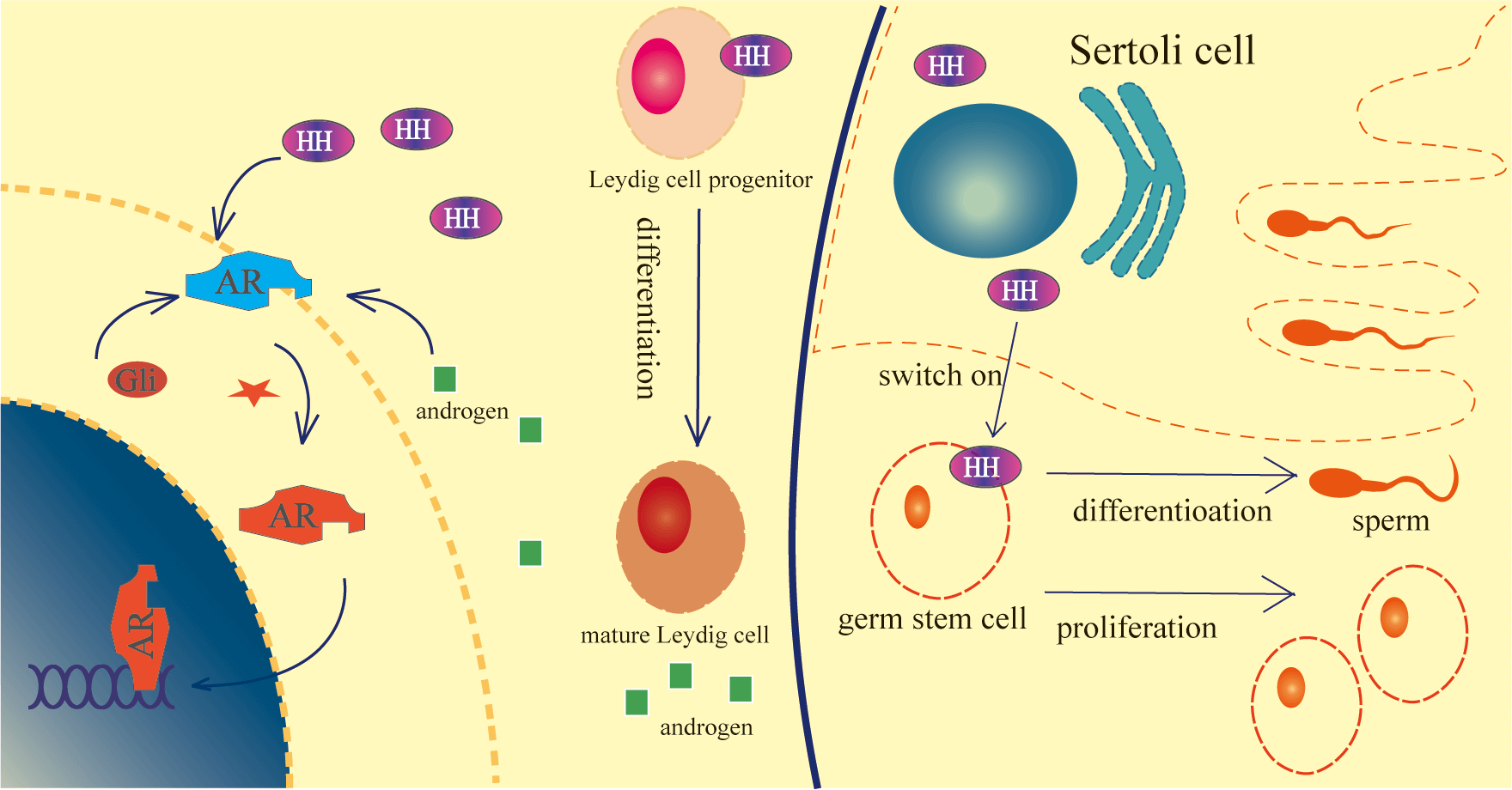
Figure 1: Distribution of HH signaling members and brief mechanism in testis.
This research suggests that Dhh is probably mainly produced by Sertoli cells and secreted to the testis microenvironment, downstream proteins of HH in the signaling pathway are expressed in the germ cells and Leydig cells, and it is Sertoli cells that make the decisions to direct these cells during testis development and function.
HH proteins are produced in the Sertoli cell’s endoplasmic reticulum and secreted to the testis. HH combines with patched proteins which are located on the cell membrane of germ cells and Leydig cells to cancel Patched inhibition of Smoothen and Smoothen could function on primary cilia to recruit Gli and primary cilia activates Gli, Gli then transferred into the nucleus to promote or starts target genes transcription. The figure was drawn using Adube Illustrater 2024 software.
HH signaling in the morphogenesis and development of testis
Testis supplies the environment for germ cells’ development. Besides germ cells, the correct function of HH signaling is needed for the formation and development of male gonads. A proper expression and secretion of HH protein is required for the correct migration of germ cells in Drosophila embryos [77]. The de novo RNA-Seq analysis of Onychostoma macrolepis testis showed that the HH signaling pathway plays an important role in testis maintenance and spermatogenesis in general [78]. With the aging of Sertoli and Leydig cells, the HH signaling shows dysregulation through single-cell RNA sequencing in human testis [79]. HH signaling inhibitor Cyclopamine or Gli1/2 suppressor GANT61 treatment on mouse testis could abolish the appearance of fetal Leydig cells [80], and lead to defects in Wolffian duct morphogenesis of testis [81], and the Gli3 loss-of-function mutation leads to cryptorchidism and hypospadias in male mice [82]. The activation of HH signaling by SAG, an agonist of Smo, promotes laminin secretion and increases the proliferation of Leydig and germ cells, which contribute to the formation of the basal membrane and embedding of germ cells into Sertoli cells during the reconstruction progression of seminiferous tubule-like structures [83]. These animals treated with an inhibitor or activator of HH signaling offered us a conclusion that the activation of HH signaling is required for male gonad development, and the inhibition of HH signaling after animals’ birth will defect testis morphogenesis and development.
What will it be if the change of HH signaling is not caused by drugs, but by the mutation of the individual fetus? Several reports could answer this question. The null mutation of Dhh in male mice could induce feminization, the polarity of Sertoli cells in their testes was lost, the basal lamina was irregular, and seminiferous cords were gapped during development [84]. In the masculinized Dhh-null adult males, the basement membrane was absent, but still exist abundant adult Leydig cells [40]. But in the Dhh null; Sf1+/− (steroidogenic factor 1) male mice, the gonad development was not as clear as in the normal mice and the adult Leydig cells failed to develop [85]. By contrast, consecutive activation of HH signaling induced the appearance of ectopic fetal Leydig cells in the ovary in female mice [86] and caused a reduction of testis and epididymis wet weight in male mice [87].
From these gene-edited or drug-treated animals, we can primarily know what happened about Dhh in testis: Dhh protein produced in testes to make sure the polarity of Sertoli cells and embed germ cells into Sertoli cells, direct laminin secretion to keep the structure of the basal membrane [88]. Testis can supply a proper environment for germ cell proliferation and differentiation in this way. Out of the seminiferous tubules, Dhh promises the development of adult and fetal Leydig cells with the help of full dose expression of Sf1. The proper expression of HH signaling is required for testis successful structural morphogenesis.
Disturbance in HH signaling influences the survival and differentiation of germ cells
Sperm production is highly correlated with mammalian reproductive security and HH signaling offers an inescapable contribution to germ cell survival according to this year’s research. After the disruption of HH signaling, safe germ cell production would be problematic.
In chicken, single-cell transcriptome analysis of germ cells showed the participation of HH signaling during germ cell mitotic arrest [89]. The inhibition of HH signaling by cyclopamine, an inhibitor of Smo, increased the number of germ cells which suffered apoptotic and spermatogonia and inhibited the proliferation of primordial germ cells [83]. This is also discovered in rat testis in vivo and Medaka SG3 spermatogonial stem cell line in vitro [90–92]. Further research suggested that Dhh could rescue cell proliferation [91], and SAG, an agonist of Smo, could also rescue the number of germ cells in a reconstruction of the tubule-like structure system [83]. Treatment with cyclopamine on mice testis in vitro culture system defected the germ cells’ entrance of meiosis [93] and mitosis [94]. The deletion of the Ptc1 gene leading to germline anatomical defects and sterile in Caenorhabditis elegans [71] gives us more evidence to emphasize the importance of HH in germ cells’ development.
In the Dhh-null masculinized mice, the spermatogonia were evident but the spermatocytes underwent cell death [41] and are absent, which suggests the process of spermatogenesis was inhibited without influencing germ stem cells. However, the knockdown of Gli1 in Gallus gallus decreased the quantity of primordial germ cells [95]. The reason for this difference could be the function of Gli, which regulates the cell cycle by binding to the regulator of the cell cycle (rgcc) directly [92].
The above evidence showed us that HH signaling is indispensable in germ cell development, but what be the outcome if more HH signaling in testes would happen? Some research showed the motility and number of sperm in the HH-continuous activated mice were reduced [87], and the overexpression of Gli1 promoted the differentiation of embryoid body cells into spermatogonial stem cells [95] while leading to the halt of spermatogenesis at the pachytene primary spermatocyte stage and apoptosis of germ cells [96]. It is obvious that overactivation of HH damages the cell cycle and physical function of germ cells. A proper activation of HH signaling was needed for germ stem cells to generate normal sperm and Gli1 plays a significant role in embryonic cells to differentiate into germ cells.
HH signaling regulates Leydig cell differentiation
As constitutions of the testis, adult and fetal Leydig cells make up the testis structurally and hold the function of androgen production and secretion [97,98]. Fetal Leydig cells develop during the tenth week of fetal life and experience 2 waves before the first year of life for humans, which occur in 3 waves in pigs. Fetal Leydig cells express hydroxysteroid dehydrogenase (3β-HSD) and Sf-1, which hold the function of steroid synthesis. While adult Leydig cells experience functional differentiation at the very beginning of fetal life (Luteinizing hormone/human chorionic gonadotropin-independent) and pubertal period (Luteinizing hormone/human chorionic gonadotropin-dependent) for humans, express several HSD (including 3β-HSD), possess the high capacity of testosterone synthesis [99,100]. During the differentiation of adult Leydig cells, the cells acquire the ability of androgen production, and the expression of relative enzymes is increased [101]. The expression of these enzymes could be regarded as a Leydig cell differentiation criterion. The damage to Leydig cell development and function could be one cause of the testis defect.
The fetal Leydig cell differentiation is initiated by HH signaling [102], and the null of HH is disrupted by upregulation failure of the Sf1 and P450 Side Chain Cleavage enzyme (P450 scc) [42]. Inhibition of HH signaling by cyclopamine also led to a significant loss of P450 scc, which suggests that the Leydig cell’s function was damaged [103].
Adult Leydig cells provided a more complex view: Adult Leydig cells were evident in Dhh-null masculinized mice [40], but lost in feminized mice and TF male pseudohermaphroditism rat [104]. Furthermore, adult Leydig cells failed to develop in the Sf1+/−; Dhh null mice gonad but were normal in Sf1+/−; Dhh+/− male mice. This research indicates that HH signaling was indispensable for fetal and adult Leydig cell development, while Sf1 expression dose is decisive for adult Leydig cells.
Activation of HH signaling increased the proliferation and differentiation of Leydig cells [83,105], upregulated Sf1 expression, and induced ectopic fetal Leydig cells developed in the ovary [86]. The differentiation of progenitors of Sf1+/3βHSD- steroidogenic cells into Sf+ or Sf−/3βHSD- cells was also upregulated in Smo consecutive expressed mice [87]. By contrast, inhibition of HH signaling decreased Leydig cell number, especially the fetal: cyclopamine reduced the number of steroidogenic Leydig cells [35], abolished the appearance of fetal Leydig cells but not its maintenance, and this effect was not mediated by Gli1/2 [80]. Loss-of-function mutation of Gli3 decreased the transcription level of Sf1 and a number of differentiated fetal Leydig cells [82], which indicates the Leydig cell differentiation-induced function is executed by Gli3 and mediated by Sf1.
To sum up, HH secreted by Sertoli cells and combined with Leydig cell progenitors to induce the expression of P450scc, leads to fetal Leydig cell differentiation by Gli3’s transcription activation, promise the generation of adult Leydig cells with enough dose of Sf1. The HH signaling functions in the testis development are shown in Fig. 2.
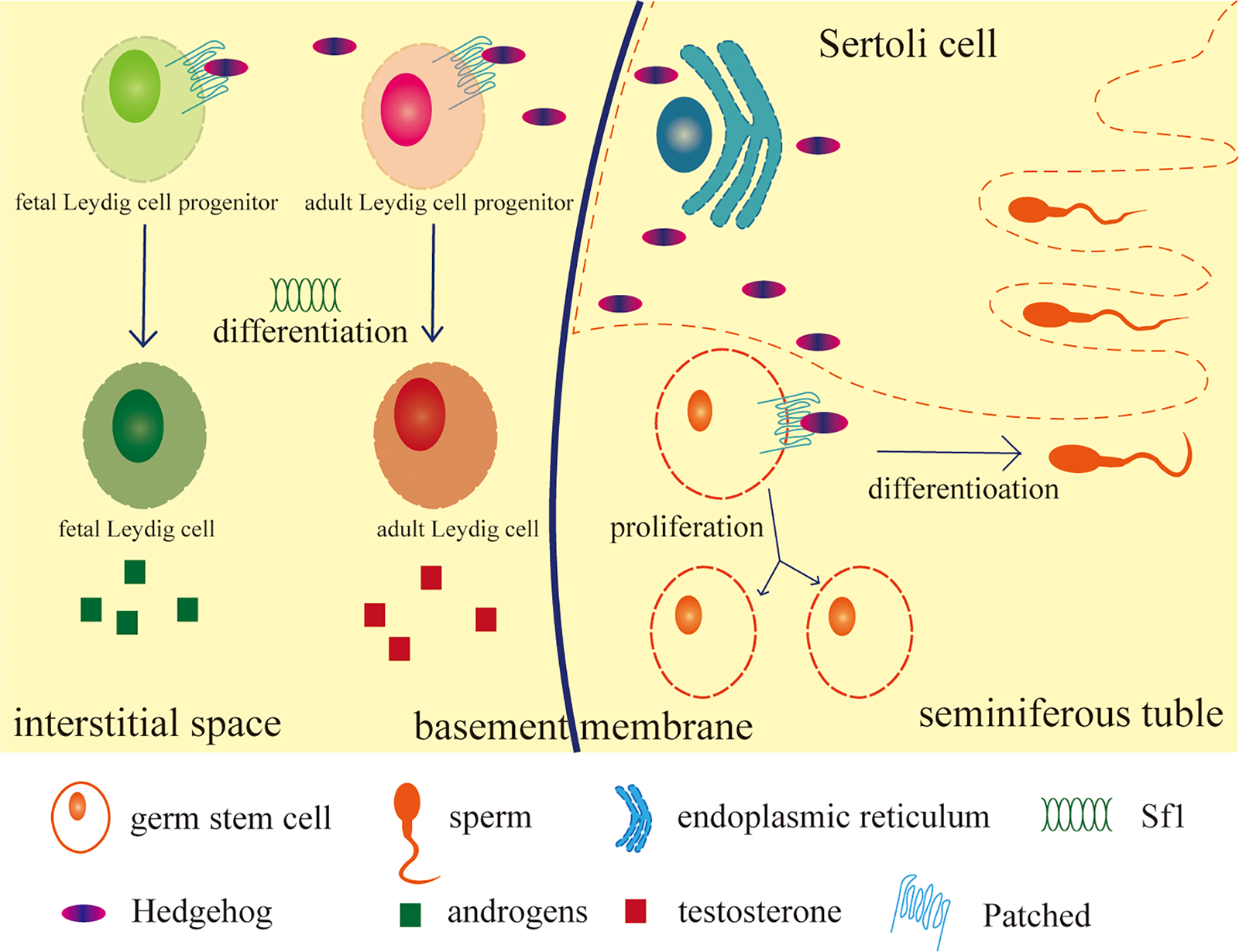
Figure 2: HH signaling function in testis.
HH ligands secreted by Sertoli cells combine with the receptor on target cells: Leydig cells and germ cells to initiate the differentiation of fetal Leydig cell progenitors and make sure the production of androgens, guarantee the differentiation of adult Leydig cell progenitors with enough expression of steroidogenic factor 1 (Sf1) and the testosterone production in adult Leydig cells. Germ stem cells keep the normal differentiation and proliferation by signals from HH and enough androgens. HH also makes sure these cells produce enough laminin to keep the structure of the testis (figure not shown). After all, HH keeps the cell types and functions developing and the testis structure forming normally during the development of the testes. The figure was drawn using Adube Illustrater 2024 software.
Hormones Interact with HH Signaling in Testis Development
Regulation of HH signaling changes steroidogenic enzyme expression
Some research proves the combination of Glis and steroidogenic enzymes gene promoters straight. Cholesterol conversion to progesterone and estradiol could be triggered by HH signaling by inducing the Gli3-controlled P450 scc and Gli2-controlled 3β-HSD1 and aromatase by combining with their promoters in human trophoblasts [43], and the mutation of Gli3 also decreased Sf1 transcription level and testosterone production [82]. Gli2 interacts with RNA polymerase II and enhances the binding to the 11β-HSD2 gene’s promoter in human trophoblast-like BeWo cells [106].
Besides the straight combination of Gli with promoters, the disturbance of HH signaling has a much deeper influence on hormone levels than we thought in mammalian animals and cells. Agonist of Smo treatment on prostate stromal cells upregulated the transcription of steroidogenesis enzymes, including Sf-1, SREBP (sterol regulatory element binding protein), CYP17A1 (a p450 cytochrome oxidase) RDH5 (retinol dehydrogenase 5) [107], and some other genes’ expression involved in lipid metabolism and steroid biosynthesis [108]. SAG treatment can also partially rescue steroidogenic genes’ expression in Wt1-deficient mice testes whose steroidogenic enzyme expression was decreased [109]. Cyclopamine treatment of in vitro cultured testis suppressed P450 scc and Hsd3β1 expression and secretion of testosterone [81]. The suppressed secretion of testosterone and impairment of P450 scc and other steroidogenic enzyme expressions would defect fetal Leydig cell differentiation and function, and that could be the reason why the researchers found adult but not fetal Leydig cells in HH-null masculinized mice which have been mentioned in the second part.
Deletion of Ptc resulted in significantly elevated ACTH (corticotrophin) release GH (growth hormone), and Prl (prolactin) expression in mice [110]. Activation of HH signaling by Shh treatment can increase GH and prolactin secretion in mice, even activate bone marrow stromal cells to steroidogenesis [111] and upregulate Sf1 expression and production of androgens in female mice which caused pseudohermaphroditism [86].
Loss-of-function mutation on Dhh in TF rats led to lower testosterone levels [104]. In Sf1+/−; Dhh+/− male mice, the Amh (anti-mullerian hormone), gonadal testosterone, and P450 scc were absent [85,94], Amh could also be reduced by cyclopamine treatment in a hanging drop gonad culture system [94].
These results indicate that HH signaling controls several enzyme’s expressions which function in hormone production, then control several hormone levels and impact the health and development of individuals.
Hormone treatment leads to changes in HH signaling members
The influence between HH signaling and some hormones is bidirectional.
Exposure to estrogen receptor (ER) or its agonists downregulates HH genes predominantly via an ERα-dependent pathway in the prostate gland of male rats [112]. In contrast, agonists or antagonists of Erβ treatment, respectively, increased or blocked the proliferation in the NCI-H295A cell line (the human adrenal cell line) [113]. What’s more, diethylstilbestrol (DES, estrogen compounds) and estrogen treatment increase expression of HH and HH signaling in mice experiment and rat SAH (subarachnoid haemorrhage) model [114,115]. Research about MLO-Y4 mouse osteocyte-like cells indicates that estrogen could influence the elongation of cilia to affect the activation of HH signaling [116].
There are also some other hormones that could regulate the HH signaling. FSH (follicle-stimulating hormone) is relevant to Dhh signaling [117], and leptin-induced Dhh signaling during human Leydig stem cell differentiation [105]. A high dosage of glucocorticoid treatment on NIH/3T3 cells (mouse fibroblast cell line) accumulated Smo in the primary cilia, conferring hypersensitivity to HH stimulation [118].
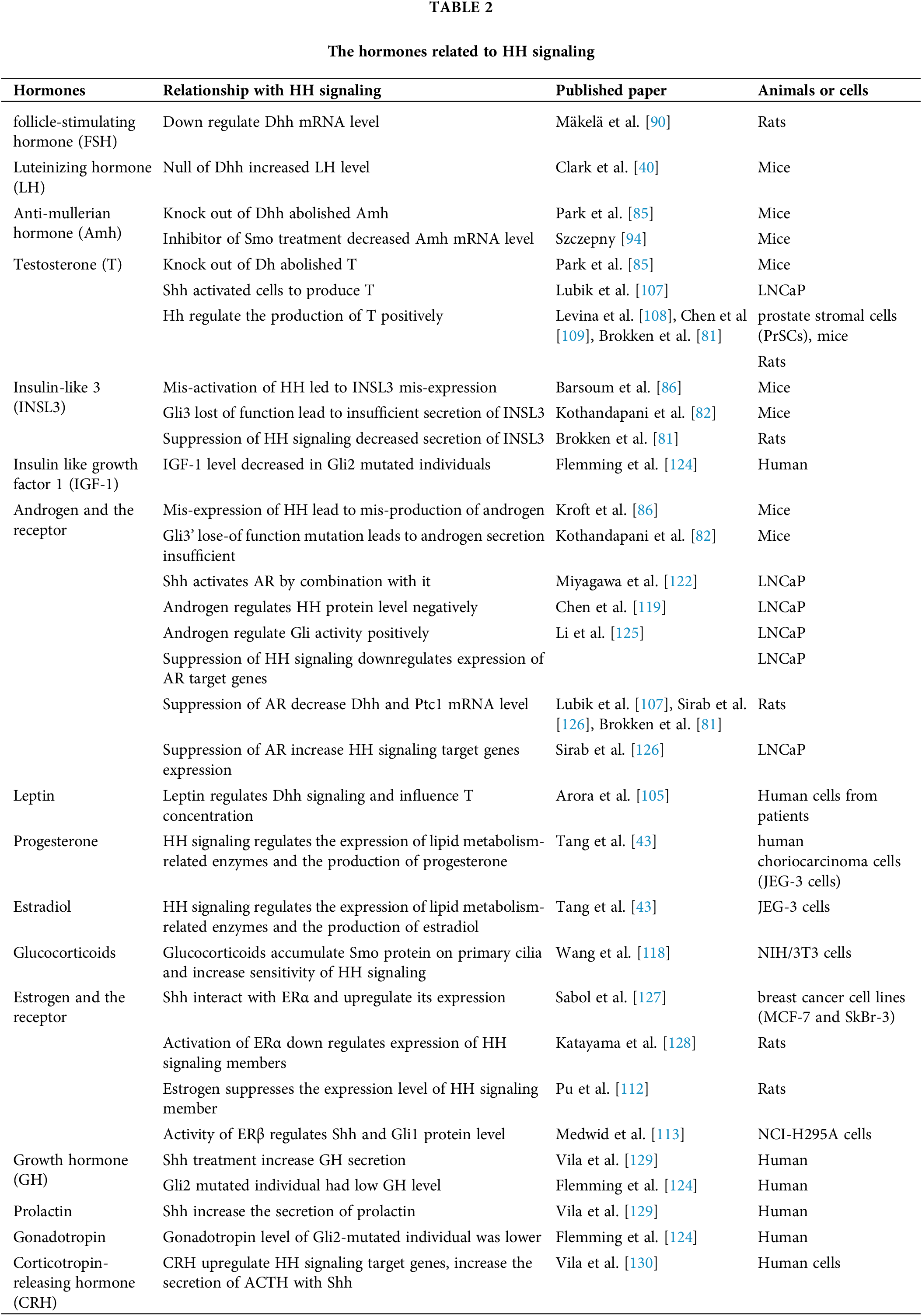
Androgens also suppress HH expression and its autocrine and paracrine activity in a dose-dependent manner [119], deprivation of androgen could rescue the Shh and Dhh expression time-dependently in LNCaP (prostate cancer cell line) cells [120,121] and HH signaling was activated in androgen-independent prostate cancer cells [122]. The antagonist of AR (androgen receptor) flutamide treatment on rat fetalis in utero significantly decreased the mRNA level of Dhh and Ptc1 [81]. The mutation of Gli2 decreased the responsiveness of androgens in mice, but Gli3 could make some compensation in the process [123]. We summarized the hormones that influence or are influenced by HH signaling in Table 2. This evidence all showed a phenomenon that changed with different hormones, especially sex hormones, will influence the HH signaling members’ transcription or protein function, and there seems to exist some relationship between the base of HH and hormone signaling that we want to explore further in the next section.
HH signaling members interact with hormones
As mentioned in this review, HH signaling has a deep relation with hormones, especially sex hormones. After years of research, it was indicated that members of HH signaling could interact with AR and some other hormones to impact hormone target genes and HH signaling.
For AR, the evidence is sufficient and convincing. The RNA sequencing analysis of androgen receptor axis inhibitors (ARPI) resistance patients emphasized the role of HH signaling primarily [131]. Other research in androgen-independent LNCaP (LNCaP-AI) cells showed Shh-activated AR (androgen receptor), and the impact could be caused by the binding of Shh-N-cholesterol through molecular dynamic simulation [122], this work primarily demonstrated the combination of HH protein and AR.
However, more research pointed to the interaction between Glis and AR. Knockdown of Smo attenuated AR signaling while overexpression of Gli1 partially reversed this inhibition in LNCaP cell culture [132]. Loss of AR elevated Shh-signaling activation in prostatic stromal cells [133]. It seems that Gli activity was needed for AR transcription activity under androgen-depleted conditions, this need will occupy Gli proteins and lead to a decrease in HH signaling activity.
The presence of dihydrotestosterone (DHT) or bicalutamide, an AR antagonist, affected the HH pathway target gene expression in different combinational treatments [126], androgen-activated AR significantly upregulated Glis transcriptional activity and the target genes expression by binding of AR to Gli2/3 [125,134], which means AR activity also impacted HH signaling target genes significantly. Counting on this research, we may find out a law: Glis combined with AR, activation of anyone will promote the other’s activity but AR’s promotion without androgen will occupy Glis and lead to a decrease of HH signaling. The three states in this hypothesis are shown in Fig. 3.
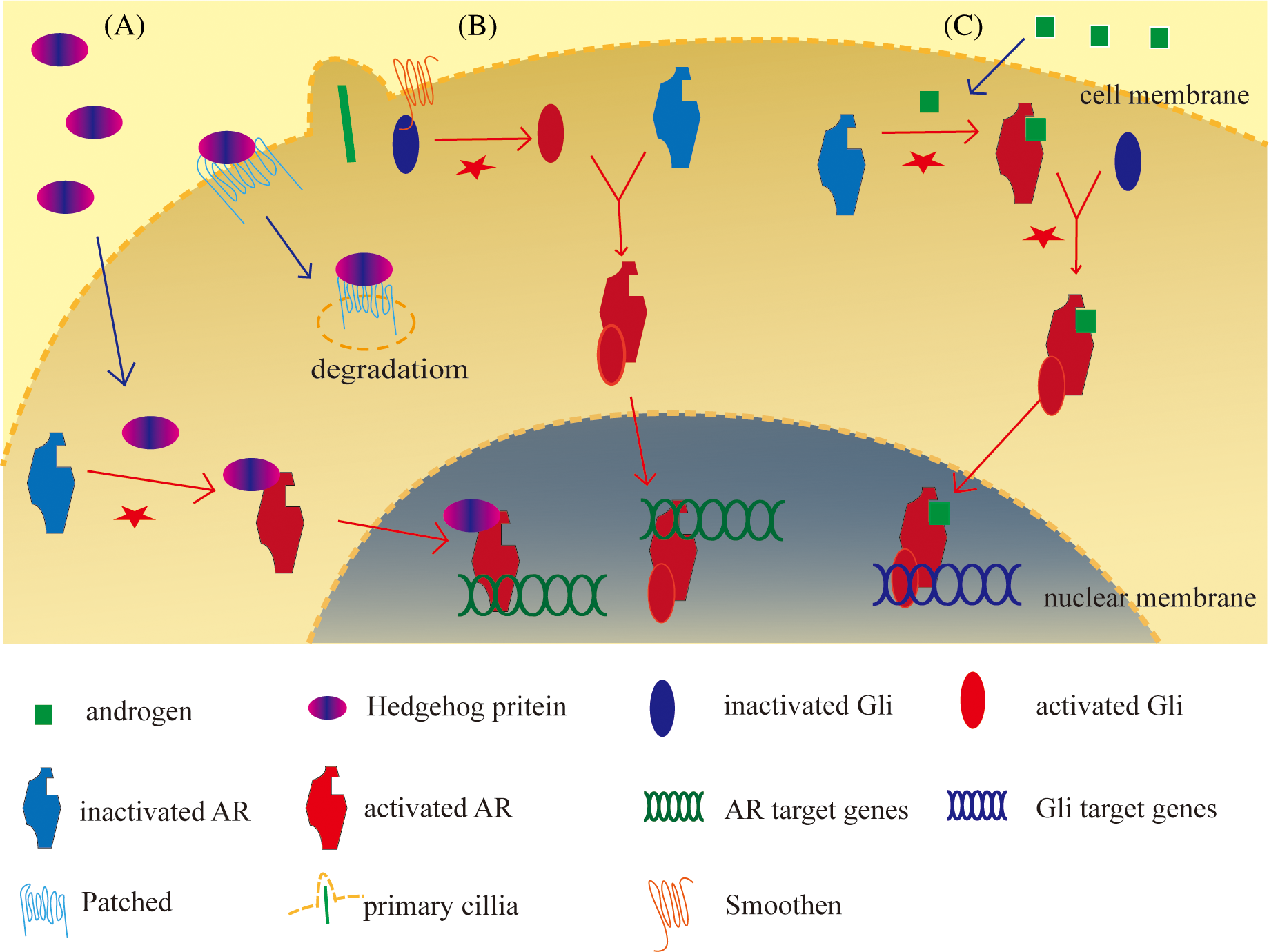
Figure 3: Three states of relation between HH signaling and androgen receptor (AR).
A: HH protein combines with and activates AR, and the complex translocates into the nucleus to start AR target gene expression; B: The combination of HH protein and patched led to the degeneration and permitted the activation of Gli proteins by Smoothen and primary cilia. Activated Gli combines with and activates AR, they are translocated into the nucleus to initiate AR target genes expression; C: Androgen activated AR, activated AR combines with and activates Gli, then the complex translocates into the nucleus to initiate Gli target genes expression. The figure was drawn using Adube Illustrater 2024 software.
The relationship among HH, Gli proteins, and hormones can explain what we have mentioned in the section ‘Disturbance on HH signaling influences survival and differentiation of germ cells’: primordial germ cell development was decreased in Gli1-knock down mice but is not affected in HH-null mice. The knockdown of Gli1 decreased the reaction of Gli proteins and hormones and led to a decrease of primordial germ cells [95], but the null of HH remained the reaction between Glis and hormones, which supports the hormones signaling and the production of primordial germ cells were not affected.
The proofs about ER or other hormones were not much but suggestable: The result of immunofluorescent and co-immunoprecipitation indicated the interaction of Shh and ERα protein in the MCF-7 cell line [127]. A computational study suggested the binding affinity of progesterone receptor and mature form of Shh and even more tendency for ERα to bind with the mature form of Shh than estradiol [135]. CRH increased Gli-dependent gene expression with Gli reporter plasmid, and the knockdown of Gli1 abolished the transcription stimulatory effect of CRH on POMC (proopiomelanocortin) [130], which suggested that Gli mediated CRH signaling.
Clinical Cases of HH Signaling Mutated Gonad
The mutation of HH signaling caused some diseases in patients, and most diseases were related to hormone production. These cases were divided into Shh and Dhh mutation and they were connected to pituitary function and gonad development, respectively.
A female patient with Gli2 transcription activity lost mutation, her GH, insulin-like growth factor I (IGF-I), thyroxine (T4), and gonadotropin were quite lower than the normal level. The GH replacement therapy helped to normalize her growth, but after the cessation, GH and IGF-I serum concentrations returned to subnormal values [124]. Research on some partial gonadal dysgenesis (PGD) and combined pituitary hormone deficiency (CPHD) patients revealed the mutation of Shh and HH interacting protein (HHIP) [136], which means HH signaling may participate in pituitary development and its hormone production.
SRY mutation causes gonadal dysgenesis in most cases [137], but in some of the other cases, mutation of Dhh may take charge. The mutations of Dhh were reported in some gonadal dysgenesis (GD) cases [138]. In some cases, the patients grew up as female but suffered from a defect of breast development, they were then found with 46, XY karyotype and homozygous mutation on the Dhh gene. The premature termination or loss of self-cleavage of Dhh protein or perturbation of the interaction of Dhh with its binding partners may contribute to GD [139], and in the other case, the patient got estrogen replacement therapy to rescue breast development [140]. The rescue of breast development was successful but had serious side effects: more obvious peripheral neuropathy, obesity, insulin resistance, fatty liver, and gastric ulcers.
One pure gonadal dysgenesis (PGD) patient with a homozygous missense mutation of Dhh was found the same heterozygous mutation in his father [141]. Two siblings with disorders of sex development (DSD) were found to compound heterozygous mutations of Dhh which were inherited from their parents [142]. The two reports suggested the risk that mutations of Dhh may be inherited and lead to the disease of offspring. Another PGD case was where a Dhh mutation altered a conserved residue among HH genes. It was found in situ seminoma and loss of Leydig cells in the peritubular, the patients with this mutation also suffered from polyneuropathy and change of peripheral structure [143]. HH homozygous mutation was also found in some individuals of PGD patients [144]. For these patients, the treatment after gonad development could not reverse the sex character but accumulated more hormone problems [124], and the hormone treatment couldn’t save the defect during external genital development at fetal time in a mice experiment but the additional DHT rescued steroidogenic function of Leydig cells to some extent [82].
HH signaling is so important for a human’s physical development, but gonadal dysgenesis patients with the mutation of HH signaling are not easy to find at their very young age before the gonad mature age. However, it is the premature stage for the patients to obtain medical aid before the gonad fails to develop since hormone therapy in adult patients has so many side effects and can’t rescue the gonadal development. So the cure of gonadal dysgenesis patients should concentrate on the younger stage and still wait for further exploration.
Since the discovery of HH signaling, more research concentrated on Shh and Ihh, and they also made some progress in some diseases. The usage of Metformin could improve bronchopulmonary dysplasia through the regulation of Shh [145], the increase and over-expression of Shh could protect the development of embryonic spinal cord neocortical, neural stem cell number and expansion of the neocortex [146,147]. Some reports on Ihh uncovered the important role of micro RNAs in the function of this signaling by targeting Ihh or the signaling [148–150]. After a dozen years of research on Dhh, the signaling function of male gonads and hormones also gradually dis-enveloped. Dhh is produced in Sertoli cells in the testes and targets germ cell and Leydig cells to ensure germ cell and Leydig cell differentiation, androgen production, and integrity of testes structure during embryonic development.
HH signaling could be used to regulate cells and organoid states in experiments on testis [151]. The interaction of HH members and hormones reminds us of the possibility that hormone therapy for prostate cancers or other diseases may not be sufficient and HH signaling should be considered. The mutation of HH signaling members in gonadal dysgenesis provides a better view of this kind of disease and how to make better therapy plans to fight for the health of patients. However, research on HH signaling mostly concentrated on vertebrate animals and the difference with invertebrates was largely ignored. How this signaling evolved in the animal kingdom may support us with a better view of evolution.
Acknowledgement: We appreciate all members of the Sperm Laboratory of Zhejiang University for providing valuable support, we also want to thank the College Students Creative Research Training Center at National Biological Demonstration Experimental Center, Zhejiang University for their service.
Funding Statement: This work was supported in part by the National Natural Science Foundation of China (Nos. 32270555 and 32072954).
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Jun-Jie Yu, Wan-Xi Yang; draft manuscript preparation: Jun-Jie Yu; review and editing: Jun-Jie Yu, Wan-Xi Yang; supervision: Wan-Xi Yang. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. John J, Lee DPVK. Secretion and localized transcription suggest a role in positional signaling for products of the segmentation gene hedgehog. Cell. 1992;71(1):33–50. doi:10.1016/0092-8674(92)90264-D. [Google Scholar] [PubMed] [CrossRef]
2. Mohler J, Vani K. Molecular organization and embryonic expression of the hedgehog gene involved in cell-cell communication in segmental patterning of drosophila. Development. 1992;115(4):957–71. doi:10.1242/dev.115.4.957. [Google Scholar] [PubMed] [CrossRef]
3. Briscoe J, Thérond PP. The mechanisms of hedgehog signalling and its roles in development and disease. Nature Reviews. Mol Cell Biol. 2013;14(7):416–29. doi:10.1038/nrm3598. [Google Scholar] [PubMed] [CrossRef]
4. Wu M, Mi J, Qu G, Zhang S, Jian Y, Gao C, et al. Role of hedgehog signaling pathways in multipotent mesenchymal stem cells differentiation. Cell Transplant. 2024;33. doi:10.1177/09636897241244943. [Google Scholar] [PubMed] [CrossRef]
5. Gamart J, Barozzi I, Laurent F, Reinhardt R, Martins LR, Oberholzer T, et al. SMAD4 target genes are part of a transcriptional network that integrates the response to bmp and shh signaling during early limb bud patterning. Development. 2021;148(23):dev200182. doi:10.1242/dev.200182. [Google Scholar] [PubMed] [CrossRef]
6. Tada R, Higashidate T, Amano T, Ishikawa S, Yokoyama C, Kobari S, et al. The shh limb enhancer is activated in patterned limb regeneration but not in hypomorphic limb regeneration in Xenopus laevis. Dev Biol. 2023;500:22–30. doi:10.1016/j.ydbio.2023.05.009. [Google Scholar] [PubMed] [CrossRef]
7. Sato Y, Fujiwara M, Nishino H, Harada R, Kawasaki E, Morimoto R, et al. Normal skeletal pattern formation in chick limb bud with a mesenchymal hole is mediated by adjustment of cellular properties along the anterior-posterior axis in the limb bud. Dev Biol. 2022;483:76–88. doi:10.1016/j.ydbio.2021.12.016. [Google Scholar] [PubMed] [CrossRef]
8. Douceau S, Deutsch GT, Ferent J. Establishing hedgehog gradients during neural development. Cells. 2023;12(2):225. doi:10.3390/cells12020225. [Google Scholar] [PubMed] [CrossRef]
9. Wang W, Shiraishi R, Kawauchi D. Sonic hedgehog signaling in cerebellar development and cancer. Front Cell Dev Biol. 2022;10:864035. doi:10.3389/fcell.2022.864035. [Google Scholar] [PubMed] [CrossRef]
10. Zhang H, Xu S, He D, Wang X, Zhu G. Spatiotemporal expression of SHH/GLI signaling in human fetal bladder development. Front Pediatr. 2021;9:765255. doi:10.3389/fped.2021.765255. [Google Scholar] [PubMed] [CrossRef]
11. Sasai N, Tada S, Ohshiro J, Kogiso C, Shinozuka T. Regulation of progenitor cell survival by a novel chromatin remodeling factor during neural tube development. Dev Growth Differ. 2024;66(1):89–100. doi:10.1111/dgd.12905. [Google Scholar] [PubMed] [CrossRef]
12. Gingrich EC, Case K, Garcia ADR. A subpopulation of astrocyte progenitors defined by sonic hedgehog signaling. Neural Dev. 2022;17(1):2. doi:10.1186/s13064-021-00158-w. [Google Scholar] [PubMed] [CrossRef]
13. Kanungo J, Twaddle NC, Silva C, Robinson B, Wolle M, Conklin S, et al. Inorganic arsenic alters the development of dopaminergic neurons but not serotonergic neurons and induces motor neuron development via sonic hedgehog pathway in zebrafish. Neurosci Lett. 2023;795:137042. doi:10.1016/j.neulet.2022.137042. [Google Scholar] [PubMed] [CrossRef]
14. Li MY, Yang XL, Chung CC, Lai YJ, Tsai JC, Kuo YL, et al. Trip6 promotes neural stem cell maintenance throughyap-mediated sonic hedgehog activation. FASEB J. 2024;38(5):e23501. doi:10.1096/fj.202301805RRR. [Google Scholar] [PubMed] [CrossRef]
15. Nocera S, Marchena MA, Fernández-Gómez B, Gómez-Martín P, Sánchez-Jiménez E, Macías-Castellano A, et al. Activation of Shh/Smo is sufficient to maintain oligodendrocyte precursor cells in an undifferentiated state and is not necessary for myelin formation and (Re)myelination. Glia. 2024. doi:10.1002/glia.24540. [Google Scholar] [PubMed] [CrossRef]
16. Xie Y, Kuan AT, Wang W, Herbert ZT, Mosto O, Olukoya O, et al. Astrocyte-neuron crosstalk through hedgehog signaling mediates cortical synapse development. Cell Rep. 2022;38(8):110416. doi:10.1016/j.celrep.2022.110416. [Google Scholar] [PubMed] [CrossRef]
17. Liu X, Pacwa A, Bresciani G, Swierczynska M, Dorecka M, Smedowski A. Retinal primary cilia and their dysfunction in retinal neurodegenerative diseases: beyond ciliopathies. Mol Med. 2024;30(1):109. doi:10.1186/s10020-024-00875-y. [Google Scholar] [PubMed] [CrossRef]
18. Yang C, Qi Y, Sun Z. The role of sonic hedgehog pathway in the development of the central nervous system and aging-related neurodegenerative diseases. Front Mol Biosci. 2021;8:711710. doi:10.3389/fmolb.2021.711710. [Google Scholar] [PubMed] [CrossRef]
19. Bhardwaj K, Jha A, Roy A, Kumar H. The crucial role of Vps35 and Shh in Parkinson’s disease: understanding the mechanisms behind the neurodegenerative disorder. Brain Res. 2024;1845:149204. doi:10.1016/j.brainres.2024.149204. [Google Scholar] [PubMed] [CrossRef]
20. Ohba S. Hedgehog signaling in skeletal development: roles of indian hedgehog and the mode of its action. Int J Mol Sci. 2020;21(18):6665. doi:10.3390/ijms21186665. [Google Scholar] [PubMed] [CrossRef]
21. Jiang Z, Derrick-Roberts ALK, Byers S. Altered Ihh signaling contributes to reduced chondrocyte proliferation in the growth plate of Mps Vii Mice. Mol Genet Metab Rep. 2020;25:100668. doi:10.1016/j.ymgmr.2020.100668. [Google Scholar] [PubMed] [CrossRef]
22. Orikasa S, Matsushita Y, Manabe H, Fogge M, Lee Z, Mizuhashi K, et al. Hedgehog activation promotes osteogenic fates of growth plate resting zone chondrocytes through transient clonal competency. JCI Insight. 2023;9(2):e165619. doi:10.1172/jci.insight.165619. [Google Scholar] [PubMed] [CrossRef]
23. Peng G, Sun H, Jiang H, Wang Q, Gan L, Tian Y, et al. Exogenous Growth hormone functionally alleviates glucocorticoid-induced longitudinal bone growth retardation in male rats by activating the Ihh/Pthrp signaling pathway. Mol Cell Endocrinol. 2022;545:111571. doi:10.1016/j.mce.2022.111571. [Google Scholar] [PubMed] [CrossRef]
24. Ghuloum FI, Johnson CA, Riobo-Del Galdo NA, Amer MH. From mesenchymal niches to engineered in vitro model systems: exploring and exploiting biomechanical regulation of vertebrate hedgehog signalling. Materials Today Bio. 2022;17:100502. doi:10.1016/j.mtbio.2022.100502. [Google Scholar] [PubMed] [CrossRef]
25. Sun Q, Huang J, Tian J, Lv C, Li Y, Yu S, et al. Key roles of Gli1 and Ihh signaling in craniofacial development. Stem Cells Dev. 2024. doi:10.1089/scd.2024.0036. [Google Scholar] [PubMed] [CrossRef]
26. Cao X, Deng S, Liu Q, Wu L, Zhuang X, Ding S. Important role of the ihh signaling pathway in initiating early cranial remodeling and morphological specialization in cromileptes altivelis. Animals. 2023;13(24). doi:10.3390/ani13243840. [Google Scholar] [PubMed] [CrossRef]
27. Pachernegg S, Georges E, Ayers K. The desert hedgehog signalling pathway in human gonadal development and differences of sex development. Sex Dev. 2022;16(2–3):98–111. doi:10.1159/000518308. [Google Scholar] [PubMed] [CrossRef]
28. Johansson HKL, Svingen T. Hedgehog signal disruption, gonadal dysgenesis and reproductive disorders: is there a link to endocrine disrupting chemicals? Current Res Toxicol. 2020;1:116–123. doi:10.1016/j.crtox.2020.10.001. [Google Scholar] [PubMed] [CrossRef]
29. Chai S, Tian R, Yang Y, Yang G, Xu S, Ren W. Enhanced negative regulation of the DHH signaling pathway as a potential mechanism of ascrotal testes in laurasiatherians. Evol Biol. 2021;48(3):335–45. doi:10.1007/s11692-021-09542-0. [Google Scholar] [CrossRef]
30. Xu J, Iyyanar PPR, Lan Y, Jiang R. Sonic hedgehog signaling in craniofacial development. Differentiation. 2023;133:60–76. doi:10.1016/j.diff.2023.07.002. [Google Scholar] [PubMed] [CrossRef]
31. Westendorp F, Karpus ON, Koelink PJ, Vermeulen JLM, Meisner S, Koster J, et al. Epithelium-derived Indian hedgehog restricts stromal expression of erbb family members that drive colonic tumor cell proliferation. Oncogene. 2021;40(9):1628–43. doi:10.1038/s41388-020-01633-0. [Google Scholar] [PubMed] [CrossRef]
32. Zotter B, Dagan O, Brady J, Baloui H, Samanta J, Salzer JL. Gli1 regulates the postnatal acquisition of peripheral nerve architecture. J Neurosci. 2022;42(2):183–201. doi:10.1523/JNEUROSCI.3096-20.2021. [Google Scholar] [PubMed] [CrossRef]
33. Moreau N, Boucher Y. Hedging against neuropathic pain: role of hedgehog signaling in pathological nerve healing. Int J Mol Sci. 2020;21(23):9115. doi:10.3390/ijms21239115. [Google Scholar] [PubMed] [CrossRef]
34. Boso F, Zanette G, Baldinotti F, Bertelloni S, Taioli F, Monaco S, et al. Convergent pathological and ultrasound features in hereditary syndromic and non-syndromic minifascicular neuropathy related to Dhh. J Peripher Nerv Syst. 2020;25(4):423–8. doi:10.1111/jns.12417. [Google Scholar] [PubMed] [CrossRef]
35. Ma L, Duan CC, Yang ZQ, Ding JL, Liu S, Yue ZP, et al. Novel insights into Dhh signaling in antler chondrocyte proliferation and differentiation: involvement of foxa. J Cell Physiol. 2020;235(9):6023–31. doi:10.1002/jcp.29528. [Google Scholar] [PubMed] [CrossRef]
36. Li B, Yan Y, He Y, Liang C, Li M, Wang Y, et al. Ihh, Shh, and primary cilia mediate epithelial-stromal cross-talk during decidualization in mice. Sci Signal. 2023;16(774):eadd0645. doi:10.1126/scisignal.add0645. [Google Scholar] [PubMed] [CrossRef]
37. Sigafoos AN, Paradise BD, Fernandez-Zapico ME. Hedgehog/Gli signaling pathway: transduction, regulation, and implications for disease. Cancers. 2021;13(14):3410. doi:10.3390/cancers13143410. [Google Scholar] [PubMed] [CrossRef]
38. Bian Y, Hahn H, Uhmann A. The hidden hedgehog of the pituitary: hedgehog signaling in development, adulthood and disease of the hypothalamic-pituitary axis. Front Endocrinol. 2023;14. doi:10.3389/fendo.2023.1219018. [Google Scholar] [PubMed] [CrossRef]
39. Bhattacharya I, Dey S. Emerging concepts on leydig cell development in fetal and adult testis. Front Endocrinol. 2023;13. doi:10.3389/fendo.2022.1086276. [Google Scholar] [PubMed] [CrossRef]
40. Clark AM, Garland KK, Russell LD. Desert Hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63(6):1825–38. doi:10.1095/biolreprod63.6.1825. [Google Scholar] [PubMed] [CrossRef]
41. Bitgood MJ, Shen L, Mcmahon AP. Sertoli cell signaling by desert hedgehog regulates the male germline. Curr Biol. 1996;6(3):298–304. doi:10.1016/S0960-9822(02)00480-3. [Google Scholar] [PubMed] [CrossRef]
42. Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal leydig cell fate in testis organogenesis. Genes Dev. 2002;16(11):1433–40. doi:10.1101/gad.981202. [Google Scholar] [PubMed] [CrossRef]
43. Tang C, Pan Y, Luo H, Xiong W, Zhu H, Ruan H, et al. Hedgehog signaling stimulates the conversion of cholesterol to steroids. Cell Signal. 2015;27(3):487–97. doi:10.1016/j.cellsig.2015.01.004. [Google Scholar] [PubMed] [CrossRef]
44. Kothandapani A, Jefcoate CR, Jorgensen JS. Cholesterol contributes to male sex differentiation through its developmental role in androgen synthesis and hedgehog signaling. Endocrinology. 2021;162(7). doi:10.1210/endocr/bqab066. [Google Scholar] [PubMed] [CrossRef]
45. Yatsenko SA, Witchel SF, Gordon CM. Primary amenorrhea and premature ovarian insufficiency. Endocrin Metab Clin. 2024;53(2):293–305. doi:10.1016/j.ecl.2024.01.009. [Google Scholar] [PubMed] [CrossRef]
46. Chakravarthi VP, Dilower I, Ghosh S, Borosha S, Mohamadi R, Dahiya V, et al. Erβ regulation of indian hedgehog expression in the first wave of ovarian follicles. Cells. 2024;13(7):644. doi:10.3390/cells13070644. [Google Scholar] [PubMed] [CrossRef]
47. Dilower I, Niloy AJ, Kumar V, Kothari A, Lee EB, Rumi MAK. Hedgehog signaling in gonadal development and function. Cells. 2023;12(3):358. doi:10.3390/cells12030358. [Google Scholar] [PubMed] [CrossRef]
48. Takabatake T, Takahashi TC, Inoue K, Ogawa M, Takeshima K. Activation of two cynops genes, fork head and sonic hedgehog, in animal cap explants1. Biochem Biophys Res Commun. 1996;218(1):395–401. doi:10.1006/bbrc.1996.0069. [Google Scholar] [PubMed] [CrossRef]
49. Avaron F, Hoffman L, Guay D, Akimenko MA. Characterization of two new zebrafish members of the hedgehog family: atypical expression of a zebrafishindian hedgehog gene in skeletal elements of both endochondral and dermal origins. Dev Dynam. 2006;235(2):478–89. doi:10.1002/dvdy.20619. [Google Scholar] [PubMed] [CrossRef]
50. Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, et al. Floor plate and motor neuron induction by Vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76(4):761–75. doi:10.1016/0092-8674(94)90514-2. [Google Scholar] [PubMed] [CrossRef]
51. Peter D, Currie PWI. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature. 1996;6590(382):452–5. doi:10.1038/382452a0. [Google Scholar] [PubMed] [CrossRef]
52. Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in drosophila. Nature. 1980;287(5785):795–801. doi:10.1038/287795a0. [Google Scholar] [PubMed] [CrossRef]
53. Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the Zpa. Cell. 1993;75(7):1401–16. doi:10.1016/0092-8674(93)90626-2. [Google Scholar] [PubMed] [CrossRef]
54. Andrea Vortkamp KLBL, Gino V.Segre HMKC. Regulation of rate of cartilage differentiation by indian hedgehog and PTH-related protein. Science. 1996;5275(273):613–22. doi:10.1126/science.273.5275.613. [Google Scholar] [PubMed] [CrossRef]
55. Chang DT, López A, Kessler DPV, Chiang C, Simandl BK, Zhao R, et al. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development. 1994;120(11):3339–53. doi:10.1242/dev.120.11.3339. [Google Scholar] [PubMed] [CrossRef]
56. Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, Mcmahon JA, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of cns polarity. Cell. 1993;75(7):1417–30. doi:10.1016/0092-8674(93)90627-3. [Google Scholar] [PubMed] [CrossRef]
57. Ekker SC, Mcgrew LL, Lai CJ, Lee JJ, von Kessler DP, Moon RT, et al. Distinct expression and shared activities of members of the hedgehog gene family of xenopus laevis. Development. 1995;121(8):2337–47. doi:10.1242/dev.121.8.2337. [Google Scholar] [PubMed] [CrossRef]
58. Ruiz i Altaba A, Jessell TM, Roelink H. Restrictions to floor plate induction by hedgehog and winged-helix genes in the neural tube of frog embryos. Mol Cell Neurosci. 1995;2(6):106–21. doi:10.1006/mcne.1995.1011. [Google Scholar] [PubMed] [CrossRef]
59. Zhang Y, Beachy PA. Cellular and molecular mechanisms of hedgehog signalling. Nat Rev Mol Cell Bio. 2023;24(9):668–87. doi:10.1038/s41580-023-00591-1. [Google Scholar] [PubMed] [CrossRef]
60. Kinnebrew M, Luchetti G, Sircar R, Frigui S, Viti LV, Naito T, et al. Patched 1 reduces the accessibility of cholesterol in the outer leaflet of membranes. elife. 2021;10:698. doi:10.7554/eLife.70504. [Google Scholar] [PubMed] [CrossRef]
61. Li Y, Sun X, Gao D, Ding Y, Liu J, Chen J, et al. Dual functions of rack1 in regulating hedgehog pathway. Cell Death Differ. 2020;27(11):3082–96. doi:10.1038/s41418-020-0563-7. [Google Scholar] [PubMed] [CrossRef]
62. Little JC, Garcia-Garcia E, Sul A, Kalderon D. Drosophila hedgehog can act as a morphogen in the absence of regulated ci processing. eLife. 2020;9. doi:10.7554/eLife.61083. [Google Scholar] [PubMed] [CrossRef]
63. Gong W, Zhao W, Liu G, Shi L, Zhao X. Curcumin analogue Bddd-721 Exhibits more potent anticancer effects than curcumin on medulloblastoma by targeting Shh/Gli1 signaling pathway. Aging. 2022;14(13):5464–77. doi:10.18632/aging.204161. [Google Scholar] [PubMed] [CrossRef]
64. He M, Subramanian R, Bangs F, Omelchenko T, Liem Jr KF, Kapoor TM. The kinesin-4 protein Kif7 regulates mammalian hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol. 2014;16(7):663–72. doi:10.1038/ncb2988. [Google Scholar] [PubMed] [CrossRef]
65. Katoh Y, Katoh M. Kif27 is one of orthologs for drosophila costal-2. Int J Oncol. 2004;25(6):1875–80. [Google Scholar] [PubMed]
66. Fulmer D, Toomer KA, Glover J, Guo L, Moore K, Moore R, et al. Desert hedgehog-primary cilia cross talk shapes mitral valve tissue by organizing smooth muscle actin. Dev Biol. 2020;463(1):26–38. doi:10.1016/j.ydbio.2020.03.003. [Google Scholar] [PubMed] [CrossRef]
67. Yue Y, Engelke MF, Blasius TL, Verhey KJ, Welch M. Hedgehog-induced ciliary trafficking of kinesin-4 Motor Kif7 requires intraflagellar transport but Not Kif7’S microtubule binding. Mol Biol Cell. 2022;33(1):br1. doi:10.1091/mbc.E21-04-0215. [Google Scholar] [PubMed] [CrossRef]
68. Zhang Y, Lu K, Wu X, Liu H, Xin J, Wang X, et al. Genetic variants in the hedgehog signaling pathway genes are associated with gastric cancer risk in a chinese han population. J Biomed Res. 2022;36(1):22. doi:10.7555/JBR.35.20210091. [Google Scholar] [PubMed] [CrossRef]
69. Yoshida T, Takizawa N, Matsuda T, Yamada H, Kitada M, Tanaka S. Gata4/6 regulate Dhh transcription in rat adrenocortical autografts. Sci Rep. 2020;10(1). doi:10.1038/s41598-019-57351-5. [Google Scholar] [PubMed] [CrossRef]
70. Qi Q, Dong Z, Zhang N, Wang L, Shao C, Xu W. Expression and functional analysis of the desert hedgehog (dhh) gene in chinese tongue sole (Cynoglossus semilaevis). Gene Expr Patterns. 2021;39:119163. doi:10.1016/j.gep.2020.119163. [Google Scholar] [PubMed] [CrossRef]
71. Kuwabara PE, Lee MH, Schedl T, Jefferis GSAC. Elegans patched gene, Ptc-1, functions in germ-line cytokinesis. Gene Dev. 2000;14(15):1933–44. doi:10.1101/gad.14.15.1933. [Google Scholar] [CrossRef]
72. Morales CR, Fox A, El Alfy M, Ni X, Argraves WS. Expression of patched-1 and smoothened in testicular meiotic and post-meiotic cells. Microsc Res Techniq. 2009;72(11):809–15. doi:10.1002/jemt.20733. [Google Scholar] [PubMed] [CrossRef]
73. Sahin Z, Szczepny A, Mclaughlin EA, Meistrich ML, Zhou W, Ustunel I, et al. Dynamic hedgehog signalling pathway activity in germline stem cells. Andrology. 2014;2(2):267–74. doi:10.1111/j.2047-2927.2014.00187.x. [Google Scholar] [PubMed] [CrossRef]
74. Fan J, Akabane H, Zheng X, Zhou X, Zhang L, Liu Q, et al. Male germ cell-specific expression of a novel patched-domain containing gene Ptchd3. Biochem Bioph Res Co. 2007;363(3):757–61. doi:10.1016/j.bbrc.2007.09.047. [Google Scholar] [PubMed] [CrossRef]
75. Szczepny A, Hime GR, Loveland KL. Expression of hedgehog signalling components in adult mouse testis. Dev Dynam. 2006;235(11):3063–70. doi:10.1002/dvdy.20931. [Google Scholar] [PubMed] [CrossRef]
76. Kothandapani A, Larsen MC, Lee J, Jorgensen JS, Jefcoate CR. Distinctive functioning of stard1 in the fetal leydig cells compared to adult leydig and adrenal cells. impact of hedgehog signaling via the primary cilium. Mol Cell Endocrinol. 2021;531:111265. doi:10.1016/j.mce.2021.111265. [Google Scholar] [PubMed] [CrossRef]
77. Deshpande G, Ng C, Jourjine N, Chiew JW, Dasilva J, Schedl P. Hedgehog signaling guides migration of primordial germ cells to thedrosophila somatic gonad. Genetics. 2023;225(3). doi:10.1093/genetics/iyad165. [Google Scholar] [PubMed] [CrossRef]
78. Cao H, Li L, Li Z, Gao H, Peng G, Zhu C, et al. Rna-Seq Analysis of ovary and testis reveals potential differentially expressed transcripts associated with gonadal unsynchronization development in onychostoma macrolepis. Gene Expr Patterns. 2023;47:119303. doi:10.1016/j.gep.2022.119303. [Google Scholar] [PubMed] [CrossRef]
79. Dong F, Ping P, Ma Y, Chen X. Application of single-cell Rna sequencing on human testicular samples: a comprehensive review. Int J Biol Sci. 2023;19(7):2167–97. doi:10.7150/ijbs.82191. [Google Scholar] [PubMed] [CrossRef]
80. Barsoum I, Yao HHC. Redundant and differential roles of transcription factors Gli1 and Gli2 in the development of mouse fetal leydig cells1. Biol Reprod. 2011;84(5):894–9. doi:10.1095/biolreprod.110.088997. [Google Scholar] [PubMed] [CrossRef]
81. Brokken LJS, Adamsson A, Paranko J, Toppari J. Antiandrogen exposure in Utero disrupts expression of desert hedgehog and insulin-like factor 3 in the developing fetal rat testis. Endocrinology. 2009;150(1):445–51. doi:10.1210/en.2008-0230. [Google Scholar] [PubMed] [CrossRef]
82. Kothandapani A, Lewis SR, Noel JL, Zacharski A, Krellwitz K, Baines A, et al. Gli3 resides at the intersection of hedgehog and androgen action to promote male sex differentiation. PLoS Genet. 2020;16(6):e1008810. doi:10.1371/journal.pgen.1008810. [Google Scholar] [PubMed] [CrossRef]
83. Min M, Song T, Sun M, Wang T, Tan J, Zhang J. Dhh signaling pathway regulates reconstruction of seminiferous tubule-like structure. Reprod Biol. 2022;22(4):100684. doi:10.1016/j.repbio.2022.100684. [Google Scholar] [PubMed] [CrossRef]
84. Pierucci-Alves F, Clark AM, Russell LD. A Developmental study of the desert hedgehog-null mouse testis. Biol Reprod. 2001;65(5):1392–402. doi:10.1095/biolreprod65.5.1392. [Google Scholar] [PubMed] [CrossRef]
85. Park SY, Tong M, Jameson JL. Distinct roles for steroidogenic factor 1 and desert hedgehog pathways in fetal and adult leydig cell development. Endocrinology. 2007;148(8):3704–10. doi:10.1210/en.2006-1731. [Google Scholar] [PubMed] [CrossRef]
86. Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HHC. Activation of the hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal leydig cells and female pseudohermaphroditism. Dev Biol. 2009;329(1):96–103. doi:10.1016/j.ydbio.2009.02.025. [Google Scholar] [PubMed] [CrossRef]
87. Barsoum IB, Kaur J, Ge RS, Cooke PS, Yao HHC. Dynamic changes in fetal leydig cell populations influence adult leydig cell populations in mice. FASEB J. 2013;27(7):2657–66. doi:10.1096/fj.12-225060. [Google Scholar] [PubMed] [CrossRef]
88. Arraf AA, Yelin R, Reshef I, Jadon J, Abboud M, Zaher M, et al. Hedgehog signaling regulates epithelial morphogenesis to position the ventral embryonic midline. Dev Cell. 2020;53(5):589–602.e6. doi:10.1016/j.devcel.2020.04.016. [Google Scholar] [PubMed] [CrossRef]
89. Choi HJ, Jung KM, Park KJ, Lee KY, Woo SJ, Han JY. Single-cell transcriptome analysis of male chicken germ cells reveals changes in signaling pathway-related gene expression profiles during mitotic arrest. Febs Open Bio. 2023;13(5):833–44. doi:10.1002/2211-5463.13600. [Google Scholar] [PubMed] [CrossRef]
90. Mäkelä J, Saario V, Bourguiba-Hachemi S, Nurmio M, Jahnukainen K, Parvinen M, et al. Hedgehog signalling promotes germ cell survival in the rat testis. Reproduction. 2011;142(5):711–21. doi:10.1530/REP-11-0110. [Google Scholar] [PubMed] [CrossRef]
91. Changle Zhao ZZXQ. Desert hedgehog mediates the proliferation of medaka spermatogonia through smoothened signaling. Reproduction. 2022;4(163):209–18. doi:10.1530/REP-21-0468. [Google Scholar] [PubMed] [CrossRef]
92. Zhao C, Liu X, Liu L, Li J, Liu X, Tao W, et al. Smoothened mediates medaka spermatogonia proliferation via Gli1-Rgcc–Cdk1 Axis. Biol Reprod. 2023;109(5):772–84. doi:10.1093/biolre/ioad090. [Google Scholar] [PubMed] [CrossRef]
93. Yao HH, Capel B. Disruption of testis cords by cyclopamine or forskolin reveals independent cellular pathways in testis organogenesis. Dev Biol. 2002;246(2):356–65. doi:10.1006/dbio.2002.0663. [Google Scholar] [PubMed] [CrossRef]
94. Szczepny A, Hogarth CA, Young J, Loveland KL. Identification of hedgehog signaling outcomes in mouse testis development using a hanging drop-culture system1. Biol Reprod. 2009;80(2):258–63. doi:10.1095/biolreprod.108.067926. [Google Scholar] [PubMed] [CrossRef]
95. Li D, Cheng S, Zhang W, Wang M, Sun C, Zhang C, et al. Hedgehog-Gli1 signaling regelates differentiation of chicken (Gallus gallus) embryonic stem cells to male germ cells. Anim Reprod Sci. 2017;182:9–20. doi:10.1016/j.anireprosci.2017.02.002. [Google Scholar] [PubMed] [CrossRef]
96. Kroft TL, Patterson J, Won Yoon J, Doglio L, Walterhouse DO, Iannaccone PM, et al. Gli1 localization in the germinal epithelial cells alternates between cytoplasm and nucleus: upregulation in transgenic mice blocks spermatogenesis in pachytene1. Biol Reprod. 2001;65(6):1663–71. doi:10.1095/biolreprod65.6.1663. [Google Scholar] [PubMed] [CrossRef]
97. Shan Y, Yu Y, Li X, Zhu Q, Wang Y, Zhao Z, et al. Arbutin inhibits androgen biosynthesis by rat immature leydig cells in vitro. Reprod Toxicol. 2023;122:108476. doi:10.1016/j.reprotox.2023.108476. [Google Scholar] [PubMed] [CrossRef]
98. Mpatsoulis D, Nieto JJ, Lonsdale R, Fisher C, Mazibrada J. Differential diagnosis of adipocytic differentiation in androgen-secreting mature ovarian teratoma with leydig cell hyperplasia. Gynecol Oncol Rep. 2021;36:100786. doi:10.1016/j.gore.2021.100786. [Google Scholar] [PubMed] [CrossRef]
99. Haider SG. Cell biology of leydig cells in the testis. Int Rev Cytol. 2004;233:181. doi:10.1016/S0074-7696(04)33005-6. [Google Scholar] [PubMed] [CrossRef]
100. Galuszka AP, Pardyak L, Tuz R, Płachno BJ, Malopolska M, et al. Leydig cells in immunocastrated polish landrace pig testis: differentiation status and steroid enzyme expression status. Int J Mol Sci. 2022;23(11):6120. doi:10.3390/ijms23116120. [Google Scholar] [PubMed] [CrossRef]
101. Stévant IP, Neirijnck Y, Rebourcet D, Darbey A, Curley MK, Françoise K, et al. Specific transcriptomic signatures and dual regulation of steroidogenesis between fetal and adult mouse leydig cells. Front Cell Dev Biol. 2021;9. doi:10.3389/fcell.2021.695546. [Google Scholar] [PubMed] [CrossRef]
102. Jiang K, Jorgensen JS. Fetal leydig cells: what we know and what we don’t. Mol Reprod Dev. 2024;91(3):e23739. doi:10.1002/mrd.23739. [Google Scholar] [PubMed] [CrossRef]
103. Rivera-González KS, Beames TG, Lipinski RJ. Examining the developmental toxicity of piperonyl butoxide as a sonic hedgehog pathway inhibitor. Chemosphere. 2021;264:128414. doi:10.1016/j.chemosphere.2020.128414. [Google Scholar] [PubMed] [CrossRef]
104. Kawai Y, Noguchi J, Akiyama K, Takeno Y, Fujiwara Y, Kajita S, et al. A missense mutation of the dhh gene is associated with male pseudohermaphroditic rats showing impaired leydig cell development. Reproduction. 2011;141(2):217–25. doi:10.1530/REP-10-0006. [Google Scholar] [PubMed] [CrossRef]
105. Arora H, Qureshi R, Khodamoradi K, Seetharam D, Parmar M, Van Booven DJ, et al. Leptin secreted from testicular microenvironment modulates hedgehog signaling to augment the endogenous function of leydig cells. Cell Death Dis. 2022;13(3):208–8. doi:10.1038/s41419-022-04658-3. [Google Scholar] [PubMed] [CrossRef]
106. Zhu H, Zou C, Fan X, Xiong W, Tang L, Wu X, et al. Up-regulation of 11β-hydroxysteroid dehydrogenase type 2 expression by hedgehog ligand contributes to the conversion of cortisol into cortisone. Endocrinology. 2016;157(9):3529–39. doi:10.1210/en.2016-1286. [Google Scholar] [PubMed] [CrossRef]
107. Lubik AA, Nouri M, Truong S, Ghaffari M, Adomat HH, Corey E, et al. Paracrine sonic hedgehog signaling contributes significantly to acquired steroidogenesis in the prostate tumor microenvironment. Int J Cancer. 2017;140(2):358–69. doi:10.1002/ijc.30450. [Google Scholar] [PubMed] [CrossRef]
108. Levina E, Chen M, Carkner R, Shtutman M, Buttyan R. Paracrine hedgehog increases the steroidogenic potential of prostate stromal cells in a Gli-dependent manner. The Prostate. 2012;72(8):817–24. doi:10.1002/pros.21500. [Google Scholar] [PubMed] [CrossRef]
109. Chen M, Wang X, Wang Y, Zhang L, Xu B, Lv L, et al. Wt1 is involved in leydig cell steroid hormone biosynthesis by regulating paracrine factor expression in mice1. Biol Reprod. 2014;90(4). doi:10.1095/biolreprod.113.114702. [Google Scholar] [PubMed] [CrossRef]
110. Pyczek J, Buslei R, Schult D, Hölsken A, Buchfelder M, Heß I, et al. Hedgehog signaling activation induces stem cell proliferation and hormone release in the adult pituitary gland. Sci Rep. 2016;6(1):24928. doi:10.1038/srep24928. [Google Scholar] [PubMed] [CrossRef]
111. Botermann DS, Brandes N, Frommhold A, Heß I, Wolff A, Zibat A, et al. Hedgehog signaling in endocrine and folliculo-stellate cells of the adult pituitary. J Endocrinol. 2021;248(3):303–16. doi:10.1530/JOE-20-0388. [Google Scholar] [PubMed] [CrossRef]
112. Pu Y, Huang L, Prins GS. Sonic hedgehog-patched Gli signaling in the developing rat prostate gland: lobe-specific suppression by neonatal estrogens reduces ductal growth and branching. Dev Biol. 2004;273(2):257–75. doi:10.1016/j.ydbio.2004.06.002. [Google Scholar] [PubMed] [CrossRef]
113. Medwid S, Guan H, Yang K. Bisphenol a stimulates adrenal cortical cell proliferation via erβ-mediated activation of the sonic hedgehog signalling pathway. J Steroid Biochem Mol Biol. 2018;178:254–62. doi:10.1016/j.jsbmb.2018.01.004. [Google Scholar] [PubMed] [CrossRef]
114. Terauchi KJ, Miyagawa S, Iguchi T, Sato T. Hedgehog signaling regulates the basement membrane remodeling during folliculogenesis in the neonatal mouse ovary. Cell Tissue Res. 2020;381(3):555–67. doi:10.1007/s00441-020-03222-9. [Google Scholar] [PubMed] [CrossRef]
115. Zhang J, Li H, Xu Z, Lu J, Cao C, Shen H, et al. Oestrogen ameliorates blood-brain barrier damage after experimental subarachnoid haemorrhage via the Shh pathway in male rats. Stroke Vasc Neurol. 2023;8(3):217–28. doi:10.1136/svn-2022-001907. [Google Scholar] [PubMed] [CrossRef]
116. Geoghegan IP, Mcnamara LM, Hoey DA. Estrogen withdrawal alters cytoskeletal and primary ciliary dynamics resulting in increased hedgehog and osteoclastogenic paracrine signalling in osteocytes. Sci Rep. 2021;11(1):9272. doi:10.1038/s41598-021-88633-6. [Google Scholar] [PubMed] [CrossRef]
117. Zhang D, Raza SHA, Du X, Wang J, Wang M, Ma J, et al. Effect of feeding corn silage on semen quality and spermatogenesis of bulls. Vet Res Commun. 2024;48(1):391–401. doi:10.1007/s11259-023-10218-7. [Google Scholar] [PubMed] [CrossRef]
118. Wang Y, Davidow L, Arvanites AC, Blanchard J, Lam K, Xu K, et al. Glucocorticoid compounds modify smoothened localization and hedgehog pathway activity. Chem Biol. 2012;19(8):972–82. doi:10.1016/j.chembiol.2012.06.012. [Google Scholar] [PubMed] [CrossRef]
119. Chen M, Tanner M, Levine AC, Levina E, Ohouo PY, Buttyan R. Androgenic regulation of hedgehog signaling pathway components in prostate cancer cells. Cell cycle. 2009;8(1):149–57. doi:10.4161/cc.8.1.7532. [Google Scholar] [PubMed] [CrossRef]
120. Azoulay S, Terry S, Chimingqi M, Sirab N, Faucon H, Diez De Medina SG, et al. Comparative expression of hedgehog ligands at different stages of prostate carcinoma progression. J Pathol. 2008;216(4):460–70. doi:10.1002/path.2427. [Google Scholar] [PubMed] [CrossRef]
121. Dixon S, Tran A, Schrier MS, Dong J, Deth RC, Castejon A, et al. Metformin-induced oxidative stress inhibits lncap prostate cancer cell survival. Mol Biol Rep. 2024;51(1):729. doi:10.1007/s11033-024-09662-8. [Google Scholar] [PubMed] [CrossRef]
122. Trnski D, Sabol M, Tomić S, štefanac I, Mrčela M, Musani V, et al. Shh-N non-canonically sustains androgen receptor activity in androgen-independent prostate cancer cells. Sci Rep. 2021;11(1). doi:10.1038/s41598-021-93971-6. [Google Scholar] [PubMed] [CrossRef]
123. Miyagawa S, Matsumaru D, Murashima A, Omori A, Satoh Y, Haraguchi R, et al. The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology. 2011;152(7):2894–03. doi:10.1210/en.2011-0263. [Google Scholar] [PubMed] [CrossRef]
124. Flemming GMC, Klammt J, Ambler G, Bao Y, Blum WF, Cowell C, et al. Functional characterization of a heterozygous Gli2 missense mutation in patients with multiple pituitary hormone deficiency. J Clin Endocr Metab. 2013;98(3):E567–75. doi:10.1210/jc.2012-3224. [Google Scholar] [PubMed] [CrossRef]
125. Li N, Truong S, Nouri M, Moore J, Al Nakouzi N, Lubik AA, et al. Non-canonical activation of hedgehog in prostate cancer cells mediated by the interaction of transcriptionally active androgen receptor proteins with Gli3. Oncogene. 2018;37(17):2313–25. doi:10.1038/s41388-017-0098-7. [Google Scholar] [PubMed] [CrossRef]
126. Sirab N, Terry S, Giton F, Caradec J, Chimingqi M, Moutereau S, et al. Androgens regulate hedgehog signalling and proliferation in androgen-dependent prostate cells. Int J Cancer. 2012;131(6):1297–306. doi:10.1002/ijc.27384. [Google Scholar] [PubMed] [CrossRef]
127. Sabol M, Trnski D, Uzarevic Z, Ozretic P, Musani V, Rafaj M, et al. Combination of cyclopamine and tamoxifen promotes survival and migration of Mcf-7 breast cancer cells-interaction of hedgehog-Gli and estrogen receptor signaling pathways. PLoS One. 2014;9(12):e114510. doi:10.1371/journal.pone.0114510. [Google Scholar] [PubMed] [CrossRef]
128. Katayama S, Ashizawa K, Gohma H, Fukuhara T, Narumi K, Tsuzuki Y, et al. The expression of hedgehog genes (Ihh, Dhh) and hedgehog target genes (Ptc1, Gli1, Coup-Tfii) is affected by estrogenic stimuli in the uterus of immature female rats. Toxicol Appl Pharm. 2006;217(3):375–83. doi:10.1016/j.taap.2006.10.003. [Google Scholar] [PubMed] [CrossRef]
129. Vila G, Theodoropoulou M, Stalla J, Tonn JC, Losa M, Renner U, et al. Expression and function of sonic hedgehog pathway components in pituitary adenomas: evidence for a direct role in hormone secretion and cell proliferation. J Clin Endocr Metab. 2005;90(12):6687–94. doi:10.1210/jc.2005-1014. [Google Scholar] [PubMed] [CrossRef]
130. Vila G, Papazoglou M, Stalla J, Theodoropoulou M, Stalla GK, Holsboer F, et al. Sonic hedgehog regulates crh signal transduction in the adult pituitary. FASEB J. 2004;19(2):1–15. doi:10.1096/fj.04-2138fje. [Google Scholar] [PubMed] [CrossRef]
131. Menssouri N, Poiraudeau L, Helissey C, Bigot L, Sabio J, Ibrahim T, et al. Genomic profiling of metastatic castration-resistant prostate cancer samples resistant to androgen receptor pathway inhibitors. Clin Cancer Res. 2023;29(21):4504–17. doi:10.1158/1078-0432.CCR-22-3736. [Google Scholar] [PubMed] [CrossRef]
132. Wang L, Li H, Li Z, Li M, Tang Q, Wu C, et al. Smoothened loss is a characteristic of neuroendocrine prostate cancer. Prostate. 2021;81(9):508–20. doi:10.1002/pros.24122. [Google Scholar] [PubMed] [CrossRef]
133. Olson AW, Le V, Wang J, Hiroto A, Kim WK, Lee D, et al. Stromal androgen and hedgehog signaling regulates stem cell niches in pubertal prostate development. Development. 2021;148(19):dev199738. doi:10.1242/dev.199738. [Google Scholar] [PubMed] [CrossRef]
134. Li N, Chen M, Truong S, Yan C, Buttyan R. Determinants of Gli2 Co-activation of wildtype and naturally truncated androgen receptors. Prostate. 2014;74(14):1400–10. doi:10.1002/pros.22855. [Google Scholar] [PubMed] [CrossRef]
135. Tomić A, čonkaš J, Ozretić P. Let’ S talk about sex hormone receptors and their physical interaction with sonic hedgehog protein: a computational study with emphasis on progesterone receptor. Appl Sci. 2024;14(2):562. doi:10.3390/app14020562. [Google Scholar] [CrossRef]
136. Baz-Redon N, Soler-Colomer L, Fernandez-Cancio M, Benito-Sanz S, Garrido M, Moline T, et al. Novel variant in hhat as a cause of different sex development with partial gonadal dysgenesis associated with microcephaly, eye defects, and distal phalangeal hypoplasia of both thumbs: case report. Front Endocrinol. 2022;13:957969. doi:10.3389/fendo.2022.957969. [Google Scholar] [PubMed] [CrossRef]
137. Elzaiat M, Mcelreavey K, Bashamboo A. Genetics of 46,Xy gonadal dysgenesis. Best Pract Res Cl En. 2022;36(1):101633. doi:10.1016/j.beem.2022.101633. [Google Scholar] [PubMed] [CrossRef]
138. Mehta P, Singh P, Gupta NJ, Sankhwar SN, Chakravarty B, Thangaraj K, et al. Mutations in the Desert Hedgehog (Dhh) gene in the disorders of sexual differentiation and male infertility. J Assist Reprod Gen. 2021;38(7):1871–8. doi:10.1007/s10815-021-02140-1. [Google Scholar] [PubMed] [CrossRef]
139. Flatters DM, Sierra-Díaz DC, Legois B, Laissue P, Veitia RA. DHH pathogenic variants involved in 46,XY disorders of sex development differentially impact protein self-cleavage and structural conformation. Hum Genet. 2020;139(11):1455–70. doi:10.1007/s00439-020-02189-5. [Google Scholar] [PubMed] [CrossRef]
140. Pan L, Li Z, Su Z, Su W, Zheng R, Chen W, et al. Case report: long-term follow-up of desert hedgehog variant caused 46,XY gonadal dysgenesis with multiple complications in a chinese child. Front Genet. 2022;13. doi:10.3389/fgene.2022.954288. [Google Scholar] [PubMed] [CrossRef]
141. Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, Mitsuya T, et al. A novel mutation of desert hedgehog in a patient with 46,XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Amer J Hum Genet. 2000;67(5):1302–5. doi:10.1016/S0002-9297(07)62958-9. [Google Scholar] [PubMed] [CrossRef]
142. Wei J, Wu J, Ru W, Chen G, Gao L, Tang D. Novel compound heterozygous mutations in the desert hedgehog (DHH) gene in cases of siblings with 46,XY disorders of sexual development. BMC Med Genomics. 2022;15(1):1. doi:10.1186/s12920-022-01334-5. [Google Scholar] [PubMed] [CrossRef]
143. Werner R, Merz H, Birnbaum W, Marshall L, Schröder T, Reiz B, et al. 46,Xy gonadal dysgenesis due to a homozygous mutation in desert hedgehog (DHH) identified by exome sequencing. J Clin Endocr Metab. 2015;100(7):E1022–E1029. doi:10.1210/jc.2015-1314. [Google Scholar] [PubMed] [CrossRef]
144. Canto P, Söderlund D, Reyes E, Méndez JP. Mutations in the desert hedgehog (DHH) gene in patients with 46,XY complete pure gonadal dysgenesis. J Clin Endocr Metab. 2004;89(9):4480–3. doi:10.1210/jc.2004-0863. [Google Scholar] [PubMed] [CrossRef]
145. Xiang X, Zhou L, Lin Z, Qu X, Chen Y, Xia H. Metformin regulates macrophage polarization via the Shh signaling pathway to improve pulmonary vascular development in bronchopulmonary dysplasia. Iubmb Life. 2022;74(3):259–71. doi:10.1002/iub.2588. [Google Scholar] [PubMed] [CrossRef]
146. Ma Z, Li C, Wang L, Xia Y, Feng C, Peng Y, et al. Microrna-138 regulates spinal cord development by activating the shh in fetal rats. Pediatr Neurosurg. 2023;57(6):407–21. doi:10.1159/000527587. [Google Scholar] [PubMed] [CrossRef]
147. Shqirat M, Kinoshita A, Kageyama R, Ohtsuka T. Sonic hedgehog expands neural stem cells in the neocortical region leading to an expanded and wrinkled neocortical surface. Genes Cells. 2021;26(6):399–410. doi:10.1111/gtc.12847. [Google Scholar] [PubMed] [CrossRef]
148. Zhang F, Zhen Y, Guo Z, Dai J, Zhu L, Liang P, et al. Mir-143 is implicated in growth plate injury by targeting ihh in precartilaginous stem cells. Int J Med Sci. 2021;18(9):1999–2007. doi:10.7150/ijms.46474. [Google Scholar] [PubMed] [CrossRef]
149. Cong L, Jiang P, Wang H, Huang L, Wu G, Che X, et al. Mir-1 is a critical regulator of chondrocyte proliferation and hypertrophy by inhibiting indian hedgehog pathway during postnatal endochondral ossification in Mir-1 overexpression transgenic mice. Bone. 2022;165:116566. doi:10.1016/j.bone.2022.116566. [Google Scholar] [PubMed] [CrossRef]
150. Chen T, Che X, Han P, Lu J, Wang C, Liang B, et al. Microrna-1 promotes cartilage matrix synthesis and regulates chondrocyte differentiation via post-transcriptional suppression of ihh expression. Mol Med Rep. 2020;22(3):2404–14. doi:10.3892/mmr.2020.11296. [Google Scholar] [PubMed] [CrossRef]
151. Goldsmith TM, Sakib S, Webster D, Carlson DF, Van der Hoorn F, Dobrinski I. A reduction of primary cilia but not hedgehog signaling disrupts morphogenesis in testicular organoids. Cell Tissue Res. 2020;380(1):191–200. doi:10.1007/s00441-019-03121-8. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF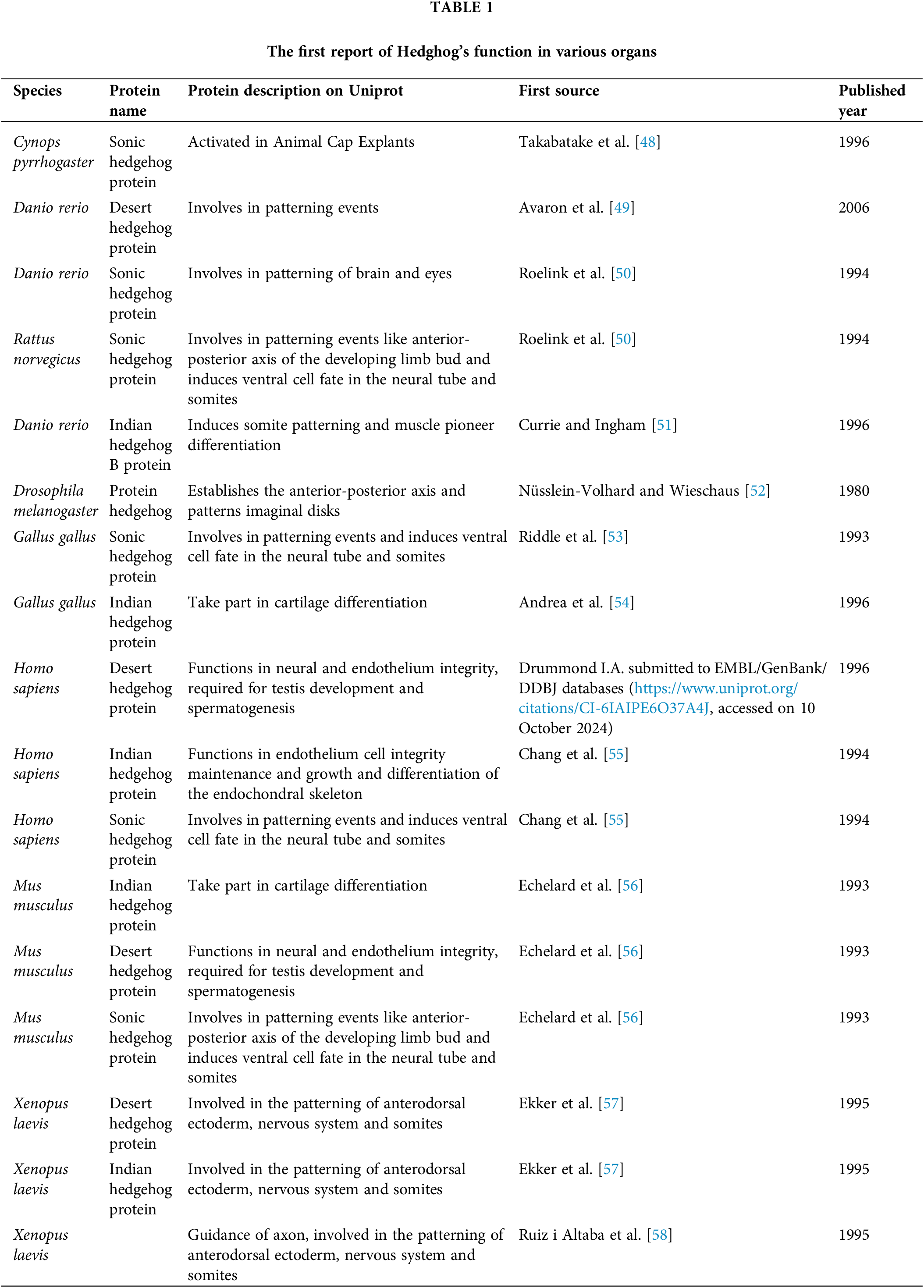
 Downloads
Downloads
 Citation Tools
Citation Tools