 Open Access
Open Access
REVIEW
Assessing the Relationship between Lactobacilli and HPV: A Decade of Research
1 Section of Microbiology and Virology, Interdisciplinary Department of Medicine, School of Medicine, University of Bari Aldo Moro, Bari, 70124, Italy
* Corresponding Authors: Paolo Veneziani. Email: ; Luigi Santacroce. Email:
# These authors contributed equally to this work
BIOCELL 2025, 49(2), 199-220. https://doi.org/10.32604/biocell.2025.059322
Received 04 October 2024; Accepted 14 January 2025; Issue published 28 February 2025
Abstract
The composition of the vaginal microbiota (VMB) influences the health of the female reproductive tract. Several studies have shown how the absence of lactobacilli causes an imbalance in the vaginal microbial community, favoring the development of infections. The present study aims to evaluate the relationship between the VMB and human papillomavirus (HPV) infection to clarify the role of the vaginal microbiota in the persistence and clearance of HPV. Many researchers have provided the scientific community with information on the composition of the microbiota and how it may also influence HPV infection and the development of cervical cancer. Studies have shown that the main Lactobacilli species that favor HPV clearance are Lactobacillus (L.) iners and L. gasseri. In this review, we focused on the general aspects of the interaction between HPV and the VMB, evaluating the influence of the microbiota on the immune system and the effect on different physiologic stages of women’s lives, like pregnancy and menopause. The diversity of the VMB worldwide was also assessed. The association between lactobacilli composition, ethnicity, and cancer must be related to other factors (age, smoking, physiological factors) and co-infections. New formulations of probiotics and innovative surgical removal systems that preserve the integrity of the VMB are also being developed to prevent and reduce the incidence of cervical cancer. More research and understanding are needed on a very complex topic such as the VMB.Keywords
Highlights:
- L. crispatus protects against HPV infection and neoplastic progression; L. gasseri and L. iners influence HPV clearance.
- A diverse vaginal microbiota with decreased lactobacilli and increased anaerobic bacteria is associated with HPV persistence and carcinogenesis.
- The composition of the vaginal microbiota and the progression of HPV infection are strongly affected by socio-demographic factors such as age, ethnicity, smoking habits, and sexual behavior.
- Emerging treatments, including probiotics and innovative surgical techniques, show promise in reducing HPV incidence and improving clinical outcomes.
The female lower reproductive tract is composed of host epithelial and immune cells, along with a diverse community of microorganisms such as bacteria, fungi, and viruses, collectively known as the vaginal microbiota (VMB). The most prevalent microorganisms in this microbiota are species of Lactobacillus (L. crispatus, L. gasseri, L. iners, and L. jensenii), which are associated with maintaining vaginal health. Conversely, their depletion is often correlated with an increased susceptibility to various infections [1].
Lactobacilli make up over 50% of the entire commensal ecosystem and, together with other bacteria, maintain a pH between 3.8 and 4.5 thanks to their lactic acid production, which is considered normal [2]. Lactobacilli are the first line of defense against dysbiosis and infection, together with their antibacterial molecules such as citric, succinic, phenylacetic acid, hydroxyphenyl acetic acid, indole lactic acid, 3-phenylacetic acid, 4-hydroxyphenyl acetic acid and 2-hydroxyisocaproic acid [2,3]. The innate immune system of the vagina consists mainly of a tissue barrier, immune cells, and innate immune molecules (IgA) [3]. The antigen-presenting cells, macrophages, and natural killer cells in the genital tract use their pattern recognition receptors to bind to various bacterial products, such as the antimicrobial acids and peptides of lactobacilli [3]. This interaction determines the transmission of inflammatory signals into the cell so that the products of vaginal lactobacilli could be considered part of innate immunity [3]. So far, over 120 species of lactobacilli have been identified, dozens of which living in vaginal environments [2].
In lactobacilli deficiency, the VMB can be dominated by different bacterial species including both aerobic species (such as Escherichia coli, Streptococcus spp., Enterococcus faecalis, and/or coagulase-negative Staphylococcus spp.) and strictly anaerobic species such as Prevotella, Mobiluncus, Gardnerella spp. and Atopobium vaginae (now Fannyhessia vaginae) [4]. These two common bacterial dysbiosis are known as Aerobic Vaginosis and Bacteroid Vaginosis. Bacteroid Vaginosis is characterized by the reduction or absence of lactobacilli and the predominance of strict anaerobes. The polymicrobial anaerobic overgrowth disrupts the ecological balance of the VMB determining an increase in pH [5]. Aerobic Vaginosis and Bacteroid Vaginosis determine a significantly different inflammatory response: Aerobic Vaginosis presents a classic inflammatory response [6], whereas Bacteroid Vaginosis may be more or less silent/subclinical [7]. The vaginal bacterial community can be categorized into different community state types (CSTs) based on the composition of Lactobacillus species in terms of type and abundance. CST I, II, III, and V are characterized by the dominance of Lactobacillus crispatus, L. gasseri, L. iners, and L. jensenii, respectively [8]. In contrast, CST IV is dominated by non-Lactobacillus bacteria and is further divided into CST IV-a and CST IV-b. CST IV-a includes moderate levels of Lactobacillus species and smaller quantities of strict anaerobes, while CST IV-b features higher levels of Atopobium, Prevotella, Sneathia, and Gardnerella [8,9].
There has been significant focus in scientific research on the associations between CSTs and HPV infection status, as well as on the connection between VMB and cervical lesions [10]. HPV is the primary cause of cervical cancer globally; however, HPV infection alone is insufficient to trigger cancer development. Other co-factors play a crucial role in facilitating the carcinogenic process [11]. Conditions like bacterial vaginosis (BV) or persistent high-risk HPV (hr-HPV, including types such as HPV 13, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, and 73, with HPV 16 and 18 being the most critical) are often associated with reduced levels of Lactobacillus and increased populations of anaerobic bacteria such as Gardnerella, Clostridiales, and Prevotella [12,13].
The relationship between HPV and the VMB remains complex and incompletely understood. Studies [14,15] suggest that when the vaginal environment is dominated by non-Lactobacillus species the probability of acquiring and maintaining HPV infection increases. This study aims to review the literature published over the last decade on the composition of the VMB, focusing on the role of Lactobacillus species and their potential impact on the development and progression of HPV infection.
A literature review was conducted of articles published on PubMed from around the world between 01 January 2014 and 31 December 2023 on the topic of the relationship between lactobacilli and HPV.
The following keywords were used for the search: “Lactobacillus spp. AND HPV”, “Lactobacillus spp. AND Human Papilloma Virus”, “Lactobacillus AND Human Papilloma Virus”, “Lactobacillus AND HPV”, “Lactobacilli AND HPV”, “Lactobacilli AND Human Papilloma Virus”. Exclusion criteria were as follows: reviews, mini-reviews, non-English articles, and unrelated topics.
In total, over the period from 2014 to 2023, our search yielded 292 results. Of these, excluding duplicates (103), reviews (29), and non-English language articles (18), we examined 142 scientific articles. Off-topic articles were also excluded (44), so 98 articles were included in our review (Fig. 1).
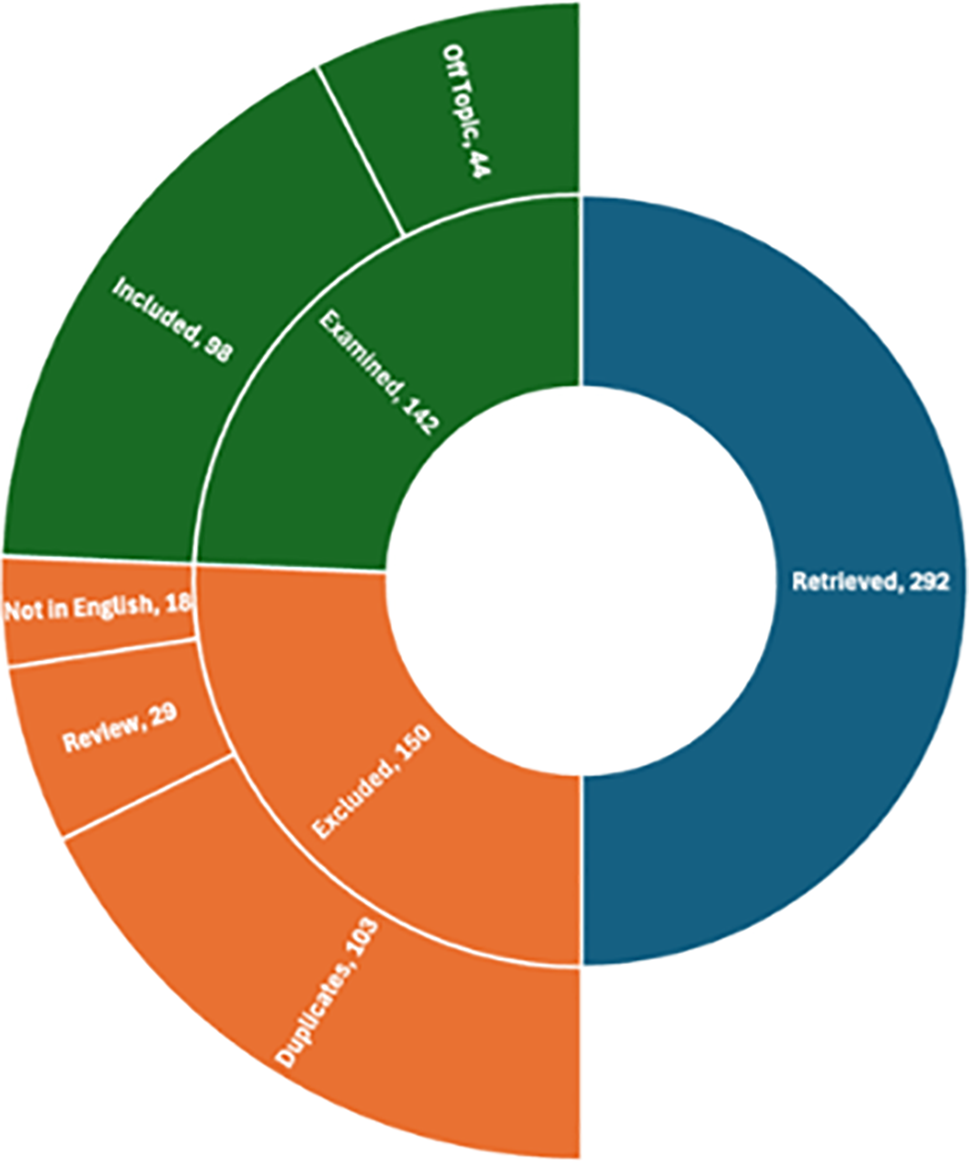
Figure 1: Results overview
3.1 Interaction of Lactobacillus spp. and HPV: From General to Particular
Over the past decade, researchers have studied abundantly the composition of the VMB, concerning Lactobacilli, and their relationship with HPV infection. Various factors such as urbanization [16], sociodemographic factors, sexual behavior, and contraceptive methods have been shown to influence cervical microbiota composition [17]. These microbiota changes were associated with abnormal cervical cytology [17–20].
As the severity of cervical pathology increased, the composition, including diversity and relative abundance, of the cervical microbiome changed accordingly, and in healthy women, lactobacilli are dominant [21].
Several studies [12,22–25] have demonstrated that HPV infection or the importance of cervical neoplasia in terms of severity are linked with changes in the species that form the VMB. Particularly, a depletion of health-associated Lactobacillus spp. and an expansion of anaerobic bacteria linked to dysbiosis, including Gardnerella vaginalis, Prevotella, Fannyhessia vaginae, Sneathia, Megasphaera, Enterobacteriaceae, Faecalibacterium prausnitzii, and others, appears.
Reduced Lactobacillus levels and increased pathogenic bacteria could directly result from early HPV infection [24].
Processes such as genital inflammation, altered cell proliferation and apoptosis, angiogenesis, hormonal dysfunction, and metabolic dysregulation, all of which are hallmarks of cancer, are connected with modifications of VMB [26,27].
Silva et al. [28] conducted a retrospective study on 3390 patients, of which 409 progressed to high-grade intraepithelial lesions. Among these, 354 were initially diagnosed with HPV infection, 27 with squamous cell undetermined atypia, 22 with low-grade intraepithelial lesions with or without cytological evidence of HPV-related disease, and six with glandular cell atypia of unknown significance. The aim was to evaluate the development, associated factors, and cytohistologic correlates of low-grade intraepithelial, squamous, and glandular lesions of uncertain significance. It was highlighted that lactobacilli, bacterial vaginosis, smoking, and immunosuppression were factors linked to development, whereas single sexual partner, use of hormonal methods of contraception, age, T. vaginalis, Candida, and cocci resulted in protection.
Comparing the VMB of healthy women vs. women with an hr-HPV-infection, it appears to be less diversified in species, with Lactobacillus predominant, making up over 80% of the microbiota [29,30]. In infected women species diversity increases, often characterized by a reduction in Lactobacillus and an increase in Gardnerella vaginalis. Moreover, the cluster of women dominated by L. crispatus is less likely to have hr-HPV, other than HIV and herpes simplex virus type 2 (HSV-2) [31]. The changes in the microbiota of hr-HPV infected women seem to occur in the early stages of HPV infection [32]. The relationship between VMB CSTs and hr-HPV infection was investigated in Nigerian women [33]. The connection between prevalent hr-HPV infection and a lower frequency of Lactobacillus spp. with a higher frequency of anaerobic microorganisms, especially Prevotella and Leptotrichia, in HPV-negative women was demonstrated. Instead, in another study [34], the evaluation of women with hr-HPV demonstrated a depletion of Lactobacillus in the women with an elevated viral load, with L. gallinarum and L. iners the most abundant species.
Further investigations [35] aimed to explore potential biomarkers of the vaginal microbiome that predict hr-HPV infection and cervical intraepithelial neoplasia (CIN) 2. Three hundred and twenty-nine women were enrolled and divided into persistent cervical HPV infection, incident HPV infection, and no HPV infection groups. The evaluation of the vaginal microbiome in the three groups showed that the predominance of Prevotella bivia, Enterococcus durans, and Porphyromonas uenonis with a simultaneous scarcity of L. iners and Prevotella disiens may be related to persistent HPV infection. Chao et al. [36] also noted that the presence of L. iners, Atopobium vaginae, and Gardnerella vaginalis was higher in HPV-positive women [as well as anaerobic bacteria (Bacteroides plebeius, Acinetobacter lwoffii, and Prevotella buccae)] than the HPV-negative women. Furthermore, L. gasseri was less present in HPV-positive women compared to HPV-negative women [36].
The study conducted by Chorna et al. [37] hypothesized that changes in the VMB induced by HPV (L. iners dominates under physiological conditions) and their metabolites can be used to develop a rapid diagnosis system for the infection by HPV.
Therefore, Liu et al. [38] suggest that L. iners, L. cripatus, and L. gasseri may act as potential biomarkers for identifying HPV infection in any cervical lesion. In addition, the genera Prevotella, Porphyromonas, and Bacteroides proved to be good microbial markers for cervical cancer, and the genus Bifidobacterium was highlighted as a reliable biomarker for precancerous conditions.
Teka et al. [39], also found in their study that Porphyromonas somerae, Porphyromonas asaccharolytica, and Prevotella timonensis were highly abundant in the cervical cancer cases compared to patients with dysplasia, in which L. iners was the predominant species. The researchers also emphasized that metagenomic sequencing technology could overcome the limitations of 16S rRNA gene sequencing, enhancing the analysis of microbiota function. So, metagenomic sequencing technology on cervical swabs from women infected with hr-HPV indicated that in uninfected women there was a significant increase in L. crispatus, L. jensenii, and L. helveticus, and these species had biomarker significance among two groups [40].
A stable lactobacilli-dominant microbiota was associated with higher HPV clearance, while a diverse microbiota with increased anaerobic bacteria was linked to HPV persistence [13,41]. CSTs dominated by L. gasseri have resulted in the fastest HPV clearance rate, while CSTs with low Lactobacillus levels and a high proportion of Atopobium spp. (CST IV-b) exhibited the slowest clearance rates compared to L. crispatus CSTs dominated [42]. Therefore, L. gasseri-dominated VMB seems to be associated with increased shedding of detectable HPV [42]. The same results were found in the study by Yang et al. [43], where L. gasseri may be associated with HPV clearance in association with other microorganisms (Streptococcus agalactiae and Prevotella timonensis). In their pilot study, they assessed the association between VMB, personal risk factors, and HPV clearance in 38 women grouped as follows HPV-negative, persistent HPV infection, and HPV status conversion. The relative abundance of VMB was assessed by a metagenomic approach and compared between the three cohorts. The results showed that L. gasseri in association with other microorganisms (S. agalactiae and Prevotella timonensis) may be associated with HPV clearance. According to Shi et al. [44], the increased abundance of L. iners has a significant risk effect on HPV clearance. In support of this, Zeng et al. [45] to clarify the role of the VMB in HPV persistence and clearance, analyzed the VMB of 90 patients with HPV infection vs 45 healthy individuals and found that the dominant species in HPV-positive women was L. iners, with L. iners, Sneathia amnii, and L. delbrueckii being the top three dominant bacteria in the HPV-persistent group.
In various studies [46,47], L. acidophilus and L. iners were the predominant bacterial species in the HPV-positive group, whereas L. crispatus, L. iners, and L. taiwanensis were abundant in the control group and L. acidophilus and Gardnerella vaginalis were not detected. Bacterial dysbiosis, characterized by a predominance of Gardnerella vaginalis and a concomitant deficiency of L. crispatus, L. iners, and L. taiwanensis may act as an HPV-dependent cofactor in the development of cervical neoplasia [47].
Contrarily, Sun et al. [48], observed a significant (p < 0.001) decrease in L. acidophilus, in women with hr-HPV-positive infection (70.28% compared to 52.33% of controls).
Frąszczak et al. [49], who compared the abundance of lactobacilli species between healthy subjects and patients with abnormal Pap test results, found that most patients harbored two dominant Lactobacillus species, mainly L. gasseri (93%) and L. crispatus (83%). The diversity of vaginal lactobacilli was influenced by age and geographic location. An increase in L. delbrueckii and a decrease in L. gasseri were observed with age, while L. acidophilus and Limosilactobacillus fermentum were more common in rural than in urban populations.
Campisciano et al. [50] suggested that L. iners, L. gasseri, Prevotella bivia, and HPV form microbiological signatures associated with adverse reproductive outcomes. Musa et al. [51] evaluated that L. iners was linked to a 3–5 times higher likelihood of detecting hr-HPV, cervical precancer, or invasive cervical cancer, compared to a VMB dominated by L. crispatus.
Using shotgun metagenomic sequencing, Yang et al. [52] provided a comprehensive analysis of microbial abundances and metabolic functions in the cervicovaginal microbiome. The study revealed that L. crispatus, L. iners, and Gardnerella vaginalis were the top three species in both HPV-positive and control groups. However, HPV16-positive women exhibited a reduced abundance of Lactobacillus spp. compared to controls. This highlights the role of Lactobacillus spp. in adhering to vaginal epithelial cells and preventing pathogenic bacterial or viral colonization. Berggrund et al. [53] compared the VMB of women with persistent and transient HPV16 infection with controls. The results showed that a VMB dominated by non-Lactobacillus species was associated with both transient and persistent HPV-16 infection (p < 0.05) compared to HPV-negative women.
Furthermore, Liu et al. [2], who studied and sequenced the VMB in single or multiple HPV genotype infections, detected an abundance of unclassified lactobacilli, although decreased in comparison to the control group. So, the authors revealed the affinity of some Lactobacillus spp. with specific strains of HPV. Gonçalves-Nobre et al. [54] demonstrated that L. crispatus is not associated with any specific HPV genotype, therefore representing a protection against HPV infection.
In contrast, cervicovaginal microbiota dominated by L. gasseri carried an elevated risk of HPV-51 infection, while L. jensenii, carried an enhanced risk of HPV-56 infection. Interestingly, L. iners, known as a transitional species, showed affinity for multiple HPV genotypes, including HPV-18, 53, and 59. However, in cases of normal cytology and low-grade lesions, the Lactobacillus species that show such affinities are predominantly associated with the most common HPV genotypes (Fig. 2).
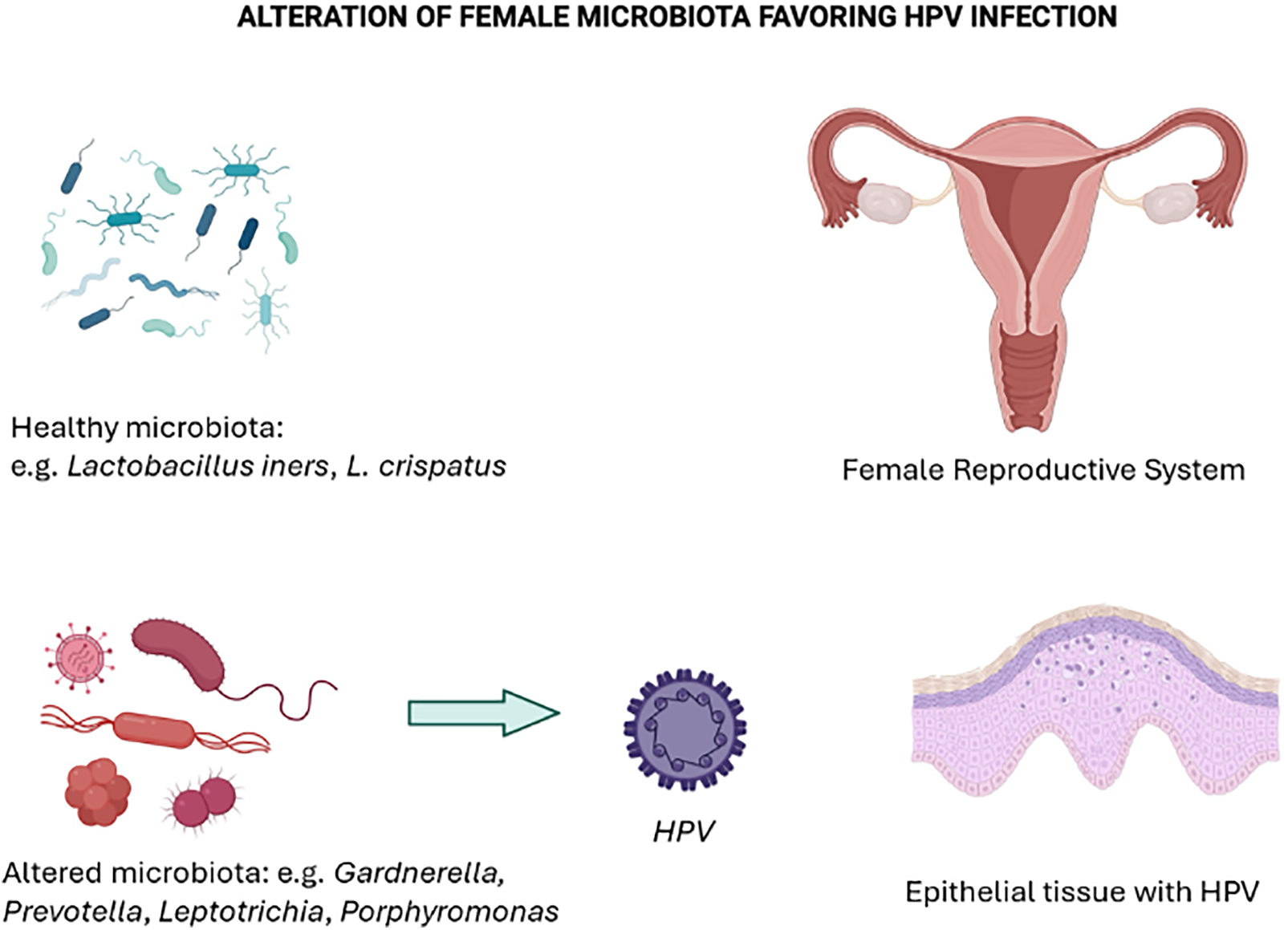
Figure 2: HPV infection as a consequence of an altered microbiota
Some authors [55,56] have studied the correlation between HPV infection and other infections, such as human immunodeficiency virus (HIV), as a function of the Lactobacillus composition. Comparing the cervicovaginal microbiota of HIV-positive and HIV-negative women, the abundance of L. crispatus resulted inversely associated with vaginal pH and with lower HPV detection [55]. The observed inverse associations between L. crispatus and HPV were independent of HIV [55]. L. crispatus may help to reduce HPV load in both HIV-infected and HIV-uninfected women [55].
To investigate how HIV and HPV infection affect vaginal microbes in women living with HIV (WLWH), Chávez-Torres et al. [56] determined the prevalence of hr-HPV and cervical cytological abnormalities in 44 WLWH and 39 seronegative women (SNW). Vaginal microbial composition was characterized by sequencing and revealed that L. iners was the dominant species in both groups. HIV and HPV status did not influence diversity or community structure.
To date, the first study to investigate the entire cervical microbiome was performed by Sasivimolrattana et al. [57], encompassing bacteria, fungi, and viruses, in women with and without HPV16 or hr-HPVs infection. In different histological groups (CIN1, CIN2, and CIN3), L. iners constituted over 60% of the total Lactobacillus spp. in both HPV-positive and HPV-negative groups. In a study [58], virome and bacteriome analyses were performed on a sample of Brazilian women infected and uninfected with HPV (HPV+ and HPV−). The results showed that HPV infection was associated with the presence of Anellovirus (AV). The bacteriome results showed that the distribution of vaginal CSTs was independent of HPV or AV status, although the distribution of bacterial phyla differed between groups. Overall, the data showed an intriguing association between HPV and AV infections, which may promote the development of cervical cancer.
Recently, innovative approaches have been employed to model infection trends. Xu et al. [59] developed an algorithm to study the microbiota of patients with HPV-related cervical disease (HRCD) and healthy controls. The study included 98 HRCD patients and 58 healthy individuals whose VMB was analyzed to construct a disease classification model using a random forest algorithm. Results showed that VMB diversity was significantly higher in HRCD patients than in controls (p < 0.05). Among species, L. iners showed marked differences. Firmicutes were dominant, with Lactobacillus identified as the genus most significantly altered between the two groups (p < 0.01) This study successfully characterized the vaginal microbiota composition of HRCD and developed a diagnostic model for the disease.
3.2 HPV Infection and Vaginal Microbiome in Pregnancy
During pregnancy, the vaginal environment is physiologically different and more complex. Overall, HPV incidence in pregnant women is still a controversial topic, with ongoing debate about whether pregnancy predisposes women to HPV infection and what factors influence vulnerability. Chen et al. [60] examined the association between hr-HPV infection and the vaginal environment in Chinese pregnant women with and without HPV infection. Vaginal bacterial composition was assessed in four groups: pregnant women infected with hr-HPV (PHR), pregnant women not infected with hr-HPV (PN), non-pregnant women infected with hr-HPV (NPHR) and non-pregnant women not infected with hr-HPV (NPN). Both pregnancy and HPV infection resulted involved in the increase of vaginal bacterial richness and variety. Lactobacillus spp. remained dominant in all samples, but NPN samples were linked to CST III, where L. iners predominated, whereas pregnancy and hrHPV infection were linked to CST I, where L. crispatus predominated. Although biomarkers of hr-HPV infection included Bifidobacterium, Bacillus, Megasphaera, Sneathia, Prevotella, Gardnerella, Fastidiosipila and Dialister, among pregnant women, different bacterial genera (Bifidobacterium, Megasphaera, Bacillus, Acidovorax, Oceanobacillus and Lactococcus) were linked with hr-HPV infection. These findings suggest that hr-HPV infection and pregnancy together alter the diversity of the vaginal microbiota, resulting in a different composition between PHR and NPHR, although hormonal influences were not assessed. Juliana et al. [5] also investigated the composition of the VMB which pathogens are more present and their connections during and after pregnancy in 90 women in Tanzania. This study showed that Lactobacilli are dominant during pregnancy, that’s why high estrogen levels during pregnancy lead to glycogen production promoting colonization by lactobacilli and their production of lactic acid, which helps to maintain a low vaginal pH. In contrast to Chen’s study [60], L. iners (CST III) is dominant in this study of pregnant women, in addition to L. crispatus (CST I). After pregnancy, lowered estrogen levels alter the VMB: in fact, the VMB is dominated by non-lactobacilli (CST IV). Such changes can persist for a year, and bacteria like G. vaginalis, Prevotella species, and Anaerococcus species have been associated with higher pH and BV [5], suggesting a negative impact on HPV infection. Vargas-Robles et al. [61] evaluated the differences in the cervicovaginal microbiota between pregnant, non-pregnant, and menopausal Puerto Rican women, divided into HPV-negative, HPV-positive, or women with cervical cancer. Across all physiological stages, L. iners was the most abundant species in the Puerto Rican cervical microbiota. Previous studies have suggested that cervicovaginal samples dominated by L. iners and samples with a higher microbial diversity are both associated with a higher risk of HPV infection and its permanence [12,15,42]. This could be attributed to several factors: first, L. iners produces very low levels of H2O2 and also produces the L-isomer of lactic acid rather than the D-isomer, which is protective. It also produces less of it, favoring colonization by pathogenic anaerobic bacteria and an inflammatory state. In non-pregnant women, Robles et al. [61] identified CST IV-b (associated with bacterial vaginosis caused by G. vaginalis), while CST IV-c (dominated by Prevotella) was predominant in menopausal women. In pregnant women, CSTs dominated by lactobacilli (L. crispatus in CST I and L. iners in CST III) were more prevalent. Notably, from the fourth month of pregnancy, alpha diversity decreases, and vaginosis-associated bacteria are reduced. High estrogen levels during pregnancy promote glycogen release in the cervicovaginal epithelium, which supports the growth of lactobacilli. For example, lactobacilli can use glycogen to produce lactic acid, which promotes a low vaginal pH and, together with their production of bacteriocins, promotes defense against pathogens, including HPV [61].
3.3 Vaginal Microbiota in Postmenopausal Cervical Squamous Intraepithelial Lesion (SIL) and Squamous Cell Carcinoma (SCC)
Avsaroglu et al. [62] showed that the number of Lactobacilli in the cervicovaginal microbiota is greatly reduced in women with HPV persistence vs. women without HPV persistence, independent of age and menopausal status. While the study had a limited sample size and the risk factors for HPV persistence are not fully understood, ongoing research highlights the interactions between the VMB and the immune system, particularly how the microbiota influences the production of pro-inflammatory cytokines. This finding is crucial for developing optimal management guidelines for women identified through HPV screening programs. Targeted interventions to modify the VMB could play a significant role in the management of HPV infection and related cervical lesions, especially given that cervical cancer is largely preventable through vaccination and early detection. Postmenopausal HPV-positive women have been shown to have less abundant cervical microbiota compared to their HPV-negative counterparts [41,63]. During menopause, decreased estrogen levels reduce mucus deposition, causing vaginal dryness and less glycogen deposition. So, the lactobacilli decrease, and consequently, the vaginal pH increases, and the defenses produced by the lactobacilli are reduced exposing the environment to infection [61]. Decreasing estrogen in postmenopausal women alters vaginal microecology, and Limosilactobacillus vaginalis acts synergistically with HPV to promote the developing of cervical squamous intraepithelial neoplasia to cervical cancer in postmenopausal women. The aetiologies of cervical squamous intraepithelial lesion (SIL) and cervical cancer in postmenopausal women are not clear but are associated with persistence of high-risk HPVs (HPV-16, HPV-18), economic level, multiple sexual partners, smoking, premature birth, and environmental factors. The expression of L. vaginalis in postmenopausal cervical SIL and cervical squamous cell carcinoma (SCC) has not been studied. In the study by Liu et al. [63], L. iners was dominant in the VMB of postmenopausal women, and postmenopausal cervical SILs developed into cervical cancer. As the grade of cervical lesions increased, the vaginal Lactobacillus decreased, the diversity of the vaginal flora increased, and the ability of the cervical epithelial cells to clear the HPV virus slowed down, which exacerbated the HPV-16 infection and promoted the occurrence of epithelial-mesenchymal transition in postmenopausal women.
3.4 Vaginal Microbiota and Ethnicity
There are many studies on the VMB in different areas of the world and different female populations and how it changes under different conditions.
Wei et al. [64] included 59 Chinese women divided into 5 groups: healthy women, hr-HPV-infected women, low-grade SIL, high-grade SIL, and cervical cancer. The study aimed to characterize the VMB in the stages from HPV infection to the development of cervical cancer. With the initiation of hr-HPV infection and the development of cervical lesions, the species diversity of the VMB increased, but the presence of Lactobacillus spp. Gradually declined. Furthermore, the abundance of Actinobacteria was higher than in healthy women. They also found that Gardnerella, Atopobium, and Dialister play a significant role both in persistent high-risk HPV infection and in the pathogenesis of cervical cancer.
Zhang et al. [65] performed a similar study on 602 Chinese women and Ivanov et al. [66] conducted a study on 140 Russian women. In these studies, the women were divided into three groups: healthy women, HPV-infected women, and a group diagnosed with CIN. Chinese women have high levels of L. crispatus and L. iners, and low levels of L. gasseri, which reduces their resistance to HPV. In Russian women, L. crispatus and L. iners are most abundant, while L. gasseri and L. jensenii are less abundant. Zhang et al. [65] also found that in all three groups, CST III and CST IV-b showed a gradual increase, while CST I and CST IV showed a gradual decrease. This suggests that the presence of HPV impacts the VMB. In fact, L. crispatus secretes lactic acid, which prevents colonization by other bacteria. Its decrease, associated with a low presence of L. gasseri, downregulates the protective effect, leading to an alteration of the VMB and the development of cervical lesions. The reason why L. gasseri is less present in Chinese women than in other countries is unclear and needs to be researched in genetic, social, dietary, and age factors. Arokiyaraj et al. [67] also found in Korean women that L. crispatus was predominant in the HPV-negative group, confirming the fact that L. crispatus is protective, while the increase in L. johnsonii and the increase in species richness were associated with HPV persistence.
Another interesting study [68] was conducted on Mexican women, all with squamous intraepithelial lesions, some with HPV infection. This study showed that the change in the composition of the VMB depended on HPV infection and not on squamous intraepithelial lesions, suggesting that the infection induces changes in the composition of the microbiota. When lactobacilli are reduced, bacterial diversity increases, and L. iners has previously been associated with a dysbiotic community. L. iners is a low producer of D-lactic acid, so the protective effect was reduced and it induced the secretion of interleukin-8, which causes proinflammatory activity in the cervix and influences the progression of cervical intraepithelial neoplasia.
Finally, Tossas et al. [69] considered, for the first time, the VMB as a potential contributor to ethnic differences in CIN3 risk. The study included 3050 black women. They divided VMB into three groups based on different compositions and kinds of microbiome: optimal (Lactobacillus crispatus, L. gasseri, and L. jensenii), moderate (L. iners), and suboptimal (Gardnerella vaginalis, Ca. Lachnocurva vaginae (formerly BVAB1), Atopobium vaginae, (and others). This study, consistent with existing literature, confirmed an independent association between ethnicity and the risk of CIN 3, with black non-Latina women having a higher risk of CIN 3 compared to white non-Latina women. In addition, black non-Latina women with optimal VMB had a significantly higher risk of developing CIN. L. crispatus was decreased in the VMB of participants with CIN3, again suggesting its protective effect. Finally, the suboptimal category was found in CIN 3-positive women.
Therefore, the composition of the VMB varies in different regions of the world, and the association between lactobacilli, ethnicity, and potential cancer must also be considered about factors such as age, smoking, physiological and biochemical conditions, and co-infection with microorganisms responsible for sexually transmitted diseases.
3.5 Lactobacillus spp. and Cervical Intraepithelial Neoplasia (CIN)
The hr-HPV causes high-grade cervical intraepithelial neoplasia (CIN 2), which is a precursor to invasive cervical cancer (ICC). However, only a small number of women with hr-HPV go on to develop CIN 2 [70]. According to the WHO Classification of Female Genital Tumorsof 2020 [71], low-grade SIL (LSIL) has the same cytological classification as high-grade SIL (HSIL); LSIL is CIN1, whereas HSIL mostly includes CIN2 and CIN3 [63]. It is important to identify factors that may alter the natural history of hr-HPV infection.
A potential association between the VMB and CIN disease progression has not been studied, although evidence suggests a link between the structure of the vaginal microbiome and HPV infection. A decrease in the relative abundance of Lactobacillus spp. was also correlated with increased disease severity. Liu et al. [11] showed that HPV shifted VMB composition and diversity, and BV amplified this trend. The relative abundance of 12 anaerobic taxa was significantly elevated in HPV-positive samples and further increased with BV infection, whereas lactobacilli showed the opposite trend, decreasing in CIN 1 and increasing in CIN 2.
The development of cancer could be divided into two phases: in the first phase Prevotella reduces the presence of lactobacilli, in the second phase Ignatzschineria and Enterococcus show a positive interaction in the development of cervical lesions, and at the same time there is a reduction of lactobacilli and Ignatzschineria [72]. The reduction of these species may lead to the development of cancer.
Brotman et al. [42] found that an L. iners-dominated VMB (CST III) was related to HPV infection, whereas L. gasseri-dominated VMB (CST II) had the fastest clearance of HPV infection. However, Mitra et al. [73], did not detect an overrepresentation of CST II or III in their CIN groups. The reduced prevalence of L. iners in CINs compared to controls, coupled with the increased prevalence of CST IV, may represent a change from an L. iners (CSTIII) to a CST IV with the development of CIN. Women whose CIN2 lesions regressed spontaneously had higher levels of lactobacilli-dominant microbiota compared to those with persistent or progressive lesions. The results suggest that a healthy VMB may promote the natural regression of CIN2 lesions, highlighting potential microbial biomarkers for predicting lesion outcomes.
At present, it is unclear whether a CST IV microbiome influences the development of CIN or a consequence of CIN. Bacterial vaginosis (BV) is a disorder diagnosed by traditional culture techniques based in part on the depletion of Lactobacillus spp. and an increased variety of opportunistic pathogenic Gram-negative bacteria. This condition is correlated with significantly higher rates of HPV infection and CIN [22].
Piyathilake et al. [74] found no association between microbiome diversity and CIN grade but demonstrated the association between cervical microbiome dominated by Lactobacillus spp. and L. iners and CIN 2 in the presence of hr-HPV infection. Microbiota characterization revealed that L. iners and unclassified Lactobacillus spp. dominated cervical mucosal community was associated with CIN 2. CIN 2 was also associated with sequence reads mapping to Lactobacillaceae, Lactobacillus, L. reuteri, and some subgenus-level of Lactobacillus OTUs. These results are in agreement with those of Stoian et al. [75], the first to be carried out in Romania, according to which inadequate screening alone cannot explain the high incidence of cervical cancer. HPV persistence and progression of cervical lesions may be linked to these microbiota characteristics. The predominance of L. iners and the absence of L. crispatus with Atopobium spp, Prevotella spp, and Gardnerella spp. may characterize the environment for severe cervical lesions. The peculiarity of this study in comparison with the literature is the almost undetectable presence of L. crispatus, also in the HPV-negative group for intraepithelial lesion or malignancy.
The study by Zhang et al. [76] investigated both direct and indirect relationships between cervical microbiota and CIN risk. Cervical biopsy samples were collected directly from CIN are as to more accurately represent the actual microbial community of these areas. This study found L. jensenii and L. crispatus in high numbers in CIN 2 and CIN 1, but no link between L. iners and CIN severity.
In addition to Lactobacillus spp., some other microbes were found to be highly abundant in the CIN 1 and CIN 2 groups. Direct associations included specific bacterial species linked to CIN, while indirect associations included microbial interactions influencing host immune responses and disease progression. This suggests that differences in the composition of the microbiota may have a direct effect on the process of cervical carcinogenesis, with an indirect effect mediated by HPV.
Higher CINs severity is associated with a reduced abundance of Lactobacillus [22,77,78] and increased bacterial diversity [22,77]. Chen et al. [79] found that regardless of CIN status, HPV infection increases vaginal bacterial richness and diversity. HPV was associated with a decreased abundance of Lactobacillus, Gardnerella, and Atopobium. Correspondingly, HPV infection leads to an increased abundance of Prevotella, Bacillus, Sneathia, Megasphaera, Streptococcus, and Anaerococcus, similar to previous studies [23,36,42,80,81].
Increased Bacillus, Anaerococcus, and decreased Gardnerella vaginalis were probably related to the progression of CIN severity, while Megasphaera was strongly associated with HPV infection without CIN or cancerous lesions. A surprising finding of this study [79] was that Gardnerella and Atopobium amounts were reduced in HPV-positive women. On the other hand, CIN-positive patients from Liu’s study [82] have a distinct vaginal microbial profile characterized by decreased Lactobacillus and Pseudomonas and increased Gardnerella, Prevotella and Dialister. Xia et al. [83] report that increased Gardnerella, the predominance of L. iners, and the presence of Shuttleworthia may be a sign of HPV infection with an LSIL. There was a significant positive association between Lactobacillus vaginalis, Ureaplasma parvum colonization, and the severity of Cervical Cancer (CC) precursor lesions [70].
3.6 Immune Mechanisms and Oncogene Expression
The importance of lactobacilli in the development and trend of bacterial vaginosis and cervical carcinoma has led scientists to research the molecular effects that these microorganisms have. Studies [84] were performed on SiHa cell line (HPV-16-transformed epithelial cell) and CaSki cell line, which are cells derived from cervical carcinoma. These ones contain 600 copies of integrated HPV-16. These two cellular types were cultured with bacterial cell supernatants or bacterial lysates: strains of L. crispatus, L. gasseri, L. iners, and L. jensenii were used, respectively as representatives of CSTI, II, III, and V. Other bacteria for CSTIV. The results showed that L. gasseri or L. jensenii were the best stimuli for inducing the production of interferon-γ (IFN-γ) by human peripheral blood mononuclear cells (PBMCs), while the highest production of IFN-γ and the lowest production of interleukin-17 (IL-17) was carried by L. gasseri. L. iners, on the other hand, served as an effective inducer of both IFN-γ and IL-17 production. Thus, a VMB predominantly composed of lactobacilli, particularly L. gasseri or L. jensenii, could support immune cells in eliminating HPV infection, overcoming viral evasion, and reestablishing immune balance. There was also a correlation between levels of vaginal inflammation and microbial composition and secreted immune checkpoint molecules, key immunoregulatory proteins that play a role in cancer immune escape. The immune checkpoint molecules were found to be highly interconnected, with many correlating with other immune checkpoint proteins. This aligns with prior findings that these proteins are frequently co-expressed on various cell types, such as T cells, antigen-presenting cells, and malignant cells [85]. Additionally, several immune checkpoint proteins were found to correlate positively with vaginal inflammation, which was markedly higher patients with cancer compared to other groups. Notably, IL-36γ emerged as a distinct immune mediator strongly associated with cervical cancer, independent of the VMB composition [23]. They also found Toll-like receptor 2 (TLR2) to be closely linked to lactobacilli dominance, while also being associated with increased genital inflammation. Evidence suggests that vaginal Lactobacillus species may leverage TLR2 signaling to influence immune responses within the cervicovaginal microenvironment [85].
Gao et al. [86] showed that, in vitro, exposure of L. gasseri LGV03 on Ect1/E6E7 cells (cell line derived from the ectocervix of a female patient with endometriosis, that expresses HPV-16 E6/E7 and cytokeratins) significantly upregulated the mRNA levels of IFN-α and IFN-β and downregulated the levels of IL-6, IL-8 and IL-1β. The first attempt to integrate microbiome and cytokine profiling was undertaken only in 2023 [87]. This study showed that elevated levels of all three cytokine groups (inflammatory, anti-inflammatory, and trafficking-related) are linked to cervical dysbiosis, characterized by a significant depletion of protective lactobacilli and the proliferation of various anaerobic taxa. Participants with negative intraepithelial lesion or malignancy (NILM)/HPV- and LSIL exhibited similar microbiome profiles, primarily dominated by L. crispatus and L. iners. However, LSIL was marked by an increase in L. iners and a reduction in L. crispatus, a trend that became more pronounced in HSIL, where L. crispatus was completely absent. This shift was accompanied by dysbiosis-related taxa such as Lachnospiraceae spp., Prevotella bivia, Atopobium vaginae, and G. vaginalis. In the study conducted by Zheng et al. [88], patients with cervical lesions showed reductions in dominant lactobacilli and dysbacteriosis in both HPV-negative and hr-HPV-positive groups. Patients with cervical lesions also exhibited changes in the concentrations of immune factors compared to healthy women. As the lesion progressed, IL-2 decreased, IL-10 increased, and IL-2/IL-10 decreased. In general, when lactobacilli are low cytokines are high. Liu et al. [89] evaluated different cytokines (IL2, IL4, IL6, IL8, IL10, IL12P70, and IL23, INF-γ, and TNF-α) showing that in HPV-infected women all of those cytokines were lower and only TNF-α was significantly (p < 0.05) down-regulated. HPV reduces host defenses and causes the downregulation of most molecules used by lactobacilli for their survival having a bad impact on VMB [90]. Under normal conditions, the host mucosa is involved in the maintenance and regulation of the bacterial composition. HPV through E7 oncoprotein induces suppression of nfkb (Nuclear Factor kappa B), and up-regulation of c-myc through Wnt/β-catenin signaling cascades [90]. Motevaseli et al. [91] studied the influence of lactobacilli on the expression of HPV oncogenes through the evaluation of the expression of CASP3 (Caspase-3) and three autophagy-related genes [Autophagy-related 14—ATG14, Beclin-1—BECN1, and the alpha 2 catalytic subunit of AMPK (PRKAA2)], as well as the HPV18 E6 and E7 genes, in HeLa cells before and after treatment with culture supernatants of L. crispatus and L. rhamnosus. Following treatment with lactobacilli, HeLa cells showed reduced expression of CASP3 and autophagy genes. However, this reduction was not significant for PRKAA2. In addition, the expression of HPV E6 was significantly reduced after treatment with lactobacilli. Lactobacilli culture supernatants can reduce the expression of ATG14 and BECN1 and the HPV E6 oncogene. Pawar et al. [92], who studied the effects of lactobacilli-derived metabolites on HeLa cell lines, also highlighted their potential as biotherapeutics for the control of HPV infection and cervical cancer. Lactobacilli showed greater inhibitory activity mainly producing L-lactic acid and hydrogen peroxide (H2O2). While the lactic acid acts by reducing pH and competing with other microorganisms for resources and inhibiting epithelial binding, H2O2 inhibits the growth of catalase-negative anaerobic organisms through the production of hydroxyl free radicals [93]. However, the amount of oxygen required to produce H2O2 is limited in the cervicovaginal tract, casting doubt on whether this protective property can be exercised [94]. Some Lactobacillus spp. produce bacteriocins, antimicrobial proteins that neutralize closely related species [93].
Exposure of SiHa cells to two probiotics, L. crispatus and L. gasseri, both of which produce D- and L-lactic acid isomers, resulted in the expression of the HPV oncogenes E6 and E7 [89]. In addition, D-lactic acid exhibited greater migration and invasion activities towards SiHa cells in a concentration-dependent manner compared to L-lactic acid [89]. Culturing cells with L. crispatus for 24 h exerted notable effects on the mRNA level of E6 and E7 genes, with inhibition rates of 63.0% and 66.6% [95]. However, L. gasseri had a less pronounced suppressive effect on E6 (19.3%) and did not significantly inhibit E7 [89]. Furthermore, the ratio of D-lactic acid to L-lactic acid in lactobacilli strains could influence the expression levels of the HPV16 E6 and E7 oncogenes [95].
Subsequently, when SiHa cells were exposed to other representative vaginal microbial communities, the expression of E6 and E7 oncogenes and the production of corresponding oncoproteins were observed [96]. L. crispatus and L. gasseri, modulators of basal E6 and E7 gene expression, influenced the level of E6 and E7 oncoprotein production, whereas bacteria associated with vaginal dysbiosis had an opposite effect on E6/E7 gene expression and protein production. In particular, the expression of E6 and E7 genes and production of their proteins were increased by G. vaginalis and Megasphaera micronuciformis. On the other hand, Prevotella bivia reduced oncogene expression and E7 protein production. In SiHa cell cultures stimulated with lactobacillus, an increased amount of p53 and pRb was found and, consequently, a lower percentage of cells entered the S-phase of the cell cycle. Based on this evidence, the downregulation of HPV E6 and E7 by lactobacilli may have therapeutic potential in the treatment of cervical cancer.
Some researchers [97,98] have investigated cervical microbiome composition in cervical intraepithelial neoplasias after surgical removal. The results showed that treatment caused significant changes in the cervical microbiota, with lactobacilli decreasing and anaerobic bacteria increasing. These changes were maintained after treatment. The results highlight the impact of surgical treatments on the cervical microbiota and suggest the need for post-treatment interventions to restore the microbial balance.
Li et al. [99] evaluated the cervicovaginal microbiota in relation to different treatments in 91 women divided into four groups: healthy control women and HPV16-infected women with CIN1, CIN2/3, and SCC. Endocervical swabs were analyzed for pyrosequencing of the bacterial 16S rRNA gene and the detection of HPV DNA 3 months before treatment (antiviral treatment with recombinant human interferon α2a vaginal suppositories for CIN 1, cervical excision for CIN 2/3, and radical hysterectomy for SCC patients) and 3 months after treatment (when women tested negative for HPV16). Lactobacillus species increased after clinical treatment, and CST profiles were shifted from dysbiotic CST II and IV to Lactobacillus-dominated CST I and III. In particular, the pre-treatment composition of Geobacter and Prevotella and post-treatment composition of L. secaliphilus may be associated with CIN 1, the pre-treatment composition of Burkholderia and the post-treatment composition of L. iners may be associated with CIN 2/3, and the pre-treatment composition of Atopobium and Aerococci and post-treatment composition of bacilli may be associated with SCC.
López-Cárdenas et al. [100] evaluated photodynamic therapy in Mexican women with CIN 1, CIN 1 + HPV, and HPV infection alone, as a strategy with few adverse events. After 6 months of treatment, HPV was eradicated in 100% of patients (p < 0.01), CIN 1 + HPV in 64.3% (p < 0.01) and CIN 1 in 57.2% (p > 0.05). Photodynamic therapy did not affect the normal microbiota, with L.iners being eliminated in only 5.8% of patients.
Wang et al. [101] investigated changes in the vaginal microbiome following focused ultrasound (FU) treatment for hr-HPV infection in 37 patients. Lactobacilli were predominant in both patients and controls, with L. iners being the most abundant species.
In the last few years, studies have been carried out to evaluate new strategies for the treatment of women with HPV infection. For example, a study [102] evaluated whether long-term oral administration of L. crispatus could re-establish eubiosis in HPV-infected women, thereby promoting the elimination of the virus. In total, 160 women were enrolled and randomly divided into two groups, one of which received oral L. crispatus M247 (Group 1) and the other a control group (Group 2). After 12 months, patients treated with long-term oral probiotics were more likely to resolve cytological abnormalities associated with HPV infection than controls. 15.3% of the patients in Group 1 had the virus completely cleared, compared to only 9.3% in Group 2.
Other authors [103] studied the effects of oral administration of L. crispatus M247 combined with Active Hexose Correlated Compound (AHCC) a mixture of alpha-glucans which can promote the eradication of HPV through the increase of interferon-γ and the decrease of interferon-β suggesting that can be a preventive therapy in subjects with a high-risk profile.
Palma et al. [104] evaluated the long-term use of vaginal lactobacilli in women with HPV infection associated with bacterial vaginosis or vaginitis to eradicate the viral infection and restore eubiosis. The women (117) were treated with metronidazole 500 mg twice daily for 7 days or fluconazole 150 mg once daily for 2 days plus short-term (3 months) and long-term (6 months) vaginal administration of L. rhamnosus BMX 54. The likelihood of resolution of HPV-related cytological abnormalities was twice that of short-term probiotic users (79.4% vs. 37.5%). HPV clearance was achieved in 11.6% of patients given short-term probiotics, compared to 31.2% of those given long-term vaginal lactobacilli. These results are in contrast with Ou et al. [105]. They evaluated the effect of oral probiotic strains L. rhamnosus GR-1 and L. reuteri RC-14 (one capsule daily until a negative hr-HPV test) on HPV clearance and cervical smear quality in a randomized placebo-controlled trial. The administration of these probiotic strains had no effect on genital hr-HPV clearance but may have decreased the rates of mildly abnormal and unsatisfactory cervical smears.
Ikeda et al. [106] developed an HPV-16 expressing E7 protein Lactobacillus-based vaccine (IGMKK16E7) to treat high-grade SIL (HSIL) in HPV-16-positive women. They started a clinical trial in 2019 to assess the safety and efficacy of IGMKK16E7. This randomized, placebo-controlled clinical trial consists of a placebo group, low dose (0.5 g/day), medium dose (1 g/day) and high dose (1.5 g/day) IGMKK16E7. IGMKK16E7 resulted in safe and pathological regression was detected after 16 weeks, with a cytological regression and immunological response to HPV-16 E7.
Early experimental data [107] suggest that IGMKK16E7 may show a remission rate in CIN3 patients.
Finally, another research conducted again by Ikeda et al. [108] studied the effects of the oral vaccine HPV-16 E7-Expressing Lactobacillus (GLBL101c) in a double-blind randomized trial in patients HPV-16 positive CIN 2 to check for possible regression of lesions, but there was no difference between treated women and placebo group.
The study highlights that although there are many published articles on the examined topic, most of them are generic. These studies evaluate the microbiota, making a general sequencing, and fail to define the actual role of the different lactobacilli species. Despite the number of studies on the vaginal microbiome and its relationship to HPV, further analyses are needed to assess sources of variability, such as variations in study populations or microbial detection methods. To date, in fact, there are no complete scientific study, that have taken into exam the multiple variables such as age, genetic factors, chronic diseases, diet, and drugs, also some studies are still lacking in some geographical areas of the world. These variables may be limitations of the research, as they may also lead to conflicting results. It would also be appropriate to consider the interaction between lactobacilli and the vaginal virome, pathogenic and non-pathogenic viruses. However, we can conclude that lactobacilli play a crucial role in vaginal microbiota health and L. crispatus is the most protective component against hr-HPV infection and neoplastic progression of infected cells. So, further studies are therefore necessary to better understand the mechanisms that regulate this complex relation between lactobacilli and HPV infection.
Acknowledgement: None.
Funding Statement: This research received no external funding.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Mara Lorusso; methodology, Francesco Triggiano, Giusy Diella; validation, Francesco Triggiano, Giusy Diella; resources, Luigi Santacroce; writing—original draft preparation, Francesco Triggiano, Giusy Diella, Mara Lorusso, Paolo Veneziani, Marilena D’Ambrosio, Daniela Nesta; writing—review and editing, Francesco Triggiano, Giusy Diella, Mara Lorusso, Paolo Veneziani, Luigi Santacroce; supervision, Luigi Santacroce. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All available data have been reported in the manuscript.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Abbreviations
| AHCC | Active Hexose Correlated Compound |
| AV | Anellovirus |
| BV | Bacterial vaginosis |
| CC | Cervical cancer |
| CIN | Cervical intraepithelial neoplasia |
| CSTs | Community status types |
| FU | Focused ultrasound |
| HIV | Human immunodeficiency virus |
| HPV | Human papillomavirus |
| HRCD | HPV-related cervical disease |
| hr-HPV | High-risk human papillomavirus |
| HSIL | High-grade SIL |
| ICC | Invasive cervical cancer |
| IFN-γ | Interferon-γ |
| IL-17 | Interleukin-17 |
| LSIL | Low-grade SIL |
| SCC | Squamous cell carcinoma |
| SIL | Squamous intraepithelial lesion |
| SNWs | Seronegative-women |
| TLR2 | Toll-like receptor 2 |
| VMB | Vaginal microbiota |
| WLWH | Women living with HIV |
| PHR | Pregnant women infected with hr-HPV |
| PN | Pregnant women not infected with hr-HPV |
| NPHR | Non-pregnant women infected with hr-HPV |
| NPN | Non-pregnant women not infected with hr-HPV |
References
1. Elovitz M, Anton L, Cristancho A, Ferguson B, Joseph A, Ravel J. Vaginal microbes alter epithelial transcriptome and induce epigenomic modifications providing insight into mechanisms for susceptibility to adverse reproductive outcomes. Res Sq. 2024;rs.3:rs-4385224. doi:10.21203/rs.3.rs-4385224/v1. [Google Scholar] [PubMed] [CrossRef]
2. Liu S, Li Y, Song Y, Wu X, Baloch Z, Xia X. The diversity of vaginal microbiome in women infected with single HPV and multiple genotype HPV infections in China. Front Cell Infect Microbiol. 2022;12:642074. doi:10.3389/fcimb.2022.642074. [Google Scholar] [PubMed] [CrossRef]
3. Heczko PB, Giemza M, Ponikiewska W, Strus M. Importance of lactobacilli for human health. Microorganisms. 2024;12(12):2382. doi:10.3390/microorganisms12122382. [Google Scholar] [PubMed] [CrossRef]
4. Nouioui I, Carro L, García-López M, Meier-Kolthoff JP, Woyke T, Kyrpides NC, et al. Genome-based taxonomic classification of the phylum actinobacteria. Front Microbiol. 2018;9:2007. doi:10.3389/fmicb.2018.02007. [Google Scholar] [PubMed] [CrossRef]
5. Juliana NCA, Deb S, Juma MH, Poort L, Budding AE, Mbarouk A, et al. The vaginal microbiota composition and genital infections during and after pregnancy among women in Pemba Island. Tanzania Microorgan. 2022;10(3):509. doi:10.3390/microorganisms10030509. [Google Scholar] [PubMed] [CrossRef]
6. Oerlemans EFM, Wuyts S, Bellen G, Wittouck S, De Boeck I, Ruban K, et al. The dwindling microbiota of aerobic vaginitis, an inflammatory state enriched in pathobionts with limited TLR stimulation. Diagnostics. 2020;10(11):879. doi:10.3390/diagnostics10110879. [Google Scholar] [PubMed] [CrossRef]
7. Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, et al. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–9. doi:10.1097/OLQ.0b013e318074e565. [Google Scholar] [PubMed] [CrossRef]
8. Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–7. doi:10.1073/pnas.1002611107. [Google Scholar] [PubMed] [CrossRef]
9. Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UM, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4(132):132ra52. doi:10.1126/scitranslmed.3003605. [Google Scholar] [PubMed] [CrossRef]
10. Mei L, Wang T, Chen Y, Wei D, Zhang Y, Cui T, et al. Dysbiosis of vaginal microbiota associated with persistent high-risk human papilloma virus infection. J Transl Med. 2022;20(1):12. doi:10.1186/s12967-021-03201-w. [Google Scholar] [PubMed] [CrossRef]
11. Liu Y, Li T, Guo R, Chen T, Wang S, Wu D, et al. The vaginal microbiota among the different status of human papillomavirus infection and bacterial vaginosis. J Med Virol. 2023;95(3):e28595. doi:10.1002/jmv.28595. [Google Scholar] [PubMed] [CrossRef]
12. Di Paola M, Sani C, Clemente AM, Iossa A, Perissi E, Castronovo G, et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci Rep. 2017;7(1):10200. doi:10.1038/s41598-017-09842-6. [Google Scholar] [PubMed] [CrossRef]
13. Usyk M, Zolnik CP, Castle PE, Porras C, Herrero R, Gradissimo A, et al. Cervicovaginal microbiome and natural history of HPV in a longitudinal study. PLoS Pathog. 2020;16(3):e1008376. doi:10.1371/journal.ppat.1008376. [Google Scholar] [PubMed] [CrossRef]
14. Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome. 2016;4(1):58. doi:10.1186/s40168-016-0203-0. [Google Scholar] [PubMed] [CrossRef]
15. Shannon B, Yi TJ, Perusini S, Gajer P, Ma B, Humphrys MS, et al. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol. 2017;10(5):1310–9. doi:10.1038/mi.2016.129. [Google Scholar] [PubMed] [CrossRef]
16. Vargas-Robles D, Morales N, Rodríguez I, Nieves T, Godoy-Vitorino F, Alcaraz LD, et al. Changes in the vaginal microbiota across a gradient of urbanization. Sci Rep. 2020;10(1):12487. doi:10.1038/s41598-020-69111-x. [Google Scholar] [PubMed] [CrossRef]
17. Onywera H, Williamson AL, Mbulawa ZZA, Coetzee D, Meiring TL. Factors associated with the composition and diversity of the cervical microbiota of reproductive-age Black South African women: a retrospective cross-sectional study. PeerJ. 2019;7(6):e7488. doi:10.7717/peerj.7488. [Google Scholar] [PubMed] [CrossRef]
18. McKee KS, Carter KA, Bassis C, Young VB, Reed B, Harper DM, et al. The vaginal microbiota, high-risk human papillomavirus infection, and cervical cytology: results from a population-based study. Gynecol Pelvic Med. 2020;3:18. doi:10.21037/gpm-20-10. [Google Scholar] [PubMed] [CrossRef]
19. So KA, Yang EJ, Kim NR, Hong SR, Lee JH, Hwang CS, et al. Changes of vaginal microbiota during cervical carcinogenesis in women with human papillomavirus infection. PLoS One. 2020;15(9):e0238705.15. doi:10.1371/journal.pone.0238705. [Google Scholar] [PubMed] [CrossRef]
20. Andrade Pessoa Morales J, Marconi C, El-Zein M, Ravel J, da Silva Pinto GV, Silveira R, et al. Vaginal microbiome components as correlates of cervical human papillomavirus infection. J Infect Dis. 2022;226(6):1084–97. doi:10.1093/infdis/jiab547. [Google Scholar] [PubMed] [CrossRef]
21. Wu S, Ding X, Kong Y, Acharya S, Wu H, Huang C, et al. The feature of cervical microbiota associated with the progression of cervical cancer among reproductive females. Gynecol Oncol. 2021;163(2):348–57. doi:10.1016/j.ygyno.2021.08.016. [Google Scholar] [PubMed] [CrossRef]
22. Mitra A, MacIntyre DA, Lee YS, Smith A, Marchesi JR, Lehne B, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep. 2015;5(1):16865. doi:10.1038/srep16865. [Google Scholar] [PubMed] [CrossRef]
23. Łaniewski P, Barnes D, Goulder A, Cui H, Roe DJ, Chase DM, et al. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci Rep. 2018;8(1):7593. doi:10.1038/s41598-018-25879-7. [Google Scholar] [PubMed] [CrossRef]
24. Liu J, Luo M, Zhang Y, Cao G, Wang S. Association of high-risk human papillomavirus infection duration and cervical lesions with vaginal microbiota composition. Ann Transl Med. 2020;8(18):1161. doi:10.21037/atm-20-5832. [Google Scholar] [PubMed] [CrossRef]
25. Hu J, Wu Y, Quan L, Yang W, Lang J, Tian G, et al. Research of cervical microbiota alterations with human papillomavirus infection status and women age in Sanmenxia area of China. Front Microbiol. 2022;13:1004664. doi:10.3389/fmicb.2022.1004664. [Google Scholar] [PubMed] [CrossRef]
26. Ilhan ZE, Łaniewski P, Thomas N, Roe DJ, Chase DM, Herbst-Kralovetz MM. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. eBioMedicine. 2019;44(2):675–90. doi:10.1016/j.ebiom.2019.04.028. [Google Scholar] [PubMed] [CrossRef]
27. Łaniewski P, Cui H, Roe DJ, Barnes D, Goulder A, Monk BJ, et al. Features of the cervicovaginal microenvironment drive cancer biomarker signatures in patients across cervical carcinogenesis. Sci Rep. 2019;9(1):7333. doi:10.1038/s41598-019-43849-5. [Google Scholar] [PubMed] [CrossRef]
28. Silva C, Almeida EC, CôboEde C, Zeferino VF, Murta EF, Etchebehere RM. A retrospective study on cervical intraepithelial lesions of low-grade and undetermined significance: evolution, associated factors and cytohistological correlation. Sao Paulo Med. 2014;132(2):92–6. doi:10.1590/1516-3180.2014.1322579. [Google Scholar] [PubMed] [CrossRef]
29. Cheng W, Xu F, Gao L, Liu J. The correlation between the determination of vaginal micro-ecological composition and the outcome of HPV infection by high-throughput metagene sequencing information technology on the illumina platform. J Infect Public Health. 2020;13(12):1961–6. doi:10.1016/j.jiph.2020.05.024. [Google Scholar] [PubMed] [CrossRef]
30. Cheng L, Norenhag J, Hu YOO, Brusselaers N, Fransson E, Ährlund-Richter A, et al. Vaginal microbiota and human papillomavirus infection among young Swedish women. npj Biofilms Microbio. 2020;6(1):39. doi:10.1038/s41522-020-00146-8. [Google Scholar] [PubMed] [CrossRef]
31. Borgdorff H, Tsivtsivadze E, Verhelst R, Marzorati M, Jurriaans S, Ndayisaba GF, et al. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014;8(9):1781–93. doi:10.1038/ismej.2014.26. [Google Scholar] [PubMed] [CrossRef]
32. Wei ZT, Chen HL, Wang CF, Yang GL, Han SM, Zhang SL. Depiction of vaginal microbiota in women with high-risk human papillomavirus infection. Front Public Health. 2021;8:587298. doi:10.3389/fpubh.2020.587298. [Google Scholar] [PubMed] [CrossRef]
33. Dareng EO, Ma B, Famooto AO, Adebamowo SN, Offiong RA, Olaniyan O, et al. Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiol Infect. 2016;144(1):123–37. doi:10.1017/S0950268815000965. [Google Scholar] [PubMed] [CrossRef]
34. Camargo M, Vega L, Muñoz M, Sánchez R, Patarroyo ME, Ramírez JD, et al. Changes in the cervical microbiota of women with different high-risk human papillomavirus loads. Viruses. 2022;14(12):2674. doi:10.3390/v14122674. [Google Scholar] [PubMed] [CrossRef]
35. Chao X, Sun T, Wang S, Tan X, Fan Q, Shi H, et al. Research of the potential biomarkers in vaginal microbiome for persistent high-risk human papillomavirus infection. Ann Transl Med. 2020;8(4):100. doi:10.21037/atm.2019.12.115. [Google Scholar] [PubMed] [CrossRef]
36. Chao XP, Sun TT, Wang S, Fan QB, Shi HH, Zhu L, et al. Correlation between the diversity of vaginal microbiota and the risk of high-risk human papillomavirus infection. Int J Gynecol Cancer. 2019;29(1):28–34. doi:10.1136/ijgc-2018-000032. [Google Scholar] [PubMed] [CrossRef]
37. Chorna N, Romaguera J, Godoy-Vitorino F. Cervicovaginal microbiome and urine metabolome paired analysis reveals niche partitioning of the microbiota in patients with human papilloma virus infections. Metabolites. 2020;10(1):36. doi:10.3390/metabo10010036. [Google Scholar] [PubMed] [CrossRef]
38. Liu H, Liang H, Li D, Wang M, Li Y. Association of cervical dysbacteriosis, HPV oncogene expression, and cervical lesion progression. Microbiol Spectr. 2022;10(5):e0015122. doi:10.1128/spectrum.00151-22. [Google Scholar] [PubMed] [CrossRef]
39. Teka B, Yoshida-Court K, Firdawoke E, Chanyalew Z, Gizaw M, Addissie A, et al. Cervicovaginal microbiota profiles in precancerous lesions and cervical cancer among ethiopian women. Microorganisms. 2023;11(4):833. doi:10.3390/microorganisms11040833. [Google Scholar] [PubMed] [CrossRef]
40. Fang B, Li Q, Wan Z, Ouyang Z, Zhang Q. Exploring the association between cervical microbiota and HR-HPV infection based on 16S rRNA gene and metagenomic sequencing. Front Cell Infect Microbiol. 2022;12:922554. doi:10.3389/fcimb.2022.922554. [Google Scholar] [PubMed] [CrossRef]
41. Ritu W, Enqi W, Zheng S, Wang J, Ling Y, Wang Y. Evaluation of the associations between cervical microbiota and HPV infection, clearance, and persistence in cytologically normal women. Cancer Prev Res. 2019;12(1):43–56. doi:10.1158/1940-6207.CAPR-18-0233. [Google Scholar] [PubMed] [CrossRef]
42. Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis. 2014;210(11):1723–33. doi:10.1093/infdis/jiu330. [Google Scholar] [PubMed] [CrossRef]
43. Yang Z, Zhang Y, Stubbe-Espejel A, Zhao Y, Liu M, Li J, et al. Vaginal microbiota and personal risk factors associated with HPV status conversion-A new approach to reduce the risk of cervical cancer? PLoS One. 2022;17(8):e0270521. doi:10.1371/journal.pone.0270521. [Google Scholar] [PubMed] [CrossRef]
44. Shi W, Zhu H, Yuan L, Chen X, Huang X, Wang K, et al. Vaginal microbiota and HPV clearance: a longitudinal study. Front Oncol. 2022;12:955150. doi:10.3389/fonc.2022.955150. [Google Scholar] [PubMed] [CrossRef]
45. Zeng M, Li X, Jiao X, Cai X, Yao F, Xu S, et al. Roles of vaginal flora in human papillomavirus infection, virus persistence and clearance. Front Cell Infect Microbiol. 2023;12:1036869. doi:10.3389/fcimb.2022.1036869. [Google Scholar] [PubMed] [CrossRef]
46. Santella B, Schettino MT, Franci G, De Franciscis P, Colacurci N, Schiattarella A, et al. The role of viralinfection on vaginalmicrobiota. J Med Virol. 2022;94(9):4478–84. doi:10.1002/jmv.27837. [Google Scholar] [PubMed] [CrossRef]
47. Kwasniewski W, Wolun-Cholewa M, Kotarski J, Warchol W, Kuzma D, Kwasniewska A, et al. Microbiota dysbiosis is associated with HPV-induced cervical carcinogenesis. Oncol Lett. 2018;16(6):7035–47. doi:10.3892/ol.2018.9509. [Google Scholar] [PubMed] [CrossRef]
48. Sun L, Li L, Xu W, Ma C. The immunomodulation role of vaginal microenvironment on human papillomavirus infection. Galen Med J. 2023;12:1–7. doi:10.31661/gmj.v12i0.2991. [Google Scholar] [PubMed] [CrossRef]
49. Frąszczak K, Barczyński B, Siwiec R, Kondracka A, Malm A, Kotarski J, et al. The analysis of Lactobacillus spp. distribution in the vaginal microbiota of Polish women with abnormal Pap smear result. Front Microbiol. 2023;14:1257587. doi:10.3389/fmicb.2023.1257587. [Google Scholar] [PubMed] [CrossRef]
50. Campisciano G, Iebba V, Zito G, Luppi S, Martinelli M, Fischer L, et al. Lactobacillus iners and gasseri, Prevotellabivia and HPV belong to the microbiological signature negatively affecting human reproduction. Microorganisms. 2020;9(1):39. doi:10.3390/microorganisms9010039. [Google Scholar] [PubMed] [CrossRef]
51. Musa J, Maiga M, Green SJ, Magaji FA, Maryam AJ, Okolo M, et al. Vaginal microbiome community state types and high-risk human papillomaviruses in cervical precancer and cancer in North-central Nigeria. BMC Cancer. 2023;23(1):683. doi:10.1186/s12885-023-11187-5. [Google Scholar] [PubMed] [CrossRef]
52. Yang Q, Wang Y, Wei X, Zhu J, Wang X, Xie X, et al. The alterations of vaginal microbiome in HPV16 infection as identified by shotgun metagenomic sequencing. Front Cell Infect Microbiol. 2020;10:286. doi:10.3389/fcimb.2020.00286. [Google Scholar] [PubMed] [CrossRef]
53. Berggrund M, Gustavsson I, Aarnio R, Lindberg JH, Sanner K, Wikström I, et al. Temporal changes in the vaginal microbiota in self-samples and its association with persistent HPV16 infection and CIN2. Virol J. 2020;17(1):147. doi:10.1186/s12985-020-01420-z. [Google Scholar] [PubMed] [CrossRef]
54. Gonçalves-Nobre JG, Matos A, Carreira M, Santos AC, Veiga LC, Ginete C, et al. The interplay between HPV, other Sexually Transmissible Infections and genital microbiome on cervical microenvironment (MicroCervixHPV study). Front Cell Infect Microbiol. 2024;13:1251913. doi:10.3389/fcimb.2023.1251913. [Google Scholar] [PubMed] [CrossRef]
55. Reimers LL, Mehta SD, Massad LS, Burk RD, Xie X, Ravel J, et al. The cervicovaginal microbiota and its associations with human papillomavirus detection in HIV-infected and HIV-uninfected women. J Infect Dis. 2016;214(9):1361–9. doi:10.1093/infdis/jiw374. [Google Scholar] [PubMed] [CrossRef]
56. Chávez-Torres M, Gómez-Palacio-Schjetnan M, Reyes-Terán G, Briceño O, Ávila-Ríos S, Romero-Mora KA, et al. The vaginal microbiota of women living with HIV on suppressive antiretroviral therapy and its relation to high-risk human papillomavirus infection. BMC Microbiol. 2023;23(1):21. doi:10.1186/s12866-023-02769-1. [Google Scholar] [PubMed] [CrossRef]
57. Sasivimolrattana T, Chantratita W, Sensorn I, Chaiwongkot A, Oranratanaphan S, Bhattarakosol P, et al. Cervical microbiome in women infected with HPV16 and high-risk HPVs. Int J Environ Res Public Health. 2022;19(22):14716. doi:10.3390/ijerph192214716. [Google Scholar] [PubMed] [CrossRef]
58. Britto AMA, Siqueira JD, Curty G, Goes LR, Policarpo C, Meyrelles AR, et al. Microbiome analysis of Brazilian women cervix reveals specific bacterial abundance correlation to RIG-like receptor gene expression. Front Immunol. 2023;14:1147950. doi:10.3389/fimmu.2023.1147950. [Google Scholar] [PubMed] [CrossRef]
59. Xu X, Rao H, Fan X, Pang X, Wang Y, Zhao L, et al. HPV-related cervical diseases: alteration of vaginal microbiotas and promising potential for diagnosis. J Med Virol. 2023;95(1):e28351. doi:10.1002/jmv.28351. [Google Scholar] [PubMed] [CrossRef]
60. Chen Y, Hong Z, Wang W, Gu L, Gao H, Qiu L, et al. Association between the vaginal microbiome and high-risk human papillomavirus infection in pregnant Chinese women. BMC Infect Dis. 2019;19(1):677. doi:10.1186/s12879-019-4279-6. [Google Scholar] [PubMed] [CrossRef]
61. Vargas-Robles D, Romaguera J, Alvarado-Velez I, Tosado-Rodríguez E, Dominicci-Maura A, Sanchez M, et al. The cervical microbiota of Hispanics living in Puerto Rico is nonoptimal regardless of HPV status. mSystems. 2023;8(4):e0035723. doi:10.1128/msystems.00357-23. [Google Scholar] [PubMed] [CrossRef]
62. Avsaroglu E, Kaleli B, Kilic D, Kaleli I, Guler T. A decrease in lactobacilli in the vaginal microbiota is independently associated with HPV persistence in women with high-risk HPV infection. Cureus. 2023;15(12):e50907. doi:10.7759/cureus.50907. [Google Scholar] [PubMed] [CrossRef]
63. Liu J, Song J, Yang Q, Wang Y. Correlation between Lactobacillus and expression of E-cadherin, β-catenin, N-cadherin, and Vimentin in postmenopausal cervical lesions. Ann Palliat Med. 2022;11(1):135–45. doi:10.21037/apm-21-3581. [Google Scholar] [PubMed] [CrossRef]
64. Wei B, Chen Y, Lu T, Cao W, Tang Z, Yang H. Correlation between vaginal microbiota and different progression stages of cervical cancer. Genet Mol Biol. 2022;45(2):e20200450. doi:10.1590/1678-4685-gmb-2020-0450. [Google Scholar] [PubMed] [CrossRef]
65. Zhang Y, Xu X, Yu L, Shi X, Min M, Xiong L, et al. Vaginal microbiota changes caused by HPV infection in chinese women. Front Cell Infect Microbiol. 2022;12:814668. doi:10.3389/fcimb.2022.814668. [Google Scholar] [PubMed] [CrossRef]
66. Ivanov MK, Brenner EV, Hodkevich AA, Dzyubenko VV, Krasilnikov SE, Mansurova AS, et al. Cervicovaginal-microbiome analysis by 16S sequencing and real-time PCR in patients from Novosibirsk (Russia) with cervical lesions and several years after cancer treatment. Diagnostics. 2023;13(1):140. doi:10.3390/diagnostics13010140. [Google Scholar] [PubMed] [CrossRef]
67. Arokiyaraj S, Seo SS, Kwon M, Lee JK, Kim MK. Association of cervical microbial community with persistence, clearance and negativity of Human Papillomavirus in Korean women: a longitudinal study. Sci Rep. 2018;8(1):15479. doi:10.1038/s41598-018-33750-y. [Google Scholar] [PubMed] [CrossRef]
68. Nieves-Ramírez ME, Partida-Rodríguez O, Moran P, Serrano-Vázquez A, Pérez-Juárez H, Pérez-Rodríguez ME, et al. Cervical squamous intraepithelial lesions are associated with differences in the vaginal microbiota of mexican women. MicrobiolSpectr. 2021;9(2):e0014321. doi:10.1128/Spectrum.00143-21. [Google Scholar] [PubMed] [CrossRef]
69. Tossas KY, Zhu B, Perera RA, Serrano MG, Sullivan S, Sayeed S, et al. Does the vaginal microbiome operate differently by race to influence risk of precervical cancer? J Womens Health. 2023;32(5):553–60. doi:10.1089/jwh.2022.0309. [Google Scholar] [PubMed] [CrossRef]
70. Condic M, Neidhöfer C, Ralser DJ, Wetzig N, Thiele R, Sieber M, et al. Analysis of the cervical microbiome in women from the German national cervical cancer screening program. J Cancer Res Clin Oncol. 2023;149(9):6489–500. doi:10.1007/s00432-023-04599-0. [Google Scholar] [PubMed] [CrossRef]
71. Höhn AK, Brambs CE, Hiller GGR, May D, Schmoeckel E, Horn LC. 2020 WHO classification of female genital tumors. GeburtshilfeFrauenheilkd. 2021;81(10):1145–53. doi:10.1055/a-1545-4279. [Google Scholar] [PubMed] [CrossRef]
72. Zhai Q, Zhang W, Zhang Z, Fu Y, Li Y, Wang X, et al. Characteristics of the cervicovaginal microenvironment in childbearing-age women with different degrees of cervical lesions and HR-HPV positivity. Pol J Microbiol. 2021;70(4):489–500. doi:10.33073/pjm-2021-046. [Google Scholar] [PubMed] [CrossRef]
73. Mitra A, MacIntyre DA, Ntritsos G, Smith A, Tsilidis KK, Marchesi JR, et al. The vaginal microbiota associates with the regression of untreated cervical intraepithelial neoplasia 2 lesions. Nat Commun. 2020;11(1):1999. doi:10.1038/s41467-020-15856-y. [Google Scholar] [PubMed] [CrossRef]
74. Piyathilake CJ, Ollberding NJ, Kumar R, Macaluso M, Alvarez RD, Morrow CD. Cervical microbiota associated with higher grade cervical intraepithelial neoplasia in women infected with high-risk human papillomaviruses. Cancer Prev Res. 2016;9(5):357–66. doi:10.1158/1940-6207.CAPR-15-0350. [Google Scholar] [PubMed] [CrossRef]
75. Stoian IL, Botezatu A, Fudulu A, Ilea CG, Socolov DG. Exploring microbiota diversity in cervical lesion progression and HPV infection through 16S rRNA gene metagenomic sequencing. J Clin Med. 2023;12(15):4979. doi:10.3390/jcm12154979. [Google Scholar] [PubMed] [CrossRef]
76. Zhang C, Liu Y, Gao W, Pan Y, Gao Y, Shen J, et al. The direct and indirect association of cervical microbiota with the risk of cervical intraepithelial neoplasia. Cancer Med. 2018;7(5):2172–9. doi:10.1002/cam4.1471. [Google Scholar] [PubMed] [CrossRef]
77. Xie Y, Feng Y, Li W, Zhan F, Huang G, Hu H, et al. Revealing the disturbed vaginal micobiota caused by cervical cancer using high-throughput sequencing technology. Front Cell Infect Microbiol. 2020;10:538336. doi:10.3389/fcimb.2020.538336. [Google Scholar] [PubMed] [CrossRef]
78. Guo C, Dai W, Zhou Q, Gui L, Cai H, Wu D, et al. Cervicovaginal microbiota significantly changed for HPV-positive women with high-grade squamous intraepithelial lesion. Front Cell Infect Microbiol. 2022;12:973875. doi:10.3389/fcimb.2022.973875. [Google Scholar] [PubMed] [CrossRef]
79. Chen Y, Qiu X, Wang W, Li D, Wu A, Hong Z, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis. 2020;20(1):629. doi:10.1186/s12879-020-05324-9. [Google Scholar] [PubMed] [CrossRef]
80. Norenhag J, Du J, Olovsson M, Verstraelen H, Engstrand L, Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG. 2020;127(2):171–80. doi:10.1111/1471-0528.15854. [Google Scholar] [PubMed] [CrossRef]
81. Wu M, Gao J, Wu Y, Li Y, Chen Y, Zhao F, et al. Characterization of vaginal microbiota in Chinese women with cervical squamous intra-epithelial neoplasia. Int J Gynecol Cancer. 2020;30(10):1500–4. doi:10.1136/ijgc-2020-001341. [Google Scholar] [PubMed] [CrossRef]
82. Liu Y, Wang S, Liu J, Su M, Diao X, Liang X, et al. Characteristics of vaginal microbiota in various cervical intraepithelial neoplasia: a cross-sectional study. J Transl Med. 2023;21(1):816. doi:10.1186/s12967-023-04676-5. [Google Scholar] [PubMed] [CrossRef]
83. Xia Y, Feng Y, Qin T, Zhao X, Lu J, Ma C. Characteristics of vaginal microbiome in reproductive-age females with HPV infection in Xinjiang, China. Evid Based Complement Alternat Med. 2022;2022(6):7332628. doi:10.1155/2022/7332628. [Google Scholar] [PubMed] [CrossRef]
84. Nicolò S, Tanturli M, Mattiuz G, Antonelli A, Baccani I, Bonaiuto C, et al. Vaginal lactobacilli and vaginal dysbiosis-associated bacteria differently affect cervical epithelial and immune homeostasis and anti-viral defenses. Int J Mol Sci. 2021;22(12):6487. doi:10.3390/ijms22126487. [Google Scholar] [PubMed] [CrossRef]
85. Łaniewski P, Cui H, Roe DJ, Chase DM, Herbst-Kralovetz MM. Vaginal microbiota, genital inflammation, and neoplasia impact immune checkpoint protein profiles in the cervicovaginal microenvironment. npj Precis Oncol. 2020;4(1):22. doi:10.1038/s41698-020-0126-x. [Google Scholar] [PubMed] [CrossRef]
86. Gao Q, Fan T, Luo S, Zheng J, Zhang L, Cao L, et al. Lactobacillus gasseri LGV03 isolated from the cervico-vagina of HPV-cleared women modulates epithelial innate immune responses and suppresses the growth of HPV-positive human cervical cancer cells. Transl Oncol. 2023;35(5):101714. doi:10.1016/j.tranon.2023.101714. [Google Scholar] [PubMed] [CrossRef]
87. Tosado-Rodríguez E, Mendez LB, Espino AM, Dorta-Estremera S, Aquino EE, Romaguera J, et al. Inflammatory cytokines and a diverse cervicovaginal microbiota associate with cervical dysplasia in a cohort of Hispanics living in Puerto Rico. PLoS One. 2023;18(12):e0284673. doi:10.1371/journal.pone.0284673. [Google Scholar] [PubMed] [CrossRef]
88. Zheng JJ, Miao JR, Wu Q, Yu CX, Mu L, Song JH. Correlation between HPV-negative cervical lesions and cervical microenvironment. Taiwan J Obstet Gynecol. 2020;59(6):855–61. doi:10.1016/j.tjog.2020.08.002. [Google Scholar] [PubMed] [CrossRef]
89. Liu CJ, Xiao WY, Fang JF, Dong YH, Ye KF, He MP, et al. Genital microbiota of women from six ethnic groups with and without human papillomavirus infection in Shangri-La, China. Front Cell Infect Microbiol. 2022;12:935068. doi:10.3389/fcimb.2022.935068. [Google Scholar] [PubMed] [CrossRef]
90. Lebeau A, Bruyere D, Roncarati P, Peixoto P, Hervouet E, Cobraiville G, et al. HPV infection alters vaginal microbiome through down-regulating host mucosal innate peptides used by Lactobacilli as amino acid sources. Nat Commun. 2022;13(1):1076. doi:10.1038/s41467-022-28724-8. [Google Scholar] [PubMed] [CrossRef]
91. Motevaseli E, Azam R, Akrami SM, Mazlomy M, Saffari M, Modarressi MH, et al. The effect of lactobacillus crispatus and lactobacillus rhamnosusculture supernatants on expression of autophagy genes and HPV E6 and E7 oncogenes in the HeLa cell line. Cell J. 2016;17(4):601–7. doi:10.22074/cellj.2016.3833. [Google Scholar] [PubMed] [CrossRef]
92. Pawar K, Aranha C. Lactobacilli metabolites restore E-cadherin and suppress MMP9 in cervical cancer cells. Curr Res Toxicol. 2022;3:100088. doi:10.1016/j.crtox.2022.100088. [Google Scholar] [PubMed] [CrossRef]
93. Amabebe E, Anumba DOC. The vaginal microenvironment: the physiologic role of lactobacilli. Front Med. 2018;5:181. doi:10.3389/fmed.2018.00181. [Google Scholar] [PubMed] [CrossRef]
94. Blaskewicz CD, Pudney J, Anderson DJ. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia1. Biol Reprod. 2011;85:97–104. doi:10.1095/biolreprod.110.090423. [Google Scholar] [PubMed] [CrossRef]
95. Li C, Jia L, Yu Y, Jin L. Lactic acid induced microRNA-744 enhances motility of SiHa cervical cancer cells through targeting ARHGAP5. Chem Biol Interact. 2019;298:86–95. doi:10.1016/j.cbi.2018.10.027. [Google Scholar] [PubMed] [CrossRef]
96. Nicolò S, Antonelli A, Tanturli M, Baccani I, Bonaiuto C, Castronovo G, et al. Bacterial species from vaginal microbiota differently affect the production of the E6 and E7 oncoproteins and of p53 and p-Rb oncosuppressors in HPV16-infected cells. Int J Mol Sci. 2023;24(8):7173. doi:10.3390/ijms24087173. [Google Scholar] [PubMed] [CrossRef]
97. Zhang H, Lu J, Lu Y, Cai Q, Liu H, Xu C. Cervical microbiome is altered in cervical intraepithelial neoplasia after loop electrosurgical excision procedure in China. Sci Rep. 2018;8(1):4923. doi:10.1038/s41598-018-23389-0. [Google Scholar] [PubMed] [CrossRef]
98. Wiik J, Sengpiel V, Kyrgiou M, Nilsson S, Mitra A, Tanbo T, et al. Cervical microbiota in women with cervical intra-epithelial neoplasia, prior to and after local excisional treatment, a Norwegian cohort study. BMC Womens Health. 2019;19(1):30. doi:10.1186/s12905-019-0727-0. [Google Scholar] [PubMed] [CrossRef]
99. Li C, Zhang Z, Yang Y, Liao H. Changes in the cervicovaginal microbiota composition of HPV16-infected patients after clinical treatment. Cancer Med. 2022;11(24):5037–49. doi:10.1002/cam4.4801. [Google Scholar] [PubMed] [CrossRef]
100. López-Cárdenas MT, Jiménez A, Espinosa-Montesinos A, Maldonado-Alvarado E, Osorio-Peralta MO, Martinez-Escobar A, et al. Elimination of human papillomavirus and cervical pathological microbiota with photodynamic therapy in women from Mexico City with cervical intraepithelial neoplasia I. PhotochemPhotobiol. 2023;99(6):1468–75. doi:10.1111/php.13791. [Google Scholar] [PubMed] [CrossRef]
101. Wang W, Liu Y, Yang Y, Ren J, Zhou H. Changes in vaginal microbiome after focused ultrasound treatment of high-risk human papillomavirus infection-related low-grade cervical lesions. BMC Infect Dis. 2023;23(1):3. doi:10.1186/s12879-022-07937-8. [Google Scholar] [PubMed] [CrossRef]
102. Di Pierro F, Criscuolo AA, Dei Giudici A, Senatori R, Sesti F, Ciotti M, et al. Oral administration of Lactobacillus crispatus M247 to papillomavirus-infected women: results of a preliminary, uncontrolled, open trial. Minerva Obstet Gynecol. 2021 Oct;73(5):621–31. doi:10.23736/S2724-606X.21.04752-7. [Google Scholar] [PubMed] [CrossRef]
103. Cazzaniga M, Cardinali M, Di Pierro F, Bertuccioli A. Ovarian microbiota, ovarian cancer and the underestimated role of HPV. Int J Mol Sci. 2022;23(24):16019. doi:10.3390/ijms232416019. [Google Scholar] [PubMed] [CrossRef]
104. Palma E, Recine N, Domenici L, Giorgini M, Pierangeli A, Panici PB. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC InfectDis. 2018;18(1):13. doi:10.1186/s12879-017-2938-z. [Google Scholar] [PubMed] [CrossRef]
105. Ou YC, Fu HC, Tseng CW, Wu CH, Tsai CC, Lin H. The influence of probiotics on genital high-risk human papillomavirus clearance and quality of cervical smear: a randomized placebo-controlled trial. BMC Womens Health. 2019;19(1):103. doi:10.1186/s12905-019-0798-y. [Google Scholar] [PubMed] [CrossRef]
106. Ikeda Y, Uemura Y, Asai-Sato M, Nakao T, Nakajima T, Iwata T, et al. Safety and efficacy of mucosal immunotherapy using human papillomavirus (HPV) type 16 E7-expressing Lactobacillus-based vaccine for the treatment of high-grade squamous intraepithelial lesion (HSILthe study protocol of a randomized placebo-controlled clinical trial (MILACLE study). Jpn J Clin Oncol. 2019;49(9):877–80. doi:10.1093/jjco/hyz095. [Google Scholar] [PubMed] [CrossRef]
107. Kawana K, Adachi K, Kojima S, Taguchi A, Tomio K, Yamashita A, et al. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine. 2014;32(47):6233–9. doi:10.1016/j.vaccine.2014.09.020. [Google Scholar] [PubMed] [CrossRef]
108. Ikeda Y, Adachi K, Tomio K, Eguchi-Kojima S, Tsuruga T, Uchino-Mori M, et al. A placebo-controlled, double-blind randomized (Phase IIB) trial of oral administration with HPV16 E7-expressing lactobacillus, GLBL101c, for the treatment of cervical intraepithelial neoplasia grade 2 (CIN2). Vaccines. 2021;9(4):329. doi:10.3390/vaccines9040329. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools