 Open Access
Open Access
REVIEW
Evaluating Oncogenic Drivers and Therapeutic Potential of the PI3K/AKT/mTOR Pathway in Hepatocellular Carcinoma: An Overview of Clinical Trials
1 Department of Hematology, School of Medical Sciences, Tarbiat Modares University, Tehran, 1411713116, Iran
2 Department of Hematology and Blood Banking, School of Allied Medical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, 1971653313, Iran
3 Gastroenterology and Liver Diseases Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, 1985714711, Iran
4 Department of Internal Medicine, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, 1985717443, Iran
* Corresponding Author: Davood Bashash. Email:
BIOCELL 2025, 49(4), 539-562. https://doi.org/10.32604/biocell.2025.059970
Received 21 October 2024; Accepted 17 February 2025; Issue published 30 April 2025
Abstract
Hepatocellular carcinoma (HCC) is the most common primary liver tumor and the third leading cause of cancer-related mortality globally. The phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway is critically involved in HCC pathogenesis, stimulating uncontrolled cell proliferation, survival, and tumor progression. The overactivation of this pathway is strongly linked to poor prognosis, making it a crucial target for therapeutic intervention. The oncogenic roles of PI3K/AKT/mTOR components in HCC have been highlighted, noting that class I PI3K deregulation, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) upregulation, and mTOR overexpression could be associated with poor HCC outcomes. To the best of our knowledge, this is the first time that the clinical trials investigating PI3K/AKT/mTOR inhibitors in HCC are analyzed. Accordingly, there is a predominance of mTOR inhibitors, with everolimus being the most frequently utilized drug. However, only 10% of studies advanced to phase III or IV, predominantly involving mTOR inhibitors. Challenges such as adverse events like hyperglycemia and bone marrow suppression, as well as the emergence of treatment resistance, have hindered the success of these therapies. Combination therapies, particularly those involving mitogen-activated protein kinase kinase (MEK) inhibitors, chemotherapy, immune checkpoint inhibitors, and vascular endothelial growth factor (VEGF) inhibitors, have shown promise in overcoming these challenges. Recent advances in nanotechnology offer the potential for improving drug delivery and reducing toxicity.Keywords
The tumors in the liver can occur in hepatocytes as well as in the bile ducts, leading to hepatocellular carcinoma (HCC) and cholangiocarcinoma, with the former comprising more than 90% of primary liver tumors [1]. According to the World Health Organization (WHO), nearly 900,000 individuals worldwide develop HCC annually, of which 69.8% are male. HCC is the third leading cause of cancer-related death globally, with more than 830,000 recorded deaths each year. Based on reports from the Global Cancer Observatory (GCO), over 72% of HCC cases are identified in Asia, followed by Africa, Oceania, North America, and Europe [2]. Numerous risk factors contribute to the development of HCC, causing fibrosis and necrosis of hepatocytes, repetitive regeneration, cirrhosis, alterations in genes, chromosomal instability (CIN), DNA damage, and altered protein expression [3]. The most well-studied risk factors include viral hepatitis (hepatitis B and hepatitis C), alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD) and its severe form, non-alcoholic steatohepatitis (NASH), obesity, diabetes, and aflatoxin B1 [4,5].
The key to success for tumors is their capacity to change their metabolic pathways to maintain the demand for energy at the highest level necessary for out-of-control growth [6]. The Warburg effect is one of the prominent events in malignant cells, referring to the generation of excessive energy by elevating the oxygen-dependent glycolysis, followed by lactic acid fermentation with lactate secretion [7]. Several signaling pathways are associated with the Warburg effect; however, the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) axis has been shown to play an imperative role in increasing Warburg effect, leading to out-of-control proliferation [8]. The activation of the PI3K signaling pathway has been demonstrated to be able to turn liver tumors into a more aggressive one as the overactivation of this axis is related to poor prognosis of HCC [9]. Conventional therapeutic strategies such as surgery, radiotherapy, chemotherapy, and liver transplantation have pros and cons and are not a guarantee for HCC treatment. Recent studies have focused on novel therapeutic approaches such as targeting oncogenic signaling pathways [1]. Concerning the oncogenic features of the PI3K/AKT/mTOR signaling pathway, the application of therapeutic inhibitors of the components of this pathway has gained popularity in preclinical and clinical studies [10]. In the present study, we provided an overview of the oncogenic characteristics of the PI3K/AKT/mTOR signaling pathway in HCC development. Furthermore, we, for the very first time, investigated the clinical trials regarding inhibitors of the PI3K axis and discussed the promising traits and shortcomings. In this review, we explore the pivotal role of the PI3K/AKT/mTOR signaling pathway in the pathogenesis and progression of HCC, highlighting its contributions to tumorigenesis and therapeutic shortcomings. Our analysis provides an in-depth overview of the clinical trials involving inhibitors of this pathway, including their efficacy, shortcomings, and the increasing interest in combination regimens to overcome resistance and adverse effects. This comprehensive evaluation aims to illuminate current advancements and inspire innovative approaches for effectively managing HCC.
2 Overview of PI3K/AKT/mTOR Signaling Pathway
The PI3K/AKT/mTOR axis is one of the fundamental signaling pathways necessary for maintaining cell growth and survival in both physiological and pathological conditions. PI3K axis could be particularly activated in cellular stress situations to regulate the survival of the cell [11]; therefore, this signaling pathway plays an imperative role in cancerous conditions as tumors exist in stressful microenvironments such as low amounts of nutrition and oxygen [12]. Three classes of PI3K have been discovered so far [13]. Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), PIK3CB, PIK3CG, and PIK3CD encode four catalytic subunits of class I PI3K, which can catalyze the phosphorylation of the phosphatidylinositol 4,5-biphosphate (PIP2) and generate phosphatidylinositol 3,4,5-triphosphate (PIP3) [14]. PI3K-C2α, PI3K-C2β, and PI3K-C2γ are three different isoforms of class II PI3K, with PI3K-C2α having roles in vesicular trafficking, endocytosis, and mitosis, PI3K-C2β in mTOR suppression and cell migration, and PI3K-C2γ in AKT2 activation and glycogen storage [15]. PIK3C3 encodes the third class of PI3K (class III) which plays an essential role in cellular events such as autophagosome formation and autophagy flux [13]. AKT family includes serine and threonine kinases, and the three isoforms are AKT1, AKT2, and AKT3. In the context of the liver, it was shown that the expression of AKT1 and AKT2 could be identified in hepatocytes. In HCC cases, the overactivation of c-MYC is tightly correlated with the expression of AKT1, and high levels of AKT1 are a risk factor for poor prognosis in HCC patients [16]. The crucial roles of mTOR are remarkably related to linking nutrient sensing to cell growth; thus, it could be a prominent agent in tumorigenesis processes [17]. A combination of some proteins including mTOR, regulatory-associated protein of mTOR (Raptor), PRAS40, mammalian lethal with sec-13 protein 8 (mLST8), telomere maintenance 2 (Tel2), TELO2 interacting protein 1 (Tti1), and DEP domain-containing mTOR-interacting protein (Deptor) compose mTORC1 complex which can influence the stimulation of glycolytic flux and mitochondrial function, synthesize proteins and lipids, block lysosomal biogenesis and autophagy [18]. Furthermore, mTORC2 complex is composed of some proteins including mTOR, rapamycin-insensitive companion of mTOR (Rictor), Deptor, mammalian stress-activated protein kinase interacting protein 1 (mSIN1), mLST8, protein observed with Rictor 1 and 2 (Protor1/2), Tel2, and Tti1. Some of the downstream targets of the mTORC2 complex have been poorly identified; however, the most well-studied targets are from the AGC protein kinase family such as AKT, protein kinase C (PKC), and serum and glucocorticoid-regulated kinase (SGK) [19]. mTORC2 could target these molecules and augment their stability, maturation, and allosteric activation [20]. In hepatocytes, mTORC2 can facilitate hepatosteatosis and induce cancer progression by de novo fatty acid and lipid synthesis [21]. The role of phosphatase and tensin homolog (PTEN) is of cardinal importance in regulating the activation of the PI3K/AKT/mTOR signaling pathway. PTEN can dephosphorylate PIP3 and terminate the axis at the initial steps; therefore, the lack of PTEN and/or its loss of function could lead to an uncontrolled activation of the PI3K/AKT/mTOR signaling pathway, contributing to tumorigenesis events [22]. It has been demonstrated that PTEN loss (like PTEN-null mice) is one of the main triggers of carcinogenesis in liver cells [23].
The overactivation of the PI3K/AKT/mTOR pathway has been demonstrated in numerous types of cancers. In the context of HCC, it has been shown that the overactivation of PI3K and AKT could be associated with the aggressiveness of tumors. Indeed, the activation of cascades related to AKT are prominent risk factor related to a poor prognosis and an earlier recurrence of the malignancy in patients with liver cancers [9]. According to positron emission tomography (PET) scans, proteins of the PI3K/AKT/mTOR pathway could be elevated in patients with HCC, implying that this signaling pathway can be activated during the pathogenesis of HCC [24].
Fig. 1 demonstrates the activation of the PI3K axis in liver cells and HCC.
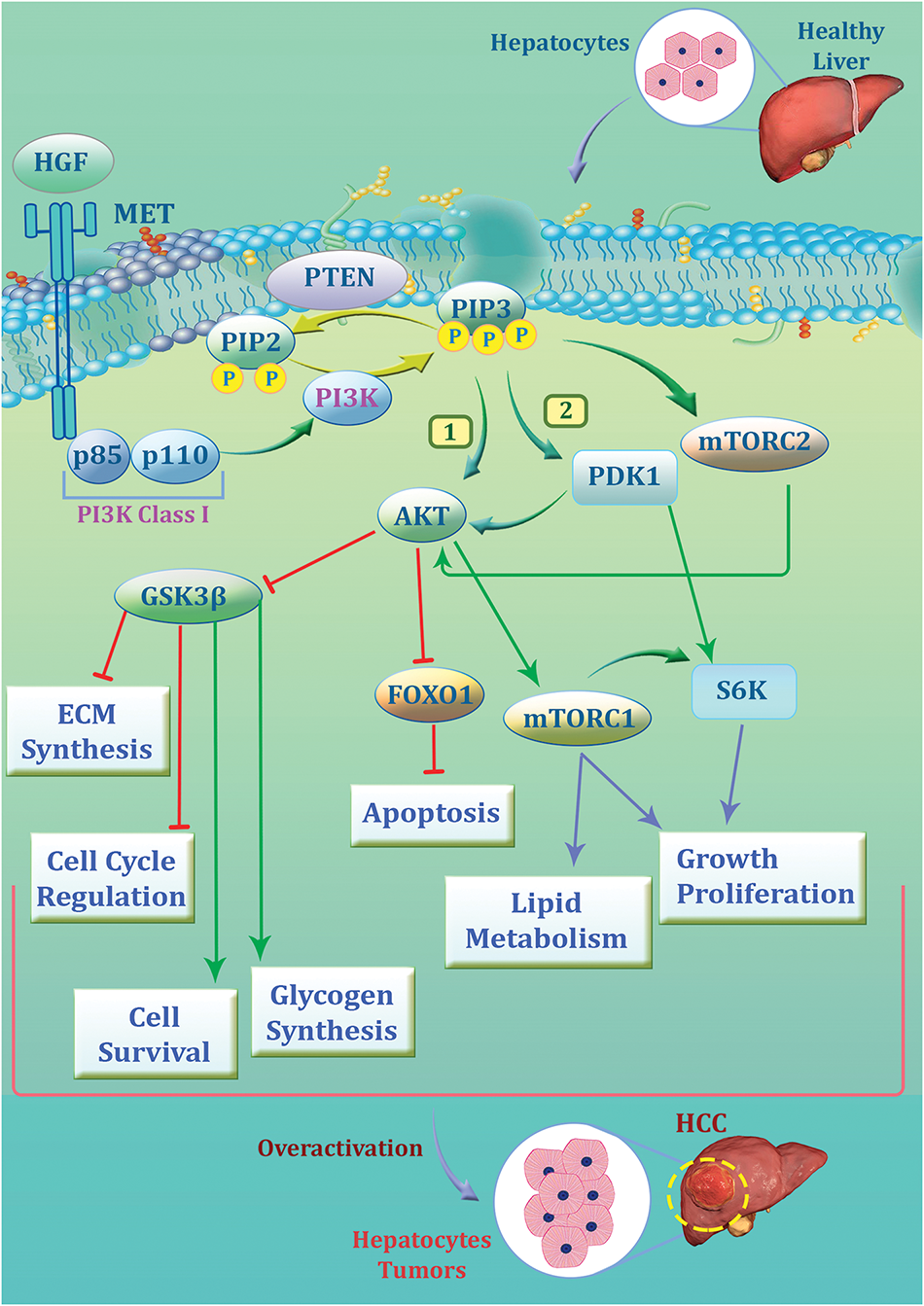
Figure 1: The signaling pathway of phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) in hepatocytes and its role in hepatocellular carcinoma (HCC) development. The pathway begins with hepatocyte growth factor (HGF) binding to its receptor MET, leading to the activation of PI3K (class I), composed of the p85 regulatory and p110 catalytic subunits. PI3K can convert phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3), leading to the activation of AKT through phosphorylation mediated by pyruvate dehydrogenase kinase 1 (PDK1) and mTORC2. Activated AKT plays a pivotal role and promotes cell survival, glycogen synthesis, and lipid metabolism while inhibiting apoptosis via suppression of forkhead box protein O1 (FOXO1). It also regulates cell cycle progression and extracellular matrix (ECM) synthesis through glycogen synthase kinase-3 beta (GSK3β) blocking. mTORC1, downstream of AKT, drives growth and proliferation by activating S6K. Furthermore, phosphatase and tensin homolog (PTEN) act as tumor suppressors by dephosphorylating PIP3 and counteracting the pathway. It could be concluded that the overactivation of the PI3K/AKT/mTOR axis contributes to the uncontrolled growth of hepatocytes, leading to HCC progression
3 Alterations of PI3K/AKT/mTOR Components in HCC
HCC is a highly heterogeneous tumor resulting from the accumulation of various genetic mutations. Several different signaling pathways, including the PI3K/AKT/mTOR, are altered in HCC, and this pathway is actively involved in the initiation, development, tumor progression, and metastasis of HCC. The PI3K/AKT/mTOR signaling pathway regulates several vital cellular processes such as cell cycle, survival, metabolism, motility, and angiogenesis in both physiological and pathological settings [25]. In HCC cases, the PI3K/AKT/mTOR signaling pathway is altered in more than half of the cases [26], with the most frequently mutated genes being PTEN (5%), PIK3CA (4%), mTOR (4%), and AKT2 (2%) [26,27], as stated by several studies.
Deregulation of the class I PI3K is frequent in tumorigenesis of HCC cases according to various studies [28]. Various reports revealed that the PI3K is highly expressed in HCC, and the PIK3CA upregulation is highly associated with HCC proliferation, poor prognosis and negatively correlated with apoptosis [29]. In addition, the PIK3CA mutations are present in HCC cases and they are highly associated with the size of the tumor. Furthermore, the reports also suggest the prognostic value of such mutations in HCC patients [30]. In addition to the PIK3CA, the high mutation rate of PIK3CB was also reported in HCC patients with an early-stage recurrence [31].
Class II PI3K also plays various important roles in HCC. According to the reports, the downregulation of PI3K-C2α in human HCC cells results in degraded vascular endothelial growth factor (VEGF)-mediated signaling and reduced angiogenesis [32]. Furthermore, the PI3K-C2β plays a vital role in the hepatitis C virus (HCV) spread in human HCC cells [33]. Like two other classes, class III PI3K like PIK3-C3 also has important roles in HCC. According to an article, Vacuolar protein sorting 34 (Vps34), also known as PI3K-C3, is significantly diminished in HCC and could be negatively correlated with the HCC invasiveness in vitro and in vivo. Furthermore, Vps34 could induce the accumulation of lysosomal juxtanuclear, leading to the reduction of HCC tumor invasiveness via the Rab7-RILP pathway. PIK3C deficiency also affected the endosome-lysosome system, contributing to improved Rab11-mediated endocytic recycling of cell surface receptors and increased invasion of HCC cells [34]. Dysregulation of AKT isoforms is responsible for a wide range of diseases including cancer [35]. The expression of both AKT1 and AKT2 is detected in hepatocytes [36]. AKT plays a significant role in tumorigenesis through apoptosis inhibition and cell proliferation. According to a report, in nearly 70% of the HCC samples, the AKT was phosphorylated at S473, which was highly related to the invasion, metastasis, and vascularization of the tumor [37]. In other studies, phosphorylated AKT was considered a poor prognosis marker as it was highly related to diminished overall survival [38,39]. Reports suggest that in HCC, the liver’s altered metabolic state often results in a significant upregulation of aldose reductase, which interacts with the catalytic domain of AKT1, thereby triggering the activation of the AKT/mTOR pathway [40]. In HCC patients, the expression of phosphorylated AKT1 contributed to the activation of c-MYC and unfavorable cellular growth [16]. The other isoform of AKT, AKT2, also has significant roles in HCC and some researchers believe that its role in HCC exceeds AKT1. AKT2 promotes cell proliferation and invasion and influences the prognosis of HCC patients [41]. Studies revealed that 38% of HCC tissues with moderate or lower AKT1 expression exhibited AKT2 overexpression. While no correlation was discovered between AKT1 and clinicopathological attributes, elevated AKT2 expression was associated with histopathological differentiation, tumor nodule count, and portal invasion [41]. In the context of tumor transformation, AKT2 plays a crucial role in metabolic regulation in HCC mice models [42]. Although AKT3 expression is primarily centralized in the brain and testis, it also plays a crucial role in HCC. Studies on miRNA profiles in HCC revealed that miRNA-144 and miRNA-582-5p were downregulated. This downregulation led to sustained expression of their downstream targets, including AKT3, promoting tumor progression and growth. These findings highlight the role of AKT3 in HCC progression [43,44].
The deregulation of mTOR is present in human malignancies and the mTOR signal is frequently altered in approximately 30% of cancers, according to the reports [45,46]. The mTOR pathway can be activated in three different ways. The first way is the activating mutations in mTORC1/2 complexes or mutations in upstream genes which could lead to the hyperactivation of the mTOR signaling. The second one is the overexpression or amplification of the components of mTORC1 and mTORC2, and the last one is the loss of function of negative regulators in the mTOR signaling cascade [47].
According to the articles, both mTORC1 and mTORC2 are upregulated in nearly half of patients with HCC, which signifies their important role as a potential therapeutic target [48,49]. Besides, elevated levels of mTOR expression are reported in poorly differentiated tumors and large tumors with earlier tumor recurrence and lower survival rates and advanced stages [50,51]. Results of a prospective study of HCC patients who underwent liver transplantation depicted that mTOR pathway expression was more intense at the tumor edge where proliferation and cellular growth were more intense [52]. While the mechanisms leading to the mTORC2 activation are not understood, the presence of p-AKT (S473) which is a biomarker for mTORC2 activation is reported in nearly more than half of human HCC samples [53]. Furthermore, no mutations in mTORC2 components have been identified. According to the articles, chromosomal gain of RICTOR was detected in nearly one-fourth of human HCC samples, and elevated levels of RICTOR expression are highly associated with poor HCC prognosis [16]. Articles suggest that mTORC1 and mTORC2 take part in controlling the migration as well as invasion of HCC cells [54], and mTORC2 can promote de novo fatty acid and lipid synthesis, causing hepatic steatosis and cancer [21].
The tumor suppressor gene PTEN, which is the negative regulator of the PI3K/AKT/mTOR, also undergoes some changes in HCC. According to the reports, PTEN heterozygosity, which reduces PTEN expression, is present in nearly 32% to 44% of HCC cases; however, PTEN mutations rarely occur in HCC [55]. Some studies revealed the relation between low expression of the PTEN gene and unsatisfactory events in HCC including poor differentiation, advanced TNM stage, intrahepatic metastasis, and decreased survival rates [37,56,57]. In HCC cells, the degradation of the PTEN protein due to miR-221 is reported to be the main reason for the PI3K/AKT/mTOR pathway activation. The overexpression of miR-221 which is an oncogene, contributes to the downregulation of PTEN expression, and its degradation and is downregulated due to the overexpression of miR-221 [58,59]. Table 1 is an overview of alterations in the PI3K/AKT/mTOR signaling pathway.
4 The Induction of Oncogenic Features as a Result of PI3K/AKT/mTOR Alterations in HCC
4.1 Apoptosis, Autophagy and Angiogenesis
Apoptosis, a programmed cell death, involves a series of molecular steps that culminate in cellular demise. Abnormalities in apoptosis are a hallmark of cancer, including HCC. The PI3K/AKT signaling pathway regulates proapoptotic and antiapoptotic downstream molecules, facilitating HCC progression. Consequently, targeting this pathway can induce apoptosis. Autophagy, on the other hand, is a cellular degradation process that eliminates unnecessary or dysfunctional proteins and components via lysosomal degradation. In this mechanism, unwanted cellular components are enclosed within autophagosomes, which then fuse with lysosomes, generating amino acids and free fatty acids that are recycled to maintain cellular homeostasis [64]. Various autophagy-related proteins (ATGs) that assemble into several complexes can mediate the mentioned mechanism, leading to the formation of autophagosomes, fusion with lysosomes, and eventual degradation [65]. Angiogenesis is defined as the formation of new vessels and it is vital for wound healing, supporting growth, and regenerating tissues [66,67]. In cancer settings including HCC, angiogenesis is important as the cancerous cells can enter the bloodstream and disseminate into different organs by angiogenesis. In the following section, we will review the role of the PI3K/AKT signaling pathway and target it in apoptosis, autophagy, and angiogenesis of the HCC.
Several different studies review the role of the pathway and targeting it in apoptosis. To begin with, it was reported that inhibition of the PI3K/AKT/mTOR signaling pathway can promote apoptosis in HCC cells by increasing the levels of Caspase-3 and Caspase-9 and decreasing the levels of Bcl-2 [68]. Besides, it was discovered that the inhibition of the signaling pathway contributed to the DNA damage and inhibition of the progression of HCC cells [69]. In another article, using 4-hydroxyderricin to target and decrease the levels of PI3K contributed to the inhibition of the pathway, apoptosis induction, and cell cycle arrest in HCC cells [70]. Furthermore, it was reported that Alpha-fetoprotein (AFP) can enhance the proliferation and invasion of HCC cells through induction of PI3K/AKT signaling by down-regulation of the PTEN. By targeting the PTEN, the pathway induction can contribute to the inhibition of apoptosis and HCC growth [71–74]. There is an evident role of the PI3K/AKT signaling pathway in the regulation of autophagy in HCC. According to an article, suppressing the activity of the PI3K/AKT by Anemoside B4, which is a saponin, leads to elevated levels of Beclin-1 and LC3 expression, inhibition of AKT and mTOR phosphorylation and results in both autophagy and apoptosis of HCC cells [75].
Some anti-cancer drugs can also target the PI3K/AKT/mTOR signaling pathway and suppress angiogenesis. To begin with, it is reported that there is an increase in the levels of both PI3K and mitogen-activated protein kinase (MAPK) in the hypoxia which induces angiogenesis. Asparagus polysaccharide (ASP) can inhibit the angiogenesis and progression of HCC cells by targeting the PI3K/AKT/mTOR signaling, reducing MAPK and PI3K expression levels to suppress the hypoxia-Inducible Factor (HIF)-1α/VEGF axis. ASP inhibitory effects are mediated by down-regulating the phosphorylation of MAPK and PI3K signaling pathway-related proteins including p-extracellular signal-regulated kinase (ERK), p-AKT, and p-mTOR in HCC cells [76]. Another study revealed that Apatinib, a tyrosine kinase inhibitor that selectively targets VEGFR2, reduced VEGF and PI3K/AKT expression to repress angiogenesis and HCC cell invasion. It inhibited the expression and activity of the pro-angiogenic factors VEGF and VEGFR2, potentially affecting the downstream activation of the PI3K/AKT signaling pathway, and ultimately leading to the suppression of angiogenesis [77]. Furthermore, another study revealed that Saponin D can induce apoptosis and suppress the growth of HCC cells in a dose-dependent manner. It can also diminish the expression of HIF-1α and VEGF, and diminish tube formation in cells. It was found that Saponin D can inhibit the tumor growth of the HCC xenograft model by inducing apoptosis and increasing the levels of Caspase-3. Additionally, it reduced the expression of VEGF and suppressed angiogenesis. Saponin D played such roles by modulating the PI3K/AKT axis in HCC by suppressing the phosphorylation of AKT, mTOR, and p70S6K both in vitro and in vivo [78]. All in all, according to the abovementioned justifications targeting the PI3K/AKT/mTOR pathway would be beneficial.
4.2 Metastasis, Invasion, and EMT
Metastasis is considered one of the hallmarks of cancer and elevated levels of tumor invasion and metastasis are significantly associated with cancer mortality. Epithelial-to-mesenchymal transition (EMT) is a phenomenon related to cancer progression, metastasis, invasion, and drug resistance, and various studies have assessed the role of EMT inducing invasiveness and oncogenic features of HCC [79,80]. In the following section, we will review the role of the PI3K/AKT/mTOR signaling pathway in metastasis, invasion, and EMT of the HCC.
To begin with, the Cartilage oligomeric matrix protein (COMP) is known to play important roles in several cancers including HCC. The serum levels of COMP in high in HCC patients which is in correlation with both malignant clinical characteristics and poor clinical outcomes. A study depicted that recombinant human COMP protein (rCOMP) contributed to increased abilities of proliferation, invasion, migration, and EMT of HCC cells via collaborating with CD36 and PI3K/AKT axis. In other words, COMP-CD36 collaboration can result in the phosphorylation of AKT and ERK, contributing to the upregulation of tumor-progressive genes including EMT markers, matrix metalloproteinases (MMPs)-2/9, Twist, and Slug in HCC cells [81]. In HCC cells, the expression of CD73 is elevated and it is responsible for aggressive clinicopathological aspects of HCC like progression, metastasis, and EMT. The mechanisms of the CD73 activity in HCC are via the PI3K/AKT axis. CD73 can activate Rap1 which recruits P110β to the plasma membrane triggers PIP3 production, and promotes AKT phosphorylation in HCC cells [82]. The abnormal expression of Protein arginine methyltransferases (PRMT) which catalyzes protein arginine methylation and plays an important role in many biological processes is present in several cancers. In HCC PRMT9 is found to be overexpressed which promotes invasion and metastasis. The overexpression of PRMT9 is significantly correlated with hepatitis B virus antigen (HBsAg) status, vascular invasion, poor tumor differentiation, and advanced TNM stage. In HCC tumor tissues harboring elevated expression of PRMT9, the expression levels of p-AKT, p-GSK-3β, and Snail were increased which consequently promoted the invasion, EMT, and metastasis of the tumor [83].
PRMT9 promotes EMT by activating the PI3K/AKT/GSK-3β/Snail signaling pathway. Mammalian sterile-20-like kinase 4 (MST4), a member of the germinal center kinase (GCK) group III, influences various physiological functions. However, its role in cancers remains poorly understood. In HCC cells, MST4 functions as a tumor suppressor, and its low expression serves as a potential biomarker for poor prognosis in HCC. Studies revealed that MST4 inactivation induces the EMT phenotype in cancer cells and enhances their invasive potential through activation of the PI3K/AKT/Snail1 signaling pathway [84]. Family member with sequence similarity 83 (FAM83A) is part of an 8-member protein family that is upregulated in HCC, and its expression is highly correlation with poor progression, invasion, metastasis, and EMT. It was reported that FAM83A could induce EMT by activating the PI3K/AKT signaling pathway, c-JUN protein, and EMT-relating proteins in HCC. Surprisingly, c-JUN was detected to make a positive feedback loop by binding to the FAM83A promotor region and inducing its expression [85]. Transcription factor activating enhancer binding protein 4 (TFAP4) is known as a regulator of tumor progression. Studies revealed that the expression of TFAP4 is upregulated in HCC, and it is responsible for invasion, metastasis, and EMT via regulating the expression of MMP-9 through activating the PI3K/AKT/mTOR signaling pathway in HCC [86]. Protein kinase D (PKD) has also been shown to contribute to invasion, metastasis, and EMT in several types of cancers including HCC. In HCC, the pyruvate dehydrogenase kinase 1 (PDK1) is overexpressed, and it is activated by the combination of TNFR1 and TRAF2 in the form of a complex. PKD2, then, attached directly to the p110α and p85 subunits and promoted the signaling cascade of PI3K/AKT/GSK-3β, leading to the stimulation of invasion and EMT [87].
4.3 Tumor Microenvironment Reprogramming
The tumor microenvironment (TME) is a critical contributor to cancerous cell growth, invasion, and metastasis [88], and according to the reports the metabolism of cell types in the TME, like immune cells, can modulate tumor progression [89]. Immune cells such as NK cells, macrophages, and T cells are an essential part of the TME and play significant roles as both prognostic and therapeutic markers [90]. In the TME, the great metabolic competition between tumor cells and immune cells could influence the growth of tumor cells and the loss of immune cell functions [91]. Notably, metabolic reprogramming following the immune cell activation or immunometabolism relies on the crosstalk between the liver kinase B1 (LKB1)/AMP-activated protein kinase (AMPK) signaling pathway and the PI3K/AKT/mTOR which is vital for regulating the metabolism of immune and nonimmune cell [92].
Macrophages deliver nutritional supplements to the cancerous cells and help both tumor progression and resistance to therapy [93]. In HCC, the AKT signaling pathway plays a vital role in the M1/M2 polarization of macrophages. To begin with, in TME of HCC tumors, the glycolysis of the cancerous cells contributes to the elevated levels of lactic acid and M2 polarization of macrophages through the AKT pathway [94–96]. Nevertheless, M1 polarized macrophages have enhanced glycolytic metabolism and damaged oxidative phosphorylation via AKT/mTOR/HIF-1α pathway [97].
T cells, particularly CD8+ T cells, are key effectors of adaptive immunity against cancers, including HCC. However, the tumor microenvironment (TME) reprograms these cells. In HCC, tumor tissues exhibit a lower density of CD8+ T cells and a higher density of Tregs, creating conditions favorable for cancer growth and intrahepatic metastasis. AKT plays a critical role in T cell reprogramming, as its inhibition reduces fatty acid oxidation and enhances the mitochondrial spare respiratory capacity of tumor-infiltrating T cells [98–100]. Interestingly, programmed death ligand 1 (PD-L1) as the immune checkpoint affects the Treg cells by elevating PTEN and inhibiting the AKT/mTOR/S6 signaling pathway [101]. Furthermore, the PD-1/PD-L1 interaction may inhibit PI3K and AKT contributing to the mTOR signaling blocking thereby decreasing the glycolysis rate of T-infiltrating cells in HCC [102].
NK cells are the main elements of innate lymphoid cells (ILCs) that have a major role in the immunity against various viral and transformed infected cells [103,104], and the PI3K/AKT/mTOR pathway is vital for promoting the development, differentiation, and activation of NK cells [105]. According to an article, PD-1/PD-L1 blockade can augment the phosphorylation of AKT, in HCC, thus PD-1 exhibits its inhibitory roles on NK cells by diminishing PI3K/AKT signaling in HCC [106].
5 Regulation of the PI3K/AKT/mTOR Signaling Pathway by Long Noncoding RNAs in HCC
Noncoding RNAs are transcription products that are not able to be translated into proteins. They normally featured with a high density of terminators and lacking effective open reading frames. Several different kinds of noncoding RNAs such as miRNA, lncRNA, piRNA, and snoRNA could have functions in many transcriptional and post-transcriptional events [107]. It has been reported that lncRNAs, whose length is generally more than 200 nucleotides, have the highest frequency among all ncRNAs. Their open reading frames are normally around 50~100 nucleotides, and RNA polymerase II mainly conducts their transcription and splicing [108,109]. By interacting with DNA, RNA, and protein, lncRNAs can serve as regulatory factors. LncRNAs control gene expression by DNA methylation, chromosomal remodeling, and histone modifications. Besides, they can also influence the transcription and translation of DNA by a series of cis- and trans-regulations. Compared with target mRNA sequences, lncRNAs can affect the editing, splicing, transport, translation, and degradation of mRNA [110–112]. lncRNAs are responsible for the initiation, development, and clinical prognosis of several different tumors including HCC. In the following table, we will review the most important lncRNAs in HCC. Table 2 represents an overview of studies assessing noncoding RNAs in the regulation of the PI3K axis in HCC.
6 Evaluating Clinical Trials in the Context of PI3K/AKT/mTOR Pathway Inhibitors in HCC
6.1 Trends of Trials Investigating PI3K/AKT/mTOR Pathway Inhibitors in HCC
In the present study, we specify over 223 keywords, which include the names of 106 PI3K inhibitors, 45 dual PI3K/mTOR inhibitors, 42 AKT inhibitors, 26 mTOR, and more than 4 other keywords to inhibitors to identify relevant clinical trials. Following a multi-step screening process, 60 studies related to HCC were included. In this section, through a conceptual analysis of these trials, we aim to present a clearer understanding of the progress made so far and identify the areas that still need attention in the future. According to our analysis, 58% of trials were completed, 7% were recruiting, and only 5% were active, not recruiting, while 13% were terminated and 7% were withdrawn.
Although there has been a reduction in the number of clinical trials concerning mTOR and AKT inhibitors in recent years, PI3K inhibitors have attracted more attention from researchers since 2017. Indeed, the trend of mTOR inhibitors and AKT inhibitors has been the same, except for the sharp rise of mTOR inhibitors in 2009–2012. In almost all investigated periods, the number of Phase I trials exceeded other phases; however, between 2009 and 2012, which was the most-studied period, the number of Phase II trials was the highest. It could be observed that the sharp rise in the trend of inhibitors in 2009–2012 was majorly related to mTOR inhibitors, while PI3K inhibitors were the least at that period. Furthermore, mTOR inhibitors have always been the most utilized drug in each phase, while in Phase I PI3K inhibitors were used more than AKT inhibitors and vice versa in Phase II. Fig. 2A–F provides an overview of the general characteristics of 60 clinical trials investigating PI3K/AKT/mTOR pathway inhibitors in HCC.
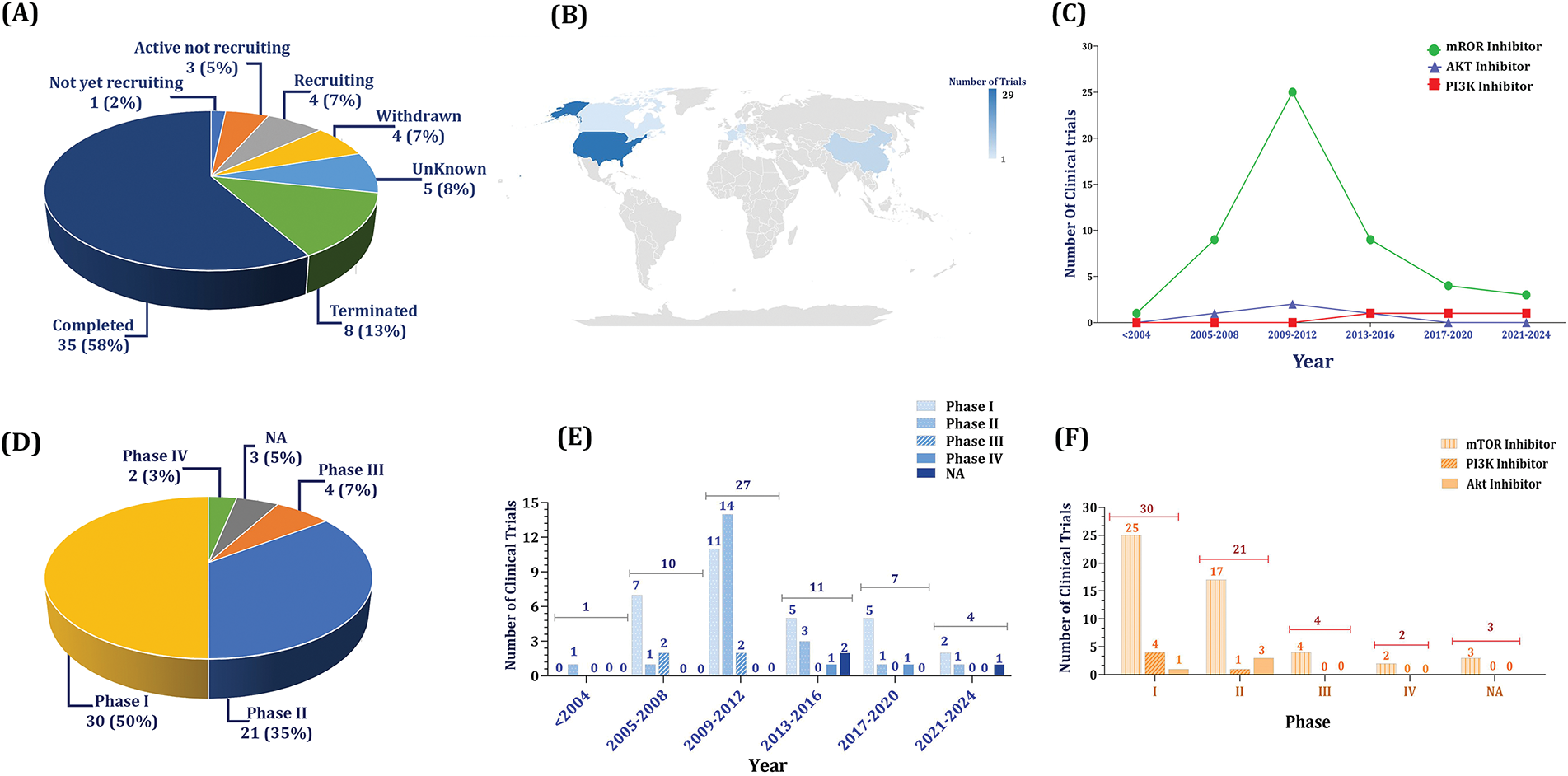
Figure 2: An overview of clinical trials investigating PI3K/AKT/mTOR pathway inhibitors in HCC. A. The status of clinical trials shows that nearly 60% of trials were completed and 7% are recruiting. B. According to the world distribution, most of the studies have been performed in the USA (29 numbers) followed by China (six numbers), and Germany and France (three numbers each). C. Generally, mammalian targets of rapamycin (mTOR) inhibitors have drawn more attention compared with phosphatidylinositol-3 kinase (PI3K) and protein kinase B (AKT) inhibitors. D. Ratio of phases. The rate of trials in phase I is higher than others (50%), while only 10% of trials have entered Phases III and IV. E. The highest numbers of clinical trials were recorded between 2009 and 2012, when, particularly, the rate of Phase II trials exceeded Phase I. F. Most of the trials have been performed in Phase I, and all phases, mTOR inhibitors have drawn more attention compared with the others, indicating their better effectiveness and tolerability
6.2 Promising Inhibitors of PI3K Axis; Analysis of Trials Entered Phases III and IV
Comparing 51 studies in Phases I and II (85%) to six in Phases III and IV (10%) demonstrates that PI3K and AKT inhibitors do not advance to Phases III and IV; however, there have been clinical trials for mTOR inhibitors in Phases III and IV. In this regard, four trials in Phase III and two trials in phase IV were recorded as mTOR inhibitors, while their trends were 25 trials in Phase I and 17 in Phase II, implying that mTOR inhibitors have been the center of attention regarding their more promising outcomes. Fig. 2A–F gives information regarding the proportion and the trends of utilized drugs in Phases III and IV compared with other phases.
6.3 The Most Promising Drugs and Therapeutic Strategies
As mentioned, the most utilized category has been mTOR inhibitors followed by PI3K inhibitors and AKT inhibitors. Among mTOR inhibitors, everolimus, rapamycin, and temsirolimus have been majorly utilized, while the tendency toward sapanisertib and onatasertib has been lower. Our assessment shows that pan-class I PI3K inhibitors have been used more, in which copanlisib has demonstrated a higher tendency. Furthermore, MK2206 was the most used AKT inhibitor followed by perifosine. Overall, it could be inferred that everolimus has been the most utilized drug regarding all PI3K/AKT/mTOR pathway inhibitors in HCC, indicating its promising clinical benefits.
Another point to be mentioned is the therapeutic strategies i.e., monotherapy and combination therapy. The promising outcomes of pre-clinical studies paved the way for clinical studies to evaluate the effectiveness of combination regimens in HCC cases [134]. For instance, the combination of everolimus plus sorafenib was assessed in patients with unresectable or metastatic HCC in a randomized Phase II clinical trial. They showed that progression-free survival at 12 weeks was 68% in patients receiving the combination regimen. Furthermore, response evaluation criteria in solid tumors (RECIST) and modified RECIST (mRECIST) response rates were 10% and 35%, respectively. While 86% of patients demonstrated grade 3/4 adverse events, the median progression-free survival (PFS) was 5.7 months and the median overall survival (OS) was 12 months [135]. Our analysis exhibits that the proportion of combination therapy has been higher in almost all categories of PI3K/AKT/mTOR pathway inhibitors. Concerning mTOR inhibitors, MEK inhibitors have been combined more followed by chemotherapy, VEGF inhibitors, and hormone therapy. Combining PI3K inhibitors has been majorly conducted using anti-PD-L1 immune checkpoint inhibitors (ICI) followed by tyrosine kinase inhibitors (TKI). Moreover, MEK inhibitors have been chiefly combined with AKT inhibitors. The contribution of PI3K/AKT/mTOR pathway inhibitors in HCC clinical trials and their therapeutic strategies are depicted in Fig. 3A,B.
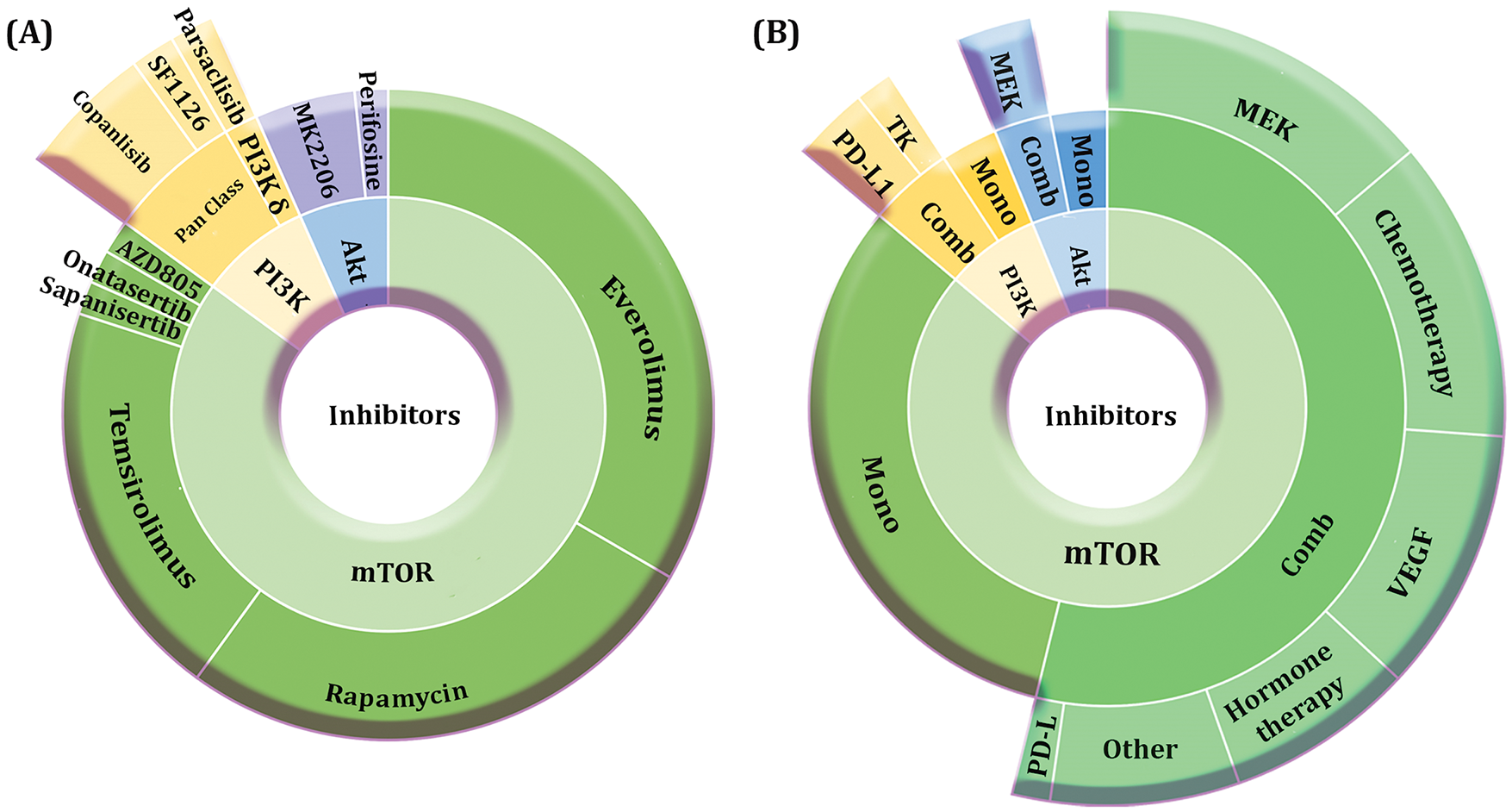
Figure 3: Contribution of PI3K/AKT/mTOR pathway inhibitors in HCC clinical trials. A. The proportion of mTOR inhibitors exceeds PI3K and AKT inhibitors. Everolimus, rapamycin, and temsirolimus are among the most utilized mTOR inhibitors, while copanlisib and MK2206 have been the most used phosphatidylinositol-3 kinase (PI3K) and protein kinase B (AKT) inhibitors, respectively. B. The contribution of combination therapy is higher compared with monotherapy when investigating all inhibitors. The combination of regimens mostly consists of MEK inhibitors, chemotherapy, and vascular endothelial growth factor (VEGF) inhibitors regarding mTOR inhibitors, anti-PD-L1 and tyrosine kinase inhibitors (TKI) regarding PI3K inhibitors, and MEK inhibitors regarding AKT inhibitors
7 Pros and Cons of Targeting PI3K/AKT/mTOR Pathway in HCC
Although targeting the PI3K/AKT/mTOR axis has been shown to be a promising therapeutic strategy to diminish the growth of liver tumors, targeting the component of this signaling pathway could be associated with the induction of severe adverse events which could terminate the conducting of the studies [10]. Indeed, one of the main causes contributing to the discontinuation of clinical trials has been the presence of uncontrollable adverse events, leading to the failure of trials. A vast range of clinical toxicities could be overserved as a result of PI3K/AKT/mTOR axis inhibition. In this regard, the mentioned therapies could induce adverse events such as hyperglycemia, stomatitis, bone marrow suppression, hyperlipidemia, pneumonitis, and hepatotoxicity [136]. Studies demonstrated that the inhibition of PI3Kα could majorly cause hyperglycemia; however, hyperlipidemia is a common side effect when mTOR is suppressed. Accordingly, pan-PI3K inhibitors, which are able to block all isoforms of PI3K, exhibit a broadened range of adverse events [137].
Another imperative challenge related to the inhibition of the PI3K/AKT/mTOR signaling pathway in HCC is the induction of treatment resistance. The induction of resistance seemed to be the main obstacle to low efficacy in clinical trials [138]. Generally, the induction of resistance to drugs in cancers could be stratified into two categories, primary and acquired resistances [139], with the former referring to the genetic heterogeneity of tumor cells and the latter referring to the activation of compensatory signaling pathways [140]. The genetic profiling of some tumors could induce the phenotype of drug resistance. Accordingly, it has been demonstrated that PDK1 overactivation could drive resistance to treatments such as radiotherapy along with the induction of EMT and cancer stemness; therefore, therapeutic inhibitors targeting PDK1 demonstrate promise in reversing these influences [141,142]. Furthermore, the bioinformatics analyses and advancements in genetic profiling pave the way for identifying molecular biomarkers related to therapy resistance. For instance, acquiring resistance to radiotherapy due to PDK1 alteration was shown to be associated with elevated flow cytometry-based Aldehyde Dehydrogenase (ALDH) activity and side-population enrichment as well as direct ALDH1-PDK1 interaction in Huh7 human HCC cell line. They exhibited that PDK1-driven resistance to radiotherapy could positively correlate with the upregulation of some biomarkers such as ALDH1A1, SOX2, PROM1, KLF4, and POU5F1 [142]. When the components of the PI3K/AKT/mTOR axis are suppressed, the tumors can activate parallel or compensatory pathways like the MAPK pathway to maintain survival and proliferation despite inhibition of the axis. Furthermore, some pathways have cross-talk between the PI3K axis; therefore, by blocking the PI3K signaling, those pathways could compensate for the survival of tumors [11,143]. As a result, understanding the cross-talk of other signaling pathways to establish methods to target them in combination regimens is of cardinal importance.
It could be inferred that there are need to develop novel drugs and/or combination regimens to increase the accuracy of treatment and overcome the drug resistance issue [11]. It has been demonstrated that the pathogenesis of HCC is derived from a complex system of signaling pathways involving PI3K/AKT/mTOR, RAS/RAF/MEK/ERK, c-MET, VEGF, as well as histone deacetylases (HDACs). Targeting the key components of these pathways could enhance the therapeutic dilemma of HCC cases [140]. It is worth mentioning that by combining inhibitors a sizable number of tumor proliferation systems could be blocked simultaneously; thus, the growth of tumors could be hindered notably; nonetheless, these methods could lead to more toxicity concerns; therefore, their utilization ought to be managed and considered [144]. As we evaluated and according to some preclinical studies, the combination of mTOR inhibitors with AKT or PI3K inhibitors as dual targeting strategies has shown promising potential in the induction of clinical benefits for HCC patients [25]. Moreover, combining mTOR inhibitors with other classes of targeted therapies such as MEK inhibitors, VEGF inhibitors, and hormone therapies as well as chemotherapy have been of great interest among studies, exhibiting their promising therapeutic potency in HCC treatment. Interestingly, novel selective mTOR inhibitors have been developed with the capacity to elevate the radiosensitization of HCC tumors [145], implying the effectiveness of combination therapies.
HCC remains a challenging concern in oncology regarding its high incidence and mortality rates worldwide. The PI3K/AKT/mTOR signaling pathway has been identified as a crucial player in the pathogenesis of HCC, primarily by promoting uncontrolled cellular proliferation. The overactivation of this pathway is strongly correlated with poor prognosis, making it a critical target for therapeutic intervention. Our comprehensive review underscores the oncogenic characteristics of the PI3K/AKT/mTOR axis in HCC. A sizable number of studies have shown the deregulation of class I PI3K in the tumorigenesis events of HCC. Indeed, the upregulation of PIK3CA is related to a poor prognosis and liver tumor proliferation. The oncogenic features are not just associated with class I PI3K, but class II and III PI3Ks have been linked with HCV spread and HCC invasiveness. Furthermore, the presence of overexpression in AKT, upregulation in mTOR, and downregulation in PTEN are related to invasiveness, metastasis, tumor recurrence, and poor survival of patients. Overall, the out-of-control activation of the PI3K axis is associated with the inhibition of apoptosis and autophagy, the stimulation of angiogenesis, and the elevation of EMT and metastasis. In addition, non-coding RNAs such as lncRNAs could play an imperative role in HCC development. On the one hand, some lncRNAs like HEIH, MALAT1, HULC, and CASC9 serve as oncogenes by activating the PI3K, AKT, and mTORC1, and suppressing PTEN. On the other hand, Meg3, FER1L4, CADM1-AS1, and F11-AS1 are sorts of tumor suppressor lncRNA able to regulate PI3K/AKT/mTOR axis and activate PTEN.
According to our analysis, 58% of clinical trials in the context of inhibiting PI3K/AKT/mTOR signaling pathway in HCC are completed, while 7% and 5% are recruiting and active, not recruiting. The tendency toward using AKT and mTOR inhibitors reduced in recent years; however, the application of PI3K inhibitors increased. In the investigated period, the number of studies between 2009 and 2012 was the highest, of which mTOR inhibitors dedicated the majority proportion. Regarding the phases, it could be concluded that mTOR inhibitors have been the most utilized drugs in each phase. Moreover, we demonstrated that only 10% of studies advanced to Phases III and IV, all of which were mTOR inhibitors, indicating the promising outcomes of these drugs compared with PI3K and AKT inhibitors. The most used drugs have been copanlisib (a pan-class I PI3K inhibitor), MK2206 (an AKT inhibitor), and everolimus (an mTOR inhibitor); nonetheless, everolimus has been the most utilized drug among all categories.
Although there are trends toward using PI3K/AKT/mTOR inhibitors, their application could be associated with challenges and shortcomings. In this regard, these drugs have been shown to stimulate out-of-control adverse events like hyperglycemia, stomatitis, and bone marrow suppression, which are one of the main causes of trial failures. The induction of resistance to these drugs is another imperative challenge affecting the outcomes of therapies in HCC. The nature of treatment resistance could be due to tumor genetic heterogeneity (the primary) and/or the activation of compensatory pathways (the secondary). In the presence of PI3K/AKT/mTOR inhibitors, some parallel signaling such as the MAPK pathway could be overactivated to compensate for the survival and proliferation of tumors.
The application of combination therapy is of cardinal importance in overcoming the mentioned challenges. Accordingly, we exhibited that combination therapy has had more proportion in almost all categories of PI3K/AKT/mTOR inhibitors compared with monotherapy, of which MEK inhibitors, chemotherapy, ICI, and VEGF inhibitors have been the most used drugs in combination regimens. Therefore, it could be inferred that combining novel approaches to target other compensatory pathways simultaneously could lead to a more successful control of HCC growth in the future. It is worth mentioning that some therapeutic agents have been particularly studied for their specific inhibitory roles on PI3K/AKT/mTOR signaling pathways such as everolimus or MK2206. Nonetheless, the fundamental functions of the PI3K/AKT/mTOR signaling pathway make it to be targeted by a variety of drugs whose main responsibility is focused on another domain. Metformin, which is commonly known for its blood glucose-lowering functions, was discussed to have an anti-tumor effect by modulating some key signaling pathways in cells including the PI3K axis [146]. Therefore, utilizing these kinds of drugs in combination regimens would be a potential strategy in future therapeutic studies.
It has been demonstrated that combining molecular inhibitors with nanocarriers enables localized delivery of the therapeutic payload and controlled drug release, potentially minimizing toxicities associated with systemic exposure to the inhibitors [147]. Nanoparticles can overcome various biological barriers, deliver drugs directly to the target site, and enable the simultaneous delivery of multiple drugs [148]. Although the application of nanoparticle-based drug delivery methods has been turned into a desirable topic in scientific studies, the use of nanoparticles in combination with inhibitors for HCC has been explored in only a limited number of studies [149–151]. Therefore, the application of nanoscience to conjugate PI3K/AKT/mTOR inhibitors into nanoparticles can pave the way for future studies to increase the accuracy of drugs and reduce their doses as well as toxicities. In a study of HCC tumor-bearing nude mice, the conjugation of miR-199a-3p, a miRNA targeting mTOR, with arginine α, β-dehydrophenylalanine (RΔF) nanoparticles resulted in the elevation of miR-199a-3p concentration over 500 fold. This event led to a remarkable downregulation of mTOR in vitro and a considerable reduction in tumor growth in vivo [152]. All in all, it could be inferred that the encapsulation of PI3K/AKT/mTOR inhibitors in nanoparticles could not only increase the local concentration of drugs but also reduce the off-target toxicities, leading to an improvement in the outcomes of therapy. Nonetheless, the application of nanoscience in the context of PI3K/AKT/mTOR inhibitors for HCC seems to be in its early stages.
Acknowledgement: The authors would like to express their gratitude to Shahid Beheshti University of Medical Sciences, Tehran, Iran for supporting this study.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm their contribution to the paper as follows: study conception and design: Ayda Baghery Saghchy Khorasani, Mahda Delshad, Davood Bashash; data collection: Ayda Baghery Saghchy Khorasani, Mohammad-Javad Sanaei; analysis and interpretation of results: Mahda Delshad, Mohammad-Javad Sanaei, Atieh Pourbagheri-Sigaroodi, Ali Pirsalehi; draft manuscript preparation: Davood Bashash. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Paskeh MDA, Ghadyani F, Hashemi M, Abbaspour A, Zabolian A, Javanshir S, et al. Biological impact and therapeutic perspective of targeting PI3K/Akt signaling in hepatocellular carcinoma: promises and challenges. Pharmacol Res. 2023;187(6):106553. doi:10.1016/j.phrs.2022.106553. [Google Scholar] [PubMed] [CrossRef]
2. Serraino D, Fratino L, Piselli P, Epidemiological aspects of hepatocellular carcinoma. Cham, Switzerland: Springer International Publishing; 2023. p. 3–9. [Google Scholar]
3. Niu M, Yi M, Li N, Wu K, Wu K. Advances of targeted therapy for hepatocellular carcinoma. Front Oncol. 2021;11:719896. doi:10.3389/fonc.2021.719896. [Google Scholar] [PubMed] [CrossRef]
4. Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. doi:10.1053/j.gastro.2019.11.312. [Google Scholar] [PubMed] [CrossRef]
5. Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26(9):2183–91. doi:10.1185/03007995.2010.506375. [Google Scholar] [PubMed] [CrossRef]
6. Hönigova K, Navratil J, Peltanova B, Polanska HH, Raudenska M, Masarik M. Metabolic tricks of cancer cells. BBA-Rev Cancer. 2022;1877(3):188705. doi:10.1016/j.bbcan.2022.188705. [Google Scholar] [PubMed] [CrossRef]
7. Kang H, Kim B, Park J, Youn H, Youn B. The Warburg effect on radioresistance: survival beyond growth. BBA-Rev Cancer. 2023;1878(6):188988. doi:10.1016/j.bbcan.2023.188988. [Google Scholar] [PubMed] [CrossRef]
8. Icard P, Simula L, Wu Z, Berzan D, Sogni P, Dohan A, et al. Why may citrate sodium significantly increase the effectiveness of transarterial chemoembolization in hepatocellular carcinoma? Drug Resist Updat. 2021;59(6):100790. doi:10.1016/j.drup.2021.100790. [Google Scholar] [PubMed] [CrossRef]
9. Buontempo F, Ersahin T, Missiroli S, Senturk S, Etro D, Ozturk M, et al. Inhibition of Akt signaling in hepatoma cells induces apoptotic cell death independent of Akt activation status. Invest New Drugs. 2011;29(6):1303–13. doi:10.1007/s10637-010-9486-3. [Google Scholar] [PubMed] [CrossRef]
10. Sun EJ, Wankell M, Palamuthusingam P, McFarlane C, Hebbard L. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Biomedicines. 2021;9(11):1639. doi:10.3390/biomedicines9111639. [Google Scholar] [PubMed] [CrossRef]
11. Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR pathway and its role in cancer therapeutics: are we making headway? Front Oncol. 2022;12:819128. doi:10.3389/fonc.2022.819128. [Google Scholar] [PubMed] [CrossRef]
12. Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4(Suppl 4):64. doi:10.3389/fonc.2014.00064. [Google Scholar] [PubMed] [CrossRef]
13. Safaroghli-Azar A, Sanaei M-J, Pourbagheri-Sigaroodi A, Bashash D. Phosphoinositide 3-kinase (PI3K) classes: from cell signaling to endocytic recycling and autophagy. Eur J Pharmacol. 2023;953:175827. doi:10.1016/j.ejphar.2023.175827. [Google Scholar] [PubMed] [CrossRef]
14. Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–41. doi:10.1038/nrm2882. [Google Scholar] [PubMed] [CrossRef]
15. Gulluni F, De Santis MC, Margaria JP, Martini M, Hirsch E. Class II PI3K functions in cell biology and disease. Trends Cell Biol. 2019;29(4):339–59. doi:10.1016/j.tcb.2019.01.001. [Google Scholar] [PubMed] [CrossRef]
16. Xu Z, Xu M, Liu P, Zhang S, Shang R, Qiao Y, et al. The mTORC2-Akt1 is crucial for c-Myc to promote hepatocarcinogenesis in mice and humans. Hepatology. 2019;70(5):1600–13. doi:10.1002/hep.30697. [Google Scholar] [PubMed] [CrossRef]
17. Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485(7396):55–61. doi:10.1038/nature10912. [Google Scholar] [PubMed] [CrossRef]
18. Wang Y, Engel T, Teng X. Post-translational regulation of the mTORC1 pathway: a switch that regulates metabolism-related gene expression. BBA-Gene Regul Mech. 2024;1867(1):195005. doi:10.1016/j.bbagrm.2024.195005. [Google Scholar] [PubMed] [CrossRef]
19. Ragupathi A, Kim C, Jacinto E. The mTORC2 signaling network: targets and cross-talks. Biochem J. 2024;481(2):45–91. doi:10.1042/BCJ20220325. [Google Scholar] [PubMed] [CrossRef]
20. Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10(14):2305–16. doi:10.4161/cc.10.14.16586. [Google Scholar] [PubMed] [CrossRef]
21. Guri Y, Colombi M, Dazert E, Hindupur SK, Roszik J, Moes S, et al. mTORC2 promotes tumorigenesis via lipid synthesis. Cancer Cell. 2017;32(6):807–23. doi:10.1016/j.ccell.2017.11.011. [Google Scholar] [PubMed] [CrossRef]
22. Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023;22(1):138. doi:10.1186/s12943-023-01827-6. [Google Scholar] [PubMed] [CrossRef]
23. Galicia VA, He L, Dang H, Kanel G, Vendryes C, French BA, et al. Expansion of hepatic tumor progenitor cells in Pten-null mice requires liver injury and is reversed by loss of AKT2. Gastroenterology. 2010;139(6):2170–82. doi:10.1053/j.gastro.2010.09.002. [Google Scholar] [PubMed] [CrossRef]
24. An J, Oh M, Kim SY, Oh YJ, Oh B, Oh JH, et al. PET-based radiogenomics supports mTOR pathway targeting for hepatocellular carcinoma. Clin Cancer Res. 2022;28(9):1821–31. doi:10.1158/1078-0432.CCR-21-3208. [Google Scholar] [PubMed] [CrossRef]
25. Grabinski N, Ewald F, Hofmann BT, Staufer K, Schumacher U, Nashan B, et al. Combined targeting of AKT and mTOR synergistically inhibits proliferation of hepatocellular carcinoma cells. Mol Cancer. 2012;11(1):85. doi:10.1186/1476-4598-11-85. [Google Scholar] [PubMed] [CrossRef]
26. Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47(5):505–11. doi:10.1038/ng.3252. [Google Scholar] [PubMed] [CrossRef]
27. The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. Pan-cancer analysis of whole genomes. Nature. 2020;578(7793):82–93. doi:10.1038/s41586-020-1969-6. [Google Scholar] [PubMed] [CrossRef]
28. Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170(4):605–35. doi:10.1016/j.cell.2017.07.029. [Google Scholar] [PubMed] [CrossRef]
29. Liu W, Zheng L, Zhang R, Hou P, Wang J, Wu L, et al. Circ-ZEB1 promotes PIK3CA expression by silencing miR-199a-3p and affects the proliferation and apoptosis of hepatocellular carcinoma. Mol Cancer. 2022;21(1):72. doi:10.1186/s12943-022-01529-5. [Google Scholar] [PubMed] [CrossRef]
30. Kim DC, Chung WJ, Lee JH, Jang BK, Hwang JS, Kang KJ, et al. Clinicopathological characteristics of PIK3CA and HBx mutations in Korean patients with hepatocellular carcinomas. APMIS. 2014;122(10):1001–6. doi:10.1111/apm.12245. [Google Scholar] [PubMed] [CrossRef]
31. Xin Z, Li J, Zhang H, Zhou Y, Song J, Chen P, et al. Cancer genomic alterations can be potential biomarkers predicting microvascular invasion and early recurrence of hepatocellular carcinoma. Front Oncol. 2022;12:783109. doi:10.3389/fonc.2022.783109. [Google Scholar] [PubMed] [CrossRef]
32. He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):425. doi:10.1038/s41392-021-00828-5. [Google Scholar] [PubMed] [CrossRef]
33. Maehama T, Fukasawa M, Date T, Wakita T, Hanada K. A class II phosphoinositide 3-kinase plays an indispensable role in hepatitis C virus replication. Biochem Biophys Res Commun. 2013;440(1):150–6. doi:10.1016/j.bbrc.2013.09.048. [Google Scholar] [PubMed] [CrossRef]
34. Qi C, Zou L, Wang S, Mao X, Hu Y, Shi J, et al. Vps34 inhibits hepatocellular carcinoma invasion by regulating endosome-lysosome trafficking via Rab7-RILP and Rab11. Cancer Res Treat. 2022;54(1):182–98. doi:10.4143/crt.2020.578. [Google Scholar] [PubMed] [CrossRef]
35. Vasudevan KM, Barbie DA, Davies MA, Rabinovsky R, McNear CJ, Kim JJ, et al. AKT-independent signaling downstream of oncogenic PIK3CA mutations in human cancer. Cancer Cell. 2009;16(1):21–32. doi:10.1016/j.ccr.2009.04.012. [Google Scholar] [PubMed] [CrossRef]
36. Wang Q, Yu W-N, Chen X, Peng X-D, Jeon S-M, Birnbaum MJ, et al. Spontaneous hepatocellular carcinoma after the combined deletion of Akt isoforms. Cancer Cell. 2016;29(4):523–35. doi:10.1016/j.ccell.2016.02.008. [Google Scholar] [PubMed] [CrossRef]
37. Chen JS, Wang Q, Fu XH, Huang XH, Chen XL, Cao LQ, et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: association with MMP-9. Hepatol Res. 2009;39(2):177–86. doi:10.1111/j.1872-034X.2008.00449.x. [Google Scholar] [PubMed] [CrossRef]
38. Schmitz KJ, Wohlschlaeger J, Lang H, Sotiropoulos GC, Malago M, Steveling K, et al. Activation of the ERK and AKT signalling pathway predicts poor prognosis in hepatocellular carcinoma and ERK activation in cancer tissue is associated with hepatitis C virus infection. J Hepatol. 2008;48(1):83–90. doi:10.1016/j.jhep.2007.08.018. [Google Scholar] [PubMed] [CrossRef]
39. Yu L, Zhang J, Guo X, Li Z, Zhang P. MicroRNA-224 upregulation and AKT activation synergistically predict poor prognosis in patients with hepatocellular carcinoma. Cancer Epidemiol. 2014;38(4):408–13. doi:10.1016/j.canep.2014.05.001. [Google Scholar] [PubMed] [CrossRef]
40. Zhao JX, Yuan YW, Cai CF, Shen DY, Chen ML, Ye F, et al. Aldose reductase interacts with AKT1 to augment hepatic AKT/mTOR signaling and promote hepatocarcinogenesis. Oncotarget. 2017;8(40):66987–67000. doi:10.18632/oncotarget.17791. [Google Scholar] [PubMed] [CrossRef]
41. Xu X, Sakon M, Nagano H, Hiraoka N, Yamamoto H, Hayashi N, et al. Akt2 expression correlates with prognosis of human hepatocellular carcinoma. Oncol Rep. 2004;11(1):25–32. doi:10.3892/or.11.1.25. [Google Scholar] [CrossRef]
42. He L, Hou X, Kanel G, Zeng N, Galicia V, Wang Y, et al. The critical role of AKT2 in hepatic steatosis induced by PTEN loss. Am J Pathol. 2010;176(5):2302–8. doi:10.2353/ajpath.2010.090931. [Google Scholar] [PubMed] [CrossRef]
43. Zhang Y, Huang W, Ran Y, Xiong Y, Zhong Z, Fan X, et al. miR-582-5p inhibits proliferation of hepatocellular carcinoma by targeting CDK1 and AKT3. Tumor Biol. 2015;36(11):8309–16. doi:10.1007/s13277-015-3582-0. [Google Scholar] [PubMed] [CrossRef]
44. Ma Y, She XG, Ming YZ, Wan QQ, Ye QF. MicroRNA-144 suppresses tumorigenesis of hepatocellular carcinoma by targeting AKT3. Mol Med Rep. 2015;11(2):1378–83. doi:10.3892/mmr.2014.2844. [Google Scholar] [PubMed] [CrossRef]
45. Kirtonia A, Pandey AK, Ramachandran B, Mishra DP, Dawson DW, Sethi G, et al. Overexpression of laminin-5 gamma-2 promotes tumorigenesis of pancreatic ductal adenocarcinoma through EGFR/ERK1/2/AKT/mTOR cascade. Cell Mol Life Sci. 2022;79(7):362. doi:10.1007/s00018-022-04392-1. [Google Scholar] [PubMed] [CrossRef]
46. Tian T, Li X, Zhang J. mTOR signaling in cancer and mTOR inhibitors in solid tumor targeting therapy. Int J Mol Sci. 2019;20(3):755. doi:10.3390/ijms20030755. [Google Scholar] [PubMed] [CrossRef]
47. Conciatori F, Ciuffreda L, Bazzichetto C, Falcone I, Pilotto S, Bria E, et al. mTOR cross-talk in cancer and potential for combination therapy. Cancers. 2018;10(1):23. doi:10.3390/cancers10010023. [Google Scholar] [PubMed] [CrossRef]
48. Matter MS, Decaens T, Andersen JB, Thorgeirsson SS. Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J Hepatol. 2014;60(4):855–65. doi:10.1016/j.jhep.2013.11.031. [Google Scholar] [PubMed] [CrossRef]
49. Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135(6):1972–83. doi:10.1053/j.gastro.2008.08.008. [Google Scholar] [PubMed] [CrossRef]
50. Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10(24):8421–5. doi:10.1158/1078-0432.CCR-04-0941. [Google Scholar] [PubMed] [CrossRef]
51. Zhou L, Huang Y, Li J, Wang Z. The mTOR pathway is associated with the poor prognosis of human hepatocellular carcinoma. Med Oncol. 2010;27(2):255–61. doi:10.1007/s12032-009-9201-4. [Google Scholar] [PubMed] [CrossRef]
52. Guerrero M, Ferrín G, Rodríguez-Perálvarez M, González-Rubio S, Sánchez-Frías M, Amado V, et al. mTOR expression in liver transplant candidates with hepatocellular carcinoma: impact on histological features and tumour recurrence. Int J Mol Sci. 2019;20(2):336. doi:10.3390/ijms20020336. [Google Scholar] [PubMed] [CrossRef]
53. Hu J, Che L, Li L, Pilo MG, Cigliano A, Ribback S, et al. Co-activation of AKT and c-Met triggers rapid hepatocellular carcinoma development via the mTORC1/FASN pathway in mice. Sci Rep. 2016;6(1):20484. doi:10.1038/srep20484. [Google Scholar] [PubMed] [CrossRef]
54. Liao H, Huang Y, Guo B, Liang B, Liu X, Ou H, et al. Dramatic antitumor effects of the dual mTORC1 and mTORC2 inhibitor AZD2014 in hepatocellular carcinoma. Am J Cancer Res. 2015;5(1):125. [Google Scholar] [PubMed]
55. Augello G, Puleio R, Emma MR, Cusimano A, Loria GR, McCubrey JA, et al. A PTEN inhibitor displays preclinical activity against hepatocarcinoma cells. Cell Cycle. 2016;15(4):573–83. doi:10.1080/15384101.2016.1138183. [Google Scholar] [PubMed] [CrossRef]
56. Su R, Nan H, Guo H, Ruan Z, Jiang L, Song Y, et al. Associations of components of PTEN/AKT/mTOR pathway with cancer stem cell markers and prognostic value of these biomarkers in hepatocellular carcinoma. Hepatol Res. 2016;46(13):1380–91. doi:10.1111/hepr.12687. [Google Scholar] [PubMed] [CrossRef]
57. Zhu X, Qin X, Fei M, Hou W, Greshock J, Bachman KE, et al. Combined phosphatase and tensin homolog (PTEN) loss and fatty acid synthase (FAS) overexpression worsens the prognosis of Chinese patients with hepatocellular carcinoma. Int J Mol Sci. 2012;13(8):9980–91. doi:10.3390/ijms13089980. [Google Scholar] [PubMed] [CrossRef]
58. Yoo BK, Santhekadur PK, Gredler R, Chen D, Emdad L, Bhutia S, et al. Increased RNA-induced silencing complex (RISC) activity contributes to hepatocellular carcinoma. Hepatology. 2011;53(5):1538–48. doi:10.1002/hep.24216. [Google Scholar] [PubMed] [CrossRef]
59. Santhekadur PK, Das SK, Gredler R, Chen D, Srivastava J, Robertson C, et al. Multifunction protein staphylococcal nuclease domain containing 1 (SND1) promotes tumor angiogenesis in human hepatocellular carcinoma through novel pathway that involves nuclear factor κB and miR-221. J Biol Chem. 2012;287(17):13952–8. doi:10.1074/jbc.M111.321646. [Google Scholar] [PubMed] [CrossRef]
60. Lim HY, Kim H, Park CK. P1-012-PIK3CA mutations in hepatocellular carcinoma in Korea. Ann Oncol. 2012;23(Supplement 11):xi133. doi:10.1016/S0923-7534(20)32385-1. [Google Scholar] [CrossRef]
61. Chai Z-T, Kong J, Zhu X-D, Zhang Y-Y, Lu L, Zhou J-M, et al. MicroRNA-26a inhibits angiogenesis by down-regulating VEGFA through the PIK3C2α/Akt/HIF-1α pathway in hepatocellular carcinoma. PLoS One. 2013;8(10):e77957. doi:10.1371/journal.pone.0077957. [Google Scholar] [PubMed] [CrossRef]
62. Kunter I, Erdal E, Nart D, Yilmaz F, Karademir S, Sagol O, et al. Active form of AKT controls cell proliferation and response to apoptosis in hepatocellular carcinoma. Oncol Rep. 2014;31(2):573–80. doi:10.3892/or.2013.2932. [Google Scholar] [PubMed] [CrossRef]
63. Wang L, Wang WL, Zhang Y, Guo SP, Zhang J, Li QL. Epigenetic and genetic alterations of PTEN in hepatocellular carcinoma. Hepatol Res. 2007;37(5):389–96. doi:10.1111/j.1872-034X.2007.00042.x. [Google Scholar] [PubMed] [CrossRef]
64. Komatsu M, Waguri S, Chiba T, Murata S, Iwata J-i, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–4. doi:10.1038/nature04723. [Google Scholar] [PubMed] [CrossRef]
65. Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nat Rev Mol Cell Biol. 2018;19(9):579–93. doi:10.1038/s41580-018-0033-y. [Google Scholar] [PubMed] [CrossRef]
66. Otrock ZK, Mahfouz RAR, Makarem JA, Shamseddine AI. Understanding the biology of angiogenesis: review of the most important molecular mechanisms. Blood Cells, Mol Dis. 2007;39(2):212–20. doi:10.1016/j.bcmd.2007.04.001. [Google Scholar] [PubMed] [CrossRef]
67. Ong SKL, Shanmugam MK, Fan L, Fraser SE, Arfuso F, Ahn KS, et al. Focus on formononetin: anticancer potential and molecular targets. Cancers. 2019;11(5):611. [Google Scholar] [PubMed]
68. Luo Y, Liu L, Zhao J, Jiao Y, Zhang M, Xu G, et al. PI3K/AKT1 signaling pathway mediates sinomenine-induced hepatocellular carcinoma cells apoptosis: an in vitro and in vivo study. Biol Pharm Bull. 2022;45(5):614–24. [Google Scholar] [PubMed]
69. Mehdi Üremiş M, Üremiş N, Tosun E, Durhan M, Çiğremiş Y, Baysar A, et al. Cucurbitacin D inhibits the proliferation of HepG2 cells and induces apoptosis by modulating JAK/STAT3, PI3K/Akt/mTOR and MAPK signaling pathways. Curr Cancer Drug Targets. 2022;22(11):931–44. [Google Scholar] [PubMed]
70. Gao X, Jiang Y, Xu Q, Liu F, Pang X, Wang M et al. 4-hydroxyderricin promotes apoptosis and cell cycle arrest through regulating PI3K/AKT/mTOR pathway in hepatocellular cells. Foods. 2021;10(9):2036. [Google Scholar] [PubMed]
71. Wang S, Zhu M, Wang Q, Hou Y, Li L, Weng H, et al. Alpha-fetoprotein inhibits autophagy to promote malignant behaviour in hepatocellular carcinoma cells by activating PI3K/AKT/mTOR signalling. Cell Death Dis. 2018;9(10):1027. [Google Scholar] [PubMed]
72. Zhu M, Guo J, Li W, Lu Y, Fu S, Xie X, et al. Hepatitis B virus X protein induces expression of alpha-fetoprotein and activates PI3K/mTOR signaling pathway in liver cells. Oncotarget. 2015;6(14):12196–208. doi:10.18632/oncotarget.2906. [Google Scholar] [PubMed] [CrossRef]
73. Li M, Li H, Li C, Wang S, Jiang W, Liu Z, et al. Alpha-fetoprotein: a new member of intracellular signal molecules in regulation of the PI3K/AKT signaling in human hepatoma cell lines. Int J Cancer. 2011;128(3):524–32. doi:10.1002/ijc.25373. [Google Scholar] [PubMed] [CrossRef]
74. Zhu M, Guo J, Xia H, Li W, Lu Y, Dong X, et al. Alpha-fetoprotein activates AKT/mTOR signaling to promote CXCR4 expression and migration of hepatoma cells. Oncoscience. 2015;2(1):59–70. doi:10.18632/oncoscience.115. [Google Scholar] [PubMed] [CrossRef]
75. Xue S, Zhou Y, Zhang J, Xiang Z, Liu Y, Miao T, et al. Anemoside B4 exerts anti-cancer effect by inducing apoptosis and autophagy through inhibiton of PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Am J Transl Res. 2019;11(4):2580–9. [Google Scholar] [PubMed]
76. Cheng W, Cheng Z, Weng L, Xing D, Zhang M. Asparagus Polysaccharide inhibits the hypoxia-induced migration, invasion and angiogenesis of hepatocellular carcinoma cells partly through regulating HIF1α/VEGF expression via MAPK and PI3K signaling pathway. J Cancer. 2021;12(13):3920–9. doi:10.7150/jca.51407. [Google Scholar] [PubMed] [CrossRef]
77. Song J, Guan Z, Song C, Li M, Gao Z, Zhao Y. Apatinib suppresses the migration, invasion and angiogenesis of hepatocellular carcinoma cells by blocking VEGF and PI3K/AKT signaling pathways. Mol Med Rep. 2021;23(6):429. doi:10.3892/mmr.2021.12068. [Google Scholar] [PubMed] [CrossRef]
78. Hong S-W, Jung KH, Lee H-S, Choi M-J, Son MK, Zheng H-M, et al. SB365 inhibits angiogenesis and induces apoptosis of hepatocellular carcinoma through modulation of PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2012;103(11):1929–37. doi:10.1111/j.1349-7006.2012.02409.x. [Google Scholar] [PubMed] [CrossRef]
79. Liu Y, Song J, Zhang H, Liao Z, Liu F, Su C, et al. EIF4A3-induced circTOLLIP promotes the progression of hepatocellular carcinoma via the miR-516a-5p/PBX3/EMT pathway. J Exp Clin Cancer Res. 2022;41(1):164. doi:10.1186/s13046-022-02378-2. [Google Scholar] [PubMed] [CrossRef]
80. Zhang Y, Cao N, Gao J, Liang J, Liang Y, Xie Y, et al. ASIC1a stimulates the resistance of human hepatocellular carcinoma by promoting EMT via the AKT/GSK3β/Snail pathway driven by TGFβ/Smad signals. J Cell Mol Med. 2022;26(10):2777–92. doi:10.1111/jcmm.17288. [Google Scholar] [PubMed] [CrossRef]
81. Li Q, Wang C, Wang Y, Sun L, Liu Z, Wang L, et al. HSCs-derived COMP drives hepatocellular carcinoma progression by activating MEK/ERK and PI3K/AKT signaling pathways. J Exp Clin Cancer Res. 2018;37(1):231. doi:10.1186/s13046-018-0908-y. [Google Scholar] [PubMed] [CrossRef]
82. Ma XL, Shen MN, Hu B, Wang BL, Yang WJ, Lv LH, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol. 2019;12(1):37. doi:10.1186/s13045-019-0724-7. [Google Scholar] [PubMed] [CrossRef]
83. Jiang H, Zhou Z, Jin S, Xu K, Zhang H, Xu J, et al. PRMT9 promotes hepatocellular carcinoma invasion and metastasis via activating PI3K/Akt/GSK-3β/Snail signaling. Cancer Sci. 2018;109(5):1414–27. doi:10.1111/cas.13598. [Google Scholar] [PubMed] [CrossRef]
84. Dian MJ, Li J, Zhang XL, Li ZJ, Zhou Y, Zhou W, et al. MST4 negatively regulates the EMT, invasion and metastasis of HCC cells by inactivating PI3K/AKT/Snail1 axis. J Cancer. 2021;12(15):4463–77. doi:10.7150/jca.60008. [Google Scholar] [PubMed] [CrossRef]
85. Liu C, Peng X, Li Y, Liu S, Hou R, Zhang Y, et al. Positive feedback loop of FAM83A/PI3K/AKT/c-Jun induces migration, invasion and metastasis in hepatocellular carcinoma. Biomed Pharmacother. 2020;123:109780. doi:10.1016/j.biopha.2019.109780. [Google Scholar] [PubMed] [CrossRef]
86. Huang T, Chen QF, Chang BY, Shen LJ, Li W, Wu PH, et al. TFAP4 promotes hepatocellular carcinoma invasion and metastasis via activating the PI3K/AKT signaling pathway. Dis Markers. 2019;2019(1):7129214–13. doi:10.1155/2019/7129214. [Google Scholar] [PubMed] [CrossRef]
87. Zhu Y, Cheng Y, Guo Y, Chen J, Chen F, Luo R, et al. Protein kinase D2 contributes to TNF-α-induced epithelial mesenchymal transition and invasion via the PI3K/GSK-3β/β-catenin pathway in hepatocellular carcinoma. Oncotarget. 2016;7(5):5327–41. doi:10.18632/oncotarget.6633. [Google Scholar] [PubMed] [CrossRef]
88. Wu Q, Zhou L, Lv D, Zhu X, Tang H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J Hematol Oncol. 2019;12(1):1–11. doi:10.1186/s13045-019-0739-0. [Google Scholar] [PubMed] [CrossRef]
89. Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21(10):669–80. doi:10.1038/s41568-021-00378-6. [Google Scholar] [PubMed] [CrossRef]
90. Ma J, Zheng B, Goswami S, Meng L, Zhang D, Cao C, et al. PD1 Hi CD8+ T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J Immunother Cancer. 2019;7:1–15. [Google Scholar]
91. Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–41. doi:10.1016/j.cell.2015.08.016. [Google Scholar] [PubMed] [CrossRef]
92. Chou WC, Rampanelli E, Li X, Ting JPY. Impact of intracellular innate immune receptors on immunometabolism. Cell Mol Immunol. 2022;19(3):337–51. doi:10.1038/s41423-021-00780-y. [Google Scholar] [PubMed] [CrossRef]
93. Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. 2019;30(1):36–50. doi:10.1016/j.cmet.2019.06.001. [Google Scholar] [PubMed] [CrossRef]
94. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–63. doi:10.1038/nature13490. [Google Scholar] [PubMed] [CrossRef]
95. Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. 2017;198(3):1006–14. doi:10.4049/jimmunol.1601515. [Google Scholar] [PubMed] [CrossRef]
96. Zhou HC, Yan XY, Yu WW, Liang XQ, Du XY, Liu ZC, et al. Lactic acid in macrophage polarization: the significant role in inflammation and cancer. Int Rev Immunol. 2022;41(1):4–18. doi:10.1080/08830185.2021.1955876. [Google Scholar] [PubMed] [CrossRef]
97. Zhang Q, Lou Y, Bai XL, Liang TB. Immunometabolism: a novel perspective of liver cancer microenvironment and its influence on tumor progression. World J Gastroenterol. 2018;24(31):3500–12. doi:10.3748/wjg.v24.i31.3500. [Google Scholar] [PubMed] [CrossRef]
98. Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75(2):296–305. doi:10.1158/0008-5472.CAN-14-2277. [Google Scholar] [PubMed] [CrossRef]
99. Basu S, Hubbard B, Shevach EM. Foxp3-mediated inhibition of Akt inhibits Glut1 (glucose transporter 1) expression in human T regulatory cells. J Leucocyte Biol. 2015;97(2):279–83. doi:10.1189/jlb.2AB0514-273RR. [Google Scholar] [PubMed] [CrossRef]
100. Yang P, Li QJ, Feng Y, Zhang Y, Markowitz GJ, Ning S, et al. TGF-β-miR-34a-CCL22 signaling-induced treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22(3):291–303. doi:10.1016/j.ccr.2012.07.023. [Google Scholar] [PubMed] [CrossRef]
101. Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–29. doi:10.1084/jem.20090847. [Google Scholar] [PubMed] [CrossRef]
102. Xia Y, Brown ZJ, Huang H, Tsung A. Metabolic reprogramming of immune cells: shaping the tumor microenvironment in hepatocellular carcinoma. Cancer Med. 2021;10(18):6374–83. doi:10.1002/cam4.4177. [Google Scholar] [PubMed] [CrossRef]
103. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–9. doi:10.1126/science.1198687. [Google Scholar] [PubMed] [CrossRef]
104. Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301. doi:10.1038/nature14189. [Google Scholar] [PubMed] [CrossRef]
105. Ali AK, Nandagopal N, Lee S-H. IL-15-PI3K–AKT-mTOR: a critical pathway in the life journey of natural killer cells. Front Immunol. 2015;6(6):355. doi:10.3389/fimmu.2015.00355. [Google Scholar] [PubMed] [CrossRef]
106. Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C, et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene. 2017;36(44):6143–53. doi:10.1038/onc.2017.209. [Google Scholar] [PubMed] [CrossRef]
107. Godet A-C, Roussel E, Laugero N, Morfoisse F, Lacazette E, Garmy-Susini B, et al. Translational control by long non-coding RNAs. Biochimie. 2024;217:42–53. doi:10.1016/j.biochi.2023.08.015. [Google Scholar] [PubMed] [CrossRef]
108. Liu N, Liu Q, Yang X, Zhang F, Li X, Ma Y, et al. Hepatitis B virus-upregulated LNC-HUR1 promotes cell proliferation and tumorigenesis by blocking p53 activity. Hepatology. 2018;68(6):2130–44. doi:10.1002/hep.30098. [Google Scholar] [PubMed] [CrossRef]
109. Liu X, Duan X, Holmes JA, Li W, Lee SH, Tu Z, et al. A long noncoding RNA regulates hepatitis C virus infection through interferon alpha-inducible protein 6. Hepatology. 2019;69(3):1004–19. doi:10.1002/hep.30266. [Google Scholar] [PubMed] [CrossRef]
110. Kambara H, Niazi F, Kostadinova L, Moonka DK, Siegel CT, Post AB, et al. Negative regulation of the interferon response by an interferon-induced long non-coding RNA. Nucleic Acids Res. 2014;42(16):10668–80. doi:10.1093/nar/gku713. [Google Scholar] [PubMed] [CrossRef]
111. Carnero E, Barriocanal M, Prior C, Pablo Unfried J, Segura V, Guruceaga E, et al. Long noncoding RNA EGOT negatively affects the antiviral response and favors HCV replication. EMBO Rep. 2016;17(7):1013–28. doi:10.15252/embr.201541763. [Google Scholar] [PubMed] [CrossRef]
112. Lau CC, Sun T, Ching AK, He M, Li JW, Wong AM, et al. Viral-human chimeric transcript predisposes risk to liver cancer development and progression. Cancer Cell. 2014;25(3):335–49. doi:10.1016/j.ccr.2014.01.030. [Google Scholar] [PubMed] [CrossRef]
113. Shen Q, Jiang S, Wu M, Zhang L, Su X, Zhao D. LncRNA HEIH confers cell sorafenib resistance in hepatocellular carcinoma by regulating miR-98-5p/PI3K/AKT pathway. Cancer Manag Res. 2020;12:6585–95. [Google Scholar] [PubMed]
114. Malakar P, Shilo A, Mogilevsky A, Stein I, Pikarsky E, Nevo Y, et al. Long noncoding RNA MALAT1 promotes hepatocellular carcinoma development by SRSF1 upregulation and mTOR activation. Cancer Res. 2017;77(5):1155–67. doi:10.1158/0008-5472.CAN-16-1508. [Google Scholar] [PubMed] [CrossRef]
115. Xin X, Wu M, Meng Q, Wang C, Lu Y, Yang Y, et al. Long noncoding RNA HULC accelerates liver cancer by inhibiting PTEN via autophagy cooperation to miR15a. Mol Cancer. 2018;17(1):1–16. doi:10.1186/s12943-018-0843-8. [Google Scholar] [PubMed] [CrossRef]
116. Zhang H, Su X, Burley SK. Zheng XS. mTOR regulates aerobic glycolysis through NEAT1 and nuclear paraspeckle-mediated mechanism in hepatocellular carcinoma. Theranostics. 2022;12(7):3518–33. doi:10.7150/thno.72581. [Google Scholar] [PubMed] [CrossRef]
117. Lin YH, Wu MH, Huang YH, Yeh CT, Cheng ML, Chi HC, et al. Taurine up-regulated gene 1 functions as a master regulator to coordinate glycolysis and metastasis in hepatocellular carcinoma. Hepatology. 2018;67(1):188–203. doi:10.1002/hep.29462. [Google Scholar] [PubMed] [CrossRef]
118. Klingenberg M, Groß M, Goyal A, Polycarpou-Schwarz M, Miersch T, Ernst AS, et al. The long noncoding RNA cancer susceptibility 9 and RNA binding protein heterogeneous nuclear ribonucleoprotein L form a complex and coregulate genes linked to AKT signaling. Hepatology. 2018;68(5):1817–32. doi:10.1002/hep.30102. [Google Scholar] [PubMed] [CrossRef]
119. Han Y, Chen M, Wang A, Fan X. STAT3-induced upregulation of lncRNA CASC11 promotes the cell migration, invasion and epithelial-mesenchymal transition in hepatocellular carcinoma by epigenetically silencing PTEN and activating PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2019;508(2):472–9. doi:10.1016/j.bbrc.2018.11.092. [Google Scholar] [PubMed] [CrossRef]
120. Liang L, Huan L, Wang J, Wu Y, Huang S, He X. LncRNA RP11-295G20.2 regulates hepatocellular carcinoma cell growth and autophagy by targeting PTEN to lysosomal degradation. Cell Discov. 2021;7(1):118. doi:10.1038/s41421-021-00339-1. [Google Scholar] [PubMed] [CrossRef]
121. Chen Z, Zhou ZY, He CC, Zhang JL, Wang J, Xiao ZY. Down-regulation of LncRNA NR027113 inhibits cell proliferation and metastasis via PTEN/PI3K/AKT signaling pathway in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2018;22(21):7222–32. [Google Scholar] [PubMed]
122. Huang JL, Cao SW, Ou QS, Yang B, Zheng SH, Tang J, et al. The long non-coding RNA PTTG3P promotes cell growth and metastasis via up-regulating PTTG1 and activating PI3K/AKT signaling in hepatocellular carcinoma. Mol Cancer. 2018;17(1):93. doi:10.1186/s12943-018-0841-x. [Google Scholar] [PubMed] [CrossRef]
123. Wei H, Hu J, Pu J, Tang Q, Li W, Ma R, et al. Long noncoding RNA HAGLROS promotes cell proliferation, inhibits apoptosis and enhances autophagy via regulating miR-5095/ATG12 axis in hepatocellular carcinoma cells. Int Immunopharmacol. 2019;73(9822):72–80. doi:10.1016/j.intimp.2019.04.049. [Google Scholar] [PubMed] [CrossRef]
124. Wu JH, Tian XY, An QM, Guan XY, Hao CY. LINC00963 promotes hepatocellular carcinoma progression by activating PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2018;22(6):1645–52. [Google Scholar] [PubMed]
125. Li W, Fu Q, Man W, Guo H, Yang P. LncRNA OR3A4 participates in the angiogenesis of hepatocellular carcinoma through modulating AGGF1/Akt/mTOR pathway. Eur J Pharmacol. 2019;849:106–14. doi:10.1016/j.ejphar.2019.01.049. [Google Scholar] [PubMed] [CrossRef]
126. Zhang L, Hu J, Hao M, Bu L. Long noncoding RNA Linc01296 promotes hepatocellular carcinoma development through regulation of the miR-26a/PTEN axis. Biol Chem. 2020;401(3):407–16. doi:10.1515/hsz-2019-0231. [Google Scholar] [PubMed] [CrossRef]
127. Cheng Z, Lei Z, Yang P, Si A, Xiang D, Zhou J, et al. Long non-coding RNA THOR promotes cell proliferation and metastasis in hepatocellular carcinoma. Gene. 2018;678:129–36. [Google Scholar] [PubMed]
128. Ye B, Hu B, Zheng Z, Zheng R, Shi Y. The long non-coding RNA AK023948 enhances tumor progression in hepatocellular carcinoma. Exp Ther Med. 2017;14(4):3658–64. [Google Scholar] [PubMed]
129. Zhang Y, Liu J, Lv Y, Zhang C, Guo S. LncRNA meg3 suppresses hepatocellular carcinoma in vitro and vivo studies. Am J Transl Res. 2019;11(7):4089. [Google Scholar] [PubMed]
130. Wang X, Dong K, Jin Q, Ma Y, Yin S, Wang S. Upregulation of lncRNA FER1L4 suppresses the proliferation and migration of the hepatocellular carcinoma via regulating PI3K/AKT signal pathway. J Cell Biochem. 2019;120(4):6781–8. [Google Scholar] [PubMed]
131. Wang F, Qi X, Li Z, Jin S, Xie Y, Zhong H. lncRNA CADM1-AS1 inhibits cell-cycle progression and invasion via PTEN/AKT/GSK-3β axis in hepatocellular carcinoma. Cancer Manag Res. 2019;11:3813–28. [Google Scholar] [PubMed]
132. Du J, Chen M, Liu J, Hu P, Guan H, Jiao X. LncRNA F11-AS1 suppresses liver hepatocellular carcinoma progression by competitively binding with miR-3146 to regulate PTEN expression. J Cell Biochem. 2019;120(10):18457–64. doi:10.1002/jcb.29163. [Google Scholar] [PubMed] [CrossRef]
133. Sui J, Yang X, Qi W, Guo K, Gao Z, Wang L, et al. Long non-coding RNA linc-USP16 functions as a tumour suppressor in hepatocellular carcinoma by regulating PTEN expression. Cell Physiol Biochem. 2017;44(3):1188–98. doi:10.1159/000485449. [Google Scholar] [PubMed] [CrossRef]
134. Son B, Lee W, Kim H, Shin H, Park HH. Targeted therapy of cancer stem cells: inhibition of mTOR in pre-clinical and clinical research. Cell Death Dis. 2024;15(9):696. doi:10.1038/s41419-024-07077-8. [Google Scholar] [PubMed] [CrossRef]
135. Koeberle D, Dufour JF, Demeter G, Li Q, Ribi K, Samaras P, et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCCa randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann Oncol. 2016;27(5):856–61. doi:10.1093/annonc/mdw054. [Google Scholar] [PubMed] [CrossRef]
136. Mishra R, Patel H, Alanazi S, Kilroy MK, Garrett JT. PI3K inhibitors in cancer: clinical implications and adverse effects. Int J Mol Sci. 2021;22(7):3464. doi:10.3390/ijms22073464. [Google Scholar] [PubMed] [CrossRef]
137. Zhang Y, Yan H, Xu Z, Yang B, Luo P, He Q. Molecular basis for class side effects associated with PI3K/AKT/mTOR pathway inhibitors. Expert Opinion Drug Metab Toxicol. 2019;15(9):767–74. doi:10.1080/17425255.2019.1663169. [Google Scholar] [PubMed] [CrossRef]
138. Bagrodia S, Smeal T, Abraham RT. Mechanisms of intrinsic and acquired resistance to kinase-targeted therapies. Pigment Cell Melanoma Res. 2012;25(6):819–31. doi:10.1111/pcmr.12007. [Google Scholar] [PubMed] [CrossRef]
139. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13(10):714–26. doi:10.1038/nrc3599. [Google Scholar] [PubMed] [CrossRef]
140. Jiang L, Li L, Liu Y, Lu L, Zhan M, Yuan S, et al. Drug resistance mechanism of kinase inhibitors in the treatment of hepatocellular carcinoma. Front Pharmacol. 2023;14:1097277. doi:10.3389/fphar.2023.1097277. [Google Scholar] [PubMed] [CrossRef]
141. Dimri M, Satyanarayana A. Molecular signaling pathways and therapeutic targets in hepatocellular carcinoma. Cancers. 2020;12(2):491. doi:10.3390/cancers12020491. [Google Scholar] [PubMed] [CrossRef]
142. Bamodu OA, Chang HL, Ong JR, Lee WH, Yeh CT, Tsai JT. Elevated PDK1 expression drives PI3K/AKT/MTOR signaling promotes radiation-resistant and dedifferentiated phenotype of hepatocellular carcinoma. Cells. 2020;9(3):746. doi:10.3390/cells9030746. [Google Scholar] [PubMed] [CrossRef]
143. Narci K, Kahraman DC, Koyas A, Ersahin T, Tuncbag N, Atalay RC. Context dependent isoform specific PI3K inhibition confers drug resistance in hepatocellular carcinoma cells. BMC Cancer. 2022;22(1):320. doi:10.1186/s12885-022-09357-y. [Google Scholar] [PubMed] [CrossRef]
144. Jin H, Wang L, Bernards R. Rational combinations of targeted cancer therapies: background, advances and challenges. Nat Rev Drug Discov. 2023;22(3):213–34. doi:10.1038/s41573-022-00615-z. [Google Scholar] [PubMed] [CrossRef]
145. Feng YQ, Gu SX, Chen YS, Gao XD, Ren YX, Chen JC et al. Virtual screening and optimization of novel mTOR inhibitors for radiosensitization of hepatocellular carcinoma. Drug Des Devel Ther. 2020;14:1779–98. [Google Scholar] [PubMed]
146. Zamanian MY, Golmohammadi M, Yumashev A, Hjazi A, Toama MA, AbdRabou MA, et al. Effects of metformin on cancers in experimental and clinical studies: focusing on autophagy and AMPK/mTOR signaling pathways. Cell Biochem Funct. 2024;42(4):e4071. doi:10.1002/cbf.4071. [Google Scholar] [PubMed] [CrossRef]
147. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24. doi:10.1038/s41573-020-0090-8. [Google Scholar] [PubMed] [CrossRef]
148. Masoumi E, Tahaghoghi-Hajghorbani S, Jafarzadeh L, Sanaei MJ, Pourbagheri-Sigaroodi A, Bashash D. The application of immune checkpoint blockade in breast cancer and the emerging role of nanoparticle. J Control Release. 2021;340(Suppl., 117):168–87. doi:10.1016/j.jconrel.2021.10.018. [Google Scholar] [PubMed] [CrossRef]
149. Metkar SP, Fernandes G, Navti PD, Nikam AN, Kudarha R, Dhas N, et al. Nanoparticle drug delivery systems in hepatocellular carcinoma: a focus on targeting strategies and therapeutic applications. OpenNano. 2023;12:100159. doi:10.1016/j.onano.2023.100159. [Google Scholar] [CrossRef]
Yoon MS. Nanotechnology-based targeting of mTOR signaling in cancer. Int J Nanomed. 2020;15:5767–81. [Google Scholar]
151. Tang H, Dilimulati D, Yang Z, Zhou K, Chen X, Sun R, et al. Chemically engineered mTOR-nanoparticle blockers enhance antitumour efficacy. eBioMedicine. 2024;103(2):105099. doi:10.1016/j.ebiom.2024.105099. [Google Scholar] [PubMed] [CrossRef]
152. Varshney A, Panda JJ, Singh AK, Yadav N, Bihari C, Biswas S, et al. Targeted delivery of microRNA-199a-3p using self-assembled dipeptide nanoparticles efficiently reduces hepatocellular carcinoma in mice. Hepatology. 2018;67(4):1392–407. doi:10.1002/hep.29643. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF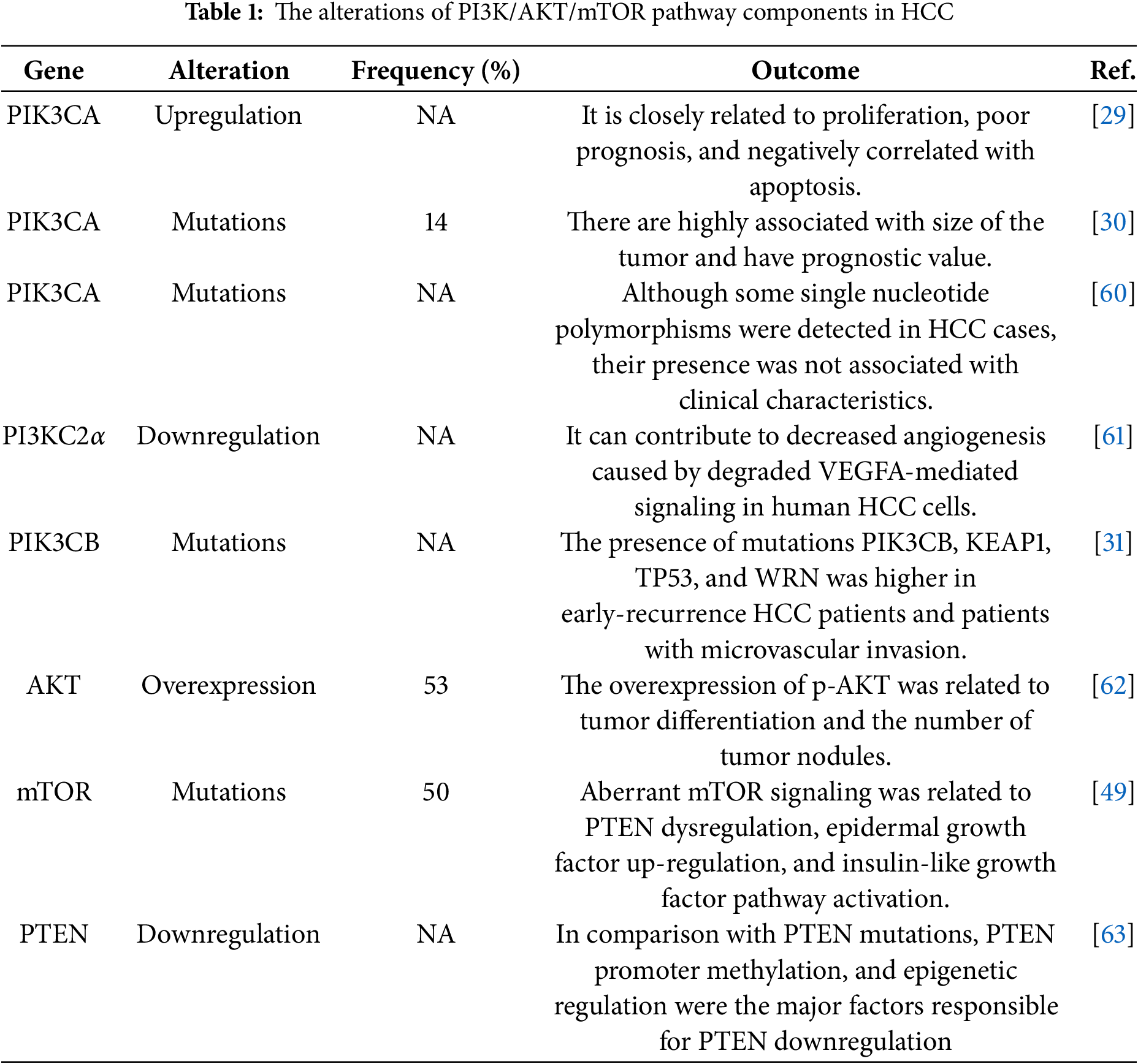

 Downloads
Downloads
 Citation Tools
Citation Tools