 Open Access
Open Access
ARTICLE
Stretch Enhances Secretion of Extracellular Vehicles from Airway Smooth Muscle Cells via Endoplasmic Reticulum Stress Signaling in Relation to Ventilator-Induced Lung Injury
Changzhou Key Laboratory of Respiratory Medical Engineering, Institute of Biomedical Engineering and Health Sciences, School of Medical and Health Engineering, Changzhou University, Changzhou, 213164, China
* Corresponding Authors: MINGZHI LUO. Email: ; LINHONG DENG. Email:
# These authors contributed equally to this work
BIOCELL 2025, 49(5), 833-855. https://doi.org/10.32604/biocell.2025.063869
Received 26 January 2025; Accepted 18 April 2025; Issue published 27 May 2025
Abstract
Background: Mechanical ventilation (MV) provides life support for patients with severe respiratory distress but can simultaneously cause ventilator-induced lung injury (VILI). However, due to a poor understanding of its mechanism, there is still a lack of effective remedies for the often-deadly VILI. Recent studies indicate that the stretch associated with MV can enhance the secretion of extracellular vesicles (EVs) and induce endoplasmic reticulum (ER) stress in airway smooth muscle cells (ASMCs), both of which can contribute to VILI. But whether MV-associated stretch enhances the secretion of EVs via ER stress in ASMCs as an underlying mechanism of VILI remains unknown. Methods: In this study, we exposed cultured human ASMCs to stretch (13% strain) and mouse models to MV at tidal volume (18 mL/kg). Subsequently, the amount of secreted EVs in the culture medium of ASMCs and the bronchoalveolar lavage fluid (BALF) of mouse models was quantitatively evaluated by ultracentrifugation, transmission electron microscopy, Western blot, flow cytometry, and nanoparticle tracking analysis. The cultured ASMCs and the lung tissues of mouse models were assessed for expression of biomarkers of EVs (cluster of differentiation antigen 63, CD63), ER stress (heat shock protein family A member 5, HSPA5), and EVs regulating molecule Rab27a by immunofluorescence microscopy, immunohistochemistry (IHC) and enzyme-linked immunosorbent assay (ELISA), respectively. MicroRNAs (miRNAs) in EVs from ASMCs were measured with miRNA whole genome sequencing (miRNA-Seq). Results: We found that stretch enhanced EV secretion from cultured ASMCs. In addition, the cultured ASMCs and the mouse models were either or not pretreated with ER stress inhibitor (tauroursodeoxycholic acid, TUDCA)/EV secretion inhibitor (GW4869) prior to stretch or MV. We found that MV-associated stretch enhanced the expression of CD63, HSPA5, and Rab27a in cultured ASMCs and BALF/lung tissues of mouse models, which could all be attenuated with TUDCA/GW4869 pretreatment. miRNA-Seq data show that differentially expressed miRNAs in EVs mainly modulate gene transcription. Furthermore, the EVs from cultured ASMCs under stretch tended to enhance detachment and expression of inflammatory cytokines, i.e., transforming growth factor-β1 (TGF-β1), interleukin-10 (IL-10) in cultured airway epithelial cells. The expression of TGF-β1 and IL-10 in BALF of the mouse models also increased in response to MV, which was attenuated together with partial improvement of lung injury by pretreatment with TUDCA, GW4869/Rab27a siRNAs. Conclusion: Taken together, our data indicate that MV-associated stretch can enhance the secretion of EVs from ASMCs via ER stress signaling to mediate airway inflammation and VILI, which provides new insight for further exploring EVs for the diagnosis and treatment of VILI.Graphic Abstract
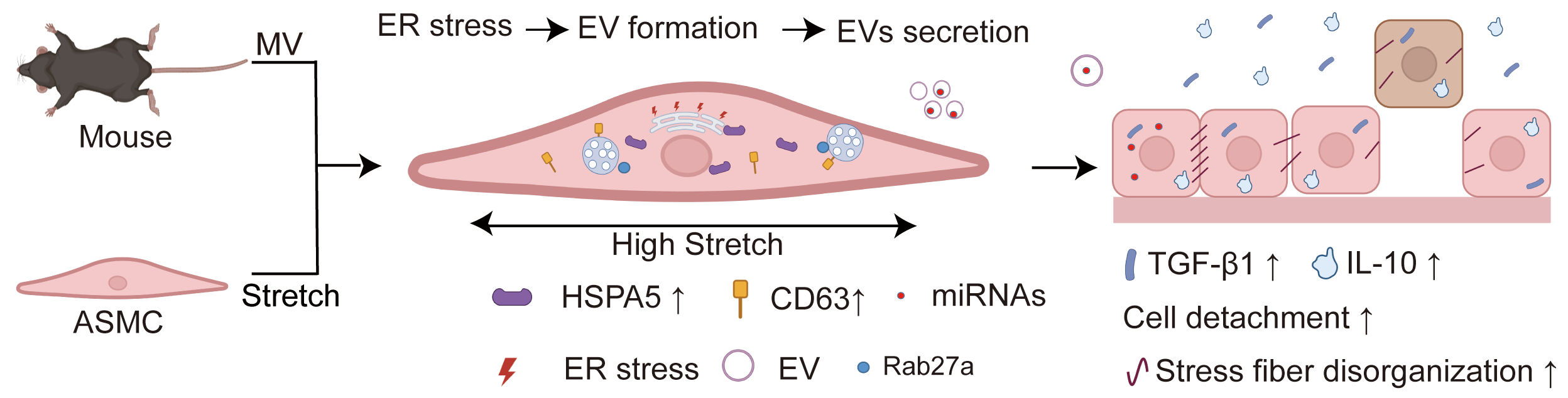
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools