 Open Access
Open Access
ARTICLE
Effectiveness and Safety of Transcatheter Closure of Various Ventricular Septal Defects Using Second-Generation Amplatzer Duct Occluders
Department of Congenital Heart Disease, General Hospital of Northern Theater Command, Shenyang, China
* Corresponding Authors: Xianyang Zhu. Email: ; Qiguang Wang. Email:
Congenital Heart Disease 2023, 18(2), 183-195. https://doi.org/10.32604/chd.2022.021855
Received 09 March 2022; Accepted 14 July 2022; Issue published 15 March 2023
Abstract
Objective: This study was designed to determine the long-term safety and efficacy of using the Amplatzer Duct Occluder II (ADO II) for the closure of various ventricular septal defects (VSDs). Methods: From January 2011 to December 2019, selected VSD patients were treated through transcatheter intervention using ADO II occluders. The closure results and complications from 188 patients, involving 167 perimembranous ventricular septal defects (pmVSDs), 9 intracristal VSDs, 11 post surgery residual shunts and 1 post closure residual shunt with the mean outlet diameter3.1 ± 0.8 mm under angiography, were enrolled in this study. Results: The success rate was 98.9% for all procedures. During the median 77-month follow-up period, no cases of complete atrioventricular block (cAVB), infective endocarditis or death occurred. One major adverse event (0.5%) was recorded: cerebrovascular accident occurred 1 day after the procedure in one patient who was transferred to the neurology department. The residual shunt rate was 44.6%, which was the most common minor adverse event. The cardiac conduction block rate was 4.3%. Specifically, one pmVSD patient developed intermittent LBBB during the 28-month follow-up. There were 3 patients (1.6%) with new-onset mild tricuspid insufficiency, and the insufficiency degree was stable during follow-up. There was no new-onset aortic insufficiency that occurred. Conclusions: Transcatheter closure of pmVSDs, some intracristal VSDs, some postsurgery or postclosure residual shunts using ADO II occluders were both safe and effective and yielded excellent long-term results in selected patients.Keywords
At the present time, transcatheter closure of ventricular septal defects (VSDs) has become an alternative to surgical repair in selected patients of perimembranous ventricular septal defects (pmVSDs) [1,2]. With the development of interventional devices, there are an increasing number of reports of successful interventional closure of not only pmVSDs but also some intracristal ventricular defects with mild aortic valve prolapse, outlet-type VSDs, and postsurgical or postclosure residual shunts [3–5]. Recently, the second generation of Amplatzer patent ductus arteriosus occluders (ADO II) have been used off-label in the treatment of VSD and exhibit promising prospects in reducing arrhythmia complications [6]. However, few studies have focused on the indications for the treatment of ventricular septal defects with ADO II occluders and the outcomes of long-term follow-up [7,8]. The purpose of this study was to investigate the safety and efficacy of ADO II occluders in the treatment of various VSDs through our retrospective study and to report the long-term follow-up results.
From January 2011 to December 2019, 188 patients with VSDs underwent transcatheter closure in the General Hospital of Northern Theater Command. Our study included 79 male and 109 female patients ranging from 2.8 to 59 years of age. All patients underwent clinical examinations, chest roentgenography, electrocardiography (ECG), and transthoracic echocardiography (TTE) before the intervention.
Based on the Chinese consensus and other references from our previous studies, the inclusion criteria for percutaneous closure included (1) a moderate or large left-to-right shunt (Qp/Qs > 1.5) causing left heart volume overload that resulted in hemodynamic changes, congestive heart failure, decreased exercise capacity, atrial dysrhythmia, or recurrent bacterial endocarditis; (2) a VSD diameter ≤6 mm as measured through TTE; (3) a distance from the aortic valve to the upper rim of the VSD of ≥1 mm as measured through TTE; (4) a pulmonary pressure of <70 mm Hg as estimated through TTE. Exclusion criteria included (1) active infective endocarditis; (2) pathologic aortic regurgitation; and (3) primary tricuspid chordae tendineae located around the rim of the pmVSD; (4) patients with other structural heart defects requiring cardiac surgery. Patients who were aged 50 years or older underwent routine coronary angiography before VSD closure. Before the intervention, each patient provided informed written consent. The ethics committees of the General Hospital of Northern Theater Command approved this study (No. XXN-VSD-01), which was conducted in accordance with all relevant Chinese laws.
ADO II occluders are the second generation of Amplatzer patent ductus arteriosus occluders (Abbott St. Jude Medical, St. Paul, MN, USA). It has been used off-label for interventional occlusion of ventricular septal defects. The frame is woven with a self-expanding and ultrafine nickel titanium alloy wire without a polyester fiber patch design. It is shaped in sequential lobes characterized by two low-profile retention discs and a connecting waist. There are 4 specifications of the waist diameter (3, 4, 5 and 6 mm) and 2 specifications of the length (4 and 6 mm). The flexibility of the articulations allows this 4F or 5F delivery system to simplify specific defect anatomies that are more challenging.
2.3 Peri-Procedure Management and Follow-Up
The transcatheter intervention procedure was described in previous reports [9]. To choose the occluder, the size of ventricular septal defect was measured mainly based on the left anterior oblique 50° head 20° projection position of angiocardiography, and echocardiography was used as an auxiliary measurement and monitoring method. For ADO II occluder, the central cylinder waist should be 1–2 mm larger than the narrowest diameter of the VSD. This minimal oversizing avoids excessive radial force to the ventricular septum yet still maintaining device stability. The left and right retention discs should match with the space in the left and right ventricles. The device length should match with the thickness of the ventricular septum and tunnel length. In most cases, a 4 mm length device is most suitable.
Demographic data before the intervention and during the follow-up period and additional baseline characteristics were collected from all patients. After the procedure, an ECG was performed on each patient daily until discharge. All patients underwent 24-h continuous electrocardiographic monitoring and TTE on the third day after the procedure. Follow-up evaluations included clinical examinations, ECG, and TTE at 1, 3, 6, and 12 months and annually thereafter. Adverse events were recorded and classified into categories of major and minor adverse events. Major adverse events included but were not limited to death, infective endocarditis, cerebrovascular accidents, complete atrioventricular block (cAVB) requiring either pacemaker implantation or surgical treatment, thromboembolism, and new-onset valvular regurgitation requiring surgical repair. Minor adverse events included but were not limited to cardiac arrhythmias requiring medication, new or exacerbated valvular regurgitation grade <2, residual shunts as trivial (<1 mm color jet width), small (1–2 mm color jet width), and moderate to large (>2 mm color jet width) under TTE [10], groin hematoma, hemolysis requiring medication, fever, and rash.
All continuous variables are expressed as either means ± SDs or medians with ranges; discrete variables are presented as either frequencies or percentages. Statistical analysis was performed using SPSS 16.0 for Windows (SPSS, Inc., Chicago, Illinois). Analysis of continuous variables was performed using Student’s unpaired t test and 1- and 2-way analyses of variance; analysis of categorical variables was performed using the chi-square test and Fisher’s exact test. A probability of <0.05 was considered statistically significant. Freedom from major or minor adverse events was assessed using the Kaplan-Meier product-limit method.
A total of 188 patients were enrolled in this study. Several basic characteristics were evaluated (Table 1). The median follow-up time was 77 months and ranged from 12 to 143 months. Patients without hemodynamic changes were enrolled primarily because they had significant symptoms, such as exercise intolerance. Three patients had a previous history of infective endocarditis and were free from fever for at least 6 months before the intervention. Of the 17 patients who underwent coronary angiography, all had normal results.
Procedural data are presented in Table 2. Deployment of the device was successful in 186 patients. The procedural success rate was 98.9%. Except for one intracristal VSD patient who developed severe aortic regurgitation, 8 patients with intracristal VSD with mild aortic prolapse were successfully treated through transcatheter intervention using ADO II (Fig. 1). The device closure failed in another pmVSD patient because of occluder embolization. After retrieval of the ADO II, the closure of pmVSD was successfully performed using the domestic symmetrical occluder. All 186 patients had no new aortic regurgitation after immediate TTE examination.
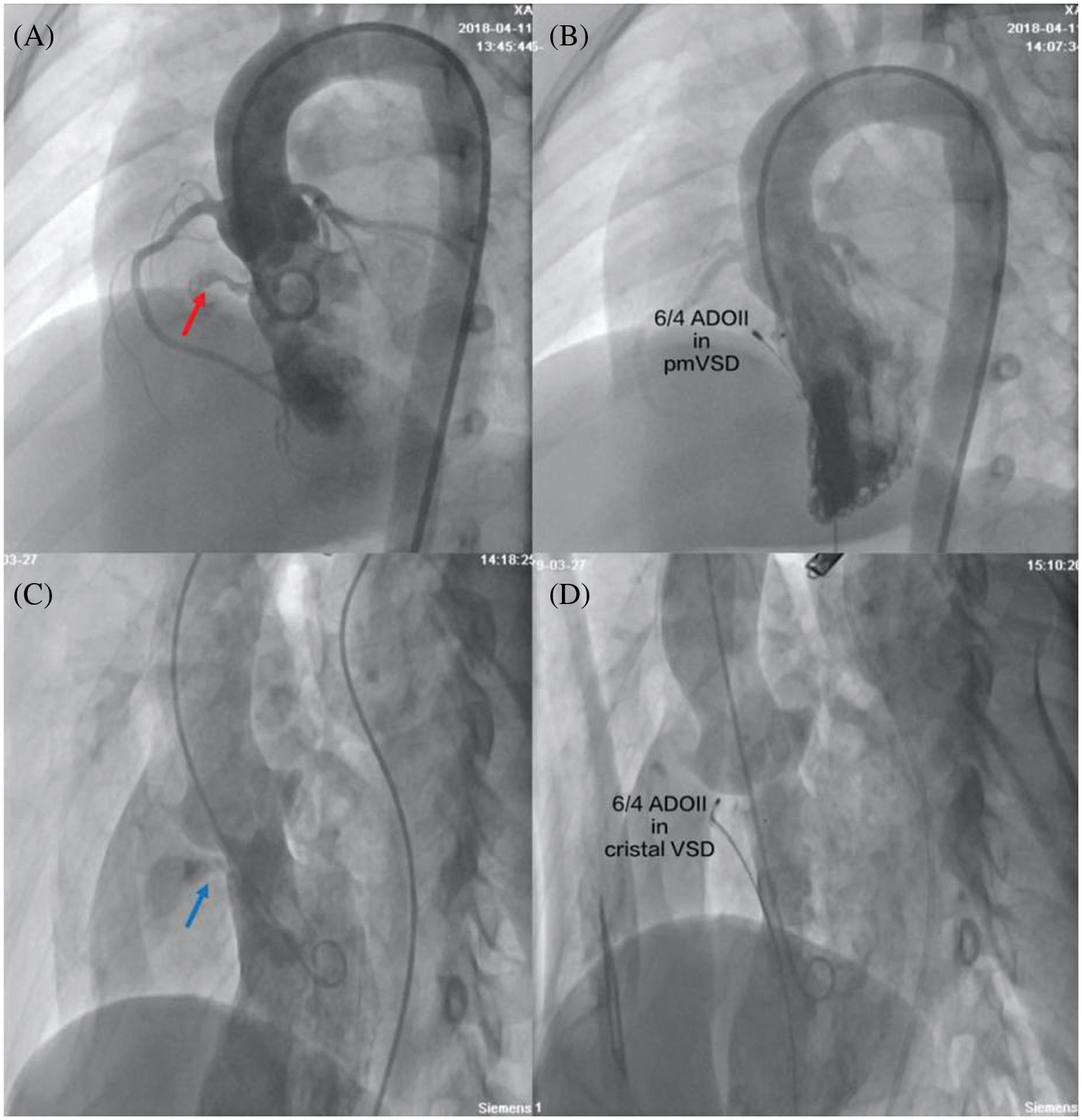
Figure 1: Closure of pmVSD and intracristal VSD with ADO II occluders. (A) Left ventriculography showed pmVSD (red arrow) with a tubular shape without septal aneurysm; (B) There was no residual shunt in left ventriculography after successful implantation of the 6/4 ADO II occluder; (C) Left ventriculography showed intracristal VSD (blue arrow) with mild aortic valve prolapse; (D) There was no residual shunt or aortic regurgitation under left ventriculography after successful implantation of the 6/4 ADO II occluder. pmVSD, perimembranous ventricular defect; cristal VSD, intracristal ventricular septal defect
The type of device was changed to ADO II in 4 patients after the initial procedure. cAVB occurred in three pmVSD patients after the placement of a double-disk symmetrical concentric occluder, but they returned to normal sinus rhythm after retrieval of symmetrical device and then the closure was successful by implantation of an ADO II. One pmVSD patient with a double-disk asymmetrical occluder developed severe aortic regurgitation, and the subsequent use of an ADO II was successful in this patient. The overall immediate complete closure rate was 55.4%. All complex congenital cardiovascular malformations (ASDs and PDAs) were successfully simultaneously closed. In one 46-year-old man with aortic arch coarctation, a 5/4 ADO II occluder was successfully implanted into the intracristal VSD with mild aortic valve prolapse, and an aortic arch coarctation stent was simultaneously implanted. The ADO II was successfully implanted in all postsurgical residual and postclosure residual patients (Fig. 2).
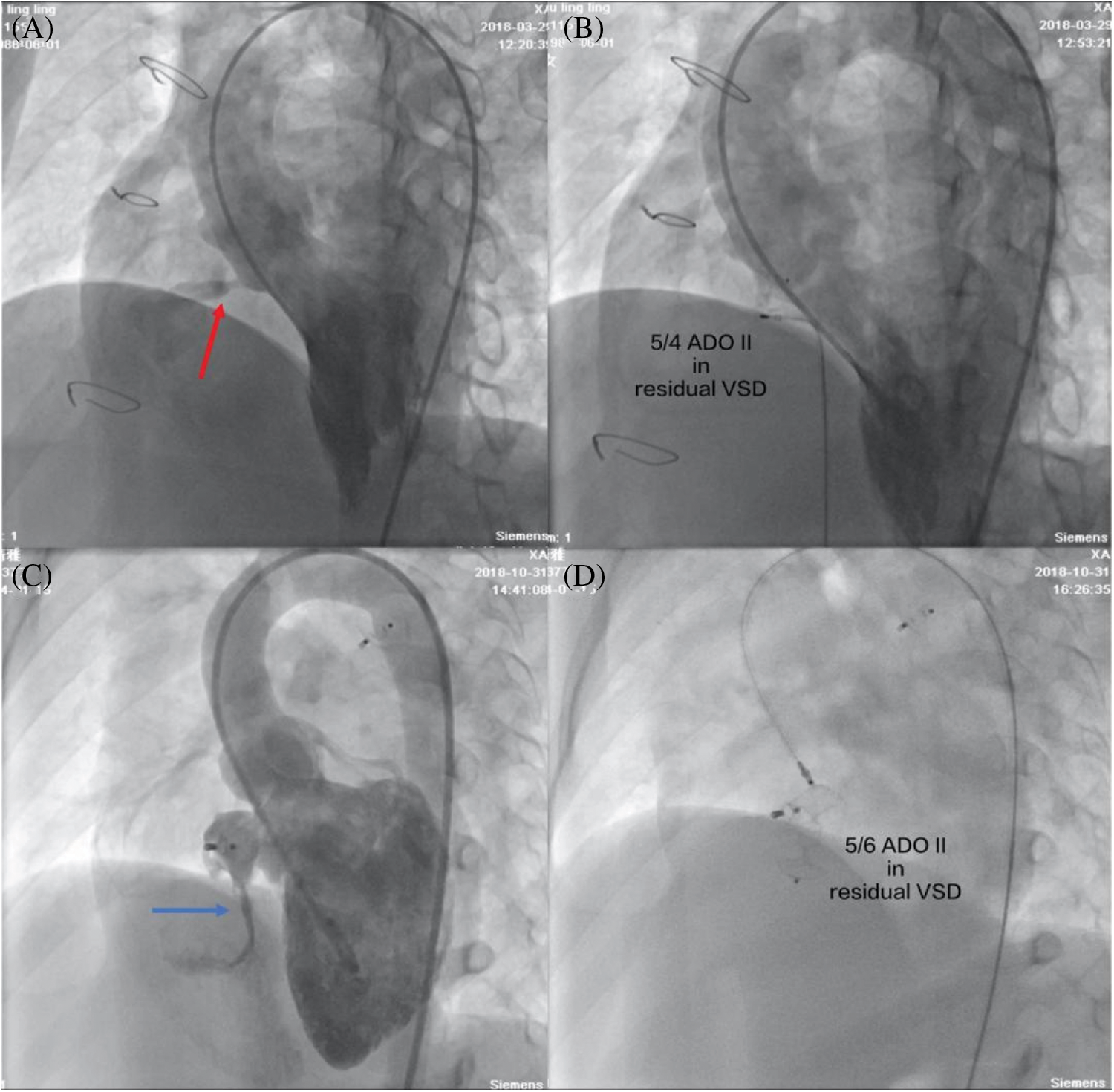
Figure 2: Transcatheter closure of postsurgical residual shunts and postclosure residual shunts. (A) Left ventriculography showed a postsurgical residual shunt (red arrow); (B) No residual shunt was found with left ventriculography after a 5/4 ADO II occluder was successfully implanted; (C) Left ventriculography showed a postclosure residual shunt (blue arrow); (D) A 5/6 ADO II occluder was successfully implanted by a retrograde approach
All patients were followed up for at least 12 months. Of these, 122 patients were followed up for >36 months, and 59 patients were followed up for >60 months.
The major and minor adverse events are summarized in Table 3. A total of one major adverse event was noted. A 47-year-old female who had diabetes and a history of cerebral infarction suffered acute cerebral embolism 1 day after closure, was transferred to the neurology department, and was discharged on the 11th day after closure. Mild residual shunting after the procedure was the most common complication, with an overall rate of 44.6% as measured through TTE. The 83 cases of immediate residual shunt after closure were mild. There were 76 cases residual shunts between disks of the occluders, and 7 cases residual shunts around disks due to multiple outlets and anatomical morphology confirmed by TTE. However, these problems did not persist because most residual shunts diminished before each patient’s 12-month follow-up visit (Fig. 3). No patients developed either trivial or small residual shunt flow, as determined through echocardiography at the latest follow-up examination.
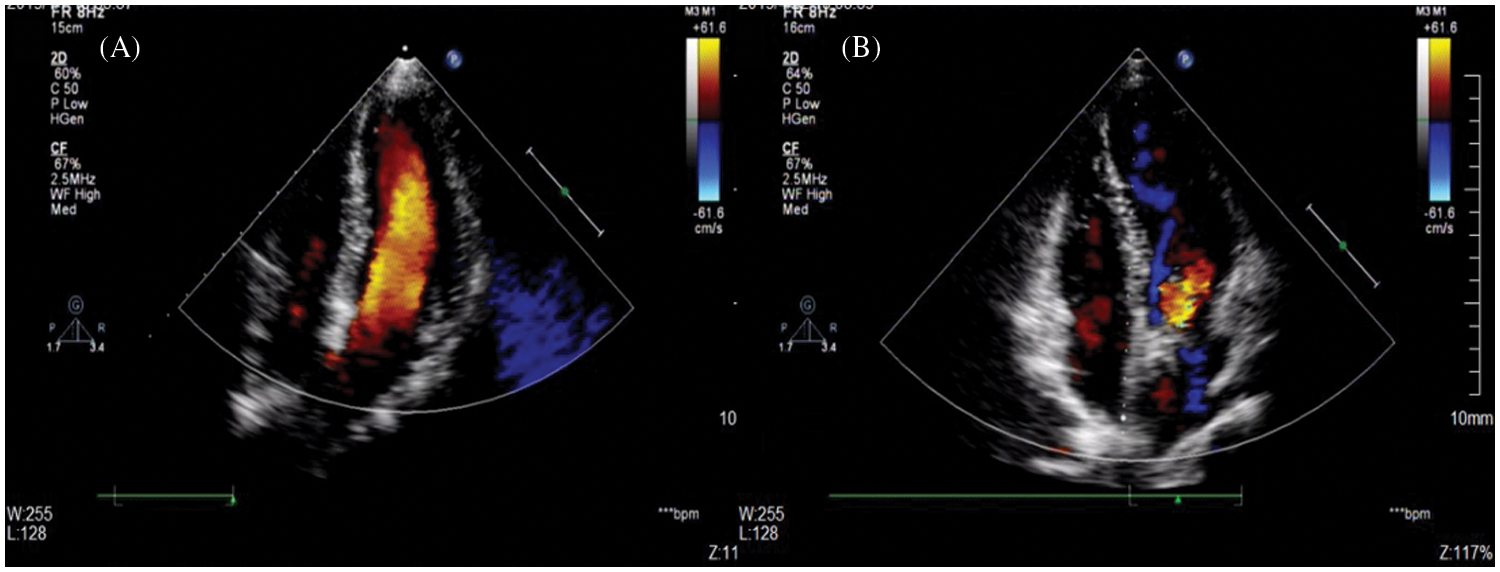
Figure 3: A mild residual shunt after the procedure did not persist. (A) TTE showed a postclosure mild residual shunt at the 1-month follow-up; (B) No residual shunt was found with TTE at the 12-month follow-up
The frequency of arrhythmia development after the procedure was 9.1% (Fig. 4). Cardiac conduction block developed in 8 patients. Right bundle branch block (RBBB) was the most common type of cardiac conduction block detected. Of the patients with conduction disturbances, a 3-year-old boy who experienced II-AVB 2 days after his intervention reverted to a normal sinus rhythm on the 11th day after the administration of intravenous hydrocortisone and phosphocreatine therapy.
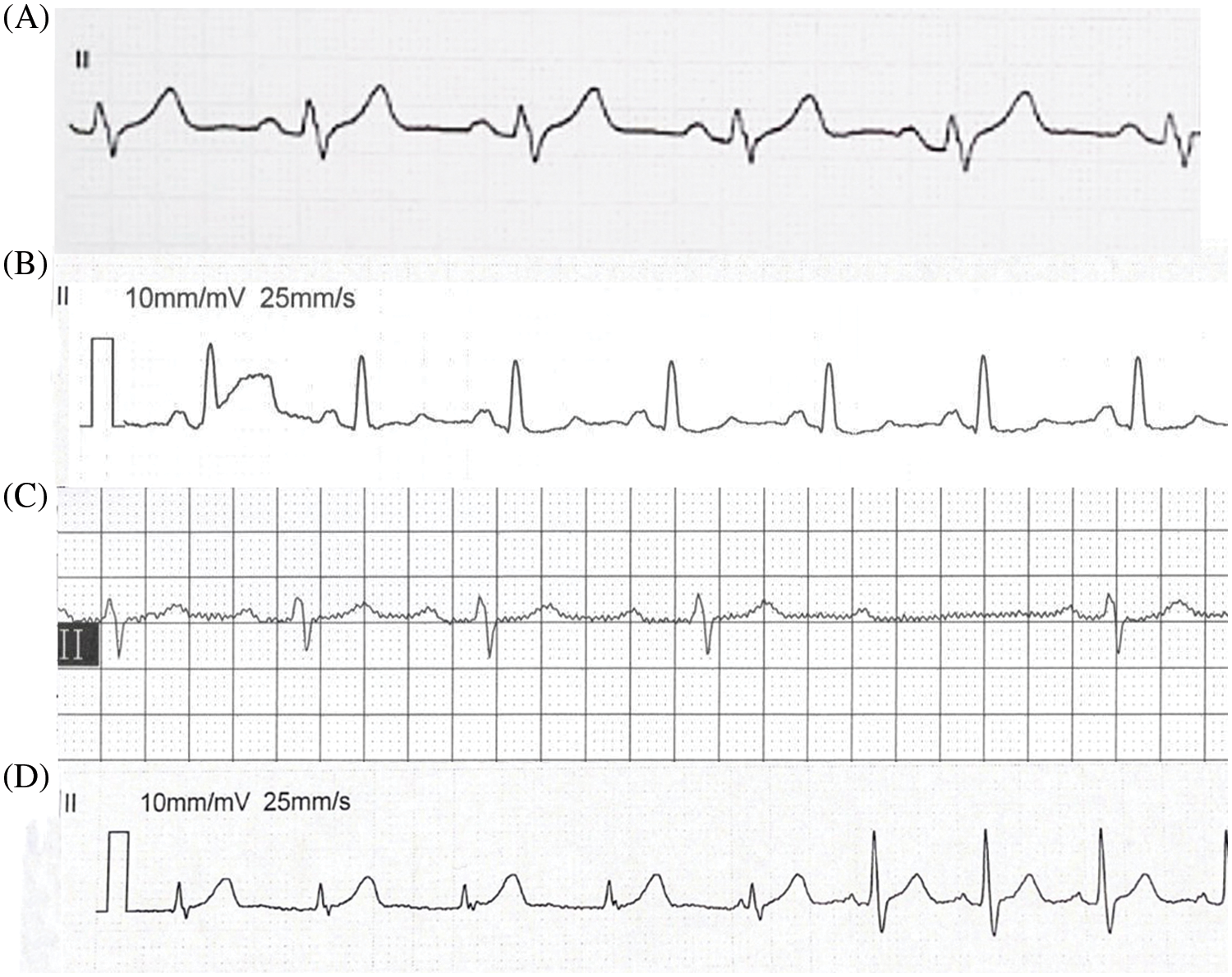
Figure 4: Arrhythmia after the procedure. (A) LBBB 8 days after the procedure in a 5-year-old girl after the placement of a 5/4 ADO II; (B) RBBB 3 days after the procedure in a 4-year-old girl after the placement of a 5/4 ADO II; (C) II-AVB 2 days after the procedure in a 3-year-old boy after the placement of a 6/4 ADO II; (D) Idiojunctional rhythm 4 days after the procedure in a 40-year-old female after the placement of a 5/4 ADO II. LBBB = left bundle branch block and RBBB = right bundle branch block
Only 1 left bundle-branch block (LBBB) event was noted in a 5-year-old girl 8 days after the placement of a 5/4 ADO II, and there was a return to a normal heart rhythm on the 11th day after the procedure. However, intermittent LBBB was still detected during follow-up without further intervention. Conduction disturbances typically developed while each patient was in the hospital after the procedure. Of the 8 patients who developed cardiac conduction block, 5 patients reverted to a normal sinus rhythm after discharge. The 3 patients continued to experience RBBB after discharge until the latest follow-up examination. As mentioned earlier, the LBBB patient continued to experience intermittent LBBB during follow-up examinations; this patient was asymptomatic and was followed for 4 years. Nine patients with either nonparoxysmal junctional tachycardia or intraventricular conduction delay reverted to a normal sinus rhythm after discharge.
The overall incidence of new-onset tricuspid valve regurgitation was 1.6%, and no new aortic valve regurgitation was observed during the follow-up period. As the latest TTE follow-up data demonstrated that no new aortic valve regurgitation in each 8 intracristal VSD patients. No new-onset tricuspid valve regurgitation more severe than second degree was noted. At their 12-month follow-up examinations, 3 patients with tricuspid valve insufficiency were deemed to be stable, and 1 patient was followed up for >5 years without intervention.
A 46-year-old female who was diagnosed with postsurgery (27 years ago) residual VSD and rehospitalized because of heart failure (NYHA II–III) was observed 43 months after closure. The other patients displayed New York Heart Association functional class I or II heart function.
After closure, no cases of infective endocarditis or death were observed during the follow-up period.
Compared with other simple congenital heart diseases (ASD or PDA), transcatheter closure of ventricular septal defects is more challenging due to its variable anatomical structure, especially for some postsurgical or post closure residual patients [10–12]. Sometimes it is difficult for the delivery sheath to pass through the relatively small defect or directly into the left ventricular apex. The ADO II occluder is a kind of double disc occluder made of extremely fine nickel titanium alloy wire without fabric material filling and was originally designed to close small patent ductus arteriosus. The delivery sheath is flexible, and the profile dimension is relatively small. All these characteristics allow the ADO II to be released through a retrograde approach (arterial side) or an antegrade approach (venous side) [13,14]. In some cases where the delivery sheath could not be directed into the left ventricular apex via the vein, the retrograde approach could be used [15]. In our one postclosure residual case, left ventriculography showed that the residual shunt direction was downward, which predicted that the traditional delivery sheath could not have crossed through the defect to the left ventricular apex due to the complex local tissue. Therefore, ADO II was successfully implanted by a retrograde approach, and postoperative angiography showed no residual shunt. In theory, the retrograde approach can simplify the procedure, which reduces the exposure time and operation time. However, we still care about the femoral artery injury especially for young age group, left ventricular side adjacent conduction system, and the potential damage to the aortic valve through retrograde approach. In our study, except for one postclosure residual case through retrograde, all other occluders were released anterograde through the femoral vein with the establishment of an arteriovenous track. Due to the small profile and super flexibility of the ADO II occluder delivery system, combined with the super elasticity of the occluder, it is possible to achieve more challenging ventricular septal defect closure.
The common characteristics of patients with various VSDs enrolled in this study were small defects without obvious symptoms, but most patients had more than 2/6 grade cardiac murmurs or signs of left heart enlargement by imaging examination. Although the defect was small, there was a risk of infective endocarditis in the future. It has been shown that 4% of patients with small ventricular defects developed infective endocarditis by 6 years of follow-up [16]. Moreover, the presence of heart murmur influenced the physical and mental health of children with VSDs. In addition, the application of ADO II occluders in patients with small ventricular defects achieved a high success rate and less trauma [17,18], so we suggest that patients with small ventricular defects should be treated in this manner.
Proper selection of the occluder is a crucial step in ensuring the success of transcatheter pmVSD closure. At the same period, we had performed other 275 pmVSD closure, mainly domestic modified double-disk symmetrical concentric pmVSD occluders (S-pmVSOs) and double-disk asymmetrical S-pmVSOs (AS-pmVSOs) were used. Aneurysmal pmVSDs are common and are often characterized by morphologic diversity, variable numbers of outlets, and complex surrounding tissues. If a multioutlet membranous aneurysm is diagnosed, the selection of an occluder should be based on the VSD number and size. The AS-pmVSO closes multiple outlets at a time because of its larger left disk. The right disk of the AS pmVSO is relatively small, which minimizes the risk of tricuspid valve impairment. The thin waist structure of this device is adapted to the diameter of the larger outlet shunt, and the 2 disks can be fully extended, avoiding over compression of the defect and the surrounding tissue. The waist lengths of both the S-pmVSO and AS pmVSO devices are longer than that of the first generation Amplatzer VSD occluder (2 and 3 mm, respectively, vs. 1.5 mm). These changes in occluder design could decrease the incidence of severe arrhythmias and gain high complete closure rate; therefore, modified double disk occluders are more favorable devices in selected patients.
Previous studies on transcatheter closure of ventricular septal defects have shown that cardiac block was the most common complication [19,20], and the incidence of cAVB was approximately 1.1% [11]. Although the exact mechanism remains unclear, one of the potential reasons may be related to the damage in the conduction system caused by the stiff disks of the occluder. In addition, younger children may be another independent risk factor for conduction damage [21,22]. The ADO II constitutes a safe and effective therapeutic alternative for morphologically varied VSDs in all pediatric age groups [23]. Our long-term follow-up study showed that the incidence rate of arrhythmia was lower than the incidence of residual shunts using ADO II after closure, and no cAVB occurred over the entire follow-up period. However, one case of intermittent complete LBBB in our study needs to be highly studied. There are a few case reports of cAVB after closure using ADO II, which indicates that the treatment of VSDs using ADO II still needs long-term follow-up [24]. In our study, we found that there was no arrhythmia after closure in postsurgical or postclosure residual patients. Local tissue hyperplasia and organization, and the anatomy of the residual shunt were far away from the conduction block, which all may be possible reasons.
Postoperative residual shunt was the most common complication in this study, with an incidence of 44.6%, which was significantly higher than that using other types of ventricular septal occluders. Previous studies have shown that residual shunts are more common after closure of membranous aneurysm pmVSDs with multiple outlets [25]. The ADO II occluder is specially designed for occlusion of the small patent ductus arteriosus (according to the instructions <5.5 mm), and there is no fabric filling in it, which may be the mechanism underlying the high incidence of postoperative residual shunts. As there is no polyester patch design in ADO IIoccluders, most residual shunts between disks of the occluders in a short time after closure. When endothelialization after 3 to 6 months post closure, the residual shunts between disks have been disappeared, and the residual shunts around disks have been gradually reduced. During the follow-up, there was no increase in the residual shunt, effect of hemodynamic changes, or mechanical hemolysis complications.
The anatomy of pmVSD is complex and adjacent to the tricuspid valve and aortic valve. Intracristal VSD with aortic valve prolapse is common and potentially leads to aortic valvular insufficiency after closure; it is still regarded as an occlusion contradiction [3]. As the risk of major complications such as unacceptable aortic valvular insufficiency and cAVB were high after closure using asymmetrical occluders [26], the ideal distance between the defect and the aortic valve was more than 3 mm according to the current research criteria. In our study, closure of the distance from the defect to the aortic valve ≥1 mm and intracristal VSD with mild aortic valve prolapse were successfully performed using the ADO II. Our long-term follow-up showed that the successful implantation of ADO II had little effect on the aortic valve. Beyond this study, ADO II can be attempted in the following conditions: some ventricular septal defects with mild aortic valve prolapse; the tubular type VSDs with small outlets; it is difficult to establish the traditional orbit under anterograde pathway, so the occluder should be implanted in the retrograde approach; high risk population of complete atrioventricular block after implantation of VSD occluders. In our study, three patients were newly diagnosed with mild tricuspid regurgitation after the procedure, and the degree of valve regurgitation was not significantly changed during the long-term follow-up. The distance between the defect and tricuspid valve and its anatomic relationship still needs to be carefully evaluated before closure. The ADO II is longer and has less stiffness than traditional symmetrical or asymmetrical occluders, which might have little influence on the membranous aneurysm and surrounding anatomy of the VSDs. Failure was observed in two patients with the ADO II in our study. Therefore, it is very important for experienced experts to strictly select the appropriate patients and monitor these patients throughout the perioperative period.
The present study had several limitations. First and foremost, as was the case during our previous study, and in accordance with a consensus reached by Chinese experts, we followed strict inclusion/exclusion criteria. Second, because this was a retrospective study, ADO II selection depended on the experience of the operators, and we lacked a controlled cohort study design with large sample sizes for testing several types of occluders. In addition, this study has not been compared with surgery at the same period. Third, patients were enrolled from only one center and therefore may not be representative of the general population. Finally, the number of patients included in the study was relatively small, and a prolonged follow-up period was not adopted for all patients.
Transcatheter closure of pmVSDs, some intracristal VSDs, some postsurgery or postclosure residual shunts using ADO II occluders were both safe and effective and yielded excellent long-term results in selected patients.
Funding Statement: This study was supported by grant from Doctoral Start-Up Foundation of Liaoning Province of China (2019-BS-266).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Xianyang Zhu and Qiguang Wang; data collection: Jianming Wang, Xiaotang Sheng and Jingsong Geng; analysis and interpretation of results: Jianming Wang and Jiawang Xiao; draft manuscript preparation: Jianming Wang. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data supporting the findings of this study are encrypted and stored in military cardiovascular intervention diagnosis and treatment management information network. Data can be made available upon request to the corresponding author.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Hoffman, J. I. (1995). Incidence of congenital heart disease: I. Postnatal incidence. Pediatric Cardiology, 16(3), 103–113. https://doi.org/10.1007/BF00801907 [Google Scholar] [PubMed] [CrossRef]
2. Wang, J., Zuo, J., Yu, S., Yi, D., Yang, X. et al. (2016). Effectiveness and safety of transcatheter closure of perimembranous ventricular septal defects in adults. The American Journal of Cardiology, 117(6), 980–987. https://doi.org/10.1016/j.amjcard.2015.12.036 [Google Scholar] [PubMed] [CrossRef]
3. Ghosh, S., Sridhar, A., Solomon, N., Sivaprakasham, M. (2018). Transcatheter closure of ventricular septal defect in aortic valve prolapse and aortic regurgitation. Indian Heart Journal, 70(4), 528–532. https://doi.org/10.1016/j.ihj.2017.11.023 [Google Scholar] [PubMed] [CrossRef]
4. Shao, S., Luo, C., Zhou, K., Hua, Y., Wang, C. (2019). What is the best management option for non-significant residual shunt after device closure of perimembranous ventricular septal defect: A case report based on the lessons from post-procedure endocarditis. Medicine, 98(42), e17347. https://doi.org/10.1097/MD.0000000000017347 [Google Scholar] [PubMed] [CrossRef]
5. Lin, H. C., Lin, M. T., Chen, C. A., Hsu, J. Y., Lin, S. M. et al. (2021). Safety and efficacy of transcatheter closure of outlet-type ventricular septal defects in children and adults with Amplatzer Duct Occluder II. Journal of the Formosan Medical Association, 120(1), 180–188. https://doi.org/10.1016/j.jfma.2020.04.015 [Google Scholar] [PubMed] [CrossRef]
6. Zhao, L. J., Han, B., Zhang, J. J., Yi, Y. C., Jiang, D. D. et al. (2018). Transcatheter closure of congenital perimembranous ventricular septal defect using the Amplatzer duct occluder 2. Cardiology in the Young, 28(3), 447–453. https://doi.org/10.1017/S1047951117002396 [Google Scholar] [PubMed] [CrossRef]
7. Lin, M. T., Chen, C. A., Hsu, J. Y., Lin, H. C., Chiu, S. N. et al. (2017). Transcatheter closure of perimembranous ventricular septal defects with Amplatzer Duct Occluders. JACC-Cardiovascular Interventions, 10(21), 2227–2228. https://doi.org/10.1016/j.jcin.2017.08.021 [Google Scholar] [PubMed] [CrossRef]
8. Esmaeili, A., Behnke-Hall, K., Schrewe, R., Schranz, D. (2019). Percutaneous closure of perimembranous ventricular septal defects utilizing almost ideal Amplatzer Duct Occluder II: Why limitation in sizes? Congenital Heart Disease, 14(3), 389–395. https://doi.org/10.1111/chd.12731 [Google Scholar] [PubMed] [CrossRef]
9. Ebeid, M. R., Batlivala, S. P., Salazar, J. D., Eddine, A. C., Aggarwal, A. et al. (2016). Percutaneous closure of perimembranous ventricular septal defects using the second-generation Amplatzer Vascular Occluders. The American Journal of Cardiology, 117(1), 127–130. https://doi.org/10.1016/j.amjcard.2015.10.010 [Google Scholar] [PubMed] [CrossRef]
10. Yang, J., Yang, L., Yu, S., Liu, J., Zuo, J. et al. (2014). Transcatheter versus surgical closure of perimembranous ventricular septal defects in children: A randomized controlled trial. Journal of the American College of Cardiology, 63(12), 1159–1168. https://doi.org/10.1016/j.jacc.2014.01.008 [Google Scholar] [PubMed] [CrossRef]
11. Santhanam, H., Yang, L., Chen, Z., Tai, B. C., Rajgor, D. D. et al. (2018). A meta-analysis of transcatheter device closure of perimembranous ventricular septal defect. International Journal of Cardiology, 254, 75–83. https://doi.org/10.1016/j.ijcard.2017.12.011 [Google Scholar] [PubMed] [CrossRef]
12. Shrestha, M., Promphan, W., Layangool, T., Roymanee, S., Wongwaitaweewong, K. et al. (2019). Feasibility and 1-year outcome of transcatheter closure of perimembranous ventricular septal defects with different devices. Catheterization and Cardiovascular Interventions, 93(1), E30–E37. https://doi.org/10.1002/ccd.27851 [Google Scholar] [PubMed] [CrossRef]
13. Narin, N., Baykan, A., Pamukcu, O., Argun, M., Ozyurt, A. et al. (2015). ADO II in percutaneous VSD closure in pediatric patients. Journal of Interventional Cardiology, 28(5), 479–484. https://doi.org/10.1111/joic.12222 [Google Scholar] [PubMed] [CrossRef]
14. Haddad, R. N., Daou, L., Saliba, Z. (2019). Device closure of perimembranous ventricular septal defect: Choosing between Amplatzer Occluders. Frontiers in Pediatrics, 7300. https://doi.org/10.3389/fped.2019.00300 [Google Scholar] [PubMed] [CrossRef]
15. Koneti, N. R., Penumatsa, R. R., Kanchi, V., Arramraj, S. K., S, J. et al. (2011). Retrograde transcatheter closure of ventricular septal defects in children using the Amplatzer Duct Occluder II. Catheterization and Cardiovascular Interventions, 77(2), 252–259. https://doi.org/10.1002/ccd.22675 [Google Scholar] [PubMed] [CrossRef]
16. Soufflet, V., van de Bruaene, A., Troost, E., Gewillig, M., Moons, P. et al. (2010). Behavior of unrepaired perimembranous ventricular septal defect in young adults. The American Journal of Cardiology, 105(3), 404–407. https://doi.org/10.1016/j.amjcard.2009.09.047 [Google Scholar] [PubMed] [CrossRef]
17. Wongwaitaweewong, K., Promphan, W., Roymanee, S., Prachasilchai, P. (2020). Effect of transcatheter closure by Amplatzer(TM) Duct Occluder II in patients with small ventricular septal defect. Cardiovascular Intervention and Therapeutics, 36(3), 375–383. https://doi.org/10.1007/s12928-020-00677-z [Google Scholar] [PubMed] [CrossRef]
18. Kanaan, M., Ewert, P., Berger, F., Assa, S., Schubert, S. (2015). Follow-up of patients with interventional closure of ventricular septal defects with Amplatzer Duct Occluder II. Pediatric Cardiology, 36(2), 379–385. https://doi.org/10.1007/s00246-014-1017-0 [Google Scholar] [PubMed] [CrossRef]
19. Zhou, D., Pan, W., Guan, L., Ge, J. (2012). Transcatheter closure of perimembranous and intracristal ventricular septal defects with the SHSMA occluder. Catheterization and Cardiovascular Interventions, 79(4), 666–674. https://doi.org/10.1002/ccd.23344 [Google Scholar] [PubMed] [CrossRef]
20. Li, Y., Zhou, K., Hua, Y. (2017). Whether heart blocks post perimembranous ventricular septal defect device closure remain threatening: How could Chinese experiences impact the world? Journal Evidence-Based Medicine, 10(1), 5–10. https://doi.org/10.1111/jebm.12214 [Google Scholar] [PubMed] [CrossRef]
21. Polat, T. B., Turkmen, E. (2016). Transcatheter closure of ventricular septal defects using the Amplatzer Duct Occluder II device: A single-center experience. Advances in Interventional Cardiology, 12(4), 340–347. https://doi.org/10.5114/aic.2016.63635 [Google Scholar] [PubMed] [CrossRef]
22. Ghaderian, M., Merajie, M., Mortezaeian, H., Aarabi, M., Mohammad, Y. et al. (2015). Efficacy and safety of using Amplatzer Ductal Occluder for transcatheter closure of perimembranous ventricular septal defect in pediatrics. Iranian Journal of Pediatrics, 25(2), e386. https://doi.org/10.5812/ijp.386 [Google Scholar] [PubMed] [CrossRef]
23. Sun, H., Luo, G., Du, Z., Ji, Z., Pan, S. (2021). Transcatheter closure of perimembranous ventricular septal defect using the Amplatzer Duct Occluder II. Congenital Heart Disease, 16(2), 151–157. https://doi.org/10.32604/CHD.2021.014770 [Google Scholar] [CrossRef]
24. Ghosh, S., Sridhar, A., Sivaprakasam, M. (2018). Complete heart block following transcatheter closure of perimembranous VSD using Amplatzer Duct Occluder II. Catheterization and Cardiovascular Interventions, 92(5), 921–924. https://doi.org/10.1002/ccd.27177 [Google Scholar] [PubMed] [CrossRef]
25. Yang, L., Tai, B. C., Khin, L. W., Quek, S. C. (2014). A systematic review on the efficacy and safety of transcatheter device closure of ventricular septal defects (VSD). Journal of Interventional Cardiology, 27(3), 260–272. https://doi.org/10.1111/joic.12121 [Google Scholar] [PubMed] [CrossRef]
26. Chen, F., Li, P., Liu, S., Du, H., Zhang, B. et al. (2015). Transcatheter closure of intracristal ventricular septal defect with mild aortic cusp prolapse using zero eccentricity ventricular septal defect occluder. Circulation Journal, 79(10), 2162–2168. https://doi.org/10.1253/circj.CJ-15-0301 [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools