 Open Access
Open Access
ARTICLE
Outcomes of Self-Expanding Transcatheter Pulmonary Valves: Extended Follow-Up of a Prospective Trial
1 Department of Structural Heart Disease, National Center for Cardiovascular Disease, China & Fuwai Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
2 Department of Structural Heart Disease, Central China Fuwai Hospital, Zhengzhou, China
3 Yunnan Fuwai Cardiovascular Hospital, Kunming, China
4 Department of Radiology, National Center for Cardiovascular Disease, China & Fuwai Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
* Corresponding Author: Gejun Zhang. Email:
Congenital Heart Disease 2023, 18(2), 219-234. https://doi.org/10.32604/chd.2023.027562
Received 07 November 2022; Accepted 09 January 2023; Issue published 15 March 2023
Abstract
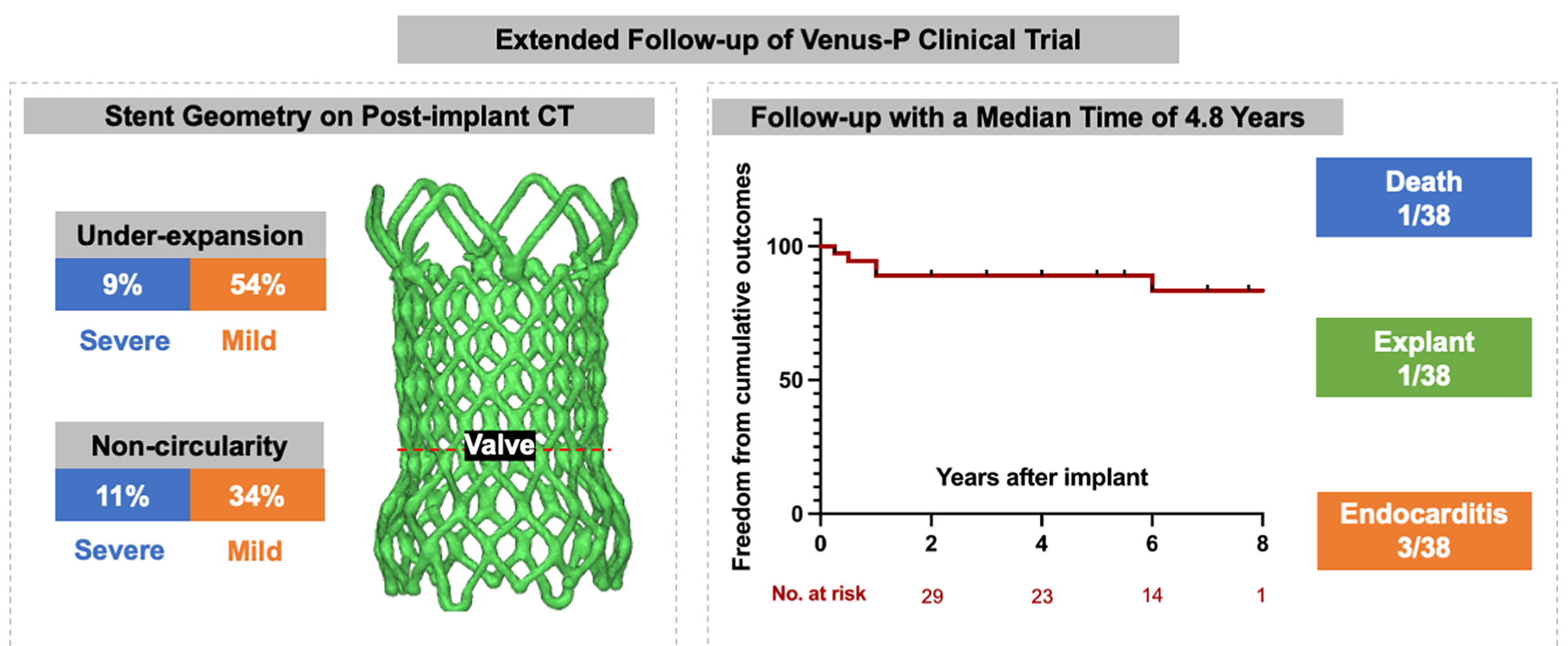
Background: The Venus-P valve was the first self-expanding valve used world-wide for transcatheter pulmonary valve replacement (TPVR) in patients with severe pulmonary regurgitation (PR). We intended to report the extended follow-up results from the prospective trial (No. NCT02590679). Methods: A total of 38 patients with severe PR (mean age 24.2 ± 13.2) were included. Follow-up data were obtained after implanted at 1, 6, and 12 months and yearly after. The frame geometry was assessed on post-implant computer tomography (CT) scanning by calculating the non-circularity [circularity ratio (minimum diameter/maximum diameter) < 0.9] and under-expansion [expansion ratio (derived external valve area/nominal external valve area) < 0.9). Adverse events (all-cause mortality, reintervention, valve dysfunction, stent fracture and endocarditis) were recorded. Results: All valves were implanted successfully with normal function at discharge. Geometric CT analysis showed under-expanded valve was detected in 22 patients (63%) and non-circular valve was seen in 16 patients (46%). During a median follow-up of 4.8 years (range 0.3–8.1), there were 1 death and 1 surgical explant, both resulting from endocarditis. Five-year freedom from valve dysfunction and stent fracture were 84.8% (95%CI 74.8–94.7) and 83.5% (95%CI 73.8–93.2). Endocarditis occurred in 3 patients at a median time of 7 months. Stent fracture was more common in patients with non-circularity stents. Conclusion: TPVR using Venus-P valve is associated with favorable outcomes at 5 years. Non-circular shapes in the valve level may have a higher risk of stent fracture.Graphic Abstract
Keywords
Abbreviations
| TOF | Tetralogy of Fallot |
| CT | Computer tomography |
| TPVR | Transcatheter pulmonary valve replacement |
| RVOT | Right ventricular outflow tract |
| MPA | Main pulmonary artery |
| TPV | Transcatheter pulmonary valve |
| CR | Circularity ratio |
| ER | Expansion ratio |
| IE | Infectious endocarditis |
| RVEDVI | Right ventricular end-diastolic volume index |
| RVEDD | Right ventricular end-diastolic diameter |
| RVEF | Right ventricular ejection fracture |
Transcatheter pulmonary valve replacement (TPVR) as a conceptualized procedure firstly reported by Bonhoeffer et al. in 2000 [1], is a less invasive and an alternative approach for surgical repair in those having cyanotic congenital heart diseases [2–4] with right ventricular outflow tract (RVOT, stenosis and/or regurgitation) dysfunction. Most of the transcatheter valves were balloon-expandable systems, but they are limited to relatively small size for a dilated RVOT [5]. Therefore, a self-expanding transcatheter pulmonary valve (TPV), namely Venus-P valve (Venus Medtech Inc., Hangzhou, China), was designed and short-term outcomes have demonstrated its safety and efficacy in the prospective trial of Venus-P valve [6–8]. However, lack of the longer follow-up results leads to concerns about the valve durability. Stent deformation of the self-expanding TPV implanted in the native RVOTs has not been reported yet but worth to report, since unfavorable stent geometry of implanted valves is associated with a series of adverse events, including elevated gradients and subclinical leaflet thrombosis [9,10], in both aortic and pulmonary position. As a result, we particularly assessed the valve eccentricity and expansion rate for a comprehensive understanding of the implanted TPV geometry. Therefore, intentions of the current study were to: (1) investigate the clinical outcomes of Venus-P valves; (2) evaluate the stent geometry of implanted Venus-P valve by calculating the circularity and expansion rate on CT scanning.
2.1 Patients and Study Protocol
The prospective trial with Venus-P valve (No. NCT02590679) was a non-randomized study that enrolled patients with severe pulmonary regurgitation after surgical repair of congenital right ventricular outflow tract obstruction with trans-annulus or RVOT patch at 5 centers in China. Patients with pulmonary stenosis were excluded. Details of the trial design and protocols were provided in the supplemental method. Procedural and short-term results were reported previously [7,8]. The initial time frame of follow-up was 1, 3, 6, and 12 months after the procedure. An extended follow-up study was conducted in our center. Thirty-eight patients were included and provided written informed consent for annually follow-up and analysis of their CT imaging data (Fig. 1). The ethics committee of our institution approved the study protocol.
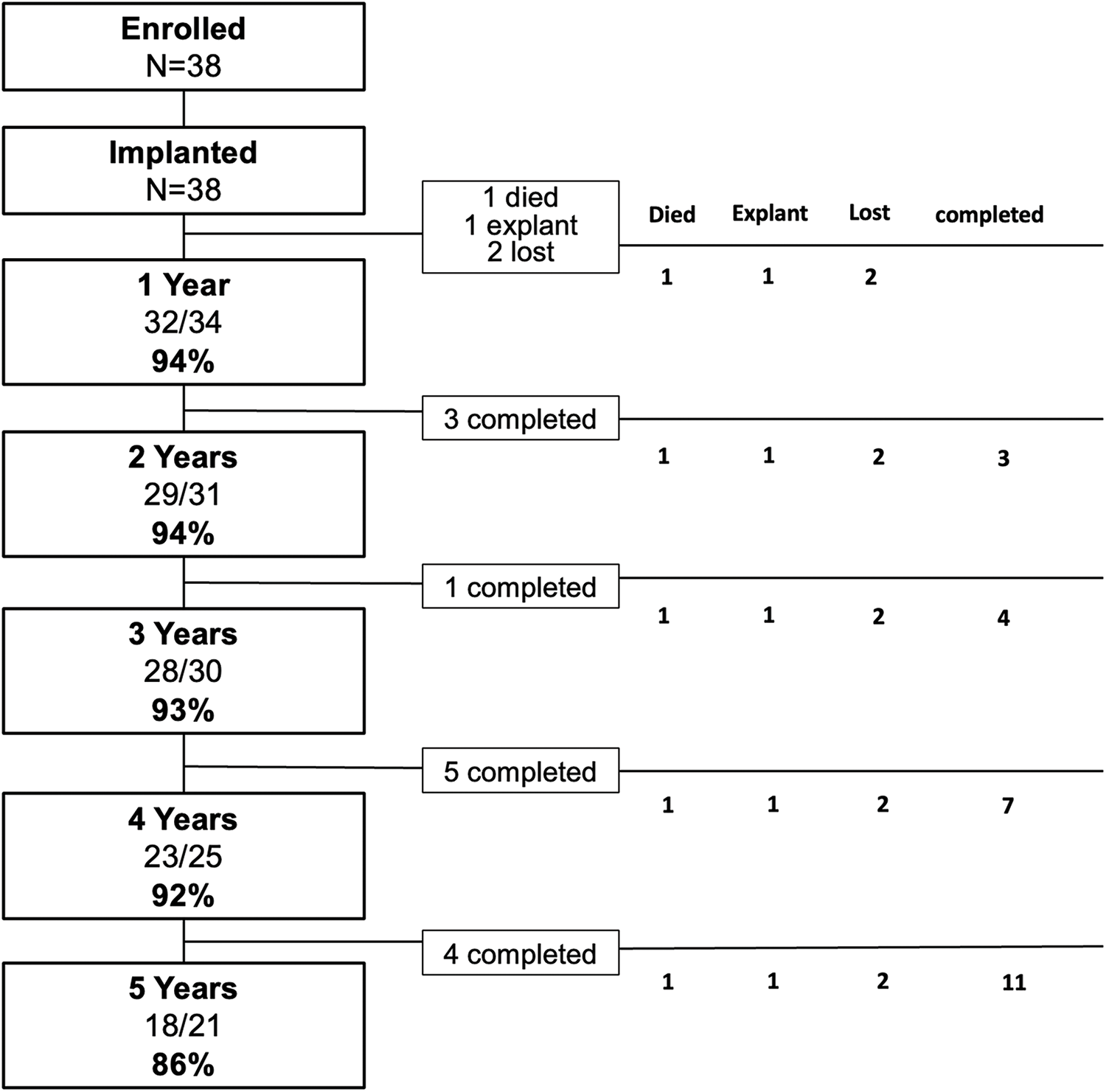
Figure 1: Patient flow. The initial time frame of follow-up was 1, 3, 6 and 12 months in the trial. The extended follow-up was performed annually. The column in the left had listed the percentage of patients with normal valve function among all active patients. Those who did not consent to longer-term follow-up, exited the study at that time were noted as “completed”. There were 1 died, 1 underwent surgical explant and 2 lost from the study at 12 months after the procedure
According to the trial protocol, follow-up was performed together with routine transthoracic echocardiography, electrocardiograph (ECG) and chest radiography at post-implant, 1 month, 3 months, 6 months, 1 year. The routine tests were also conducted in the additional follow-up study.
A 12-lead ECG was used to detect the new-onset arrhythmia, which was defined as any atrial or ventricular arrhythmia documented on 12-road ECG during routine follow-up. Only the first event was counted. Transthoracic echocardiography (TTE) was performed with commercially available machines to assess the hemodynamic performance of implanted bioprosthesis. Images of the RVOT were obtained from the parasternal long-and short-axis views. Pulmonary regurgitation (PR) was assessed with color Doppler grading as none, trace/trivial, mild, moderate, or severe. Continuous wave and color Doppler were used to measure transvalvular velocities and gradients across the RVOT. Tricuspid regurgitation (TR), assessing in the four-chamber view, was graded as none, trace/trivial, mild, moderate, or severe. Chest radiograph with anteroposterior and lateral views was obtained for the surveillance of stent fracture. Cardiac CT scanning were performed at 3 months after the implantation for the evaluation of stent shape and further geometric analysis. Cardiac magnetic resonance (CMR) was performed at 6 months after the implantation for assessment of changes in right ventricular ejection fracture (RVEF), right ventricular end-diastolic volume index (RVEDVI) and right ventricular end-diastolic diameter (RVEDD).
Adverse events including all-cause mortality, TPV reintervention, valve dysfunction, stent fracture and endocarditis were recorded. TPV reintervention was defined as any catheter or surgical procedures performed on the implanted TPVs. Valve dysfunction was defined as an increase in pulmonary mean gradient over 10 mmHg compared with baseline or more-than-moderate regurgitation evaluated by echocardiography. Stent fracture was diagnosed by chest X-ray and classified into 3 types [11]. Type I, one strut fracture without loss of stent integrity. Type II, fracture with loss of stent integrity. Type III, fracture associated with the separation of fragments or embolization. Endocarditis was diagnosed according to the modified Duke criteria.
CT was performed on a 64-slice scanner, Discovery HD 750 (GE Healthcare, Waukesha, Wisconsin). A contrast-enhanced protocol with 80 to 120 ml of iodixanol at 5 ml/s followed by 30 ml of normal saline. The detector collimation width was 0.625 mm. Detector coverage was 40 mm with 1.25 mm section reconstruction and the slice interval was 1.25 mm. Phase and angle-dependent dose modulation was used with titration of radiation dose. Images were reconstructed at 0.6 mm slices with 0.3 mm overlap and iterative reconstruction for evaluation at 10% intervals within the 0%–90% RR range. For optimal image quality, measurements were taken in diastole at 75% of the R-R interval in the local reconstruction software (AW server 2.0, GE Healthcare, Waukesha, Wisconsin).
Deformation was analyzed according to 2 geometrical aspects, stent expansion and circularity. The circularity ratio (CR), and expansion ratio (ER) were calculated [10] at the valve level where the three leaflets coaptate and one third of the stent struts are allocated to each of the three leaflets. CR was defined as the ratio of minimum to maximum external stent diameter (Fig. 2). The circularity ratio of <0.9 was considered as non-circular TPV. TPV expansion rate was the ratio of CT-derived external valve area to the nominal external valve area, and <0.9 was labeled as an under-expansion valve. Under-expansion or non-circularity was considered as severe if the ER or CR was under 0.8, and mild if the ER or CR was between 0.8 and 0.9. Geometrical details were analyzed with repeated measurements and the agreement for the deformed TPV was satisfying (Pearson’s correlation coefficient > 0.9).
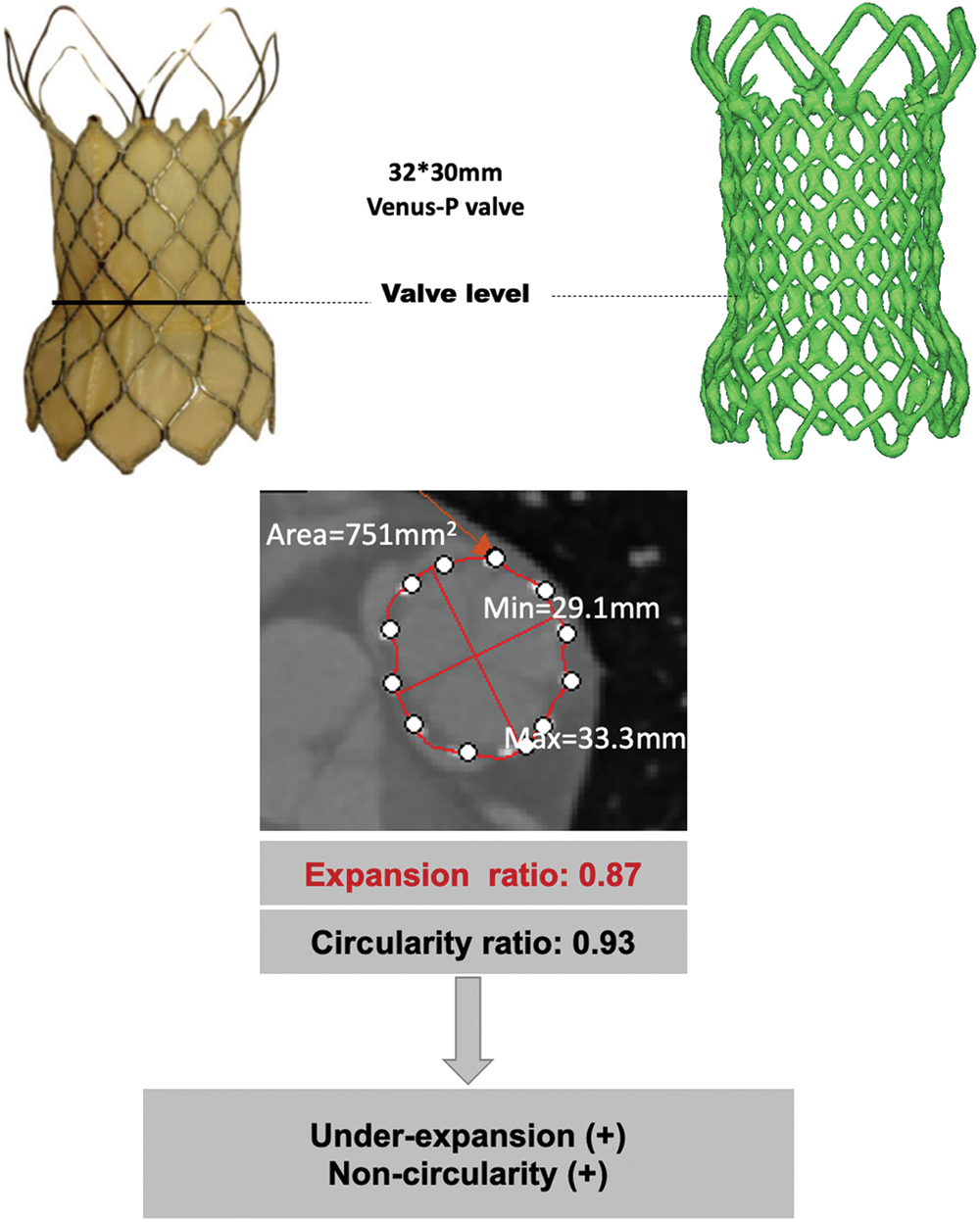
Figure 2: Geometrical analysis of post-implant CT. The minimum external stent diameter, maximum external stent diameter and external valve area were obtained for calculation of CR and ER. ER, expansion rate; CR, circularity rate
Continuous variables were presented as mean and standard deviation, and categorical parameters were presented as the frequency with percentages as appropriate. Clinical and imaging variables were compared using student-t-test. Categorical variables were compared using the χ² test. Freedom-from-event estimates and curves were from the Kaplan-Meier method and presented as rate and 95% confidential interval. A p value < 0.05 was considered statistically significant. All calculations were performed using SPSS software (SPSS version 22; IBM Corp., Armonk, NY, USA).
3.1 Procedural and Short-Term Outcomes
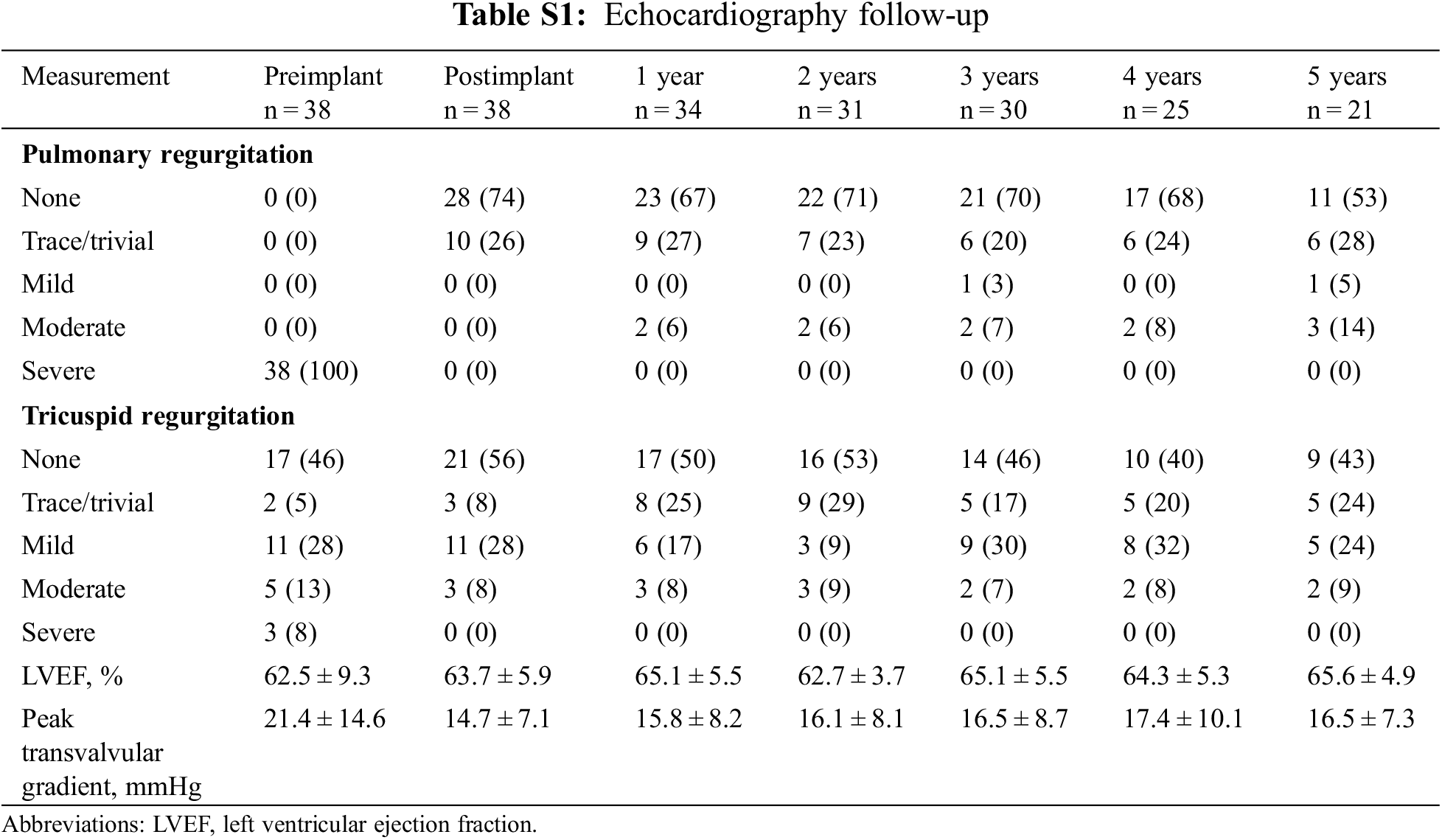
Table 1 lists the demographic data of the cohort. The mean age at time of valve deployment was 24.5 ± 12.8 years and 2 patients were over 50 years of age. Most patients (92%) were diagnosed with TOF and had patch repaired RVOTs. All patients were successfully implanted with Venus-P valves in the native RVOT and were discharged with the valve in the desired location. There was no procedural complication occurred. Normal pulmonary valve function was achieved in all patients. More-than-moderate TR was present in 8 patients at baseline and 3 after the implantation (21.1% vs. 7.9%, p < 0.05) (Fig. 3). There was no significant difference in transvalvular gradient before and after the procedure. New York Heart Association Class I or II at baseline accounted for 21 patients and 37 after implantation (55.3% vs. 97.4%, p < 0.05). Favorable changes of right heart overload were demonstrated by CMR in 25 patients (Supplemental Figure S1), with decreases in RVEDVI and RVEDD, from 146.9 to 101.9 ml/m2, and 40.9 to 34.1 mm, respectively (p < 0.05). No significant difference was seen in RVEF.
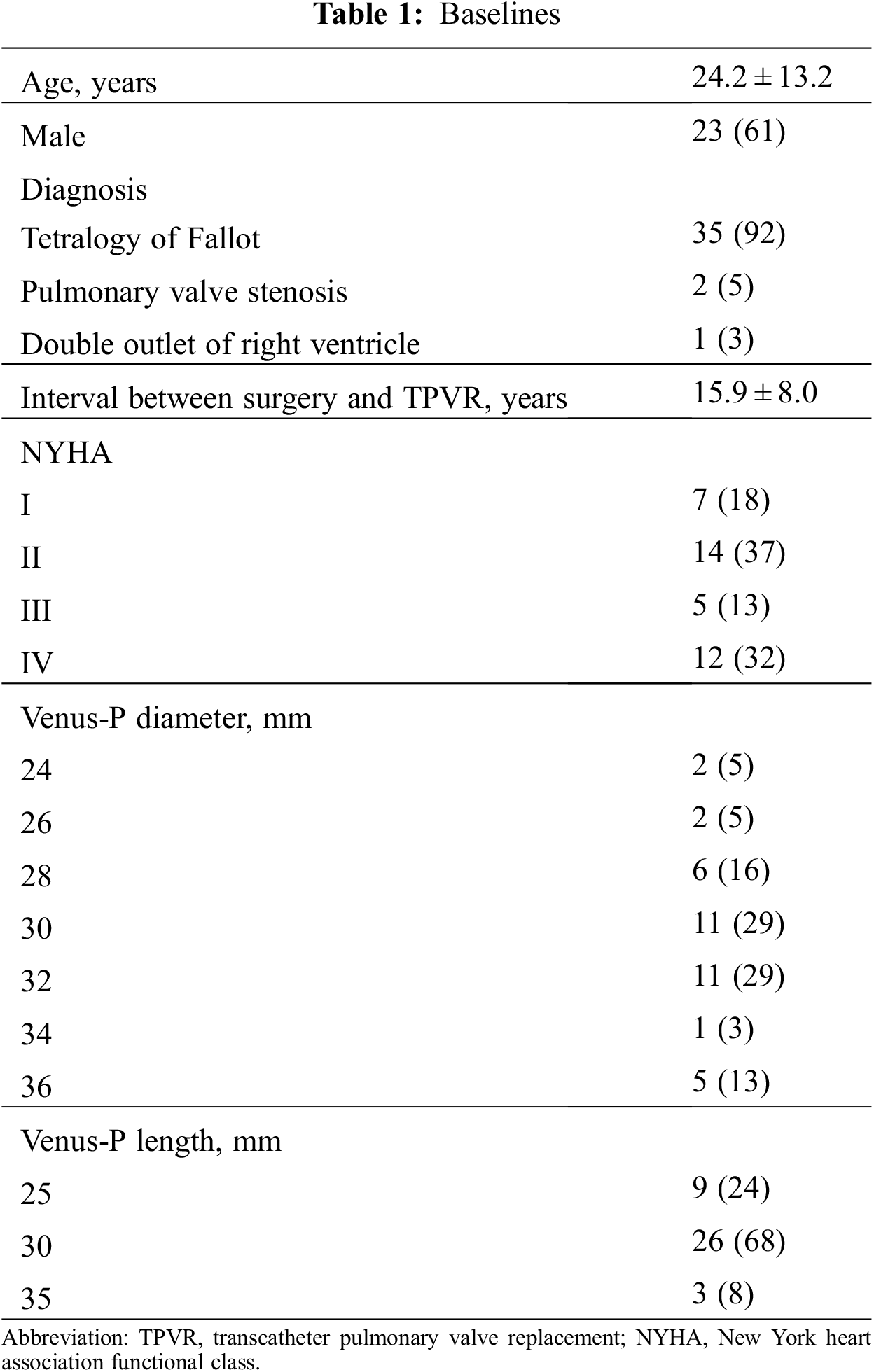
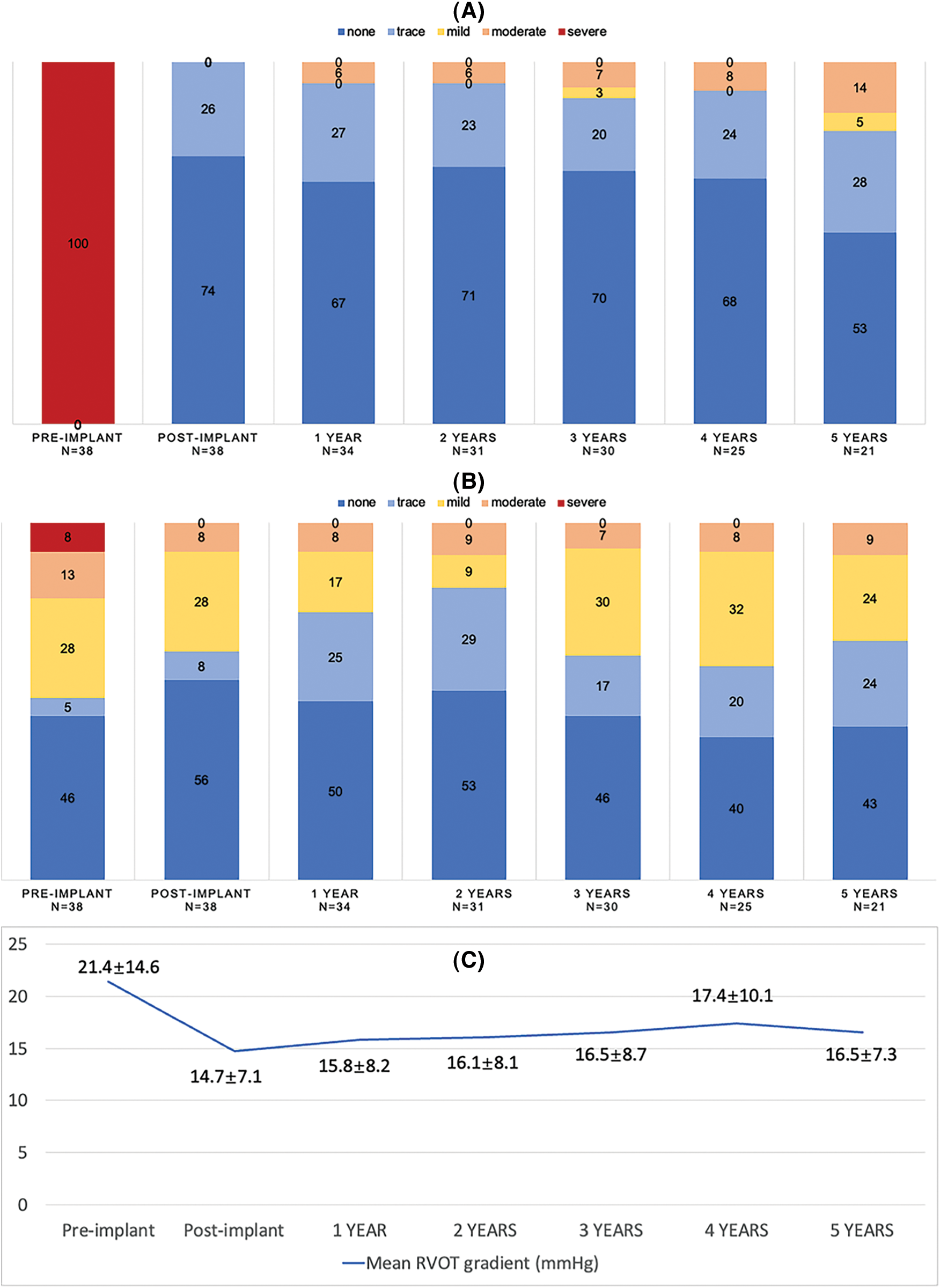
Figure 3: Echocardiography outcomes. (A) Pulmonary regurgitation; (B) Tricuspid regurgitation; (C) Transvalvular gradient. Abbreviations: RVOT, right ventricular outflow tract
Detail CT analysis were performed in 35 patients except for 1 death and 2 lost from follow-up at 3 months. Under-expansion valve was detected in 22 patients (63%) and non-circular stent shape was found in 16 patients (46%). Severe under-expansion or non-circularity (ER or CR < 0.8) was detected in 3 and 4 patients, respectively. Characteristics between valves with normal shapes and non-circular/under-expanded shapes were compared in the Table 2. Under-expansion is more common in patients implanted with larger valves (>30 mm) (p = 0.04).
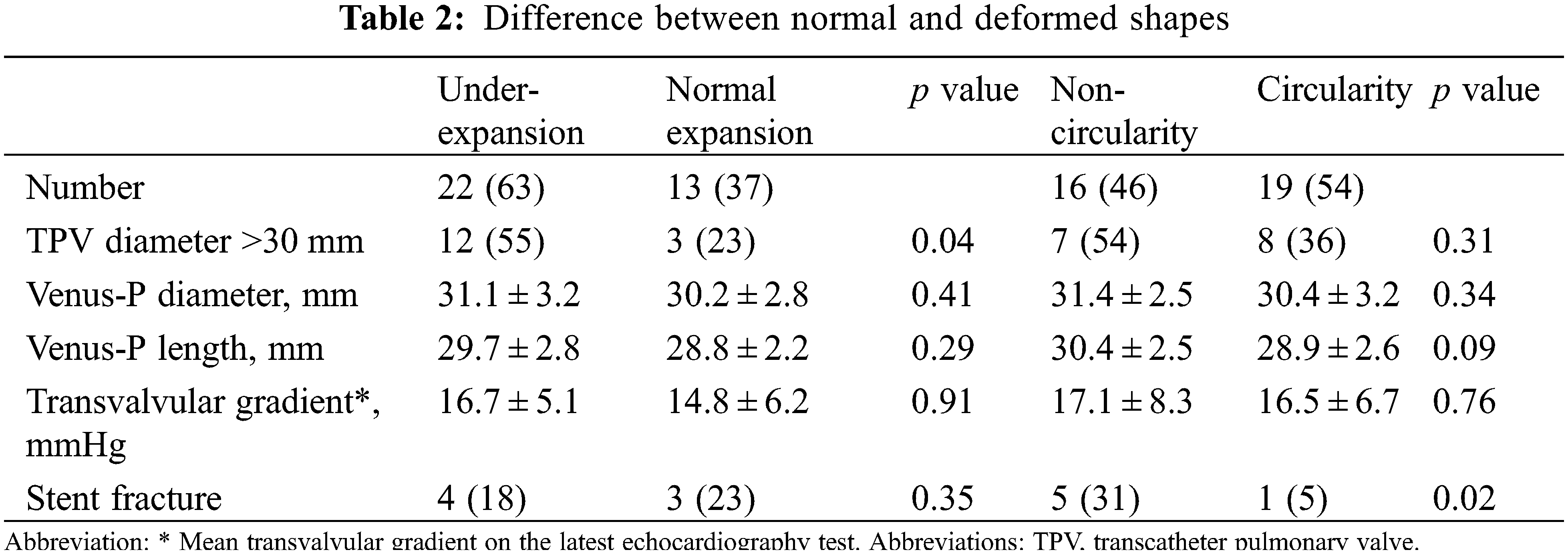
3.2 Death, Reintervention, and Valve Dysfunction
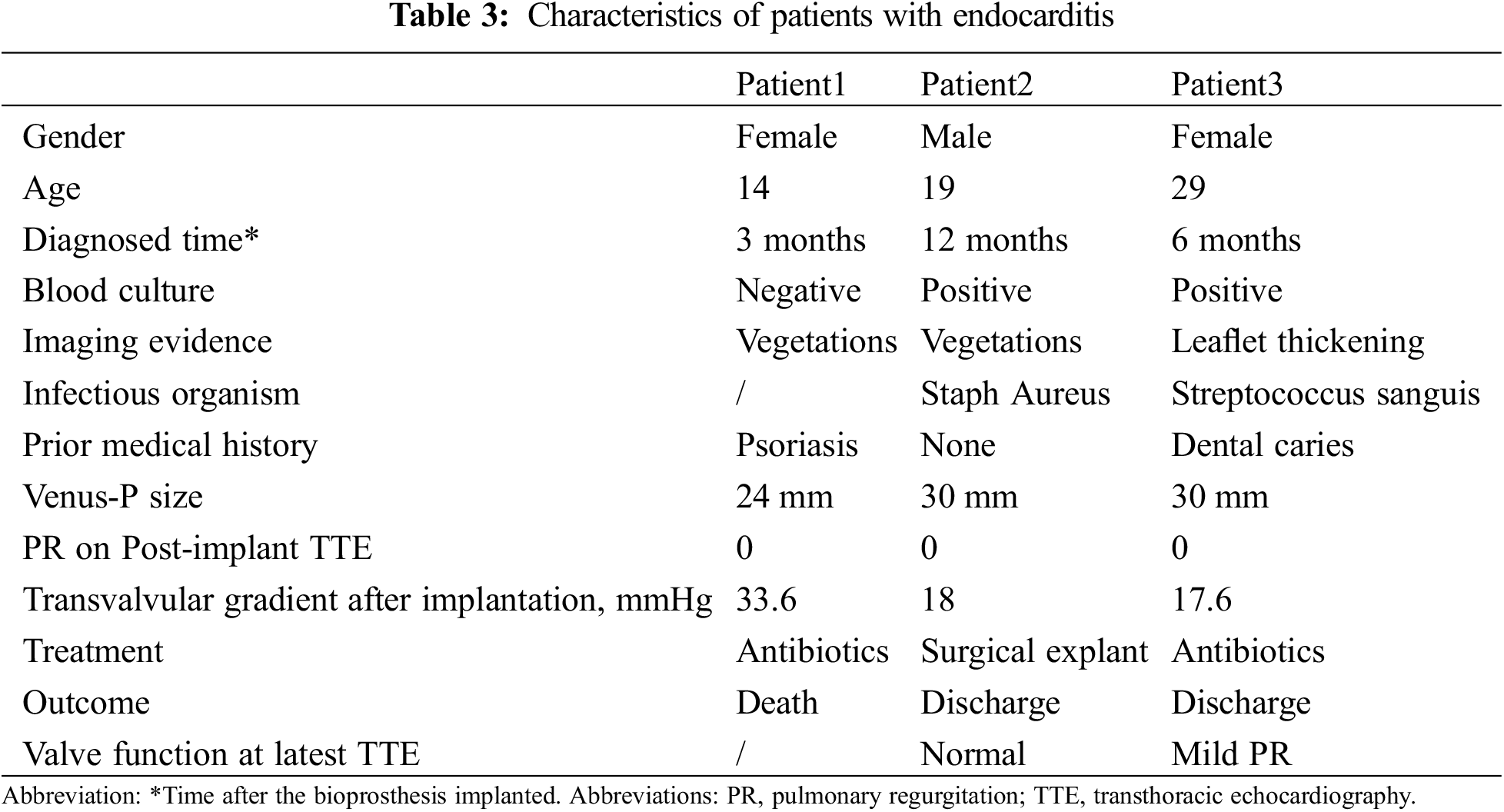
During a median follow-up of 4.8 years (range 0.3–8.1), 1 patient died of the multiorgan failure resulting from endocarditis at 3 months, who was a 14-year female with a history of psoriasis. One patient underwent the surgical explant at 1 year for the vegetations grown around the TPV and the histology demonstrated the diagnosis of endocarditis. Recurrence of significant PR occurred in 3 patients at a mean time of 26 months. All those 3 patients were classified as more-than-moderate PR on echocardiography and recommend to the close visit without any intervention. Five-year freedom from valve dysfunction was 89.3% (95%CI 81.1–97.6) (Fig. 4). Endocarditis occurred in 3 patients at a mean time of 7 months (Table 3). Except the 2 endocarditis cases mentioned above, the third patient was admitted to the hospital at 6 months because of a low-grade fever which brought down after 3-week antibiotic treatment.
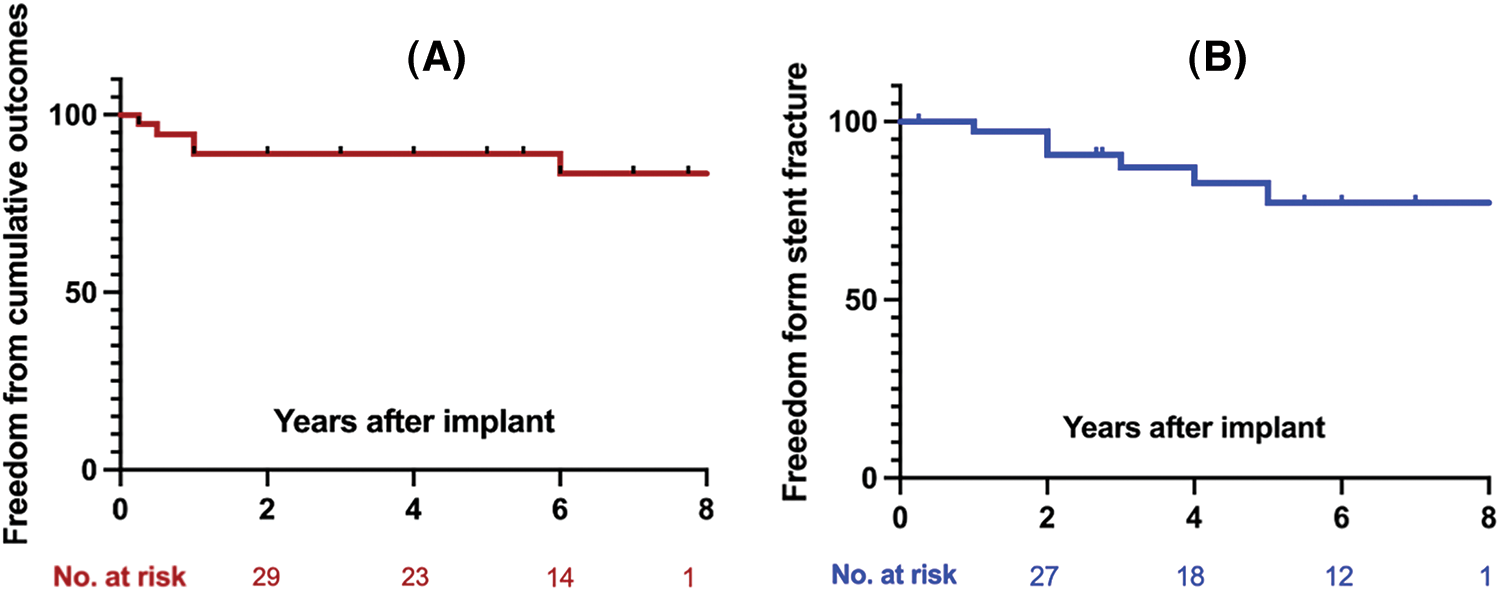
Figure 4: (A) Kaplan-Meier curves depicted the freedom from valve dysfunction; (B) Kaplan-Meier curves depicted the freedom from any stent fracture (SF)
Stent fracture was diagnosed in 7 patients at a median time of 2 years. All stent fracture cases were categorized in to type I according to the risk stratification, while the type II/III SF were absent. Freedom from any SF at 5-year was 83.5% (95%CI 73.8–93.2) (Fig. 4). SFs mainly occurred at the RVOT flare in 6 patients and 1 was detected at both the RVOT flare and the distal pulmonary flare. All SFs at RVOT flare were adjacent to the sternum (Fig. 5). Patients with non-circular valves showed a higher rate of stent fracture (31% vs. 5%, p = 0.02), while the prevalence of stent fracture was similar between groups with or without valve under-expansion (18% vs. 23%, p = 0.35).

Figure 5: Stent fracture in the proximal flare (type I)
Frequent ventricular premature beats occurred in 1 patient after implanted and disappeared spontaneously 1 week later. No extra arrhythmia or sudden heart death was seen during follow-up. One patient with pre-existing atrial fibrillation underwent went transcatheter ablation at 3 years after TPV implantation.
Although this study provides important new data, it nevertheless suffers from important limitations. This is a small study with limited numbers followed to 5 years and serial CT and CMR scans were not obtained throughout the follow-up period to better understand the development of frame changes of the Venus-P valve as well as the right ventricular remodeling. It is also not clear if the Venus-P valve also performs well in patients with elevated gradients in RVOT who were initially excluded from the trial.
This report updates the favorable outcomes of Venus-P valve through an extended follow-up of the prospective clinical trial. It is the first study to detail the geometrical information of the implanted Venus-P valves in the native RVOT. The normal valve function was maintained in valves with non-circularity and under-expansion.
The clinical results of Venus-P valve are comparable with either balloon expanding TPVR or surgical pulmonary valve replacement for treating the similar patient population [12]. As to other self-expanding TPVs, the Harmony valve (Medtronic, Minneapolis, Minnesota) was recently approved for commercial use by FDA and the 5-year outcomes showed normal valve function maintained in 75% of the patients (15/20) [13,14]. However, the availability of only 2 valve sizes (22 and 25 mm) at present may limit its application to patients with larger RVOT size. The Alterra adaptive pre-stent (Edwards Lifesciences, Irvine, California) was a self-expanding stent designed for the implantation of 29 mm Sapien3 (Edwards Lifesciences) valve in the large RVOT. The extended design was able to accommodate a RVOT diameter up to 40 mm [15]. Six-month follow-up demonstrated its safety and favorable hemodynamic performance [16]. There was 1 patient with new-onset ventricular premature after valve implanted, which was disappeared into 1 week without any intervention. Deice-related arrhythmia was also reported in Harmony valve and Alterra adaptive pre-stent both with a high rate of 30% [15,16], which was also self-limited without any further intervention reported. It seems the extended design of self-expanding TPV that led to the excessive tissue-stent interaction in RVOT, and therefore cause arrhythmia. Assessing the potential risk of device-related arrhythmia in self-expanding TPVs requires more specific study design with larger sample size.
We systematically depicted the self-expanding TPV deformation from the aspects of expansion and circularity. The valve function functioned normal in patients with stent deformation during a median of 4.8-year follow-up. Overall, Venus-P valve demonstrated a favorable stent integrity with mild deformation in most of patients. According to the Muller’s study that evaluated the geometry of sapien valve in pulmonary position [9], a lower value of ER (0.7) was detected in patients with reintervention comparing with the non-reintervention group (0.9). In a vitro experiment, eccentric and incomplete stent deployment of transcatheter valve altered the leaflet stress distribution and leaflet kinematic [17]. Impaired leaflet kinematics increased the blood stasis and consequently led to leaflet thrombosis and valve degeneration [17,18]. The sample size in our study is not enough to analysis the effect non-circularity and under-expansion can make on outcomes. However, for the exploratory purposes, this result may help to expand the application of self-expanding TPVs to more variate anatomy-types of the right ventricular outflow tract.
Recent multicenter registry reports suggested younger age, higher residual gradient, and previous IE history were all predictors for TPVR-IE and more attention should be paid during and after the intervention in patients at risks [19]. Some studies suggest that lifelong antibiotic prophylaxis may be appropriate in PVR recipients despite the lack of sufficient evidence.
Non-circularity is associated shorter freedom from type I stent fracture. Considering the position of SF, we consider that the non-circularity is a result of compression at pulmonary conus and subsequently causing stent fracture. Stent fracture is common in Melody valves, especially those implanted without pre-stent, and was the main cause of reintervention after TPVR [20]. But it was no associated with valve dysfunction in this self-expandable cohort. However, since patients with RVOT stenosis were excluded from this study, the counterpart data is scanty.
TPVR using Venus-P valve is associated with favorable long-term outcomes. Non-circular shapes in the valve level may have a higher risk of stent fracture. However, longer follow-up is still needed.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: All listed authors have contributed to the manuscript and have approved the final submitted version. GJZ and XBP were responsible for the design of the study. JNZ wrote the first draft of the report. JNZ, JYW, YHL, YH, FF, JHP, SLJ were responsible for the acquisition of data. JNZ did the analysis and interpreted the results in collaboration with JYW. All authors critically revised the report and approved the final version.
Availability of Data and Materials: This statement should make clear how readers can access the data used in the study and explain why any unavailable data cannot be released.
Ethics Approval: This study involves human participants and was approved by Ethic Committee of Fuwai Hospital. Medical devices were also allowed to be used under compassionate grounds after passing the test of National Medical Products Administration and the permission of the Ethics Committee of Fuwai Hospital. The study was conducted in compliance with the Declaration of Helsinki. Participants gave informed consent to participate in the study before taking part.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Bonhoeffer, P., Boudjemline, Y., Saliba, Z., Merckx, J., Aggoun, Y. et al. (2000). Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet, 356(9239), 1403–14055. https://doi.org/10.1016/s0140-6736(00)02844-0 [Google Scholar] [PubMed] [CrossRef]
2. Apitz, C., Webb, G. D., Redington, A. N. (2009). Tetralogy of Fallot. Lancet, 374(9699), 1462–1471. https://doi.org/10.1016/s0140-6736(09)60657-7 [Google Scholar] [PubMed] [CrossRef]
3. Baumgartner, H., de Backer, J., Babu-Narayan, S. V., Budts, W., Chessa, M. et al. (2021). 2020 ESC guidelines for the management of adult congenital heart disease. European Heart Journal, 42(6), 563–645. https://doi.org/10.1093/eurheartj/ehaa554 [Google Scholar] [PubMed] [CrossRef]
4. Villafañe, J., Feinstein, J. A., Jenkins, K. J., Vincent, R. N., Walsh, E. P. et al. (2013). Hot topics in tetralogy of Fallot. Journal of the American College of Cardiology, 62(23), 2155–2166. https://doi.org/10.1016/j.jacc.2013.07.100 [Google Scholar] [PubMed] [CrossRef]
5. Schievano, S., Coats, L., Migliavacca, F., Norman, W., Frigiola, A. et al. (2007). Variations in right ventricular outflow tract morphology following repair of congenital heart disease: Implications for percutaneous pulmonary valve implantation. Journal of Cardiovascular Magnetic Resonance, 9(4), 687–695. https://doi.org/10.1080/10976640601187596 [Google Scholar] [PubMed] [CrossRef]
6. Husain, J., Praichasilchai, P., Gilbert, Y., Qureshi, S. A., Morgan, G. J. (2016). Early european experience with the Venus P-valve®: Filling the gap in percutaneous pulmonary valve implantation. EuroIntervention, 12(5), e643–e651. https://doi.org/10.4244/eijv12i5a105 [Google Scholar] [PubMed] [CrossRef]
7. Morgan, G., Prachasilchai, P., Promphan, W., Rosenthal, E., Sivakumar, K. et al. (2019). Medium-term results of percutaneous pulmonary valve implantation using the Venus P-valve: International experience. EuroIntervention, 14(13), 1363–1370. https://doi.org/10.4244/eij-d-18-00299 [Google Scholar] [PubMed] [CrossRef]
8. Garay, F., Pan, X., Zhang, Y. J., Wang, C., Springmuller, D. (2017). Early experience with the Venus pvalve for percutaneous pulmonary valve implantation in native outflow tract. Netherlands Heart Journal, 25(2), 76–81. https://doi.org/10.1007/s12471-016-0932-5 [Google Scholar] [PubMed] [CrossRef]
9. Muller, B., Ghawi, H., Heitschmidt, M. G., Fogg, L., Hibbeln, J. et al. (2016). Medium-term CT evaluation of stent geometry, integrity, and valve function of the edwards SAPIEN transcatheter heart valve in the pulmonary position. Catheterization and Cardiovascular Interventions: Official Journal of the Society for Cardiac Angiography & Interventions, 87(3), E97–E103. https://doi.org/10.1002/ccd.26074 [Google Scholar] [PubMed] [CrossRef]
10. Fuchs, A., de Backer, O., Brooks, M., de Knegt, M. C., Bieliauskas, G. et al. (2017). Subclinical leaflet thickening and stent frame geometry in self-expanding transcatheter heart valves. EuroIntervention, 13(9), e1067–e1075. https://doi.org/10.4244/EIJ-D-17-00373 [Google Scholar] [PubMed] [CrossRef]
11. Nordmeyer, J., Khambadkone, S., Coats, L., Schievano, S., Lurz, P. et al. (2007). Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation, 115(11), 1392–1397. https://doi.org/10.1161/circulationaha.106.674259 [Google Scholar] [PubMed] [CrossRef]
12. Buber, J., Assenza, G. E., Huang, A., Valente, A. M., Emani, S. M. et al. (2014). Durability of large diameter right ventricular outflow tract conduits in adults with congenital heart disease. International Journal of Cardiology, 175(3), 455–463. https://doi.org/10.1016/j.ijcard.2014.06.023 [Google Scholar] [PubMed] [CrossRef]
13. Gillespie, M. J., Bergersen, L., Benson, L. N., Weng, S., Cheatham, J. P. (2021). 5-year outcomes from the harmony native outflow tract early feasibility study. JACC. Cardiovascular Interventions, 14(7), 816–817. https://doi.org/10.1016/j.jcin.2021.01.046 [Google Scholar] [PubMed] [CrossRef]
14. Shahanavaz, S., Balzer, D., Babaliaros, V., Kim, D., Dimas, V. et al. (2020). Alterra adaptive prestent and SAPIEN 3 THV for congenital pulmonic valve dysfunction: An early feasibility study. JACC. Cardiovascular Interventions, 13(21), 2510–2524. https://doi.org/10.1016/j.jcin.2020.06.039 [Google Scholar] [PubMed] [CrossRef]
15. U.S Food & Drug Administration (2021). P2000015/S011: Summary of safety and effectiveness. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma_pas.cfm [Google Scholar]
16. U.S Food & Drug Administration (2022). P200046/PAS001: Summary of safety and effectiveness. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma_pas.cfm [Google Scholar]
17. Sirois, E., Mao, W., Li, K., Calderan, J., Sun, W. (2018). Simulated transcatheter aortic valve flow: Implications of elliptical deployment and under-expansion at the aortic annulus. Artificial Organs, 42(7), E141–E152. https://doi.org/10.1111/aor.13107 [Google Scholar] [PubMed] [CrossRef]
18. Willson, A. B., Webb, J. G., Gurvitch, R., Wood, D. A., Toggweiler, S. et al. (2012). Structural integrity of balloon-expandable stents after transcatheter aortic valve replacement: Assessment by multidetector computed tomography. JACC. Cardiovascular Interventions, 5(5), 525–532. https://doi.org/10.1016/j.jcin.2012.03.007 [Google Scholar] [PubMed] [CrossRef]
19. McElhinney, D. B., Zhang, Y., Aboulhosn, J. A., Morray, B. H., Biernacka, E. K. et al. (2021). Multicenter study of endocarditis after transcatheter pulmonary valve replacement. Journal of the American College of Cardiology, 78(6), 575–589. https://doi.org/10.1016/j.jacc.2021.05.044 [Google Scholar] [PubMed] [CrossRef]
20. Cheatham, J. P., Hellenbrand, W. E., Zahn, E. M., Jones, T. K., Berman, D. P. et al. (2015). Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation, 131(22), 1960–1970. https://doi.org/10.1161/circulationaha.114.013588 [Google Scholar] [PubMed] [CrossRef]
Supplemental Materials
Supplemental method: Study protocol
Brief Summary:
Venus-P valve (Venus Medtech, Hangzhou, China) is the first self-expandable interventional pulmonary valve, which is composed of a Nitinol multilevel support frame with a tri-leaflet porcine pericardial tissue valve, and a 14-22 Fr delivery catheter. The purpose of the multi-center trial is to evaluate the safety and efficacy of the Venus-P valve for percutaneous pulmonary valve implantation (PPVI) in patients with pulmonary regurgitation and native right ventricular outflow tract (RVOT) after surgical repair of RVOT.
Detailed Description:
Clinical experience to date with transcatheter pulmonary valve replacement has been limited to two balloon expandable systems, namely the Melody Valve (Medtronic Inc., Minneapolis, MN) and the Sapien valve (Edwards Lifesciences, Irvine, CA). Both valves have undergone clinical trials with good medium-term valve durability. Although both the Melody and the Sapien valves have been used in native outflow tracts with good success, limitations to the extended application of these valves have generally centered on the maximum diameter of the RVOT. Indeed, in the majority of patients requiring pulmonary valve replacement (PVR), these balloon expandable systems are not large enough to maintain stable valve position within the dilated native RVOT. Therefore, more recent efforts have concentrated on a self-expanding system to provide valve competence despite significant dilation of the native RVOT.
Study Design:
Study Type: Interventional (Clinical Trial)
Estimated Enrollment: 44 participants.
Allocation: N/A
Intervention Model: Single Group Assignment
Masking: None (Open Label)
Primary Purpose: Treatment
Official Title: Multi-Center Trial of Percutaneous Pulmonary Valve Implantation Using Venus-P Valve for Patients with Severe Pulmonary Regurgitation and Native Right Ventricular Outflow Tract After Previous Surgical Repair
Study Start Date: May 2013
Estimated Primary Completion Date: July 2016
Estimated Study Completion Date: February 2017
Outcome Measures:
Primary Outcome Measures:
Right ventricular end diastolic volume index [Time Frame: 6 months]
Secondary Outcome Measures:
1. Incidence of adverse cardiovascular events [Time Frame: 48 h] (death, severe arrhythmia, pericardial tamponade, emergency surgery, RVOT rupture, pulmonary artery perforation, cardiac shock, endocarditis, bleeding, and other complications caused by procedure).
2. Incidence of deaths or strokes [Time Frame: 12 months] All cause deaths (cardiac death, and non-cardiac death) or strokes.
3. Pulmonary pressure gradient [Time Frame: 1, 3, 6, 12 months] Max pressure gradient (PG).
4. Grade of pulmonary regurgitation [Time Frame: 1, 3, 6, 12 months] New York Heart Association (NYHA) class [Time Frame: 1, 3, 6, 12 months], 6 min-walk distance [Time Frame: 1, 3, 6, 12 months].
Contacts and Locations:
Contact: Wenzhi Pan, M.D., peden@sina.com
Location1:
Zhongshan Hopital of Fudan University
Shanghai, China, 200032
Contact: Wenzhi Pan, M.D., peden@sina.com
Location2:
Beijing Fuwai Hospital
Beijing, China
Contact: Gejun Zhang, M.D.
Location3:
Shanghai Chest Hospital
Shanghai, China
Contact: Xin Pan, M.D.
Sponsors and Collaborators:
Shanghai Zhongshan Hospital
Fu Wai Hospital, Beijing, China
Huaxi Hospital
Shanghai Chest Hospital
Shanghai Children’s Medical Center
Investigators:
Study Chair: Junbo Ge, M.D. Fudan University
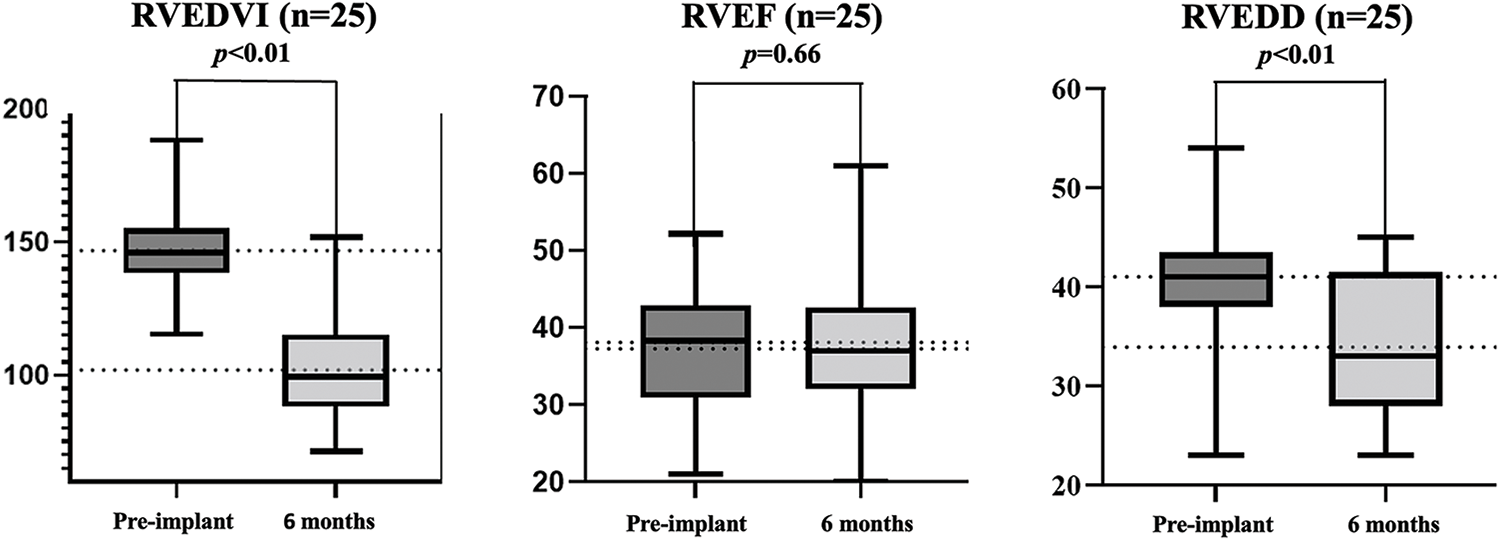
Supplemental Figure 1: Cardiac magnetic resonance data
Abbreviation: RVEDVI, right ventricular end-diastole volume index; RVEF, right ventricular ejection fractions; RVEDD, right ventricular end-diastole diameter.
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools