 Open Access
Open Access
REVIEW
Transcatheter Closure vs. Surgical Ligation in Preterm Infants with Patent Ductus Arteriosus: A Systematic Review and Meta-Analysis
1 St George’s, University of London, London, UK
2 University of Nicosia Medical School, University of Nicosia, Nicosia, 2417, Cyprus
3 Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, 55905, USA
4 School of Biomedical Sciences, The University of Queensland, St Lucia, Brisbane, 4072, Australia
5 Cardiac Innovation Center of Apollonion Private Hospital, Nicosia, Cyprus
* Corresponding Author: Ioannis Tzanavaros. Email:
Congenital Heart Disease 2023, 18(2), 245-265. https://doi.org/10.32604/chd.2023.027596
Received 05 November 2022; Accepted 09 January 2023; Issue published 15 March 2023
Abstract
Background: Persistent patent ductus arteriosus (pPDA) is a common condition in preterm infants. This meta-analysis aimed to assess the safety and efficacy of transcatheter closure (TC) when compared to surgical ligation (SL) in preterm infants with pPDA. Methods: A literature search of Ovid Cochrane Library, Medline, Embase, Epub, Scopus, PMC Preprints, and was conducted from inception to May 06, 2022. Eligible studies reported infants diagnosed with pPDA born at ≤2000 g birth weight or at ≤37 weeks’ who underwent TC or SL as treatment. This review was registered in PROSPERO (CRD42022325944). Results: From 97 studies screened, 8 studies met the eligibility criteria, with a total of 756 preterm infants undergoing either TC (n = 366) or SL (n = 390). Compared to TC, SL had higher mortality rates (OR = 0.32, 95% CI: 0.16, 0.66, I2 = 0%). No difference was seen in post-procedural complication rate (OR = 0.90, 95% CI: 0.18, 4.44, I2 = 79%), mean duration of post-procedural mechanical ventilation (MD = −2.21 days, 95% CI: −4.88, 0.47, I2 = 60%), hospital stay length (MD = −8.30 days, 95% CI: −17.03, 0.44, I2 = 0%) or neonatal intensive care unit stay length (MD = −3.50 days, 95% CI: −10.27, 3.27, I2 = 0%). Conclusion: Our meta-analysis demonstrated TC as a viable alternative option in managing preterm infants with pPDA in the context of SL. Despite the promising trends demonstrated in this meta-analysis, further studies with larger sample size and controlled baseline characteristics are needed to evaluate the safety and efficacy of TC and SL for preterm infants with pPDA.Keywords
Nomenclature
| PDA | Patent Ductus Arteriosus |
| pPDA | Persistent Patent Ductus Arteriosus |
| SL | Surgical Ligation |
| TC | Transcatheter Closure |
| NICU | Neonatal Intensive Care Unit |
| SD | Standard Deviation |
| BPD | Bronchopulmonary Dysplasia |
| NEC | Necrotising Enterocolitis |
| IVH | Intraventricular Haemorrhage |
| ELBW | Extremely Low Body Weight |
Patent ductus arteriosus (PDA) (i.e., an open ductus arteriosus beyond the first 3 postnatal days), represents the most common cardiovascular condition of preterm infants [1], defined as babies born <37 weeks’ gestation [2]. Incidence of a patent ductus arteriosus is inversely proportional to gestational age [3]. The prevalence of persistent patent ductus arteriosus (pPDA) in preterm infants is approximately 20%–50% of neonates born <32 weeks’ gestation, and exceeds 50% of neonates born <28 weeks’ gestation [4,5]. Historically, 60%–70% of preterm infants failing spontaneous closure were closed with either medical or surgical therapy [4]. Conversely, current guidelines trend towards watchful waiting without intervention, however, the medium- and long-term consequences of prolonged pPDA without therapeutic intervention remain unclear [4]. While conservative management with supportive care alone is increasingly preferred, pharmacological closure treatments such as indomethacin and ibuprofen have shown unpredictable responses, and when failed, interventional techniques need to be sought [6].
While closure of haemodynamically significant pPDA has been considered the standard of care over the past decades, there is no equivocal data to determine the optimal management in preterm infants, and which infants will benefit the most from treatment [7]. When pharmacological closure is unsuccessful or contraindicated, surgical ligation (SL) and percutaneous transcatheter closure (TC) are reserved for preterm infants with pPDA [6]. Once a popular approach, SL rates of pPDA in the US cohort of preterm infants dropped between 2006–2015, with prophylactic and early ligation approaches no longer indicated [4]. This could be in large due to the complications associated with this procedure, which may be present in up to 44% of preterm infants [8], including cardiorespiratory instability, pneumothorax, phrenic nerve palsy, wound infection, and vocal cord paralysis [9,10].
Not until very recently, TC, which is a common place in pPDA closure in children and adults, has emerged as a new alternative to SL for selected preterm infants [11]. TC involves catheter insertion through either the femoral or umbilical vein, and occlusion of the pPDA using the appropriate mesh device [12]. Furthermore, advancements in the procedure have resulted in successful closure in premature infants as small as 700 g [12,13]. In comparing SL and TC, recent studies support that TC is associated with reduced mortality and length of hospital stay [14], while providing equivalent efficiency and safety [15,16]. Furthermore, previous literature demonstrated infants who underwent TC had earlier recoveries and less need for short-term respiratory support [17–20].
While the role of interventional procedure has significantly declined in many contemporary neonatal intensive care units (NICU), it remains a necessary approach for selected cases in the management of preterm infants with pPDA. To our knowledge, no meta-analyses have investigated the mortality rates, length of hospital stays and complications of the TC procedure in the context of the traditional SL approach. As such, this paper aims to investigate the safety and efficacy of TC when compared with SL for the management of preterm infants with pPDA.
2.1 Search Strategy and Data Sources
A comprehensive search of several databases from database inception to May 06, 2022, was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21]. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Scopus, PMC Preprints, and ClinicalTrials.Gov. The search strategy was designed and conducted by an experienced librarian with input from the study’s principal investigator. Controlled vocabulary supplemented with keywords was used to search for Transcatheter, Surgical ligation and Patent ductus arteriosus. The actual strategy listing all search terms used and how they are combined is available in Appendix A. This review was registered prospectively with PROSPERO (CRD42022325944).
2.2 Eligibility Criteria and Quality Assessment
Eligible studies must have met all the following participant inclusion criteria: i) <2000 g weight at birth or born at 37 weeks’ or earlier; ii) diagnosed with PDA that meet the criteria for either TC or SL and iii) compared the outcome of post-procedural mortality, length of hospital stay and post-procedural complications between TC and SL. Case reports, case series, abstracts, conference abstracts, and articles that were not reported in English were excluded from the study. This meta-analysis also excluded studies if a sample size was less than 10 for either group. The quality of each study was independently evaluated by two authors (RSD and KS) using the Newcastle-Ottawa Scale [22].
The pooled means and proportions of our data were analysed using an inverse variance method for continuous data and Mantel-Haenszel method for dichotomous data, which assigns the weight of each study based on its variance. A direct comparison between the two techniques was conducted by assessing studies that reported outcomes of both treatments (two-arm analysis). The heterogeneity of effect size estimates across the studies was quantified using the Q statistic and I2. A value of I2 of 0%–25%, indicates insignificant statistical heterogeneity, 26%–50%, low heterogeneity and 51%–100%, high heterogeneity [23]. The Random-effects model was used when the value of I2 was >50% and the fixed-effects model was used for I2 < 50%. Furthermore, a leave-one-out sensitivity analysis was conducted to assess each study’s influence on the pooled estimate by omitting one study at a time and recalculating the combined estimates for the remaining studies. Publication bias was assessed using a funnel plot [24]. If mean and standard deviation (SD) were not available, the median was converted to mean using the formulas from the Cochrane Handbook for Systematic Reviews of Interventions [25]. Additionally, if mean and SD were only depicted in figures, mean and SD were digitized from figures using WebPlotDigitizer version 4.4 (https://automeris.io/WebPlotDigitizer/). Data analysis was performed using RevMan software version 5.4 (Review Manager (RevMan) [Computer program] The Cochrane Collaboration, 2020, Copenhagen, Denmark).
3.1 Study Selection and Characteristics
The initial literature search of the electronic databases yielded 96 studies. After title and abstract screening, eleven articles remained for full-text review. Those articles were then assessed for eligibility using specified inclusion and exclusion criteria. Finally, 8 studies (n = 756) met the eligibility criteria and were included. 3 of the studies [14,15,19] were conducted at multicentre facilities. All the included studies were retrospective. The mean gestational age of included studies ranged from 25 to 28.6 weeks, of which 338 (44.7%) patients were female. Of the 756 patients, 366 underwent the TC procedure and 390 had the SL procedure. PRISMA flowchart of the study selection process is depicted in Appendix B. The baseline characteristics of the included studies are comprehensively described in Table 1.
Results of the quality assessment of all included studies are shown in Appendix C. All the studies were judged to be of moderate quality according to the Newcastle-Ottawa quality assessment scale. The exposure and outcomes were adequately ascertained, and length of follow-up was sufficient for outcomes to occur.
All 8 studies reported patient comorbidities in both groups (TC: n = 366, SL: n = 390) prior to the procedure. 2 studies [14,28] reported bronchopulmonary dysplasia (BPD) as a pre-procedural comorbidity, with the data showing no difference between the TC group (n = 60, 51.7%) and the SL group (n = 59, 48.4%) (OR = 1.21, 95% CI: 0.71, 2.08, I2 = 0%) (Fig. 1A). Similarly, necrotising enterocolitis (NEC) was reported in 5 studies [14,15,19,27,28] and our study illustrated a comparable baseline characteristic between the TC (n = 31, 13.6%) and SL cohorts (n = 30, 11.6%) (OR = 1.22, 95% CI: 0.20, 7.33, I2 = 80%) (Fig. 1B). Additionally, 5 studies [14,15,18,27,28] reported intraventricular haemorrhage (IVH) as a pre-procedural comorbidity, demonstrating no difference between the TC group (n = 24, 12.7%) and the SL group (n = 58, 21.7%) (OR = 0.63, 95% CI: 0.21, 1.86, I2 = 60%) (Fig. 1C). The pre-procedural clinical characteristics of the included studies are comprehensively described in Appendix D.
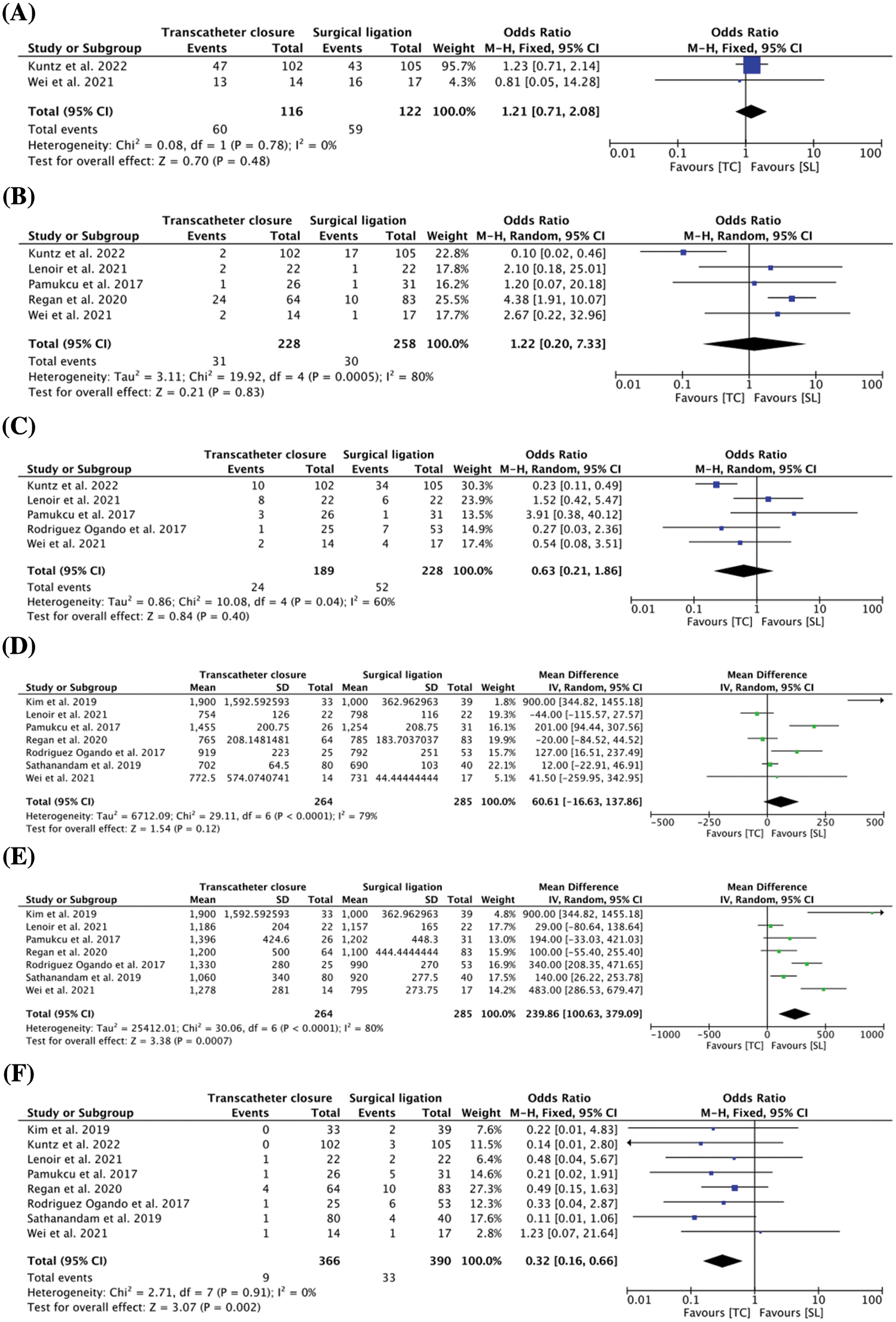
Figure 1: (A) Pooled estimate of pre-operation bronchopulmonary dysplasia for transcatheter closure vs. surgical ligation (B) Pooled estimate of pre-operation necrotizing enterocolitis for transcatheter closure vs. surgical ligation (C) Pooled estimate of pre-operation intraventricular haemorrhage for transcatheter closure vs. surgical ligation (D) Pooled estimate of birth weight for transcatheter closure vs. surgical ligation (E) Pooled estimate of weight at procedure for transcatheter closure vs. surgical ligation (F) Pooled estimate of overall mortality for transcatheter closure vs. surgical ligation
Birth weight was reported in 5 studies [15,18–20,28]. There was no difference in birth weight between the TC and SL groups (MD = 60.61 g, 95% CI: −16.63, 137.86, I2 = 79%) (Fig. 1D). Additionally, 7 studies [15,18–20,26–28] reported the pre-procedural weight of patients, and our results demonstrated the TC cohort with a higher average weight compared to the SL cohort (MD = 239.86 g, 95% CI: 100.63, 379.09, I2 = 80%) (Fig. 1E).
3.4 Surgical and Related Outcomes (Peri-Procedural)
Surgical outcomes were evaluated between the two groups. 9 incidences of device embolization (2.46%) were recorded across 4 studies [18,19,26,27] during the TC procedure, and 1 study described a case of device migration [28]. 6 procedural failures (1.64%) were recorded in the TC group [15,26,27] and none in the SL group [15,26]. Concerning the transcatheter procedure, the device most used across our studies [15,18,19,27] was the Amplatzer Piccollo Occluder, previously known as Amplatzer Duct Occluder II Additional Size, making up 54% of all reported catheter devices. Surgical and related outcomes are detailed in Table 2.
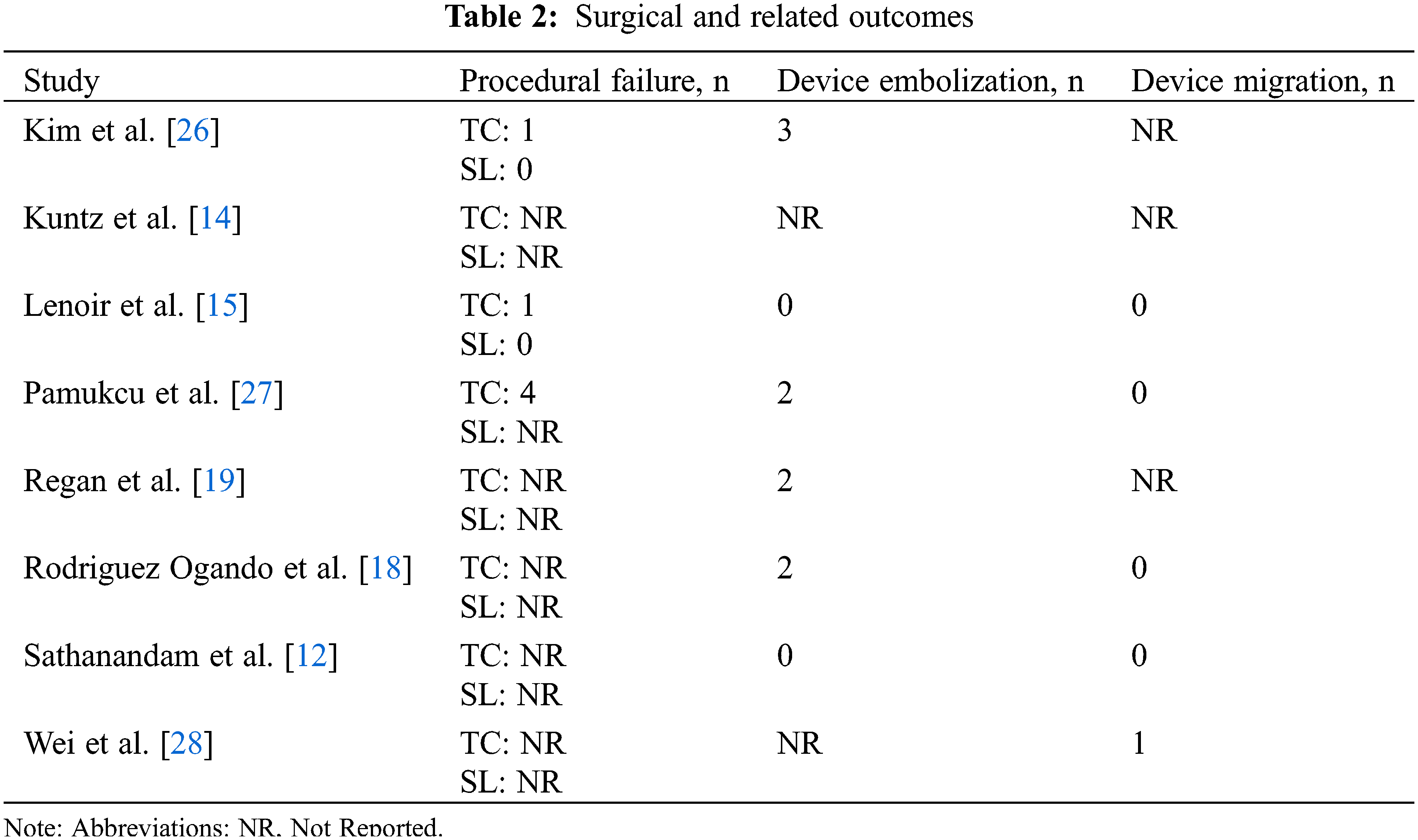
Mortality was reported in all 8 comparative studies, and the results demonstrated TC with a lower overall mortality than SL (OR = 0.32, 95% CI: 0.16, 0.66, I2 = 0%) (Fig. 1F). In total, 42 deaths (5.55%) were recorded across all studies from both groups. The TC group had 9 (2.46%) reported cases of deaths, with 2 studies [14,26] reporting no deaths in their respective TC group. 5 (1.36%) of the 9 deaths were within 30 days post-procedure. In the SL groups, 33 deaths were recorded (8.46%), of which, 15 cases (3.85%) occurring within 30 days post-procedure. The data concerning 30-day post-procedural, late, and overall mortality is comprehensively illustrated in Appendix E.
All the studies reported post-procedural complications, and the results demonstrated no difference in post-procedural complications between the TC group (n = 43, 11.75%) and the SL group (n = 60, 15.38%) (OR = 0.90, 95% CI: 0.18, 4.44, I2 = 79%) (Fig. 2A). Furthermore, the following complications were most frequently encountered between the two groups: BPD (TC: n = 14, 56%; SL: n = 17, 32.1%), sepsis (TC: n = 12, 25.5%; SL: n = 13, 12.4%), and NEC (TC: n = 9, 10.1%; SL: n = 14, 10.3%).
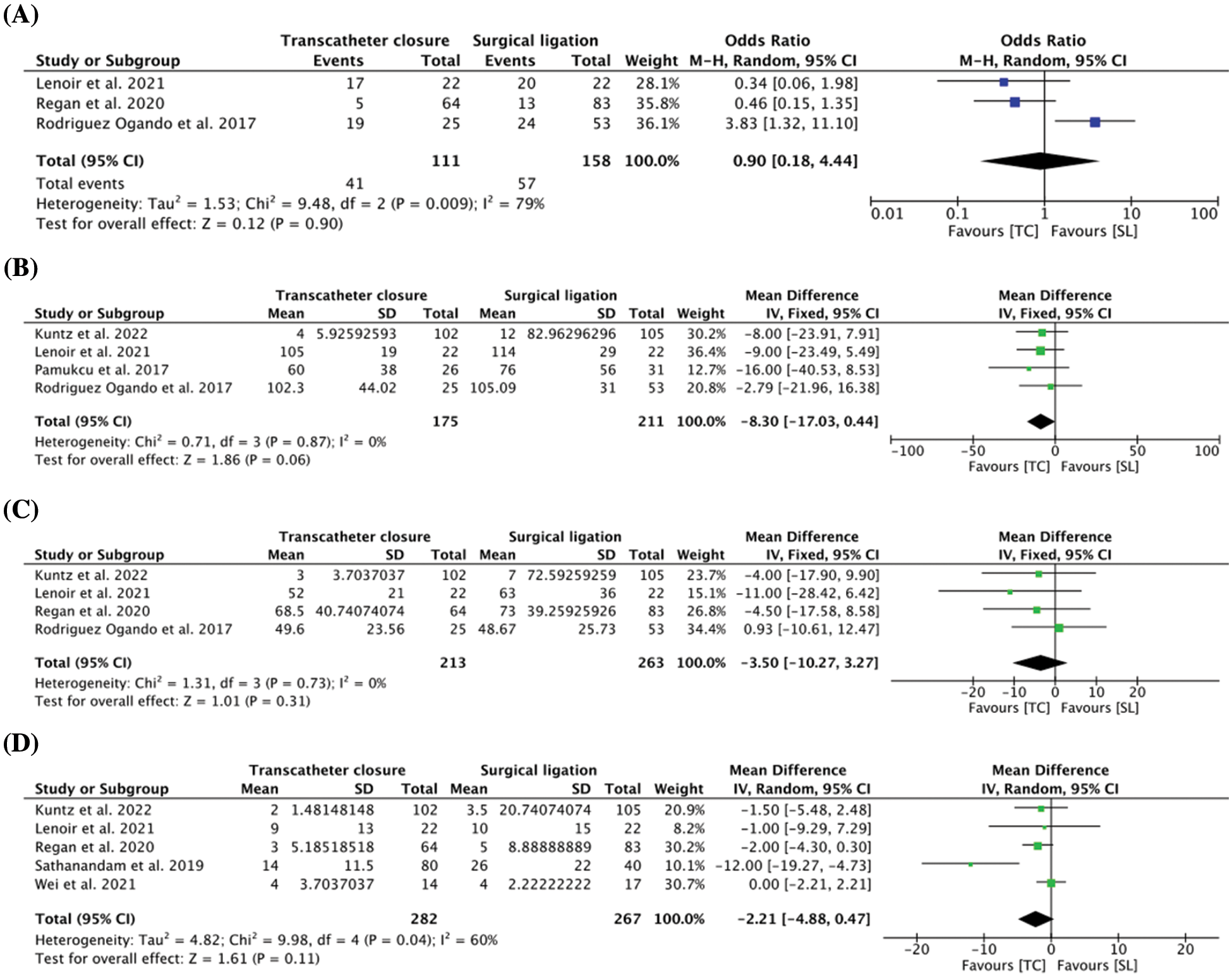
Figure 2: (A) Pooled estimate of post-operation complications for transcatheter closure vs. surgical ligation (B) Pooled estimate of length of hospital stay for transcatheter closure vs. surgical ligation (C) Pooled estimate of length of neonatal intensive care unit (NICU) stay for transcatheter closure vs. surgical ligation (D) Pooled estimate of duration of post-procedural mechanical ventilation for transcatheter closure vs. surgical ligation
In 4 comparative studies [14,15,18,27], there was no difference in the mean length of hospital stay between the two groups (MD = −8.30 days, 95% CI: −17.03, 0.44, I2 = 0%) (Fig. 2B). Similarly, length of NICU stay was reported in 4 studies [14,15,18,19] and no difference was observed between the TC and SL groups (MD = −3.50 days, 95% CI: −10.27, 3.27, I2 = 0%) (Fig. 2C). 5 studies [14,15,19,20,28] reported the mean duration of post-procedural mechanical ventilation, and the result demonstrated comparable outcome between the two groups (MD = −2.21 days, 95% CI: −4.88, 0.47, I2 = 60%) (Fig. 2D), which ranged from 2 to 14 days in the TC group and 3.5 to 26 days in the SL group. The post-procedural outcomes of included studies are comprehensively illustrated in Appendix E.
While surgical closure is increasingly uncommon in many NICUs, it remains a necessary option in the arsenal for treating preterm infants with pPDA in selected cases [29]. Furthermore, advancements in the TC procedure have expanded the applicability of interventional pPDA treatment for premature infants as small as 700 g [12,13]. The primary aim of this meta-analysis was to evaluate the efficacy of TC and SL in the management of preterm infants with pPDA. Based on 8 studies included in this meta-analysis, the following have been examined and supported. Firstly, the TC group had lower overall mortality rates compared to the SL group. Secondly, there was no difference in the duration of hospital, or NICU stay between infants in the two intervention groups. Thirdly, SL and TC had a similar incidence of post-procedural complications, including BPD, sepsis, and NEC. Lastly, our results demonstrated no difference between the two groups in the mean duration of post-procedural mechanical ventilation.
This meta-analysis is the first to evaluate overall mortality rates between TC and SL in preterm infants, and our result demonstrated the TC group with a lower overall mortality (2.46%) compared to the SL group (8.46%). Overall mortality was defined as the sum of 30-day post-procedural mortality and late mortality. In addition, the TC group also reported a lower rate of 30-day post-procedural mortality of 1.36%, compared to 3.85% in infants who underwent SL. These numbers were in concordance with the 0.3% incidence of procedure-related mortality among >1500 cases following TC, reported in a recent literature review by Garg et al. [30]. In comparison, the 30-day mortality rate for SL has been reported around 5%–8% in literature [31,32]. Kuntz et al. [14] postulated that this difference could be attributed to the higher rate of comorbid conditions in SL patients. Alternatively, differences in mortality rates seen in this meta-analysis could also coincide with patient weight, as Pamukcu et al. [27] described the bodyweight and gestational ages of patients reported in their SL group to be significantly lower than their TC group. Moreover, this hypothesis seems to align with our meta-analysis’ finding of lower pre-procedural weights for patients in the SL group. Tashiro et al. [33] suggested this link with mortality by stating that birth weight, rather than gestational age, is more strongly associated with the survival of preterm infants undergoing SL. In addition, the younger infants who were correspondingly smaller, and requiring more preprocedural inotropic and ventilatory support were allocated to the SL group in Kim et al. [26], reflecting the current limitation in TC technology, where extremely low birth weight (ELBW) infants present a challenge for percutaneous device closure. Nonetheless, as evidenced in our meta-analysis, in preterm infants of comparable weight, TC appears to demonstrate lower mortality. However, further research is required to elaborate on whether mortality can be attributed to prior weight or comorbidity predisposition between the two techniques. Thus, this gap in the current literature highlights a dire need for thorough baseline characteristic reporting and propensity score matching in future literature.
Our study evaluated the duration of hospital stay, and NICU stay, however, we observed no difference in those outcomes between the two cohorts. Contrastingly, Kuntz et al. [14] reported that duration of post-procedural intensive care or NICU admission was longer for preterm infants after SL compared to TC. However, the discrepancy between the outcomes may be secondary to some patients being referred to tertiary care centres to undergo TC and returning to their original facility, thereafter, potentially resulting in an under detection of post-procedural details [14]. Whilst this meta-analysis demonstrated comparable results, other studies found a longer length of stay after SL, in comparison to TC [34–38]. Interestingly, post-NICU discharge mortality among ELBW and <27 weeks’ gestational age infants have been shown to be of significantly increased risk following prolonged NICU stay ≥120 days [39]. Therefore, it is important to anticipate the length of post-procedural stay when selecting the intervention between the two groups.
Similar to hospital stay, no difference was found in the total number of post-procedural complications between the TC and SL groups. The most frequently encountered complications in both groups were BPD, sepsis, and NEC. However, numerous completed trials failed to demonstrate any improvement in long-term outcomes following closure of pPDA, such as death or BPD [40]. This seems to demonstrate that adverse outcomes may be associated with pPDA through prematurity or other common antecedents, such as intrauterine infection or inflammation, rather than the procedural techniques [41,42]. The non-efficacy of pPDA closure is further supported by studies from Abdel-Hady et al. [43], Kluckow et al. [44] whom did not observe any long-term benefit of early pPDA closure on morbidities including BPD, NEC, IVH, retinopathy of prematurity, periventricular leukomalacia, and long-term neurodevelopment status. However, various confounding factors may have played a role in this result. Firstly, the volume of the shunt is a significant measure in deciding the course of action for preterm infants [45,46]. The North West Neonatal Operational Delivery Network [47] classifies the diagnosis of a haemodynamically significant PDA as requiring at least 3 of the following echocardiography indices: i) PDA diameter ≥2.0 mm (either using 2D or colour Doppler); ii) Ductal flow pattern with Vmax < 2 m/s and Vmax/Vmin > 2; iii) Retrograde post-ductal aortic/coeliac/superior mesenteric artery diastolic flow; iv) Left atrial/Aortic root ratio > 2; v) Left ventricular output > 300 ml/kg/min; vi) Mitral valve E/A ratio > 1. However, at present, there is no standardised technique for quantifying shunts, with clinical assessment, echocardiography, and other indicators of systemic hypoperfusion or pulmonary over-circulation all being used but varying across institutions [45,46]. In addition, the larger the left-to-right shunt, the greater the risk of NEC, IVH, acute kidney injury, and death [48–50]. The lack of clinical benefit after ductal closure could explain the absence of any difference in the number of adverse outcomes recorded in the TC and SL groups. Moreover, this could be attributed to a few explanations: i) The lack of a standardised definition or method of quantifying a haemodynamically significant pPDA; ii) Closure alone will not ameliorate any morbidities or mortalities, despite a causal relationship between a haemodynamically significant pPDA and adverse outcomes [51]. Therefore, despite the promising results, establishing standardised reporting and measuring criteria are essential to evaluate the complication rates between the two groups.
pPDA closure with TC or SL showed no difference in duration of post-procedural mechanical ventilation, consistent to that described in other studies [15,26]. Interestingly, Kuntz et al. [14] indicated that the rate of initiating mechanical ventilation was higher in the TC cohort. This may be attributed to patients undergoing SL were more often intubated before the procedure, therefore, a direct comparison in terms of duration of post-procedural mechanical ventilation would be difficult to interpret [14,26]. Furthermore, only some studies [18] included in this paper utilised a pulmonary score, which involves the use of descriptors of respiratory support. A recent study evaluating the respiratory parameters associated with prolonged mechanical ventilation after SL in preterm infants showed that the use of greater accumulative doses of pre-procedural furosemide was observed in infants successfully extubated ≤14 days, suggesting that the pre-procedural respiratory illness severity is an important factor associated with prolonged mechanical ventilation after SL [52]. Therefore, when deciding the management plan for pPDA in preterm infants, pre-procedural respiratory status should be used to assess the risk of adverse pulmonary outcomes prior to the procedure.
Limitations of this meta-analysis were, firstly, the design of the studies included, which only consisted of retrospective (cohort) studies, and lacked randomisation of treatment groups. Additionally, most of the studies were conducted in a single centre setting, thus analysing with additional multicentre studies could improve the sample size and generalizability of this meta-analysis. Only 3 of the included studies conducted propensity score matching, and the differences in baseline characteristics between the two groups could have ultimately contributed to heterogeneity in our outcomes. Likewise, the recommended approach to pPDA in preterm infants has recently evolved, therefore, it could have introduced a potential selection bias when mixing earlier studies with more recent studies. Similarly, it is challenging to account for the differences among studies in how they regulate protocols, choice of equipment, surgical technique, and surgeons’ experience, therefore also influencing heterogeneity. Additionally, there was heterogeneity in reported outcomes among included studies, which could significantly impact the number of actual events. An example is the lack of specific reporting of 30-day post-procedural mortality compared to late mortality in the included studies. Lastly, there was considerable heterogeneity in the outcomes, such as birth weight, complication rates, and duration of post-procedural mechanical ventilation. As such, it would be beneficial for future studies to address these limitations in continuing their efforts in evaluating the safety and efficacy of TC and SL in the management of pPDA. In addition, a subgroup analysis separating infants into separate pre-procedural weight categories may also be valuable.
In comparison to SL, our study demonstrated TC as an alternative approach in the management of preterm infants with pPDA for selected cases. Our study depicted TC with lower overall mortality than SL while illustrating no difference in length of hospital stay or NICU stay, post-procedural complications, and duration of post-procedural mechanical ventilation. Despite the promising trends demonstrated in this meta-analysis, further studies with larger sample size and controlled baseline characteristics are needed to evaluate the safety and efficacy of TC in the context of SL in managing preterm infants with pPDA.
Acknowledgement: We would like to thank M. L. S Hassett Leslie C. for the literature search.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: RSD, HN, KS, IT; data collection, analysis and interpretation of results, draft manuscript preparation: RSD, HN, KS, IT, GS, GKS, MNM, DKV, RHM, CAT. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: With publication, the data set used for this meta-analysis will be shared upon request from the study authors.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Mitchell, S. C., Korones, S. B., Berendes, H. W. (1971). Congenital heart disease in 56,109 births. Incidence and natural history. Circulation, 43(3), 323–332. [Google Scholar] [PubMed]
2. Quinn, J. A., Munoz, F. M., Gonik, B., Frau, L., Cutland, C. et al. (2016). Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine, 34(49), 6047–6056. [Google Scholar] [PubMed]
3. Semberova, J., Sirc, J., Miletin, J., Kucera, J., Berka, I. et al. (2017). Spontaneous closure of patent ductus arteriosus in infants </= 1500 g. Pediatrics, 140(2), e20164258. https://doi.org/10.1542/peds.2016-4258 [Google Scholar] [PubMed] [CrossRef]
4. Hamrick, S. E. G., Sallmon, H., Rose, A. T., Porras, D., Shelton, E. L. et al. (2020). Patent ductus arteriosus of the preterm infant. Pediatrics, 146(5), e20201209. https://doi.org/10.1542/peds.2020-1209 [Google Scholar] [PubMed] [CrossRef]
5. Sellmer, A., Bjerre, J. V., Schmidt, M. R., McNamara, P. J., Hjortdal, V. E. et al. (2013). Morbidity and mortality in preterm neonates with patent ductus arteriosus on day 3. Archives of Disease in Childhood-Fetal and Neonatal Edition, 98(6), F505–F510. [Google Scholar] [PubMed]
6. Backes, C. H., Hill, K. D., Shelton, E. L., Slaughter, J. L., Lewis, T. R. et al. (2022). Patent ductus arteriosus: A contemporary perspective for the pediatric and adult cardiac care provider. Journal of the American Heart Association, 11(17), e025784. https://doi.org/10.1161/JAHA.122.025784 [Google Scholar] [PubMed] [CrossRef]
7. Ibrahim, T. K., Haium, A. A., Chandran, S., Rajadurai, V. S. (2014). Current controversies in the management of patent ductus arteriosus in preterm infants. Indian Pediatrics, 51(4), 289–294. https://doi.org/10.1007/s13312-014-0403-2 [Google Scholar] [PubMed] [CrossRef]
8. Foster, M., Mallett, L. H., Govande, V., Vora, N., Castro, A. et al. (2021). Short-term complications associated with surgical ligation of patent ductus arteriosus in ELBW infants: A 25-year cohort study. American Journal of Perinatology, 38(5), 477–481. https://doi.org/10.1055/s-0039-1698459 [Google Scholar] [PubMed] [CrossRef]
9. Malviya, M. N., Ohlsson, A., Shah, S. S. (2013). Surgical versus medical treatment with cyclooxygenase inhibitors for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database of Systematic Reviews, 3, CD003951. https://doi.org/10.1002/14651858 [Google Scholar] [CrossRef]
10. Noori, S. (2012). Pros and cons of patent ductus arteriosus ligation: Hemodynamic changes and other morbidities after patent ductus arteriosus ligation. Seminars in Perinatology, 36(2), 139–145. https://doi.org/10.1053/j.semperi.2011.09.024 [Google Scholar] [PubMed] [CrossRef]
11. Zahn, E. M., Peck, D., Phillips, A., Nevin, P., Basaker, K. et al. (2016). Transcatheter closure of patent ductus arteriosus in extremely premature newborns: Early results and midterm follow-up. JACC: Cardiovascular Interventions, 9(23), 2429–2437. [Google Scholar] [PubMed]
12. Sathanandam, S., Agrawal, H., Chilakala, S., Johnson, J., Allen, K. et al. (2019). Can transcatheter PDA closure be performed in neonates ≤ 1000 grams? The Memphis experience. Congenital Heart Disease, 14(1), 79–84. https://doi.org/10.1111/chd.12700 [Google Scholar] [PubMed] [CrossRef]
13. Sathanandam, S. K., Gutfinger, D., O’Brien, L., Forbes, T. J., Gillespie, M. J. et al. (2020). Amplatzer piccolo occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients >/= 700 grams. Catheterization and Cardiovascular Interventions, 96(6), 1266–1276. [Google Scholar] [PubMed]
14. Kuntz, M. T., Staffa, S. J., Graham, D., Faraoni, D., Levy, P. et al. (2022). Trend and outcomes for surgical versus transcatheter patent ductus arteriosus closure in neonates and infants at US children’s hospitals. Journal of the American Heart Association, 11(1), e022776. [Google Scholar] [PubMed]
15. Lenoir, M., Wanert, C., Bonnet, D., Meot, M., Tosello, B. et al. (2021). Anterior minithoracotomy vs. transcatheter closure of patent ductus arteriosus in very preterm infants. Frontiers in Pediatrics, 9, 700284. [Google Scholar] [PubMed]
16. Abu Hazeem, A. A., Gillespie, M. J., Thun, H., Munson, D., Schwartz, M. C. et al. (2013). Percutaneous closure of patent ductus arteriosus in small infants with significant lung disease may offer faster recovery of respiratory function when compared to surgical ligation. Catheterization and Cardiovascular Interventions, 82(4), 526–533. https://doi.org/10.1002/ccd.25032 [Google Scholar] [PubMed] [CrossRef]
17. Serrano, R. M., Madison, M., Lorant, D., Hoyer, M., Alexy, R. (2020). Comparison of ‘post-patent ductus arteriosus ligation syndrome’ in premature infants after surgical ligation vs. percutaneous closure. Journal of Perinatology, 40(2), 324–329. https://doi.org/10.1038/s41372-019-0513-8 [Google Scholar] [PubMed] [CrossRef]
18. Rodriguez Ogando, A., Planelles Asensio, I., de la Blanca, A. R. S., Ballesteros Tejerizo, F., Sanchez Luna, M. et al. (2018). Surgical ligation versus percutaneous closure of patent ductus arteriosus in very low-weight preterm infants: Which are the real benefits of the percutaneous approach? Pediatric Cardiology, 39(2), 398–410. [Google Scholar] [PubMed]
19. Regan, W., Benbrik, N., Sharma, S. R., Auriau, J., Bouvaist, H. et al. (2020). Improved ventilation in premature babies after transcatheter versus surgical closure of patent ductus arteriosus. International Journal of Cardiology, 311, 22–27. https://doi.org/10.1016/j.ijcard.2020.03.040 [Google Scholar] [PubMed] [CrossRef]
20. Sathanandam, S., Balduf, K., Chilakala, S., Washington, K., Allen, K. et al. (2019). Role of transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheterization and Cardiovascular Interventions, 93(1), 89–96. https://doi.org/10.1002/ccd.27808 [Google Scholar] [PubMed] [CrossRef]
21. Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 6(7), e1000097. [Google Scholar] [PubMed]
22. Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology, 25(9), 603–605. https://doi.org/10.1007/s10654-010-9491-z [Google Scholar] [PubMed] [CrossRef]
23. Higgins, J. P., Thompson, S. G., Deeks, J. J., Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. [Google Scholar] [PubMed]
24. Sterne, J. A., Egger, M. (2001). Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. Journal of Clinical Epidemiology, 54(10), 1046–1055. https://doi.org/10.1016/S0895-4356(01)00377-8 [Google Scholar] [PubMed] [CrossRef]
25. Higgins, J. P. T., Cochrane Collaboration (2020). Cochrane handbook for systematic reviews of interventions. 2nd edition. Hoboken, NJ: Wiley-Blackwell. [Google Scholar]
26. Kim, H. S., Schechter, M. A., Manning, P. B., Eghtesady, P., Balzer, D. T. et al. (2019). Surgical versus percutaneous closure of PDA in preterm infants: Procedural charges and outcomes. Journal of Surgical Research, 243, 41–46. https://doi.org/10.1016/j.jss.2019.04.069 [Google Scholar] [PubMed] [CrossRef]
27. Pamukcu, O., Tuncay, A., Narin, N., Baykan, A., Korkmaz, L. et al. (2018). Patent ductus arteriosus closure in preterms less than 2kg: Surgery versus transcatheter. International Journal of Cardiology, 250(3), 110–115. https://doi.org/10.1016/j.ijcard.2017.10.020 [Google Scholar] [PubMed] [CrossRef]
28. Wei, Y. J., Chen, Y. J., Lin, Y. C., Kan, C. D., Hsieh, M. L. et al. (2021). Respiratory trajectory after invasive interventions for patent ductus arteriosus of preterm infants. Children, 8(5), 398. https://doi.org/10.3390/children8050398 [Google Scholar] [PubMed] [CrossRef]
29. Yan, H., Ma, F., Li, Y., Zhou, K., Hua, Y. et al. (2020). The optimal timing of surgical ligation of patent ductus arteriosus in preterm or very-low-birth-weight infants: A systematic review and meta-analysis. Medicine, 99(9), e19356. https://doi.org/10.1097/MD.0000000000019356 [Google Scholar] [PubMed] [CrossRef]
30. Garg, R., Zahn, E., Sathanandam, S., Johnson, J. N. (2021). Transcatheter patent ductus arteriosus closure in extremely premature infants. Progress in Pediatric Cardiology, 61(2), 101366. https://doi.org/10.1016/j.ppedcard.2021.101366 [Google Scholar] [CrossRef]
31. Lee, L. C., Tillett, A., Tulloh, R., Yates, R., Kelsall, W. (2006). Outcome following patent ductus arteriosus ligation in premature infants: A retrospective cohort analysis. BMC Pediatrics, 6(15), 545. https://doi.org/10.1186/1471-2431-6-15 [Google Scholar] [PubMed] [CrossRef]
32. Hutchings, K., Vasquez, A., Price, D., Cameron, B. H., Awan, S. et al. (2013). Outcomes following neonatal patent ductus arteriosus ligation done by pediatric surgeons: A retrospective cohort analysis. Journal of Pediatric Surgery, 48(5), 915–918. https://doi.org/10.1016/j.jpedsurg.2013.02.003 [Google Scholar] [PubMed] [CrossRef]
33. Tashiro, J., Wang, B., Sola, J. E., Hogan, A. R., Neville, H. L. et al. (2014). Patent ductus arteriosus ligation in premature infants in the United States. Journal of Surgical Research, 190(2), 613–622. [Google Scholar] [PubMed]
34. Animasahun, B. A., Adekunle, M. O., Falase, O., Gidado, M. T., Kusimo, O. Y. et al. (2018). Is transcatheter closure superior to surgical ligation of patent ductus arteriosus among Nigerian children? African Journal of Paediatric Surgery, 15(2), 100–103. https://doi.org/10.4103/ajps.AJPS_53_17 [Google Scholar] [PubMed] [CrossRef]
35. Liem, N. T., Tung, C. V., van Linh, N., Tuan, T. M., Quang, Le, H. et al. (2014). Outcomes of thoracoscopic clipping versus transcatheter occlusion of patent ductus arteriosus: Randomized clinical trial. Journal of Pediatric Surgery, 49(2), 363–366. https://doi.org/10.1016/j.jpedsurg.2013.09.007 [Google Scholar] [PubMed] [CrossRef]
36. Lin, C. C., Hsieh, K. S., Huang, T. C., Weng, K. P. (2009). Closure of large patent ductus arteriosus in infants. American Journal of Cardiology, 103(6), 857–861. https://doi.org/10.1016/j.amjcard.2008.11.044 [Google Scholar] [PubMed] [CrossRef]
37. Wang, K., Pan, X., Tang, Q., Pang, Y. (2014). Catheterization therapy vs surgical closure in pediatric patients with patent ductus arteriosus: A meta-analysis. Clinical Cardiology, 37(3), 188–194. [Google Scholar] [PubMed]
38. Zulqarnain, A., Younas, M., Waqar, T., Beg, A., Asma, T. et al. (2016). Comparison of effectiveness and cost of patent ductus arteriosus device occlusion versus surgical ligation of patent ductus arteriosus. Pakistan Journal of Medical Sciences, 32(4), 974–977. https://doi.org/10.12669/pjms.324.10048 [Google Scholar] [PubMed] [CrossRef]
39. De Jesus, L. C., Pappas, A., Shankaran, S., Kendrick, D., Das, A. et al. (2012). Risk factors for post-neonatal intensive care unit discharge mortality among extremely low birth weight infants. Journal of Pediatrics, 161(1), 70–74.E2. https://doi.org/10.1016/j.jpeds.2011.12.038 [Google Scholar] [PubMed] [CrossRef]
40. Benitz, W. E. (2010). Treatment of persistent patent ductus arteriosus in preterm infants: Time to accept the null hypothesis? Journal of Perinatology, 30(4), 241–252. https://doi.org/10.1038/jp.2010.3 [Google Scholar] [PubMed] [CrossRef]
41. Gonzalez, A., Sosenko, I. R., Chandar, J., Hummler, H., Claure, N. et al. (1996). Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. Journal of Pediatrics, 128(4), 470–478. https://doi.org/10.1016/S0022-3476(96)70356-6 [Google Scholar] [PubMed] [CrossRef]
42. Hammerman, C., Strates, E., Valaitis, S. (1986). The silent ductus: Its precursors and its aftermath. Pediatric Cardiology, 7(3), 121–127. [Google Scholar] [PubMed]
43. Abdel-Hady, H., Nasef, N., Shabaan, A. E., Nour, I. (2013). Patent ductus arteriosus in preterm infants: Do we have the right answers? BioMed Research International, 2013(9), 676192. https://doi.org/10.1155/2013/676192 [Google Scholar] [PubMed] [CrossRef]
44. Kluckow, M., Jeffery, M., Gill, A., Evans, N. (2014). A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Archives of Disease in Childhood-Fetal and Neonatal Edition, 99(2), F99–F104. https://doi.org/10.1136/archdischild-2013-304695 [Google Scholar] [PubMed] [CrossRef]
45. El-Khuffash, A., Levy, P. T., Gorenflo, M., Frantz III, I. D. (2019). The definition of a hemodynamically significant ductus arteriosus. Pediatric Research, 85(6), 740–741. [Google Scholar] [PubMed]
46. Bischoff, A. R., Giesinger, R. E., Bell, E. F., McNamara, P. J. (2020). Precision medicine in neonatal hemodynamics: Need for prioritization of mechanism of illness and defining population of interest. Journal of Perinatology, 40(9), 1446–1449. https://doi.org/10.1038/s41372-020-0741-y [Google Scholar] [PubMed] [CrossRef]
47. North West, North Wales & Isle of Man Children’s Heart Network with Comments from all NW Neonatal Clinical Leads (2020). Guideline for the management of patent ductus arteriosus (PDA). https://www.neonatalnetwork.co.uk/nwnodn//wp-content/uploads/2021/02/GL-ODN-09-NW-Guideline-for-the-Management-of-PDA-.pdf [Google Scholar]
48. Brown, E. R. (1979). Increased risk of bronchopulmonary dysplasia in infants with patent ductus arteriosus. Journal of Pediatrics, 95(5), 865–866. https://doi.org/10.1016/S0022-3476(79)80454-0 [Google Scholar] [PubMed] [CrossRef]
49. Lipman, B., Serwer, G. A., Brazy, J. E. (1982). Abnormal cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatrics, 69(6), 778–781. [Google Scholar] [PubMed]
50. Dollberg, S., Lusky, A., Reichman, B. (2005). Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: A population-based study. Journal of Pediatric Gastroenterology and Nutrition, 40(2), 184–188. https://doi.org/10.1097/00005176-200502000-00019 [Google Scholar] [PubMed] [CrossRef]
51. Parkerson, S., Philip, R., Talati, A., Sathanandam, S. (2020). Management of patent ductus arteriosus in premature infants in 2020. Frontiers in Pediatrics, 8, 590578. https://doi.org/10.3389/fped.2020.590578 [Google Scholar] [PubMed] [CrossRef]
52. Seo, Y. M., Sung, I. K., Yum, S. K. (2022). Risk factors associated with prolonged mechanical ventilation after surgical patent ductus arteriosus ligation in preterm infants. Journal of Maternal-Fetal & Neonatal Medicine, 35(19), 3714–3721. https://doi.org/10.1080/14767058.2020.1839044 [Google Scholar] [PubMed] [CrossRef]
Appendix A: Search Strategy
Ovid
Database(s): EBM Reviews-Cochrane Central Register of Controlled Trials April 2022, EBM Reviews-Cochrane Database of Systematic Reviews 2005 to May 05, 2022, Embase 1974 to 2022 May 05, Ovid MEDLINE(R) and Epub Ahead of Print, In-Process, In-Data-Review & Other Non-Indexed Citations, Daily and Versions 1946 to May 05, 2022 Search Strategy:

Scopus
1. TITLE-ABS-KEY ((patency W/4 “ductus arteriosus”) OR “patent ductus arteriosus”)
2. TITLE-ABS-KEY (ligation OR Minithoracotom* OR open OR surg* OR thoracotom*)
3. TITLE-ABS-KEY (“Amplatzer Piccolo Occluder*” OR Percutaneous* OR Transcatheter*)
4. TITLE-ABS-KEY (prematur* OR “pre-matur*” OR preterm* OR “pre-term*”)
5. LANGUAGE (english)
6. 1 and 2 and 3 and 4 and 5
7. DOCTYPE (ab) OR DOCTYPE (ed) OR DOCTYPE (bk) OR DOCTYPE (er) OR DOCTYPE (no) OR DOCTYPE (sh)
8. 6 and not 7
9. INDEX (embase) OR INDEX (medline) OR PMID (0* OR 1* OR 2* OR 3* OR 4* OR 5* OR 6* OR 7* OR 8* OR 9*)
10. 8 and not 9
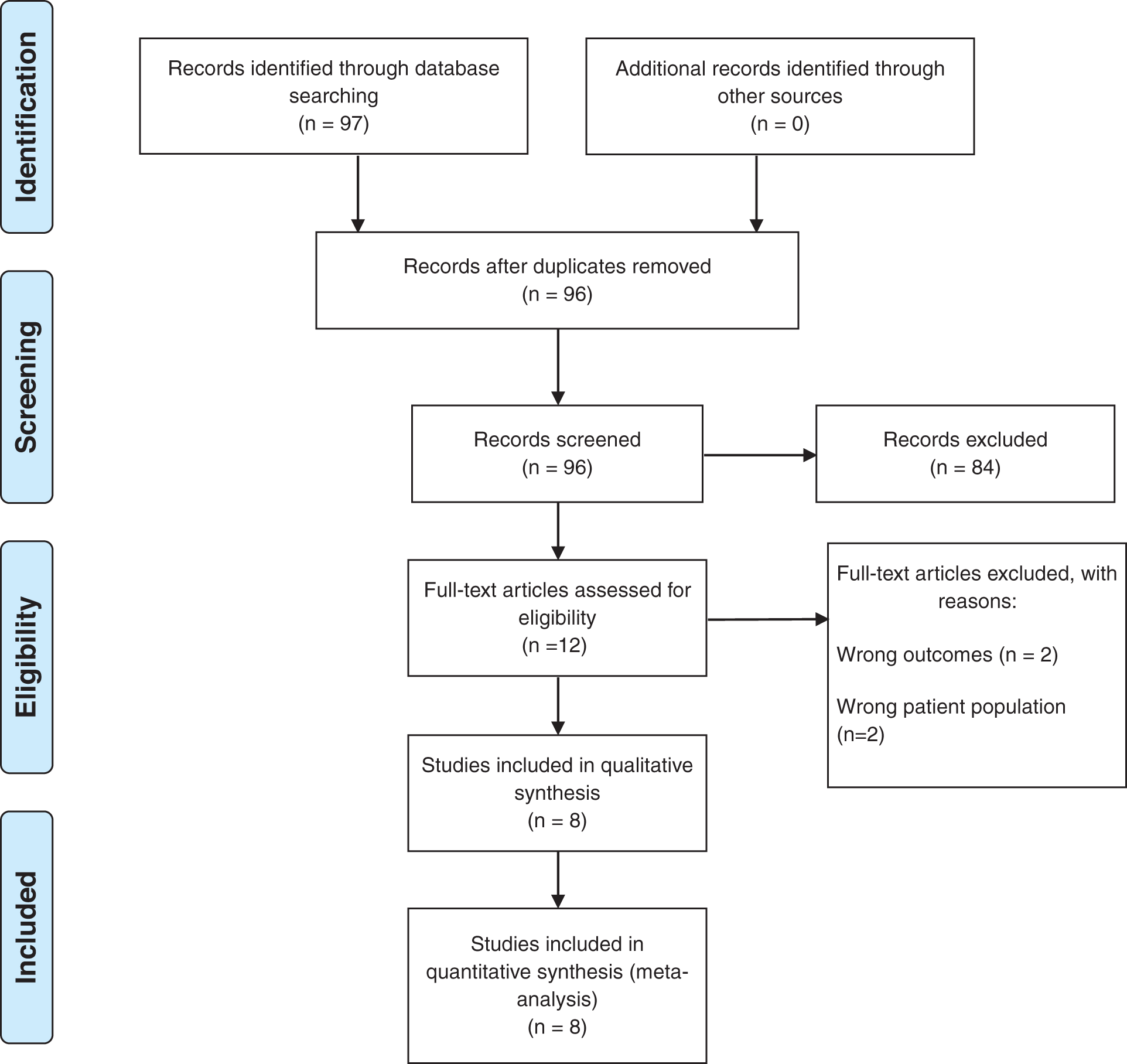
Appendix B: Preferred reporting items for systematic reviews and meta-analyses flow diagram (PRISMA)
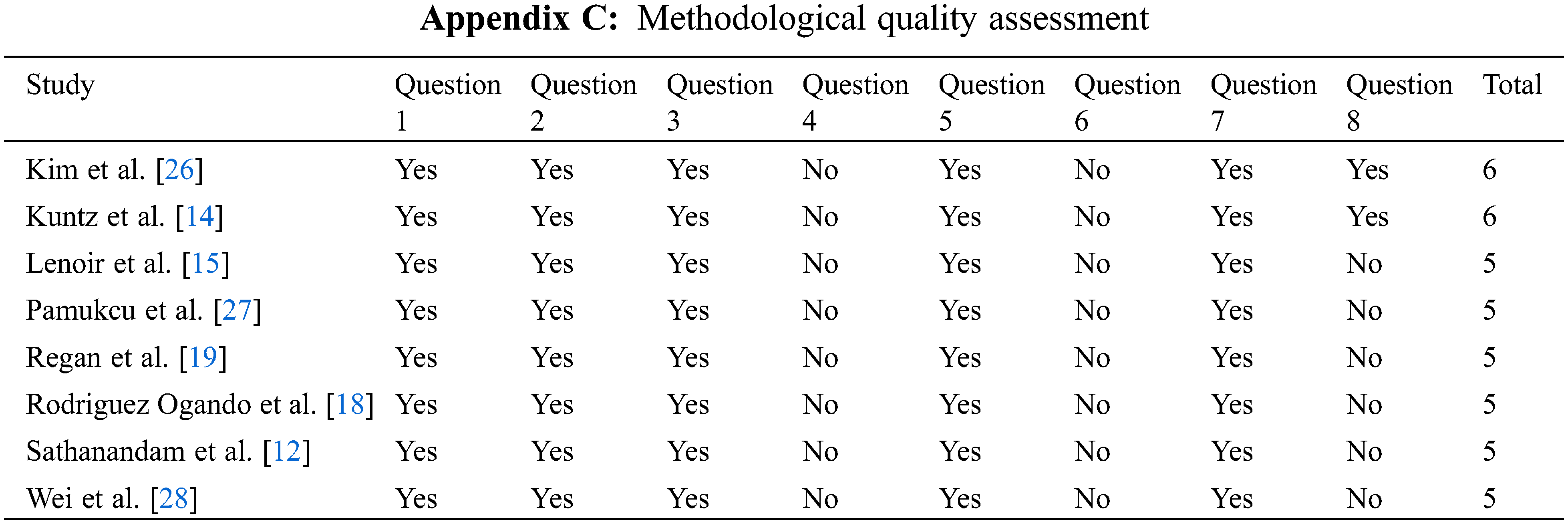
Question 1: Is the study truly/somewhat representative of what is described in the community?
Question 2: Was the non-exposed cohort drawn from the same community as the exposed cohort?
Question 3: Was exposure ascertained using a secure record or structured interview?
Question 4: Is it clearly demonstrated that the outcome of interest was not present at the start of the study?
Question 5: Are the cohorts comparable based on design and analysis?
Question 6: Are outcomes assessed via independent blinding/record linkage?
Question 7: Was follow-up long enough for outcomes to occur?
Question 8: Are all subjects of the cohort accounted for at the end of follow-up?
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools