 Open Access
Open Access
REVIEW
Advances in Pediatric Heart Valve Replacement: A State-of-the-Art Review
1 Department of Cardiovascular Diseases, Sidra Medicine, Doha, 2699, Qatar
2 Department of Cardiothoracic and Vascular Surgery, Institut Jantung Negara (National Heart Institute), Kuala Lumpur, 50400, Malaysia
* Corresponding Author: Ziyad M. Hijazi. Email:
Congenital Heart Disease 2025, 20(2), 143-179. https://doi.org/10.32604/chd.2025.064599
Received 19 February 2025; Accepted 07 April 2025; Issue published 30 April 2025
Abstract
Pediatric heart valve replacement (PHVR) remains a challenging procedure due to the unique anatomical and physiological characteristics of children, including growth and development, as well as the long-term need for durable valve function. This review provides an overview of both surgical and transcatheter options for aortic, mitral, pulmonary, and tricuspid valve replacements in pediatric patients, highlighting the indications, outcomes, and advancements in technology and technique. Surgical valve replacement traditionally involves the implantation of biological or mechanical prosthetic valves, with biological valves being preferred in children to reduce the need for lifelong anticoagulation therapy. However, the limitation of biological prostheses, namely their inability to grow with the child, necessitates the frequent need for reoperations. Recent innovations in valve engineering, such as the development of tissue-engineered and expandable valves, aim to address these issues. Transcatheter valve replacement (TVR) has emerged as a promising alternative, particularly for patients with complex anatomy or those who are high-risk for traditional surgery. While the use of transcatheter devices in children remains limited due to the smaller vascular size and limited long-term data, several studies have demonstrated the feasibility and safety of the procedure in certain patient populations. Despite these advancements, challenges related to valve size, durability, and the need for individualized treatment planning persist. The future of pediatric heart valve replacement will likely involve a multidisciplinary approach combining surgical, transcatheter, and regenerative medicine strategies, aimed at optimizing outcomes, reducing the need for reinterventions, and improving long-term quality of life for pediatric patients with valvular heart disease. This article discusses all options available for patients with valvular dysfunction, making it easy for parents/patients to go to as a reference source of information.Keywords
The treatment of valvular heart diseases in children has evolved significantly in recent years. Without a doubt, valvular repair is the primary goal, because preserving the native valve anatomy and function allows for its growth and possibly has a better long-term outcome. However, when valve repair fails or is not feasible, valve replacement becomes necessary. Due to several unique factors, pediatric valve replacements represent a complex medical challenge distinct from adult interventions. Children’s rapid somatic growth, the need to accommodate this growth in valve selection, and the limitations imposed by smaller anatomical structures necessitate innovative approaches in pediatric cardiac surgery. Furthermore, the potential for complications such as thrombosis, infection, rapid calcification, and the likelihood of multiple reinterventions throughout a patient’s lifetime add layers of complexity to clinical decision-making. Nowadays, Transcatheter valve replacement (TVR) has become one of the most exciting new advancements in the management of congenital heart disease. While it has not replaced surgical valve replacement, TVR has emerged as an important alternative, especially for patients who are high-risk or not suitable candidates for surgery.
Significant advancements in pediatric heart valve replacements have been made in recent years, including developing novel biomaterials, improved surgical techniques, and the emergence of transcatheter interventions. However, the ideal heart valve implant for pediatric patients remains elusive. Current options must be carefully tailored to balance survival outcomes against foreseeable complications, considering factors such as the child’s age, size, and specific cardiac anatomy.
In this review, we will examine the current heart valve replacement options for each valve position in the pediatric age group. We aim to provide a thorough overview to assist clinicians in making informed decisions for their pediatric patients who require valve replacement. Furthermore, we will highlight emerging solutions and technological advancements that show potential in addressing the unique challenges of pediatric heart valve replacements.
2.1 Pulmonary Valve Replacement in Children
Right ventricular outflow tract (RVOT) obstruction is a common congenital heart disease (CHD) that includes a wide spectrum of diseases) ranging from isolated pulmonary valve stenosis to complex lesions, such as tetralogy of Fallot (TOF) and pulmonary atresia [1]. Of all RVOT obstructive lesions, Tetralogy of Fallot is the most common and accounts for about 7%–10% of CHD [2]. The main goal of TOF repair is to relieve the RVOT obstruction which can be achieved by either valve sparing repair or transannular patch technique [3]. Inserting a valved conduit from the RV to the main pulmonary artery (PA) is an alternative surgical method. Unfortunately following TOF repair, long-term complications may develop including, chronic pulmonary regurgitation (PR) which is linked to development of right ventricle (RV) dilatation, RV dysfunction, and arrhythmias, residual RVOT obstruction, endocarditis and sudden death [4]. Approximately one-third of patients may require reoperation in the early to mid-term follow-up, most commonly for pulmonary valve replacement (PVR). However, the cumulative reoperation rate over a lifetime is higher, particularly due to the development of pulmonary valve dysfunction and other long-term complications [5].
PVR in children presents significant challenges due to patient growth and the need for multiple reoperations. Current options include bioprosthetic, mechanical, homografts, and transcatheter valves, each with specific advantages and drawbacks. Factors influencing valve choice include patient size, right ventricular outflow tract anatomy, and potential for growth accommodation. While valve repair is preferred, replacement becomes necessary in cases of irreparable congenital abnormalities, failed previous repairs, or severe valve dysfunction causing life-threatening symptoms. The ideal prosthesis should offer excellent hemodynamics, growth potential, minimal complications, and longevity; regrettably, no current option meets all these criteria.
2.2 Surgical Pulmonary Valve Replacement (SPVR)
Pulmonary homografts represent a significant advancement in right ventricular outflow tract reconstruction for pediatric patients, demonstrating superior intermediate-term outcomes compared to alternative conduits. The hemodynamic profile of pulmonary homografts is particularly favorable, offering optimal flow characteristics without necessitating anticoagulation therapy. They exhibit notable advantages over aortic homografts, including enhanced durability, reduced calcification rates, and lower reoperation frequency [6,7]. When compared with bovine jugular vein conduits, pulmonary homografts demonstrate superior outcomes in patients under 20 years of age with conduit sizes exceeding 15 mm, specifically showing improved freedom from reintervention and reduced risk of infective endocarditis [8]. While some comparative studies suggest the potential superiority of bioprosthetic valves over homografts, these findings lack statistical significance [9].
Despite these advantages, significant limitations persist in pediatric applications. Early-onset homograft stenosis can develop within the initial two postoperative years [10]. Its durability is particularly critical in younger populations where reoperation rates may reach 60% [11,12]. Current research focuses on enhancing homograft durability through innovative preservation techniques and structural modifications, with preliminary investigations yielding promising results in extending conduit longevity and improving patient outcomes.
2.2.2 Decellularized Pulmonary Homografts (DPH)
DPH has emerged as the preferred option for pulmonary valve replacement in pediatric and young adult congenital heart disease patients, demonstrating significant advantages over conventional cryopreserved homografts and bovine jugular vein conduits. Multiple clinical studies have established DPH superiority through reduced reoperation rates [13,14], decreased valve dysfunction [15], and improved freedom from explantation [16,17]. This is attributed to decellularization, which diminishes immunogenicity while preserving structural integrity [14,16]. DPH demonstrate remarkable hemodynamic performance and potential for adaptive growth with valve annulus diameters approaching normal z-values over time, and exceptional resistance to endocarditis [13,16,17]. The multicenter ESPOIR trial further validated these findings, documenting excellent freedom from structural valve degeneration at 10-year follow-up with consistently low gradients [16,17]. While additional long-term data in very young patients remains necessary, the substantial body of evidence supports the emerging consensus that decellularized pulmonary homografts represent the new gold standard for pulmonary valve replacement in this patient population.
2.2.3 Bovine Jugular Vein Grafts
The Contegra bovine jugular vein graft (Medtronic, Minneapolis, MN, USA) has emerged as a significant alternative to homografts for right ventricular outflow tract reconstruction in pediatric patients with congenital heart defects. Its practical advantages include excellent off-the-shelf availability in various sizes and favorable handling characteristics, including ease of tailoring and suturing. Multiple studies have demonstrated encouraging short to medium-term outcomes, featuring low mortality rates and satisfactory hemodynamic performance [18,19,20]. Contemporary research has further validated the long-term efficacy of these grafts, suggesting durability comparable to, and in some instances superior to, traditional homografts [11].
However, important considerations demand attention in clinical decision-making. The main complication associated with Contegra grafts is conduit stenosis, particularly at the distal anastomosis site [21]. While outcomes may be comparable to or better than those of homografts, there is a significant concern regarding higher rates of infective endocarditis [22,23,24]. These findings indicate that while the Contegra graft represents a promising option for pediatric pulmonary valve replacement, careful patient selection and diligent long-term monitoring remain crucial for optimal outcomes. In addition, smaller patient populations, complex regulations, and high research and development costs create a challenging commercial landscape for manufacturers. This situation raises concerns about the availability of essential pediatric conduits, such as the Contegra conduit from Medtronic Inc., USA, where there are indications regarding product sustainability that could potentially affect the options available to congenital cardiac surgeons.
Bioprosthetic pulmonary valve replacement (bPVR) in children demonstrates excellent early outcomes. However, it also presents challenges, including rapid degeneration and the eventual need for reoperation. Despite these challenges, bioprosthetic valves provide several advantages, such as low valve-related morbidity and favorable long-term survival rates [25]. The incidence of infective endocarditis (IE) following bPVR is low, at 333 cases per 100,000 person-years, with generally good outcomes following intravenous antibiotics alone and neither deaths nor recurrence of IE after treatment [26]. Consequently, the freedom from endocarditis is high, reaching 97% over a 15-year period [26].
The risk of structural valve degeneration increases significantly over time, with only 32.8% of patients remaining free from dysfunction after 10 years [27]. This issue is particularly pronounced in younger patients, who often face higher reoperation rates [28]. Therefore, it is crucial to weigh the benefits and risks carefully, especially for this demographic. Careful monitoring is vital due to the risk of rapid progression toward valve failure, highlighting the need for advancements in valve design to improve effectiveness and long-term performance in pediatric age group.
2.2.5 Hancock Porcine-Valved Dacron Conduit
The Hancock conduit represents a significant option for RVOT reconstruction in pediatric patients, demonstrating satisfactory mid-term performance with 96% freedom from failure at 3 years and 83% at 5 years [29]. Its overall function remains adequate for 5–10 years with relatively rare early valve failure. However, long-term durability remains a concern, as conduit failure becomes inevitable with longer follow-up, primarily due to valve leaflet fibrocalcification and exuberant pseudointimal proliferation in the proximal conduit causing obstructions [30]. Despite these limitations, the Hancock conduit offers significant advantages, including the absence of RVOT-aneurysm formation and distal conduit stenoses, availability in various sizes enhancing surgical utility, and comparable or superior mid-term results relative to alternative options [29,31]. While it remains valuable in pediatric pulmonary valve replacement, its application necessitates careful consideration of patient age, growth potential, and anticipated reoperations.
Recent studies have explored innovative techniques for pulmonary valve management during tetralogy of Fallot repair to prevent pulmonary insufficiency. A novel approach involves creating a neo-pulmonary valve using autologous right atrial appendage (RAA) tissue [32,33,34]. This technique has shown promising short- to mid-term results, with most patients experiencing trivial to mild pulmonary insufficiency post-operatively [33,34]. The RAA valve construction is particularly beneficial for patients with small pulmonary valve z-scores (<−2), who may not be suitable candidates for valve-sparing repair [34]. This approach offers advantages such as right heart protection and the potential for growth with the patient, potentially reducing the need for future interventions [34]. While longer follow-up is needed to confirm long-term outcomes, the RAA valve technique is a safe and effective alternative for implanting a pulmonary valve substitute in tetralogy of Fallot repair [32,33].
2.2.7 Expanded Polytetrafluoroethylene Valved Conduits (ePTFE-VC)
Expanded polytetrafluoroethylene valved conduits (ePTFE-VC) have emerged as a promising solution to address the historically limited options for RVOT reconstruction in pediatric patients. Both laboratory and clinical investigations have validated the efficacy of ePTFE trileaflet-valved conduits, with ex vivo and in vivo studies demonstrating acceptable functional outcomes [35]. Longitudinal research has revealed impressive survival metrics, with rates of 96.7% at 3 years and 96% at 10 years, complemented by robust freedom from reintervention, reaching 90.2% at 5 years [12,36]. Medium-term follow-up studies indicate superior performance and reduced reintervention rates compared to alternative conduit options, with recent standardized placement techniques yielding favorable clinical and echocardiographic outcomes [12,37]. A novel ePTFE-based valve design has demonstrated positive right ventricular remodeling without significant complications [38,39].
While ePTFE-VC demonstrates performance comparable to homografts in infants and young children [40], important limitations persist in younger pediatric populations, primarily related to conduit stenosis and restricted growth potential [12]. Despite these challenges, ePTFE-VC stands as a viable solution for pediatric pulmonary valve replacement and presents potential as a platform for future transcatheter interventions, highlighting the importance of ePTFE-VC in the changing landscape of pediatric cardiac care surgery.
2.3 Transcatheter Pulmonary Valve Replacement (TPVR)
It has been 24 years since the first successful percutaneous implantation of a balloon expandable pulmonary valve and interestingly that it was implanted in a child rather than an adult [41]. This was a groundbreaking development in the field of transcatheter heart valve implantation.
Initially, balloon-expandable valve technologies (Medtronic’s Melody valve and Edwards Lifescience’s SAPIEN) were the main focus of percutaneous pulmonary valve advancement. These valves were approved and primarily used in patients with conduits and bioprosthetic valve malfunction. Nowadays, a number of companies are offering larger self-expanding valves, which are primarily intended to include patients with native and patched RVOT. Presently available technologies comprise balloon expandable valves, self-expanding valves and self-expanding prestents (Right ventricular outflow tract reducers).
2.3.1 Balloon Expandable Valves
The US Food and Drug Administration (FDA) and CE have authorized two balloon-expandable Transcatheter pulmonary valves, Melody and SAPIEN valves 3.
- (1) Medtronic Melody Valve
The Medtronic Melody valve (Medtronic, Minneapolis, MN, USA) was the first transcatheter valve to be implanted in humans by Bonhoeffer et al. [41]. The Melody transcatheter pulmonary valve (TPV) is made up of a heterologous (bovine) jugular vein valve sutured within a laser-welded, platinum-iridium stent and has its welds brazed with gold. There are two sizes available for the Melody TPV, a 16 mm bovine jugular vein (nominal length of 30 mm) which can be expanded to 20 mm, and 18 mm bovine jugular vein (nominal length of 28 mm) that can be expanded to 22 mm. The valve is delivered through the Medtronic Ensemble delivery system which uses a “balloon-in-balloon” (NuMED Inc., Hopkinton, NY, USA) available in three sizes, 18, 20, and 22 mm. The valve is delivered through a 22-Fr Ensemble Transcatheter Delivery system (Medtronic).
As in all balloon-expandable pulmonary valves, all patients receiving TPV implantation should be evaluated for the possibility of coronary artery compression with either aortography or selective coronary angiography before deployment of the TPV [42,43]. In the current practice, the procedure involves first inserting stents inside the outflow tract or conduit to provide a robust, rigid landing zone for the Melody valve to prevent frame fractures because the Melody valve frame alone lacks the radial strength to withstand fracture [42].
Interestingly, in 2017, Boudjemline Y innovated a one-step procedure that enables interventionists to pre-stent (up to three stents) and implant the melody valve simultaneously. This approach resulted in a reduction in procedure time, fluoroscopic time, and radiation exposure when compared to the usual 2-step approach [44].
The issue of endocarditis following TPVR with the Medtronic Melody valve has received significant attention, as its incidence rate is approximately 3% per patient-year. This is 3 to 6 times greater than the 0.5–1% per patient-year observed with other valve alternatives. [45,46].
- (2) Edwards SAPIEN 3
Originally intended to be inserted into the aortic position, the SAPIEN Transcatheter Heart Valve (THV) was found to have excellent success rates when implanted into the pulmonary position; the first pulmonary implantation was accomplished successfully in 2006 [47].
There are different versions of SAPIEN THV, however, SAPIEN 3 is the one currently being used in the pulmonary position.
The components of the Edwards SAPIEN 3 (Edwards Lifesciences LLC, Irvine, CA, USA) THV include a trileaflet bovine pericardial tissue valve, a balloon-expandable, radiopaque, cobalt-chromium frame, and a skirt made of polyethylene terephthalate (PET). With four rows and four columns, the biomedical-grade chromium-cobalt frame offers high radial strength to prevent fractures.
It is available in four sizes, 20, 23, 26, and 29 mm permitting implantation of the valve in larger RVOTs. Additionally, SAPIEN 3 THV features uniquely designed externally sealed PET skirts that may reduce paravalvular leakage [47] (Fig. 1).
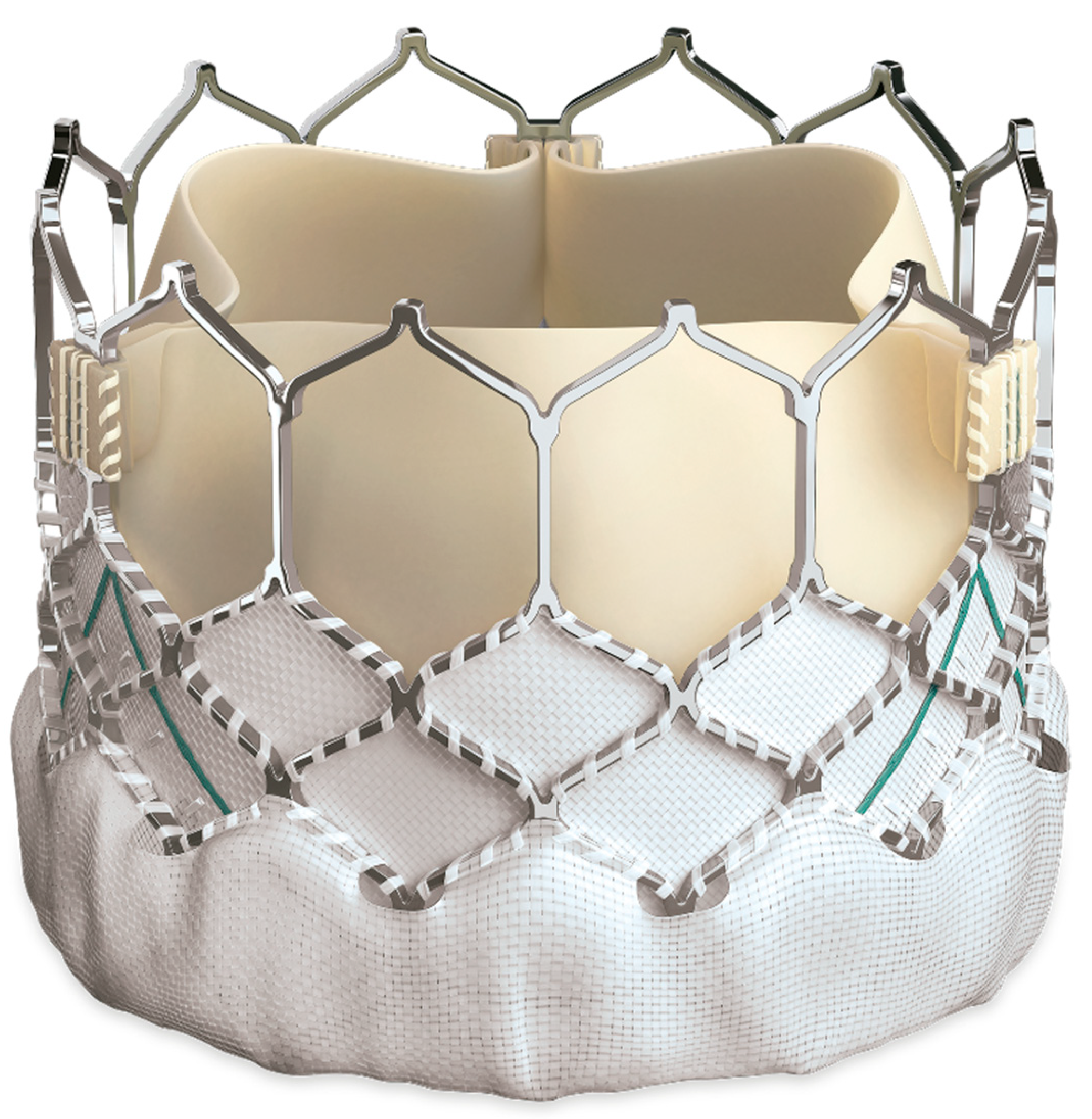
Figure 1: SAPIEN 3 valve.
The valve is crimped with an Edwards crimper to decrease its diameter so that it can be mounted onto the delivery system (Edwards Commander Delivery system) (Fig. 2). It is advised to introduce the Edwards Sapien valve delivery system using the 14 Fr (for 20, 23, 26 mm valves) or 16 Fr (for 29 mm valves) Edwards expandable sheaths (e-sheaths).

Figure 2: SAPIEN-3 delivery system (Edwards Commander Delivery system).
Recommendations for valve size are dependent on the size of the native valve annulus, using transesophageal echocardiography (TEE) or CT scan measurements.
TPVR in pediatric patients using the SAPIEN 3 valve has demonstrated promising outcomes. Studies have shown that the procedure success rates are exceptionally high, ranging from 96.4% to 98.6% for different cohorts [48,49].
In spite of the excellent outcomes of the TPVR with SAPIEN valve, tricuspid valve injury during valve delivery has been one of the most concerning complications associated with SAPIEN TPVR [50,51]. In their study, Butera et al. observed that tricuspid valve injury occurred in 3 out of 50 patients during SAPIEN 3 valve implantation. Therefore, modifications made on the delivery technique using long Gore DrySeal sheath resulted in a safer and faster SAPIEN TPVR procedure [52,53,54]. In a study comparing two groups, 8% (2 out of 25) of patients using standard delivery techniques (group I) developed severe tricuspid regurgitation, while no injuries were reported (0 out of 23) when a Gore DrySeal sheath was utilized (Group II) [52].
- (3) c. Myval
Myval (developed by Meril Life Sciences) is a CE marked device designated for percutaneous aortic valve implantation in 2019. It is distinguished by a nickel-cobalt alloy frame made of a single hexagon. The hexagons are organized in a hybrid honeycomb pattern, with 53% of the frame consisting of open cells at the aortic end so that the coronary arteries are well perfused and 47% of the frame consisting of closed cells at the ventricular end, which provides a larger annular radial force. Moreover, to reduce paravalvular leakage, the lower “closed cell” segment is covered by a protective PET sealing cuff externally (Fig. 3).
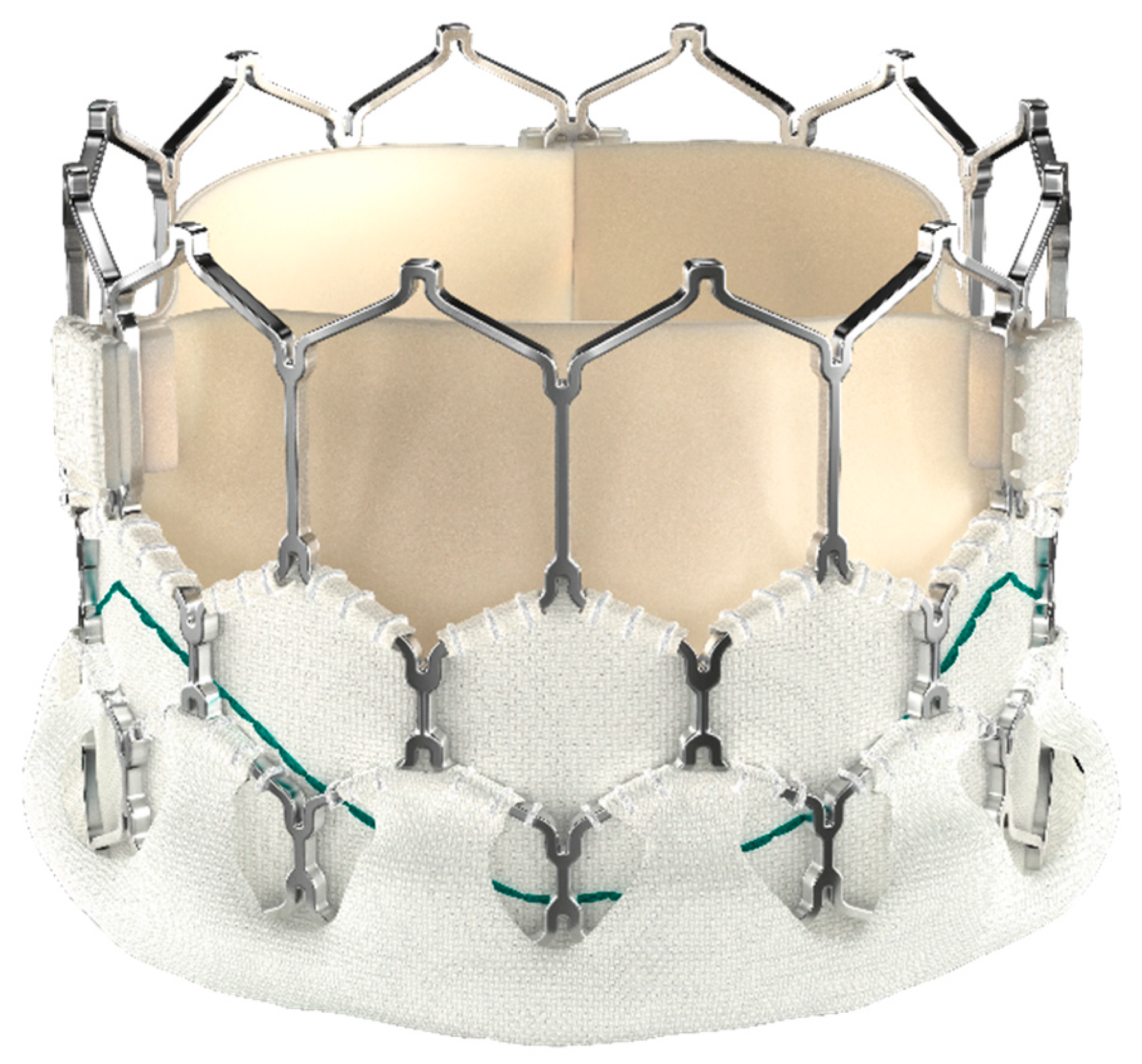
Figure 3: MyVal Valve.
The available sizes of the valve range from 20 to 32 mm in 1.5-mm increments. The availability of these wide range size options is one of the main advantages of the valve over other existing valves.
The Myval valve is delivered through a unique delivery system, named Navigator (Meril Life Sciences Pvt. Ltd., Gujarat, India). Two stoppers are included in the Navigator delivery system to avoid embolization while the valve is being advanced. The valve is crimped directly onto the balloon rather than the catheter shaft. Therefore, the valve does not need to be repositioned [55].
Prior to the procedure, patient should be assessed thoroughly with clinical examination, blood testing, electrocardiography, chest radiography, and transthoracic echocardiography. Important echocardiographic information should include RVOT gradient, severity of PR, right ventricular systolic pressure, systolic and diastolic function of both ventricles. Cardiac magnetic resonance imaging is advised to measure ventricular volumes and function and PR fraction, especially for patients with significant PR. It is important also to perform conduit and selective coronary angiogram and balloon interrogation to assess the diameter of the valve and coronary compression risk. Prestenting is an important step to provide a proper landing zone for the valve. However, many cases have been performed without prior pre-stenting. The selected size of the valve diameter should be 1–2 mm greater than the stented conduit diameter based on the pre-procedural assessment [56,57].
After obtaining the vascular access, typically through the femoral vein, and advancing a catheter to the right heart, hemodynamics and angiograms should be recorded. Heparin should be given to achieve activated clotting time more than 250 s and antibiotic prophylaxis should be received. Myval THV of a proper size is crimped using Navigator THV balloon-expandable delivery system. Then, the Navigator THV delivery system with the crimped Myval THV is advanced using the Python sheath over a Lunderquist wire (Cook Medical, Bloomington, IN, USA). The valve is then positioned across the conduit stent, and deployed via balloon inflation. Following deployment, RV angiogram is performed to evaluate the function of implanted valve [57].
Transthoracic echocardiography is performed after the procedure to verify valve function, relief of RVOT obstruction, and RV contractility.
Patient should be started on antiplatelet therapy with aspirin for at least 6 months. Some operators may add Clopidogrel for 2–3 months.
Patients should be followed at intervals of 3–6 months with echocardiography to assess valve function, gradient and presence of regurgitation, and electrocardiography for arrhythmias.
According to a recent multicenter study, the early and mid-term outcomes of MyVal transcatheter pulmonary valve implantation are encouraging. The study involved fifty-three pediatric patients (median age of 15 years) from seven cardiac centers. The results of the study showed significant reduction in the peak instantaneous gradient across the RVOT from a pre-procedure median of 23.5 mmHg (IQR 10–53 mmHg) to a post-procedure median of 10 mmHg (IQR 5–16 mmHg). After a follow up period of 360 days (IQR 164–525 days), no tricuspid valve regurgitation was reported. Only three cases of moderate neo-pulmonary valve regurgitation were reported [58].
The commonly used balloon-expandable valves are not appropriate for the native outflow tracts, particularly enlarged RVOT, especially in patients treated with transannular patch approach.
- (1) Venus P-Valve
The Venus P-Valve (Venus MedTech, Shanghai, China) is intended to treat patients with patched RVOTs, particularly with dilated RVOTs. The self-expanding Venus P-Valve (Venus MedTech, China) received the CE certification in April 2022, making it a viable substitute for the largest native tracts. It is a trileaflet porcine pericardial tissue valve that is sutured onto a self-expandable nitinol support frame. It is characterized by a flared design at both distal and proximal ends. While the proximal inflow (RVOT) end is covered, the distal outflow (PA) end is left uncovered at the pulmonary bifurcation to ensure unobstructed branch pulmonary artery flow. The flared part of both ends measure about 10–14 mm in length and the width is 10 mm larger than the diameter of the middle (straight) part. The presence of proximal and distal flares creates an hourglass orientation which aids in stabilizing the valve in the RVOT and main PA. The valve position, proximal flare, and distal flare are identified by three sets of radiopaque platinum marker bands which help fluoroscopic positioning of the valve especially at the time of deployment (Fig. 4).

Figure 4: Venus P Valve.
The delivery system is made up of a 16 F 100-cm-long shaft catheter and a 20–22 Fr capsule with a rotating handle for controlled deployment of the valve. In order to load the valve, it must first be immersed in a sterile iced saline solution, which helps to reduce the nitinol’s memory qualities. The valve is then crimped using a specialized crimping instrument. A 22–24 F Gore DrySeal sheath is used to advance the delivery system to the target area.
The valve is readily available in diameters ranging from 28 mm to 36 mm in 2 mm increments, and lengths of 25 and 30 mm of the middle straight part of the stent.
A thorough assessment of the right ventricular outflow tract is essential for patient selection, as well as for the effective preparation and execution of Venus P-Valve implantation. It is vital to evaluate the candidate’s anatomy for the valve implantation using cardiac MR (CMR) with or without CT scan. Cardiac catheterization is also recommended to improve valve selection. To determine the appropriate size of the valve, the balloon waist is measured and subsequent oversizing by 2–4 mm is the standard. The valve length that is selected ought to be comparable to the distance measured by the RVOT between the predicted valve annulus position and the PA bifurcation.
Following tPVR using Venus P-Valve, patient should be started on Aspirin for at least 6 months. It is important to follow-up patients in the clinic at 1-, 3-, and 6-month intervals, and annually after that. Moreover, it is advisable to perform CMR to evaluate the RV function and size between 6–12 months from implantation.
Excellent early outcome results have been reported in various studies [59,60,61].
A recent study has reported the five-year follow up outcomes of the Venus P-Valve implantation from six hospitals in China [62]. It included 55 patients with moderate to severe pulmonary regurgitation. Valve dislodgement/migration to right ventricle occurred in only one patient two days after implantation and a surgical intervention was needed to relocate and suture the valve back into the pulmonary annulus. One patient died due to infective endocarditis 6 months after implantation. After that, no more death incidents were reported. Total of five patients developed infective endocarditis. With the exception of one mortality and one surgical reintervention, three patients were treated with 6-weeks course of antibiotics. The authors linked the increased risk of endocarditis to the lack of strict clinical guidelines in administrating antibiotic prophylaxis after surgical or percutaneous interventions in China. Five patients developed atrial tachyarrhythmias, however, no ventricular arrhythmia was reported. During the five years follow up there were no stent fracture or paravalvular leak. 6 months following implantation, there was a significant improvement in the New York Heart Association (NYHA) functional class compared to baseline. On echocardiographic follow up assessment, only one patient progressed to moderate PR, but the other patients continued to have ≤mild PR at five years. There was no significant change in the peak transpulmonary gradient or stent orifice diameter over time. Longer-term follow-up data remain necessary [62].
- (2) Harmony Valve
The Medtronic Harmony valve is known to be the first FDA-approved self-expanding valve in native and patched RVOTs. It is constructed from a nitinol frame with a woven polyester cloth covering and a porcine pericardial tissue valve sewn into its center. To reduce leaflet calcification, the Harmony valve is treated with an alpha-amino oleic acid anti mineralization procedure. Then, 0.2% glutaraldehyde is used to sterilize the material. It is available in two sizes (PV 22, model number HARMONY-22 and TPV 25, model number HARMONY-25), depending on the valve housing diameter. The TPV 22 is longer but smaller in diameter (outflow and inflow diameters) compared to TPV 25. Both valve sizes are delivered using a 25-Fr delivery system with a coil loading catheter [43].
Choosing the proper size Harmony TPV bioprosthesis is dependent on the patient main PA sizes that is measured using ECG-gated CTA, with reconstructions done in both the systolic and diastolic phases. To make screening easier, Medtronic created the “perimeter plot,” which visually shows the anatomy of the RVOT. This plot helps compare the average diameter of the valve frame with the RVOT anatomy during both phases of the cardiac cycle. Suitable candidates for Harmony valve implantation should have a proper overlap of the RVOT anatomy with the valve frame. Recently, Medtronic has emphasized the importance of assessing both systolic and diastolic anatomy for a good valve fit. The valve should be larger than the patient’s RVOT anatomy at both ends to ensure a proper fit [63].
The Harmony device is not meant for those who have received treatment with an RV-PA conduit or who have had a bioprosthesis implanted in the past.
Interestingly, Mejia et al. described a novel approach by implanting a self-expanding transcatheter valve (Harmony valve-TPV22) in the right PA position with excellent outcome [64].
Recently, Agasthi et al. described a TPVR using a Harmony valve through Transjugular approach in a patient with no femoral access [65].
According to Gillespie et al., the 5-year outcome from the Harmony TPV Early Feasibility Study showed stable Harmony TPV position (18 out of 20 patients). Two patients underwent surgical explants: one before discharge (because the device was undersized), and the other one developed obstruction due to valve frame fracture at one-month. There was only one fatality, after the 3-year follow-up with no device-related relationship-severe valve or perivalvular leak [66].
In a recent multicenter registry study of 243 patients who underwent TPVR using Harmony device, with 7 patients under the age of 15 years, ventricular arrhythmia was commonly observed, affecting about one-fourth of patients. Of note, a diagnosis of valvular pulmonary stenosis (PS) was noted to be associated with ventricular arrhythmia in this registry [67].
- (3) Pulsta® Valve
Pulsta Valve (TaeWoong Medical Co., Ltd., Gimpo-si, Gyeonggi-do, South Korea)) is a self-expandable transcatheter heart valve designed for percutaneous pulmonary valve implantation in patients with native RVOT lesions. It is composed of a nitinol stent frame and Porcine pericardium leaflets that are sewn to the Nitinol stent wall. The porcine valve leaflets are treated with decellularization, α galactosidase to eliminate the α-gal xenoantigen, space filler, glutaraldehyde fixing, organic solvent treatment, and detoxification. The available valve diameters range in increments of 2 mm from 18 to 32 mm to accommodate diverse RVOT geometries. The valve has flares on both sides and is 4 mm wider than its outer diameter. The valve’s overall length possibilities range from 28, 31, 33, and 38 mm depending on the length of the RVOT. With its cylindrical shape and uncovered proximal and distal ends, the Pulsta valve allows for accurate positioning without obstructing the blood flow (Fig. 5) [68,69,70].

Figure 5: Pulsta valve.
A low-profile (18 or 20 Fr) catheter can be used to load the Pulsta valve directly into the venous system with simple pre-crimping at room temperature with a certain device is necessary (Fig. 6).
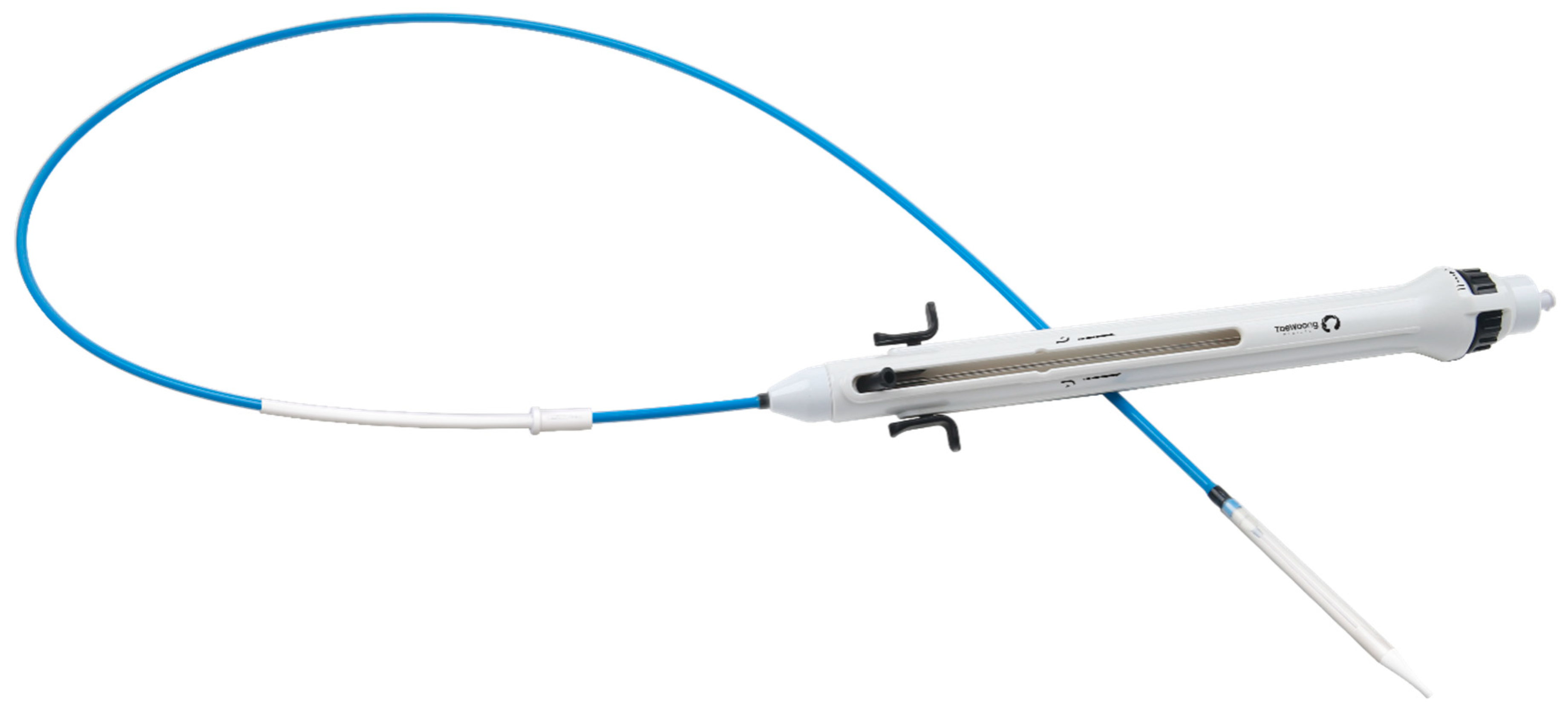
Figure 6: Pulsta valve delivery system.
In clinical settings, the main area of interest for Pulsta valve implantation in transcatheter pulmonary valve replacement procedures is usually the main PA. RVOT, including the main PA, is measured prior to the procedure using various imaging techniques like echocardiography, cardiac CT scans, and cardiac MRI. During the actual procedure, angiography and balloon sizing are used to determine the RVOT diameter and length. Measurements of the main PA are taken at its most dilated phase, considering parameters like the PA annulus, mid-PA section, and length. The length of the RVOT, including the main PA, is measured from the proximal annulus to the distal bifurcation site, helping in planning and executing TPVR procedures accurately.
A multi-center clinical trial in South Korea involved 25 patients with severe pulmonary regurgitation (PR) and enlarged RV volume. The mean age was 21.6 ± 6.6 years old (range of 11.2–38.5 years). The Pulsta valves were successfully implanted in all patients using 18 or 20 French delivery catheters. At 6-month follow-up, the indexed RV end-diastolic volume dropped significantly to 126.9 mL/m2. The mean pressure gradient across the Pulsta valve at the average follow-up duration of 33.1 ± 14.3 months was 6.5 mmHg, showing efficient valve function without significant PR [71].
According to Park et al., the Pulsta valve is adaptable to various shapes of the native RVOT. They identified different shapes of main PA including pyramidal, straight, reverse pyramidal, convex, and concave. They reported a high success rate of 98.4% (179 out of 182 patients) for Pulsta valve implantation, suggesting it is effective across diverse RVOT anatomies. The mean follow-up duration was 29 months, with significant reductions in right ventricle volume from 163.1 mL/m2 to 123.0 mL/m2 after one year. However, two patients needed surgical intervention to remove their Pulsta valves after they embolized to the RV, while one patient needed surgery to correct their valve after it migrated to the distal main PA [70].
- (4) Alterra Adaptive Prestent
The Alterra Adaptive Prestent (Edwards Lifesciences, Irvine, CA, USA) was first described by Zahn et al. in 2018. The purpose of the Alterra Adaptive Prestent is to serve as a docking adaptor for the 29 mm SAPIEN 3 THV within the RVOT. It is made up of a self-expanding, radiopaque, nitinol frame assembly, with PET fabric covering, and has distinct inflow and outflow ends. The inflow section has two triangular tabs that are connected to the delivery system and circumferential covering of all cells, while the outflow section has open cells to allow blood flow into branch pulmonary arteries. The PET fabric is sutured to the inside surface of the frame to make a seal at the inflow section. The device has a symmetrical frame design with 40 mm inflow and outflow diameters and a 27 mm central section for THV placement. The total device length is 48 mm, with a covered length of 30 mm at the outflow [72].
The Alterra Adaptive Prestent is delivered using a custom delivery system with a handle, retractable outer shaft, inner delivery shaft, prestent connector, and tapered tip for easy tracking through blood vessels. The delivery handle has a single knob for slow, controlled deployment, recapture, and a flush port for flushing the guidewire lumen. The system can fit through a 16 Fr eSheath (Edwards Lifesciences, Irvine, CA, USA) [72].
3.1 Aortic Valve Replacement (AVR)
Aortic valve disease is a common congenital heart defect that affects approximately 6% of children with congenital heart disease [73]. The most common form of aortic valve disease is aortic stenosis accounting for about 71% of patients [74]. Repair of aortic valve disease surgically is the recommended initial treatment option to allow the patient to grow for more definitive solution. Nevertheless, in children with extensive valve destruction following repair, or failure of intervention, aortic valve replacement may become essential [75,76,77]. The most common indications for AVR are severe aortic valve stenosis and severe aortic valve regurgitations.
Developing an ideal heart valve implant remains an unmet goal. Current options must be carefully tailored to balance survival outcomes against foreseeable complications. Surgical AVR options in pediatric patients include mechanical valves, bioprosthetic valves, aortic valve homografts, or the Ross procedure. Table 1 demonstrates the currently available mechanical and bioprosthetic valves used in pediatric patients.
Table 1: Comparative Analysis of Valve Options in Pediatric Cardiac Surgery: Key Parameters at a Glance.
| Parameters | Ross Procedure | Mechanical Valve | Bioprosthetic Valve | Ozaki Procedure | Homografts | Contegra® |
|---|---|---|---|---|---|---|
 |  |  |  |  |  | |
| Durability | Good | Excellent | Poor | Variable | Variable | Moderate |
| Re-intervention Rate | High | Moderate to High | High | Low | Moderate to High | Moderate to High |
| Growth potential | Yes | No | No | Yes | No | No |
| Anticoagulation | Not required | Required | Not required | Not required | Not required | Not required |
| Suitability | All ages | >5 years preferred | Older adolescents | All ages | All ages | All ages |
Mechanical aortic valve replacement (mAVR) in children with congenital heart disease demonstrates concerning long-term outcomes despite relatively favorable initial results, with survival rates of 90–96% at 1 year declining to 81–90% at 10 years [78,79,80]. It offers excellent long-term hemodynamic performance, especially with valve sizes >21 mm in the aortic position [79]. Although, patient-prosthesis mismatch and lack of growth potential remain concerns [80], a miniature mechanical valve of 15 mm is available as an option in infants when alternatives are unavailable [81]. Younger age and smaller valve size are also associated with increased risk of adverse events and reoperation [78,79]. Reoperations were necessary for up to 33% of patients, and freedom from reoperation at 90% at 7 years, 78% at 10 years and 28.5% at 20 years [78,80,82]. For these reasons, mAVR is typically reserved for children over five years and is supported by data demonstrating improved outcomes in this age group [79,83].
Mechanical valves require lifelong anticoagulation therapy. As a consequence, children with mechanical aortic valves experience substantial morbidity, with a composite linearized event rate of 3.2% per valve-year, comprising 0.41% of valve thrombosis, 1.6% of thromboembolic complications including transient ischemic attacks and strokes and 1.2% bleeding events per valve-year [76]. The hemorrhagic complications are two to three times higher than the rates associated with the Ross or homograft AVR procedures, raising concerns for pediatric patients and young females considering future pregnancies [78,84].
Bioprosthetic aortic valve replacement (bAVR) is less frequently used in children due to the rapid structural degeneration of the valves seen in younger patients. Reports indicate that reoperation rates can be as high as 18% within three years for certain types of valves, with as low as a 33% freedom from reoperation at 10 years, underscoring the challenges associated with this approach [85,86]. Despite these limitations, bioprosthetic valves may be considered for older adolescents or patients with contraindications to anticoagulation. Advances in valve design and anti-calcification treatments hold promise for enhancing durability. Thus, bioprosthetic valves represent an alternative option, although their utility in pediatric populations remains uncertain and limited.
The Ross procedure involves auto-transplanting the patient’s pulmonary valve to the aortic position, with a biological conduit replacing the original valve. This technique offers a unique growth potential, but it comes with significant challenges, such as increased technical complexity and a higher likelihood of needing future interventions. It is an appealing option due to its somatic growth capabilities, excellent hemodynamic performance, and favorable long-term outcomes, with survival rates exceeding 90% at 20 years in several studies [87,88]. The primary advantage of this procedure is that it provides a living, autologous valve replacement that can grow alongside the patient, thus avoiding complications associated with prosthetic materials. The pulmonary autograft positioned in the aorta has shown remarkable hemodynamic properties, such as low transvalvular gradients, larger effective orifice areas, and enhanced left ventricular function [89,90].
The Ross procedure offers a significant advantage by eliminating the need for anticoagulation, which is particularly beneficial for pediatric patients. Children undergoing this procedure experience fewer valve-related complications than other valve replacement methods. Notably, the procedure reduces the risks of thromboembolism and infective endocarditis [91]. Research shows that the long-term survival rate for children receiving the Ross procedure is between 88% and 94% at 20 years, and in some cases, it is comparable to that of the general population [88,90].
The Ross procedure has its limitations. Its technical complexity, especially in converting a single-valve disease to a double-valve disease, requires a high level of surgical expertise and must be carried out in specialized centers by experienced teams. Furthermore, there is a risk of autograft dilatation and failure, with freedom from reoperation rate of approximately 70–80% at 20 years [92]. The procedure may not be appropriate for patients with certain connective tissue disorders, such as Marfan syndrome or Ehlers-Danlos syndrome, due to the increased risk of autograft dilatation. A recent meta-analysis from the Netherlands, utilizing microsimulation modeling to examine the effectiveness of aortic valve interventions in children, found that while the Ross procedure offers better survival rates compared to mAVR, it has a notably higher risk of reintervention, at 42% after 20 years [93]. The overall reintervention rate following the Ross procedure is about 3.4% per year, surpassing the approximately 1.2% per year rate for other interventions.
Despite various challenges, the Ross procedure can be performed on patients of any age, including neonates. A UK national database study found it to be the most common AVR in neonates, infants, and children under 16, comprising 78.5% of cases [90]. This procedure is unique in achieving survival rates comparable to those of matched general populations. However, it presents a significantly elevated mortality rate in neonates and infants, with early mortality ranging from 18.3% to 23.3%, mainly due to procedural complications like coronary transfer issues [94]. There is also a concerning late mortality rate of 9.7% to 15% in infants, underscoring the serious risks associated with coronary manipulation during pulmonary autograft transfer in this vulnerable population [95].
The Ozaki procedure creates customized neo-cusps from either autologous pericardium or synthetic materials and has emerged as a promising alternative for treating aortic valve disease in children [96]. This technique preserves the aortic valve complex while effectively replacing the leaflets [97]. Short-term results in pediatric patients show acceptable outcomes, including low mortality and good valve function [98]. Early clinical studies indicate the procedure offers excellent hemodynamics and low reoperation rates, with some studies reporting over 90% freedom from reoperation at 5 years for older children [99]. Like the Ross procedure, the Ozaki technique demonstrates similar effectiveness but potentially fewer adverse effects. These include reduced risks of thromboembolism and infection, shorter hospital stays due to its less invasive nature, and quicker postoperative recovery [100]. The Ozaki procedure also serves as a valuable alternative or bridge to the Ross procedure, particularly for children with aortic stenosis and small annuli. It can effectively stage patients for eventual aortic valve replacement with prostheses to accommodate somatic growth into adulthood. Various clinical scenarios illustrate the benefits of aortic valve repair using the Ozaki technique. For instance, it is particularly suitable for patients with anatomical variations such as truncal arteriosus [101], where the Ross procedure may not be feasible. Additionally, it can serve as a preparatory step for patients at high risk of root dilation, paving the way for a reinforced Ross procedure in a larger conduit.
Some studies advise caution regarding the Ozaki procedure, citing complications and reinterventions in young patients [102]. Additionally, the long-term durability of this approach remains uncertain, particularly in small children, mainly due to the materials used for neo-cusps. Currently, no ideal substitute completely overcomes the limitations of existing materials. The Ozaki procedure typically uses glutaraldehyde-treated autologous pericardium to create customized neo-aortic valve cusps. Other reported materials include Photofix (CryoLife Inc., Kennesaw, GA, USA), CardioCel (Admedus Regen Pty Ltd., Perth, WA, Australia), synthetic options like ePTFE and collagen-coated knitted Dacron graft (Haemasheld–Getinge AB), and porcine intestinal mucosa (Co-Matrix, Auto Tissue Berlin GmbH) [103]. Each alternative has distinct advantages and disadvantages concerning biocompatibility, immunogenicity, and long-term outcomes, with no significant advancements over autologous pericardium. Thus, while the Ozaki procedure shows promise as an alternative to traditional valve replacements, further research with larger patient cohorts and extended follow-up is essential to assess its long-term success in pediatric populations.
Homografts provide distinct advantages as aortic valve substitutes for children, including no anticoagulation requirement and a low risk of thromboembolism. Despite a reported surgical risk of about 2.5% [104], a meta-analysis by Notenboom et al. highlighted a concerning mortality rate of up to 10.6% [6,93]. These valves are particularly useful for complex aortic root issues, especially in endocarditis cases or when other treatments are not viable. However, they have drawbacks, including limited durability. For instance, one study showed an 81% freedom from reoperation at 60 months for homografts in the pulmonary position [6], while another indicated about 85% of patients remained reoperation-free at 10 years with aortic homografts [104]. Notably, the lifespan of homografts in younger patients is often shorter due to accelerated calcification and degeneration [11,105].
Pulmonary homografts are more durable and show less wall calcification than aortic homografts when used in the pulmonary position [6]. However, this benefit may not apply to aortic valve replacements in children. Studies in adults also indicate that pulmonary homografts used for aortic valve replacement have poorer outcomes, with significantly higher reoperation rates due to severe aortic insufficiency (35% in pulmonary homografts versus 0% in aortic homografts at 3.3 years) and reduced survival rates after 15 years (68.7% for pulmonary homografts compared to 79.9% for aortic homografts) [106,107]. Additionally, there is a lower rate of freedom from reoperation in pulmonary homografts (57.4% versus 77.7%) [107]. These performance differences occur because pulmonary tissue degenerates quickly under systemic pressure. Despite these differences, considerations such as size compatibility and availability often precede the choice of graft source in clinical practice. Current research is focused on improving homograft durability and performance through advanced preservation and decellularization techniques, which may help to overcome the specific limitations found in traditional homograft preparations [108].
3.1.6 Decellularized Aortic Homografts (DAH)
DAH have emerged as a promising option for pediatric aortic valve replacement, offering lower immunogenicity than traditional cryopreserved homografts. DAH yield significantly lower early mortality (2.2%) compared with conventional cryopreserved homografts (4.2%) in pediatric aortic valve replacement [109]. Additional reports suggest that DAH offers a higher rate of freedom from explantation and improved survival compared to standard homografts [11,105]. A multicenter study involving 143 DAH implants in children demonstrated excellent mid-term survival and adverse event rates comparable to the Ross procedure [110,111]. The study found that DAHs maintained normal hemodynamics without necessitating the removal of the pulmonary valve, making them a viable alternative for children who are not suitable candidates for the Ross procedure. Although the Ross procedure may provide the possibility for growth, it nevertheless carries a comparatively higher reintervention rate, around 42% over 20 years. Bioprosthetic valves used in AVR have a median reoperation interval of less than five years for pediatric patients [85]. In contrast, decellularized aortic heart valves (DAHs) may provide greater durability, potentially decreasing the frequency of surgical interventions in this population.
Although the decellularization process effectively reduces immunogenicity, it does not completely eliminate immune responses or cytokine-related inflammation [112,113]. Thus, while mid-term data suggest improved durability, long-term monitoring remains essential to evaluate their performance and growth potential in pediatric patients.
3.2 Transcatheter Aortic Valve Implantation (TAVI)
The outcome of SAVR in pediatric population has been well documented thoroughly in the literature, however, Transcatheter aortic valve implantation (TAVI) is rarely described. TAVI is widely used in the adult population, but there is limited data regarding its application in children. Generally speaking, TAVI is typically not an option for younger children due to limitations of the available valve sizes as well as delivery system size requirements via transfemoral arterial approach. Till now, there is no FDA-approved transcatheter valve for treating aortic valve dysfunction in the pediatric age group. However, from the limited case reports and small case series there is a trend to use SAPIEN 3 and to less extent melody valve as a transcatheter valve in the aortic position. In their retrospective study, Robertson et al. described the use of SAPIEN 3 valve in 17 patients who underwent TAVI using a transfemoral arterial approach with low morbidity and mortality at short-term outcomes. The youngest patient who underwent TAVI in this study was 13 years of age, and weight range between 54 and 67 kg (119 and 147 Ibs). The main factor that restricts adoption of the transfemoral arterial approach is the size of the patient [114]. In their study of patients who underwent TAVI, Sinha et al. did not approve the use of transfemoral approach for pediatric patients weighing less than 30 kg (66 Ibs). The transfemoral approach was used in two patients and both were of adult size. This important aspect hopefully will be addressed by the advancing technology so we can have a downsized delivery system that can accommodate for smaller size pediatric patients. This study also involved two patients with Melody valves placed surgically, but within two years of the procedure Melody valves were explanted surgically due to moderate to severe aortic regurgitation. There was a clear leaflet malfunction of the Melody valve, without any fractures. This study demonstrated failure of Melody valve in the aortic environment and suggested to use SAPIEN 3 valve whenever possible. SAPIEN 3 valves used in aortic position are at increased risk of embolization if implanted in non-calcified annuli so that oversizing by 2 mm larger than the annulus is recommended [115,116]. Calcified annuli help secure the valve in position. The presence of the cuff of tissue on the lower part of the valve helps reduce the paravalvar leak.
Recently, Barfuss et al. retrospectively investigated the effects of TAVI on left ventricular (LV) reverse remodeling in pediatric patients (22 patients under 21 years old) in a single center. Results showed that after six months, there were significant improvements in LV volume, mass, end-diastolic and end-systolic dimensions, and sphericity index. Both EF and strain remained normal throughout the study [117].
4.1 Surgical Mitral Valve Replacement
Due to the small size of the annulus and the delicate nature of pediatric cardiac anatomy, mitral valve replacement (MVR) in pediatric patients presents unique technical challenges. While mitral valve repair remains the preferred intervention in children, offering the advantages of annular growth potential, preserved ventricular function, and freedom from anticoagulation, replacement becomes necessary in cases where repair is not feasible. The selection between mechanical and bioprosthetic valves mirrors the decision-making processes used in aortic valve replacement, with careful consideration of patient-specific factors [118].
Recent evidence demonstrates that mechanical mitral valve replacement (m-MVR) achieves acceptable short-term outcomes with manageable in-hospital morbidity and mortality rates, particularly among older pediatric patients and adolescents. However, significant age-related outcome disparities exist. Notably, neonates and infants experience higher rates of reduced hospital survival, extended hospitalization periods, and non-home discharge dispositions compared to older children [119]. A primary challenge in mechanical valve placement, particularly in young children, is the risk of patient-prosthesis mismatch (PPM) as somatic growth occurs, frequently necessitating subsequent operations for valve upsizing [120]. These age-dependent outcome variations, combined with persistent PPM risks, underscore the critical importance of optimal surgical timing, as clinicians must carefully balance the urgency of surgical intervention against risks associated with operating on smaller, more vulnerable cardiac structures.
Mechanical prostheses are the most common choice for MVR in children due to their excellent durability, favorable hemodynamics, and long-term survival rates approaching 80% at 20 years [119,121]. These valves come in small sizes suitable for pediatric patients, with recent reports highlighting positive outcomes, particularly with the 15-mm mechanical valve. This specific valve has a reported mean time to repeat MVR of 23 months [122,123,124]. The sustained success, however, is critically contingent upon the dimensions of the valve. When the valve diameter is 1–2 times the patient’s weight in kilograms, an optimal reoperation-free survival rate of 96% at one year can be achieved; however, this rate drops to 46% for patients whose valve size is outside this range [125].
Outcomes vary with age, as studies indicate better results in older children, while neonates and infants face significantly higher mortality rates. Specifically, mortality rates are reported at 10% for neonates and 11.8% for infants, in contrast to just 3.2% in older children [119,126]. Long-term outcomes are especially poor for neonates and infants, with 10-year survival rates falling below 50%. [83,127]. Additionally, lifelong anticoagulation therapy is necessary, which increases the risks of bleeding and thromboembolic complications. Due to the lack of growth potential, PPM is also a concern [120,128,129]. Strategies such as supra-annular valve placement and innovative annular and subvalvular enlargement techniques have been suggested to mitigate PPM [124,130]. Nonetheless, reoperation for valve upsizing remains almost inevitable in small children undergoing MVR [131].
Bioprosthetic mitral valve replacements (bMVR) in children are still challenging. They are infrequently performed due to high early failure rates stemming from rapid valve degeneration caused by pannus deposition and calcification [132], which leads to a frequent need for reoperations. This limitation is further highlighted by studies indicating a median time to reoperation of less than two years [85,121]. Despite these obstacles, bioprosthetic valves may be considered in specific cases, such as older adolescents or patients with contraindications to anticoagulation. Reports indicate no difference in transplant-free survival after bioprosthetic and mechanical mitral valve replacement in children [129]. Recent advancements in anti-calcification treatments and bioprosthetic valve designs seek to enhance their durability and expand their applicability in pediatric populations. The Perimount Magna-Ease (Edwards Lifesciences Corp., Irvine, CA, USA) pericardial bioprostheses is an example of this; however, it has also shown accelerated structural degeneration in children, necessitating vigilant monitoring [133].
The pulmonary autograft procedure, known as Ross II, had emerged as a compelling option for mitral valve replacement within the pediatric population, proffering two principal advantages: the potential for growth adaptation and the absence of the need for long-term anticoagulation therapy [134,135]. Longitudinal surveillance studies have elucidated encouraging outcomes concerning valve functionality and patient survival; however, the necessity for subsequent surgical interventions remains a considerable consideration [136,137]. Noteworthy cases have illustrated the procedure’s potential durability, epitomized by a documented instance of sustained valve competence in an infant over a 12-year follow-up period [138]. Nevertheless, the procedure presents specific challenges, particularly the risk of autograft dysfunction and the possible requirement for re-operative surgery. Despite these limitations and declining use, the Ross II procedure remains an essential surgical alternative for carefully selected pediatric candidates. This is particularly relevant in cases where anticoagulation therapy is contraindicated or poses significant challenges to effective management [137].
4.1.4 Modified Bovine Jugular Vein Valve (Melody Valve)
The Melody valve (Medtronic Inc., USA), originally designed for pulmonary valve replacement, has shown promising outcomes in off-label surgical MVR for pediatric patients, particularly addressing a critical need in infants with small mitral annuli and irreparable mitral valve disease. Recent studies have reported favorable outcomes in children under one year of age, with notably competent valve function and low gradient profiles [139,140,141]. A key advantage of the Melody valve is its unique ability for catheter-based expansion to accommodate somatic growth [142]. However, significant concerns remain regarding the risk of late infective endocarditis and relatively high reintervention rates [141]. Alternative options in the evolving pediatric mitral valve replacement landscape include the Edwards Sapien 3 valve (Edwards Lifesciences Corp., USA), which has demonstrated promising early safety and feasibility in infant populations [143]. These emerging data suggest an expanding array of surgical options for this challenging patient population, though long-term outcomes and comparative effectiveness studies are essential areas for future research.
4.2 Transcatheter Mitral Valve Replacement (TMVR)
Although Transcatheter Mitral valve replacement (TMVR) is a well-established intervention in high-risk surgical adults, there is limited experience with TMVR in pediatric patients. Only a few pediatric valve-in-valve (ViV) case reports have been published and were performed through a hybrid, transapical approach in all but one patient [144].
Murphy M et al. published a case report of an 11-year-old boy with St. Jude mechanical prosthesis with severe mitral stenosis that was replaced through a hybrid technique with a 26 mm Edwards Sapien 3 valve, with excellent outcome after one year [145]. In 2019, Momenah et al. published a case report of an 11 year old girl who underwent a transcatheter valve-in-valve implantation using a 23 mm Edwards Sapien 3 to replace a stenotic mitral valve prosthesis with satisfactory outcome [146].
Transcatheter Mitral Valve Replacement in pediatric patients presents several limitations and challenges that hinder its widespread application. These challenges stem from technical difficulties, limited device options, and the unique physiological considerations of pediatric patients. Compared with adults, the challenges of TMVR in children are mainly related to the relatively small size of the patients and the small dimensions of the LA and LV, which not only make the procedure more likely to be technically challenging but also may mean there is a higher risk of causing left ventricular outflow tract obstruction (LVOTO) obstruction with the newly placed valve [146].
It is preferred to use the Sapien 3 valve in pediatric patients because it is shorter compared to other transcatheter valves such as the Melody valve, and therefore it does not go beyond the struts of the defected surgical valve. This avoids LVOTO, which is one of the complications of transcatheter mitral valve implantation [147].
All of the studies in pediatrics used the SAPIEN valve, through transseptal approach except one case report. In a recent systemic review of 2990 adults, Al-Tawil et al. suggested that transseptal approach of MVR is superior to the transapical, due to lower mortality and complication rates [148,149].
Transseptal Valve-in-Valve Transcatheter Mitral Valve Replacement (ViV-TMVR) with the Edwards SAPIEN 3 valve is a complex procedure requiring careful patient selection. Multidisciplinary discussions involving pediatric cardiologists, interventional cardiologists, cardiac surgeons, and anesthesiologists are essential. Contraindications include infective endocarditis, bioprosthetic thrombosis, valve dehiscence, and interrupted IVC. Patients with a narrow left ventricular outflow tract, repaired ASDs, or prior ViV-TMVR are at higher risk of LVOTO and are considered poor or relative candidates. The procedure is performed under TEE and fluoroscopic guidance, with careful hemodynamic monitoring and appropriate anticoagulation. Post-procedure, patients should receive clopidogrel and aspirin for six months.
After the transcatheter mitral valve-in-valve implantation (TMVI) procedure, the transmitral mean gradient decreased significantly to a mean of 1 mmHg (range 0–3 mmHg). All patients’ functional status improved from NYHA class IV to class I during follow-up. No major complications occurred, with only one patient developing a small pericardial effusion that resolved spontaneously. The authors conclude that TMVI can be performed safely in the pediatric population with dysfunctional bioprosthetic mitral valves, with favorable early and mid-term outcomes.
AlNasef et al. reported the outcomes of TMVI in four symptomatic pediatric patients with dysfunctional bioprosthetic mitral valves. The study showed favorable early and mid-term outcomes, with a significant reduction in transmitral mean gradient from 19.75 mmHg (ranging from 15 to 22 mmHg) to 1 mmHg (ranging from 0 to 3 mmHg). The average length of hospital stay for the patients was 4 days. Following the procedure, the patients showed marked improvement in their functional status, with their NYHA class improving from class IV (severe symptoms) to class I (no symptoms) during the follow-up period [147].
Another study from Boston Children’s Hospital included eight high surgical risk pediatric patients with a median age of 9 years (ranging from 8 to 15 years). All patients underwent successful implantation of the MVR. The procedure resulted in reduction of the size of the left atrium and relieved pulmonary hypertension, with statistically significant improvements (p = 0.012 and 0.043, respectively) [144].
The long-term effects and possible need for reintervention in young patients are still important factors to take into account, even with the positive outcomes of TMVR. Additional multicenter research is required to determine long-term results.
5.1 Tricuspid Valve Replacement
Of all cardiac valves, the tricuspid valve (TV) is the least likely to require replacement in children. Data on tricuspid valve replacement (TVR) in children is very limited. TVR is only performed when the repair of TV is not amenable. Indications of TVR in children include congenital heart disease such as Ebstein’s TVs and TV dysplasia, and acquired TV diseases such as TV endocarditis, or rheumatic. Data on tricuspid valve replacement come from adult studies. Even with improvements in perioperative care, TVR is associated with high mortality rates [150]. Current surgical valve replacement options include mechanical, bioprosthetic, and homograft valves, each presenting unique advantages and challenges. It is important to note that no dedicated prosthesis has been specifically designed for the tricuspid position among the available mechanical and bioprosthetic options [151].
Meta-analyses indicate that mechanical and bioprosthetic valves have similar mortality, reoperation rates, and long-term survival [152]. Nevertheless, mechanical valves present a heightened thrombosis risk, requiring anticoagulation therapy, which complicates their application in younger patients [153,154]. Although bioprosthetic valves are prone to rapid structural failure and high postoperative regurgitation rates, they can be a viable option for children due to their predictable failure patterns and lack of anticoagulation needs [151,155,156].
Homografts may be suitable for cases involving IE, especially when valve preservation is not feasible [157]. For example, mitral homografts have been effectively used for TVR in children with IE [158,159]. Tissue-engineered valves present an intriguing alternative, utilizing autologous cells and biodegradable scaffolds to create living, adaptable valve constructs [152,160]. New options, including tubular valves made from porcine-derived extracellular matrix, show potential for improved growth and performance [161]. Furthermore, researchers are investigating allogeneic valve transplantation as a possible solution to current limitations in substitute growth [152].
The choice of valve for TVR remains highly debatable, underscoring the need for ongoing research to improve patient outcomes. Ultimately, valve selection should be customized to individual patient circumstances, considering the patient’s age and associated risk factors [153].
5.2 Transcatheter Tricuspid Valve Replacement
Reviewing the literature, the use of Melody valve is supported for the tricuspid valve position [162]. In their study, Roberts et al. described the first series of transcatheter TVR using the Melody percutaneous pulmonary valve (Medtronic, Inc., Minneapolis, Minnesota) in patients with TV disease. Of note, 2 out of 15 were pediatric patients (8 and 9 years old). This study demonstrated a high procedural success rate, with only one patient developed a third-degree heart block needing pacemaker insertion. Within 4 months of follow-up, there was one patient who developed endocarditis, and another patient with pre-replacement multiorgan failure died 20 days after the procedure [162].
Interestingly, Saini et al. recently published a unique case report of successful transcatheter Melody valve (Medtronic, Inc., Minneapolis, Minnesota) implantation in the tricuspid area for a 14-year-old boy with progressive bioprosthetic tricuspid valve stenosis post heart transplant with a satisfactory outcome after 11 years of follow-up [163].
According to Webb et al., a hybrid technique was used to implant an Edwards Sapien valve (Edwards Lifesciences, Irvine, CA, USA) using thoracotomy access in a patient with a TV bioprosthesis [164].
6 Common Atrio-Ventricular Valve Replacement
Due to the complex anatomical variations and the need to address mitral and tricuspid valve functions, atrioventricular valve (AVV) replacement requires a tailored approach. Valve replacement may be indicated in severe regurgitation or stenosis that is unresponsive to repair, particularly when associated with significant hemodynamic compromise. Options for replacement, including mechanical and bioprosthetic valves, are reasonably similar to those available for mitral and tricuspid valve disease in patients with other biventricular physiologies.
Patients with single ventricle physiology face unique challenges related to AVV disease, especially when repair strategies are unsuccessful, necessitating valve replacement. Current data reveal concerning outcomes, with in-hospital mortality rates for atrioventricular valve replacement reaching 42%, and only 66% of patients surviving three years post-procedure [165,166]. Complications associated with this procedure are significant, including a 25% likelihood of requiring pacemaker implantation and a notable risk of needing redo valve replacement, with a cumulative incidence of 20% within three years [166]. Several factors can influence these outcomes, such as the patient’s age at the time of replacement, the specific type of valve used (with tricuspid valve replacement being particularly risky), and the timing of the procedure.
Clinicians must carefully evaluate valvular replacements and alternative palliation strategies for children with single ventricle physiology and significant valve regurgitation. These strategies may include prophylactic valve repair performed concurrently with other staged palliative procedures, such as the bidirectional Glenn operation, or opting for staged palliation without valve intervention [167]. Early heart transplantation may also be considered [165]. While some centers utilize valve replacement techniques, including the Melody valve [168], as a bridge to transplantation, the literature indicates that outcomes remain challenging, irrespective of the chosen approach. Therefore, management strategies should be individualized, considering crucial factors like ventricular function, timing within the staged palliation sequence, and the patient’s candidacy for Fontan completion.
7 Emerging Solutions and Advancements
The Heart Valve Collaboratory identifies significant challenges in managing pediatric and congenital heart valve disease [169]. To improve outcomes, their multidisciplinary approach emphasizes innovation, patient-centred solutions, and regulatory collaboration. Key areas for development include enhancing valve durability, accommodating somatic growth, and advancing transcatheter technologies.
7.1 Autus Size-Adjustable Valve
The Autus Size-Adjustable Valve represents an innovative approach to pulmonary valve replacement in pediatric patients, offering a key advantage over conventional fixed-size prostheses. This investigational device’s distinguishing feature is its capacity for balloon-mediated expansion, which accommodates somatic growth. Clinical evidence from initial trials demonstrates encouraging results. report successful valve implantation and subsequent expansion procedures, with favorable hemodynamic performance maintained post-expansion [170]. Additionally, these investigations document minimal calcification and hold particular promise for reducing the burden of repeated surgical interventions, potentially improving patient quality of life. However, critical questions persist regarding the valve’s long-term performance, particularly concerning structural integrity following multiple expansion procedures.
7.2 Allogeneic Valve Transplantation
Allogeneic valve transplantation emerges as a solution, providing living tissue that grows with the child but requires immunosuppression [152]. Allogeneic valve transplantation involves replacing damaged native valves with fresh, living allografts from size-matched donor hearts. This technique provides a unique advantage by offering growth potential, potentially reducing the need for multiple reoperations over the patient’s lifetime. Reports highlighted the feasibility of allogeneic valve transplantation, demonstrating good hemodynamic outcomes and somatic growth [152,160]. However, significant challenges persist, including the limited availability of suitable donors and the requirement for long-term immunosuppression to prevent rejection. The latter poses risks such as increased susceptibility to infections and potential impacts on the child’s overall growth and development.
Tissue-engineered heart valves show promise in pre-clinical and clinical studies, offering the potential for growth and integration [170,171]. Research into tissue-engineered valves seeks to create living replacements capable of somatic growth. These innovative valves aim to address the limitations of existing prosthetic options by facilitating remodeling, growth, and self-repair, thereby providing a long-term solution for pediatric patients. Studies demonstrate preclinical success in using decellularized scaffolds seeded with autologous cells to develop functional valve constructs [152,160]. Advances in biomaterial engineering have also introduced synthetic biodegradable scaffolds that mimic native valve architecture, offering a framework for cellular integration and extracellular matrix production. Contemporary innovations also concentrate on genetically modified porcine tissues lacking xenogeneic antigens, potentially enhancing longevity and diminishing calcification processes [172,173].
Despite these promising developments, significant challenges remain in translating these technologies into clinical practice. Critical hurdles include ensuring long-term durability, preventing calcification, and achieving uniform cell seeding. Furthermore, the scalability of tissue-engineered valves for widespread clinical use poses logistical and manufacturing challenges. Continued research and collaboration among bioengineers, cardiac surgeons, and material scientists are essential to refining these technologies and bringing them closer to becoming viable options for pediatric valve replacement.
7.4 Xeltis Pulmonary Valved Conduit (XPV)
The XPV represents a novel bioabsorbable technology for reconstructing the RVOT. Preclinical sheep models demonstrated favourable haemodynamics with minimal gradients and regurgitation at 24-month follow-up [174,175], demonstrating progressive tissue replacement and ongoing polymer resorption at 12 months [176]. Initial pediatric clinical trials reported no surgical reinterventions at 24 months, despite some instances of insufficiency due to leaflet prolapse [177]. While the XPV offers a promising alternative to conventional bioprosthetics [178], significant questions remain regarding resorption dynamics and the long-term functionality of the resultant autologous tissue [179].
7.5 “GrOwnValve” Bioprosthesis
While tissue-engineered heart valves show promise for pediatric patients, they face challenges in long-term durability and structural integrity [180,181]. The GrOwnValve, developed at German Heart Center Berlin, represents an innovative autologous tissue-engineered pulmonary valve replacement derived from patients’ own pericardial tissues. It promises self-repair capacity, growth potential, and functional adaptability and is designed to overcome the limitations of traditional valve replacement options in pediatric cardiac patients [182].
Pediatric valve replacement remains a complex and evolving field, driven by the need to address the unique challenges of valvular heart disease in children. Surgical valve replacement options as illustrated in Table 2, including biological and mechanical prostheses, have long been the standard of care; however, they often require frequent reoperations due to the growth demands of pediatric patients. Recent advancements in TAVR have significantly expanded treatment options, offering less invasive alternatives that can be particularly beneficial in high-risk or complex cases. Despite these advances, challenges remain regarding valve size limitations, long-term durability, and the optimal timing for intervention. Ongoing research, technological innovations, and multi-disciplinary approaches will continue to shape the future of pediatric valve replacement. Ultimately, a personalized treatment approach, considering the specific needs of each patient, is crucial for optimizing outcomes and improving the quality of life for pediatric patients with valvular heart disease. In addition to its clinical implications, this review provides valuable information for parents, helping them understand the treatment options available for their child and guiding them in finding the appropriate physician and heart center tailored to their child’s specific needs.
Table 2: Overview of Valve Replacement Choices for Children: Advantages and Disadvantages.
| Valve Type | Description | Advantages | Disadvantages | Best for |
|---|---|---|---|---|
| Ross Procedure | Aortic valve replaced by pulmonary valve, pulmonary replaced with homograft. | - Autograft grows with child. - No anticoagulation. - Excellent long-term outcomes. | - Complex surgery. - Two valves involved. - Risk of autograft dilatation/homograft degeneration. | Active children needing growth potential. |
| Mechanical Valve | Durable artificial valve. | - Excellent durability. - Stable hemodynamics. - Good for older children/adults. | - Lifelong anticoagulation (risk of bleeding/thrombosis). - No growth potential. - INR monitoring. | Older children/adolescents requiring durability. |
| Bioprosthetic Valve | Bioprosthetic (bovine/porcine). | - No anticoagulation (long term). - Quiet. - Good hemodynamics. | - Limited durability (5–10 years). - Rapid degeneration in children. - Risk of calcification. | Younger patients avoiding anticoagulation. |
| Ozaki Procedure | Reconstruction of aortic valve using autologous pericardium. | - No anticoagulation. - Growth potential. - Customized fit. - Good hemodynamics. | - Limited long-term data. - Requires expertise. - Risk of degeneration/calcification. | Children needing aortic valve repair. |
| Homograft | Human donor valve (pulmonary/aortic). | - Good hemodynamics. - No anticoagulation. - Lower immune response than Contegra. | - Limited durability (10–15 years). - No growth potential. - Donor-dependent availability. | Pulmonary valve replacement in complex repairs. |
| Contegra Graft | Bovine jugular vein graft for RVOT. | - No anticoagulation. - Good for RVOT reconstructions. - Good availability. | - Limited durability. - Risk of stenosis/calcification. - Not for systemic valves. | Congenital RVOT anomalies. |
Acknowledgement:
Funding Statement: The authors received no specific funding for this study.
Author Contributions: The authors confirm contribution to the paper as follows: Conceptualization, Ziyad M. Hijazi; writing—transcatheter intervention section, Baker M. Ayyash; writing—surgical section, Yen Chuan Chen and Ahmad Sallehuddin; validation, Ziyad M. Hijazi and Ahmad Sallehuddin; resources, Baker M. Ayyash and Yen Chuan Chen; supervision, Ziyad M. Hijazi and Ahmad Sallehuddin. Ziyad M. Hijazi is the corresponding author. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare the following conflict of interest. Dr. Hijazi is a consultant to Venus MedTech. The remaining authors declare no conflicts of interest to report regarding the present study.
References
1. Arunamata A, Goldstein BH. Right ventricular outflow tract anomalies: neonatal interventions and outcomes. Semin Perinatol. 2022;46(4):151583. [Google Scholar]
2. Banjoko A, Seyedzenouzi G, Ashton J, Hedayat F, Smith NN, Nixon H, et al. Tetralogy of fallot: stent palliation or neonatal repair? Cardiol Young. 2021;31(10):1658–66. doi:10.1017/S1047951121000846. [Google Scholar] [CrossRef]
3. Vanderlaan RD, Barron DJ. Optimal surgical management of tetralogy of fallot. CJC Pediatr Congenit Heart Dis. 2023;2(6 Part A):352–60. [Google Scholar]
4. Bakhtiary F, Dähnert I, Leontyev S, Schröter T, Hambsch J, Mohr FW, et al. Outcome and incidence of re-intervention after surgical repair of tetralogy of fallot. J Card Surg. 2013;28(1):59–63. doi:10.1111/jocs.12030. [Google Scholar] [CrossRef]
5. Park CS, Lee JR, Lim HG, Kim WH, Kim YJ. The long-term result of total repair for tetralogy of Fallot. Eur J Cardiothorac Surg. 2010;38(3):311–7. doi:10.1016/j.ejcts.2010.02.030. [Google Scholar] [CrossRef]
6. Yankah AC, Alexi-Meskhishvili V, Weng Y, Berger F, Lange P, Hetzer R. Performance of aortic and pulmonary homografts in the right ventricular outflow tract in children. J Heart Valve Dis. 1995;4(4):392–5. [Google Scholar]
7. Raja J, Menon S, Mohammed S, Ramanan S, Baruah SD, Gopalakrishnan A, et al. Midterm results of homografts in pulmonary position: a retrospective single-center study. Indian J Thorac Cardiovasc Surg. 2021;37(2):129–37. doi:10.1007/s12055-020-01065-1. [Google Scholar] [CrossRef]
8. Marathe SP, Hussein N, Wallace FRO, Bell D, Yong M, Betts KS, et al. Comparison of homografts and bovine jugular vein conduits in the pulmonary position in patients <20 years of age. J Thorac Cardiovasc Surg. 2022;164(3):752–62.e8. doi:10.1016/j.jtcvs.2021.11.087. [Google Scholar] [CrossRef]
9. Abbas JR, Hoschtitzky JA. Which is the best tissue valve used in the pulmonary position, late after previous repair of tetralogy of Fallot? Interact Cardiovasc Thorac Surg. 2013;17(5):854–60. [Google Scholar]
10. Hörer J, Hanke T, Stierle U, Takkenberg JJM, Bogers AJJC, Hemmer W, et al. Homograft performance in children after the ross operation. Ann Thorac Surg. 2009;88(2):609–15. [Google Scholar]
11. Sandica E, Boethig D, Blanz U, Goerg R, Haas N, Laser K, et al. Bovine jugular veins versus homografts in the pulmonary position: an analysis across two centers and 711 patients—conventional comparisons and time status graphs as a new approach. Thorac Cardiovasc Surg. 2015;64(1):25–35. [Google Scholar]
12. Ootaki Y, Welch AS, Walsh MJ, Quartermain MD, Williams DA, Ungerleider RM. Medium-term outcomes after implantation of expanded polytetrafluoroethylene valved conduit. Ann Thorac Surg. 2018;105(3):843–50. [Google Scholar]
13. Cebotari S, Tudorache I, Ciubotaru A, Boethig D, Sarikouch S, Goerler A, et al. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation. 2011;124(11 Suppl 1):S115–23. doi:10.1161/CIRCULATIONAHA.110.012161. [Google Scholar] [CrossRef]
14. Konuma T, Devaney EJ, Bove EL, Gelehrter S, Hirsch JC, Tavakkol Z, et al. Performance of Cryo Valve SG decellularized pulmonary allografts compared with standard cryopreserved allografts. Ann Thorac Surg. 2009;88(3):849–55. doi:10.1016/j.athoracsur.2009.06.003. [Google Scholar] [CrossRef]
15. Ruzmetov M, Shah JJ, Geiss DM, Fortuna RS. Decellularized versus standard cryopreserved valve allografts for right ventricular outflow tract reconstruction: a single-institution comparison. J Thorac Cardiovasc Surg. 2012;143(3):543–9. doi:10.1016/j.jtcvs.2011.12.032. [Google Scholar] [CrossRef]
16. Sarikouch S, Horke A, Tudorache I, Beerbaum P, Westhoff-Bleck M, Boethig D, et al. Decellularized fresh homografts for pulmonary valve replacement: a decade of clinical experience. Eur J Cardiothorac Surg. 2016;50(2):281–90. doi:10.1093/ejcts/ezw050. [Google Scholar] [CrossRef]
17. Boethig D, Horke A, Hazekamp M, Meyns B, Rega F, Van Puyvelde J, et al. A European study on decellularized homografts for pulmonary valve replacement: initial results from the prospective ESPOIR trial and ESPOIR registry data. Eur J Cardiothorac Surg. 2019;56(3):503–9. [Google Scholar]
18. Carrel T, Berdat P, Pavlovic M, Pfammatter JP. The bovine jugular vein: a totally integrated valved conduit to repair the right ventricular outflow. J Heart Valve Dis. 2002;11(4):552–6. [Google Scholar]
19. Breymann T, Boethig D, Goerg R, Thies WR. The contegra bovine valved jugular vein conduit for pediatric RVOT reconstruction: 4 years experience with 108 patients. J Card Surg. 2004;19(5):426–31. doi:10.1111/j.0886-0440.2004.04083.x. [Google Scholar] [CrossRef]
20. Sfyridis PG, Papagiannis JK, Avramidis DP, Zavaropoulos PN. The contegra® valved heterograft conduit for right ventricular outflow tract reconstruction: a reliable solution. Hell J Cardiol. 2011;52:501–8. [Google Scholar]
21. Schoenhoff FS, Loup O, Gahl B, Banz Y, Pavlovic M, Pfammatter JP, et al. The Contegra bovine jugular vein graft versus the Shelhigh pulmonic porcine graft for reconstruction of the right ventricular outflow tract: a comparative study. J Thorac Cardiovasc Surg. 2011;141(3):654–61. [Google Scholar]
22. Mery CM, Guzmán-Pruneda FA, De León LE, Zhang W, Terwelp MD, Bocchini CE, et al. Riskfactorsfor development of endocarditis and reintervention in patients undergoing right ventricle to pulmonary artery valved conduit placement. J Thorac Cardiovasc Surg. 2016;151(2):432–41.e2. doi:10.1016/j.jtcvs.2015.10.069. [Google Scholar] [CrossRef]
23. Gröning M, Tahri NB, Søndergaard L, Helvind M, Ersbøll MK, Ørbæk Andersen H. Infective endocarditis in right ventricular outflow tract conduits: a register-based comparison of homografts, contegra grafts and melody transcatheter valves. Eur J Cardiothorac Surg. 2019;56(1):87–93. [Google Scholar]
24. Stammnitz C, Huscher D, Bauer UMM, Urban A, Nordmeyer J, Schubert S, et al. Nationwide registry-based anal-ysis of infective endocarditis risk after pulmonary valve replacement. J Am Heart Assoc. 2022;11(5):e022231. [Google Scholar]
25. Alsoufi B, Manlhiot C, McCrindle BW, Canver CC, Sallehuddin A, Al-Oufi S, et al. Aortic andmitralvalve replacement in children: is there any role for biologic and bioprosthetic substitutes. Eur J Cardiothorac Surg. 2009;36(1):84–90. doi:10.1016/j.ejcts.2009.02.048. [Google Scholar] [CrossRef]
26. Robichaud B, Hill G, Cohen S, Woods R, Earing M, Frommelt P, et al. Bioprosthetic pulmonary valve endocarditis: incidence, risk factors, and clinical outcomes. Congenit Heart Dis. 2018;13(5):734–9. doi:10.1111/chd.12639. [Google Scholar] [CrossRef]
27. Shinkawa T, Lu CK, Chipman C, Tang X, Gossett JM, Imamura M. The midtermoutcomesofbioprosthetic pulmonary valve replacement in children. Semin Thorac Cardiovasc Surg. 2015;27(3):310–8. doi:10.1053/j.semtcvs.2015.07.010. [Google Scholar] [CrossRef]
28. Chen XJ, Smith PB, Jaggers J, Lodge AJ. Bioprosthetic pulmonary valve replacement: contemporary analysis of a large, single-center series of 170 cases. J Thorac Cardiovasc Surg. 2013;146(6):1461–6. doi:10.1016/j.jtcvs.2012.09.081. [Google Scholar] [CrossRef]
29. Ruffer A, Wittmann J, Potapov S, Purbojo A, Glockler M, Koch AM, et al. Mid-term experience with the Hancock porcine-valved Dacron conduit for right ventricular outflow tract reconstruction. Eur J Cardiothorac Surg. 2012;42(6):988–95. doi:10.1093/ejcts/ezs103. [Google Scholar] [CrossRef]
30. Miller DC, Stinson EB, Oyer PE, Billingham ME, Pitlick PT, Reitz BA, et al. The durability of porcine xenograft valves and conduits in children. Circulation. 1982;66(2 Pt 2):I172–85. [Google Scholar]
31. Yuan SM, Mishaly D, Shinfeld A, Raanani E. Right ventricular outflow tract reconstruction: valved conduit of choice and clinical outcomes. J Cardiovasc Med. 2008;9(4):327–37. doi:10.2459/JCM.0b013e32821626ce. [Google Scholar] [CrossRef]
32. Onan IS, Ergün S, Özturk E, Çelik EC, Ayyıldız P, Onan B. Early results of neopulmonary valve creation technique using right atrial appendage tissue. J Card Surg. 2020;35(10):2640–8. doi:10.1111/jocs.14860. [Google Scholar] [CrossRef]
33. Amirghofran A, Edraki F, Edraki M, Ajami G, Amoozgar H, Mohammadi H, et al. Surgical repair of tetralogy of Fallot using autologous right atrial appendages: short- to mid-term results. Eur J Cardiothorac Surg. 2021;59(3):697–704. doi:10.1093/ejcts/ezaa374. [Google Scholar] [CrossRef]
34. Schulte LJ, Miller PC, Miller JR, Nath D, Eghtesady P. Technique for neo-pulmonary valve creation with living tissue for repair of atrioventricular septal defect and tetralogy of fallot. World J Pediatr Congenit Heart Surg. 2022;13(4):499–502. doi:10.1177/21501351221096048. [Google Scholar] [CrossRef]
35. Kan CD, Wang JN, Chen WL, Lu PJ, Chan MY, Lin CH, et al. Applicability of handmade expanded polytetraflu-oroethylene trileaflet-valved conduits for pulmonary valve reconstruction: an ex vivo and in vivo study. J Thorac Cardiovasc Surg. 2018;155(2):765–74.e3. [Google Scholar]
36. Chang TI, Hsu KH, Li SJ, Chuang MK, Luo CW, Chen YJ, et al. Evolution of pulmonary valve reconstruction with focused review of expanded polytetrafluoroethylene handmade valves. Interact Cardiovasc Thorac Surg. 2021;32(4):585–92. [Google Scholar]
37. Tocharoenchok T, Siriprompatr S, Subtaweesin T, Tantiwongkosri K, Nitiyarom E, Sriyoschati S. Early outcome of simplified standardized trileaflet polytetrafluoroethylene valved conduit placement. World J Pediatr Congenit Heart Surg. 2022;13(6):723–30. [Google Scholar]
38. Baird CW, Chávez M, Backer CL, Galantowicz ME, Del Nido PJ. Preliminary results with a novel expanded polytetrafluoroethylene-based pulmonary valved conduit. Ann Thorac Surg. 2022;114(6):2314–21. [Google Scholar]
39. Galantowicz ME, Baird C, Backer C, Del Nido PJ. Abstract 14086: early results of a novel EPTFE-based pulmonary valved conduit in a pediatric patient population. Circulation. 2020;142(Suppl 3):A14086. [Google Scholar]
40. Mercer CW, West SC, Sharma MS, Yoshida M, Morell VO. Polytetrafluoroethylene conduits versus homografts for right ventricular outflow tract reconstruction in infants and young children: an institutional experience. J Thorac Cardiovasc Surg. 2018;155(5):2082–91.e1. doi:10.1016/j.jtcvs.2017.11.107. [Google Scholar] [CrossRef]
41. Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356(9239):1403–5. doi:10.1016/S0140-6736(00)02844-0. [Google Scholar] [CrossRef]
42. Zablah JE, Morgan GJ. Current treatment options for catheter-based pulmonary valve replacement in children. Curr Treat Options Pediatr. 2020;6(4):274–82. doi:10.1007/s40746-020-00209-0. [Google Scholar] [CrossRef]
43. Patel ND, Levi DS, Cheatham JP, Qureshi SA, Shahanavaz S, Zahn EM. Transcatheter pulmonary valve replace-ment: a review of current valve technologies. J Soc Cardiovasc Angiogr Interv. 2022;1(6):100452. doi:10.1016/j.jscai.2022.100452. [Google Scholar] [CrossRef]
44. Boudjemline Y. A new one-step procedure for pulmonary valve implantation of the melody valve: simultaneous prestenting and valve implantation. Catheter Cardiovasc Interv. 2018;91(1):64–70. doi:10.1002/ccd.27332. [Google Scholar] [CrossRef]
45. McElhinney DB, Sondergaard L, Armstrong AK, Bergersen L, Padera RF, Balzer DT, et al. Endocarditis after transcatheter pulmonary valve replacement. J Am Coll Cardiol. 2018;72(22):2717–28. doi:10.1016/j.jacc.2018.09.039. [Google Scholar] [CrossRef]
46. Haas NA, Bach S, Vcasna R, Laser KT, Sandica E, Blanz U, et al. The risk of bacterial endocarditis after percutaneous and surgical biological pulmonary valve implantation. Int J Cardiol. 2018;268(1):55–60. doi:10.1016/j.ijcard.2018.04.138. [Google Scholar] [CrossRef]
47. Garay F, Webb J, Hijazi ZM. Percutaneous replacement of pulmonary valve using the Edwards-Cribier percuta-neous heart valve: first report in a human patient. Catheter Cardiovasc Interv. 2006;67(5):659–62. doi:10.1002/ccd.20753. [Google Scholar] [CrossRef]
48. Lehner A, Dashkalova T, Ulrich S, Fernandez Rodriguez S, Mandilaras G, Jakob A, et al. Intermediateoutcomes of transcatheter pulmonary valve replacement with the Edwards Sapien 3 valve—German experience. Expert Rev Med Devices. 2019;16(9):829–34. doi:10.1080/17434440.2019.1653180. [Google Scholar] [CrossRef]
49. Hascoet S, Bentham JR, Betrian-Belasco P, Houeijeh A, Jones M, Biernacka EK, et al. Long-term outcomes following transcatheter pulmonary valve implantation with the sapien 3 valve: an international multicentre registry. Eur Heart J. 2022;43(Suppl 2):ehac544-1659. [Google Scholar]
50. Faccini A, Butera G. Tricuspid regurgitation as a complication of Edwards Sapien XT valve implantation in pulmonary position a problem to deal with. Catheter Cardiovasc Interv. 2018;91(5):927–31. doi:10.1002/ccd.27527. [Google Scholar] [CrossRef]
51. Butera G, Hansen JH, Jones MI. Tricuspid regurgitation complicating SAPIEN 3 valve implantation in pulmonary position. Catheter Cardiovasc Interv. 2019;94(6):894. doi:10.1002/ccd.28083. [Google Scholar] [CrossRef]
52. Stapleton GE, Gowda ST, Bansal M, Khan A, Qureshi AM, Justino H. SAPIEN S3 valve deployment in the pulmonary position using the gore DrySeal sheath to protect the tricuspid valve. Catheter Cardiovasc Interv. 2020;96(6):1287–93. doi:10.1002/ccd.29120. [Google Scholar] [CrossRef]
53. Fukuda T, Tan W, Sadeghi S, Lin J, Salem M, Levi D, et al. Utility of the long DrySeal sheath in facilitating transcatheter pulmonary valve implantation with the Edwards Sapien 3 valve. Catheter Cardiovasc Interv. 2020;96(6):E646–52. doi:10.1002/ccd.28776. [Google Scholar] [CrossRef]
54. Hascoet S, Karsenty C, Tortigue M, Watkins AC, Riou JY, Boet A, et al. A modified procedure for percutaneous pulmonary valve implantation of the Edwards SAPIEN 3 valve. EuroIntervention. 2019;14(13):1386–8. doi:10.4244/EIJ-D-18-00530. [Google Scholar] [CrossRef]
55. Odemis E, Yenidogan I. First experiences with Myval transcatheter heart valve system in the treatment of severe pulmonary regurgitation in native right ventricular outflow tract and conduit dysfunction. Cardiol Young. 2022;32(10):1609–15. doi:10.1017/S1047951121004650. [Google Scholar] [CrossRef]
56. Rodríguez Ogando A, Ballesteros F, Martínez JLZ. Pulmonary percutaneous valve implantation in large native right ventricular outflow tract with 32 mm Myval transcatheter heart valve. Catheter Cardiovasc Interv. 2022;99(1):E38–42. doi:10.1002/ccd.29985. [Google Scholar] [CrossRef]
57. Sivaprakasam MC, Reddy JRV, Gunasekaran S, Sivakumar K, Pavithran S, Rohitraj GR, et al. Early multicen-ter experience of a new balloon expandable MyVal transcatheter heart valve in dysfunctional stenosed right ventricular outflow tract conduits. Ann Pediatr Cardiol. 2021;14(3):293–301. doi:10.4103/apc.apc_242_20. [Google Scholar] [CrossRef]
58. Al Nasef M, Erdem A, Aldudak B, Yildirim A, Hijazi ZM, Boudjemline Y, et al. Multicenter experience for early and mid-term outcome of MyVal transcatheter pulmonary valve implantation. Pediatr Cardiol. 2024;45(3):570–9. doi:10.1007/s00246-023-03398-1. [Google Scholar] [CrossRef]
59. Zhou D, Pan W, Jilaihawi H, Zhang G, Feng Y, Pan X, et al. A self-expanding percutaneous valve for patients with pulmonary regurgitation and an enlarged native right ventricular outflow tract: one-year results. EuroIntervention. 2019;14(13):1371–7. doi:10.4244/EIJ-D-18-00715. [Google Scholar] [CrossRef]
60. Cao QL, Kenny D, Zhou D, Pan W, Guan L, Ge J, et al. Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2014;84(7):1131–7. doi:10.1002/ccd.25544. [Google Scholar] [CrossRef]
61. Morgan GJ, Sivakumar K, Promphan W, Goreczny S, Prachasilchai P, Qureshi S. Early clinical experience with the straight design of Venus P-valveTM in dysfunctional right ventricular outflow tracts. Catheter Cardiovasc Interv. 2020;96(6):E653–9. [Google Scholar]
62. Jin Q, Long Y, Zhang G, Pan X, Chen M, Feng Y, et al. Five-year follow-up after percutaneous pulmonary valve implantation using the Venus P-valve system for patients with pulmonary regurgitation and an enlarged native right ventricular outflow tract. Catheter Cardiovasc Interv. 2024;103(2):359–66. doi:10.1002/ccd.30916. [Google Scholar] [CrossRef]
63. Gillespie MJ, Benson LN, Bergersen L, Bacha EA, Cheatham SL, Crean AM, et al. Patient selection process for the harmony transcatheter pulmonary valve early feasibility study. Am J Cardiol. 2017;120(8):1387–92. doi:10.1016/j.amjcard.2017.07.034. [Google Scholar] [CrossRef]
64. Mejia E, O’Neill K, Lozier JS, Bocks ML. Self-expanding transcatheter pulmonary valve implant in the right pulmonary artery. JACC Case Rep. 2023;14:101823. doi:10.1016/j.jaccas.2023.101823. [Google Scholar] [CrossRef]
65. Agasthi P, Cabalka AK, Cetta F, Anderson JH. Harmony transcatheter pulmonary valve successfully implanted via transjugular approach. J Soc Cardiovasc Angiogr Interv. 2023;3(2):101207. [Google Scholar]
66. Gillespie MJ, Bergersen L, Benson LN, Weng S, Cheatham JP. 5-year outcomes from the harmony native outflow tract early feasibility study. JACC Cardiovasc Interv. 2021;14(7):816–7. [Google Scholar]
67. Goldstein BH, McElhinney DB, Gillespie MJ, Aboulhosn JA, Levi DS, Morray BH, et al. Early outcomes from a multicenter transcatheter self-expanding pulmonary valve replacement registry. J Am Coll Cardiol. 2024;83(14):1310–21. [Google Scholar]
68. Kim GB, Song MK, Bae EJ, Park EA, Lee W, Lim HG, et al. Successful feasibility human trial of a new self-expandable percutaneous pulmonary valve (Pulsta Valve) implantation using knitted nitinol wire backbone and trileaflet α-gal-free porcine pericardial valve in the native right ventricular outflow tract. Circ Cardiovasc Interv. 2018;11(6):e006494. [Google Scholar]
69. Odemis E, Yenidogan I, Kizilkaya MH. Early results of Pulsta Rtranscatheter heart valve in patients with enlarged right ventricular outflow tract and severe pulmonary regurgitation due to transannular patch. Cardiol Young. 2023;33(10):1926–34. [Google Scholar]
70. Park WY, Kim GB, Lee SY, Kim AY, Choi JY, Jang SI, et al. The adaptability of the Pulsta valve to the diverse main pulmonary artery shape of native right ventricular outflow tract disease. Catheter Cardiovasc Interv. 2024;103(4):587–96. [Google Scholar]
71. Lee SY, Kim GB, Kim SH, Jang SI, Choi JY, Kang IS, et al. Mid-term outcomes of the Pulsta transcatheter pulmonary valve for the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2021;98(5):E724–32. doi:10.1002/ccd.29865. [Google Scholar] [CrossRef]
72. Zahn EM, Chang JC, Armer D, Garg R. First human implant of the Alterra Adaptive PrestentTM: a new self-expanding device designed to remodel the right ventricular outflow tract. Catheter Cardiovasc Interv. 2018;91(6):1125–9. doi:10.1002/ccd.27581. [Google Scholar] [CrossRef]
73. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890–900. doi:10.1016/S0735-1097(02)01886-7. [Google Scholar] [CrossRef]
74. Kitchiner D, Jackson M, Malaiya N, Walsh K, Peart I, Arnold R. Incidence and prognosis of obstruction of the left ventricular outflow tract in Liverpool (1960–91): a study of 313 patients. Br Heart J. 1994;71(6):588–95. doi:10.1136/hrt.71.6.588. [Google Scholar] [CrossRef]
75. Vergnat M, Asfour B, Arenz C, Suchowerskyj P, Bierbach B, Schindler E, et al. Contemporary results of aortic valve repair for congenital disease: lessons for management and staged strategy. Eur J Cardiothorac Surg. 2017;52(3):581–7. doi:10.1093/ejcts/ezx172. [Google Scholar] [CrossRef]
76. Schlein J, Simon P, Wollenek G, Base E, Laufer G, Zimpfer D. Aortic valve replacement in pediatric patients: 30 years single center experience. J Cardiothorac Surg. 2021;16(1):259. doi:10.1186/s13019-021-01636-2. [Google Scholar] [CrossRef]
77. Alsoufi B. Aortic valve replacement in children: options and outcomes. J Saudi Heart Assoc. 2014;26(1):33–41. doi:10.1016/j.jsha.2013.11.003. [Google Scholar] [CrossRef]
78. Myers PO, Mokashi SA, Horgan E, Borisuk M, Mayer JE, Del Nido PJ, et al. Outcomes after mechanical aortic valve replacement in children and young adults with congenital heart disease. J Thorac Cardiovasc Surg. 2019;157(1):329–40. doi:10.1016/j.jtcvs.2018.08.077. [Google Scholar] [CrossRef]
79. Kim JY, Cho WC, Kim DH, Choi ES, Kwon BS, Yun TJ, et al. Outcomes after mechanical aortic valve replacement in children with congenital heart disease. J Chest Surg. 2023;56(6):394–402. doi:10.5090/jcs.23.071. [Google Scholar] [CrossRef]
80. Zhu MZL, Buratto E, Wu DM, Ishigami S, Schulz A, Brizard CP, et al. Long-term outcomes of mechanical aortic valve replacement in children. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2024;27:52–60. [Google Scholar]
81. Mikulski MF, Well A, Beckerman Z, Fraser CD. A 15-mm mechanical aortic prosthesis in a small infant. JTCVS Tech. 2022;12:157–8. doi:10.1016/j.xjtc.2022.01.013. [Google Scholar] [CrossRef]
82. Popov A, Coskun KO, Tirilomis T, Schmitto JD, Hinz J, Kriebel T, et al. Mechanical aortic valve replacement in children and adolescents after previous repair of congenital heart disease. Artif Organs. 2009;33(11):915–21. doi:10.1111/j.1525-1594.2009.00886.x. [Google Scholar] [CrossRef]
83. Ibezim C, Sarvestani AL, Knight JH, Qayum O, Alshami N, Turk E, et al. Outcomesof mechanicalmitralvalve replacement in children. Ann Thorac Surg. 2019;107(1):143–50. doi:10.1016/j.athoracsur.2018.07.069. [Google Scholar] [CrossRef]
84. Shanmugam G, MacArthur K, Pollock J. Mechanical aortic valve replacement: long-term outcomes in children. J Heart Valve Dis. 2005;14(2):166–71. [Google Scholar]
85. Saleeb SF, Newburger JW, Geva T, Baird CW, Gauvreau K, Padera RF, et al. Accelerated degeneration of a bovine pericardial bioprosthetic aortic valve in children and young adults. Circulation. 2014;130(1):51–60. doi:10.1161/CIRCULATIONAHA.114.009835. [Google Scholar] [CrossRef]
86. Ahmed A, Aziz TAA, Al Asaad MMR, Majthoob M, Altahmody KA. Early and late clinical outcomes and cost-effectiveness of aortic valve replacement using the Inspiris Resilia bioprosthesis: a systematic review and meta-analysis. J Cardiothorac Surg. 2025;20(1):117. doi:10.1186/s13019-024-03269-7. [Google Scholar] [CrossRef]
87. Alsoufi B, Knight JH, Louis JS, Raghuveer G, Kochilas L. Are mechanical prostheses valid alternatives to the Ross procedure in young children under 6 years old? Ann Thorac Surg. 2022;113(1):166–73. doi:10.1016/j.athoracsur.2020.12.014. [Google Scholar] [CrossRef]
88. Moroi MK, Bacha EA, Kalfa DM. The ross procedure in children: a systematic review. Ann Cardiothorac Surg. 2021;10(4):420–32. doi:10.21037/acs-2020-rp-23. [Google Scholar] [CrossRef]
89. Takkenberg JJM, Klieverik LMA, Schoof PH, Van Suylen RJ, Van Herwerden LA, Zondervan PE, et al. The ross procedure: a systematic review and meta-analysis. Circulation. 2009;119(2):222–8. doi:10.1161/CIRCULATIONAHA.107.726349. [Google Scholar] [CrossRef]
90. Sharabiani MTA, Dorobantu DM, Mahani AS, Turner M, Peter Tometzki AJ, Angelini GD, et al. Aortic valve replacement and the ross operation in children and young adults. J Am Coll Cardiol. 2016;67(24):2858–70. doi:10.1016/j.jacc.2016.04.021. [Google Scholar] [CrossRef]
91. Galzerano D, Kholaif N, Al Amro B, Al Admawi M, Eltayeb A, Alshammari A, et al. Therossprocedure:imaging, outcomes and future directions in aortic valve replacement. J Clin Med. 2024;13(2):630. doi:10.3390/jcm13020630. [Google Scholar] [CrossRef]
92. Laudito A, Brook MM, Suleman S, Bleiweis MS, Thompson LD, Hanley FL, et al. The Ross procedure in children and young adults: a word of caution. J Thorac Cardiovasc Surg. 2001;122(1):147–53. doi:10.1067/mtc.2001.113752. [Google Scholar] [CrossRef]
93. Notenboom ML, Schuermans A, Etnel JRG, Veen KM, Van De Woestijne PC, Rega FR, et al. Pediatricaorticvalve replacement: a meta-analysis and microsimulation study. Eur Heart J. 2023;44(34):3231–46. doi:10.1093/eurheartj/ehad370. [Google Scholar] [CrossRef]
94. Buratto E, Wallace F, Schulz A, Zhu M, Ishigami S, Brizard CP, et al. The ross procedure in children: defining the optimal age. Heart Lung Circ. 2023;32(6):745–9. doi:10.1016/j.hlc.2023.04.005. [Google Scholar] [CrossRef]
95. Kallio M, Pihkala J, Sairanen H, Mattila I. Long-term results of the Ross procedure in a population-based follow-up. Eur J Cardiothorac Surg. 2015;47(5):e164–70. doi:10.1093/ejcts/ezv004. [Google Scholar] [CrossRef]
96. Wang K, Zhang H, Jia B. Current surgical strategies and techniques of aortic valve diseases in children. Transl Pediatr. 2018;7(2):83–90. doi:10.21037/tp.2018.02.03. [Google Scholar] [CrossRef]
97. Wiggins LM, Mimic B, Issitt R, Ilic S, Bonello B, Marek J, et al. The utility of aortic valve leaflet reconstruction techniques in children and young adults. J Thorac Cardiovasc Surg. 2020;159(6):2369–78. doi:10.1016/j.jtcvs.2019.09.176. [Google Scholar] [CrossRef]
98. Baird CW, Marathe SP, Del Nido PJ. Aortic valve neo-cuspidation using the Ozaki technique for acquired and congenital disease: where does this procedure currently stand? Indian J Thorac Cardiovasc Surg. 2020;36(S1):113–22. doi:10.1007/s12055-019-00917-9. [Google Scholar] [CrossRef]
99. Badalyan SS. Outcomes of ozaki procedure/aortic valve neocuspidization for aortic valve diseases: a systematic review. Anatol J Cardiol. 2023:619–27. doi:10.14744/AnatolJCardiol.2023.3477. [Google Scholar] [CrossRef]
100. Halder V, Mishra A, Ghosh S, Singh H, Barwad P, Thingnam SK, et al. Effectiveness and safety of the ozaki procedure for aortic valve disease in pediatric patients: a systematic review and meta-analysis. Cureus. 2023;15(9):e45269. [Google Scholar]
101. Baird CW, Cooney B, Chávez M, Sleeper LA, Marx GR, Del Nido PJ. Congenital aortic and truncal valve reconstruction using the Ozaki technique: short-term clinical results. J Thorac Cardiovasc Surg. 2021;161(5):1567–77. doi:10.1016/j.jtcvs.2020.01.087. [Google Scholar] [CrossRef]
102. Chivers SC, Pavy C, Vaja R, Quarto C, Ghez O, Daubeney PEF. The Ozaki procedure with cardiocel patch for children and young adults with aortic valve disease: preliminary experience—a word of caution. World J Pediatr Congenit Heart Surg. 2019;10(6):724–30. doi:10.1177/2150135119878108. [Google Scholar] [CrossRef]
103. Sun M, La Sala VR, Giuglaris C, Blitzer D, Jackman S, Ustunel S, et al. Cardiovascular patches applied in congenital cardiac surgery: current materials and prospects. Bioeng Transl Med. 2024;10(1):e10706. [Google Scholar]
104. Doty DB. Aortic valve replacement with homograft and autograft. Semin Thorac Cardiovasc Surg. 1996;8(3):249–58. [Google Scholar]
105. Ohye RG, Bove EL. Advances in congenital heart surgery. Curr Opin Pediatr. 2001;13(5):473–81. [Google Scholar]
106. Naegele H, Bohlmann M, Döring V, Kalmar P, Rödiger W. Results of aortic valve replacement with pulmonary and aortic homografts. J Heart Valve Dis. 2000;9(2):215–20. discussion 220–221. [Google Scholar]
107. Vogt F, Kowert A, Beiras-Fernandez A, Oberhoffer M, Kaczmarek I, Reichart B, et al. Pulmonary homografts for aortic valve replacement: long-term comparison with aortic grafts. Heart Surg Forum. 2011;14(4):237. doi:10.1532/HSF98.20101162. [Google Scholar] [CrossRef]
108. Van Den Heever JJ, Jordaan CJ, Lewies A, Goedhals J, Bester D, Botes L, et al. Impact of three different processing techniques on the strength and structure of juvenile ovine pulmonary homografts. Polymers. 2022;14(15):3036. doi:10.3390/polym14153036. [Google Scholar] [CrossRef]
109. Horke A, Tudorache I, Laufer G, Andreas M, Pomar JL, Pereda D, et al. Early results from a prospective, single-arm European trial on decellularized allografts for aortic valve replacement: the ARISE study and ARISE Registry data. Eur J Cardiothorac Surg. 2020;58(5):1045–53. doi:10.1093/ejcts/ezaa100. [Google Scholar] [CrossRef]
110. Horke A, Bobylev D, Avsar M, Meyns B, Rega F, Hazekamp M, et al. Pediatric aortic valve replacement using decellularized allografts. Eur J Cardiothorac Surg. 2020;58(4):817–24. doi:10.1093/ejcts/ezaa119. [Google Scholar] [CrossRef]
111. Horke A, Bobylev D, Avsar M, Cvitkovic T, Meyns B, Rega F, et al. Pediatric aortic valve replacement using decellularized allografts: a multicentre update following 143 implantations and five-year mean follow-up. Eur J Cardiothorac Surg. 2024;65(4):ezae112. doi:10.1093/ejcts/ezae112. [Google Scholar] [CrossRef]
112. Ebken J, Mester N, Smart I, Ramm R, Goecke T, Jashari R, et al. Residual immune response towards decellularized homografts may be highly individual. Eur J Cardiothorac Surg. 2021;59(4):773–82. doi:10.1093/ejcts/ezaa393. [Google Scholar] [CrossRef]
113. Kasravi M, Ahmadi A, Babajani A, Mazloomnejad R, Hatamnejad MR, Shariatzadeh S, et al. Immunogenicity of decellularized extracellular matrix scaffolds: a bottleneck in tissue engineering and regenerative medicine. Biomater Res. 2023;27(1):10. doi:10.1186/s40824-023-00348-z. [Google Scholar] [CrossRef]
114. Robertson DM, Boucek DM, Martin MH, Gray RG, Griffiths ER, Eckhauser AW, et al. Transcatheter and surgical aortic valve implantation in children, adolescents, and young adults with congenital heart disease. Am J Cardiol. 2022;177:128–36. doi:10.1016/j.amjcard.2022.04.056. [Google Scholar] [CrossRef]
115. Tannous P, Nugent A. Transcatheter pulmonary valve replacement in native and nonconduit right ventricle outflow tracts. J Thorac Cardiovasc Surg. 2021;162(3):967–70. doi:10.1016/j.jtcvs.2020.07.126. [Google Scholar] [CrossRef]
116. Sinha S, Khan A, Qureshi AM, Suh W, Laks H, Aboulhosn J, et al. Application of transcatheter valves for aortic valve replacement in pediatric patients: a case series. Catheter Cardiovasc Interv. 2020;95(2):253–61. doi:10.1002/ccd.28505. [Google Scholar] [CrossRef]
117. Barfuss SB, Boucek DM, McFarland CA, Martin MH, Lu Ann Minich L, Eckhauser AW, et al. Short-term left ventricular reverse remodeling after transcatheter aortic valve replacement in children. J Am Soc Echocardiogr. 2022;35(10):1077–83. doi:10.1016/j.echo.2022.05.009. [Google Scholar] [CrossRef]
118. Kidane AG, Burriesci G, Cornejo P, Dooley A, Sarkar S, Bonhoeffer P, et al. Current developments and future prospects for heart valve replacement therapy. J Biomed Mater Res B Appl Biomater. 2009;88B(1):290–303. doi:10.1002/jbm.b.31151. [Google Scholar] [CrossRef]
119. Elsisy MF, Dearani JA, Ashikhmina E, Aganga DO, Taggart NW, Todd A, et al. National in-hospital outcomes of mechanical mitral valve replacement in the pediatric population. World J Pediatr Congenit Heart Surg. 2024;15(1):37–43. doi:10.1177/21501351231185118. [Google Scholar] [CrossRef]
120. Nakamura Y, Hoashi T, Imai K, Okuda N, Komori M, Kurosaki K, Ichikawa H. Patient-Prosthesis Mismatch Associated With Somatic Growth After Mechanical Mitral Valve Replacement in Small Children: Metrics for Reoperation and Outcomes. Semin Thiracic Cardiovasc Surg. 2023;35(2):348–57. doi:10.1053/j.semtcvs.2022.01.010. [Google Scholar] [CrossRef]
121. Schumacher K, Marin-Cuartas M, Aydin MI, De La Cuesta M, Meier S, Borger MA, et al. Long-term outcomes following mitral valve replacement in children at heart center Leipzig: a 20-year analysis. J Cardiothorac Surg. 2024;19(1):419. doi:10.1186/s13019-024-02904-7. [Google Scholar] [CrossRef]
122. Eltayeb OM, Readdy WJ, Mongé MC, Forbess JM, Sarwark AE, Patel A, et al. Mitral valve replacement in infants using a 15-mm mechanical valve. Ann Thorac Surg. 2019;108(2):552–7. doi:10.1016/j.athoracsur.2019.02.061. [Google Scholar] [CrossRef]
123. IJsselhof RJ, Slieker MG, Hazekamp MG, Accord R, Van Wetten H, Haas F, et al. Mitral valve replacement with the 15-mm mechanical valve: a 20-year multicenter experience. Ann Thorac Surg. 2020;110(3):956–61. doi:10.1016/j.athoracsur.2019.11.050. [Google Scholar] [CrossRef]
124. Mills M, John M, Tang R, Fundora MP, Keesari R, Kanter K, et al. Mitral valve replacement in infants and children: experience using a 15-mm mechanical valve. Ann Thorac Surg. 2023;116(2):322–9. doi:10.1016/j.athoracsur.2023.04.035. [Google Scholar] [CrossRef]
125. Ibarra C, Spigel Z, John R, Thomason AB, Binsalamah Z, Adachi I, et al. Mechanical mitral valve replacements in the pediatric population. Ann Thorac Surg. 2021;112(2):626–31. doi:10.1016/j.athoracsur.2020.06.068. [Google Scholar] [CrossRef]
126. Lehenbauer DG, Tweddell JS, Winlaw DS. Debate-replacement of the mitral valve under one year of age: mechanical valves should be used. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2021;24:44–56. doi:10.1053/j.pcsu.2021.03.005. [Google Scholar] [CrossRef]
127. Shaw FR, Kogon B, Chen J, Mitchell MB, Fraser C, Kanter K. Mitral valve replacement in infants and children: five-year outcomes of the HALO clinical trial. Ann Thorac Surg. 2024;118(2):449–57. doi:10.1016/j.athoracsur.2024.04.025. [Google Scholar] [CrossRef]
128. Gottlieb Sen D. Between a rock and a hard place—challenges of mitral valve replacement in infants. Ann Thorac Surg. 2023;116(2):329–30. doi:10.1016/j.athoracsur.2023.05.006. [Google Scholar] [CrossRef]
129. Van Puyvelde J, Meyns B, Rega F, Gewillig M, Eyskens B, Heying R, et al. Mitral valve replacement in children: balancing durability and risk with mechanical and bioprosthetic valves. Interdiscip Cardiovasc Thorac Surg. 2024;38(3):ivae034. [Google Scholar]
130. Carroll ND, Beers KM, Maldonado EM, Calhoon JH, Husain SA. Novel annular and subvalvular enlargement in congenital mitral valve replacement. Ann Thorac Surg. 2016;102(3):e277–9. [Google Scholar]
131. Attisani M, Pellegrini A, Sorrentino P, Rinaldi M. Enlargement of mitral valve ring in a young woman with severe prosthesis-patient mismatch. Heart Surg Forum. 2014;17(2):61. [Google Scholar]
132. Gellis L, Baird CW, Emani S, Borisuk M, Gauvreau K, Padera RF, et al. Morphologic and histologic findings in bioprosthetic valves explanted from the mitral position in children younger than 5 years of age. J Thorac Cardiovasc Surg. 2018;155(2):746–52. [Google Scholar]
133. Philip R, Kumar TKS, Waller BR, Mc Coy M, Knott-Craig CJ. Near catastrophic accelerated structural degeneration of the perimount magna pericardial bioprosthesis in children. Ann Thorac Surg. 2016;102(1):308–11. [Google Scholar]
134. Kanzaki T, Yamagishi M, Yashima M, Yaku H. Seven-year outcome of pulmonary valve autograft replacement of the mitral valve in an infant. J Thorac Cardiovasc Surg. 2011;141(5):e33–5. doi:10.1016/j.jtcvs.2011.01.049. [Google Scholar] [CrossRef]
135. Blitzer D, Herrmann JL, Brown JW. Pulmonary autograft mitral valve replacement (Ross II): long-term follow-up of a US center. World J Pediatr Congenit Heart Surg. 2018;9(6):645–50. doi:10.1177/2150135118792196. [Google Scholar] [CrossRef]
136. Brown JW, Fiore AC, Ruzmetov M, Eltayeb O, Rodefeld MD, Turrentine MW. Evolution of mitral valve replace-ment in children: a 40-year experience. Ann Thorac Surg. 2012;93(2):626–33. doi:10.1016/j.athoracsur.2011.08.085. [Google Scholar] [CrossRef]
137. Kabbani SS, Sabbagh NA, Kudsi AY, Nabhani F, Jamil H. Update on the mitral pulmonary autograft. Asian Cardiovasc Thorac Ann. 2011;19(3–4):253–9. doi:10.1177/0218492311409631. [Google Scholar] [CrossRef]
138. Moreau De Bellaing A, Mathiron A, Lecompte Y, Vouhé P. Mitral valve replacement with a pulmonary autograft: long-term follow-up in an infant. Interact Cardiovasc Thorac Surg. 2019;28(5):828–9. doi:10.1093/icvts/ivy322. [Google Scholar] [CrossRef]
139. Malik T, Jaquiss RDB, Harirah O, Davies RR, Andersen N, Leonard S, et al. Themelodyvalveinsmall children undergoing first mitral valve replacement: better than mechanical? Ann Thorac Surg Short Rep. 2024;2(3):359–63. [Google Scholar]
140. Honjo O, Chetan D, Fan CPS, Kadowaki S, Marshall AC, Chaturvedi RR, et al. Surgical melody mitral valve: a paradigm shift for infants with unrepairable mitral valve disease. Ann Thorac Surg. 2024;118(3):623–32. doi:10.1016/j.athoracsur.2024.04.037. [Google Scholar] [CrossRef]
141. Padovani P, Jalal Z, Fouilloux V, Benbrik N, Grunenwald C, Thambo JB, et al. Riskofinfectiveendocarditisafter hybrid melody mitral valve replacement in infants: the French experience. Interdiscip Cardiovasc Thorac Surg. 2024;38(4):ivae046. doi:10.1093/icvts/ivae046. [Google Scholar] [CrossRef]
142. Kantzis M, Shebani S, Yong S, Saeed I. Transapical access for dilatation of a melody valve in the mitral position using large balloons, in a small child: a case description with midterm follow-up and literature review. Cardiol Young. 2021;31(8):1336–9. doi:10.1017/S104795112100041X. [Google Scholar] [CrossRef]
143. Chai PJ, George I, Nazif TM, Kalfa DM, Kodali SK, Torres AJ, et al. Useofstented bovine pericardialvalve for surgical mitral valve replacement in infants. J Thorac Cardiovasc Surg. 2016;151(3):e51–2. doi:10.1016/j.jtcvs.2015.09.050. [Google Scholar] [CrossRef]
144. Maschietto N, Prakash A, Del Nido P, Porras D. Acute and short-term outcomes of percutaneous tran-scatheter mitral valve replacement in children. Circ Cardiovasc Interv. 2021;14(4):e009996. doi:10.1161/CIRCINTERVENTIONS.120.009996. [Google Scholar] [CrossRef]
145. Murphy M, Austin C, Bapat V, Morgan GJ. Use of an edwards sapien S3 valve to replace a dysfunctional mechanical mitral valve in an 11-year old boy: another small step for surgical and interventional collaboration. Eur J Cardiothorac Surg. 2016;50(3):577–9. doi:10.1093/ejcts/ezw076. [Google Scholar] [CrossRef]
146. Momenah TS, Alsahari A, Ahmed E, Al Khalaf K. Transcatheter mitral valve-in-valve implantation in a pediatric patient. Catheter Cardiovasc Interv. 2020;95(5):1062–5. doi:10.1002/ccd.28390. [Google Scholar] [CrossRef]
147. Al Nasef M, Alsahari A, Eltayeb A, Ahmad S, Al Khalaf K, Al Otaiby M, et al. Transcathetermitralvalve-in-valve implantation in pediatric patients. CJC Open. 2021;4(1):20–7. doi:10.1016/j.cjco.2021.08.007. [Google Scholar] [CrossRef]
148. Al-Tawil M, Butt S, Reap S, Duric B, Harahwa T, Chandiramani A, et al. Transseptal vs. transapical transcatheter mitral valve-in-valve and valve-in-ring implantation: a systematic review and meta-analysis. Curr Probl Cardiol. 2023;48(7):101684. doi:10.1016/j.cpcardiol.2023.101684. [Google Scholar] [CrossRef]
149. Urena M, Himbert D, Brochet E, Carrasco JL, Iung B, Nataf P, et al. Transseptal transcatheter mitral valve replacement using balloon-expandable transcatheter heart valves: a step-by-step approach. JACC Cardiovasc Interv. 2017;10(19):1905–19. doi:10.1016/j.jcin.2017.06.069. [Google Scholar] [CrossRef]
150. Filsoufi F, Anyanwu AC, Salzberg SP, Frankel T, Cohn LH, Adams DH. Long-term outcomes of tricuspid valve replacement in the current era. Ann Thorac Surg. 2005;80(3):845–50. doi:10.1016/j.athoracsur.2004.12.019. [Google Scholar] [CrossRef]
151. Wiedemann D, Rupprechter V, Mascherbauer J, Kammerlander A, Mora B, Dimitrov K, et al. Tricuspid valve replacement: results of an orphan procedure—which is the best prosthesis? J Cardiovasc Surg. 2018;59(4):626–32. doi:10.23736/S0021-9509.18.10392-2. [Google Scholar] [CrossRef]
152. Nguyen SN, Vinogradsky AV, Ferrari G, Sykes M, Bacha EA, Richmond ME, et al. Pitfalls and future directions of contemporary pediatric valve surgery: the case for living valve substitutes. Curr Pediatr Rep. 2023;11(4):180–92. doi:10.1007/s40124-023-00295-2. [Google Scholar] [CrossRef]
153. Cheng Z, Fang T, Wang D, Guo Y. Tricuspid valve replacement: mechanical or biological prostheses? A systematic review and meta-analysis. Heart Surg Forum. 2021;24(2):E209–14. doi:10.1532/hsf.3531. [Google Scholar] [CrossRef]
154. Kawahira Y, Yagihara T, Uemura H, Yoshizumi K, Yoshikawa Y, Kitamura S. Replacement of the tricuspid valve in children with congenital cardiac malformations. J Heart Valve Dis. 2000;9(5):636–40. [Google Scholar]
155. Redondo Palacios A, López Menéndez J, Miguelena Hycka J, Martín García M, Varela Barca L, Ferreiro Marzal A, et al. Which type of valve should we use in tricuspid position? Long-term comparison between mechanical and biological valves. J Cardiovasc Surg. 2017;58(5):739–46. [Google Scholar]
156. Solomon NAG, Lim RCH, Nand P, Graham KJ. Tricuspid valve replacement: bioprosthetic or mechanical valve? Asian Cardiovasc Thorac Ann. 2004;12(2):143–8. [Google Scholar]
157. Vaidyanathan K, Agarwal R, Johari R, Cherian KM. Tricuspid valve replacement with a fresh antibiotic preserved tricuspid homograft. Interact Cardiovasc Thorac Surg. 2010;10(6):1061–2. doi:10.1510/icvts.2010.234757. [Google Scholar] [CrossRef]
158. Nozar JV, Anzibar R, Picarelli D, Tambasco J, Leone RW. Mitral homograft replacement of tricuspid valve in children. J Thorac Cardiovasc Surg. 2000;120(4):822–3. doi:10.1067/mtc.2000.108694. [Google Scholar] [CrossRef]
159. Hvass U, Lansac E, Chatel D, Henri I. Mitral homograft for tricuspid valve endocarditis complicating a congenital fistula between the right coronary artery and right ventricle. J Heart Valve Dis. 1996;5(5):564–6. [Google Scholar]
160. Vogel AD, Kwon JH, Mitta A, Sherard C, Brockbank KGM, Rajab TK. Immunogenicityofhomologousheart valves: mechanisms and future considerations. Cardiol Rev. 2024;32(5):385–91. doi:10.1097/CRD.0000000000000519. [Google Scholar] [CrossRef]
161. Zafar F, Morales DLS. Living and growing valve replacements for children: so near yet so far. J Thorac Cardiovasc Surg. 2017;154(3):e63–4. doi:10.1016/j.jtcvs.2017.05.081. [Google Scholar] [CrossRef]
162. Roberts PA, Boudjemline Y, Cheatham JP, Eicken A, Ewert P, Mc Elhinney DB, et al. Percutaneous tricuspid valve replacement in congenital and acquired heart disease. J Am Coll Cardiol. 2011;58(2):117–22. doi:10.1016/j.jacc.2011.01.044. [Google Scholar] [CrossRef]
163. Saini A, Kim DW, Maher KO, Deshpande SR. Melody valve implantation in the tricuspid position after pediatric heart transplantation—a case report. J Soc Cardiovasc Angiogr Interv. 2024;3(5):101354. doi:10.1016/j.jscai.2024.101354. [Google Scholar] [CrossRef]
164. Webb JG, Wood DA, Ye J, Gurvitch R, Masson JB, Rodés-Cabau J, et al. Transcatheter valve-in-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121(16):1848–57. doi:10.1161/CIRCULATIONAHA.109.924613. [Google Scholar] [CrossRef]
165. Alshami N, Sarvestani AL, Thomas AS, St Louis J, Kochilas L, Raghuveer G. Valve replacement in children with single ventricle physiology. Pediatr Cardiol. 2020;41(1):129–33. doi:10.1007/s00246-019-02234-9. [Google Scholar] [CrossRef]
166. Sughimoto K, Hirata Y, Hirahara N, Miyata H, Suzuki T, Murakami A, et al. Mid-term result of atrioventricular valve replacement in patients with a single ventricle. Interact Cardiovasc Thorac Surg. 2018;27(6):895–900. [Google Scholar]
167. Misumi Y, Hoashi T, Kagisaki K, Kitano M, Kurosaki K, Shiraishi I, et al. Long-term outcomes of common atrioventricular valve plasty in patients with functional single ventricle. Interact Cardiovasc Thorac Surg. 2014;18(3):259–65. doi:10.1093/icvts/ivt508. [Google Scholar] [CrossRef]
168. Luo S, Honjo O. A bridge to heart transplant: systemic atrioventricular valve replacement with a Melody valve in an infant with a single-ventricle physiology. J Thorac Cardiovasc Surg. 2019;158(2):e73–4. doi:10.1016/j.jtcvs.2019.01.111. [Google Scholar] [CrossRef]
169. Bauser-Heaton H, Barry OM, Hofferberth SC, Tretter JT, Ma M, Goldstone A, et al. Challenges and priorities for children with congenital valvar heart disease. JACC Adv. 2024;3(10):101191. doi:10.1016/j.jacadv.2024.101191. [Google Scholar] [CrossRef]
170. Konsek H, Sherard C, Bisbee C, Kang L, Turek JW, Rajab TK. Growing heart valve implants for children. J Cardiovasc Dev Dis. 2023;10(4):148. doi:10.3390/jcdd10040148. [Google Scholar] [CrossRef]
171. Dijkman PE, Fioretta ES, Frese L, Pasqualini FS, Hoerstrup SP. Heart valve replacements with regenerative capacity. Transfus Med Hemotherapy. 2016;43(4):282–90. doi:10.1159/000448181. [Google Scholar] [CrossRef]
172. Mc Gregor C, Byrne G, Rahmani B, Chisari E, Kyriakopoulou K, Burriesci G. Physical equivalency of wild type and galactose α 1,3 galactose free porcine pericardium; a new source material for bioprosthetic heart valves. Acta Biomater. 2016;41:204–9. doi:10.1016/j.actbio.2016.06.007. [Google Scholar] [CrossRef]
173. Smood B, Hara H, Cleveland DC, Cooper DKC. In search of the ideal valve: optimizing Genetic modifications to prevent bioprosthetic degeneration. Ann Thorac Surg. 2019;108(2):624–35. doi:10.1016/j.athoracsur.2019.01. [Google Scholar] [CrossRef]
174. Soliman O, Miyazaki Y, Abdelghani M, Brugmans M, Witsenburg M, Onuma Y, et al. Midterm performanceofa novel restorative pulmonary valved conduit: preclinical results. EuroIntervention. 2017;13(12):e1418–27. doi:10.4244/EIJ-D-17-00553. [Google Scholar] [CrossRef]
175. Bennink G, Torii S, Brugmans M, Cox M, Svanidze O, Ladich E, et al. A novel restorative pulmonary valved conduit in a chronic sheep model: mid-term hemodynamic function and histologic assessment. J Thorac Cardiovasc Surg. 2018;155(6):2591–601.e3. doi:10.1016/j.jtcvs.2017.12.046. [Google Scholar] [CrossRef]
176. Brugmans M, Serrero A, Cox M, Svanidze O, Schoen FJ. Morphology and mechanisms of a novel absorbable polymeric conduit in the pulmonary circulation of sheep. Cardiovasc Pathol. 2019;38:31–8. doi:10.1016/j.carpath.2018.10.008. [Google Scholar] [CrossRef]
177. Prodan Z, Mroczek T, Sivalingam S, Bennink G, Asch FM, Cox M, et al. Initial clinical trial of a novel pulmonary valved conduit. Semin Thorac Cardiovasc Surg. 2022;34(3):985–91. doi:10.1053/j.semtcvs.2021.03.036. [Google Scholar] [CrossRef]
178. Serruys P, Miyazaki Y, Katsikis A, Abdelghani M, Leon M, Virmani R, et al. Restorative valve therapy by endogenous tissue restoration: tomorrow’s world? Reflection on the EuroPCR 2017 session on endogenous tissue restoration. EuroIntervention. 2017;13(AA):AA68–77. doi:10.4244/EIJ-D-17-00509. [Google Scholar] [CrossRef]
179. Alsoufi B. Commentary: is the vanishing conduit the answer for the lack of ideal conduit? Semin Thorac Cardiovasc Surg. 2022;34(3):992–3. [Google Scholar]
180. Reimer J, Syedain Z, Haynie B, Lahti M, Berry J, Tranquillo R. Implantation of a tissue-engineered tubular heart valve in growing lambs. Ann Biomed Eng. 2017;45(2):439–51. doi:10.1007/s10439-016-1605-7. [Google Scholar] [CrossRef]
181. Baker RS, Zafar F, Moore RA, Taylor MD, Morales DLS. Tubular bioprosthetic tricuspid valve implant demon-strates chordae formation and no calcification. J Am Coll Cardiol. 2017;70(19):2456–8. doi:10.1016/j.jacc.2017.09.012. [Google Scholar] [CrossRef]
182. Schmitt B, Sun X, Steitz M, Breitenstein A. A novel regenerative autologous heart valve for adults and children: from bench to bedside: an interim report. Thorac Cardiovasc Surg. 2023;71(S2):S73–106. doi:10.1055/s-0043-1761865. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools