 Open Access
Open Access
ARTICLE
Real-Time Prediction Algorithm for Intelligent Edge Networks with Federated Learning-Based Modeling
1 Department of Software Convergence, Soonchunhyang University, Asan, 31538, Korea
2 Department of Telecommunication and Electronic Engineering, Royal University of Phnom Penh, Phnom Penh, 12156, Cambodia
3 Department of Computer Software Engineering, Soonchunhyang University, Asan, 31538, Korea
* Corresponding Author: Seokhoon Kim. Email:
Computers, Materials & Continua 2023, 77(2), 1967-1983. https://doi.org/10.32604/cmc.2023.045020
Received 14 August 2023; Accepted 27 September 2023; Issue published 29 November 2023
Abstract
Intelligent healthcare networks represent a significant component in digital applications, where the requirements hold within quality-of-service (QoS) reliability and safeguarding privacy. This paper addresses these requirements through the integration of enabler paradigms, including federated learning (FL), cloud/edge computing, software-defined/virtualized networking infrastructure, and converged prediction algorithms. The study focuses on achieving reliability and efficiency in real-time prediction models, which depend on the interaction flows and network topology. In response to these challenges, we introduce a modified version of federated logistic regression (FLR) that takes into account convergence latencies and the accuracy of the final FL model within healthcare networks. To establish the FLR framework for mission-critical healthcare applications, we provide a comprehensive workflow in this paper, introducing framework setup, iterative round communications, and model evaluation/deployment. Our optimization process delves into the formulation of loss functions and gradients within the domain of federated optimization, which concludes with the generation of service experience batches for model deployment. To assess the practicality of our approach, we conducted experiments using a hypertension prediction model with data sourced from the 2019 annual dataset (Version 2.0.1) of the Korea Medical Panel Survey. Performance metrics, including end-to-end execution delays, model drop/delivery ratios, and final model accuracies, are captured and compared between the proposed FLR framework and other baseline schemes. Our study offers an FLR framework setup for the enhancement of real-time prediction modeling within intelligent healthcare networks, addressing the critical demands of QoS reliability and privacy preservation.Keywords
By enabling the interaction of exchanging model parameters using local on-device computation and global server aggregation, federated learning (FL) presents a collaborative and privacy-preserving framework, which is cooperative for future digital healthcare systems that need assistance from machine learning and deep learning [1,2]. FL has been widely adopted in privacy-sensitive fields like smart healthcare services, where local participant devices such as the Internet of Healthcare Things (IoHT) utilize the sharing-restricted data to compute the models. By keeping patient data locally, healthcare institutions can uphold privacy regulations and maintain the trust of the patients while still benefiting from collective knowledge through scalable parties/organizations gained with collaborative model training. The main functionality phases of FL include 3 primary entities, including the parameter server, edge aggregator node, and IoHT devices. The framework starts by distributing the global model structure and parameters to the local participant, and then after the local computation, the model aggregation between local and edge is executed to assist the resource-constrained IoHT devices before updating to the global parameter server. The active status of local participants from the previous learning iteration requires requesting establishment for next-iteration model learning. The framework iteratively trains the model through multiple round communications until reaching the final convergence points. FL ensures five primary beneficial factors as follows for digital healthcare systems:
• Healthcare Privacy Protection: FL allows data to remain on local devices, ensuring internal confidentiality. Since the data is not shared in a centralized manner, the model can be trained on personal data locally without exposure to other parties.
• Sensitive Data Security: By keeping data decentralized, FL reduces the risk of data breaches or unauthorized access. The risk of data exposure during transmission or storage is minimized. FL safeguards patient information and protects against potential security vulnerabilities, which is particularly important when dealing with sensitive data such as medical records or financial information.
• Collaboration and Integration: FL enables the pooling of knowledge and data resources from different parties and healthcare organizations. Each party can contribute their local knowledge to the model while maintaining data control. The collaborative approach enhances the accuracy and robustness of predictive models, allowing for improved decision-making.
• Massive Scalability: As the number of participating institutions increases, the collective dataset grows larger, allowing for more diverse and representative training data. The scalability of FL helps in developing more accurate and generalized models that can be applied to a wider range of healthcare scenarios and populations.
• Regulatory Compliance: FL aligns with regulations such as the general data protection regulation (GDPR) and other data privacy and security standards [3,4]. The compliance factor ensures that healthcare institutions can leverage the benefits of FL without compromising legal and ethical obligations related to patient data handling.
In future digital healthcare systems, real-time disease prediction is one of the cutting-edge approaches that need support from the FL architecture [5,6]. However, achieving an accurate final learning model requires attention to several key aspects for sufficient real-time performance metrics, such as communication and computation resource placement, client selection, model aggregation scheduling, and offloading strategies. One of the key challenges in healthcare applications is the need for real-time predictions with high accuracy and reliability. Quality-of-service (QoS) reliability is a vital factor in healthcare because it directly affects the quality and timeliness of healthcare services and decision-making.
Therefore, this paper aims to design a well-formulated objective function with system models of complete FL interactions, termed federated logistic regression (FLR), that can deploy a real-time prediction model in intelligent healthcare networks and ensure maximum reliability with weighted high-quality data contribution of IoHT participants. The integration of FLR in edge networks enhances the accuracy and robustness of predictive models, thereby significantly impacting QoS reliability in healthcare. The key contribution can be summarized as follows:
• We define the final predictive modeling problem by specifying three key components for each healthcare service, including target variables, input variables, and optimization parameters. After specifying the key components, edge aggregation is pairing for optimizing each service following their QoS expectations and upper-bound threshold.
• Our system architecture provides an overview of the communication flow within the FLR system in healthcare networks, spanning three crucial phases, including framework setup, iterative round communications, and model evaluation and deployment. Furthermore, we employ a dataset from the 2019 annual data (Version 2.0.1) of the Korea Medical Panel Survey, a collaboration between the Korea Institute for Health and Social Affairs (KIHASA) and the National Health Insurance Service (NHIS).
• The experiment of our prediction model was conducted on a Mininet testbed to represent the model flows in communication networks, and from another perspective, we focused on the final model on hypertension prediction, considering factors such as age, gender, diabetes status, physical activity, smoking habits, occupation, and education level to develop a comprehensive policy.
Healthcare institutions deal with highly confidential information related to patient’s medical conditions, treatments, and personal details. Therefore, it is crucial to safeguard this data and ensure its privacy protection throughout the learning process. Practical FL needs to be flexible in a resource-constrained and scalable environment, which provides self-organizing capabilities in terms of resource awareness, personalization, incentive awareness, etc. [7–9]. Distributed edge FL plays a vital role in offering the mentioned capabilities by leveraging edge aggregation processes within micro data centers or other access points (equipped computing servers) [10–12]. These edge aggregators act as intermediaries that facilitate the aggregation of locally computed updates without exposing the raw data.
The healthcare domain has witnessed extensive exploration of FL, particularly in real-time medical data processing and applications such as brain tumor segmentation [13,14]. The ability to learn from distributed data sources while preserving privacy has opened up new avenues for improving medical diagnostics and treatment outcomes. FL harnesses the concept of learning over networks, allowing healthcare institutions to collaborate and share knowledge intelligently. Furthermore, bandwidth efficiency is a key aspect, especially when integrating FL with the message queuing telemetry transport (MQTT) protocol. MQTT, known for its lightweight and efficient messaging capabilities, facilitates seamless communication between the central server and local clients. This protocol minimizes the overhead associated with data transmission, enabling faster and more efficient model updates [13].
The integration of real-time medical data processing within the FL architecture has proven to be highly signification. By continuously incorporating subsets of medical data with varying timestamps and conditions, the models can adapt to dynamic healthcare scenarios and improve their diagnostic capabilities [14]. The workflow procedures involve multiple data rounds, where streaming data is collected in each iteration. The model stages leverage the FL paradigm to aggregate and average between the old-timeslot and new-timeslot models, allowing for incremental learning and continuous improvement. Exemplar stages help establish a new set of exemplars based on the previous diagnosis model, contributing to the ongoing refinement and accuracy of the model [14].
The proposed solution in [6] leverages homomorphic encryption to implement logistic regression for vertical FL and model prediction. By designing a privacy-preserving logistic regression training scheme based on homomorphic encryption in vertical FL, the paper contributes to improving security while maintaining an acceptable level of efficiency. The scheme overcomes limitations of existing approaches, such as protecting gradient information during training, avoiding the leakage of party labels, and significantly safeguarding data features of the host. The authors also introduce a multi-party vertical FL framework that eliminates the need for a third-party to address the multi-party logistic regression problem. The proposed framework enables effective model training among multiple participants while ensuring privacy and data protection. By removing the reliance on a trusted third-party coordinator, the proposed framework simplifies the complexity and enhances security.
The aforementioned studies collectively emphasize the critical significance of FL in the context of real-time healthcare services. The ability to leverage distributed data while maintaining privacy and confidentiality enables healthcare networks to unlock new possibilities for improving patient care, medical research, and decision-making processes. However, to harness the full potential of FL in prediction modeling within healthcare systems, it is essential to converge the FL system architecture with logistic regression. Logistic regression is a well-established and interpretable machine learning technique, particularly suited for binary classification tasks common in healthcare, such as disease prediction. Combining FL with logistic regression allows us to leverage the strengths of each approach: (1) FL facilitates collaborative learning from distributed data while preserving privacy, and (2) logistic regression provides transparent and interpretable models crucial for gaining medical professional trust. Therefore, the concept of convergence enables real-time and incremental learning, which is crucial in healthcare where data continually evolves. Logistic regression within FL ensures that models adapt incrementally to changing healthcare scenarios while maintaining accuracy.
3 System Architectures and Models for Federated Logistic Regression
Resource-constrained and mission-critical healthcare environments pose unique challenges to the FL framework, requiring efficient management of communication resources and modification of real-time prediction performance. In this section, the network setup, system architectures, and models for the proposed FLR are presented. Table 1 presents the key notations and its description used in this paper.

The proposed system defined the final predictive modeling problem by determining the 3-tuple information for each healthcare modeling in service-
For input variables from multi-participants, all the features are mostly not complete/matching and consist of null values, which requires a collaborative normalization process from experience feature batch from global server. Healthcare feature normalization is formulated as a problem with the solutions by standard scaling or imputation technique. Edge-assisted data feature filtering can be used to expedite the preprocessing. At the output layer of FLR, sigmoid function is employed, which requires the utilization of optimal
The objectives of this proposed prediction algorithm using FLR are expressed in Eqs. (8) and (9) by optimizing the reliability of prediction output measuring by cost function, normalization of collaborative input features per services, and optimization of argument
This section introduces the communications flow for FLR systems in healthcare networks, encompassing three key phases: framework setup, iterative round communications, and model evaluation and deployment. Framework setup introduces the involved entities and controlling policies for optimal FLR initialization. Iterative round communications include the global model distribution, local model computation (missing input values handling), local model transmission, secure aggregation and updates, and global model re-distribution in the next round of communication. Finally, model evaluation and deployment cover the efficiency weighting of trained FLR models and decide based on evaluation metrics before whether to re-train or compress for final implementation. This section primarily presents the objective in communication networking perspectives in the execution of FLR for reliable healthcare QoS requirements, which essentially aims for minimizing the latency in constructing the final converged FLR model. Fig. 1 illustrates the interactions between the key entities and execution functions in the FLR system, which describes the overall functionalities formulated in the following sub-sections.
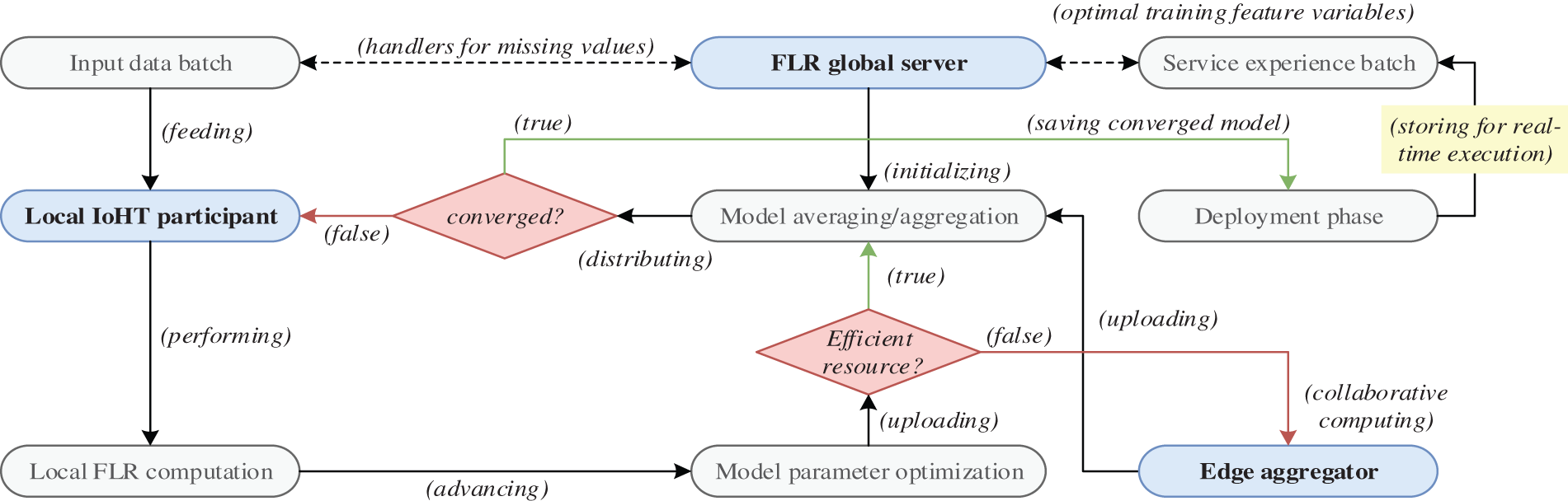
Figure 1: Working flow of the proposed FLR framework
The collaborative FLR framework involves two essential entities, namely (1) the locally selected participants that compute the local model
The decision on whether to perform central model aggregation and averaging in the cloud, at the edge, or through a hybrid approach requires adaptability from the controller. In order to accommodate the heterogeneity of the IoHT environment, where participants and services may have varying features and labels, multi-service containers are employed. To be selected as an IoHT participant, certain criteria must be met, including computation capabilities, secure communications, and the ability to provide high-quality data. If these requirements are not met, edge-assisted model computation is employed to aid in the training and computing processes, ensuring effective FL. The setup of the FLR framework includes the following major procedures:
• Network Topology: In the context of FLR, network topology refers to the structure of connections among the participating IoHT devices and other nodes, which interact by two topology settings. 1) Centralized topology, where the central server acts as the coordinator of the FLR execution. The participating IoHT devices n communicate with the central server G to exchange model updates
• Participant Selection: The framework controller determines which IoHT devices will participate in communication rounds based on various selection metrics, such as device heterogeneity, healthcare data privacy, connectivity and availability, and representativeness. These metrics ensure that the chosen devices meet specific criteria to ensure the effectiveness of the FLR process for healthcare prediction services.
• Data Batch Partitioning: FLR policy settings ensure the completion time of local training to avoid heavy delays on model updates and later affect the global convergence speed. The partition process divides the training data batches into smaller subsets and assigns each subset
• Model Initialization:
3.2.2 Global Model Distribution
The central server fine-tunes the model architectures, hyperparameters, and target use cases as a global learning model during the initial iteration, aiming to distribute it to chosen IoHT participants. When it comes to multi-service IoHT prediction, the diverse models endure the deployment process by considering participant clustering, service prioritization, and aggregation strategies. The models are distributed to selected IoHT participants with matching services and enable the structure for the next phase of local model computation.
3.2.3 Local Model Distribution
Once the chosen IoHT nodes receive the distributed model
3.2.4 Local Model Transmission
Once the optimal parameter
3.2.5 Global Model Aggregation/Updates and Re-Distribution
In the aggregation process, a weighted metric
This phase of the proposed FLR system aims to achieve several functionalities, including (1) promotes collaboration and knowledge sharing among the IoHT participating devices leading to a collectively improved global model, (2) allows for privacy-preserving learning and adaptive exchanges of model updates, and (3) enables continuous learning and adaptation, as the global model is refined over multiple iterations, capturing weight factors from various IoHT participants and data sources.
3.2.6 Model Evaluation and Deployment
Once the FLR process is complete, the system evaluates the performance of the trained global model using appropriate metrics and saves the converged model for the deployment phase, which can be handled using a held-out validation set. If the model is not converged following the expectation metrics of particular healthcare service requirements, false conditions lead to the distribution of the global model to local IoHT participants for next-round communication training.
The optimal trained global model
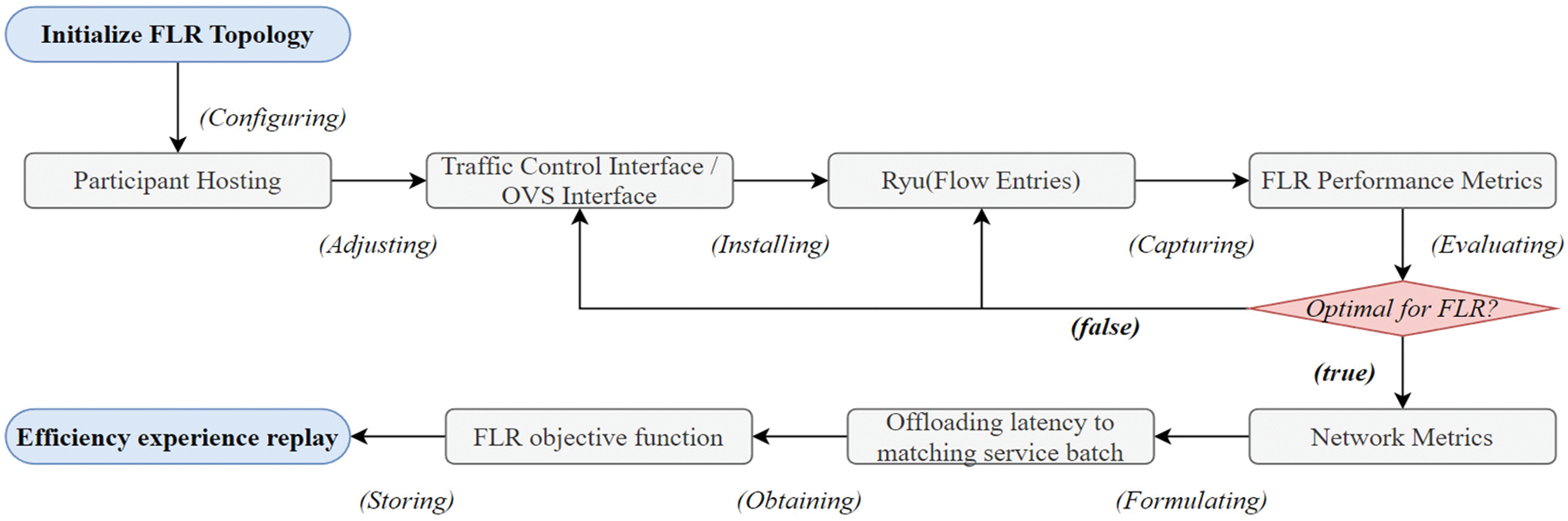
Figure 2: Flow for evaluation and primary deployment execution
The dataset utilized in this research is derived from the 2019 annual data (Version 2.0.1) of the Korea Medical Panel Survey, a collaborative effort between KIHASA and NHIS. The focus of the prediction model developed in this study is on hypertension, incorporating factors such as age, gender, diabetes status, physical activity, smoking status, occupation, and education level. By using this dataset and selected features, we aim to measure and identify the coefficient weights in each category and the high/low capability of current risk analysis, providing a foundation and recommendation for future health improvements. The selection of features is partially influenced by previous studies addressing risk factors related to hypertension prediction [15–17], which highlights the importance of critically examining the performance of existing hypertension risk models.
The proposed FLR method is compared to two baseline approaches that utilize computation-intensive mechanisms to guide the policies of FL networks, termed CI-FL and Conv-FL. Fig. 3 is given as a FLR network topology representation. The employed topology is primarily based on Mininet software-defined networking (SDN) emulator and RYU controller (using Python-based custom scripts) [18–22], utilizing a testbed that supports programmable networking as follows:
• CPULimitedHost is used to configure the 5 participants (P1 to P5) and 1 global parameter server (G1) with IoHT capacities for simulating resource-constrained hosts with limited CPU capacity. The setting parameter determines the maximum CPU utilization allowed for the host and specifies the 70% of CPU resources that the host is allowed to utilize in the FLR execution.
• Open vSwitch (OVS) and RYU adjusts the functionalities and uses TCIntf with OVSIntf in topology settings and flow entry management. OVSIntf offers the creation and management of virtual switches, ports, and flows within the proposed FLR topology. The bridge name, ports, VLANs, flows, and QoS settings are assigned. TCIntf configures the control parameters on bandwidth, delay, loss, jitter, txo, rxo, and max_queue_size. The port configurations and other OVS-specific settings are set within OVSIntf. This functionality serves as a crucial component in our framework, simulating the behavior and functionality of edge devices in a controlled environment. While it may not replicate all aspects of real-world devices, it allows us to assess the impact of edge-structured functionality on the FLR framework and evaluate the contribution to real-time aspects.
• FL Setting partially follows the integration process with TensorFlow-Federated to capture the results and execute the FL aspect. However, this paper extends the contribution by introducing FLR models and IoHT nodes to evaluate the resource-constrained and mission-critical healthcare metrics with real-time prediction models and new dataset distribution as non-IID. The hyperparameters of forecasting and deep learning [23–25], applied for the proposed FLR method are set to 0.01 for the learning rate α, and
• Hosting Server in the experiments is equipped with an Intel(R) Xeon(R) Silver 4280 CPU @ 2.10 GHz, 128 GB memory, and an NVIDIA Quadro RTX 4000 GPU.
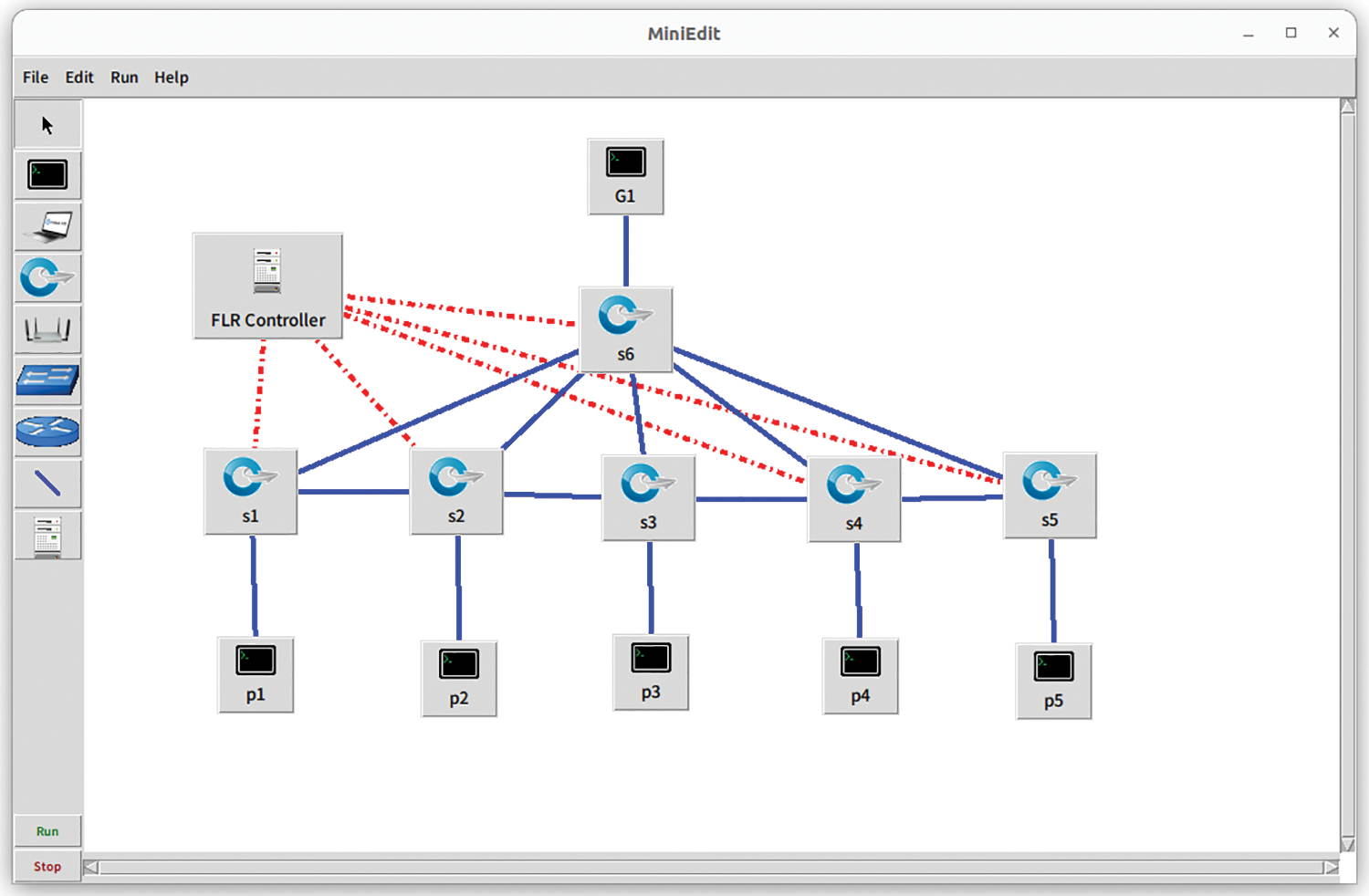
Figure 3: Representation of FLR network topology
Data preprocessing involves the use of an imputation technique, resulting in a reduction of null target classes from 14,741 to 13,834. Then, FLR is employed to determine the essential coefficients. The selected features, descriptions, and coefficients after the final FLR prediction model are given in Table 2. The results of coefficients represent the relationship between the independent variables (features of patient information) and the log-odds of the dependent variable (target variable of hypertension status) being in a particular category. The coefficient value indicates the direction and strength of the association between a specific independent variable from a given patient condition and the log-odds of the target variable. A positive coefficient suggests the value increment of the independent variable is related to the increment of target variable log-odds being in the hypertension status class. Otherwise, a negative coefficient suggests that the value increment of the independent variable is related to the decrement of the target instead. The magnitude of the coefficient indicates the strength of the relationship. Larger coefficient values indicate a stronger association between the independent variable and the log-odds. In other words, a larger coefficient implies a larger impact of the independent variable on the predicted hypertension outcome (0 to 1). Additionally, the coefficient values can be used to interpret the odds ratio associated with each independent variable. The odds ratio is calculated by exponentiating the coefficient value. For example, if the coefficient of physical activity is −0.2326, the odds ratio would be exp(−0.2326), which represents the multiplicative change in the odds of the target variable for a one-unit increase in the independent variable.

Overall, the coefficient values from FLR provide insights into the direction, strength, and magnitude of the relationship between the independent variables in the general dataset features and the log-odds of the predicted target variable of hypertension status, allowing for an understanding of the impact of each variable on predicted disease likelihood.
In FLR communication perspective, IoHT nodes in the Mininet network represent resource-constrained devices, and their configuration is designed to exceed the maximum resource threshold. The proposed offloading strategies and fast-convergence FLR aim to highlight the significance of reliable model flows by incorporating congestion and resource limitations. Selected performance metrics include the local model update delivery/drop ratios, end-to-end execution latencies, and final accuracies per round communication. Each metric is presented as follows:
• Local model update delivery/drop ratio: the delivery ratio refers to the ratio of model updates that are successfully transmitted or delivered from the local IoHT nodes (P1 to P5) to the central server G1 during the FL process. This indicator measures the effectiveness of communication and data transmission between the local devices and the central server. A higher drop ratio indicates a higher rate of unsuccessful transmission, which can be due to network congestion, limited resources, or other computation-intensive modeling issues. A higher delivery ratio suggests a reliable and efficient transmission of local model updates and leads to applicable FL in healthcare.
• End-to-end execution latencies: this metric measures the time the system takes for the entire round communication to complete, from the initialization to the ending round point, until the model reaches a satisfactory level of accuracy. The end-to-end latency
• Final model accuracies: to evaluate the final FL model performance in predicting the target hypertension condition, the training and testing phases are captured for accuracy measurement. Higher model accuracies indicate a better predictive performance and a more reliable model. Assessing the final model accuracies helps evaluate the effectiveness of the FL approach in improving the predictive performance of healthcare.
In FLR architectures, the consideration of local-global model communications leads to the possible drop of local model updates based on simulated resource efficiency and offloading scheduling, which severely affects the reliability of the system, particularly in real-time healthcare applications. The development of optimal prediction modeling and network topology setup have to be balanced for practical systems in real-world scenarios that can be highly congested, communicating/computing resource constraints, multi-personalized systems, or energy limitations. Fig. 4a presents the results on delivery ratios within 270 s of simulation throughout 3 different congestion states, from 30 to 270. The results show an average output of 99.96% for the proposed FLR, which is 0.06% and 0.1% higher than CI-FL and Conv-FL, respectively. The primary reason of CI-FL for aiming high delivery ratio is the target setting on resource-intensive computations. CI-FL prioritized delivering updates from resource-efficient nodes or with less computational load. While CI-FL ensures efficient communication, it leads to a high delivery ratio by favoring nodes that can handle computation-intensive tasks effectively. However, delivery efficiency comes at the expense of scalability and adaptability to resource-constrained devices, which may not perform well in real-time healthcare scenarios. Conv-FL demonstrates a high delivery ratio, but it appears to perform the least efficiently among the three approaches. The reason behind the delivery ratio of Conv-FL is attributed to its conventional communication strategies. Conv-FL is not adapted as well to network congestion or resource limitations. Consequently, while it maintains a high delivery ratio, it might be less suitable for real-time healthcare applications due to longer communication latencies. In the context of FL model communications, a high delivery ratio refers to a high proportion of successfully transmitted or delivered model updates, which indicates that the majority of the model updates sent by the local devices have reached the intended destination for aggregation/averaging without being lost or dropped during transmission. A high delivery ratio is desirable and achieved in the proposed FLR because the controlling policies and weight
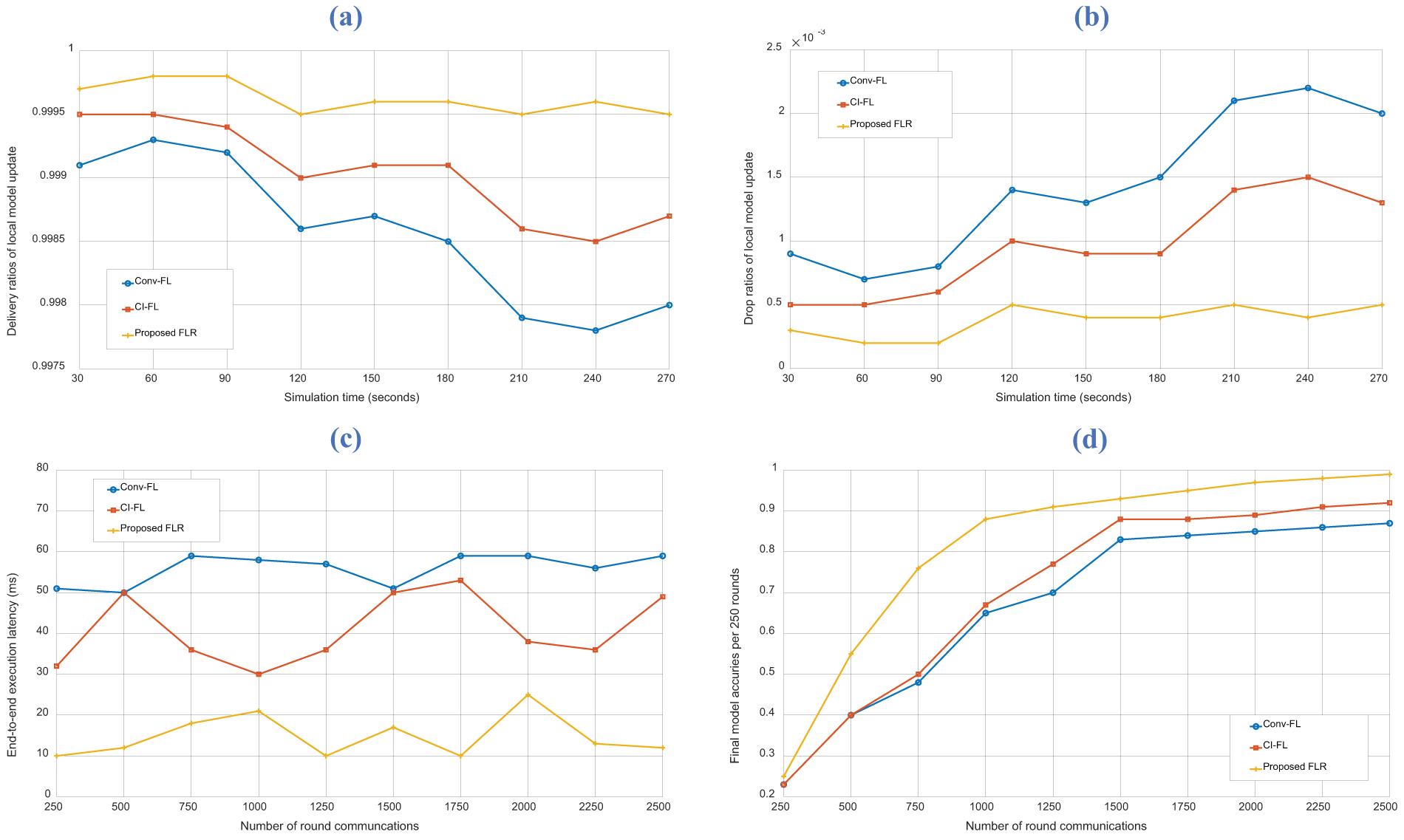
Figure 4: Results of proposed and baseline schemes on (a) delivery ratios of local model update, (b) drop ratios of local model update, (c) end-to-end execution latency, and (d) final model accuracies
The end-to-end execution latency is illustrated in Fig. 4c, which determines the total time for the FL operation to finish within each round of communication. The proposed scheme achieved an average latency of 14.8 ms, which is 26.2 and 41.1 ms faster than CI-FL and Conv-FL, respectively. This low latency output can be attributed to several factors. Firstly, FLR employs efficient controlling policies and weight placement (
Low end-to-end execution latency in completing FL model communications in each round iteration refers to the short amount of time it takes for the entire process of transmitting and updating the model between the local devices and the central server to occur. This metric improvement indicates that the communication and computational tasks involved in healthcare FL systems are performed efficiently, enabling rapid progress in each round of iteration. The proposed FLR allows a better connection between healthcare entities for a seamless and fast exchange of information, facilitating the aggregation and integration of the local models into the global prediction model. The proposed FLR is particularly significant in real-time applications of healthcare networks, where the availability of up-to-date models is crucial for timely decision-making and accurate predictions.
Fig. 4d shows the final model accuracies per 250 rounds of the proposed and baseline schemes. The proposed FLR reached the final accuracy at the 2500-th round with 99.97%, which is attributed to the efficient communication and aggregation strategies. The baseline schemes, namely CI-FL and Conv-FL, can only reach 92.92% and 87.09% only for this hypertension prediction problem, which is not efficient enough. The resource-intensive computation of CI-FL contributed to lower accuracy compared to FLR, as it could prioritize computation over edge placement and aggregation methods. For Conv-FL, it obtained less efficient data exchange and model aggregation. Conv-FL struggled to adapt to the evolving data landscape and real-time healthcare demands, which resulted in reduced accuracy. These results highlight the effectiveness of the proposed FLR architecture in terms of accuracy, convergence time, and resource utilization in the restricted healthcare environment.
This paper aims to minimize the latency in constructing the final converged FLR model while ensuring reliable healthcare QoS requirements. The interactions between key entities and execution functions in the FLR system were illustrated, highlighting the functionalities and the topology deployment phase. The results demonstrated the effectiveness of the proposed FLR architecture in terms of drop/delivery ratios of local model update, end-to-end execution latency, and final model accuracies. The proposed scheme achieved significantly lower latency compared to baseline schemes, with an average latency of 14.8 ms. We indicated that the communication and computational tasks involved in healthcare FL systems are performed efficiently by enabling rapid progress in each round of iteration. The proposed FLR architecture facilitated a seamless and fast exchange of information allowing for the aggregation and integration of local models into a reliable and real-time global prediction model. Furthermore, the proposed FLR architecture achieved better accuracy compared to baseline schemes. The final accuracy of the proposed FLR reached 99.97% after 2500 rounds, surpassing the baseline schemes, which achieved 92.92% and 87.09% for this particular hypertension prediction dataset. Overall, the proposed scheme offered beneficial factors in healthcare networks in terms of latency reduction, improved accuracy, and efficient utilization of resources. The integration of FLR into healthcare networks has the potential to enhance real-time prediction-based applications, ensuring timely decision-making and accurate modeling.
In future studies, the testbed platform for federated servers will be further developed to integrate our systems to serve more deep learning-based modeling in IoHT services. Furthermore, we will deploy fine-tuning edge aggregator components to better emulate real-world edge devices and patterns within healthcare networks. Performance metrics on (1) convergence speed, (2) resource consumption, and (3) energy consumption will be optimized in further study as a joint reward function in deep reinforcement learning agents. Autonomy and long-term sufficiency will be appended to the FLR framework.
Acknowledgement: Not applicable.
Funding Statement: This work was supported by Institute of Information & Communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) (No. RS-2022-00167197, Development of Intelligent 5G/6G Infrastructure Technology for the Smart City), in part by the National Research Foundation of Korea (NRF), Ministry of Education, through Basic Science Research Program under Grant NRF-2020R1I1A3066543, in part by BK21 FOUR (Fostering Outstanding Universities for Research) under Grant 5199990914048, and in part by the Soonchunhyang University Research Fund.
Author Contributions: Study conception and design: S. Kang, S. Ros, S. Kim; data collection: I. Song, P. Tam; analysis and interpretation of results: S. Kang, I. Song, S. Kim; draft manuscript preparation: S. Kang. S. Ros, I. Song, P. Tam, S. Math, S. Kim. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. M. H. Brendan, E. Moore, D. Ramage, S. Hampson and A. B. Agüera, “Communication-efficient learning of deep networks from decentralized data,” arXiv:1602.05629, 2016. [Google Scholar]
2. F. Cremonesi, V. Planat, V. Kalokyri, H. Kondylakis, T. Sanavia et al., “The need for multimodal health data modeling: A practical approach for a federated-learning healthcare platform,” Journal of Biomedical Informatics, vol. 141, pp. 104338–104338, 2023. [Google Scholar] [PubMed]
3. N. Truong, K. Sun, S. Wang, F. Guitton and Y. Guo, “Privacy preservation in federated learning: Insights from the gDPR perspective,” arXiv:2011.05411, 2020. [Google Scholar]
4. A. Brauneck, L. Schmahorst, M. Majdabadi, M. Bakhtiari, U. Völker et al., “Federated machine learning in data-protection-compliant research,” Nature Machine Intelligence, vol. 5, no. 1, pp. 2–4, 2023. [Google Scholar]
5. N. Rieke, J. Hancox, W. Li, F. Milletari, H. Roth et al., “The future of digital health with federated learning,” arXiv:2003.08119, 2020. [Google Scholar]
6. D. He, R. Du, S. Zhu, M. Zhang, K. Laing et al., “Secure logistic regression for vertical federated learning,” IEEE Internet Computing, vol. 26, no. 2, pp. 61–68, 2022. [Google Scholar]
7. M. G. Crowson, D. Moukhheiber, A. Arévalo, B. Lam, S. Mantena et al., “A systematic review of federated learning applications for biomedical data,” PLoS Digital Health, vol. 1, no. 5, pp. e0000033, 2022. [Google Scholar] [PubMed]
8. A. Z. Tan, H. Yu, L. Cui and Q. Yang, “Towards personalized federated learning,” arXiv:2103.00710, 2021. [Google Scholar]
9. S. Ros, P. Tam and S. Kim, “Modified deep reinforcement learning agent for dynamic resource placement in IoT network slicing,” Journal of Internet Computing and Services, vol. 23, no. 5, pp. 17–23, 2022. [Google Scholar]
10. L. U. Khan, S. Pandey, N. Tran, W. Saad, Z. Han et al., “Federated learning for edge networks: Resource optimization and incentive mechanism,” IEEE Communications Magazine, vol. 58, no. 10, pp. 88–93, 2020. [Google Scholar]
11. X. Wang, Y. Han, C. Wang, Q. Zhao, X. Chen et al., “In-edge AI: Intelligentizing mobile edge computing, caching and communication by federated learning,” IEEE Network, vol. 33, no. 5, pp. 156–165, 2019. [Google Scholar]
12. Y. Ye, S. Li, F. Liu, Y. Tang and W. Hu, “EdgeFed: Optimized federated learning based on edge computing,” IEEE Access, vol. 8, pp. 209191–209198, 2020. [Google Scholar]
13. B. Camajori Tedeschini, S. Savazzi, R. Stoklasa, L. Barbieri, L. Stathopoilos et al., “Decentralized federated learning for healthcare networks: A case study on tumor segmentation,” IEEE Access, vol. 10, pp. 8693–8708, 2022. [Google Scholar]
14. K. Guo, T. Chen, S. Ren, N. Li, M. Hu et al., “Federated learning empowered real-time medical data processing method for smart healthcare,” IEEE/ACM Transactions on Computational Biology and Bioinformatics, pp. 1–12, 2022. [Google Scholar]
15. J. B. Echouffo-Tcheugui, G. D. Batty, M. Kivimäki and A. P. Kengne, “Risk models to predict hypertension: A systematic review,” PLoS One, vol. 8, no. 7, pp. e67370, 2013. [Google Scholar] [PubMed]
16. L. A. AlKaabi, L. S. Ahmed, M. F. Al Attiyah and M. E. Abdel-Rahman, “Predicting hypertension using machine learning: Findings from Qatar biobank study,” PLoS One, vol. 15, no. 10, pp. e0240370, 2020. [Google Scholar] [PubMed]
17. M. Alotaibi and M. A. Uddin, “Effectiveness of e-health systems in improving hypertension management and awareness: A systematic review,” KSII Transactions on Internet and Information Systems, vol. 16, no. 1, pp. 173–187, 2022. [Google Scholar]
18. B. Lantz, B. Heller and N. McKeown, “A network in a laptop,” in Proc. of the 9th ACM SIGCOMM Workshop on Hot Topics in Networks, Monterey, California, pp. 1–6, 2010. [Google Scholar]
19. “Ryu controller,” [Online]. Available: https://github.com/faucetsdn/ryu [Google Scholar]
20. Y. Ren, A. Guo and C. Song, “Multi-slice joint task offloading and resource allocation scheme for massive MIMO enabled network,” KSII Transactions on Internet and Information Systems, vol. 17, no. 3, pp. 794–815, 2023. [Google Scholar]
21. Y. Zhu, C. Liu, Y. Zhang and W. You, “Research on 5G core network trust model based on NF interaction behavior,” KSII Transactions on Internet and Information Systems, vol. 16, no. 10, pp. 3333–3354, 2022. [Google Scholar]
22. R. L. S. de Oliveira, C. M. Schweitzer, A. A. Shinoda and L. R. Prete, “Using mininet for emulation and prototyping software-defined networks,” in 2014 IEEE Colombian Conf. on Communications and Computing (COLCOM), Bogota, Colombia, pp. 1–6, 2014. [Google Scholar]
23. A. Parizad and C. Hatziadoniu, “Deep learning algorithms and parallel distributed computing techniques for high-resolution load forecasting applying hyperparameter optimization,” IEEE Systems Journal, vol. 16, no. 3, pp. 3758–3769, 2022. [Google Scholar]
24. K. E. Hoque and H. Aljamaan, “Impact of hyperparameter tuning on machine learning models in stock price forecasting,” IEEE Access, vol. 9, pp. 163815–163830, 2021. [Google Scholar]
25. G. Peter and M. Matskevichus, “Hyperparameters tuning for machine learning models for time series forecasting,” in 2019 Sixth Int. Conf. on Social Networks Analysis, Management and Security (SNAMS), Granada, Spain, pp. 328–332, 2019. [Google Scholar]
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools