 Open Access
Open Access
ARTICLE
Fluoride Ion Adsorption Effect and Adsorption Mechanism of Self-Supported Adsorbent Materials Based on Desulfurization Gypsum-Aluminate Cement
College of Materials Science and Engineering, Xi’an University of Architecture and Technology, Xi’an, 710055, China
* Corresponding Author: Xuefeng Song. Email:
(This article belongs to the Special Issue: Eco-Friendly Waste-Base Materials for Pollution Control Sustainable Technologies)
Journal of Renewable Materials 2023, 11(12), 4079-4095. https://doi.org/10.32604/jrm.2023.028885
Received 13 January 2023; Accepted 07 March 2023; Issue published 10 November 2023
Abstract
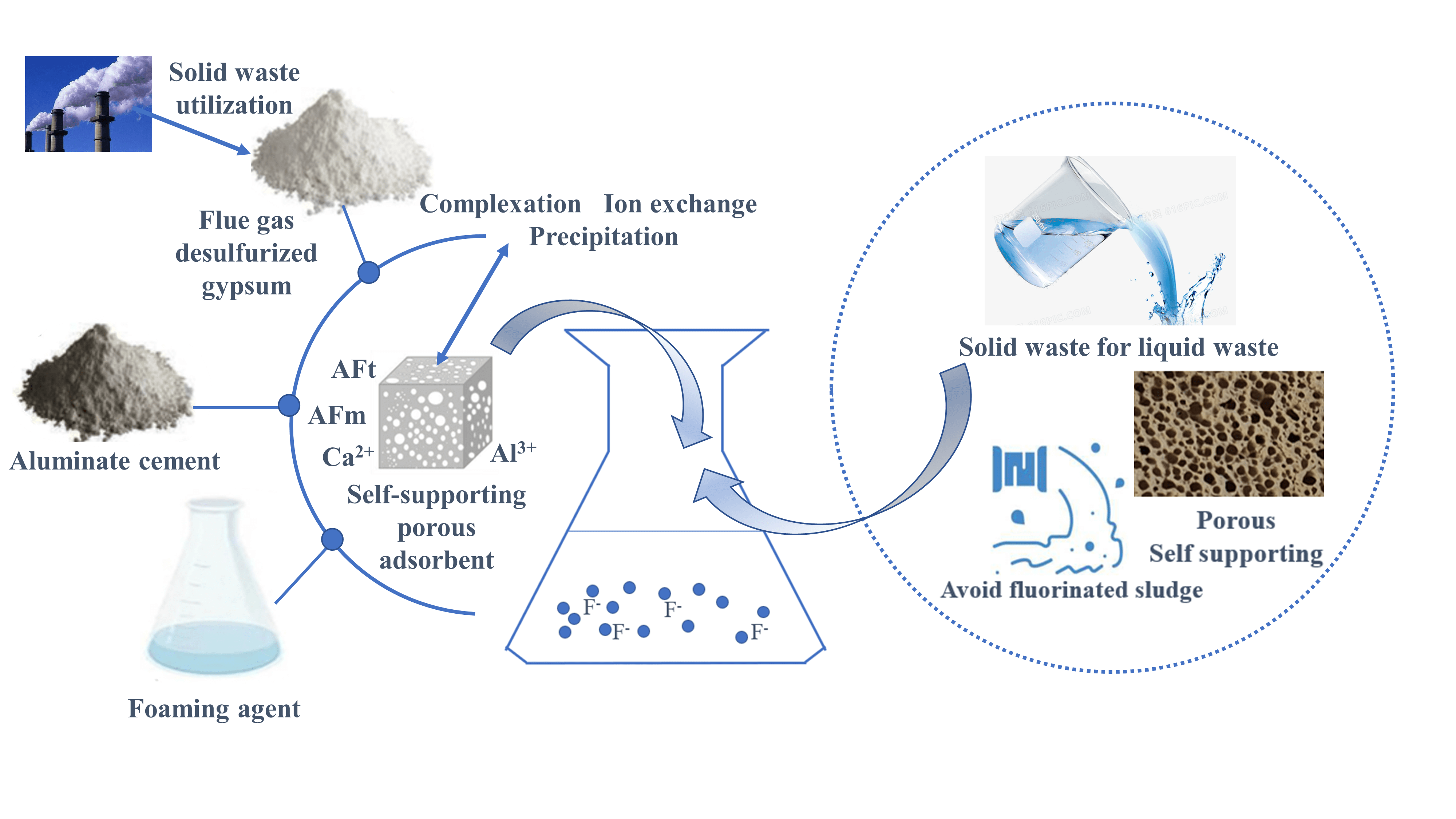
The adsorption method has the advantages of low cost, high efficiency, and environmental friendliness in treating fluorinated wastewater, and the adsorbent material is the key. This study combines the inherent anion-exchange adsorption properties of layered double hydroxides (LDHs). Self-supported porous adsorbent materials loaded with AFm and AFt were prepared from a composite cementitious system consisting of calcium aluminate cement (CAC) and flue gas desulfurization gypsum (FGDG) by chemical foaming technique. The mineral composition of the adsorbent material was characterized by X-ray diffraction (XRD) and Scanning electron microscopy (SEM). Through the static adsorption experiment, the adsorption effect of the mineral composition of the adsorbent on fluoride ions was deeply analyzed, and the adsorption mechanism was revealed. XRD and SEM showed that the main hydration phases of the composite cementitious system consisting of CAC and FGDG are AFm, AFt, AH3, and CaSO4·2H2O. FGDG accelerates the hydration process of CAC and inhibits the transformation of AFt to AFm. The AFt content increased, and the AFm content decreased or even disappeared as the amount of FGDG increased. Static adsorption experiment results showed that AFm and AFt in adsorbent materials could signifi- cantly enhance the adsorption of fluoride ions. The adsorption of F− in aqueous solution by PAG tends more towards monolayer adsorption with a theoretical maximum capacity of 108.70 mg/g and is similar to the measured value of 112.77 mg/g.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2023 The Author(s). Published by Tech Science Press.
Copyright © 2023 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools