 Open Access
Open Access
ARTICLE
The Influence of Tartaric Acid in the Silver Nanoparticle Synthesis Using Response Surface Methodology
1 Chemistry Department, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember, Surabaya, Indonesia
2 Chemistry Department, Faculty of Science and Technology, Airlangga University, Surabaya, Indonesia
3 Department Clinical Microbiology, Faculty of Medicine, Brawijaya University, Malang, Indonesia
* Corresponding Author: Suprapto Suprapto. Email:
(This article belongs to the Special Issue: Special Issue in Celebration of JRM 10 Years)
Journal of Renewable Materials 2024, 12(2), 245-258. https://doi.org/10.32604/jrm.2023.045514
Received 29 August 2023; Accepted 11 December 2023; Issue published 11 March 2024
Abstract
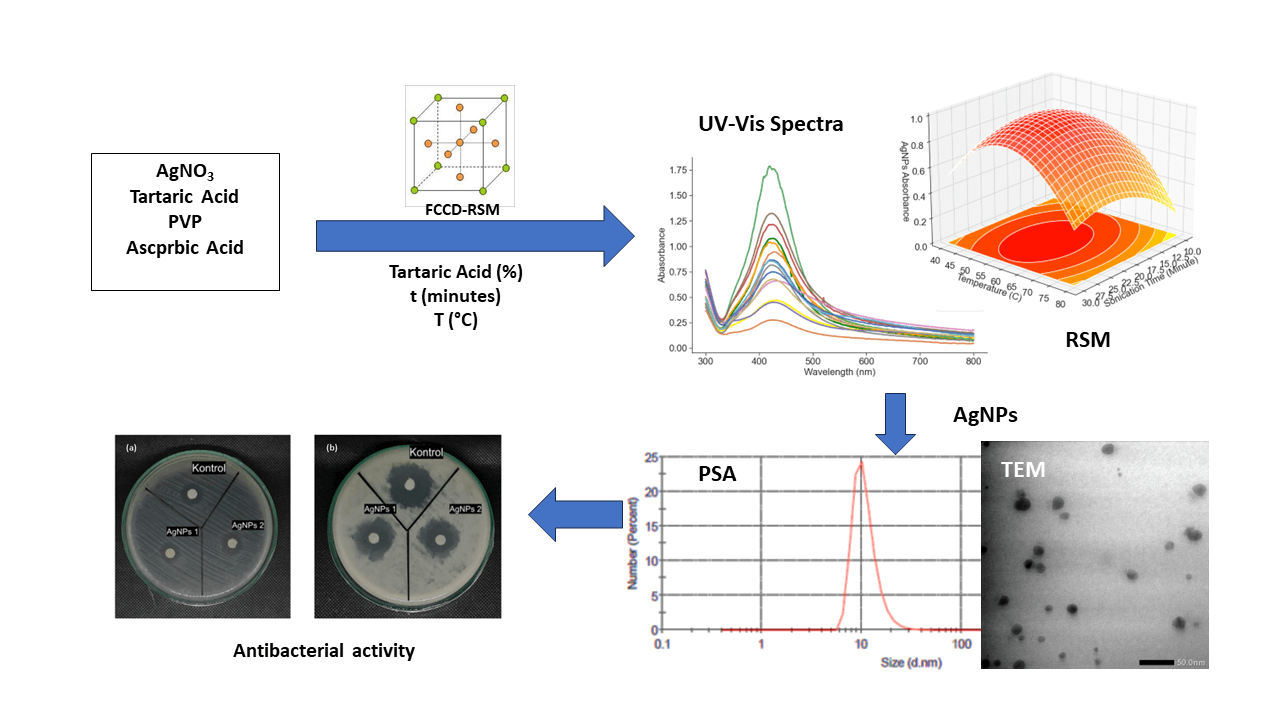
Silver nanoparticles (AgNPs) synthesized using tartaric acid as a capping agent have a great impact on the reaction kinetics and contribute significantly to the stability of AgNPs. The protective layer formed by tartaric acid is an important factor that protects the silver surface and reduces potential cytotoxicity problems. These attributes are critical for assessing the compatibility of AgNPs with biological systems and making them suitable for drug delivery applications. The aim of this research is to conduct a comprehensive study of the effect of tartaric acid concentration, sonication time and temperature on the formation of silver nanoparticles. Using Response Surface Methodology (RSM) with Face-Centered Central Composite Design (FCCD), the optimization process identifies the most favorable synthesis conditions. UV-Vis spectrum regression analysis shows that AgNPs stabilized with tartaric acid are more stable than AgNPs without tartaric acid. This highlights the increased stability that tartaric acid provides in AgNP ssssynthesis. Particle size distribution analysis showed a multimodal distribution for AgNPs with tartaric acid and showed the smallest size peak with an average size of 20.53 nm. The second peak with increasing intensity shows a dominant average size of 108.8 nm accompanied by one standard deviation of 4.225 nm and a zeta potential of −11.08 mV. In contrast, AgNPs synthesized with polyvinylpyrrolidone (PVP) showed a unimodal particle distribution with an average particle size of 81.62 nm and a zeta potential of −2.96 mV. The more negative zeta potential of AgNP-tartaric acid indicates its increased stability. Evaluation of antibacterial activity showed that AgNPs stabilized with tartaric acid showed better performance against E. coli and B. subtilis bacteria compared with AgNPs-PVP. In summary, this study highlights the potential of tartaric acid in AgNP synthesis and suggests an avenue for the development of stable AgNPs with versatile applications.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2024 The Author(s). Published by Tech Science Press.
Copyright © 2024 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools