 Open Access
Open Access
ARTICLE
The Potential of Wacapou (Vouacapoua americana) Extracts to Develop New Biobased Protective Solutions for Low-Durability Wood Species
1 CIRAD, Ecologie de Forêts de Guyane (EcoFoG), AgroparisTech, CNRS, INRAE, Université de Guyane, Kourou, 97379, France
2 CIRAD, UPR BioWooEB, Montpellier, F-34398, France
3 BioWooEB, Université de Montpellier, CIRAD, Montpellier, F-34398, France
4 CNRS, Ecologie de Forêts de Guyane (EcoFoG), AgroparisTech, CIRAD, INRAE, Université de Guyane, Kourou, Guyane Française, 97379, France
5 Sorbonne Université, CNRS, Laboratoire de Biodiversité et Biotechnologies Microbiennes, LBBM, Observatoire Océanologique, Banyuls-sur-Mer, 66650, France
* Corresponding Author: Kévin Candelier. Email:
Journal of Renewable Materials 2025, 13(1), 79-100. https://doi.org/10.32604/jrm.2024.056731
Received 29 July 2024; Accepted 27 September 2024; Issue published 20 January 2025
Abstract
The valorization of Amazonian wood residues into active chemical compounds could be an eco-friendly, cost-effective and valuable way to develop wood preservative formulations to enhance the decay and termite resistance of low-durable wood species. Wacapou (Vouacapoua americana., Fabaceae) is a well-known Guianese wood species commonly used in local wood construction due to its outstanding natural durability, which results from the presence of a large panel of extractives compounds. In addition, its industrial processing generates large amounts of residues. Wacapou residues were extracted by maceration using four different solvents (water/ethanol, ethyl acetate, hexane and dichloromethane/methanol), separately and successively. The yield of each extractive fraction was determined, and their chemical compositions were analyzed by Liquid Chromatography-Mass Spectrometry (LC-MS). Ethyl acetate led to the highest extraction yield, and the active compounds were identified in the obtained extractive fraction. In this sense, the fungicidal and termite-repellent properties of these extractives were then tested using a screening laboratory (with temperate and tropical microorganisms), according to the solution concentration (1%, 2.5%, 5%, 8% and 10%). Finally, Virola michelii Heckel wood samples (low durable species) were impregnated with the 8% concentration solution. The impregnated wood samples were then exposed to a soil bed test. The results highlighted that the nature of the solvent used during wood maceration affects the content of the obtained extractive fractions. Ultra-Performance Liquid Chromatography–High-Resolution Mass Spectrometry (UHPLC-HRMS) analyses showed the influence of extraction parameters on the nature of the extracted molecules. Wacapou extracts (from ethyl acetate maceration) showed good anti-fungal and anti-termite activities. Additionally, the concentration in extractives had an impact on the anti-termite activity level for Reticulitermes flavipes and Cryptotermes sp. Formulations based on Wacapou extractives showed a good potential for valorization in eco-friendly preservatives, aiming to confer better durability to local low-durability wood species.Graphic Abstract
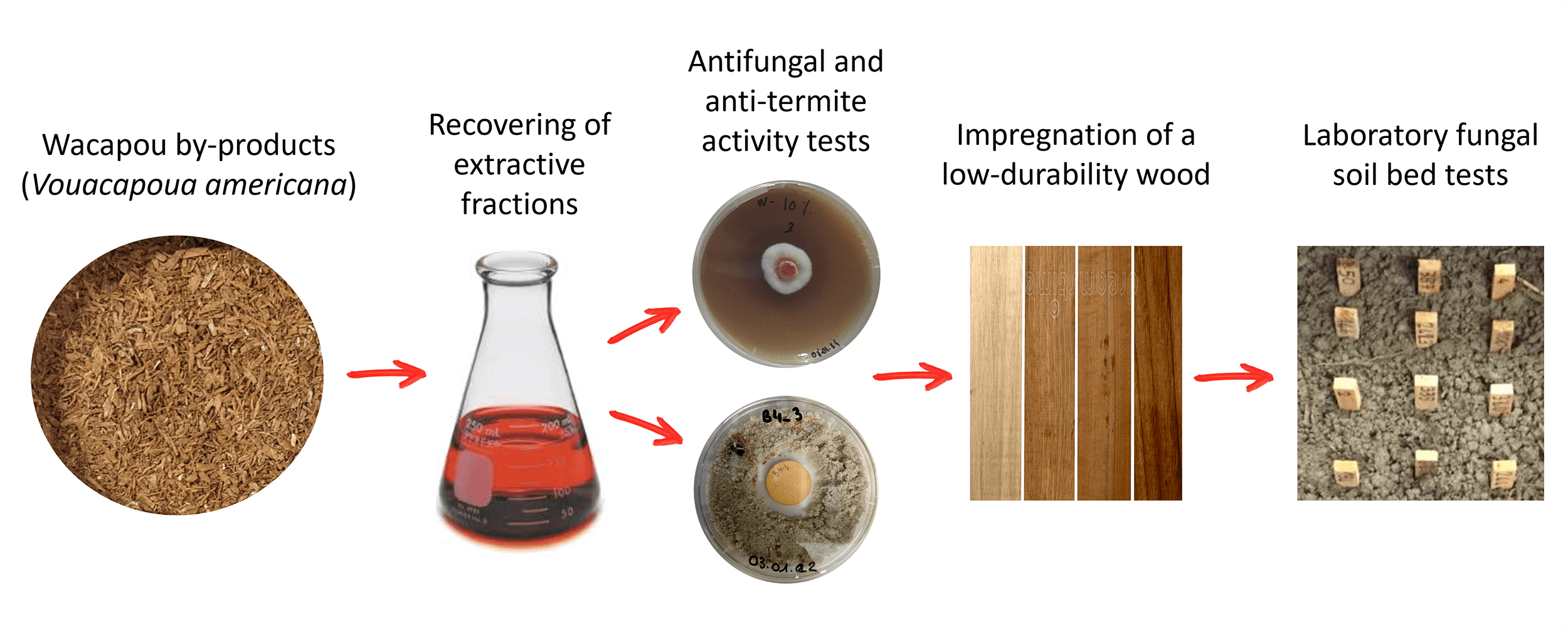
Keywords
Nomenclature
| AcET | Ethyl Acetate |
| CCA | Copper Chrome Arsenate |
| GT | Gloeophyllum trabeum |
| CTBFG | Technical Center for Wood and Forestry of French Guiana |
| DCM | Dichloromethane |
| EtOH | Ethanol |
| Ext (%) | Extractives yield in % |
| H2O | Distilled water |
| Inhibition (%) | Fungal growth inhibition in % |
| IR | Impregnation rate in % |
| m | Mass in g |
| MeOH | Methanol |
| PS | Pycnoporus sanguineus |
| RH | Relative Humidity |
| R × T × L | Orientation of the wood sample dimensions Radial × Tangential × Longitudinal |
| S | Area in cm2 |
| Sterm | Area degraded by termite attacks in cm2 |
| T°C | Temperature |
| TV | Trametes versicolor |
| UHPLC-HRMS | Ultra-Performance Liquid Chromatography–High-Resolution Mass Spectrometry |
| Vol | Volume |
| v/v | Volume percentage |
| WHC | Water Holding Capacity |
| wt | Weight percentage |
| WL | Weight loss in % |
Wood-destroying fungi (including cubic, fibrous, and soft rots) and termites cause extensive damage to wood and (ligno) cellulosic products in temperate and tropical climates. Numerous past preservatives have been forbidden or highly restricted, such as synthetic preservatives like lindane, dieldrin, pentachlorophenol, mineral copper chrome arsenate (CCA) (banned in many countries; in the UE and USA since 2003), or heavy-duty coal tar creosote [1]. More recent treatments based on water-borne copper systems associated with organic fungicides and/or quaternary amines have been developed and are used mainly in Northern Hemisphere countries [2]. These treatments remain affordable in terms of price (otherwise, they wouldn’t be viable on the market), especially when compared with new wood modification alternatives. In this context, various solutions involving non-biocidal treatments are also possible, potentially including thermal treatments [3] or wood chemical modifications [4,5]. Another possibility could be the exploitation of active extractives present in wood that are naturally durable [6–8].
Consequently, many studies have investigated alternative active ingredients derived from natural renewable materials. These studies focused on wood extractives [9–12] or essential oils [13,14], some of which have properties comparable to synthetic preservatives [11,15–18]. Some wood species are naturally resistant to termite attack due to their high content of extractive compounds, which are part of their natural defence systems [11,16]. Many studies have shown that very durable wood species can be valorised through extraction of the active components responsible for durability [19–22]. These can then be used in preservative formulations to confer higher durability to susceptible wood species through impregnation processes [23–27].
Wacapou (Vouacapoua americana, Fabaceae), a Guianese wood species, produces a highly durable wood, widely used in building and outdoor applications [28,29]. Its high natural durability is mainly due to the high content of its extractive compounds, synthetized during the heartwood formation process, which may reach a yield of (wt.) around 17.5% [30].
The chemistry of extractives from Wacapou includes mainly diterpenoid compounds such as methyl vouacapenate, vouacapenic acid and vouacapenol [31,32]. Meurer-Grimes et al. [33] showed that the chemical profile of Wacapou extractives could be summarized by the presence of two dominant non-polar compounds corresponding to methyl vouacapane diterpenoïds and one phenylpropanoid. Additionally, Kido et al. [34] isolated furanic diterpenoids of the cassane family, such as methyl vouacapenate, from the wood of Wacapou. More recently, Çiçek et al. [35] highlighted 15 compounds constituting the major part of essential oil recovered by the hydro-distillation process of Wacapou bark, including (+)-vouacapenic acid, and (+)-methyl vouacapenate, which showed high antimicrobial and cytotoxicity activities, respectively. It has been demonstrated that these compounds possess antibacterial and antioxidant activities [36,37].
Moreover, Wacapou is fairly well-harvested and processed in French Guiana, with an annual production volume of around 1430 m3/year (average yield between 2010 and 2020, data from the observatory of the Observatory of the Guianese Timber Industry (CTBFG). Although Wacapou has been on the IUCN Red List since 1998 as a Critically Endangered species under criteria A1cd+2cd [38], it does not represent an endangered species in French Guiana [39]. It should be noted that this annual production volume currently represents only 20% of the exploitable volume. The Guianese National Forest Office has estimated, based on inventories, a potential future resource of 10,415 m3 of exploitable Wacapou in the exploited forest area. Finally, because Wacapou has a heterogeneous trunk aspect, its transformation into building materials generates between 20% and 55% of residues from harvesting and sawing operations [38]. These data highlight that a higher Wacapou production volume can be envisaged in the coming years, with the objective of diversifying tree species in the Guianese wood sector, generating a large volume of co-products.
Polyphenols and diterpenoids are prevalent secondary specialized metabolites in Vouacapoua americana (wood and bark fractions) that have been extensively characterized for their noteworthy anti-inflammatory, antioxidant, anticarcinogenic, antiviral and antiseptic properties [40,41]. However, very few studies have investigated either the termicidal or fungicidal properties of Wacapou extractives, despite evidence that they could have a potentially wide range of applications [21,42]. Previous studies have shown that among other Guianese wood species, methanolic extractives from Wacapou heartwood were effective in improving the durability of Scot pine sapwood (Pinus sylvestris Thunb. Pinaceae) and Beech (Fagus sylvatica L., Fagaceae) through impregnation processes, against Trametes versicolor and Gloeophyllum trabeum, respectively. In addition, methanolic extractives showed slightly better activity than those from ethyl acetate extraction.
Nevertheless, few studies have investigated the activity of Wacapou extractives against local tropical wood-destroying fungal strains and Guianese termite species, in order to use them as a basis for new wood-protectant formulations for non-durable local wood species that could be fullfill tropical climate requirements.
The present work aims to evaluate the properties of Wacapou’s extractive fractions as potential antifungal and anti-termite agents, identifying the most interesting molecules to develop more acceptable wood protection systems based on the use of natural products sourced from Guyanese renewable resources. For this purpose, extractives were recovered from Wacapou heartwood residues through separate or successive maceration processes using different solvents with various polarities. Each fraction was analysed using Ultra-Performance Liquid Chromatography–High-Resolution Mass Spectrometry (UHPLC-HRMS), and the most promising was tested at various concentrations in bioassays to evaluate its anti-termite and antifungal properties. Finally, Wacapou extractives at a concentration of 8% (v/v) were applied through an impregnation process to low-durability wood species (Virola surinamensis (Rol. ex Rottb.) Warb, Myristicaceae) [28,29] in order to evaluate the conferred durability through unsterile soil bed tests.
A mixture of wood residues from Wacapou (Vouacapoua americana, Fabaceae) was collected from Kourou’s sawmill (Scierie Dégrad Saramaca, Kourou, French Guiana) in December 2022. The initial moisture content of the recovered wood residues, with granulometry ranging between 2 to 4 mm, was approximately 56%. The sawdust mixture was spread out and air-dried over a month in a ventilated attic until mass stabilization (wood residues moisture content of 14 ± 1%), and stored in an air-conditioned room (20 ± 2°C and 65 ± 5% RH).
Wacapou is a wood with differentiated heartwood, where the color is distinctly darker than that of the sapwood. Although special care was taken during the harvesting of the wood at the sawmill, the chips were carefully reselected based on color to recover only the heartwood part of the wood, for the study.
Heartwood chips were then ground to reach a granulometry of 0.5 mm and kept in the same air-conditioned room for 2 months until it was submitted to various extraction processes.
The maceration process was selected for wood extraction. This technique is simple, involving immersing the wood sawdust for a prolonged period in a solvent to extract the soluble components [43]. The greatest advantages of this method are its simplicity, a key prerequisite to enable industrial application, particularly in tropical areas, and its ability to produce satisfactory yields [16] without risking the degradation of thermolabile compounds. However, it requires large volumes of solvent and does not allow the bioactive compounds to be totally removed from the ligno-cellulosic material.
Wacapou powder was macerated for 48 h under magnetic agitation in water/ethanol, ethyl acetate, hexane and dichloromethane/methanol solvents (with a purity of 99.9% for each solvent), in separate or successive steps (Table 1).

For all maceration processes, the biomass/solvent ratio was 10 g (m0)/100 mL. After filtering the macerate, the solvent containing the extractives was removed using a vacuum rotary evaporator (40°C, 200 mbar). Then recovered extractives were then oven-dried at 103 ± 2°C, before being weighed (m1).
Extractive yields were determined by Eq. (1), according to the solvent used for maceration:
where, m0 and m1 are the anhydrous mass (oven-dried at 103 ± 2°C) of the biomass before and after each solvent maceration, respectively.
2.3 Extractives Chemical Composition by UHPLC-HRMS Analyses
The chemical compositions of the extractive fractions obtained from water/ethanol, ethyl acetate, hexane and dichloromethane/methanol separate or successive maceration processes were analyzed by UHPLC-HRMS. The analyses were conducted using a Thermo UHPLC-HRMS system. Crude extracts were dissolved in acetonitrile/methanol/isopropanol 2:2:1 via sonication to achieve a final concentration of 1 mg/mL, and 2 µL were injected into the column (Phenomenex Luna Omega polar C-18 150 mm × 2.1 mm, 1.6 μm). The analyses were performed as described in Stien et al. [44] with modifications.
The solvent system was a mixture of water (Solution A) with increasing proportions of acetonitrile (Solution B), both modified with 0.1% formic acid. The gradient was as follows: 5% B 3 min before injection, then from 1 to 13 min, a linear gradient increase of B up to 100% followed by 100% B for 7 min. The column temperature was set to 42°C, and the flow rate was 0.5 mL·min−1.
Mass spectrometry analyses were performed in electrospray positive ionization mode in the range of 133.4–2000 Da in centroid mode. The mass detector used was an Orbitrap MS/MS FT Q-Exactive focus mass spectrometer. The analyses were performed in FullMS data-dependent MS2 mode. In FullMS, the resolution was set to 70,000, and the AGC target was 3 × 106. In MS2, the resolution was 17,500, AGC target 105, isolation window 0.4 Da, and stepped normalized collision energy 15/30/45 was used, with 15 s dynamic exclusion. The lock mass option was set for the ion at m/z 144.98215, corresponding to Cu (CH3CN)2+.
2.4.1 Antifungal Activity Tests
Antifungal activities against the growth of Pycnoporus sanguineus (PS) [Pycnoporus sanguineus (L.) Murrill, 1904] were tested as per Boer et al. [45] with some variations.
The Pycnoporus sanguineus strain was chosen due to its prevalence and virulence in French Guiana and its frequent use in the scientific literature on wood decay resistance [46]. Each Petri dish (9 cm diameter) was filled with 10 mL of malt-agar medium containing 1200 μL of the diluted extracts in acetone [Merck company, Darmstadt, Germany] (C = 1, 2.5, 5, 8 and 10% m/m) and left to solidify. These dilutions were chosen based on previous studies [22,45–47] and aligned with concentrations used for anti-termite activity tests. A 1-cm diameter portion of a seven-day-old culture of Pycnoporus sanguineus was placed in the center of the Petri dish and incubated in a climatic chamber (27 ± 2°C, >75% RH) for seven days’ incubation. Three replicates were carried out per diluted wood extract sample. A culture medium free of extractives and solvent was used (in triplicate) as a control (Sc), and an additional control (in triplicate) was set up using a culture medium with added acetone to verify solvent effects on fungal growth. Following the fungal exposure period, the mycelium growth surface was measured in mm2, for the control medium (Sc) and the extract-supplemented medium (St). To be noted that the initial 1-cm diameter of a seven-day-old culture of Pycnoporus sanguineus was not counted within the mycelium growth surface (Fig. 1). The mycelium growth surface was measured through the ImageJ 1.53 k software, following the same protocol used for the anti-termite activity tests, which is detailed below (in Section 2.4.2).

Figure 1: Steps of image processing by ImageJ software, from original picture (A) to color threshold and segmented image (B) and binary image (C), using for the determination of the Pycnoporus sanguineus mycelium growing area
The percentage of mycelium growth inhibition was determined using Eq. (2):
where, Sc is the mycelium growth surface on the control medium, and St is the mycelium growth surface on the tested extract-supplemented medium.
2.4.2 Anti-Termite Activity Tests
Termite non-choice tests were previously carried out on the extractive fractions from the different solvent maceration processes, with a concentration of 8% (diluted in pure acetone solution), in order to select the extractive fraction with the highest anti-termite activity. Then, the same tests were carried out using only the extractive fractions from ethyl acetate maceration, with different solution concentrations (diluted in pure ethyl acetate solution). Although the anti-termite activity of the extractive fractions from ethyl acetate maceration is higher than those from water/ethanol, it was not significantly different from the fractions from hexane and dichloromethane/methanol. However, ethyl acetate was selected for the next step of the study because the latter solvents do not allow for reliable industrial and ecological applications [48]. The non-choice tests were conducted against Reticulitermes flavipes (Kollar, 1837, ex. santonensis) as subterranean termites, and Cryptotermes sp. as dry-wood termites frequently found in French Guiana. These two termite species were tested because of their different biological and ecological features. The anti-termite activity tests were carried out using a Joseph filter paper (grammage of 25 g.m−2) made of pure cellulose (diameter of 42.5 mm). The tests using Reticulitermes flavipes were carried out in the CIRAD’s Wood Preservation Laboratory in Montpellier (France), whereas those using Cryptotermes sp. were performed in the wood science technical platform of the EcoFoG joint research unit (French Guiana). Solid wood extractive powders were diluted in ethyl acetate, reaching the following concentration (in m/m): 1%, 2%, 5%, 5%, 8% and 10%. The tested papers were impregnated with 80 µL of each extractive solution (Fig. 2). One set of controls was also prepared by impregnating the filter paper with 80 µL of ethyl acetate. A second set of controls consisted of tested cellulosic paper without any impregnation. Three replicates were performed for each treatment based on extractives, and six replicates were used for each control modality. Before termite exposure, all of the treated filter papers were air-dried at 20 ± 2°C and 65 ± 5% RH for at least 12 h.

Figure 2: Impregnation of cellulosic papers prior to termite attacks
A 9-cm diameter Petri dish was filled with 25 g of wet Fontainebleau sand (4 vol. of sand/1 vol. of deionized water) for Reticulitermes flavipes and 25 g of air-dried sand and 1 g of agglomerated faeces for Cryptotermes sp. The tested papers were placed in the middle of the device, on the plastic mesh to avoid direct contact with sand. The test devices are illustrated in Fig. 3. Then, a total of 20 termite workers were introduced in each Petri dish and put in a dark climatic chamber at 27 ± 2°C, >75% RH. Six diet control set-ups containing only 25 of wet (for Reticulitermes flavipes) or dried (for Cryptotermes sp.) sand and 20 termites were used to check termite survival without any trophic sources, in order to determine the end of the test. Every two days, each test set-up was observed to check sand humidity, add water if needed (for Reticulitermes flavipes) and keep track of termite behaviour and activity. When all the termites contained in the diet control set-ups had died, the test was stopped (17 days). The termite survival rate (TSR, in %) was then determined, and the paper was air-dried at 20 ± 2°C and 65 ± 5% RH for 24 h. Afterwards, the final area of the cellulose papers was measured (cm2) by image analysis tool and the degraded area (Sterm%) due to termite attack was calculated by the following Eq. (3):

Figure 3: Termites test devices for the non-choice test using one Joseph filter paper (diameter of Petri-dish = 9 cm) against Reticulitermes flavipes (A) and Cryptotermes sp. (B)
where, S1 is the initial surface of the filter paper (14.18 cm2), and S2 is the final surface of the filter paper after the termite exposure test.
The quick method of measuring the remaining paper area consumed by termites is described in Fig. 4.

Figure 4: Steps of image processing, from original picture (A) to colour threshold and segmented image (B) and binary image (C), using for the determination of the paper area consumed by termites
The total remaining paper area consumed by termites was measured through the ImageJ 1.53 k software. The automated segmentation protocol and paper area measurement are illustrated in Fig. 4. The area of all the filter paper (S2) exposed to termites was then compared to that of the original filter paper (S1), which was determined using the same analysis procedure, to calculate the paper area consumed by termites (Sterm%).
2.5 Bio-Impregnation Process to Valorize and Protect Low-Durable Wood Species
Conditioned Virola (Virola surinamensis) wood samples (20 ± 2°C and 65 ± 5% RH) were cut into dimensions of 10 × 5 × 100 mm3 (R × T × L). Before impregnation, the samples were oven-dried at 103°C until they reached their constant mass (mi). Anhydrous Virola wood samples (48 replicates) were impregnated with ethyl acetate extractive fraction of Wacapou at a concentration of 8% (v/v) (diluted in a solution of ethanol (70%), distilled water (15%) in ethyl acetate (15%), in vol.) using a single vacuum pressure impregnation. The wood samples, along with the extractive solution, were placed in a desiccator. The samples were kept immersed during the treatment (0.01 MPa vacuum for 48 h, followed by 2 h at atmospheric pressure). Additionally, 48 Virola samples were impregnated with the commercial preservative “Universal treatment-AXTON” (and consumer product) (Cypermethrin (0.17%), Propiconazole (0.13%), Tebuconazole (0.14%), in oil-in-water emulsion), using the same impregnation process to serve as a positive control for soil bed tests.
The samples were then removed and blotted with tissue paper to remove the excess product from the wood surface, before being oven-dried at 103 ± 2°C and weighed after mass stabilization (mw). The anhydrous impregnation rates (IR in %) of Wacapou extractives and AXTON preservatives were determined using Eq. (4):
where, mi is the initial anhydrous mass of the wood sample prior to the impregnation process, and mw is the anhydrous mass of the impregnated wood sample.
The durability of impregnated Virola (Virola surinamensis) wood samples against soil rot micro-fungi was determined following the protocol of Beauchêne et al. [49], according to the guidelines of XP ENV 807 [50], with adjustments to the number of the tested samples and incubation room conditions. 48 specimens, from both native and bio-impregnated Virola wood samples, were dried at 103 ± 2°C until their mass stabilization and weight (mw), before being placed into containers with unsterile forest soil from Kourou. pH–H2O [1:2], determined according to NF ISO 10390 [51], was 6.6. The water holding capacity (WHC) of the soil used for these tests, determined according to XP CEN/TS 15083-2 [52], was 95%. Some water was added to the soil throughout the test, if necessary, to maintain a WHC value of 95% at all times. The incubation times were 7, 14, 28, 56, 91 and 120 days at ambient conditions in Kourou (French Guiana). Each week, 9 replicates of each modality were picked and analyzed. In addition, 250 ml of water were added to each container each month to maintain the soil humidity.
After each unsterile soil incubation period, 9 specimens from each wood material were picked up, cleaned of soil with a brush, washed in a dishwashing machine (1 h–40°C), then dried at 103 ± 2°C and weighed (mf) in order to determine their weight losses due to soil degradation agents (WLsbt%), according to Eq. (5):
where, mw is the initial anhydrous mass of native and bio-impregnated Virola wood samples prior soil bed tests, and mf initial anhydrous mass of native and bio-impregnated Virola wood samples after soil bed test exposure.
Nine untreated or impregnated with AXTON commercial solution Virola wood samples were placed in the soil bed for each tested time exposure (7, 14, 28, 56, 91 and 120 days) (Fig. 5) in order to assess the virulence of degrading microorganisms present in the soil, and to have a positive reference with a commercialized preservative, respectively. Untreated Virola wood samples were inserted in each container to compare their activities. No significant differences could be observed based on statistical analysis.

Figure 5: Laboratory test device for the unsterile soil exposures of native and impregnated Virola woods, according to the test duration
3.1 Influence of the Solvent Used for Maceration in Wacapou Extractive Yields
All the solvents used for extraction maceration exhibited pronounced differences in terms of extractive yields for both separate and successive maceration processes.
The results presented in Table 2 show that the highest extraction yields from Wacapou-separated macerations were recorded using dichloromethane/methanol (7.00%), followed by ethyl acetate (5.90%). Regarding the successive macerations process, the highest extractive fraction was obtained with ethyl acetate (4.44%), after water/ethanol maceration.

According to Verpoorte et al. [53], dichloromethane/methanol is well-known to be the most effective solvent in metabolomic extractions. In this sense, dichloromethane/methanol was used in this study as a control solvent allowing to reach the maximal extractives rate from the Wacapou maceration process. Our results confirm this statement, as in separate extractions, dichloromethane/methanol provides the highest extractive yields. For this reason, and also due to its toxic and pollutant properties, dichloromethane/methanol was not used for successive macerations, as a yield of 6.78% of extractives was already achieved using water/ethanol followed by Ethyl acetate and hexane.
Ethyl acetate is recognized as a relevant solvent candidate for wood extraction, allowing for the high yield of extractives, primarily composed of flavonoid and phenolic compounds [54,55]. Moreover, Murugan et al. [56] highlighted that among different solvent extracts, maceration with ethyl acetate shows higher antioxidant activity, mainly due to its composition in alkaloids, phenolics, flavonoids, and terpenoids [57]. The most active extracts from olive wood (Olea europaea L., cultivar Picual) were obtained with ethyl acetate either through direct extraction or successive liquid-liquid partitioning procedures [58]. Based on a study conducted on the chemical composition of the specialized metabolite compounds isolated from the ethyl acetate extract of bark from Lannea coromandelica (Houtt.) Merr., Sudding et al. [59] highlighted that this fraction was mainly composed of steroid group compounds (i.e., lipids derived from triterpenoids). Kameri et al. [60] showed that ethyl acetate extract from plants has a high antifungal activity compared with other commercial products. These results are comparable to the results previously reported by Rodrigues [42] for heartwood specimens of Wacapou extracted in successive macerations of 48h in ethyl acetate and methanol. However, despite the identical solvent/matter ratio, time of maceration and method of evaporation, the author measured a yield of 8.67% in ethyl acetate, which is superior to the yield obtained in our study (5.90%). It is worth noting that the value indicated in Rodrigues [42] corresponds to external heartwood samples when our study was conducted on industrial residuals with no control over the exact type of tissues used for extraction. In addition, the variations in extraction yields can be attributed to the biological variability of the specimens of the same species, which has not been characterized so far.
3.2 Influence of the Solvent Used for Maceration in Wacapou Extractive Yields
Fig. 6 shows the chemical composition of extractive fractions, according to the peak area recording and annotation by UHPLC-HRMS analyses, from separate maceration processes using water/ethanol, ethyl acetate, hexane and dichloromethane/methanol. It reveals that for ethyl acetate, hexane and dichloromethane/methanol macerations, the three main components were identified as methyl vouacapenate, vouacapenic acid and vouacapenol (Fig. 7). The identification of these three major compounds from Wacapou extractive fractions aligns with previous studies [31–34].
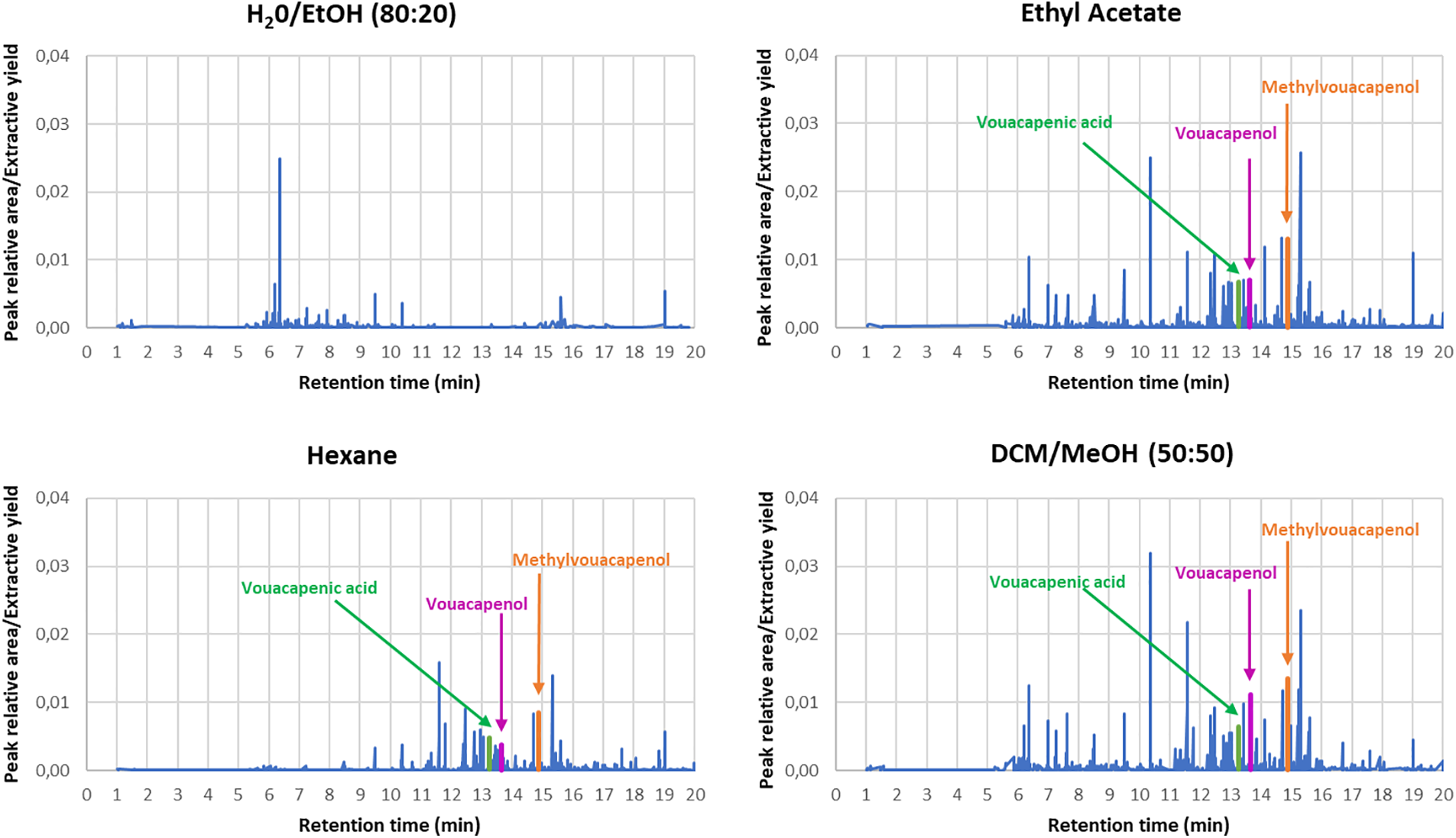
Figure 6: Chemical composition of extractive fractions from separate maceration processes using water/ethanol, ethyl acetate, hexane and dichloromethane/methanol, annotated by UHPLC-HRMS analyses
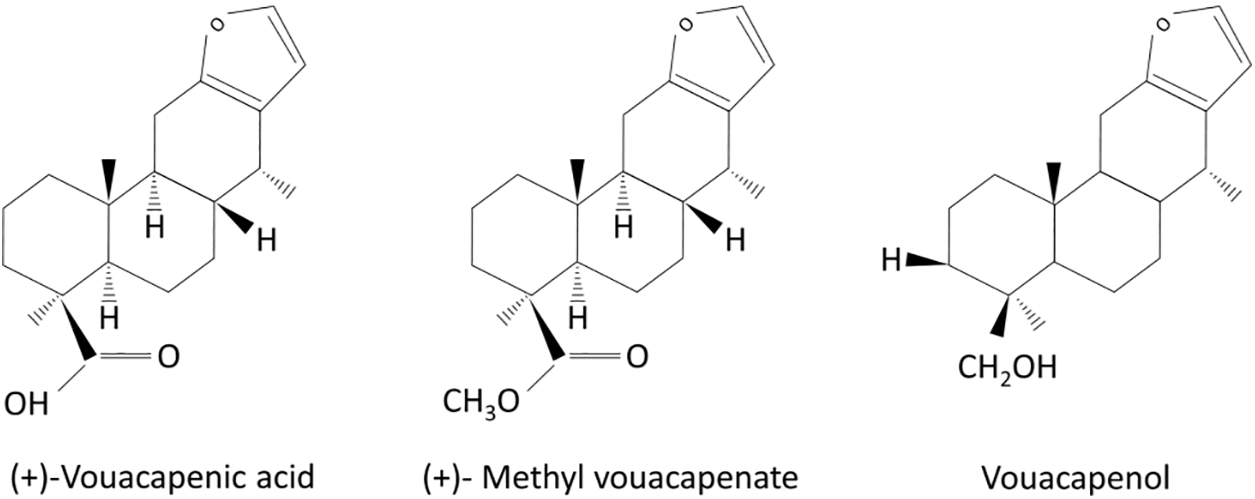
Figure 7: Chemical structures of isolated compounds (+)-vouacapenic acid, (+)-methyl vouacapenate, and vouacapenol (adapted from Çiçek et al. [35] and Godoy et al. [64]). Copyright © 1989 Published by Elsevier Ltd
These cassane-type diterpenoids possess a wide range of biological activities, such as antimicrobial and antioxidant properties, as well as pharmacological activities like anti-inflammatory, antimalarial, antiplasmodial and antiviral properties, mainly due to numerous oxygenated groups present in these terpene compounds [61]. Dickson et al. [36] also highlighted the antibacterial and antioxidant properties of the molecules from the methyl vouacapenate family, while Çiçek et al. [35] demonstrated the pronounced effects of vouacapenic acid in antimicrobial and cytotoxicity assays. Despite numerous studies focusing on the antifungal activities of cassane-type diterpenoids, most have been conducted for medical purposes [62,63]. To our knowledge, no studies have specifically adressed the activity of these compounds, particularly methyl vouacapenate, vouacapenic acid, and vouacapenol, on wood-destroying fungi and insects.
3.3 Antitermites Activities of Wacapou Extractives Coming from Maceration Processes Using the Four Different Solvents, against Reticulitermes flavipes
As shown in Fig. 8, control paper samples, both untreated and treated with acetone solution, showed significant paper area loss (52.74 ± 9.89% and 72.80 ± 8.90%, respectively) with a high termite survival rate (88.3 ± 2.9% and 95.0 ± 5.0%, respectively).
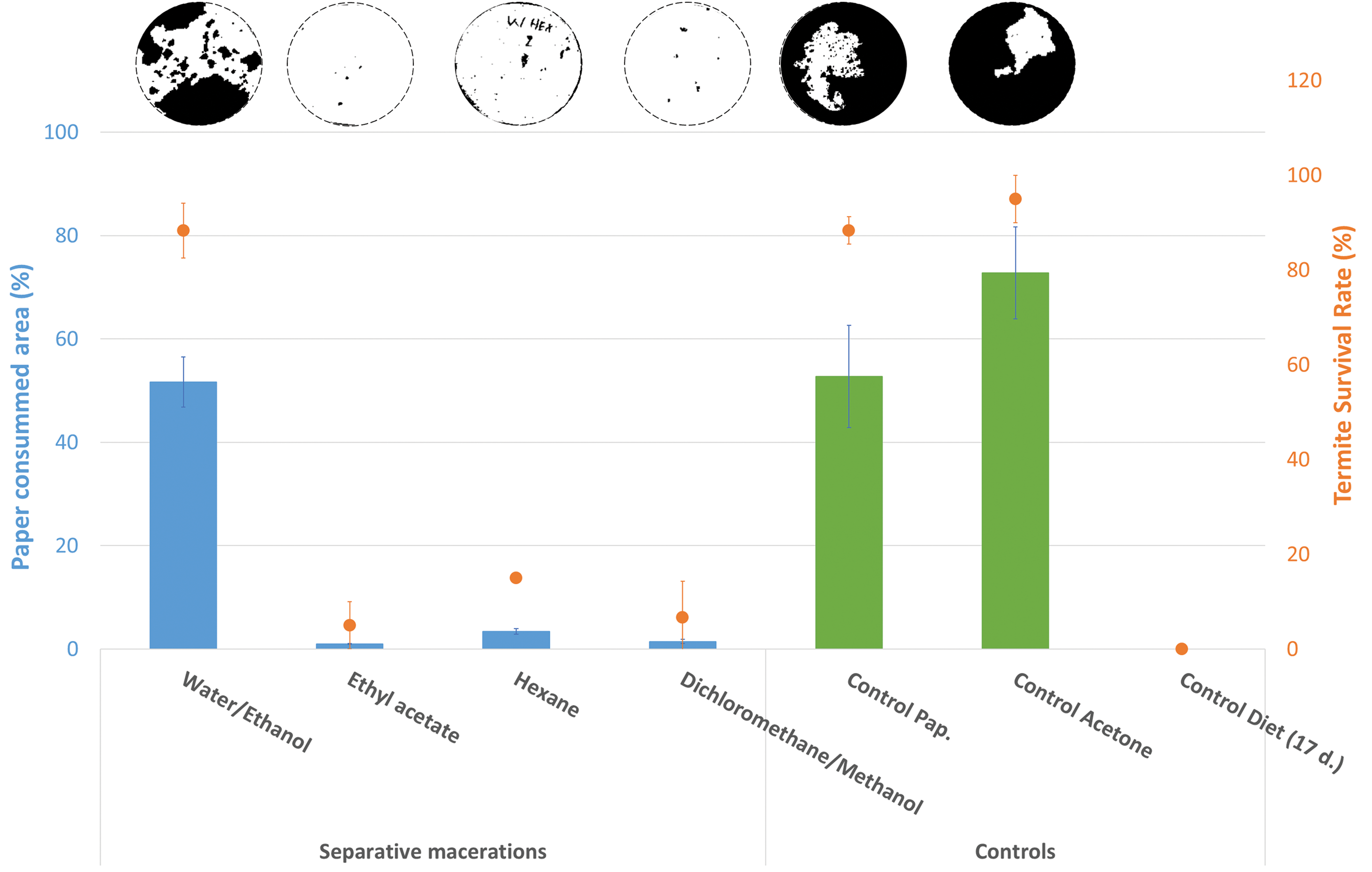
Figure 8: Average values of the percentage of paper loss area (barplot) and termite survival rates (point), according to the solvent used during the Wacapou wood maceration processes (tested concentration of each extractive fraction was 8%, diluted in an acetone solution), against Reticulitermes flavipes. The visual aspects of the most representative papers, after exposure to termites and processed by image analysis, are shown at the top of the figure for each treatment modality. White represents the remaining part of the paper and black represents the part degraded by termites
The results clearly indicate that the paper loss area and the termite survival rate depend greatly on the nature of the solvent used during the maceration process. It appears that the least effective Wacapou extractive fraction is obtained from water/ethanol maceration, with a paper area loss of 51.68 ± 4.84% and a termite survival rate of 88.3 ± 5.8%. For the other solvent, the Wacapou extractive fractions show similar anti-termite activities. However, extractives from ethyl acetate maceration presented the best anti-termite activity against Reticulitermes flavipes, with a paper area loss of 1.00 ± 0.07% and a very low termite survival rate. These results are consistent with the chemical compositions of the Wacapou extractive fraction, notably due to the high proportion of (+)-vouacapenic acid, (+)-methyl vouacapenate, and vouacapenol in the ethyl acetate, hexane and dichloromethane/methanol extractive fraction, whereas water:ethanol extracts did not contain these active compounds (Fig. 6).
Given the high extraction yields from Wacapou maceration using ethyl acetate, its efficiency against Reticulitermes flavipes, and the eco-friendly nature of the solvent, ethyl acetate extractives of Wacapou were selected for further study in wood preservative applications.
3.4 Biological Activities of Wacapou Extractives Coming from Ethyl Acetate Maceration Process
3.4.1 Anti-Termite Activities against Reticulitermes flavipes and Cryptotermes sp.
According to Fig. 9, Cryptotermes sp. appears to be slightly less aggressive in terms of paper surface loss than Reticulitermes flavipes, but more resistant to Wacapou extracts. These termite behaviors could be attributed to their differing biology and lifestyle differing biology and lifestyle (dry-wood vs. subterranean termites).
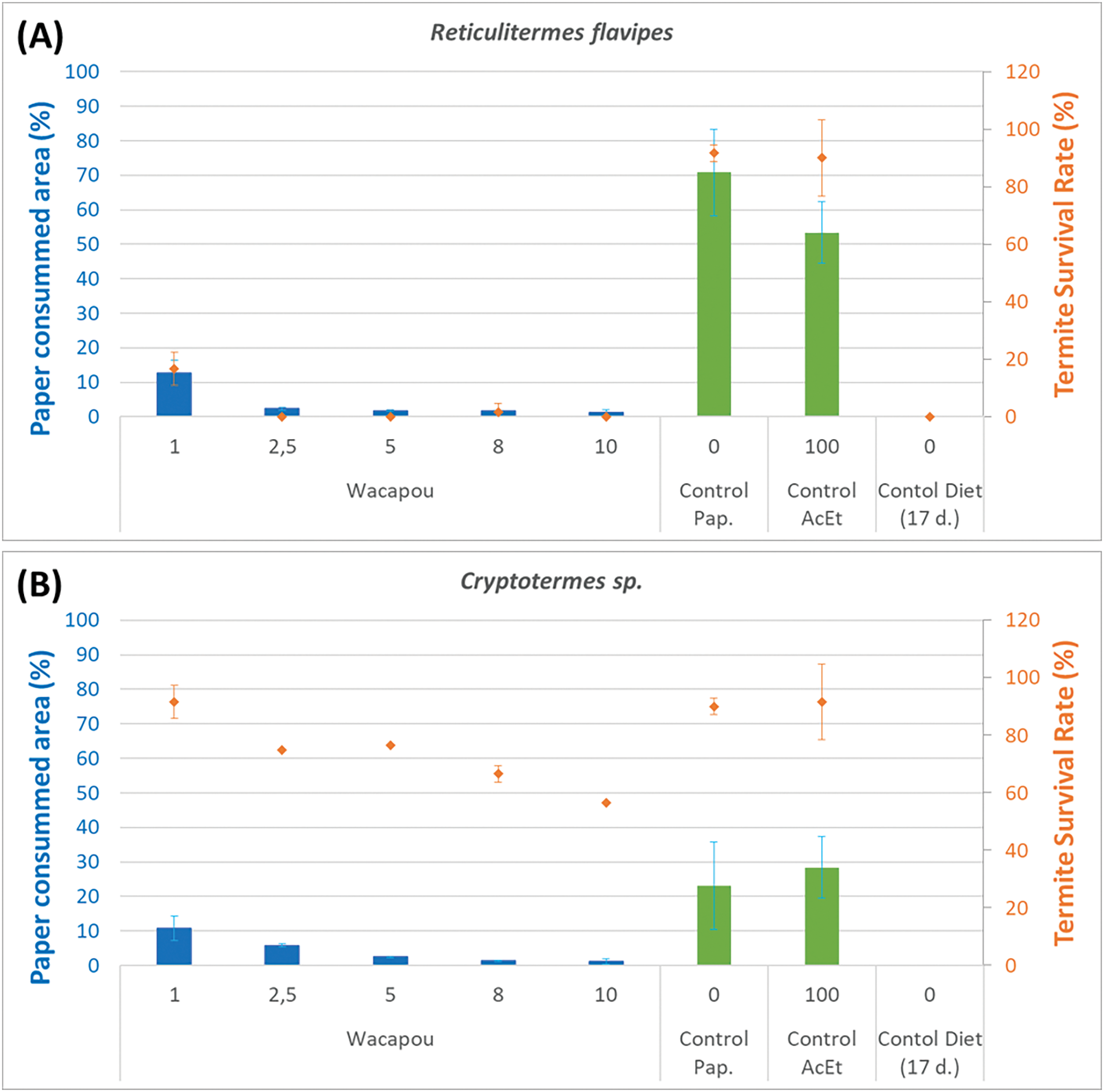
Figure 9: Average values of the percentage of paper loss area (barplot) and termite survival rates (point), according to the concentration of the Wacapou extractives (from ethyl acetate maceration) solution, against (A) Reticulitermes flavipes and (B) Cryptotermes sp.
Indeed, for all test modalities (impregnated or not impregnated paper samples, and not included control papers impregnated with acetone), the recorded average value of paper area loss was always slightly higher (but not in a significant way) with Reticulitermes flavipes than those of Cryptotermes sp., whereas an opposite significant trend was observed concerning the termite survival rates. Moreover, Fig. 9 shows clearly that the paper loss area and the termite survival rate depend greatly on the concentration level of Wacapou extractives solution impregnated on the cellulosic paper discs. For all tested modalities, and for both termite species, the higher the extract concentration, the higher the termite’s survival rate and the lower the degraded surface paper area (Fig. 10). The lowest paper loss area was found with the Wacapou wood extracts at a 10% concentration level for Reticulitermes flavipes (1.26 ± 0.80%) as well as Cryptotermes sp. (1.09 ± 0.58%). However, starting at a concentration of 2.5% in Wacapou extracts, all the Reticulitermes flavipes were dead at the end of the test, while many Cryptotermes sp. were still alive, whatever the extractive concentration level (56.57 ± 14.43% for the concentration of 10%). A previous study conducted on the determination of the termite resistance of tropical wood (Paraserianthes falcataria(L.) I. C. Nielsen, Cryptomeria japonica (Thunb. ex. L.) D. Don, and Alstonia sp.) also highlighted that the wood mass loss due to termite attack was lower with dry-wood termites (Cryptotermes cynocephalus Light, 1921), than subterranean termites (Coptotermes curvignathus Holmgren) [65].
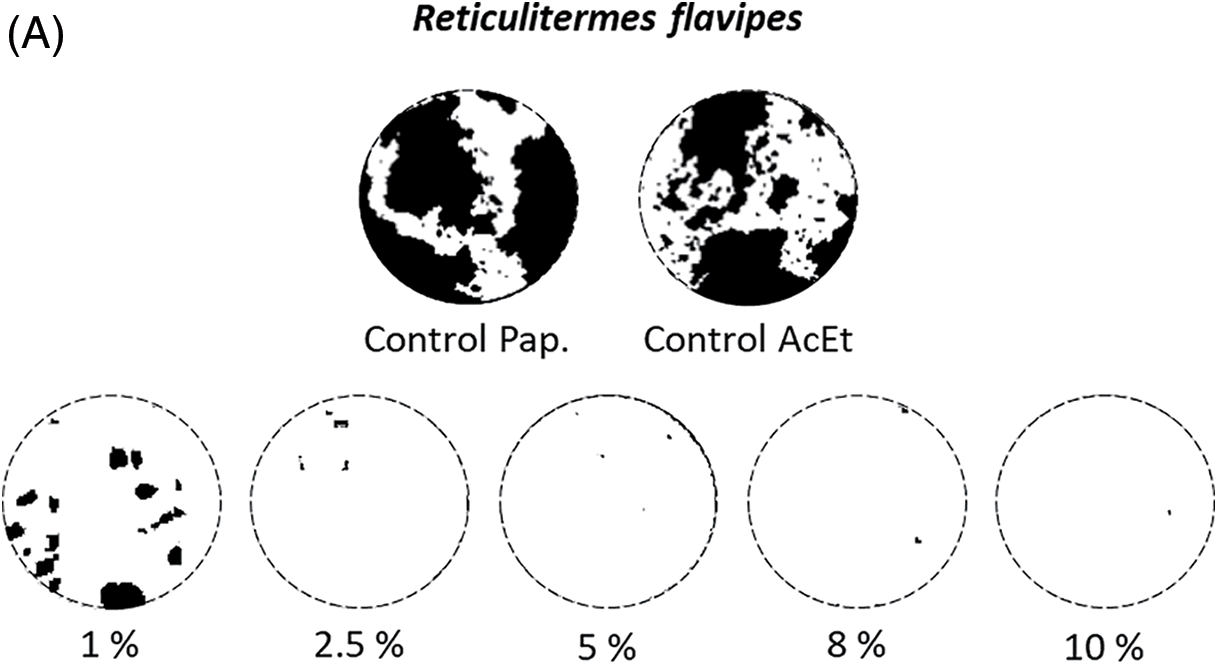

Figure 10: Visual aspects of the most representative papers, after exposure to termites and processed by image analysis, according to the concentration of the Wacapou extractives (from ethyl acetate maceration) solution, against (A) Reticulitermes flavipes and (B) Cryptotermes sp. White color represents the remaining part of the paper and black color represents the part degraded by termites
3.4.2 Antifungal Activity against Pycnoporus sanguineus
Whatever the concentration, Wacapou extracts present an important delaying effect on the growth of Pycnoporus sanguineus fungus (Figs. 11 and 12). According to the results in Fig. 11, representing the fungal inhibition efficiency of Wacapou extractives according to their concentration level incorporated within the culture medium, it appears that the effect of ethyl acetate Wacapou extractives seems more fungistatic than fungicidal against white rot, such as Pycnoporus sanguineus. This fungistatic behaviour could be explained by the capacity of white rot to limit the toxicity and therefore the efficacy of the extracts to inhibit the growth of the mycelium. Indeed, the mechanism involved in wood polymer degradation by white rot is based on the secretion of oxidative enzymes such as laccases and peroxidases, which may be able to degrade or interfere with phenolic compounds by oxidation and then limit their antifungal activities [66].
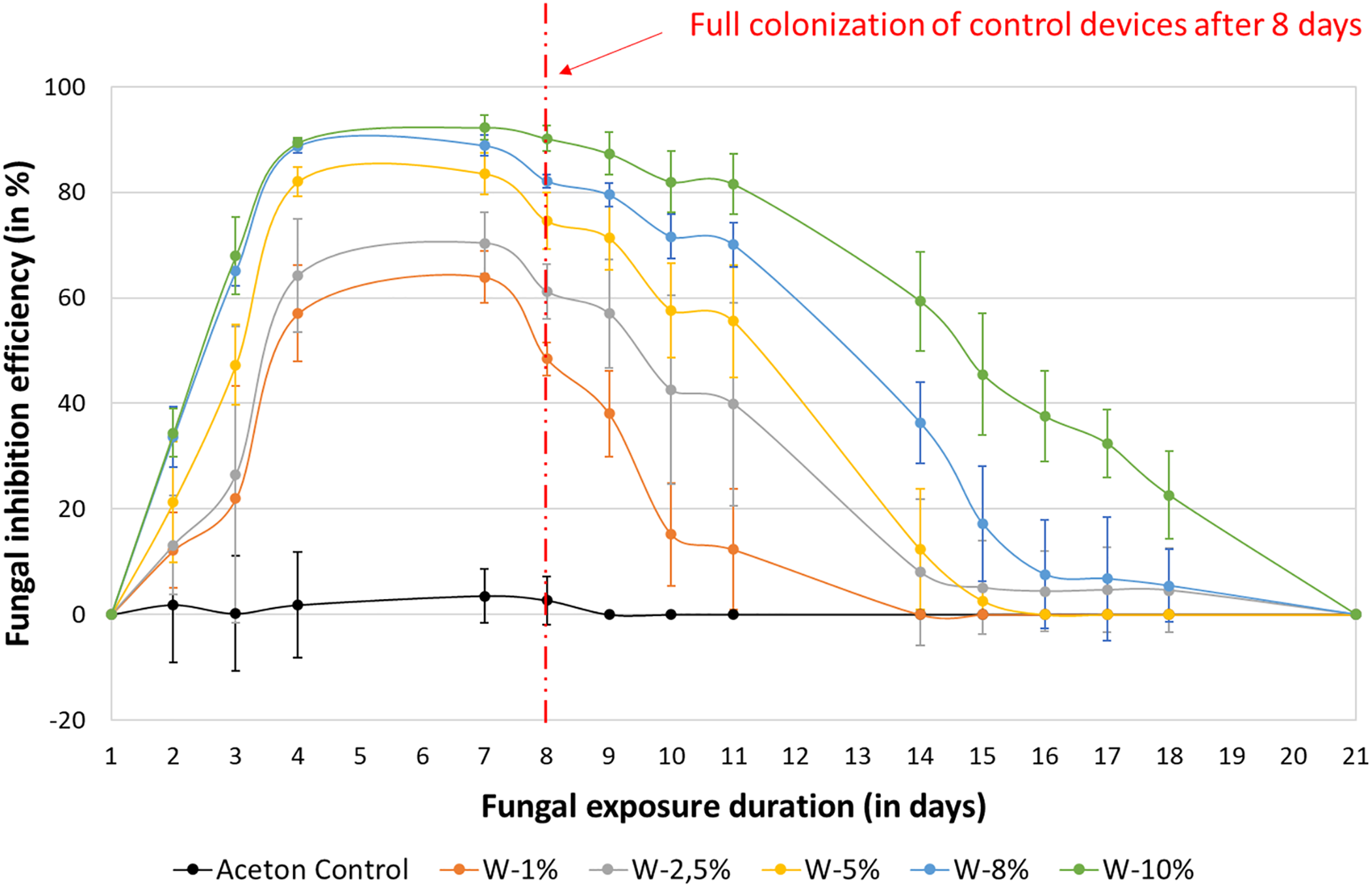
Figure 11: Fungal inhibition (in %) of Wacapou extracts from ethyl acetate maceration (1200 μL) against Pycnoporus sanguineus, according to the fungal exposure duration (in days)

Figure 12: Visual aspect of the fungal inhibition kinetic (in %) of Wacapou extracts from ethyl acetate maceration (1200 μL), according to the extractive’s concentration, after 8, 14 and 21 days of exposure to Pycnoporus sanguineus
These results and the visual representation from Figs. 11 and 12 clearly indicate that ethyl acetate extractives from Wacapou wood inhibit the growth of during the first 8 days following fungal exposure, which corresponds to the time needed for the mycelium to fully colonize the control sample. Moreover, the higher the extract concentration, the higher the efficiency of reducing the fungal growth. However, after 21 days, fungal growth was fully recovering the medium in the petri dishes for all concentrations.
In brief, the literature has recently shown that phenolic acids and flavonoids naturally protect plants against phytopathogenic fungi and, therefore, plant extracts containing phenolic compounds are considered natural alternatives to conventional antifungal molecules [67]. In addition, terpenoid compounds have been proven to be active against termites and wood-destroying fungi [68]. In fact, according to the literature, terpenes and terpenoids have been reported to present toxic, antifeeding and repellent properties against termites and other insects [14,69].
3.5 Potential of Wacapou Extractives in Preservatives
3.5.1 Anhydrous Impregnation Rates
After being impregnated into 48 samples of Virola wood, the average anhydrous impregnation retention value of Wacapou extractive (by ethyl acetate maceration process) solution (concentration of 8%) was 10.69 ± 2.66% (Table 3). For the 48 samples of Virola wood impregnated with the commercial preservative (AXTON Universal treatment), the average anhydrous impregnation retention value was 3.98 ± 1.39%.

3.5.2 Durability in Soil Bed Test Conditions
As shown in Fig. 13, the degree of weight loss (WLsbt%) due to soft rot degradation in soil bed tests increased as a function of the exposure time to soil-inhabiting micro-organisms, as well as for Virola control sample than commercial or Wacapou’s extractives treated woods. Nevertheless, it appears that the weight loss due to soil exposure is significantly reduced by impregnation treatment. Both commercial preservative-impregnated and Wacapou extractive-impregnated Virola wood samples showed similar degradation kinetics in soil bed test conditions. However, it should be noted that the active compound retention rate was much lower for the commercial product than for wood impregnated with Wacapou extractives. In this sense, the different average values of the anhydrous impregnation retention of Wacapou extractive and the commercial preservative need to be taken into consideration when both treated wood durability properties are compared together. After 120 days of soil bed test exposure, both treatments conferred Virola wood a class 4 durability (Slightly durable).
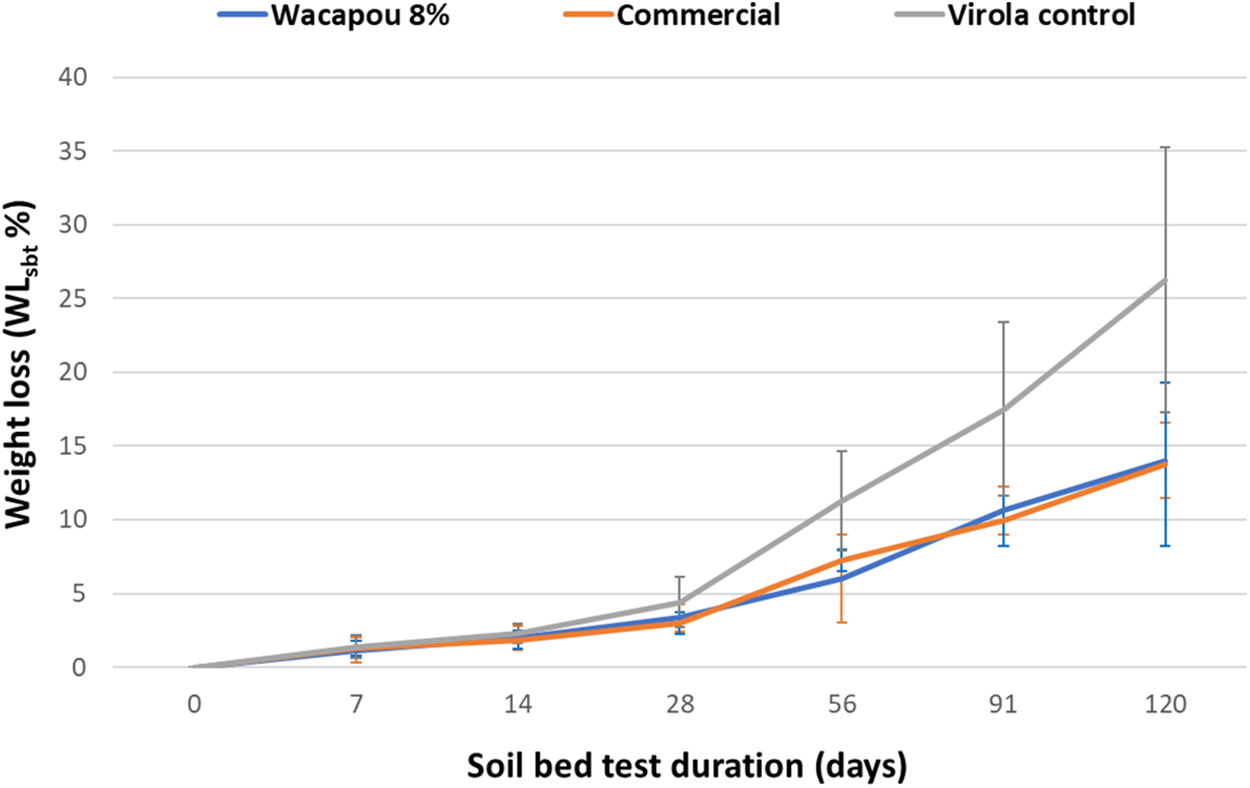
Figure 13: Weight loss (WLsbt%) due to unsterile soil exposure of native and impregnated Virola woods, according to the test duration
Past studies showed that treatments containing ethanol extractives (from teak wood) conferred significant resistance gains against basidiomycetes (brown and white-rot fungi) to teak sapwood and Pinus sp. whereas no durability difference, between these native and treated wood was observed after exposure to soft-rots in soil bed tests [70].
In addition, the oven drying (at 103 ± 2°C) of the treated Virola samples, just after impregnation processes, could result in the volatilization and degradation of some active extractive compounds previously impregnated into the wood. Such a degradation under heat exposure could therefore lead to a loss of the biobased protective solutions treatment efficacy [6].
The wood degradation mechanisms are different depending on the type of fungi involved [71]. According to these results, the resistance of Virola samples impregnated by Wacapou extractives could perform better against white and brown rots that against soft rot. Additional durability tests against basidiomycetes are needed to confirm this hypothesis and assess the potential of impregnated Virola with Wacapou extractives used above-ground [72].
Wacapou extracts (from ethyl acetate maceration) showed the best antifungal and anti-termite activities. In addition, the concentration level of extractives had an impact on the anti-termite activity against Reticulitermes flavipes and Cryptotermes sp., as well as on the fungal growth inhibition of Pycnoporus sanguineus. The biological activities of Wacapou extractives are primarily due to the presence of cassane-type diterpenoids such as methyl vouacapenate, vouacapenic acid and vouacapenol in their chemical composition.
Finally, a formulation containing 8% Wacapou extractives was selected for impregnation into the Virola wood sample. The impregnated Virola samples showed similar resistance to soil micro-organisms in the laboratory after 120 days, comparable to Virola treated with a commercial preservative, achieving a class 4 durability rating.
In this regard, the formulation based on Wacapou extractives demonstrated interesting potential as eco-friendly treatment, aiming to confer better durability to local low-durability wood species. Further tests on the impregnated Virola samples, focusing on resistance to basidiomycete attacks according to European Standard EN EN 113-2 [73], will determine whether this material can achieve sufficient durability for outdoor exposure according in Use Class 3.1 or 3.2.
Despite their potential, the use of Wacapou extractives as preservatives may face challenges related to stability, solubility, and regulatory approval. Further research is needed to optimize formulations, understand their mechanisms of action, and ensure their safety and efficacy for commercial use. Current research focuses on the volatility, leachability and eco-toxicity of extractive-based preservatives to improve formulations and impregnation processes for the optimal use of treated woods.
In conclusion, Wacapou extractives hold significant promise as natural preservatives due to their antifungal and anti-termite properties. With ongoing research and development, sustainable alternatives to synthetic preservatives could be offered in various industries, contributing to safer and more environmentally friendly products.
Acknowledgement: The authors would like to thank the BIO2MAR platform (http://bio2mar.obsbanyuls.fr) (accessed on 26 September 2024) for providing access to instrumentation.
Funding Statement: This work was conducted as part of the PhD thesis of Emma Kieny, which is financially supported by Ademe (Environmental and Energy Management Agency). These researches take part from the project PROTEXTWOOD (ID 2202-102) funded through LabEx AGRO ANR-10-LABX-0001-01 (under I-Site Université de Montpellier framework) and the project PANTHER2-Guyane funded through Agence Nationale de la Recherche (ANR-22-CE43-0019). In addition, this work has benefited from an “Investissement d’Avenir” grant managed by Agence Nationale de la Recherche (CEBA, ref. ANR-10-LABX-25-01), and it was also supported by the FEDER (European Regional Development Fund) research project “EcovaloBois” (Project number: GY0015430), and by the CNRS peps INSIS 2018 research project “GuyavaloFibres”.
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Kévin Candelier, Julie Bossu, Romain Lehnebach; data collection: Emma Kieny, Louis Milhe, Yannick Estevez, Cyrielle Sophie, Emeline Houël; analysis and interpretation of results: Emma Kieny, Kévin Candelier, Julie Bossu, Romain Lehnebach, Jérémie Damay, Daniela Florez, Marie-France Thévenon; draft manuscript preparation: Emma Kieny, Kévin Candelier, Julie Bossu, Marie-France Thévenon. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The detailed data obtained through this study and presented in this article can be requested from the corresponding author.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare that they have no conflicts of interest to report regarding the present study.
References
1. Boucard P, Denize C. Potential for substitution of substances used in wood preservatives (PT8). Issues related to future approval decisions-October 2022, INERIS; 2022. Document Ineris-207016-2757679-v1.0. Available from: https://www.ineris.fr/sites/ineris.fr/files/contribution/Documents/INERIS-207016-2757679-Potential%20for%20substitution%20of%20substances%20used%20in%20wood%20preservatives%20%28PT8%29%20v1_0.pdf. [Accessed 2024]. [Google Scholar]
2. Khademibami L, Bobadilha GS. Recent developments studies on wood protection research in academia: a review. Front For Glob Change. 2022;5:793177. doi:10.3389/ffgc.2022.793177. [Google Scholar] [CrossRef]
3. Jones D, Sandberg D, Goli G, Todaro L. Wood modification in Europe: a state-of-the-art about processes, products and applications. Italia: Firenze University Press, Proceedings e Report; 2019. vol. 124. [Google Scholar]
4. Gérardin P. New alternatives for wood preservation based on thermal and chemical modification of wood—a review. Ann For Sci. 2016;73:559–70. doi:10.1007/s13595-015-0531-4. [Google Scholar] [CrossRef]
5. Mubarok M, Gérardin-Charbonnier C, Azadeh E, Obounou Akong F, Dumarçay S, Pizzi A, et al. Modification of wood by Tannin-Furfuryl Alcohol resins-effect on dimensional stability, mechanical properties and decay durability. J Renew Mater. 2023;11(2):505–21. doi:10.32604/jrm.2022.024872. [Google Scholar] [CrossRef]
6. Kirker GT, Hassan B, Mankowski ME, Eller FJ. Critical review on the use of extractives of naturally durable woods as natural wood protectants. Insects. 2024;15(1):69. doi:10.3390/insects15010069. [Google Scholar] [PubMed] [CrossRef]
7. Ayilara MS, Adeleke BS, Akinola SA, Fayose CA, Adeyemi UT, Gbadegesin LA, et al. Biopesticides as a promising alternative to synthetic pesticides: a case for microbial pesticides, phytopesticides, and nanobiopesticides. Front Microbiol. 2023;14:1040901. doi:10.3389/fmicb.2023.1040901. [Google Scholar] [PubMed] [CrossRef]
8. N’Guessan JLL, Niamke FB, N’Guessan JCY, Amusant N. Wood extractives: main families, functional properties, fields of application and interest of wood. Forest Prod J. 2023;73(3):194–208. doi:10.13073/FPJ-D-23-00015. [Google Scholar] [CrossRef]
9. Wagner K, Musso M, Kain S, Willför S, Petutschnigg A, Schnabel T. Larch wood residues valorization through extraction and utilization of high value-added products. Polymers. 2020;12(2):359. doi:10.3390/polym12020359. [Google Scholar] [PubMed] [CrossRef]
10. Hussein HS, Salem MZM, Soliman AM, Eldesouky SE. Comparative study of three plant-derived extracts as new management strategies against Spodoptera littoralis (Boisd.) (Lepidoptera: noctuidae). Sci Rep. 2023;13:3542. doi:10.1038/s41598-023-30588-x. [Google Scholar] [PubMed] [CrossRef]
11. Mishra P, Verma M, Jha S, Tripathi A, Pandey A, Dikshit A, et al. Biological approaches of termite management: a review. Curr Bot. 2021;12:121–31. [Google Scholar]
12. Ahmed S, Tabassum MH, Hassan B. Evaluation of Antitermite properties of wood extracts from Pongamia pinnata (L.) Pierre (Leguminosae) against subterranean termites. An Acad Bras Cienc. 2022;94:e20190591. doi:10.1590/0001-3765202220190591. [Google Scholar] [PubMed] [CrossRef]
13. Bédounguindzi WF, Candelier K, Edou Engonga P, Dumarçay S, Thévenon MF, Gérardin P. Anti-termite and antifungal bio-sourced wood preservation ingredients from Dacryodes edulis (G. Don) H.J. Lam resin. Holzforschung. 2020;74(8):745–53. doi:10.1515/hf-2019-0106. [Google Scholar] [CrossRef]
14. Šimůnková K, Htfytfsek Š., Reinprecht L, Šobotník J, Lišková T, Pánek M. Lavender oil as eco-friendly alternative to protect wood against termites without negative effect on wood properties. Sci Rep. 2022;12:1909. doi:10.1038/s41598-022-05959-5. [Google Scholar] [PubMed] [CrossRef]
15. Kadir R, Babar H. Toxicity and repellent effects of wood extractives of five Malaysian wood species on Asian subterranean termite Coptotermes gestroi Wasmann. Eur J Wood Wood Prod. 2020;78:1249–62. doi:10.1007/s00107-020-01592-z. [Google Scholar] [CrossRef]
16. Santos MB, Sillero L, Darci Alberto Gatto DA, Labidi J. Bioactive molecules in wood extractives: methods of extraction and separation, a review. Ind Crops Prod. 2022;186:115231. doi:10.1016/j.indcrop.2022.115231. [Google Scholar] [CrossRef]
17. Isman MB. Bioinsecticides based on plant essential oils: a short overview. Zeitschrift für Naturforschung C. 2020;75(7–8):179–82. [Google Scholar]
18. Gupta I, Singh R, Muthusamy S, Sharma M, Grewal K, Singh HP, et al. Plant essential oils as biopesticides: applications, mechanisms, innovations, and constraints. Plants. 2023;12:2916. doi:10.3390/plants12162916. [Google Scholar] [PubMed] [CrossRef]
19. Sogabe A, Kinjo K, Abe F, Yamauchi T, Yaga S. Termiticidal substances from the heartwood of Cryptomeria japonica D. Don. Mokuzai Gakkaishi. 2020;46:24–131. [Google Scholar]
20. Vij T, Anil PP, Shams R, Dash KK, Kalsi R, Pandey VK, et al. A comprehensive review on bioactive compounds found in Caesalpinia sappan. Molecules. 2023;28(17):6247. doi:10.3390/molecules28176247. [Google Scholar] [PubMed] [CrossRef]
21. Rodrigues AMS, Stien D, Eparvier V, Espindola LS, Beauchêne J, Amusant N, et al. The wood preservative potential of long-lasting Amazonian wood extracts. Int Biodeterior Biodegradation. 2012;75:146–9. doi:10.1016/j.ibiod.2012.03.014. [Google Scholar] [CrossRef]
22. Candelier K, Jay-Allemand C, Dijoux R, Ducruet R, Kieny E, Aznar D, et al. Repellent activities against four Ascomycota species and Reticulitermes flavipes of acetonic extractives from sapwood to inner heartwood fractions of Cedrus atlantica. Bois for Trop. 2023;355:87–98. doi:10.19182/bft2023.355.a37002. [Google Scholar] [CrossRef]
23. Broda M. Natural compounds for wood protection against Fungi—a review. Molecules. 2020;25(15):3538. doi:10.3390/molecules25153538. [Google Scholar] [PubMed] [CrossRef]
24. Asamoah A, Frimpong-Mensah K, Antwi-Boasiako C. Efficacy of Tectona grandis (Teak) and Distemonanthus benthamianus (Bonsamdua) water extractives on the durability of five selected ghanaian less used timber species. Pak J Chem. 2011;1:28–31. doi:10.15228/issn.2220-2625. [Google Scholar] [CrossRef]
25. Kirker GT, Bishell AB, Lebow PK. Laboratory evaluations of durability of southern pine pressure treated with extractives from durable wood species. J Econ Entomol. 2016;109:259–66. doi:10.1093/jee/tov286. [Google Scholar] [PubMed] [CrossRef]
26. Sablík P, Giagli K, Pařil P, Baar J, Rademacher P. Impact of extractive chemical compounds from durable wood species on fungal decay after impregnation of non-durable wood species. Eur J Wood Wood Prod. 2016;74:231–6. doi:10.1007/s00107-015-0984-z. [Google Scholar] [CrossRef]
27. Vek V, Balzano A, Poljanšek I, Humar M, Oven P. Improving fungal decay resistance of less durable sapwood by impregnation with scots pine knotwood and black locust heartwood hydrophilic extractives with antifungal or antioxidant properties. Forests. 2020;11:1024. doi:10.3390/f11091024. [Google Scholar] [CrossRef]
28. Gérard J, Guibal D, Paradis S, Cerre JC, Chalon I, Thévenon MF, et al. Tropical timber atlas. In: Technological characteristics and uses. Versailles: Ed. Quae; 2017. [Google Scholar]
29. EN 350. Durability of wood and wood-based products–testing and classification of the durability to biological agents of wood and wood-based materials. Brussels, Belgium: European Committee for Standardization (CEN); 2016. [Google Scholar]
30. Amusant N, Nigg M, Thibaut B, Beauchêne J. Diversity of decay resistance strategies of durable tropical woods species: Bocoa prouacencsis Aublet, Vouacapoua americana Aublet, Inga alba (Sw.) Wild. Int Biodeterior Biodegradation. 2014;94:103–8. doi:10.1016/j.ibiod.2014.06.012. [Google Scholar] [CrossRef]
31. Hegnauer R. Chemotaxonomie der Pf1anzen. Band Il a. Leguminosae. Basel: Birkhauser Verlag; 1994. [Google Scholar]
32. King FE, Godson DH, King TJ. The chemistry of extractives from hardwoods. Part XXII. The structure of diterpenes from Vouacapoua species. J Chem Soc; 1955;1117–25. doi:10.1039/jr9550001117. [Google Scholar] [CrossRef]
33. Meurer-Grimes B, Tavakilian G. Chemistry of cerambycid host plants. Part 1: survey of Leguminosae—a study in adaptive radiation. Bot Rev. 1997;64(4):356–94. [Google Scholar]
34. Kido T, Taniguchi M, Baba K. Diterpenoids from Amazonian crude drug of Fabaceae. Chem Pharm Bull. 2003;51:207–8. doi:10.1248/cpb.51.207. [Google Scholar] [PubMed] [CrossRef]
35. Çiçek SS, Pfeifer Barbosa AL, Wenzel-Storjohann A, Segovia JFO, Bezerra RM, Sönnichsen F, et al. Chemical and biological evaluation of amazonian medicinal plant Vouacapoua americana Aubl. Plants. 2023;12(1):99. [Google Scholar]
36. Dickson RA, Houghton PJ, Hylands PJ. Antibacterial and antioxidant cassane diterpenoids from Caesalpinia benthamiana. Phytochem. 2007;68(10):1436–41. doi:10.1016/j.phytochem.2007.03.008. [Google Scholar] [PubMed] [CrossRef]
37. Zhang Z, Wang P, Chen M, Xie L, Zhang X, Shi Y, et al. Antibacterial activity of two new cassane diterpenoids from Caesaplinia pulcherrima against Bacillus cereus by damage to cell membrane. Int J Mol Sci. 2023;24(5):4917. doi:10.3390/ijms24054917. [Google Scholar] [PubMed] [CrossRef]
38. Varty N, Guadagnin DL. Vouacapoua americana. The IUCN Red List of Threatened Species 1998: e.T33918A9820054; 2015. doi:10.2305/IUCN.UK.1998.RLTS.T33918A9820054.en. [Google Scholar] [CrossRef]
39. Grébic H, Bougé F, Guitet S. Etude de sensibilité de Vouacapoua Americana. ONF report 2010; 100. 2010; Available from: https://www.guyane.developpement-durable.gouv.fr/IMG/pdf/Wacapou_Eval_sensibilit__ONF_2010.pdf. [Accessed 2024]. [Google Scholar]
40. da Silva Rios MN, Pastore Júnior F. Plants of the Amazon: 450 species for general use. Biblioteca Central: Universidade de Brasília; 2011 (In Portuguese). [Google Scholar]
41. Fournier M, Amusant N, Beauchêne J, Mouras S. Qualité des bois de Guyane. Revue forestière française. 2003;55:340–51. [Google Scholar]
42. Rodrigues A. Analysis and bio-inspired valorisation of secondary metabolites responsible for the natural durability of exploited woods from Guiana (Ph.D. Thesis). University of Antilles-Guyane: Cayenne, France; University of Brasilia: Brasilia, Brazil; 2010 (In French). [Google Scholar]
43. Rodríguez De Luna SL, Ramírez-Garza RE, Serna Saldívar SO. Environmentally friendly methods for flavonoid extraction from plant material: impact of their operating conditions on yield and antioxidant properties. Sci World J. 2020;2020(1):6792069. [Google Scholar]
44. Stien D, Clergeaud F, Rodrigues AMS, Lebaron K, Pillot R, Romans P, et al. Metabolomics reveal that octocrylene accumulates in Pocillopora damicornis tissues as fatty acid conjugates and triggers coral cell mitochondrial dysfunction. Anal Chem. 2019;91:990–5. doi:10.1021/acs.analchem.8b04187. [Google Scholar] [PubMed] [CrossRef]
45. Boer FD, Pignolet L, Valette J, Candelier K, Commandré JM, Fournier M, et al. Efficacy of slow pyrolysis liquid from sugarcane bagasse for wood protection and its leaching properties. Eur J Wood Prod. 2024;19. [Google Scholar]
46. Leroy M, Candelier K, Damay J, Bossu J, Lehnebach R, Thévenon MF, et al. Natural durability of 8 tropical species suitable Bois for. trop. and termites. Bois for Trop. 2023;358:15–29. doi:10.19182/bft2023.358.a37217. [Google Scholar] [CrossRef]
47. Zalsabila A, Syafii W, Priadi T, Syahidah. Anti-termite activity of Tamanu Bark extract (Calophyllum inophyllum L.). J Korean Wood Sci Technol. 2024;52(2):134–44. doi:10.5658/WOOD.2024.52.2.134. [Google Scholar] [CrossRef]
48. Prasad W, Wani AD, Khamrui K, Hussain SA, Khetra Y. Green solvents, potential alternatives for petroleum-based products in food processing industries. Cleaner Chem Eng. 2022;3:100052. doi:10.1016/j.clce.2022.100052. [Google Scholar] [CrossRef]
49. Beauchêne J, Amusant N, Cigna J, Koese S, Thibaut B. Using specimens from the CIRAD Kourou wood collection to build a database of properties. Bois et Forêts des Tropiques. 2022;352:61–70. doi:10.19182/bft2022.352.a36936. [Google Scholar] [CrossRef]
50. XP ENV 807. Wood preservatives-determination of the effectiveness against soft rotting micro-fungi and other soil inhabiting micro-organisms. Brussels, Belgium: European Committee for Standardization (CEN); 2021. [Google Scholar]
51. ISO 10390. Soil, treated biowaste and sludge-determination of pH. Paris, France: International Organization for Standardization (AFNOR); 2021. [Google Scholar]
52. XP CEN/TS 15083-2. Durability of wood and wood-based products-determination of the natural durability of solid wood against wood destroying fungi-test methods—part: soft rotting micro-fungi. Brussels, Belgium: European Committee for Standardization (CEN); 2006. [Google Scholar]
53. Verpoorte R, Kim HK, Choi YH. Trivialities in metabolomics: artifacts in extraction and analysis. Front Mol Biosci. 2022;9:972190. doi:10.3389/fmolb.2022.972190. [Google Scholar] [PubMed] [CrossRef]
54. Malik J, Santoso A, Mulyana Y, Ozarska B. Characterization of merbau extractives as a potential wood-impregnating material. BioResources. 2016;11(3):7737–53. [Google Scholar]
55. Shalev Y, Hadaya O, Bransi-Nicola R, Landau SY, Azaizeh H, Muklada H, et al. Entourage effect for phenolic compounds on production and metabolism of mammary epithelial cells. Heliyon. 2022;8(3):e09025. doi:10.1016/j.heliyon.2022.e09025. [Google Scholar] [PubMed] [CrossRef]
56. Murugan R, Parimelazhagan T. Comparative evaluation of different extraction methods for antioxidant and anti-inflammatory properties from Osbeckia parvifolia Arn.—an in vitro approach. J King Saud Univ Sci. 2014;26(4):267–75. doi:10.1016/j.jksus.2013.09.006. [Google Scholar] [CrossRef]
57. Alawiyah A, Senania A. Antioxidant activity and bioactive compounds of ethyl acetate fractions from Syzygium cumini wood stem. ALKIMIA: Jurnal Ilmu Kimia Dan Terapan. 2022;5(1):93–101. doi:10.19109/alkimia.v5i1. [Google Scholar] [CrossRef]
58. Pérez-Bonilla M, Salido S, Sánchez A, Van Beek TA, Altarejos J. Effect of extraction conditions on the antioxidant activity of olive wood extracts. Int J Food Sci. 2013;2013:719593. [Google Scholar]
59. Sudding, Salempa P, Nurhikmah. Isolation and identification of ethyl acetate extract secondary metabolite compound of Kayu Jawa Bark (L. Coromandelica). J Phys: Conf Ser. 2021;1899:012035. doi:10.1088/1742-6596/1899/1/012035. [Google Scholar] [CrossRef]
60. Kameri A, Koçani F, Hashani Z, Kurteshi K, Kamberi B, Kurti A, et al. Antifungal and synergistic effects of the ethyl acetate extract of Tanacetum vulgare (L.) against Candida albicans. Med Sci Monit Basic Res. 2019;25:179–86. doi:10.12659/MSMBR.917394. [Google Scholar] [PubMed] [CrossRef]
61. Zentar H, Jannus F, Medina-O’Donnell M, Lupiáñez JA, Justicia J, Alvarez-Manzaneda R, et al. Synthesis and biological evaluation of Cassane Diterpene (5α)-Vuacapane-8(149(11)-diene and of some related compounds. Molecules. 2022;27(17):5705. doi:10.3390/molecules27175705. [Google Scholar] [PubMed] [CrossRef]
62. Maurya R, Ravi M, Singh S, Yadav PP. A review on cassane and norcassane diterpenes and their pharmacological studies. Fitoterapia. 2012;83(2):272–80. doi:10.1016/j.fitote.2011.12.007. [Google Scholar] [PubMed] [CrossRef]
63. Tu WC, Ding LF, Peng LY, Song LD, Wu XD, Zhao QS. Cassane diterpenoids from the seeds of Caesalpinia bonduc and their nitric oxide production and α-glucosidase inhibitory activities. Phytochemistry. 2022;193:112973. doi:10.1016/j.phytochem.2021.112973. [Google Scholar] [PubMed] [CrossRef]
64. Godoy RLO, Lima PDDB, Pinto AC, Neto FRAN. Diterpenoids from Dypterix odorata. Phytochem. 1989;28(2):642–4. doi:10.1016/0031-9422(89)80073-1. [Google Scholar] [CrossRef]
65. Hadi YS, Nurhayati T, Jasni, Yamamoto H, Kamiya N. Resistance of smoked wood to subterranean and dry-wood termite attack. Int Biodeterior Biodegradation. 2012;70:79–81. doi:10.1016/j.ibiod.2011.06.010. [Google Scholar] [CrossRef]
66. Gochev VK, Krastanov AI. Fungal laccases. Bulg J Agric Sci. 2007;13(1):75–83. [Google Scholar]
67. Filippi D, Rodrigues LB, Boscutti F, Priamo WL, Chiomento JLT, Friedrich MT. Phenolic compounds in fisalis (Physalis peruviana Linneus) extracts and action of the extracts on the phytopathogen Botrytis cinerea Pers. Braz J Develop, Curitiba. 2020;6(10):78370–85. doi:10.34117/bjd. [Google Scholar] [CrossRef]
68. Nisar MS, Nazir T, Zaman S, Hussain SI, Khan NA, Aslam HU, et al. Toxicity and repellency of plant extract and termiticide against fungus growing subterranean termites (Blattodea: termitidae). J Bioresour Manage. 2022;9(2):119–32. [Google Scholar]
69. Patel KK, Narasimhacharya AVRL. Potentiality of phytochemical compounds isolated and identified from Jatropha curcas L. against Odontotermes obesus Rambur. J Nat Pestic Res. 2022;2:100009. doi:10.1016/j.napere.2022.100009. [Google Scholar] [CrossRef]
70. Fassina Brocco V, Benigno Paes J, da Costa L G, Brazolin S. Potential of teak heartwood extracts as a natural wood preservative. J Clean Prod. 2017;142(4):2093–9. [Google Scholar]
71. Goodell B, Qian Y, Jellison J. Fungal decay of wood: soft rot—brown rot—white rot. ACS Symp Ser Am Chem Soc. 2008;982:9–31. [Google Scholar]
72. EN 460. Durability of wood and wood-based materials—a guide to determining performance. Brussels, Belgium: European Committee for Standardization (CEN); 2023. [Google Scholar]
73. EN 113-2. Durability of wood and wood-based products—test method against wood destroying basidiomycetes—part 2: Assesment of inherent or enhanced durability. Brussels, Belgium: European Committee for Standardization (CEN); 2020. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools