 Open Access
Open Access
REVIEW
Transforming Sawdust Waste into Renewable Energy Resources: A Comprehensive Review on Sustainable Bio-Oil and Biochar Production via Thermochemical Processes
1 Department of Chemistry, Nile University of Nigeria, FCT, Abuja, 9000001, Nigeria
2 Waste to Wealth Research Group, Nile University of Nigeria, FCT, Abuja, 9000001, Nigeria
3 Department of Mechanical Engineering, Nile University of Nigeria, FCT, Abuja, 9000001, Nigeria
4 Department of Chemical Engineering, Bucknell University, Lewisburg, PA 17837, USA
* Corresponding Author: Adekunle Adeleke. Email:
Journal of Renewable Materials 2025, 13(12), 2375-2430. https://doi.org/10.32604/jrm.2025.02025-0109
Received 02 June 2025; Accepted 01 September 2025; Issue published 23 December 2025
Abstract
The increasing need for sustainable energy and the environmental impacts of reliance on fossil fuels have sparked greater interest in biomass as a renewable energy source. This review provides an in-depth assessment of bio-oil and biochar generation through the pyrolysis of sawdust, a significant variety of lignocellulosic biomass. The paper investigates different thermochemical conversion methods, including fast, slow, catalytic, flash, and co-pyrolysis, while emphasizing their operational parameters, reactor designs, and effects on product yields. The influence of temperature, heating rate, and catalysts on enhancing the quality and quantity of bio-oil and biochar is thoroughly analyzed. Additionally, the review examines advanced reactor technologies such as fluidized beds, fixed beds, auger reactors, and plasma pyrolysis systems. It also discusses recent progress in catalyst innovation and product enhancement techniques to overcome the challenges posed by bio-oil, including its high oxygen content and low stability. By synthesizing experimental results and conducting comparative analyses, the paper identifies existing research gaps and provides insights into future paths for effective biomass utilization, thereby aiding in the creation of economically viable and environmentally responsible bioenergy systems.Graphic Abstract

Keywords
1.1 Overview of Renewable Energy
Research into alternative energy sources has intensified due to the environmental impacts of fossil fuel use and increasing global commitment to sustainability. According to the International Energy Agency (IEA), total energy consumption is projected to rise from 12,000 to 16,800 Mtoe between 2007 and 2030, with an annual growth rate of 1.5% [1]. However, fossil fuels such as coal and petroleum remain the dominant energy sources and account for approximately 56.6% of global greenhouse gas (GHG) emissions [1]. To address this, the United Nations (UN) Climate Panel has proposed reducing GHG emissions by 50%–80% by 2050 [1]. Achieving these goals requires a significant transition from fossil fuels to renewable energy sources, which offer environmental benefits and long-term economic viability. Among renewable options, biomass has gained prominence due to its availability and environmental advantages. Biomass, primarily in the form of lignocellulosic material from forestry waste, aquatic plants, crop residues, and energy crops, is available in large quantities, estimated at around 220 billion tons annually [1]. These residues often require significant processing and disposal space; however, converting them into energy not only reduces environmental load but also generates economic value [2]. Recycling biomass waste into energy and fuel supports a bio-based economy, promotes resource efficiency, and contributes to job creation [3]. Biomass is chemically diverse, and its energy potential depends heavily on its composition. For instance, grain and animal waste are rich in starch, while plant-based residues contain varying proportions of cellulose, hemicellulose, and lignin [4]. These variations directly affect how biomass behaves under thermochemical conversion processes such as pyrolysis, gasification, combustion, and hydrothermal liquefaction. Among these, pyrolysis stands out for its ability to convert biomass into three valuable products bio-oil, biochar, and syngas within a short reaction time and under limited oxygen supply [5]. Pyrolysis has gained increasing attention for biofuel production due to its versatility, efficiency, and lower power requirements compared to other thermochemical methods. The liquid product, bio-oil, is considered one of the most promising carbon-neutral fuels for the future [6]. Its use significantly reduces emissions of sulfur dioxide and nitrogen oxides, and it can be easily stored and transported using existing infrastructure [7,8]. These features make bio-oil a more practical alternative to biogas or charcoal in many energy applications.
Various woody biomasses have been investigated for pyrolysis-based fuel production. For example, Siddiqi et al. [1] reported that the physicochemical characteristics of Shorea robusta biomass show strong potential for producing liquid fuel and biochar [9]. Similarly, Ameh et al. [10] studied Sal wood sawdust and found its derived fuel to be a promising substitute for fossil fuels. However, thermal pyrolysis often produces bio-oil with high viscosity, oxygen content, and acidity, limiting its direct application as a transportation fuel. These issues can be mitigated through catalytic pyrolysis, which enhances oil quality by promoting deoxygenation and reducing unwanted compounds. This review aims to provide a comprehensive analysis of the pyrolysis of bio-oil and biochar derived from sawdust, with a focus on the influence of feedstock composition, pyrolysis conditions, and catalyst application. It critically evaluates recent advancements in characterization techniques, product optimization, and potential applications, while identifying research gaps and future prospects for sustainable bioenergy and biochar utilization.
1.2 The Role of Biomass in Addressing Environmental and Energy Issues
The globe is searching for renewable energy sources due to the depletion of fossil fuel reserves and rising expenses. To balance the production and consumption of energy resources, a variety of biomass sources including both lignocellulosic and non-lignocellulosic biomass are used. Woody biomass, agricultural residues, and plants that contain industrial byproducts like sewage sludge and animal dung, bones, microorganisms like algae, and various other sources are classified as non-lignocellulosic biomass that is used in catalysis, pollutant removal, and energy production. The main components of lignocellulosic biomass are cellulose, hemicellulose, and lignin, whereas the constituents of non-lignocellulosic biomass are proteins, carbohydrates, saccharides, and different inorganic substances [11].
Researchers are interested in biomass as an alternative energy source since it is a renewable source of organic solid waste [12]. Because of a number of issues, such as excessive humidity, low density, and milling challenges, the original form of biomass is very challenging to use directly as fuel. Therefore, a variety of thermal techniques are used to transform the initial biomass samples into more workable material. Biomass undergoes chemical and physical changes during thermal degradation. When a material’s porosity increases, its physical and chemical properties alter as a result of thermal breakdown at various rates of temperature and variable product composition and characteristics [13]. Various sources of biomass waste are categorized based on origin and composition, as shown in Fig. 1. Through a variety of conversion pathways, lignocellulosic biomass, a renewable and carbon-neutral resource, can be transformed into biofuel and other intermediate compounds [14]. It is made up of biopolymers like cellulose (40%–60%), hemicellulose (20%–40%), and lignin (15%–30%) [15].
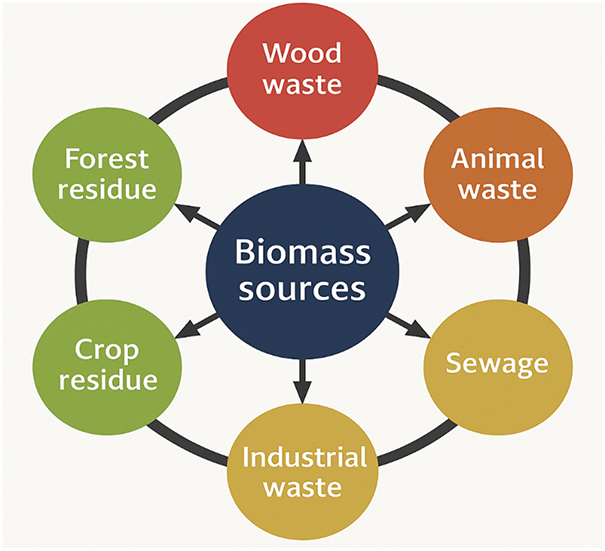
Figure 1: Various sources of bimass waste
Cellulose, a crystalline polymer has the generic formula (C6H10O5) n and is made up of b-linked chains. Hydrogen bonds between the oxygen molecules in cellulose and the hydroxyl groups of glucose produce microfibrils that are joined in a carbohydrate matrix, giving a plant’s cell wall its strength and rigidity [16]. Compared to cellulose, hemicellulose is a complex amorphous polymer with a lower molecular mass and varied degree of branching. It shares structural and chemical similarities with cellulose. The kind and quantity of monosaccharides that make up its structure, which often consists of xylose (the most prevalent), galactose, glucose, arabinose, mannose, and sugar acids, set it apart from cellulose [17]. Hemicellulose should be eliminated during pretreatment because it binds to lignin, pectin, and cellulose micro-fibrils to form a cross-linked network that supports the structural integrity of cell walls [16]. Lignin, after cellulose and hemicellulose, is the most prevalent natural polymer on the planet [18]. This amorphous polymer matrix, which is the third major component of lignocellulosic biomass, is created by the random polymerization of three main phenylpropane monomers: coumaryl, coniferyl, and sinapyl alcohols [19]. According to their origin, these three lignin precursors cause the H (p-hydroxyphenyl), G (guaiacyl), and S (syringyl) to be acylated and exhibit varying abundances [20]. Determining the extent of lignin’s natural polymerization is challenging because it is always broken up during extraction and is made up of several substructures that repeat randomly together [21]. These three key components of lignocellulose material are arranged with acetyl groups, minerals, and phenolic substituents to form an extremely complicated structure [22]. Additionally, the components of lignocellulosic biomass determine its usage because cellulose, hemicellulose, and lignin fractions interact differently in complex and wide-ranging molecular systems [22]. Pretreatment is required to dissolve the intricate bonds between these three main biomass constituents. The next step after pretreatment is to transform them into the desired chemical products. Fig. 2 displays the process’s schematic diagram.
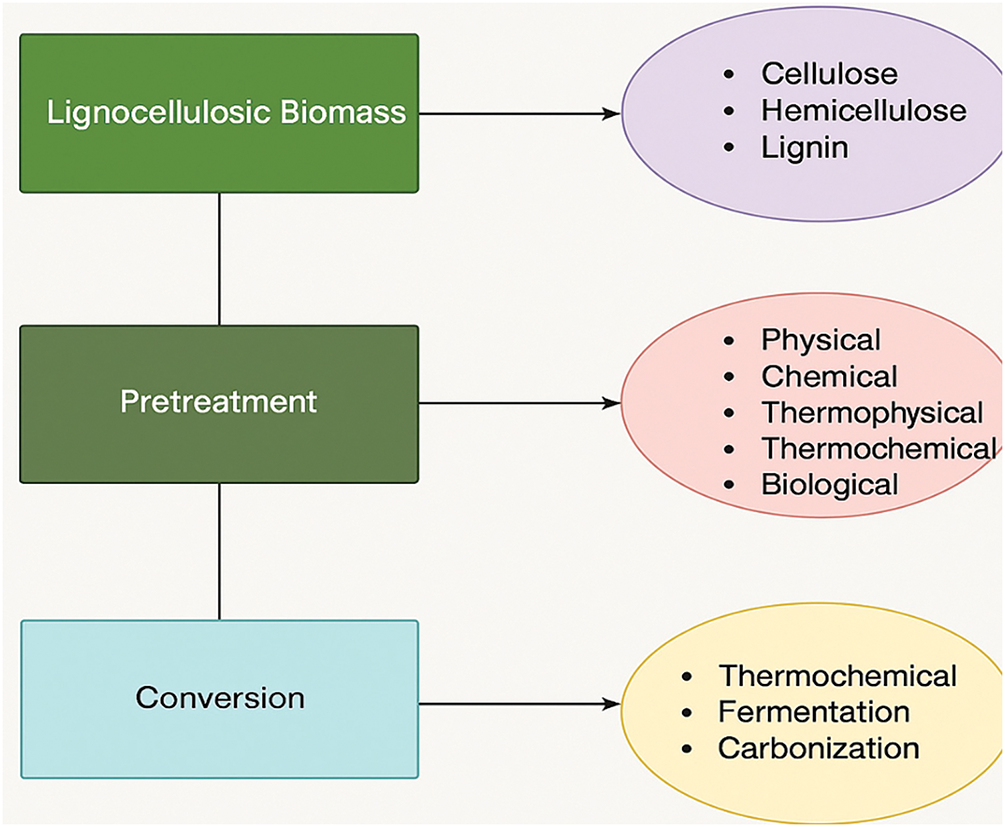
Figure 2: Schematic diagram showing utilization of lignocellulose biomass
The main ways to characterize biomass are to examine its carbon, hydrogen, and oxygen contents to ascertain its elemental composition [23]. The primary reasons of the variation in the chemical composition and ash levels of biomass are the varying moisture concentrations, ash yields, and the presence of different kinds of inorganics in biomass. The elements that are most prevalent in biomass are Carbon (C), Hydrogen (H), Oxygen (O), Nitrogen (N), Calcium (Ca), Potassium (K), Silicon (Si), Magnesium (Mg), Aluminium (Al), Silicon (S), Iron (Fe), Phophorus (P), Chlorine (Cl), Sodium (Na), Manganese (Mn), and Titanium (Ti) [24]. The kinds of species found in biomass vary depending on whether it originates from agriculture, industrial waste, or waste from plants or animals [25]. Although in extremely little amounts, biomass also contains sulfur and nitrogen, indicating its amiable nature. Since the emission of Nitrogen oxides (NOx) and Sulfur oxides (SOx) is caused by the presence of nitrogen and sulfur, its low concentration is beneficial to the environment and does not harm it in any way [26]. According to literature, the most common components in biomasses are C, O, and H, with smaller amounts of K, P, and Si present in some of them [27]. The morphology and surface characteristics of biomass and biochar have been determined using a variety of characterization techniques in addition to elemental composition analysis, such as Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Spectrometer (EDX), and Surface Area Analyzer (SAA). According to the findings of these characterizations, lignocellulosic biomass has the potential to be used globally as a sustainable energy source [28–32]. Understanding the physicochemical characteristics of biomass, such as elemental composition and surface morphology, provides essential insights into its suitability for various thermochemical conversion technologies.
2 Thermochemical Biomass Conversion Pathways
Building upon the feedstock properties discussed earlier, understanding the available thermochemical conversion routes is critical to selecting appropriate methods for transforming sawdust into valuable bio-products. Biomass can be transformed into a variety of beneficial products and energy sources, including heat, natural gas, biochar, bio-oil, and thermal and electrical energy, by employing a number of thermochemical processes [33,34]. Thermochemical conversion is essentially the thermal breakdown of organic biomass components to produce useful compounds. It is a successful alternative method of creating bioenergy that includes carefully regulated biomass oxidation or heating [32]. However, a variety of factors, including the type and quantity of biomass, its accessibility, the cost of the manufacturing process, options for the final products, and environmental concerns, influence the choice of conversion technique [33–35]. For example, the biomass feedstock should be low in moisture and in solid form. Likewise, using the wrong technology could lead to inefficiencies [36,37]. Common thermochemical technology categories often comprise combustion, hydrothermal liquefaction (HTL), torrefaction, gasification, and pyrolysis [32]. Among these, torrefaction and pyrolysis have received substantial attention due to their suitability for lignocellulosic feedstocks like sawdust. The next subsection explores torrefaction, particularly as a thermal pretreatment technique that enhances the efficiency of downstream processes such as pyrolysis.
Torrefaction serves as an essential first step in improving the thermal characteristics of biomass. It involves the drying of feedstock in an inert atmosphere, usually at temperatures between 200°C and 300°C. Torrefaction is characterized as light (200°C–235°C), mild (235°C–275°C), or severe (275°C–300°C) depending on the temperature range [34]. It may also be thought of as the gradual heating of biomass without the presence of oxygen. Hot vapors from the torrefaction process carry the volatile materials to the condenser for condensation. Torrefaction is a clean and promising thermochemical process frequently used as a thermal pretreatment for biomass resources [35]. Torrefaction has been shown to improve the efficiency of thermochemical conversion processes including gasification, liquefaction, and pyrolysis by eliminating moisture and partially breaking down the biomass [36]. Torrefaction has attracted significant attention because, in addition to the improved qualities of torrefied and densified biomass, the technologies associated with torrefaction are almost ready for commercialization. Compared to raw biomass, torrefied biomass exhibits several key advantages including enhanced hydrophobicity, improved grindability, increased higher heating value, reduced moisture content, and superior pelletizability stemming from structural changes like fiber degradation, reduced hemicellulose, and increased brittleness that facilitate densification and handling [37]. By increasing the proportion of carbon and lowering the oxygen level, torrefaction improves biomass’s heating value. Along with traces of aldehydes, ketones, ethers, esters, and alcohols, the condensed liquid product of torrefaction also contains water and carboxylic acids [38]. The non-condensable gases consist of Carbon Dioxide (CO2), Carbon Monoxide (CO), Methane (CH4) and traces of Hydrogen (H2). The torrefied solids left behind have properties similar to coal and hence can be used as green alternatives. One of the advantages of using torrefaction is that it does not emit any GHGs; instead, it fixes the carbon in the form of densified and dehydrated biomass for further thermochemical processing.
The torrefaction of biomass feedstocks, including cotton stalk, sugarcane bagasse, rice straw, rice husk, and oats, has been extensively studied by [39]. Under processing conditions involving temperatures of 250°C–300°C and residence times ranging from 15 to 60 min, significant changes in both the structural and chemical properties of the biomass were observed. Microwave drying, employed as a pretreatment, caused visible surface cracking in the biomass, facilitating the release of additional volatile compounds. This surface alteration also contributed to a reduction in crystallinity, enhancing the reactivity of the feedstocks. Such modifications are crucial for improving the efficiency of biomass utilization in thermochemical processes. The elemental analysis revealed that torrefied rice husk, sugarcane bagasse, and cotton stalk exhibited increased carbon content and heating values. These values were found to be comparable to those of bituminous coal, emphasizing the potential of these materials as high-energy-density fuels. In contrast, torrefied rice straw showed distinctive structural properties, with carbon exhibiting a high crystallinity of approximately 50%. Conversely, the carbon in rice husk and sugarcane bagasse predominantly displayed an amorphous structure. This variability in crystallinity across feedstocks indicates the influence of inherent material properties on the outcomes of torrefaction. These findings highlight the versatility and efficacy of torrefaction in enhancing biomass properties, making it a promising approach for converting agricultural residues into energy-dense, coal-like fuels.
Torrefied biomass is very compatible with dry pyrolysis, which prefers pre-dried feedstocks and usually takes place at 350°C–600°C in inert atmospheres. On the other hand, wet pyrolysis, also known as hydrothermal pyrolysis, processes biomass with a high moisture content without the requirement for drying because it works at pressures between 5 and 20 MPa and temperatures between 200°C and 350°C [40]. Torrefaction, a mild pyrolysis process for thermal pretreatment, aims to improve higher heating values and energy densities, reduce atomic O/C and H/C ratios, lower moisture content, enhance water resistance, increase grindability and reactivity, and achieve uniform properties. Dry torrefaction (DT) takes place between 200°C and 300°C in an oxygen-free atmosphere with low heating rates (usually less than 50°C/min). Often referred to as hydrothermal torre faction, wet torrefaction (WT) is a high-pressure (up to 4.6 MPa) thermal pretreatment procedure that is conducted in hot compressed water under inert circumstances, usually at temperatures between 180°C and 260°C [41]. WT has a number of benefits over DT. In order to achieve comparable solid yields, WT needs significantly lower temperatures and shorter holding times than DT. This leads to higher energy yields, a higher heating value and enhanced hydrophobicity. Consequently, when it comes to biomass energy densification and conservation, WT outperforms DT at this point. Furthermore, WT has a lot of promise as an affordable method of turning cheap, low-quality biomass materials into cleaner biomass fuels. WT can effectively process biomass materials with high moisture content, even when they are very wet, a condition that typically poses significant challenges for dry torrefaction. Overall, torrefaction enhances the fuel properties and thermal stability of biomass, making it an effective pretreatment that significantly improves the efficiency and product quality of subsequent pyrolysis processes.
Following torrefaction, gasification presents a more advanced thermochemical route, particularly for producing clean syngas from sawdust and other lignocellulosic feedstocks. The thermochemical process known as “gasification” has the capacity to convert any carbonaceous material into syngas [42]. Gasification offers the ability to produce char and gaseous products (such as H2, CO, CO2, and CH4) from a range of feedstocks. Energy can be produced from syngas in a variety of ways, including heat, electricity, biofuel, biomethane, chemicals, and hydrogen [43]. Hydrothermal gasification can function at relatively moderate temperatures (400°C–700°C), but thermal gasification of coal and complex biomass requires high temperatures (800°C–1200°C). The primary gaseous products of gasification are accompanied by condensable liquids that are high in biochemicals and water. The gas can be cleaned and recovered quite easily because of the limited tar development caused by the higher temperature [42]. Furthermore, high gasification temperatures promote endothermic reactions such as water-gas shift, methanation, and steam reforming, which result in a quicker reaction rate and almost total breakdown of biomass into gases. By using Fischer-Tropsch catalysis, the syngas can be transformed into clean fuels and goods with additional value [44]. In order to create syngas, hydrothermal gasification (HTG) uses subcritical or supercritical water as a reaction medium. Below the critical temperature of water (374°C) and above the critical pressure of water (22.1 MPa), respectively, subcritical and supercritical water exist. HTG’s primary advantage is its ability to effectively convert biomass with high moisture content, which would otherwise need expensive and energy-intensive drying processes prior to gasification [45]. On the other hand, because the heat of moisture evaporation typically exceeds the heat of biomass combustion, converting wet biomass through pyrolysis, liquefaction, and gasification is typically inefficient. Applications for the HTG product include chemical synthesis, electricity generation, and hydrogen-producing fuel cells. Recent studies have explored the gasification of agricultural waste biomass, such as cotton stalks, corn stalks, and rice straw, using various catalysts such as Marley clay, calcium hydroxide, dolomite, and cement kiln dust were evaluated in a bench-scale fixed-bed reactor operating at temperatures ranging from 700°C–800°C. Among these, calcium hydroxide and cement kiln dust were found to be particularly effective in increasing gas yields while significantly reducing tar and char formation [46]. While gasification is effective for generating gaseous fuels, it is less suitable for solid or liquid fuel production. The next section explores combustion a simpler, yet highly established thermochemical process widely used for direct heat and power generation.
In contrast to gasification, combustion relies on complete oxidation of biomass to generate thermal energy and is commonly employed for large-scale heating and electricity production. The combustion process consists of an exothermic series of processes. When chemical bonds break, the energy they contain is released. In the majority of industries and power plants, this energy is used to generate the steam required for turbines, which in turn generate heat and electricity. When it comes to alternative fuels like biomass, combustion is defined as the burning of organic materials. Wood is the most commonly used fuel for burning biomass [47]. Biomass combustion systems come in a variety of sizes, ranging from small domestic units of just a few kilowatts to large power plants with capacities up to hundreds of megawatts. These systems can provide heat and power with relatively high efficiency and at reasonable costs, with large-scale applications being particularly significant for the cost-effectiveness of steam plants [48]. Generally, there are two types of biomass combustion models: macroscopic and microscopic. The macroscopic characteristics of biomass specifically, its moisture content, heating value, density, particle size, and ash fusion temperature are given for macroscopic study. Among the characteristics that can be investigated under a microscope are thermal, chemical kinetics, and mineral data [49]. The interplay between fuel, energy, and ambient elements can be viewed as the three fundamental combustion operations. Biomass fuel burns in the boiler to produce flammable vapors that volatilize and burn like flames. With more air present, the residual material which is still a carbon char will subsequently catch fire. The heat produced during combustion can be utilized as a source for other conversion procedures that produce electrical energy, albeit this again depends on a number of other factors [50]. Although combustion is well established and cost-efficient, it is primarily limited to heat and power production. Hydrothermal liquefaction (HTL) emerges as an alternative that offers the potential to produce liquid biofuels, particularly from wet biomass feedstocks.
2.4 Hydrothermal Liquefaction (HTL)
Following combustion, which mainly produces heat and electricity through complete oxidation, hydrothermal liquefaction (HTL) presents a more advanced approach for converting wet biomass into energy-dense liquid fuels. HTL thermochemically converts biomass into liquid biocrude using sub- or supercritical water under elevated temperature and pressure. Typical operating conditions range between 523 and 647 K and 4 to 22 MPa, depending on feedstock and reactor design [51]. This method enables any energy-intensive drying process to directly convert wet biomass material into liquid fuels [52]. Additionally, the resultant oil has a significant calorific value. Water serves as a solvent in this process and has several benefits, including improved biomass solubility, a low dielectric constant and viscosity, and support for acid-base reactions. Through decarboxylation and dehydration processes, some of the oxygen in biomass is eliminated. Carbon dioxide, carbon monoxide, and water are the products of these reactions. To produce biofuels, biomass is subjected to a number of chemical reactions using hydrothermal processing technologies. These procedures include gasification, reforming, condensation, depolymerization, hydrolysis, and pyrolysis.
Sivabalan et al. [53] investigated the use of high moisture content waste from the tobacco processing sector for HTL in the manufacture of bio crude oil. The study investigated on optimized effects of operating circumstances to produce the most liquid bio crude oil possible. For HTL operating conditions, 280°C–340°C temperatures and 15–45 min residence times were considered, with a fixed biomass to deionized water ratio of 1:3. The yield of liquid bio crude oil at 310°C and 15 min was more than 52% w/w, which was the maximum. Additionally, the HTL of oak wood was investigated by [54] in order to create premium bio crude. They investigated the effects of using iron (Fe), zinc (Zn), and other metals as hydrogen generators and nickel (Ni) and cobalt (Co) as hydrodeoxygenation catalysts on the quality of bio crude. Supercritical water oxidizes Fe and Zn to produce active hydrogen. When Ni and Co are present, this hydrogen is subsequently used to power hydrodeoxygenation activities and stabilize biomass pieces during the process. The results show that bio crude yields are significantly impacted by the deployment of hydrogen generators. In summary, HTL offers a promising route for converting wet biomass into high-quality liquid bio-crude without the need for drying, making it energy-efficient for moisture-rich feedstocks. However, its reliance on high-pressure systems, complex reactor design, and catalyst requirements may limit its scalability and economic feasibility in certain contexts. The next section explores pyrolysis, a versatile thermochemical process that operates at atmospheric pressure and accommodates a wide variety of biomass types, making it more practical for scalable bio-oil and biochar production.
Among thermochemical conversion methods, pyrolysis stands out as a flexible and efficient process for converting lignocellulosic biomass into valuable energy products such as bio-oil, biochar, and syngas. It involves thermal decomposition of biomass in the absence or near absence of oxygen, offering significant potential for bioenergy and material applications. Pyrolysis is a process that converts biomass into energy by heating (not burning) at a high temperature and certain pressure with little or no oxygen. The most common byproduct of pyrolysis is charcoal, which is used extensively in metallurgical operations. Other by-products of pyrolysis include liquids (water, tar, and oil) and gases (methane, hydrogen, and carbon monoxide) [55]. Pyrolysis is a versatile and efficient process that turns solid biomass into a liquid that is easy to transport and store, enabling the efficient production of heat, power, and chemicals. But before pyrolysis, drying is essential for high moisture waste biomass, such as sludge and waste from meat production [56]. It is a complex process that pyrolyzes biomass using both sequential and simultaneous processes. When biomass is heated in an inert atmosphere, the components begin to thermally decompose at 350 to 550 degrees Celsius, which speeds up to 700°C–800°C when air is not present. Long chains of carbon, hydrogen, and oxygen compounds break down into smaller molecules during pyrolysis as a result of the decomposition of biomass, producing gases and condensable vapors such as tars, oils, and solid charcoal. Depending on the pyrolysis process conditions, each of these components decomposes differently and to varying degrees [7]. Additionally, pyrolysis is further divided into many categories, including fast pyrolysis, catalytic pyrolysis, fast pyrolysis, and slow pyrolysis.
The low molecular weight lignin produced during pyrolysis exhibits significant physicochemical complexity, including high oxygen content, thermal instability, and molecular heterogeneity. As a result, detailed characterization of pyrolysis products has become a key focus to better understand and manage this complexity. The pyrolysis process occurs in two stages: primary pyrolysis, involving depolymerization, fragmentation, and char formation; and secondary pyrolysis, where further cracking and condensation reactions take place. Due to the poor physical and chemical properties of raw bio-oil such as acidity, viscosity, and low heating value upgrading is essential before it can be commercially utilized [57]. An immense diversity of pyrolysis processes, including flash pyrolysis, catalytic pyrolysis, slow pyrolysis, quick pyrolysis, can be employed depending on the operational parameters, each offering distinct product yields and characteristics. In summary, Pyrolysis is a key thermochemical pathway for converting biomass into bio-oil, biochar, and syngas. Its adaptability through fast, slow, flash, catalytic, or microwave-assisted modes allows for customized product yields. While raw bio-oil often needs upgrading due to its instability, advancements in catalysts and reactor design are enhancing its viability, making pyrolysis a leading option for sustainable biomass valorization.
Fast pyrolysis is the rapid heating of biomass at a high temperature in an oxygen-deficient atmosphere. When biomass undergoes thermal decomposition, it releases vapors, aerosols, and solid residues such as char. During the cooling and condensation stages, condensable vapors and aerosols form a dark brown bio-oil that typically has a high heating value due to its rich organic content. The char remains as a solid by-product. Depending on the feedstock and conditions, fast pyrolysis typically yields 60–70 wt % liquid products (bio-oil), 15–30 wt % solid products (biochar), and 10–20 wt % non-condensable gases [58]. Fast pyrolysis is an effective conversion technique because of four key features: it uses high heating rates, controlled temperature conditions (typically between 430°C and 500°C), a very short vapor residence time (less than 2 s), and rapid cooling of vapors and aerosols to produce bio-oil. In addition, this method uses less energy and is less costly than alternative approaches [59].
Other researches in the literature also address the integration of fast pyrolysis with other processes within the setting of a bio refinery. For example, to assess product yields and energy efficiency, Jafri et al. [58] examined six commercial methods for biofuel generation from forest leftovers. Most biofuel manufacturing paths involve integrating it with a pulp mill or a crude oil refinery. Thermochemical conversion processes included gasification, fast pyrolysis, and lignin depolymerization. According to Afraz et al. [60], they studied the fast pyrolysis of sugarcane bagasse and the upgrading of bio-oil using nickel-based catalysts (Nickel on Silica-Ni/SiO2 and Chromium doped Nickel on silica-Ni–Cr/SiO2). The upgraded bio-oil showed water and oxygen contents that were 63.3% and 43.3% lower, respectively, than those of the raw bio-oil, and its higher heating value (HHV) was 63% higher than that of the bagasse [60]. The upgraded bio-oil’s carbon recovery from sugarcane bagasse was 41.9% when using Ni/SiO2 and 32.5% when using Ni–Cr/SiO2. Additionally, a study by [61] conducted an experimental investigation on the quick pyrolysis of Miscanthus in a fluidized bed reactor, and then hydrolyzed the bio-oil that was produced over a Palladium on Carbon (Pd/C) catalyst in a fixed bed reactor. The phenol, ketone, aldehyde, alcohol, and acid components of the photolytic bio-oil were found to be transformed into over 41% of the alkanes in the upgraded fuel, according to the results [62].
The literature has a number of studies that concentrate on environmental assessments in addition to technical and economic ones. Shemfe et al. [63] for example, assessed the greenhouse gas emissions resulting from the quick pyrolysis and bio-oil upgrading of Miscanthus in the manufacture of bio-hydrocarbons. Similarly, Ref. [64] carried out a Life Cycle Assessment (LCA) to assess the techno-economic and environmental aspects of producing renewable diesel through two methods: catalytic fast pyrolysis and fast pyrolysis followed by multiple catalytic hydro-treating and hydrocracking using U.S. forest residues [63]. A considerable amount of research has focused on the quick pyrolysis of biomass and upgrading of bio-oil to produce biofuel in recent years. This demonstrates the increasing attention and significance of this topic, given that the utilization of second-generation biofuels derived from lignocellulosic residues plays a major role in lowering greenhouse gas emissions and decarbonizing the transportation sector.
The method has been used for centuries, mostly in the manufacture of charcoal. As opposed to fast pyrolysis, slow conventional pyrolysis involves heating biomass to 500°C at a significantly slower rate, which allows the fumes produced to be continually removed [64]. As seen in Fig. 3, the production of char is particularly well-suited to the prolonged residence period and low temperature of slow pyrolysis. But because of its numerous drawbacks, this method is not suitable for producing high-quality bio-oil. Long-term residence times might cause original product fragmentation, which can negatively impact oil amount and quality. Additionally, the longer stay necessitates more energy [65]. A popular thermochemical method for turning lignocellulosic biomass into bio-oil is slow pyrolysis, which involves depolymerizing three-building biopolymers including cellulose, hemicellulose, and lignin using heat and an inert atmosphere. Slow pyrolysis is a less energy-intensive method of producing bio oil than other thermal processes like hydrothermal liquefaction [66]. Furthermore, following catalytic upgrading, bio-oil from slow pyrolysis may be combined with other transportation fuels.
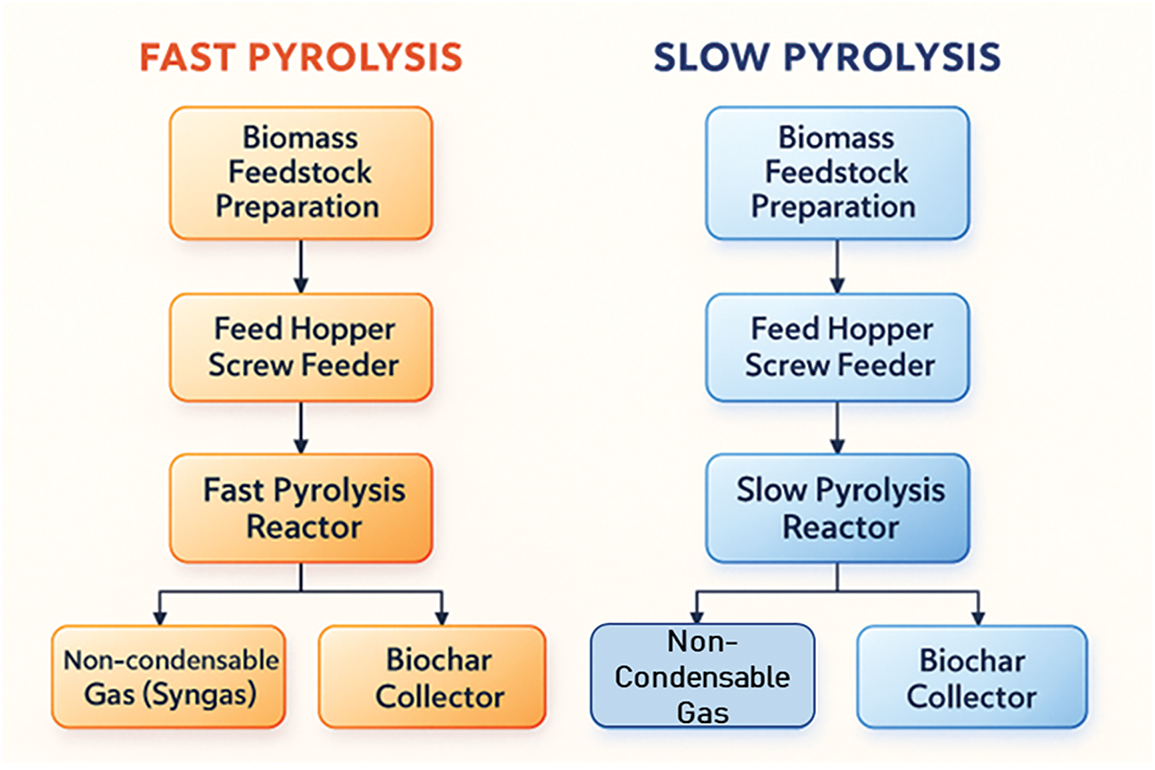
Figure 3: Fast and slow pyrolysis processes
The liquid bio-oil obtained from slow pyrolysis is dark brown in color and comprises several complex organic compounds such as alcohols, water, phenolics, ketones, esters, aldehydes, and carboxylic acids. Scholars have endeavored to synthesize pyrolysis bio-oil from diverse waste materials and assessed the properties of the bio-oil. About 42% bio-oil was created by the slow pyrolysis of linseed residue. This bio-oil was then analyzed using Fourier Transform Infrared Spectroscopy (FTIR) and Nuclear Magnetic Resonance (NMR) methods, which mostly revealed the aliphatic and aromatic groups [67]. Similarly, 45% bio-oil was produced by slow pyrolysis of Prunus Armeniaca seed waste, and column chromatography mostly revealed the existence of polar and aliphatic chemicals, which are soluble in methanol and n-hexane, respectively [68]. Experimental pyrolysis of Pongamia pinnata (karanja) de-oiled seed cake yielded appreciable bio-oil, with Gas Chromatography-Mass Spectrometry (GC–MS)/FTIR indicating mixtures of aliphatic and aromatic compounds alongside char and gas fractions [69]. The authors also looked into the slow pyrolysis of sawdust using a batch approach, and they found a bio-oil yield of roughly 45%. By using 1H and 13C NMR investigations, it was determined that the sawdust bio oil composition contained alkyl and aromatic groups [70]. Pyrolysis was carried out by Qing et al. [71] at different temperatures (400°C–700°C) on food waste that was collected from a food waste treatment factory in China. N-compounds contribute 40% (highest) of the total bio-oil at 500°C, according to the researchers’ GC-MS investigation, which showed that the production of bio-oil increases with temperature. A different study on food waste used leftover fruits and vegetables (banana, orange, onion, bell pepper peels, etc.) as feedstock for pyrolysis at different temperatures, times of reaction, and rates of heating in order to maximize the yield of biochar; the highest yields of biochar and bio-oil were found at 500°C and 300°C, respectively, and the bio-oil was enhanced with phenols, acids, and alcohols [72]. The comparison study conducted by Pascarella et al. [73] revealed that the middle level of bio-oil produced by slow pyrolysis of cherry seed biomass was 21 weight percent, whereas the high bio-oil yield of around 44% was obtained by fast pyrolysis of the same feedstock. It can be concluded from this that slow pyrolysis is essentially less effective than quick pyrolysis in terms of bio-oil yield and high levels of char generation. It follows that a change in the type of biomass or waste causes a noticeable variation in the composition of the bio-oil produced by slow pyrolysis.
This method typically differs from traditional and rapid pyrolysis in that it uses big biomass pieces that have a temperature range of 1100°C–1300°C, a heating rate of more than 1000 Ks−1, and a relatively brief residence time, or 0.5–1 s. In comparison to quick pyrolysis, this method is better at producing high-quality goods [74]. The rapid heating rate is connected with high temperature and short residence time result in high bio-oil yield with little generation of char. As can be seen in the schematic in Fig. 4, the main issue with flash pyrolysis is that the liquid products contain biochar, which catalyzes the polymerization events inside the bio-oil and increases the viscosity of the oil. The minimum feedstock size is required for flash pyrolysis due to its fast reaction time and rapid heating [64]. Flash pyrolysis, however, has inadequate temperature stability. Additionally, the char has a catalytic action that produces more viscous oil with solid residue occasionally, rendering it economically unfeasible due to the additional costs associated with upgrading to improve the quality [75].
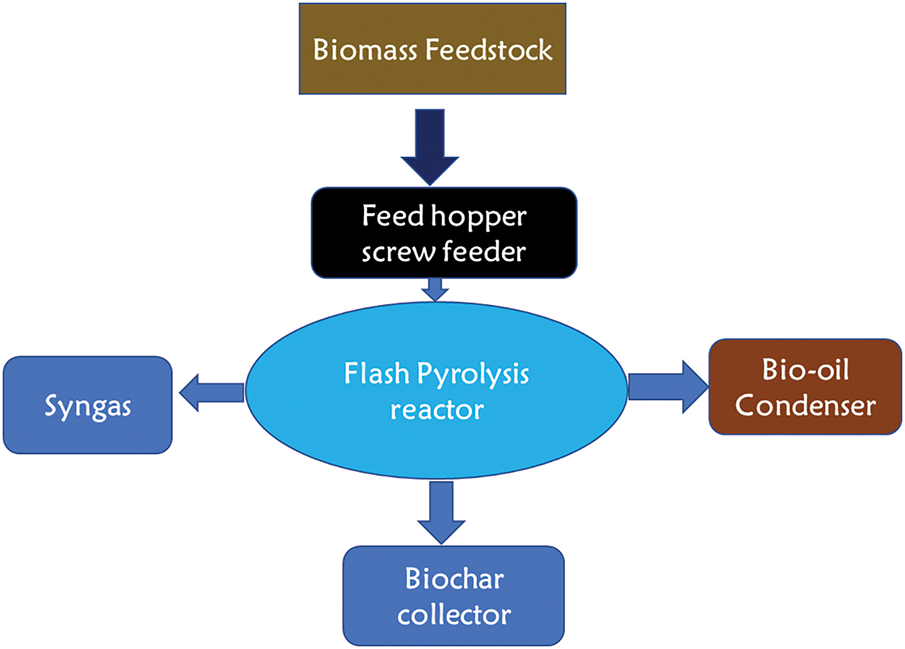
Figure 4: Flash pyrolysis process
The bio-oil derived via flash pyrolysis differs from traditional fossil oil derived from petroleum sources. In contrast to fossil fuel, bio-oil still has several drawbacks despite its many advantageous qualities. Because of its high concentration of oxygen-containing functional groups, the bio-oil has poor properties like high acidity and viscosity, non-volatility, thermal instability, lower energy density, and a tendency to polymerise in the presence of air. These characteristics depend on the raw material and the conditions of the pyrolysis process. Its heating value is about half that of conventional fossil fuel [76].
Catalytic pyrolysis is the most appropriate process for converting a variety of goods into premium liquid fuel since increased bio-oil quality and quantity are necessary for more effective fuel product utilization. The use of an effective catalyst is necessary to remove the reactive species because the liquid product created by fast py-rolysis contains more reactive oxygenated molecules. A catalyst added to rapid pyrolysis offers a greater chance of directly producing higher-quality hydrocarbons from biomass [77]. Fig. 5 shows the catalytic conversion of biomass feedstock exposed to pre-treatment process to ensure a separation between bio-oil and biochar. Numerous investigations have demonstrated that a synthetic catalyst may be used to effectively convert a variety of organic species into fuel-grade hydrocarbons. The unwanted characteristic of oxygenated molecules in bio-oil is their presence, which limits the applications of bio-oil. These must be removed in order to make the oil viable and economically appealing [78].
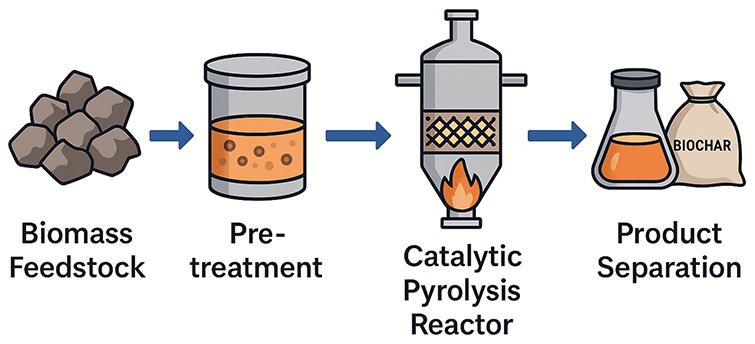
Figure 5: Catalytic pyrolysis process
Bio-oil frequently contains oxidized substances, such as lignin derivatives, which lower the fuel’s quality in a variety of ways. Its heating value can be lowered, stability can be increased, volatility can be decreased, and viscosity may be increased. The quality of the fuel would be increased by adding a catalyst to the biomass in the pyrolysis chamber prior to the start of the process. During catalytic pyrolysis, several reactions are coordinated, such as cracking, decarboxylation, aromatic side chain scission, dehydration, etc. Deoxygenation and the disintegration of bigger molecules into smaller ones are the outcomes of these processes. Changes in catalyst type, biomass type, processing parameters (e.g., temperature and time), and other variables can affect the resultant pyrolysates [79]. The amount of cellulose, hemicelluloses, and lignin in the biomass, together with some inorganic content, affect the characteristics of the bio-oil [80]. In addition to improving product quality, the catalyst lowers the activation energy and degradation temperature of the decomposition process. By efficiently lowering the oxygen concentration and raising the hydrogen to carbon ratio, catalysts improve the pyrolysis of biomass, which in turn raises the oil’s heating value and combustion efficiency. The principal factors influencing the quality and yield of pyrolysates are the biomass’s composition, reaction mechanism, and operating parameters, which include temperature, biomass particle size, type of catalyst, heating rate, and residence time [81].
Numerous studies have been conducted on the catalytic hydro treatment of bio-oils to upgrade them, using different types of catalysts and reaction conditions. For instance, Oh et al. [82] used a variety of metal catalysts (Ni/C, Ni/mesoporous silica(SBA-15), Ni/alumimium-modified silica(Al-SBA-15), Ni-Manganese(Mn)/SBA-15, platinum(Pt)/C) to experimentally assess the catalytic hydrodeoxygenation of bio-oil generated from fast pyrolysis of woody biomass. The properties of hydro treated bio-oil, such as its miscibility in gasoline and diesel, were significantly improved by adding an emulsifier to each blend. The pyrolysis process and yields were significantly influenced by a number of parameters, such as temperature, heating rate, residence time, biomass particle size, catalyst presence, etc. Table 1 provides a comparative overview of key thermochemical conversion methods summarizing their typical operating conditions, product yields, and primary applications in bioenergy production.
3 Pyrolysis Reactors for Effective Biomass Conversion
The pyrolysis process relies heavily on reactors. Many different reactor layouts have been attempted in experimental settings in an effort to create reactors that may be used in commercial settings [89]. The most popular designs are those that can achieve large rates of heat and mass transfer, including circulation fluidized bed (CFB) and bubbling fluidized bed (BFB) reactors. Other types of reactors include plasma reactors, melting containers, and autoclaves [90], rotary kilns [91], cyclonic reactors [92], and reactors using an ablative process [93]. It is crucial to remember that the type of particle interaction depends on the reactor setup and increases the complexity of the pyrolysis process [94]. Regarding techno-economic criteria, each reactor architecture has pros and cons. Numerous research into the techno-economics of these reactors have been published in the literature [95–99]. Reactors for pyrolysis can be categorized according to a variety of factors, including the way the heat is supplied, how they operate, and how the particles travel within the reactor. Reactor design for the pyrolysis process involves a number of factors, including reaction temperature, inert gas flow rate, vapor residence time, heating rate, and heating type. For biomass to be converted into the intended product, each of these factors is crucial. For example, inert gas prevents the oxidation of organic materials by acting as a shield. Additionally, it assists the reactor’s temperature to be distributed evenly and releases the volatiles [99]. In general, pyrolysis reactors fall into one of two types: those with mechanical charge transport or those with a pneumatically displaced bed. For the former, there are fixed bed, fluidized bed, and circulating fluidized bed reactors; for the latter, there are rotating cone, ablative, auger, and stirred reactors.
The most popular pyrolysis reactors are fluidized beds because of their many benefits, including superior temperature control, high heat transfer rates, ease of construction and operation, and the ability to scale up [100,101]. These reactors are typically used to investigate secondary tar cracking processes under long residence times and to analyze and comprehend the behavior of biomass during fast and flash pyrolysis [102]. These reactors are said to be suitable for pyrolyzing waste polymers [103], When considering other types of waste biomass, such as municipal solid waste (MSW), there are other challenges. First, the reactor’s feedstock needs to be easily fed in terms of size. Second, separating char from the bed material is very challenging [104]. Numerous researchers have tried to pyrolyze waste biomass, including lignocellulosic materials, both catalytically and non-catalytically using fluidized bed reactors [105–107], aquatic biomass [108,109], in addition to plastic garbage [110,111], tyre wastes [112,113] and MSW [114,115]. In most set ups, it requires the use of a rotameter and pump to ensure seamless movement of the biomass wastes (Fig. 6). Fluidized bed reactors are now the best choice for catalytic pyrolysis of waste biomass, according to data accessible in the literature. This is because the catalyst may be utilized again, whereas the cost of the catalyst could otherwise be a limiting factor [116].
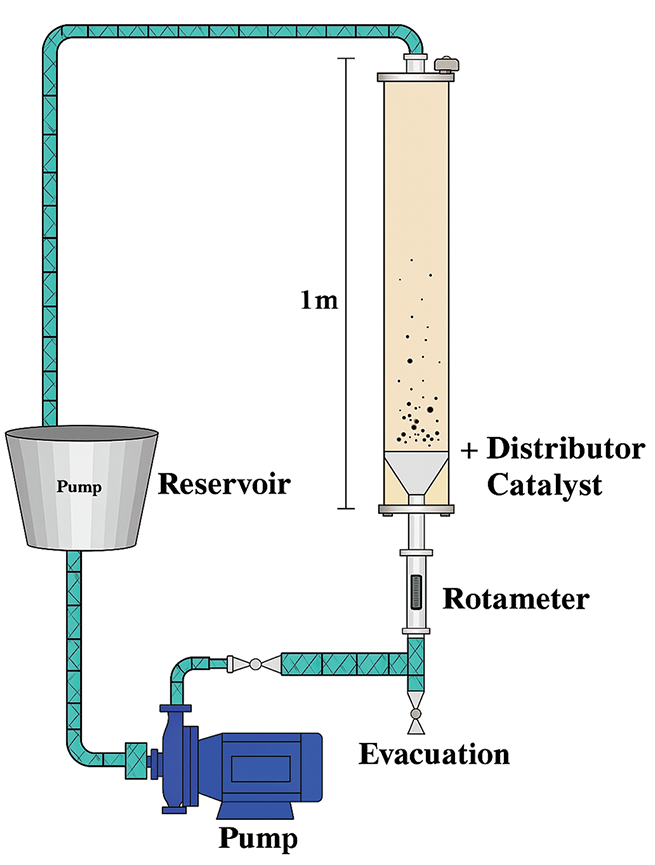
Figure 6: Fluidized bed reactor. Reprinted/adapted with permission from Reference [116] Copyright 2016, Elsevier
Furthermore, the reactor can be operated continuously, and circulating fluidized bed reactors are especially well-suited for large-scale operations from an economic standpoint. The pyrolysis of biomass and other wastes is commonly accomplished using two types of fluidized bed reactors: bubbling (BFB) and circulating (CFB) [95]. BFB are utilized for particles that are between 0.5 and 2 mm in size and yield a high liquid yield of 70 to 75 percent. However, fine char contamination of the liquid products can occur from this sort of reactor up to 15% of the weight, depending on the reactor’s design and the velocity of the fluidizing gas [117]. A significant distinction between BFB and CFB is the vapor residence time, which is normally 0.5–1 s for BFB and 2–3 s for CFB [118]. Fluidized-bed reactors are highly suitable for sawdust due to their excellent heat and mass transfer characteristics. They enable continuous operation, uniform temperature distribution, and higher bio-oil yields. Despite their complexity and need for more control systems, they are the most commercially viable for sawdust pyrolysis.
Fixed bed reactors are frequently chosen for lab-scale investigations due to their straightforward design and convenience of use. Large-scale fixed bed reactors haven’t yet shown their promise, while a lot of research is being done to find ways to distribute heat within the reactor uniformly [99,118]. Typically, a solid stationary bed is used for the reactions in a fixed bed reactor [119] and a thermocouple inside the bed regulates the amount of heat needed and at times a temperature controller is deployed (Fig. 7) for this purpose. This kind of reactor uses an inert gas to flush out the volatiles and keep the surroundings inert while receiving heat from an external source [110]. The fixed bed category has several variations, including two-stage fixed bed reactors, vertical fixed bed reactors, and horizontal fixed bed reactors [117,118]. Xiong et al. [120] investigated the formation of coke by pyrolyzing rice husks using a fixed bed system and varying the temperature between 300°C and 800°C. Despite their ease of design and operation, fixed bed reactors are known for their low heat transfer rate [121,122]. There are two ways to operate fixed bed reactors: batch mode and semi-batch mode. However, the results can vary greatly from batch to batch. These systems’ relatively low specific capacity restricts their use on a large scale, and they do not enable continuous operation [123]. Moreover, the need for a lengthy solid residency period and the challenges associated with eliminating char from the system can be considered additional significant drawbacks [122]. Fixed bed reactors can also be used as secondary pyrolysis reactors since they can readily receive the initial pyrolysis products, which typically consist of liquid and gaseous phases [124]. The fixed-bed reactor is easy to use, inexpensive, and ideal for sawdust pyrolysis on a modest scale. In contrast to more sophisticated designs, it has poor heat transfer and limited scalability, which lowers product yields. It is less suitable for continuous and effective bio-oil production due to its batch operation and uneven heating.
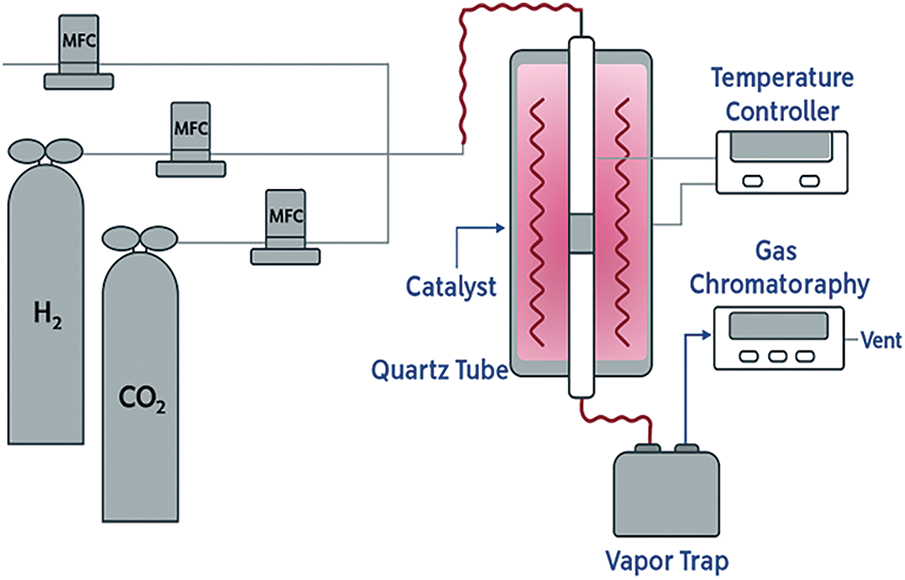
Figure 7: Fixed bed reactor. Reprinted/adapted with permission from Reference [123] Copyright 2020, Elsevier
The “National Renewable Energy Laboratory (NREL)” in Colorado, USA, invented the idea of ablative pyrolysis in the 1980s. Ablative pyrolysis operates on a very different principle than other pyrolysis methods. The process by which ablative technology works is comparable to that of melting butter on a hot skillet [95]. The biomass “melts” into leftover oil and evaporates when exposed to external pressure and heat from the reactor’s surface, creating pyrolytic vapors that can be collected via a cooling system (Fig. 8). The biomass’s surface exhibits a sharp temperature gradient, which causes a thin layer of reacting solids to form on the surface. Instead of occurring in the biomass itself, the reaction occurs at the surface layer in the ablative reactor, and heat transfer within the particle does not slow down the rate of reaction [125], however, the process is very dependent on the reactor’s surface temperature, the pressure that is delivered, and the contact between the biomass and the hot surface [126].
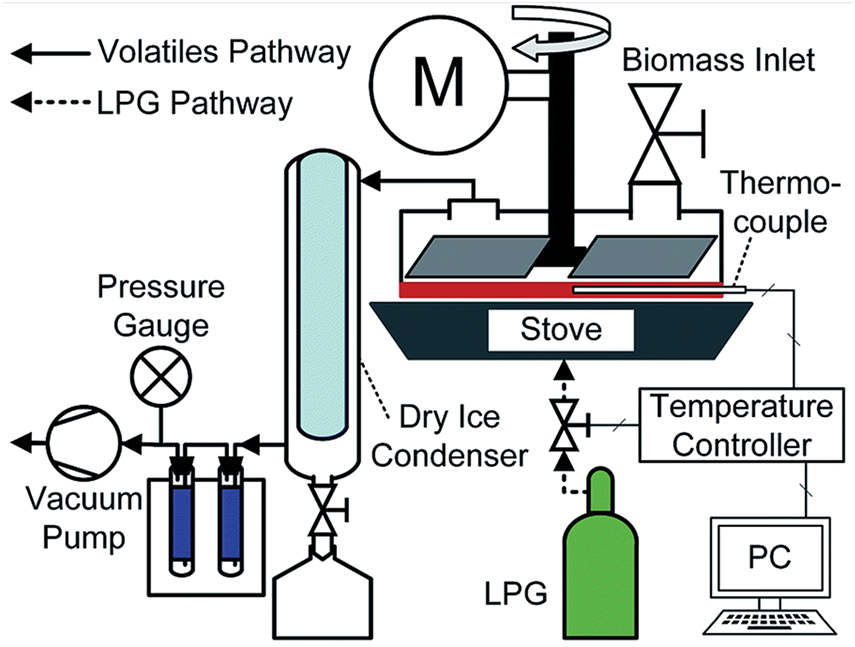
Figure 8: Ablative reactor. Reprinted/adapted with permission from Reference [126]. Copyright, 2020 The Royal Society of Chemistry
Biomass is typically pyrolyzed in an ablative reactor using one of two fundamental methods: direct exposure to focused radiation (radiant ablative pyrolysis of biomass, or RAPB) or contact with a hot surface (contact ablative pyrolysis of biomass, or CAPB) [127]. The cost of producing bio-oil is further increased by the fact that all pyrolysis reactors currently on the market require energy-intensive grinding procedures to reduce the biomass feedstock to microscopic particle sizes, often less than 1 mm. For example, about 7%–9% of the total costs associated with producing bio-oil come from the expense of grinding wood into tiny particles [128]. Although ablative reactors might not seem appealing from an energy standpoint, they could be useful for transportable applications, which could be crucial to lowering the cost of bio-oil [129]. Ablation reactors frequently have low mechanical reliability and heat loss to the environment since the hot plate is exposed to the surrounding air. Furthermore, a post-treatment procedure is required since the intermediate liquid product becomes contaminated due to the char that forms on the hot plate’s surface [126]. Ablative reactors lower pre-processing expenses by doing away with the necessity for extremely tiny sawdust particles. High heat transfer rates are possible with them, but maintaining surface contact necessitates mechanical complexity. They are less popular for commercial sawdust pyrolysis due to their specialized uses and constrained scalability.
Pyrolysis in an auger (screw) reactor operates on a straightforward concept in contrast to many other methods. The biomass is forced to undergo pyrolysis in a heated tube. To maintain an inert atmosphere inside the reactor and supply the pressure needed for the pyrolysis vapors to be transferred, the tube is flushed with an inert gas [118]. In industry, solid biomass products are frequently transported and processed using augers. In addition to supplying the reactor with feedstock, the screw also extracts solid leftovers from the reactor. Furthermore, a reactor with the right design can enhance the heat transfer between heat carriers and biomass particles [130]. To transfer the energy needed for pyrolysis, the reactor wall is heated to a temperature higher than the pyrolysis temperature. To improve heat transfer from the wall to the biomass particles, the screw mixes the biomass feedstock as needed. Theoretically, the auger speed and inert gas flow rate regulate the residence duration of the solid and gaseous intermediate products once the biomass fed to the reactor has been fully volatilized [131]. Currently, auger reactors come in two different layouts depending on how many screws are used. These include pyrolizers with a single auger and those with two or three augers. A twin-auger system is far more effective at mixing biomass and granular heat carriers than a single-auger reactor. Furthermore, the twin-auger reactor can guarantee the full devolatilization of feedstock while reducing the power needed to operate the augers. Similar to BFB reactors, twin-auger pyrolyzers produce large quantities of liquid products [132,133]. Fig. 9 shows a schematic of a typical auger reactor with the feed screw, fuel gas outlet and the rotary valves quite evident. Note that the desired end-products determine the choice of technology and operational parameters. The heat needed for the pyrolysis can be provided directly through heat carriers or indirectly through the reactor walls. It is possible to use the first method for slow or intermediate pyrolysis and the second method for rapid pyrolysis. It is possible to use inert solid materials as heat carriers, including ceramic balls, silicon carbide, steel shot, sand, etc. [133,134]. Additionally, recycled char is employed as a heat carrier to promote higher gas fractions, improve secondary processes, and prevent the production of tar [135,136].
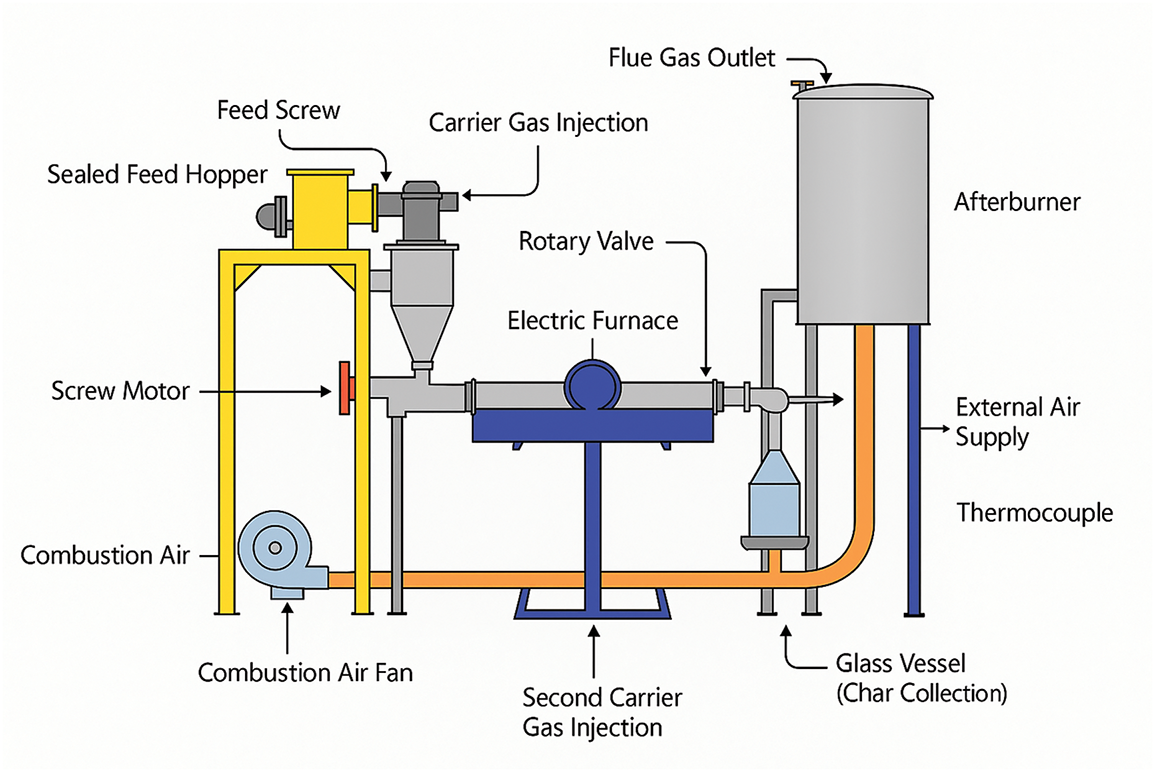
Figure 9: Auger’s reactor. Reprinted/adapted with permission from Reference [131]. Copyright 2019, Elsevier
Auger reactors are a viable option for dry sawdust because of their small size and controlled residence period. However, when feedstock is poorly prepared, they are prone to clogging and usually have lower heating rates. They are better suited for lab and pilot-scale settings due to their moderate performance.
In conclusion, fixed-bed and auger reactors, although simple and low-cost, suffer from limited heat transfer capacity and scaling challenges when processing fine biomass like sawdust, as seen in fixed-bed gasification of woody feedstocks [121] and auger reactor reviews [131]. Although they provide excellent heat transfer and lower grinding costs, ablative reactors are still less marketed and mechanically difficult. On the other hand, fluidized-bed reactors are the best choice for sawdust pyrolysis because they combine technical efficiency with commercial scalability. They do this by providing greater thermal performance, enhanced mixing, and continuous operation.
Several more established and planned reactor configurations have been extensively discussed in the literature in addition to the ones already mentioned. University of Twente created the rotating cone reactor (RCR) and Biomass Technology Group (BTG) later developed it [137] in the Netherlands in 1998. This reactor is relatively new and as efficient as a transport bed reactor, except instead of gas flow, centrifugal forces influence transport in a spinning cone. The main characteristics of the rotating cone reactor are (i) hot sand or other biomass and inert are forced up a rotating cone by centrifugation; (ii) char is gathered and burned in a fluidized bed to heat sand particles, which are then recalculated back into the reactor; and (iii) normally, 60%–70% of liquid yields on dry feed can be obtained. A more recent invention that works well for flash pyrolysis is the Pyros reactor. This technology can be used to turn wood biomass and biomass residues into high-quality, solid-free bio-oil [118]. This process is patented and involves pyrolysis in a cyclone where biomass is fed from the top and combined with a heated inert carrier, usually sand. The char is separated by centrifugal forces and gathered at the bottom of the cyclone, while the pyrolysis vapor is pulled off from the top.
Additionally, plasma-assisted pyrolysis is increasingly compelling because it delivers ultra-rapid heating rates and precise control over syngas composition. This results in cleaner gas with favorable heating value compared to conventional systems, which typically produce tar-laden syngas requiring downstream cleaning. Recent insights highlight plasma’s superior performance in achieving controlled syngas characteristics and reduced tar formation [138]. Utilizing a plasma reactor has the benefit of having a direct, independent heating source in the form of the plasma torch. This enables the use of various reactants and improves temperature control [139]. Furthermore, the high energy-density and temperature linked to thermal plasma and consequently fast reactions can overcome the possible issues with traditional pyrolysis. Combustible gases and manageable solid residues are produced by thermal plasma pyrolysis of waste biomass, including organic wastes [104]. According to a study on the plasma pyrolysis of polypropylene, the solid residue’s calorific value was nearly equal to that of elemental carbon, meaning that elemental carbon made up the majority of the residue. The authors also observed some unique carbon structures, which suggests the potential for the production of multiple high-value products [104]. The commercial growth of plasma technology has been hindered by technological challenges in many regions of the world. On the other hand, a recent review by Sikarwar et al. [140] highlighted the growing global popularity of plasma-assisted biomass treatment. The researchers attribute its increasing appeal to several advantages, including compact system design, versatility in processing various types of waste biomass, and its environmentally friendly nature.
Zeng et al. [141] considered a solar irradiation-based pyrolysis reactor to process beech wood, which presented an intriguing solution to the heating of a pyrolysis reactor The technology of solar biomass pyrolysis is dominated by fixed bed reactors, however a variety of reactor layouts have been investigated for solar pyrolysis. Both vertical and horizontal orientations are possible for reactors used in solar pyrolysis [142]. It’s crucial to remember that the majority of the study in this field has only been done at the laboratory level. The largest technological obstacle to solar pyrolysis techniques is still reactor operation and scaling up. To achieve optimal conversion of the feedstock, a reactor with improved hydrodynamics for reactants moving through the focus zone is required [143]. In general, the unique circumstances involved in solar pyrolysis make the gas-solid reactor types that are frequently considered in chemical engineering unsuitable. Since solar pyrolysis reactors will probably differ significantly from conventional reactor designs in the future, new concepts must be developed [144]. To better illustrate the influence of reactor design on pyrolysis performance, a detailed comparison of commonly used reactor types is presented in Table 2.
Reaction Conditions in Other Thermochemical Processes
Although a lot of research has been done on the design of pyrolysis reactors, it is equally crucial to comprehend how operating conditions impact other thermochemical processes like gasification and hydrothermal liquefaction (HTL). In gasification, syngas composition and process efficiency are greatly impacted by operating factors like temperature, equivalency ratio (ER), and residence duration. An ER of 0.2 to 0.3 maximizes the yield of combustible gas and reduces the generation of tar, while raising the temperature from 400°C to 1000°C can increase biomass conversion from 46% to 82%, according to open-access studies. The choice of the gasifying agent (air, steam, or O2) further influences the syngas output; for example, mixes of oxygen and steam at about 800°C produce syngas with over 90% carbon conversion and H2 volume fractions above 43% [152]. Similarly, HTL uses sub- or supercritical water conditions to transform wet biomass into biocrude by using high temperature (250°C–374°C) and pressure (5–25 MPa). While working in this range improves hydrolysis and bio-crude yields, higher temperatures or pressures might cause secondary oil splitting into gas and char. Further studies indicate that prolonging residence time and keeping water and temperature constant maximizes the generation of biocrude, but too lengthy exposures or too high temperatures can reduce yield because of repolymerization [40]. These results collectively demonstrate that, similar to pyrolysis, precise control of temperature, pressure, ER, and residence time is essential for optimizing product quality, yield, and energy efficiency in gasification and HTL. Such information guarantees a thorough and impartial examination of all thermochemical pathways.
3.6 Advanced Pyrolysis Technologies
The goal of recent developments in pyrolysis technologies is to increase the yield, quality, and efficiency of pyrolysis products, supporting waste management and sustainable energy production. The most cutting-edge biomass pyrolysis technologies, such as co-pyrolysis, catalytic pyrolysis, microwave pyrolysis, hydrothermal pyrolysis, and plasma pyrolysis, are thoroughly examined in this section. Every technology is analyzed in terms of its workings, benefits, and possible uses.
Co-pyrolysis refers to the simultaneous pyrolysis of biomass with other feedstocks like plastics or coal. This process often enhances bio-oil yield and quality via synergistic thermal cracking and hydrogen transfer from co-reactants (e.g., plastics) that help suppress char formation and oxygenated byproducts [153]. This process improves the quantity and quality of the pyrolysis products without requiring the costly addition of catalyst, solvent, or significant hydrogen. For example, co-pyrolysis with polymers can boost liquid hydrocarbon output, increasing the process’s economic viability. Co-pyrolysis would make it possible to produce stable pyrolysis bio-oils from multiple feeds, something that is frequently impossible to accomplish independently. For instance, oils made from plastic fractions cannot be completely mixed with those made from biomass pyrolysis, which eventually results in the formation of several phases. Co-pyrolysis’s synergistic effects can result in better product dispersion and thermal degradation. According to studies, co-pyrolysis can greatly increase the bio-oil’s calorific value and lower its oxygen content, increasing its stability and energy density. Bio-oil’s high oxygen concentration makes it difficult to utilize as a fossil fuel substitute since it lowers its calorific value and increases instability and corrosion problems [154]. Co-pyrolysis requires intricate interactions between the co-reactant and the feedstock. For instance, the presence of plastics may contribute to the deoxygenation of bio-oil by supplying more hydrogen, increasing the hydrocarbon yields. The efficiency and product distribution of co-pyrolysis are greatly influenced by the process parameters, which include temperature, heating rate, and the ratio of biomass to co-reactant. Co-pyrolysis is also a cost-effective way to upgrade biomass pyrolysis systems because it can be readily implemented into already-existing pyrolysis units with little modification.
Catalysts are used in catalytic pyrolysis to increase the output and quality of bio-oil and gas. Biomass contains metallic minerals that can act as natural catalysts to change the pyrolysis products. Certain catalysts, like metal oxides, zeolites, and mesoporous materials, can be used to lower the amount of oxygen in bio-oil, producing fuel of higher quality. The selective synthesis of useful compounds is another benefit of this technique. Catalysts can improve the pyrolysis process’s deoxygenation, cracking, and reforming reactions, producing hydrocarbons and other useful compounds [155]. Both in situ and ex situ catalytic therapy are possible. There are various methods of implementation, even if we only consider the in situ alternative. In the reactor where the biomass is pyrolyzed, the catalyst can be added straight. This choice streamlines the catalytic pyrolysis procedure and may considerably lower the temperature at which the biomass components decompose [155]. The drawback is that it complicates the process of separating the catalyst from the leftover pyrolysis solid and, consequently, its subsequent reuse. Furthermore, the pyrolysis reactor’s operating parameters must fall halfway between those that are most appropriate for catalysis and pyrolysis.
In case the catalytic treatment is done online in a second stage [156], only the volatiles produced by the pyrolysis process would undergo treatment. As a result, the catalyst will be able to be used for longer. It is possible to pyrolyze several biomass batches while keeping the same catalytic bed. Furthermore, based on the kind of catalyst being used, the kind of solid that is wanted, the type of liquid, and the process’s target gas, the operating parameters for both pyrolysis and volatile treatment can be optimized independently. Actually, the yields and caliber of the pyrolysis products produced depend heavily on the catalyst and operation parameters used [157]. Catalytic pyrolysis has advanced recently, with an emphasis on creating new catalysts with excellent stability, selectivity, and activity. For example, the quantity and quality of bio-oil have been proven to be improved by modified natural zeolites. Acid and heat activation procedures can be used to increase these materials’ catalytic activity even more. Particularly, the acid’s strength, type, and number of sites all have a significant impact on the catalytic activity of acid catalysts [158]. ZSM-5 zeolite has been used extensively, either exactly as supplied or modified, to produce bio-oils that contain higher aromatic and olefin molecules [159,160].
The quality of the bio-oil can be further improved by combining catalytic pyrolysis with other procedures, like hydrodeoxygenation (HDO), which qualifies it for use as a fuel for vehicles. High coke output, stability, catalyst deactivation, and regeneration are still major obstacles, though. The primary deactivation mechanism of zeolites that shortens their lifespan is coke formation. In the event that a fixed bed reactor the most straightforward and adaptable kind of catalytic reactor is employed, this coke may also raise the pressure drop across the bed [161]. These days, there are technical ways to counteract the quick deactivation caused by coke, like the ongoing regeneration of catalysts in the oil refining process known as Fluidized Catalytic Cracking (FCC). The production of coke, which quickly deactivates the catalyst, makes this regeneration a necessary step. However, these systems are expensive and complicated to build, operate, and maintain. Building on recent progress in catalyst design and performance evaluation, it is essential to compare how various catalyst types influence the yield, composition, and quality of bio-oil and biochar. Table 3 summarizes key findings from recent studies, focusing on catalysts commonly used in the catalytic pyrolysis of sawdust and other lignocellulosic feedstocks.
The comparison study shows that the kind of catalyst employed significantly affects the physicochemical characteristics of biochar as well as the yield and composition of bio-oil. CaO and other basic oxides greatly improve deoxygenation and raise the heating value of bio-oil. While metal-doped versions (like Fe/HZSM-5) enhance performance through synergistic effects, zeolitic catalysts like HZSM-5 encourage the synthesis of aromatic hydrocarbons with good selectivity. In particular, urea and other carbon-based and nitrogen-modified catalysts show great promise for enhancing the properties of biochar by providing functionalized surfaces and possible repurposing in energy storage or environmental remediation. Consequently, the catalyst selection should be in line with the intended result, be it optimizing fuel-grade bio-oil, improving biochar for cutting-edge uses, or striking a balance between the two under economical and environmentally friendly processing circumstances.
In microwave pyrolysis, the biomass is heated using microwave radiation. Numerous benefits of this approach include homogeneous temperature distribution, quick heating, and increased energy efficiency. Using less energy than traditional procedures, microwave pyrolysis can yield high-quality biochar and bio-oil. The biomass and microwave energy interact directly in the microwave heating mechanism, producing uniform and effective heating. There are two possible outcomes from microwave dielectric heating: thermal and non-thermal. Different temperature regimes produced by microwave heating are the cause of thermal effects. Situations when the outcome of a synthesis carried out under microwave settings deviates from the outcome of conventional heating at the same temperature are referred to as non-thermal effects (e.g., changes in reactivity and selectivity or acceleration). Nonetheless, the existence of certain (non-thermal) effects of microwaves is currently a contentious scientific topic [166,167]. When employing microwaves as a heating medium to pyrolyze biomass, the operating parameters are absolutely crucial. Both the nature of the biomass and the energy transfer that is accomplished will depend heavily on the size of the particles. There may need to be minimal moisture content because water is frequently the primary component of the biomass that absorbs microwaves. By introducing heating aids (microwave adsorbers), this deficiency in moisture can be addressed [168,169]. The lignocellulosic biomass components exhibit distinct breakdown under microwave-assisted heating and traditional electrical heating due to their disparate heating properties [170]. For example, inadequate absorption of microwave irradiation would limit microwave pyrolysis if microalgae biomass were utilized in place of cellulosic biomass [171]. In order to get around this restriction, researchers have combined algae with sorbents including coals, metal oxides, and activated carbons to produce high temperatures [172,173]. This technique has demonstrated the ability to scale up and integrate with other processes to improve the conversion of biomass.
Plasma pyrolysis efficiently converts biomass into syngas and charcoal by using plasma arcs to create extraordinarily high temperatures [174]. This process is quite effective and can process a variety of biomass feedstocks, even ones with a lot of moisture. Reactive species produced by the plasma arc speed up and complete the breakdown of biomass. High-purity syngas generation and waste-to-energy applications have both showed potential with plasma pyrolysis. Plasma pyrolysis works by creating a plasma arc, which raises the temperature to over 10,000°C and produces a highly reactive environment. Because of this high temperature, biomass can be completely broken down into its component parts, mainly carbon dioxide, hydrogen, and carbon monoxide [139,175]. Even with high moisture content, the plasma arc’s high energy density guarantees effective biomass conversion. Systems for plasma pyrolysis can be integrated with other waste treatment procedures and are generally small, which makes them appropriate for decentralized waste management.
4 Influence of Operating Conditions on Sawdust Pyrolysis
Temperature plays a crucial role in biomass pyrolysis by supplying the heat required to break the many different types of connections found in lignocellulosic biomass. Temperature is therefore one of the most crucial factors since it has a direct impact on the distribution of products, the extension of biomass decomposition, and calorific values [176]. Numerous published researches examining the impact of temperature on the ultimate yield of bio-oil have demonstrated that higher yields can only be achieved at temperatures between 450°C and 550°C. However, the feedstock and other operational conditions also have a significant impact on the ultimate bio-oil outputs [177–179]. Sohaib et al. [180] examined how temperature affected the rapid pyrolysis of sugarcane bagasse. At 500°C, the authors reported a bio-oil production of 60.4%, although modest outputs of gas and charcoal were attained, Lin and Chen found that when sugarcane bagasse was treated to pyrolysis at 500°C, identical bio-oil yields (55%) were obtained after 30 min of reaction time [181]. Furthermore, a study by [182] found that when rice husks were pyrolyzed at a temperature of 400°C to 500°C, the output of bio-oil increased from 11.3% to 35.9%.
Temperature affects not just the yield of bio-oil but also the distribution of the product overall. Excellent indicators for assessing the quality of the finished product are the levels of carbon and hydrogen. Therefore, the composition of high-quality bio-oil should be high in carbon and hydrogen, and as low in oxygen as feasible [182]. Additionally, according to [183,184] examined the effects of different pyrolysis temperatures on food waste and food waste solid digestate, finding that higher temperatures encourage the reduction of oxygen content while increasing carbon and hydrogen content. This is a significant finding because it appears that higher temperatures cause the hydrocarbons in the biomass to crack, which lowers the oxygen content in the bio-oil, which is expected and very desirable.
Another study that looked at the effect of temperature on the distribution of the final product was Alvarez et al. [185]. They found that when lignin broke down at 350°C, phenols (such as catechols, guaiacols, and alkyl-phenols) were produced when the temperature at which rice husks were pyrolyzed was raised. Temperatures above 600°C were also associated with a decrease in the concentration of ketones (including cyclopentenones and open-chain ketones) due to the condensation processes of the feedstock carbohydrates. Higher pyrolysis temperatures (>600°C) promote gasification reactions because they lead to the creation of lighter compounds, which in turn promotes cracking events. Interestingly, it appeared that temperature had no effect on the concentration of ethers, aldehydes, or water.
The heating rate is another important factor in biomass pyrolysis since it affects the kinds of breakdown products that are produced. Increased heating rates have the potential to quickly fracture biomass and encourage the production of volatiles [186]. The impact of varying heating rates on the ultimate yields of bio-oil has been extensively documented in scholarly works [182]. Furthermore, Sensöz et al. [187] used a fixed bed reactor to study various heating rates in the pyrolysis of olive bagasse. The scientists found that a paltry 8.4% increase in bio-oil yield resulted from raising the heating rate from 10 to 50 °C/min. Another study by the same team found that the heating rate had no effect on the bio-oil output from hornbeam shell pyrolysis [188]. It is important to note that raising the heating rates from 10 to 50 °C/min might not have a substantial enough effect on the yield of the finished product. It is therefore advised to use higher heating rate ranges, such as 10 to 100–300 °C/min, to get around any or both of the mass transfer and heat constraints. Raising the heating rates won’t produce any more gains in bio-oil yields when the mass transfer and heat constraints are overcome. For instance, Bhoi et al. [189] found increases in bio-oil yield when using a faster residence period (<2 s) and a greater heating rate (>1000 °C/s). Additionally, Xiong et al. [190] found that the final bio-oil yield from rice husk increased by 10% when heating at a high rate of 200 °C/s as opposed to medium (≉20 °C/s) and slow (≈0.33 °C/s) heating rates. It is important to note that the final bio-oil yields are impacted by a variety of factors, including temperature, reactor configuration, and biomass type, composition, and particle size, in addition to the combined effects of these variables. Furthermore, heating rates can affect the ideal temperature to optimize the yields of bio-oil in the end.
Catalytic pyrolysis is a well-known technology for the thermal conversion of biomass into chemicals and fuels by selectively favoring decarboxylation reactions. The bio-oils with better stability and fewer oxygenated components (e.g., acids, ketones, and carbonyl compounds) and water content are desirable for direct use or additional processing of the bio-oil [191–193]. Catalyst development for biomass pyrolysis often involves controlling the formation of catalyst particle size and optimizing features including acidity, basicity, porosity, and metal-support interactions [194,195]. Another key feature to consider is their thermal stability, such that new catalysts are robust enough to resist deactivation while being easy to regenerate [196]. Zeolites and other microporous acidic catalysts are known to catalyze the pyrolysis process, which converts heavier oxygenates into lighter ones by cleaving the carbon-carbon bond in biomass. Furthermore, the quantity of aromatic compounds in the bio-oils produced by catalytic pyrolysis of biomass using zeolites has increased. Numerous research groups have looked into acidic zeolite catalysts, with Zeolite Secondary Mobil-5 (ZSM-5) being the most studied zeolite for pyrolytic bio-oil upgrading [197]. Ref. [198] studied the catalytic pyrolysis performance on pine wood and reported that differing proton forms of zeolite catalysts Hydrogen Beta Zeolite ratio 25 (H-Beta-25), Hydrogen-form Y Zeolite (H-Y-12), Hydrogen-form Zeolite Socony Mobil-5 (H-ZSM-5), and Hydrogen-form Mordenite (H-MOR-20) resulted in variable bio-oil yields. H-ZSM-5-23 zeolite resulted in a bio-oil yield of 20.7 wt %, while 15.1, 9.0, and 17.6 wt % were achieved for H-Beta-25, H-Y-12, and H-MOR-20, respectively. In addition, compared to other catalysts, ZSM-5 caused a greater synthesis of phenols and ketones and a decreased amount of acids and alcohols in the bio-oil. Furthermore, Ref. [199] examined the effects of several zeolite structures, such as ZSM-5, H-Y, and Ultrastable Y-type Zeolite (USY), on maize stalk pyrolysis. It was discovered that ZSM-5 produced the best yields of bio-oil. Interestingly, they also observed that the H-Y zeolite produced a larger content of aliphatic hydrocarbons, whereas USY produced a higher concentration of aromatics.
In addition to the benefits of using zeolite catalysts such as selectivity and deoxygenation two significant drawbacks are rapid deactivation due to coke deposition and increased gasification/water formation. For instance, HZSM-5 catalysts in plastic pyrolysis suffer from fast deactivation due to “soft” coke formation, which requires thermal or oxidative regeneration to restore activity [200]. As a result, larger pore-sized catalysts have been studied in comparison to zeolite materials because they are thought to promote the larger bio-oil molecules’ diffusion, reactivity, and extraction from the catalyst matrix while reducing the possibility of coke buildup in the pores. Apart from acidic microporous and mesoporous materials, the literature has extensively examined solid basic catalysts, including Zinc Oxide (ZnO), Calcium Oxide (CaO) and Calcium-oxide-on-silica sand (CaO-sand/silica), Magnesium Oxide (MgO), and Iron(III) Oxide (Fe2O3), among others [201]. Zhang et al. [202] examined the impact of several metal oxides on the catalytic pyrolysis of poplar at 600°C. They found that CaO greatly enhanced the production of cyclopentanones, hydrocarbons, and light compounds while considerably reducing the amounts of phenol and anhydrosugar in the bio-oils. Additionally, Ref. [201] evaluated the effects of several kinds of catalysts on the catalytic pyrolysis of rice straw, including metal basic oxides, metal sites, acidic metals, and zeolite catalysts. When compared to basic (MgO), acidic Cerium(IV) Oxide (CeO2), and metal salt Magnesium Chloride (MgCl2) catalysts, Y-zeolite produced more phenolic monomers and yielded the maximum amount of bio-oil (55.2 weight percent).
The amount of time a substance remains in a medium or place is known as its length of stay, and it has a major impact on output yield. The term “vapor residence time” refers to how long it takes for the vaporized gas inside the reactor to evaporate [203]. The majority of significant outcomes, including biomass conversion, product production, and composition, are impacted by retention time. The best retention time is determined by a number of factors, primarily temperature, heating rate, and catalysts. In the end, a longer retention time is not necessary because using a low heating rate will take longer to achieve the required temperature and give more time for oil creation and biomass conversion. Similar to this, the presence of alkalis and the critical temperature zone speed up hydrolysis, which eliminates the need for a longer retention period. Numerous researches have been carried out to ascertain the impact of retention duration across a broad spectrum of biomass. Ref. [204] observed that the HTL of Cunninghamia lanceolate decreased in bio-oil with an increase in retention time. This was mostly because of condensation and the repolymerization of intermediates to create solid products as a result of the intermediates being exposed to high temperatures over an extended period of time. Other studies have shown that longer retention times are more beneficial for increasing yield, thereby supporting extended durations in contrast to the aforementioned results.
Particle size affects product yield; greater particle sizes result in lower gas yields and higher yields of tar and char because of their reduced surface area and increased thermal resistance [204]. When smaller particles undergo pyrolysis, the temperature remains constant throughout the particle, resulting in a complete conversion and increased production of liquids and gases. Nevertheless, larger particles stay in the solid phase because the interior of them heats more slowly and produces a greater mass of solids at lower temperatures. Smaller particles are greatly preferred for bigger amounts of bio-oils [204]. The ease of heat transport to the biomass components influences how easily the particles break down into distinct products. In their investigation into the impact of particle size on the flash pyrolysis of cotton shell, Ref. [205] reported a maximum bio-oil yield of 51.25 weight percent at a particle size of 1 mm and a constant sweep gas flow rate, with a corresponding decline in product yield at 1.25 mm particle size. This pattern can be linked to the biomass material’s resistance to mass and heat transfer, which is made easier by the greater distance between the material’s surface and center. This resistance makes it more difficult to break biomass bonds and produce higher product yields, in this case bio-oil. Consequently, there is a decrease in volatile matter and an increase in char formation [206]. From an alternative perspective, an adequately small particle size makes it easier for heat to be transferred uniformly to biomass materials, which maximizes the quantity of bio-oil produced [207] and significant non-condensable gaseous component production (a maximum gas yield of 40.03 weight percent was achieved at 0.6 mm) [205].
4.6 Feedstock Moisture Content
The amount of water in the biomass is known as its moisture content (MC), and it is essential to the thermochemical conversion process. The MC of native biomass ranges from 25% to 60%, which is deemed excessively high for pyrolysis [208]. High moisture content might affect the biomass’s energy content since the heat produced during burning is lost when the moisture evaporates [83]. Furthermore, an excessive amount of water in the bio-oil may be caused by high moisture content. For the biomass pyrolysis process, a moisture level of less than 10% is advised; any higher number produces bio-oil in two distinct phases, an aqueous phase and an oil phase [83]. Although the bio-oil’s high MC can increase stability, it can also lower the oil’s calorific value and viscosity [64]. The yields of hydrogen, on the other hand, increased when the moisture content increased; according to Hu et al. [209], the pyrolysis of biomass with 47.4% moisture content produced larger hydrogen yields than the pyrolysis of dry biomass with 7.9% moisture content, according to the scientists.
The type of pyrolysis technique used, however, determines how moisture affects the process. For example, moisture has a favorable impact on the products that are produced during microwave pyrolysis. During the drying stage, water has high microwave absorbability; therefore the MC in the feedstock makes sure the biomass rises in temperature quickly. But as the temperature rises, the feedstock loses moisture and becomes less microwave-absorptive, which also slows down the rate of temperature rise [210].
5 Characterization of Bio-Oil and Biochar Products
Bio-oil is a liquid fuel produced through the process of pyrolysis, which involves the thermal decomposition of organic materials in the absence of oxygen. This process breaks down complex organic molecules into smaller, simpler compounds, producing bio-oil [211]. Bio-oil is a viscous, dark brown liquid that smells strongly [212], and the type of feedstock and operating conditions determine its composition. A complex mixture of hundreds of different groups of organic chemicals, bio-oil mostly consists of acids, aldehydes, alcohols, ketones, esters, guaiacols, and phenols [213]. Bio-oil from pyrolysis has a number of unfavorable characteristics that restrict its application when compared to heavy petroleum fuel oil, including a high water and oxygen content, high ash content, a high viscosity, high corrosivity (acidity), and a poor heating value. Bio-oil has a moisture content of 15%–30% and an oxygen content of 35%–40% [79].
Numerous reactions, such as aromatization, coking, dehydration, dehydrogenation, hydrolysis and isomerization, and retro-condensation, are anticipated to take place during the creation of bio-oil. The precise composition of bio-oil is dependent on the following variables: (i) feedstock, (ii) heat transfer rate and final char temperature during pyrolysis, (iii) vapor dilution degree, residence time, and reactor temperature, (iv) the effectiveness of the char removal system, (v) feedstock water content, (vi) storage duration, (vii) storage temperature, and (viii) degree of contamination during storage [214]. To comprehend the composition of bio-oil, it is imperative to characterize it. Bio-oil is generally utilized as fuel because its low quality has so far prevented it from being used in diesel engines for extended periods of time. It is well acknowledged that before bio-oil can be used in diesel engines, it needs to be further upgraded [215]. Hydrotreating [216], hydrocracking [217], application of supercritical fluid [218], catalytic cracking [219], are some of the proven techniques for upgrading bio-oil [220].
Potential Application of Bio-oil
Bio-oil finds use in a variety of static applications, such as boilers, engines, turbines, and power generation furnaces, where it can be used in place of fuel oil or diesel. On the other hand, bio-oil can be used as a raw material to make a variety of chemicals and other useful goods, like resins of the phenol-formaldehyde type [221], adhesives [222]. Furthermore, after being upgraded, bio-oil can be utilized as a fuel for vehicles and to produce anhydro-sugars, such as levoglucosan, which may be used to make biopolymers and medications [223]. Numerous studies have documented the upgrading of bio-oil in petroleum refineries through co-processing with fossil feedstocks [224,225]. The product quality can be altered by co-refining bio-oils in a fluid catalytic cracking (FCC) unit, and the end products are more like those typically associated with the FCC unit [226]. Using data from the Petrobras demonstration facility, Talmadge et al. [225] conducted a recent techno-economic study on the co-processing of vacuum gasoil and raw bio-oil. The authors stated that, under the assumption that the cost of bio-oil and the size of the biomass pyrolysis plant are $80–84/bbl and 400 dry tons/day, respectively, co-processing up to 5% bio-oil would be economically feasible in the near future. The break-even point for 5 weight percent co-processing corresponds to the intermediate crude price of $54/bbl in west Texas, where bio-oil prices range from $80 to 84/bbl. The authors added that a blending of up to 10% can be anticipated to be economically possible in the near future given technological advancements and the assumption of a plant capacity of 2000 dry tons d−1 and a bio-oil cost of 50–60/bbl. However, in order to market bio-oils, further challenges must be resolved. Bio-oils gradually polymerize and condense a problem that is made worse by rising temperatures, oxygen exposure, and ultraviolet light. Furthermore, the primary obstacle to the commercialization of bio-oil is the economics of its manufacture. Other frequently mentioned obstacles to the usage of bio-oil include a lack of specialized fuel handling equipment, incompatibility with traditional fuels, and ignorance of bio-oil [214].
Char, also known as biochar, is a solid residue that is rich in carbon, with a carbon content that ranges from 65% to 90%. It has many pores and surfaces that are rich in oxygen functional groups and aromatic species [227]. When compared to terrestrial biomass, aquatic biomass produces more biochar because it has a higher fixed carbon content and more ash [228]. The composition of biochar is often determined by the process conditions used; for example, high temperatures raise the carbon content, while the kind of biomass feedstock dictates the amount of ash in the biochar [229]. Carbon, hydrogen, nitrogen, oxygen, inorganics including potassium, sodium, calcium, and magnesium, and trace amounts of sulfur are the usual components of biomass [207,228]. The heating process in biochar alters the carbon’s chemical environment to create aromatic rings that are difficult for microbes to break down, allowing for long-term carbon storage in soil [7]. Biochar stability increases with pyrolysis temperature, as more condensed aromatic structures form enhancing persistence in soil environments. Pore structure spans macropores, mesopores, and micropores, contributing to both physical stability and microbial habitat provision. Nutrient retention varies with temperature: elements such as Phosphorus (P), Mn, and Fe tend to be retained in biochar, while Ca, Mg, and Si may volatilize or be liberated at higher temperatures [230]. Furthermore, the availability of the biomass’s primary structural elements and other macromolecules in the solid residue declines as the pyrolysis temperature rises. This lessens the polarity of biochar as well, which lowers its hydrophilicity [231]. The surface area of the biochar greatly rises with temperature, regardless of the type of feedstock employed [232]. Nevertheless, pore expansion and/or coalescence with neighboring pores may result in a decrease in the number of pores over 900-degree Celsius. Furthermore, the collapse of micropores and the reduction of surface area might be caused by secondary reactions between volatiles and coke [231].
Potential Application of Biochar
Among its many uses are soil amendment to boost agricultural productivity, soil fertility, and water retention; its role as a feedstock in bioenergy production (bio-oil, syngas); and its function in reducing greenhouse gas emissions and sequestering carbon [233]. In the wastewater treatment process, biochar is widely desired as an adsorbent to eliminate heavy metals, organic and inorganic pollutants, etc. [234–236]. Additionally, a few research documented using biochar as an additive to boost microorganisms’ biological activity during the biomethanation processes [219,237]. Through activation or modification, the properties of the biochar can be further improved [213]. There are two methods of activation: (i) physical activation, which is accomplished with steam or gas, enhances the volume, pore structure, and chemical characteristics of biochar (such as polarity, functional groups, and hydrophobicity); and (ii) chemical activation, which involves treating biochar with chemical reagents (such as oxidants, bases, and acids) to enhance the pore characteristics of biochar [238].
A wide range of applications, including environmental remediation, use modified biochar as catalysts for energy production (electrocatalysis, catalytic reforming/cracking, transesterification/esterification), value-added chemicals (hydrolysis, oxidation, isomerization, hydroxylation, and deoxygenation), and other processes [239]. Modified biochar has recently been shown to be a viable substitute for supercapacitor electrodes [240]. Therefore, by turning waste products into useful resources, pyrolysis has the potential to support a more circular and sustainable economy.
5.3 Sawdust as a Renewable Feedstock for Thermochemical Bio-Oil and Biochar Production
Sawdust is a by product of wood processing that is produced in significant amounts, particularly in the wood-processing and forestry sectors. It is a low-cost and abundant feedstock that has the potential to be converted into bio-oil and bio- char through thermochemical processes. It is created when wood is cut or processed, and sawmills, furniture manufacturers, and other wood-based businesses frequently produce it in huge numbers. Sawdust can present several health and environmental risks if improperly handled [241]. Additionally, burning wood sawdust adds to climate change and deforestation [242]. Furthermore, if sawdust is not properly managed, it may damage soil and water sources. All things considered, sawdust pyrolysis offers a viable way to address the sawdust waste issue in Nigeria and other regions of the world. Pyrolysis can lessen the environmental impact of sawdust waste while simultaneously producing a sustainable energy source by turning it into valuable products like bio-oil, gas, and char.
Sawdust is a lignocellulosic material made up of the three main components of plant biomass: lignin, cellulose, and hemicellulose [243,244]. These components give sawdust its unique physical and chemical properties, which make it a great feedstock for the creation of biochar and bio-oil. Sawdust’s high cellulose and hemicellulose concentration makes it extremely flammable, but the lignin presence offers a source of fixed carbon that can raise the biochar’s carbon content [70]. They usually have heteroatoms and low mineral compositions, which burn well when burned directly [245]. The highest concentrations of carbon (44%–64%) and oxygen (28%–50%) have been found in sawdust derived from other types of wood. Traces of sulfur, nitrogen, and hydrogen have also been found to be present in the samples, along with small levels of hydrogen (about 6%). Additionally, wood sawdust is known to have low quantities of ash (0.1%–1.6%) and large levels of volatile matter (above 70%). Often called the calorific value or energy content, the heating value of biomass is a crucial metric that quantifies the amount of energy contained in a specific volume of biomass material. This figure is crucial for evaluating biomass’s potential as a renewable energy source and contrasting it with alternative energy sources, such as fossil fuels [246,247]. A number of variables, such as the type of biomass, its moisture content, and the presence of contaminants like ash content, affect its heating value [248]. Low-moisture dry biomass typically has a higher heating value because less energy is lost during combustion due to water evaporation.
Due to its large surface area and porosity, sawdust is highly reactive and subject to thermochemical change [249]. Physical pre-treatment techniques like milling, screening, or grinding can further improve the sawdust’s surface area and porosity. By increasing the surface area of the sawdust particles, these techniques improve the feedstock-conversion medium interaction, resulting in a more effective conversion process [250].
Sawdust is produced in vast quantities all over the world and is a plentiful and accessible feedstock. A number of variables, including regional forestry practices, woodworking techniques, and the demand for wood-based products, affect its availability for the production of bio-oil and biochar. It is comparatively easy to obtain sawdust from sawmills and timber processing plants in areas where the timber industry is thriving [251]. Moreover, significant volumes of sawdust are commonly produced by carpentry workshops, furniture manufacturers, and other woodworking businesses. Over 1 billion cubic meters of sawdust are thought to be produced annually worldwide, mostly in nations like the US, Canada, China, and Russia that have sizable forestry and wood-processing sectors [252,253]. A growing number of organizations now recognize the benefits of converting sawdust and other wood biomass into high-performance biochar not just as a waste valorization pathway, but also as a strategy for designing value-added carbon materials using various thermochemical techniques [254]. However, rivalry for the use of sawdust may exist even in areas where it is plentiful. Sawdust, for example, is frequently used as animal bedding and can also be used as fuel. Therefore, depending on the unique needs of a given location, sawdust availability for the creation of biochar may vary. Generally speaking, sawdust is an easily obtained raw material for the synthesis of biochar, with possible advantages like soil improvement and carbon sequestration.
5.4 Role of Catalysts in Enhancing Pyrolysis Processes
Pyrolysis of lignocellulosic biomass is one potential method for creating sustainable chemicals and fuels to replace products made from fossil fuels. However, because lignin, cellulose, and hemicellulose molecules are complex, a variety of chemicals are often produced, making practical implementation difficult. The use of a catalyst during reactions, which permits the production of specific molecules, is one of the most significant developments in pyrolysis [148]. Furthermore, the large number of available catalysts allows for a wide range of possibilities for controlling the reaction network. Alkali and alkaline earth oxides, transition metals, carbonaceous materials, zeolites, and hierarchical zeolites have all been used to study the pyrolysis of various biomasses. Catalytic pyrolysis increases the yield, speeds up the reaction, and improves the characteristics of the final products. Additionally, using catalysts effectively raises the intended product yield, decreases the rate at which tars are generated, and improves transformation efficiency [255]. Various catalysts, including copper oxide (CuO) and calcium oxide (CaO), have been used to improve product properties and yield. According to a study, employing CaO as a reactant enhanced the ketones in the pyrolytic oil by lowering the acids, while increasing the hydrogen percentage by lowering the carbon dioxide [148]. By lowering the ester and anhydrosugar levels, CaO also raised the furans and hydrocarbons. Additionally, it has been demonstrated experimentally that adding CuO during pyrolysis produced a consistent product distribution. Furthermore, it raised the relative concentration of phenols, decreased the polycyclic aromatic hydrocarbons (PCAH), and enhanced tar output [148]. When biomass pyrolyzes in the presence of CaO, the high-oxygen-containing acids may be removed, but more hydrocarbons can be produced in bio-oil [256].
5.5 Pretreatment Technologies for Bio-Oil and Biochar Production
Pretreatment technologies play a crucial role in enhancing the efficiency and yield of bio-oil and biochar production through pyrolysis. Pyrolysis, a thermal decomposition process, converts biomass into bio-oil, biochar, and syngas.
5.5.1 Physical/ Mechanical Pretreatment
It is commonly recognized that cellulose’s crystallinity prevents lignocellulose material from dissolving. A common approach to break the crystallinity of biomass is size reduction. Numerous studies have documented the impact of biomass particle size distribution on biofuel conversion [257]. Although it also depends on the properties of the biomass, size reduction raises the specific surface area of biomass and decreases the degree of polymerization and cellulose crystallinity [258]. However, the initial and ultimate sizes of biomass, as well as its moisture content, determine the power input required for mechanical size reduction. In order to improve enzyme digestibility, Liu et al. [259] investigated the impact of steam explosion pretreatment on corn stover particle size. They found that larger biomass particle sizes resulted in higher byproduct amounts and lower sugar recovery, but also higher yields and sugar conversion during enzymatic hydrolysis. Both specific surface area and crystallinity decrease as particle size increases. Additionally, Khullar et al. [260] investigated how particle size affected the enzymatic hydrolysis of Miscanthus that had been pretreated. The lowest conversion was attained at a particle size of 6 mm, followed by the smallest size of 0.08 mm, which produced the best overall conversion of biomass. Lignocellulosic biomass can also be physically pretreated using microwave irradiation. This pretreatment method has been honed over time and is well known for its superior heating efficiency and simplicity of usage. The pretreatment of rice straw was investigated by Shangdiar et al. [261] using microwave irradiation without the use of catalysts. In order to evaluate the effect of microwave irradiation on the recalcitrant structure, they found that cellulose increased from 33.4% to 41.8% and acid-soluble lignin decreased from 2.1% to 1.9%. This finding suggests that microwave radiation may break down the lignin–hemicellulose complex, partially remove silicon and lignin, disturb the silicified waxy surface, and reveal additional cellulose surface area that is accessible.
The use of chemicals to separate lignocellulose is a well-known pretreatment technique that offers more benefits than physical pretreatment [262]. Eliminating hemicellulose or lignin during chemical pretreatment can increase the production of glucose [263]. The removal of lignin and hemicellulose during chemical pretreatment with an alkaline or acidic solution depends on pH. More lignin is often removed by alkaline pretreatment with Sodium hydroxide (NaOH) than by acid pretreatment with Hydrochrolic acid (HCl) and Sulfuric acid (H2SO4) [264]. There are no byproducts from alkaline pretreatment, while acid pretreatment yielded 2-furfuraldehyde and 5-hydroxymethyfurfural [264]. Alkaline hydrogen peroxide pretreatment is becoming more popular since it breaks down lignin into oxygen and water and leaves no leftovers in the pretreated biomass [264]. Temperature had very little effect on the removal of lignin in the alkali-based pretreatment employing NaOH. The lignin removal rose from 41% to 72% at 140°C with increasing alkali dosage, while it only increased by 1% at the same alkali dosage of 7% Weight per volume (w/v) [265]. According to Yang et al., [266], the addition of a sodium carbonate and sodium sulfate mixture stopped the breakdown of carbohydrates during low temperature pretreatment. Additionally, lignin can be efficiently removed by peracetic acid pretreatment, which also degrades some hemicellulose and exposes cellulose. Additionally, practically all carboxylic acid changes, including acetyl groups and uronic acids, were eliminated by both acid and alkaline pretreatments [267]. Chemical pretreatment process is widely used for industrial pulp and paper production.
Nearly all of the biomass that is taken from the source contains water. For example, sawdust might include as much as 50.23%–53.49% moisture [268]. Bio-oil, the liquid product of pyrolysis, would re-condense if the biomass contained too much moisture. Even while a higher liquid yield from biomass pyrolysis could result from higher moisture content in the feedstock [269]. However, moisture primarily contributed to the aqueous phase, which will dilute and impair the quality of the bio-oil [170]. Drying is a thermophysical process that mostly takes place between room temperature and 150°C and uses hot air or flue gas to remove the water from biomass. In summary, biomass particles contract and lose porosity when dried at temperatures ranging from 50°C to 100°C. After rewetting, the structure can be reversed. The lignin in biomass softens and starts to flow when dried at 120°C to 150°C, especially when pressure is applied [270]. Biomass drying before pyrolysis can improve the energy efficiency of the process and the quality of the bio-oil because water is removed beforehand. Among the drying methods are microwave, hot air, and natural drying.
According to Chen et al. [271], analysis of drying’s effects on biomass properties and the devolatilization process, moisture has no effect on the chemical structure or constituents of straw. The dried samples’ grain size shrank as a result, though, and tiny surface fissures developed. Thus, it helps improve heat and mass transfer for bio-mass pyrolysis, which raises pyrolysis rates and, ultimately, the yield of volatile materials. Microwave and conventional drying methods significantly influence the properties of biomass prior to pyrolysis. Faster drying rates, such as those achieved by microwave methods, can improve heat and mass transfer, enhance the pore structure of the biomass, and promote the release of volatile compounds. These improvements help increase the yield of liquid bio-oil and solid char while minimizing gas production. Overall, the choice of pretreatment and drying method directly impacts the efficiency of pyrolysis and the composition of its products bio-oil [272].
5.6 Feedstock Influence on Product Yield Quality
The physicochemical properties of bio-oil and biochar are strongly influenced by the composition and characteristics of the biomass feedstock. Key components such as lignin, cellulose, and hemicellulose play critical roles in determining product yield and quality. Typically, lignin-rich biomass tends to produce more biochar and less bio-oil, while cellulose- and hemicellulose-rich materials favor higher bio-oil yields. Moisture content is another major factor influencing pyrolysis performance. High moisture levels increase energy requirements and can suppress char formation, whereas low-moisture feedstocks enhance thermal efficiency and promote biochar production [6]. Additionally, physical characteristics such as particle size and density affect heat transfer and reaction rates. Smaller, less dense feedstocks generally facilitate bio-oil formation, while denser materials favor solid residue production. Selecting appropriate feedstock based on chemical composition and physical properties is essential for optimizing pyrolysis conditions and achieving desired product outcomes.
6 Technological Challenges in Pyrolysis of Sawdust
6.1 Challenges in Feedstock Preparation
The composition of feedstock plays a significant role in determining the make-up and production of pyrolytic products. Technologies for pyrolysis are typically tested at pilot and laboratory scales due to the complexity of the feedstock’s amount and heat transfer quality, scaling up to commercial production might be difficult. With significant variations in moisture content, particle size, and chemical makeup, biomass feedstocks are frequently heterogeneous in composition. This causes uneven heating and reaction rates during pyrolysis, which lowers product yield and quality. Woody biomass is distinguished by its low moisture, ash, and volatile content, as well as its high bulk density and calorific value. High moisture and ash content, low bulk density, poor calorific value, and volatile content are characteristics of non-woody biomass [273]. The final yield of pyrolytic products is determined by the different proportions of each component of feedstock, which are mainly made up of lignin, cellulose, and hemicellulose with trace amounts of minerals and other substances [274,275].
6.2 Reactor Design and Scaling Issues
Typically, approximately 10%–25% of the overall capital cost is attributed to pyrolysis reactors. Nonetheless, the overall yield, process configuration, and energy requirements are significantly impacted by the reactor’s design and operation. Ablative pyrolysis, auger reactors, revolving cones, circulating fluidized beds, and microwave-assisted reactors are some examples of commercial and development reaction systems. These designs vary greatly in terms of how they transfer heat as well as how well they remove solids, collect liquids, and scale up. Transportation phenomena severely limit the pyrolysis processes [276]. Specifically, heat transport presents the most challenge among several design factors. Because the pyrolysis reactions are endothermic by nature, a greater heating rate, the reduction of the water content (e.g., below 10%), and the grinding of biomass into finer particles (e.g., 2–3 mm) all increase the yield of bio-oil. A carrier gas, reactor surfaces, transportable bed materials (such as sand), or partial combustion with a tiny addition of air can all provide the necessary energy. The size of the equipment and its capacity for expansion are greatly impacted by the need for carrier gas. The design is further complicated by the need to move mechanical components at high temperatures and by the need to enter feed from the bottom (in the case of circulating fluidized bed (CFB) relays). The distribution of residence times of gases is also influenced by the mode of biomass delivery, such as fluidization, centrifugal force, or mechanical drive. Because local hotspots and excessive heating would lower product yields, particle size reduction is frequently required.
6.3 Economic and Environmental Barriers
While pyrolysis of sawdust presents a promising route for renewable fuel and material production, its commercial deployment is still hindered by several economic and environmental challenges. Sawdust, as a lignocellulosic biomass by-product from wood processing industries, offers feedstock consistency and improved reactivity. However, despite these advantages, large-scale implementation remains limited due to infrastructure, financial, and ecological concerns.
One of the primary economic constraints is the high capital cost associated with establishing pyrolysis facilities. Investment is required not only for reactor construction but also for feedstock pre-treatment systems, catalysts, emission controls, and analytical equipment. These costs can range from hundreds of thousands to several million United State Dollars (USD), depending on the scale and technology sophistication. This financial burden is particularly challenging for small-scale operators and startups especially in developing countries due to limited access to green financing and credit. Market uncertainty also stifles commercialization: inconsistent product quality, unstable fossil fuel prices, and fragmented supply chains deter investor confidence. Significant policy gaps such as a lack of unified standards and unclear regulatory frameworks further inhibit biochar market growth. On top of this, scale-up complexities from process energy balance to continuous operation design necessitate expensive retrofitting or redesign. These commercialization challenges are recognized globally, with stakeholders pointing to inadequate standards, insufficient industrial-scale validation, weak policy frameworks, and fragmented supply chains as critical obstacles to widespread adoption [243].
Although pyrolysis is generally regarded as cleaner than combustion, environmental concerns still persist, particularly if the process is not optimized. Emissions of greenhouse gases (GHGs), volatile organic compounds (VOCs), and particulates can occur during feedstock drying, volatile release, or char handling. Without proper gas cleaning and combustion systems, these emissions may offset the environmental benefits of bio-oil and biochar production [63]. The chemical safety of biochar is another concern. Depending on the feedstock and temperature, biochar may contain heavy metals, PAHs (polycyclic aromatic hydrocarbons), or dioxins, which limit its use in agriculture or water treatment unless thoroughly tested and treated. Ensuring biochar quality requires costly post-processing, certification, and regulation compliance. In addition, waste management challenges arise from the disposal of by-products such as tar, spent catalysts, and non-condensable gases. If not treated or recycled, these residues can contribute to soil and water contamination, defeating the purpose of environmental sustainability. To address these environmental concerns, the adoption of emission control systems, carbon capture integration, and green catalyst alternatives is essential [73]. Moreover, comprehensive life cycle assessment (LCA) is needed to quantify the net environmental benefits of the pyrolysis process across its full value chain.
6.4 Mitigation Strategies and Research Gaps
The production of bio-oil and biochar from sawdust pyrolysis presents several challenges that require targeted solutions. One approach involves optimizing the feedstock properties to enhance chemical balance and improve pyrolysis efficiency [277]. Pretreatment methods like drying, torrefaction, and milling enhance uniformity and energy efficiency, reducing moisture content and improving product yield. Effective catalysts and process optimization are also crucial. Cost-effective catalysts like calcium oxide (CaO) and aluminum oxide (Al2O3) enhance bio-oil quality and increase biochar surface area. Adjusting key pyrolysis parameters, such as temperature, residence time, and heating rate, further improves yield and selectivity. Bio-oil and biochar quality can be enhanced through upgrading techniques. Hydrodeoxygenation, emulsification, and catalytic cracking improve bio-oil stability and reduce oxygen content, making it more suitable for fuel applications. Biochar activation and functionalization increase its adsorption capacity, making it more effective in environmental and agricultural applications. Life cycle assessments (LCA) and cost-benefit analyses are essential for determining the sustainability and commercial viability of sawdust pyrolysis [49]. These evaluations provide insights into environmental impacts and economic feasibility, guiding large-scale implementation. The integration of pyrolysis with circular economy principles enhances sustainability by utilizing waste-derived catalysts, reducing environmental impact, and maximizing resource efficiency. Despite these advancements, several research gaps remain. Limited studies focus on hybrid sawdust pyrolysis, with most research centered on single biomass types. Understanding the synergistic effects of different wood compositions on product yield and quality requires further investigation. Additionally, variations in heating rates, residence times, and temperatures lead to inconsistent results, highlighting the need for standardized pyrolysis conditions. The development of cost-effective catalysts remains a challenge, as many are expensive and non-renewable. Research into biochar-based and waste-derived catalysts presents a promising alternative [278]. Bio-oil upgrading also requires further attention due to its high oxygen content and instability, necessitating affordable techniques such as low-cost hydrogenation and catalytic reforming.
Addressing these challenges will drive advancements in bio-oil and biochar production. Optimizing feedstock selection, refining pyrolysis conditions, and integrating sustainability measures will unlock the full potential of hybrid sawdust as a renewable energy source. Future research should focus on bridging knowledge gaps, standardizing processes, and developing innovative solutions to enhance the commercial viability of pyrolysis technology.
6.5 Comparative Evaluation of Techno-Economic for Thermochemical Conversion Processes
There are significant disparities in the technical and financial feasibility of the four main thermochemical conversion technologies gasification, combustion, hydrothermal liquefaction (HTL), and pyrolysis. Because pyrolysis can handle a variety of biomass feedstocks, it is a fairly capital-intensive process that works well for decentralized systems. It has a moderate efficiency and reasonably low operating costs, producing both charcoal and bio-oil. Although gasification is technically sophisticated and may produce clean syngas for chemical synthesis or electricity, its high operating temperatures and intricate gas cleanup systems result in greater capital expenditures [42]. HTL produces high-quality bio-crude and is beneficial for wet biomass feedstocks, it is the most costly thermochemical process and necessitates high-pressure, corrosion-resistant equipment. The most straightforward and widely utilized method, on the other hand, is combustion, which is mostly employed for the production of direct heat and electricity. Despite its limited product versatility and inability to produce liquid fuels or charcoal, its operational simplicity and cost feasibility make it appropriate for large-scale energy systems. In terms of cost-effectiveness and technical versatility, pyrolysis and gasification stand out as the most promising processes for producing bio-oil and biochar, especially when combined with upgrading and emission control technologies.
The techno-economic feasibility of thermochemical conversion technologies including pyrolysis, gasification, hydrothermal liquefaction (HTL), and combustion varies significantly depending on operating conditions, feedstock, and end-use goals. Pyrolysis is moderately capital intensive but well-suited for decentralized systems due to its feedstock flexibility and relatively simple operation. It offers moderate energy efficiency (∼50%) and produces both bio-oil and biochar, although the raw bio-oil requires upgrading before use. Gasification is technically more complex but enables clean syngas production, which can be used for electricity, fuels, or chemicals. This method achieves high energy efficiency (∼42%) but incurs higher capital and operational costs due to elevated temperatures and extensive gas cleanup systems. HTL is the most costly thermochemical process, requiring high-pressure reactors and corrosion-resistant equipment, yet it works well for wet biomass and produces high-quality bio-crude. In the meantime, combustion is still the most developed and extensively used technique, mostly for producing heat and power. Although its product variety is limited, its cost-effectiveness and ease of use make it perfect for large-scale energy production.
In a recent study, Ref. [279] used municipal solid waste (MSW) as fuel to evaluate chemical looping and updraft gasification. Due to cheaper capital investment, chemical looping showed a better economic advantage with a lower levelized cost of substitute natural gas (SNG) (€1.76/kg vs. €2.26/kg), despite gasification offering higher SNG productivity (16.3 t/h) and energy efficiency (42%). The capacity factor and power price were highlighted in the study as crucial sensitivity elements affecting economic performance. A comparative techno-economic analysis of the four major thermochemical conversion technologies pyrolysis, gasification, hydrothermal liquefaction (HTL), and combustion is presented in Table 4. The comparison highlights key performance criteria, including capital and operating costs, feedstock flexibility, energy efficiency, product types, process complexity, environmental impact, scalability, technology readiness level (TRL), and operating temperature. As shown in the table, pyrolysis and gasification demonstrate balanced technical and economic performance, making them attractive options for decentralized bioenergy systems. HTL, while efficient for wet biomass, remains less mature due to its high-pressure operating requirements and limited scalability. In contrast, combustion is the most technologically mature and widely deployed process, though it offers limited product flexibility. This comparative assessment supports the selection of appropriate technologies based on resource availability, target products, and economic constraints.
7 Kinetics of Pyrolysis Studies
7.1 Overview of Pyrolysis Kinetics
Pyrolysis, a thermochemical process, involves the decomposition of organic materials at elevated temperatures in the absence of oxygen. It is a key step in various thermal conversion technologies, including bioenergy production, waste management, and materials recovery. A critical aspect of pyrolysis is its kinetics, which refers to the rates at which the chemical reactions occur during the process [277]. Understanding pyrolysis kinetics is vital for designing efficient reactors, optimizing operating conditions, and predicting the distribution of pyrolysis products such as gases, liquids, and solid char. The kinetics of pyrolysis refer to the rates and mechanisms by which the thermal decompositions occur, which essentially optimize reactor design, enhance energy efficiency, and control product yields and properties. Kinetic modeling is necessary to accurately forecast biomass breakdown performance under various operating conditions. Thousands of events occur simultaneously, in tandem, in seconds, or within minutes (depending on the operating parameters) during the pyrolysis of biomass, making it a tremendously complex process. Because of the intricacies of pyrolytic reactions, there is a substantial amount of literature on the many methods used to explain biomass breakdown. The experimental methods include derivative thermogravimetric analysis (DTG), evolved gas analysis (EGA), differential scanning calorimetry (DSC), differential thermal analysis (DTA), thermogravimetric analysis (TGA), and others.
The two most common models used in TGA-based biomass reaction kinetics analysis are the iso-thermal and the non-isothermal models. Because the isothermal process necessitates both holding time and holding rates, the non-isothermal procedures have a smaller margin of error than the isothermal [278]. Additionally, it takes less time to get data from non-isothermal techniques throughout the experimental or investigative processes. The kinetics may be evaluated continuously throughout a wide temperature range, which lowers the likelihood of errors in thermo-chemical induction procedures. The two most popular non-isothermal models are model fitting and model-free. Since model-free approaches result in the least amount of error, they are widely accepted [279]. Since greater heating rates produce turbulence because of longer residence times, non-isothermal approaches have been shown to be the most suitable models for predicting the kinetics of pyrolysis reactions that operate at lower and dynamic heating rates [280]. Long intervals were also avoided since short intervals produced more accurate results due to the shifting of the peaks during TGA pyrolysis [281]. The activation energy is determined via the iso-conversional model by assuming that it is a function of temperature and heating rate. In essence, these models operated under the premise that the heating process and activation energy remained constant throughout. To ascertain kinetic devolatilization, they were employed [204], the primary function of temperature in the physicochemical character of biomass. To explain reaction kinetics, including the single-step reaction model, which does not predict how operational parameters will affect product yield, some studies have employed TGA techniques for pyrolysis. The exact kinetics of the synthesis of tar, gases, and biochar can be explained by the two-step or parallel reaction models.
Understanding thermodynamic parameters Gibbs free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) is essential for deciphering pyrolysis energetics and estimating process energy demands. For example, recent TGA-based kinetic studies on Nitella hyalina revealed ΔH values ranging from 187 to 363 kJ/mol, ΔG between 146 and 278 kJ/mol, and ΔS spanning –51 to +94 J/mol·K, highlighting the feasibility and temperature sensitivity of the reaction pathway. Such data are invaluable for developing, scaling, and optimizing commercial pyrolysis systems [282]. Moreover, the iso-conversional models were thought to be the most effective techniques for calculating frequency factor and activation energy (Ea), producing more accurate findings because they were used without making any assumptions [278].
Several kinetic techniques, such as the Friedman, Horowitz–Metzger, Van Krevelen, Coats–Redfern (CR), Flynn–Wall–Ozawa (FWO), Vyazovkin and Kissinger–Akahira–Sunose (KAS) models, can be used to ascertain the parameters of lignocellulosic biomass. These techniques were all developed to ascertain the most efficient use of energy derived from thermal processes, including pyrolysis, the thermal conversion reaction that converts biomass into gases and liquids of oxygenated organic compounds [283]. They may use any kind of biomass, even cattle manure [284], incense sticks [285], cornstalks [286], pine waste [287], and microalgal- bacterial [288] alongside numerous other substances and leftovers.
The development of mathematical models and the explanation of the properties of pyrolysis reactions depend heavily on chemical kinetics [277]. The kinetics of biomass pyrolysis has been the subject of in-depth research in recent decades in order to create a variety of kinetic models [11]. The majority of kinetic models are referred to as lumped models since they are predicated on the yields of lumped products, such as gas, tar, and char. The most common types of kinetic models include the distributed activation energy model (DAEM), nucleation growth model, one-step global kinetic model, parallel and competitive reactions model, secondary tar cracking model, and detailed lumped kinetic model.
The simplest models that depict the conversion of biomass into volatiles and char as a first-order single-step reaction are one-step global kinetic models. Since the only products of pyrolysis are char and volatiles [289], With the help of suitable heat transport and volume reaction models, the majority of studies have employed the global reaction model [12]. The kinetic parameters of these models can be calculated experimentally or with the aid of other models, like the Flynn-Wall-Ozawa (FWO), Kissenger Model, and Kissenger-Akahira Sunose (KAS) [290]. Global kinetic models, however, have certain drawbacks because they only take into account main reactions and lack complex reaction mechanisms. As a result, further kinetic models were later developed to incorporate the secondary reactions [137] The kinetic model incorporated the production of gases from the breakdown of tar and the polymerization process that turns tar into char as secondary reactions [291].
The Shafizadeh and Bradbury model which included both primary and secondary decomposition reactions, predicted the product yield well. However, because the active material’s composition was unknown, it was challenging to determine the activation energy. In this model, cellulose was first converted to active cellulose, which was then further broken down to other secondary products. Researchers have used this model extensively in both its extended and original forms. Ref. [292] presented a comprehensive lumped kinetic model for biomass pyrolysis that comprised 15 reactions with 30 lumped species. This mechanistic model was developed by [293] to explain the gas phase reaction, devolatilization, and pyrolysis of biomass from the three reference components of biomass.
7.3 Influence of Heating Rate and Temperature on Kinetics Models
It is crucial for material synthesis and fabrication, industrial production, biomedical, energy storage, catalysis, and other fields to use thermal analysis techniques to determine kinetic parameters including activation energy (Ea), pre-exponential factor (A), rate constant (r), and reaction order (n) [294–299]. To determine kinetic parameters, several non-isothermal kinetic techniques have been developed in recent decades. These techniques rely on correlations between heating rates and temperatures at which the reaction reaches its maximum value. The most popular methods are Kissinger [300] and Ozawa [301] methods. The Kissinger method was developed by Homer E. Kissinger in 1957 [300]. The technique has grown to be one of the most widely used methods for kinetic parameter determination using thermal analysis due to its speed and ease of usage. As of now, 31 May 2020, the initial document [300] according to which more than 5600 publications have mentioned the Kissinger model (5605 in Web of Science, 9757 in Scopus, and 12505 in Google Scholar).
The Kissinger technique is based on a set of tests that use thermal analysis tools like DTA to heat samples at multiple (usually three or four) distinct heating rates. The kinetic parameters are then calculated by recording the reaction exothermic peak temperature at each heating rate. Two independent functions, f(x) and k(T), can be used to characterize the rate of conversion for a thermal reaction [300]. Various kinetics paremeters for different biomass types are presented in Table 5.
8 Future Works and Implications
8.1 Comparative Future Directions across Thermochemical Technologies
Pyrolysis is still a viable process for producing bio-oil and biochar, but there is still a lot of promise in alternative thermochemical conversion techniques that need more research. For gasification, future studies should concentrate on improving the production of hydrogen-rich syngas, decreasing tar formation, and optimizing gas cleaning systems [189]. Additionally, in order to optimize energy recovery, gasification must be integrated with downstream synthesis and carbon capture. For hydrothermal liquefaction (HTL), more research is needed to reduce operating pressures, enhance reactor design, and expand biocrude upgrading techniques in order to satisfy transportation fuel regulations. Although combustion is technically advanced, it still has problems controlling emissions; future research should look into ash valorization and low-NOx technology. Torrefaction and slow pyrolysis are two carbonization processes that provide high-yield biochar; nevertheless, in order to enhance carbon stability and useful qualities, process parameters must be standardized [179]. For various biomass kinds and local requirements, the best conversion pathway should be chosen based on comparative life cycle assessments, cost-benefit analysis, and techno-economic studies. Addressing these shortcomings in all thermochemical techniques is essential to developing scalable, adaptable, and sustainable biomass valorization systems.
8.2 Future Research Directions
Future studies should focus on optimizing the pyrolysis conditions for sawdust to enhance process efficiency and reproducibility. Investigating the influence of sawdust properties on product yield and quality will provide valuable insights for process improvement. Additionally, the development of standardized pyrolysis protocols will promote consistency across research efforts and support broader commercial applications. Cost-effective catalyst development is another crucial field. Biochar-based and waste-derived catalysts should be investigated in research as environmentally friendly substitutes for traditional catalysts. Additionally, research on the regeneration and reusability of catalysts will improve economic feasibility and cut waste [215]. The development of upgrading methods for bio-oil is also essential. To increase the stability of bio-oils and lower their oxygen concentration, future research should investigate low-cost hydrogenation, catalytic cracking, and emulsification. Its commercial application will be further supported by looking into ways to combine bio-oil with current fuel infrastructures.
Resolving these issues and research gaps will propel the production of biochar and bio-oil forward. Choosing the right feedstock, improving the pyrolysis process, and incorporating sustainability practices will maximize hybrid sawdust’s potential as a renewable energy source. The goal of future research should be to improve the commercial viability of pyrolysis technology by filling in knowledge gaps, standardizing procedures, and creating creative solutions.
8.3 Implications for Industry and Policy
The industry and politicians will be significantly impacted by the commercialization of pyrolysis technology for the generation of bio-char and bio-oil. The economic viability and scalability of sawdust pyrolysis are two significant industrial implications. Industries will be able to move from laboratory-scale research to large-scale manufacturing by standardizing pyrolysis settings and improving catalyst consumption [134]. Industries can profit from bio-based substitutes for traditional fossil fuels by increasing process efficiency and optimizing product yield. Another crucial industrial factor is incorporating bio-oil into current energy systems. Reliance on non-renewable resources can be decreased by refining bio-oil to meet fuel criteria and offering an alternative to petroleum-based fuels. Additionally, there are numerous industrial uses for biochar’s potential in soil amendment, carbon sequestration, and water purification, opening up new revenue streams for companies who invest in biochar technologies [227]. Another important factor in industrial sustainability is the economical use of catalysts. The creation of waste-derived and biochar-supported catalysts can lessen their negative effects on the environment and operational expenses. These sustainable catalysts can be used by industries looking to boost productivity and profitability while also enhancing their green credentials. Furthermore, by reducing biomass waste and producing value-added goods, including sawdust pyrolysis into circular economy models can support sustainable waste management practices.
Waste-to-energy policies should promote the use of agricultural residues and sawdust as viable feedstocks for energy generation. Implementing biomass-friendly regulations can reduce landfill dependency and foster emerging markets for bio-based energy products. Aligning pyrolysis technologies with global climate goals requires adherence to sustainability frameworks and environmental regulations. Addressing these policy and industrial factors is essential for unlocking the full potential of bio-oil and biochar production. A collaborative approach between industry stakeholders and policymakers driven by innovation and supportive legislation can accelerate the adoption of pyrolysis as a sustainable energy solution.
This review presents a comprehensive overview of sawdust pyrolysis as a viable thermochemical route for the sustainable production of bio-oil and biochar. The study examined the influence of feedstock composition, pyrolysis conditions, reactor types, and upgrading strategies on product yield and quality. Biochar has shown promising applications in soil amendment, carbon sequestration, and pollutant removal, while bio-oil holds potential as a renewable energy carrier, although further upgrading is required to improve its fuel properties and economic value. Despite its potential, widespread adoption of pyrolysis technology is still constrained by economic and environmental barriers. High capital costs, limited infrastructure, market instability, and regulatory uncertainty continue to affect large-scale implementation. Additionally, environmental concerns related to emissions, biochar safety, and waste management require more robust mitigation strategies. Improving the economic and environmental sustainability of pyrolysis will require innovations in reactor design, catalyst development, and integration with renewable energy systems. Life cycle assessments (LCA), AI-driven process optimization, and standardization of product quality will further support scalability. Stronger policy frameworks, industrial collaboration, and targeted investment will be critical to advancing commercial adoption. With continued technological advancement and regulatory support, pyrolysis can play a significant role in circular bioeconomy development, sustainable energy production, and effective biomass waste valorization.
Acknowledgement: The authors would like to thank Nile University of Nigeria, Abuja for providing the resources and the atmosphere to successfully put together this review.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: : Conceptualization, Hauwau Kaoje and Adekunle Adeleke; validation, Adekunle Adeleke, Temitayo Ogedengbe and Esther Anosike-Francis; resources, Hauwa Rasheed, Seun Jesuloluwa and Jude Okolie; writing—original draft preparation, Hauwau Kaoje; writing—review and editing, Adekunle Adeleke, Temitayo Ogedengbe, Esther Anosike-Francis and Hauwa Rasheed; supervision, Adekunle Adeleke. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The data that support the findings of this study are available from the Corresponding Author, [Adekunle Adeleke], upon reasonable request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Siddiqi H, Bal M, Kumari U, Meikap BC. In-depth physiochemical characterization and detailed thermo-kinetic study of biomass wastes to analyze its energy potential. Renew Energy. 2020;148:756–71. doi:10.1016/j.renene.2019.10.162. [Google Scholar] [CrossRef]
2. Abedin T, Pasupuleti J, Paw JKS, Tak YC, Islam MR, Basher MK, et al. From waste to worth: advances in energy recovery technologies for solid waste management. Clean Technol Environ Policy. 2025;15(23):6915. doi:10.1007/s10098-025-03204-x. [Google Scholar] [CrossRef]
3. Kumar A, Mishra RK, Jaglan S. Pyrolysis of low-value waste Miscanthus grass: physicochemical characterization, pyrolysis kinetics, and characterization of pyrolytic end products. Process Saf Environ Prot. 2022;163:68–81. doi:10.1016/j.psep.2022.05.022. [Google Scholar] [CrossRef]
4. de Dieu Marcel Ufitikirezi J, Filip M, Ghorbani M, Zoubek T, Olšan P, Bumbálek R, et al. Agricultural waste valorization: exploring environmentally friendly approaches to bioenergy conversion. Sustainability. 2024;16(9):3617. doi:10.3390/su16093617. [Google Scholar] [CrossRef]
5. Ganesan A, Rezazgui O, Burgos JB, Mangin PJ, Barnabé S. Valorization of lignocellulosic biomass forest residues in Quebec via the integrated hydropyrolysis and hydroconversion (IH2) technology: a review. Biomass Bioenergy. 2025;193(21):107516. doi:10.1016/j.biombioe.2024.107516. [Google Scholar] [CrossRef]
6. Jayakumar M, Hamda AS, Abo LD, Daba BJ, Venkatesa Prabhu S, Rangaraju M, et al. Comprehensive review on lignocellulosic biomass derived biochar production, characterization, utilization and applications. Chemosphere. 2023;345:140515. doi:10.1016/j.chemosphere.2023.140515. [Google Scholar] [PubMed] [CrossRef]
7. Al-Rumaihi A, Shahbaz M, McKay G, MacKey H, Al-Ansari T. A review of pyrolysis technologies and feedstock: a blending approach for plastic and biomass towards optimum biochar yield. Renew Sustain Energy Rev. 2022;167:112715. doi:10.1016/j.rser.2022.112715. [Google Scholar] [CrossRef]
8. Ippolito JA, Cui L, Kammann C, Wrage-Mönnig N, Estavillo JM, Fuertes-Mendizabal T, et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: a comprehensive meta-data analysis review. Biochar. 2020;2(4):421–38. doi:10.1007/s42773-020-00067-x. [Google Scholar] [CrossRef]
9. Abnisa F, Arami-Niya A, Wan Daud WMA, Sahu JN, Noor IM. Utilization of oil palm tree residues to produce bio-oil and bio-char via pyrolysis. Energy Convers Manag. 2013;76:1073–82. doi:10.1016/j.enconman.2013.08.038. [Google Scholar] [CrossRef]
10. Ameh VI, Ayeleru OO, Nomngongo PN, Ramatsa IM. Bio-oil production from waste plant seeds biomass as pyrolytic lignocellulosic feedstock and its improvement for energy potential: a review. Waste Manag Bull. 2024;2(2):32–48. doi:10.1016/j.wmb.2024.03.002. [Google Scholar] [CrossRef]
11. Hameed S, Sharma A, Pareek V, Wu H, Yu Y. A review on biomass pyrolysis models: kinetic, network and mechanistic models. Biomass Bioenergy. 2019;123:104–22. doi:10.1016/j.biombioe.2019.02.008. [Google Scholar] [CrossRef]
12. Kaczor Z, Buliński Z, Werle S. Modelling approaches to waste biomass pyrolysis: a review. Renew Energy. 2020;159:427–43. doi:10.1016/j.renene.2020.05.110. [Google Scholar] [CrossRef]
13. Leng E, Guo Y, Chen J, Liu S, E J, Xue Y. A comprehensive review on lignin pyrolysis: mechanism, modeling and the effects of inherent metals in biomass. Fuel. 2022;309(5):122102. doi:10.1016/j.fuel.2021.122102. [Google Scholar] [CrossRef]
14. Osman AI, Lai ZY, Farghali M, Yiin CL, Elgarahy AM, Hammad A, et al. Optimizing biomass pathways to bioenergy and biochar application in electricity generation, biodiesel production, and biohydrogen production. Environ Chem Lett. 2023;21(5):2639–705. doi:10.1007/s10311-023-01613-2. [Google Scholar] [CrossRef]
15. Thiviyanathan VA, Ker PJ, Leong YS, Abdullah F, Ismail A, Jamaludin MZ. Power transformer insulation system: a review on the reactions, fault detection, challenges and future prospects. Alex Eng J. 2022;61(10):7697–713. doi:10.1016/j.aej.2022.01.026. [Google Scholar] [CrossRef]
16. Jarvis MC. Hydrogen bonding and other non-covalent interactions at the surfaces of cellulose microfibrils. Cellulose. 2023;30(2):667–87. doi:10.1007/s10570-022-04954-3. [Google Scholar] [CrossRef]
17. Bonifacio A, Bonetti L, Piantanida E, De Nardo L. Plasticizer design strategies enabling advanced applications of cellulose acetate. Eur Polym J. 2023;197(10):112360. doi:10.1016/j.eurpolymj.2023.112360. [Google Scholar] [CrossRef]
18. Duran S, Şolpan D, Güven O. Synthesis and characterization of acrylamide-acrylic acid hydrogels and adsorption of some textile dyes. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater At. 1999;151(1–4):196–9. doi:10.1016/S0168-583X(99)00151-2. [Google Scholar] [CrossRef]
19. Beaucamp A, Muddasar M, Amiinu IS, Moraes Leite M, Culebras M, Latha K, et al. Lignin for energy applications-state of the art, life cycle, technoeconomic analysis and future trends. Green Chem. 2022;24(21):8193–226. doi:10.1039/d2gc02724k. [Google Scholar] [CrossRef]
20. Rosado MJ, Rencoret J, Marques G, Gutiérrez A, Del Río JC. Structural characteristics of the guaiacyl-rich lignins from rice (Oryza sativa L.) husks and straw. Front Plant Sci. 2021;12:640475. doi:10.3389/fpls.2021.640475. [Google Scholar] [PubMed] [CrossRef]
21. Zhang W, Qiu X, Wang C, Zhong L, Fu F, Zhu J, et al. Lignin derived carbon materials: current status and future trends. Carbon Res. 2022;1(1):14. doi:10.1007/s44246-022-00009-1. [Google Scholar] [CrossRef]
22. Lynd LR, Beckham GT, Guss AM, Jayakody LN, Karp EM, Maranas C, et al. Toward low-cost biological and hybrid biological/catalytic conversion of cellulosic biomass to fuels. Energy Env Sci. 2022;15(3):938–90. doi:10.1039/D1EE02540F. [Google Scholar] [CrossRef]
23. Inthalaeng N, Barker RE, Dugmore TIJ, Matharu AS. Microwave-assisted production of defibrillated lignocelluloses from blackcurrant pomace via citric acid and acid-free conditions. Molecules. 2024;29(23):5665. doi:10.3390/molecules29235665. [Google Scholar] [PubMed] [CrossRef]
24. Onokwai AO, Ajisegiri ESA, Okokpujie IP, Ibikunle RA, Oki M, Dirisu JO. Characterization of lignocellulose biomass based on proximate, ultimate, structural composition, and thermal analysis. Mater Today Proc. 2022;65(5):2156–62. doi:10.1016/j.matpr.2022.05.313. [Google Scholar] [CrossRef]
25. Ding L, Cheng MH, Lin Y, Lin KT, Sale KL, Sun N, et al. Understanding the impacts of inorganic species in woody biomass for preprocessing and pyrolysis—a review. Energy. 2025;322(5760):135697. doi:10.1016/j.energy.2025.135697. [Google Scholar] [CrossRef]
26. Varma AK, Mondal P. Physicochemical characterization and pyrolysis kinetics of wood sawdust. Energy Sources Part A Recovery Util Environ Eff. 2016;38(17):2536–44. doi:10.1080/15567036.2015.1072604. [Google Scholar] [CrossRef]
27. González Martínez M, Floquet P, Dupont C, da Silva Perez D, Meyer XM. Assessing the impact of woody and agricultural biomass variability on its behaviour in torrefaction through Principal Component Analysis. Biomass Bioenergy. 2020;134(3):105474. doi:10.1016/j.biombioe.2020.105474. [Google Scholar] [CrossRef]
28. Yallappa S, Deepthi DR, Yashaswini S, Hamsanandini R, Chandraprasad M, Ashok Kumar S, et al. Natural biowaste of Groundnut shell derived nano carbons: synthesis, characterization and its in vitro antibacterial activity. Nano-Struct Nano-Objects. 2017;12(7):84–90. doi:10.1016/j.nanoso.2017.09.009. [Google Scholar] [CrossRef]
29. Garba A. Biomass conversion technologies for bioenergy generation: an introduction. In: Biotechnological applications of biomass. London, UK: IntechOpen; 2021. doi:10.5772/intechopen.93669. [Google Scholar] [CrossRef]
30. Matsumura Y. Hydrothermal gasification of biomass. In: Recent advances in thermo-chemical conversion of biomass. Amsterdam, The Netherlands: Elsevier; 2015. p. 251–67. doi:10.1016/b978-0-444-63289-0.00009-0. [Google Scholar] [CrossRef]
31. Perera SMHD, Wickramasinghe C, Samarasiri BKT, Narayana M. Modeling of thermochemical conversion of waste biomass-a comprehensive review. Biofuel Res J. 2021;8(4):1481–528. doi:10.18331/brj2021.8.4.3. [Google Scholar] [CrossRef]
32. Guran S. Thermochemical conversion of biomass. Green energy and technology. In: Mitra M, Nagchaudhuri A, editors. Practices and perspectives in sustainable bioenergy. New Delhi, India: Springer; 2020. doi:10.1007/978-81-322-3965-9_8. [Google Scholar] [CrossRef]
33. Zhang J, Zhang X. The thermochemical conversion of biomass into biofuels. In: Biomass, biopolymer-based materials, and bioenergy. Amsterdam, The Netherlands: Elsevier; 2019. p. 327–68. doi:10.1016/b978-0-08-102426-3.00015-1. [Google Scholar] [CrossRef]
34. Gururani P, Bhatnagar P, Bisht B, Jaiswal KK, Kumar V, Kumar S, et al. Recent advances and viability in sustainable thermochemical conversion of sludge to bio-fuel production. Fuel. 2022;316(14):123351. doi:10.1016/j.fuel.2022.123351. [Google Scholar] [CrossRef]
35. Bhatnagar A, Barthen R, Tolvanen H, Konttinen J. Bio-oil stability through stepwise pyrolysis of groundnut shells: role of chemical composition, alkali and alkaline earth metals, and storage conditions. J Anal Appl Pyrolysis. 2021;157:105219. doi:10.1016/j.jaap.2021.105219. [Google Scholar] [CrossRef]
36. Sarker TR, Nanda S, Dalai AK, Meda V. A review of torrefaction technology for upgrading lignocellulosic biomass to solid biofuels. BioEnergy Res. 2021;14(2):645–69. doi:10.1007/s12155-020-10236-2. [Google Scholar] [CrossRef]
37. Tumuluru JS, Ghiasi B, Soelberg NR, Sokhansanj S. Biomass torrefaction process, product properties, reactor types, and moving bed reactor design concepts. Front Energy Res. 2021;9:728140. doi:10.3389/fenrg.2021.728140. [Google Scholar] [CrossRef]
38. Acharya B, Dutta A, Minaret J. Review on comparative study of dry and wet torrefaction. Sustain Energy Technol Assess. 2015;12(5):26–37. doi:10.1016/j.seta.2015.08.003. [Google Scholar] [CrossRef]
39. Amer M, Nour M, Ahmed M, Ookawara S, Nada S, Elwardany A. The effect of microwave drying pretreatment on dry torrefaction of agricultural biomasses. Bioresour Technol. 2019;286(1):121400. doi:10.1016/j.biortech.2019.121400. [Google Scholar] [PubMed] [CrossRef]
40. Ghavami N, Özdenkçi K, Salierno G, Björklund-Sänkiaho M, De Blasio C. Analysis of operational issues in hydrothermal liquefaction and supercritical water gasification processes: a review. Biomass Convers Biorefinery. 2023;13(14):12367–94. doi:10.1007/s13399-021-02176-4. [Google Scholar] [CrossRef]
41. Kostyniuk A, Likozar B. Catalytic wet torrefaction of biomass waste into bio-ethanol, levulinic acid, and high quality solid fuel. Chem Eng J. 2024;485:149779. doi:10.1016/j.cej.2024.149779. [Google Scholar] [CrossRef]
42. Makwana JP, Pandey J, Mishra G. Improving the properties of producer gas using high temperature gasification of rice husk in a pilot scale fluidized bed gasifier (FBG). Renew Energy. 2019;130(4):943–51. doi:10.1016/j.renene.2018.07.011. [Google Scholar] [CrossRef]
43. Centi G, Perathoner S. Chemistry and energy beyond fossil fuels. A perspective view on the role of syngas from waste sources. Catal Today. 2020;342:4–12. doi:10.1016/j.cattod.2019.04.003. [Google Scholar] [CrossRef]
44. dos Santos RG, Alencar AC. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: a review. Int J Hydrog Energy. 2020;45(36):18114–32. doi:10.1016/j.ijhydene.2019.07.133. [Google Scholar] [CrossRef]
45. Mansuri SQ, Shekhawat VPS. Hydrothermal liquefaction: exploring feedstock for sustainable biofuel production. Environ Exp Biol. 2024;22(3):135–47. doi:10.22364/eeb.22.13. [Google Scholar] [CrossRef]
46. Hamad MA, Radwan AM, Heggo DA, Moustafa T. Hydrogen rich gas production from catalytic gasification of biomass. Renew Energy. 2016;85(8):1290–300. doi:10.1016/j.renene.2015.07.082. [Google Scholar] [CrossRef]
47. Ali Yousefi Rizi H, Shin D. Development of ammonia combustion technology for NOx reduction. Energies. 2025;18(5):1248. doi:10.3390/en18051248. [Google Scholar] [CrossRef]
48. Nunes LJR, Causer TP, Ciolkosz D. Biomass for energy: a review on supply chain management models. Renew Sustain Energy Rev. 2020;120(2):109658. doi:10.1016/j.rser.2019.109658. [Google Scholar] [CrossRef]
49. Osman AI, Mehta N, Elgarahy AM, Al-Hinai A, Al-Muhtaseb AH, Rooney DW. Conversion of biomass to biofuels and life cycle assessment: a review. Env Chem Lett. 2021;19(6):4075–118. doi:10.1007/s10311-021-01273-0. [Google Scholar] [CrossRef]
50. Saengsuriwong R, Onsree T, Phromphithak S, Tippayawong N. Conversion of tobacco processing waste to biocrude oil via hydrothermal liquefaction in a multiple batch reactor. Clean Technol Environ Policy. 2023;25(2):397–407. doi:10.1007/s10098-021-02132-w. [Google Scholar] [PubMed] [CrossRef]
51. Usman M, Cheng S, Boonyubol S, Cross JS. From biomass to biocrude: innovations in hydrothermal liquefaction and upgrading. Energy Convers Manag. 2024;302:118093. doi:10.1016/j.enconman.2024.118093. [Google Scholar] [CrossRef]
52. Kumar V, Jaiswal KK, Vlaskin MS, Nanda M, Tripathi MK, Gururani P, et al. Hydrothermal liquefaction of municipal wastewater sludge and nutrient recovery from the aqueous phase. Biofuels. 2022;13(5):657–62. doi:10.1080/17597269.2020.1863627. [Google Scholar] [CrossRef]
53. Sivabalan K, Hassan S, Ya H, Pasupuleti J. A review on the characteristic of biomass and classification of bioenergy through direct combustion and gasification as an alternative power supply. J Phys Conf Ser. 2021;1831(1):012033. doi:10.1088/1742-6596/1831/1/012033. [Google Scholar] [CrossRef]
54. Tai L, de Caprariis B, Scarsella M, De Filippis P, Marra F. Improved quality bio-crude from hydrothermal liquefaction of oak wood assisted by zero-valent metals. Energy Fuels. 2021;35(12):10023–34. doi:10.1021/acs.energyfuels.1c00889. [Google Scholar] [CrossRef]
55. Osman AI, Fang B, Zhang Y, Liu Y, Yu J, Farghali M, et al. Life cycle assessment and techno-economic analysis of sustainable bioenergy production: a review. Environ Chem Lett. 2024;22(3):1115–54. doi:10.1007/s10311-023-01694-z. [Google Scholar] [CrossRef]
56. Wang G, Dai Y, Yang H, Xiong Q, Wang K, Zhou J, et al. A review of recent advances in biomass pyrolysis. Energy Fuels. 2020;34(12):15557–78. doi:10.1021/acs.energyfuels.0c03107. [Google Scholar] [CrossRef]
57. Nisar J, Ali F, Malana MA, Ali G, Iqbal M, Shah A, et al. Kinetics of the pyrolysis of cobalt-impregnated sesame stalk biomass. Biomass Convers Biorefinery. 2020;10(4):1179–87. doi:10.1007/s13399-019-00477-3. [Google Scholar] [CrossRef]
58. Jafri Y, Wetterlund E, Anheden M, Kulander I, Håkansson Å, Furusjö E. Multi-aspect evaluation of integrated forest-based biofuel production pathways: part 1. Product yields & energetic performance. Energy. 2019;166:401–13. doi:10.1016/j.energy.2018.10.008. [Google Scholar] [CrossRef]
59. Guedes RE, Luna AS, Torres AR. Operating parameters for bio-oil production in biomass pyrolysis: a review. J Anal Appl Pyrolysis. 2018;129(40):134–49. doi:10.1016/j.jaap.2017.11.019. [Google Scholar] [CrossRef]
60. Afraz M, Muhammad F, Nisar J, Shah A, Munir S, Ali G, et al. Production of value added products from biomass waste by pyrolysis: an updated review. Waste Manag Bull. 2024;1(4):30–40. doi:10.1016/j.wmb.2023.08.004. [Google Scholar] [CrossRef]
61. Schmitt CC, Moreira R, Neves RC, Richter D, Funke A, Raffelt K, et al. From agriculture residue to upgraded product: the thermochemical conversion of sugarcane bagasse for fuel and chemical products. Fuel Process Technol. 2020;197:106199. doi:10.1016/j.fuproc.2019.106199. [Google Scholar] [CrossRef]
62. Wang WC, Lee AC. The study of producing “drop-in” fuels from agricultural waste through fast pyrolysis and catalytic hydro-processing. Renew Energy. 2019;133:1–10. doi:10.1016/j.renene.2018.10.022. [Google Scholar] [CrossRef]
63. Shemfe MB, Whittaker C, Gu S, Fidalgo B. Comparative evaluation of GHG emissions from the use of Miscanthus for bio-hydrocarbon production via fast pyrolysis and bio-oil upgrading. Appl Energy. 2016;176:22–33. doi:10.1016/j.apenergy.2016.04.113. [Google Scholar] [CrossRef]
64. Spatari S, Larnaudie V, Mannoh I, Wheeler MC, Macken NA, Mullen CA, et al. Environmental, exergetic and economic tradeoffs of catalytic- and fast pyrolysis-to-renewable diesel. Renew Energy. 2020;162(12):371–80. doi:10.1016/j.renene.2020.08.042. [Google Scholar] [CrossRef]
65. Suriapparao DV, Vinu R. Biomass waste conversion into value-added products via microwave-assisted Co-Pyrolysis platform. Renew Energy. 2021;170:400–9. doi:10.1016/j.renene.2021.02.010. [Google Scholar] [CrossRef]
66. Bayat H, Dehghanizadeh M, Jarvis JM, Brewer CE, Jena U. Hydrothermal liquefaction of food waste: effect of process parameters on product yields and chemistry. Front Sustain Food Syst. 2021;5:658592. doi:10.3389/fsufs.2021.658592. [Google Scholar] [CrossRef]
67. Sarkar JK, Wang Q. Characterization of pyrolysis products and kinetic analysis of waste jute stick biomass. Processes. 2020;8(7):837. doi:10.3390/pr8070837. [Google Scholar] [CrossRef]
68. Fadhil AB. Evaluation of apricot (Prunus armeniaca L.) seed kernel as a potential feedstock for the production of liquid bio-fuels and activated carbons. Energy Convers Manag. 2017;133(5):307–17. doi:10.1016/j.enconman.2016.12.014. [Google Scholar] [CrossRef]
69. Patil SA, Hebbal OD, Hotti SR. Bio-oil production by pyrolysis of Hibiscus cannabinus (Deccan Hemp) and Pongamia pinnata (Karanja) seed cake and its characterization. Int J Adv Tecnol Eng Explor. 2023;10(98):87. doi:10.19101/ijatee.2021.876172. [Google Scholar] [CrossRef]
70. Soni B, Karmee SK. Towards a continuous pilot scale pyrolysis based biorefinery for production of biooil and biochar from sawdust. Fuel. 2020;271(11):117570. doi:10.1016/j.fuel.2020.117570. [Google Scholar] [CrossRef]
71. Qing M, Long Y, Liu L, Yi Y, Li W, He R, et al. Pyrolysis of the food waste collected from catering and households under different temperatures: assessing the evolution of char structure and bio-oil composition. J Anal Appl Pyrolysis. 2022;164(1):105543. doi:10.1016/j.jaap.2022.105543. [Google Scholar] [CrossRef]
72. Patra BR, Nanda S, Dalai AK, Meda V. Slow pyrolysis of agro-food wastes and physicochemical characterization of biofuel products. Chemosphere. 2021;285:131431. doi:10.1016/j.chemosphere.2021.131431. [Google Scholar] [PubMed] [CrossRef]
73. Pascarella AE, Coppola A, Marrone S, Chirone R, Sansone C, Salatino P. Critical assessment of machine learning prediction of biomass pyrolysis. Fuel. 2025;394:135000. doi:10.1016/j.fuel.2025.135000. [Google Scholar] [CrossRef]
74. Guo S, Li Y, Wang Y, Wang L, Sun Y, Liu L. Recent advances in biochar-based adsorbents for CO2 capture. Carbon Capture Sci Technol. 2022;4:100059. doi:10.1016/j.ccst.2022.100059. [Google Scholar] [CrossRef]
75. Dada TK, Sheehan M, Murugavelh S, Antunes E. A review on catalytic pyrolysis for high-quality bio-oil production from biomass. Biomass Convers Biorefinery. 2023;13(4):2595–614. doi:10.1007/s13399-021-01391-3. [Google Scholar] [CrossRef]
76. Ighalo JO, Iwuozor KO, Ogunfowora LA, Abdulsalam A, Iwuchukwu FU, Itabana B, et al. Regenerative desulphurisation of pyrolysis oil: a paradigm for the circular economy initiative. J Environ Chem Eng. 2021;9(6):106864. doi:10.1016/j.jece.2021.106864. [Google Scholar] [CrossRef]
77. Shah MP, Kaur P,editors. Biomass energy for sustainable development. 1st ed. Boca Raton, FL, USA: CRC Press; 2024. doi:10.1201/9781003406501. [Google Scholar] [CrossRef]
78. Cortazar M, Santamaria L, Lopez G, Alvarez J, Zhang L, Wang R, et al. A comprehensive review of primary strategies for tar removal in biomass gasification. Energy Convers Manag. 2023;276:116496. doi:10.1016/j.enconman.2022.116496. [Google Scholar] [CrossRef]
79. Blasi A, Verardi A, Lopresto CG, Siciliano S, Sangiorgio P. Lignocellulosic agricultural waste valorization to obtain valuable products: an overview. Recycling. 2023;8(4):61. doi:10.3390/recycling8040061. [Google Scholar] [CrossRef]
80. Sun C, Tan H, Zhang Y. Simulating the pyrolysis interactions among hemicellulose, cellulose and lignin in wood waste under real conditions to find the proper way to prepare bio-oil. Renew Energy. 2023;205:851–63. doi:10.1016/j.renene.2023.02.015. [Google Scholar] [CrossRef]
81. Kan T, Strezov V, Evans T, He J, Kumar R, Lu Q. Catalytic pyrolysis of lignocellulosic biomass: a review of variations in process factors and system structure. Renew Sustain Energy Rev. 2020;134:110305. doi:10.1016/j.rser.2020.110305. [Google Scholar] [CrossRef]
82. Oh S, Lee JH, Choi JW. Hydrodeoxygenation of crude bio-oil with various metal catalysts in a continuous-flow reactor and evaluation of emulsion properties of upgraded bio-oil with petroleum fuel. Renew Energy. 2020;160(4):1160–7. doi:10.1016/j.renene.2020.07.051. [Google Scholar] [CrossRef]
83. Aboelela D, Saleh H, Attia AM, Elhenawy Y, Majozi T, Bassyouni M. Recent advances in biomass pyrolysis processes for bioenergy production: optimization of operating conditions. Sustainability. 2023;15(14):11238. doi:10.3390/su151411238. [Google Scholar] [CrossRef]
84. Ahmed A, Abu Bakar MS, Sukri RS, Hussain M, Farooq A, Moogi S, et al. Sawdust pyrolysis from the furniture industry in an auger pyrolysis reactor system for biochar and bio-oil production. Energy Convers Manag. 2020;226:113502. doi:10.1016/j.enconman.2020.113502. [Google Scholar] [CrossRef]
85. Gupta A, Thengane SK, Mahajani S. Kinetics of pyrolysis and gasification of cotton stalk in the central parts of India. Fuel. 2020;263(1207):116752. doi:10.1016/j.fuel.2019.116752. [Google Scholar] [CrossRef]
86. Wang Y, Mu J, Zhang X, Ding X, Zheng M, Guo T. Optimized torrefaction of corn straw in a screw reactor: energy balance analysis and biochar production enhancement. Processes. 2025;13(5):1302. doi:10.3390/pr13051302. [Google Scholar] [CrossRef]
87. Mahima J, Sundaresh RK, Gopinath KP, Rajan PSS, Arun J, Kim SH, et al. Effect of algae (Scenedesmus obliquus) biomass pre-treatment on bio-oil production in hydrothermal liquefaction (HTLbiochar and aqueous phase utilization studies. Sci Total Env. 2021;778:146262. doi:10.1016/j.scitotenv.2021.146262. [Google Scholar] [PubMed] [CrossRef]
88. Rahib Y, Sarh B, Bostyn S, Bonnamy S, Boushaki T, Chaoufi J. Non-isothermal kinetic analysis of the combustion of argan shell biomass. Mater Today Proc. 2020;24:11–6. doi:10.1016/j.matpr.2019.07.437. [Google Scholar] [CrossRef]
89. Qureshi KM, Kay Lup AN, Khan S, Abnisa F, Wan Daud WMA. A technical review on semi-continuous and continuous pyrolysis process of biomass to bio-oil. J Anal Appl Pyrolysis. 2018;131(1):52–75. doi:10.1016/j.jaap.2018.02.010. [Google Scholar] [CrossRef]
90. Wang B, Lan J, Bo C, Gong B, Ou J. Adsorption of heavy metal onto biomass-derived activated carbon: review. RSC Adv. 2023;13(7):4275–302. doi:10.1039/D2RA07911A. [Google Scholar] [PubMed] [CrossRef]
91. Lasek J, Głód K, Słowik K, Cygan A, Li YH. Static and dynamic characteristics of rotary kiln reactor during processing of biomass and municipal solid waste. Powder Technol. 2022;404:117476. doi:10.1016/j.powtec.2022.117476. [Google Scholar] [CrossRef]
92. Zhang Y, Yi W, Fu P, Li Z, Bai X, Tian C, et al. Flow and reaction characteristics on catalytic upgrading of biomass pyrolysis vapors in novel cyclone reactors. Energy. 2019;189:116052. doi:10.1016/j.energy.2019.116052. [Google Scholar] [CrossRef]
93. Fakayode OA, Aboagarib EAA, Zhou C, Ma H. Co-pyrolysis of lignocellulosic and macroalgae biomasses for the production of biochar—a review. Bioresour Technol. 2020;297(4):122408. doi:10.1016/j.biortech.2019.122408. [Google Scholar] [PubMed] [CrossRef]
94. Dhyani V, Bhaskar T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew Energy. 2018;129:695–716. doi:10.1016/j.renene.2017.04.035. [Google Scholar] [CrossRef]
95. Fekhar B, Zsinka V, Miskolczi N. Thermo-catalytic co-pyrolysis of waste plastic and paper in batch and tubular reactors for in situ product improvement. J Env Manag. 2020;269:110741. doi:10.1016/j.jenvman.2020.110741. [Google Scholar] [PubMed] [CrossRef]
96. Ida H, Ohtani H, Karagöz S. Online fast pyrolysis of cellulose over titanium dioxide using tandem micro-reactor-GC-MS. Sustain Chem Pharm. 2020;16:100268. doi:10.1016/j.scp.2020.100268. [Google Scholar] [CrossRef]
97. Mkhize NM, Danon B, Alvarez J, Lopez G, Amutio M, Bilbao J, et al. Influence of reactor and condensation system design on tyre pyrolysis products yields. J Anal Appl Pyrolysis. 2019;143:104683. doi:10.1016/j.jaap.2019.104683. [Google Scholar] [CrossRef]
98. Qi F, Wright MM. A DEM modeling of biomass fast pyrolysis in a double auger reactor. Int J Heat Mass Transf. 2020;150(2):119308. doi:10.1016/j.ijheatmasstransfer.2020.119308. [Google Scholar] [CrossRef]
99. Lewandowski WM, Januszewicz K, Kosakowski W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—a review. J Anal Appl Pyrolysis. 2019;140(011001):25–53. doi:10.1016/j.jaap.2019.03.018. [Google Scholar] [CrossRef]
100. Ding K, Xiong Q, Zhong Z, Zhong D, Zhang Y. CFD simulation of combustible solid waste pyrolysis in a fluidized bed reactor. Powder Technol. 2020;362(13):177–87. doi:10.1016/j.powtec.2019.12.011. [Google Scholar] [CrossRef]
101. Park JY, Kim JK, Oh CH, Park JW, Kwon EE. Production of bio-oil from fast pyrolysis of biomass using a pilot-scale circulating fluidized bed reactor and its characterization. J Environ Manag. 2019;234:138–44. doi:10.1016/j.jenvman.2018.12.104. [Google Scholar] [PubMed] [CrossRef]
102. Chen D, Yin L, Wang H, He P. Pyrolysis technologies for municipal solid waste: a review. Waste Manag. 2014;34(12):2466–86. doi:10.1016/j.wasman.2014.08.004. [Google Scholar] [PubMed] [CrossRef]
103. Zheng X, Shang M, Zhang B, Li Y, Wang S, Huang Z. The co-combustion and pollutant emission characteristics of lignite and lake sediment. Fuel Process Technol. 2025;274:108240. doi:10.1016/j.fuproc.2025.108240. [Google Scholar] [CrossRef]
104. Czajczyńska D, Anguilano L, Ghazal H, Krzyżyńska R, Reynolds AJ, Spencer N, et al. Potential of pyrolysis processes in the waste management sector. Therm Sci Eng Prog. 2017;3:171–97. doi:10.1016/j.tsep.2017.06.003. [Google Scholar] [CrossRef]
105. Chen J, Fang D, Duan F. Pore characteristics and fractal properties of biochar obtained from the pyrolysis of coarse wood in a fluidized-bed reactor. Appl Energy. 2018;218:54–65. doi:10.1016/j.apenergy.2018.02.179. [Google Scholar] [CrossRef]
106. Ly HV, Park JW, Kim SS, Hwang HT, Kim J, Woo HC. Catalytic pyrolysis of bamboo in a bubbling fluidized-bed reactor with two different catalysts: hZSM-5 and red mud for upgrading bio-oil. Renew Energy. 2020;149:1434–45. doi:10.1016/j.renene.2019.10.141. [Google Scholar] [CrossRef]
107. Pielsticker S, Schlögel K, Kreitzberg T, Hatzfeld O, Kneer R. Biomass pyrolysis kinetics in a fluidized bed reactor: measurements and plausibility verification for reaction conditions. Fuel. 2019;254(2):115589. doi:10.1016/j.fuel.2019.05.172. [Google Scholar] [CrossRef]
108. Ly HV, Choi JH, Woo HC, Kim SS, Kim J. Upgrading bio-oil by catalytic fast pyrolysis of acid-washed Saccharina Japonica Alga in a fluidized-bed reactor. Renew Energy. 2019;133(Part A):11–22. doi:10.1016/j.renene.2018.09.103. [Google Scholar] [CrossRef]
109. Yang C, Li R, Zhang B, Qiu Q, Wang B, Yang H, et al. Pyrolysis of microalgae: a critical review. Fuel Process Technol. 2019;186:53–72. doi:10.1016/j.fuproc.2018.12.012. [Google Scholar] [CrossRef]
110. Xue Y, Zhou S, Brown RC, Kelkar A, Bai X. Fast pyrolysis of biomass and waste plastic in a fluidized bed reactor. Fuel. 2015;156:40–6. doi:10.1016/j.fuel.2015.04.033. [Google Scholar] [CrossRef]
111. Kaminsky W. Chemical recycling of plastics by fluidized bed pyrolysis. Fuel Commun. 2021;8(6):100023. doi:10.1016/j.jfueco.2021.100023. [Google Scholar] [CrossRef]
112. Edwin Raj R, Robert Kennedy Z, Pillai BC. Optimization of process parameters in flash pyrolysis of waste tyres to liquid and gaseous fuel in a fluidized bed reactor. Energy Convers Manag. 2013;67:145–51. doi:10.1016/j.enconman.2012.11.012. [Google Scholar] [CrossRef]
113. Wang J, Zhong Z, Ding K, Li M, Hao N, Meng X, et al. Catalytic fast co-pyrolysis of bamboo sawdust and waste tire using a tandem reactor with cascade bubbling fluidized bed and fixed bed system. Energy Convers Manag. 2019;180:60–71. doi:10.1016/j.enconman.2018.10.056. [Google Scholar] [CrossRef]
114. Liu Y, Ran C, Ali Siyal A, Song Y, Jiang Z, Dai J, et al. Comparative study for fluidized bed pyrolysis of textile dyeing sludge and municipal sewage sludge. J Hazard Mater. 2020;396:122619. doi:10.1016/j.jhazmat.2020.122619. [Google Scholar] [PubMed] [CrossRef]
115. AlDayyat EA, Saidan MN, Al-Hamamre Z, Al-Addous M, Alkasrawi M. Pyrolysis of solid waste for bio-oil and char production in refugees’ camp: a case study. Energies. 2021;14(13):3861. doi:10.3390/en14133861. [Google Scholar] [CrossRef]
116. Anuar Sharuddin SD, Abnisa F, Wan Daud WMA, Aroua MK. A review on pyrolysis of plastic wastes. Energy Convers Manag. 2016;115:308–26. doi:10.1016/j.enconman.2016.02.037. [Google Scholar] [CrossRef]
117. Bridgwater AV. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy. 2012;38(2):68–94. doi:10.1016/j.biombioe.2011.01.048. [Google Scholar] [CrossRef]
118. Guda VK, Steele PH, Penmetsa VK, Li Q. Chapter 7 fast pyrolysis of biomass recent advances in fast pyrolysis technology. Recent Adv Thermo-Chem Convers Biomass. 2015;2015(1):177–211. doi:10.1016/B978-0-444-63289-0.00007-7. [Google Scholar] [CrossRef]
119. Kakku S, Rathod W, Chakinala AG, Joshi J, Thota C, Somasundaram M, et al. Utilization of customized fixed bed system for bio-oil production from agricultural residue. Biomass Convers Biorefinery. 2025;15(11):16931–43. doi:10.1007/s13399-024-06421-4. [Google Scholar] [CrossRef]
120. Xiong Z, Syed-Hassan SSA, Xu J, Wang Y, Hu S, Su S, et al. Evolution of coke structures during the pyrolysis of bio-oil at various temperatures and heating rates. J Anal Appl Pyrolysis. 2018;134(6008):336–42. doi:10.1016/j.jaap.2018.06.023. [Google Scholar] [CrossRef]
121. Van de steene L, Tagutchou JP, Mermoud F, Martin E, Salvador S. A new experimental Continuous Fixed Bed Reactor to characterise wood char gasification. Fuel. 2010;89(11):3320–9. doi:10.1016/j.fuel.2010.03.035. [Google Scholar] [CrossRef]
122. Chew JJ, Soh M, Sunarso J, Yong ST, Doshi V, Bhattacharya S. Gasification of torrefied oil palm biomass in a fixed-bed reactor: effects of gasifying agents on product characteristics. J Energy Inst. 2020;93(2):711–22. doi:10.1016/j.joei.2019.05.010. [Google Scholar] [CrossRef]
123. Arabiourrutia M, Lopez G, Artetxe M, Alvarez J, Bilbao J, Olazar M. Waste tyre valorization by catalytic pyrolysis—a review. Renew Sustain Energy Rev. 2020;129:109932. doi:10.1016/j.rser.2020.109932. [Google Scholar] [CrossRef]
124. Rokhman BB, Vyfatnyuk VG. The two-stage process of thermochemical processing of lignin in a fixed bed. 3. Simulation and numerical analysis of the nonstationary process of pyrolysis in a retort. J Eng Phys Thermophys. 2023;96(2):506–12. doi:10.1007/s10891-023-02711-2. [Google Scholar] [CrossRef]
125. Mankeed P, Khuenkaeo N, Malik FR, Tippayawong N. Temperature evolution and heating rates of biomass undergoing ablative pyrolysis. Eng Technol Appl Sci Res. 2023;13(2):10301–5. doi:10.48084/etasr.5621. [Google Scholar] [CrossRef]
126. Khuenkaeo N, MacQueen B, Onsree T, Daiya S, Tippayawong N, Lauterbach J. Bio-oils from vacuum ablative pyrolysis of torrefied tobacco residues. RSC Adv. 2020;10(58):34986–95. doi:10.1039/d0ra06014c. [Google Scholar] [PubMed] [CrossRef]
127. Bridgwater T. Challenges and opportunities in fast pyrolysis of biomass: part I. Johns Matthey Technol Rev. 2018;62(1):118–30. doi:10.1595/205651318X696693. [Google Scholar] [CrossRef]
128. Sullivan TP, Sullivan DS, Klenner W. Fate of postharvest woody debris, mammal habitat, and alternative management of forest residues on clearcuts: a synthesis. Forests. 2021;12(5):551. doi:10.3390/f12050551. [Google Scholar] [CrossRef]
129. Ibitoye SE, Mahamood RM, Olayemi OA, Jen TC, Omoniyi PO, Loha C, et al. Design and construction of low-cost biomass pyrolysis reactor for research and teaching in universities and colleges. Biomass Convers Biorefinery. 2025;15(12):18887–903. doi:10.1007/s13399-024-06375-7. [Google Scholar] [CrossRef]
130. Altundoğan HS, Tanyıldızı MŞ, Kalender M, Elçiçek S, Pehlivan D. A novel screw-type pyrolysis system for selectively producing value-added chemicals from lignocellulosic biomass. Biomass Convers Biorefinery. 2025;15(6):9193–204. doi:10.1007/s13399-024-05791-z. [Google Scholar] [CrossRef]
131. Campuzano F, Brown RC, Martínez JD. Auger reactors for pyrolysis of biomass and wastes. Renew Sustain Energy Rev. 2019;102:372–409. doi:10.1016/j.rser.2018.12.014. [Google Scholar] [CrossRef]
132. Liaw SS, Zhou S, Wu H, Garcia-Perez M. Effect of pretreatment temperature on the yield and properties of bio-oils obtained from the auger pyrolysis of Douglas fir wood. Fuel. 2013;103(2):672–82. doi:10.1016/j.fuel.2012.08.016. [Google Scholar] [CrossRef]
133. Yu Y, Yang Y, Cheng Z, Blanco PH, Liu R, Bridgwater AV, et al. Pyrolysis of rice husk and corn stalk in auger reactor. 1. characterization of char and gas at various temperatures. Energy Fuels. 2016;30(12):10568–74. doi:10.1021/acs.energyfuels.6b02276. [Google Scholar] [CrossRef]
134. Daugaard TJ, Dalluge DL, Brown RC, Wright MM. Effect of thermophysical properties of heat carriers on performance of a laboratory-scale auger pyrolyzer. Fuel Process Technol. 2018;176:182–9. doi:10.1016/j.fuproc.2018.03.024. [Google Scholar] [CrossRef]
135. Raza M, Inayat A, Ahmed A, Jamil F, Ghenai C, Naqvi SR, et al. Progress of the pyrolyzer reactors and advanced technologies for biomass pyrolysis processing. Sustainability. 2021;13(19):11061. doi:10.3390/su131911061. [Google Scholar] [CrossRef]
136. Tahir MH, Vasudev V, Ibrahim M, Alotaibi MO, Abbas FM, Asseri TAY, et al. Optimizing waste valorization: catalytic co-pyrolysis of cabbage waste and tire waste for enhanced bio-oil and syngas production utilizing char as a reforming catalyst. RSC Adv. 2025;15(25):19937–46. doi:10.1039/d5ra03554f. [Google Scholar] [PubMed] [CrossRef]
137. Sharifzadeh M, Sadeqzadeh M, Guo M, Borhani TN, Murthy Konda NVSN, Garcia MC, et al. The multi-scale challenges of biomass fast pyrolysis and bio-oil upgrading: review of the state of art and future research directions. Prog Energy Combust Sci. 2019;71:1–80. doi:10.1016/j.pecs.2018.10.006. [Google Scholar] [CrossRef]
138. Elhambakhsh A, Van Duc Long N, Lamichhane P, Hessel V. Recent progress and future directions in plasma-assisted biomass conversion to hydrogen. Renew Energy. 2023;218:119307. doi:10.1016/j.renene.2023.119307. [Google Scholar] [CrossRef]
139. Muvhiiwa RF, Sempuga B, Hildebrandt D, Van Der Walt J. Study of the effects of temperature on syngas composition from pyrolysis of wood pellets using a nitrogen plasma torch reactor. J Anal Appl Pyrolysis. 2018;130(3):159–68. doi:10.1016/j.jaap.2018.01.014. [Google Scholar] [CrossRef]
140. Sikarwar VS, Hrabovský M, Van Oost G, Pohořelý M, Jeremiáš M. Progress in waste utilization via thermal plasma. Prog Energy Combust Sci. 2020;81:100873. doi:10.1016/j.pecs.2020.100873. [Google Scholar] [CrossRef]
141. Zeng K, Minh DP, Gauthier D, Weiss-Hortala E, Nzihou A, Flamant G. The effect of temperature and heating rate on char properties obtained from solar pyrolysis of beech wood. Bioresour Technol. 2015;182:114–9. doi:10.1016/j.biortech.2015.01.112. [Google Scholar] [PubMed] [CrossRef]
142. Ullah F, Hasrat K, Mu M, Wang S, Kumar S. Optimizing solar-biomass pyrolysis: innovations in reactor design and thermal-solar system efficiency. Energies. 2025;18(10):2640. doi:10.3390/en18102640. [Google Scholar] [CrossRef]
143. Emran M, El-Gamal EH, Mokhiamar O, Elsamni O, Rashad M. A novel solar disk chamber reactor for agricultural waste recycling and biochar production. Clean Technol Environ Policy. 2024;26(2):467–79. doi:10.1007/s10098-023-02635-8. [Google Scholar] [CrossRef]
144. Gouda A, Merhi N, Hmadeh M, Cecchi T, Santato C, Sain M. Sustainable strategies for converting organic, electronic, and plastic waste from municipal solid waste into functional materials. Glob Chall. 2025;9(4):2400240. doi:10.1002/gch2.202400240. [Google Scholar] [PubMed] [CrossRef]
145. Bieniek A, Sieradzka M, Jerzak W, Magdziarz A. Fast pyrolysis of agricultural biomass in drop tube reactor for bio-oil production: numerical calculations. J Anal Appl Pyrolysis. 2023;176:106241. doi:10.1016/j.jaap.2023.106241. [Google Scholar] [CrossRef]
146. Jung SH, Kang BS, Kim JS. Production of bio-oil from rice straw and bamboo sawdust under various reaction conditions in a fast pyrolysis plant equipped with a fluidized bed and a char separation system. J Anal Appl Pyrolysis. 2008;82(2):240–7. doi:10.1016/j.jaap.2008.04.001. [Google Scholar] [CrossRef]
147. Elhenawy Y, Fouad K, Bassyouni M, Al-Qabandi OA, Majozi T. Yield and energy outputs analysis of sawdust biomass pyrolysis. Energy Convers Manag X. 2024;22(14):100583. doi:10.1016/j.ecmx.2024.100583. [Google Scholar] [CrossRef]
148. Mishra RK, Mohanty K. Thermal and catalytic pyrolysis of pine sawdust (Pinus ponderosa) and Gulmohar seed (Delonix regia) towards production of fuel and chemicals. Mater Sci Energy Technol. 2019;2(2):139–49. doi:10.1016/j.mset.2018.12.004. [Google Scholar] [CrossRef]
149. Prasetiawan H, Fardhyanti DS, Fatrisari W, Hadiyanto H. Preliminary study on the bio-oil production from multi feed-stock biomass waste via fast pyrolysis process. J Adv Res Fluid Mech Therm Sci. 2023;103(2):216–27. doi:10.37934/arfmts.103.2.216227. [Google Scholar] [CrossRef]
150. Le Brech Y, Jia L, Cissé S, Mauviel G, Brosse N, Dufour A. Mechanisms of biomass pyrolysis studied by combining a fixed bed reactor with advanced gas analysis. J Anal Appl Pyrolysis. 2016;117:334–46. doi:10.1016/j.jaap.2015.10.013. [Google Scholar] [CrossRef]
151. Phan BMQ, Duong LT, Nguyen VD, Tran TB, Nguyen MHH, Nguyen LH, et al. Evaluation of the production potential of bio-oil from Vietnamese biomass resources by fast pyrolysis. Biomass Bioenergy. 2014;62(12):74–81. doi:10.1016/j.biombioe.2014.01.012. [Google Scholar] [CrossRef]
152. Wang L, Zhou T, Hou B, Yang H, Hu N, Zhang M. A comprehensive review of biomass gasification characteristics in fluidized bed reactors: progress, challenges, and future directions. Fluids. 2025;10(6):147. doi:10.3390/fluids10060147. [Google Scholar] [CrossRef]
153. Mo F, Ullah H, Zada N, Shahab A. A review on catalytic co-pyrolysis of biomass and plastics waste as a thermochemical conversion to produce valuable products. Energies. 2023;16(14):5403. doi:10.3390/en16145403. [Google Scholar] [CrossRef]
154. Abnisa F, Wan Daud WMA. A review on co-pyrolysis of biomass: an optional technique to obtain a high-grade pyrolysis oil. Energy Convers Manag. 2014;87:71–85. doi:10.1016/j.enconman.2014.07.007. [Google Scholar] [CrossRef]
155. Sharma A, Pareek V, Zhang D. Biomass pyrolysis—a review of modelling, process parameters and catalytic studies. Renew Sustain Energy Rev. 2015;50(2009):1081–96. doi:10.1016/j.rser.2015.04.193. [Google Scholar] [CrossRef]
156. Iisa K, Mukarakate C, French RJ, Agblevor FA, Santosa DM, Wang H, et al. From biomass to fuel blendstocks via catalytic fast pyrolysis and hydrotreating: an evaluation of carbon efficiency and fuel properties for three pathways. Energy Fuels. 2023;37(24):19653–63. doi:10.1021/acs.energyfuels.3c03239. [Google Scholar] [PubMed] [CrossRef]
157. Pang S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol Adv. 2019;37(4):589–97. doi:10.1016/j.biotechadv.2018.11.004. [Google Scholar] [PubMed] [CrossRef]
158. Sun W, Yan Y, Wei Y, Ma J, Niu Z, Hu G. Catalytic pyrolysis of biomass: a review of zeolite, carbonaceous, and metal oxide catalysts. Nanomater. 2025;15(7):493. doi:10.3390/nano15070493. [Google Scholar] [PubMed] [CrossRef]
159. Jideani T, Chukwuchendo E, Khotseng L. An approach towards the conversion of biomass feedstocks into biofuel using a zeolite socony mobil-5-based catalysts via the hydrothermal liquefaction process: a review. Catalysts. 2023;13(11):1425. doi:10.3390/catal13111425. [Google Scholar] [CrossRef]
160. Butt W, Cabello JH, Hedlund J, Shafaghat H. Structure-modified zeolites for an enhanced production of bio jet fuel components via catalytic pyrolysis of forestry residues. Catal Lett. 2025;155(3):116. doi:10.1007/s10562-025-04958-1. [Google Scholar] [CrossRef]
161. Cai W, Luo Z, Zhou J, Wang Q. A review on the selection of raw materials and reactors for biomass fast pyrolysis in China. Fuel Process Technol. 2021;221:106919. doi:10.1016/j.fuproc.2021.106919. [Google Scholar] [CrossRef]
162. Khelfa A, Rodrigues, Augusto F, Koubaa, Mohamed, Vorobiev E. Microwave-assisted pyrolysis of pine wood sawdust. Processes. 2020;8:1–12. [Google Scholar]
163. Wang X, Wu F, Li C, Chen M, Wang J. High quality bio-oil production from catalytic microwave-assisted pyrolysis of pine sawdust. BioResources. 2018;13(3):5479–90. doi:10.15376/biores.13.3.5479-5490. [Google Scholar] [CrossRef]
164. Mishra RK, Mohanty K. Pyrolysis of low-value waste sawdust over low-cost catalysts: physicochemical characterization of pyrolytic oil and value-added biochar. Biofuel Res J. 2022;9(4):1736–49. doi:10.18331/brj2022.9.4.4. [Google Scholar] [CrossRef]
165. Bello SI, Uthman TO, Surgun S, Sokoto AM. Urea enhances the yield and quality of bio-oil produced from corncob through pyrolysis. Sci Rep. 2025;15(1):2990. doi:10.1038/s41598-025-86800-7. [Google Scholar] [PubMed] [CrossRef]
166. Ke L, Zhou N, Wu Q, Zeng Y, Tian X, Zhang J, et al. Microwave catalytic pyrolysis of biomass: a review focusing on absorbents and catalysts. npj Mater Sustain. 2024;2(1):24. doi:10.1038/s44296-024-00027-7. [Google Scholar] [CrossRef]
167. Li Y, Campos LC, Hu Y. Microwave pretreatment of wastewater sludge technology—a scientometric-based review. Environ Sci Pollut Res. 2024;31(18):26432–51. doi:10.1007/s11356-024-32931-9. [Google Scholar] [PubMed] [CrossRef]
168. Ramírez Cabrera PA, Lozano Pérez AS, Guerrero Fajardo CA. Design of a semi-continuous microwave system for pretreatment of microwave-assisted pyrolysis using a theoretical method. Inventions. 2025;10(2):24. doi:10.3390/inventions10020024. [Google Scholar] [CrossRef]
169. Singh R, Lindenberger C, Chawade A, Vivekanand V. Unveiling the microwave heating performance of biochar as microwave absorber for microwave-assisted pyrolysis technology. Sci Rep. 2024;14(1):9222. doi:10.1038/s41598-024-59738-5. [Google Scholar] [PubMed] [CrossRef]
170. Zhang Y, Chen P, Liu S, Peng P, Min M, Cheng Y, et al. Effects of feedstock characteristics on microwave-assisted pyrolysis—a review. Bioresour Technol. 2017;230(23):143–51. doi:10.1016/j.biortech.2017.01.046. [Google Scholar] [PubMed] [CrossRef]
171. Sekar M, Mathimani T, Alagumalai A, Chi NTL, Duc PA, Bhatia SK, et al. A review on the pyrolysis of algal biomass for biochar and bio-oil—bottlenecks and scope. Fuel. 2021;283(8):119190. doi:10.1016/j.fuel.2020.119190. [Google Scholar] [CrossRef]
172. Mohd Mokhta Z, Ong MY, Salman B, Nomanbhay S, Salleh SF, Chew KW, et al. Simulation studies on microwave-assisted pyrolysis of biomass for bioenergy production with special attention on waveguide number and location. Energy. 2020;190(112):116474. doi:10.1016/j.energy.2019.116474. [Google Scholar] [CrossRef]
173. Ellison CR, Hoff R, Mărculescu C, Boldor D. Investigation of microwave-assisted pyrolysis of biomass with char in a rectangular waveguide applicator with built-in phase-shifting. Appl Energy. 2020;259(1):114217. doi:10.1016/j.apenergy.2019.114217. [Google Scholar] [CrossRef]
174. Tamošiūnas A, Gimžauskaitė D, Aikas M, Uscila R, Snapkauskienė V, Zakarauskas K, et al. Biomass gasification to syngas in thermal water vapor arc discharge plasma. Biomass Convers Biorefin. 2023;13(18):1–12. doi:10.1007/s13399-023-03828-3. [Google Scholar] [PubMed] [CrossRef]
175. Messerle VE, Ustimenko AB, Lavrichshev OA, Nugman MK. The gasification and pyrolysis of biomass using a plasma system. Energies. 2024;17(22):5594. doi:10.3390/en17225594. [Google Scholar] [CrossRef]
176. Toscano Miranda N, Lopes Motta I, Maciel Filho R, Wolf Maciel MR. Sugarcane bagasse pyrolysis: a review of operating conditions and products properties. Renew Sustain Energy Rev. 2021;149(2):111394. doi:10.1016/j.rser.2021.111394. [Google Scholar] [CrossRef]
177. Modak S, Katiyar P, Talukdar D, Gole B. Pyrolytic evaluation of essential oil industry waste: effect of pyrolysis temperature on bio-oil composition. J Environ Manag. 2025;392:126757. doi:10.1016/j.jenvman.2025.126757. [Google Scholar] [PubMed] [CrossRef]
178. Zhao A, Liu S, Yao J, Huang F, He Z, Liu J. Characteristics of bio-oil and biochar from cotton stalk pyrolysis: effects of torrefaction temperature and duration in an ammonia environment. Bioresour Technol. 2022;343(1):126145. doi:10.1016/j.biortech.2021.126145. [Google Scholar] [PubMed] [CrossRef]
179. Fu J, Liu J, Xu W, Chen Z, Evrendilek F, Sun S. Torrefaction, temperature, and heating rate dependencies of pyrolysis of coffee grounds: its performances, bio-oils, and emissions. Bioresour Technol. 2022;345(4):126346. doi:10.1016/j.biortech.2021.126346. [Google Scholar] [PubMed] [CrossRef]
180. Sohaib Q, Muhammad A, Younas M. Fast pyrolysis of sugarcane bagasse: effect of pyrolysis conditions on final product distribution and properties. Energy Sources Part A Recovery Util Environ Eff. 2017;39(2):184–90. doi:10.1080/15567036.2016.1212292. [Google Scholar] [CrossRef]
181. Lin BJ, Chen WH. Sugarcane bagasse pyrolysis in a carbon dioxide atmosphere with conventional and microwave-assisted heating. Front Energy Res. 2015;3:4. doi:10.3389/fenrg.2015.00004. [Google Scholar] [CrossRef]
182. Tsai WT, Lee MK, Chang YM. Fast pyrolysis of rice husk: product yields and compositions. Bioresour Technol. 2007;98(1):22–8. doi:10.1016/j.biortech.2005.12.005. [Google Scholar] [PubMed] [CrossRef]
183. Huang S, Qin J, He Q, Wen Y, Huang S, Li B, et al. Torrefied herb residues in nitrogen, air and oxygen atmosphere: thermal decomposition behavior and pyrolytic products characters. Bioresour Technol. 2021;342(3):125991. doi:10.1016/j.biortech.2021.125991. [Google Scholar] [PubMed] [CrossRef]
184. Zhao J, Wang Z, Li J, Yan B, Chen G. Pyrolysis of food waste and food waste solid digestate: a comparative investigation. Bioresour Technol. 2022;354(2):127191. doi:10.1016/j.biortech.2022.127191. [Google Scholar] [PubMed] [CrossRef]
185. Alvarez J, Lopez G, Amutio M, Bilbao J, Olazar M. Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel. 2014;128:162–9. doi:10.1016/j.fuel.2014.02.074. [Google Scholar] [CrossRef]
186. Wang J, Zhang B, Shujaa Aldeen A, Mwenya S, Cheng H, Xu Z, et al. Enhancing production of hydrocarbon-rich bio-oil from biomass via catalytic fast pyrolysis coupled with advanced oxidation process pretreatment. Bioresour Technol. 2022;359(9):127450. doi:10.1016/j.biortech.2022.127450. [Google Scholar] [PubMed] [CrossRef]
187. Şensöz S, Demiral İ, Ferdi Gerçel H. Olive bagasse (Olea europea L.) pyrolysis. Bioresour Technol. 2006;97(3):429–36. doi:10.1016/j.biortech.2005.03.007. [Google Scholar] [PubMed] [CrossRef]
188. Moralı U, Şensöz S. Pyrolysis of hornbeam shell (Carpinus betulus L.) in a fixed bed reactor: characterization of bio-oil and bio-char. Fuel. 2015;150:672–8. doi:10.1016/j.fuel.2015.02.095. [Google Scholar] [CrossRef]
189. Bhoi PR, Ouedraogo AS, Soloiu V, Quirino R. Recent advances on catalysts for improving hydrocarbon compounds in bio-oil of biomass catalytic pyrolysis. Renew Sustain Energy Rev. 2020;121:109676. doi:10.1016/j.rser.2019.109676. [Google Scholar] [CrossRef]
190. Xiong Z, Wang Y, Syed-Hassan SSA, Hu X, Han H, Su S, et al. Effects of heating rate on the evolution of bio-oil during its pyrolysis. Energy Convers Manag. 2018;163:420–7. doi:10.1016/j.enconman.2018.02.078. [Google Scholar] [CrossRef]
191. Wang Y, Akbarzadeh A, Chong L, Du J, Tahir N, Awasthi MK. Catalytic pyrolysis of lignocellulosic biomass for bio-oil production: a review. Chemosphere. 2022;297:134181. doi:10.1016/j.chemosphere.2022.134181. [Google Scholar] [PubMed] [CrossRef]
192. Carrasco Díaz A, Abdelouahed L, Brodu N, Montes-Jiménez V, Taouk B. Upgrading of pyrolysis bio-oil by catalytic hydrodeoxygenation, a review focused on catalysts, model molecules, deactivation, and reaction routes. Molecules. 2024;29(18):4325. doi:10.3390/molecules29184325. [Google Scholar] [PubMed] [CrossRef]
193. Rowland SM, Martinez R, Wrasman CJ, Iisa K, Nimlos MR, Griffin MB. Thermal reactivity of bio-oil produced from catalytic fast pyrolysis of biomass. Energy Fuels. 2024;38(20):19626–38. doi:10.1021/acs.energyfuels.4c03430. [Google Scholar] [PubMed] [CrossRef]
194. Ryu HW, Kim DH, Jae J, Lam SS, Park ED, Park YK. Recent advances in catalytic co-pyrolysis of biomass and plastic waste for the production of petroleum-like hydrocarbons. Bioresour Technol. 2020;310:123473. doi:10.1016/j.biortech.2020.123473. [Google Scholar] [PubMed] [CrossRef]
195. Su G, Ong HC, Gan YY, Chen WH, Chong CT, Ok YS. Co-pyrolysis of microalgae and other biomass wastes for the production of high-quality bio-oil: progress and prospective. Bioresour Technol. 2022;344(12):126096. doi:10.1016/j.biortech.2021.126096. [Google Scholar] [PubMed] [CrossRef]
196. Lachos-Perez D, César Torres-Mayanga P, Abaide ER, Zabot GL, De Castilhos F. Hydrothermal carbonization and Liquefaction: differences, progress, challenges, and opportunities. Bioresour Technol. 2022;343(7):126084. doi:10.1016/j.biortech.2021.126084. [Google Scholar] [PubMed] [CrossRef]
197. Yang Q, Wu Q, Zhou N, Ke L, Fan L, Zeng Y, et al. Continuous catalytic pyrolysis of waste oil to aromatics: exploring the structure-performance relations of ZSM-5 based on different scale-up forms. Chem Eng J. 2023;475(5):146259. doi:10.1016/j.cej.2023.146259. [Google Scholar] [CrossRef]
198. Chen D, Li Y, Deng M, Wang J, Chen M, Yan B, et al. Effect of torrefaction pretreatment and catalytic pyrolysis on the pyrolysis poly-generation of pine wood. Bioresour Technol. 2016;214(7):615–22. doi:10.1016/j.biortech.2016.04.058. [Google Scholar] [PubMed] [CrossRef]
199. Sun T, Li Z, Zhang Z, Wang Z, Yang S, Yang Y, et al. Fast corn stalk pyrolysis and the influence of catalysts on product distribution. Bioresour Technol. 2020;301(1):122739. doi:10.1016/j.biortech.2020.122739. [Google Scholar] [PubMed] [CrossRef]
200. Daligaux V, Richard R, Manero MH. Deactivation and regeneration of zeolite catalysts used in pyrolysis of plastic wastes—a process and analytical review. Catalysts. 2021;11(7):770. doi:10.3390/catal11070770. [Google Scholar] [CrossRef]
201. Cao Z, Niu J, Gu Y, Zhang R, Liu Y, Luo L. Catalytic pyrolysis of rice straw: screening of various metal salts, metal basic oxide, acidic metal oxide and zeolite catalyst on products yield and characterization. J Clean Prod. 2020;269(7):122079. doi:10.1016/j.jclepro.2020.122079. [Google Scholar] [CrossRef]
202. Zhang C, Zhang Z, Zhang L, Li Q, Li C, Chen G, et al. Evolution of the functionalities and structures of biochar in pyrolysis of poplar in a wide temperature range. Bioresour Technol. 2020;304(5):123002. doi:10.1016/j.biortech.2020.123002. [Google Scholar] [PubMed] [CrossRef]
203. Talwar P, Agudelo MA, Nanda S. Pyrolysis process, reactors, products, and applications: a review. Energies. 2025;18(11):2979. doi:10.3390/en18112979. [Google Scholar] [CrossRef]
204. Gao N, Wang F, Quan C, Santamaria L, Lopez G, Williams PT. Tire pyrolysis char: processes, properties, upgrading and applications. Prog Energy Combust Sci. 2022;93(5):101022. doi:10.1016/j.pecs.2022.101022. [Google Scholar] [CrossRef]
205. Madhu P, Kanagasabapathy H, Neethi Manickam I. Cotton shell utilization as a source of biomass energy for bio-oil by flash pyrolysis on electrically heated fluidized bed reactor. J Mater Cycles Waste Manag. 2016;18(1):146–55. doi:10.1007/s10163-014-0318-y. [Google Scholar] [CrossRef]
206. Tu P, Zhang G, Wei G, Li J, Li Y, Deng L, et al. Influence of pyrolysis temperature on the physicochemical properties of biochars obtained from herbaceous and woody plants. Bioresour Bioprocess. 2022;9(1):131. doi:10.1186/s40643-022-00618-z. [Google Scholar] [PubMed] [CrossRef]
207. Amenaghawon AN, Anyalewechi CL, Okieimen CO, Kusuma HS. Biomass pyrolysis technologies for value-added products: a state-of-the-art review. Environ Dev Sustain. 2021;23(10):14324–78. doi:10.1007/s10668-021-01276-5. [Google Scholar] [CrossRef]
208. Ambaye TG, Vaccari M, Bonilla-Petriciolet A, Prasad S, van Hullebusch ED, Rtimi S. Emerging technologies for biofuel production: a critical review on recent progress, challenges and perspectives. J Environ Manag. 2021;290(1):112627. doi:10.1016/j.jenvman.2021.112627. [Google Scholar] [PubMed] [CrossRef]
209. Hu G, Huang H, Li Y. Hydrogen-rich gas production from pyrolysis of biomass in an autogenerated steam atmosphere. Energy Fuels. 2009;23(3):1748–53. doi:10.1021/ef800988r. [Google Scholar] [CrossRef]
210. Al-Qahtani AM. A comprehensive review in microwave pyrolysis of biomass, syngas production and utilisation. Energies. 2023;16(19):6876. doi:10.3390/en16196876. [Google Scholar] [CrossRef]
211. Dyer AC, Nahil MA, Williams PT. Catalytic co-pyrolysis of biomass and waste plastics as a route to upgraded bio-oil. J Energy Inst. 2021;97(3):27–36. doi:10.1016/j.joei.2021.03.022. [Google Scholar] [CrossRef]
212. Saber M, Nakhshiniev B, Yoshikawa K. A review of production and upgrading of algal bio-oil. Renew Sustain Energy Rev. 2016;58:918–30. doi:10.1016/j.rser.2015.12.342. [Google Scholar] [CrossRef]
213. Peng X, Jiang Y, Chen Z, Osman AI, Farghali M, Rooney DW, et al. Recycling municipal, agricultural and industrial waste into energy, fertilizers, food and construction materials, and economic feasibility: a review. Environ Chem Lett. 2023;21(2):765–801. doi:10.1007/s10311-022-01551-5. [Google Scholar] [CrossRef]
214. Hart A, Onwudili JA, Yildirir E, Hashemnezhad SE. Energy-dense sustainable aviation fuel-range hydrocarbons from cyclohexanone as a biomass-derived feedstock via sequential catalytic aldol condensation and hydrodeoxygenation. Chem Eng J. 2025;509(16):161494. doi:10.1016/j.cej.2025.161494. [Google Scholar] [CrossRef]
215. Zhao C, Xu Q, Gu Y, Nie X, Shan R. Review of advances in the utilization of biochar-derived catalysts for biodiesel production. ACS Omega. 2023;8(9):8190–200. doi:10.1021/acsomega.2c07909. [Google Scholar] [PubMed] [CrossRef]
216. Doukeh R, Bombos D, Bombos M, Oprescu EE, Dumitrascu G, Vasilievici G, et al. Catalytic hydrotreating of bio-oil and evaluation of main noxious emissions of gaseous phase. Sci Rep. 2021;11(1):6176. doi:10.1038/s41598-021-85244-z. [Google Scholar] [PubMed] [CrossRef]
217. Bergvall N, Molinder R, Johansson AC, Sandström L. Continuous slurry hydrocracking of biobased fast pyrolysis oil. Energy Fuels. 2021;35(3):2303–12. doi:10.1021/acs.energyfuels.0c03866. [Google Scholar] [CrossRef]
218. Ibrahim A, Elsayed I, Hassan EB. Catalytic upgrading of rice straw bio-oil via esterification in supercritical ethanol over bimetallic catalyst supported on rice straw biochar. Energies. 2024;17(2):407. doi:10.3390/en17020407. [Google Scholar] [CrossRef]
219. Lahijani P, Mohammadi M, Mohamed AR, Ismail F, Lee KT, Amini G. Upgrading biomass-derived pyrolysis bio-oil to bio-jet fuel through catalytic cracking and hydrodeoxygenation: a review of recent progress. Energy Convers Manag. 2022;268(3):115956. doi:10.1016/j.enconman.2022.115956. [Google Scholar] [CrossRef]
220. Lachos-Perez D, Martins-Vieira JC, Missau J, Anshu K, Siakpebru OK, Thengane SK, et al. Review on biomass pyrolysis with a focus on bio-oil upgrading techniques. Analytica. 2023;4(2):182–205. doi:10.3390/analytica4020015. [Google Scholar] [CrossRef]
221. Celikbag Y, Nuruddin M, Biswas M, Asafu-Adjaye O, Via BK. Bio-oil-based phenol-formaldehyde resin: comparison of weight- and molar-based substitution of phenol with bio-oil. J Adhes Sci Technol. 2020;34(24):2743–54. doi:10.1080/01694243.2020.1784540. [Google Scholar] [CrossRef]
222. Zimmer A, Angie Lunelli Bachmann S. Challenges for recycling medium-density fiberboard (MDF). Results Eng. 2023;19:101277. doi:10.1016/j.rineng.2023.101277. [Google Scholar] [CrossRef]
223. Itabaiana Junior I, Avelar do Nascimento M, de Souza ROMA, Dufour A, Wojcieszak R. Levoglucosan: a promising platform molecule? Green Chem. 2020;22(18):5859–80. doi:10.1039/D0GC01490G. [Google Scholar] [CrossRef]
224. Gueudré L, Chapon F, Mirodatos C, Schuurman Y, Venderbosch R, Jordan E, et al. Optimizing the bio-gasoline quantity and quality in fluid catalytic cracking co-refining. Fuel. 2017;192:60–70. doi:10.1016/j.fuel.2016.12.021. [Google Scholar] [CrossRef]
225. Talmadge M, Kinchin C, Li Chum H, de Rezende Pinho A, Biddy M, de Almeida MBB, et al. Techno-economic analysis for co-processing fast pyrolysis liquid with vacuum gasoil in FCC units for second-generation biofuel production. Fuel. 2021;293(4):119960. doi:10.1016/j.fuel.2020.119960. [Google Scholar] [CrossRef]
226. Mallapragada DS, Dvorkin Y, Modestino MA, Esposito DV, Smith WA, Hodge BM, et al. Decarbonization of the chemical industry through electrification: barriers and opportunities. Joule. 2023;7(1):23–41. doi:10.1016/j.joule.2022.12.008. [Google Scholar] [CrossRef]
227. Qambrani NA, Rahman MM, Won S, Shim S, Ra C. Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: a review. Renew Sustain Energy Rev. 2017;79(10):255–73. doi:10.1016/j.rser.2017.05.057. [Google Scholar] [CrossRef]
228. Aravind S, Kumar PS, Kumar NS, Siddarth N. Conversion of green algal biomass into bioenergy by pyrolysis. A review. Environ Chem Lett. 2020;18(3):829–49. doi:10.1007/s10311-020-00990-2. [Google Scholar] [CrossRef]
229. Anca-Couce A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog Energy Combust Sci. 2016;53(1):41–79. doi:10.1016/j.pecs.2015.10.002. [Google Scholar] [CrossRef]
230. Saffari N, Hajabbasi MA, Shirani H, Mosaddeghi MR, Mamedov AI. Biochar type and pyrolysis temperature effects on soil quality indicators and structural stability. J Env Manag. 2020;261(2):110190. doi:10.1016/j.jenvman.2020.110190. [Google Scholar] [PubMed] [CrossRef]
231. Li Y, Xing B, Ding Y, Han X, Wang S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour Technol. 2020;312(1):123614. doi:10.1016/j.biortech.2020.123614. [Google Scholar] [PubMed] [CrossRef]
232. Suman S, Gautam S. Pyrolysis of coconut husk biomass: analysis of its biochar properties. Energy Sources Part A Recovery Util Environ Eff. 2017;39(8):761–7. doi:10.1080/15567036.2016.1263252. [Google Scholar] [CrossRef]
233. Singh Yadav SP, Bhandari S, Bhatta D, Poudel A, Bhattarai S, Yadav P, et al. Biochar application: a sustainable approach to improve soil health. J Agric Food Res. 2023;11:100498. doi:10.1016/j.jafr.2023.100498. [Google Scholar] [CrossRef]
234. Jayawardhana Y, Mayakaduwa SS, Kumarathilaka P, Gamage S, Vithanage M. Municipal solid waste-derived biochar for the removal of benzene from landfill leachate. Env Geochem Health. 2019;41(4):1739–53. doi:10.1007/s10653-017-9973-y. [Google Scholar] [PubMed] [CrossRef]
235. Jiang B, Lin Y, Mbog JC. Biochar derived from swine manure digestate and applied on the removals of heavy metals and antibiotics. Bioresour Technol. 2018;270:603–11. doi:10.1016/j.biortech.2018.08.022. [Google Scholar] [PubMed] [CrossRef]
236. Sophia AC, Lima EC. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol Env Saf. 2018;150(5326):1–17. doi:10.1016/j.ecoenv.2017.12.026. [Google Scholar] [PubMed] [CrossRef]
237. Song J, Wang Y, Zhang S, Song Y, Xue S, Liu L, et al. Coupling biochar with anaerobic digestion in a circular economy perspective: a promising way to promote sustainable energy, environment and agriculture development in China. Renew Sustain Energy Rev. 2021;144(14):110973. doi:10.1016/j.rser.2021.110973. [Google Scholar] [CrossRef]
238. Wang J, Wang S. Preparation, modification and environmental application of biochar: a review. J Clean Prod. 2019;227(12):1002–22. doi:10.1016/j.jclepro.2019.04.282. [Google Scholar] [CrossRef]
239. Shan R, Han J, Gu J, Yuan H, Luo B, Chen Y. A review of recent developments in catalytic applications of biochar-based materials. Resour Conserv Recycl. 2020;162(8):105036. doi:10.1016/j.resconrec.2020.105036. [Google Scholar] [CrossRef]
240. Huang S, Ding Y, Li Y, Han X, Xing B, Wang S. Nitrogen and sulfur co-doped hierarchical porous biochar derived from the pyrolysis of mantis shrimp shell for supercapacitor electrodes. Energy Fuels. 2021;35(2):1557–66. doi:10.1021/acs.energyfuels.0c04042. [Google Scholar] [CrossRef]
241. Owoyemi JM, Zakariya HO, Elegbede IO. Sustainable wood waste management in Nigeria. Environ Socio-Econ Stud. 2016;4(3):1–9. doi:10.1515/environ-2016-0012. [Google Scholar] [CrossRef]
242. Waswa F, Mcharo M, Mworia M. Declining wood fuel and implications for household cooking and diets in tigania Sub-county Kenya. Sci Afr. 2020;8(53):e00417. doi:10.1016/j.sciaf.2020.e00417. [Google Scholar] [CrossRef]
243. Senadheera SS, Withana PA, You S, Tsang DCW, Hwang SY, Ok YS. Sustainable biochar: market development and commercialization to achieve ESG goals. Renew Sustain Energy Rev. 2025;217(10):115744. doi:10.1016/j.rser.2025.115744. [Google Scholar] [CrossRef]
244. Zou S, Li H, Wang S, Jiang R, Zou J, Zhang X, et al. Experimental research on an innovative sawdust biomass-based insulation material for buildings. J Clean Prod. 2020;260:121029. doi:10.1016/j.jclepro.2020.121029. [Google Scholar] [CrossRef]
245. Krylova A, Krysanova K, Kulikova M, Kulikov A. Non-catalytic dissolution of biochar obtained by hydrothermal carbonization of sawdust in hydrogen donor solvent. Energies. 2021;14(18):5890. doi:10.3390/en14185890. [Google Scholar] [CrossRef]
246. Emenike EC, Iwuozor KO, Agbana SA, Otoikhian KS, Adeniyi AG. Efficient recycling of disposable face masks via co-carbonization with waste biomass: a pathway to a cleaner environment. Clean Environ Syst. 2022;6(6):100094. doi:10.1016/j.cesys.2022.100094. [Google Scholar] [CrossRef]
247. Adeniyi AG, Amusa VT, Iwuozor KO, Emenike EC. Thermal recycling strategy of Coca-Cola PVC label films by its co-carbonization with Terminalia ivorensis leaves. Clean Eng Technol. 2022;11(6):100564. doi:10.1016/j.clet.2022.100564. [Google Scholar] [CrossRef]
248. Emenike EC, Iwuozor KO, Okwu KC, Qudus AH, Egbemhenghe AU, Adeniyi AG. Composition and morphology of biomass-based soot from updraft gasifier system. J Renew Sustain Energy. 2023;15(4):043101. doi:10.1063/5.0154780. [Google Scholar] [CrossRef]
249. Ben Salem M, Abdelouahed L, Taouk B, Bouaziz M. Comparative study of the pyrolysis of sawdust contaminated with hydraulic oil and metals from industrial activities and raw sawdust for the production of biochar and bio-oil. J Anal Appl Pyrolysis. 2025;191:107203. doi:10.1016/j.jaap.2025.107203. [Google Scholar] [CrossRef]
250. Adeniyi AG, Abdulkareem SA, Iwuozor KO, Ogunniyi S, Abdulkareem MT, Emenike EC, et al. Effect of salt impregnation on the properties of orange albedo biochar. Clean Chem Eng. 2022;3(1):100059. doi:10.1016/j.clce.2022.100059. [Google Scholar] [CrossRef]
251. Solarte-Toro JC, González-Aguirre JA, Poveda Giraldo JA, Cardona Alzate CA. Thermochemical processing of woody biomass: a review focused on energy-driven applications and catalytic upgrading. Renew Sustain Energy Rev. 2021;136(2):110376. doi:10.1016/j.rser.2020.110376. [Google Scholar] [CrossRef]
252. Trada A, Chaudhary A, Patel D, Upadhyay DS. An alternative fuel production from sawdust through batch-type pyrolysis reactor: fuel properties and thermodynamic analysis. Process Saf Environ Prot. 2022;167:332–42. doi:10.1016/j.psep.2022.09.023. [Google Scholar] [CrossRef]
253. Chang G, Huang Y, Xie J, Yang H, Liu H, Yin X, et al. The lignin pyrolysis composition and pyrolysis products of palm kernel shell, wheat straw, and pine sawdust. Energy Convers Manag. 2016;124(6):587–97. doi:10.1016/j.enconman.2016.07.038. [Google Scholar] [CrossRef]
254. Zhang Y, Zhang X, Zhou Z, Liu G, Wang C. A review of the conversion of wood biomass into high-performance bulk biochar: pretreatment, modification, characterization, and wastewater application. Sep Purif Technol. 2025;361:131448. doi:10.1016/j.seppur.2025.131448. [Google Scholar] [CrossRef]
255. Wang Q, Zhang X, Sun S, Wang Z, Cui D. Effect of CaO on pyrolysis products and reaction mechanisms of a corn stover. ACS Omega. 2020;5(18):10276–87. doi:10.1021/acsomega.9b03945. [Google Scholar] [PubMed] [CrossRef]
256. Chen X, Li S, Liu Z, Cai N, Xia S, Chen W, et al. Negative-carbon pyrolysis of biomass (NCPB) over CaO originated from carbide slag for on-line upgrading of pyrolysis gas and bio-oil. J Anal Appl Pyrolysis. 2021;156:105063. doi:10.1016/j.jaap.2021.105063. [Google Scholar] [CrossRef]
257. Islam S, Jameel H, Cullen JM. Multi-stage MFA for evaluating sustainable waste potential for bioplastics conversion in the circular economy: an examination of UK wastes to produce cellulose nanofibre. J Clean Prod. 2024;482(2):144166. doi:10.1016/j.jclepro.2024.144166. [Google Scholar] [CrossRef]
258. Boumediri H, Bezazi A, Del Pino GG, Haddad A, Scarpa F, Dufresne A. Extraction and characterization of vascular bundle and fiber strand from date palm rachis as potential bio-reinforcement in composite. Carbohydr Polym. 2019;222(2–3):114997. doi:10.1016/j.carbpol.2019.114997. [Google Scholar] [PubMed] [CrossRef]
259. Liu L, Zhang Z, Wang J, Sun Q, Shi W, Liu X. Combination pretreatment of steam explosion and NaOH enhances enzymatic saccharification of corn stove. BioResources. 2019;14(1):1157–73. doi:10.15376/biores.14.1.1157-1173. [Google Scholar] [CrossRef]
260. Khullar E, Dien BS, Rausch KD, Tumbleson ME, Singh V. Effect of particle size on enzymatic hydrolysis of pretreated Miscanthus. Ind Crops Prod. 2013;44:11–7. doi:10.1016/j.indcrop.2012.10.015. [Google Scholar] [CrossRef]
261. Shangdiar S, Cheng PC, Chen SC, Amesho KTT, Ponnusamy VK, Lin YC. Enhancing sugar yield for bioconversion of rice straw: optimization of Microwave-assisted Pretreatment using dilute acid hydrolysis. Env Technol Innov. 2023;32(8):103313. doi:10.1016/j.eti.2023.103313. [Google Scholar] [CrossRef]
262. Kalak T. Potential use of industrial biomass waste as a sustainable energy source in the future. Energies. 2023;16(4):1783. doi:10.3390/en16041783. [Google Scholar] [CrossRef]
263. Bai Y, Tian M, Dai Z, Zhao X. Improving the cellulose enzymatic digestibility of sugarcane bagasse by atmospheric acetic acid pretreatment and peracetic acid post-treatment. Molecules. 2023;28(12):4689. doi:10.3390/molecules28124689. [Google Scholar] [PubMed] [CrossRef]
264. Abolore RS, Jaiswal S, Jaiswal AK. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: a review. Carbohydr Polym Technol Appl. 2024;7(29):100396. doi:10.1016/j.carpta.2023.100396. [Google Scholar] [CrossRef]
265. Saelor S, Kongjan P, Prasertsan P, Mamimin C, O-Thong S. Enhancing thermophilic methane production from oil palm empty fruit bunches through various pretreatment methods: a comparative study. Heliyon. 2024;10(20):e39668. doi:10.1016/j.heliyon.2024.e39668. [Google Scholar] [PubMed] [CrossRef]
266. Yang L, Cao J, Mao J, Jin Y. Sodium carbonate-sodium sulfite pretreatment for improving the enzymatic hydrolysis of rice straw. Ind Crops Prod. 2013;43:711–7. doi:10.1016/j.indcrop.2012.08.027. [Google Scholar] [CrossRef]
267. Puiţel AC, Balan CD, Nechita MT. Corn stalks-derived hemicellulosic polysaccharides: extraction and purification. Polysaccharides. 2025;6(1):2. doi:10.3390/polysaccharides6010002. [Google Scholar] [CrossRef]
268. Silva J, Ferreira AC, Teixeira S, Martins L, Ferreira E, Teixeira JC. Sawdust drying process in a large-scale pellets facility: an energy and exergy analysis. Clean Env Syst. 2021;2(14–15):100037. doi:10.1016/j.cesys.2021.100037. [Google Scholar] [CrossRef]
269. Bassey U, Ibrahaim H, Edet E, Narra S, Beck G, Nelles M, et al. Exergy and energy analysis of pyrolysis of pretreated single-use waste plastics. Sustain Chem Pharm. 2025;45(12):102020. doi:10.1016/j.scp.2025.102020. [Google Scholar] [CrossRef]
270. Börcsök Z, Pásztory Z. The role of lignin in wood working processes using elevated temperatures: an abbreviated literature survey. Eur J Wood Wood Prod. 2021;79(3):511–26. doi:10.1007/s00107-020-01637-3. [Google Scholar] [CrossRef]
271. Chen D, Li D, Zhu X. Effect of moisture content on the devolatilization and kinetics of corn straw. Energy Sources Part A Recovery Util Environ Eff. 2015;37(9):1005–11. doi:10.1080/15567036.2011.596904. [Google Scholar] [CrossRef]
272. Jamil F, Inayat A, Hussain M, Akhter P, Abideen Z, Ghenai C, et al. Valorization of waste biomass to biofuels for power production and transportation in optimized way: a comprehensive review. Adv Energy Sustain Res. 2024;5(10):2400104. doi:10.1002/aesr.202400104. [Google Scholar] [CrossRef]
273. Jafri N, Wong WY, Doshi V, Yoon LW, Cheah KH. A review on production and characterization of biochars for application in direct carbon fuel cells. Process Saf Environ Prot. 2018;118:152–66. doi:10.1016/j.psep.2018.06.036. [Google Scholar] [CrossRef]
274. Antal MJ, Grønli M. The art, science, and technology of charcoal production. Ind Eng Chem Res. 2003;42(8):1619–40. doi:10.1021/ie0207919. [Google Scholar] [CrossRef]
275. Shahbaz M, AlNouss A, Parthasarathy P, Abdelaal AH, MacKey H, McKay G, et al. Investigation of biomass components on the slow pyrolysis products yield using Aspen Plus for techno-economic analysis. Biomass Convers Biorefinery. 2022;12(3):669–81. doi:10.1007/s13399-020-01040-1. [Google Scholar] [CrossRef]
276. Klinger J, Westover T, Saha N, Sibbett C, Williams CL. Thermal properties of native and densified hardwood, softwoods, and agricultural residue. Biomass Bioenergy. 2025;195(2):107711. doi:10.1016/j.biombioe.2025.107711. [Google Scholar] [CrossRef]
277. Adeleke AA, Odusote JK, Lasode OA, Ikubanni PP, Madhurai M, Paswan D. Evaluation of thermal decomposition characteristics and kinetic parameters of melina wood. Biofuels. 2021;13(1):117–23. doi:10.1080/17597269.2019.1646541. [Google Scholar] [CrossRef]
278. Heydari M, Rahman M, Gupta R. Kinetic study and thermal decomposition behavior of lignite coal. Int J Chem Eng. 2015;2015(1):481739. doi:10.1155/2015/481739. [Google Scholar] [CrossRef]
279. Plota A, Masek A. Lifetime prediction methods for degradable polymeric materials-a short review. Materials. 2020;13(20). doi:10.3390/ma13204507.doi:. [Google Scholar] [CrossRef]
280. Navarro MV, López JM, Veses A, Callén MS, García T. Kinetic study for the co-pyrolysis of lignocellulosic biomass and plastics using the distributed activation energy model. Energy. 2018;165(5):731–42. doi:10.1016/j.energy.2018.09.133. [Google Scholar] [CrossRef]
281. Mishra RK, Mohanty K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour Technol. 2018;251(3):63–74. doi:10.1016/j.biortech.2017.12.029. [Google Scholar] [PubMed] [CrossRef]
282. Iqbal A, Imran M, Alves JLF, Hadi F, Yao Z, da Silva JCG, et al. Pyrolysis of macroalgae biomass from Nitella hyalina and its thermokinetics. Biomass Convers Biorefinery. 2025;15(10):15211–23. doi:10.1007/s13399-024-06242-5. [Google Scholar] [CrossRef]
283. Wang S, Dai G, Yang H, Luo Z. Lignocellulosic biomass pyrolysis mechanism: a state-of-the-art review. Prog Energy Combust Sci. 2017;62:33–86. doi:10.1016/j.pecs.2017.05.004. [Google Scholar] [CrossRef]
284. Zhang J, Liu J, Evrendilek F, Zhang X, Buyukada M. TG-FTIR and Py-GC/MS analyses of pyrolysis behaviors and products of cattle manure in CO2 and N2 atmospheres: kinetic, thermodynamic, and machine-learning models. Energy Convers Manag. 2019;195:346–59. doi:10.1016/j.enconman.2019.05.019. [Google Scholar] [CrossRef]
285. Wen S, Yan Y, Liu J, Buyukada M, Evrendilek F. Pyrolysis performance, kinetic, thermodynamic, product and joint optimization analyses of incense sticks in N2 and CO2 atmospheres. Renew Energy. 2019;141:814–27. doi:10.1016/j.renene.2019.04.040. [Google Scholar] [CrossRef]
286. Cai J, He Y, Yu X, Banks SW, Yang Y, Zhang X, et al. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew Sustain Energy Rev. 2017;76:309–22. doi:10.1016/j.rser.2017.03.072. [Google Scholar] [CrossRef]
287. Xu L, Zhou J, Ni J, Li Y, Long Y, Huang R. Investigating the pyrolysis kinetics of Pinus sylvestris using thermogravimetric analysis. BioResources. 2020;15(3):5577–92. doi:10.15376/biores.15.3.5577-5592. [Google Scholar] [CrossRef]
288. Cui B, Chen Z, Guo D, Liu Y. Investigations on the pyrolysis of microalgal-bacterial granular sludge: products, kinetics, and potential mechanisms. Bioresour Technol. 2022;349(1):126328. doi:10.1016/j.biortech.2021.126328. [Google Scholar] [PubMed] [CrossRef]
289. Suresh Babu KKB, Nataraj M, Tayappa M, Vyas Y, Mishra RK, Acharya B. Production of biochar from waste biomass using slow pyrolysis: studies of the effect of pyrolysis temperature and holding time on biochar yield and properties. Mater Sci Energy Technol. 2024;7(22):318–34. doi:10.1016/j.mset.2024.05.002. [Google Scholar] [CrossRef]
290. Papari S, Hawboldt K. A review on the pyrolysis of woody biomass to bio-oil: focus on kinetic models. Renew Sustain Energy Rev. 2015;52(5):1580–95. doi:10.1016/j.rser.2015.07.191. [Google Scholar] [CrossRef]
291. Zhang D, Song Q, Hou B, Zhang M, Teng D, Zhang Y, et al. Experimental study on microwave pyrolysis of decommissioned wind turbine blades based on silicon carbide absorbents. Processes. 2024;12(6):1065. doi:10.3390/pr12061065. [Google Scholar] [CrossRef]
292. Ong HC, Yu KL, Chen WH, Pillejera MK, Bi X, Tran KQ, et al. Variation of lignocellulosic biomass structure from torrefaction: a critical review. Renew Sustain Energy Rev. 2021;152:111698. doi:10.1016/j.rser.2021.111698. [Google Scholar] [CrossRef]
293. Wang W, Lemaire R, Bensakhria A, Luart D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J Anal Appl Pyrolysis. 2022;163(1):105479. doi:10.1016/j.jaap.2022.105479. [Google Scholar] [CrossRef]
294. Ren D, Liu X, Feng X, Lu L, Ouyang M, Li J, et al. Model-based thermal runaway prediction of lithium-ion batteries from kinetics analysis of cell components. Appl Energy. 2018;228(57):633–44. doi:10.1016/j.apenergy.2018.06.126. [Google Scholar] [CrossRef]
295. Yao Z, Yu S, Su W, Wu D, Wu W, Tang J. Kinetic modeling study on the combustion treatment of cathode from spent lithium-ion batteries. Waste Manag Res. 2020;38(1):100–6. doi:10.1177/0734242x19879224. [Google Scholar] [PubMed] [CrossRef]
296. Li W, Yang YZ, Xu J. Crystallization and soft magnetic properties of metalloid-free Fe89Hf7Al3Zr1 amorphous alloy. J Non-Cryst Solids. 2017;461(9):93–7. doi:10.1016/j.jnoncrysol.2017.01.047. [Google Scholar] [CrossRef]
297. Weng W, Chen G, Wu D. Crystallization kinetics and melting behaviors of nylon 6/foliated graphite nanocomposites. Polymer. 2003;44(26):8119–32. doi:10.1016/j.polymer.2003.10.028. [Google Scholar] [CrossRef]
298. Miyagawa Y, Yoshida M, Nakagawa K, Adachi S. Kinetic analysis of rapeseed oil crystallization during isothermal storage. Cryst Growth Des. 2018;18(2):642–50. doi:10.1021/acs.cgd.7b00789. [Google Scholar] [CrossRef]
299. Boopathi AA, Sampath S, Narasimhaswamy T. Isothermal and non-isothermal cold crystallization of tetrabenzofluorene (TBF) molecules. New J Chem. 2019;43(24):9500–6. doi:10.1039/c8nj06514d. [Google Scholar] [CrossRef]
300. Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29(11):1702–6. doi:10.1021/ac60131a045. [Google Scholar] [CrossRef]
301. Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38(11):1881–6. doi:10.1246/bcsj.38.1881. [Google Scholar] [CrossRef]
302. Zhang X. Applications of kinetic methods in thermal analysis: a review. Eng Sci. 2021;14:13. doi:10.30919/es8d1132. [Google Scholar] [CrossRef]
303. Pudasainee D, Kurian V, Gupta R. Application status of post-combustion CO2 capture. In: post-combustion Carbon Dioxide Capture Materials. Royal Soc Chem. 2018;51(9):259–89. doi:10.1039/9781788013352-00259. [Google Scholar] [CrossRef]
304. Mehmood MA, Ye G, Luo H, Liu C, Malik S, Afzal I, et al. Pyrolysis and kinetic analyses of Camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour Technol. 2017;228:18–24. doi:10.1016/j.biortech.2016.12.096. [Google Scholar] [PubMed] [CrossRef]
305. Mishra RK, Mohanty K. Kinetic analysis and pyrolysis behaviour of waste biomass towards its bioenergy potential. Bioresour Technol. 2020;311(13):123480. doi:10.1016/j.biortech.2020.123480. [Google Scholar] [PubMed] [CrossRef]
306. Gogoi M, Konwar K, Bhuyan N, Borah RC, Kalita AC, Nath HP, et al. Assessments of pyrolysis kinetics and mechanisms of biomass residues using thermogravimetry. Bioresour Technol Rep. 2018;4(9):40–9. doi:10.1016/j.biteb.2018.08.016. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF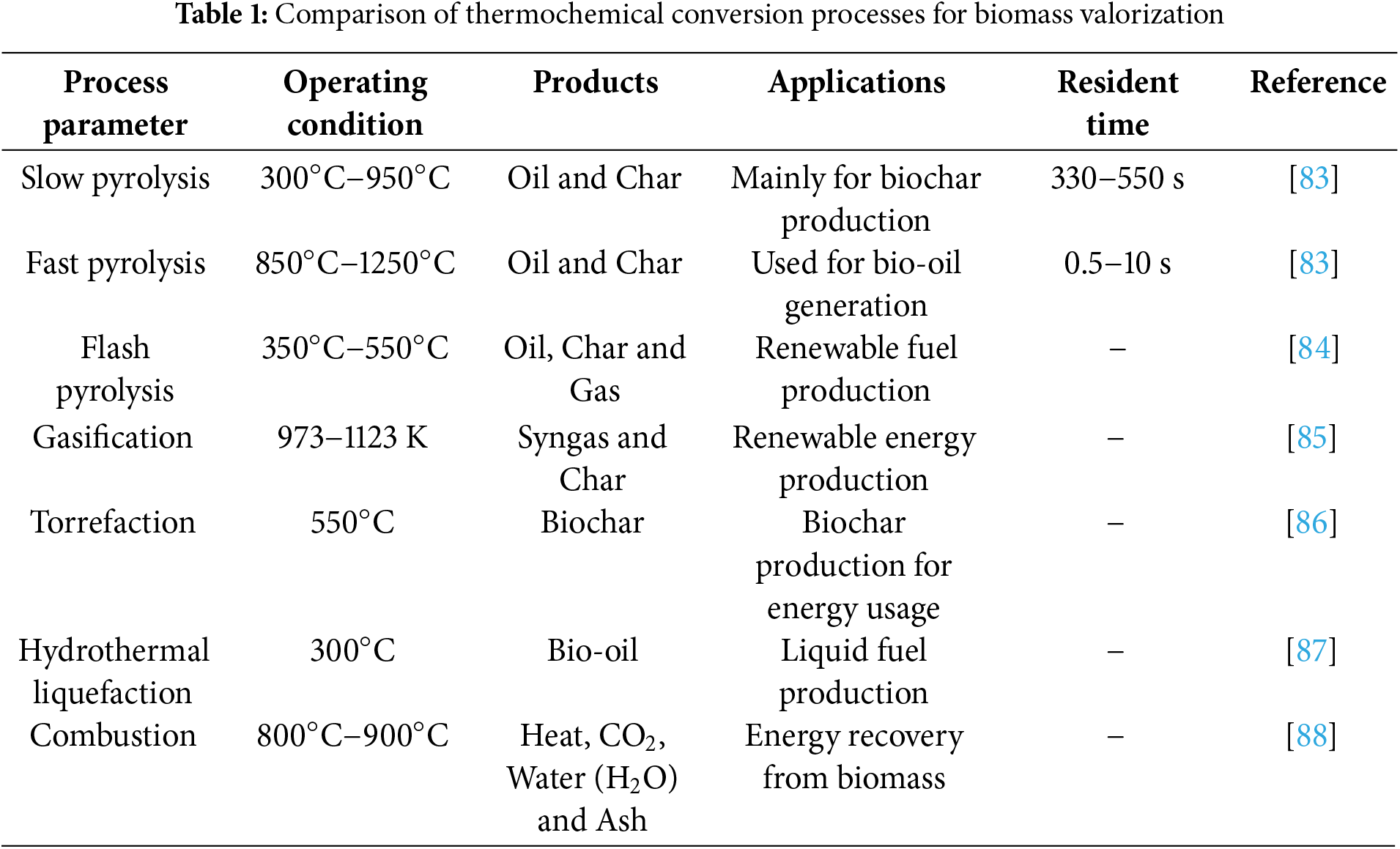
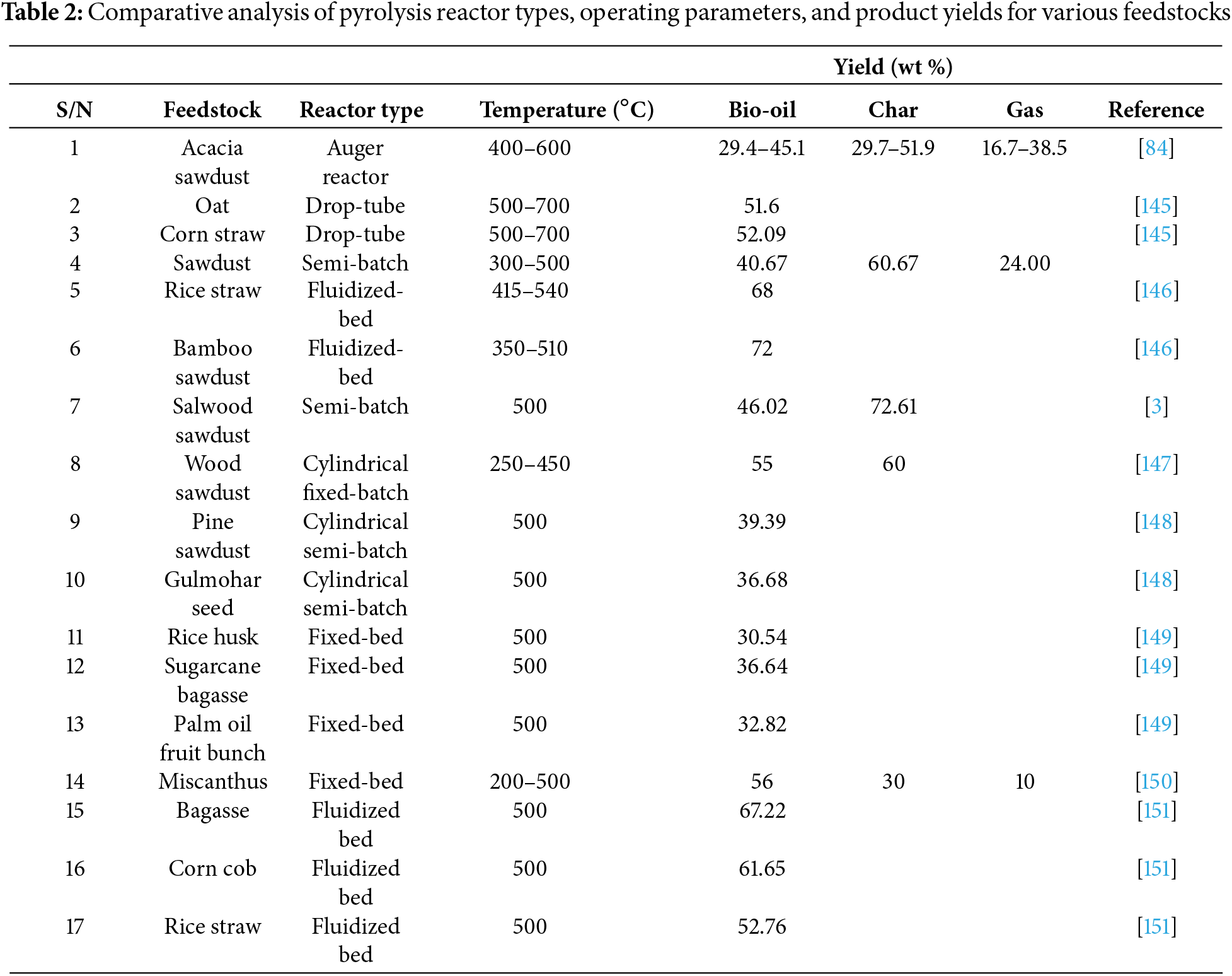
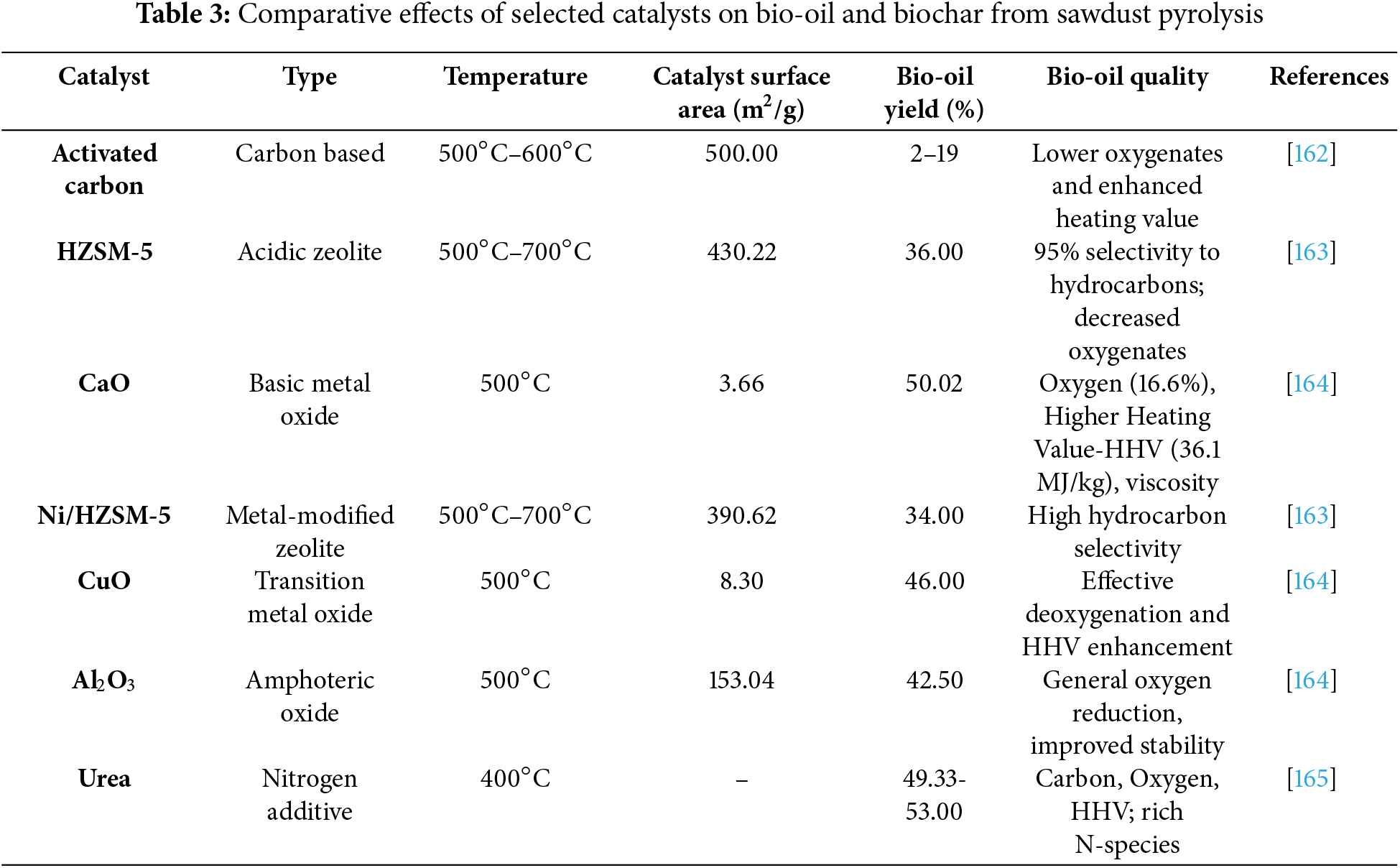
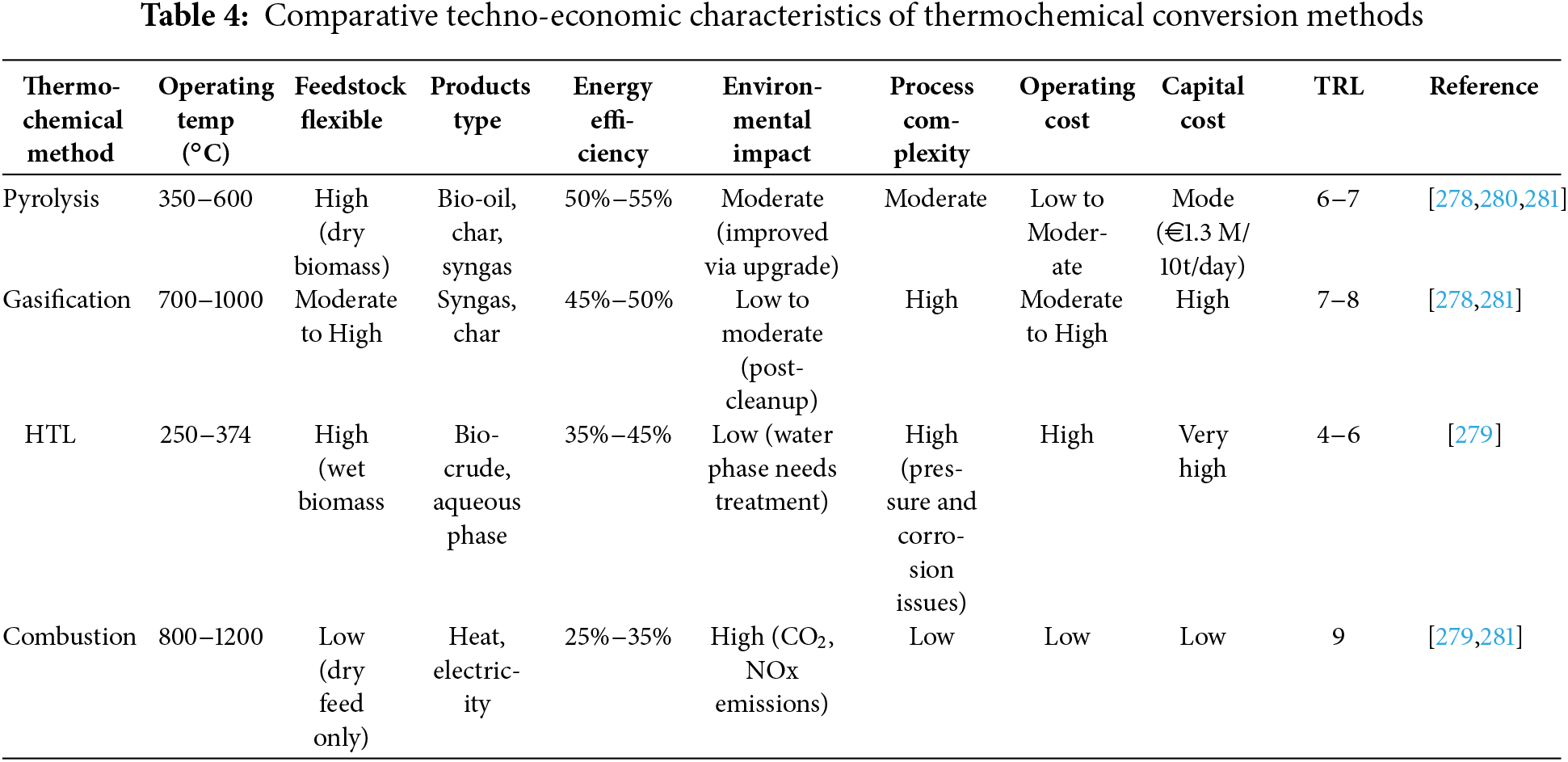
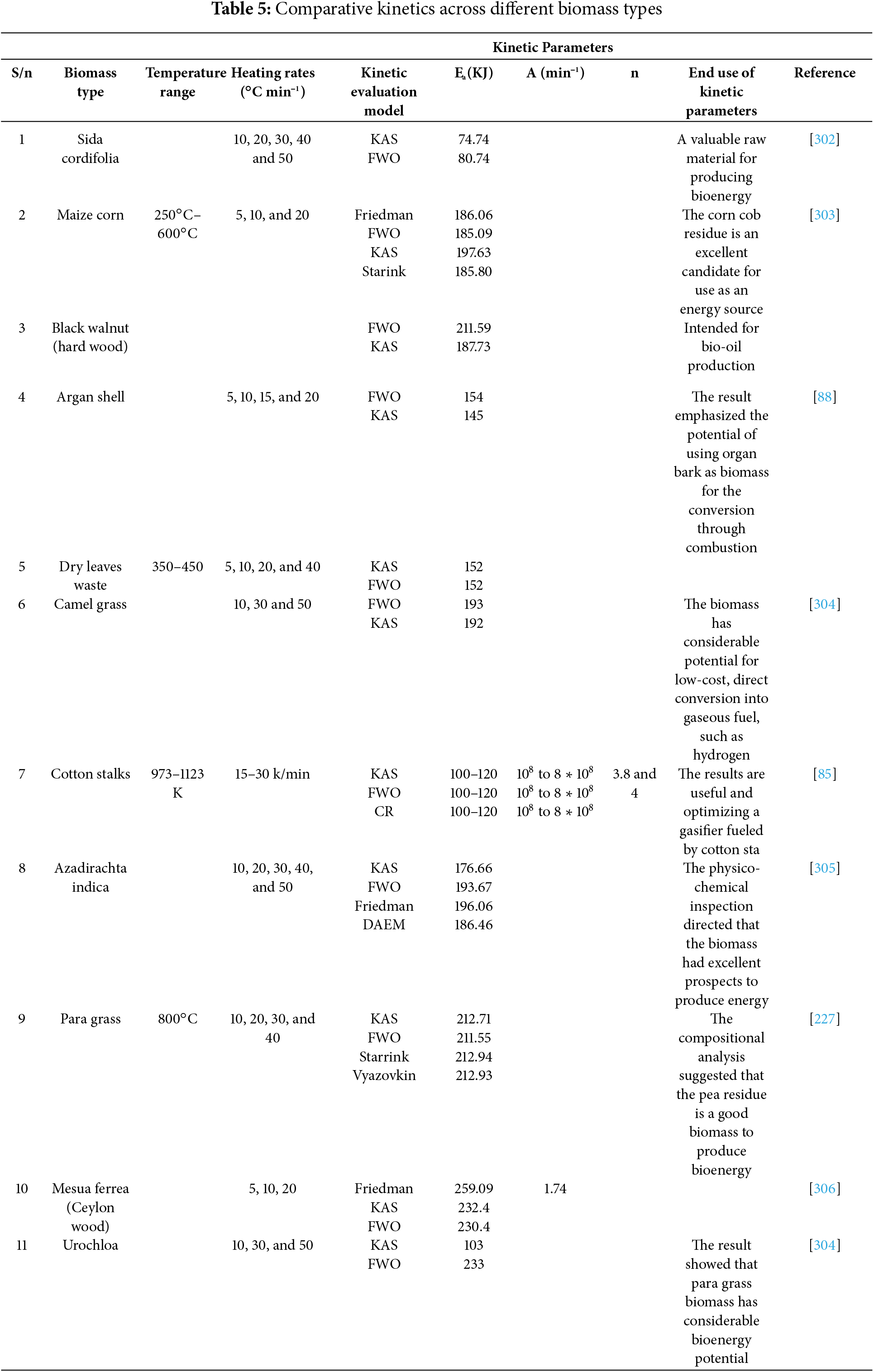
 Downloads
Downloads
 Citation Tools
Citation Tools