 Open Access
Open Access
ARTICLE
Green Natural Rubber Foam and Enhanced Physical Properties from Sugarcane Bagasse Ash
Division of Chemistry (Materials Science), Faculty of Science, Buriram Rajabhat University, Buriram, 31000, Thailand
* Corresponding Author: Bualoy Chanpaka. Email:
Journal of Renewable Materials 2025, 13(4), 753-772. https://doi.org/10.32604/jrm.2025.057590
Received 21 August 2024; Accepted 14 February 2025; Issue published 21 April 2025
Abstract
Natural rubber (NR) foams are widely used. However, further studies are required for preparing eco-friendly NR foam and determining the optimum physical properties appropriate for application. This study aims to create an NR foam from rubber reinforced with sugarcane bagasse ash (SCBA) and sodium alginate. The results showed that the SCBA was primarily composed of silica or silicon dioxide (87.52% by weight) and carbon (11.41% by weight). This study investigated the influence of the amount of sodium alginate (0–5 phr) used in the NR foam formation. The addition of SCBA on the NR foam affected the density, swelling behavior, and crosslink density of the foam. The results identified an optimal loading level that improved the density and morphology of the foam. The hardness and modulus of the NR foam increased with increasing amounts of SCBA, suggesting insufficient reinforcement. The NR exhibited the highest compressive stress at the SCBA concentration of 5 phr. This study facilitates the development of NR as green material and other support materials.Graphic Abstract
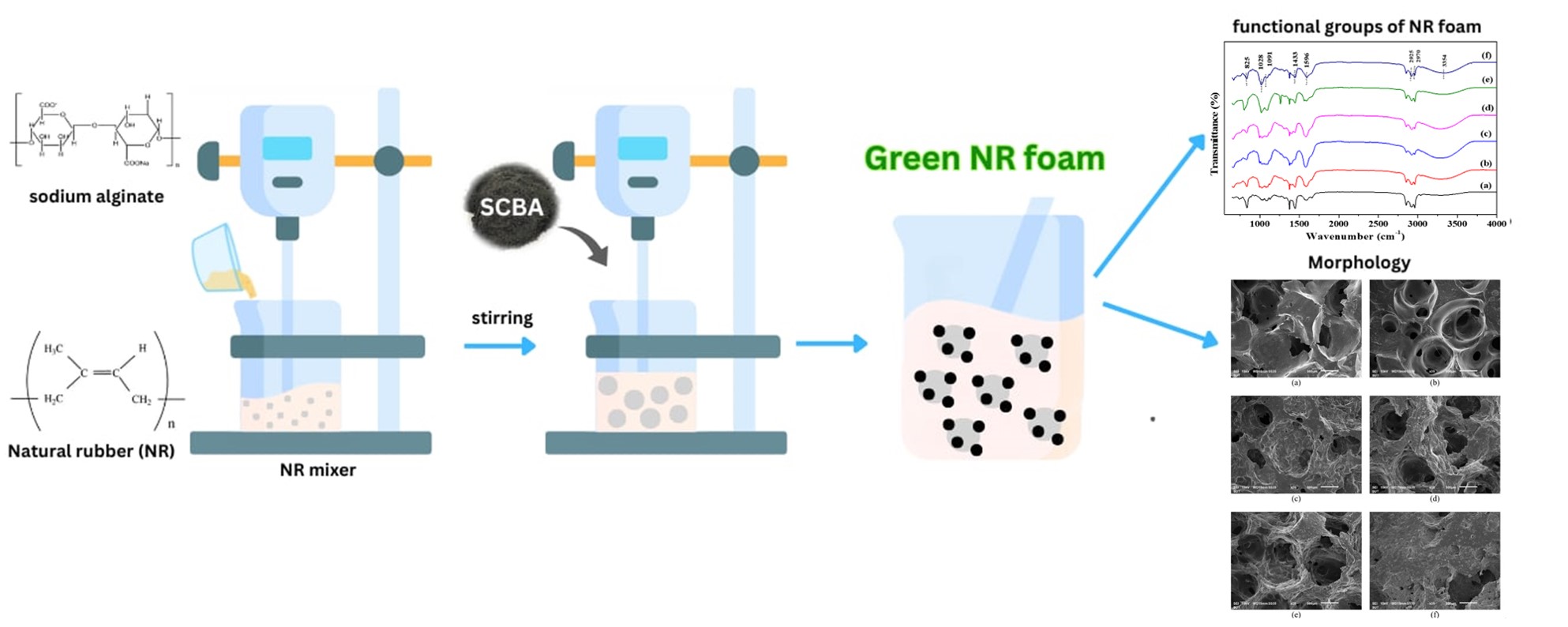
Keywords
Supplementary Material
Supplementary Material FileA global survey found that the countries that produced the highest amounts of bagasse in 2020 were Brazil (31.16), India (15.14), China (6.54), and Thailand (5.69 thousand metric tons) [1]. Sugarcane bagasse ash (SCBA) is a byproduct of bagasse combustion, which is obtained from electrical biomass. It is renewable and widely available. SCBA is primarily composed of silica, an alkaline element, alkaline earth oxide, and residue carbon [2]. SCBA has gained considerable attention from various industries owing to its potential applications, which include increasing the pozzolanic and compressive strengths of cement [3,4] and zeolite synthesis [5,6]. However, agriculture has also been used for soil amendment [7]. Water treatment is also used to remove dyes and pollutants from water [8]. Currently, significant quantities of SCBA are utilized, but considerable amounts may remain unused. SCBA can be added to natural rubber (NR). SCBA is burned to produce ash mixed with inorganic substances. SCBA contains large amounts of silicon dioxide and carbon black [9], which can be used as reinforcing agents in industrial rubber production. Research has shown that NR can be added to agricultural wastes containing silica and carbon to obtain desired tensile strength, tear strength, and scratch resistance [10,11]. Additionally, Santos et al. [12] found that when SCBA was burned until it was reduced to ashes, up to 70%–90% of the majority of the constituent inorganic substances were retained, with 82% silica or silicon dioxide (SiO2) [13]. Silica, which occurs naturally in living organisms, has a large specific surface area, and it is used as a desiccant, absorbent, and strengthening agent. It can improve various mechanical properties of rubber, such as the hardness, modulus, and tensile strength [14]. Furthermore, silica is commonly used as a reinforcing agent. It aids in enhancing adhesion to other ingredients during rubber processing, thereby increasing the strength of the rubber material [15].
Currently, this waste is an alternative and eco-friendly material for the rubber industry. It offers the advantage of being economical and has undergone continuous development for rubber processing. NR consists of blowing agents, vulcanizing agents, catalysts, stimulants, antioxidants, and gelling agents in various ratios. These constituents create a mixed latex that influences the physical and mechanical properties of NR [16,17]. Generally, NR foam is porous and flexible, with a ventilated surface. This characteristic can be achieved using gelling agents. Currently, the commonly used gelling agents are hazardous upon skin contact (irritant), eye contact (irritant), ingestion, or inhalation, and severe overexposure can result in death [18]. Additionally, there is a growing awareness regarding the environmental friendliness of industrial manufacturing procedures. Research has found that a developed product strategy has sustainable impacts. Consequently, using non-hazard, inexpensive methods to create highly effective materials without adverse manufacturing effects may be strategic. In particular, sodium alginate is a water-soluble material used as a gelling agent. It is extracted from brown seaweed, a negatively charged polysaccharide [19,20]. It is a low-risk, harmless, cost-effective, and renewable material [21,22]. In a previous study, sodium alginate was added to concentrated latex. The results showed no significant differences between fresh natural latex and creamy concentrated natural latex, and it was found to have a neutralizer, total solid content (TSC), and dry rubber content (DRC) [23]. However, this chemical is used to form thin films of aerogels or foam [24,25]. In addition, its development will involve an approach aimed at raising awareness of simple and low-risk toxic procedures for producing NR foams.
This study also highlights the influences of SCBA on the mechanical properties of NR foam prepared using sodium alginate. The study aimed to examine the feasibility of using various alternative and green materials for preparing NR foams.
Preparation SCBA
SCBA was obtained from an electrical biomass power plant and waste from a sugar factory in Buriram Province, Thailand. The SCBA was washed, dried at 110°C for 3 h, milled, and sieved through a 270-mesh sieve, as shown in Fig. 1. The chemical composition of the SCBA was characterized via X-ray fluorescence spectrophotometry (XRF; Horiba MESA-500 W). The amount of carbon residue was determined through analysis (CHNS/O Analyzer; Thermo Scientific FLASH-2000). The Brunauer–Emmett–Teller (BET) method based on the N2 adsorption isotherm (Tristar-ll 3020; Micromeritics, 3Flex) was used to determine the specific surface area and pore-size distribution of the SCBA. The mineral composition of the SCBA was determined via X-ray diffraction (XRD; D500, Siemens, Germany).

Figure 1: Sugarcane bagasse ash (SCBA)
2.2 Formula for NR Foam Preparation
Fresh NR latex was collected from rubber trees in Buriram Province; Thailand, which contained 34.2% dry rubber. It was preserved using 0.3% ammonium hydroxide. The NR latex was placed in a beaker and stirred at 100 rpm for 1 min to evaporate ammonia and render it homogeneous. Subsequently, sodium alginate was added and the mixture was stirred at 200 rpm for 10 min to obtain a foam. The speed was then decreased to 100 rpm while adding sodium fluoride, potassium oleate solution, sulfur, potassium hydroxide, SCBA, and zinc oxide. The mixture was stirred for 30 min to disperse the various substances. Then, zinc diethyldithiocarbamate and 1, 3-diphenyl guanidine were added to the mixture. The constituents of the NR foam are obtained in Table 1. The NR foam mixture was stirred at 200 rpm until the gel point was reached. The mixture was then closed with a parafilm lid and kept overnight for crosslinking. The mixture was then placed in a metallic tray (25 × 35 cm2) and dried at 80°C for 12 h. The foam sheet was washed with distilled water and dried at 70°C for 4 h. The aforementioned procedure was repeated to prepare additional NR foams for studying the influence of drying temperatures at 80°C, 90°C, and 110°C. The optimal drying temperature was then used in subsequent experiments.

2.2.1 Characteristics of NR Foam
Density
The NR foam was then cut, weighed, and replaced with water. The density of the NR foam was calculated from its mass-to-volume ratio using Eq. (1) [26].
where m and V are the mass (g) and volume (cm3) of the rubber, respectively.
Swelling Index
For assessing toluene uptake, dried NR foam specimens (2 × 2 cm2) of thickness 0.5–0.6 mm were immersed in toluene at room temperature for 24 h. The weight change was measured using the weights of the dry and swollen specimens. All tests were performed in triplicates. The percentage of swelling was calculated using the following formula in Eq. (2).
where W1 and W2 are the weights of the dry and swollen NR foam, respectively. Furthermore, the swelling data were used to determine the crosslink density and volume fraction of the NR foam samples using the Flory–Rehner equation, as shown in Eq. (3) [27].
Crosslink Density
The crosslink density (ν) can be calculated from Eq. (3).
where Vr is the volume fraction of rubber in the swollen phase, Vs is the molar volume of toluene (106.2 cm3/mol), and χ is the rubber–solvent interaction parameter (0.38 in this study) [28].
The volume fraction of rubber in the swollen phase, Vr, was calculated using Eq. (4) [29].
where S was calculated from weights of the NR foam and swollen NR foam.
where W1 and W2 are the weights of the NR foam and swollen NR foams, respectively.
Gibbs Free Energy and Entropy
The changes in Gibbs free energy (ΔG) and entropy (ΔS) were calculated using the Flory–Huggins equation. They also used the swelling data, as shown in Eqs. (6) and (7) [30,31].
where R is the ideal gas constant (8.3145 J/mol/K), and T is the test temperature (298.15 K).
Characteristics of Functional Group
The functional groups in the NR foam were analyzed using attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR; Perkin Elmer brand, model Spectrum GX-1). They were analyzed in terms of percent of transmission in the wavenumber range of 4000–550 cm−1.
Characteristics of Morphology
The NR foam was coated with gold and its morphology was determined using scanning electron microscopy (SEM; FE-SEM, ZEISS brand, Auriga Model, as shown in Fig. 7 and JEOL brand, model JSM-6010LV, as shown in Figs. 11 and 15).
Hardness
Hardness tests were performed using a digital dynamometer (Model TA300A) on a Shore C scale according to ASTM D 2240 [32].
Compression Test
A compressive force was applied until the thickness of the foam was reduced to 50% of the initial thickness, according to the ISO 1856 method B. The NR foam was cut to the dimensions of 5.0 × 5.0 × 2.5 cm3. A universal testing machine (Instron, model 5965) was used for the compression test.
The chemical composition and carbon residues of the SCBA are presented in Tables 2 and 3, respectively. SCBA was primarily composed of silicon dioxide (SiO2) (87.52%) and other oxide elements (12.48%), as presented in Table 2. This result was well-consistent with previous studies [3]. The SCBA exhibited 11.41% carbon residue (Table 3) and corresponded to that reported previously [9]. It exhibited a specific surface area of 68.41 m2/g after being passed through a 270-mesh sieve, which is close to that of carbon black (industrial grade) [2]. However, the SCBA demonstrated a density of 1.90 g/cm3 as shown in Table 3, which is in the range of 1.69 to 2.52 g/cm3 [11,33,34]. The material exhibited an H4 hysteresis loop, which featured both type I and type II isotherms as shown in Fig. 2. Specifically, the adsorption branch showed both types I and II adsorption. However, the adsorption was more pronounced at low relative pressures, which was attributed to the presence of both micropores and mesopores. H4 loops are commonly exhibited by zeolite crystal aggregates, mesoporous zeolites, and micro-mesoporous carbon [25,35].


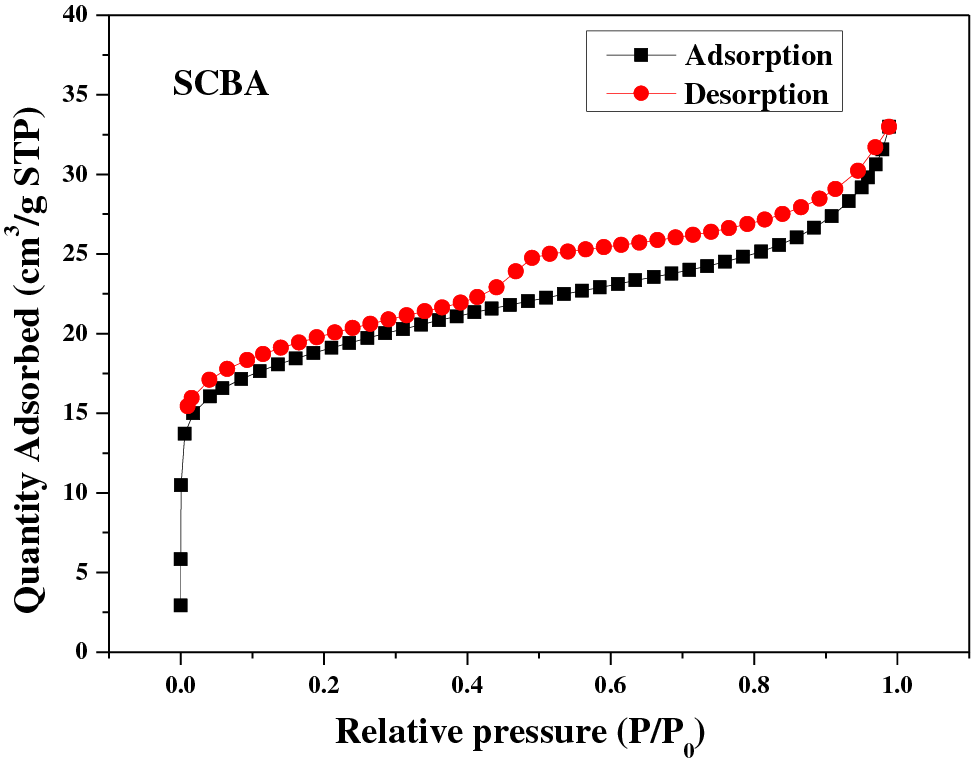
Figure 2: N2-adsorption/desorption isotherms of SCBA
X-Ray Diffraction Pattern (XRD) of SCBA
The XRD pattern of SCBA indicated that it was crystalline. An orderly arrangement of the molecules in the silica structure was indicated at the 2theta (2θ) values of 20.90°, 26.68°, 36.59°, 39.48°, and 50.22°, as shown in Fig. 3 and it corresponded to silica JCPDS number 00-046-1045. The XRD pattern exhibited peaks representing quartz (SiO2), cristobalite (SiO2), and iron oxide (Fe2O3). The XRD pattern is consistent with the XRF results evidenced in Table 2. This is consistent with the results of previous research [3,36].
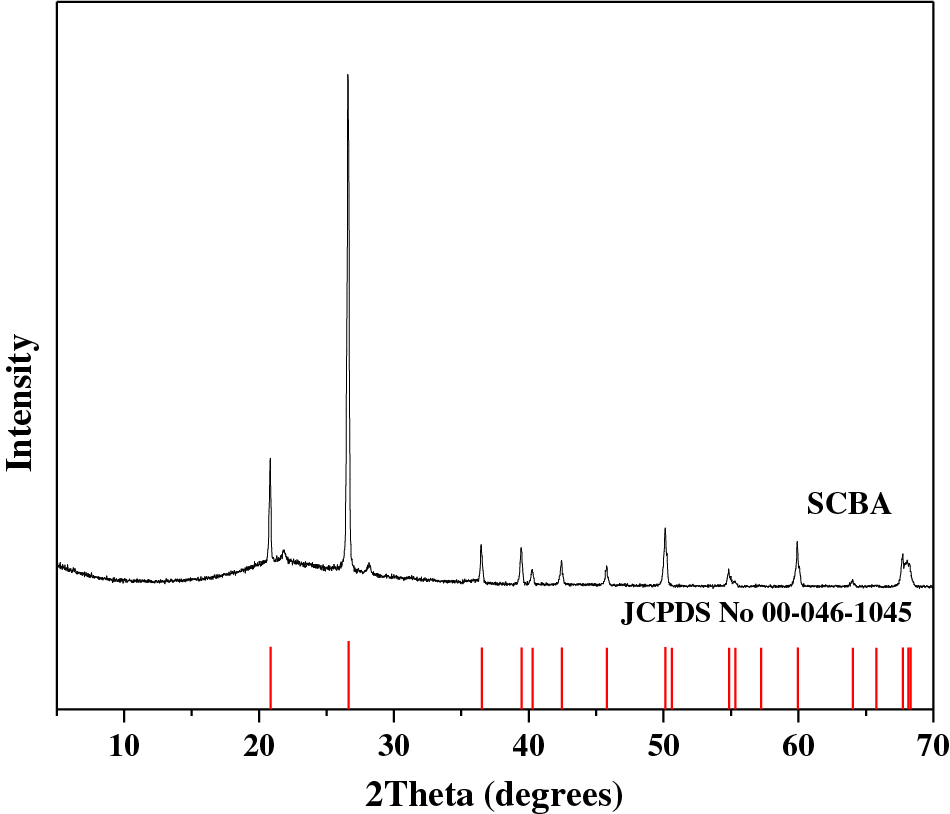
Figure 3: XRD pattern of SCBA
3.2 Characterization of NR Foam
3.2.1 Influence of Drying Temperature on NR Foam Formation
The control formula was obtained by adding 0.5 phr sodium alginate and 0.5 phr SCBA. The influence of drying temperature on NR foam characteristics were investigated after drying the foam in 80°C, 90°C, and 110°C. The results revealed that the density increased with increasing temperature, as shown in Fig. 4. Upon analyzing the physical properties of the foam, we discovered that increasing the drying temperature reduced the swelling of the foam in toluene, as shown in Fig. 5. This result corresponds to the influence of the drying temperature on the crosslink density of the NR foam (Fig. 6). However, the crosslink density was increased to represent the drying temperature induced by the crosslink rubber network. These results are consistent with those reported by a study conducted on the influence of the drying temperature on the crosslink densities of waste rubber blends. Prolonged exposure to high drying temperatures slightly increased the average crosslink density [37]. Fig. 7 shows the morphological structure of the NR foam for various drying temperatures at a magnification of 50×. The images were obtained using a SEM. Increasing the drying temperature reduced the size. As evident from the number of pores inside the NR foam, the drying temperature increase resulted in a high water-evaporation rate, increasing the shrinkage of the NR foam. Therefore, the density of the foam increased, as shown in Fig. 7b,c. As shown in Fig. 7a, a drying temperature of 80°C was chosen for preparing the NR foam, because this temperature ensured the optimum porosity for preparing NR foam.

Figure 4: Influence of drying temperature on the density of NR foam

Figure 5: Influence of drying temperature on the swelling index of NR foam

Figure 6: Influence of drying temperature on crosslink density

Figure 7: SEM image of NR foam under various drying temperatures at 50× magnification (a) 80°C, (b) 90°C, (c) 110°C
3.2.2 Influence of Sodium Alginate on the Characteristics of NR Foam
The formula was adjusted to make it well-suited for preparing the NR foam. When mixing the NR foam, the controlled formula was found to be a latex-mixing formula without the addition of SCBA. This formula was used to construct the NR foam. The NR foam was prepared and dried at 80°C. Subsequently, we studied the dependence of the characteristics of the NR foam on the formula and the addition of sodium alginates at various concentrations. The formulas held that serving the preparation was the purpose for consistency in foaming condition. These investigations were aimed at identifying the optimal conditions conductive to foaming. The density, swelling index, and crosslink density of the NR foam were evaluated to elucidate the influence of sodium alginate on the NR foam characteristics under various foaming conditions. The addition of sodium alginate reduced the density of the NR foam. The results showed a gradual decrease in the density with increasing concentration of sodium alginate, as shown in Fig. 8. The density exhibited the lowest value when the concentration of the added sodium alginate was 5 phr. Additionally, the swelling index increased with increasing concentration of sodium alginate, as shown in Fig. 9. However, the optimum amount of sodium alginate was determined to be approximately 2 phr, as shown in Fig. 10. At this value, the crosslink density was approximately 4 × 10−4/cm3 owing to the presence of 4,4′-oxybis benzene sulfonyl hydrazide (OBSH). When OBSH is added as a foaming agent, an excess amount of OBSH could lead to excessive crosslinking, leading to disrupted crosslinking [38]. Therefore, ensuring proper foaming conditions is essential for attaining suitable mechanical properties in NR foam. The results of this study are consistent with those reported by a previous study that used sodium alginate as a creaming agent, a highly polar substance, mannuronate, and glucuronate groups. The hydroxyl group (−OH) in sodium alginate may have caused it to disperse in the NR and react with the rubber particles at the negatively charged positions on the surface. During the reaction, the NR was covered with surface particles and the branched portions of the sodium alginate molecules were bonded to the rubber particles. Consequently, the rubber particles became larger in size. This is because the high-molecular-weight sodium alginate has a hydroxyl group (−OH) that reacts with the amino acids in the rubber particles, which causes the NR particles to move more slowly and increases its viscosity [23,39]. Therefore, the rubber became more viscous and thicker with increasing concentration of sodium alginate. Further, the low density increased the swelling index and decreased crosslink density. We examined the morphology of the NR foam for various amounts of sodium alginate at a magnification of 35× using a SEM. The porosity of the NR foam remained unchanged with the addition of 0.5 phr of sodium alginate, as shown in Fig. 11a. However, the number of pores increased with increasing concertation of sodium alginate, as shown in Fig. 11b–d. Large and uniform pores were observed when the amount of sodium alginate was 5 phr. Additionally, the material exhibited a rough morphology, which could be due to the disorder caused during the foaming process. Therefore, the density was reduced, and high swelling was observed, as shown in Fig. 11d. The results illustrate the influence of the filler, SCBA, on the mechanical properties of NR.

Figure 8: Influence of sodium alginate on density

Figure 9: Influence of sodium alginate on swelling

Figure 10: Influence of sodium alginate on crosslink density

Figure 11: SEM of NR adjusted with various amounts of sodium alginate: (a) 0.5 phr, (b) 1 phr, (c) 2 phr, and (d) 5 phr at 35× magnification
3.2.3 Influence of SCBA on the Properties of NR Foam
Next, the influence of SCBA concentration on the properties of NR foam containing 2 phr sodium alginate was studied. Although SCBA contained silica and carbon, which are reinforcing fillers [36], the density of the NR foam slightly changed upon increasing the amount of SCBA. The density of the NR foam increased when the lowest amount of SCBA was added to NR foam. This was because the addition of a small amount of SCBA compensated for the weight loss of NR foam during drying. In addition, SCBA obstructed foaming, causing the NR foam to become increasingly compact. The density of NR foam increased with increasing amount of SCBA. This may be attributed to the dispersion of SCBA in the liquid state during the NR latex-mixing process. When dried to obtain a solid foam, SCBA compensated the weight loss in the NR foam during the drying process. Subsequently, the density of the foam increased owing to the enhanced compactness of the NR foam. Consequently, the density of NR foam increased for a certain range of SCBA concentration, as shown in Fig. 12. This led to a decrease in the swelling index, as shown in Fig. 13. This result is consistent with that of the experimental study on the addition of nanoparticles as reinforcing fillers (graphene and carbon black), which improved the particle distribution in the NR matrix with respect to the static and dynamic mechanical properties and swelling. It was found that the optimum filler content was 3 phr [40]. From this condition, it was found that the nature of SCBA, when sorted through a 270 mesh sieve, showed agglomerated micron particle size in this research. However, pores were detected inside the structure, affecting the total specific surface area [41]. Therefore, the amount of SCBA added to this foam must be higher because it is not well distributed in the matrix. When the amount of SCBA was increased, its effect on the physical properties gradually improved. However, when the amount of SCBA was increased to 10 phr, the compactness of the NR foam declined as a result of the gradual increase in density. The addition of excess amounts of SCBA decreased the sample porosity. Natural fillers are good nucleating agents for foams. However, the use of fibers as reinforcing agents can result in uneven cell nucleation and inconsistent cell sizes [42]. This leads to the deterioration of the physical and mechanical properties of the composite foam in terms of swelling index and crosslink density (hardness and modulus). High concentrations of SCBA effectively obstructed the alignment of SCBA during the compensation process in solid foam formation, which is consistent with the reduced physical properties observed in most commercial silica at 10 phr [43]. Moreover, the density of NR foams, similar to the foams based on the formulations, was enhanced using various micro and nanofibrillated cellulose materials, yielding densities in the range of 0.15–0.35 g/cm3 [44]. Additionally, NR foam composites incorporating mica waste exhibited densities in the range of 0.20–0.42 g/cm3 [45]. As shown in Fig. 14, the crosslink density of the NR foam increased slightly with increasing SCBA concentration. The increasing crosslink density affected SCBA because the penetration of SCBA enlarged the NR molecules. The bonding between SCBA and NR molecules could be occluded within the cavities on the surface of SCBA, leading to carbon–sulfur linkages and enhanced interactions [17]. Fig. 15a–f shows the SEM results (35× magnification) that reveal different intercell foams and the effect of the filler on the distribution of SCBA in the NR matrix. Fig. 15a shows that the sample without SCBA was found to have a cell size between 300 and 500 μm. When the amount of SCBA was increased (0.5–5 phr), as shown in Fig. 15b–e, filler particles were observed that were not covered by the NR matrix. The cell size was not uniform, and more SCBA particles agglomerated on the surface with increasing amounts of SCBA. However, the slightly different porosities of the loaded SCBA indicated a non-uniform distribution of SCBA particles in the NR matrix. All formulations of the NR foam exhibited a heterogeneous pore size in the open-cell structure, consistent with the compressive stress analysis results. However, the literature reports that open-cell foam drives flexibility [46]. Therefore, it can be explained that sodium alginate is properly proportioned and used with SCBA [47,48] except for the NR foam loaded with 10 phr. The cell size decreased suggestively at high filler concentrations, which was related to poor adhesion between the filler particles and the NR matrix. The higher loading of SCBA resulted in a more disordered surface of the NR foam, as shown in Fig. 15f. In addition, this was consistent with the results of other physical property analyses, namely, density, swelling, and crosslink density. This affects the compressive stress of the sample. As shown in Fig. 15a–e, the NR matrix covers the filler particles because of the good affinity between the filler and NR. In this case, the strong adhesion between the components prevented the escape of gas, which is consistent with the FTIR analysis results. Therefore, the foam cells are similar in size to those in the samples. It similarly affected the response, density, swelling, crosslink density, and mechanical properties.

Figure 12: Influence of SCBA on density

Figure 13: Influence of SCBA on swelling

Figure 14: Influence of SCBA on crosslink density

Figure 15: SEM of NR foam adjusted with different amounts of SCBA; (a) =0 phr, (b) =0.5 phr, (c) =1 phr, (d) =2 phr, (e) =5 phr, and (f) =10 phr, respectively, at 35× magnification
The results of the swelling test of the NR foam, particularly the volume fraction and crosslink density of the NR, led to negative changes in Gibbs free energy (ΔG). The entropy (ΔS) change showed positive values (Table 4), corresponding to the spontaneous nature of all foaming processes. Other thermodynamic analyses established identical behaviors. Suethao et al. [46] prepared the NR foam via the Dunlop method using NR latex and chemical agents exhibited ΔS in the range of 0.1195–0.1231 J/mol/K and ΔG between −35.6386 to −36.7143 J/mol. Comparable results were obtained for the NR-based nanocomposites using different amounts of nanofillers. The effect of the used nanofiller exhibited a wide range of ΔS that is in the range of 0.0971–0.1553 J/mol/K and ΔG between −29.15 to −46.61 J/mol [17]. On the other hand, Suethao et al. [49] used NR latex to produce a porous elastic rubber foam, which showed a ΔS in the range of 0.1134–0.1218 J/mol/K and ΔG of −33.8061 to −36.3233 J/mol, respectively. The negative values of ΔG indicated the occurrence of a spontaneous process. It can be implied that the condition of the preparation affects the variation of nature in thermodynamic function.

The FTIR spectra of the NR foam for various amounts of SCBA showed peaks at the wavenumbers of 825, 1433, and 1596, which corresponded to the stretching of C=C, C−H, and C=O, respectively. The adsorption bands of –CH3 and –CH2 in the NR structure were observed at 2925 and 2970 cm−1, respectively. This indicated that NR consisted of double bonds. An asymmetric vibration of the Si–O–Si bonds of 1028 and 1091 cm−1 appears in SiO2 of SCBA [36]. SiO2 showed the vibrations of Si–OH at 3354 cm−1 due to water adsorbed on the surface and are presented in Fig. 16 and Table 5. Using SCBA as a filler in NR foams can affect their mechanical properties. The interaction between SCBA and NR, particularly when enhanced by the interaction between SCBA and the curing system, can improve mechanical performance.

Figure 16: FTIR spectra of NR foam at various SCBA contents: (a) =0 phr, (b) =0.5 phr, (c) =1 phr, (d) =2 phr, (e) =5 phr, and (f) =10 phr
The hardness and modulus of the NR are listed in Table 6. The hardness and modulus also showed the same trend of the NR foam gradually increased with increasing concentration of SCBA, as shown in Table 5. The compression test showed the relationship between compressive stress and compressive strain, as shown in Fig. 17. When the compressive strain was in the range of 0–10%, the rubber foam with and without SCBA produced a low compressive stress. Initially, the NR foam exhibited a low modulus (low compressive stress ability); therefore, it collapsed easily. However, at a compressive strain of 50%, the NR foam without SCBA behaved nearly the same as the NR foam added with 0.5 phr of SCBA and showed the lowest compressive stress. This indicated that the NR foam without SCBA had only the rubber component to receive the compressive force. The small amount of 0.5 phr SCBA was insufficient to distribute the compressive force. Therefore, the NR foam shrank rapidly. The NR foam added with 5 phr SCBA showed the highest modulus and compressive stress, whereas the NR foams added with 1, 2, and 10 phr SCBA showed lower compressive stresses than the foam added with 5 phr SCBA. The crosslinking during vulcanization affected the hardness [34] of the NR foam, which increased with an increase in the amount of the filler to 5 phr. When NR foam was loaded with 10 phr of SCBA, the excess SCBA was combined with a reduced proportion of rubber. Therefore, the compressive force of the NR foam decreased because the filler agglomerated, as shown in the SEM results. SCBA, which is a natural filler for internal reinforcement, effectively dispersed within the rubber matrix. This reduced the flexibility of the rubber chain, impeding the vulcanization [54,55].


Figure 17: Compressive stress on compressive strain of NR foam various of SCBA
This study demonstrated the use of sodium alginate in the preparation of NR foams to improve the properties of SCBA-based NR foams. This study suggests that sodium alginate affects the density, swelling behavior, crosslink density, and morphology of NR foams. At 2 phr of sodium alginate, enlarged and consistent pores were observed, along with increased roughness. Additionally, an increase in the concentration of SCBA resulted in a decrease in the percentage swelling of the foam. The influence of SCBA concentration on the modulus of NR foam was investigated, with optimal loading levels required to achieve the desired mechanical properties. The negative ΔG values, as obtained in various studies, highlight the spontaneous nature of these processes. Furthermore, the reinforcing potential of the unmodified SCBA was emphasized, which contributed to the improved hardness and compression properties of the NR foam. This study holds potential applications for developing other support materials and foaming processes and for harnessing the reinforcing potential of fillers to improve the performance of other foams.
Acknowledgement: The authors thank the Thailand Science Research and Innovation, National Science Research and Innovation Fund (Fundamental Fund, FF 2023) for supporting this study and gratefully acknowledge the financial support received from Buriram Rajabhat University for publishing this article. We would like to thank Athitiya Yaikrathok and Panumart Yindee for preliminary preparation of NR foam.
Funding Statement: This research was supported by the Thailand Science Research and Innovation, National Science Research and Innovation Fund and the Fundamental Fund (FF 2023).
Author Contributions: Conceptualization, formal analysis, funding acquisition, investigation, methodology, resources, writing—original draft and editing: Pattaranun Thuadaij. Conceptualization, formal analysis, funding acquisition, investigation, methodology, resources, writing—original draft and editing: Bualoy Chanpaka. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The detailed data for the analysis results of this research can be obtained from the supplementary file and requested from the corresponding author.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://doi.org/10.32604/jrm.2025.057590.
References
1. Sugar-producing-countries. (s.p.c.). [cited 2025 Jan 25]. Available from: https://worldpopulationreview.com/country-rankings/sugar-producing-countries. [Google Scholar]
2. Pérez-Casas JA, Zaldívar-Cadena AA, Álvarez-Mendez A, Ruiz-Valdés JJ, Parra-Arciniega SM, López-Pérez DC, et al. Sugarcane bagasse ash as an alternative source of silicon dioxide in sodium silicate synthesis. Materials. 2023;16(18):6327. doi:10.3390/ma16186327. [Google Scholar] [PubMed] [CrossRef]
3. Prabhath N, Kumara BS, Vithanage V, Samarathunga AI, Sewwandi N, Damruwan HH, et al. Investigation of pozzolanic properties of sugarcane bagasse ash for commercial applications. ACS Omega. 2023;8(13):12052–61. doi:10.1021/acsomega.2c07844. [Google Scholar] [PubMed] [CrossRef]
4. Gudia SEL, Go AW, Giduquio MB, Juanir RG, Jamora JB, Gunarto C, et al. Sugarcane bagasse ash as a partial replacement for cement in paste and mortar formulation–a case in the Philippines. J Build Eng. 2023;76(4):107221. doi:10.1016/j.jobe.2023.107221. [Google Scholar] [CrossRef]
5. Sriatun S, Taslimah T, Suyati L. Synthesis of zeolite from sugarcane bagasse ash using cetyltrimethylammonium bromide as structure directing agent. Indones J Chem. 2018;18(1):159–65. doi:10.22146/IJC.22197. [Google Scholar] [CrossRef]
6. Tobarameekul P, Sangsuradet S, Worathanakul P. Comparative study of Zn loading on advanced functional zeolite NaY from bagasse ash and rice husk ash for sustainable CO2 adsorption with ANOVA and factorial design. Atmosphere. 2022;13(2):314. doi:10.3390/atmos13020314. [Google Scholar] [CrossRef]
7. Dombinov V, Herzel H, Meiller M, Müller F, Willbold S, Zang JW, et al. Sugarcane bagasse ash as fertilizer for soybeans: effects of added residues on ash composition, mineralogy, phosphorus extractability and plant availability. Front Plant Sci. 2022;13:1041924. doi:10.3389/fpls.2022.1041924. [Google Scholar] [PubMed] [CrossRef]
8. Kerrou M, Bouslamti N, Raada A, Elanssari A, Mrani D, Slimani MS. The use of sugarcane bagasse to remove the organic dyes from wastewater. Int J Anal Chem. 2021;2021(1):5570806. doi:10.1155/2021/5570806. [Google Scholar] [PubMed] [CrossRef]
9. Katare VD, Madurwar MV. Experimental characterization of sugarcane biomass ash—a review. Constr Build Mater. 2017;152(6):1–15. doi:10.1016/j.conbuildmat.2017.06.142. [Google Scholar] [CrossRef]
10. Oyetunji A, Umunakwe R, Madueke CI. A review on agricultural wastes as fillers for vulcanized natural rubber. J Inst Polym Eng. 2017;1:23–34. [Google Scholar]
11. Sattayanurak S, Sahakaro K, Kaewsakul W, Dierkes WK, Reuvekamp LAEM, Blume A, et al. Synergistic effect by high specific surface area carbon black as secondary filler in silica reinforced natural rubber tire tread compounds. Polym Test. 2020;81:106173. doi:10.1016/j.polymertesting.2019.106173. [Google Scholar] [CrossRef]
12. Santos RJD, Agostini DLDS, Cabrera FC, Reis EAPD, Ruiz MR, Budemberg ER, et al. Sugarcane bagasse ash: new filler to natural rubber composite. Polímeros. 2014;24(6):646–53. doi:10.1590/0104-1428.1547. [Google Scholar] [CrossRef]
13. Thuadaij P. Synthesis and characterization of zeolite derived from Buriram sugarcane bagasse ash and Narathiwat kaolinite. SNRU J Sci Technol. 2017;8:320–6. [Google Scholar]
14. Choophun N, Chaiammart N, Sukthavon K, Veranitisagul C, Laobuthee A, Watthanaphanit A, et al. Natural rubber composites reinforced with green silica from rice husk: effect of filler loading on mechanical properties. J Compos Sci. 2022;6(12):369. doi:10.3390/jcs6120369. [Google Scholar] [CrossRef]
15. Jong L. Improved mechanical properties of silica reinforced rubber with natural polymer. Polym Test. 2019;79(6):106009. doi:10.1016/j.polymertesting.2019.106009. [Google Scholar] [CrossRef]
16. Morita J, Ando Y, Komatsu S, Matsumura K, Okazaki T, Asano Y, et al. Mechanical properties and reliability of parametrically designed architected materials using urethane elastomers. Polymers. 2021;13(5):842. doi:10.3390/polym13050842. [Google Scholar] [PubMed] [CrossRef]
17. Prasopdee T, Smitthipong W. Effect of fillers on the recovery of rubber foam: from theory to applications. Polymers. 2020;12(11):2745. doi:10.3390/polym12112745. [Google Scholar] [PubMed] [CrossRef]
18. Material safety data sheet sodium silicofluoride MSDS. [cited 2025 Jan 1]. Available from: https://jcichem.com/images/MSDS/Sodium-Silicofluoride.pdf. [Google Scholar]
19. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1):106–26. doi:10.1016/j.progpolymsci.2011.06.003. [Google Scholar] [PubMed] [CrossRef]
20. Wang C, Yokota T, Someya T. Natural biopolymer-based biocompatible conductors for stretchable bioelectronics. Chem Rev. 2021;121(4):2109–46. doi:10.1021/acs.chemrev.0c00897. [Google Scholar] [PubMed] [CrossRef]
21. Hajiali H, Heredia-Guerrero JA, Liakos I, Athanassiou A, Mele E. Alginate nanofibrous mats with adjustable degradation rate for regenerative medicine. Biomacromolecules. 2015;16(3):936–43. doi:10.1021/bm501834m. [Google Scholar] [PubMed] [CrossRef]
22. Thakur S, Sharma B, Verma A, Chaudhary J, Tamulevicius S, Thakur VK. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J Clean Prod. 2018;198:143–59. doi:10.1016/j.jclepro.2018.06.259. [Google Scholar] [CrossRef]
23. Suksup R, Imkaew C, Smitthipong W. Cream concentrated latex for foam rubber products. IOP Conf Ser: Mater Sci Eng. 2017;272:12025. doi:10.1088/1757-899X/272/1/012025. [Google Scholar] [CrossRef]
24. Tang S, Yang J, Lin L, Peng K, Chen Y, Jin S, et al. Construction of physically crosslinked chitosan/sodium alginate/calcium ion double-network hydrogel and its application to heavy metal ions removal. Chem Eng J. 2020;393:124728. doi:10.1016/j.cej.2020.124728. [Google Scholar] [CrossRef]
25. Shamsudin MS, Azha SF, Sellaoui L, Badawi M, Bonilla-Petriciolet A, Ismail S. Performance and interactions of diclofenac adsorption using Alginate/Carbon-based films: experimental investigation and statistical physics modelling. Chem Eng J. 2022;428(5):131929. doi:10.1016/j.cej.2021.131929. [Google Scholar] [CrossRef]
26. Suksup R, Sun Y, Sukatta U, Smitthipong W. Foam rubber from centrifuged and creamed latex. J Polym Eng. 2019;39(4):336–42. doi:10.1515/polyeng-2018-0219. [Google Scholar] [CrossRef]
27. Paulin JA, Lopez-Aguilar JE, Fouconnier B, Vargas RO, Lopez-Serrano F. Revisiting the flory-rehner equation: taking a closer look at the flory-Huggins interaction parameter and its functionality with temperature and concentration with NIPA as a case example. Polym Bull. 2022;79(8):6709–32. doi:10.1007/s00289-021-03836-1. [Google Scholar] [CrossRef]
28. Mathai AE, Thomas S. Transport of aromatic hydrocarbons through crosslinked nitrile rubber membranes. J Macromol Sci Part B. 1996;35(2):229–53. doi:10.1080/00222349608212383. [Google Scholar] [CrossRef]
29. Yatsuyanagi F, Suzuki N, Ito M, Kaidou H. Effect of secondary structure on mechanical properties. Polymer. 2001;42(23):9523–9. doi:10.1016/S0032-3861(01)00472-4. [Google Scholar] [CrossRef]
30. Sperling LH. Introduction to physical polymer science. 4th ed. Hoboken, NJ, USA: Wiley-Interscience; 2005. [Google Scholar]
31. Pojanavaraphan T, Magaraphan R. Prevulcanized natural rubber latex/clay aerogel nanocomposites. Eur Polym J. 2008;44(7):1968–77. doi:10.1016/j.eurpolymj.2008.04.039. [Google Scholar] [CrossRef]
32. Yoon YC, Lee JS, Park SU, Kwon JH, Hong TH, Kim DG. Quantitative assessment of liver fibrosis using shore durometer. Ann Surg Treat Res. 2017;93(6):300–4. doi:10.4174/astr.2017.93.6.300. [Google Scholar] [PubMed] [CrossRef]
33. Avasthi AA, Joshi P, Nanaware S, Patil P, Kundlikar V. Use of sugarcane bagasse ash to manufacture light weight sustainable and economical concrete. Int J Adv Res Sci Commun Technol. 2022;2022:30–5. doi:10.48175/ijarsct-2496. [Google Scholar] [CrossRef]
34. Prabhath N, Kumara BS, Vithanage V, Samarathunga AI, Sewwandi N, Maduwantha K, et al. A review on the optimization of the mechanical properties of sugarcane-bagasse-ash-integrated concretes. J Compos Sci. 2022;6(10):283. doi:10.3390/jcs6100283. [Google Scholar] [CrossRef]
35. Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem. 2015;87(9–10):1051–69. doi:10.1515/pac-2014-1117. [Google Scholar] [CrossRef]
36. Barrera Torres G, Dognani G, da Silva Agostini DL, dos Santos RJ, Camargo Cabrera F, Gutierrez Aguilar CM, et al. Potential eco-friendly application of sugarcane bagasse ash in the rubber industry. Waste Biomass Valori. 2021;12(8):4599–613. doi:10.1007/s12649-020-01309-6. [Google Scholar] [CrossRef]
37. Candau N, Zimny A, Vives E, Maspoch ML. Elastocaloric waste/natural rubber materials with various crosslink densities. Polymers. 2023;15(11):2566. doi:10.3390/polym15112566. [Google Scholar] [PubMed] [CrossRef]
38. Rostami-Tapeh-Esmaeil E, Ahmad H, Kazemi H, Rodrigue D. Optimization of natural rubber foams: effect of foaming agent content and processing conditions on the cellular structure and mechanical properties. Cell Polym. 2024;43(2–4):57–75. doi:10.1177/02624893241241680. [Google Scholar] [CrossRef]
39. Yumae N, Kaesaman A, Rungvichaniwat A, Thepchalerm C, Nakason C. Novel creaming agent for preparation of creamed concentrated natural rubber latex. J Elastomers Plast. 2010;42(5):453–70. doi:10.1177/0095244310374227. [Google Scholar] [CrossRef]
40. Strommer B, Battig A, Schulze D, Jácome LA, Schartel B, Böhning M. Shape, orientation, interaction, or dispersion: valorization of the influence factors in natural rubber nanocomposites. Rubber Chem Technol. 2023;96(1):40–58. doi:10.5254/rct.23.77961. [Google Scholar] [CrossRef]
41. Hussaini SR, Dvorkin J. Specific surface area versus porosity from digital images: high-porosity granular samples. J Petrol Sci Eng. 2021;206:108961. doi:10.1016/j.petrol.2021.108961. [Google Scholar] [CrossRef]
42. Nampitch T. Mechanical, thermal and morphological properties of polylactic acid/natural rubber/bagasse fiber composite foams. Results Mater. 2021;12(1):100225. doi:10.1016/j.rinma.2021.100225. [Google Scholar] [CrossRef]
43. Mohamad KK. Study and development of green elastomeric compounds. J Biotechnol Biores. 2019;1(4):516. doi:10.31031/jbb.2019.01.000516. [Google Scholar] [CrossRef]
44. Phomrak S, Nimpaiboon A, Newby BZ, Phisalaphong M. Natural rubber latex foam reinforced with micro- and nanofibrillated cellulose via Dunlop method. Polymers. 2020;12(9):1959. doi:10.3390/polym12091959. [Google Scholar] [PubMed] [CrossRef]
45. Dananjaya SAV, Somarathna YR, Karunanayake L, Siriwardena S. Waste Mica as filler for natural rubber latex foam composites. J Polym Res. 2022;29(3):71. doi:10.1007/s10965-022-02930-w. [Google Scholar] [CrossRef]
46. Suethao S, Ponloa W, Phongphanphanee S, Wong-Ekkabut J, Smitthipong W. Current challenges in thermodynamic aspects of rubber foam. Sci Rep. 2021;11(1):6097. doi:10.1038/s41598-021-85638-z. [Google Scholar] [PubMed] [CrossRef]
47. Garrison TF, Murawski A, Quirino RL. Bio-based polymers with potential for biodegradability. Polymers. 2016;8(7):262. doi:10.3390/polym8070262. [Google Scholar] [PubMed] [CrossRef]
48. Ergün ME, Kurt R, Can A, Özlüsoylu İ, Ersoy Kalyoncu E. Optimized eco-friendly foam materials: a study on the effects of sodium alginate, cellulose, and activated carbon. Polymers. 2024;16(17):2511. doi:10.3390/polym16172511. [Google Scholar] [PubMed] [CrossRef]
49. Suethao S, Phongphanphanee S, Wong-Ekkabut J, Smitthipong W. The relationship between the morphology and elasticity of natural rubber foam based on the concentration of the chemical blowing agent. Polymers. 2021;13(7):1091. doi:10.3390/polym13071091. [Google Scholar] [PubMed] [CrossRef]
50. Keawkumay C, Krukkratoke P, Youngjan S, Osakoo N, Deekamwong K, Khemthong P, et al. Extraction of silica from sugarcane bagasse ash and its utilization in zeolite 4A synthesis for CO2 adsorption. RSC Adv. 2024;14(27):19472–82. doi:10.1039/d4ra02207f. [Google Scholar] [PubMed] [CrossRef]
51. Pereira AM, Moraes JCB, Moraes MJB, Akasaki JL, Tashima MM, Soriano L, et al. Valorisation of sugarcane bagasse ash (SCBA) with high quartz content as pozzolanic material in Portland cement mixtures. Mater Constr. 2018;68(330):e153. doi:10.3989/mc.2018.00617. [Google Scholar] [CrossRef]
52. Ngamsurach P, Nemkhuntod S, Chanaphan P, Praipipat P. Modified beaded materials from recycled wastes of bagasse and bagasse fly ash with iron(III) oxide-hydroxide and zinc oxide for the removal of reactive blue 4 dye in aqueous solution. ACS Omega. 2022;7(39):34839–57. doi:10.1021/acsomega.2c03250. [Google Scholar] [PubMed] [CrossRef]
53. Reowdecha M, Dittanet P, Sae-Oui P, Loykulnant S, Prapainainar P. Film and latex forms of silica-reinforced natural rubber composite vulcanized using electron beam irradiation. Heliyon. 2021;7(6):e07176. doi:10.1016/j.heliyon.2021.e07176. [Google Scholar] [PubMed] [CrossRef]
54. Osarenmwinda JO, Abode SI. Potential of carbonized bagasse filler in rubber products. J Emerg Trends Eng Appl Sci. 2010;7016:2141. [Google Scholar]
55. Zafeer MK, Prabhu R, Rao S, Mahesha GT, Bhat KS. Mechanical characteristics of sugarcane bagasse fibre reinforced polymer composites: a review. Cogent Eng. 2023;10(1):2200903. doi:10.1080/23311916.2023.2200903. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF
 Downloads
Downloads
 Citation Tools
Citation Tools