 Open Access
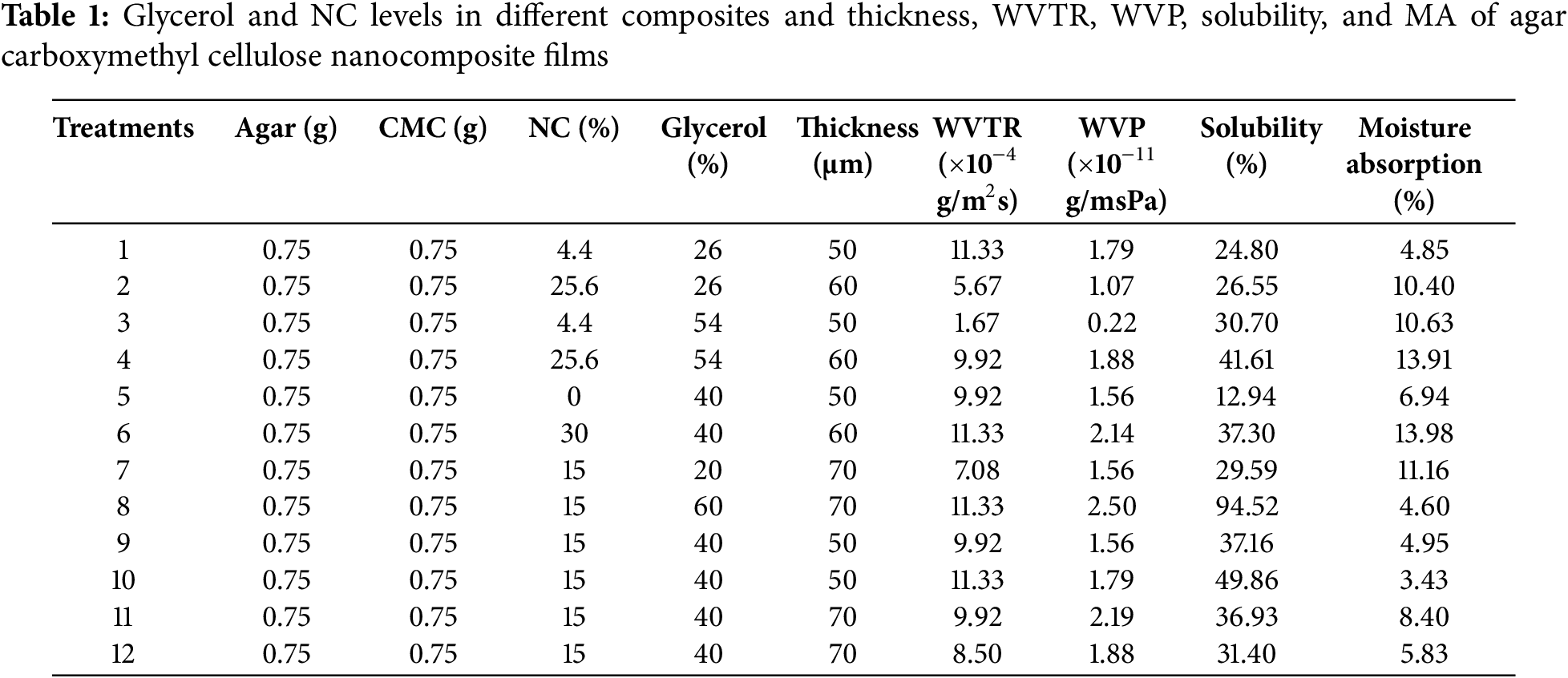
Open Access
ARTICLE
Plasticized Agar-Carboxymethyl Cellulose Based Composites Properties Reinforced with Nanocellulose
1 Department of Food Science and Technology, Sofian Branch, Islamic Azad University, Sofian, Iran
2 Industrial Nanotechnology Research Center, Tabriz Branch, Islamic Azad University, Tabriz, Iran
* Corresponding Author: Farid Amidi-Fazli. Email:
Journal of Renewable Materials 2025, 13(5), 915-929. https://doi.org/10.32604/jrm.2025.02024-0009
Received 07 October 2024; Accepted 08 January 2025; Issue published 20 May 2025
Abstract
Biodegradable packaging has emerged as a viable alternative to non-biodegradable polymers. This study explores different treatments of agar-carboxymethyl cellulose (CMC) nanocomposites developed via the casting method. We investigated the effects of varying glycerol levels (20%–60%) as a plasticizer and nanocellulose levels (0%–30%) as a filler on the properties of agar-CMC nanocomposites. Key properties analyzed included water vapor permeability, solubility in water, moisture absorption, water contact angle, color properties, and mechanical properties. The films exhibited low water vapor permeability, ranging from 2.50 × 10−11 g/msPa to 2.23 × 10−12 g/msPa. Water solubility of the films was below 40%, except for the film containing 60% glycerol. Despite this, all samples demonstrated very low moisture absorption. The water droplet contact angle tests indicated that the film surfaces were moderately hydrophilic. Increasing the nanocellulose content further enhanced the hydrophilicity of the surfaces. The maximum tensile strength and elongation at break recorded were 31.09 MPa and 46.2%, respectively. While adding glycerol reduced tensile strength, it improved elongation at break. Visual observations and color evaluation revealed that the films were transparent, with a low total color difference and a low yellow index. The simultaneous use of both agar and carboxymethyl cellulose polymers in biocomposite formulation successfully mitigated the disadvantages associated with each biopolymer when used alone, thereby enhancing their functional properties.Graphic Abstract
Keywords
Waste burning leads to the release of harmful gases (e.g., carbon dioxide, carbon monoxide, chlorine, furans, amines, and dioxins), which degrade air quality, increase global warming threats and pose health risks. The growing issues related to waste disposal and the detrimental environmental and public health impacts of non-degradable synthetic polymers have heightened global concerns about finding alternative, environmentally friendly materials [1].
Nanomaterials exhibit higher solubility and absorption. However, public acceptance issues due to fears of unknown or potential side effects related to dairy and food products based on nanomaterials, must be resolved for large-scale commercialization [2]. Nanomaterials can create barriers against oxygen and moisture, significantly prolonging food freshness. Nanopackaging reduces microbial contamination, ensuring safer food products for consumers [3,4].
To achieve biodegradability and transparency in composites, marine-origin biopolymer agar can be used. Agar is an inert biopolymer, allowing it to interact easily with various biological compounds and be used as a surface coating for food products. The film-forming ability of agar is based on its gelling properties. Dissolving agar powder in hot water forms a viscous liquid that, when cooled below the gel point, transforms into a reversible gel through hydrogen bonding between agarose molecules. However, agar cannot produce desirable films on its own due to some disadvantages [5]. Agar-based composites exhibit several limitations regarding their mechanical properties, which can hinder their practical applications. While agar can enhance certain characteristics, it also introduces challenges that need to be addressed for optimal performance. Agar composites often demonstrate moderate tensile strength, which can be insufficient for demanding applications. For instance, the tensile strength of reed leaf fiber/agar composites was only 1.81 MPa [6]. Incorporating materials like collagen into agar can enhance its flexibility and strength, making it effective for wound healing applications [7]. Agar films can be enhanced by incorporating gelatin, which improves tensile strength [8]. Moreover, agar composites tend to absorb moisture, which can lead to reduced mechanical performance. The moisture absorption values increased with higher agar content in thermoplastic sago starch composites [9].
In the film-forming process, the temperature of the film solution must be higher than the agar gel temperature to prevent premature gelation. When dried, the non-ionic and linear structure of agarose produces a strong film. The concentration of agar required depends on its type, source, production method, and other components in the film matrix [5]. Agar film presents a promising sustainable alternative to traditional plastic films in the food industry, primarily due to its biodegradable properties. Agar-based films exhibit excellent biodegradability, with significant weight loss observed in soil degradation studies, indicating their potential to reduce plastic waste [10,11].
Modified agar/gelatin composite films reinforced with cellulose nanocrystals, agar/gellan gum composite films, and agar/carrageenan composite films reinforced with cellulose nanocrystals and calcium chloride were prepared. These nanocomposite films exhibited dense and smooth surface structures, tensile strength greater than 60 MPa, elongation at break of 21%, and light transmittance of 80% at 660 nm. Water swelling rate, solubility, and vapor permeability were less than 155%, 33%, and 6.8 × 10−11 g/mPas, respectively. These films demonstrated good edibility, light transmittance, and thermal properties and outperformed plastic-based packaging films in preserving strawberries [12].
The plant cell wall contains polysaccharides such as cellulose, hemicellulose, and pectin, which are dietary fibers with significant potential to replace petroleum-based polymers. Carboxymethyl cellulose (CMC) incorporation into various matrices has been shown to substantially increase tensile strength, breaking stress, and elongation at break, making it a valuable additive in composite formulations. CMC composites exhibit remarkable improvements in mechanical properties. For instance, paper composites with 3 wt.% CMC showed breaking stress and strain increases of 2.0- and 3.9-fold, respectively, compared to untreated paper [13].
Nanocellulose presents a promising sustainable alternative to traditional plastics in packaging materials, particularly due to its biodegradable nature and favorable mechanical properties. Nanocellulose is fully biodegradable, addressing the critical issue of plastic waste [14]. Nanocellulose can provide effective barriers against moisture and gases, essential for food preservation [15]. Its integration with other materials, such as PLA, can lead to innovative packaging solutions that improve shelf life and reduce waste [16].
Plasticizers are low-molecular-weight non-volatile compounds extensively used in the composite and polymer industries as additives [17]. Glycerol and sorbitol plasticizers at different concentrations in polysaccharide-based biocomposites have improved film flexibility and moisture absorption, enhancing their insulation against cold and heat [18,19].
This study aims to investigate the properties of plasticized agar-CMC composites reinforced by nanocellulose. By evaluating the mechanical, hydrophilic, and morphological characteristics of these nanocomposites, we aim to provide insights into their potential applications, particularly in biodegradable packaging and biomedical engineering. This research could significantly contribute to the growing body of knowledge on sustainable materials and their applications.
Numerous studies have explored the individual properties of agar and CMC, as well as their composites. However, limited literature has focused specifically on the synergistic effects of integrating glycerol as a plasticizer and incorporating nanocellulose as a reinforcing agent within agar-CMC matrices. Considering the mentioned limitations for pure agar films, it is crucial to balance the benefits and drawbacks of agar in composite materials. By combining agar with carboxymethyl cellulose and optimizing the levels of glycerol and nanocellulose, the current study aimed to create nanocomposites with improved mechanical properties and reduced moisture absorption.
Carboxymethyl cellulose (practical grade) was obtained from Caragum Parsian Corporation (Tehran, Iran). Agar was purchased from Biolife (Milan, Italy). Glycerol, sulfuric acid, and sodium hydroxide were sourced from Dr. Mojallali Chemical Industry Complex Company (Tehran, Iran). Calcium sulfate and potassium sulfate (analytical grade) were procured from Merck (Darmstadt, Germany).
Carboxymethyl cellulose (practical grade) was provided by Caragum Parsian Corporation (Tehran, Iran). Agar was purchased from Biolife (Milan, Italy). Glycerol, sulfuric acid, and sodium hydroxide were purchased from Dr. Mojallali Chemical Industry Complex Company (Tehran, Iran). Calcium sulfate and potassium sulfate (analytical grade) were purchased from Merck (Darmstadt, Germany).
Nanocellulose fibers (NC) were extracted from cotton using a modified chemical method based on Cao et al. [20]. Cotton was immersed in a 2% (w/v) sodium hydroxide solution for 24 h at room temperature to remove impurities. The fibers were then washed with distilled water until the sodium hydroxide was completely eliminated (confirmed by phenolphthalein reagent). Chemical hydrolysis of the purified cotton was performed using a 65% (w/v) sulfuric acid solution with an acid-to-cotton ratio of 20:1. The hydrolysis process continued for 3 h at 50°C with continuous agitation at 600 rpm. Neutralization was then performed using a 10% (w/v) sodium hydroxide solution until the pH of the nanocellulose suspension reached 5–5.5. Finally, the nanocellulose was washed with distilled water for 1 h.
Twelve agar-carboxymethyl cellulose composites containing varying levels of glycerol and nanocellulose were produced. The agar and carboxymethyl cellulose amounts were constant in all composites, with a 1:1 blend ratio. Initially, 0.75 g of agar and 0.75 g of CMC were dissolved in distilled water. Glycerol (20% to 60% of polymer weight) was added as a plasticizer, and the solution was rested for 15 min. The gelatinization procedure was conducted at 90°C for 60 min. Nanocellulose (0%–30% of polymer weight) was added to the cooled blend (70°C). The final volume of all composites was 150 mL. The film-making solution was stirred at 200 rpm for 30 min at 70°C, then 25 mL of the prepared solution was poured into polystyrene plates with a diameter of 8 cm and left to dry at ambient temperature for 24 h [21].
2.4 Scanning Electron Microscopy (SEM)
The size of the nanocellulose was determined using a XL30 scanning electron microscope (Philips, Netherlands). The electron acceleration voltage was 28 kV, and images were taken at various magnifications.
2.5 X-Ray Diffraction (XRD) Analysis
The crystallinity of nanocellulose was determined using Xpert Pro MPD XRD equipment (Panalytical Xpert PRO X-Ray, Almelo, Netherlands) with a copper anode. The X-ray generator was set to 30 mA and 40 kV, with samples exposed to X-rays at a wavelength of 1.5405 Å at ambient temperature. The scanning speed was 1 s, with a step size of 2θ = 0.05°. Refractive radiation was collected within the range of 2θ = 10°−80°. The Segal method [22] was used to calculate the crystallinity index (CI) of the obtained nanocellulose:
where CI: crystallinity index, I002: peak intensity at 2θ = 22.7° and IAM: peak intensity at 2θ = 18°.
2.6 Film Thickness Measurements
Film thickness was measured using a caliper (Mitutoyo, Nakatsugawa, Japan) with a precision of 0.01 mm. Thickness measurements were taken at five different parts of each film, and the average was reported as the nanocomposite thickness.
2.7 Water Vapor Permeability (WVP)
Water vapor flux (g/m2s) and water vapor permeability (g/msPa) through the film samples were determined according to ASTM E96-95 with modifications [23]. Film samples were kept for 24 h at 50% relative humidity at ambient temperature. Vials containing 3 g of calcium sulfate (0% RH inside) were placed in containers with saturated potassium sulfate solution (97% RH) at 25°C. The water vapor transmission rate (WVTR) and water vapor permeability (WVP) were calculated using the following equations:
where WVTR: water vapor transmission rate (g/sm2), WVP: water vapor permeation (g/msPa), Δw: weight gain (g), t: time (s), A: film area exposed to water vapor flux (m2), X: film thickness (m), P: water vapor pressure (Pa), R1: relative humidity in the container (%) and R2: relative humidity in the vial (%).
Film solubility in water was defined as the amount of film dissolved after 24 h in distilled water. The dry matter of 20 mm × 20 mm film samples was determined [24]. Samples were immersed in 50 mL of distilled water at 25°C for 24 h, then removed and dried. Solubility was calculated using equation below [25]:
where WS: solubility in water (%), Dm1: initial film dry matter (%) and Dm2: film dry matter after immersion in water (%).
To measure moisture absorption, 20 mm × 20 mm film pieces were dried at 50°C for 24 h, then placed in a container with saturated potassium sulfate solution (97% RH) at 25°C for 4 days. Moisture absorption was calculated using the equation suggested by [26]:
where MA: moisture absorption (%), W1: initial film weight (g) and W2: final film weight (g).
2.10 Surface Hydrophobicity of the Film
The sessile drop method was used to determine the contact angle. A drop of distilled water was placed on the sample surface [27], and the shape was imaged after 5 s using a 16 MP digital camera (Canon IXY 10s, Nagano, Japan). The contact angle was measured using ImageJ 1.50i software. The angle between the tangent line on the droplet and the horizontal line indicates the contact angle.
The Mechanical properties were determined using a CT3 instrument (Brookfield, WI, USA) according to ASTM D882-91 [28]. Samples were conditioned at 50% RH for 24 h before testing. Belt-shaped samples (8 cm × 1 cm) were stretched at 1 mm/s with a 6 cm grip distance. Ultimate tensile strength (maximum stress of a material that can be sustained without permanent strain) and elongation at break (the percentage of length increase to the initial length of the film) were measured.
Color and transparency were evaluated in a black box. Samples were placed on standard white paper (L* = 87.99, a* = −1.64, b* = −9.09) and imaged from 10 cm using a Canon digital camera. RGB values were extracted using ImageJ 1.50i software and converted to CIELab values in terms of brightness (L* = 0−100), red-green (a* = (−127) − 128), and yellow-blue (b* = (−127) − 128). Total color difference (ΔE) [29], yellow index (YI), and white index (WI) [30] were calculated using:
where ΔE: total color difference, Yi: yellow index, WI: white index, L, a and b: color parameters in CIELab color space and Standard: standard background color parameters.
Twelve agar-carboxymethyl cellulose nanocomposites with different levels of glycerol and nanocellulose were prepared according to central composite design (CCD) (Table 1). Statistical analysis was performed using the response surface method (RSM) and ANOVA with Minitab 18 Software.
The dry matter content of the nanocellulose suspension was 2.4%. The produced nanocellulose was evaluated using scanning electron microscopy (SEM) and X-ray diffraction (XRD). The SEM image of nanocellulose is shown in Fig. 1. As seen in the image, the size of the produced nanocellulose ranges between 54 and 108 nm, confirming the effectiveness of the applied procedure in producing nanomaterial since the size is below 100 nm.

Figure 1: SEM micrograph of nanocellulose
The XRD pattern of the produced nanocellulose is shown in Fig. 2, where three peaks are observed. Peaks in an XRD pattern indicate the presence of crystalline regions in the tested sample. The observed peaks were located at 2θ = 14.6°, 16.6°, and 22.7°. According to the Segal equation (Eq. (1)), the crystallinity index (CI) of cellulose is the ratio of the crystalline region to the total material (including both crystalline and amorphous regions). The peak at 2θ = 22.7 represents both crystalline and amorphous regions, while the peak at 2θ = 16.6 represents the amorphous region.
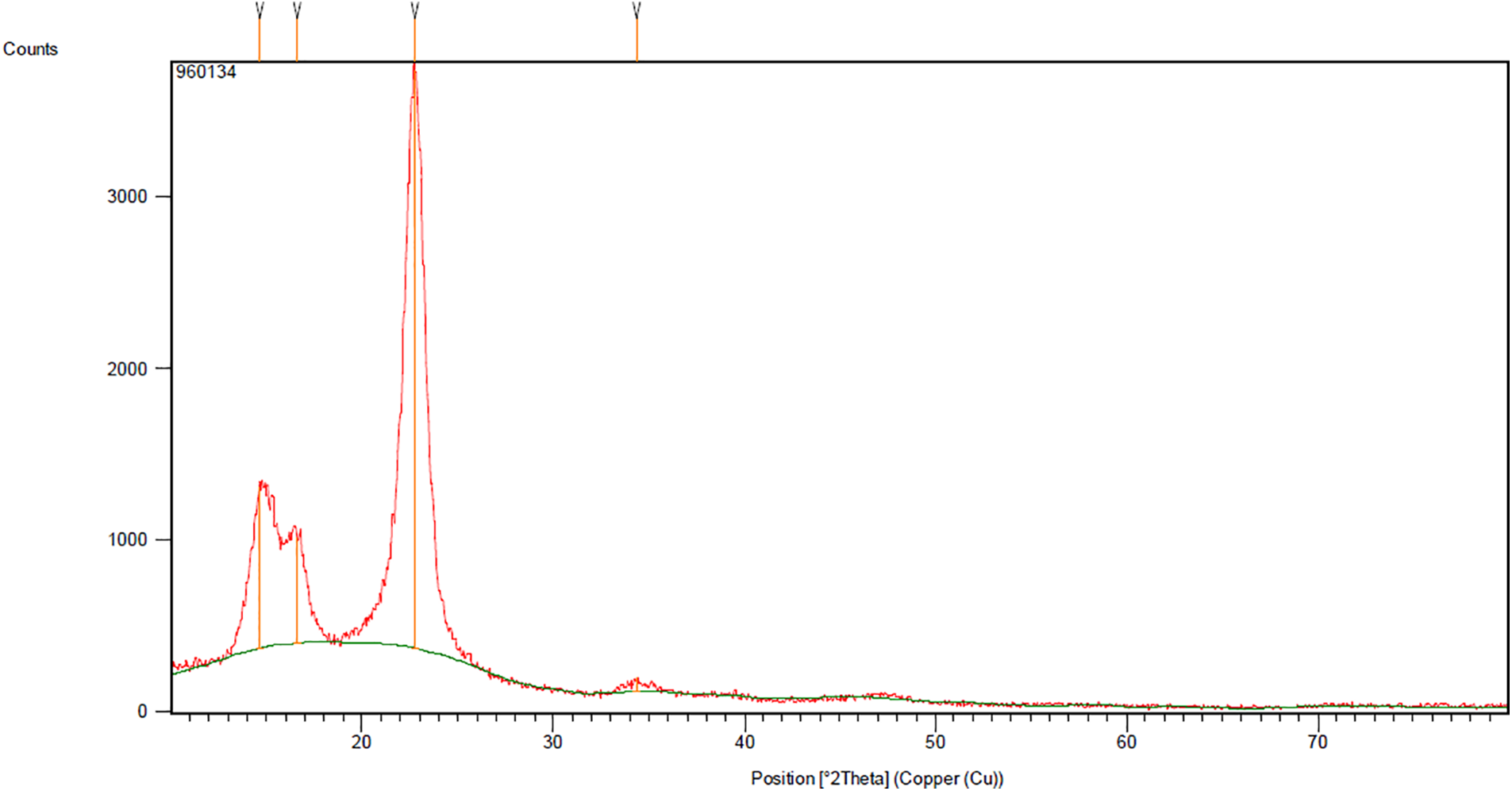
Figure 2: XRD diagram of produced nanocellulose
The crystallinity of the nanocellulose was calculated to be 82.1%. This crystallinity is higher than that of cellulose obtained from corn stalks (47%), Norwegian pigeons (47%), and kenaf fibers (77%), and is approximately equal to the crystallinity of filtered paper cellulose (83%) [31]. Each glucose unit in cellulose, as its primary structural unit, includes three hydroxyl groups. These groups and their ability to form hydrogen bonds play a crucial role in the formation of the crystalline structure of cellulose and its physical properties.
One of the important characteristics of food packaging films is to minimize moisture transmission between the packaged food and the surrounding environment. Thus, food packaging films must be capable of preventing high moisture exchange between the food and the environment. The average thickness of the prepared samples, based on five measurements, is presented in Table 1. The permeability of the prepared nanocomposites to water vapor ranged from 2.5 × 10−11 g/msPa to 2.23 × 10−12 g/msPa (Table 1). This is lower than the WVP of cellophane (6.89 × 10−11 g/msPa) [32].
According to ANOVA results, water vapor permeability (WVP) was significantly affected by the independent variables: the interaction between glycerol and nanocellulose (p < 0.01) and the second order of glycerol (p < 0.05). The following equation (Eq. (9)) demonstrates the correlation between WVP and the applied factors (R2 = 88.41%):
The WVP of carboxymethyl cellulose film containing 50% (v/w) glycerol was reported to be 2.98 × 10−10 g/msPa [33]. In the present study, the WVP of agar-carboxymethyl cellulose composite containing 40% glycerol without nanocellulose was found to be 1.56 × 10−11 g/msPa. The lower WVP of the composite in this study can be attributed to the lower glycerol level, as glycerol, acting as a plasticizer, increases the WVP of films. Polyol compounds like glycerol enhance the movement of polymeric chains and increase the film’s hydrophilicity. On the other hand, adding agar to carboxymethyl cellulose promoted interaction between the two biopolymers, reducing the availability of hydrophilic groups. As a result, the WVP of the developed composite decreased compared to the pure carboxymethyl cellulose film.
Nanocellulose did not improve the WVP property of the film at this glycerol level. The high nanocellulose content in the formulation led to increased WVP, likely due to filler accumulation, especially at high levels of NC, such as 25% and 30%. The WVP of the composite containing 40% glycerol and 30% NC was calculated to be 2.14 × 10−11 g/msPa, which is higher than the WVP of the film without NC, but still lower than the WVP of pure carboxymethyl cellulose film as reported by Dashipour et al. [33].
The findings showed that a high concentration of glycerol (up to 60%) and the use of 15% nanocellulose as reinforcement significantly increased the water vapor permeability (WVP), with the highest WVP of 2.50 × 10−11 g/msPa observed for this composite. Generally, glycerol as a plasticizer weakened the barrier properties to water vapor in films. The interaction between glycerol and starch in Starch/Clay Composites enhances plasticization but leads to phase separation at high glycerol concentrations, which can increase WVP due to the presence of free glycerol [34]. Similarly, in carboxymethyl cellulose films, increasing glycerol concentration raises the monolayer water content and WVP, with values ranging from 10.383 × 10−5 to 10.826 × 10−5 g/m2mmHgday as glycerol concentration increases [35]. This phenomenon is consistent with our findings where higher glycerol levels in the agar-carboxymethyl cellulose composites also resulted in increased WVP, highlighting the plasticizing effect of glycerol, which facilitates the movement of polymer chains and increases film hydrophilicity. Conversely, filler accumulation at high levels was linked to the increased WVP in the mentioned composite. Hence, nanocellulose, used at low levels, had noticeable effects on the WVP of the developed nanocomposites. It improved the barrier properties by creating a tortuous path for water vapor molecules. The composite containing 54% glycerol and 4.4% NC had the lowest WVP at 2.23 × 10−12 g/msPa.
Additionally, the water vapor permeability for pure carboxymethyl cellulose film was reported to be 3.60 × 10−10 g/msPa [36]. Large side anionic groups of carboxymethyl cellulose increase the free space in the polymer matrix, resulting in high WVP. Therefore, in this study, adding nanocellulose fills the empty spaces, slows the movement and diffusion of water molecules, and thus decreases the WVP. It should be noted that adding glycerol also led to a significant increase in water vapor permeability, as glycerol, being a polyol compound, facilitates the smooth movement of polymeric chains and increases film hydrophilicity. Consequently, the diffusion rate of water molecules in the polymer matrix increased [36]. In this case, using high levels of nanocellulose inhibited the plasticizing effect of glycerol.
Agar, as a packaging film, has advantages such as high mechanical strength, but its permeability to water vapor is high. Films made of agar containing 30% glycerol were found to have a WVP of 1.32 × 10−9 g/msPa [37,38]. In this study, using carboxymethyl cellulose and agar in composite production without adding nanocellulose improved barrier properties compared to films made of each pure biopolymer. The WVP of agar film containing 66% glycerol was reported to be 2.0 × 10−10 g/msPa, but it decreased to 1.90 × 10−10 g/msPa when one-third of the agar was replaced by fish skin gelatin with the same amount of glycerol [39]. Other researchers have reported WVP for agar film as 1.6 × 10−10 g/msPa [40].
The solubility in water of the composites was less than 40%, except for the composite containing 60% glycerol, which exhibited high solubility (Table 1). The low solubility of the prepared films can be attributed to the intermolecular interactions. The presence of hydroxyl and carboxyl groups in carboxymethyl cellulose and agar enhances the formation of strong hydrogen and ester bonds between the two polymers. These intermolecular interactions increase the homogeneity of the composite matrix and reduce solubility in water. The formation of such bonds between carboxymethyl cellulose and starch has also been reported [41]. The lowest solubility in water (12.94%) was observed in the composite containing 40% glycerol without nanocellulose (NC).
In the current study, the high amount of glycerol increased the solubility of composites due to its plasticizing effect on the polymer chain movement. Glycerol significantly affects the solubility in water of composites (p < 0.05). Although NC reduced the water vapor permeability, it increased the solubility in water of composites containing NC. This may be due to the replacement of NC between polymer chains, preventing suitable interaction between them. The solubility of carboxymethyl cellulose film containing Zataria multiflora was reported to be 79.84% (28). The solubility of the agar film was 27.3%, which increased to 31.2% by adding lignin [42]. This increase could be attributed to the high solubility of lignin compared to agar.
Generally, the prepared agar-carboxymethyl cellulose films exhibited low moisture absorption (MA), with most cases demonstrating MA values of less than 7%. The low moisture absorption was observed in composites containing low levels of nanocellulose (NC), while the addition of up to 15% NC had a negligible effect on the MA of the agar-carboxymethyl cellulose nanocomposites (Table 1). However, it was also observed that NC levels exceeding 15% increased the moisture absorption of the films up to two times.
The MA of a nanocomposite sample containing 40% glycerol and 15% NC was 5.65%, while the MA of a composite with the same amount of glycerol and 30% NC was as high as 13.98%. Compared to this study, high moisture absorption was observed for pure agar and agar-starch films. The addition of agar to the starch film increased the moisture absorption due to the hydrophilic nature of agar [43]. The researchers also concluded that agar is much more hygroscopic than starch.
As low moisture absorption is a crucial requirement for food packaging films, the MA values obtained for the prepared agar-carboxymethyl cellulose films seem appropriate. The low MA can be attributed to the low amount and the inaccessibility of hydrophilic groups resulting from intermolecular interactions between carboxymethyl cellulose and agar. In contrast, high amounts of nanocellulose dramatically increase the MA. The increased MA observed in some composites can also be attributed to the natural hydrophilic characteristics of NC, which contain numerous hydrophilic groups. Additionally, the limited linkage between agar and carboxymethyl cellulose due to the presence of nanocellulose increases the free hydrophilic groups of polymers in the matrix, leading to increased MA in composites containing high levels of NC. Accumulation of NC also increases the free space in the polymer matrix and decreases the linkage between agar and carboxymethyl cellulose.
3.5 Surface Hydrophobicity of the Films
As shown in Fig. 3, the contact angle of the water drop on the surfaces of the films is approximately 60 degrees in most composites. These results align well with the moisture absorption findings. Moderate levels of nanocellulose used in film preparation resulted in a high contact angle, indicating low surface hydrophilicity. However, as the nanocellulose content increased, the contact angle of the produced composites decreased, and the surfaces of the films exhibited higher hydrophilicity. This is because the presence of hydrophilic groups increases the interaction between water molecules and these groups, reducing the contact angle and increasing moisture absorption in the nanocomposites.
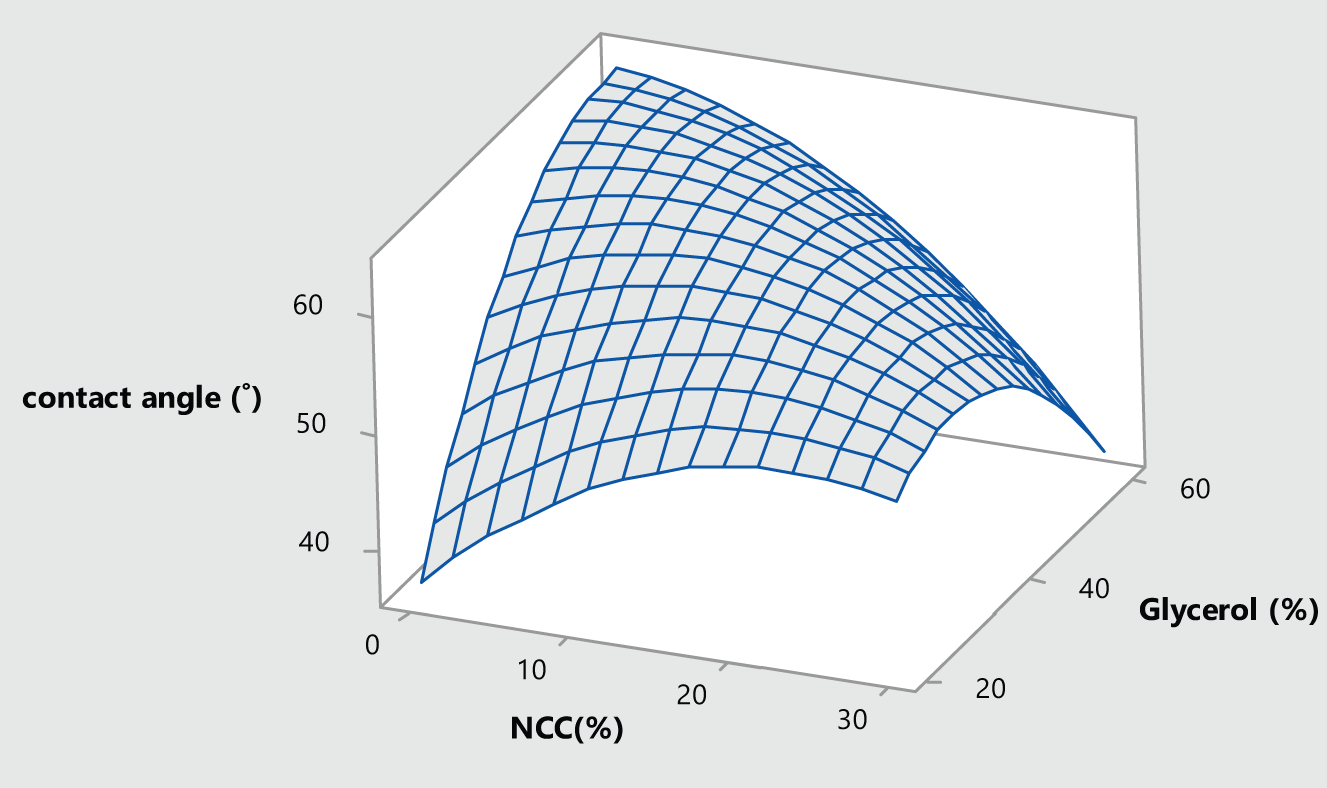
Figure 3: Surface plot of water contact angle vs. glycerol and nanocellulose
Statistical analysis indicated that all factors used in the preparation of composites had a significant effect on the contact angle (p < 0.05). The following equation (Eq. (10)) describes the correlation between the contact angle (CA) and the applied factors (R2 = 93.57%):
A decrease in the contact angle was observed with the addition of montmorillonite to the carboxymethyl cellulose film. The contact angle for the carboxymethyl cellulose film was 45.37°, which decreased to 36.53° with the addition of 10% montmorillonite [44]. This decrease was attributed to the increased hydrophilic properties of the films. The agar film initially presented a high contact angle (52.3°), but it decreased with increasing nanocellulose content, reaching 44.4° with the addition of 10% nanocellulose [45]. Additionally, cellulose sulfonation during nanocellulose preparation by the sulfuric acid hydrolysis method increased the hydrophilic properties of NC [46].
The high contact angle measured in this work for nanocomposites, compared to the carboxymethyl cellulose film, can be attributed to the addition of agar to the formulations. However, the contact angle decreased with the addition of more nanocellulose. The increase in contact angle with the addition of agar to protein-based films has also been reported [40].
Films made for food packaging often require high tensile strength, but they should also demonstrate appropriate flexibility. Various mechanical behaviors were observed in the composites depending on their different formulations. Ultimate tensile strength and elongation at break results for different nanocomposites are shown in Table 2. As shown, the ultimate tensile strength decreased as the amount of plasticizer increased, with the highest ultimate tensile strength (31.09 MPa) obtained in the film containing 26% glycerol. When the glycerol level increased to 60% in the composite formulation, the ultimate tensile strength decreased by one third (10.3 MPa). Statistically, glycerol was observed to have a significant effect on ultimate tensile strength (p < 0.05).
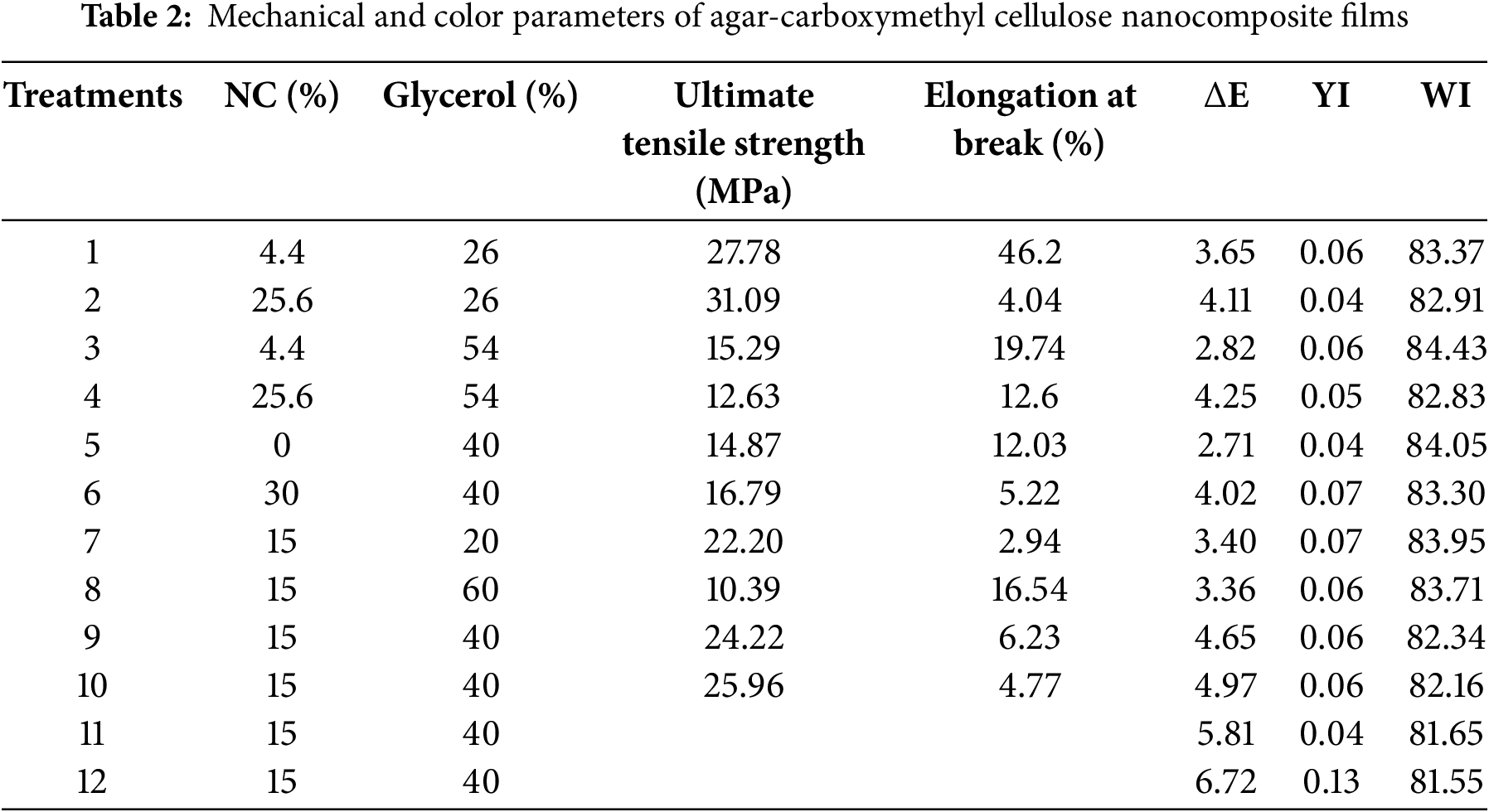
The absence of nanocellulose or a high amount of nanocellulose also had negative effects on the ultimate tensile strength of composites containing moderate levels of glycerol. Meanwhile, adding 15% nanocellulose to those composites (15% NC and 40% glycerol) enhanced the tensile strength. The high ultimate tensile strength of films containing nanocellulose could be due to the high surface area of the nanomaterials, which likely increased hydrogen or ionic bonds between the filler and the matrix, resulting in an enhanced nanocomposite structure [47].
In this study, as expected, glycerol increased the elongation at break, while nanocellulose caused the films to become brittle. This is evident when comparing composite 5 (0% NC and 40% glycerol) and composite 6 (30% NC and 40% glycerol). The ultimate tensile strength for composites 5 and 6 were 14.87 and 16.795 MPa, respectively, which are low compared to other composites. The elongation at break for composites 5 and 6 were 12.03% and 5.225%, respectively, indicating flexible behavior for composite 5 due to the absence of NC, while composite 6 exhibited brittle behavior with 30% NC. Notably, both composites contained the same level of glycerol (40%). Hence, elongation at break reflects the film’s flexibility, and the low flexibility at high filler levels can be due to the limited movement of polymer chains and increased film hardness [45].
It should also be noted that the addition of 4% nanocellulose significantly improved elongation at break, leading to an increase up to 46.2% in the composite containing 26% glycerol. This sample also had a high ultimate tensile strength of 31.09 MPa. High filler content led to heterogeneous dispersion and aggregation of the filler, resulting in uneven mechanical stress distribution between the matrix and the filler, causing a decrease in ultimate tensile strength [48].
In plasticized agar films with 30% glycerol, the use of nanocellulose up to 3% increased tensile strength, but higher amounts of NC weakened this property, similar to the current study [38]. The ultimate tensile strength and elongation at break of pure agar films containing 40% glycerol were 45.5 MPa and 26.7%, respectively. Adding lignin up to 5% increased these values, but later on, the ultimate tensile strength and elongation at break decreased [49].
The weak ultimate tensile strength for glycerol-plasticized carboxymethyl cellulose films has also been reported by other researchers [33,50]. In this study, low ultimate tensile strength and elongation at break values obtained for the composite without filler can be attributed to the weak mechanical properties of carboxymethyl cellulose. This failure was overcome by adding NC. Additionally, agar acted as a reinforcing agent by forming a strong three-dimensional gel network [51]. The use of agar also strengthened the mechanical properties of gelatin-made films [40].
In the present study, the total color difference (ΔE) of the samples ranged from 2.7 to 5.5 (Table 2), while the ΔE of pure agar films has been reported to be 3.6 [52]. Pure carboxymethyl cellulose films also exhibited a low ΔE of 5.18 [44]. Since both pure agar film and pure carboxymethyl cellulose film had low ΔE values, the composite of these two polymers in this study demonstrated a low total color difference. Color is considered an effective characteristic of food appearance and is one of the key factors in the food packaging industry.
Increasing the amount of nanocellulose to more than 15% also increased the ΔE, likely due to the crystalline structure of the nanocellulose. The second order of NC significantly affected the ΔE (p < 0.01). The lowest ΔE (2.7) was observed in the composite without the addition of nanocellulose. However, the transparency of the films decreased as the NC content in the formulations increased, and ΔE reached 2.8 and 3.6 in composites 3 and 1, respectively, which contain 4.4% NC.
A rise in ΔE due to the addition of NC to pure agar film has also been reported by Reddy et al. [45]. Similarly, other studies have shown an increase in ΔE with the addition of nanoparticles. The ΔE of pure agar film was measured to be 2.98, and it dramatically increased with the addition of nanoparticles [53].
The different levels of glycerol and NC used in film preparation did not significantly affect the yellow index (YI) of the samples. The calculated YI for all samples was less than 0.07 (Table 2). Conversely, the calculated white index (WI) for all composites was more than 81 (Table 2), indicating the transparency of the produced films. Statistical analysis results showed that nanocellulose (p < 0.05), as well as the second order of nanocellulose and the second order of glycerol (p < 0.01), significantly affected the white index.
Carboxymethyl cellulose (CMC) has a good inhibitory effect on water vapor permeability but exhibits weak mechanical strength. In contrast, agar is permeable to water vapor but demonstrates high mechanical strength. Utilizing these two polymers in the production of nanocomposites proved to be an effective way to mitigate the disadvantages of each biopolymer when used alone. As a result, films with enhanced permeability and mechanical properties were produced. This study demonstrates that using medium glycerol levels and low nanocellulose (NC) levels can improve the hydrophobic properties of the produced films. Additionally, these amounts positively affect the mechanical properties of the tested samples. Further research is needed to reduce the solubility in water and surface hydrophilicity of the composites beyond the levels achieved in this study.
Acknowledgement: None.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Vahideh Pourghasemi-Soufiani: Investigation, Methodology, Data curation. Farid Amidi-Fazli: Conceptualization, Methodology, Writing—original draft preparation, Reviewing, Validation, and Editing. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Shaikh S, Yaqoob M, Aggarwal P. An overview of biodegradable packaging in food industry. Curr Res Food Sci. 2021;4:503–20. [Google Scholar] [PubMed]
2. Malik S, Muhammad K, Waheed Y. Nanotechnology: a revolution in modern industry. Molecules. 2023;28(2):661. [Google Scholar] [PubMed]
3. Kumar S. Prospects and challenges of nanomaterials in sustainable food preservation and packaging: a review. Discover Nano. 2024;19(1):178. doi:10.1186/s11671-024-04142-1. [Google Scholar] [PubMed] [CrossRef]
4. Jamshaid A, Ibrahim S, Ali A, Basim M, Atta A, Haseeb MA, et al. Nanotechnology in food: processing, packaging, and preservation. DIET FACTOR (J Nutrit Food Sci). 2024;5(3):2–11. doi:10.54393/df.v5i03.122. [Google Scholar] [CrossRef]
5. Mostafavi FS, Zaeim D. Agar-based edible films for food packaging applications—a review. Int J Biol Macromol. 2020;159(2):1165–76. doi:10.1016/j.ijbiomac.2020.05.123. [Google Scholar] [PubMed] [CrossRef]
6. Shi K, Li X, Wang Q, He C. The interfacial and mechanical properties of plant leaf fibers combined with agar composites. J Nat Fibers. 2023;20(1):2150357. [Google Scholar]
7. Surendran G, Sherje AP. Biomedical applications of agar and its composites: a mini-review. Nat Prod J. 2022;13(5):e150622206016. doi:10.2174/2210315512666220615113320. [Google Scholar] [CrossRef]
8. Nissa RC, Hanifah A, Nurhamiyah Y, Firdiana B, Christian D, Veridianti DD, et al. The processability and properties of agar/gelatin film in a melt-mixing process. Green Mater. 2024;1–9. doi:10.1680/jgrma.24.00002. [Google Scholar] [CrossRef]
9. Taharuddin NH, Jumaidin R, Ilyas RA, Kamaruddin ZH, Mansor MR, Md Yusof FA, et al. Effect of agar on the mechanical, thermal, and moisture absorption properties of thermoplastic sago starch composites. Materials. 2022;15(24):8954. [Google Scholar] [PubMed]
10. Sable S, Vairal A, Bhosale V, Surve S, Raza U, Thengre M. Bioplastic from agar powder: preparation and its characterization. Sci Adv Mater. 2024;16(10):1040–6. doi:10.1166/sam.2024.4713. [Google Scholar] [CrossRef]
11. Wagh P, Vaidya V, Nawani N. Physical characterization of agar-based biodegradable films derived from nonhazardous laboratory waste. Energy Environ. 2024;8:121385. doi:10.1177/0958305x241282606. [Google Scholar] [CrossRef]
12. Zhao J, Liu T, Xia K, Liu X, Zhang X. Preparation and application of edible agar-based composite films modified by cellulose nanocrystals. Food Packag Shelf Life. 2022;34:100936. [Google Scholar]
13. Kobayashi J, Kaneko M, Supachettapun C, Takada K, Kaneko T, Kim JY, et al. Mechanical properties and reinforcement of paper sheets composited with carboxymethyl cellulose. Polymers. 2023;16(1):80. [Google Scholar] [PubMed]
14. Shanmugam K. Nanocellulose as sustainable eco-friendly nanomaterials: production, characterization, and applications. Dermatol Health. 2024;2(3):8–33. [Google Scholar]
15. Nath PC, Sharma R, Mahapatra U, Mohanta YK, Rustagi S, Sharma M, et al. Sustainable production of cellulosic biopolymers for enhanced smart food packaging: an up-to-date review. Int J Biol Macromol. 2024;273:133090. [Google Scholar] [PubMed]
16. Jacob J, Linson N, Mavelil-Sam R, Maria HJ, Pothan LA, Thomas S, et al. Poly(lactic acid)/nanocellulose biocomposites for sustainable food packaging. Cellulose. 2024;31(10):5997–6042. [Google Scholar]
17. Giroto AS, Garcia RHS, Colnago LA, Klamczynski A, Glenn GM, Ribeiro C. Role of urea and melamine as synergic co-plasticizers for starch composites for fertilizer application. Int J Biol Macromol. 2020;144:143–50. [Google Scholar] [PubMed]
18. Nagy S, Fekete E, Móczó J, Koczka K, Csiszár E. Heat-induced changes in cellulose nanocrystal/amino-aldehyde biocomposite systems. J Therm Anal Calorim. 2020;142(6):2371–83. [Google Scholar]
19. Rojas-Bringas PM, De-la-Torre GE, Torres FG. Influence of the source of starch and plasticizers on the environmental burden of starch-Brazil nut fiber biocomposite production: a life cycle assessment approach. Sci Total Environ. 2021;769:144869. [Google Scholar] [PubMed]
20. Cao X, Dong H, Li CM. New nanocomposite materials reinforced with flax cellulose nanocrystals in waterborne polyurethane. Biomacromolecules. 2007;8(3):899–904. [Google Scholar]
21. Golmohammadzadeh S, Amidi-Fazli F. The effect of glycerol and nanocellulose on hydrophilic and mechanical properties of gelatin-carboxymethyl cellulose composites. Iran Food Sci Technol Res J. 2022;18(5):681–97. [Google Scholar]
22. Segal L, Creely JJ, Martin AE, Conrad CM. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J. 1959;29(10):786–94. doi:10.1177/004051755902901003. [Google Scholar] [CrossRef]
23. ASTM. Annual book of ASTM standards. In: Standard test methods for water vapor transmission of materials (Vol. Designation E 96-95). Philadelphia: American Society for Testing and Materials; 1995. [Google Scholar]
24. Latimer GW. Official methods of analysis of AOAC INTERNATIONAL. New York: Oxford University Press; 2023. [Google Scholar]
25. Siddique N, Din MI, Hussain Z, Khalid R, Alsafari IA. Syzgium cumini seed/poly vinyl alcohol based water resistant biodegradable nano-cellulose composite reinforced with zinc oxide and silver oxide nanoparticles for improved mechanical properties. Int J Biol Macromol. 2024;277:134218. doi:10.1016/j.ijbiomac.2024.134218. [Google Scholar] [PubMed] [CrossRef]
26. Rahmadiawan D, Abral H, Railis RM, Iby IC, Mahardika M, Handayani D, et al. The enhanced moisture absorption and tensile strength of PVA/uncaria gambir extract by boric acid as a highly moisture-resistant, anti-UV, and strong film for food packaging applications. J Compos Sci. 2022;6(11):337. doi:10.3390/jcs6110337. [Google Scholar] [CrossRef]
27. Rahman S, Konwar A, Majumdar G, Chowdhury D. Guar gum-chitosan composite film as excellent material for packaging application. Carbohydr Polym Technol Appl. 2021;2(5):100158. doi:10.1016/j.carpta.2021.100158. [Google Scholar] [CrossRef]
28. ASTM. Standard test methods for tensile properties of thin plastic sheeting. D882-10 annual book of ASTM. Philadelphia, PA: American Society for Testing and Materials; 1995. [Google Scholar]
29. Zheng T, Tang P, Li G. Development of composite film based on collagen and phenolic acid-grafted chitosan for food packaging. Int J Biol Macromol. 2023;241(3):124494. doi:10.1016/j.ijbiomac.2023.124494. [Google Scholar] [PubMed] [CrossRef]
30. El Mouzahim M, Eddarai EM, Eladaoui S, Guenbour A, Bellaouchou A, Zarrouk A, et al. Food packaging composite film based on chitosan, natural kaolinite clay, and Ficus. carica leaves extract for fresh-cut apple slices preservation. Int J Biol Macromol. 2023;233:123430. doi:10.1016/j.ijbiomac.2023.123430. [Google Scholar] [PubMed] [CrossRef]
31. Thygesen A, Oddershede J, Lilholt H, Thomsen AB, Ståhl K. On the determination of crystallinity and cellulose content in plant fibres. Cellulose. 2005;12(6):563–76. doi:10.1007/s10570-005-9001-8. [Google Scholar] [CrossRef]
32. Bhatia S, Shah YA, Al-Harrasi A, Jawad M, Koca E, Aydemir LY. Novel applications of black pepper essential oil as an antioxidant agent in sodium caseinate and chitosan based active edible films. Int J Biol Macromol. 2024;254(1):128045. doi:10.1016/j.ijbiomac.2023.128045. [Google Scholar] [PubMed] [CrossRef]
33. Dashipour A, Razavilar V, Hosseini H, Shojaee-Aliabadi S, German JB, Ghanati K, et al. Antioxidant and antimicrobial carboxymethyl cellulose films containing Zataria multiflora essential oil. Int J Biol Macromol. 2015;72:606–13. doi:10.1016/j.ijbiomac.2014.09.006. [Google Scholar] [PubMed] [CrossRef]
34. Yu J, Wang J, Wu X, Zhu P. Effect of glycerol on water vapor sorption and mechanical properties of starch/clay composite films. Starch-Stärke. 2008;60(5):257–62. doi:10.1002/star.200700695. [Google Scholar] [CrossRef]
35. Rachtanapun P, Tongdeesoontorn W. Effect of glycerol concentration on sorption isotherms and water vapour permeability of carboxymethyl cellulose films from waste of mulberry paper. Asian J Food Agro-Ind. 2009;2:478–88. [Google Scholar]
36. Tong Q, Xiao Q, Lim LT. Preparation and properties of pullulan-alginate–carboxymethylcellulose blend films. Food Res Intern. 2008;41(10):1007–14. [Google Scholar]
37. Kanmani P, Rhim JW. Antimicrobial and physical-mechanical properties of agar-based films incorporated with grapefruit seed extract. Carbohydr Polym. 2014;102:708–16. [Google Scholar] [PubMed]
38. Shankar S, Rhim JW. Preparation of nanocellulose from micro-crystalline cellulose: the effect on the performance and properties of agar-based composite films. Carbohydr Polym. 2016;135:18–26. [Google Scholar] [PubMed]
39. Giménez B, López de Lacey A, Pérez-Santín E, López-Caballero ME, Montero P. Release of active compounds from agar and agar-gelatin films with green tea extract. Food Hydrocoll. 2013;30(1):264–71. [Google Scholar]
40. Mohajer S, Rezaei M, Hosseini SF. Physico-chemical and microstructural properties of fish gelatin/agar bio-based blend films. Carbohydr Polym. 2017;157:784–93. doi:10.1016/j.carbpol.2016.10.061. [Google Scholar] [PubMed] [CrossRef]
41. Tongdeesoontorn W, Mauer LJ, Wongruong S, Sriburi P, Rachtanapun P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem Cent J. 2011;5(1):6. doi:10.1186/1752-153X-5-6. [Google Scholar] [PubMed] [CrossRef]
42. Shankar S, Rhim JW. Preparation and characterization of agar/lignin/silver nanoparticles composite films with ultraviolet light barrier and antibacterial properties. Food Hydrocoll. 2017;71:76–84. doi:10.1016/j.foodhyd.2017.05.002. [Google Scholar] [CrossRef]
43. Wu Y, Geng F, Chang PR, Yu J, Ma X. Effect of agar on the microstructure and performance of potato starch film. Carbohydr Polym. 2009;76(2):299–304. doi:10.1016/j.carbpol.2008.10.031. [Google Scholar] [CrossRef]
44. Quilaqueo Gutiérrez M, Echeverría I, Ihl M, Bifani V, Mauri AN. Carboxymethylcellulose-montmorillonite nanocomposite films activated with murta (Ugni molinae Turcz) leaves extract. Carbohydr Polym. 2012;87(2):1495–502. doi:10.1016/j.carbpol.2011.09.040. [Google Scholar] [CrossRef]
45. Reddy JP, Rhim JW. Characterization of bionanocomposite films prepared with agar and paper-mulberry pulp nanocellulose. Carbohydr Polym. 2014;110:480–8. [Google Scholar] [PubMed]
46. Morais JPS, de Rosa MF, de Souza Filho M de sá M, Nascimento LD, do Nascimento DM, Cassales AR. Extraction and characterization of nanocellulose structures from raw cotton linter. Carbohydr Polym. 2013;91(1):229–35. [Google Scholar] [PubMed]
47. Siqueira G, Bras J, Dufresne A. Cellulosic bionanocomposites: a review of preparation, properties and applications. Polymers. 2010;2(4):728–65. [Google Scholar]
48. Agustin MB, Ahmmad B, De Leon ERP, Buenaobra JL, Salazar JR, Hirose F. Starch-based biocomposite films reinforced with cellulose nanocrystals from garlic stalks. Polym Compos. 2013;34(8):1325–32. [Google Scholar]
49. Shankar S, Reddy JP, Rhim JW. Effect of lignin on water vapor barrier, mechanical, and structural properties of agar/lignin composite films. Int J Biol Macromol. 2015;81:267–73. [Google Scholar] [PubMed]
50. Arık Kibar EA, Us F. Thermal, mechanical and water adsorption properties of corn starch-carboxymethylcellulose/methylcellulose biodegradable films. J Food Eng. 2013;114(1):123–31. [Google Scholar]
51. Tian H, Xu G, Yang B, Guo G. Microstructure and mechanical properties of soy protein/agar blend films: effect of composition and processing methods. J Food Eng. 2011;107(1):21–6. [Google Scholar]
52. Orsuwan A, Shankar S, Wang LF, Sothornvit R, Rhim JW. Preparation of antimicrobial agar/banana powder blend films reinforced with silver nanoparticles. Food Hydrocoll. 2016;60:476–85. [Google Scholar]
53. Arfat YA, Ahmed J, Jacob H. Preparation and characterization of agar-based nanocomposite films reinforced with bimetallic (Ag-Cu) alloy nanoparticles. Carbohydr Polym. 2017;155:382–90. [Google Scholar] [PubMed]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools