 Open Access
Open Access
ARTICLE
Development of Filter Composites Based on Eucalyptus Cellulosic Nanofibers, Sugarcane Bagasse Fibers and Soybean Hulls Applied in Biodiesel Purification
1 Institute of Chemistry, Federal University of Uberlândia, Uberlândia, 38400-902, Brazil
2 Institute of Exact and Natural Sciences of Pontal, Federal University of Uberlândia, Ituiutaba, 38304-402, Brazil
3 Exact Sciences, Natural and Education Institute, Federal University of Triângulo Mineiro, Uberaba, 38064-200, Brazil
* Corresponding Authors: Daniel Pasquini. Email: ; Luís Carlos de Morais. Email:
Journal of Renewable Materials 2025, 13(5), 957-980. https://doi.org/10.32604/jrm.2025.02024-0014
Received 11 October 2024; Accepted 31 December 2024; Issue published 20 May 2025
Abstract
Alternative methods for biodiesel purification that focus on ease of operation, cost reduction, and elimination of contaminated residues or that are easier to treat have received more attention. The dry wash route was used as an alternative to the wet route in biodiesel production. Filter membranes were developed based on cellulose nanofibers as the matrix and sugarcane bagasse fibers or soy hulls, as reinforcement to the matrix, before and after two chemical treatments (carboxymethylation and regeneration with sulfuric acid). The filters were characterized by permeability capacity, morphology, wettability, porosity, SEM and mechanical properties. The filtered biodiesel was also completely characterized. One of the major impacts of dry purification of biodiesel was the glycerin content after filtration. The filters CNFBR 20–28, CNFSR 5–28, CNFSR 5–35, and CNFBC 5–28 produced purified biodiesel with glycerin content below 0.02% (200 mg/L). Another relevant fact is related to the best results for acidity index, combined alkalinity, and glycerin content, obtained by the regenerated filter CNFBR 20–28, which presented a considerable permeate flow rate value above 4145 L h−1 m−2, which can be related to compacted lamellar layers observed by SEM. The produced filters were applied to biodiesel purification using a low-pressure filtration system and a simple vacuum pump, which resulted in an appreciable reduction in cost. The produced filter with sugarcane bagasse fiber carboxymethylated at 28 mesh of granulometry was efficient for biodiesel purification, including the efficient removal of free glycerin, in agreement with the standards defined by the national controlling agencies.Graphic Abstract
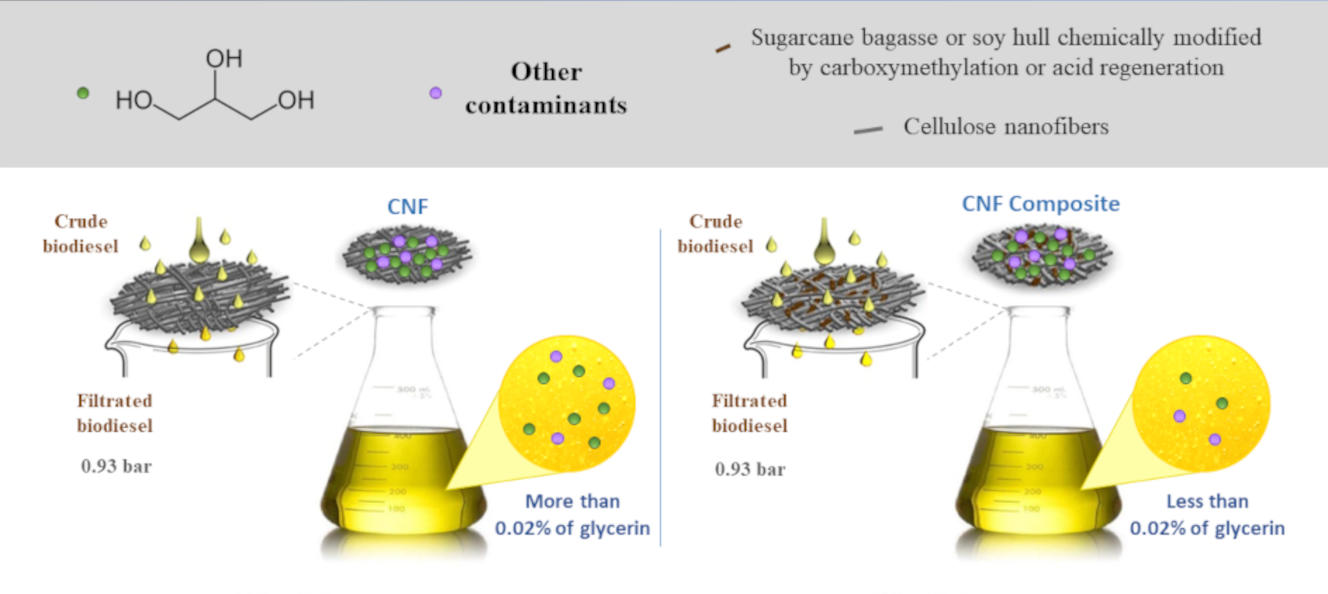
Keywords
Nowadays, there is a great need for technological solutions to supply the high energy demand, especially for eco-friendly alternative energy sources like ethanol and biodiesel, which are renewable and contribute to sustainable development with minimal environmental impacts [1].
Specifically, biodiesel may be produced from oil extracted from different vegetable species, like sunflower, soy, cotton, and corn. Brazil has a great diversity of these crops and holds technology for the extraction and processing of these oils [2]. Moreover, the production of biodiesel as an energetic matrix is encouraged by the National Program of Production and Use of Biodiesel (PNPB) and by Law N°11.097/2005, which determined the mandatory mixture of biodiesel with fossil diesel [3].
Biodiesel is mainly produced by transesterification. This reaction occurs at the molar proportion of 1:3 between triacylglycerol and methyl alcohol in the presence of a basic catalyst, like sodium hydroxide [4]. Considering complete esterification reactions, the products are free esters and glycerin as subproducts. The crude ester, however, cannot be commercialized as biodiesel, because it contains impurities such as glycerin residues, salts, and alcohol. Therefore, a purification process is required to fit the quality standards of the Brazilian Agency for Petroleum, Natural Gas and Biofuels (ANP) resolution N°45/2014 [5].
The most used method for biodiesel purification is the wet route, which consists of washing the biodiesel successively with water until all the impurities are removed. Although it is an efficient process, it can produce emulsions and uses a high volume of water, generating high quantities of effluents, which may cause negative impacts on the environment and economy [6].
An alternative method to purify biodiesel is the dry wash route, which avoids the excessive use of water and wastewater production. However, the conventional materials often used to construct filtering membranes are expensive and produced from non-renewable materials [7]. Bio-based membranes could be used for biodiesel purification to obtain a high level of purity, in accordance with the quality standards, with reduced consumption and energy costs, especially by the use of a vacuum pump connected to the polymeric membranes system [8]. However, some factors, like fragility and yield rates, must be addressed to optimize biodiesel purification. This information is the basis on which this scientific work was prepared and carried out.
Some authors have been studying alternative methods to purify the biodiesel to adapt it to the quality standards and at the same time make the process less expensive. Gomes et al. [9] showed that cellulose is an efficient adsorbent for glycerin removal from biodiesel. Squissato et al. [10] used a column filled with cellulose and obtained a good purification of biodiesel produced from sunflower oil, with reduced concentrations of free glycerin, methanol, and water.
Several studies have explored the use of adsorbents to purify biodiesel. Notably, a recent study by Konovalov et al. [11] examined various adsorbents, including activated anthracite, synthetic carbon (Chemviron), colloidal silica, meta-kaolin, talc, and bentonite, as part of a dry washing purification method. According to the authors themselves, the conclusion “as it does not remove the high molecular weight components originating from partially polymerized triglycerides” highlights the limits that adsorption methods impose. In addition, it involves longer times to improve purification than filtration methods.
One noteworthy study is by Camilo et al. [12], who utilized olive pits as adsorbents for biodiesel purification. Their findings indicate that the variables of time and temperature should be greater than those used in conventional filtration methods. This highlights the necessity of developing low-cost composite filters that possess the appropriate physical and chemical properties for use in crude biodiesel filtration processes.
Munoz et al. [13] investigated the use of natural fibers—specifically banana peel (BP), coconut fiber (CF), coffee husk (CH), and sugarcane bagasse (SB)—as adsorbents for the removal of glycerol in the purification of biodiesel. However, it is important to note that their evaluation method was indirect. They used an ethanolic glycerol solution for the removal studies, which does not fully replicate the actual conditions for glycerol removal present in biodiesel after its synthesis. However, the results demonstrated that the residual glycerol levels did not meet the specifications of biodiesel quality control agencies that specify values <0.02%. This work highlights an interesting fact in comparison with the current proposal presented by us, which demonstrates that by transforming fibers and natural sources of biomass into composites for filtration, the purification effect of biodiesel is enhanced, combined with the flow properties and reduction of steps to obtain quality biodiesel.
In a recent review of the literature, Abed et al. [14] highlight the advantages of dry-washing technologies that utilize filtration processes for biodiesel purification. They emphasize how these methods offer benefits compared to others, particularly in reducing environmental impacts.
In this context, agro-industrial residues, which are often discarded as lignocellulosic materials, are rich in cellulose and can be used for biodiesel purification. These lignocellulosic residues are available in high amounts and can be obtained from renewable and biodegradable sources [7]. These lignocellulosic residues are composed of cellulose, hemicellulose, and lignin, which are responsible for the reduction of acidity and the removal of glycerol and other contaminants from biodiesel [15].
However, the use of pure lignocellulosic materials for biodiesel purification has some drawbacks, such as low mechanical properties and inadequate wettability. To overcome these issues, the combination of lignocellulosic materials with other materials, like nanofibers may generate filter composites with enhanced biodiesel filtration yield and mechanical properties [5,16].
In this work, lignocellulosic composite filters were produced to be applied for biodiesel purification. The filters were composed of lignocellulosic materials and fibers obtained from agro-industrial residues, like sugarcane bagasse and soy hulls. In addition, the fibers were physically and chemically modified by carboxymethylation and acid regeneration to improve the interaction between the components of the composite filters.
The carboxymethylation aims at the insertion of -CH2COOX groups. The X can be a cation of metallic or acid hydrogen [17]. The acid regeneration consists of the sulfuric acid treatment to promote rupture of the aggregation of the vegetable cells, which allows the recovery by the precipitation in cold water [18]. This acid regeneration of the cellulose, lignin, and hemicelluloses generates fibers aggregated with morphologies different from the original, which contributes to improving the potential for the removal of biodiesel contaminants.
According to the National Supply Company [19], in Brazil, the sugarcane harvest for 2023/2024 was estimated at 713.2 million tons, and for soy (2023/2024) was estimated at approximately 294.1 million tons. These data reflect the high generation of these crop residues. Therefore, the residues from sugarcane and soy were chosen for the production of the composite lignocellulosic filters.
In this work, the “membrane” term is related to cellulosic filters, since these filters showed similar properties to the membranes. Cellulosic and lignocellulosic filters were produced based on cellulose nanofibers as matrix and sugarcane bagasse fibers and soy hulls, as reinforcement for the matrix. The produced filters were applied to biodiesel purification using a low-pressure filtration system and a simple vacuum pump to meet the requirements of the Brazilian controlling agency, ANP.
The main points of innovation of this work include i) the innovative use of filters based on lignocellulosic components successfully applied in the purification of biodiesel, ii) the filters require a vacuum system below one atmosphere to purify the biodiesel, iii) short times, from seconds to minutes, were sufficient to offer high purity to the biodiesel, iv) chemical treatment with sulfuric acid (regeneration) and carboxymethylation produced the best cellulosic filters, and v) the final composition of the biodiesel after only one filtration allowed for the qualification of biodiesel as acceptable by the National Agency of Petroleum, Natural Gas and Biofuels.
2.1 Preparation of Agro-Industrial Residues and of Cellulose Nanofiber
The cellulose nanofibers (CNF) from eucalyptus were supplied by the Suzano Pulp and Paper Company (São Paulo, Brazil) as aqueous dispersion with 3% solid mass. The sugarcane bagasse and soy hulls were supplied by Usina Vale do Tijuco (Uberaba, Brazil) and Algar Agro S.A. (Uberlândia, Brazil), respectively.
Sugarcane bagasse and soy hulls were first washed and dried at 80°C for 6 h. Then, the materials were crushed in an industrial blender for 2 min. The residues were separated by granulometry (28 and 35 mesh). Once obtained in the desired granulometry, the residues were washed again with water at 70°C under filtration three times. The materials were dried at 75°C for 4 h and at 105°C for 2 h. After drying, the samples were stored appropriately until use.
2.2.1 Regeneration (Sulfuric Acid Treatment)
For the regeneration, a stainless-steel cylinder with external water circulation was connected to a thermostatic bath at 45°C + 1°C. The cylinder was coupled to a mechanical stirring of 730 rpm. It was used 5 g of the washed sugarcane bagasse fibers and soy hulls to 250 mL of 1:1 (v/v) solution of sulfuric acid: water. After 90 min, the cylinder content was transferred to a beaker with water and ice (less than 5°C). The materials were washed successively until neutrality. The fibers were stored in water. With this technique, it is expected to obtain partial disorganization of the plant fibers, exposing the lignin and restructuring it on the surfaces of the fibers, and soy hulls, thus producing a modified material.
The carboxymethylation reaction was performed at the same stainless-steel cylinder. The reaction was performed at two steps at a temperature of 60°C, and 660 rpm of stirring. Firstly, 5 g of the sugarcane bagasse fibers (or soy hulls) were added to the 500 mL flat bottom flask with reflux column. Then, 250 mL of isopropyl alcohol, and sodium hydroxide solution (8 g in 25 mL of water) were added to the cylinder and the reaction occurred for 30 min. Secondly, a mixture of 50 mL of isopropyl alcohol and monochloroacetic acid (3.5 g dissolved in 12.5 mL of water) was added to the flask and stirred for 75 min at 60°C. After this, the samples were washed several times with ethyl alcohol: water (9:1, v/v). Then, the samples were dried at 70°C for 4 h and 105°C for 2 h. With this technique, it is expected to insert carboxymethyl groups to increase the potential of negative charges on the surfaces of vegetable fibers, and soy hulls, in such a way that they remove metal cations from biodiesel, and also increase the interaction with glycerin, aiming to promote better removal of glycerin and purification of biodiesel.
2.2.3 Determination of Ion Exchange Capacity (IEC)
The determination of the acid groups of carboxymethylated samples was performed according to Fras et al. [20]. Briefly, the samples were dispersed in 0.1 M of NaOH under constant stirring. After the addition of every 1 mL of titrant, the conductivity measurement was performed, at conductivity meter AC-200P. With the obtained data, the total of the acid groups was calculated using the Eq. (1).
where, X = total of acid groups (mol/g); COH = sodium hydroxide solution concentration (mol/L); Vt = volume of sodium hydroxide solution used in the intersection of the phases (L); m = dry mass of the sample (g).
40 g of CNF, sugarcane bagasse fibers, or soy hulls were added to 360 mL of distilled water and dispersed with mechanical stirring for 10 min. Then, the dispersion was vacuum-filtered. The residual solid mass was dried. The amount of sugarcane bagasse fibers or soy hulls added to the CNF were 2.5%, 5%, 7.5%, 10%, and 20% when the samples were pure, and 5% and 20% when the residues were modified chemically. A commercial cellulosic filter (CCF) with a grammage of 80 g m−2 and porous of 8 µm was cut in the same diameter as the filters produced in this work and used as a reference. The samples were labeled as CNF = cellulose nanofiber; B = bagasse; S = soy hull; CNFB 5–28 = 5% in mass of bagasse fibers and 28 mesh; R = regeneration treatment and C = carboxymethylated sample. With this filter preparation technique, it is possible to form layers upon layers in such a way that the final filter presents a good biodiesel filtration flow and, even with a short contact time, is able to remove the contaminants present in the biodiesel.
Biodiesel was produced following the methodology of Gomes et al. [9] with modification. The syntheses were performed by transesterification by methyl Route in a basic medium, using sodium hydroxide as a catalyst and soy oil as a source of triglycerides. 460 g of oil was stirred at 300 rpm at 60°C. After stabilization, a solution of 2.75 g NaOH dissolved in 92.06 g of methanol was added to the system and kept under agitation for 30 min. Then, the solution was kept in repose for phase separation for 2 h. After the process, glycerin was removed and biodiesel was obtained.
2.5.1 Biodiesel Filtration and Permeability
Biodiesel was filtered in a system similar to a Manifold, connected to a vacuum pump. The cellulosic and lignocellulosic filters were cut to a diameter of 48 mm and positioned between the container with oil and the base connected to the kitassato. The filtration kinetics was determined and the permeability was calculated using Eq. (2).
where, FP = Permeate flux; V = Volume of the eluate (L); T = Elution time (h); A = Filter area (m2).
2.5.2 Scanning Electron Microscopy (SEM)
Scanning electron microscopy (SEM) images were acquired using ZEISS microscope model EVO MA10. Energy-dispersive X-ray spectroscopy (EDS) analysis was performed at Oxford model 51-ADD0048. The samples were cryogenically fractured and covered with gold. The images obtained by SEM are expected to confirm the lamellar aspects involved in the formation of the filters observed in the cross-section after cold fracture. As well as to highlight possible defects inherent to the preparation method.
2.5.3 Infrared Spectroscopy (FTIR)
Fourier transform infrared (FTIR) analyses were performed using a Perkin Elmer spectrometer model Spectrum Two at a spectral resolution of 4 cm−1, in the range of 500–4000 cm−1 by ATR method. Small pieces of samples are placed in the reading cell and pressed for reading in the FTIR. The FTIR spectra are expected to verify the main chemical groups present in the vegetable fibers and soybean hulls, as well as confirm the presence of signals related to carboxymethyl groups. And also to evaluate how the regeneration process with sulfuric acid affects the chemical groups present in the vegetable fibers and hulls.
The hydrophilic/hydrophobic surface characteristics of the filters were investigated by contact angle measurements using a contact angle meter (Theta Lite Optical Tensiometer TL100). Analysis was made using water, dimethylformamide and ethylene glycol, pure glycerin, crude biodiesel, and filtered biodiesel droplets (estimated volume of 5 μL) were dripped onto the sample surfaces with dimensions of 10 mm by 10 mm images acquired with a CCD video camera of 60 frames per second. The initial (t = 0 s) and equilibrium (t = 25 s) contact angles were obtained as averages of three individual measurements (n = 3). Calculations of the solid surface tension (γs) and the dispersive (γsd) and polar (γsp) components were done by using the Owens-Wendt equation. The CA technique aims to evaluate the surface wettability of the filters before and after chemical modifications by regeneration and carboxymethylation. It also seeks to obtain energy parameters to correlate them with the purification process by filtration of biodiesel.
Compression tests were carried out on the universal testing machine Instron model 5982, equipped with a 5 kN load cell. The proof bodies had 1 cm × 6 cm (width × length). The 4-point method was used with the automatic speed of 2.0 mm min−1. The arrow in the center span was measured with an Instron LVDT, with a 5 mm course. The mechanical test was performed with at least 10 replicates. The mechanical assays aim to verify whether the filters are resistant to handling and whether they can be subjected to forces perpendicular to their surfaces.
2.5.6 Surface Area and Porosity
The analysis of specific surface area was executed by the method of Brunauer-Emmett-Teller (BET) at Micromeritics, model ASAP 2020 Plus with nitrogen adsorption and desorption at −195.5°C. The total pore volume was determined by the method Barrett-Joyner-Halenda (BJH). For the assay, approximately 300 to 500 mg of sample previously dried at 130°C for 2 h under vacuum, was required to eliminate solvents and unclog pores.
2.6 Biodiesel Characterization
Biodiesel produced in this work was characterized as crude phase and after filtration, allowing comparative analysis of quality by the following analysis.
The acid value was determined according to the EN 14104 standard, using potassium hydroxide for titration. The measurements were made in triplicates. The acid value was determined by titration with potassium hydroxide (KOH) in triplicate. The volume of 0.1 mol L−1 KOH used to turn a mixture of 5 g of biodiesel, 3 drops of 1% phenolphthalein, and 10 mL of neutralized ethyl alcohol pink was recorded, and the acid value was calculated according to Eq. (3) [9].
where, AI = acid number (mg of KOH.g−1 of biodiesel); V = volume of KOH spent in the titration (mL); C = concentration of the KOH solution (mol L−1); Fc = KOH solution correction factor; m = mass of the biodiesel sample (g).
2.6.2 Combined Alkalinity (CAK)
The AOCS method Cc 17-19, with modifications, was followed for combined alkalinity analysis [9]. The process was made in triplicates. The process consisted of two steps, to determine the presence of catalyst, in the first, and soap, in the second. The first step consisted of determining the volume of 0.01 N HCl used to titrate a solution of 5 g of biodiesel, 100 mL of acetone and 2 mL of phenolphthalein until the pink color disappeared. For the second step, 1 mL of 0.1% bromophenol blue was then added and the new mixture was once again titrated with 0.01 N HCl until the blue color became yellowish. The alkalinity was calculated employing the Eq. (4).
where, CAK = Combined Alkalinity (ppm Na g−1 sample); V = volume of HCl spent in the titration (mL); C = HCl solution concentration (mol/L); m = mass of the biodiesel sample (g).
Free glycerin was determined from 3 g of biodiesel sample, 20 mL of distilled water, and 0.5 mL of aqueous solution of sulfuric acid 1:4 (v/v) in a separatory funnel. The mixture was allowed to rest until the phase separation. The lower fraction was transferred to an Erlenmeyer and 50 mL of sodium periodate solution (5.5 g/L) was added, and it was kept in repose for 10 min. Then 4 g of sodium bicarbonate and 1.5 g of potassium iodide were added and stirred. The mixture was titrated with sodium arsenite until the color changed to orangish. 3 drops of starch solution (1%) were added and titrated again until the solution became colorless. The glycerin-free value was calculated employing the Eq. (5).
where, m = mass of the biodiesel sample; Vb = volume spent with blank titration (mL); Vt = volume spent on the titration of the biodiesel sample (mL); T = Titer of sodium arsenite solution (mg of glycerin/mL); N = normality of the sodium arsenite solution; f = dilution factor: if there is no dilution during the analysis, f = 1.
ANP establishes the maximum of 0.02% in mass of free glycerin in biodiesel for its commercialization.
3.1 Fibers Chemical Modification
The chemical process for regeneration and carboxymethylation was evaluated (Table 1).

At the chemical treatment by regeneration in strong acid, lignocellulosic raw materials, such as soy hull and fibers from sugarcane bagasse, both in natura, showed partial degradation of cellulose and hemicelluloses [21], which are solubilized and lead to the decrease of efficiency observed. However, the lower particle size of sugarcane bagasse was more susceptible to degradation in an acid medium than soy hull.
To sugarcane bagasse and soy hull in natura, the carboxymethylation may occur at the cellulose, lignin, or even at the hemicellulose structure. In general, there was a mass gain, despite the losses that may occur during the purification phases with water, due to the solubilization of the components with lower molar mass [22]. The mass gain of the carboxymethylated samples was evidenced indirectly by the values of acid groups shown in Table 1.
Fras et al. [20] found values between 20 to 43 µmol/g for acid groups in different regenerated celluloses. In carboxymethylated pulp, Junka et al. [23] found 0.32 mmol/g of carboxylic groups in carboxymethylated pulp with a replacement degree of 0.052. Zemljic et al. [24] found a maximum of 2.26 mmol/g of carboxymethylated cotton fibers.
3.2 Synthesis and Biodiesel Filtration
The yield of biodiesel synthesis from soy, related to vegetable oil, was 85.4%. Quessada et al. [25], Leite [26], and Gomes et al. [22] also produced biodiesel by transesterification of soy and sunflower oil, using methanol and NaOH as catalysts found yields of 94.5%, 75.0%, 95.1% and 87.0%. Table 2 presents the relative parameters for the produced biodiesel filtration.

The commercial filter, CNF, CNFB and CNFS, CNFBR-20, and CNFSR 20–35 exhibited filtration times lower than 63 s. The other filters, showed filtration times ranging from 630 to 1580 s. The filters CNF, CNFB 20–28 and CNFS 20–35 had permeate fluxes above 10,000 L h−1 m−2.
Atadashi et al. [27] obtained the best-permeated flux with a ceramic membrane with pores of 0.2 μm and pressure of 2 bar and the observed flux was 78,4 L h−1 m−2. Wang et al. [28] determined flux of 360, 480 e 675 L h−1 m−2 using ceramic membranes with pores of 0.1, 0.2 and 0.6 μm, respectively, for biodiesel filtration at 1.5 bar. In comparison to the values reported in the literature [27,28], the values obtained in this work for the permeated flux were superior.
3.3 Scanning Electron Microscopy (SEM)
The sugarcane bagasse and soy hulls were analyzed by SEM, both raw and chemically modified samples (regenerated and carboxymethylated), and are presented in Figs. 1 and 2.
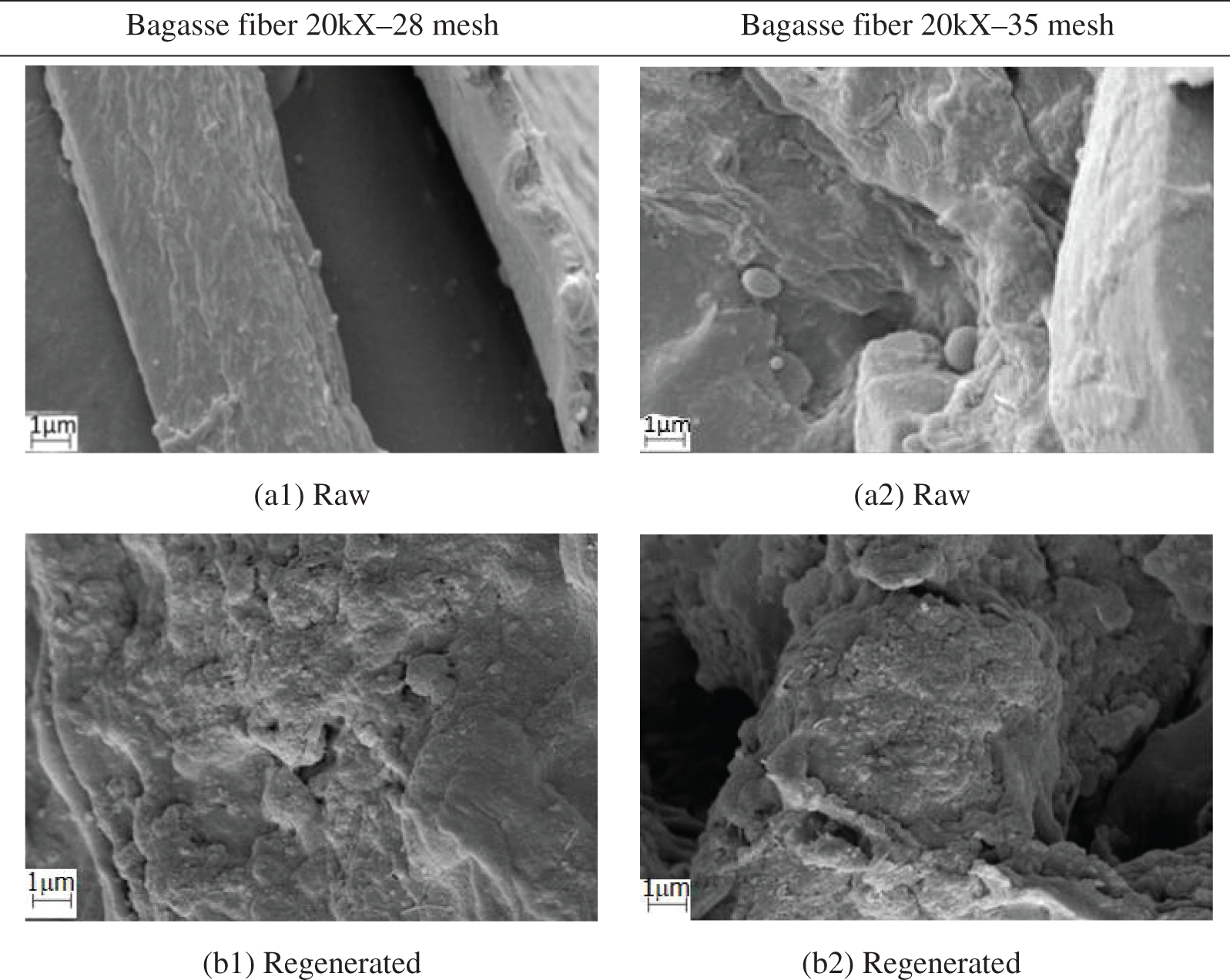

Figure 1: SEM images for sugarcane bagasse fiber at granulometries 28 (left) and 35 mesh (right). Images of raw fiber (a1, a2), regenerated (b1, b2), and carboxymethylated (c1, c2)

Figure 2: SEM images for soy hulls. at granulometries 28 (left) and 35 mesh (right). Images of raw fiber (a1, a2), regenerated (b1, b2), and carboxymethylated (c1, c2)
The images in Fig. 1a1,a2 showed similar surface texture; however, the morphological aspects are different due to the different particle sizes. The raw sugarcane bagasse fibers present a smooth surface, probably due to the presence of lignin, wax, or oil [29]. After the chemical modification by regeneration (Fig. 1b1,b2) with concentrated sulfuric acid (50%), the surfaces became wrinkled and different from the raw materials, possibly because the acid treatment caused the removal of non-cellulosic elements [30]. On the other hand, the chemical modification by carboxymethylation produced smoother surfaces, in comparison to the raw material in Fig. 1c1, while the sample in Fig. 1c2 showed an irregular and wrinkled surface. Similar outcomes were found in other works [31,32].
The raw fiber with 28 mesh exhibited a wrinkled and porous surface (Fig. 2a1), while the raw fiber with 35 mesh was smooth and pleated (Fig. 2a2). After the regeneration (Fig. 2b1,b2) the sample surfaces were similar, showing a smooth surface, without visible porous. On the other hand, the samples obtained after carboxymethylation (Fig. 2c1,c2) presented an open and defibrillated structure. According to Balla et al. [33], the soy hulls after chemical treatment became finer due to the removal of waxes, hemicellulose, pectin and other impurities from the hulls. The removal of hemicellulose also contributes to the increase of the surface area [34].
The comparison between the sugarcane bagasse fiber and soy hull morphology showed differences between the modified samples and the raw materials. For both fibers, the chemical treatments and granulometries show differences that may be significant in the production of the lignocellulosic filters.
Lignocellulosic composite filters were produced using CNF as matrix and sugarcane bagasse fiber and soy hull were incorporated as fillers (both at 28 and 35 mesh; raw, regenerated or carboxymethylated). The SEM images of the fractured surface of the prepared filters are presented in Fig. 3.


Figure 3: SEM images for de CCF filter, and for the filters prepared with only CNF, and CNF containing soy hull, sugarcane bagasse fiber, and regenerated and carboxymethylated samples
The commercial filter (Fig. 3a) showed irregular layers of deposition and free spaces were observed. However, the CNF and CNF-based filters (Fig. 3b to l) showed compacted lamellar layers, except for CNFB 20–28 (Fig. 3d), which exhibited regions with large holes, but lower layers compacted. These compressions could improve the contact between the biodiesel and filter surface and the diffusion process until the eluted exit.
The FTIR analysis showed that the filters had a cellulosic and lignocellulosic composition (Fig. 4).
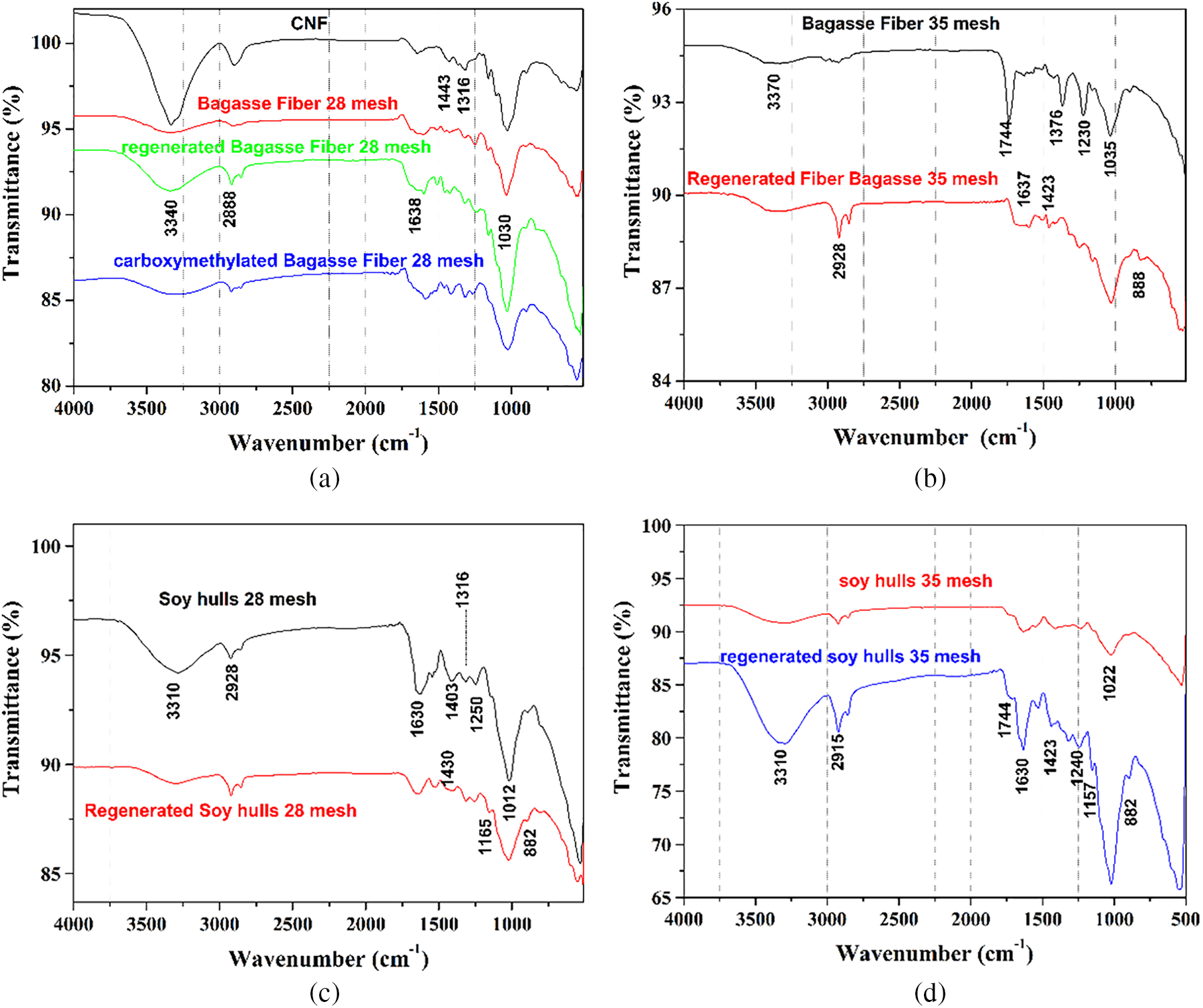
Figure 4: FTIR spectra of components used for filter production (a, b) sugarcane bagasse fibers (raw, regenerated and carboxymethylated), (c, d) soy hulls (raw and regenerated)
All the samples showed typical signals of cellulosic and lignocellulosic components. The broad band around 3440 to 3550 cm−1 is related to OH- stretch at cellulose, hemicelluloses, and lignin. A band around 2900 cm−1 is assigned to C-H stretching. The band at 1742 cm−1 is attributed to the acetyl and uronic ester groups of hemicelluloses [35,36]. The typical band approximately at 1640 cm−1 is assigned to water [37,38]. Carboxylate groups show bands around 1610, 1207 and 1032 cm−1 [39,40].
Lignin exhibits a band around 1520 cm−1 and is attributed to the aromatic vibration of C=C [36,41]. The band at 1230 cm−1 is related to the asymmetric deformation of =C-O-C, typical of phenol, ether, and esters [42]. The band approximately at 1320 cm−1 can be attributed to stretches of -CH2 groups and to syringyl units [43]. After regeneration, due to the partial destructuring of vegetal fibers, it is possible that the regrouping of lignin on the surface of the fibers occured. The band around 850 to 1190 cm−1 was more intense due to couplings C-O-C, C-H and C-O present in glycosidic bonds [44,45].
Fig. 5 shows the results of tension at break and strain obtained in the mechanical tests carried out for the filters.
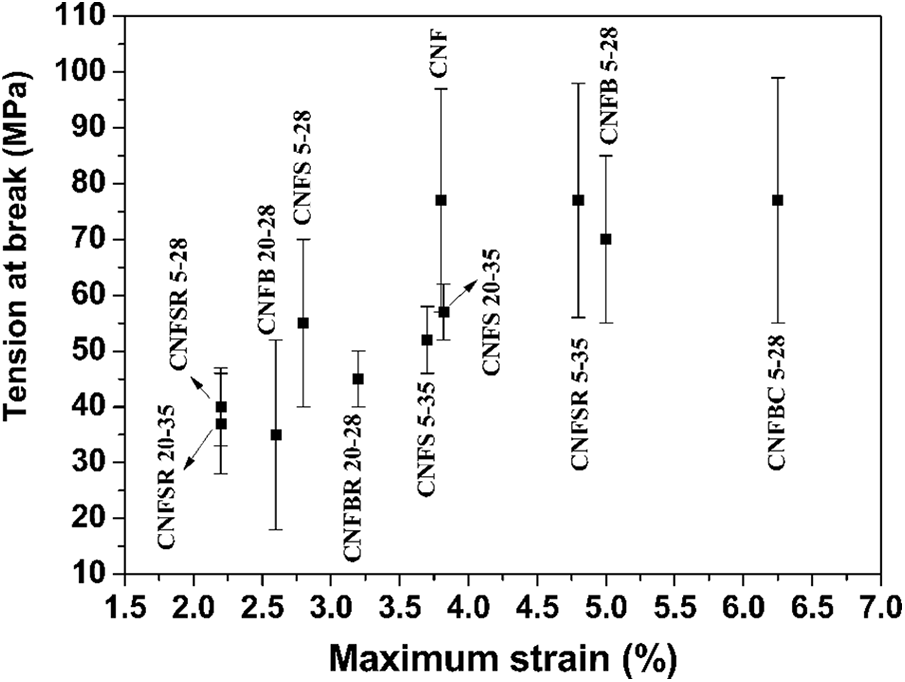
Figure 5: Mechanical properties for the filters
The filters are resistant to tension. The tensile elastic modulus is significant, since these filters will require mechanical resistance at a perpendicular direction due to the flow involved during filtrations. Considering the mean value for the tensile rupture, the more resistant filters were CNF, CNFSR 5–35, and CNFBC 5–28. The mean value was 77 MPa. This result shows that for soy hulls the regeneration treatment had a positive effect on the increase of the mechanical resistance. A similar effect was observed in sugarcane bagasse that was carboxymethylated. The less resistant filter to tensile strength was CNFB 20–28 with 35 MPa. The mechanical studies of sugarcane bagasse filters revealed that the lower addition of fibers to the CNF matrix increases the filter resistance, achieving values around 70 MPa of tensile strength, while the addition of 20% of fibers reduces the tensile strength to 35 MPa. In these cases, the reduction in the tensile strength may be associated with the agglomeration of the filler due to the increase in its concentration in the filter. The filler aggregation and the heterogeneous dispersion of the filler in the CNF matrix prevent the transmittance of stress from the filler to the matrix [46]. Similar outcomes were found by Sahu et al. [47]; when the authors increased the filler concentration to 20%, the tensile strength was reduced.
Regarding the elongation at break, the CNFBC 5–28 filter presented higher values, around 6.25%, followed by CNFB 5–28 with 5%. The filters with lower elongation at break were CNFSR 5–28 and CNFSR 20–35 both with 2.2%. Ma et al. [48] observed that the increase in filler material concentration drastically reduced the elongation at break. This means that the filters are less resistant to changes in shape without crack formation [49]. In other words, the materials became more fragile.
To evaluate the surface energy and the contact angle of the filter’s surface different solvents were employed for contact angle measurements. The obtained values are presented in Table 3.

Only the samples regenerated and carboxymethylated were evaluated with respect to the contact angle, since these samples showed better results for biodiesel purification. The CNF filter was used as a reference, considering that CNF was used as a matrix for all the samples.
Biodiesel has an apolar nature; therefore, solvents with lower surface tension tend to produce drops with lower contact angles. From Table 3, the lowest contact angles were obtained using DMF solvent (surface tension of 36.42 mN/m). This shows that the filter surfaces became hydrophobic in the presence of sugarcane bagasse and soy hulls, considering both regenerated.
Considering the presence of sugarcane bagasse on CNF matrix, the surface energy (γs = 38.8 mN/m) of the filters increased by approximately 29% and 36% for the filters CNFBR 20–28 and CNFBC 5–28, respectively. Since 20% and 5% of sugarcane bagasse regenerated and carboxymethylated, respectively, were used in these filter samples, it is possible to conclude that carboxymethylation has more effect on surface energy than regeneration. Possibly, during the concentrated sulfuric acid attack process, cellulose and hemicelluloses were solubilized, followed by lignin recondensation over the fiber surface, contributing to the observed effect. For the other samples, the γs decreased to 26%.
Gassan et al. [50] evaluated the surface tension values of lignin and some sugars of hemicellulose, estimating individually the wettability characteristics. According to the authors, cellulose has a total surface energy of 35.5 mJ m−2, lignin of 37.0 mJ m−2, and sugars ranging from 33.0 to 36.5 mJ m−2.
To evaluate biodiesel interaction with the regenerated and carboxymethylated filter surface, new contact angle measurements were performed using pure glycerin, raw biodiesel, and filtrated biodiesel by using the filter CNFSR 5–35. The values of the measurements are represented in Table 4.

The presence of glycerin increases the contact angle values since it is one of the most hydrophilic components. All the filters presented a hydrophobic surface, except for CNF. Considering the process for raw biodiesel obtaining, there is a previous step of decantation and separation of free glycerin, the amount of residues on raw biodiesel is low, but enough to change the contact angles of the raw biodiesel. These conclusions were made after a comparison of the contact angle of raw and filtrated biodiesel.
The polar and dispersive energy of glycerin is 34 and 30 mJ m−2, respectively, indicating that this liquid interacts well with membranes with both hydrophilic and hydrophobic surfaces [51].
The specific surface area values and the porous information of the materials were determined by the BET method and by the BJM technique, as shown in Table 5. According to the International Union of Pure and Applied Chemistry (IUPAC), a membrane is classified according to its porous size: a microporous membrane if the porous diameter is smaller than 2 nm; if the diameter is between 2 and 50 nm the membrane is mesoporous, while membranes with a diameter higher than 50 nm are considered as macroporous.

According to the results, the filters CNF, CNFBR 20–28, and CNFS 5–28 are classified as macroporous, while the other filters are classified as mesoporous.
Except for the filters CNFBR 20–28 and CNFS 5–28, the addition of sugarcane bagasse fibers and soy hulls regenerated led to an increase of 92-fold in the surface area. The carboxymethylated bagasse filter (CNFBC 5–28) showed an increase in the surface area of 71-fold.
Comparing the two filters, CNFS 5–28 to CNFS 5–35, the values of SBET 0.0214 and 6.7929 show that a reduction of the soy hull particle size favors the increase of the filter surface area, by 317-fold. The analysis of the filters with the same particle size (CNFS 5–35 and CNFS 20–35) showed an increase of 4-fold in soy hull particles leading to a decrease of the specific surface area by 2 fold.
3.8 Biodiesel Characterization
According to ANP n°45, 2014, the maximum acidity index allowed is 0.5 mg KOH g−1 of biodiesel. This parameter is used because it is directly related to the hydrolysis and oxidation process of biodiesel. In high values, it can lead to engine corrosion, because it affects the thermal and oxidative stability of the oil [52].
Fig. 6 shows that the pre-step purification of biodiesel after the synthesis is enough to biodiesel achieve the standard values of acidity. However, it can be observed that after the filtration, the acidity values decrease even more, increasing the quality standards of the biodiesel. For the better filters, the acidity index was reduced in 75%. The filters of soy hulls, regenerated and carboxymethylated, lead to a lower acidity index. Another important fact is that for some filters times lower than 40 s already led to acidity reduction, regardless of the particle size (28 or 35 mesh).
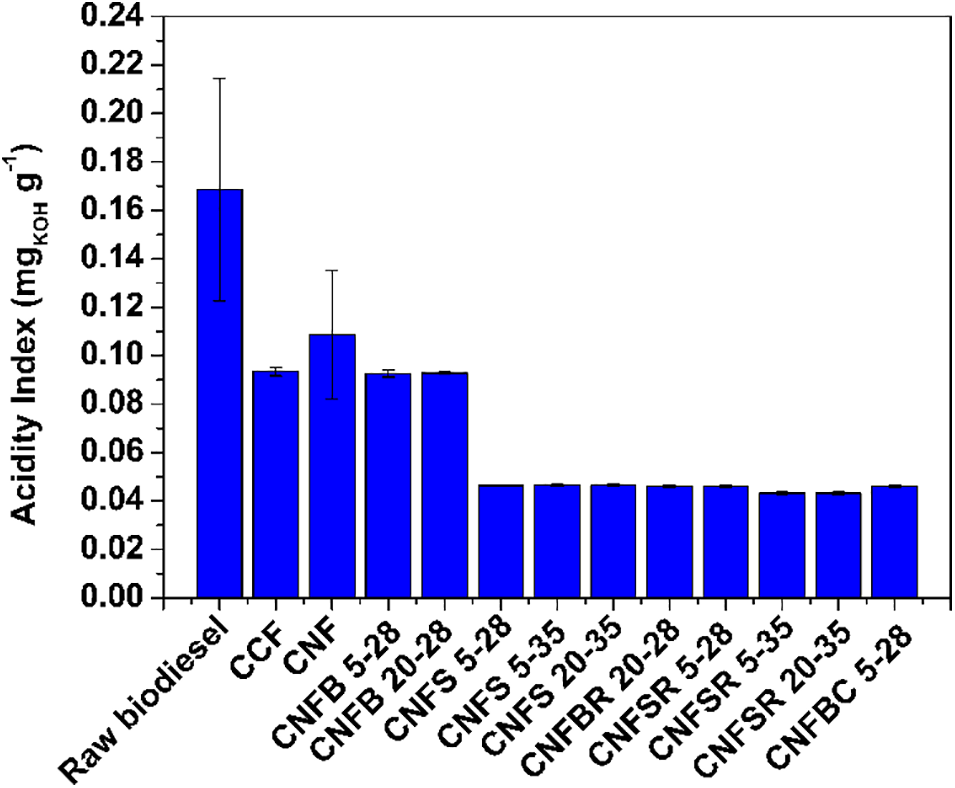
Figure 6: Acidity index values for raw biodiesel after the filtration
Hazmi et al. [53] produced biodiesel from used cooking oil using a bifunctional magnetic nano-catalyst and found that the acid value for the produced biodiesel was 0.24 mg/KOH, therefore, corrosion of metal parts can be slowed down. In comparison to these data, the values found in this work are extremely lower, indicating the good quality of the produced biodiesel and the possibility of detaining the metal corrosion.
Other studies used a commercial quantitative filter paper and obtained biodiesel from black mustard seed with 0.4 mg KOH.g−1 [54], a value acceptable by ANP but higher in comparison to the values obtained with the developed films in this work. These results indicate the great efficiency of biodiesel purification of the developed filter used in this study in comparison to other commercial filters.
The alkalinity content is useful to verify the presence of soaps, and catalyst residues at the same time allowing to monitor the biodiesel production process. A high alkalinity content may contribute to generating carbon residues that accumulate during the injection process and may poison the system of emission control [55].
All the filters contributed to the reduction of sodium index after filtration, as exhibited in Fig. 7. However, four filters (CNFBR 20–28, CNFSR 5–28, CNFSR 20–35, CNFBC 5–28) reduced the sodium index values below the values required by the ASTM D6751 and EN 14214 international standards. This dictates the quality and reliability of the final biodiesel product, where the maximum allowable content of alkali metals must be less than 5 (mg/kg) [56].
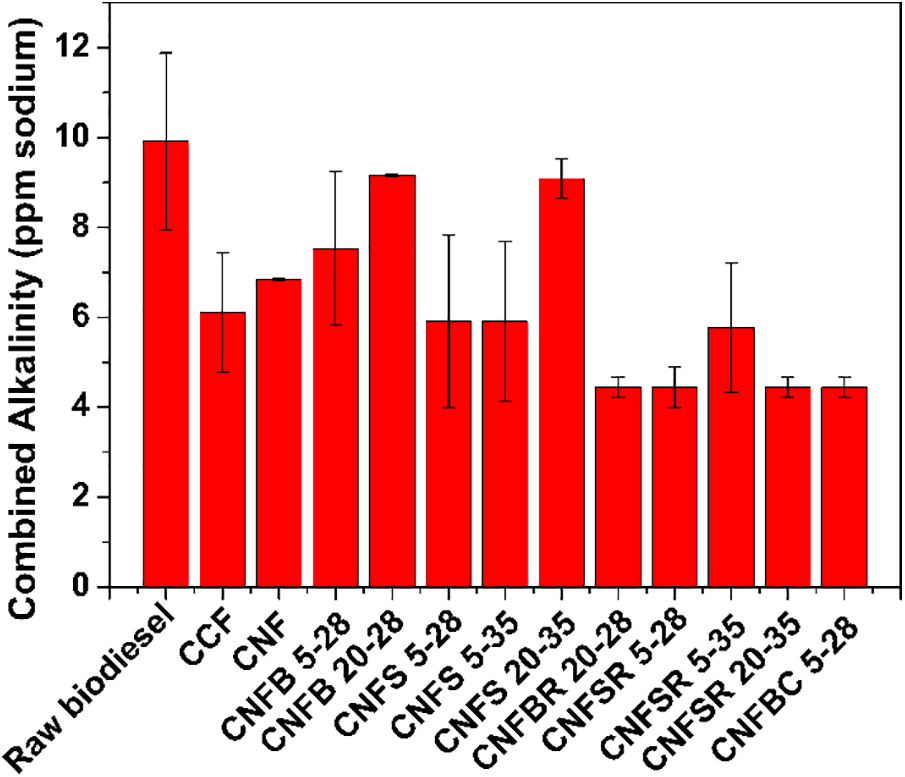
Figure 7: Alkalinity values combined with raw biodiesel after filtration
Sodium ion in the medium have a weak interaction with binders, but even with the low-pressure system during filtration, the filter promoted a 58% reduction in this ion.
Linglin et al. [57] used an ion exchange resin for biodiesel purification and achieved a sodium content reduction to 0.53 mg/kg. However, the biodiesel before the purification had a sodium content of 2 mg/kg. That means the efficiency of the process was 26.5%. Therefore, the use of cellulosic filters had better efficiency in sodium removal from biodiesel in comparison to other methodologies.
Glycerin is the main residue for raw biodiesel. It is undesired on the motor because it causes problems of clogging of engine injection nozzles and separation in storage tanks. This occurs because glycerin is insoluble in biodiesel, causing phase separation when present or being found as dispersed droplets. The quantification of glycerin is indispensable to evaluate the transesterification process efficiency and the removal of residual glycerin during the purification process, as well as the interaction degree between the filtering membranes components and glycerin [58].
According to ANP, the minimum acceptable glycerin value in biodiesel must be lower than 0.02%, represented by the dashed line in Fig. 8. Therefore, it is possible to verify that raw biodiesel and some purified biodiesels do not meet the quality of the specifications required by ANP standards.
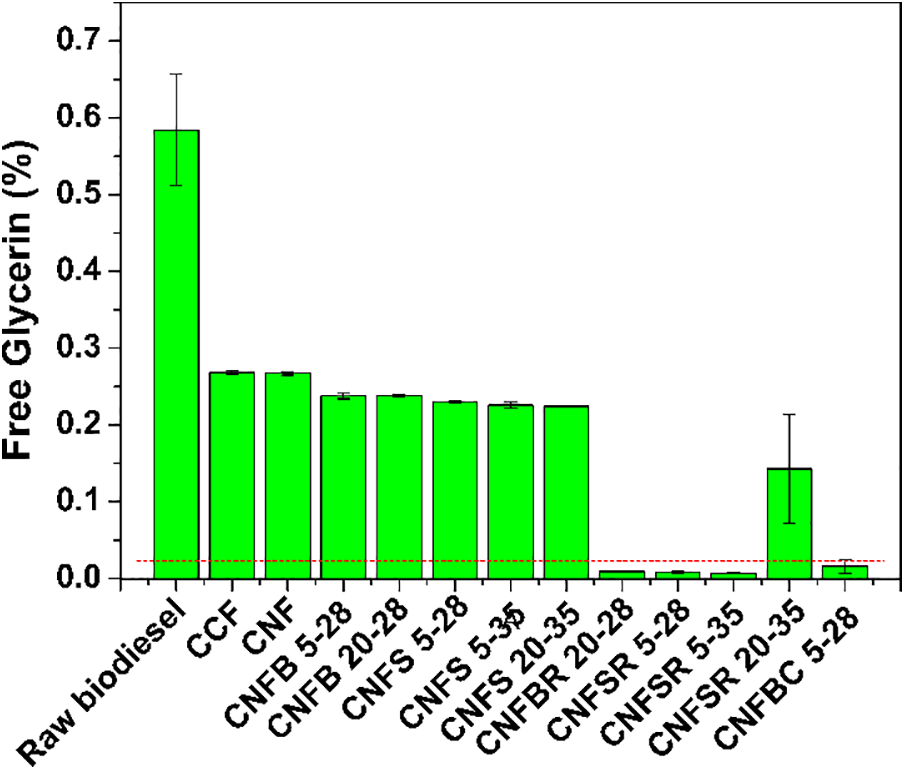
Figure 8: Free glycerin values of raw biodiesel and after filtration
From this, it is possible to verify that four filters (CNFBR 20–28, CNFSR 5–28, CNFSR 5–35, and CNFBC 5–28) produced purified biodiesel with glycerin content below the levels specified by ANP standards. The particle size and the amount of the regenerated components do not cause differences in the glycerin removal. The filter with only 5% of carboxymethylated sugarcane bagasse at 28 mesh granulometry was efficient in removing free glycerin.
Igreja et al. [59] obtained values that meet the free glycerin quality standards, but the acidity index was higher using ultrafiltration with ceramic membranes (α-Al2O3/TiO2). Faccini et al. [60] purified soy biodiesel using 2% silica and were able to promote purification that met the quality standards for acidity index, but not meet the standards for free glycerin.
Sokac et al. [61] studied several membrane compositions for biodiesel purification, and the membrane composed of regenerated cellulose showed the second-best efficiency on the glycerin removal (around 83%) from biodiesel produced by lipase-catalyzed transesterification. On the other hand, Alves et al. [62] used a microfiltration method using a cellulose membrane and the purified biodiesel obtained after the filtration retained around 0.03% of free glycerol. In contrast, the developed filter in this work was able to reduce the levels of free glycerin to acceptable levels as per regulatory agencies. Therefore, the new filters developed in this work have great potential to be applied to biodiesel purification.
To explain the enhancement in the performance of the filters, we need to refer to the characterization data set. The information presented in Table 2 indicates that the presence of soybean hulls (S) and the sugarcane bagasse fibers (B) in the CNF matrices increases the contact time between the raw biodiesel and the filters, compared to the CNF filter alone. Additionally, the SEM images in Fig. 3 reveal that both the CNF filter and the filters containing bagasse (B) and hulls (S) exhibit lamellar structures. This structural characteristic allows filtration to occur in several stages, in contrast to polymeric membranes, which have a single dense structure throughout their thickness.
By examining the specific surface area data (Table 5), we can see that the inclusion of S and B increases the specific surface area by up to 71-fold compared to the SBET data for the CNF filter. This dataset demonstrates that the physical and chemical parameters change in the presence of S and B, leading to improved removal of contaminants, as observed.
The biodiesel purification by dry route using lignocellulosic filters was efficient for biodiesel purification.
The mechanical resistance of the three filters, CNF, CNFSR 5–35, and CNFBC 5–28, showed a mean value of 77 MPa, which allows us to classify them as very strong filters.
The filtration flux was considered relevant, because values up to 18,894 L h−1 m−2 were observed. However, the best results for acidity index, combined alkalinity and glycerin content for the regenerated and carboxymethylated filters were achieved with the higher permeated flow value of 4145 L h−1 m−2. Filtration times of less than 40 s were observed with good performances in the filtration, since only one filtration cycle was performed.
The glycerin index for four filters, CNFBR 20–28, CNFSR 5–28, CNFSR 5–35, and CNFBC 5–28, after filtration remained below 0.02%, which is the value established by the ANP resolution, demonstrating the potential of filters to remove this contaminant from Biodiesel.
For acidity index, eight filters, CNFS 5–28, CNFS 5–35, CNFS 5–35, CNFBR 20–28, CNFSR 5–28, CNFSR 5–35, CNFSR 20–35, and CNFBC 5–28, had acidity index values below the maximum value of 0.5 mg KOH g−1 of biodiesel established by ANP resolution.
Finally, for combined alkalinity index, four filters, CNFBR 20–28, CNFSR 5–28, CNFSR 20–35, and CNFBC 5–28, achieved sodium index values lower than 5 mg/kg, which is the requirement according to ASTM D6751 and EN 14214 international standards.
The obtained data showed that the development of filter technology is relevant for biodiesel purification. Besides the reduction of process costs and lower environmental pollution, they are derived from renewable sources and the purification process involved is dry.
Acknowledgement: The authors thank the Minas Gerais State’s Foundation for Research Support (FAPEMIG, Brazil), CAPES, CNPq and FINEP for their financial support.
Funding Statement: This work was supported by the Minas Gerais State’s Foundation for Research Support (FAPEMIG, Brazil, Process CEX-APQ-01651-17, RED-00224-23, and PPM-00645-17).
Author Contributions: The authors confirm contribution to the paper as follows: study conception and design: Flávia Naves Ferreira do Prado, Daniel Pasquini, Luís Carlos de Morais; data collection: Flávia Naves Ferreira do Prado, Michelle Garcia Gomes; analysis and interpretation of results: Flávia Naves Ferreira do Prado, Daniel Pasquini, Anízio Márcio de Faria, Luís Carlos de Morais; draft manuscript preparation: Flávia Naves Ferreira do Prado, Michelle Garcia Gomes, Marcela Piassi Bernardo, Daniel Pasquini, Luís Carlos de Morais. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: The authors confirm that the data supporting the findings of this study are available within the article.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Malik K, Capareda SC, Kamboj BR, Malik S, Singh K, Arya S, et al. Biofuels production: a review on sustainable alternatives to traditional fuels and energy sources. Fuels. 2024;5(2):157–75. doi:10.3390/fuels5020010. [Google Scholar] [CrossRef]
2. Pires VD, Cavalcante RM, Young AF. Process simulation and economic evaluation of biodiesel production from several feedstocks in Brazil. Braz J Chem Eng. 2023;3:5. doi:10.1007/s43153-023-00412-0. [Google Scholar] [CrossRef]
3. Barros S. A new model for the biodiesel market. United States Dep Agric. 2021;1(1):1–5. [Google Scholar]
4. Anil N, Rao PK, Sarkar A, Kubavat J, Vadivel S, Manwarf NR, et al. Advancements in sustainable biodiesel production: a comprehensive review of bio-waste derived catalysts. Energy Convers Manag. 2024;318:118884. doi:10.1016/j.enconman.2024.118884. [Google Scholar] [CrossRef]
5. Agência Nacional do Petróleo Gás Natual e Biocombustíveis. RESOLUÇÃO ANP Nº 45, DE 25 DE AGOSTO DE 2014. 2023 [cited 2024 Nov 15]. Available from: https://atosoficiais.com.br/anp/resolucao-n-45-2014?origin=instituicao&q=45/2014. [Google Scholar]
6. Osman WNAW, Rosli MH, Mazli WNA, Samsuri S. Comparative review of biodiesel production and purification. Carbon Capture Sci Technol. 2024;13(2):100264. doi:10.1016/j.ccst.2024.100264. [Google Scholar] [CrossRef]
7. Abdullah A, Ahmed A, Akhter P, Razzaq A, Zafar M, Hussain M, et al. Bioenergy potential and thermochemical characterization of lignocellulosic biomass residues available in Pakistan. Korean J Chem Eng. 2020;37(11):1899–906. doi:10.1007/s11814-020-0624-0. [Google Scholar] [CrossRef]
8. Camilo GL, Queiroz A, Ribeiro AE, Gomes MCS, Brito P. Review of biodiesel production using various feedstocks and its purification through several methodologies, with a specific emphasis on dry washing. J Ind Eng Chem. 2024;136:1–15. doi:10.1016/j.jiec.2024.02.016. [Google Scholar] [CrossRef]
9. Gomes MG, Santos DQ, De Morais LC, Pasquini D. Purification of biodiesel by dry washing, employing starch and cellulose as natural adsorbents. Fuel. 2015 Sep 1;155:1–6. doi:10.1016/j.fuel.2015.04.012. [Google Scholar] [CrossRef]
10. Squissato AL, Fernandes DM, Sousa RMF, Cunha RR, Serqueira DS, Richter EM, et al. Eucalyptus pulp as an adsorbent for biodiesel purification. Cellulose. 2015;22(2):1263–74. doi:10.1007/s10570-015-0557-7. [Google Scholar] [CrossRef]
11. Konovalov SV, Zubenko SO, Patrylak LK, Povazhnyi VA, Davitadze DZ. Evaluation of available adsorbents for the dry washing of the wasted frying oil based crude ethyl esters. Voprosy khimii i khimicheskoi tekhnologii. 2023;6(6):114–23. doi:10.32434/0321-4095-2023-151-6-114-123. [Google Scholar] [CrossRef]
12. Camilo GL, Garção MIL, Milani EC, Queiroz A, Ribeiro AE, Sérgi Gomes MC, et al. Study of biodiesel production from waste cooking oil by ethyl transesterification and its purification using olive pits. 2023. doi:10.2139/ssrn.4675775. [Google Scholar] [CrossRef]
13. Munoz ADT, Lucca G, Damasceno KG, del Roveri C, Carvalho AKF, Paula AV, et al. Agro-industrial wastes as solid adsorbents for glycerol removal: potential use in biodiesel dry purification. Indus Crops Prod. 2024;222(3):119697. doi:10.1016/j.indcrop.2024.119697. [Google Scholar] [CrossRef]
14. Abed KM, Hayyan A, Hizaddin HF, Hashim MA, Basirun WJ, Saleh J, et al. Superiority of liquid membrane-based purification techniques in biodiesel downstream processing. Renew Sustain Energ Rev. 2025;207(1):114911. doi:10.1016/j.rser.2024.114911. [Google Scholar] [CrossRef]
15. Ojo AO. An overview of lignocellulose and its biotechnological importance in high-value product production. Fermentation. 2023;9(11):990. doi:10.3390/fermentation9110990. [Google Scholar] [CrossRef]
16. Chingakham C, Manaf O, Sujith A, Sajith V. Hydrophobic nano-bamboo fiber-reinforced acrylonitrile butadiene styrene electrospun membrane for the filtration of crude biodiesel. Appl Nanosci. 2020;10(3):795–806. doi:10.1007/s13204-019-01140-z. [Google Scholar] [CrossRef]
17. Rahman MS, Hasan MS, Nitai AS, Nam S, Karmakar AK, Ahsan MS, et al. Recent developments of carboxymethyl cellulose. Polymers. 2021;13(8):1345. doi:10.3390/polym13081345. [Google Scholar] [PubMed] [CrossRef]
18. Wu S, Shi S, Liu R, Wang C, Li J, Han L. The transformations of cellulose after concentrated sulfuric acid treatment and its impact on the enzymatic saccharification. Biotechnol Biofuels Bioprod. 2023;16(1):1–10. doi:10.1186/s13068-023-02293-4. [Google Scholar] [PubMed] [CrossRef]
19. CONAB-Companhia Nacional de Abastecimento. [cited 2024 Nov 15]. Available from: https://www.conab.gov.br/. [Google Scholar]
20. Fras L, Laine J, Stenius P, Stana-Kleinschek K, Ribitsch V, Doleček V. Determination of dissociable groups in natural and regenerated cellulose fibers by different titration methods. J Appl Polym Sci. 2004;92(5):3186–95. doi:10.1002/app.20294. [Google Scholar] [CrossRef]
21. Woiciechowski AL, Neto CJD, Vandenberghe LPS, Neto DPC, Sydney ACN, Letti LAJ, et al. Lignocellulosic biomass: acid and alkaline pretreatments and their effects on biomass recalcitrance—conventional processing and recent advances. Bioresour Technol. 2020;304(3):122848. doi:10.1016/j.biortech.2020.122848. [Google Scholar] [PubMed] [CrossRef]
22. Gomes MG, Santos DQ, de Morais LC, Pasquini D. Purification of biodiesel by dry washing and the use of starch and cellulose as natural adsorbents: part II—study of purification times. Biofuels. 2018;12(5):579–87. doi:10.1080/17597269.2018.1510721. [Google Scholar] [CrossRef]
23. Junka K, Filpponen I, Lindström T, Laine J. Titrimetric methods for the determination of surface and total charge of functionalized nanofibrillated/microfibrillated cellulose (NFC/MFC). Cellulose. 2013;20(6):2887–95. doi:10.1007/s10570-013-0043-z. [Google Scholar] [CrossRef]
24. Zemljič LF, Bračič M, Ristić T, Šauperl O, Strnad S, Peršin Z. Functionalization of polymer materials for medical applications using chitosan nanolayers. Polym Nanomater Nanotherapeutics. 2018;76(22):333–58. doi:10.1016/B978-0-12-813932-5.00009-1. [Google Scholar] [CrossRef]
25. Quessada TP, Guedes CLB, Borsato D. Obtenção De Biodiesel a Partir De Óleo De Soja E Milho Utilizando Catalisadores Básicos E Catalisador Ácido. Encicl Biosf. 2010;6:1–25. [Google Scholar]
26. Leite PFS. Melhoria do Processo de Produção de Biodiesel: Avaliação de Vias de Valorização do Glicerol (Portuguese). Porto, Portugal: Universidade do Porto; 2011. [Google Scholar]
27. Atadashi IM, Aroua MK, Abdul Aziz AR, Sulaiman NMN. Membrane biodiesel production and refining technology: a critical review. Renew Sustain Energy Rev. 2011;15(9):5051–62. doi:10.1016/j.rser.2011.07.051. [Google Scholar] [CrossRef]
28. Wang Y, Wang X, Liu Y, Ou S, Tan Y, Tang S. Refining of biodiesel by ceramic membrane separation. Fuel Process Technol. 2009;90(3):422–7. doi:10.1016/j.fuproc.2008.11.004. [Google Scholar] [CrossRef]
29. Gupta MK, Gond RK, Bharti A. Effects of treatments on the properties of polyester based hemp composite. Indian J Fibre Text Res. 2018;43(3):313–9. [Google Scholar]
30. Gond RK, Gupta MK, Jawaid M. Extraction of nanocellulose from sugarcane bagasse and its characterization for potential applications. Polym Compos. 2021;42(10):5400–12. doi:10.1002/pc.26232. [Google Scholar] [CrossRef]
31. Makhanya FM. Conversion of sugarcane bagasse into carboxymethyl cellulose. Durban, South Africa: Aculty of Applied Sciences at the Durban University of Technology; 2020. 95 p. [Google Scholar]
32. Babaladimath G, Rayar A, Chapi S. Sugarcane bagasse valorized superabsorbent graft copolymer for efficient deposition of crystal violet and indigo carmine dyes from aqueous solutions. Emergent Mater. 2022;5(5):1485–93. doi:10.1007/s42247-021-00333-z. [Google Scholar] [CrossRef]
33. Balla VK, Tadimeti JGD, Sudan K, Satyavolu J, Kate KH. First report on fabrication and characterization of soybean hull fiber: polymer composite filaments for fused filament fabrication. Prog Addit Manuf. 2021;6(1):39–52. doi:10.1007/s40964-020-00138-2. [Google Scholar] [CrossRef]
34. Herde ZD, Dharmasena R, Sumanasekera G, Tumuluru JS, Satyavolu J. Impact of hydrolysis on surface area and energy storage applications of activated carbons produced from corn fiber and soy hulls. Carbon Resour Convers. 2020;3(6):19–28. doi:10.1016/j.crcon.2019.12.002. [Google Scholar] [CrossRef]
35. Singh SS, Lim LT, Manickavasagan A. Imaging and spectroscopic techniques for microstructural and compositional analysis of lignocellulosic materials: a review. Biomass Convers Biorefinery. 2023;13(1):499–517. doi:10.1007/s13399-020-01075-4. [Google Scholar] [CrossRef]
36. Rodrigues RCLB, Rodrigues BG, Canettieri EV, Martinez EA, Palladino F, Rodrigues DJr, et al. Comprehensive approach of methods for microstructural analysis and analytical tools in lignocellulosic biomass assessment—a review. Bioresour Technol. 2022;348(2):126627. doi:10.1016/j.biortech.2021.126627. [Google Scholar] [PubMed] [CrossRef]
37. Díaz GSC, Aburto AR, Lima EV, Santos LF, Hernández MAV, Luna MGH, et al. Determination of the composition of lignocellulosic biomasses from combined analyses of thermal, spectroscopic, and wet chemical methods. Ind Eng Chem Res. 2021;60(9):3502–15. doi:10.1021/acs.iecr.0c05243. [Google Scholar] [CrossRef]
38. Oh SY, Dong IY, Shin Y, Hwan CK, Hak YK, Yong SC, et al. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr Res. 2005;340(15):2376–91. doi:10.1016/j.carres.2005.08.007. [Google Scholar] [PubMed] [CrossRef]
39. He F, Zhao D, Liu J, Roberts CB. Stabilization of Fe-Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater. Ind Eng Chem Res. 2007;1(1):29–34. doi:10.1021/ie0610896. [Google Scholar] [CrossRef]
40. Konduri MK, Kong F, Fatehi P. Production of carboxymethylated lignin and its application as a dispersant. Eur Polym J. 2015;70(4):371–83. doi:10.1016/j.eurpolymj.2015.07.028. [Google Scholar] [CrossRef]
41. Xiao B, Sun XF, Sun R. Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym Degrad Stab. 2001;73:307–19. doi:10.1016/S0141-3910(01)00163-X. [Google Scholar] [CrossRef]
42. Siqueira G, Bras J, Dufresne A. Cellulosic bionanocomposites: a review of preparation, properties and applications. Polymers. 2010;2(4):728–65. doi:10.3390/polym2040728. [Google Scholar] [CrossRef]
43. Łojewska J, Miśkowiec P, Łojewski T, Proniewicz LM. Cellulose oxidative and hydrolytic degradation: in situ FTIR approach. Polym Degrad Stab. 2005;88(3):512–20. doi:10.1016/j.polymdegradstab.2004.12.012. [Google Scholar] [CrossRef]
44. Pavithra R, Gunasekaran S, Sailatha ES, Kamatchi S. Investigations on paper making raw materials and determination of paper quality by FTIR-UATR and UV-Vis DRS spectroscopy. Int J Curr Res Acad Rev. 2015;3(11):42–59. [Google Scholar]
45. Alemdar A, Sain M. Isolation and characterization of nanofibers from agricultural residues-Wheat straw and soy hulls. Bioresour Technol. 2008;99(6):1664–71. doi:10.1016/j.biortech.2007.04.029. [Google Scholar] [PubMed] [CrossRef]
46. Boey JY, Lee CK, Tay GS. Factors affecting mechanical properties of reinforced bioplastics: a review. Polymers. 2022;14(18):3737. doi:10.3390/polym14183737. [Google Scholar] [PubMed] [CrossRef]
47. Sahu G, Rajput MS, Mahapatra SP. Polylactic acid nanocomposites for biomedical applications: effects of calcium phosphate, and magnesium phosphate nanoparticles concentration. Plast Rubber Compos. 2021;50(5):228–40. doi:10.1080/14658011.2021.1871818. [Google Scholar] [CrossRef]
48. Ma X, Yu J, Kennedy JF. Studies on the properties of natural fibers-reinforced thermoplastic starch composites. Carbohydr Polym. 2005;62(1):19–24. doi:10.1016/j.carbpol.2005.07.015. [Google Scholar] [CrossRef]
49. Djafari Petroudy SR. Physical and mechanical properties of natural fibers. In: Advanced high strength natural fibre composites in construction. Elsevier Ltd.; 2017. p. 59–83. doi:10.1016/B978-0-08-100411-1.00003-0. [Google Scholar] [CrossRef]
50. Gassan J, Gutowski VS, Bledzki AK. About the surface characteristics of natural fibres. Macromol Mater Eng. 2000;283:132–9. doi:10.1002/1439-2054(20001101)283:1<132::AID-MAME132>3.0.CO;2-B. [Google Scholar] [CrossRef]
51. Mantel M, Wightman JP. Influence of the surface chemistry on the wettability of stainless steel. Surf Interface Anal. 1994;21(9):595–605. doi:10.1002/sia.740210902. [Google Scholar] [CrossRef]
52. Chen YH, Chen JH, Luo YM, Shang NC, Chang CH, Chang CY, et al. Property modification of jatropha oil biodiesel by blending with other biodiesels or adding antioxidants. Energy. 2011;36(7):4415–21. doi:10.1016/j.energy.2011.04.001. [Google Scholar] [CrossRef]
53. Hazmi B, Rashid U, Ibrahim ML, Nehdi IA, Azam M, Al-Resayes SI. Synthesis and characterization of bifunctional magnetic nano-catalyst from rice husk for production of biodiesel. Environ Technol Innov. 2021;21(264):101296. doi:10.1016/j.eti.2020.101296. [Google Scholar] [CrossRef]
54. Aslan V, Eryilmaz T. Polynomial regression method for optimization of biodiesel production from black mustard (Brassica nigra L.) seed oil using methanol, ethanol, NaOH, and KOH. Energy. 2020;209(9):118386. doi:10.1016/j.energy.2020.118386. [Google Scholar] [CrossRef]
55. Shahbaz K, Mjalli FS, Hashim MA, Alnashef IM. Eutectic solvents for the removal of residual palm oil-based biodiesel catalyst. Sep Purif Technol. 2011;81(2):216–22. doi:10.1016/j.seppur.2011.07.032. [Google Scholar] [CrossRef]
56. Conshohocken AS for T, MW. ASTM D-6751-02. Standard Specification for Biodiesel Fuel (B100) Blend Stock for Distillate Fuels, Designation D-6751-02; West Conshohocken, PA, USA; 2002. [Google Scholar]
57. Linglin L, Fashe L, Huicong Z, Yaozong D, Wenchao W. Efficient removal of alkali and alkaline earth metals from biodiesel using Ion-exchange resin: performance and mechanism. Sep Purif Technol. 2023;323(68):124485. doi:10.1016/j.seppur.2023.124485. [Google Scholar] [CrossRef]
58. Tajziehchi K, Sadrameli SM. Optimization for free glycerol, diglyceride, and triglyceride reduction in biodiesel using ultrafiltration polymeric membrane: effect of process parameters. Process Saf Environ Prot. 2021;148(4):34–46. doi:10.1016/j.psep.2020.09.047. [Google Scholar] [CrossRef]
59. Igreja G, Medeiros JF, Moreira WM, Gomes MCS, Pereira NC. Purificação De Biodiesel Etílico De Óleo De Soja Utilizando Ultrafiltração Com Membrnas Cerâmicas. In: XX Congresso Brasileiro de Engenharia Química; 2013. p. 1–8. [Google Scholar]
60. Faccini CS, Cunha ME, Moraes MSA, Krause LC, Manique MC, Rodrigues MRA, et al. Dry washing in biodiesel purification: a comparative study of adsorbents. J Braz Chem Soc. 2011;22(3):558–63. doi:10.1590/S0103-50532011000300021. [Google Scholar] [CrossRef]
61. Sokač T, Gojun M, Tušek AJ, Šalić A, Zelić B. Purification of biodiesel produced by lipase catalysed transesterification by ultrafiltration: selection of membranes and analysis of membrane blocking mechanisms. Renew Energy. 2020;159:642–51. doi:10.1016/j.renene.2020.05.132. [Google Scholar] [CrossRef]
62. Alves MJ, Nascimento SM, Pereira IG, Martins MI, Cardoso VL, Reis M. Biodiesel purification using microand ultrafiltration membranes. Renew Energy. 2013;58:15–20. doi:10.1016/j.renene.2013.02.035. [Google Scholar] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools