 Open Access
Open Access
ARTICLE
Facile Crosslinking of Hardwood Kraft Lignin for Sustainable Bio-Based Wood Adhesives
1 Department of Wood and Paper Science, Kyungpook National University, Daegu, 41566, Republic of Korea
2 Yunnan Province Key Lab of Wood Adhesives and Glued Products, International Joint Research Center for Biomass Materials, Southwest Forestry University, Kunming, 650224, China
* Corresponding Author: Byung-Dae Park. Email:
(This article belongs to the Special Issue: Renewable and Biosourced Adhesives-2023)
Journal of Renewable Materials 2025, 13(5), 829-848. https://doi.org/10.32604/jrm.2025.02024-0056
Received 10 December 2024; Accepted 07 February 2025; Issue published 20 May 2025
Abstract
As the most abundant aromatic bio-based polymer, lignin has great potential as a sustainable feedstock for building crosslinked thermoset polymers as bio-based adhesives. However, the potential of hardwood kraft lignin (HKL) is limited due to its poor crosslinking reactivity. Hence, for the first time, the present study reports the facile oxidation of HKL involving a redox reaction with silver-ammonia complexes ([(AgNH3)2]+), primarily focusing on oxidation to produce reactive quinones and promote C–C linkages during reaction. This study aims to increases reactivity of oxidized HKL for effective crosslinking with monoethanolamine (MEA) for the development of bio-based wood adhesives. The characterization, including 13C-nuclear magnetic resonance (NMR) and Fourier transform infrared (FT-IR) spectroscopy, confirms the oxidation reaction, such as the formation of quinones (C=O) and subsequent crosslinking between the oxidized HKL molecules and MEA. Additionally, gel permeation chromatography (GPC) confirms the C–C and C–O linkages with increased molecular weight after oxidation, and is supported by differential scanning calorimetry (DSC) which shows the exothermic reaction due to the crosslinking of the oxidized HKL molecules via condensation to form C–C and C–O linkages. The crosslinked HKL/MEA-based adhesives underwent mild reaction and achieved a maximum dry shear strength of 0.77 MPa, which exceeds the standard requirement of 0.6 MPa. These findings demonstrate not only a one-pot oxidation for improving the reactivity of HKL using silver complexes, but also its facile crosslinking with MEA for sustainable bio-based wood adhesives.Graphic Abstract
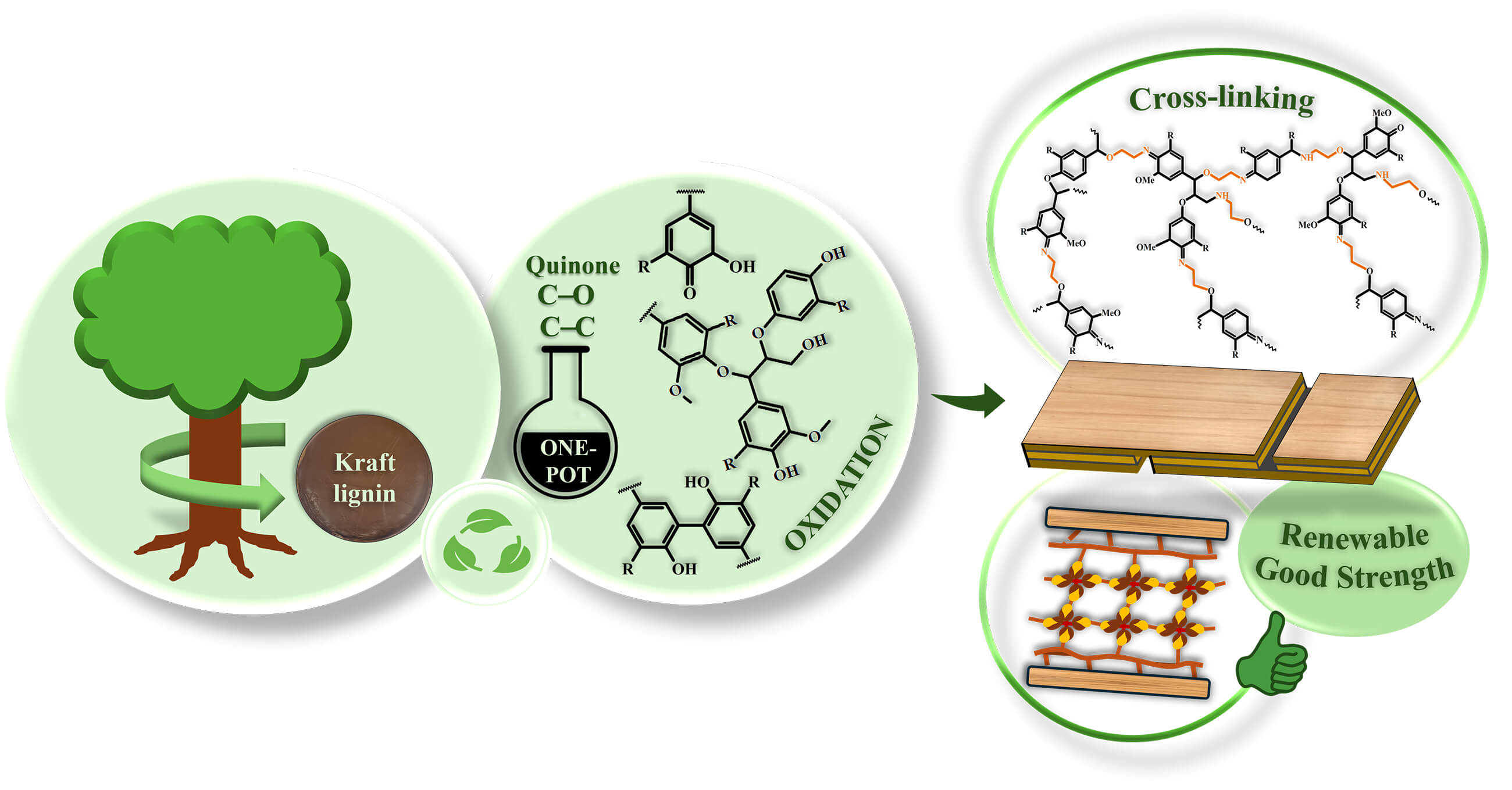
Keywords
The petroleum-based adhesives have been recognized as non-sustainable due to their toxicity and the emissions they produce. In recent years, research into alternative biomass-based adhesives such as cellulose [1,2], plant oils [3,4], starch [5,6], soy protein [7,8], and lignin [9] has been widely reported. However, despite its potential, lignin is significantly under-utilized and is primarily incinerated for energy recovery. Approximately 130 million tons of lignin are burned annually, with an additional 50 million tons generated as a byproduct [10]. Due to inherent steric hindrance, lignin requires modification to enhance its reactivity and solubility. Consequently, various functionalization and modification techniques have been developed to improve its strength and make it compatible with industrial standards for applications in adhesives [2]. Recent modifications for producing lignin-based adhesives include hydrodeoxygenation [11], phenolation [12], amination [13], and carboxymethylation [14]. Utilizing lignin not only offers a low-cost and environmentally-friendly option, but also helps to reduce formaldehyde emissions, which are a primary source of toxicity associated with formaldehyde-based resins. In some cases, lignin can replace up to 80% of these resins [15] owing to its primary monolignols, which include sinapyl alcohol, coniferyl alcohol, and p-coumaryl alcohol. These monolignols play a crucial role in the chemical modification of lignin, particularly in applications such as crosslinking for adhesives [16]. To obtain a more homogeneous lignin fraction for improved modification, lignin is subjected to fractionation, with acetone as an effective and volatile solvent [17]. Acetone offers several advantages over other solvents, including low cost, biodegradability, a low boiling point, minimal waste generation, and high efficiency [18–21]. Indeed, a previous study by the present research group demonstrated promising results in obtaining both soluble and insoluble lignin fractions by this method [22]. Although these fractions exhibit distinct structural differences, they have potential applications in adhesive formulations. Notably, the soluble fraction was found to have a higher content of phenolic hydroxyl groups and has been shown to replace 100% of the phenol in phenolic resin formulations, thereby achieving a high dry adhesion strength [20].
The modification of lignin to alter its structural composition, particularly its monolignols, enables specific and valuable reactions. However, the oxidation for lignin-based adhesives has not been widely recognized. Hence, the present study builds on established chemical modifications that enable the formation of complex aromatic compounds with enhanced functionalities or allow the direct conversion of lignin into targeted fine chemicals [23]. Despite the potential of such modifications, their effectiveness has often been considered modest [24]. Nevertheless, oxidation processes have been used to promote depolymerization and generate selective reaction products, including hydroxyl and carboxyl groups [25]. It has also been used for lignin degradation through electro-reductive fragmentation [26]. Moreover, chelator-mediated Fenton (CMF) processes involving oxidation have demonstrated success in the development of lignin-based hydrogels [27].
Interestingly, lignin can be synthesized via oxidation reactions involving silver-ammonia complexes, specifically [(AgNH3)2]+ (commonly known as the Tollen’s reagent) [28,29] which serves the dual roles of reducing agent and stabilizing agent for lignin silver nanoparticles [30–32]. Despite its utility in the selective detection of aldehydes and its potential in green synthesis, it is important to note the environmental concerns associated with Tollen’s reagent [33]. The silver ions in the reagent can be toxic to aquatic life and may accumulate in ecosystems if not properly handled or disposed of. Notably, as the primary silver complex in the Tollen’s reagent, AgNO3 can oxidize aldehydes to carboxylic acids without the need for ligands or additional additives [28]. In the Tollen’s reaction, the silver complex oxidizes the aldehyde group to a carboxyl group while concurrently reducing Ag+ to form a characteristic silver mirror. Moreover, this silver complex facilitates oxidative C–C coupling with the reduction of Ag+ to Ag0 [29]. The concept of C–C coupling entails the joining of two nucleophilic centers, which may include carbon, oxygen, nitrogen, or other elements. Understanding these oxidative processes is crucial for enhancing the reactivity of lignin, particularly in the development of crosslinking agents for adhesives.
Building on this phenomenon, lignin, with its abundant monolignol structures, has the potential to react with silver-ammonia complexes, leading to novel modifications suitable for adhesive applications. Further, it is suggested that process may involve reaction of the C–O bond with the free-radical compound of aliphatic lignin, as well as the C–C coupling of free-radical compound of aromatic. By leveraging the unique properties of oxidized lignin, this research aims to create innovative adhesive formulations that offer improved reactivity, performance, and sustainability. This work has the potential to contribute significantly to the field of bio-based materials, promoting the use of lignin as a renewable resource in adhesive applications. To this end, the present study explores the crosslinking reactions between oxidized hardwood kraft lignin (HKL) and an amine, specifically monoethanolamine (MEA). Notably, MEA has been primarily studied for CO2 separation from natural gas [34,35], and has not been widely reported for crosslinking applications, except in prior work by the present research group involving lignin crosslinking with aldehydes [36]. The present study aims to expand the existing understanding of MEA’s potential by investigating its role in forming a three-dimensional crosslinked network with oxidized lignin. It is anticipated that the amine will react with the C=O groups of the oxidized lignin, thus leading to the formation of amide linkages. Consequently, the modifications applied prior to crosslinking are expected to enhance the reactivity and improve the adhesive performance by generating additional carbonyl groups. To the author’s knowledge, this approach of focusing on lignin oxidation with silver-ammonia complexes for bio-based adhesives has not been previously reported.
Hence, a novel oxidation method is introduced herein, whereby silver complexes oxidize lignin to facilitate the formation of quinones at the C4 position of the hydroxyl aromatic unit. The study is conducted at various temperatures over several trials, with the resulting products being analyzed by GPC to evaluate the molecular weight, 13C NMR to assess the chemical changes, FT-IR to identify the functional group changes, and DSC to evaluate the thermal behavior. Notably, after the addition of MEA, both the FT-IR and DSC analyses provide insights into the crosslinking reactions, and the dry shear strength is used to evaluate the adhesion strength.
Industrial black liquor of mixed hardwood species from kraft pulping was obtained from a local pulp mill (Moorim P&P, Ulsan, Korea). The reagents sulfuric acid (98%), acetone, and monoethanolamine (MEA) were purchased from Daejung Chemicals, Korea. Pyridine, deuterated dimethyl sulfoxide (DMSO-d6), chromium (III) acetylacetone, and trioxane were obtained from Sigma Aldrich (Seoul, Korea). Hydrochloric acid (HCl), acetyl anhydride, ethyl alcohol (EtOH), and ammonia water (NH4OH) were obtained from Duksan Chemical (Ansan, Korea) Chemical, Korea. Silver nitrate (AgNO3) was sourced from Samchun Pure Chemical, Korea.
2.2.1 Lignin Extraction from Black Liquor and Acetone Fractionation
As shown schematically in Fig. 1a, mixed HKL was isolated from black liquor by adjusting the pH to 9 using a 4 N sulfuric acid solution. The lignin precipitate was then subjected to stirring at 70°C for 1 h and subsequently filtered through Whatman No. 41 filter paper. The precipitated lignin was washed with 2 wt.% sulfuric acid in a lignin-to-acid ratio of 1:10 to remove any impurities. This was followed by an additional hour of stirring at 70°C and another round of filtration. The lignin was then washed a second time with distilled water, maintaining the same 1:10 ratio. The purified lignin was then dried at 60°C for 72 h to produce HKL powder. The HKL was then fractionated using acetone to separate it into acetone-soluble HKL (designated as AS-HKL) and acetone-insoluble HKL (AI-HKL). For this procedure, the HKL powder was washed with distilled water and stirred at 300 rpm at room temperature (RT; 21°C–30°C) for 2 h. After that, the product was filtered through Whatman No. 1 filter paper and then dried at 50°C for 36 h. The dried HKL was then stirred at 300 rpm and RT in an acetone solution with an acetone-to-HKL ratio of 12:1 for 24 h, followed by filtration. Finally, the AI-HKL fraction was obtained by vacuum filtration through Whatman filter paper, while the AS-HKL fraction was recovered via rotary evaporation at 45°C to remove the acetone. Both the AS-HKL and AI-HKL were then dried at 50°C for 48 h. This study will focus on AS-HKL as raw material for further modification and will be designated as HKL material.
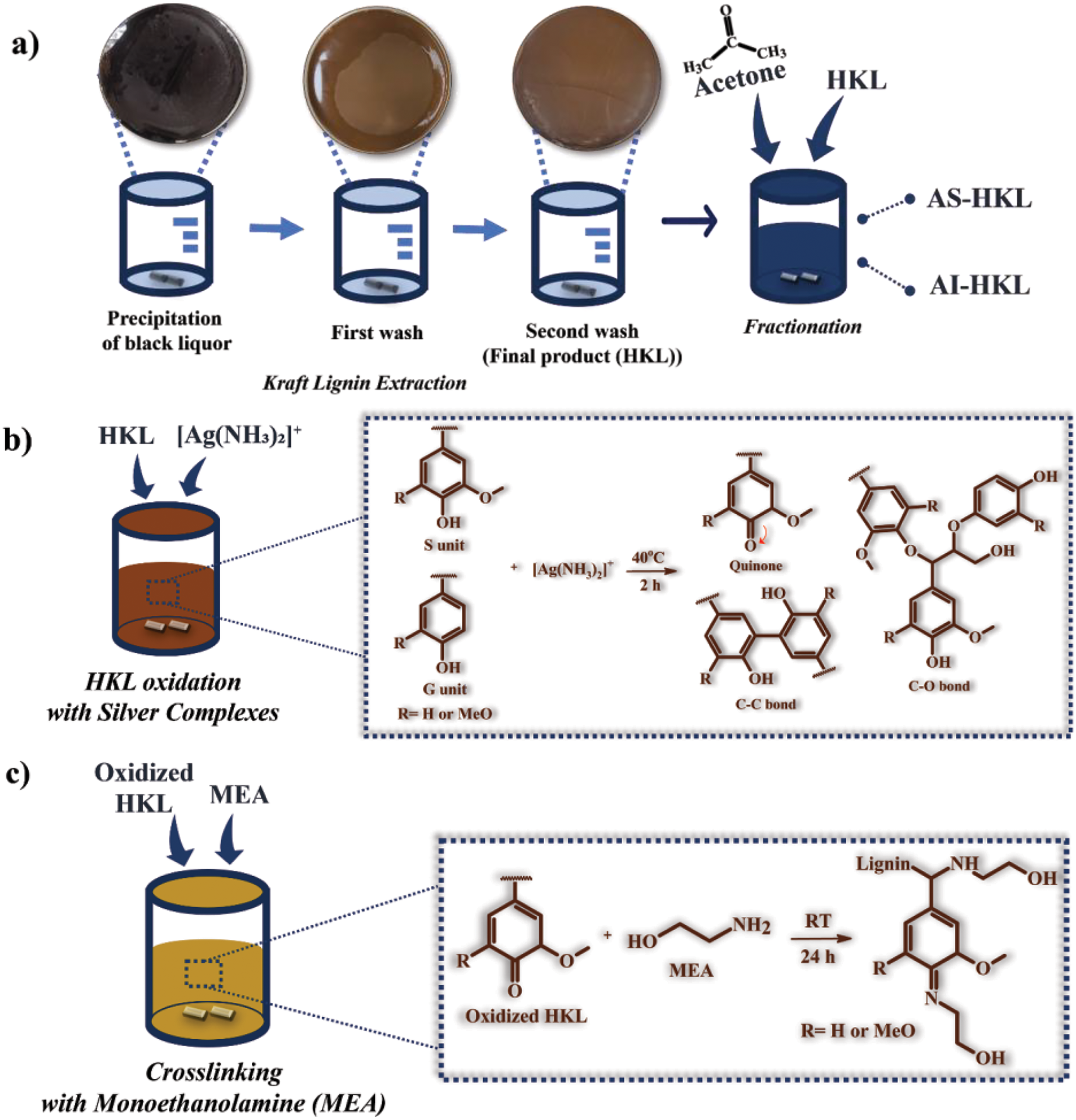
Figure 1: Schematic diagrams showing (a) the extraction and acetone fractionation of HKL, (b) the one-pot oxidation with silver complexes, and (c) a method of crosslinking the oxidized HKL with MEA
2.2.2 One-Pot Oxidation and Formation of Silver-Ammonia Complexes with HKL
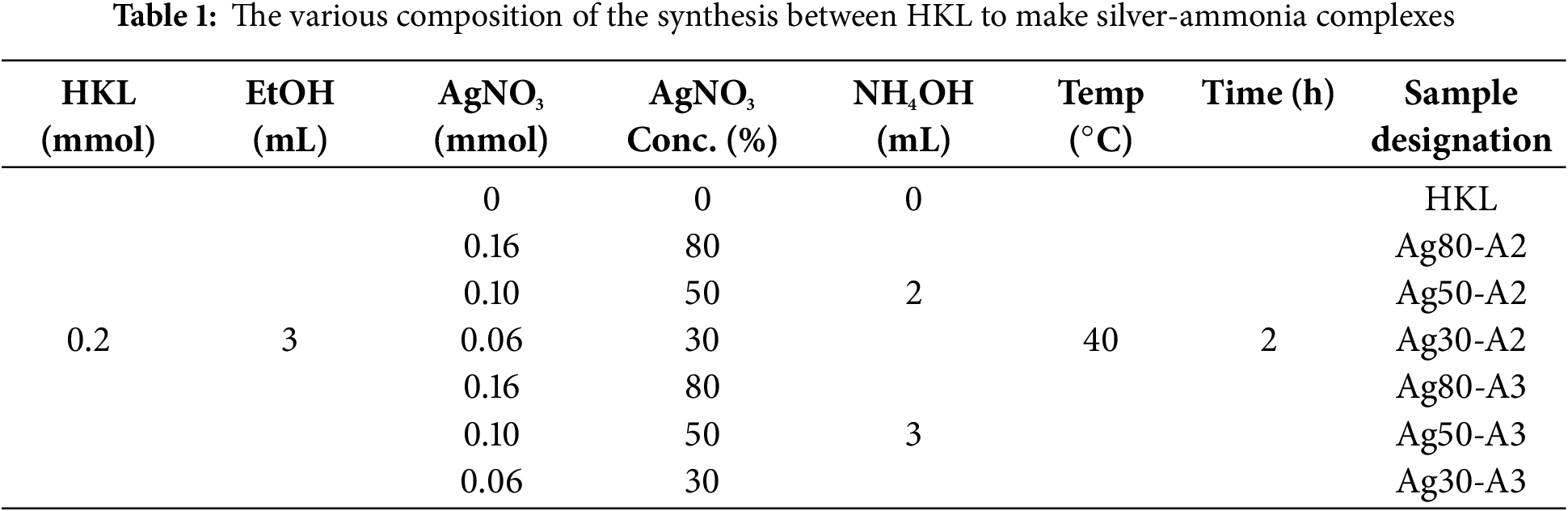
For the one-pot oxidation and synthesis of silver-ammonia complexes with HKL, the HKL (0.2 mmol) was mixed with ethanol (3 mL) in a 1:15 ratio, while the silver reagents were separately prepared by mixing various concentrations of silver nitrate (0–0.1 mmol) and NH4OH (0–3 mmol) to form silver-ammonia solution ([(AgNH3)2]+). The concentration of AgNO3 was calculated by dividing the desired amount of AgNO3 (in mmol) with the amount of HKL (in mmol) and multiplying the result by 100 to express the concentration as a percentage. The HKL and [(AgNH3)2]+ solutions were then combined in a round-bottomed flask and mixed at 40°C for 2 h to obtain the samples designated as Ag80-A2, Ag50-A2, Ag30-A2 (80%, 50%, and 30% AgNO3 with 2 mL NH4OH) as well as those designated as Ag80-A3, Ag50-A3, Ag30-A3 (80%, 50%, and 30% AgNO3 with 3 mL NH4OH) as summarized in Table 1. As shown schematically in Fig. 1b, this synthesis process involves the following three key reactions: (i) the reduction of Ag+ and concurrent oxidation of the hydroxyl (–OH) group, to result in the formation of a quinone group, (ii) the reaction of the C–O bond between the quinone group and the –OH group of the aliphatic chain, (iii) C–C coupling between free aromatic units, which allows them to react either with another aromatic unit or with a reactive aliphatic unit. The changes in lignin properties due to oxidation with the silver complexes were subsequently elucidated by various techniques, including GPC, 13C-NMR, and FT-IR, as detailed below.
2.2.3 Molar Mass Measurement of the Oxidized HKL via Gel Permeation Chromatography (GPC)
Molecular weight analysis was conducted via GPC (Waters Alliance e2695, Milford, MA, USA). For this purpose, approximately 2 mg of the sample was dissolved in 1 mL of tetrahydrofuran (THF), sonicated for 5 s, and filtered through a 0.45-μm polytetrafluoroethylene syringe filter. The GPC was operated at a column temperature of 35°C with an isocratic THF flow rate of 1 mL/min, using polystyrene as an internal standard [37]. To enhance the sample solubility in THF, 300 mg of each sample was subjected to acetylation by stirring in 18 mL of a 1:1 pyridine:anhydride at RT for 48 h. The resulting solution was then added to 250 mL of 0.1 N HCl to precipitate the product, which was filtered through a 0.45 μm Whatman nylon membrane. The lignin was further purified by repeated washing with 0.01 N HCl and water, followed by filtration. Successful acetylation of the lignin was confirmed by FTIR spectroscopy, which indicated the replacement of hydroxyl groups with acetyl groups.
The chemical bonds of the samples were identified by attenuated total reflectance infrared spectroscopy (ALPHA, Bruker Optics GmbH, Ettilingen, Germany). The FT-IR spectra were recorded over the range of 4000 to 400 cm−1 with a resolution of 4 cm−1 and a total of 32 scans.
2.2.5 Differential Scanning Calorimetry (DSC)
The thermal curing behavior of the adhesives was analyzed via DSC (Discovery 25, TA Instruments, New Castle, DE, United States). The glass transition temperature (Tg) was measured using 3–5 mg of dried sample placed in an aluminum pan with a lid. Samples were annealed by heating to 105°C at a rate of 10°C/min, followed by isothermal conditioning for 30 min before cooling to 0°C. The DSC thermogram was then recorded by gradually increasing the temperature to 250°C. For curing measurements, a dried sample (3–5 mg) was equilibrated to 30°C prior to heating to 250°C. Additionally, crosslinked oxidized lignin with MEA in liquid form was analyzed using a high-pressure pan to prevent water evaporation during the test, utilizing approximately 18–20 mg of the sample. Each sample was equilibrated to 30°C and then heated from 0 to 200°C at a rate of 10°C/min under a nitrogen flow rate of 50 mL/min.
2.2.6 Quantitative Carbon Nuclear Magnetic Resonance (13C-NMR) Spectroscopy
Quantitative 13C-NMR measurements were performed using a Bruker Avance Neo 700 MHz NMR spectrometer, following a method adapted from the literature [38]. An 80 mg sample was dissolved in 500 μL of DMSO-d6, with the addition of 50 μL of chromium(III) acetylacetone (5 mg/mL) as a relaxation agent and 50 μL of trioxane (15 mg/mL) as an internal standard. An inverse-gated proton decoupling sequence was employed with a 90° pulse width, a relaxation delay of 2 s, and an acquisition time of 1.4 s, with a total of 20,000 to 25,000 scans.
Oxidized lignin was prepared via one-pot synthesis with 10%, 15%, and 25% monoethanolamine (MEA), as shown schematically in Fig. 1c. The mixture was stirred for approximately 24 h to achieve the target viscosity of 200 mPa·s. The adhesive mixture was prepared for application to plywood and analysis of properties such as pH, solid content, and viscosity. The pH of each resin was measured at RT using a digital pH meter (Mettler-Toledo GmbH, 8603 Schwerzenbach, Switzerland). The solid content of the samples was determined in triplicate. For this procedure, approximately 1 g of resin was placed in each pre-weighed, labeled pan and dried in an oven at 105°C for 3 h. The weight of the oven-dried resin was then recorded. The percentage of solid content was calculated by dividing the weight of the dried resin by the initial weight of the resin and multiplying by 100. Viscosity measurements of the resin were performed using a Brookfield RVDV-II+ programmable viscometer with LV spindles at RT.
2.2.8 Determination of Adhesion Strength
The adhesive application was evaluated by preparing three veneers (150 mm × 150 mm × 2 mm) from Radiata pine. The adhesive was applied to the veneer surface at a spreading rate of 180 g/m² using a rubber roller. The plywood was cold-pressed at 8.8 kPa for 10 min prior to pressing at 160°C under a pressure of 1 MPa for 8 min. Subsequently, all samples were conditioned for a minimum of 8 h before being cut and tested. The dry tensile shear strength (TSS) of the plywood bonded with the adhesive was measured according to the standard procedure (KS F 3101). Three specimens were tested at a crosshead speed of 2 mm/min using the peak load of a universal testing machine (H50KS, Hounsfield, Redhill, England).
3.1 Properties of the Oxidized HKL
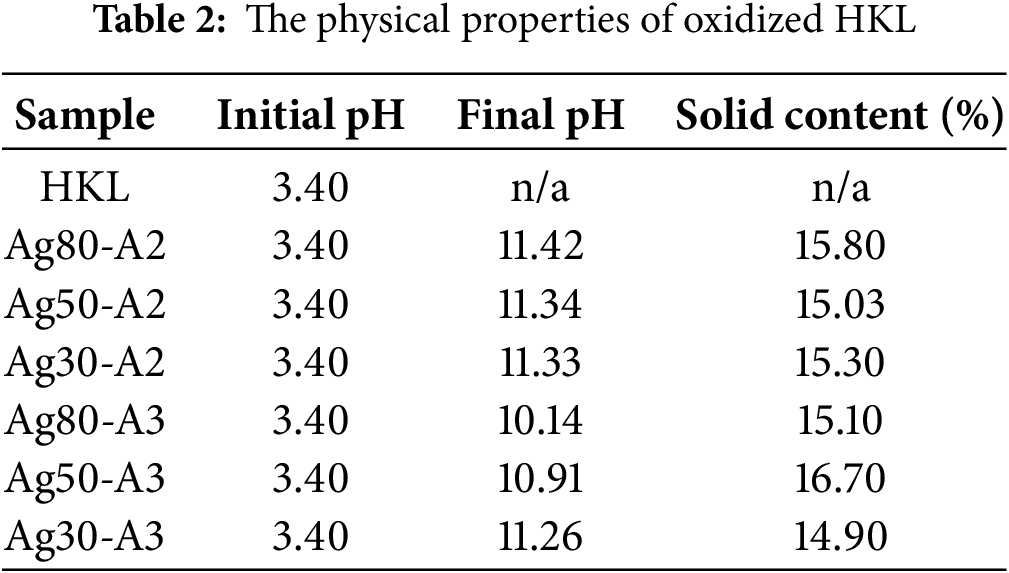
From the acetone fractionation of extracted HKL, acetone soluble HKL and acetone insoluble HKL samples with distinct characteristics were obtained. As discussed in the previous studies [20,21], the acetone soluble HKL features fewer polymeric chains and contains an abundance of newly-formed unsaturated linkages and phenolic hydroxyl groups for conversion to quinone/hydroquinone groups using silver complexes [31]. Hence, the acetone soluble HKL was specifically selected for the oxidation and complexation reaction. The initial and final pH values and solid contents of the various samples are listed in Table 2. Thus, while all of the samples have an initial pH of 3.4, samples Ag80-A2, Ag50-A2, and Ag30-A2 exhibit pH values of 11.42, 11.34, and 11.33, respectively, compared to 10.14, 10.91, and 11.26 for Ag80-A3, Ag50-A3, and Ag30-A3, respectively, after the oxidation reactions. These results indicate that while increasing the solvent volume from 2 mL and 3 mL generally results in lower pH values for Ag80-A3 relative to Ag80-A2, and Ag50-A3 relative to Ag50-A2, the effect is less marked for Ag30-A3 vs. Ag30-A2, which exhibit a similar pH of 11.33 and 11.26, respectively. These findings suggest that increasing the solvent volume influences the pH value, especially at higher silver concentrations. However, the solid content of all samples is relatively low, which is insufficient for use as an adhesive, especially for specific glue applications [39]. The highest solid contents are observed for sample Ag50-A3, at 16.7%, which is higher than all 3 mL NH4OH samples. This could be due to the higher crosslinking efficiency of this composition compared to the others, which leads to a reduction in the amount of vaporized water during adhesive application.
3.2 Molecular Weight of the Oxidized HKL
The molecular weight is a critical parameter in evaluating adhesives, as it significantly influences their mechanical properties and overall performance, and is closely associated with the physico-chemical characteristics of lignin [40,41]. It provides insights into the differences in molecular structure before and after oxidation. Previous research has demonstrated that lignin has limited solubility in organic solvents [42], thus leading to the use of acetylation to improve the solubility by replacing the hydroxyl groups with acetyl groups via reactions with acid anhydride and pyridine [43]. In the present study, the successful acetylation reaction is demonstrated by the FTIR spectra in Fig. 2a where the adsorption due to the hydroxyl group is observed in the 3600–3200 cm−1 range before the reaction and is replaced by that due to acetyl groups at 1763 cm−1, after the reaction. Further, the number average molecular weight (Mn), weight average molecular weight (Mw), and polydispersity (PDI) values of the initial HKL and oxidized HKL samples are compared in Fig. 2b. These results clearly demonstrate that varying the amounts of AgNO3 and NH4OH significantly affect the molecular weight of the product (Table 3).
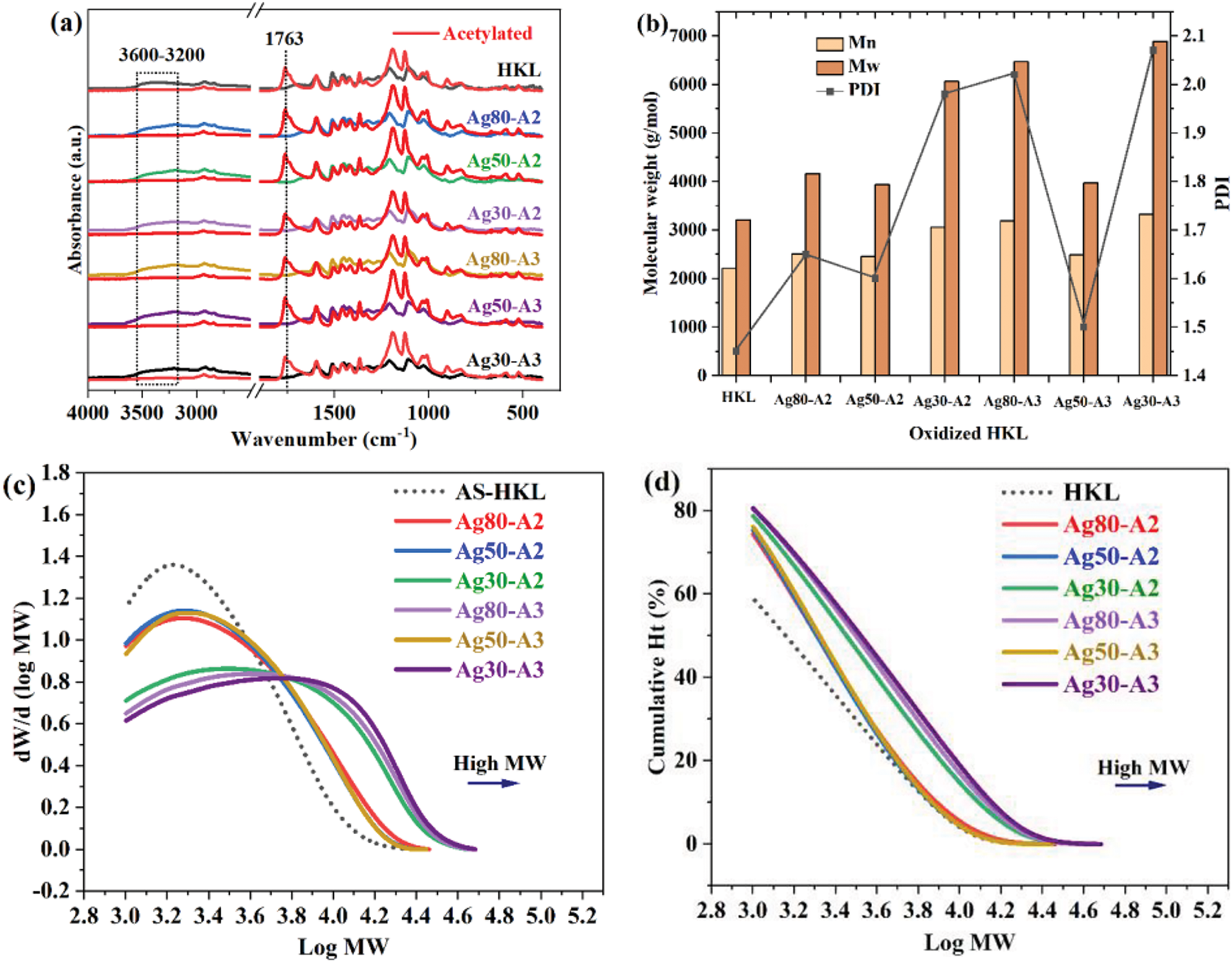
Figure 2: (a) The FT-IR spectra of the various samples before and after acetylation, (b) the comparison of Mn, Mw, and PDI values of the oxidized HKL, (c, d) the molecular weight distributions of the oxidized HKL samples (A shift to the right indicates a high molecular weight)
In all cases, an increase in the molecular weight of the HKL is observed after the oxidation. Notably, the highest molecular weight (6880 g/mol) is observed for sample Ag30-A3 with 30% AgNO3 and 3 mL of NH4OH. Additionally, Ag30-A2 with the same proportion of AgNO3 shows the highest molecular weight among the samples prepared using 2 mL NH4OH at 6061 g/mol. Nevertheless, these results suggest that the 30% AgNO3 concentration consistently provides a high molecular weight at both solvent concentrations, thus indicating the potential of this AgNO3 for an increased molecular weight across varying solvent volumes. Meanwhile, all the investigated AgNO3 concentrations give higher molecular weight with 3 mL NH4OH compared to the corresponding values with 2 mL of NH4OH. The high molecular weights of these samples suggest the formation of a crosslinked network or condensation reaction between the C–C and C–O bonds in the oxidized HKL, as condensed lignin is believed to exhibit increased molecular weight due to the formation of new C–C bonds [44,45].
By contrast, the lowest molecular weights are observed at both NH4OH concentrations with 50% AgNO3, with Ag50-A2 and Ag50-A3 exhibiting comparably low values of 3940 and 3974 g/mol, respectively. Notably, these low molecular weights can be expected to provide stronger surface wetting and, hence, adhesion than the higher molecular weights [46–48]. Overall, the present results have demonstrated the condensation of HKL via oxidation with silver-ammonia complexes. This is important because the oxidized HKL can form new bonds that will lead to self-condensation at high temperature, thereby improving its reactivity in the presence of a crosslinking agent.
For further understanding, the change in the molecular weight distributions (MWD) are plotted in Fig. 2c,d, respectively. Here, both types of MWD show a shift towards higher log Mw values for Ag30-A2, Ag80-A3, and Ag30-A3 compared to Ag80-A2, Ag50-A2, and Ag50-A3, thereby indicating an increase in molecular weight. This is relevant because the MWD of a polymer plays a crucial role in determining its mechanical strength, morphological phase behavior, and processability. This fundamental relationship across various polymers has led to the development of numerous synthetic and processing techniques [49–51].
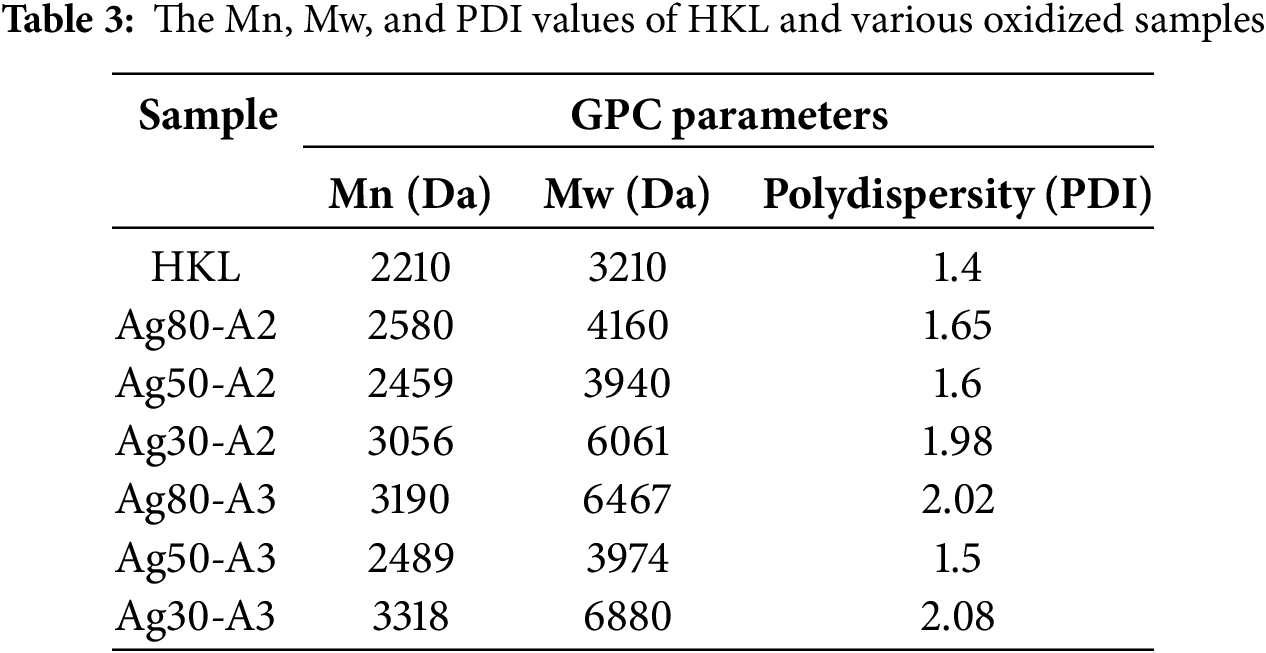
3.3 The Structure and Potential of the Oxidized HKL for Wood Bonding
An understanding of the structure of lignin, especially after oxidation, is crucial in order to select the best conditions for lignin cross-linking in adhesives. The hydroxyl group contents of HKL were determined via 31P-NMR in a previous study [52] and are illustrated as a pie chart in Fig. 3a. These hydroxyl groups can enhance the efficacy of the oxidation reaction by providing binding sites for Ag+ ions [53]. These results are further validated by comparing the FT-IR spectra of the various lignin samples before and after oxidation in Fig. 3b. Thus, while most of the peaks occupy the same positions for oxidized samples, significant changes are observed relative to HKL due to the oxidation. In particular, oxidized samples, each exhibit increasing spectra related to the vibrations of the new O–H group at 3200 cm−1 [32]. This suggests that the O–H group is directly involved in bonding with the carbonyl groups. Furthermore, the C=O stretching peak at 1732 cm−1 in the HKL is seen to disappear after the oxidation, while new peak appears at 1654 cm−1. This new peak may relate to the C=O stretching vibration of the quinone, which is probably generated alongside the reduction of Ag+ to Ag [54]. Additionally, the band at 1109 cm−1 due to the C–O stretching vibration in aliphatic lignin is seen to have slightly decreased in intensity. This suggests that the aliphatic chains have been disrupted, to form either carboxylic acids or C–O bonds during oxidation, which may have contributed to the above mentioned high molecular weights. In brief, the FT-IR spectra confirm that oxidation with silver-ammonia complex occurs under all investigated conditions.
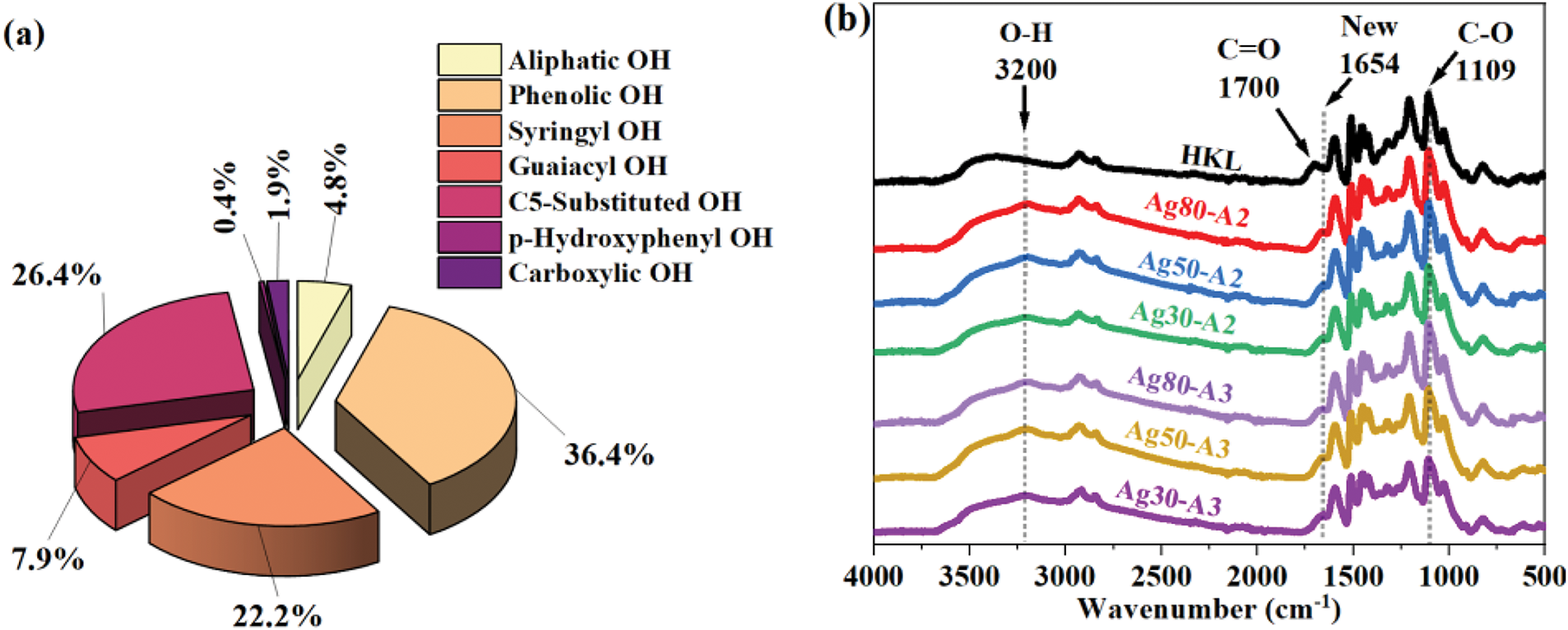
Figure 3: (a) A pie chart showing the composition of various –OH of the HKL according to 31P NMR [52] and (b) the FT-IR spectra of the oxidized HKL samples
Further, the quantitative 13C-NMR spectra of the HKL and the oxidized HKL (Ag50-A2) are presented in Fig. 4a. As expected, a new peak corresponding to C=O is observed at 163 ppm for the oxidized sample, which is consistent with the above FT-IR results. This peak indicates that the lignin is successfully oxidized during the reaction [55]. Additionally, the peak associated with C4–O bond of the aromatic ring in the HKL is seen to have disappeared after oxidation, thereby indicating that oxidation has occurred at the hydroxyl group in the C4 position. In addition, several new peaks have emerged, such as those in the aromatic region corresponding to C–C bonds, which may indicate radical coupling between the free positions of the coniferyl and p-hydroxycinnamyl alcohol groups, as supported by the high molecular weights. Meanwhile, the new peaks around 63 and 73 ppm may correspond to the C–O bonds of oxygenated side-chains, such as aliphatic chain linkages. This suggests that some cleavage or disruption has occurred [30] and new C–O bonds have formed between the reactive aliphatic sites and the oxidized HKL during oxidation. By contrast, the methoxy group observed around 56 ppm remains unchanged after oxidation, which is in contrast to previously-published findings indicating that the oxidation of lignin with AgNO3 and NH4OH can affect both the hydroxyl and methoxy groups [31,32,54]. These results are further supported by the upfield shift in the CH2 peak from 30 ppm to 18 ppm. However, the varying additions of silver complexes have resulted in different intensities across all oxidized samples, as shown in Fig. 4b. In particular, the C=O peaks exhibit similar trends across all samples, thereby indicating that the formation of quinones from the –OH groups has occurred in each case. Conversely, the peak corresponding to C–O shows significant differences among the various samples, which may explain the variations in molecular weight. As noted above, this peak is associated with aliphatic bonding with the formed quinone. Consequently, only samples Ag80-A2 and Ag50-A2 exhibit high intensities for the C–O peak at 63 ppm.
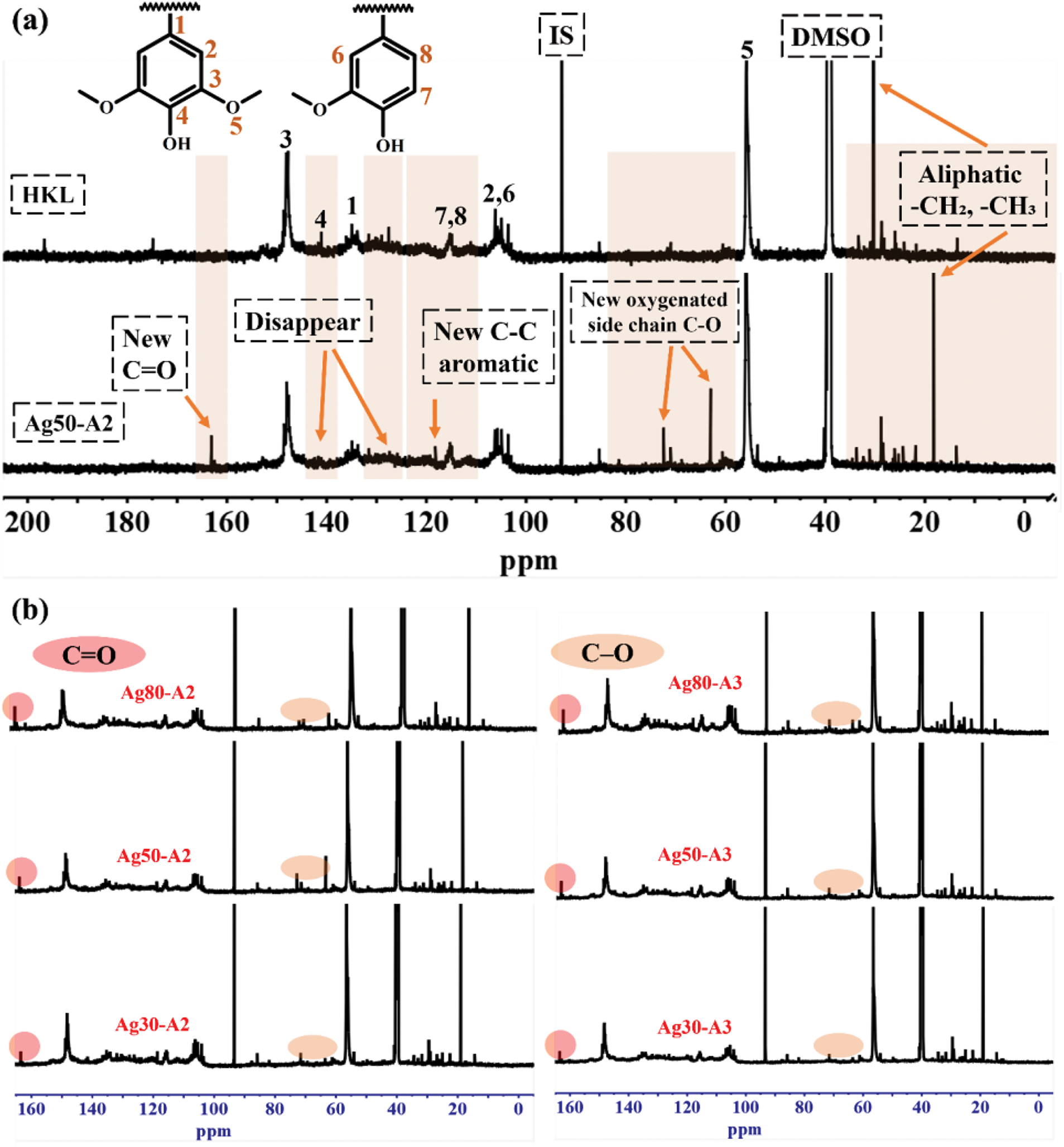
Figure 4: 13C-NMR spectrum of (a) HKL and oxidized HKL (Ag50-A2) and (b) oxidized HKL samples
The thermal behavior and particularly the glass transition temperature (Tg), is critical for understanding the material behavior, as it significantly influences the processing temperatures and mechanical properties that are essential for adhesive applications. Hence, the potential of oxidized HKL is further elucidated by the DSC results in Fig. 5. First, the differences between the Tg characteristics of the extracted HKL and acetone soluble fraction HKL are revealed in Fig. 5a, while the Tg values of the various oxidized HKL samples are compared in Fig. 5b. Here, the Tg of the HKL is decreased from ~150°C to 120°C for the soluble fraction. These results demonstrate the effectiveness of acetone fractionation in reducing the Tg of HKL. This reduction in the Tg of the HKL upon fractionation can be attributed to an increase in the free volume of the molecular structure [56].
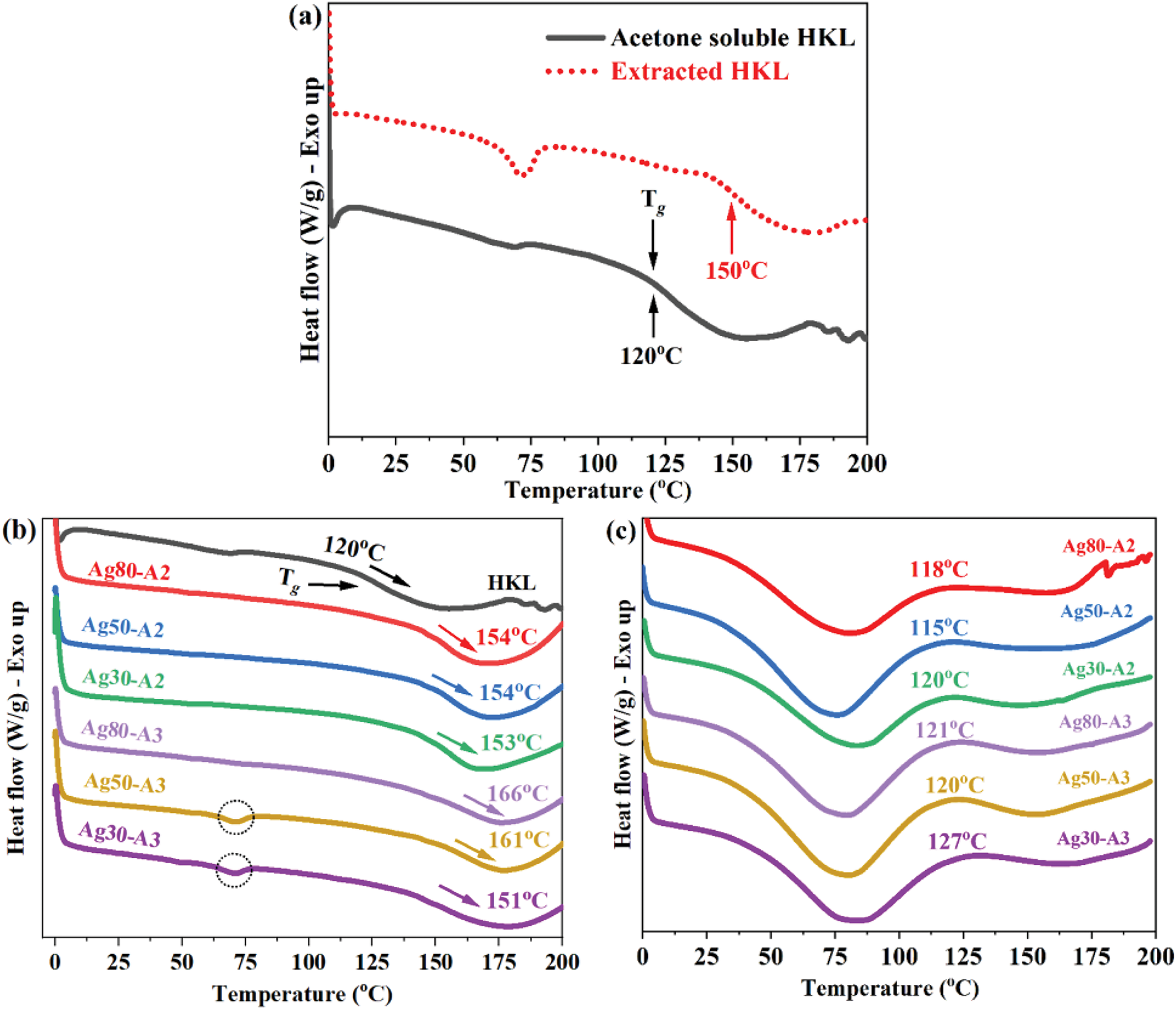
Figure 5: (a) The comparative Tg values of the extracted HKL, the acetone soluble HKL. (b) The Tg values of the various oxidized HKL samples. (c) The DSC curves of the various oxidized HKL samples
Notably, after oxidation, the oxidized HKL samples exhibit further increases in Tg (Fig. 5b), and the specific trend mirrors that observed in the molecular weights. Thus, Ag80-A2 and Ag50-A2 which were treated with 2 mL NH4OH, each have a low Tg value of 154°C, along with lower molecular weights, than those of the equivalent samples treated with 3 mL NH4OH (Ag80-A3 and Ag50-A3), with Tg values of 166°C and 161°C. However, the trend is reversed for Ag30-A2 and Ag30-A3, with Tg values of 153 and 151°C, respectively. This general correlation is consistent with the fact that Tg is influenced by molecular weight, along with factors such as the presence of water and solvent, thermal history, and hydrogen bonding interactions [57,58]. Specifically, a higher molecular weight typically leads to a higher Tg due to increased chain entanglement, which suggests that the adhesive material will have greater crosslinking potential and brittleness [59]. Notably, samples Ag50-A3 and Ag30-A3 each exhibit a modest endothermic peak at around 67°C (indicated by the dotted circles in Fig. 5b). This peak is also observed in the extracted HKL in Fig. 5a, which suggests that the peak may be associated with carbohydrate impurities [21]. By contrast, the acetone soluble HKL does not display this peak, and the peak remains absent or barely detectable after oxidation for the remaining samples. Thus, it can be concluded that the specific concentration of AgNO3 and NH4OH significantly influences the thermal behavior of the oxidized lignin.
Further, the thermal curing of each sample via both exothermic and endothermic processes is illustrated in Fig. 5c. Here, the oxidized HKL samples not only exhibit an increase in Tg but also display altered peaks at varying temperatures. These exothermic reactions suggest that crosslinking or the formation of a more rigid structure may have occurred. This suggests that oxidation with AgNO3 may facilitate C–C bonding or hydrogen bonding, thereby contributing to condensation at high temperatures [29]. Thus, the presence of exothermic peaks confirms the effectiveness of enhanced lignin as a crosslinking agent for wood bonding. Notably, the lowest exothermic peak is observed at 115°C for sample Ag50-A2, while the highest is 127°C for sample Ag30-A3. Consequently, Ag50-A2 is evaluated as a crosslinking agent in combination with MEA to produce lignin-based wood adhesives in the following section.
3.4 Structure and Physical Properties of the Crosslinked Oxidized HKL Resin
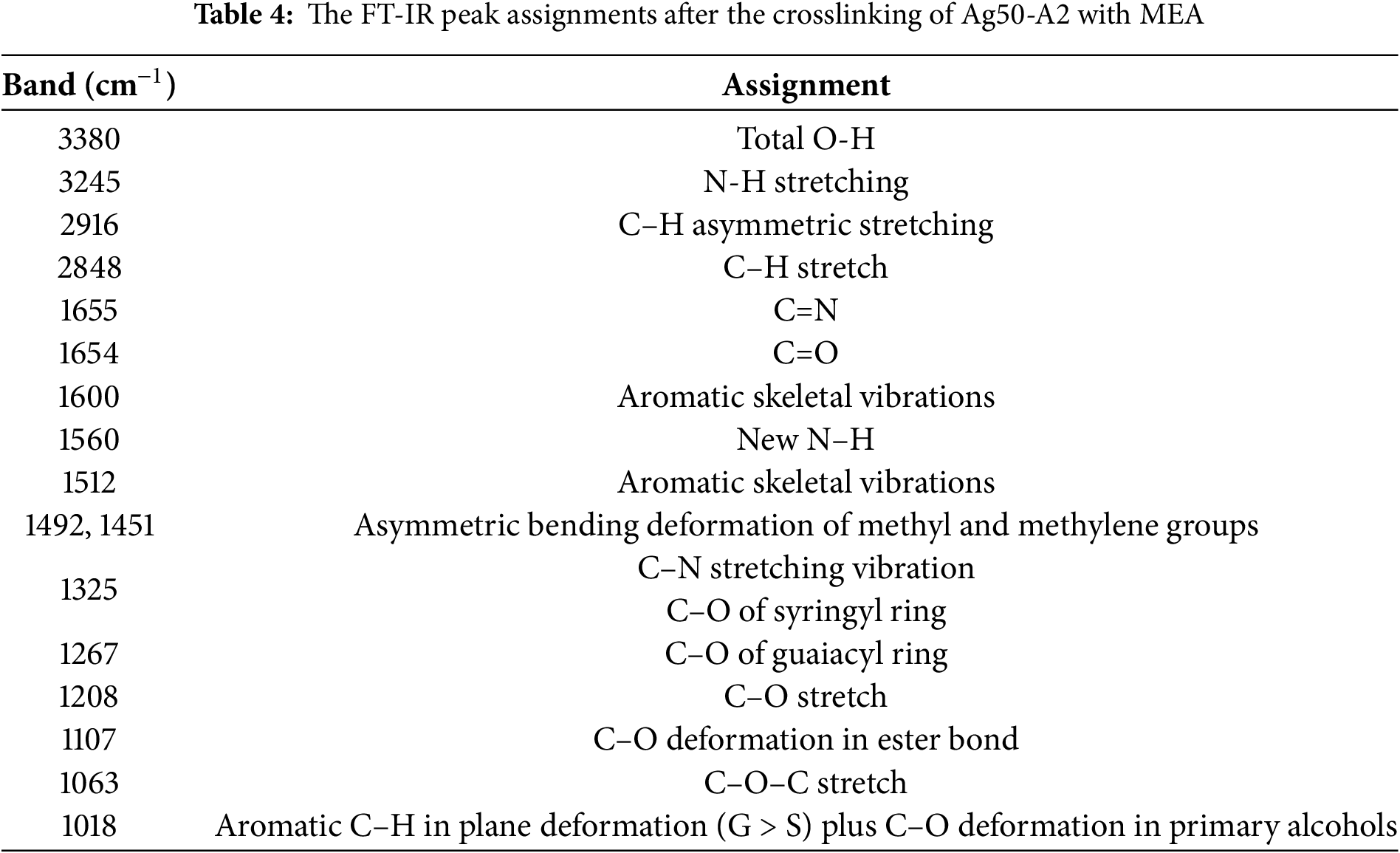
The FT-IR spectra of the Ag50-A2 sample before and after crosslinking with 10%, 15%, and 25% MEA are presented in Fig. 6a, and the various peak assignments according to the literature are summarized in Table 4 [36,60,61]. Here, the peak at 3200 cm–1 due to the O–H bonds of Ag50-A2 is seen to have disappeared after the crosslinking reaction, and a new band has appeared, which corresponds to the formation of N–H. In addition, new bands are observed at 1655; 1560; 1492 and 1063 cm–1, due to the respective C=N, N–H, C–H and C–O–C stretching vibrations. These results, especially the presence of N–H, confirm the crosslinking reaction between the oxidized HKL and the MEA.
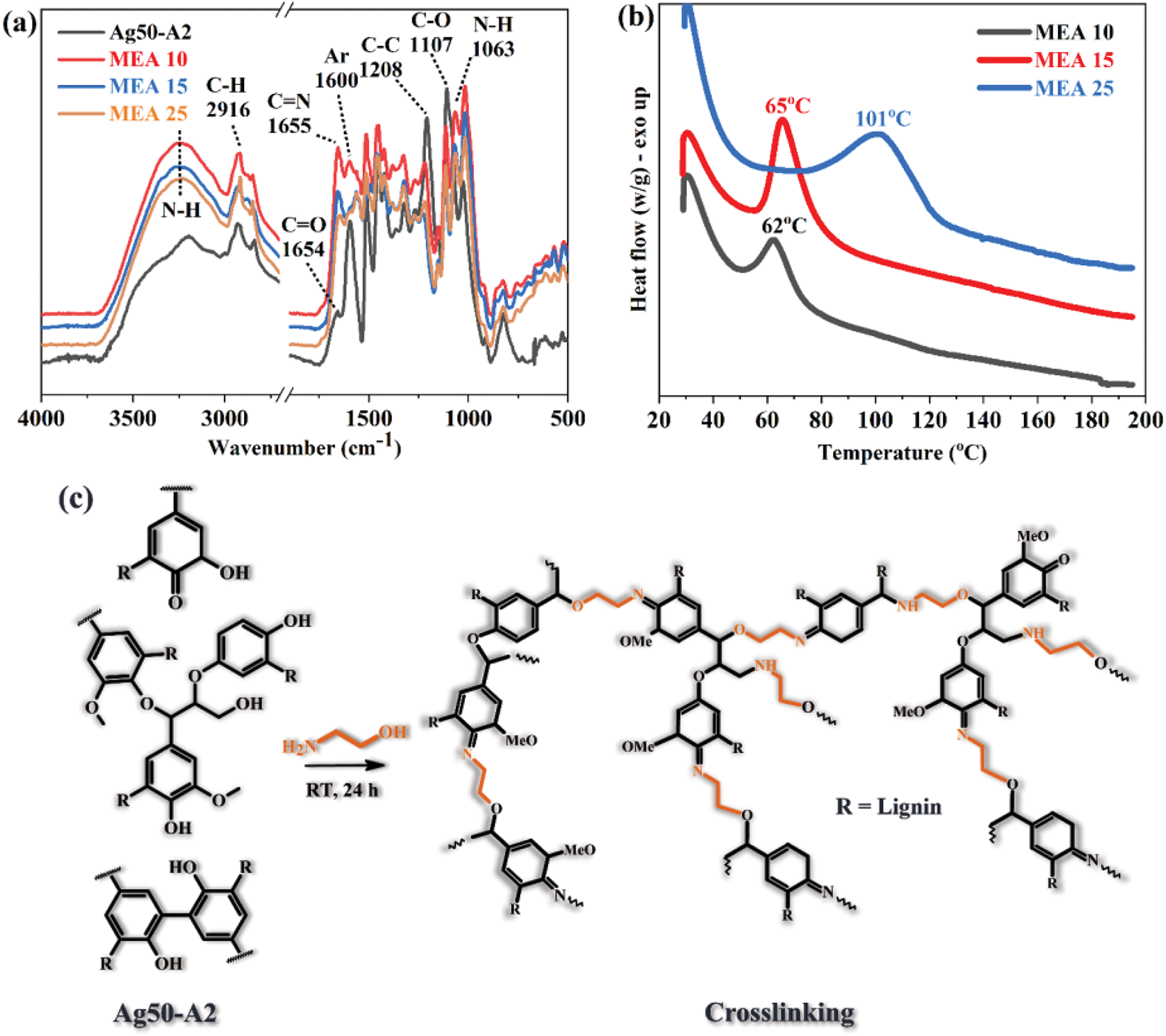
Figure 6: (a, b) The FT-IR spectra and DSC curves of the Ag50-A2 sample before and after crosslinking with 10%, 15%, and 25% MEA. (c) A schematic diagram showing the crosslinking reaction of Ag50-A2 and MEA
The DSC curves of the three crosslinked samples are presented in Fig. 6b. Here, each sample exhibits a well-defined exothermic peak with curing temperatures of 62, 65, and 101°C for the MEA 10, MEA 15, and MEA 25, respectively. This trend suggests that an increased quantity of crosslinker requires more time for effective curing, while a lower concentration allows for faster curing due to the reduced material density. This is important because curing should take place within an appropriate timeframe and under controlled temperature conditions that enhance the curing process [62]. Interestingly, the curing temperatures identified in this study are lower than those previously reported for crosslinked lignin [9,63–65], thereby indicating that the oxidation of lignin with silver-ammonia complexes may enhance the subsequent curing reaction with a crosslinker. The exothermic peak of each sample can be attributed to either the polymerization of macromolecular double bonds, or the heat released during the curing reaction [66], which contribute to the structural changes shown schematically in Fig. 6c.
Typically, the characterization of lignin-based adhesives involves measuring the pH, viscosity, and solid contents in order to ensure optimal adhesion performance. Hence, the pH, viscosity, and solid contents of the Ag50-A2 resin before and after crosslinking with 10%, 15%, and 25% MEA are summarized in Table 5. Here, the pH of the initial Ag50-A2 is seen to decrease from 11.34 before to 10.40 after reaction with 10% MEA (MEA 10). However, the pH subsequently increases slightly as the amount of MEA increases, so that the MEA 15 and MEA 25 samples exhibit pH values of 10.43 and 10.83, respectively. Importantly, while the viscosity of the initial Ag50-A2 (~20 mPa·s) is inadequate for effective application on the veneer substrates, the viscosity of each crosslinked sample has increased significantly to ~200 mPa·s. The low viscosity of the initial sample facilitates rapid penetration of resin into the veneer, thus resulting in reduced surface resin and insufficient interlocking after curing, leading to delamination [67]. The viscosity of the Ag50-A2 and Ag50-A2-MEA 25 samples compared with that of water in Fig. 7a. Meanwhile, as anticipated, the solid content of the initial sample increases significantly from 15.03% to 22.90% upon reaction with 10% MEA, and continues to increase slightly with increasing quantity of crosslinker.
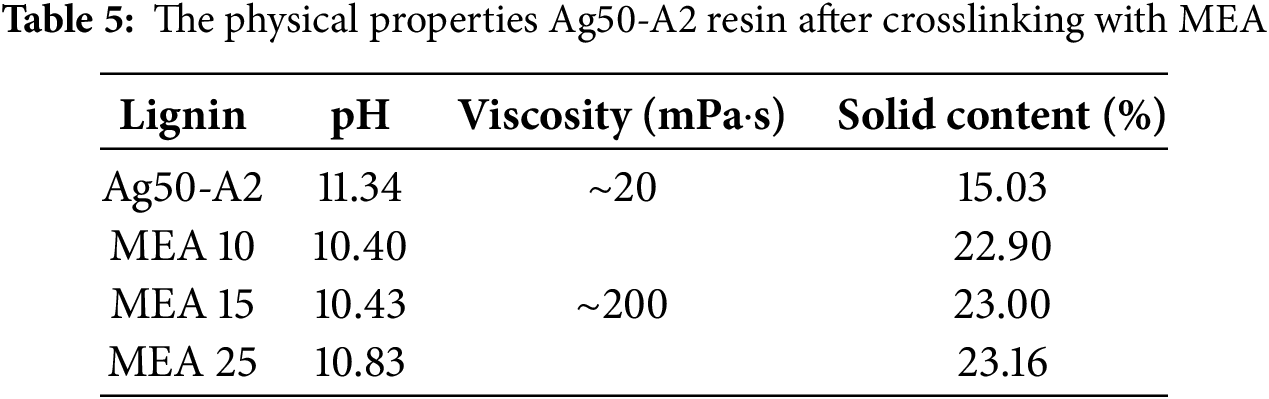
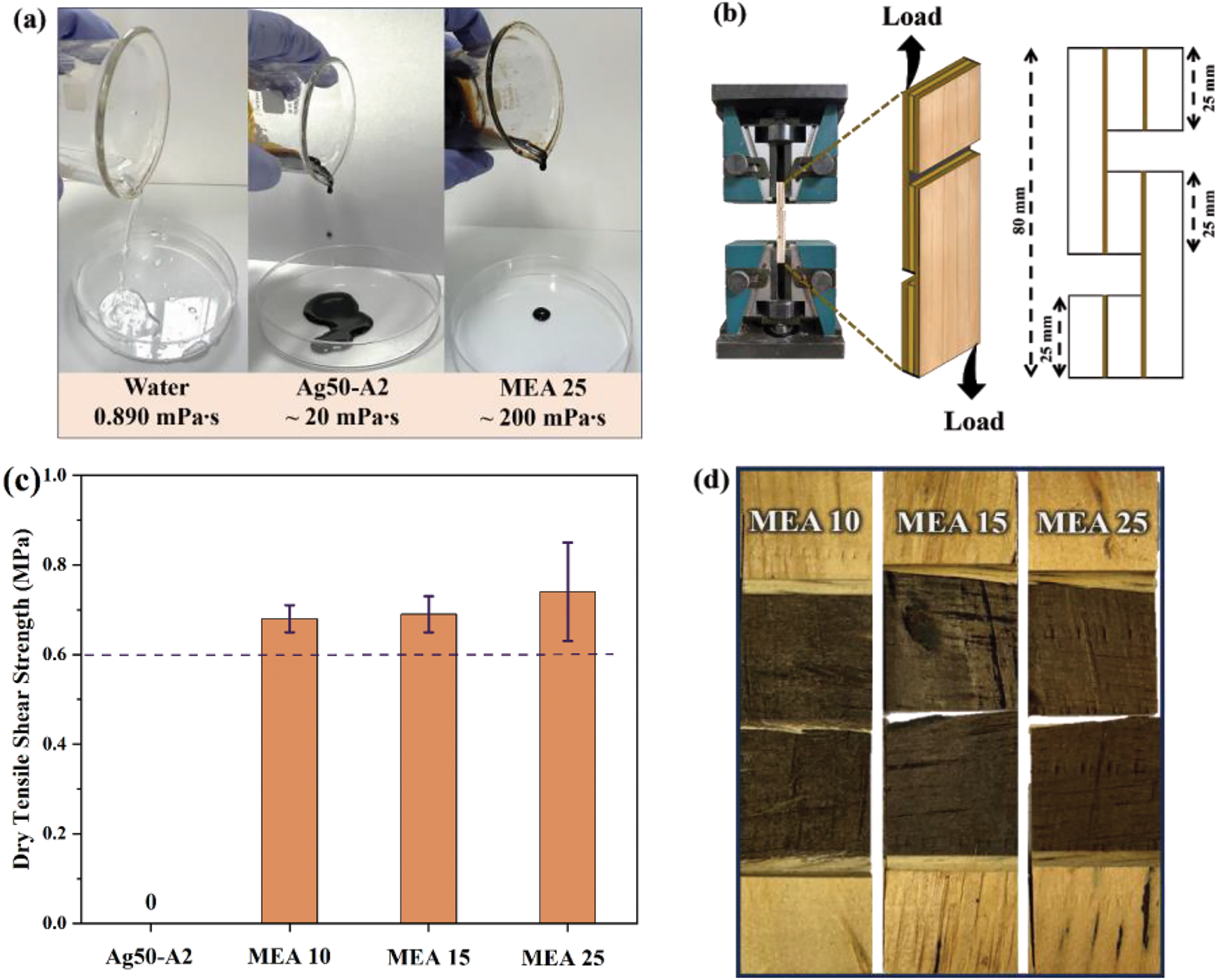
Figure 7: (a) A comparison of the viscosities of the Ag50-A2 and Ag50-A2-MEA 25 samples with that of water. (b) A schematic illustration of the sample dimensions for the dry TSS. (c) A bar chart comparing the dry TSS results of the various samples. (d) Photographic images of the samples after the dry TSS
The adhesion performance of each sample is revealed by the TSS results in Fig. 7. Specifically, the dimensions of the test samples are illustrated in Fig. 7b, and the results are presented as a bar chart in Fig. 7c. Here, the MEA 25 sample exhibits the greatest strength, at 0.77 MPa, while the MEA 10 and MEA 15 exhibit strengths of 0.63 and 0.65 MPa, respectively. With reference to the above-mentioned DSC results (Fig. 6b), these results indicate that the enhanced adhesion strength of the MEA 25 corresponds to its high curing temperature (101°C) while the sample with lower curing temperatures exhibits lower adhesion strength. Moreover, compared to a previous study in which the dry shear strength of unmodified lignin on a 3-layered wood panel is around 0.42 MPa [68], the present results clearly demonstrate that the oxidation and subsequent crosslinking reactions suggested herein provide greatly enhanced performance. Moreover, all of the crosslinker concentrations examined herein provide adhesive strength that exceeds the value of 0.60 MPa required by the KS F 3101 standard. In addition, there are few important limitations that should be considered, particularly with regard to scalability and long-term stability. Regarding scalability, the use of silver-ammonia complexes and MEA, though effective, could present cost challenges if scaled up for industrial applications. In addition, optimizing the process to ensure consistent quality and reducing the use of silver may help to address these concerns. Further, the potential environmental impact of silver remains an important consideration. In terms of long-term stability, while preliminary testing suggests that the adhesives perform well, further studies are needed to assess their durability under various conditions, such as exposure to moisture, heat, and UV radiation. These factors will be crucial for evaluating the adhesive’s suitability for widespread industrial use.
The adhesive strength of the MEA 10, MEA 15, and MEA 25 samples are further revealed by the photographic images of the samples after TSS testing in Fig. 7d where no signs of fracture are observed. In general, the wood failure measurement is initially assessed by examination with the naked eyes, after which the fractured surface is colored using oil-borne ink and the captured image is subjected to computer processing [69]. However, the black color of the lignin covered the fracture, so that it was hard to determine visually. Hence, it will be important to check the surface condition more thoroughly in the future. To solve this problem, maximum wood failure could be reached by treating or further modifying the veneer surface [70]. This finding suggests the need for further development of the crosslinked lignin conditions in future studies.
Herein, a novel modification of hardwood kraft lignin (HKL) was demonstrated via oxidation with silver-ammonia complexes ([(AgNH3)2]+) followed by crosslinking with monoethanolamine (MEA) for use as an adhesive. Compared to the use of 30% or 80% AgNO3, the incorporation of 50% AgNO3 resulted in a lower molecular weight and a reduced curing temperature of the oxidized fractionated soluble HKL (AS-HKL). The oxidation reaction was confirmed by FT-IR and 13C-NMR analyses, with spectra indicating the presence of C=O groups due to the anticipated quinone formation. The presence of C–C and C–O bonds after the oxidation was further supported by the high molecular weight and exothermic peak. The oxidized HKL showed increased reactivity and potential for the MEA crosslinking reactions, thus leading to stronger adhesive bonds. The crosslinking reaction was evidenced by the formation of N-H bonds in the FT-IR spectra, along with the exothermic peaks observed in the DSC analysis. Additionally, the dry shear strength was found to increase with the increased amount of crosslinker, with 25% MEA giving the highest strength of 0.77 MPa. Notably, there was no visible wood failure or fracture after testing. Future research based on the optimum condition should focus on the oxidation process, and exploring crosslinking with surface-treated wood or bio-crosslinker. These efforts could further solidify the role of modified lignin as a sustainable alternative in the adhesive industry, potentially reducing the reliance on synthetic adhesives and contributing to more eco-friendly manufacturing practices.
Acknowledgement: All the authors are grateful to Moorim P&P Co., Ltd., Ulsan, Republic of Korea for the donation of black liquor of kraft pulping.
Funding Statement: This work was supported by the National Research Foundation (NRF) of Korea, funded by the Korean Government (MSIT) (Grant No. RS-2023-00240043).
Author Contributions: Ega Cyntia Watumlawar prepared methodology, investigations, conceptualization, original draft. Byung-Dae Park supervised and acquired funding, prepared methodology, conceptualization, validated data, reviewed, and edited original articles. Long Yang provided critical review on the manuscript. Guanben Du provided critical review on the manuscript. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data is available upon request.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
References
1. Liu S, Du G, Yang H, Su H, Ran X, Li J, et al. Developing high-performance cellulose-based wood adhesive with a cross-linked network. ACS Sustain Chem Eng. 2021;9(49):16849–61. doi:10.1021/acssuschemeng.1c07012. [Google Scholar] [CrossRef]
2. Yang H, Tan X, Du G, Ni K, Wu Y, Li Z, et al. Development of biomass adhesives based on aminated cellulose and oxidized sucrose reinforced with epoxy functionalized wood interface. Compos Part B Eng. 2023;263(16):110872. doi:10.1016/j.compositesb.2023.110872. [Google Scholar] [CrossRef]
3. Addis CC, Koh RS, Gordon MB. Preparation and characterization of a bio-based polymeric wood adhesive derived from linseed oil. Int J Adhes Adhes. 2020;102:102655. doi:10.1016/j.ijadhadh.2020.102655. [Google Scholar] [CrossRef]
4. Zhang L, Chen Y, Geng S, Huang Z, Qiu R, Chen T, et al. Development of plant oil-based adhesives for formaldehyde-free bamboo particleboards. Ind Crops Prod. 2024;210:118146. doi:10.1016/j.indcrop.2024.118146. [Google Scholar] [CrossRef]
5. Zhang Y, Ding L, Gu J, Tan H, Zhu L. Preparation and properties of a starch-based wood adhesive with high bonding strength and water resistance. Carbohydr Polym. 2015;115:32–7. doi:10.1016/j.carbpol.2014.08.063. [Google Scholar] [PubMed] [CrossRef]
6. Boussetta A, Benhamou AA, Charii H, Ablouh E, Mennani M, Kasbaji M, et al. Formulation and characterization of chitin-starch bio-based wood adhesive for the manufacturing of formaldehyde-free composite particleboards. Waste Biomass Valorization. 2023;14(11):3671–87. doi:10.1007/s12649-023-02091-x. [Google Scholar] [CrossRef]
7. Zhao X, Liu T, Ou R, Hao X, Fan Q, Guo C, et al. Fully biobased soy protein adhesives with integrated high-strength, waterproof, mildew-resistant, and flame-retardant properties. ACS Sustain Chem Eng. 2022;10(20):6675–86. doi:10.1021/acssuschemeng.2c00742. [Google Scholar] [CrossRef]
8. Hao Z, Xi X, Hou D, Lei H, Li C, Xu G, et al. A fully bio-based soy protein wood adhesive modified by citric acid with high water tolerance. Int J Biol Macromol. 2023;253:127135. doi:10.1016/j.ijbiomac.2023.127135. [Google Scholar] [PubMed] [CrossRef]
9. Siahkamari M, Emmanuel S, Hodge DB, Nejad M. Lignin-glyoxal: a fully biobased formaldehyde-free wood adhesive for interior engineered wood products. ACS Sustain Chem Eng. 2022;10(11):3430–41. doi:10.1021/acssuschemeng.1c06843. [Google Scholar] [CrossRef]
10. Bruijnincx PCA, Rinaldi R, Weckhuysen BM. Unlocking the potential of a sleeping giant: lignins as sustainable raw materials for renewable fuels, chemicals and materials. Green Chem. 2015;17(11):4860–1. doi:10.1039/C5GC90055G. [Google Scholar] [CrossRef]
11. Yang G, Gong Z, Zhou B, Luo X, Liu J, Du G, et al. Hydrodeoxygenation of condensed lignins followed by acid-mediated methylolation enables preparation of lignin-based wood adhesives. Green Chem. 2024;26(2):753–9. doi:10.1039/D3GC03935H. [Google Scholar] [CrossRef]
12. Liu LY, Chiang WS, Chang HM, Yeh TF. Phenolation to improve hardwood kraft lignin for wood adhesive application. Polymers. 2024;16(13):1923. doi:10.3390/polym16131923. [Google Scholar] [PubMed] [CrossRef]
13. Zheng Q, Nong G, Li N. Preparation and structural analysis of a water-soluble aminated lignin. Polymers. 2024;16(9):1237. doi:10.3390/polym16091237. [Google Scholar] [PubMed] [CrossRef]
14. Sun C, Cao F, Xu Y, Feng J, Wang K, Fang Z, et al. Carboxymethylated lignin reinforcement of spi adhesive: Enhancing strength, antimicrobial, and flame-retardant properties without excessive alkali introduction. ACS Omega. 2024;9(21):22703–10. doi:10.1021/acsomega.4c00452. [Google Scholar] [PubMed] [CrossRef]
15. Tao Y, Zhou S, Luo Q, Song F, Gong Z, Wang Z, et al. Replacing phenol and formaldehyde for green adhesive synthesis by lignin and furfural. ACS Appl Polym Mater. 2024;6(12):6897–905. doi:10.1021/acsapm.3c03087. [Google Scholar] [CrossRef]
16. Gadhave RV, Srivastava S, Mahanwar PA, Gadekar PT. Lignin: renewable raw material for adhesive. Open J Polym Chem. 2019;9(2):27–38. doi:10.4236/ojpchem.2019.92003. [Google Scholar] [CrossRef]
17. Liu R, Smeds A, Wang L, Pranovich A, Hemming J, Willfor S, et al. Fractionation of lignin with decreased heterogeneity: based on a detailed characteristics study of sequentially extracted softwood kraft lignin. ACS Sustain Chem Eng. 2021;9(41):13862–73. doi:10.1021/acssuschemeng.1c04725. [Google Scholar] [CrossRef]
18. Li H, Yuan Z, Xing Y, Li J, Fang J, Chang L, et al. Acetone fractionation: a simple and efficient method to improve the performance of lignin for dye pollutant removal. RSC Adv. 2019;9(61):35895–903. doi:10.1039/C9RA07017F. [Google Scholar] [PubMed] [CrossRef]
19. Karaaslan MA, Cho MJ, Liu LY, Wang H, Renneckar S. Refining the properties of softwood kraft lignin with acetone: effect of solvent fractionation on the thermomechanical behavior of electrospun fibers. ACS Sustain Chem Eng. 2021;9(1):458–70. doi:10.1021/acssuschemeng.0c07634. [Google Scholar] [CrossRef]
20. Arefmanesh M, Nikafshar S, Master ER, Nejad M. From acetone fractionation to lignin-based phenolic and polyurethane resins. Ind Crops Prod. 2022;178:114604. doi:10.1016/j.indcrop.2022.114604. [Google Scholar] [CrossRef]
21. Wibowo ES, Park BD. The role of acetone-fractionated kraft lignin molecular structure on surface adhesion to formaldehyde-based resins. Int J Biol Macromol. 2023;225:1449–61. doi:10.1016/j.ijbiomac.2022.11.202. [Google Scholar] [PubMed] [CrossRef]
22. Watumlawar EC, Park BD. Synthesis of acetone-fractionated hardwood kraft lignin-based adhesive crosslinked with epichlorohydrin. J Adhes Sci Technol. 2024;38(3):442–57. doi:10.1080/01694243.2023.2236400. [Google Scholar] [CrossRef]
23. Chatel G, Rogers RD. Review: oxidation of lignin using ionic liquids-an innovative strategy to produce renewable chemicals. ACS Sustain Chem Eng. 2014;2(3):322–39. doi:10.1021/sc4004086. [Google Scholar] [CrossRef]
24. Collinson SR, Thielemans W. The catalytic oxidation of biomass to new materials focusing on starch, cellulose and lignin. Coord Chem Rev. 2010;254(15–16):1854–70. doi:10.1016/j.ccr.2010.04.007. [Google Scholar] [CrossRef]
25. Ma R, Guo M, Zhang X. Recent advances in oxidative valorization of lignin. Catal Today. 2018;302:50–60. doi:10.1016/j.cattod.2017.05.101. [Google Scholar] [CrossRef]
26. Yang C, Magallanes G, Maldonado S, Stephenson CRJ. Electro-reductive fragmentation of oxidized lignin models. J Org Chem. 2021;86(22):15927–34. doi:10.1021/acs.joc.1c00346. [Google Scholar] [PubMed] [CrossRef]
27. Hwang JH, Martinez DV, Martinez EJ, Metavarayuth G, Goodlett D, Wang Q, et al. Highly swellable hydrogels prepared from extensively oxidized lignin. Giant. 2022;10(11):100106. doi:10.1016/j.giant.2022.100106. [Google Scholar] [CrossRef]
28. Chakraborty D, Gowda RR, Malik P. Silver nitrate-catalyzed oxidation of aldehydes to carboxylic acids by H2O2. Tetrahedron Lett. 2009;50(47):6553–6. doi:10.1016/j.tetlet.2009.09.044. [Google Scholar] [CrossRef]
29. Bhanderi K, Ghalsasi PS, Inoue K. Nonconventional driving force for selective oxidative C-C coupling reaction due to concurrent and curious formation of Ag0. Sci Rep. 2021;11(1):1568. doi:10.1038/s41598-021-81020-1. [Google Scholar] [PubMed] [CrossRef]
30. Zhang Q, Chen C, Wan G, Lei M, Chi M, Wang S, et al. Solar light induced synthesis of silver nanoparticles by using lignin as a reductant, and their application to ultrasensitive spectrophotometric determination of mercury(II). Microchim Acta. 2019;186(11):727. doi:10.1007/s00604-019-3832-8. [Google Scholar] [PubMed] [CrossRef]
31. Zhang L, Lu H, Chu J, Ma J, Fan Y, Wang Z, et al. Lignin-directed control of silver nanoparticles with tunable size in porous lignocellulose hydrogels and their application in catalytic reduction. ACS Sustain Chem Eng. 2020;8(33):12655–63. doi:10.1021/acssuschemeng.0c04298. [Google Scholar] [CrossRef]
32. Yu J, Ran F, Li C, Hao Z, He H, Dai L, et al. A lignin silver nanoparticles/polyvinyl alcohol/sodium alginate hybrid hydrogel with potent mechanical properties and antibacterial activity. Gels. 2024;10(4):240. doi:10.3390/gels10040240. [Google Scholar] [PubMed] [CrossRef]
33. Montazer M, Alimohammadi F, Shamei A, Rahimi MK. In situ synthesis of nano silver on cotton using Tollens’ reagent. Carbohydr Polym. 2012;87(2):1706–12. doi:10.1016/j.carbpol.2011.09.079. [Google Scholar] [CrossRef]
34. Xie HB, Zhou Y, Zhang Y, Johnson K. Reaction mechanism of monoethanolamine with CO2 in aqueous solution from molecular modeling. J Phys Chem A. 2010;114(43):11844–52. doi:10.1021/jp107516k. [Google Scholar] [PubMed] [CrossRef]
35. Song Y, Cao L, Yu J, Zhang S, Chen S, Jiang Y. Amino-functionalized graphene oxide blend with monoethanolamine for efficient carbon dioxide capture. J Alloys Compd. 2017;704:245–53. doi:10.1016/j.jallcom.2017.01.310. [Google Scholar] [CrossRef]
36. Ghahri S, Park BD. Amination and crosslinking of acetone-fractionated hardwood kraft lignin using different amines and aldehydes for sustainable bio-based wood adhesives. Bioresour Technol. 2024;399:130645. doi:10.1016/j.biortech.2024.130645. [Google Scholar] [PubMed] [CrossRef]
37. Liu LY, Cho M, Sathitsuksanoh N, Chowdhury S, Rennecker S. Uniform chemical functionality of technical lignin using ethylene carbonate for hydroxyethylation and subsequent greener esterification. ACS Sustain Chem Eng. 2018;6(9):12251–60. doi:10.1021/acssuschemeng.8b02649. [Google Scholar] [CrossRef]
38. Xia Z, Akim LG, Argyropoulos DS. Quantitative 13C NMR analysis of lignins with internal standards. J Agric Food Chem. 2001;49(8):3573–8. doi:10.1021/jf010333v. [Google Scholar] [PubMed] [CrossRef]
39. Zheng G, Pan A, Xu Y, Zhang X. Preparation of a superior soy protein adhesive with high solid content by enzymatic hydrolysis combined with cross-linking modification. Ind Crops Prod. 2024;213:118446. doi:10.1016/j.indcrop.2024.118446. [Google Scholar] [CrossRef]
40. Solt P, Jääskeläinen AS, Lingenfelter P, Konnerth J, Herwijnen HWGV. Impact of molecular weight of kraft lignin on adhesive performance of lignin-based phenol-formaldehyde resins. For Prod J. 2019;68(4):365–71. doi:10.13073/FPJ-D-17-00079. [Google Scholar] [CrossRef]
41. Ma Q, Zhang X. An integral method for determining the molecular composition of lignin and its application. Sci Rep. 2022;12(1):19136. doi:10.1038/s41598-022-23884-5. [Google Scholar] [PubMed] [CrossRef]
42. Balakshin M, Capanema E. On the quantification of lignin hydroxyl groups with 31P and 13C NMR spectroscopy. J Wood Chem Technol. 2015;35(3):220–37. doi:10.1080/02773813.2014.928328. [Google Scholar] [CrossRef]
43. Johansson M, Skrifvars M, Kadi N, Dhakal HN. Effect of lignin acetylation on the mechanical properties of lignin-poly-lactic acid biocomposites for advanced applications. Ind Crops Prod. 2023;202:117049. doi:10.1016/j.indcrop.2023.117049. [Google Scholar] [CrossRef]
44. Ma R, Zhang X, Wang Y, Zhang X. New insights toward quantitative relationships between lignin reactivity to monomers and their structural characteristics. ChemSusChem. 2018;11(13):2146–55. doi:10.1002/cssc.201800550. [Google Scholar] [PubMed] [CrossRef]
45. Chen X, Liu Z, Zhou Z, Li R, Li L, Cao Y. The synergetic reduction of the condensation degree of dissolved lignin (DL) during the refining process of wheat straw biomass based on the MA/O3 system. Molecules. 2024;29(13):3228. doi:10.3390/molecules29133228. [Google Scholar] [PubMed] [CrossRef]
46. Siebert HM, Wilker JJ. Improving the molecular weight and synthesis of a renewable biomimetic adhesive polymer. Eur Polym J. 2019;113:321–7. doi:10.1016/j.eurpolymj.2019.01.063. [Google Scholar] [CrossRef]
47. Choi GY, Zurawsky W, Ulman A. Molecular weight effects in adhesion. Langmuir. 1999;15(24):8447–50. doi:10.1021/la990312j. [Google Scholar] [CrossRef]
48. Peppas NA, Buri PA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J Control Release. 1985;2:257–75. doi:10.1016/0168-3659(85)90050-1. [Google Scholar] [CrossRef]
49. Talebi S, Duchateau R, Rastogi S, Kaschta J, Peters GWM, Lemstra PJ. Molar mass and molecular weight distribution determination of UHMWPE synthesized using a living homogeneous catalyst. Macromolecules. 2010;43(6):2780–8. doi:10.1021/ma902297b. [Google Scholar] [CrossRef]
50. Walsh DJ, Schinski DA, Schneider RA, Guironnet D. General route to design polymer molecular weight distributions through flow chemistry. Nat Commun. 2020;11(1):3094. doi:10.1038/s41467-020-16874-6. [Google Scholar] [PubMed] [CrossRef]
51. Yang J, Sun M, Jiao L, Dai H. Molecular weight distribution and dissolution behavior of lignin in Alkaline solutions. Polymers. 2021;13(23):4166. doi:10.3390/polym13234166. [Google Scholar] [PubMed] [CrossRef]
52. Ghahri S, Park BD. Self-crosslinking of acetone-fractionated and glyoxalated hardwood kraft lignin as bio-adhesives for wood bonding. Ind Crops Prod. 2023;206:117711. doi:10.1016/j.indcrop.2023.117711. [Google Scholar] [CrossRef]
53. Richter AP, Brown JS, Bharti B, Wang A, Gangwal S, Houck K, et al. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat Nanotechnol. 2015;10(9):817–23. doi:10.1038/NNANO.2015.141. [Google Scholar] [PubMed] [CrossRef]
54. Ran F, Li C, Hao Z, Zhang X, Dai L, Si C, et al. Combined bactericidal process of lignin and silver in a hybrid nanoparticle on E. coli. Adv Compos Hybrid Mater. 2022;5(3):1841–51. doi:10.1007/s42114-022-00460-z. [Google Scholar] [CrossRef]
55. Shen Z, Luo Y, Wang Q, Wang X, Sun R. High-value utilization of lignin to synthesize ag nanoparticles with detection capacity for Hg2+. ACS Appl Mater Interfaces. 2014;6(18):16147–55. doi:10.1021/am504188k. [Google Scholar] [PubMed] [CrossRef]
56. Sain S, Matsakas L, Rova U, Christakopoulos P, Öman T, Skrifvars M. Spruce bark-extracted lignin and tannin-based bioresin-adhesives: effect of curing temperatures on the thermal properties of the resins. Molecules. 2021;26(12):3523. doi:10.3390/molecules26123523. [Google Scholar] [PubMed] [CrossRef]
57. El Mansouri NE, Yuan Q, Huang F. Synthesis and characterization of kraft lignin-based epoxy resins. BioResources. 2011;6(3):2492–503. doi:10.15376/biores.6.3.2492-2503. [Google Scholar] [CrossRef]
58. Choi JH, Cho SM, Kim JC, Park SW, Cho YM, Koo B, et al. Thermal properties of ethanol organosolv lignin depending on its structure. ACS Omega. 2021;6(2):1534–46. doi:10.1021/acsomega.0c05234. [Google Scholar] [PubMed] [CrossRef]
59. Abenojar J, de Armentia SL, Martinez MA, del Real JC. Development of a green epoxy adhesive for cork by adding lignin: thermal and bonding properties. Wood Sci Technol. 2022;56(3):721–42. doi:10.1007/s00226-022-01374-1. [Google Scholar] [CrossRef]
60. Li R, Gao B, Sun S, Yue Q, Li M, Yang X, et al. Amine-cross-linked lignin-based polymer: modification, characterization, and flocculating performance in humic acid coagulation. ACS Sustain Chem Eng. 2015;3(12):3253–61. doi:10.1021/acssuschemeng.5b00844. [Google Scholar] [CrossRef]
61. Oh DH, Heo JW, Xia Q, Kim MS, Kim YS. Amine-crosslinked lignin for water pollution attributable to organic dye remediation: versatile adsorbent for selective dye removal and reusability. Heliyon. 2024;10(17):e37497. doi:10.1016/j.heliyon.2024.e37497. [Google Scholar] [PubMed] [CrossRef]
62. Bae SG, Gim MJ, Kim W, Oh MK, Yun JH, Kim JS. Effect of cross-linking characteristic on the physical properties and storage stability of acrylic rubber. Elastomers Compos. 2023;58(3):136–41. doi:10.7473/EC.2023.58.3.136. [Google Scholar] [CrossRef]
63. Saito T, Perkins JH, Jackson DC, Trammel NE, Hunt MA, Naskar AK. Development of lignin-based polyurethane thermoplastics. RSC Adv. 2013;3(44):21832–40. doi:10.1039/c3ra44794d. [Google Scholar] [CrossRef]
64. Song Y, Wang Z, Zhang X, Zhang R, Li J. Synthetic process of bio-based phenol formaldehyde adhesive derived from demethylated wheat straw alkali lignin and its curing behavior. J Renew Mater. 2021;9(5):943–57. doi:10.32604/jrm.2021.014131. [Google Scholar] [CrossRef]
65. Younesi-Kordkheili H. Ionic liquid modified lignin-phenol-glyoxal resin: a green alternative resin for production of particleboards. J Adhes. 2019;95(12):1075–87. doi:10.1080/00218464.2018.1471994. [Google Scholar] [CrossRef]
66. Dimonie D, Dimonie M, Munteanu V, Iovu H, Couve J, Abadie MJ. Nature of the first exothermic peak on the DSC curve of linear polydicyclopentadiene obtained with a special catalytic system. Polym Degrad Stab. 2000;70(3):319–24. doi:10.1016/S0141-3910(00)00093-8. [Google Scholar] [CrossRef]
67. Xu Y, Han Y, Shi SQ, Gao Q, Li J. Preparation of a moderate viscosity, high performance and adequately-stabilized soy protein-based adhesive via recombination of protein molecules. J Clean Prod. 2020;255(6):120303. doi:10.1016/j.jclepro.2020.120303. [Google Scholar] [CrossRef]
68. Chen X, Xi X, Pizzi A, Fredon E, Du G, Gerardin C, et al. Oxidized demethylated lignin as a bio-based adhesive for wood bonding. J Adhes. 2021;97(9):873–90. doi:10.1080/00218464.2019.1710830. [Google Scholar] [CrossRef]
69. Kim M, Park BD. A method of measuring wood failure percentage of wood specimens bonded with melamine-urea-formaldehyde resins using image analysis. J Korean Wood Sci Technol. 2021;49(3):274–82. doi:10.5658/WOOD.2021.49.3.274. [Google Scholar] [CrossRef]
70. Rowell RM. Understanding wood surface chemistry and approaches to modification: a review. Polymers. 2021;13(15):2558. doi:10.3390/polym13152558. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools