 Open Access
Open Access
ARTICLE
An Investigation into the Cationic Dye Adsorption Capacity of Prickly Pear Cactus-Derived Cellulose
Laboratory of Materials and Environment for Sustainable Development, LR18ES10, University of Tunis El Manar, 9, Avenue Dr. Zoheir Safi, Tunis, 1006, Tunisia
* Corresponding Author: Rached Ben Hassen. Email:
(This article belongs to the Special Issue: Biobased Materials for Advanced Applications )
Journal of Renewable Materials 2025, 13(7), 1389-1411. https://doi.org/10.32604/jrm.2025.02025-0022
Received 29 January 2025; Accepted 07 May 2025; Issue published 22 July 2025
Abstract
This research aims to investigate the potential of a plant cellulose developed from Opuntia ficus-indica (OFI) cladode as a sustainable and renewable adsorbent for the removal of neutral red (NR), a cationic dye pollutant, from aqueous environments. Analysis of raw and treated OFI using X-ray diffraction (XRD), scanning electron microscopy (SEM), and Fourier Transform Infrared Spectroscopy (FTIR) demonstrated the successful extraction of type cellulose. The Brunauer–Emmett–Teller (BET) analysis of the nitrogen adsorption-desorption isotherm revealed an improved specific surface area of 12.4 m2/g after treatment. A systematic study of key parameters in batch adsorption experiments revealed removal rates greater than 90% at pH = 3, an adsorbent dosage of 3 g/L and an initial dye concentration of 100 mg/L with equilibrium achieved within 2 h. The high correlation coefficient (R2 = 0.98) obtained with the Langmuir isotherm model suggests that the adsorption behavior is consistent with monolayer surface adsorption. A maximum adsorption capacity (Qm) of 357.1 mg/g for neutral red dye was achieved, demonstrating a significant adsorption capacity relative to other materials such as chitosan-modified activated carbon and halloysite nanotubes. The pseudo-second-order model effectively described the kinetics of the adsorption phenomena. Thermodynamic analysis revealed an exothermic and spontaneous adsorption process, with an enthalpy change (ΔH) of −24.886 kJ/mol, indicative of predominantly physisorption-driven interactions. Moreover, the regenerated cellulose exhibited a retention of over 70% efficiency after multiple adsorption-desorption cycles, highlighting its potential as an excellent reusable adsorbent. The outcomes of this research present an environmentally conscious alternative to synthetic adsorbents, facilitating the effective NR dye removal through renewable and sustainable means.Graphic Abstract
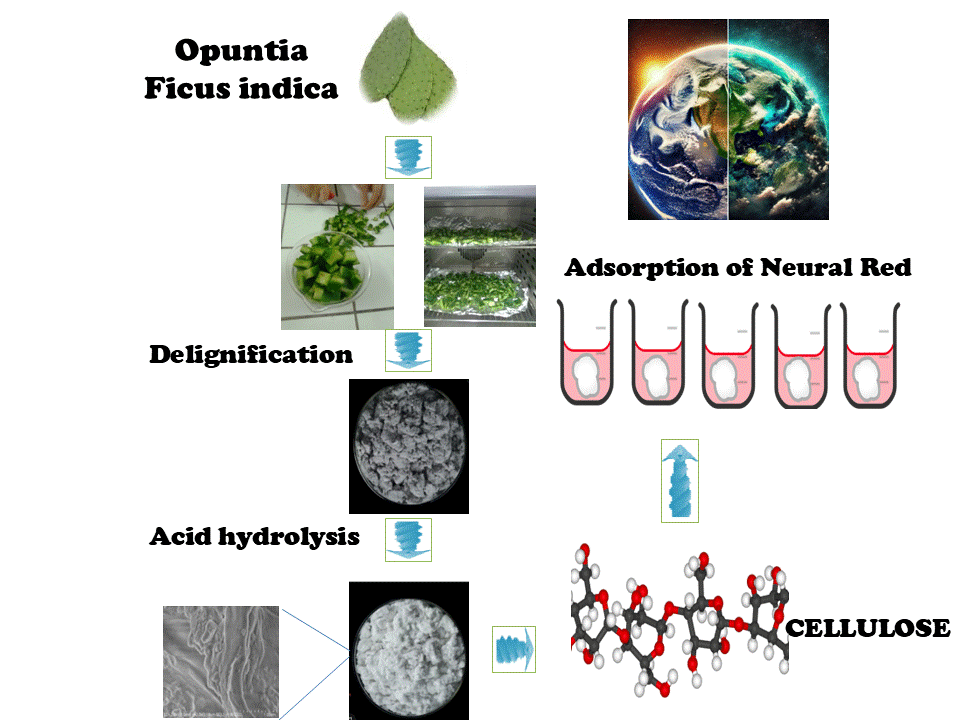
Keywords
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools