 Open Access
Open Access
REVIEW
Pharmacological Phase I Clinical Trials in Pediatric Brain Tumors (1990–2024): A Historical Perspective
1 Scuola di Specializzazione di Farmacologia e Tossicologia Clinica, Dipartimento di Ricerca Traslazionale e delle Nuove Tecnologie in Medicina e Chirurgia, University of Pisa, Pisa, 56126, Italy
2 Azienda Ligure Sanitaria–Alisa, Regione Liguria, Piazza della Vittoria 15, Genova, 16121, Italy
3 Centro di Farmacologia Clinica per la Sperimentazione dei Farmaci, Azienda Ospedaliera Universitaria Pisana, Pisa, 56126, Italy
* Corresponding Author: Guido Bocci. Email:
# These authors contributed equally to this work
(This article belongs to the Special Issue: Cutting-edge strategies for pediatric solid tumors: diagnostic and therapeutic insights)
Oncology Research 2025, 33(10), 2603-2656. https://doi.org/10.32604/or.2025.066260
Received 02 April 2025; Accepted 05 August 2025; Issue published 26 September 2025
Abstract
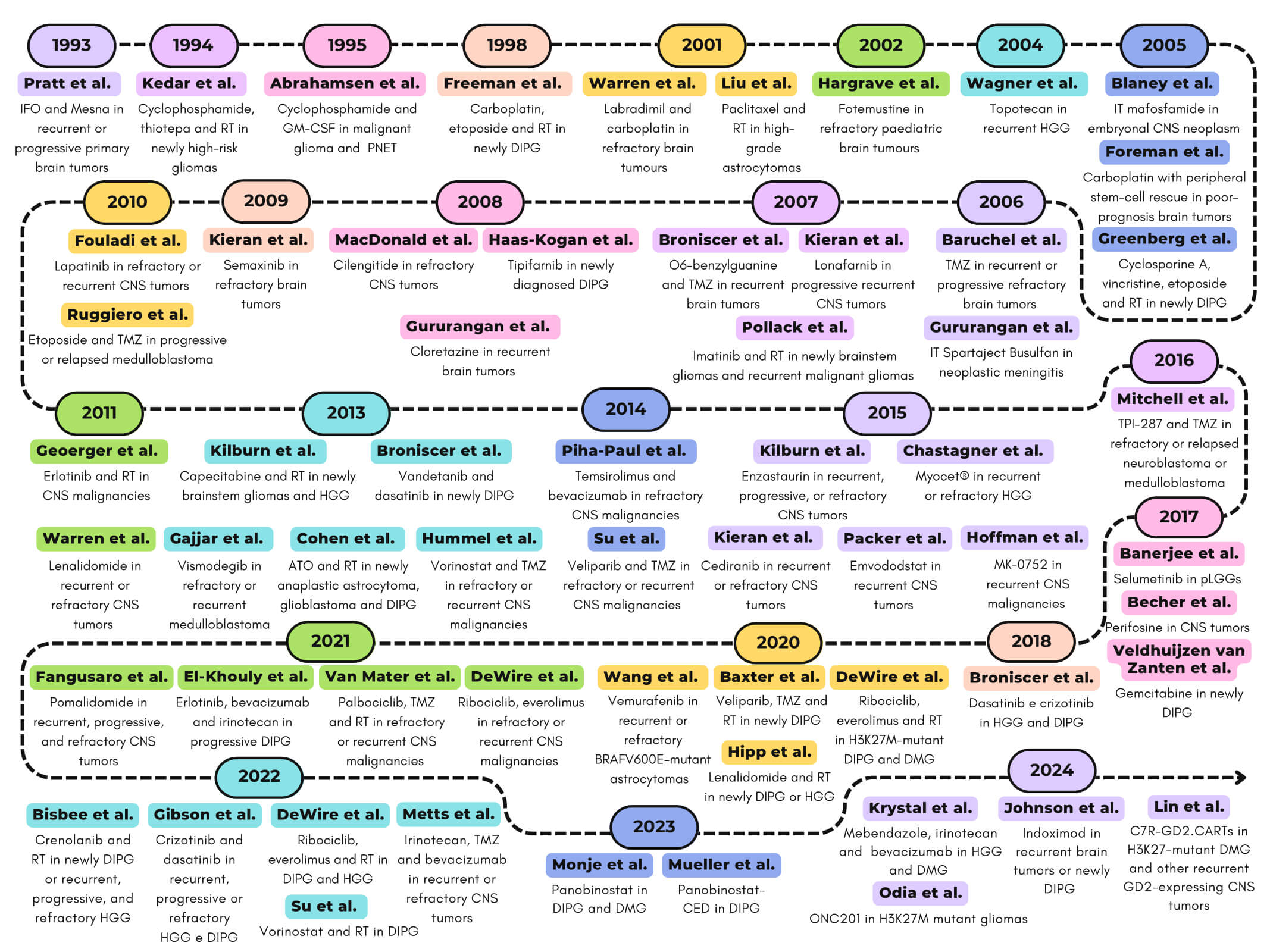
Central nervous system (CNS) tumors are the most common solid tumors in pediatric patients and the leading cause of childhood cancer-related mortality. Their rarity compared to adult cancers has made enrolling sufficient cases for clinical trials challenging. Consequently, pediatric CNS tumors were long treated with adult protocols despite distinct biological and clinical characteristics. This review examines key aspects of phase I pediatric oncology trials, including study design, primary outcomes, and pharmacological approaches, along with secondary considerations like clinical responses and ethical aspects. Firstly, we evaluated all phase I trial protocols focusing on pediatric CNS tumors with accessible results published in scientific databases (Pubmed, Scopus, Embase, Web of Science, and Google Scholar) from 1990 to November 2024. Secondly, we searched EudraCT and ClinicalTrials.gov on 30 November 2024 for ongoing trials. Our search yielded 60 completed phase I studies and 15 trials in progress. Dividing them by chronological order revealed that study designs and the response assessments evolved as the understanding of CNS tumor biology increased. Despite advancements improving diagnosis, management, and prognostication, mortality remains high, and morbidity persists. Notably, pediatric pharmacokinetics and pharmacodynamics differ from adults, complicating trial comparisons and dosage optimization. Future efforts should focus on large-scale clinical data collection to enhance trial efficiency.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools