 Open Access
Open Access
REVIEW
Targeting AMPK for Cancer Therapy: Metabolic Reprogramming as a Therapeutic Strategy
School of Life Science, Handong Global University, Pohang, 37554, Republic of Korea
* Corresponding Author: Jea-Hyun Baek. Email:
Oncology Research 2025, 33(10), 2699-2724. https://doi.org/10.32604/or.2025.067487
Received 05 May 2025; Accepted 05 August 2025; Issue published 26 September 2025
Abstract
AMP-activated protein kinase (AMPK) is a highly conserved serine/threonine kinase that functions as a central regulator of cellular energy status. In cancer, where metabolic reprogramming enables rapid proliferation and survival under stress, AMPK functions as a metabolic checkpoint that restrains tumor growth by inhibiting biosynthetic pathways and promoting catabolic processes, such as autophagy and fatty acid oxidation. Given its role in opposing many hallmarks of cancer metabolism, AMPK has attracted significant interest as a therapeutic target. This review examines the molecular mechanisms by which AMPK influences tumor progression and evaluates the preclinical and clinical evidence for pharmacological AMPK activation using agents such as metformin, phenformin, and canagliflozin. While promising anti-tumor effects have been reported in specific contexts—such as HER2-positive breast cancer, colorectal cancer, and metabolically distinct lung cancer subtypes—clinical efficacy remains variable. Limitations include indirect activation mechanisms, low tissue penetrance, tumor heterogeneity, and lack of reliable biomarkers for patient selection. We discuss emerging strategies to overcome these challenges, including combination therapies, metabolic stratification, and the development of direct AMPK activators or mRNA-based delivery platforms. Together, these insights support a renewed focus on AMPK as a modifiable node in cancer metabolism and a candidate for integration into precision oncology frameworks.Keywords
Cancer remains one of the leading causes of death globally, with incidence and mortality rates continuing to rise. According to the World Health Organization (WHO), cancer accounted for nearly 10 million deaths in 2020, and by 2023, it became the second leading cause of death worldwide [1]. Beyond its health impact, cancer also imposes a growing economic burden with the global cost between 2020 and 2050 projected to reach $25.2 trillion [2]. Despite significant advancements in targeted therapy, chemotherapy, and immunotherapy, major clinical limitations persist, particularly in the form of drug resistance, off-target toxicity, and tumor heterogeneity [3]. These challenges present the need for alternative therapeutic strategies that target cancer’s core vulnerabilities.
One such vulnerability is metabolic reprogramming, now recognized as a hallmark of cancer. Tumor cells undergo profound shifts in energy metabolism to support rapid proliferation and survival under hypoxic or nutrient-deprived conditions. A prime example is the Warburg effect, in which cancer cells preferentially utilize glycolysis even in the presence of oxygen, generating ATP less efficiently but enabling biosynthetic flexibility [4,5]. Furthermore, clinical data suggest strong correlations between metabolic diseases, such as obesity, type 2 diabetes, and dyslipidemia, and increased cancer risk, mediated through chronic inflammation, oxidative stress, and insulin resistance [6]. These findings highlight the therapeutic potential of targeting tumor metabolism as a broadly applicable strategy across diverse cancer types.
At the core of cellular energy regulation lies AMP-activated protein kinase (AMPK), a highly conserved serine/threonine kinase that acts as a master regulator of metabolic balance. AMPK senses energy stress through changes in the AMP: ATP ratio and responds by inhibiting ATP-consuming anabolic processes (e.g., lipid and protein synthesis) while activating catabolic pathways (e.g., fatty acid oxidation and autophagy) [7].
In glucose metabolism, AMPK directly activates phosphofructokinase (PFK)-2, leading to an increased cellular concentration of fructose-2,6-bisphosphate (F-2,6-BP) [8]. F-2,6-BP is a potent allosteric activator of PFK-1, a rate-limiting enzyme and the first committed step of glycolysis [9]. Additionally, AMKP stimulates glycogen breakdown by inducing the fusion of glucose transporter 4 (GLUT4) vesicles with the plasma membrane and activating glycogen phosphorylase, thereby mobilizing stored glucose for immediate energy needs [10]. Furthermore, AMPK inhibits transcription factors, which are required for expression of gluconeogenic enzymes [11].
In fatty acid oxidation, AMPK inactivates acetyl-CoA carboxylase (ACC), the enzyme responsible for the synthesis of malonyl-CoA [12]. Since malonyl-CoA allosterically inhibits carnitine palmitoyltransferase 1 (CPT1)—the transporter of long-chain acyl-CoA into mitochondria—AMPK indirectly facilitates the import of long-chain acyl-CoA into mitochondria [13]. Beyond this role, AMPK regulates triglyceride hydrolysis by phosphorylating hormone-sensitive lipase and suppresses triglyceride esterification by inhibiting mitochondrial glycerol-3-phosphate acyltransferase [14]. This combined action leads to an increased release of free fatty acids into circulation from adipose tissue and enhances fatty acid oxidation in muscle.
In protein metabolism, AMPK inhibits protein biosynthesis by targeting key components of the translation initiation pathway (e.g., tuberous sclerosis complex 2 [TSC2], raptor, transcription initiation factor 1A.66, and eukaryotic elongation factor [eEF] 2 kinase) [15]. It can also induce a shift from cap-dependent translation to cap-independent translation, a more energy-efficient mechanism [16]. Furthermore, AMPK activation strongly suppressed the activity of the master regulator of growth, mammalian target of rapamycin complex 1 (mTORC1). Inhibition of mTORC1 leads to the activation of unc-51-like autophagy-activating kinase 1 (ULK1), a kinase essential for autophagy induction [17].
Overall, AMPK-mediated metabolic shifts not only restore homeostasis but also suppress oncogenic pathways and promote tumor cell death under energetic stress [18,19].
Structurally, AMPK is a heterotrimeric complex comprising α (catalytic), β (scaffold and carbohydrate-binding module), and γ (nucleotide-sensing) subunits [20,21]. The liver kinase B1 (LKB1)–AMPK axis is a primary signaling route for AMPK activation, with LKB1 directly phosphorylating AMPK at Thr172, a modification essential for its full activity [22] (Fig. 1). Once activated, AMPK can inhibit tumor-promoting pathways, notably mTORC1, thereby reducing cell proliferation and biosynthetic activity. In addition, AMPK suppresses cyclooxygenase-2 (COX-2), a pro-inflammatory enzyme associated with tumorigenesis, and promotes the phosphorylation of the tumor suppressor p53, leading to cell cycle arrest (Fig. 2). Loss of LKB1 is frequently observed in several cancer types and is associated with AMPK inactivation, metabolic dysregulation, and enhanced tumor progression [23]. Notably, tumors with LKB1 loss are more vulnerable to AMPK-inducing agents, while LKB1-wildtype tumors may resist such stress, suggesting a context-dependent therapeutic window [24,25]. Thus, restoring or enhancing AMPK activity via the LKB1 pathway represents a rational therapeutic goal in metabolically dysregulated tumors.
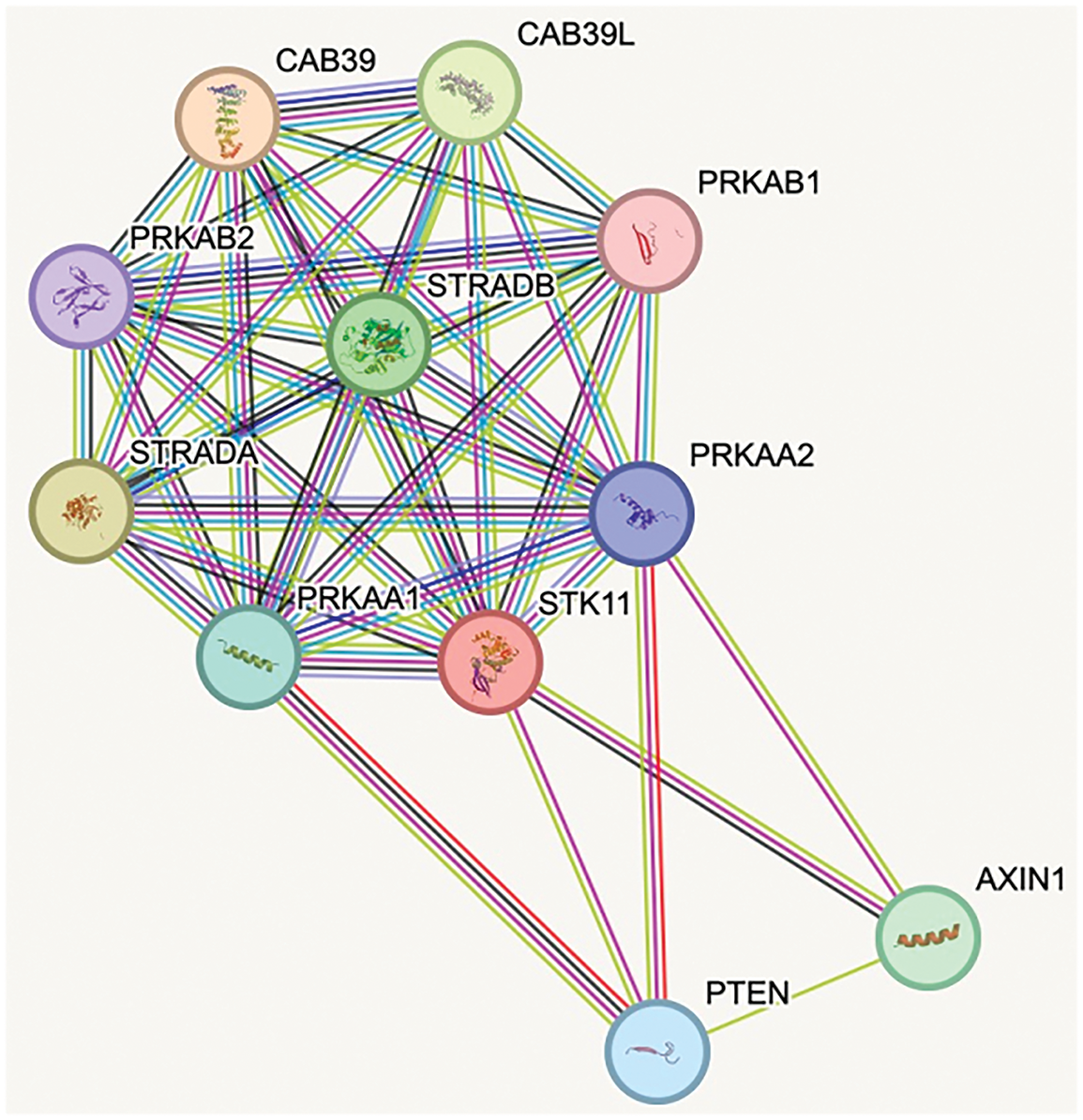
Figure 1: STRING analysis [26]: Protein-protein interaction network highlighting the associations between STK11 (LKB1) and AMPK subunits. The network illustrates direct interactions between STK11 and the AMPK α-subunits PRKAA1 and PRKAA2, as well as the β-subunit PRKAB1. Each protein is represented as a node, and the connecting lines indicate known or predicted interactions. CAB39, calcium-binding protein 39; CAB39L, calcium-binding protein 39-like; PRKAB1, 5′-AMP-activated protein kinase subunit beta-1; PRKAB2, 5′-AMP-activated protein kinase subunit beta-2; PRKAA1, 5′-AMP-activated protein kinase catalytic subunit alpha-1; PRKAA2, 5′-AMP-activated protein kinase catalytic subunit alpha-2; PTEN, phosphatase and tensin homolog; STK11, serine/thereonine kinase 11; STRADA, STE20-related kinase adaptor alpha; STRADB, STE20-related kinase adaptor beta (Source: https://string-db.org)
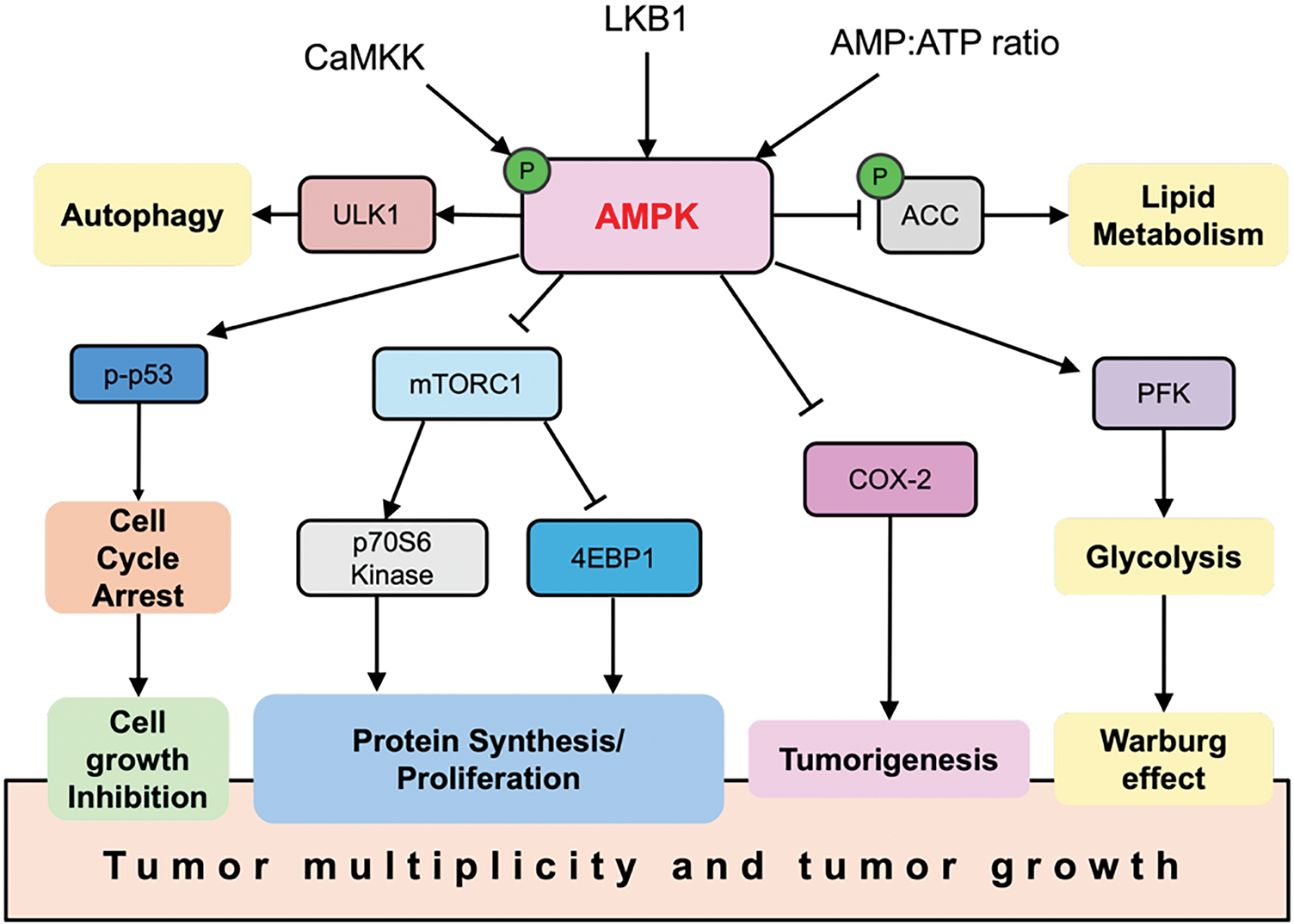
Figure 2: AMPK functions as a tumor suppressor. When activated, AMPK regulates multiple pathways that contribute to its anti-cancer effects. Arrows indicate activation of molecular targets, while bars represent inhibition. 4EBP1, Eukaryotic translation initiation factor 4E-binding protein 1; CaMKK, Calcium/calmodulin-dependent protein kinase kinase 2
Various pharmacological AMPK activators—such as metformin [27,28], phenformin [29], and canagliflozin [30,31]—have been studied in both preclinical and clinical settings. These agents induce AMPK activation indirectly through mitochondrial inhibition or glucose modulation and have shown potential in select cancer subtypes. However, their clinical efficacy is limited by factors such as low tumor-specific bioavailability, systemic side effects, and variability in AMPK-dependence among tumor types [3,32]. These challenges highlight the need for more precise and sustained activation methods that selectively target cancer metabolism without affecting normal tissues. Enhancing AMPK signaling within tumor cells will help reprogram aberrant metabolism, inhibit tumor growth, and augment existing therapeutic regimens.
This review will examine the central role of AMPK in tumor metabolism, critically assess the clinical landscape of AMPK-targeted therapies.
2 AMPK Activation as a Therapeutic Strategy in Cancer
2.1 Molecular Mechanisms Underlying the Antitumor Effects of AMPK
AMPK is a master regulator of cellular energy homeostasis, and its activation in tumor cells acts as a countermeasure against the metabolic reprogramming characteristic of cancer, commonly referred to as the Warburg effect [33]. Upon activation, AMPK conserves energy by shutting down energy-consuming anabolic processes. Specifically, it phosphorylates and inactivates ACC1 and ACC2, thereby halting fatty acid synthesis, and also targets 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase to suppress the cholesterol/mevalonate biosynthetic pathway [34]. These changes deprive tumor cells of critical lipids and building blocks required for rapid proliferation. Additionally, AMPK inhibits the growth-promoting kinase mTORC1 through phosphorylation of TSC2 or raptor, leading to reduced protein synthesis and suppressed glycolytic flux. Collectively, these actions induce a metabolic shift away from the high-glucose, high-biosynthesis state of tumor cells toward a more quiescent phenotype effectively reversing the Warburg effect [35]. This energy stress, combined with mTORC1 inhibition, ultimately slows tumor cell growth and may even trigger an energy crisis that compromises cancer cell viability.
In addition to its role in tumor metabolism, AMPK also exerts antitumor effects by modulating immune cell function, particularly in T cells [36]. T cell-mediated responses are central to effective tumor immunosurveillance [37,38]; however, in the nutrient-depleted tumor microenvironment (TME), T cells face intense metabolic competition with tumor cells, especially for glucose. Glucose limitation impairs T cell activation and induces expression of inhibitory receptors such as programmed cell death protein 1 (PD-1), driving metabolic exhaustion and a shift from glycolysis to fatty acid oxidation [36]. Furthermore, immunosuppressive cytokines like interleukin (IL)-10 can suppress glycolytic metabolism in T cells through AMPK activation. While such activation may, in some cases, attenuate effector T cell responses, accumulating evidence suggests a supportive role for AMPK in sustaining T cell function under metabolic stress. For example, mice lacking AMPKα1 in T cells exhibit accelerated tumor growth [39], whereas treatment with metformin [31,40]; an indirect activator of AMPK has been shown to enhance CD8+ T cell infiltration and promote tumor rejection, particularly when used in combination with PD-1 checkpoint blockade. These findings highlight AMPK’s potential in maintaining T cell metabolic fitness and bolstering antitumor immunity within the hostile conditions of the TME [36].
AMPK also exerts its regulatory effects on ion channels and transporters in cancer cells [41]. It can directly phosphorylate various transport proteins, thereby altering their function, activity and cellular localization. For instance, AMPK-mediated direct phosphorylation inhibits the activity of cystic fibrosis transmembrane conductance regulators (CFTR), a chloride channel; reduces the activity of the epithelial sodium channel (ENaC); and slows the inactivation of the voltage-gated sodium channel Nav1.5 [41]. In addition, AMPK downregulates several potassium channels through direct phosphorylation, including Ca2+-activated potassium channels (e.g., KCa3.1), inwardly rectifying potassium channels (e.g., Kir1.1, Kir2.1), and voltage-gated potassium channels (e.g., Kv1.5, Kv2.1, Kv7.1, Kv11) [42]. Beyond direct phosphorylation, AMPK also modulates transport protein indirectly through several mechanisms: stimulation of ubiquitin ligase, inhibition of Rab GTPase activating protein (GAP) tre-2/BUB2/cdc 1 domain family (TBC1D1), stimulation of phosphatidylinositol 3-phosphate 5-kinase (PIKfyve), inhibition of Phosphatase and tensin homolog (PTEN) via glycogen synthase kinase 3β (GSK3β), F-actin modulation, and downregulation of transcription factor NF-κB [42]. Through these pathways, AMPK’s regulation of ion channels and transporters has complex, context-dependent effects on cancer cell behaviors—including proliferation, apoptosis, migration, and multidrug resistance—by modulating metabolic pathways, autophagy, and ABC transporter expression.
One of the most clinically significant aspects of AMPK activation is its ability to enhance tumor sensitivity to conventional therapies such as chemotherapy and radiation. By suppressing DNA repair mechanisms and pro-survival signaling, AMPK activation lowers the threshold for therapy-induced cancer cell death. As a result, co-treatment with AMPK activators and traditional anti-cancer agents has demonstrated synergistic effects in various preclinical models [34]. For instance, 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR)-induced AMPK activation sensitizes colorectal cancer cells to 5-fluorouracil (5-FU), leading to greater apoptotic responses than 5-FU alone [43]. In pancreatic cancer, metformin enhances the efficacy of gemcitabine, likely by altering tumor metabolism and affecting the tumor stroma [44]. Similarly, AMPK activators such as phenformin and AICAR have been reported to increase tumor radiosensitivity by disrupting cellular energy supply and impairing DNA repair capacity [34]. In essence, AMPK activation generates a metabolically unfavorable environment for tumor survival under therapeutic stress: it suppresses growth, promotes apoptosis, and diminishes the tumor’s ability to recover post-treatment. These insights have led to growing interest in repurposing AMPK activators like metformin as adjuvant therapies to improve treatment outcomes in cancers such as colorectal, breast, and lung cancer.
In cancer therapy, the activation of AMPK plays a crucial role in disrupting tumor proliferation and metabolic plasticity [45]. Various agents, including metformin, phenformin, salicylate, berberine, resveratrol, and quercetin have been explored for their ability to activate AMPK and exert anti-cancer effects (Table 1).
2.2 AMPK Activators Proposed in Cancer Therapy
Interestingly, a variety of anti-diabetic drugs are being tested for their potential use in cancer therapy beyond their primary use. Biguanides, particularly metformin and phenformin, are being explored in cancer therapy for their potential to inhibit tumor growth by altering cellular metabolism and activating AMPK. Metformin indirectly activates AMPKα1/α2 by inhibiting mitochondrial Complex I [27]. This activation leads to the suppression of protein prenylation and an increase in reactive oxygen species (ROS), culminating in the downregulation of p70 S6 kinase (p70S6K) and eIF4E-binding protein 1 (4EBP1), critical downstream effectors of the mTOR pathway [46]. In acute myeloid leukemia (AML), metformin induces G0/G1 arrest mediated by p21/p27 and reduces levels of cyclin-dependent kinase (CDK) 4 and cyclin D1 [47–49]. In breast cancer, particularly human epidermal growth factor receptor (HER2)-positive and triple-negative breast cancer (TNBC) subtypes, both metformin and phenformin are found to suppress tumor growth and metastasis by downregulating c-MYC and reducing invasiveness through the suppression of hyaluronan synthase 2 (HAS2) [50–52]. Furthermore, phenformin enhances apoptosis in bladder cancer [53,54] and increases vulnerability in glioblastoma [55] and hepatocellular carcinoma [56,57] via AMPK-mediated nutrient deprivation. Phenformin also enhances non-small cell lung cancer (NSCLC) radiosensitivity by impairing DNA repair mechanisms [58]. However, phenformin was removed from most markets in the 1970s due to a high risk of fatal lactic acidosis [59].
2.2.2 Non-Biguanide Anti-Diabetic Drugs
Non-biguande anti-diabetic drugs, such as canagliflozin and pioglitazone, have also been tested in cancer therapy. Canagliflozin, an oral antidiabetic drug that inhibits sodium-glucose co-transporter 2 (SGLT2), inhibits mitochondrial Complex I, thereby inducing oxidative stress and promoting cancer cell death. This mechanism enhances the efficacy of radiotherapy, positioning canagliflozin as a potential adjunct in cancer treatment strategies [30]. Pioglitazone, a peroxisome proliferator-activated receptor gamma (PPARγ) agonist, exhibits anti-proliferative and pro-differentiation effects in several cancer models. It is also currently under investigation for its interaction with AMPK and therapeutic synergy with metformin. This combination has been shown to suppress desmoplasia and stromal support by inhibiting pancreatic stellate cell activation and downregulating fibrogenic cytokines [27,60].
2.2.3 Synthetic mTORC1 Inhibitors
Several anti-cancer and immunomodulatory agents were developed targeting mTORC1. The combination of everolimus and octreotide long-acting release (LAR) has demonstrated clinical benefits in neuroendocrine tumors (NETs) through effective inhibition of mTORC1 signaling [61]. Everolimus directly targets the phosphoinositide 3-kinase (PI3K)/AKT/mTOR pathway to suppress tumor growth and proliferation. Octreotide is a synthetic analog of somatostatin, a hormone that inhibits the release of several other hormones and is used primarily for its ability to reduce the symptoms of hormone-secreting tumors [62]. Additional multi-cancer agents such as LY3023414, a PI3K/mTOR inhibitor; zotatifin, an eIF4A inhibitor affecting translation; and sirolimus, which inhibits mTORC1 downstream of AMPK activation, are being explored [63–65].
Natural compounds, artificially synthesized but originally found in nature, are widely considered for their potential roles in cancer prevention and treatment due to their diverse biological activities and relatively low toxicity [66]. Berberine, another plant-derived compound, reduces the viability of colon and lung cancer cells by inducing apoptosis and activating caspase-3 and the miR-21/programmed cell death protein 4 (PDCD4) axis associated with tumor progression [67–69]. Resveratrol, a natural compound found in the skin of grapes and associated with red wine, is an AMPK activator extensively studied for its cancer-preventive properties in clinical trials and preclinical models [70]. Quercetin, a flavonoid found in many fruits and vegetables, inhibits mTOR and reduces glycolysis through AMPK, inhibiting tumor proliferation and inducing apoptosis [71–73]. In colorectal cancer cells, quercetin increases p-AMPK levels and downregulates cyclin D1 and the anti-apoptotic Bcl-2 [74,75]. Isoquercetin, studied for melanoma, indirectly activates AMPKα, contributing to angiogenesis inhibition and apoptosis [76]. Furthermore, vitamin C has been shown to activate AMPK by disrupting glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and inducing oxidative stress, particularly in KRAS/BRAF-mutant tumors [77].
2.2.5 Non-Canonical Inhibitors of AMPK
Antifungal medication itraconazole has been found to indirectly activate AMPKα1 by inhibiting mTOR, beyond its antifungal properties, and is in Phase II trials for NSCLC [78]. In liver cancer, simvastatin, a cholesterol-lowering agent, contributes to its role in lipid regulation by modulating the AMPK-mTOR axis [61]. Additionally, NSCLC treatments involve erlotinib, which targets the epidermal growth factor receptor (EGFR) pathway and intersects with AMPK-regulated metabolism [79]. Together, these agents highlight the diversity of strategies leveraging AMPK pathways in cancer therapy, from metabolic reprogramming to immunomodulation and cytotoxic synergy.
3 Current Clinical Development of AMPK Activation in Anti-Cancer Therapy
AMPK activators have shown promise in preventing tumor growth and migration. Many of these activators have been approved to treat cancers [80]. Moreover, several AMPK activators are currently undergoing preclinical or clinical trials as potential anti-cancer drugs. Existing drugs that activate AMPK are also being repurposed for cancer treatment, highlighting ongoing research and development in clinical trials (Table 1).
3.1 Hepatobiliary and Digestive System
Recent trials in CRC have explored whether targeting metabolism can improve patient outcomes [109]. One notable approach involves exercise: the Phase 2 EDICT trial investigated whether an exercise program in CRC patients could trigger AMPK-related metabolic changes that might slow tumor growth [110]. While results are still pending, this reflects growing interest in non-pharmacologic strategies to activate AMPK [110].
Another promising avenue is the use of aspirin, which indirectly activates AMPK by inhibiting prostaglandin synthesis. Long-term follow-ups of the CAPP trials and findings from the recent Phase 3 ALASCCA trial provide evidence that aspirin can reduce CRC recurrence in certain molecularly defined patient groups. For instance, in the ALASCCA trial, adjuvant aspirin therapy in patients with stage III CRC and PIK3CA-mutated tumors halved the 3-year recurrence risk compared to placebo [111]. This suggests that targeting the PI3K–AMPK–mTOR axis with a repurposed drug like aspirin offers a meaningful benefit in a subset of CRC patients.
Metformin has mostly been evaluated in observational settings, with some reports indicating that CRC patients with diabetes on metformin have better outcomes. However, dedicated trials are limited. One small Phase 1 study combined metformin with high-dose vitamin C, which induces ROS, in KRAS-mutant colorectal and pancreatic cancers. This was based on preclinical evidence that vitamin C can selectively kill KRAS-mutant cells by inducing metabolic stress [88]. Early results showed some tumor metabolic responses, but no definitive efficacy yet.
Overall, in CRC, the most impactful metabolic intervention to date is aspirin in the adjuvant setting for genetically selected patients, while trials of metformin remain exploratory. Current research is increasingly focused on integrating metabolic therapies with molecular profiling, such as applying AMPK activators or metabolic diets in patients with obesity, insulin resistance, or specific mutations that confer metabolic vulnerabilities.
Hepatocellular carcinoma (HCC) often develops in the context of diabetes and nonalcoholic fatty liver disease (NAFLD), positioning metformin as an attractive candidate for both chemoprevention and therapy. Epidemiological studies have indicated that metformin use is associated with a significantly lower risk of developing HCC in diabetic patients [112]. However, clinical trials directly targeting AMPK in HCC have been limited.
A planned Phase 1/2 trial of resveratrol—a nutraceutical that activates AMPK and sirtuins—aimed to assess its effects on liver tumor cell growth and metabolism [86,95] but was withdrawn before enrollment, likely due to feasibility challenges [95]. Consequently, no clinical data on resveratrol in liver cancer were collected [95]. Another small pilot study combined metformin with transarterial chemoembolization (TACE) in HCC, but the results were inconclusive regarding tumor response, and concerns about lactic acidosis limited metformin use in cirrhotic patients [86].
Due to a lack of interventional trial data in liver cancer, current insights come mostly from retrospective analyses. These suggest metformin might improve survival in HCC patients, particularly those receiving immune checkpoint inhibitors, by modifying TME [113]. There is also evidence that metformin, when used after curative HCC treatment like surgery or ablation, may reduce recurrence rates. Nevertheless, HCC is a complex disease where liver function and hepatitis status can confound outcomes, making it challenging to isolate the effect of AMPK activation [114–116].
In summary, while liver cancer is biologically amenable to AMPK-targeting strategies, their clinical application remains largely theoretical. Ongoing preventative trials in high-risk patients, such as those with nonalcoholic steatohepatitis (NASH) with fibrosis, will determine if metformin can lower HCC incidence. For active HCC therapy, future studies might explore combining metformin or aspirin with newer systemic treatments like anti-PD-1 immunotherapy or tyrosine kinase inhibitors to assess potential additive benefits.
Despite strong epidemiological links, prospective trials of AMPK activators in pancreatic ductal adenocarcinoma (PDAC) have largely yielded disappointing. A notable Phase 2 trial in advanced pancreatic cancer tested the addition of metformin to standard gemcitabine plus erlotinib therapy [102]. Unfortunately, metformin did not improve six-month survival or median overall survival (OS) [102]. Similarly, other trials in metastatic disease—including combinations of metformin with FOLFIRINOX chemotherapy—were negative, leading investigators to conclude that conventional-dose metformin offers no survival advantage in unselected pancreatic cancer patients [102].
A Phase 2 randomized clinical trial in the Netherlands also assessed the addition of metformin to standard chemoradiation in locally advanced pancreatic cancer. The study found no improvement in OS, resulting in early termination of the trial due to futility [117]. These results suggest that pancreatic tumors—often characterized by hypoxia and dense desmoplasia—may not respond to indirect metabolic interventions like metformin unless patients have metabolic profiles, such as hyperinsulinemia.
There is, however, ongoing interest in combining AMPK activators with targeted therapies for well-differentiated NETs. The Phase 2 MetNET-1 trial is currently evaluating the combination of everolimus, octreotide, and metformin in advanced pancreatic NETs [101], aiming to enhance mTOR pathway blockade via AMPK activation. While final results are pending, a prior single-arm study of metformin monotherapy in 28 patients with metastatic NET reported a six-month disease control rate of 46%, but no objective tumor responses and a median progression-free survival (PFS) of approximately 6.2 months, essentially equivalent to historical placebo rates [101]. This suggests metformin alone has minimal activity in NETs. Combination strategies may offer better tumor suppression, as hinted by retrospective data showing that diabetic NET patients receiving metformin and everolimus experienced longer PFS [101].
In summary, the consensus for pancreatic malignancies is that metformin alone is ineffective for the general patient population. Future trials are focusing on specific niches—pairing phenformin or metformin with immunotherapy or targeting patients with metabolic syndrome—in hopes of identifying subgroups that might derive meaningful clinical benefit.
Large later-phase trials have investigated the use of metformin in the adjuvant setting of breast cancer. The Phase 3 MA.32 trial, which included over 3600 patients, found that adding metformin to standard therapy did not improve invasive disease-free survival or OS in early breast cancer. This result was consistent for both hormone receptor–positive and TNBC subsets, leading researchers to conclude that metformin should not be used as adjuvant therapy in unselected breast cancer patients [118].
Short-term preoperative “window” studies have demonstrated some biological effects. For example, a UK Phase 2 trial of 2–3 weeks of neoadjuvant metformin observed changes in tumor metabolism and increased tumor fluorodeoxyglucose (FDG) uptake, though there was variability in metabolic responses between tumors [119]. Some neoadjuvant studies also reported modest reductions in the proliferation marker Ki-67 with metformin treatment, although these findings were not always consistent [120].
In HER2-positive metastatic breast cancer, metformin has been combined with therapies like lapatinib and endocrine treatment in Phase 2 trials, but without definitive efficacy signals; the results have been mixed or not significant [84]. In the case of TNBC, there is considerable interest in targeting metabolic vulnerabilities, although few trials of AMPK activators have been completed [84,85]. Other metabolic interventions have shown promise: notably, the addition of carboplatin to standard neoadjuvant chemotherapy significantly improved event-free and overall survival in TNBC. A Phase 3 trial, involving 720 patients, achieved higher pathologic response rates and established platinum-based chemotherapy as a standard treatment in TNBC [121]. While not directly targeting AMPK, this highlights the importance of targeting cancer metabolism/stress in TNBC [121].
Ongoing studies are exploring metformin in combination with standard neoadjuvant chemotherapy or immunotherapy in TNBC. However, as of now, no Phase 3 data have shown a clear benefit of metformin in this subtype [121]. In summary, despite a strong preclinical rationale, metformin’s clinical impact in breast cancer has been limited. Future approaches may require better biomarkers, such as tumor metabolic profiles or insulin levels, to identify patient subsets that are more likely to respond.
3.2.2 Endometrial and Ovarian Cancers
Endometrial carcinoma is closely associated with obesity and hyperinsulinemia, making AMPK activation an appealing therapeutic strategy. Small preoperative trials have demonstrated that metformin can modulate tumor biology in endometrial cancer [122]. In an early-phase window study, obese endometrial cancer patients took metformin for a short period before surgery. The tumors exhibited an average decrease of 11%–15% in the Ki-67 proliferation index and significant inhibition of the mTOR pathway signaling, indicated by reduced levels of p-AKT, p-S6, and p-4EBP1 [91]. Patients who responded metabolically to metformin showed serum changes, such as increased lipolysis, fatty acid oxidation, suggesting that metformin’s antitumor effect was linked to systemic metabolic improvements [91]. These findings support further investigation of metformin in endometrial cancer, particularly in metabolically abnormal patients. Although a Phase 2 trial was planned to administer metformin pre-surgery and measure changes in tumor pS6 and other AMPK/mTOR biomarkers, it was withdrawn before completion possibly due to slow accrual [91].
Ongoing Phase 2 studies are exploring metformin in combination with other treatments. The RESOLVE trial is testing a three-drug combination in recurrent endometrial and ovarian cancers: letrozole, abemaciclib (a CDK4/6 inhibitor), with or without the addition of metformin [92]. The rationale is that metformin’s insulin-lowering and AMPK-activating effects might enhance hormone therapy and CDK4/6 inhibition, especially in obese or insulin-resistant patients with endometrial cancer [92]. Another approach in ovarian cancer was evaluated in a Phase 2 study in advanced-stage disease, where neoadjuvant chemotherapy was combined with metformin [92]. The metformin-treated cohort showed promising results, indicating a higher than expected 18-month relapse-free survival (~59%) and a significant reduction in ALDH+CD133+ cancer stem cells in tumors. Although that was a single-arm translational study, it suggested that metformin may improve chemotherapy responses and alter TME [92]. Retrospective data for ovarian cancer have also hinted at improved survival in diabetic patients on metformin during chemotherapy.
Currently, no Phase 3 trials have definitively proven metformin’s benefit in gynecologic cancers, but the wealth of Phase 2 data suggests it is well-tolerated and potentially beneficial when combined with other therapies. Recent preclinical studies have highlighted the potential of metformin to enhance antitumor immunity and overcome immune evasion in ovarian cancer by modulating the tumor microenvironment and augmenting the efficacy of programmed death-ligand 1 (PD-L1) blockade [123]. Future larger trials may focus on obese diabetic patients or use metformin as a maintenance therapy to delay recurrence.
Prostate cancer has been the focus of several Phase 2 trials investigating metformin, but the results have been largely negative. In a randomized pre-prostatectomy trial, patients received metformin for approximately one month before surgery to assess its effects on prostate tissue. While metformin was detectable at measurable levels within the prostate, it did not significantly change tumor cell proliferation, apoptosis, or mTOR pathway markers compared to placebo [106]. Similarly, serum insulin, insulin-like growth factor 1 (IGF-1), and other systemic biomarkers remained largely unchanged over this short treatment period, suggesting no immediate antitumor effects.
A larger Phase 3 trial evaluated metformin in patients on active surveillance for low-risk prostate cancer. The findings showed that metformin failed to slow cancer progression, with similar rates of biopsy progression to higher-grade disease or need for treatment in both the metformin vs. placebo groups. Notably, an exploratory subgroup analysis suggested that men with higher body mass index (BMI) on metformin had slightly worse progression outcomes than those on placebo, although the sample size was small. Additionally, metformin’s known side effects—mainly gastrointestinal upset—were common, with about 30% of men experiencing gastrointestinal discomfort or fatigue [106]. This trial, presented in 2024, provided strong (level 1) evidence that metformin does not benefit early prostate cancer, dampening enthusiasm for its off-label use in this setting.
Metformin has also been studied in advanced prostate cancer, particularly in combination with hormonal therapies. One randomized Phase 2 trial examined adding metformin to androgen deprivation therapy in hormone-sensitive prostate cancer, but results were inconclusive. In castration-resistant prostate cancer, a Swiss Phase 2 pilot trial (MetAb-Pro) tested metformin in combination with ongoing abiraterone therapy for men who had prostate-specific antigen (PSA) progression on abiraterone [104]. The primary endpoint—disease control at 12 weeks—was achieved in only 12% of patients, falling below the predefined threshold for efficacy [104]. Nearly all patients continued to experience PSA progression despite metformin, indicating no meaningful clinical benefit. Although toxicity was generally mild, one patient had to stop metformin due to diarrhea.
These findings align with larger observational analyses suggesting that while metformin (and sometimes concurrent statins) may appear to improve outcomes in men on abiraterone or enzalutamide, prospective confirmation is lacking [103]. Overall, the available evidence indicates that although metformin is safe to combine with prostate cancer treatments, it does not meaningfully enhance their efficacy in most patients. As a result, ongoing trials have become scarce, and research attention has shifted to other metabolic targets, such as targeting obesity-related inflammation, and incorporating exercise and diet interventions for prostate cancer management.
Bladder cancer is another malignancy associated with metabolic syndrome, attracting interest in repurposing metabolic drugs for its treatment. A Phase 2 trial in non-muscle-invasive bladder cancer (NMIBC) is currently evaluating the combination of metformin and simvastatin during intravesical therapy [83]. The aim is to determine whether dual AMPK activation by metformin and inhibition of cholesterol pathway by statins can reduce tumor cell proliferation and recurrence. The primary outcome being measured is the tumor proliferation rate during therapy [83]. As of now, no efficacy results have been reported.
There is some indirect clinical evidence supporting this approach: retrospective studies have shown that diabetic bladder cancer patients on metformin have lower recurrence rates, and statin use has been associated with improved outcomes in bladder cancer. This makes the combination of metformin and simvastatin particularly intriguing [83]. However, it is worth noting that a previous Phase 2 trial involving metformin for muscle-invasive bladder cancer was closed early due to a lack of benefit [83]. Therefore, while AMPK activators are being tested in bladder cancer, their role remains unproven. The ongoing trial will provide insights into whether metabolic manipulation can enhance standard bladder cancer treatments or prevent disease progression.
In renal cell carcinoma (RCC), a Phase 1/2 trial has evaluated the addition of isoquercetin to first-line sunitinib for metastatic RCC [93]. Sunitinib, a vascular endothelial growth factor (VEGF)-targeted therapy, is the standard of care, and the hypothesis is that isoquercetin’s antioxidant and AMPK-activating properties could enhance antitumor activity using positron emission tomography (PET)-based metabolic response, response evaluation criteria in solid tumors (RECIST), and patient-reported quality of life outcomes [93]. Although results are not yet available, this study reflects a broader strategy of combining metabolic therapies with targeted agents in RCC.
While there have been no large prospective trials of metformin specifically in RCC, observational studies suggest that diabetic patients on metformin may experience prolonged survival when receiving immunotherapy or targeted therapies for kidney cancer [114]. Preclinical studies further support the rationale, showing that AMPK activation can inhibit renal carcinoma cell growth and mTOR signaling, particularly in tumors with metabolic dysregulation [124]. In clinical practice, some physicians have occasionally used metformin off-label for insulin-resistant RCC patients, though without strong supporting evidence [125,126]. The ongoing isoquercetin study and upcoming trials investigating other AMPK activators, such as phenformin, are expected to clarify whether metabolic modulation offers a meaningful benefit in RCC [93]. For now, standard treatments—including tyrosine kinase inhibitors (TKIs) and immunotherapies—remain the mainstay, with metabolic drugs considered experimental adjuncts.
3.4 Respiratory and Upper Aerodigestive System
Several Phase 2 trials have tested adding metformin to standard therapy. In one study of metastatic NSCLC, metformin combined with carboplatin/paclitaxel/bevacizumab showed a higher 1-year PFS (47% vs. 15% historical control) and a slight OS improvement, suggesting a potential benefit. However, a larger randomized trial in stage III NSCLC found no improvement in 1- or 2-year PFS or OS from adding metformin to chemoradiation. Metformin was well-tolerated in that study but did not enhance outcomes [96,108].
Ongoing Phase 3 research is focusing on specific subsets. For example, the METLUNG trial is evaluating EGFR-mutant NSCLC patients on EGFR tyrosine kinase inhibitors ± metformin (312 patients, Stage IIIB-IV) to see if metformin prolongs PFS [96,99]. Another approach combined metformin with a carbohydrate-restricted diet alongside platinum chemotherapy in advanced NSCLC; this Phase 2 trial was terminated early, underscoring the challenges of dietary interventions [96,127]. Overall, in lung cancer, the clinical efficacy of AMPK activation via metformin remains uncertain; promising signals in small studies have been tempered by larger trials showing no clear benefit, indicating a need for better patient selection or combination strategies.
3.4.2 Head and Neck Squamous Cell Carcinoma (HNSCC)
In head and neck squamous cell carcinoma (HNSCC), metformin has been tested in the preoperative setting. A Phase 2 “window of opportunity” trial tested a combination of metformin and pioglitazone (ACTO plus Met XR) in patients with oral or oropharyngeal squamous cell carcinoma [100]. Patients took the drug combination before surgery to assess biologic effects on the tumor, with the primary endpoint being changes in the tumor Ki-67 proliferation index [100]. Although the trial was terminated early, likely due to poor accrual, it demonstrated the feasibility of short-term metabolic therapy in HNSCC. Additionally, retrospective studies have reported that diabetic head and neck cancers who were metformin during chemoradiation experienced improved locoregional control and survival, suggesting a potential radio-sensitization effect worth further investigating [100]. There is also growing interest in using metformin for oral premalignant lesions, such as dysplasia to prevent progression—based on its capacity to activate AMPK and potentially trigger autophagy in damaged mucosal cells [128]. While still experimental, this represents a promising direction for future cancer prevention strategies.
3.5 Integumentary System (Skin)
Metabolic therapy in melanoma has focused more on aspirin rather than metformin [97]. A Phase 2 trial conducted in Utah enrolled individuals at elevated risk for melanoma to receive either low-dose or high-dose aspirin [97]. This completed study primarily assessed aspirin’s effects on systemic metabolism and tissue biomarkers, including AMPK activation in nevi and immune cells [97].
While there have been some case reports and small studies examining metformin use in melanoma, particularly among diabetic patients, no major clinical trials have been conducted [98]. Interestingly, melanoma cells often exhibit high basal AMPK activity, raising questions about whether further AMPK activation would provide therapeutic benefits. Instead, current research interest has shifted toward dietary interventions, such as ketogenic diets or calorie restriction, which may modulate melanoma metabolism and potentially enhance the effects of checkpoint blockade therapies. These strategies remain experimental [129,130].
The key clinical takeaway is that commonly used drugs like aspirin may offer chemo-preventive benefits for high-risk melanoma patients, with a favorable safety profile [98]. If the ongoing trial results demonstrate that aspirin beneficially alters skin or blood biomarkers—for example, by reducing inflammatory mediators or increasing tissue AMPK activation—this could pave the way for larger studies investigating whether aspirin can reduce melanoma incidence or improve patient outcomes.
Early clinical evidence suggests that metformin can improve outcomes in high-risk groups of ALL. A randomized trial in pediatric ALL reported that adding metformin to chemotherapy reduced the relapse risk by approximately 56%. Another Phase 2 study focused on ALL patients with high ATP-binding cassette subfamily B member 1 (ABCB1) expression [81,82]. In this subgroup, the combination of metformin and standard chemotherapy improved survival rates and lowered the incidence of treatment failure and early recurrence, with odds ratios between 0.05 and 0.07 favoring metformin [89]. These findings suggest that metformin may enhance the sensitivity of leukemia cells to chemotherapy, possibly by affecting AMPK and drug transporter pathways.
A pilot Phase 2 trial is currently underway, examining metformin as a therapy to delay disease progression in patients with relapsed or untreated chronic lymphocytic leukemia (CLL), a type of indolent adult leukemia. While results are not yet published, preclinical data support the rationale that metformin targets leukemia cell metabolism and self-renewal [87]. In the context of ALL, the clinical implication is that metformin could serve as a low-toxicity adjunct to enhance chemotherapy efficacy, particularly in patients with chemo-resistant profiles [87]. Future larger trials will be needed to confirm the survival benefits and identify which ALL patients benefit most from AMPK activation therapy.
Among all cancer types, diffuse large B-cell lymphoma (DLBCL) has provided one of the strongest indications for metformin’s efficacy. In a randomized Phase 2 trial, metformin was added to standard rituximab-cyclophosphamide/hydroxydaunorubicin/oncovin/prednisone (R-CHOP) immunochemotherapy in previously untreated DLBCL patients. The metformin group achieved a remission rate of 92% compared to 74% in the control group receiving R-CHOP alone. Two-year relapse rates were significantly lower with metformin and the 2-year OS was higher [89]. The only notable side effect was a slightly higher incidence of nausea in metformin-treated patients [89]. Importantly, multivariate analysis indicated that metformin use was an independent predictor of better response and lower relapse risk. This compelling evidence suggests that AMPK activation and metabolic interference can sensitize aggressive lymphoma to chemotherapy. It is hypothesized that metformin may inhibit lymphoma cell oxidative metabolism or mTOR signaling, as DLBCL cells often have high PI3K/mTOR activity, enhancing chemo-induced apoptosis. Based on these results, larger Phase 3 trials are being considered to confirm the benefit and potentially establish metformin as part of front-line DLBCL therapy [89].
In CLL, a more indolent lymphoma, metabolic interventions are being explored to prolong remission and overcome drug resistance. A Phase 2 pilot trial at the University of Michigan investigated metformin in relapsed/refractory CLL patients who are not yet on definitive therapy [87]. The rationale was that metformin might delay the need for cytotoxic treatment by suppressing CLL cell proliferation [87]. Clinically, a Spanish trial in CLL observed that diabetic patients on metformin had longer treatment-free survival. Metformin could serve as a low-cost maintenance therapy in CLL, keeping the disease in check by activating AMPK and forcing the leukemic cells out of their quiescent, protective metabolic state [87]. If successful, this could inaugurate a new strategy for managing early-stage CLL or enhancing the effect of emerging treatments like BTK inhibitors by tackling the leukemia’s metabolic adaptability.
3.7 AMPK-Targeted Novel Therapeutic Approaches
Across cancer types, an emerging theme is the combination of AMPK activators with immunotherapies. Metformin has been shown to modulate the tumor immune microenvironment by lowering immunosuppressive factors like IL-6 and VEGF and reprogramming T-cell metabolism toward memory/effector phenotypes [131]. Early clinical data in lung and liver cancers suggest that diabetic patients on metformin may respond better to PD-1 checkpoint inhibitors [132]. Building on these observations, new trials are being designed to formally test whether adding metformin can improve immunotherapy outcomes; for example, a study combining metformin with anti-PD1 in melanoma is under consideration. Moreover, other AMPK-activating agents, such as phenformin, are being evaluated in Phase 1 trials alongside immunotherapy, particularly in melanoma and NSCLC [133]. This strategy aims to leverage metabolic stress to make cancer cells more immunogenic or to relieve tumor-induced immunosuppression, offering an exciting avenue to potentially amplify the effectiveness of existing treatments.
Beyond pharmacologic agents, dietary interventions that activate AMPK are also being explored in oncology. For instance, a pilot trial in breast cancer examined short-term fasting around the time of chemotherapy to assess whether it could enhance treatment response [134]. Early small-scale studies have reported that fasting is safe and may reduce chemotherapy side effects, though larger trials are needed to determine its impact on tumor outcomes [135]. In glioblastoma and other difficult cancers, ketogenic diets have been proposed to stress tumor metabolism and activate AMPK in normal tissues, potentially slowing tumor growth [136,137]. While preclinical data are intriguing, these diets are difficult for patients to maintain and have yet to show clear clinical benefit, remaining a niche but noteworthy approach within the broader landscape of AMPK-focused cancer therapy.
4 Current Challenges and Therapeutic Limitations of AMPK-Targeted Strategies
A major challenge in leveraging AMPK for cancer therapy lies in its context-dependent, dualistic role-it can both suppress tumors and, in some cases, support their survival under stress. On one hand, AMPK activation inhibits cell growth and proliferation, functioning as a tumor suppressor [35]. The AMPK pathway is often downregulated or disrupted through loss of LKB1 in many cancers. On the other hand, tumor cells experiencing metabolic stress frequently rely on a baseline AMPK activity to adapt and survive. In such contexts, AMPK acts as a survival mechanism for cancer cells [24] (Fig. 2).
For example, in a KRAS-driven lung cancer model, treatment with phenformin significantly prolonged survival in mice-but only when the tumors lacked LKB1. Tumors with intact LKB1-AMPK signaling were more resistant to the metabolic stress induced by phenformin [35]. This suggests that cancer cells with a functional AMPK pathway may use it to endure hostile conditions like hypoxia or chemotherapy. These findings complicate AMPK-targeting therapies: while AMPK activation can initially inhibit tumor growth, in some cases, inhibiting AMPK may help eliminate highly stressed cancer cells. Therefore, AMPK activation is not always the clear therapeutic goal.
Current AMPK-targeting treatments face limitations in selectivity, bioavailability, and toxicity. Metformin, although widely used and safe for diabetes, is a relatively weak AMPK activator [138]. It requires high concentrations to significantly stimulate AMPK and works indirectly by inhibiting mitochondrial Complex I [139]. Moreover, tumor uptake of metformin is variable, and in non-diabetic cancer patients, its dosing is constrained by gastrointestinal side effects and the risk of lactic acidosis [140]. Phenformin, a more potent analog, activates AMPK more effectively through mitochondrial inhibition. However, it was withdrawn from clinical use in the 1970s due to cases of life-threatening lactic acidosis. This risk makes systemic phenformin use particularly dangerous, especially in patients with comorbidities [141].
Even metformin, while safer, can rarely cause lactic acidosis, especially with long-term use. Other AMPK activators also come with off-target effects. For instance, salicylate activates AMPK but also inhibits COX-1 and COX-2, which can lead to gastrointestinal bleeding at chemo-preventive doses [142]. The SGLT2 inhibitor canagliflozin activates AMPK indirectly by inducing glucose starvation, but its use may cause systemic effects such as ketoacidosis or weight loss [143]. A common issue with many of these compounds is a lack of specificity-they often activate AMPK as part of a broader metabolic disturbance rather than directly targeting the pathway.
This lack of selectivity can harm normal tissues: widespread AMPK activation may impair normal cell growth or lead to side effects such as fatigue and hypoglycemia [144]. Therefore, there is a critical need for more selective AMPK activators that specifically target tumor cells or the AMPK pathway without causing widespread metabolic disruption. Although some new allosteric AMPK activators and prodrugs are under investigation, challenges related to their bioavailability and efficacy have so far limited their clinical translation (Table 1).
AMPK plays a central role in maintaining cellular energy homeostasis, acting as a metabolic checkpoint that suppresses tumor growth by inhibiting anabolic pathways and promoting catabolic processes. Since cancer cells often depend on dysregulated metabolism to support rapid proliferation, AMPK activation has emerged as a promising strategy to counteract tumor progression. Both clinical and preclinical studies using activators such as metformin, phenformin, and canagliflozin have shown encouraging results in specific cancer types, including HER2-positive breast cancer, colorectal cancer, and certain metabolic subtypes of lung cancer.
However, their clinical utility of these agents remains limited by several factors. Many AMPK activators act indirectly and require high concentrations to achieve therapeutic effects, raising concerns about off-target actions and systemic toxicity. Additionally, tumor heterogeneity, differences in metabolic dependency, and the absence of robust biomarkers complicate patient stratification and treatment outcomes. While some cancers appear responsive to AMPK modulation, others may resist or adapt to energy stress, highlighting the need for better mechanistic insights.
To overcome these barriers, future research should prioritize combination therapies, precision targeting based on tumor metabolic profiles, and the development of more direct and selective AMPK modulators. mRNA-based approaches represent an innovative avenue for achieving controlled and tumor-specific AMPK activation, although challenges related to delivery and immunogenicity remain to be addressed. Ultimately, refining AMPK-targeted strategies may provide a powerful tool in metabolic cancer therapy, particularly when integrated into personalized treatment frameworks.
Acknowledgement: Not applicable.
Funding Statement: This research was supported by the National Research Foundation of Korea (NRF) through the Ministry of Education (2021R1I1A3059820) (to Jea-Hyun Baek).
Author Contribution: Conceptualization, Minseo Hong, Jea-Hyun Baek; writing—original draft preparation, Minseo Hong; writing—review and editing, Jea-Hyun Baek; supervision, Jea-Hyun Baek. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Ethics Approval: Not applicable.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Abbreviations
| 4EBP1 | Eukaryotic translation initiation factor 4E-binding protein 1 |
| 5-FU | 5-Fluorouracil |
| ABCB1 | ATP-binding cassette sub-family B member 1 |
| ACC | Acetyl-CoA carboxylase |
| AICAR | 5-Aminoimidazole-4-carboxamide ribonucleoside |
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myeloid leukemia |
| AMPK | AMP-activated protein kinase |
| BMI | Body mass index |
| CAB39 | Calcium-binding protein 39 |
| CAB39L | Calcium-binding protein 39-like |
| CaMKK | Calcium/calmodulin-dependent protein kinase kinase 2 |
| CLL | Chronic lymphocytic leukemia |
| COX-2 | Cyclooxygenase-2 |
| CPT1 | Carnitine palmitoyltransferase 1 |
| CRC | Colorectal cancer |
| DLBCL | Diffuse large B cell lymphoma |
| EGFR | Epidermal growth factor receptor |
| F-2,6-BP | Fructose-2,6,-bisphosphate |
| FDG | Fluorodeoxyglucose |
| GLUT4 | Glucose transporter 4 |
| HAS2 | Hyaluronan synthase 2 |
| HCC | Hepatocellular carcinoma |
| HMG-CoA | 3-hydroxy-3-methylglutaryl coenzyme A |
| HNSCC | Head and neck squamous cell carcinoma |
| LAR | Long-acting release |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| NET | neuroendocrine tumor |
| NMIBC | Non-muscle-invasive bladder cancer |
| NSCLC | Non-small cell lung cancer |
| OS | Overall survival |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDCD4 | Programmed cell death protein 4 |
| PET | Positron emission tomography |
| PFS | Progression-free survival |
| PPARγ | Peroxisome proliferator-activator gamma |
| PRKAB1 | 5′-AMP-activated protein kinase subunit beta-1 |
| PRKAB2 | 5′-AMP-activated protein kinase subunit beta-2 |
| PRKAA1 | 5′-AMP-activated protein kinase catalytic subunit alpha-1 |
| PRKAA2 | 5′-AMP-activated protein kinase catalytic subunit alpha-2 |
| PTEN | Phosphatase and tensin homolog |
| RCC | Renal cell carcinoma |
| RECIST | Response evaluation criteria in solid tumors |
| ROS | Reactive oxygen species |
| SGLT2 | Sodium-glucose co-transporter 2 |
| STK11 | Serine/Thereonine Kinase 11 |
| STRADA | STE20-related kinase adaptor alpha |
| STRADB | STE20-related kinase adaptor beta |
| TACE | Transarterial chemoembolization |
| TKI | Tyrosine kinase inhibitor |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
| ULK1 | Unc-51-like autophagy-activating kinase 1 |
| WHO | World Health Organization |
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 May;71(3):209–49. doi:10.3322/caac.21660. [Google Scholar] [PubMed] [CrossRef]
2. Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023 Apr 1;9(4):465–72. doi:10.1001/jamaoncol.2022.7826. [Google Scholar] [PubMed] [CrossRef]
3. Cai Z, Peng D, Lin HK. AMPK maintains TCA cycle through sequential phosphorylation of PDHA to promote tumor metastasis. Cell Stress. 2020 Nov 25;4(12):273–7. doi:10.15698/cst2020.12.238. [Google Scholar] [PubMed] [CrossRef]
4. Cheng Y, Ji Y, Tong J. Triple stimuli-responsive supramolecular nanoassembly with mitochondrial targetability for chemophotothermal therapy. J Control Release off J Control Release Soc. 2020 Nov 10;327:35–49. doi:10.1016/j.jconrel.2020.08.006. [Google Scholar] [PubMed] [CrossRef]
5. Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016 Jan 12;23(1):27–47. doi:10.1016/j.cmet.2015.12.006. [Google Scholar] [PubMed] [CrossRef]
6. Scully T, Ettela A, LeRoith D, Gallagher EJ. Obesity, type 2 diabetes, and cancer risk. Front Oncol. 2021 Feb 2;10:615375. doi:10.3389/fonc.2020.615375. [Google Scholar] [PubMed] [CrossRef]
7. Trefts E, Shaw RJ. AMPK: restoring metabolic homeostasis over space and time. Mol Cell. 2021 Sep 16;81(18):3677–90. doi:10.1016/j.molcel.2021.08.015. [Google Scholar] [PubMed] [CrossRef]
8. Muraleedharan R, Dasgupta B. AMPK in the brain: its roles in glucose and neural metabolism. FEBS J. 2022;289(8):2247–62. doi:10.1111/febs.16151. [Google Scholar] [PubMed] [CrossRef]
9. Wang M, Flaswinkel H, Joshi A, Napoli M, Masgrau-Alsina S, Kamper JM, et al. Phosphorylation of PFKL regulates metabolic reprogramming in macrophages following pattern recognition receptor activation. Nat Commun. 2024 Jul 31;15(1):6438. doi:10.1038/s41467-024-50104-7. [Google Scholar] [PubMed] [CrossRef]
10. Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2021 Dec 30;46(1):15–37. [Google Scholar]
11. Goel S, Singh R, Singh V, Singh H, Kumari P, Chopra H, et al. Metformin: activation of 5′ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front Genet. 2022 Oct 31;13:1022739. doi:10.3389/fgene.2022.1022739. [Google Scholar] [PubMed] [CrossRef]
12. Wang Y, Yu W, Li S, Guo D, He J, Wang Y. Acetyl-CoA carboxylases and diseases. Front Oncol. 2022 Mar 11;12:836058. doi:10.3389/fonc.2022.836058. [Google Scholar] [PubMed] [CrossRef]
13. Szrok-Jurga S, Czumaj A, Turyn J, Hebanowska A, Swierczynski J, Sledzinski T, et al. The physiological and pathological role of Acyl-CoA oxidation. Int J Mol Sci. 2023 Jan;24(19):14857. doi:10.3390/ijms241914857. [Google Scholar] [PubMed] [CrossRef]
14. Grabner GF, Xie H, Schweiger M, Zechner R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat Metab. 2021 Nov;3(11):1445–65. doi:10.1038/s42255-021-00493-6. [Google Scholar] [PubMed] [CrossRef]
15. Wu X, Xie W, Xie W, Wei W, Guo J. Beyond controlling cell size: functional analyses of S6K in tumorigenesis. Cell Death Dis. 2022 Jul 25;13(7):1–19. doi:10.1038/s41419-022-05081-4. [Google Scholar] [PubMed] [CrossRef]
16. Kim YS, Kimball SR, Piskounova E, Begley TJ, Hempel N. Stress response regulation of mRNA translation: implications for antioxidant enzyme expression in cancer. Proc Natl Acad Sci. 2024 Nov 12;121(46):e2317846121. doi:10.1073/pnas.2317846121. [Google Scholar] [PubMed] [CrossRef]
17. Panwar V, Singh A, Bhatt M, Tonk RK, Azizov S, Raza AS, et al. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct Target Ther. 2023 Oct 2;8(1):1–25. doi:10.1038/s41392-023-01608-z. [Google Scholar] [PubMed] [CrossRef]
18. Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018 Feb;19(2):121–35. doi:10.1038/nrm.2017.95. [Google Scholar] [PubMed] [CrossRef]
19. Suchacki KJ, Thomas BJ, Ikushima YM, Chen KC, Fyfe C, Tavares AA, et al. The effects of caloric restriction on adipose tissue and metabolic health are sex- and age-dependent. eLife. 2023 Apr 25;12:e88080. [Google Scholar]
20. Carling D. The AMP-activated protein kinase cascade—a unifying system for energy control. Trends Biochem Sci. 2004 Jan;29(1):18–24. doi:10.1016/j.tibs.2003.11.005. [Google Scholar] [PubMed] [CrossRef]
21. Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007 Sep 27;449(7161):496–500. doi:10.1038/nature06161. [Google Scholar] [PubMed] [CrossRef]
22. Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009 Aug;9(8):563–75. doi:10.1038/nrc2676. [Google Scholar] [PubMed] [CrossRef]
23. Li W, Saud SM, Young MR, Chen G, Hua B. Targeting AMPK for cancer prevention and treatment. Oncotarget. 2015 Mar 20;6(10):7365–78. doi:10.18632/oncotarget.3629. [Google Scholar] [PubMed] [CrossRef]
24. Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013 Feb 11;23(2):143–58. doi:10.1016/j.ccr.2012.12.008. [Google Scholar] [PubMed] [CrossRef]
25. Tsakiridis EE, Broadfield L, Marcinko K, Biziotis OD, Ali A, Mekhaeil B, et al. Combined metformin-salicylate treatment provides improved anti-tumor activity and enhanced radiotherapy response in prostate cancer; drug synergy at clinically relevant doses. Transl Oncol. 2021 Nov;14(11):101209. doi:10.1016/j.tranon.2021.101209. [Google Scholar] [PubMed] [CrossRef]
26. Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, et al. The STRING database in 2023: protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023 Jan 6;51(D1):D638–46. doi:10.1093/nar/gkac1000. [Google Scholar] [PubMed] [CrossRef]
27. Pollak M. Metformin and other biguanides in oncology: advancing the research agenda. Cancer Prev Res Phila Pa. 2010 Sep;3(9):1060–5. doi:10.1158/1940-6207.capr-10-0175. [Google Scholar] [PubMed] [CrossRef]
28. Villani LA, Smith BK, Marcinko K, Ford RJ, Broadfield LA, Green AE, et al. The diabetes medication canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration. Mol Metab. 2016 Oct;5(10):1048–56. doi:10.1016/j.molmet.2016.08.014. [Google Scholar] [PubMed] [CrossRef]
29. Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996 Feb;270(2 Pt 1):E299–304. doi:10.1152/ajpendo.1996.270.2.e299. [Google Scholar] [PubMed] [CrossRef]
30. Biziotis OD, Tsakiridis EE, Ali A, Ahmadi E, Wu J, Wang S, et al. Canagliflozin mediates tumor suppression alone and in combination with radiotherapy in non-small cell lung cancer (NSCLC) through inhibition of HIF-1α. Mol Oncol. 2023 Aug 27;17(11):2235. doi:10.1002/1878-0261.13508. [Google Scholar] [PubMed] [CrossRef]
31. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001 Oct;108(8):1167–74. doi:10.1172/jci13505. [Google Scholar] [PubMed] [CrossRef]
32. Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016 Apr 1;48(4):e224. doi:10.1038/emm.2016.16. [Google Scholar] [PubMed] [CrossRef]
33. Pokhrel RH, Acharya S, Mishra S, Gu Y, Manzoor U, Kim JK, et al. AMPK alchemy: therapeutic potentials in allergy, aging, and cancer. Biomol Ther. 2024 Mar 1;32(2):171–82. doi:10.4062/biomolther.2023.222. [Google Scholar] [PubMed] [CrossRef]
34. Keerthana CK, Rayginia TP, Shifana SC, Anto NP, Kalimuthu K, Isakov N, et al. The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment. Front Immunol. 2023 Feb 15;14:1114582. doi:10.3389/fimmu.2023.1114582. [Google Scholar] [PubMed] [CrossRef]
35. Hardie DG. Molecular pathways: is AMPK a friend or a foe in cancer? Clin Cancer Res. 2015 Sep 1;21(17):3836–40. doi:10.1158/1078-0432.CCR-14-3300. [Google Scholar] [PubMed] [CrossRef]
36. Ma EH, Poffenberger MC, Wong AHT, Jones RG. The role of AMPK in T cell metabolism and function. Curr Opin Immunol. 2017 Jun;46:45–52. doi:10.1016/j.coi.2017.04.004. [Google Scholar] [PubMed] [CrossRef]
37. Jung S, Baek JH. The potential of T cell factor 1 in sustaining CD8+ T lymphocyte-directed anti-tumor immunity. Cancers. 2021 Jan 29;13(3):515. doi:10.3390/cancers13030515. [Google Scholar] [PubMed] [CrossRef]
38. Rashad AA, Elshafie MF, Mangoura SA, Akool ES. Modulatory effect of metformin and its transporters on immune infiltration in tumor microenvironment: a bioinformatic study with experimental validation. Discov Oncol. 2025 May 31;16(1):973. doi:10.1007/s12672-025-02766-y. [Google Scholar] [PubMed] [CrossRef]
39. Pokhrel RH, Acharya S, Ahn JH, Gu Y, Pandit M, Kim JO, et al. AMPK promotes antitumor immunity by downregulating PD-1 in regulatory T cells via the HMGCR/p38 signaling pathway. Mol Cancer. 2021 Oct 14;20(1):133. doi:10.1186/s12943-021-01420-9. [Google Scholar] [PubMed] [CrossRef]
40. Zhou J, Huang W, Tao R, Ibaragi S, Lan F, Ido Y, et al. Inactivation of AMPK alters gene expression and promotes growth of prostate cancer cells. Oncogene. 2009 May 7;28(18):1993–2002. doi:10.1038/onc.2009.63. [Google Scholar] [PubMed] [CrossRef]
41. Lang F, Föller M. Regulation of ion channels and transporters by AMP-activated kinase (AMPK). Channels Austin Tex. 2014;8(1):20–8. doi:10.4161/chan.27423. [Google Scholar] [PubMed] [CrossRef]
42. Li M, Tian P, Zhao Q, Ma X, Zhang Y. Potassium channels: novel targets for tumor diagnosis and chemoresistance. Front Oncol. 2023 Jan 10;12:1074469. doi:10.3389/fonc.2022.1074469. [Google Scholar] [PubMed] [CrossRef]
43. Wu Y, Qi Y, Liu H, Wang X, Zhu H, Wang Z. AMPK activator AICAR promotes 5-FU-induced apoptosis in gastric cancer cells. Mol Cell Biochem. 2016 Jan;411(1–2):299–305. doi:10.1007/s11010-015-2592-y. [Google Scholar] [PubMed] [CrossRef]
44. Koltai T, Reshkin SJ, Carvalho TMA, Di Molfetta D, Greco MR, Alfarouk KO, et al. Resistance to gemcitabine in pancreatic ductal adenocarcinoma: a physiopathologic and pharmacologic review. Cancers. 2022 May 18;14(10):2486. doi:10.3390/cancers14102486. [Google Scholar] [PubMed] [CrossRef]
45. Tufail M, Jiang CH, Li N. Altered metabolism in cancer: insights into energy pathways and therapeutic targets. Mol Cancer. 2024 Sep 18;23(1):203. doi:10.1186/s12943-024-02119-3. [Google Scholar] [PubMed] [CrossRef]
46. Seo Y, Kim J, Park SJ, Park JJ, Cheon JH, Kim WH, et al. Metformin suppresses cancer stem cells through AMPK activation and inhibition of protein prenylation of the mevalonate pathway in colorectal cancer. Cancers. 2020 Sep;12(9):2554. doi:10.3390/cancers12092554. [Google Scholar] [PubMed] [CrossRef]
47. Zhou X, Kuang Y, Liang S, Wang L. Metformin inhibits cell proliferation in SKM-1 cells via AMPK-mediated cell cycle arrest. J Pharmacol Sci. 2019 Dec;141(4):146–52. doi:10.1016/j.jphs.2019.10.003. [Google Scholar] [PubMed] [CrossRef]
48. Gotlib J, Kluin-Nelemans HC, George TI, Akin C, Sotlar K, Hermine O, et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N Engl J Med. 2016 Jun 30;374(26):2530–41. doi:10.1056/nejmoa1513098. [Google Scholar] [PubMed] [CrossRef]
49. DeAngelo DJ, George TI, Linder A, Langford C, Perkins C, Ma J, et al. Efficacy and safety of midostaurin in patients with advanced systemic mastocytosis: 10-year median follow-up of a phase II trial. Leukemia. 2018 Feb;32(2):470–8. doi:10.1038/leu.2017.234. [Google Scholar] [PubMed] [CrossRef]
50. Beckers A, Organe S, Timmermans L, Vanderhoydonc F, Deboel L, Derua R, et al. Methotrexate enhances the antianabolic and antiproliferative effects of 5-aminoimidazole-4-carboxamide riboside. Mol Cancer Ther. 2006 Sep;5(9):2211–7. doi:10.1158/1535-7163.mct-06-0001. [Google Scholar] [PubMed] [CrossRef]
51. Fodor T, Szántó M, Abdul-Rahman O, Nagy L, Dér Á, Kiss B, et al. Combined treatment of MCF-7 cells with AICAR and methotrexate, arrests cell cycle and reverses warburg metabolism through AMP-activated protein kinase (AMPK) and FOXO1. PLoS One. 2016;11(2):e0150232. doi:10.1371/journal.pone.0150232. [Google Scholar] [PubMed] [CrossRef]
52. Orecchioni S, Reggiani F, Talarico G, Mancuso P, Calleri A, Gregato G, et al. The biguanides metformin and phenformin inhibit angiogenesis, local and metastatic growth of breast cancer by targeting both neoplastic and microenvironment cells. Int J Cancer. 2015 Mar 15;136(6):E534–544. doi:10.1002/ijc.29193. [Google Scholar] [PubMed] [CrossRef]
53. Huang Y, Zhou S, He C, Deng J, Tao T, Su Q, et al. Phenformin alone or combined with gefitinib inhibits bladder cancer via AMPK and EGFR pathways. Cancer Commun. 2018 Jul 27;38(1):50. doi:10.1186/s40880-018-0319-7. [Google Scholar] [PubMed] [CrossRef]
54. Peng M, Deng J, Zhou S, Xiao D, Long J, Zhang N, et al. Dual inhibition of pirarubicin-induced AKT and ERK activations by phenformin sensitively suppresses bladder cancer growth. Front Pharmacol. 2019 Oct 8;10:1159. doi:10.3389/fphar.2019.01159. [Google Scholar] [PubMed] [CrossRef]
55. Jiang W, Finniss S, Cazacu S, Xiang C, Brodie Z, Mikkelsen T, et al. Repurposing phenformin for the targeting of glioma stem cells and the treatment of glioblastoma. Oncotarget. 2016 Jul 29;7(35):56456–70. doi:10.18632/oncotarget.10919. [Google Scholar] [PubMed] [CrossRef]
56. Wu T, Zhou S, Qin M, Tang J, Yan X, Huang L, et al. Phenformin and ataxia-telangiectasia mutated inhibitors synergistically co-suppress liver cancer cell growth by damaging mitochondria. FEBS Open Bio. 2021;11(5):1440–51. doi:10.1002/2211-5463.13152. [Google Scholar] [PubMed] [CrossRef]
57. Chen G, Li MY, Yang JY, Zhou ZH. Will AMPK be a potential therapeutic target for hepatocellular carcinoma? Am J Cancer Res. 2024 Jul 15;14(7):3241–58. [Google Scholar]
58. Wang J, Xia S, Zhu Z. Synergistic effect of phenformin in non-small cell lung cancer (NSCLC) ionizing radiation treatment. Cell Biochem Biophys. 2015 Mar;71(2):513–8. doi:10.1007/s12013-014-0283-z. [Google Scholar] [PubMed] [CrossRef]
59. García Rubiño ME, Carrillo E, Ruiz Alcalá G, Domínguez-Martín A, Marchal JA, Boulaiz H. Phenformin as an anticancer agent: challenges and prospects. Int J Mol Sci. 2019 Jul 5;20(13):3316. doi:10.3390/ijms20133316. [Google Scholar] [PubMed] [CrossRef]
60. Storer PD, Xu J, Chavis J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J Neuroimmunol. 2005 Apr;161(1–2):113–22. doi:10.1016/j.jneuroim.2004.12.015. [Google Scholar] [PubMed] [CrossRef]
61. Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011 Feb 10;364(6):514–23. doi:10.1056/nejmoa1009290. [Google Scholar] [PubMed] [CrossRef]
62. Navalkissoor S, Grossman A. Somatostatin receptor-linked α-particle therapy in neuroendocrine tumours. J Neuroendocrinol. 2025 Mar;37(3):e13463. doi:10.1111/jne.13463. [Google Scholar] [PubMed] [CrossRef]
63. Bendell JC, Varghese AM, Hyman DM, Bauer TM, Pant S, Callies S, et al. A first-in-human phase 1 study of LY3023414, an oral PI3K/mTOR dual inhibitor, in patients with advanced cancer. Clin Cancer Res. 2018 Jul 15;24(14):3253–62. doi:10.1158/1078-0432.ccr-17-3421. [Google Scholar] [PubMed] [CrossRef]
64. Boyer JA, Sharma M, Dorso MA, Mai N, Amor C, Reiter JM, et al. eIF4A controls translation of estrogen receptor alpha and is a therapeutic target in advanced breast cancer. BioRxiv Prepr Serv Biol. 2024 May 11;3:480. doi:10.1101/2024.05.08.593195. [Google Scholar] [PubMed] [CrossRef]
65. Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003 May;35(3 Suppl):S7–14. doi:10.1016/s0041-1345(03)00211-2. [Google Scholar] [PubMed] [CrossRef]
66. Chunarkar-Patil P, Kaleem M, Mishra R, Ray S, Ahmad A, Verma D, et al. Anticancer drug discovery based on natural products: from computational approaches to clinical studies. Biomedicines. 2024 Jan 16;12(1):201. doi:10.3390/biomedicines12010201. [Google Scholar] [PubMed] [CrossRef]
67. Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, et al. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006 Jun 1;47(6):1281–8. doi:10.1194/jlr.m600020-jlr200. [Google Scholar] [PubMed] [CrossRef]
68. Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006 Aug;55(8):2256–64. doi:10.2337/db06-0006. [Google Scholar] [PubMed] [CrossRef]
69. Xu X, He Y, Liu J. Berberine: a multifaceted agent for lung cancer treatment-from molecular insight to clinical applications. Gene. 2025 Jan 20;934(21):149021. doi:10.1016/j.gene.2024.149021. [Google Scholar] [PubMed] [CrossRef]
70. Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, et al. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res. 2011 Sep 4;4(9):1419–25. doi:10.1158/1940-6207.capr-11-0148. [Google Scholar] [PubMed] [CrossRef]
71. Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008 Sep 5;373(4):545–9. doi:10.1016/j.bbrc.2010.11.062. [Google Scholar] [CrossRef]
72. Lotfi N, Yousefi Z, Golabi M, Khalilian P, Ghezelbash B, Montazeri M, et al. The potential anti-cancer effects of quercetin on blood, prostate and lung cancers: an update. Front Immunol. 2023 Feb 28;14:1077531. doi:10.3389/fimmu.2023.1077531. [Google Scholar] [PubMed] [CrossRef]
73. Buonerba C, De Placido P, Bruzzese D, Pagliuca M, Ungaro P, Bosso D, et al. Isoquercetin as an adjunct therapy in patients with kidney cancer receiving first-line sunitinib (QUASARresults of a phase I trial. Front Pharmacol. 2018 Mar 16;9:189. doi:10.3389/fphar.2018.00189. [Google Scholar] [PubMed] [CrossRef]
74. Neamtu AA, Maghiar TA, Alaya A, Olah NK, Turcus V, Pelea D, et al. A comprehensive view on the quercetin impact on colorectal cancer. Molecules. 2022 Mar 14;27(6):1873. doi:10.3390/molecules27061873. [Google Scholar] [PubMed] [CrossRef]
75. Asgharian P, Tazekand AP, Hosseini K, Forouhandeh H, Ghasemnejad T, Ranjbar M, et al. Potential mechanisms of quercetin in cancer prevention: focus on cellular and molecular targets. Cancer Cell Int. 2022 Aug 15;22(1):257. doi:10.1186/s12935-022-02677-w. [Google Scholar] [PubMed] [CrossRef]
76. Wang G, Wang Y, Yao L, Gu W, Zhao S, Shen Z, et al. Pharmacological activity of quercetin: an updated review. Evid Based Complement Alternat Med. 2022;2022(1):3997190. [Google Scholar] [PubMed]
77. Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015 Dec 11;350(6266):1391–6. doi:10.1126/science.aaa5004. [Google Scholar] [PubMed] [CrossRef]
78. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017 Aug 3;377(5):454–64. doi:10.1056/nejmoa1614359. [Google Scholar] [PubMed] [CrossRef]
79. Gridelli C, Bareschino MA, Schettino C, Rossi A, Maione P, Ciardiello F. Erlotinib in non-small cell lung cancer treatment: current status and future development. Oncologist. 2007 Jul;12(7):840–9. doi:10.1634/theoncologist.12-7-840. [Google Scholar] [PubMed] [CrossRef]
80. Steinberg GR, Carling D. AMP-activated protein kinase: the current landscape for drug development. Nat Rev Drug Discov. 2019 Jul;18(7):527–51. doi:10.1038/s41573-019-0019-2. [Google Scholar] [PubMed] [CrossRef]
81. Ramos-Peñafiel C, Olarte-Carrillo I, Cerón-Maldonado R, Rozen-Fuller E, Kassack-Ipiña JJ, Meléndez-Mier G, et al. Effect of metformin on the survival of patients with ALL who express high levels of the ABCB1 drug resistance gene. J Transl Med. 2018 Sep 3;16(1):245. doi:10.1186/s12967-018-1620-6. [Google Scholar] [PubMed] [CrossRef]
82. Morales DO. Effect of metformin on ABCB1 and AMPK expression in adolescents with newly diagnosed acute lymphoblastic leukemia [Internet]; 2022 Apr. Report No.: NCT05326984. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT05326984. [Google Scholar]
83. Izawa DJ. A window of opportunity study to evaluate the role of the combination of metformin and simvastatin as a neoadjuvant therapy in invasive bladder cancer [Internet]; 2016 Jul . Report No.: NCT02360618. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT02360618. [Google Scholar]
84. Lord SR, Cheng WC, Liu D, Gaude E, Haider S, Metcalf T, et al. Integrated pharmacodynamic analysis identifies two metabolic adaption pathways to metformin in breast cancer. Cell Metab. 2018 Nov 6;28(5):679–688.e4. doi:10.1016/j.cmet.2018.08.021. [Google Scholar] [PubMed] [CrossRef]
85. Michelangelo F. Modulation of response to hormonal therapy with lapatinib and/or metformin in patients with HER2-negative, ER and/or PgR positive metastatic breast cancer with progressive disease after first-line therapy [Internet]; 2014 Feb. Report No.: NCT01477060. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT01477060. [Google Scholar]
86. Sehdev A, Zha Y, Karrison TG, Janisch LA, Cohen EEW, Maitland ML, et al. A pharmacodynamic study of sirolimus and metformin in patients with advanced solid tumors. J Clin Oncol. 2017 May 20;35(15_suppl):TPS11628. doi:10.1200/jco.2017.35.15_suppl.tps11628. [Google Scholar] [CrossRef]
87. University of Michigan Rogel Cancer Center. A phase II pilot study of metformin therapy in patients with relapsed chronic lymphocytic leukemia and untreated CLL patients with genomic deletion 11q [Internet]; 2025 Feb . Report No.: NCT01750567. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT01750567. [Google Scholar]
88. Weill Medical College of Cornell University. A phase II study of high dose vitamin C intravenous infusion in patients with resectable or metastatic solid tumor malignancies [Internet]; 2024 Apr . Report No.: NCT03146962. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT03146962. [Google Scholar]
89. Rush University Medical Center. A phase II study evaluating the efficacy and safety of metformin in combination with standard induction therapy (RM-CHOP) for previously untreated aggressive diffuse large B-cell lymphoma [Internet]; 2022 Sep . Report No.: NCT02531308. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT02531308. [Google Scholar]
90. UNC Lineberger Comprehensive Cancer Center. Preoperative window study of metformin for the treatment of endometrial cancer [Internet]; 2013 Jul . Report No.: NCT01911247. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT01911247. [Google Scholar]
91. Salvador S. A clinical trial to evaluate endometrial cancer biomarker changes following exposure to the insulin sensitizer metformin [Internet]; 2016 Aug. Report No.: NCT02042495. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT02042495. [Google Scholar]
92. Konstantinopoulos PA, Lee EK, Xiong N, Krasner C, Campos S, Kolin DL, et al. A Phase II, two-stage study of letrozole and abemaciclib in estrogen receptor-positive recurrent endometrial cancer. J Clin Oncol. 2023 Jan 20;41(3):599–608. doi:10.1200/jco.22.00628. [Google Scholar] [PubMed] [CrossRef]
93. Consorzio Oncotech. Isoquercetin as an adjunct therapy in patients with kidney cancer receiving first-line sunitinib: a phase I/II trial [Internet]; 2017 Jan. Report No.: NCT02446795. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT02446795. [Google Scholar]
94. Paschka P, Marcucci G, Ruppert AS, Mrózek K, Chen H, Kittles RA, et al. Adverse prognostic significance of KIT mutations in adult acute myeloid leukemia with inv(16) and t(8;21a cancer and leukemia group B study. J Clin Oncol. 2006 Aug 20;24(24):3904–11. doi:10.1200/jco.2006.06.9500. [Google Scholar] [PubMed] [CrossRef]
95. Harbrecht BG. Resveratrol and human hepatocyte function in cancer [Internet]; 2017 Mar. Report No.: NCT02261844. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT02261844. [Google Scholar]
96. Beth Israel Medical Center. Metformin with a carbohydrate restricted diet in combination with platinum based chemotherapy in stage IIIB/IV non-squamous non-small cell lung cancer (NS-NSCLC)-METRO study [Internet]; 2018 Apr. Report No.: NCT02019979. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT02019979. [Google Scholar]
97. University of Utah. Pilot studies assessing the metabolomic and inflammatory effects of oral aspirin (ASA) in human subjects at risk for melanoma [Internet]; 2022 Feb. Report No.: NCT04062032. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT04062032. [Google Scholar]
98. Centre Hospitalier Universitaire de Nice. Pilot study evaluating the efficacy and safety of metformin in melanoma [Internet]; 2022 Nov. Report No.: NCT01840007. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT01840007. [Google Scholar]
99. Rodríguez OGA. Effect of metformin plus tyrosine kinase inhibitors compared with tyrosine kinase inhibitors alone for patients with advanced non-small cell lung cancer and EGFR mutations: phase 3 randomized clinical trial [Internet]; 2023 Jun. Report No.: NCT05445791. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT05445791. [Google Scholar]
100. National Cancer Institute (NCI). Phase IIB randomized, placebo-controlled trial of ACTOplus Met® XR in subjects with stage I-IV squamous cell carcinoma of the oral cavity or oropharynx prior to definitive treatment [Internet]; 2023 Feb. Report No.: NCT02917629. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT02917629. [Google Scholar]
101. Fondazione IRCCS Istituto Nazionale dei Tumori, Milano. Activity and safety of everolimus in combination with octreotide LAR and metformin in patients with advanced pancreatic well-differentiated neuroendocrine tumors (pWDNETsa phase II, open, monocentric, prospective study [Internet]; 2021 Sep. Report No.: NCT02294006. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT02294006. [Google Scholar]
102. Wilmink JW. A phase II, randomized, placebo controlled study to evaluate the efficacy of the combination of gemcitabine, erlotinib and metformin in patients with locally advanced and metastatic pancreatic cancer [Internet]; 2021 Apr. Report No.: NCT01210911. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT01210911. [Google Scholar]
103. Memorial Sloan Kettering Cancer Center. A phase 2, randomized, 3-arm study of abiraterone acetate alone, abiraterone acetate plus degarelix, a GnRH antagonist, and degarelix alone for patients with prostate cancer with a rising PSA or a rising PSA and nodal disease following definitive radical prostatectomy [Internet]; 2021 Oct. Report No.: NCT01751451. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT01751451. [Google Scholar]
104. The University of Texas Health Science Center at San Antonio. Phase II, randomized, placebo controlled, double blind, prospective study of castration compared to castration plus metformin as first line treatment for patients with advanced prostate cancer [Internet]; 2018 May. Report No.: NCT01620593. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT01620593. [Google Scholar]
105. National Cancer Institute (NCI). Phase II study of metformin in a pre-prostatectomy prostate cancer cohort [Internet]; 2017 Dec. Report No.: NCT01433913. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT01433913. [Google Scholar]
106. Joshua DA. A phase II, open label assessment of neoadjuvant intervention with metformin against tumour expression of signaling [Internet]; 2012 Jun. Report No.: NCT00881725. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT00881725. [Google Scholar]
107. Guy’s and St Thomas’ NHS Foundation Trust; NHS Foundation Trust. METformin and longevity (METALa window of opportunity study investigating biological effects of metformin in localised prostate cancer [Internet]; 2015 Jul. Report No.: NCT02511665. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT02511665. [Google Scholar]
108. Phillips JD. Metformin pharmacology in human cancers [Internet]; 2022 Nov . Report No.: NCT03477162. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT03477162. [Google Scholar]
109. Pang B, Wu H. Metabolic reprogramming in colorectal cancer: a review of aerobic glycolysis and its therapeutic implications for targeted treatment strategies. Cell Death Discov. 2025 Jul 14;11(1):321. doi:10.1038/s41420-025-02623-5. [Google Scholar] [PubMed] [CrossRef]
110. The Royal Bournemouth Hospital. Clinical and biological effects of a preoperative exercise programme in colorectal tumour and skeletal muscle tissues (exercise inDuced changes in colorectal cancer tissues) [Internet]; 2018 Mar. Report No.: NCT02056691. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT02056691. [Google Scholar]
111. Martling A, Lindberg J, Hed Myrberg I, Nilbert M, Mayrhofer M, Gronberg H, et al. Low-dose aspirin to reduce recurrence rate in colorectal cancer patients with PI3K pathway alterations: 3-year results from a randomized placebo-controlled trial. J Clin Oncol. 2025 Feb;43(4_suppl):LBA125–LBA125. doi:10.1200/jco.2025.43.4_suppl.lba125. [Google Scholar] [CrossRef]
112. Teng PC, Huang DQ, Lin TY, Noureddin M, Yang JD. Diabetes and risk of hepatocellular carcinoma in cirrhosis patients with nonalcoholic fatty liver disease. Gut Liver. 2023 Jan 15;17(1):24–33. doi:10.5009/gnl220357. [Google Scholar] [PubMed] [CrossRef]
113. Lin H, Yiu DCY, Chin S, Liu K, Yip TCF. Metformin in patients with hepatocellular carcinoma receiving immunotherapy. J Hepatol. 2023 May 1;78(5):e180–2. doi:10.1016/j.jhep.2022.12.011. [Google Scholar] [PubMed] [CrossRef]
114. Chiang CH, Chen YJ, Chiang CH, Chen CY, Chang YC, Wang SS, et al. Effect of metformin on outcomes of patients treated with immune checkpoint inhibitors: a retrospective cohort study. Cancer Immunol Immunother CII. 2023 Jun;72(6):1951–6. doi:10.1007/s00262-022-03363-6. [Google Scholar] [PubMed] [CrossRef]
115. Yuan B, Ma J, Wang J, Hao J. The effect of metformin usage on survival outcomes for hepatocellular carcinoma patients with type 2 diabetes mellitus after curative therapy. Front Endocrinol. 2022 Dec 13;13:1060768. doi:10.3389/fendo.2022.1060768. [Google Scholar] [PubMed] [CrossRef]
116. Papadakos SP, Ferraro D, Carbone G, Frampton AE, Vennarecci G, Kykalos S, et al. The emerging role of metformin in the treatment of hepatocellular carcinoma: is there any value in repurposing metformin for HCC immunotherapy? Cancers. 2023 Jan;15(12):3161. [Google Scholar]
117. Kordes S, Pollak MN, Zwinderman AH, Mathôt RA, Weterman MJ, Beeker A, et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015 Jul;16(7):839–47. doi:10.1016/s1470-2045(15)00027-3. [Google Scholar] [PubMed] [CrossRef]
118. Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ, Ennis M, Lemieux J, et al. Effect of metformin versus placebo on new primary cancers in canadian cancer trials group MA.32: a secondary analysis of a phase III randomized double-blind trial in early breast cancer. J Clin Oncol. 2023 Dec 10;41(35):5356–62. doi:10.1200/jco.23.00296. [Google Scholar] [PubMed] [CrossRef]
119. Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. Gut microbiome, adverse effects, and markers through MEtabolic reprogramming (GAMMER) study in early stage breast cancer receiving chemotherapy [Internet]; 2024 Aug. Report No.: NCT06536881. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT06536881. [Google Scholar]
120. Corleto KA, Strandmo JL, Giles ED. Metformin and breast cancer: current findings and future perspectives from preclinical and clinical studies. Pharmaceuticals. 2024 Mar 19;17(3):396. doi:10.3390/ph17030396. [Google Scholar] [PubMed] [CrossRef]
121. Badwe DRA. A randomized controlled trial of neoadjuvant weekly paclitaxel versus weekly paclitaxel plus weekly carboplatin in women with large operable or locally advanced, triple negative breast cancer [Internet]; 2023 Nov. Report No.: NCT03168880. [cited 2025 Mar 11]. Available from: https://clinicaltrials.gov/study/NCT03168880. [Google Scholar]
122. Kitson SJ, Maskell Z, Sivalingam VN, Allen JL, Ali S, Burns S, et al. PRE-surgical metformin in uterine malignancy (PREMIUMa multi-center, randomized double-blind, placebo-controlled phase III trial. Clin Cancer Res. 2019 Apr 15;25(8):2424–32. doi:10.1158/1078-0432.ccr-18-3339. [Google Scholar] [PubMed] [CrossRef]
123. Zhang Q, Han S, Zhang X, Wang Y, Li T, Yang B. Metformin enhances PD-L1 inhibitor efficacy in ovarian cancer by modulating the immune microenvironment and RBMS3 expression. FASEB J. 2025 Jun 15;39(11):e70705. doi:10.1096/fj.202500906r. [Google Scholar] [PubMed] [CrossRef]
124. Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 2023 Aug 18;22(1):138. doi:10.1186/s12943-023-01827-6. [Google Scholar] [PubMed] [CrossRef]
125. Fiala O, Ostašov P, Rozsypalová A, Hora M, Šorejs O, Šustr J, et al. Metformin use and the outcome of metastatic renal cell carcinoma treated with sunitinib or pazopanib. Cancer Manag Res. 2021 May 21;13:4077–86. doi:10.2147/cmar.s305321. [Google Scholar] [PubMed] [CrossRef]
126. Hamieh L, McKay RR, Lin X, Moreira RB, Simantov R, Choueiri TK. Effect of metformin use on survival outcomes in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer. 2017 Apr;15(2):221–9. doi:10.1016/j.clgc.2016.06.017. [Google Scholar] [PubMed] [CrossRef]
127. Mohamed AW, Elbassiouny M, Elkhodary DA, Shawki MA, Saad AS. The effect of itraconazole on the clinical outcomes of patients with advanced non-small cell lung cancer receiving platinum-based chemotherapy: a randomized controlled study. Med Oncol. 2021 Feb 9;38(3):23. doi:10.1007/s12032-021-01475-0. [Google Scholar] [PubMed] [CrossRef]
128. Gutkind JS, Molinolo AA, Wu X, Wang Z, Nachmanson D, Harismendy O, et al. Inhibition of mTOR signaling and clinical activity of metformin in oral premalignant lesions. JCI Insight. 2021 Sep 8;6(17):e147096. doi:10.1172/jci.insight.147096. [Google Scholar] [PubMed] [CrossRef]
129. Weber DD, Aminzadeh-Gohari S, Thapa M, Redtenbacher AS, Catalano L, Capelôa T, et al. Ketogenic diets slow melanoma growth in vivo regardless of tumor genetics and metabolic plasticity. Cancer Metab. 2022 Jul 18;10(1):12. doi:10.1186/s40170-022-00288-7. [Google Scholar] [PubMed] [CrossRef]
130. Ferrere G, Tidjani Alou M, Liu P, Goubet AG, Fidelle M, Kepp O, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021 Jan 25;6(2):e145207. doi:10.1172/jci.insight.145207. [Google Scholar] [PubMed] [CrossRef]
131. Wu Z, Zhang C, Najafi M. Targeting of the tumor immune microenvironment by metformin. J Cell Commun Signal. 2022 Sep;16(3):333–48. doi:10.1007/s12079-021-00648-w. [Google Scholar] [PubMed] [CrossRef]
132. Kang S, Khalil L, McCook-Veal A, Liu Y, Galvin J, Draper A, et al. Impact of metformin on clinical outcomes in advanced hepatocellular carcinoma treated with immune checkpoint inhibitors. Liver Cancer Int. 2023;4(2):77–88. doi:10.1002/lci2.71. [Google Scholar] [CrossRef]
133. Han P, Zhou J, Xiang J, Liu Q, Sun K. Research progress on the therapeutic effect and mechanism of metformin for lung cancer. Oncol Rep. 2022 Nov 7;49(1):3. [Google Scholar]
134. Gabel K, Cares K, Varady K, Gadi V, Tussing-Humphreys L. Current evidence and directions for intermittent fasting during cancer chemotherapy. Adv Nutr. 2022 Mar 1;13(2):667–80. doi:10.1093/advances/nmab132. [Google Scholar] [PubMed] [CrossRef]
135. Tiwari S, Sapkota N, Han Z. Effect of fasting on cancer: a narrative review of scientific evidence. Cancer Sci. 2022 Oct;113(10):3291–302. doi:10.1111/cas.15492. [Google Scholar] [PubMed] [CrossRef]
136. Valerio J, Borro M, Proietti E, Pisciotta L, Olarinde IO, Fernandez Gomez M, et al. Systematic review and clinical insights: the role of the ketogenic diet in managing glioblastoma in cancer neuroscience. J Pers Med. 2024 Aug 31;14(9):929. doi:10.3390/jpm14090929. [Google Scholar] [PubMed] [CrossRef]
137. Basheer HA, Salman NM, Abdullah RM, Elsalem L, Afarinkia K. Metformin and glioma: targeting metabolic dysregulation for enhanced therapeutic outcomes. Transl Oncol. 2025 Mar 1;53(2):102323. doi:10.1016/j.tranon.2025.102323. [Google Scholar] [PubMed] [CrossRef]
138. Klein JD, Khanna I, Pillarisetti R, Hagan RA, LaRocque LM, Rodriguez EL, et al. An AMPK activator as a therapeutic option for congenital nephrogenic diabetes insipidus. JCI Insight. 2021 Apr 22;6(8):e146419. doi:10.1172/jci.insight.146419. [Google Scholar] [PubMed] [CrossRef]
139. Triggle CR, Mohammed I, Bshesh K, Marei I, Ye K, Ding H, et al. Metformin: is it a drug for all reasons and diseases? Metab Clin Exp. 2022 Aug 1;133(8):155223. doi:10.1016/j.metabol.2022.155223. [Google Scholar] [PubMed] [CrossRef]
140. Amengual-Cladera E, Morla-Barcelo PM, Morán-Costoya A, Sastre-Serra J, Pons DG, Valle A, et al. Metformin: from diabetes to cancer—unveiling molecular mechanisms and therapeutic strategies. Biology. 2024 Apr 27;13(5):302. doi:10.3390/biology13050302. [Google Scholar] [PubMed] [CrossRef]
141. Tarasiuk O, Miceli M, Di Domizio A, Nicolini G. AMPK and diseases: state of the art regulation by AMPK-targeting molecules. Biology. 2022 Jul;11(7):1041. doi:10.3390/biology11071041. [Google Scholar] [PubMed] [CrossRef]
142. Steinberg GR, Dandapani M, Hardie DG. AMPK: mediating the metabolic effects of salicylate-based drugs? Trends Endocrinol Metab TEM. 2013 Oct;24(10):481–7. [Google Scholar]
143. Koutentakis M, Kuciński J, Świeczkowski D, Surma S, Filipiak KJ, Gąsecka A. The ketogenic effect of SGLT-2 inhibitors—beneficial or harmful? J Cardiovasc Dev Dis. 2023 Nov 16;10(11):465. doi:10.3390/jcdd10110465. [Google Scholar] [PubMed] [CrossRef]
144. Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009 May 6;9(5):407–16. doi:10.1016/j.cmet.2009.03.012. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF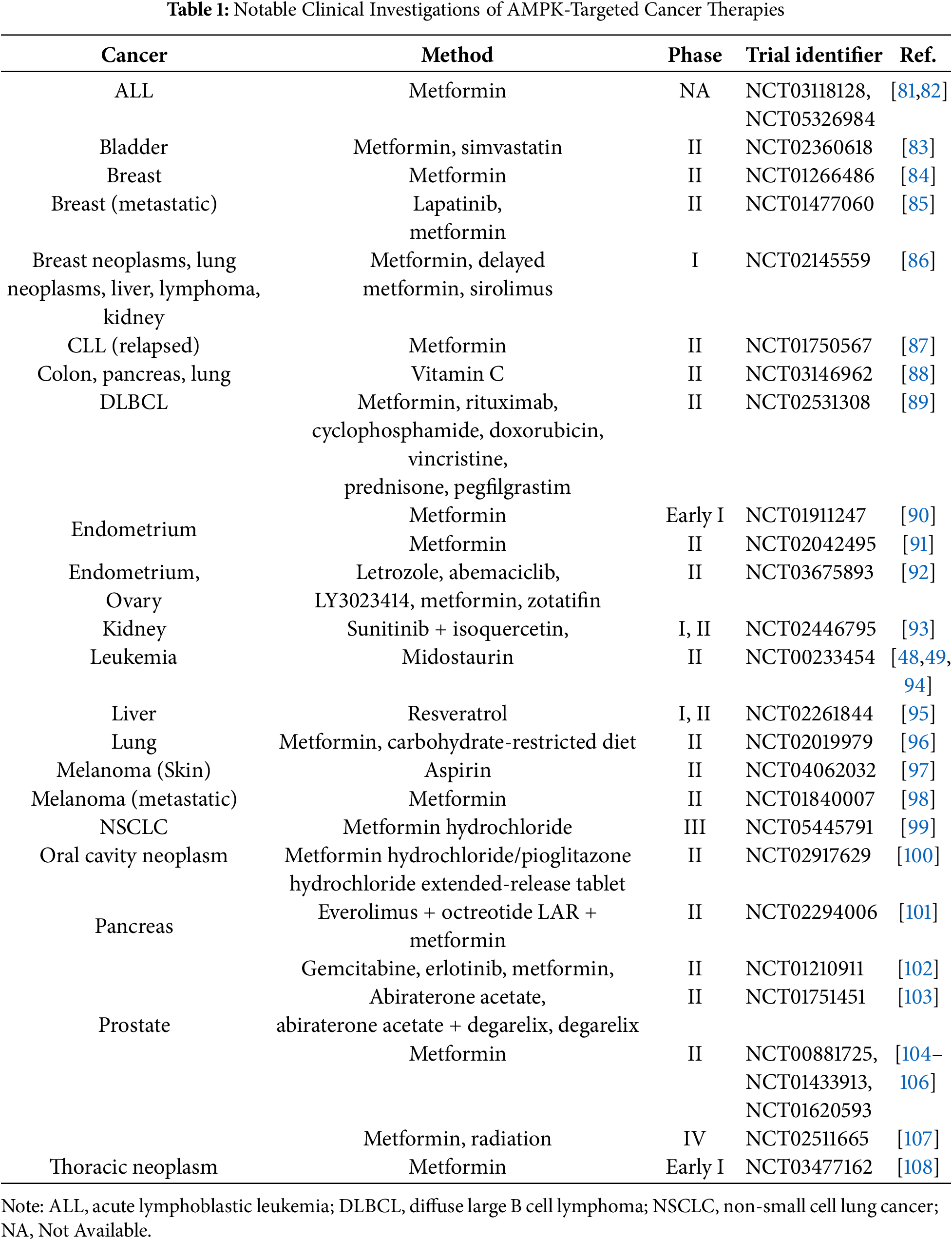
 Downloads
Downloads
 Citation Tools
Citation Tools