 Open Access
Open Access
ARTICLE
MINDY1 Induces PD-L1 Deubiquitination to Promote Immune Escape in Hepatocellular Carcinoma by the Wnt/β-Catenin Pathway
1 Department of Hepatobiliary and Pancreatic Surgery, The Affiliated Xuzhou Municipal Hospital of Xuzhou Medical University, Xuzhou, 221116, China
2 Clinical Medical College, Tianjin Medical University, Tianjin, 300270, China
* Corresponding Author: Chunyan Tian. Email:
#These authors contributed equally to this work
(This article belongs to the Special Issue: Signaling Pathway Crosstalk in Malignant Tumors: Molecular Targets and Combinatorial Therapeutics)
Oncology Research 2025, 33(11), 3583-3603. https://doi.org/10.32604/or.2025.067638
Received 08 May 2025; Accepted 31 July 2025; Issue published 22 October 2025
Abstract
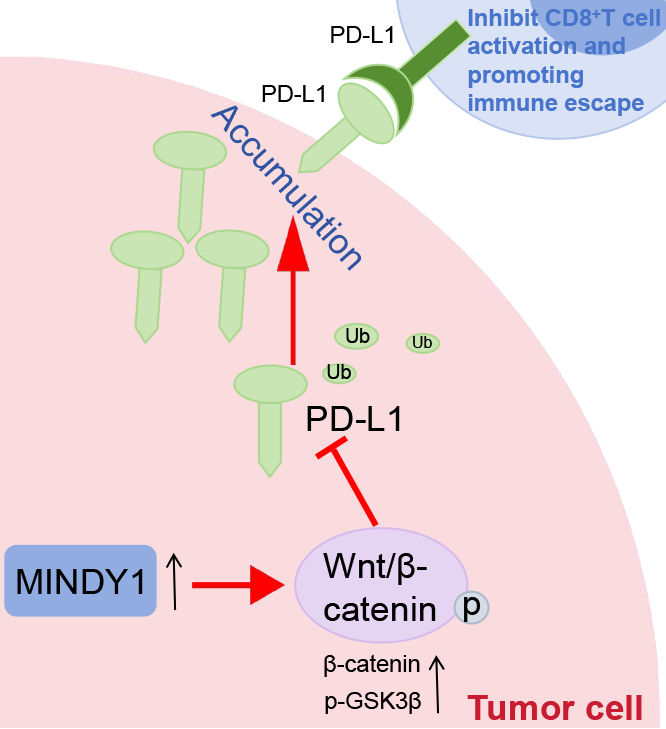
Background: Motif interacting with ubiquitin-containing novel DUB family-1 (MINDY1) could enhance the stability of programmed death-ligand 1 (PD-L1). The study aimed to investigate whether MINDY1 regulates the immune escape of hepatocellular carcinoma (HCC) mediated by PD-L1. Methods: MINDY1 and PD-L1 levels were detected through Western blot. The link between MINDY1 and PD-L1 was validated using the co-immunoprecipitation assay. The malignant biology of HCC cells was assessed through Cell Counting Kit-8, Carboxyfluorescein Succinimidyl Ester staining, transwell, and wound healing assay. CD8+ T cells were isolated and then co-cultured with HCC cells. Enzyme-linked immunosorbent Assay kits detected CD8+ T cytokine content. CD8+ T cell activation markers, PD-L1 ubiquitination levels, and Wnt/β-catenin pathway-associated protein levels were detected through Western blot. A HCC nude mouse model was developed, Ki-67 positivity and CD8+ T-cell infiltration were assessed through pathological staining and flow cytometry. Results: MINDY1 and PD-L1 levels were elevated in HCC. Overexpression of MINDY1 increased migrating and invading cells, elevated cell viability, and decreased apoptosis in HCC cells, leading to PD-L1 deubiquitination. Knockdown of MINDY1 reversed all of these indicators. Co-culturing with HCC cells overexpressing MINDY1 resulted in decreased proliferative capacity and cytotoxicity of CD8+ T cells, increased apoptosis, and decreased levels of cytokines and activation markers in CD8+ T cells. MINDY1 triggered Wnt/β-catenin pathway, Wnt activators further promoted PD-L1 deubiquitination and suppressed CD8+ T cell activation. MINDY1 overexpression increased PD-L1 and Ki67 positivity level in HCC tumors, suppressed CD8+ T-cell infiltration. Conclusion: MINDY1 promotes PD-L1 deubiquitination and inhibits CD8+ T cell activation by stimulating the Wnt/β-catenin pathway, consequently promoting HCC tumor immune escape.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools