 Open Access
Open Access
REVIEW
Advances in Tissue-Agnostic Targeting in Cancer Therapeutics: Current Approvals, Challenges, and Future Directions
1 College of Osteopathic Medicine, Touro University, Henderson, NV 89014, USA
2 Department of Internal Medicine, Kirk Kerkorian School of Medicine at UNLV, Las Vegas, NV 89106, USA
3 Department of Internal Medicine, One Brooklyn Health—Interfaith Medical Center Campus, Brooklyn, NY 11213, USA
4 Division of Hematology and Medical Oncology, Comprehensive Cancer Centers of Nevada, Central Valley, Las Vegas, NV 89169, USA
* Corresponding Author: Matthew Rubinstein. Email:
Oncology Research 2025, 33(11), 3161-3183. https://doi.org/10.32604/or.2025.067791
Received 13 May 2025; Accepted 19 August 2025; Issue published 22 October 2025
Abstract
The ever-expanding development of tissue-agnostic therapies which target malignancies based on specific mutations rather than tissue origin have transformed the landscape of oncology. The purpose of this review is to explore the impact, safety, and challenges of tissue-agnostic therapies including pembrolizumab, dostarlimab, larotrectinib, entrectinib, repotrectinib, dabrafenib plus trametinib, selpercatinib, and trastuzumab deruxtecan. As the therapeutic arsenal continues to grow, it is crucial to understand how these therapies truly benefit patients and to address the barriers that stand in the way of making them more widely available. Although these therapies have shown effectiveness across multiple cancer types, substantial challenges persist, including overcoming the burden of intratumoral heterogeneity and resistance mechanisms that reduce therapeutic efficacy. We discuss emergence of pan-histological biomarkers, such as neoantigen burden, current updates on trials as well as trial outlining strategies to refining patient selection, while also supporting broader access to biomarker testing. Collectively, these insights underscore the transformative role of tissue-agnostic therapies in precision oncology while emphasizing the ongoing need for research to optimize their application and overcome current barriers.Keywords
Tissue-agnostic therapies represent a groundbreaking shift in oncology, marking a departure from the traditional approach of treating cancer based on its tissue of origin. Instead, these therapies focus on the molecular and genetic characteristics underlying the disease [1–3]. In the past, treatments were guided by the tumor’s location, which often left patients with rare or treatment-resistant cancers with limited to no options (Fig. 1). However, advances in genomics and precision medicine have changed this narrative. By targeting the genetic drivers shared across various cancers, these therapies address the mechanisms of tumor growth rather than their physical location, offering new hope for patients with complex and diverse cancer diagnoses [2,3].
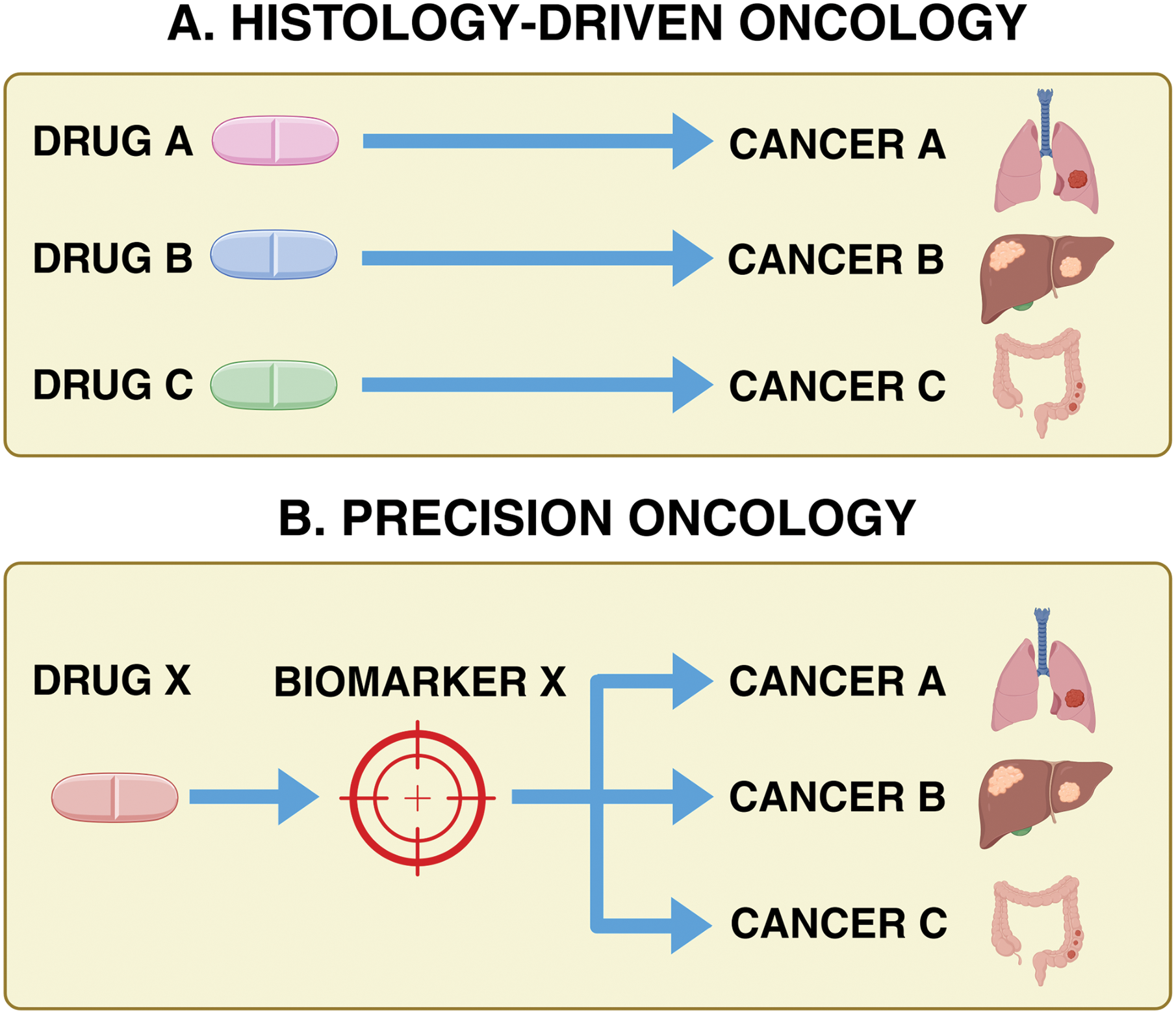
Figure 1: Histology-driven targeting of cancer therapeutics vs. tissue-agnostic targeting of cancer therapeutics (precision oncology).
Historically, oncology treatments were guided by tumor histology. Tumor-agnostic therapies, however, target cancers based on shared biomarkers, such as mutations or fusions, regardless of tissue origin. Basket trials play a crucial role in evaluating their efficacy across diverse cancer types, expanding treatment options for patients, including those with rare malignancies who might not otherwise receive targeted therapies. Created in BioRender. Thein, K. (2025) https://BioRender.com/s80z131 (accessed on 18 August 2025)
This progress has been fueled by two main strategies: targeted therapies and immunotherapies. Targeted therapies are designed to inhibit specific molecular alterations, such as mutations, gene fusions, or overexpressed proteins, that are critical to tumor growth and survival [2–4]. For instance, drugs like larotrectinib and entrectinib block neurotrophic tyrosine kinase (NTRK) fusions, while dabrafenib and trametinib inhibit the mitogen-activated protein kinase (MAPK) pathway in cancers with BRAFV600E mutations. By precisely targeting the genetic drivers of cancer, these treatments are often more effective and associated with fewer side effects compared to traditional chemotherapy [3].
Immunotherapies, on the other hand, harness the body’s immune system to combat cancer. Immune checkpoint inhibitors, such as pembrolizumab and dostarlimab, block pathways like Programmed Cell Death Protein-1/Programmed Cell Death Ligand-1 (PD-1/PD-L1) that tumors use to evade immune detection [5,6]. By reactivating T cells, these therapies enable the immune system to recognize and attack cancer cells. Immunotherapies have shown particular success in tumors with high immunogenicity, such as those characterized by microsatellite instability-high (MSI-H) or high tumor mutational burden [3].
Despite their differing mechanisms, these approaches share a common goal: improving patient outcomes by targeting cancer’s biology. Targeted therapies disrupt the pathways that fuel tumor growth, while immunotherapies restore the immune system’s ability to mount a defense. These advancements have led to the 2017 approval of pembrolizumab for MSI-H cancers marked a pivotal moment, signaling a shift toward biomarker-driven therapies rather than treatments based solely on anatomical classification [2,3,7].
2 Immunotherapy: Tissue-Agnostic Targeting of Cancer Therapeutics
The mismatch repair system utilizes several genes, including mutL homolog 1 (MLH1), mutL homolog 2 (MSH2), mutL homolog 6 (MSH6), and post-meiotic segregation increased 2 (PMS2) to repair errors made during DNA replication [6]. Defects in the mismatch repair (dMMR) system can lead to high mutation rates, known as DNA microsatellite instability. The continued inability to correct these mismatches leads to the introduction of more errors into the affected DNA, which may be used as potential targets for the development of new chemotherapies [8].
2.1.1 Pembrolizumab in MSI-H Solid Tumors
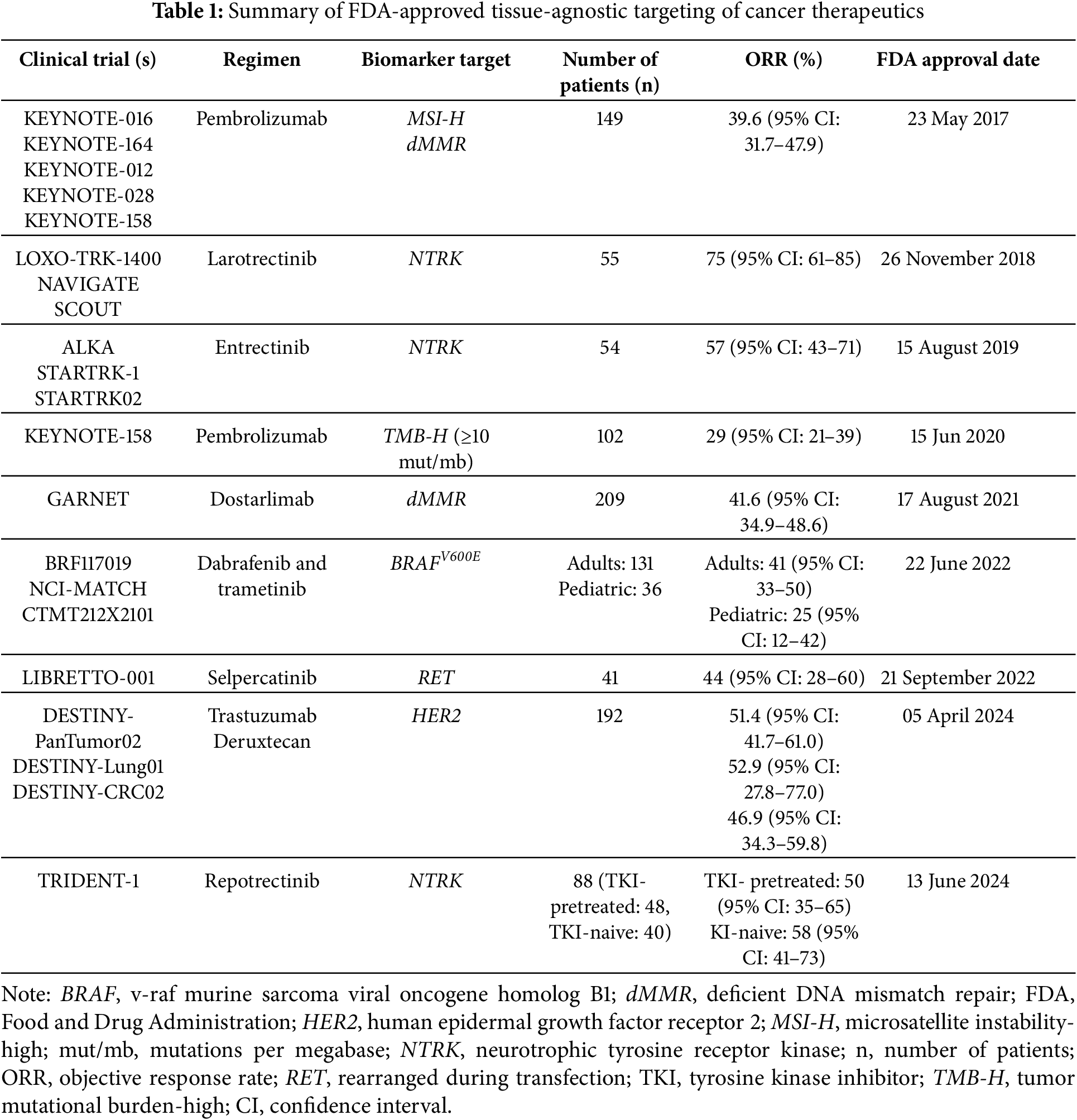
Pembrolizumab has transformed cancer treatment as the first FDA-approved therapy that works across multiple cancer types based on specific genetic markers, rather than the tumor’s location (Fig. 2). Nevertheless, pembrolizumab targets tumors with MSI-H or dMMR [7,8]. The primary endpoint of the KEYNOTE trials was objective response rate (ORR) assessed with RECIST criteria, and several secondary endpoints including duration of response (DOR), progression-free survival (PFS), and overall survival (OS) [7,8]. As depicted in Table 1, evidence from the pivotal KEYNOTE trials demonstrated its effectiveness, with an impressive objective response rate (ORR) of 39.6% across 15 different tumor types, such as colorectal, endometrial, and gastric cancers (Table 1). Even more striking, non-colorectal cancers showed a higher ORR of 46%, underscoring pembrolizumab’s broad potential. Additionally, the therapy achieved durable responses lasting over six months in 78% of patients, with some even experiencing complete remission [9].
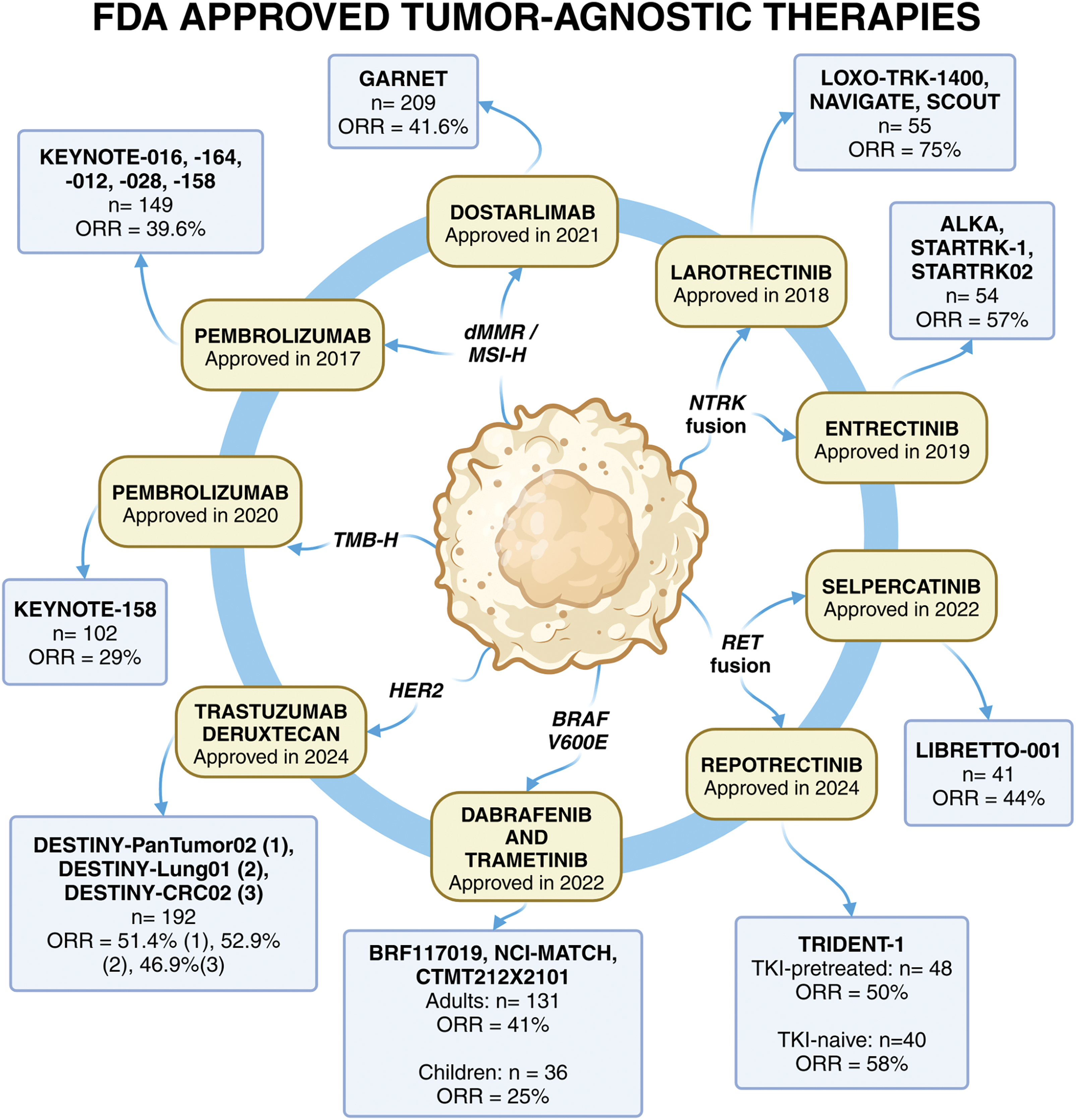
Figure 2: FDA approved tissue-agnostic targeting of cancer therapeutics. There are a total of eight FDA-approved tumor-agnostic therapies across nine different indications. The year of approval, as well as the clinical trials, the number of patients in the efficacy population and the ORR leading to the approval are summarized in the figure. These treatments include immunotherapy, such as the PD-L1 inhibitors, pembrolizumab and dostarlimab.Pembrolizumab was approved for both dMMR/MSI-H and TMB-H cancers, whereas dostarlimab was approved for dMMR cancers. Targeted therapies for oncogenic fusions include larotrectinib and entrectinib for NTRK fusions, as well as selpercatinib and repotrectinib for RET fusions. Dabrafenib (BRAF inhibitor) and trametinib (MEK inhibitor) represent the first combinational biomarker-driven treatment that was approved. Lastly, trastuzumab deruxtecan is an antibody-drug conjugate targeting HER2-positive solid tumors, which was approved based on three clinical trials. The continued development of tumor-agnostic therapies highlights the shift toward biomarker-driven precision oncology, expanding treatment options for patients across diverse cancer types, with more targeted therapies expected to emerge as research advances
Note: BRAF, v-raf murine sarcoma viral oncogene homolog B1; dMMR, deficient DNA mismatch repair; FDA, Food and Drug Administration; HER2, human epidermal growth factor receptor 2; MEK, mitogen-activated extracellular signal-regulated kinase; MSI-H, microsatellite instability-high; NTRK, neurotrophic tyrosine receptor kinase; n, number of patients; ORR, objective response rate; RET, rearranged during transfection; TKI, tyrosine kinase inhibitor; TMB-H, tumor mutational burden-high. Created in BioRender. Thein, K. (2025) https://BioRender.com/s80z131.
Pembrolizumab works by blocking the PD-1 receptor on T cells, preventing it from interacting with its ligands PD-L1 and PD-L2 [6,9]. This mechanism, known as immune checkpoint blockade, reinvigorates the immune system’s ability to recognize and attack cancer cells, a hallmark of immunotherapy [6,9,10]. While its benefits are well-documented, pembrolizumab is not without challenges. Immune-related adverse events (irAEs) like colitis, pneumonitis, and endocrinopathies can occur, but they are typically manageable with timely treatment [11–13].
Despite its remarkable impact, pembrolizumab has some limitations. It has shown limited effectiveness in primary brain tumors due to the distinct tumor environment and genetic complexity [14]. Additionally, the high cost of genomic testing for MSI-H status can make access difficult for some patients [15]. Nevertheless, pembrolizumab represents a major milestone in oncology, showcasing the promise of immunotherapy in treating cancers defined by specific biomarkers [6,7].
2.1.2 Dostarlimab in MSI-H-Solid Tumors
Dostarlimab is an IgG4 monoclonal antibody that functions to target and inhibit the PD-1/PD-L1 pathway similarly to pembrolizumab [16,17]. Dostarlimab has made significant strides in cancer treatment, particularly for patients with tumors characterized by MSI-H or dMMR. The KEYNOTE-158 and PHAEDRA studies yielded promising results in patients with endometrial cancer (EC) with the use of monoclonal antibodies [16]. The data from these studies lead to the approval of dostarlimab by the FDA in 2021 for its remarkable promise in advanced or recurrent endometrial cancer, offering a much-needed option for patients with limited alternatives [16–18].
The GARNET trial highlighted the drug’s efficacy in phases and cohorts of solid neoplasms, highlighting its utility in not only treating advanced/recurrent EC, but also colorectal and other solid tumor types. The overall ORR among all cohorts seen by this trial was 41.6 (95% Confidence Interval (CI): 34.9–48.6) [16]. The response rates are comparable to those observed with pembrolizumab, and in some cases, patients have achieved prolonged remission. Its success in refractory cases, where other treatments have failed, underscores dostarlimab’s potential to address unmet needs in oncology. However, most of the available data focuses on endometrial cancer, and while early findings suggest it may be effective against a range of tumor types, further studies are needed to confirm its broader applicability [16]. Additional ongoing research has revealed the ability to replace chemotherapy, radiation and surgery, in patients with rectal cancer with ICIs, to preserve organ function and maintain quality of life by avoiding permanent -ostomy creation [16].
The safety profile of dostarlimab has been another focus of clinical research. irAEs, such as colitis and dermatitis, are relatively common but generally mild and manageable with timely care [16,18]. These side effects stem from the drug’s mechanism of action, which activates the immune system and can inadvertently lead to inflammation in healthy tissues [16]. Despite these challenges, most patients tolerate the drug well when irAEs are managed appropriately, allowing them to continue treatment and benefit from its therapeutic effects.
Dostarlimab’s approval has opened new doors in cancer therapy, but it also comes with ongoing discussions. Its use beyond endometrial cancer remains an area of active research, as data on other tumor types is still limited. Comparisons with pembrolizumab, which has more extensive clinical evidence, also raise questions about how best to integrate dostarlimab into existing treatment frameworks. Additionally, the financial barriers associated with immune checkpoint inhibitors and the cost of genomic testing to identify MSI-H or dMMR status present challenges for broader access [16,17].
Despite these considerations, dostarlimab has already proven to be a valuable addition to the immunotherapy toolkit, offering hope to patients with MSI-H or dMMR cancers. Its ability to deliver durable responses and manage refractory cases highlights its potential to improve outcomes for many patients, even as research continues to expand its applications.
2.1.3 Resistance Mechanisms in MSI-H Cancers
Mechanisms of resistance for MSI-H tumors involve the activation and upregulation of alternative signaling pathways that allow for bypassing the effects of immune checkpoint inhibitors (ICIs) [19]. Mutations in the janus kinases 1 and 2 have led to the inactivation of the JAK-STAT pathway which directly decreases a cell’s ability to induce proteasomes and decreasing MHC-I antigen presentation while increasing tumor cell survivability [20]. This resistance can lead to clonal evolution and the further emergence of subpopulations that are less responsive to ICIs [20]. Other means of altering the efficacy of ICIs have been thought to be based on intratumoral microenvironment, epigenetic modifications, and dysbiosis of the gut microbiome [20]. Patients with a higher diversity of the gut microbiome, specifically with Faecalibacterium and Ruminococaceae, have enhanced antigen presentation and anti-tumoral immune responses, whereas patients with an abundance of Bacteriodales, demonstrated a decreased immune response [21]. Ongoing research into these mechanisms may provide further insight into the development of a range of therapeutic options for treating MSI-H cancers.
2.2 Tumor Mutational Burden-High (TMB-H) Solid Cancers
Tumor mutational burden (TMB) refers to the number of somatic mutations per megabase of coding DNA. TMB-H was previously defined as having ≥175 mutations per exome [22]. Since the FDA approval of the FoundationOne CDx genomic profiling assay, which demonstrated the ability to identify patients with TMB-H solid cancers as ≥10 mutations/megabase that may benefit from immunotherapy such as pembrolizumab [23]. These high rates of mutations lead to the formation of neoantigens, which prompt a stronger immune response [24]. This mutational burden can act as a biomarker for the development of immune checkpoint inhibitors [24,25].
2.2.1 Pembrolizumab in TMB-H Solid Cancers
Pembrolizumab’s approval for treating cancers with TMB-H has broadened its role in oncology, offering a critical option for patients with challenging tumor profiles [1,26,27]. The abundance of neoantigens makes TMB-H tumors particularly responsive to immunotherapies like pembrolizumab. This mechanism has been instrumental in delivering durable responses for patients across multiple tumor types, even in cases where other treatments have failed [26,27].
Clinical trials, most notably KEYNOTE-158, have demonstrated pembrolizumab’s effectiveness in TMB-H cancers, showing significant and lasting responses in a variety of tumors with an ORR of 29% (95% CI: 21–39) [5], (Table 1). This led to another approved indication by the FDA for treating TMB-H solid cancers in patients who did not have other alternative treatment options [26,27]. These results highlight pembrolizumab’s broad applicability, providing new hope for patients with cancers that were previously difficult to treat. However, while its efficacy has been impressive, the question of who stands to benefit the most remains a topic of ongoing research. Determining the best threshold for defining TMB-H has been challenging, with different studies and real-world practices often using inconsistent cutoffs [28]. Standardizing these criteria is essential to ensure that patients most likely to benefit from pembrolizumab can be accurately identified [28]. In terms of safety, pembrolizumab’s use in TMB-H cancers is consistent with its established profile in MSI-H tumors [11–13]. The drug’s overall tolerability in clinical trials and real-world settings has provided reassurance, making it a viable option for many patients [5].
Despite these advancements, discussions about pembrolizumab’s role in TMB-H cancers continue. The confounding biology of MSI-H status with TMB-H status in colorectal cancer has demonstrated that MSI-H status is the primary determinant of benefit from immunotherapy [29]. The variability in defining TMB-H poses challenges not only for clinical trials but also for ensuring equitable access to treatment [30]. The high cost of genomic testing needed to identify TMB-H status can limit access for some patients, further complicating its implementation [30]. Addressing these issues is critical for maximizing the drug’s potential and ensuring that it reaches all eligible patients [30].
Pembrolizumab’s approval for TMB-H cancers has marked a significant milestone in precision medicine. Its ability to deliver durable responses across a range of tumors reaffirms its importance in cancer immunotherapy. While refining patient selection and addressing barriers to access remain areas for improvement, pembrolizumab has already made a profound impact on the lives of many patients with biomarker-driven cancers.
2.2.2 Resistance Mechanisms in TMB-H Cancers
TMB-H cancers have also been shown to have several means of resisting therapies. One mechanism of resistance has been shown to reduce the expression of antigen-presenting major histocompatibility complex class I molecules, leading to the failure of the immune system to recognize those cancer cells and deficiencies in antigen processing machinery [24,25,31]. Additionally, intratumor heterogeneity contributes to the diversity within tumor profiles themselves, which may result in the emergence of resistant clones that can survive and proliferate [32].
3 Targeted Therapy: Tissue-Agnostic Targeting of Cancer Therapeutics
3.1 NTRK-Fusion Positive Solid Cancers
The NTRK genes code for the tropomyosin receptor kinase (TRK) receptor proteins. NTRK1, NTRK2, and NTRK3 code for TRK-A, TRK-B, and TRK-C, which activate the phosphoinositide 3-kinase (PI3K), rat sarcoma viral oncogene homolog/mitogen-activated protein kinase (RAS/MAPK), and phospholipase C (PLC) pathways, respectively [33]. These cellular pathways play a vital role in overall cell growth, proliferation, and survival. NTRK fusions are chromosomal rearrangements that are highly prevalent in rare malignancies, such as thyroid cancer, salivary gland cancer, and mammary analogue secretory carcinoma [33,34]. In contrast, NTRK gene fusions occur in less than 5% of common cancers, including pulmonary carcinoma, colorectal carcinoma, and cutaneous melanoma [35]. This molecular targeting has proved useful and effective in treating diverse tumor types [34].
3.1.1 Larotrectinib in NTRK-Fusion Positive Solid Cancers
Laroctretinib is a first-generation TRK inhibitor approved in 2018 to treat advanced NTRK fusion-positive solid tumors in both adults and children [36–38]. Laroctretinib works by competitively inhibiting the ATP-binding site on the TRK receptor intracellular kinase domain and subsequently disrupting the tumor [39].
To assess the clinical efficacy of larotrectinib, multiple studies have been conducted to examine its impact on NTRK fusion-positive tumors. A pooled analysis of clinical trials LOXO-TRK-14001, SCOUT, and NAVIGATE demonstrated that larotrectinib effectively treated NTRK-positive tumors, with ORR as the primary endpoint [40]. LOXO-TRK-14001 included patients with advanced solid tumors, SCOUT included pediatric patients with advanced solid tumors, and NAVIGATE included patients with NTRK fusion-positive tumors [41,42]. The most common cancer types treated were salivary gland cancer, soft tissue carcinoma, infantile fibrosarcoma, and thyroid cancer [43]. This initial pooled analysis yielded an ORR of 75% (95% CI: 61–85) with a median duration of response (mDOR) of 35.2 months, while an updated pooled analysis produced an ORR of 66% with a median progression-free survival time of 46.2 months [36,43]. Additionally, a post hoc analysis including patients with brain metastases revealed an ORR of 68% and an mDOR of 48.7 months [43]. This pooled data not only reinforces larotrectinib’s clinical utility but also provides detailed insights into its optimal therapeutic impact.
While larotrectinib has shown diverse clinical applications, it is also important to evaluate its safety profile and tolerability. The most common adverse events include elevated liver enzymes, anemia, neutropenia, cough, constipation, diarrhea, and nausea. Because larotrectinib metabolism occurs primarily via hepatic cytochrome P450 3A4, drug-drug interactions must be carefully considered when dosing [39]. These adverse effects and drug interactions require careful monitoring and further investigation, so that patients can safely reap the therapeutic effects [39].
NTRK fusions have been detected with various testing methods, including fluorescence in situ hybridization (FISH), immunohistochemistry, and next-generation sequencing. Due to poor mapping quality and long intronic regions found in the NTRK genes, DNA-based next-generation sequencing does not yield reliable results for detection [44]. Additional efforts have been to circumvent this with the use of RNA-based next-generation sequencing, but has also shown to be limiting due to the labile nature of RNA [44,45]. However, more analysis must be performed on which method most optimally balances cost, accuracy, and turnaround time. Furthermore, the number of possible NTRK fusion partners may be infinite, which may complicate NTRK detection. Reliable and standardized diagnostic methods will expand patient accessibility and TRK inhibitor applicability to cancer treatment [44].
Larotrectinib provides effective treatment and durable response for numerous NTRK-fusion positive tumors. With continued adverse effect evaluation and evolving NTRK-fusion detection, larotrectinib stands as a groundbreaking option in precision oncology.
3.1.2 Entrectinib in NTRK-Fusion Positive Solid Cancers
Shortly after larotrectinib, another first-generation TRK inhibitor known as entrectinib was approved by the FDA in 2019 [46]. Entrectinib uses the metabolite M5 to competitively inhibit NTRK, ROS proto-oncogene 1, tyrosine kinase (ROS1), and anaplastic lymphoma kinase (ALK) [47]. Entrectinib uniquely remains in the central nervous system as a weak p-glycoprotein substrate and demonstrates weak interaction with efflux transporters, which allows for better blood-brain barrier penetration than larotrectinib [48]. Because of its versatile multikinase properties, entrectinib is approved both for the treatment of advanced or metastatic NTRK fusion-positive tumors in adult and pediatric patients with disease progression following treatment or without satisfactory alternative therapy and for the treatment of ROS1-positive non-small cell lung cancer (NSCLC) [49,50]. These approvals are rooted in the numerous clinical trials exploring entrectinib’s efficacy across different tumor types [49,50].
Extensive analyses have evaluated entrectinib’s therapeutic impact. ALKA-371-001, STARTRK-1, and STARTRK-2 each included patients with advanced or metastatic NTRK fusion-positive tumors with ORR as the primary endpoint [47,51]. The most common cancer types included sarcoma, NSCLC, mammary analogue secretory carcinoma, breast cancer, thyroid cancer, and colorectal cancer. A pooled analysis of these three trials demonstrated an ORR of 57.4% (95% CI: 43.2–70.8) with a mDOR of 10.4 months (95% CI: 7.1–Not Estimable (NE)), while an updated pooled analysis showed an ORR of 61.2% (95% CI: 51.9–69.9) and mDOR of 20 months (95% CI: 13–38.2) [47,51]. An analysis of patients with brain metastasis yielded an intracranial ORR of 63.6% [45]. Additionally, a pooled analysis of the STARTRK-NG and phase II TAPISTRY trials demonstrated efficacy in pediatric patients with an ORR 70% (95% CI: 51–84) and mDOR of 25.4 months (95% CI: 14.3–NE) [46]. These promising results have established entrectinib as a reliable choice in cancer treatment.
As with most targeted therapies, entrectinib administration has specific safety concerns. A variety of adverse effects may be expected, including dizziness, diarrhea, constipation, cough, and weight gain [47]. More serious adverse events include QT interval prolongation, vision changes, and neurotoxicity [47]. Additionally, entrectinib metabolism by cytochrome P450 3A4 and CA5 indicates potential drug-drug interactions that must be accounted for when dosing. Further research must be conducted to fully characterize these safety concerns and their long-term implications [49].
Entrectinib has proven itself an important multikinase inhibitor with durable response against ROS1-positive NSCLC and NTRK fusion-positive tumors with CNS metastatic potential. If balanced with potential adverse effects, entrectinib’s versatility allows it to optimize patient outcomes.
3.1.3 Repotrectinib in NTRK-Fusion Positive Solid Cancers
As tumors developed resistance to first-generation TRK inhibitors, second-generation TRK inhibitors, such as repotrectinib, were created. Repotrectinib is a small-molecule kinase inhibitor that specifically targets several tropomyosin receptor kinases including ROS1, TRKA, TRKB, and TRKC [52–54]. Repotrectinib was approved in 2023 for locally advanced or metastatic ROS1-positive NSCLC and in 2024 for advanced or metastatic NTRK fusion-positive solid tumors in adults and pediatric patients 12 years and older [55]. These recent approvals were supported by clinical trials demonstrating repotrectinib’s unique tumor applications.
The primary clinical trial for repotrectinib, TRIDENT-1, provided critical evidence supporting repotrectinib’s clinical benefits. TRIDENT-1 included patients with advanced solid tumors expressing ALK, ROS, and NTRK rearrangements. Akin to the trials that led to the approval of first-generation TRK inhibitors, the primary endpoint of TRIDENT-1 was ORR. This study yielded an ORR of 58% in tyrosine kinase inhibitor (TKI)-naive patients and an ORR of 50% in TKI-pretreated patients. Additionally, a response was detected in each of the 5 patients with CNS metastatic disease [56–58]. Repotrectinib provides an effective second-generation treatment option for advanced tumors that may not be treatable by other means.
Repotrectinib has proven effective in treating rare tumors, but not without notable side effects. Adverse effects of repotrectinib include dizziness, nausea, constipation, peripheral neuropathy, ataxia, cognitive impairment, dyspnea, muscular weakness, and fatigue [59]. Because of repotrectinib’s novelty, more research is needed to balance efficacy with tolerability.
3.1.4 Resistance Mechanisms in NTRK-Fusion Positive Solid Tumors
The approval of NTRK inhibitors has revolutionized cancer therapy but also opened many discussions. As tumors progress, they may acquire resistance to TRK inhibitors. Repotrectinib has shown efficacy in targeting tumors with solvent front mutations (SFM), ALK, and other non-SFM mutations. Although no specific NTRK fusion gene partners have been found resistant to therapies, mechanisms of resistance include NTRK solvent front mutations, xDFG motif, gatekeeper residue mutations, which directly affect drug binding [60]. Additionally, tumors may utilize alternative signaling pathways or downstream pathway mutations, allowing the tumor to bypass the effects of NTRK inhibition [61,62]. Meanwhile, other second-generation TRK inhibitors, such as selitrectinib and taletrectinib, are currently under development. Combination therapy, such as selitrectinib with crizotinib, may also prove useful to bypass resistance mechanisms [63]. However, the rarity of NTRK fusions poses significant challenges to the continued evaluation of new therapies [33,47]. Although further research and collaborative efforts are necessary to address these limitations, repotrectinib has already secured itself as a cornerstone therapy supported by promising clinical results.
3.2 V-Raf Murine Sarcoma Viral Oncogene Homolog B (BRAF V600E)-Altered Solid Cancers
3.2.1 Dabrafenib Plus Trametinib in BRAFV600E-Altered Solid Cancers
BRAF Class I mutations possess strong kinase activity compared to BRAF Class II and Class III and are solely found at the V600 locus, making this mutation class a target for new cancer therapies [64]. The combination therapy with dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase kinase (MEK) inhibitor, targets the MAPK signaling cascade for phosphorylating several proteins, including RAS, BRAF, MEK, and extracellular signal-regulated kinase (ERK) [65,66]. In combination, this therapy regulates several processes in the tumor life cycle, including cell proliferation, survival, and differentiation in cancers with BRAFV600E mutations. Blocking the BRAF protein and inhibiting MEK in combination effectively disrupts the activation of the MAPK pathway [65–67]. This combination therapy was approved in June 2022 for the treatment of unresectable or metastatic solid tumors, mainly biliary tree cancer (BTC), high-grade glioma (HGG), and low-grade glioma (LGG), which exhibited BRAFV600E mutations in adult or pediatric patients ≥6 years of age [65–67].
One of the greatest upsides to using dabrafenib is that it targets mutations in rare cancer types, including gliomas, biliary tract cancer, and gastrointestinal stromal tumors, among others [68]. An important drawback to using dabrafenib monotherapy is the paradoxical activation of the MAPK pathway, hence why trametinib is utilized in combination for disrupting the MAPK downstream proteins MEK1 and MEK2 [68].
Three trials (ROAR, NCI-MATCH, and Study X2101) evaluated the effectiveness of dabrafenib and trametinib focused on treating various cancer types exhibiting BRAFV600E mutations with the primary endpoint being ORR; ROAR and NCI-MATCH (adult patient population), and Study X2101 trials (pediatric patient population as young as six years old) [65,68,69]. The adult populations demonstrated an ORR of 46% in biliary tract cancer (95% CI: 31–61), 33% for high-grade glioma (95% CI: 20–48), and 50% for low-grade glioma (95% CI: 23–77) [65,68,69]. Comparably, Study X2101 demonstrated an ORR was 25% (95% CI: 12–42) and a duration of response (DOR) of ≥6 months for 78% of patients and ≥24 months for 44% of patients [65].
Among the adult populations, the most frequent adverse effects with this combination therapy included pyrexia, headache, hemorrhage, fatigue, rash, nausea, vomiting, diarrhea, constipation, cough, myalgia, arthralgia, and edema [65]. The pediatric population in Study X2101 exhibited similar adverse effects with the addition of dry skin, dermatitis, acneiform, abdominal pain, and paronychia [68].
3.2.2 Resistance Mechanisms in BRAFV600E-Altered Solid Cancers
One mechanism of resistance that the BRAFV600E mutation utilizes is driven by alterations in MAPK-dependent and MAPK independent pathways, thereby evading selective inhibition [66]. Other alterations in these signaling pathways involve the hyperactivation of tyrosine kinases seen in the PI3K/AKT pathway [70,71]. This leads to secondary mutations that alter downstream pathways to circumvent inhibition, seen with mutations in components including NRAS [70,71]. Although proven effective for inhibiting the MAPK signaling cascade in cancers with BRAFV600E mutations, further research is necessary to improve the current effectiveness of dabrafenib + trametinib combination therapy while also limiting the common adverse effects.
3.3 Rearranged during Transfection (RET)-Altered Solid Cancers
Rearranged during transfection (RET) is a transforming proto-oncogene which encodes a receptor tyrosine kinase initially discovered in a patient with T-cell lymphoma [72]. Since its discovery, it has been shown to be involved in the fetal development of several body systems, including the nervous, genitourinary, hematopoietic, and gastrointestinal systems [73–75]. Due to its involvement in the embryogenesis of several body systems, RET fusions and mutations are found in a diverse set of cancers including lung, colorectal, breast, and thyroid cancers [76,77]. Activation of this oncogenic driver is done mainly through gene fusions and point mutations with >35 different RET fusion genes, more commonly seen with NSCLC and papillary thyroid cancer (PTC) [78–80]. In contrast to activation by fusion mutations seen in NSCLC and PTC cancers, multiple endocrine neoplasia type 2 (MEN2) syndromes display RET point mutations that cluster in the cysteine-rich domain in exons 10, 11, and the tyrosine kinase domains in exons 13 and 16 [81,82]. The RETM918T point mutation is the most common and most aggressive within the kinase domain of the RET gene [83].
3.3.1 Selpercatinib in RET-Altered Solid Cancers
Selpercatinib is a tyrosine kinase inhibitor directed towards inhibiting the rearranged during transfection (RET) gene, a proto-oncogenic transmembrane receptor kinase [84,85]. Selpercatinib functions to inhibit downstream signaling cascades, further disrupting important processes for tumor growth and proliferation. Selpercatinib’s expedited approval in 2020 has provided another treatment option for patients with metastatic RET fusion-positive NSCLC and metastatic RET mutant medullary thyroid cancers [84,85]. It received further FDA approval for its utilization in unresectable or metastatic RET-positive cancers, locally advanced or metastatic solid tumors in patients ≥ 12 years old, in September 2022 [84,85].
Early efficacy data in the LIBRETTO-001 phase I/II recommended dosing at 120 mg twice daily (<50 kg) or 160 mg twice daily (≥50 kg) [86]. Further evaluation of the efficacy of selpercatinib was done in an ongoing multicenter, open-label multi-cohort trial in 41 patients with RET fusion-positive tumors beyond NSCLC and thyroid cancers. These tumor types included pancreatic adenocarcinoma, colorectal, salivary, unknown primary, breast, soft tissue sarcoma, bronchial carcinoid, ovarian, small intestine, and cholangiocarcinoma [87]. The ORR was 44% (95% CI: 41–87) with a DOR of 24.5 months (95% CI: 9.2–NE). At 6 months, 67% (95% CI: 41–87) were continuing to respond positively to selpercatinib, and 56% at 12 months (95% CI: 31–78) [87].
Although selpercatinib has shown incredible benefit to many cancer types, patients in these early trials experienced a range of adverse reactions including nausea, dry mouth, fatigue, edema and rash; more serious adverse effects being hypertension and elevated liver aminotransferases [86,87].
Recent updates to LIBRETTO-001 have included 52 more patients and 16 months of longer follow-up. Most patients in this update had GI RET-positive cancers (n = 31), with pancreatic and colorectal being the most common. Among this group of patients, the ORR was 44.2% (95% CI: 30.5–58.7), with pancreatic tumors being 53.8% and 30.8% in colorectal tumors [88]. The median DOR among this group across all tumor types was 37.2 months [88]. A significant number of colorectal cancer (CRC) patients also had MSI-H status, suggesting that combinational therapies may provide further benefit [88]. This updated data further demonstrates the vast anti-tumor activity against RET-positive tumors and further provides hope in impacting the lives of patients with several types of solid-organ, metastatic, RET-positive cancers.
3.3.2 Resistance Mechanisms in RET-Altered Solid Cancers
RET-altered cancers utilize gatekeeper mutants as a mechanism of resistance to treatments, as seen in RETV804M/L [89–91]. These mutations interrupt the ability to bind to the RET kinase domains by creating steric hindrance and preventing binding due to the V804 gatekeeper and invariant gatewall K758 residues [89–91]. Solvent front mutations (SFMs) are variations in the surface-exposed amino acid sequence which exist in close proximity to the ATP-binding site in kinases, causing electrostatic forces and reorientation of the surrounding residues [92]. These RETG810 SFMs demonstrated the first described recurrent mechanism by which tumor cells have shown to be resistant against selpercatinib, emerging as early as 3 months after starting treatment [93]. Further research directed at inhibiting gatekeeper mutations, while maintaining activity against SFMs can prove to be the key in treating RET resistance [93].
4 Antibody Drug Conjugate: Tissue-Agnostic Targeting of Cancer Therapeutics
Trastuzumab Deruxtecan in HER2-Positive Solid Cancers
Trastuzumab deruxtecan has brought a new wave of optimism to the treatment of HER2-positive solid tumors. This innovative therapy combines the precision of HER2-targeting trastuzumab with a powerful chemotherapy payload, deruxtecan, delivered directly to cancer cells expressing HER2 receptors [94–96]. The result is a targeted yet potent attack on tumors, sparing healthy cells and minimizing many of the broader side effects seen with traditional chemotherapies; a remarkable step forward for patients who often face limited options when their cancer progresses.
In the DESTINY-PanTumor02 trials, trastuzumab-deruxtecan (T-DXd) demonstrated exceptional efficacy, particularly in patients with advanced or metastatic HER2-positive breast cancer. Among those who had undergone extensive prior treatments, this therapy achieved an ORR of 51.4% (95% CI: 41.7–61.0) and a mDOR of 19.4 months, offering many patients durable and meaningful responses [94,95]. Importantly, this therapy has shown promise beyond breast cancer, extending to HER2-positive gastric and non-small cell lung cancers [95].
However, as with any powerful treatment, there are risks. One of the more serious concerns with trastuzumab deruxtecan is interstitial lung disease (ILD), which, although rare, requires close monitoring and swift intervention if symptoms develop [95,96]. Other side effects, such as nausea, fatigue, and reduced blood cell counts, are more common but generally manageable with supportive care [94–96]. Despite these challenges, the benefits of the therapy often outweigh the risks for patients who have run out of options [96].
The FDA’s approval of T-DXd for HER2-positive solid cancers represents a significant shift in how we think about HER2-driven diseases. While issues like its high cost (as much as $4281/cycle) remain, ongoing research is looking to refine its use and extend its reach to other tumor types [97]. For patients and their families, trastuzumab deruxtecan offers hope where little existed before—a testament to the power of combining cutting-edge science with compassionate care.
Resistance Mechanisms in HER2-Positive Solid Cancers
Several mechanisms of resistance have been seen in the use of current treatments for HER2-positive solid cancers including mutations within the HER2 receptor leading to alterations in structure, thereby reducing the ability for therapies to bind and inhibit tumor development [98]. Epigenetic alterations have also shown to play a role in resistance, allowing for tumor cells to rely on constitutive activation of parallel signaling pathways involving MET proto-oncogene receptor tyrosine kinase (MET), Human Epidermal growth factor Receptor 3 (HER3) and fibroblast growth factor receptor (FGFR) to bypass HER2 inhibition [98,99]. The involvement and activation of the cyclin D1-CDK4/6 proteins that regulate the G1-S transition in the cell cycle have also been implicated in the resistance of treatment, enhancing the ability to escape from antibody-dependent cellular cytotoxicity [98–100].
5 Challenges and Future Directions
Tissue-agnostic therapies, while groundbreaking, face significant challenges that limit their potential. One of the key obstacles is the development of resistance. Tumors often adapt to treatment by acquiring secondary mutations, reactivating alternative signaling pathways, or evading immune system detection—all of which are hallmarks of cancer—which can undermine the effectiveness of these therapies over time [101]. To overcome these resistance mechanisms, researchers are exploring innovative solutions, including combination therapies that target multiple pathways simultaneously, aiming to improve treatment durability and outcomes.
Another major hurdle is access to the advanced diagnostics required to identify patients who could benefit from these therapies. Molecular testing, essential for detecting biomarkers like MSI-H or TMB-H, can be prohibitively expensive and is often unavailable in resource-limited regions [16,17,30]. This disparity creates significant barriers to equitable treatment access. Addressing this issue will require expanding diagnostic infrastructure, lowering the costs of testing, and ensuring that these advancements are accessible to patients everywhere.
Adaptive clinical trial designs, such as basket trials, have shown promise in evaluating tissue-agnostic therapies across diverse tumor types. These trials allow researchers to test treatments based on genetic markers rather than tumor location, accelerating the development of new therapies. However, they face challenges, including small sample sizes and the variability of patient populations, which can make it difficult to draw definitive conclusions [102]. To maximize their effectiveness, these trials require careful design and collaboration among researchers, clinicians, and regulatory agencies.
Despite these obstacles, tissue-agnostic therapies represent a significant leap forward in precision medicine. By addressing resistance, improving access to diagnostics, and refining clinical trial methods, the field can continue to advance and bring these transformative treatments to more patients in need [2]. Adaptive clinical trial designs will require collaboration among clinicians, governmental regulatory agencies, and the pharmaceutical industry in order to continue to bring promise and hope for patients and their families.
6 Future Tissue-Agnostic Targeting of Cancer Therapeutics
Advancements in precision oncology continue to expand as more tumor-agnostic treatments are approved. KRASG12C inhibitors, fibroblast growth factor receptor (FGFR) inhibitors, and neuregulin 1 (NRG1) fusion inhibitors show promise across multiple cancers, with ongoing trials evaluating their efficacy.
Kirsten rat sarcoma viral oncogene homolog (KRAS) serves as a prominent oncogenic target due to its prevalence in human cancers. Despite its early discovery, researchers initially considered it undruggable [103]. The identification of the switch II pocket in the KRASG12C isotype enabled targeted therapies to bind the site, yielding promising results for pan-cancer populations, especially in patients with NSCLC [103]. Covalent KRASG12C inhibitors, such as sotorasib and adagrasib, have shown success in the CodeBreaK and KRYSTAL trials, respectively. In a pan-cancer cohort in the KRYSTAL-1 trial, 64 patients who received adagrasib had an ORR of 35.1%, mDOR of 5.3 months, and median progression-free survival (mPFS) of 7.4 months [104]. Sotorasib demonstrated its potential as a tumor-agnostic agent in the CodeBreaK 100 trial, with 59 patients with NSCLC showing an ORR of 32.2%, and 42 patients with CRC showing an ORR of 7.1% [105]. Divarasib is another KRASG12C inhibitor that exhibited an ORR of 53.1% in NSCLC and 29.1% in CRC patients [106]. Although cross-trial data are limited, divarasib demonstrates numerically superior efficacy compared to sotorasib and adagrasib. The study included tumor types beyond NSCLC and CRC, but was constrained by the patient population. Variations in efficacy across cancers may stem from biological differences, the distribution of mutations, and resistance mechanisms. For example, KRASG12D inhibitors are under investigation but face challenges due to the absence of a cysteine residue adjacent to the switch II pocket, thus, a new approach was required to access the binding site. Additionally, the KRASG12D mutation is more prevalent in cancers like pancreatic ductal adenocarcinoma (PDAC), distinguishing it from KRASG12C, which occurs in other tumor types [107]. A Phase 1 trial using zoldonrasib (RMC-9805) is currently being studied as a promising treatment for patients with the KRASG12D mutation, and works as a molecular-glue to target the constitutively-activated KRAS protein [108].
FGFR is a family of tyrosine kinases involved in cellular proliferation and survival. The RAGNAR trial demonstrated that erdafitinib achieved an ORR of 64% in patients with FGFR1-3 alterations (mutations and fusions) across 16 solid tumor types, outperforming other FGFR inhibitors with lower response rates [109]. In contrast, the NCI-MATCH trial reported a more modest ORR of 16% among 25 patients with FGFR alterations, with a median duration of response of less than six months. Erdafitinib’s toxicity profile may restrict its pan-cancer use, as it showed higher rates of dose reduction (63%), interruption (75%), and discontinuation (10%) compared to other FGFR inhibitors [110]. Comparable to the RAGNAR trial, the phase 2 trial, FIGHT-207, examined the tumor-agnostic capabilities of pemigatinib as a FGFR1-FGFR3 inhibitor, in three cohorts (Cohort A: FGFR fusions/rearrangements, Cohort B: FGFR actionable single nucleotide variants (SNVs), and Cohort C: FGFR kinase domain mutations and variants of uncertain significance) [111]. The ORR in cohort A, B, and C was 26.5%, 9.4%, and 3.8%, respectively [111]. Further investigation efforts are necessary to examine the challenges in targeting FGFR alterations including mutation diversity, diagnostic limitations, and tumor heterogeneity.
NRG1 is a ligand that binds to HER receptors. NRG1 fusions result in overexpression of the NRG1 protein, which can promote oncogenesis via upregulation of proliferative pathways [112]. The recent development of zenocutuzumab represents a unique mechanism of action as a bispecific antibody. It targets NRG1 fusions by binding to both HER2 and HER3 on the surface of cancer and immune cells [113]. This interaction brings cancer cells closer to immune cells, promoting antibody-dependent cytotoxicity through immune activation. In the eNRGy trial evaluating zenocutuzumab, patients with NRG1 fusion-positive solid tumors demonstrated clinical efficacy. On 4 December 2024, zenocutuzumab received FDA approval for NSCLC and pancreatic adenocarcinomas based on data from the eNRGy trial. In an updated follow-up, 158 patients across 12 unique cancer types had an ORR of 30% and a mPFS of 6.8 months [114]. The side effects were tolerable, with only one patient who discontinued due to treatment-related adverse events.
Ongoing global efforts to examine the efficacy of immunotherapy through a tumor-agnostic approach are continually being evaluated through the multicenter, open-label, multi-cohort TAPISTRY platform study [115]. Several of the aforementioned therapies including (and not limited to) entrectinib, trastuzumab, and divarasib are being studied in patients with unresectable, locally advanced, or metastatic solid tumors [115]. The TAPISTRY platform study is still underway with the primary completion estimated to be on 25 September 2032, and finalized results are expected to follow once these trials are completed [115].
The development of KRASG12C inhibitors, FGFR inhibitors, and NRG1-targeted therapies marks a significant step forward in tumor-agnostic treatments. With ongoing research, these agents have the potential to offer new, more effective treatment options for patients with diverse and difficult-to-target cancers. Additionally, other agents with novel mechanisms of action are likely to be approved in the future, further advancing precision oncology. The future of tumor-agnostic therapies is promising, with the potential for more effective and personalized cancer care.
7 Impact of Trial Designs in Tissue-Agnostic Targeting of Cancer Therapeutics
Tumor-agnostic therapy development is driven by innovative clinical trial designs that prioritize molecular characteristics over anatomical tumor origin. These designs enable researchers to evaluate the efficacy of therapies across diverse cancer types unified by shared genomic alterations. Unlike traditional site-specific trials, tumor-agnostic trials employ frameworks that allow for patient recruitment based on biomarkers, which accelerates drug development and enhances clinical relevance in precision oncology settings [114,116].
Master protocols have emerged as a cornerstone in the development of tumor-agnostic therapies. These include basket trials, which test a single therapy across various tumor types with the same genetic alteration (e.g., NCI-MATCH, KEYNOTE-158) [9,117], umbrella trials, which focus on one cancer type but assign patients to treatment arms based on specific molecular profiles (e.g., National Lung Matrix Trial) [118], and platform trials, which are adaptive, multi-arm studies allowing therapies to enter or exit dynamically (e.g., I-SPY 2, GBM AGILE) [119,120]. Additionally, N-of-1 trials offer highly individualized testing strategies, making them ideal for rare or ultra-rare cancers where traditional enrollment is impractical [121,122].
The NCI-MATCH trial (Molecular Analysis for Therapy Choice) is a landmark basket trial that matched patients with actionable genetic mutations to targeted therapies regardless of cancer type [9,123]. It validated the feasibility of tumor-agnostic strategies using large-scale genomic sequencing and flexible trial structures, enrolling over 1200 patients across 38 treatment arms. Importantly, it demonstrated that patients with rare cancers often have a higher prevalence of actionable mutations, supporting the inclusion of underserved populations in precision oncology [51].
Expanding on this approach, the ComboMATCH trial (launched in 2023) seeks to improve outcomes by evaluating combinatorial therapies—dual-targeted regimens or targeted drugs plus chemotherapy—to address resistance mechanisms observed in monotherapies [124]. Its trial architecture builds on NCI-MATCH but incorporates more extensive patient cohorts and adaptive mechanisms, with a particular focus on achieving durable responses. This model enhances treatment personalization and reflects a broader shift in clinical oncology toward combination-based strategies.
The TAPUR study, initiated by ASCO in 2015, complements these studies by assessing the off-label use of FDA-approved therapies in real-world clinical settings [125]. By enrolling patients with advanced cancers who have exhausted standard treatments, TAPUR evaluates molecularly targeted drugs across various tumor types. A significant outcome of TAPUR was the identification of TMB as a predictive biomarker, influencing the FDA’s approval of pembrolizumab for TMB-high cancers [8]. Collectively, these trials—NCI-MATCH, ComboMATCH, and TAPUR—demonstrate the transformative potential of trial design innovations in expanding access to and the effectiveness of tumor-agnostic therapies [124].
Tissue-agnostic therapies have truly transformed the way we think about cancer treatment. For decades, treatments were largely determined by where a tumor started, which often left patients with rare or difficult-to-treat cancers without many options. Now, by focusing on the genetic and molecular drivers behind cancer growth, we are not only providing more precise care but also giving hope to patients who once had very few paths forward. This shift reflects how far we have come in understanding the biology of cancer and how to fight it effectively.
That said, the journey is far from over. One of the biggest challenges is that cancers can adapt and find ways to resist even the most advanced treatments. Tumors can develop secondary mutations or activate alternative pathways, making therapies less effective over time. On top of that, the cost of these treatments and the advanced testing needed to identify eligible patients can put them out of reach for many, especially in places with limited healthcare resources. To truly make these therapies accessible to everyone who needs them, we must find ways to make testing and treatments more affordable and widely available.
The future of tissue-agnostic therapies is exciting. Researchers are working hard to discover new genetic targets, and advancements in technology like artificial intelligence are making it easier to identify which patients are likely to benefit the most. Combination therapies, which attack cancer from multiple angles, are also showing promise in overcoming resistance and improving long-term outcomes.
Tissue-agnostic therapies are a powerful reminder of what’s possible when science focuses on the unique needs of individuals. They have given us a new way to think about and treat cancer, offering personalized, targeted options that have already transformed countless lives. Once believed to be undruggable cancers have now become druggable, all thanks to the continued efforts and funding of researchers globally. Expanding the global collaborative efforts through the funding of trials will not only hasten the approval of current tissue-agnostic therapies, but acts to further preserve hope for patients without other therapeutic options. As we continue to innovate and expand access, these therapies will no doubt shape the future landscape of oncology, bringing hope and better outcomes to patients everywhere.
Acknowledgement: Not applicable.
Funding Statement: The authors declare that no funding was provided to perform this research activity.
Author Contributions: Conceptualization, Matthew Rubinstein, Madeline Lauren Hong, Kyaw Zin Thein; methodology, Kyaw Zin Thein; software, Kyaw Zin Thein; validation, Kyaw Zin Thein; formal analysis, Kyaw Zin Thein; investigation, Matthew Rubinstein, Madeline Lauren Hong, Rishi Kumar Nanda, Daniel Thomas Jones, Kyaw Zin Thein; resources, Matthew Rubinstein, Madeline Lauren Hong, Rishi Kumar Nanda, Daniel Thomas Jones, Kyaw Zin Thein; writing—original draft preparation, Matthew Rubinstein, Madeline Lauren Hong, Rishi Kumar Nanda, Daniel Thomas Jones, Hazem Aboaid, Yin Mon Myat, Kyaw Zin Thein; writing—review and editing, Matthew Rubinstein, Madeline Lauren Hong, Kyaw Zin Thein; visualization, Yin Mon Myat, Kyaw Zin Thein; supervision, Kyaw Zin Thein; project administration, Kyaw Zin Thein; funding acquisition, Kyaw Zin Thein. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: All relevant data is contained within the manuscript: All datasets analyzed for this study are included in the manuscript.
Ethics Approval: Not applicable.
Conflicts of Interest: Kyaw Z. Thein received honoraria from Targeted Oncology, MD Outlook, Curio Science, Aptitude Health, Inizio, OMNI-Oncology, and Onviv Expert Network and served on the Advisory Board for EMD Serono and on the Speaker Bureau for Eisai, not related to this manuscript. All other authors declare that they have no conflicts of interest.
References
1. Mansinho A, Fernandes RM, Carneiro AV. Histology-agnostic drugs: a paradigm shift—a narrative review. Adv Ther. 2023 Apr;40(4):1379–92. doi:10.1007/s12325-022-02362-4. [Google Scholar] [PubMed] [CrossRef]
2. Gouda MA, Subbiah V. Tissue-agnostic cancer therapy approvals. Surg Oncol Clin N Am. 2024;33(2):243–64. doi:10.1016/j.soc.2023.12.001. [Google Scholar] [PubMed] [CrossRef]
3. Subbiah V, Gouda MA, Ryll B, Burris HA3rd, Kurzrock R. The evolving landscape of tissue-agnostic therapies in precision oncology. CA Cancer J Clin. 2024;74(5):433–52. doi:10.3322/caac.21844. [Google Scholar] [PubMed] [CrossRef]
4. Flaherty KT, Le DT, Lemery S. Tissue-agnostic drug development. Am Soc Clin Oncol Educ Book. 2017;37(37):222–30. doi:10.1200/EDBK_173855. [Google Scholar] [PubMed] [CrossRef]
5. Subbiah V, Kurzrock R. The marriage between genomics and immunotherapy: mismatch meets its match. Oncologist. 2019 Jan;24(1):1–3. doi:10.1634/theoncologist.2017-0519. [Google Scholar] [PubMed] [CrossRef]
6. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med. 2020;383(23):2207–18. doi:10.1056/NEJMoa2017699. [Google Scholar] [PubMed] [CrossRef]
7. U.S. Food and Drug Administration. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication [Internet]. [cited 2025 Apr 6]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication. [Google Scholar]
8. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65. doi:10.1016/S1470-2045(20)30445-9. [Google Scholar] [PubMed] [CrossRef]
9. Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753–8. doi:10.1158/1078-0432.CCR-18-4070. [Google Scholar] [PubMed] [CrossRef]
10. Alkholifi FK, Alsaffar RM. Dostarlimab an inhibitor of PD-1/PD-L1: a new paradigm for the treatment of cancer. Medicina. 2022;58(11):1572. doi:10.3390/medicina58111572. [Google Scholar] [PubMed] [CrossRef]
11. Patel AB, Pacha O. Skin reactions to immune checkpoint inhibitors. Adv Exp Med Biol. 2018;995(5):117–29. doi:10.1007/978-3-319-53156-4_9. [Google Scholar] [PubMed] [CrossRef]
12. Baraibar I, Melero I, Ponz-Sarvise M, Castanon E. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf. 2019;42(2):281–94. doi:10.1007/s40264-018-0774-8. [Google Scholar] [PubMed] [CrossRef]
13. Vaddepally R, Doddamani R, Sodavarapu S, Madam NR, Katkar R, Kutadi AP, et al. Review of immune-related adverse events (irAEs) in non-small-cell lung cancer (NSCLC)-their incidence, management, multiorgan irAEs, and rechallenge. Biomedicines. 2022;10(4):790. doi:10.3390/biomedicines10040790. [Google Scholar] [PubMed] [CrossRef]
14. Reardon DA, Gokhale PC, Klein SR, Ligon KL, Rodig SJ, Ramkissoon SH, et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol Res. 2016 Feb;4(2):124–35. doi:10.1158/2326-6066.CIR-15-0151. [Google Scholar] [PubMed] [CrossRef]
15. Shimozaki K, Hayashi H, Tanishima S, Horie S, Chida A, Tsugaru K, et al. Concordance analysis of microsatellite instability status between polymerase chain reaction based testing and next generation sequencing for solid tumors. Sci Rep. 2021 Oct 8;11(1):20003. doi:10.1038/s41598-021-99364-z. [Google Scholar] [PubMed] [CrossRef]
16. Cicala CM, Musacchio L, Scambia G, Lorusso D. Dostarlimab: from preclinical investigation to drug approval and future directions. Hum Vaccin Immunother. 2023;19(1):2178220. doi:10.1080/21645515.2023.2178220. [Google Scholar] [PubMed] [CrossRef]
17. André T, Berton D, Curigliano G, Sabatier R, Tinker AV, Oaknin A, et al. Antitumor activity and safety of dostarlimab monotherapy in patients with mismatch repair deficient solid tumors: a nonrandomized controlled trial. JAMA Netw Open. 2023;6(11):e2341165. doi:10.1001/jamanetworkopen.2023.41165. [Google Scholar] [PubMed] [CrossRef]
18. U.S. Food and Drug Administration. FDA grants accelerated approval to dostarlimab-gxly for dMMR advanced solid tumors [Internet]. [cited 2025 Apr 6]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-advanced-solid-tumors. [Google Scholar]
19. Bhamidipati D, Subbiah V. Impact of tissue-agnostic approvals for patients with gastrointestinal malignancies. Trends Cancer. 2023;9(3):237–49. doi:10.1016/j.trecan.2022.11.003. [Google Scholar] [PubMed] [CrossRef]
20. Wang B, Han Y, Zhang Y, Zhao Q, Wang H, Wei J, et al. Overcoming acquired resistance to cancer immune checkpoint therapy: potential strategies based on molecular mechanisms. Cell Biosci. 2023;13(1):1–23. doi:10.1186/s13578-023-01073-9. [Google Scholar] [PubMed] [CrossRef]
21. Wu Z, Zhang S, Li L, Huang Z, Huang D, Hu Y. The gut microbiota modulates responses to anti-PD-1 and chemotherapy combination therapy and related adverse events in patients with advanced solid tumors. Front Oncol. 2022 Oct 25;12:887383. doi:10.3389/fonc.2022.887383. [Google Scholar] [PubMed] [CrossRef]
22. Cristescu R, Aurora-Garg D, Albright A, Xu L, Liu XQ, Loboda A, et al. Tumor mutational burden predicts the efficacy of pembrolizumab monotherapy: a pan-tumor retrospective analysis of participants with advanced solid tumors. J Immunother Cancer. 2022;10(1):e003091. doi:10.1136/jitc-2021-003091. [Google Scholar] [PubMed] [CrossRef]
23. Lin DI, Quintanilha JCF, Danziger N, Lang L, Levitan D, Hayne C, et al. Pan-tumor validation of a NGS fraction-based MSI analysis as a predictor of response to Pembrolizumab. npj Precis Oncol. 2024 Sep 14;8(1):204. doi:10.1038/s41698-024-00679-7. [Google Scholar] [PubMed] [CrossRef]
24. Gao C, Li H, Liu C, Xu X, Zhuang J, Zhou C, et al. Tumor mutation burden and immune invasion characteristics in triple negative breast cancer: genome high-throughput data analysis. Front Immunol. 2021;12:650491. doi:10.3389/fimmu.2021.650491. [Google Scholar] [PubMed] [CrossRef]
25. Wang P, Chen Y, Wang C. Beyond tumor mutation burden: tumor neoantigen burden as a biomarker for immunotherapy and other types of therapy. Front Oncol. 2021;11:672677. doi:10.3389/fonc.2021.672677. [Google Scholar] [PubMed] [CrossRef]
26. U.S. Food and Drug Administration. FDA approves pembrolizumab for adults and children with TMB-H solid tumors. [cited 2025 Apr 6]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors. [Google Scholar]
27. Marcus L, Fashoyin-Aje LA, Donoghue M, Yuan M, Rodriguez L, Gallagher PS, et al. FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin Cancer Res. 2021;27(17):4685–9. doi:10.1158/1078-0432.CCR-21-0327. [Google Scholar] [PubMed] [CrossRef]
28. Merino DM, McShane LM, Fabrizio D, Funari V, Chen SJ, White JR, et al. Establishing guidelines to harmonize tumor mutational burden (TMBin silico assessment of variation in TMB quantification across diagnostic platforms: phase I of the Friends of Cancer Research TMB Harmonization Project. J Immunother Cancer. 2020;8(1):e000147. doi:10.1136/jitc-2019-000147. [Google Scholar] [PubMed] [CrossRef]
29. Rousseau B, Foote MB, Maron SB, Diplas BH, Lu S, Argilés G, et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N Engl J Med. 2021 Mar 25;384(12):1168–70. doi:10.1056/NEJMc2031965. [Google Scholar] [PubMed] [CrossRef]
30. Ahmed J, Das B, Shin S, Chen A. Challenges and future directions in the management of tumor mutational burden-high (TMB-H) advanced solid malignancies. Cancers. 2023;15(24):5841. doi:10.3390/cancers15245841. [Google Scholar] [PubMed] [CrossRef]
31. Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8(1):1136. doi:10.1038/s41467-017-01062-w. [Google Scholar] [PubMed] [CrossRef]
32. McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, et al. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell. 2017;171(6):1259–71.e11. doi:10.1016/j.cell.2017.10.001. [Google Scholar] [PubMed] [CrossRef]
33. Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–47. doi:10.1038/s41571-018-0113-0. [Google Scholar] [PubMed] [CrossRef]
34. Gouda MA, Nelson BE, Buschhorn L, Wahida A, Subbiah V. Tumor-agnostic precision medicine from the AACR GENIE database: clinical implications. Clin Cancer Res. 2023 Aug 1;29(15):2753–60. doi:10.1158/1078-0432.CCR-23-0090. [Google Scholar] [PubMed] [CrossRef]
35. Manea CA, Badiu DC, Ploscaru IC, Zgura A, Bacinschi X, Smarandache CG, et al. A review of NTRK fusions in cancer. Ann Med Surg. 2022 Sep;79:103893. doi:10.1016/j.amsu.2022.103893. [Google Scholar] [PubMed] [CrossRef]
36. U.S. Food and Drug Administration. FDA approves larotrectinib for solid tumors with NTRK gene fusions [Internet]; 2018. [cited 2025 Apr 6]. Available from: https://www.fda.gov/drugs/fda-approves-larotrectinib-solid-tumors-ntrk-gene-fusions. [Google Scholar]
37. Filippi R, Depetris I, Satolli MA. Evaluating larotrectinib for the treatment of advanced solid tumors harboring an NTRK gene fusion. Expert Opin Pharmacother. 2021 Apr;22(6):677–84. doi:10.1080/14656566.2021.1876664. [Google Scholar] [PubMed] [CrossRef]
38. Berger S, Martens UM, Bochum S. Larotrectinib (LOXO-101). Recent Results Cancer Res. 2018;211(16):141–51. doi:10.1007/978-3-319-91442-8_10. [Google Scholar] [PubMed] [CrossRef]
39. Bayer HealthCare Pharmaceuticals Inc. VITRAKVI® (larotrectinib) capsules, for oral use: FDA packaging insert [Internet]; 2022. [cited 2025 Apr 6]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/210861s010lbl.pdf. [Google Scholar]
40. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018 Feb 22;378(8):731–9. doi:10.1056/NEJMoa1714448. [Google Scholar] [PubMed] [CrossRef]
41. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020 Apr;21(4):531–40. doi:10.1016/S1470-2045(19)30856-3. [Google Scholar] [PubMed] [CrossRef]
42. Scott LJ. Larotrectinib: first global approval. Drugs. 2019 Feb;79(2):201–6. doi:10.1007/s40265-018-1044-x. [Google Scholar] [PubMed] [CrossRef]
43. Hong DS, Lassen UN, Jahr S, Kramer D, Schöffski P, Darabi K, et al. Larotrectinib long-term efficacy and safety in adult patients with tropomyosin receptor kinase (TRK) fusion cancer. J Clin Oncol. 2023;41(16_suppl):3141. doi:10.1200/JCO.2023.41.16_suppl.3141. [Google Scholar] [CrossRef]
44. Solomon JP, Benayed R, Hechtman JF, Ladanyi M. Identifying patients with NTRK fusion cancer. Ann Oncol. 2019 Oct;30(Suppl_8):viii16–22. doi:10.1093/annonc/mdz384. [Google Scholar] [CrossRef]
45. Murphy DA, Ely HA, Shoemaker R, Boomer A, Culver BP, Hoskins I, et al. Detecting gene rearrangements in patient populations through a 2-step diagnostic test comprised of rapid IHC enrichment followed by sensitive next-generation sequencing. Appl Immunohistochem Mol Morphol. 2017 Aug;25(7):513–23. doi:10.1097/PAI.0000000000000360. [Google Scholar] [PubMed] [CrossRef]
46. U.S. Food and Drug Administration. FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC [Internet]; 2019. [cited 2025 Apr 6]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc. [Google Scholar]
47. Marcus L, Donoghue M, Aungst S, Myers CE, Helms WS, Shen G, et al. FDA approval summary: entrectinib for the treatment of NTRK gene fusion solid tumors. Clin Cancer Res. 2021 Feb 1;27(4):928–32. doi:10.1158/1078-0432.CCR-20-2771. [Google Scholar] [PubMed] [CrossRef]
48. Fischer H, Ullah M, de la Cruz CC, Hunsaker T, Senn C, Wirz T, et al. Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein. Neuro Oncol. 2020 Jun;22(6):819–29. doi:10.1093/neuonc/noaa052. [Google Scholar] [PubMed] [CrossRef]
49. Genentech, Inc. ROZLYTREK (entrectinib) capsules, for oral use: FDA packaging insert [Internet]; 2022. [cited 2025 Apr 6]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/212725s011lbl.pdf. [Google Scholar]
50. Rolfo C, Passiglia F, Ostrowski M, Farracho L, Ondøichová T, Dolcan A, et al. Improvement in lung cancer outcomes with targeted therapies: an update for family physicians. J Am Board Fam Med. 2015 Jan–Feb;28(1):124–33. doi:10.3122/jabfm.2015.01.140072. [Google Scholar] [PubMed] [CrossRef]
51. Demetri GD, De Braud F, Drilon A, Siena S, Patel MR, Cho BC, et al. Updated integrated analysis of the efficacy and safety of entrectinib in patients with NTRK fusion-positive solid tumors. Clin Cancer Res. 2022 Apr 1;28(7):1302–12. doi:10.1158/1078-0432.CCR-21-3597. [Google Scholar] [PubMed] [CrossRef]
52. Liu Z, Yu P, Dong L, Wang W, Duan S, Wang B, et al. Discovery of the next-generation pan-TRK kinase inhibitors for the treatment of cancer. J Med Chem. 2021 Jul 22;64(14):10286–96. doi:10.1021/acs.jmedchem.1c00712. [Google Scholar] [PubMed] [CrossRef]
53. Drilon A, Ou SI, Cho BC, Kim DW, Lee J, Lin JJ, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent-front mutations. Cancer Discov. 2018 Oct;8(10):1227–36. doi:10.1158/2159-8290.CD-18-0484. [Google Scholar] [PubMed] [CrossRef]
54. Drilon A, Camidge DR, Lin JJ, Kim SW, Solomon BJ, Dziadziuszko R, et al. Repotrectinib in ROS1 fusion-positive non-small-cell lung cancer. N Engl J Med. 2024 Jan 11;390(2):118–31. doi:10.1056/NEJMoa2302299. [Google Scholar] [PubMed] [CrossRef]
55. Bristol Myers Squibb. AUGTYROTM (repotrectinib) capsules, for oral use: FDA packaging insert [Internet]; 2024. [cited 2025 Apr 6]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/218213s001lbl.pdf. [Google Scholar]
56. Besse B, Springfeld C, Baik C, Hervieu A, Solomon B, Moreno V, et al. Update from the ongoing phase 1/2 registrational trial of repotrectinib: results in TKI-naïve and TKI-pretreated patients with NTRK fusion-positive advanced solid tumors (TRIDENT-1). Eur J Cancer. 2022 Oct;174(Suppl 1):S75–6. doi:10.1016/S0959-8049(22)01000-0. [Google Scholar] [CrossRef]
57. Solomon BJ, Drilon A, Lin JJ, Bazhenova L, Goto K, De Langen J, et al. 1372P Repotrectinib in patients (pts) with NTRK fusion-positive (NTRK+) advanced solid tumors, including NSCLC: update from the phase I/II TRIDENT-1 trial. Ann Oncol. 2023 Sep;34(Suppl 2):S787–8. doi:10.1016/j.annonc.2023.09.2405. [Google Scholar] [CrossRef]
58. Chen MF, Yang SR, Shia J, Girshman J, Punn S, Wilhelm C, et al. Response to repotrectinib after development of NTRK resistance mutations on first- and second-generation TRK inhibitors. JCO Precis Oncol. 2023;7(7):e2200697. doi:10.1200/PO.22.00697. [Google Scholar] [PubMed] [CrossRef]
59. U.S. Food and Drug Administration. FDA grants accelerated approval to repotrectinib for adult and pediatric patients with NTRK gene fusion-positive solid tumors [Internet]; 2023. [cited 2025 Apr 6]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-repotrectinib-adult-and-pediatric-patients-ntrk-gene-fusion-positive. [Google Scholar]
60. Xiang S, Lu X. Selective type II TRK inhibitors overcome xDFG mutation mediated acquired resistance to the second-generation inhibitors selitrectinib and repotrectinib. Acta Pharm Sin B. 2024;14(2):517–32. doi:10.1016/j.apsb.2023.11.010. [Google Scholar] [PubMed] [CrossRef]
61. Yang Y, Li S, Wang Y, Zhao Y, Li Q. Protein tyrosine kinase inhibitor resistance in malignant tumors: molecular mechanisms and future perspective. Signal Transduct Target Ther. 2022;7(1):329. doi:10.1038/s41392-022-01168-8. [Google Scholar] [PubMed] [CrossRef]
62. Liu F, Wei Y, Zhang H, Jiang J, Zhang P, Chu Q. NTRK fusion in non-small cell lung cancer: diagnosis, therapy, and TRK inhibitor resistance. Front Oncol. 2022;12:864666. doi:10.3389/fonc.2022.864666. [Google Scholar] [PubMed] [CrossRef]
63. Cocco E, Schram AM, Kulick A, Misale S, Won HH, Yaeger R, et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med. 2019;25(10):1422–7. doi:10.1038/s41591-019-0542-z. [Google Scholar] [PubMed] [CrossRef]
64. Owsley J, Stein MK, Porter J, In GK, Salem M, O’Day S, et al. Prevalence of class I–III BRAF mutations among 114,662 cancer patients in a large genomic database. Exp Biol Med. 2021 Jan;246(1):31–9. doi:10.1177/1535370220959657. [Google Scholar] [PubMed] [CrossRef]
65. FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. [cited 2025 Apr 6]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid. [Google Scholar]
66. Gouda MA, Subbiah V. Expanding the benefit: dabrafenib/trametinib as tissue-agnostic therapy for BRAF V600E-positive adult and pediatric solid tumors. Am Soc Clin Oncol Educ Book. 2023;43(43):e404770. doi:10.1200/EDBK_404770. [Google Scholar] [PubMed] [CrossRef]
67. Barbato MI, Nashed J, Bradford D, Ren Y, Khasar S, Miller CP, et al. FDA approval summary: dabrafenib in combination with trametinib for BRAFV600E mutation-positive low-grade glioma. Clin Cancer Res. 2024;30(2):263–8. doi:10.1158/1078-0432.CCR-23-1503. [Google Scholar] [PubMed] [CrossRef]
68. Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8(2):100788. doi:10.1016/j.esmoop.2023.100788. [Google Scholar] [PubMed] [CrossRef]
69. Subbiah V, Kreitman RJ, Wainberg ZA, Gazzah A, Lassen U, Stein A, et al. Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: the phase 2 ROAR trial. Nat Med. 2023;29(9):1103–12. doi:10.1038/s41591-023-02321-8. [Google Scholar] [PubMed] [CrossRef]
70. Sanchez JN, Wang T, Cohen MS. BRAF and MEK inhibitors: use and resistance in BRAF-mutated cancers. Drugs. 2018;78(5):549–66. doi:10.1007/s40265-018-0884-8. [Google Scholar] [PubMed] [CrossRef]
71. Corcoran RB, André T, Atreya CE, Schellens JHM, Yoshino T, Bendell JC, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov. 2018;8(4):428–43. doi:10.1158/2159-8290.CD-17-1226. [Google Scholar] [PubMed] [CrossRef]
72. Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42(2):581–8. doi:10.1016/0092-8674(85)90115-1. [Google Scholar] [PubMed] [CrossRef]
73. Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009;17(2):199–209. doi:10.1016/j.devcel.2009.07.013. [Google Scholar] [PubMed] [CrossRef]
74. de Graaff E, Srinivas S, Kilkenny C, D.’Agati V, Mankoo BS, Costantini F, et al. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 2001;15(18):2433–44. doi:10.1101/gad.205001. [Google Scholar] [PubMed] [CrossRef]
75. Tsuzuki T, Takahashi M, Asai N, Iwashita T, Matsuyama M, Asai J. Spatial and temporal expression of the ret proto-oncogene product in embryonic, infant and adult rat tissues. Oncogene. 1995;10(1):191–8. doi:10.1038/sj.onc.1200813. [Google Scholar] [CrossRef]
76. Kohno T, Tabata J, Nakaoku T. REToma: a cancer subtype with a shared driver oncogene. Carcinogenesis. 2020;41(2):123–9. doi:10.1093/carcin/bgz184. [Google Scholar] [PubMed] [CrossRef]
77. Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET aberrations in diverse cancers: next-generation sequencing of 4,871 patients. Clin Cancer Res. 2017;23(8):1988–97. doi:10.1158/1078-0432.CCR-16-1679. [Google Scholar] [PubMed] [CrossRef]
78. Liu X, Hu X, Shen T, Li Q, Mooers BHM, Wu J. RET kinase alterations in targeted cancer therapy. Cancer Drug Resist. 2020;3(3):472–81. doi:10.20517/cdr.2020.15. [Google Scholar] [PubMed] [CrossRef]
79. Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000;85(3):1170–5. doi:10.1210/jcem.85.3.6472. [Google Scholar] [PubMed] [CrossRef]
80. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7510):543–50. doi:10.1038/nature13385. [Google Scholar] [PubMed] [CrossRef]
81. Donis-Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993;2(7):851–6. doi:10.1093/hmg/2.7.851. [Google Scholar] [PubMed] [CrossRef]
82. Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363(6428):458–60. doi:10.1038/363458a0. [Google Scholar] [PubMed] [CrossRef]
83. Subbiah V, Yang D, Velcheti V, Drilon A, Meric-Bernstam F. State-of-the-art strategies for targeting RET-dependent cancers. J Clin Oncol. 2020;38(11):1209–21. doi:10.1200/JCO.19.02551. [Google Scholar] [PubMed] [CrossRef]
84. Bradford D, Larkins E, Mushti SL, Rodriguez L, Skinner AM, Helms WS, et al. FDA approval summary: selpercatinib for the treatment of lung and thyroid cancers with RET gene mutations or fusions. Clin Cancer Res. 2021;27(8):2130–5. doi:10.1158/1078-0432.CCR-20-3558. [Google Scholar] [PubMed] [CrossRef]
85. U.S. Food and Drug Administration. FDA approves selpercatinib for locally advanced or metastatic RET fusion-positive solid tumors. [cited 2025 Apr 6]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-selpercatinib-locally-advanced-or-metastatic-ret-fusion-positive-solid-tumors. [Google Scholar]
86. Subbiah V, Wolf J, Konda B, Kang H, Spira A, Weiss J, et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001a phase 1/2, open-label, basket trial. Lancet Oncol. 2022;23(10):1261–73. doi:10.1016/S1470-2045(22)00541-1. [Google Scholar] [CrossRef]
87. Duke ES, Bradford D, Marcovitz M, Amatya AK, Mishra-Kalyani PS, Nguyen E, et al. FDA approval summary: selpercatinib for the treatment of advanced RET fusion-positive solid tumors. Clin Cancer Res. 2023;29(18):3573–8. doi:10.1158/1078-0432.CCR-23-0459. [Google Scholar] [PubMed] [CrossRef]
88. Subbiah V, Drilon AE, Sukrithan V, Spira AI, Robinson B, Deschler-Baier B, et al. Durable efficacy of selpercatinib in patients with RET fusion+ solid tumors, with a focus on GI tumors: lIBRETTO-001. J Clin Oncol. 2024;42(3_suppl):746. doi:10.1200/JCO.2024.42.3_suppl.746. [Google Scholar] [CrossRef]
89. Li GG, Somwar R, Joseph J, Smith RS, Hayashi T, Martin L, et al. Antitumor activity of RXDX-105 in multiple cancer types with RET rearrangements or mutations. Clin Cancer Res. 2017;23(12):2981–90. doi:10.1158/1078-0432.CCR-16-1887. [Google Scholar] [PubMed] [CrossRef]
90. Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, et al. Precision targeted therapy with BLU-667 for RET-driven cancers. Cancer Discov. 2018;8(7):836–49. doi:10.1158/2159-8290.CD-18-0338. [Google Scholar] [PubMed] [CrossRef]
91. Knowles PP, Murray-Rust J, Kjaer S, Scott RP, Hanrahan S, Santoro M, et al. Structure and chemical inhibition of the RET tyrosine kinase domain. J Biol Chem. 2006;281(44):33577–87. doi:10.1074/jbc.M605604200. [Google Scholar] [PubMed] [CrossRef]
92. New England Journal of Medicine Illustrated Glossary. Solvent front variant. [cited 2025 Apr 6]. Available from: https://illustrated-glossary.nejm.org/term/solvent_front_variant. [Google Scholar]
93. Solomon BJ, Tan L, Lin JJ, Wong SQ, Hollizeck S, Ebata K, et al. RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET-driven malignancies. J Thorac Oncol. 2020 Apr;15(4):541–9. doi:10.1016/j.jtho.2020.01.006. [Google Scholar] [PubMed] [CrossRef]
94. Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-expressing solid tumors: DESTINY-PanTumor02 (DP-02) interim results. J Clin Oncol. 2023;41(17_suppl):LBA3000. doi:10.1200/JCO.2023.41.17_suppl.LBA3000. [Google Scholar] [CrossRef]
95. Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol. 2024;42(1):47–58. doi:10.1200/JCO.23.02005. [Google Scholar] [PubMed] [CrossRef]
96. U.S. Food and Drug Administration. FDA grants accelerated approval to fam-trastuzumab deruxtecan-Nxki for unresectable or metastatic HER2-positive solid tumors. [cited 2025 Apr 6]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2. [Google Scholar]
97. Yang J, Han J, Zeng N, Yan X. Cost-effectiveness of trastuzumab deruxtecan in previously treated human epidermal growth factor receptor 2-low metastatic breast cancer. Ther Adv Med Oncol. 2023 May 21;15:17588359231169983. doi:10.1177/17588359231169983. [Google Scholar] [PubMed] [CrossRef]
98. Vernieri C, Milano M, Brambilla M, Mennitto A, Maggi C, Cona MS, et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: current knowledge, new research directions and therapeutic perspectives. Crit Rev Oncol Hematol. 2019;139(15_suppl):53–66. doi:10.1016/j.critrevonc.2019.05.001. [Google Scholar] [PubMed] [CrossRef]
99. Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–37. doi:10.1016/s1535-6108(02)00097-1. [Google Scholar] [PubMed] [CrossRef]
100. Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006 Jan 30;94(2):259–67. doi:10.1038/sj.bjc.6602930. [Google Scholar] [PubMed] [CrossRef]
101. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022 Jan;12(1):31–46. doi:10.1158/2159-8290.CD-21-1059. [Google Scholar] [PubMed] [CrossRef]
102. Hobbs BP, Pestana RC, Zabor EC, Kaizer AM, Hong DS. Basket trials: review of current practice and innovations for future trials. J Clin Oncol. 2022 Oct 20;40(30):3520–8. doi:10.1200/JCO.21.02285. [Google Scholar] [PubMed] [CrossRef]
103. Huang L, Guo Z, Wang F, Fu L. KRAS mutation: from undruggable to druggable in cancer. Signal Transduct Target Ther. 2021 Nov 15;6(1):386. doi:10.1038/s41392-021-00780-4. [Google Scholar] [PubMed] [CrossRef]
104. Bekaii-Saab TS, Yaeger R, Spira AI, Pelster MS, Sabari JK, Hafez N, et al. Adagrasib in advanced solid tumors harboring a KRASG12C mutation. J Clin Oncol. 2023;41(25):4097–106. doi:10.1200/JCO.23.00434. [Google Scholar] [PubMed] [CrossRef]
105. Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207–17. doi:10.1056/NEJMoa1917239. [Google Scholar] [PubMed] [CrossRef]
106. Sacher A, LoRusso P, Patel MR, Miller WHJr, Garralda E, Forster MD, et al. Single-agent divarasib (GDC-6036) in solid tumors with a KRAS G12C mutation. N Engl J Med. 2023;389(8):710–21. doi:10.1056/NEJMoa2303810. [Google Scholar] [PubMed] [CrossRef]
107. Tang D, Kang R. Glimmers of hope for targeting oncogenic KRAS-G12D. Cancer Gene Ther. 2023;30(3):391–3. doi:10.1038/s41417-022-00561-3. [Google Scholar] [PubMed] [CrossRef]
108. Khan N, Raza U, Ali Zaidi SA, Nuer M, Abudurousuli K, Paerhati Y, et al. Drugging the ‘undruggable’ KRAS: breakthroughs, challenges, and opportunities in pancreatic cancer. Cancer Biol Med. 2025 Jul 7:1–27. doi:10.20892/j.issn.2095-3941.2025.0122. [Google Scholar] [PubMed] [CrossRef]
109. Pant S, Schuler M, Iyer G, Witt O, Doi T, Qin S, et al. Erdafitinib in patients with advanced solid tumours with FGFR alterations (RAGNARAn international, single-arm, phase 2 study. Lancet Oncol. 2023;24(8):925–35. doi:10.1016/S1470-2045(23)00275-9. [Google Scholar] [PubMed] [CrossRef]
110. Ellis H, Goyal L. Are FGFR fusions and mutations the next tumor-agnostic targets in oncology? JCO Precis Oncol. 2024;8(8):e2400113. doi:10.1200/PO.24.00113. [Google Scholar] [PubMed] [CrossRef]
111. Rodón J, Damian S, Furqan M, García-Donas J, Imai H, Italiano A, et al. Pemigatinib in previously treated solid tumors with activating FGFR1-FGFR3 alterations: phase 2 FIGHT-207 basket trial. Nat Med. 2024;30(6):1645–54. doi:10.1038/s41591-024-02934-7. [Google Scholar] [PubMed] [CrossRef]
112. Laskin J, Liu SV, Tolba K, Heining C, Schlenk RF, Cheema P, et al. NRG1 fusion-driven tumors: biology, detection, and the therapeutic role of afatinib and other ErbB-targeting agents. Ann Oncol. 2020;31(12):1693–703. doi:10.1016/j.annonc.2020.08.2335. [Google Scholar] [PubMed] [CrossRef]
113. Schram AM, Goto K, Kim DW, Macarulla T, Hollebecque A, O’Reilly EM, et al. Efficacy of Zenocutuzumab in NRG1 fusion-positive cancer. N Engl J Med. 2025;392(6):566–76. doi:10.1056/NEJMoa2405008. [Google Scholar] [PubMed] [CrossRef]
114. Park JJH, Hsu G, Siden EG, Thorlund K, Mills EJ. An overview of precision oncology basket and umbrella trials for clinicians. CA Cancer J Clin. 2020;70(2):125–37. doi:10.3322/caac.21600. [Google Scholar] [PubMed] [CrossRef]
115. Tumor-Agnostic Precision Immuno-Oncology and Somatic Targeting Rational for You (TAPISTRY) Platform Study. Bethesda (MDNational Library of Medicine (US). Identifier NCT04589845. [cited 2025 Jul 7]. Available from: https://www.clinicaltrials.gov/study/NCT04589845. [Google Scholar]
116. Janiaud P, Serghiou S, Ioannidis JPA. New clinical trial designs in the era of precision medicine: an overview of definitions, strengths, weaknesses, and current use in oncology. Cancer Treat Rev. 2019;73:20–30. doi:10.1016/j.ctrv.2018.12.003. [Google Scholar] [PubMed] [CrossRef]
117. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. doi:10.1056/NEJMoa1500596. [Google Scholar] [PubMed] [CrossRef]
118. Middleton G, Fletcher P, Popat S, Savage J, Summers Y, Greystoke A, et al. The National Lung Matrix Trial of personalized therapy in lung cancer. Nature. 2020;583(7817):807–12. doi:10.1038/s41586-020-2481-8. [Google Scholar] [PubMed] [CrossRef]
119. Meyer EL, Mesenbrink P, Dunger-Baldauf C, Fulle HJ, Glimm E, Li Y, et al. The evolution of masterprotocol clinical trial designs: a systematic literature review. Clin Ther. 2020;42(8):1330–60. doi:10.1016/j.clinthera.2020.05.010. [Google Scholar] [PubMed] [CrossRef]
120. Parmar MK, Sydes MR, Cafferty FH, Choodari-Oskooei B, Langley RE, Brown L, et al. Testing many treatments within a single protocol over 10 years at MRC Clinical Trials Unit at UCL: multi-arm, multi-stage platform, umbrella and basket protocols. Clin Trials. 2017;14(5):451–61. doi:10.1177/1740774517725697. [Google Scholar] [PubMed] [CrossRef]
121. Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377(1):62–70. doi:10.1056/NEJMra1510062. [Google Scholar] [PubMed] [CrossRef]
122. Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med. 2019;381(17):1644–52. doi:10.1056/NEJMoa1813279. [Google Scholar] [PubMed] [CrossRef]
123. O’Dwyer PJ, Gray RJ, Flaherty KT, Chen AP, Li S, Wang V, et al. The NCI-MATCH trial: lessons for precision oncology. Nat Med. 2023 Jun;29(6):1349–57. doi:10.1038/s41591-023-02379-4. [Google Scholar] [PubMed] [CrossRef]
124. Harris LN, Blanke CD, Erba HP, Ford JM, Gray RJ, LeBlanc ML, et al. The new NCI precision medicine trials. Clin Cancer Res. 2023 Dec 1;29(23):4728–32. doi:10.1158/1078-0432.CCR-23-0917. [Google Scholar] [PubMed] [CrossRef]
125. American Society of Clinical Oncology. TAPUR: testing the use of food and drug administration (FDA) approved drugs that target a specific abnormality in a tumor gene in people with advanced stage cancer (TAPUR). [cited 2025 Apr 6]. Available from: https://www.cancer.gov/research/participate/clinical-trials-search/v?id=NCT02693535&r=1. [Google Scholar]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools