 Open Access
Open Access
ARTICLE
Real-World Data on Stage III Non-Small Cell Lung Cancer in Vietnam
1 Department of Medical Oncology 2, Nghe An Oncology Hospital, Vinh City, 43000, Vietnam
2 Department of Oncology, Hanoi Medical University, Ha Noi City, 10000, Vietnam
* Corresponding Author: Khanh Toan Nguyen. Email:
(This article belongs to the Special Issue: Novel Biomarkers and Treatment Strategies in Solid Tumor Diagnosis, Progression, and Prognosis)
Oncology Research 2025, 33(12), 4013-4028. https://doi.org/10.32604/or.2025.069281
Received 19 June 2025; Accepted 19 September 2025; Issue published 27 November 2025
Abstract
Objective: Patients with stage III non-small cell lung cancer (NSCLC) present with a heterogeneous disease profile and often require multifaceted treatment strategies. This research aimed to investigate the demographic features, therapeutic patterns, and survival outcomes of such patients in Vietnam. Methods: A retrospective descriptive study was conducted on 731 patients diagnosed with stage III NSCLC American Joint Committee on Cancer (AJCC) 8th edition, at Nghe An Oncology Hospital from January 2018 to August 2024. Descriptive statistics summarized baseline and treatment characteristics. We calculated progression-free survival (PFS) and overall survival (OS) through the Kaplan–Meier approach and compared survival curves with the log-rank test. Prognostic variables were assessed using Cox regression analysis. Results: Patients had a median age of 64 years, and the majority (84%) were male. Disease stages IIIA, IIIB, and IIIC accounted for 26.0%, 49.9%, and 24.1% of cases, respectively. Adenocarcinoma (60.7%) was the most common histological subtype. Initial treatments included surgery (8.5%), concurrent chemoradiotherapy (38.6%), sequential chemoradiotherapy (2.2%), radiotherapy alone (1.4%), systemic therapy (37.3%), and palliative care (12.0%). From 2018 to 2024, the use of systemic therapy declined (88.5% to 21.7%), while concurrent chemoradiotherapy rose significantly (1.1% to 51.5%). Median progression-free survival (mPFS) and median overall survival (mOS) were 8.9 months and 20.5 months, respectively. Patients with stage IIIA had significantly better outcomes (mPFS: 12.6 months; mOS: 32.4 months; p < 0.001). Surgical treatment yielded the longest survival (mPFS: 13.5 months; mOS: 42.8 months). Favorable prognostic factors included adenocarcinoma subtype, presence of driver mutations, stage IIIA, and good performance status. Conclusion: For stage III NSCLC, concurrent chemoradiotherapy is still considered the standard treatment, whereas surgery can provide the highest survival advantage in carefully selected cases. Histology, molecular profile, and disease stage are key prognostic indicators.Keywords
Supplementary Material
Supplementary Material FileLung cancer remains one of the most significant causes of global cancer burden. In 2022, lung cancer ranked as the leading cancer worldwide in both new cases and deaths, with an estimated 2,480,301 incident cases (12.4% of all cancers) and 1,817,172 deaths (18.7% of all cancer-related deaths) [1]. In Asia, lung cancer was responsible for 1,142,397 deaths (62.9% of total cancer deaths) in 2022 [2]; a similarly high mortality rate was observed in Vietnam, with 22,597 deaths, accounting for 18.8% of cancer-related mortality [2]. Roughly 85% of lung cancer diagnoses are classified as non-small cell lung cancer (NSCLC) and are typically divided into three primary histological forms: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma [3,4].
Recent trends show a higher proportion of early-stage diagnoses, with the 5-year survival rate improving notably to 26.4% [5]. Between one-fifth and one-third of lung cancer patients are diagnosed at stage III, the 5-year survival rate decreases from 36% in stage IIIA to 26% in stage IIIB, and further declines to 13% in stage IIIC [6]. Stage III NSCLC is considered a heterogeneous condition characterized by locally advanced tumors and/or mediastinal lymph node involvement without evidence of distant metastasis [7].
Management of stage III NSCLC requires multiple therapeutic strategies tailored to disease stage, leading to different management strategies and therapeutic outcomes depending on disease stage [8]. Multimodal treatment includes surgery, radiotherapy, and systemic therapies. For patients with resectable stage IIIA NSCLC, a combination of surgery and neoadjuvant therapy remains a potentially curative option [9]. In contrast, platinum-based concurrent chemoradiotherapy (cCRT) continues to be the cornerstone of treatment for individuals who are not candidates for surgery. Patients without disease progression after cCRT may benefit from consolidation with durvalumab, which has been shown to improve survival compared with cCRT alone [10–12]. More recently, osimertinib has provided additional benefit as consolidation therapy in patients with EGFR-mutated, unresectable stage III disease [13]. Other treatment approaches, including sequential chemoradiotherapy, chemotherapy or radiotherapy alone, and targeted agents, can be appropriate depending on patient characteristics and comorbidities [14]. Meanwhile, emerging strategies such as neoadjuvant chemo-immunotherapy have demonstrated promising early outcomes [15–17].
The relatively high prevalence of actionable gene mutations among Vietnamese lung cancer patients presents further opportunities for targeted therapy, contributing to improved quality of life and survival outcomes [18–20].
We performed a retrospective descriptive analysis to investigate the demographic characteristics, therapeutic patterns, and results among patients with stage III NSCLC in North-Central Vietnam’s clinical practice.
2.1 Study Design and Patient Population
This retrospective descriptive study included 731 patients diagnosed with stage III NSCLC at Nghe An Oncology Hospital from January 2018 to August 2024. Diagnosis and treatment adhered to national guidelines and international standards [14,21,22]. Eligible patients had histologically confirmed NSCLC according to the WHO 2015 classification [23] and staged III based on the AJCC 8th edition guidelines [24]. Patients with unclear histology, multiple primary tumors, or incomplete records were excluded.
While positron emission tomography (PET/CT) and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) are not available, staging procedures are carried out using the diagnostic modalities that are available at our institution, such as computed tomography (CT), magnetic resonance imaging (MRI), bone scan, and single photon emission computed tomography (SPECT/CT), which are routine [14].
Patient data were collected retrospectively from medical records, with a cut-off date of 1 November 2024. Demographics, smoking status, comorbidities, TNM stage, histology, genetic mutation status, Eastern Cooperative Oncology Group performance status (ECOG PS), and initial treatment modality were among the extracted data. Prognostic factors for OS, overall survival (OS), and progression-free survival (PFS) were evaluated as outcomes.
Genetic testing was performed selectively, mainly for EGFR mutations via real-time Polymerase Chain Reaction (PCR), which is reimbursed by national insurance. Other mutations (ALK, ROS1, MET) were assessed using PCR or next-generation sequencing (NGS), depending on availability and patient affordability.
Treatment modalities were classified by primary therapeutic intent as surgery (with or without neoadjuvant/adjuvant therapy), concurrent chemoradiotherapy (cCRT), sequential chemoradiotherapy (sCRT), radiotherapy alone, or systemic therapy (chemotherapy, targeted therapy, or immunotherapy). In cases of combined treatment, classification was based on the primary therapeutic intent.
Progression-free survival (PFS) was measured from the start of treatment until disease progression, recurrence, or the end of the study period, whichever came first. Overall survival (OS) was determined from treatment initiation to death from any cause or the conclusion of the study.
Covariates included age (<70 vs. ≥70), sex, smoking status, ECOG PS (<2 vs. ≥2), histology (adenocarcinoma vs. others), stage (IIIA, IIIB, IIIC), mutation status (positive vs. negative), and comorbidities (yes vs. no). Comorbidities were grouped by organ system.
Comorbidities were categorized based on organ systems to facilitate analysis and interpretation. The major groups included cardiovascular diseases (e.g., hypertension, coronary artery disease), respiratory diseases (e.g., COPD), endocrine disorders (e.g., diabetes mellitus), hepatobiliary diseases, and musculoskeletal disorders. This grouping approach was adopted due to the wide range of comorbid conditions and their relatively low individual frequencies.
Analyses were performed using SPSS v25.0, with p < 0.05 as the significance threshold. Survival outcomes were analyzed using Kaplan–Meier curves, with differences between groups assessed by the log-rank test. To identify independent prognostic factors, univariate and multivariate Cox proportional hazards regression analyses were applied. Variables with a p-value < 0.05 in the univariate analysis were subsequently included in the multivariate model. Additionally, a sensitivity analysis was performed, excluding patients whose mutation status was unknown.
In line with the Declaration of Helsinki, the research protocol was examined and approved by the Ethics Committee of Nghe An Oncology Hospital (Decision No. 344/QD-BVUB, dated 28 February 2025).
As this was a retrospective study using anonymized medical records, informed consent was waived for all participants, including those who were deceased, in accordance with institutional ethical guidelines. All personal data was kept strictly confidential, used solely for research purposes, and no individual identifiers were disclosed.
3.1 General Characteristics of the Study Population
The median age among the patients was 64 years (ranging from 26 to 88), with over 94% aged above 50. The predominant age bracket was 60–69 years (43.4%), with 26.3% aged more than 70 years. Males made up 84.0% of the cohort, and 74.1% had a smoking history, either former or current.
The majority of participants exhibited good performance status (PS 0–1) at 79.5%, and 62.9% had no comorbidities. Among those with comorbidities, cardiovascular diseases were the most frequent (17.9%), followed by respiratory diseases (7.9%).
Of the 731 patients included in the study, 190 (26.0%) were diagnosed with stage IIIA disease, 365 (49.9%) with stage IIIB, and 176 (24.1%) with stage IIIC. Among the entire cohort of 731 patients, only 279 (38.2%) underwent molecular testing. Of these, 111/279 patients (39.8%) tested positive for actionable driver mutations.
Regarding histology, adenocarcinoma was the predominant subtype (60.7%), followed by squamous cell carcinoma (28.9%). Only four cases (0.5%) were classified as large cell carcinoma, while the remaining cases (9.9%) were grouped under other histological subtypes.
During the study period from 2018 to 2024, there were no significant differences in demographic characteristics such as sex, age, performance status, or disease stage. However, there was a notable change in histological patterns: the proportion of adenocarcinoma in stage III NSCLC patients significantly increased from 44.8% to 69.7%. Conversely, the proportion of other histological types decreased from 25.3% to 6.1%, while the prevalence of squamous cell carcinoma showed a slight decline from 29.9% to 24.2%. The characteristics of the patients are summarized in Table 1.
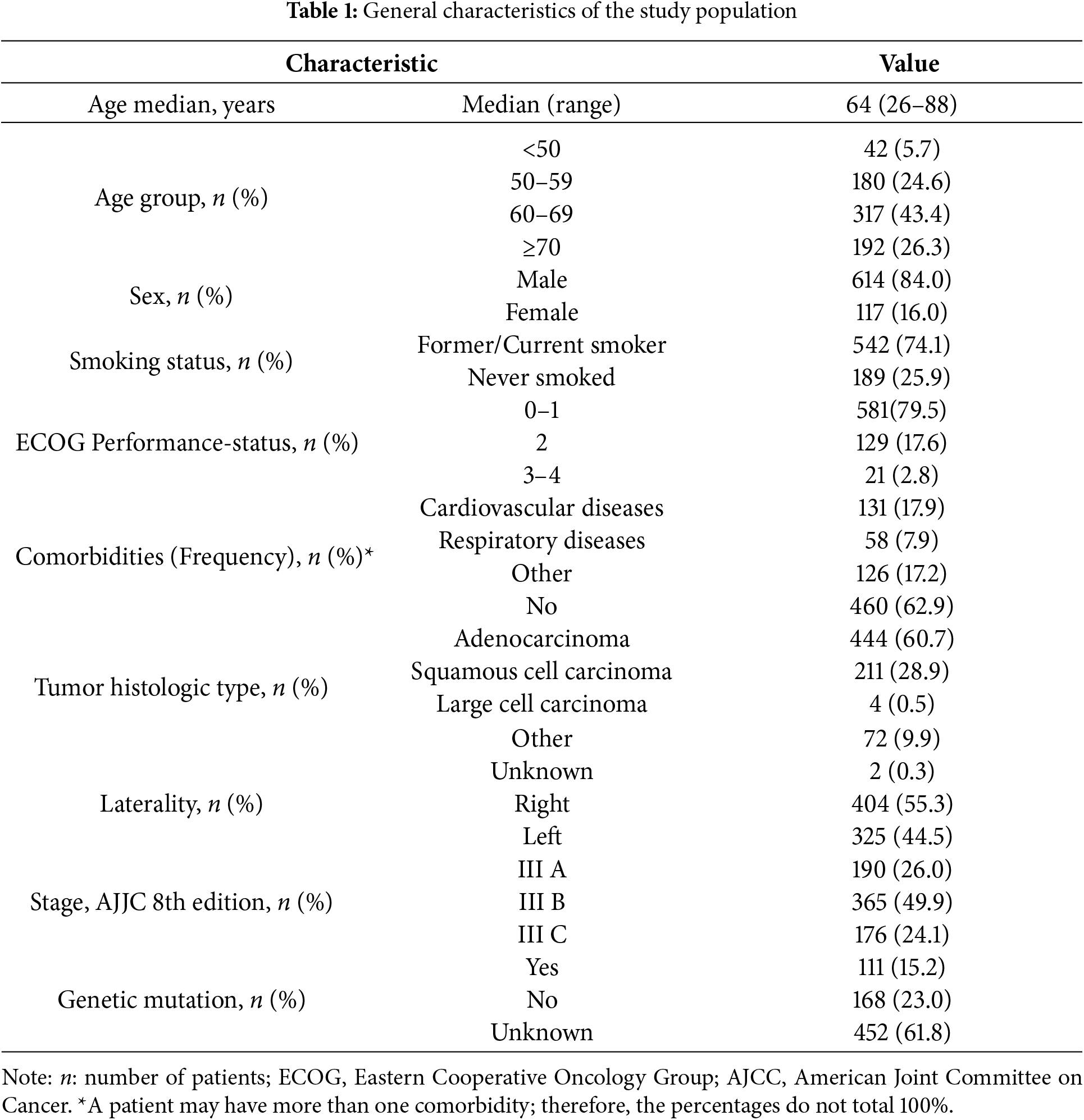
In addition, evaluation of tumor (T) and nodal (N) status revealed a heterogeneous distribution across the cohort. The majority of patients presented with T2–T4 tumors and N2–N3 nodal involvement, consistent with the locally advanced nature of stage III disease. A detailed breakdown of T and N classification is provided in Table S1.
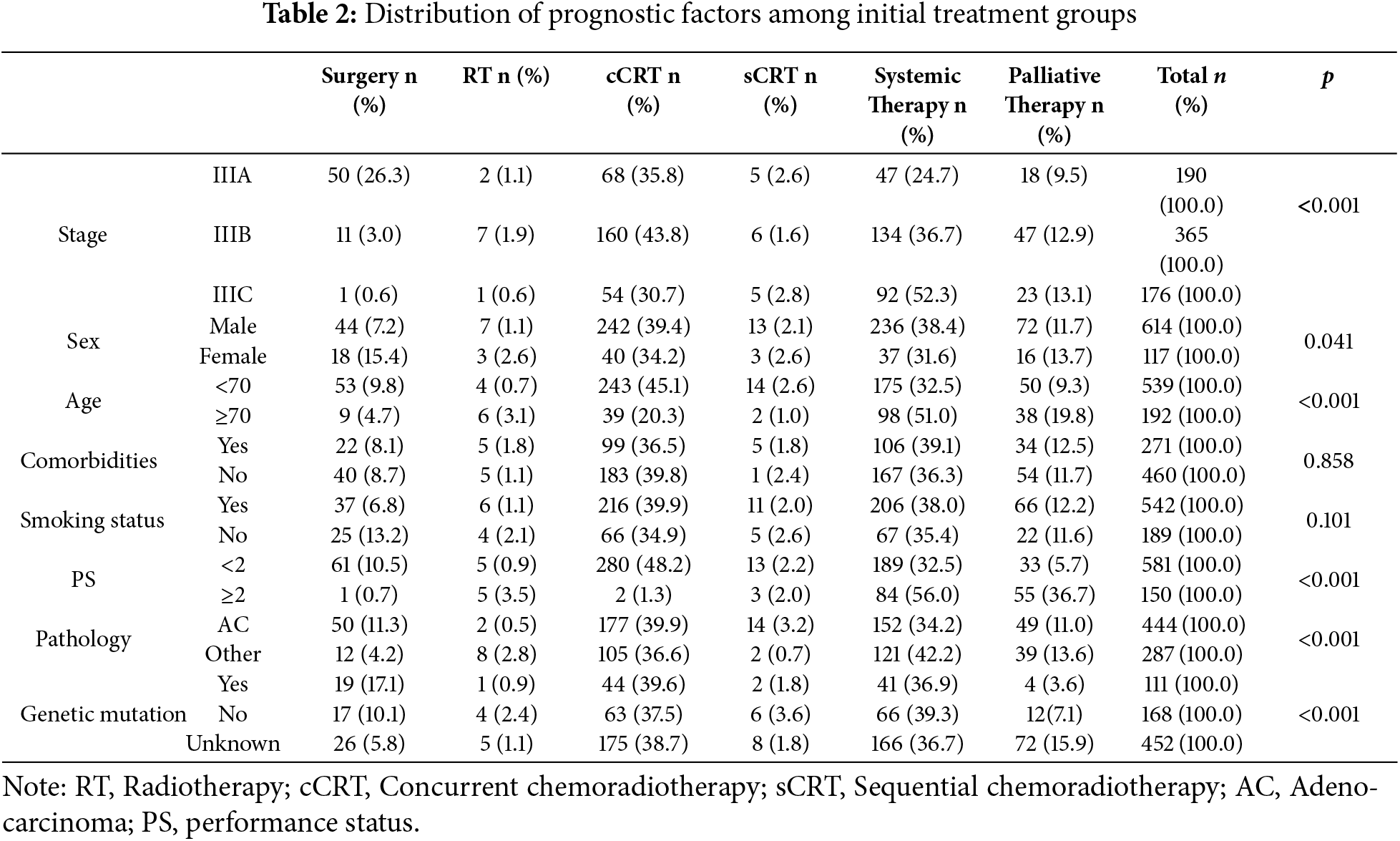
3.2 Initial Treatment Modalities
Although stage III NSCLC is heterogeneous in terms of anatomical classification (T/N status) and therapeutic intent (curative vs. palliative), we chose to present our data primarily according to initial treatment modality. This approach reflects real-world clinical decision-making, where treatment choices are influenced by a combination of stage, patient performance status, comorbidities, molecular profile, and resource availability.
Furthermore, grouping by treatment allows a clearer comparison of clinical outcomes across modalities (e.g., surgery, chemoradiotherapy, systemic therapy), which aligns with our study’s aim of evaluating treatment patterns and real-world survival. To address potential selection bias, we provide detailed baseline characteristics by stage and other prognostic variables for each treatment group.
Surgery was employed as the initial treatment modality in 8.5% of stage III NSCLC patients. Among these, 2 patients (3.2%) received neoadjuvant therapy prior to surgery, while 44 patients (71.0%) underwent adjuvant therapy postoperatively. The most common surgical procedure was lobectomy with regional lymph node dissection (n = 58, 93.5%), followed by segmentectomy and bilobectomy in a small number of cases.
Radiotherapy was utilized in 308 patients as part of the initial treatment approach. Among these, 96.8% received radical-intent radiotherapy, predominantly using intensity-modulated radiotherapy (IMRT). The standard curative dose was 60 Gy, delivered in 2 Gy fractions. A small proportion (3.2%) received radiotherapy alone, with a mean total dose of 54 Gy (2 Gy/fraction).
3.5 Systemic Therapy Chemotherapy
Chemot herapy was administered to 541 patients as part of initial treatment. Among them, 55.1% received chemotherapy in combination with definitive radiotherapy (concurrent chemoradiotherapy), while 44.9% received palliative chemotherapy. The most frequently used regimens in the concurrent setting were paclitaxel–carboplatin (42.6%), etoposide–cisplatin (32.2%), pemetrexed–platinum (20.1%), and vinorelbine–cisplatin (5.1%). For patients receiving chemotherapy alone, the most common regimens included paclitaxel–carboplatin (42.4%) and single-agent vinorelbine (17.7%).
In patients with EGFR-mutated tumors, targeted therapy was administered either after surgery as adjuvant therapy, post-cCRT as consolidation, or as initial systemic treatment for those deemed inoperable. Specifically, 2 patients (5.9%) received adjuvant osimertinib following surgery, 3 patients (8.8%) received consolidation osimertinib post-cCRT, and 29 patients (85.3%) received EGFR-TKIs as primary treatment. The most commonly used agents were erlotinib (41.3%), gefitinib (31.0%), and afatinib (20.7%).
Immunotherapy was used either as consolidation or systemic therapy in selected patients. Eleven patients received durvalumab as consolidation following definitive cCRT, and one patient received pembrolizumab as first-line systemic therapy.
3.8 Initial Treatment Modalities
In our study, there were six primary treatment methods used for stage III lung cancer patients, including: surgery (8.5%), radiotherapy (1.4%), concurrent chemoradiotherapy (38.6%), sequential chemoradiotherapy (2.2%), systemic therapy (37.3%), and palliative therapy (12.0%). Curative surgery was primarily applied to stage IIIA patients with good performance status (26.3%). Concurrent chemoradiotherapy was used across all stages, most commonly in stage IIIB (43.8%). Systemic therapy was used most frequently in stage IIIC patients (52.3%). The other treatment methods, including radiotherapy, sequential chemoradiotherapy, and palliative therapy, showed similar rates across the different stages.
Concurrent chemoradiotherapy was the most common treatment method for both men (39.4%) and women (34.2%), followed by systemic therapy (38.4% for men and 31.6% for women). Among patients aged under 70 years, concurrent chemoradiotherapy was the predominant method (45.1%), while systemic therapy was most commonly used in patients aged 70 or older (51%). For patients with an ECOG performance status (PS) of <2, concurrent chemoradiotherapy (48.2%) and systemic therapy (32.5%) were the most commonly used methods. Conversely, systemic therapy (56.0%) and palliative therapy (36.7%) were the most prevalent methods for patients with an ECOG PS ≥ 2. Among subgroups based on comorbidities, smoking status, histology (adenocarcinoma or other types), and genetic mutation status, concurrent chemoradiotherapy and systemic therapy were the most commonly used treatments (Table 2).
3.9 Trends in Disease Patterns over the Years
From 2018 to 2024, the incidence rate of stage IIIB disease was significantly higher than that of stage IIIA and IIIC. This rate exhibited considerable fluctuations, with a marked increase in 2019 and 2021, followed by a slight decrease and a subsequent increase again in 2024. The incidence of stage IIIA disease showed a rising trend from 2018 to 2020, followed by a slight decrease from 2020 to 2022, reaching its highest point of 31.8% in 2023. In contrast, the incidence of stage IIIC steadily decreased from 35.6% in 2018 to 21.2% in 2024 (Fig. 1A).
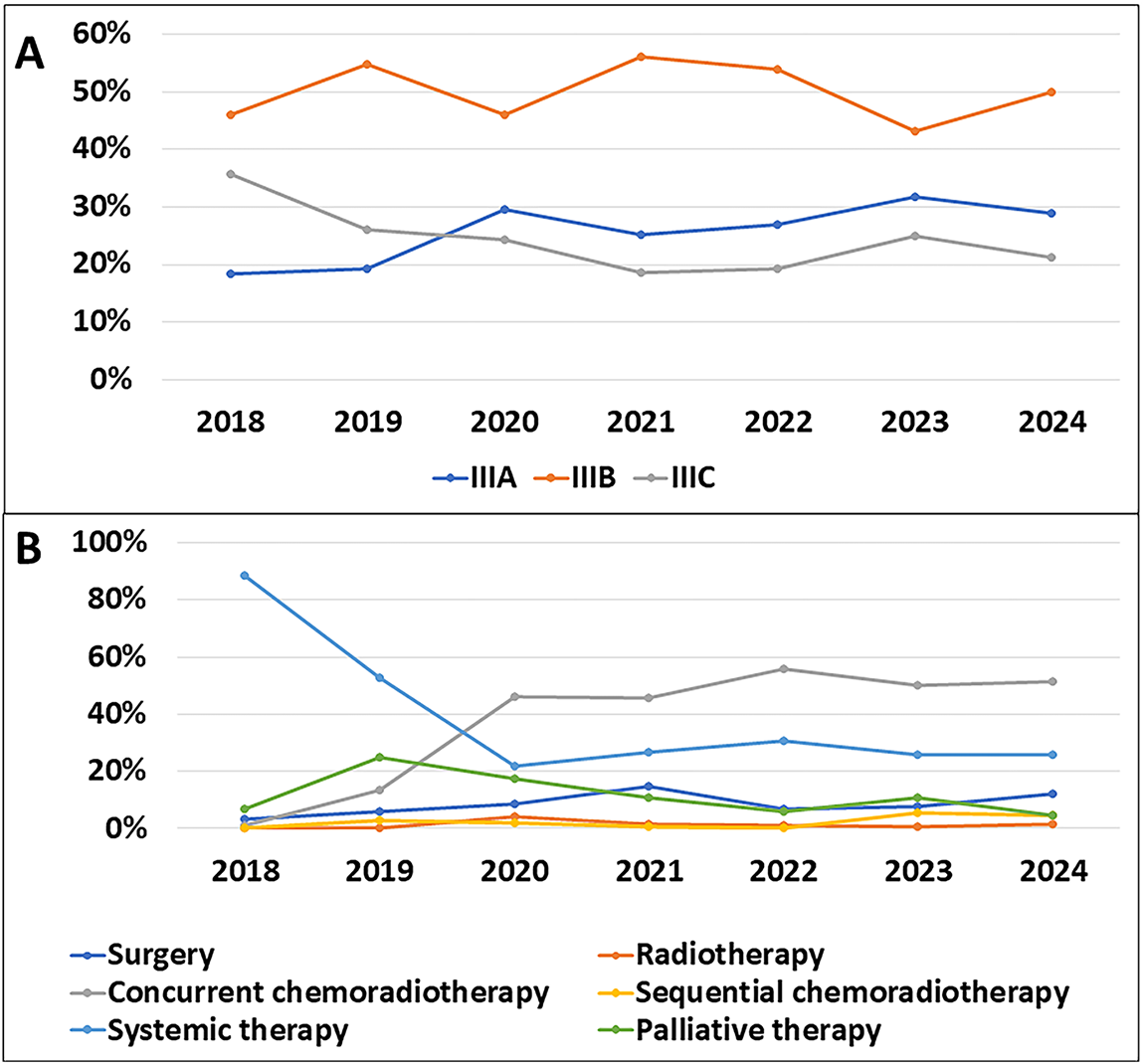
Figure 1: Temporal evolution of stage distribution and treatment selection during the study period. (A): New incidence rates by stage over the years. (B): Changes in treatment methods over the years
A graph illustrating the changes in treatment methods from 2018 to 2024 shows a significant shift in treatment preferences. In 2018, systemic therapy was the predominant treatment with a rate of 88.5%. However, this method saw a sharp decline over the years, dropping to just 25.8% in 2024. Concurrent chemoradiotherapy, on the other hand, exhibited a notable increase, rising from 1.1% in 2018 to 51.5% in 2024, making it the most commonly used treatment method. Surgery also increased from 3.4% in 2018 to 12.1% in 2024, reaching its peak in 2021 at 14.6%. Meanwhile, radiotherapy and sequential chemoradiotherapy remained relatively stable and consistently accounted for a small proportion of the treatments throughout this period (Fig. 1B).
3.10 Progression-Free Survival (PFS)
The median progression-free survival (PFS) was 8.9 months, with a 95% confidence interval (CI) ranging from 8.0 to 9.7 months. The PFS rates at 1, 2, 3, and 4 years were 35.9%, 19.3%, 14.7%, and 12.2%, respectively, while the 5-year PFS was not reached. Patients with stage IIIA disease had a median PFS of 12.6 months (95% CI: 10.2–15.0), which was significantly longer than those with stage IIIB (8.6 months; 95% CI: 8.4–9.7) and stage IIIC (6.3 months; 95% CI: 4.6–8.0) (p < 0.001) (Fig. 2A,B).
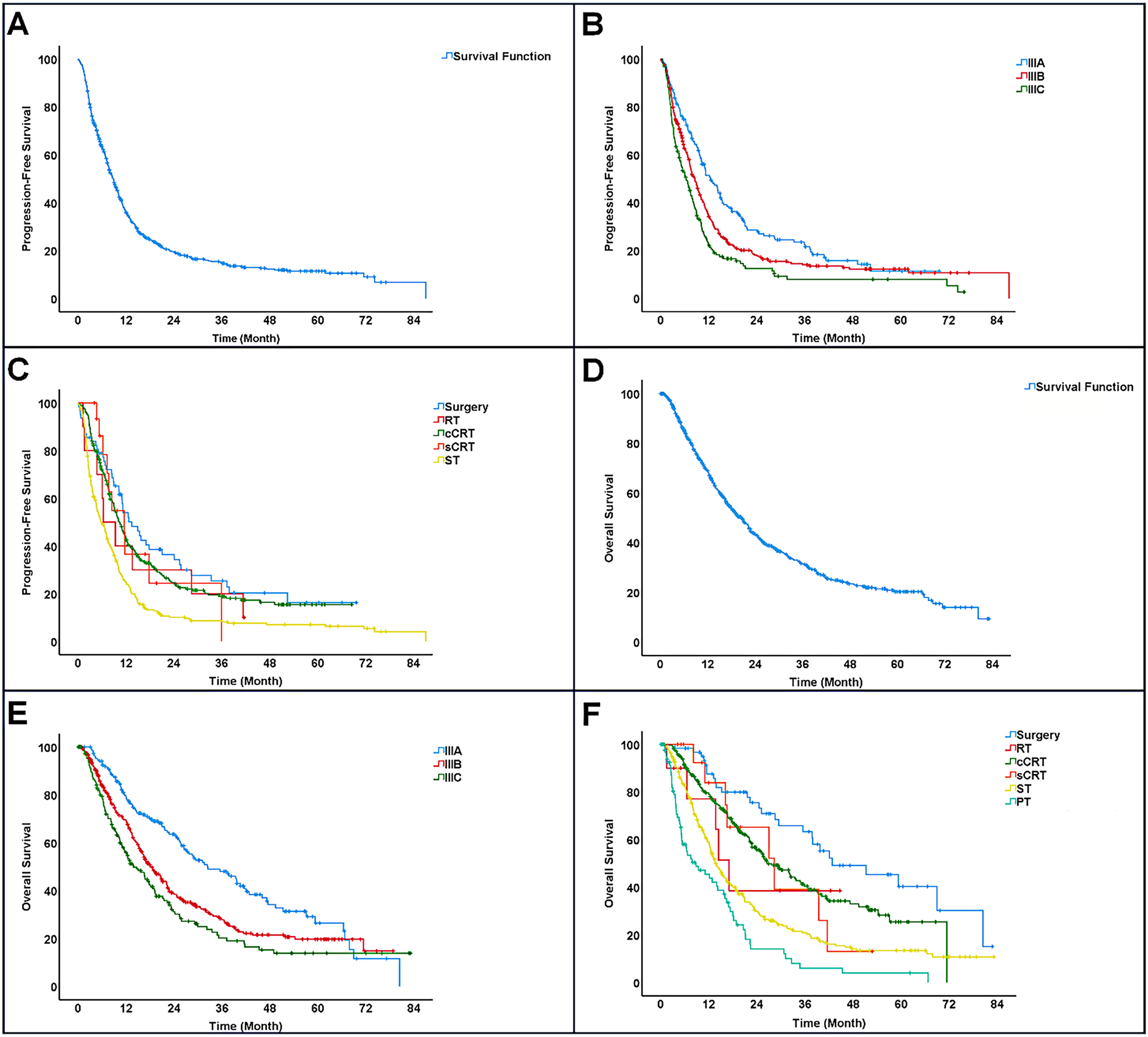
Figure 2: Survival outcomes of patients with stage III NSCLC. (A): Progression-free survival (PFS). (B): Progression-free survival (PFS) by disease stage. (C): Progression-free survival (PFS) by treatment. (D): Overall survival (OS). (E): Overall survival (OS) by disease stage. (F): Overall survival (OS) by treatment. Note: RT, Radiotherapy; cCRT, Concurrent chemoradiotherapy; sCRT, Sequential chemoradiotherapy; ST, Systemic therapy; PT, Palliative therapy
Treatment modalities showed varying median PFS outcomes: surgery yielded the longest at 13.5 months (95% CI: 8.7–18.2), followed by sequential chemoradiotherapy with 11.6 months (95% CI: 7.3–15.8), and concurrent chemoradiotherapy at 10.4 months (95% CI: 9.0–11.8). Patients treated solely with radiotherapy had a median PFS of 6.3 months (95% CI: 1.3–11.4), whereas those on systemic therapy alone had the shortest duration of 5.7 months (95% CI: 4.3–7.0). Surgical treatment appeared to offer the greatest benefit in prolonging progression-free survival (Fig. 2C).
The median overall survival (OS) was 20.5 months (95% CI: 18.3–22.7). OS rates at 1, 2, 3, and 4 years were 68.8%, 43.3%, 31.4%, and 23.4%, respectively, with the 5-year OS not reached. OS varied significantly among different disease stages: patients with stage IIIA had a median OS of 32.4 months (95% CI: 23.6–41.2), compared to 18.1 months (95% CI: 15.6–20.6) for stage IIIB and 13.9 months (95% CI: 10.6–17.2) for stage IIIC (p < 0.001). The 5-year OS rate reached 26.5% only in stage IIIA patients (Fig. 2D,E).
The longest median overall survival (42.8 months; 95% CI: 22.7–62.9) was observed in patients who underwent surgery as their initial treatment, while those receiving only palliative care showed the shortest median OS of 8.8 months (95% CI: 2.9–14.8). Among other treatment modalities, sequential chemoradiotherapy was associated with a median OS of 28.3 months (95% CI: 13.6–43.0), closely followed by concurrent chemoradiotherapy at 26.5 months (95% CI: 21.7–31.4). Radiotherapy alone had a median OS of 17.1 months (95% CI: 12.7–21.5), and systemic therapy alone had the lowest median OS of 13.7 months (95% CI: 12.0–15.5). These results indicate that surgical intervention offers a marked survival benefit compared to other treatments (Fig. 2F).
3.12 The Relationship between the OS and Various Factors
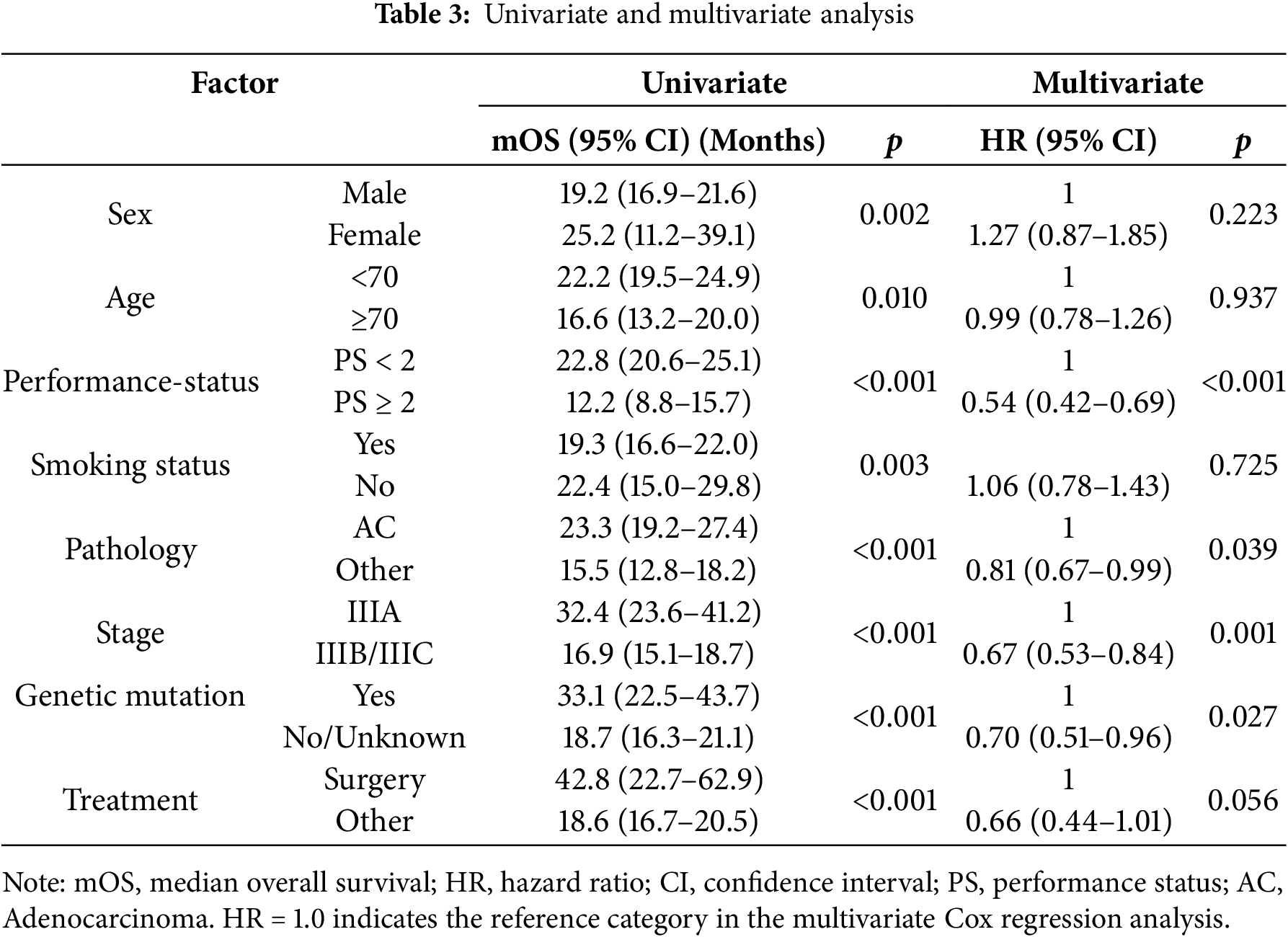
Univariate analysis showed that a higher median overall survival (mOS) was significantly associated with female sex, age under 70 years, ECOG performance status <2, non-smoking status, adenocarcinoma histology, stage IIIA disease, presence of genetic mutations, and initial treatment with surgery, with all differences being statistically significant (p < 0.05). In the multivariate Cox regression model, four independent prognostic factors associated with a reduced risk of death were identified: good performance status (PS < 2), adenocarcinoma histology, stage IIIA disease, and presence of genetic mutations, all with statistical significance (p < 0.05). In contrast, sex, age, smoking status, and surgical treatment were not significantly associated with overall survival (Table 3). To further validate the prognostic role of driver mutations, a sensitivity analysis was performed after excluding patients with unknown mutation status. This analysis confirmed that mutation-positive patients had a significantly better OS than mutation-negative patients, supporting the robustness of mutation status as an independent prognostic factor (Table S2; Fig. S1).
This real-world study analyzed treatment patterns and survival in 731 Vietnamese stage III NSCLC patients over six years. Our findings provide insights into the heterogeneity of this disease and reflect both local practices and global treatment trends.
The median age of 64 years (range: 26–88) observed in our study is lower than the global median age for lung cancer, which is approximately 71 years [25]. The majority of patients were older than 60 years (69.7%), predominantly male (84%), and had a history of smoking (74.1%). Adenocarcinoma was the most common histological subtype (60.7%), and the rate of genetic mutations in stage III patients was 39.8%. Compared to the KINDLE Asia study, the median age was similar at 63 years, while the proportions of male patients (74.8%), smoking history (72%), and adenocarcinoma subtype (55.7%) were slightly lower [26]. Recent studies from China further support the notion that NSCLC populations in Asia exhibit distinct demographic and molecular characteristics compared with Western counterparts [27].
The diversity in initial treatment approaches highlights the challenges posed by the heterogeneity of stage III NSCLC and the complexity of optimizing treatment decisions for individual patients. Similar patterns have been described in other Asian real-world cohorts. For example, the MOOREA study in China reported comparable diversity, with cCRT and systemic therapy as the most frequently adopted strategies [28]. In our study, the most common initial treatment was chemoradiotherapy (40.8%), primarily concurrent chemoradiotherapy (38.6%), applied across stages IIIA, IIIB, and IIIC with rates of 35.8%, 43.8%, and 30.7%, respectively. This was followed by systemic therapy at 37.3% (24.7% in stage IIIA, 36.7% in stage IIIB, and 52.3% in stage IIIC). These results are consistent with findings from the KINDLE Asia study [26] and are in line with general treatment guidelines [29]. Surgery was mainly employed for stage IIIA disease, with a rate of 26.3%, which is lower compared to the KINDLE Asia (37.5%) and KINDLE global (32%) studies. This difference may be attributed to the lower proportion of stage IIIA patients in our study compared to those studies [26,30].
There was also a notable shift in treatment trends for stage III NSCLC patients at our hospital over the study period. Specifically, the use of systemic therapy has decreased, while definitive, locoregional treatment modalities have become more prevalent. This trend mirrors those reported by other cancer centers and aligns with the global efforts to improve lung cancer control. According to an analysis conducted in the United Kingdom and Canada, the use of definitive treatments, particularly concurrent chemoradiotherapy, increased significantly between 2000 and 2007 among patients with stage III lung cancer [31]. Similarly, an analysis of the Surveillance, Epidemiology, and End Results (SEER) Program database showed a marked decrease in the proportion of patients diagnosed with distant metastatic disease between 2000 and 2022 [32]. International experience similarly shows that consolidation immunotherapy has transformed outcomes. Real-world studies have demonstrated that durvalumab following cCRT significantly improves survival in patients with unresectable stage III disease [11,33], thereby consolidating the PACIFIC trial findings and establishing immunotherapy consolidation as a global standard of care [34]. These trends likely reflect improvements in the TNM classification system, which has become more detailed and precise over time, as well as expanded access to advanced surgical and radiotherapy techniques, enabling more patients to benefit from curative-intent therapies. At our institution, diagnostic imaging capabilities have been gradually enhanced with the implementation of CT, SPECT/CT, MRI, and bone scintigraphy. However, the lack of PET-CT imaging and EBUS remains a limitation, potentially affecting the accuracy of staging and subsequently influencing the selection of optimal treatment strategies [35–37].
Previous reports, including the KINDLE Global and KINDLE Vietnam cohorts, demonstrated superior mPFS in stage IIIA compared with IIIB disease. Our findings align with this trend, with stage IIIA patients showing the most favorable survival outcomes (12.6 months), followed by IIIB (8.6 months) and IIIC (6.3 months) [30,38]. Regarding initial treatment modalities, patients undergoing surgery achieved a significantly longer mPFS of 13.5 months compared to non-surgical patients (p = 0.007). In our cohort, 26.3% of patients received curative surgery, primarily in stage IIIA. Concurrent chemoradiotherapy (cCRT) was the most commonly used initial therapy for stage III NSCLC at our institution, with administration rates of 35.8%, 43.8%, and 30.7% in stages IIIA, IIIB, and IIIC, respectively, and a steady increase from 2018 to 2024. Patients who underwent surgery had a median PFS of 13.5 months (95% CI: 8.7–18.2), notably longer than those receiving cCRT (10.4 months; 95% CI: 9.0–11.8) or sequential chemoradiotherapy (11.6 months; 95% CI: 7.3–15.8). These findings are consistent with results reported in the KINDLE Taiwan and KINDLE Vietnam studies [38,39].
The median overall survival (mOS) in our study was 20.5 months (95% CI: 18.3–22.7), which is lower than reported in KINDLE Asia (42.3 months; 95% CI: 38.1–46.8) and KINDLE Global (34.9 months; 95% CI: 32.0–38.0) [26,30]. This discrepancy likely reflects limited access to immunotherapy and advanced surgical or radiotherapy techniques in our setting. Recent perioperative immunotherapy trials, such as KEYNOTE-671 and CheckMate 816, have demonstrated substantial improvements in pathological response and survival [16,17]. Early-phase studies of atezolizumab also support the feasibility of IO-based neoadjuvant strategies [15,40]. Incorporating these advances into Vietnamese practice could help narrow the survival gap with international cohorts. In our cohort stage IIIA patients, the 5-year OS rate was 26.5%, whereas it remained unreached for stages IIIB and IIIC, possibly due to the relatively short median follow-up time. Significant differences in median OS were observed across stages: 32.4 months (95% CI: 23.6–41.2) for IIIA, 18.1 months (95% CI: 15.6–20.6) for IIIB, and 13.9 months (95% CI: 10.6–17.2) for IIIC (p < 0.001). Among a subgroup of 50 stage IIIA patients undergoing curative surgery, the longest median OS was observed at 42.8 months (95% CI: 22.7– 62.9), though this was lower than reported in a Korean cohort (52.5 months; 95% CI: 43.1–61.9) and KINDLE Asia (51.4 months; 95% CI: 43.8–64.1) [26,30]. The difference may be attributed to the smaller proportion of stage IIIA patients in our cohort (26%) compared to KINDLE Asia (54.7%).
For non-surgical patients receiving definitive chemoradiotherapy, median OS was 28.3 months (95% CI: 13.6–43.0) for sequential chemoradiotherapy, slightly higher than 26.5 months (95% CI: 21.7–31.4) for concurrent chemoradiotherapy, despite not being statistically significant (p = 0.747). This contrasts with the KINDLE Global study, which reported superior survival with concurrent chemoradiotherapy [30].
Among non-surgical candidates, survival outcomes were significantly better for patients treated with concurrent or sequential chemoradiotherapy compared to radiotherapy alone (median OS: 17.1 months; 95% CI: 12.7–21.5), systemic therapy (13.7 months; 95% CI: 12.0–15.5), or palliative care (8.8 months; 95% CI: 2.9–14.8) (p < 0.001).
Finally, when comparing treatment modalities, surgical patients had a significantly longer median OS of 42.8 months (95% CI: 22.7–62.9) vs. 18.6 months (95% CI: 16.7–20.5) for non-surgical patients (p < 0.001), consistent with a U.S. real-world study of 478 unresected stage III NSCLC patients reporting a median OS of 20 months [41]. Comparative real-world data reinforce this observation, showing that surgery confers a survival benefit in selected stage III patients [42]. Furthermore, emerging evidence suggests that neoadjuvant chemo-immunotherapy can downstage tumors and enable conversion surgery, offering promising outcomes for initially unresectable patients [43]. Retrospective analyses also suggest that chemo-immunotherapy followed by surgery may provide superior disease control compared with definitive CRT [44], supporting a paradigm shift toward multimodality regimens in resectable stage III NSCLC.
Statistical analyses confirmed independent prognostic factors linked with better survival outcomes, including ECOG performance status <2, adenocarcinoma histology, stage IIIA disease, and the presence of driver mutations. Patients with good PS <2 experienced a 46% reduction in mortality risk, aligning with findings from previous studies [6,31,39]. Adenocarcinoma histology was associated with a 19% survival advantage compared to other subtypes [6,31], potentially attributable to the higher prevalence of targetable mutations. Similarly, the presence of driver mutations conferred a 30% lower risk of death [26], underscoring the importance of molecular profiling even in stage III disease. Although female sex and younger age (<70 years) were associated with longer OS in univariate analysis, these factors were not retained in the multivariate models. Findings are consistent with KINDLE studies, which identified stage IIIA disease, good performance status, adenocarcinoma histology, and definitive treatment as favorable prognostic factors [26,30,39].
In multivariate analysis, ECOG PS < 2, stage IIIA disease, adenocarcinoma histology, and the presence of genetic mutations were identified as independent predictors of reduced mort. Surgery was associated with better OS but did not reach statistical significance (p = 0.056). No significant impact was observed for variables such as sex, age, and smoking history. This is consistent with the KINDLE studies, where stage IIIA, favorable performance status, adenocarcinoma subtype, and definitive therapies were identified as predictors of better outcomes [26,30,39].
There are some important limitations to acknowledge. The retrospective, single-center approach may result in selection bias and reduce the extent to which these findings can be generalized to other regions in Vietnam or to healthcare settings in different countries. Second, although clinical and treatment data were collected comprehensively, genetic testing was performed in only a subset of patients, leading to potential misclassification when grouping mutation-negative and mutation-unknown cases. Third, treatment allocation was not randomized, and decisions were influenced by patient preferences, comorbidities, and local resource availability, which may have confounded survival outcomes. Fourth, information on certain prognostic variables, such as PD-L1 expression or detailed toxicity profiles, was unavailable, preventing further subgroup analyses. Finally, the relatively short median follow-up time for some patients may have led to an underestimation of long-term survival outcomes.
Despite these limitations, our findings provide valuable real-world evidence on treatment patterns and outcomes of stage III NSCLC in a Vietnamese setting. The results highlight the heterogeneity of treatment approaches, the underutilization of concurrent chemoradiotherapy, and the survival benefits of multimodal strategies, consistent with international studies. The relatively high prevalence of actionable mutations underscores the need to expand routine molecular testing to guide targeted therapy. The results can provide evidence to guide national health policies, optimize resource distribution, and support clinical decision-making, particularly in settings with constrained healthcare infrastructure. To validate these results and enhance treatment approaches for stage III NSCLC in Vietnam, future work should include multicenter prospective studies with extended follow-up and broad genetic testing to validate these findings.
Our research presents a detailed analysis of first-line treatment patterns for stage III NSCLC within Vietnam, representing real-world medical practices. Six distinct treatment modalities were identified, withconcurrent chemoradiotherapy remaining the most frequently applied approach. Patients who underwent surgery experienced better progression-free and overall survival compared to those receiving non-surgical therapies. Better outcomes were observed in patients with adenocarcinoma subtype, actionable genetic alterations, earlier stage IIIA disease, and preserved performance status (ECOG < 2).
Acknowledgement: The authors thank the Department of Medical Oncology 2, Nghe An Oncology Hospital, for their support in data collection.
Funding Statement: The authors received no specific funding for this study.
Author Contributions: Study conception and design: Khanh Toan Nguyen; Data collection: Khanh Toan Nguyen, Thi Huong Pham, Van Lam Ngo, Thi Thuy My Nguyen, Thi Dao Nguyen, Khanh Hung Truong, Van Nhat Nguyen, Van Thanh Le, Ba Duc Ho, Thi Phuong Thao Nguyen, Thi Ha Phuong Nguyen, Thi My Linh Dinh, Thi Hong Anh Vo, Thi Thuy Phan, Thi Hai Yen Le, Thi Nhung Ngo, Khanh Ha Nguyen; Analysis and interpretation of results: Khanh Toan Nguyen, Thi Thuy My Nguyen; Draft manuscript preparation: Khanh Toan Nguyen, Thi Thuy My Nguyen. All authors reviewed the results and approved the final version of the manuscript.
Availability of Data and Materials: Upon reasonable request, the corresponding author will provide access to the data used in this study.
Ethics Approval: In line with the Declaration of Helsinki, the research protocol was examined and approved by the Ethics Committee of Nghe An Oncology Hospital (Decision No. 344/QD-BVUB, dated 28 February 2025). As this was a retrospective study using anonymized medical records, informed consent was waived for all participants, including those who were deceased, in accordance with institutional ethical guidelines. All personal data was kept strictly confidential, used solely for research purposes, and no individual identifiers were disclosed.
Conflicts of Interest: The authors declare no conflicts of interest to report regarding the present study.
Supplementary Materials: The supplementary material is available online at https://www.techscience.com/doi/10.32604/or.2025.069281/s1.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. doi:10.3322/caac.21660. [Google Scholar] [PubMed] [CrossRef]
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi:10.3322/caac.21660. [Google Scholar] [PubMed] [CrossRef]
3. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–54. doi:10.1038/nature25183. [Google Scholar] [PubMed] [CrossRef]
4. Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022;17(3):362–87. doi:10.1016/j.jtho.2021.11.003. [Google Scholar] [PubMed] [CrossRef]
5. Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824–32. doi:10.1001/jamaoncol.2021.4932. [Google Scholar] [PubMed] [CrossRef]
6. Casal-Mourino A, Ruano-Ravina A, Lorenzo-Gonzalez M, Rodriguez-Martinez A, Giraldo-Osorio A, Varela-Lema L, et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res. 2021;10(1):506–18. doi:10.21037/tlcr.2020.03.40. [Google Scholar] [PubMed] [CrossRef]
7. Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. Epidemiology of lung cancer. Contemp Oncol. 2021;25(1):45–52. doi:10.5114/wo.2021.103829. [Google Scholar] [PubMed] [CrossRef]
8. Asmara OD, Hardavella G, Ramella S, Petersen RH, Tietzova I, Boerma EC, et al. Stage III NSCLC treatment options: too many choices. Breathe. 2024;20(3):240047. doi:10.1183/20734735.0047-2024. [Google Scholar] [PubMed] [CrossRef]
9. Verma S, Breadner D, Mittal A, Palma DA, Nayak R, Raphael J, et al. An updated review of management of resectable stage III NSCLC in the era of neoadjuvant immunotherapy. Cancers. 2024;16(7):1302. doi:10.3390/cancers16071302. [Google Scholar] [PubMed] [CrossRef]
10. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–29. [Google Scholar] [PubMed]
11. Filippi AR, Bar J, Chouaid C, Christoph DC, Field JK, Fietkau R, et al. Real-world outcomes with durvalumab after chemoradiotherapy in patients with unresectable stage III NSCLC: interim analysis of overall survival from PACIFIC-R. ESMO Open. 2024;9(6):103464. doi:10.1016/j.esmoop.2024.103464. [Google Scholar] [PubMed] [CrossRef]
12. Zhang Y, Tian Y, Zheng L, Sun X, Zhao Z, Zheng Y, et al. Efficacy and safety of consolidation durvalumab after chemoradiation therapy for stage III non-small-cell lung cancer: a systematic review, meta-analysis, and meta-regression of real-world studies. Front Pharmacol. 2023;14:1103927. doi:10.3389/fphar.2023.1103927. [Google Scholar] [PubMed] [CrossRef]
13. Lu S, Kato T, Dong X, Ahn MJ, Quang LV, Soparattanapaisarn N, et al. Osimertinib after chemoradiotherapy in stage III EGFR-mutated NSCLC. N Engl J Med. 2024;391(7):585–97. doi:10.1056/nejmoa2402614. [Google Scholar] [PubMed] [CrossRef]
14. Riely GJ, Wood DE, Ettinger DS, Aisner DL, Akerley W, Bauman JR, et al. Non-small cell lung cancer, version 4, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2024;22(4):249–74. doi:10.6004/jnccn.2004.0010. [Google Scholar] [PubMed] [CrossRef]
15. Chaft JE, Oezkan F, Kris MG, Bunn PA, Wistuba II, Kwiatkowski DJ, et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm phase II trial. Nat Med. 2022;28(10):2155–61. doi:10.1038/s41591-022-01962-5. [Google Scholar] [PubMed] [CrossRef]
16. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–85. doi:10.1056/nejmoa2202170. [Google Scholar] [PubMed] [CrossRef]
17. Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, et al. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024;404(10459):1240–52. [Google Scholar] [PubMed]
18. Dang AH, Tran VU, Tran TT, Thi Pham HA, Le DT, Nguyen L, et al. Actionable mutation profiles of non-small cell lung cancer patients from vietnamese population. Sci Rep. 2020;10(1):2707. doi:10.1038/s41598-020-59744-3. [Google Scholar] [PubMed] [CrossRef]
19. Pham CP, Nguyen TTH, Do AT, Nguyen TK, Hoang TAT, Le TA, et al. A real-world cohort study of first-line afatinib in patients with EGFR-mutant advanced non-small cell lung cancer in vietnam. BMC Cancer. 2024;24(1):176. doi:10.1186/s12885-024-11891-w. [Google Scholar] [PubMed] [CrossRef]
20. Van Luan P, Tien ND, Hai NM, Tien ND, Duyen TT. Real-world analysis of the effect of gefitinib as a first-line therapy in patients with advanced non-small cell lung cancer with EGFR mutations. Ther Adv Med Oncol. 2021;13:1758835921992977. doi:10.1177/1758835921992977. [Google Scholar] [PubMed] [CrossRef]
21. Daly ME, Singh N, Ismaila N, Antonoff MB, Arenberg DA, Bradley J, et al. Management of stage III non-small-cell lung cancer: ASCO guideline. J Clin Oncol. 2022;40(12):1356–84. doi:10.1200/jco.21.02528. [Google Scholar] [PubMed] [CrossRef]
22. Eberhardt WE, De Ruysscher D, Weder W, Le Pechoux C, De Leyn P, Hoffmann H, et al. 2nd ESMO consensus conference in lung cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol. 2015;26(8):1573–88. doi:10.1093/annonc/mdv187. [Google Scholar] [PubMed] [CrossRef]
23. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243–60. doi:10.1097/jto.0000000000000630. [Google Scholar] [PubMed] [CrossRef]
24. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. doi:10.3322/caac.21388. [Google Scholar] [PubMed] [CrossRef]
25. National Cancer Institute. Age as a risk factor for cancer. 2024. [cited 2025 Sep 4]. Available from: https://www.cancer.gov/about-cancer/causes-prevention/risk/age. [Google Scholar]
26. Prabhash K, Tan DSW, Soo RA, Sitthideatphaiboon P, Chen YM, Voon PJ, et al. Real-world clinical practice and outcomes in treating stage III non-small cell lung cancer: KINDLE-Asia subset. Front Oncol. 2023;13:1117348. doi:10.3389/fonc.2023.1117348. [Google Scholar] [PubMed] [CrossRef]
27. Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun. 2022;42(10):937–70. [Google Scholar]
28. Li J, Zhang H, Zhou Y, Chen X, Xu C, Wang J, et al. 111P Real-world treatment patterns and clinical outcomes in Chinese stage III non-small cell lung cancer (NSCLC) patients: results of MOOREA study. ESMO Immuno-Oncol Congr. 2024;24:100854. doi:10.1016/j.iotech.2024.100854. [Google Scholar] [CrossRef]
29. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLCESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv1–21. doi:10.1093/annonc/mdx222. [Google Scholar] [PubMed] [CrossRef]
30. Jazieh AR, Onal HC, Tan DSW, Soo RA, Prabhash K, Kumar A, et al. Real-world treatment patterns and clinical outcomes in patients with stage III NSCLC: results of KINDLE, a multicountry observational study. J Thorac Oncol. 2021;16(10):1733–44. doi:10.1016/j.jtho.2021.05.003. [Google Scholar] [PubMed] [CrossRef]
31. Vinod SK, Wai E, Alexander C, Tyldesley S, Murray N. Stage III non-small-cell lung cancer: population-based patterns of treatment in British Columbia, Canada. J Thorac Oncol. 2012;7(7):1155–63. doi:10.1097/jto.0b013e31824fea07. [Google Scholar] [PubMed] [CrossRef]
32. Surveillance Research Program National Cancer Institute. SEER*Explorer: an interactive website for SEER Cancer Statistics. 2025 [cited 2025 Sep 4]. Available from: https://seer.cancer.gov/statistics-network/explorer/application.html?site=1&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&hdn_stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion0. [Google Scholar]
33. Huang Y, Zhao JJ, Soon YY, Wong A, Aminkeng F, Ang Y, et al. Real-world experience of consolidation durvalumab after concurrent chemoradiotherapy in stage III non-small cell lung cancer. Thorac Cancer. 2022;13(22):3152–61. doi:10.1111/1759-7714.14667. [Google Scholar] [PubMed] [CrossRef]
34. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–11. doi:10.1200/jco.21.01308. [Google Scholar] [PubMed] [CrossRef]
35. Farsad M. FDG PET/CT in the staging of lung cancer. Curr Radiopharm. 2020;13(3):195–203. doi:10.2174/1874471013666191223153755. [Google Scholar] [PubMed] [CrossRef]
36. Inage T, Nakajima T, Yoshino I. Staging lung cancer: role of endobronchial ultrasound. Lung Cancer. 2014;5:67–72. doi:10.2147/lctt.s46195. [Google Scholar] [PubMed] [CrossRef]
37. Navani N, Nankivell M, Lawrence DR, Lock S, Makker H, Baldwin DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med. 2015;3(4):282–9. doi:10.1016/s2213-2600(15)00029-6. [Google Scholar] [PubMed] [CrossRef]
38. Van Dao T, Diep TB, Le Phuong T, Huggenberger R, Kumar A. Real-world treatment patterns and clinical outcomes in patients with stage III non-small-cell lung cancer: results of KINDLE-vietnam cohort. Front Oncol. 2022;12:842296. doi:10.3389/fonc.2022.842296. [Google Scholar] [PubMed] [CrossRef]
39. Su PL, Chang GC, Hsiao SH, Hsia TC, Lin MC, Lin MH, et al. An observational study on treatment outcomes in patients with stage III NSCLC in Taiwan: the KINDLE study. JTO Clin Res Rep. 2022;3(3):100292. doi:10.1016/j.jtocrr.2022.100292. [Google Scholar] [PubMed] [CrossRef]
40. Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):786–95. doi:10.1016/s1470-2045(20)30140-6. [Google Scholar] [PubMed] [CrossRef]
41. Ryan KJ, Skinner KE, Fernandes AW, Punekar RS, Pavilack M, Walker MS, et al. Real-world outcomes in patients with unresected stage III non-small cell lung cancer. Med Oncol. 2019;36(3):24. doi:10.1007/s12032-019-1249-1. [Google Scholar] [PubMed] [CrossRef]
42. Shigenobu T, Taniguchi Y, Suzuki T, Tabuchi Y, Sato M, Odagiri K, et al. Surgery versus concurrent chemoradiotherapy for stage III non-small cell lung cancer: a retrospective study with propensity score matching. BMC Cancer. 2025;25(1):121. doi:10.1186/s12885-025-13550-0. [Google Scholar] [PubMed] [CrossRef]
43. Qi Y, Sun Y, Hu Y, Zhu H, Guo H. Clinical outcomes of conversion surgery following neoadjuvant chemoimmunotherapy in potentially resectable stage IIIA/IIIB non-small cell lung. Sci Rep. 2025;15(1):18422. doi:10.1038/s41598-025-99571-y. [Google Scholar] [PubMed] [CrossRef]
44. Zhao J, Miao D, Zhou J, Guo S, Tang Y, Lan F, et al. A retrospective comparison of induction chemoimmunotherapy versus chemotherapy followed by concurrent chemoradiotherapy and consolidation immunotherapy in stage III non-small cell lung cancer. Front Oncol. 2024;14:1432954. doi:10.3389/fonc.2024.1432954. [Google Scholar] [PubMed] [CrossRef]
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools