 Open Access
Open Access
ARTICLE
CXCR1 and CXCR2 Antagonism with G31P Attenuates Chemotherapy-Induced Lung Inflammation and Augments the Gefitinib Therapeutic Response in Lung Cancer
1 Henan University People’s Hospital, Henan Provincial People’s Hospital, Zhengzhou, 450003, China
2 College of Basic Medical Sciences, Dalian Medical University, Dalian, 116011, China
3 Unani Medicine Research Laboratory, Faculty of Eastern Medicine, Hamdard University, Karachi, 74600, Pakistan
4 Division of Respirology, Critical Care and Sleep Medicine, Royal University Hospital, University of Saskatchewan, Saskatoon, SK S7N 5E5, Canada
* Corresponding Authors: Muhammad Noman Khan. Email: ; Song-Ze Ding. Email:
(This article belongs to the Special Issue: The Evolving Landscape of Cancer Treatment: Molecular Insights and Immunotherapeutic Breakthroughs)
Oncology Research 2025, 33(12), 3837-3854. https://doi.org/10.32604/or.2025.069408
Received 22 June 2025; Accepted 30 September 2025; Issue published 27 November 2025
Abstract
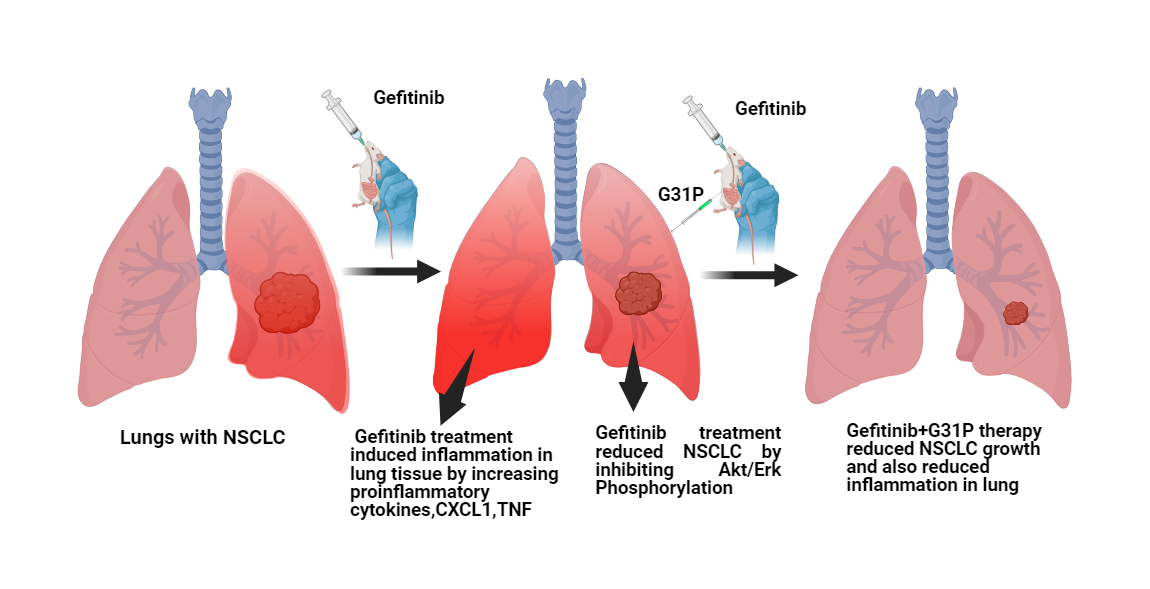
Objectives: Chemotherapy-induced lung inflammation limits the efficacy of anticancer therapies such as gefitinib in non-small cell lung cancer (NSCLC). Glutamic acid-leucine-arginine positive (ELR+) CXC chemokines and their receptors, CXC chemokine receptor 1 and 2 (CXCR1 and CXCR2), mediate both inflammatory responses and tumor progression. This study evaluated the effects of CXCR1/2 antagonism by G31P, a CXC motif chemokine ligand 8 (CXCL8)-mutated peptide, alone or in combination with gefitinib, on lung cancer growth and chemotherapy-induced pulmonary inflammation. Methods: Human NSCLC cell lines (A549 and H460) were treated with gefitinib and/or G31P. Cell proliferation, apoptosis, and signaling pathways, including protein kinase B (AKT) and extracellular signal-regulated kinase (ERK) phosphorylation, were evaluated by cell counting kit-8 (CCK-8) assay, flow cytometry, and Western blotting. An orthotopic lung tumor xenograft model was established in BALB/c nude mice to evaluate tumor growth, metastasis, cytokine expression, and lung histopathology. A bleomycin-induced lung injury model was also used to assess the anti-inflammatory effects of G31P, with or without gefitinib, by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and flow cytometry of inflammatory markers. Results: G31P and Gefitinib, either alone or combined, inhibited proliferation and migration of A549 and H460 cells in vitro. Combination treatment effectively reduced AKT and ERK phosphorylation in both cell lines. In vivo, G31P with gefitinib significantly suppressed tumor growth, metastasis, and increased apoptosis. G31P decreased CXCL1 and CXCL2, and tumor necrosis factor-alpha (TNF-α) mRNA levels, lung hydroxyproline content, and myeloperoxidase (MPO) activity in the lungs of mice. In the bleomycin-induced lung injury model, G31P similarly reduced inflammatory responses. Conclusion: CXCR1/2 antagonism by G31P attenuates chemotherapy-induced pulmonary inflammation and enhances the anti-tumor efficacy of gefitinib in NSCLC. These findings support the therapeutic potential of G31P as an adjuvant to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) to improve clinical outcomes by limiting inflammation.Graphic Abstract
Keywords
Cite This Article
 Copyright © 2025 The Author(s). Published by Tech Science Press.
Copyright © 2025 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools