 Open Access
Open Access
ARTICLE
ELK4 Promotes Vasculogenic Mimicry in Oral Squamous Cell Carcinoma via Driving DHFR Transcriptional Activation
1 Department of Stomatology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, 050000, China
2 Department of Oral Surgery, The Second Hospital of Hebei Medical University, Shijiazhuang, 050000, China
3 Department of Stomatology, Qinhuangdao Haigang Hospital, Qinhuangdao, 066000, China
* Corresponding Author: Jie Guo. Email:
# These authors contribute equally to this work
(This article belongs to the Special Issue: Advances in Oral Cancer Treatment)
Oncology Research 2026, 34(1), 21 https://doi.org/10.32604/or.2025.069612
Received 26 June 2025; Accepted 31 October 2025; Issue published 30 December 2025
Abstract
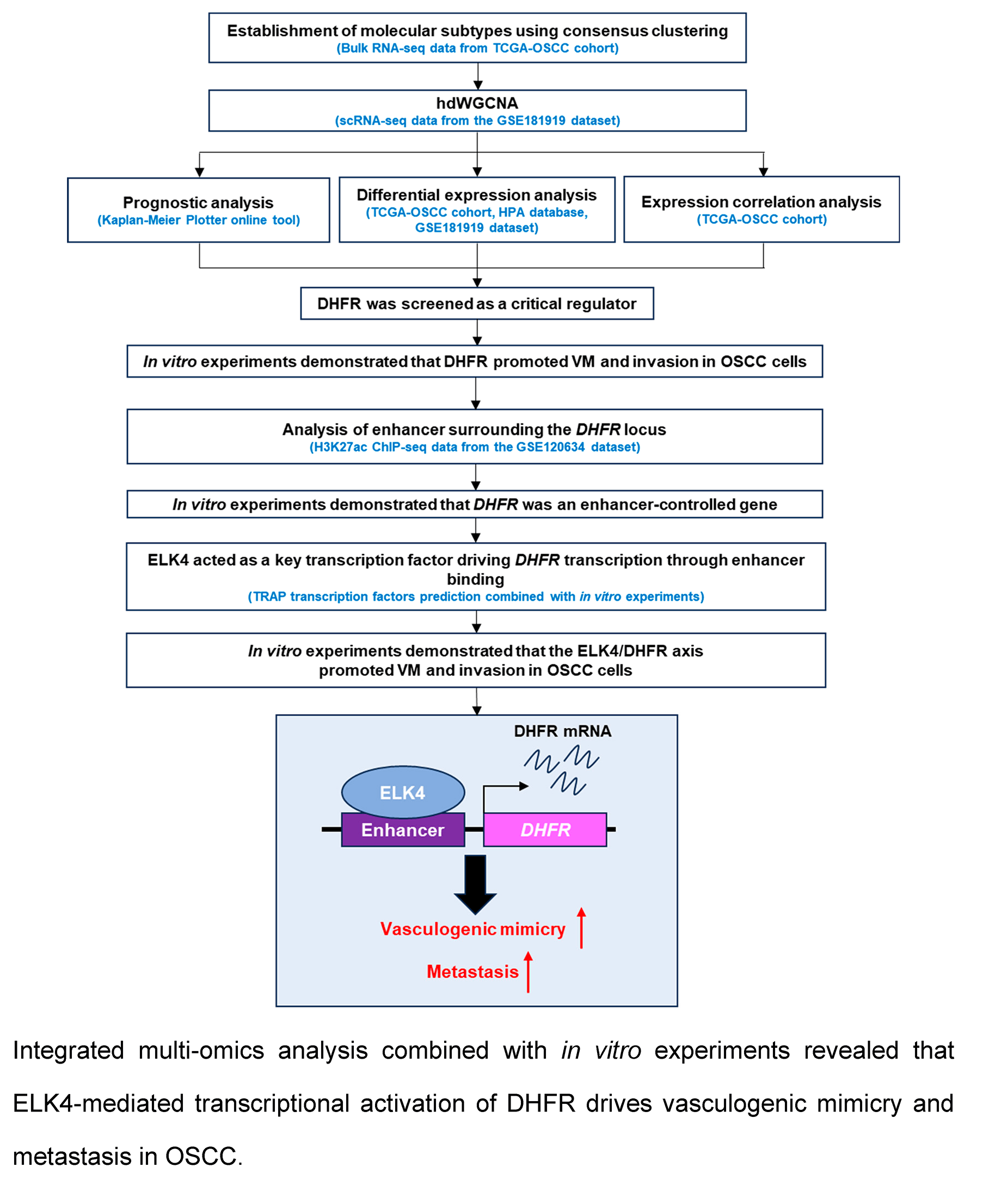
Background: The regulatory mechanisms governing vasculogenic mimicry (VM) in oral squamous cell carcinoma (OSCC) remain largely undefined. This study aimed to identify critical factors and elucidate the epigenetic mechanisms underlying VM in OSCC. Methods: Bioinformatics analysis was performed utilizing single-cell RNA-seq, bulk RNA-seq, and histone H3 lysine 27 acetylation (H3K27ac) Chromatin Immunoprecipitation (ChIP)-seq data obtained from The Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO) databases. ChIP-qPCR was used to validate the binding of ETS transcription factor ELK4 (ELK4) to the dihydrofolate reductase (DHFR) enhancer. In vitro VM formation and invasion of OSCC cells were assessed using Matrigel-based tube formation and Transwell assays, respectively. Results: Elevated expression of VM-related genes predicts unfavorable prognosis in OSCC patients. High-dimensional weighted gene co-expression network analysis (hdWGCNA) identified epithelial subcluster C4 as most strongly associated with VM and metastasis. Three co-expression modules within this subcluster exhibited significant positive correlations with both phenotypic traits. Among the 30 eigengenes from the three modules, DHFR emerged as a key regulator of VM and metastasis. Knockdown or inhibition of DHFR significantly suppressed VM formation and invasion in OSCC cells. Mechanistically, ELK4 activated DHFR transcription through direct binding to its enhancer. DHFR overexpression rescued VM and invasion impairment induced by ELK4 knockdown. Conclusion: DHFR was a pivotal enhancer-regulated gene driving VM and metastasis in OSCC. ELK4 directly binds to DHFR enhancer regions to activate its transcription, thereby promoting these malignant phenotypes. These findings identified the ELK4/DHFR axis as a promising therapeutic target for anti-angiogenic intervention in OSCC.Graphic Abstract
Keywords
Supplementary Material
Supplementary Material FileCite This Article
 Copyright © 2026 The Author(s). Published by Tech Science Press.
Copyright © 2026 The Author(s). Published by Tech Science Press.This work is licensed under a Creative Commons Attribution 4.0 International License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.


 Submit a Paper
Submit a Paper Propose a Special lssue
Propose a Special lssue View Full Text
View Full Text Download PDF
Download PDF Downloads
Downloads
 Citation Tools
Citation Tools